Abstract
Background:
The Movement Disorder Society–sponsored Nonmotor Rating Scale is an update of the existing Parkinson’s disease Nonmotor Symptoms Scale modified to address some limitations in Nonmotor Symptoms Scale scoring, structure, and symptom coverage.
Methods:
PD patients were recruited from movement disorder centers in an international, multicenter study. The Movement Disorder Society Nonmotor Rating Scale, consisting of 13 domains plus a subscale for nonmotor fluctuations, was rater administered, along with the Nonmotor Symptoms Scale and other clinical assessments. Standard reliability and validity testing were conducted.
Results:
Four hundred and two PD patients were recruited (mean age ± standard deviation, 67.42 ± 9.96 years; mean age at PD onset ± standard deviation, 59.27 ± 10.67 years; median Hoehn and Yahr stage 2 (interquartile range 2–3). Data quality was satisfactory for all Movement Disorder Society Nonmotor Rating Scale domains except sexual (6.7% missing data). There were no floor or ceiling effects for the Movement Disorder Society Nonmotor Rating Scale and nonmotor fluctuations total score; domains had no ceiling effects, but some floor effects (13.5%–83.5%). The Movement Disorder Society Nonmotor Rating Scale and nonmotor fluctuations total score internal consistency were acceptable (average Cronbach’s alpha, 0.66 and 0.84, respectively); interrater reliability was excellent (intraclass correlation coefficient, >0.95); for test-retest reliability, the intraclass correlation coefficient was 0.84 for the Movement Disorder Society Nonmotor Rating Scale and 0.70 for Movement Disorder Society nonmotor fluctuations total score, and precision was excellent for the Movement Disorder Society Nonmotor Rating Scale (standard error of measurement, 25.30) and fair for nonmotor fluctuations (standard error of measurement, 7.06). Correlations between Movement Disorder Society Nonmotor Rating Scale score and the corresponding Nonmotor Symptoms Scale and Movement Disorder Society UPDRS scores were high. There were no significant sex or age effects. The Movement Disorder Society Nonmotor Rating Scale score increased with increasing PD duration, disease severity, and PD medication dose (all P < 0.001).
Conclusions:
The Movement Disorder Society Nonmotor Rating Scale is a valid measure for measuring the burden of a wide range of Nonmotor Rating Scale scores, including nonmotor fluctuations, in PD patients.
Keywords: movement disorders, nonmotor fluctuations, nonmotor symptoms, Parkinson’s disease, scales
Nonmotor symptoms (NMSs) collectively have emerged as key features of Parkinson’s disease (PD), evident from the prodromal period to the palliative stage.1 Measurement of individual NMSs in PD and their overall burden has been made possible by the development and validation of instruments such as the Nonmotor Symptoms Scale (NMSS).2 Assessment of NMSs in PD are now key to value-based health care and recommended by patient-led organizations and the International Parkinson and Movement Disorder Society (IPMDS).3 The availability of the NMSS has also allowed NMSs to be assessed in many clinical trials.4–12 The impact of NMS burden on quality of life across all stages of PD is now well established, and the NMSS has been used in PD subtyping studies.13,14
The NMSS was developed approximately 15 years ago. Since then some deficiencies in NMSS scoring and structure have been noted (eg, grouping of items such as depression, anxiety, and apathy in the same domain, as well as sleep disorders and fatigue), there was recognition of limited coverage of crucial cognitive deficits, and there was lack of assessment of more recently described NMSs (eg, nonmotor fluctuations [NMFs] and impulse control disorders).
The issues listed above led to the development and validation of a new, updated rater-administered scale, the IPMDS Nonmotor Rating Scale (MDS-NMS), based on the NMSS and supported by the IPMDS. Data on acceptability and reliability of the preliminary version of the MDS-NMS in a study population of neurologists, PD patients and healthy controls as part of a cognitive pretesting study have been reported.15 Here we report the clinimetric properties of the MDS-NMS from a formal validation study.
Methods
Study Design and Patients
This was an international multicenter cross-sectional study. English-speaking patients with a diagnosis of PD based on MDS criteria16 were included. Exclusion criteria were parkinsonism because of other neurodegenerative diseases or secondary causes, moderate or greater cognitive impairment, defined as Montreal Cognitive Assessment (MoCA)17 score < 21,18 and active medical or psychiatric disorders or treatment that precluded accurate assessments. Patients were recruited from 6 movement disorders units in England (n = 5) and the United States (n = 1) from October 2016 to September 2018. Patients provided their own answers to questions, and no informed others were involved in the validation.
Sample Size
A sample size of 400 allowed for factor analysis and provided a sufficient number of cases for other aspects of instrument validation.19–21
Assessments
In addition to collecting sociodemographic data, the following rating scales were administered:
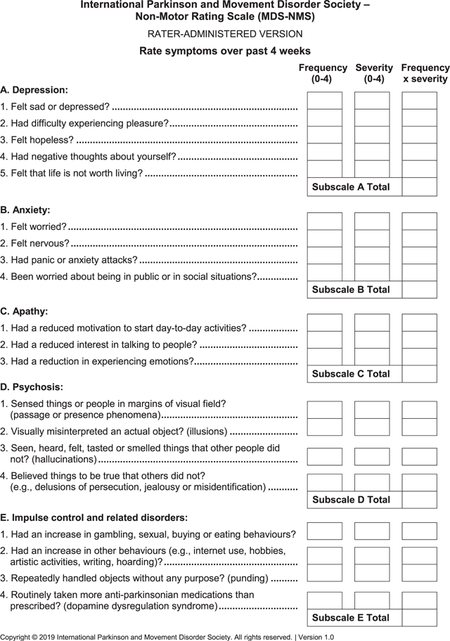
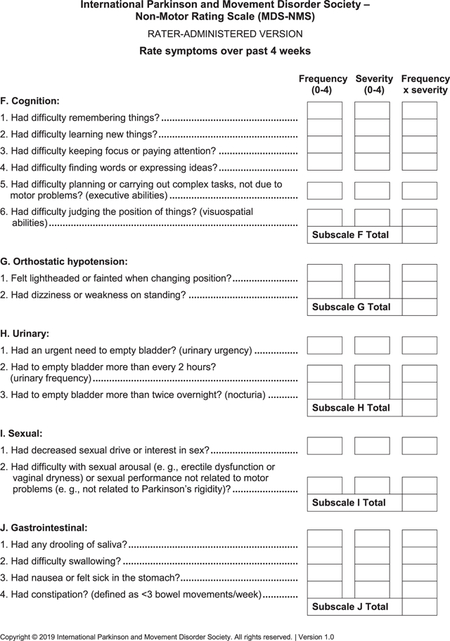
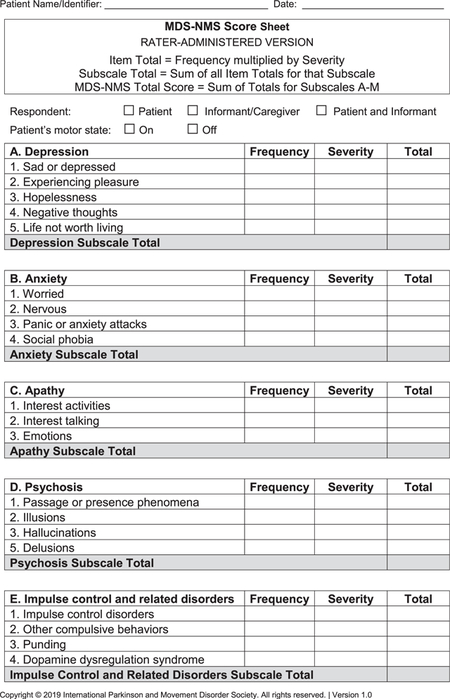
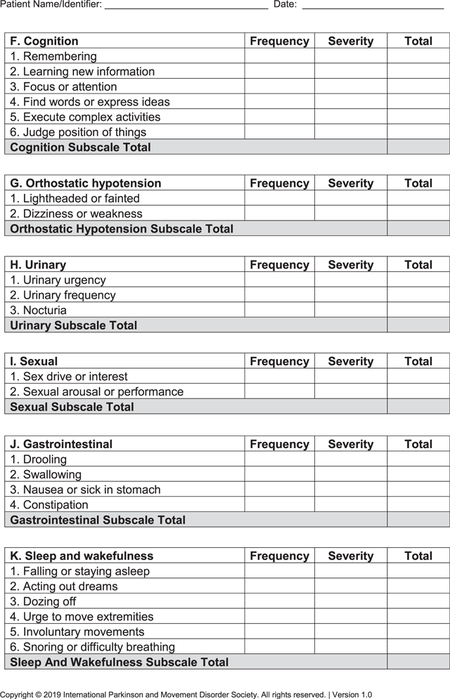
- MDS-NMS (see Appendix): After the pilot study,15 the final version of the MDS-NMS has 52 items, grouped according to clinical content into 13 domains: (1) depression (5 items), (2) anxiety (4 items), (3) apathy (3 items), (4) psychosis (4 items), (5) impulse control and related disorders (4 items), (5) cognition (6 items), (7) orthostatic hypotension (2 items), (8) urinary (3 items), (9) sexual (2 items), (10) gastrointestinal (4 items), (11) sSleep and wakefulness (6 items), (12) pain (4 items), and (13) other (5 items; unintentional weight loss, decreased smell, physical fatigue, mental fatigue, and excessive sweating). Items are scored for frequency (from 0 [never] to 4 [majority of time]) and severity (from 0 [not present] to 4 [severe]), which are multiplied to generate the item total score. Scores for each domain and the total rating scale (maximum, 832 points) are calculated by summing the corresponding items.The NMF subscale has 8 items: depression, anxiety, thinking or cognitive abilities, bladder symptoms, restlessness, pain, fatigue, and excessive sweating. Each item is scored for typical degree of change from “on” to “off” periods, from 0 (no change) to 4 (large). The sum of degree of change for the 8 items is multiplied by the amount of time spent in the “off” state with NMSs, which ranges from 1 (rarely) to 4 (majority of time).22 Maximum possible score is 128.
NMSS: Composed of 30 items, grouped into 9 domains (cardiovascular, sleep/fatigue, mood/apathy, perceptual problems/hallucinations, attention/memory, gastrointestinal tract, urinary, sexual function, and miscellaneous). Item scores for severity (from 0 to 3) are multiplied by scores for frequency (from 1 to 4), reaching a maximum item score of 12 (range, 0–12). Total score for domains and the full scale are obtained by sum of the corresponding items (0–360 points for total score).23
MoCA: For global cognition.17
MDS-Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): The MDS-UPDRS includes 4 parts: part I, Nonmotor Experiences of Daily Living (nM-EDL); part II, motor experiences of daily living (M-EDL); part III, motor examination (ME); and part IV, motor complications (MCompl). In addition, it contains the Hoehn and Yahr scale (HY), a tool for grading severity of PD.24
Clinical Impression of Severity Index for PD (CISI-PD): A rater-based global severity assessment specific for PD, with 4 items (motor signs, disability, cognition, and complications), each rated from 0 (normal) to 6 (very severe/severely disabled).25
Patient Global Impression of Severity (PGIS): A self-assessed severity rating scored from 0 (no symptoms) to 5 (very severe).26,27
Information on current PD treatment was obtained. Levodopa-equivalent daily dose (LEDD) was calculated.28
Procedures
The study was approved by the institutional review boards of the participant centers, and the study conducted according to Good Clinical Practice.29 Patients meeting inclusion criteria signed informed consent before study participation. Assessments were performed during the “on” state when possible. All patients completed the MDS-NMS at baseline, a subset of 123 patients completed retest evaluation within 7–14 days of baseline, and a subset of 164 patients underwent interrater evaluation. Patient assessments between US and UK clinics were harmonized.
Data Analysis
Data were stored and analyzed centrally at the National Center of Epidemiology (Madrid, Spain). Descriptive statistics were used to describe sample characteristics and assessment scores. The primary variables in the study had nonnormal distribution (Shapiro-Francia test, all <0.001). After checking for missing data (acceptable, <5%),30 the following clinimetric properties were assessed:
Acceptability: Floor and ceiling effect (satisfactory threshold, ≤15%)31; skewness (criterion values, from −1 to +1)32; and range of observed versus theoretical values.
Internal consistency: For each domain: (1) interitem correlation (standard values, 0.20–0.75)33; (2) item homogeneity coefficient (standard, 0.15 for broad domains)34; (3) corrected item-total correlation (standard, ≥0.20)30; and (4) Cronbach’s alpha (standard, ≥0.70).30
Reproducibility: Test-retest (baseline and 7–14 days later) and interrater (2 raters) reliability were analyzed with percentage of agreement and weighted kappa (kappaw) with quadratic weights for items and intraclass correlation coefficient (ICC, 1-, and 2-way, random effect) for domains and total scores. Kappa values > 0.60 (substantial agreement)35 and ICC ≥ 0.7030 were deemed satisfactory.
Precision: Estimated by standard error of measurement (SEM) based on agreement in test-retest, according to the formula , where and rxx are the ICC of the test-retest. SEM values lower than half SDpooled were considered acceptable (ICC ≥ 0.75).
Hypotheses testing: For convergent validity, we hypothesized that MDS-NMS domains would be highly associated (Spearman rank correlation coefficient value, rS > 0.50)36 with corresponding components of the MDS-UPDRS part 1 and NMSS and moderate or weak correlation (rS = 0.20–0.50) with other PD severity measures. The known-groups validity of the MDS-NMS and NMF was tested by determining the difference in total scores for subgroups based on sex, age, HY, PGIS, PD duration, and LEDD (the latter 2 groups stratified by tertiles).
Results
Cohort Characteristics
The sample included 402 PD patients, 234 (58.2%) from England and 168 (41.8%) from the United States. Of these 62.2% were men and had mean age ± SD of 67.42 ± 9.96 years and an average age at PD onset ± SD of 59.3 ± 10.7 years. Median HY stage was 2 (interquartile range 2–3; range, 1–4). Sociodemographic and clinical characteristics are detailed in Table 1.
TABLE 1.
Descriptive statistics of the sample
| n | % | |
|---|---|---|
| Sex | ||
| Men | 250 | 62.2 |
| Women | 152 | 37.8 |
| Civil status | ||
| Singled | 52 | 12.94 |
| Married | 297 | 73.88 |
| Widowed | 29 | 7.21 |
| Divorced | 24 | 5.97 |
| Activity: | ||
| Employee | 91 | 22.7 |
| Retired/pensioner | 295 | 73.6 |
| Housewife | 2 | 0.5 |
| Other | 13 | 3.2 |
| Mean (SD) | Range | |
| Age | 67.42 (9.96) | 35–93 |
| Age at onset | 59.27 (10.67) | 26–93 |
| Disease duration | 8.20 (5.93) | 0–35 |
| LEDD | 735.29 (554.46) | 0–3180 |
| Education (years) | 15.11 (3.80) | 4–30 |
| MoCA total score | 26.74 (2.48) | 21–30 |
| NMSS | ||
| Domain 1. Cardiovascular | 1.25 (2.33) | 0–18 |
| Domain 2. Sleep/fatigue | 7.85 (8.36) | 0–48 |
| Domain 3. Mood/apathy | 5.89 (9.39) | 0–51 |
| Domain 4. Perceptual problems/hallucinations | 1.04 (2.75) | 0–21 |
| Domain 5. Attention/memory | 4.29 (5.87) | 0–30 |
| Domain 6. Gastrointestinal | 3.78 (5.22) | 0–30 |
| Domain 7. Urinary | 6.45 (7.86) | 0–36 |
| Domain 8. Sexual | 3.30 (6.16) | 0–24 |
| Domain 9. Miscellaneous | 7.39 (7.53) | 0–36 |
| Total score | 41.39 (35.20) | 0–197 |
| MDS-UPDRS | ||
| Part I | 10.58 (6.72) | 0–35 |
| Part II | 12.12 (8.16) | 0–38 |
| Part III | 29.45 (13.73) | 3–76 |
| Part IV | 3.15 (3.73) | 0–19 |
| Patient Global Impression of Severity | ||
| Normal | 5 | 1.3 |
| Minimal/mild | 256 | 65.0 |
| Moderate | 112 | 28.4 |
| Severe/very severe | 21 | 5.3 |
| CISI-PD | 6.91 (3.64) | 0–21 |
SD, standard deviation; LEDD, levodopa-equivalent daily doses; MoCA, Montreal Cognitive Assessment; NMSS, Nonmotor Symptoms Scale; MDS-UPDRS, Movement Disorders Society Unified Parkinson’s Disease Rating Scale; CISI-PD, Clinical Impression of Severity Index Parkinson’s Disease.
Data Quality and Acceptability
Table 2 shows the data quality and acceptability statistics of the total score and each domain; for the NMF subscale, results are presented for both the total sample and the subset of patients who reported NMF (n = 165). The mean ± SD MDS-NMS total score was 79.33 ± 65.87), observed range of 0–334. Fully computable data were 92.8% for the MDS-NMS total score and 99.8% to 100% for all domains except sexual dysfunction, which had missing data for 6.7% of patients. There were missing data for 1.2% of patients with NMF on that subscale. The MDS-NMS and the NMF total scores showed no significant floor effects (0.3% and 3.7%, respectively) or ceiling effects. For individual domains, there were no ceiling effects, but floor effects ranged from a low of 13.5% (sleep and wakefulness) to a high of 83.5% (impulse control and related disorders). As a whole, there was a positive skewness that was higher than the standard, mirroring the floor effect.
TABLE 2.
Data quality and acceptability of the MDS-NMS domains and total score
| Missing | Mean | Median | SD | Skewness | Min | Max | Floor (%) | Ceiling (%) | |
|---|---|---|---|---|---|---|---|---|---|
| Total sample (n = 402) | |||||||||
| A. Depression | 1 | 5.76 | 1 | 10.60 | 3.00 | 0 | 72 | 43.6 | 0 |
| B. Anxiety | 0 | 5.95 | 2 | 8.45 | 2.28 | 0 | 54 | 32.8 | 0 |
| C. Apathy | 0 | 4.00 | 0 | 7.12 | 2.28 | 0 | 36 | 53.0 | 0 |
| D. Psychosis | 0 | 1.64 | 0 | 4.01 | 4.06 | 0 | 36 | 68.9 | 0 |
| E. IC and related disorders | 1 | 0.99 | 0 | 3.09 | 4.39 | 0 | 25 | 83.5 | 0 |
| F. Cognition | 0 | 9.57 | 5 | 11.92 | 1.83 | 0 | 66 | 19.9 | 0 |
| G. Orthostatic hypotension | 0 | 2.24 | 0 | 4.26 | 2.66 | 0 | 24 | 58.7 | 0 |
| H. Urinary | 0 | 7.50 | 4 | 9.23 | 1.80 | 0 | 48 | 25.9 | 0.8 |
| I. Sexual | 27 | 4.48 | 0 | 8.01 | 2.01 | 0 | 32 | 60.0 | 2.9 |
| J. Gastrointestinal | 1 | 6.09 | 4 | 7.83 | 2.00 | 0 | 47 | 27.9 | 0 |
| K. Sleep and wakefulness | 1 | 11.08 | 8 | 11.34 | 1.78 | 0 | 76 | 13.5 | 0 |
| L. Pain | 0 | 8.05 | 5 | 9.44 | 1.75 | 0 | 53 | 24.1 | 0 |
| M. Other | 0 | 13.09 | 12 | 11.92 | 0.97 | 0 | 56 | 19.9 | 0 |
| MDS-NMS total score | 29 | 79.33 | 61 | 65.87 | 1.33 | 0 | 334 | 0.3 | 0 |
| NMF Change | 2 | 3.28 | 0 | 5.33 | 1.85 | 0 | 30 | 58.8 | 0 |
| Time in “off” | 1 | 0.86 | 0 | 1.08 | 1.01 | 0 | 4 | 53.6 | 2.2 |
| NMF total score | 2 | 6.87 | 0 | 13.17 | 2.73 | 0 | 88 | 59.3 | 0 |
| Sample restricted to patients with fluctuations (n = 165) | |||||||||
| NMF change | 1 | 8.00 | 7 | 5.61 | 1.03 | 1 | 30 | 6.7 | 0.6 |
| Time in “off” | 1 | 1.92 | 2 | 0.83 | 0.73 | 1 | 4 | 32.9 | 5.5 |
| NMF total score | 2 | 16.87 | 12 | 16.06 | 1.71 | 1 | 88 | 3.7 | 0.0 |
SD, standard deviation; IC, impulse control; NMF, nonmotor fluctuations.
Reliability
Internal consistency
Internal consistency results are shown in Table 3. Some items in 5 MDS-NMS domains and in the NMF subscale had an interitem (within-domain) correlation less than the 0.20 standard value, but the item homogeneity coefficient was more than the 0.15 threshold value for all domains except impulse control and related disorders. Only 3 of 52 items showed a corrected item-total domain correlation below the standard criterion. Average Cronbach’s alpha was 0.66 for domains and 0.84 for the NMF subscale.
TABLE 3.
Reliability of the MDS-NMS domains
| Domain | Interitem correlation | Item homogeneity coefficient | Item-total correlation | Cronbach’s alpha |
|---|---|---|---|---|
| A. Depression | 0.40–0.67 | 0.51 | 0.63–0.72 | 0.86 |
| B. Anxiety | 0.22–0.58 | 0.38 | 0.43–0.68 | 0.73 |
| C. Apathy | 0.37–0.58 | 0.46 | 0.30–0.64 | 0.68 |
| D. Psychosis | 0.19–0.48 | 0.33 | 0.38–0.64 | 0.72 |
| E. IC and related disorders | 0.00–0.21 | 0.12 | 0.05–0.31 | 0.32 |
| F. Cognition | 0.25–0.50 | 0.40 | 0.47–0.61 | 0.81 |
| G. Orthostatic hypotension | 0.62 | 0.62 | 0.62b | 0.76 |
| H. Urinary | 0.36–0.43 | 0.39 | 0.53–0.61 | 0.74 |
| I. Sexual | 0.57 | 0.57 | 0.57b | 0.75 |
| J. Gastrointestinal | 0.06–0.27 | 0.16 | 0.21–0.36 | 0.45 |
| K. Sleep and wakefulness | 0.08–0.37 | 0.19 | 0.27–0.50 | 0.61 |
| L. Pain | 0.11–0.38 | 0.22 | 0.24–0.50 | 0.59 |
| M. Other | 0.01–0.53 | 0.19 | 0.12–0.47 | 0.50 |
| Nonmotor Fluctuations Subscalea | ||||
| NMF change | 0.07–0.52 | 0.19 | 0.22–0.54 | 0.84 |
ICC, Intraclass correlation coefficient; IC, impulse control; NMF, nonmotor fluctuations.
In patients with fluctuations (n = 165).
= These domains have only two items; therefore, values are like inter-item correlation.
Test-retest reliability
Test-retest agreement was >95% for 39 of 52 items on the MDS-NMS and for 5 of 8 items on the NMF subscale. Weighted kappa index ranged from 0.26 to 0.86 for MDS-NMS items and from 0.48 to 0.68 for the NMF subscale items (Table 4). ICC was 0.84 for MDS-NMS and 0.70 for the MDS-NMF subscale and ranged from 0.50 to 0.85 for domains.
TABLE 4.
Reproducibility parameters of the MDS-NMS
| Domain | Test-retest (n = 123) |
Interrater (n = 164) |
Precision SEM | ||
|---|---|---|---|---|---|
| Kappaw | ICC | Kappaw | ICC | ||
| A. Depression | 0.45–0.74 | 0.73 | 0.91–1.0b | 0.99 | 4.91 |
| B. Anxiety | 0.39–0.66 | 0.66 | 0.93–0.99 | 0.99 | 4.53 |
| C. Apathy | 0.48–0.53 | 0.62 | 0.89–1.0b | 0.99 | 4.04 |
| D. Psychosis | 0.26–0.68 | 0.66 | 0.98–1.0b | 0.99 | 2.35 |
| E. IC and related disorders | 0.36–0.94 | 0.50 | 0.94–1.0b | 1.0 | 1.57 |
| F. Cognition | 0.58–0.74 | 0.74 | 0.94–0.98 | 0.99 | 5.80 |
| G. Orthostatic hypotension | 0.58–0.62 | 0.56 | 0.94–0.95 | 0.97 | 2.61 |
| H. Urinary | 0.68–0.78 | 0.78 | 0.96–0.99 | 0.99 | 4.52a |
| I. Sexual | 0.59–0.71 | 0.70 | 0.96–1.0b | 0.99 | 4.12 |
| J. Gastrointestinal | 0.59–0.79 | 0.77 | 0.98–0.99 | 0.99 | 3.37a |
| K. Sleep and wakefulness | 0.56–0.74 | 0.81 | 0.94–0.99 | 0.97 | 4.74a |
| L. Pain | 0.45–0.73 | 0.75 | 0.99 | 1.0 | 4.78a |
| M. Other | 0.65–0.86 | 0.85 | 0.95–0.98 | 0.98 | 4.39a |
| MDS-NMS total score | — | 0.84 | — | 1.0 | 25.30a |
| NMF change | 0.48–0.68 | 0.76 | 0.90–0.99 | 0.99 | 2.61 |
| Time in off | 0.70 | — | 0.97 | — | |
| NMF total score | — | 0.70 | 1.0 | 7.06 | |
SEM, standard error of measurement.
SEM < ½SDpooled.
Rounded figures when weighted kappa value was >0.999.
Interrater reliability
Interrater agreement was >99% for all but 1 item. Weighted kappa index ranged from 0.89 to 1.00, and ICC ranged from 0.97 to 1.00 (Table 4). Similar results were obtained for the NMF subscale (agreement > 99%; weighted kappa, 0.90–0.99; ICC, 1.00).
Precision
The SEM for the MDS-NMS total score was 25.30, lower than the corresponding ½ SDpooled (31.63), demonstrating good precision. However, the SEM for the NMS subscale was 7.06, slightly higher than the corresponding ½ SDpooled (6.45); see Table 4. SEM values for individual domains are also presented in Table 4.
Convergent Validity
MDS-NMS domains correlated 0.57–0.87 with the corresponding NMSS domains (Table 5). The correlation between MDS-NMS total score and MDS-UPDRS part I was 0.75 and with NMSS total score was 0.88. Correlation coefficients of MDS-NMS domains with the corresponding items of the MDS-UPDRS part I ranged between 0.31 and 0.68. There was also high correlation between NMF subscale and MDS-UPDRS motor fluctuations scores (rS = 0.72).
TABLE 5.
Convergent validity of the MDS-NMS
| MDS-UPDRS | MDS-NMS domains | Spearman R |
|---|---|---|
| 1.1 Cognitive | F. Cognition | 0.50 |
| 1.2 Hallucination/psychosis | D. Psychosis | 0.49 |
| 1.3 Depression | A. Depression | 0.67 |
| 1.4 Anxiety | B. Anxiety | 0.59 |
| 1.5 Apathy | C. Apathy | 0.49 |
| 1.6 Dopamine dysregulation syndrome | E. IC and related disorders | 0.31 |
| 1.7 Sleep problems | K. Sleep and wakefulness | 0.54 |
| 1.8 Daytime sleepiness | K. Sleep and wakefulness | 0.38 |
| 1.9 Pain | L. Pain | 0.60 |
| 1.10 Urinary problems | H. Urinary | 0.68 |
| 1.11 Constipation | J. Gastrointestinal | 0.45 |
| 1.12 Lightheadedness | G. Orthostatic hypotension | 0.61 |
| 1.13 Fatigue | M. Other | 0.48 |
| 2.2 Saliva and drooling | J. Gastrointestinal | 0.54 |
| 2.3 Swallowing | J. Gastrointestinal | 0.44 |
| 2.9 Turning in bed | K. Sleep and wakefulness | 0.31 |
| NMSS | MDS-NMS domains | Spearman R |
| 1. Cardiovascular | G. Orthostatic hypotension | 0.72 |
| 2. Sleep/fatigue | K. Sleep and wakefulness | 0.73 |
| 3. Mood/apathy | A. Depression | 0.73 |
| 3. Mood/apathy | B. Anxiety | 0.64 |
| 3. Mood/apathy | C. Apathy | 0.59 |
| 4. Halluc./perceptual | D. Psychosis | 0.57 |
| 5. Attention/memory | F. Cognition | 0.73 |
| 6. Gastrointestinal | J. Gastrointestinal | 0.81 |
| 7. Urinary | H. Urinary | 0.87 |
| 8. Sexual | I. Sexual | 0.87 |
| 9. Miscellaneous | M. Other | 0.39 |
| 9. Miscellaneous (Paina) | L. Pain | 0.72 |
MDS-NMS, Movement Disorder Society–sponsored Nonmotor Symptoms Rating Scale; MDS-UPDRS, Movement Disorder Society–sponsored Unified Parkinson’s Disease Rating Scale; NMSS, Nonmotor Symptoms Rating Scale.
Pain item from Miscellaneous domain.
Spearman rank correlation coefficients. All, P < 0.0001.
Correlations with other variables in the study are displayed in Supplementary Table 1S.
Known-Groups Validity
The MDS-NMS total and NMF subscale scores showed no significant differences between subgroups defined by sex or age. However, MDS-NMS score increased significantly with increasing HY stage, PD duration, LEDD, and PGIS (Kruskal-Wallis test, P < 0.001 for all); see Supplementary Table 2S.
Discussion
The results presented here represent the primary clinimetric validation of the English version of the MDS-NMS from an international multicenter study. The data indicate that the MDS-NMS has acceptable clinimetric properties to capture in a single instrument a broad range of NMSs that occur commonly in PD. The overall MDS-NMS had no ceiling or floor effects, acceptable internal consistency, and satisfactory interrater and test-retest reliability. The good reliability plus acceptable precision support its use as an outcome measure in clinical trials.
Data quality was satisfactory for all items and domains, except for the sexual domain, which had 6.7% missing data. This is not surprising, as some patients may either have believed that items regarding sexual performance did not apply to them because of a lack of sexual activity (but there was no “not applicable” response option), or they have been reluctant to answer questions about intimate sexual behavior.
For the overall MDS-NMS and the NMF subscale, there were no floor and ceiling effects, and there were no ceiling effects for any domain. The lack of ceiling effects suggests that even the highest severity of any NMS in PD will be captured by this scale. However, there were moderate to high floor effects for many domains, indicating that some NMSs, although important and requiring assessment, do not occur universally. This may currently include impulse control disorders, which perhaps are less common now given changes in PD medication prescribing practices (ie, less dopamine agonist prescribing). The floor effects observed also help to explain the high skewness values observed in domain scores and are similar to what was reported in the original NMSS validation studies.23,25
Most domains (8 of 13) showed good internal consistency. The lower internal consistency in 5 domains indicates a weak relationship between items within those domains. This was expected, but we had decided in advance that it was important to group items into domains based on clinical considerations. As an example, the impulse control and related disorders domain includes gambling, punding, and dopamine dysregulation syndrome, which are distinct disorders, but also overlapping and best considered together. Similarly, the gastrointestinal domain includes dribbling of saliva and constipation, which are distinct and largely unrelated disorders. Thus, we think the results reflect the complexity of the underlying disease rather than a deficiency of the scale and again are consistent with findings reported previously for the NMSS.23,25
For test-retest reliability, most of the MDS-NMS domains and the NMF showed adequate test-retest results. Five MDS-NMS domains (impulse control and related disorders, orthostatic hypotension, anxiety, apathy, and psychosis) reached ICC values under the standard 0.70, results that were slightly worse than corresponding findings for the NMSS.23,25 These suboptimal test-retest results for some domains may be explained, at least in part, by the short-term changes in NMSs, so test-retest variability may reflect real differences in the frequency or severity of some symptoms (52 items assessed, or 22 more than the NMSS).
The interrater reliability analyses showed excellent results, with all ICC values from 0.97 to 1. Interrater agreement was >95% in 39 of 52 items for the MDS-NMS, whereas for the NMF subscale, 5 of 8 items reached >95% of agreement. These results are indicative of excellent reproducibility of the MDS-NMS, including the NMF subscale.
The overall MDS-NMS had adequate precision. This parameter is dependent on reliability coefficient (ICC of the test-retest) and was satisfactory for the components with ICC > 0.75, thus reflecting the stability of the measure. The NMF subscale obtained a SEM value slightly higher than the criterion.
In relation to convergent validity, the MDS-NMS domains and total score correlated strongly with the corresponding elements of the NMSS and MDS-UPDRS. The NMF subscale showed a close association with the MDS-UPDRS part IV, suggesting that it is a good complement to the assessment of motor fluctuations in PD.
The MDS-NMS and NMF subscale showed no significant differences in the sample grouped by sex or age, results similar to those for the NMSS.23,25 A significant increase in MDS-NMS scores happened in parallel with increasing LEDD, perceived disease severity, HY stage, and PD duration, as occurred with the latter 2 with the NMSS.25
Taking into account the data from the clinimetric testing essential for the evaluation of the performance of a modern scale, the MDS-NMS appears to be an effective measure for addressing the severity and frequency of a wide range of NMSs that occurs in PD. The new scale is enriched with domains to evaluate impulse control and related disorders, while adding depth to domains assessing cognition and other neuropsychiatric aspects of PD, therapeutic challenges that PD clinicians are confronted with commonly. The new NMF subscale and its satisfactory performance allow for evaluation of a distinct and important nonmotor syndrome in PD and make the instrument potentially valuable to test the efficacy of treatment for patients with fluctuations, still a major unmet need.
Several limitations have to be recognized in the present study. First, the sample included patients predominantly with mild to moderate disease severity and with at most mild cognitive deficits. Second, MDS-NMS concurrent validity with diagnostic criteria and other measures has not been tested yet. Third, sensitivity to change, either with disease progression or because of therapeutic intervention, has not been assessed. Future studies will need to address these issues, as well as validate a self-rated version of the instrument for use in clinical care and some clinical research studies.
This new scale complements the MDS-UPDRS for use in epidemiological and clinical trials, allowing users and policy makers to obtain an in-depth assessment of the effect of the disease and the impact of investigational agents. This is especially relevant as recent views suggest that motor complications in PD may be less prominent than in the past, whereas evidence for effective treatments of many NMSs in PD is still quite limited.37,38 In addition, we hope that the MDS-NMS will help policy makers decide on the impact of a drug on value-based health care, as well as help in creating national registries using postmarketing surveillance. Finally, in academic centers, the MDS-NMS and NMF subscale can help researchers to design clinical translational studies addressing NMSs in PD.
The need for and importance of a global measure of NMS rating in PD is evident from the widespread use of the NMSS over the past decade in clinical trials, global clinical registries, and epidemiological cohort studies in PD. Such needs are likely to grow, given that our understanding of the impact of NMSs in PD, ranging from prodromal to palliative phases, has increased substantially over time, and treatment developers are eager to explore global NMS burden as an end point in clinical trials. The MDS-NMS is well poised, offering a timely, up-to-date, and state-of-the-art option for the assessment of these issues.
Supplementary Material
Acknowledgments:
The authors acknowledge grant support from the International Parkinson and Movement Disorder Society to plan, develop, and validate the English version of the MDS-NMS in an international study. K.R.C. acknowledges support from the NIHR clinical research network and CRN-supported staff (Dhaval Trivedi, Aleksandra Podlewska, Corinne Borley) for data collection in NHS clinics. A.S. was supported by the National Institute for Health Research UCLH Biomedical Research Centre. D.W. acknowledges the support of the Parkinson’s Disease and Movement Disorders Center at the University of Pennsylvania School of Medicine, and the involvement of Dr. Jacqui Rick, Sam Rudovsky, and Benjamin Deck in the conduct of the study. The authors thank Dr. Mubasher A. Qamar for assistance in preparing the article and coordination.
This article also presents independent research part funded by the National Institute for Health Research (NIHR) Mental Health Biomedical Research Centre and Dementia Unit at South London and Maudsley NHS Foundation Trust and King’s College London as KRC is funded by these institutions. The views expressed are those of the author and not necessarily those of the NHS, the NIHR or the Department of Health.
Relevant conflicts of interest/financial disclosures: The project was supported by a 2-stage academic peer reviewed grant from the International Parkinson’s and Movement Disorder’s Society (IPMDS), as well as the Clinical Research Network (CRN), London South and London North Thames, National Institute of Health Research (for the UK sites).
International Parkinson and Movement Disorder Society–Non-Motor Rating Scale (MDS-NMS): Glossary of Terms
- A. Depression
a mood disorder characterized by sustained change in emotions (sadness, decreased interest or pleasure), cognition (negative thoughts about life or self, such as hopelessness, helplessness, indecisiveness, or death or suicide ideation) or behavior (isolative, withdrawn, sleep disturbances, appetite disturbances)
- B. Anxiety
an affective disorder characterized by sustained excessive worrying which can be (1) generalized and include symptoms such as restlessness, being easily fatigued, mind going blank or trouble concentrating, irritability, and muscle tension; (2) specific anxiety or panic attacks; (3) fear of being in public (agoraphobia); or (4) fear of being in social situations (social phobia)
- Anxiety or panic attack
abrupt surge of intense fear or intense discomfort, can include shortness of breath, heart beating fast, upset stomach, sweating, dizziness or faintness, sensation of chill or heat, or sense something bad is going to happen or even a sense of dying
- C. Apathy
a disorder characterized by decreased motor activity (less initiation of motor activity not due to parkinsonism), emotional expression (less emotional engagement separate from decreased facial expression due to parkinsonism) or speech (less likely to initiate or engage in conversation)
- D. Psychosis
a disorder characterized by changes in perception (passage or presence phenomena, illusions or hallucinations) or thought (delusions)
- Passage phenomenon
visual sensation of something moving in periphery of visual field
- Presence phenomenon
visual sensation of person being in periphery of visual field
- Illusions
visual misinterpretation of an actual object
- Hallucinations
a sensory (visual, auditory, taste, smell, or feeling) experience that is not real or experienced by other people
- Delusions
a belief that something is true for which there is no objective evidence and which other persons do not hold true
- E. Impulse control disorders
a failure to resist an impulse or drive that leads to repeated engagement in activities that become harmful to self or others; compared with pre-PD behavior
- Hoarding
the needless collection of objects and an inability to get rid of them
- Punding
the needless or purposeless repetition of a simple motor activity
- Dopamine dysregulation syndrome
taking an excess (beyond what is prescribed) of Parkinson’s medications for their motor or psychological effects, often with significant mood changes during “on” (irritability, hypomania) or “off’ (dysphoria) states
- F. Cognition
the activities of thinking, understanding, learning, and remembering
- Attention
concentrating on one part of the environment while ignoring other things
- Executive abilities
cognitive processes involved in maintaining multiple pieces of information in the mind at the same time, reasoning, task flexibility, problem solving, and task planning and execution
- Visuospatial abilities
ability relating to visual perception of spatial relationships among objects
- G. Orthostatic hypotension
a drop in blood pressure, severe enough to cause symptoms, when changing from sitting to standing position or from lying to sitting position
- H. Urinary
- Nocturia
excessive urination at night, defined as more than 2 times overnight
- I. Sexual
- Erectile dysfunction
inability of a man to maintain an erection sufficient for satisfying sexual activity
- J. Gastrointestinal
relating to the stomach and intestines
- Saliva
watery liquid secreted into the mouth by glands, providing lubrication for chewing and swallowing, and aiding digestion
- Swallowing
difficulty swallowing including liquids & solids, as well as choking while swallowing
- Nausea
a feeling of sickness with a tendency to vomit
- Constipation
infrequent bowel movements (usually less than three bowel movements per week) or difficult passage of stools
- K. Sleep and wakefulness
- Insomnia
difficultly falling asleep or staying asleep
- Rapid eye movement (REM) sleep
a stage in the normal sleep cycle during which dreams occur and the body undergoes marked changes including rapid eye movement, loss of reflexes, and increased pulse rate and brain activity
- L. Pain
- Dystonia
a state of abnormal muscle tone resulting in muscular spasm and abnormal posture
- Nocturnal pain
pain overnight
- Orofacial pain
pain which is felt in the mouth, jaws or face
- M. Other
- Olfaction
the action or capacity of smelling
- Fatigue (physical)
state of excessive physical weariness or exhaustion (after physical exertion), different from sleepiness
- Fatigue (mental)
state of excessive mental weariness or exhaustion, different from sleepiness
Appendix



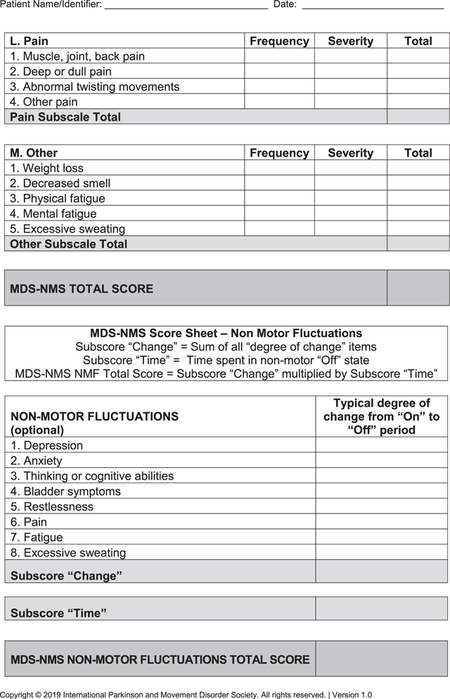
Footnotes
Supporting Data
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site.
References
- 1.Schapira AHV, Chaudhuri KR, Jenner P. Non-motor features of Parkinson disease. Nat Rev Neurosci 2017;18(7):435–450. [DOI] [PubMed] [Google Scholar]
- 2.Titova N, Chaudhuri KR. Non-motor Parkinson disease: new concepts and personalised management. Med J Aust 2018;208(9):404–409. [DOI] [PubMed] [Google Scholar]
- 3.Martinez-Martin P, Ray Chaudhuri K. Comprehensive grading of Parkinson’s disease using motor and non-motor assessments: addressing a key unmet need. Expert Rev Neurother 2018;18(1):41–50. [DOI] [PubMed] [Google Scholar]
- 4.Dafsari HS, Martinez-Martin P, Rizos A, et al. EuroInf 2: Sub-thalamic stimulation, apomorphine, and levodopa infusion in Parkinson’s disease. Mov Disord 2019;34(3):353–365. [DOI] [PubMed] [Google Scholar]
- 5.Poewe W, Chaudhuri KR, Bergmann L, Antonini A. Levodopa-carbidopa intestinal gel in a subgroup of patients with dyskinesia at baseline from the GLORIA Registry. Neurodegener Dis 2019;9(1):39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Katzenschlager R, Poewe W, Rascol O, et al. Apomorphine subcutaneous infusion in patients with Parkinson’s disease with persistent motor fluctuations (TOLEDO): a multicentre, double-blind, randomised, placebo-controlled trial. Lancet Neurol 2018;17(9):749–759. [DOI] [PubMed] [Google Scholar]
- 7.Trenkwalder C, Kies B, Rudzinska M, et al. Rotigotine effects on early morning motor function and sleep in Parkinson’s disease: a double-blind, randomized, placebo-controlled study (RECOVER). Mov Disord 2011;26(1):90–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bhidayasiri R, Phokaewvarangkul O, Sringean J, et al. Evaluation of nocturnal hypokinesia in Parkinson’s disease using a novel patient/-proxy questionnaire and correlations with objective monitoring. Parkinsonism Relat Disord 2019;61:219–223. [DOI] [PubMed] [Google Scholar]
- 9.Martinez-Martin P, Leentjens AF, de Pedro-Cuesta J, Chaudhuri KR, Schrag AE, Weintraub D. Accuracy of screening instruments for detection of neuropsychiatric syndromes in Parkinson’s disease. Mov Disord 2016;31(3):270–279. [DOI] [PubMed] [Google Scholar]
- 10.Chaudhuri KR, Antonini A, Robieson WZ, Sanchez-Solino O, Bergmann L, Poewe W. Burden of non-motor symptoms in Parkinson’s disease patients predicts improvement in quality of life during treatment with levodopa-carbidopa intestinal gel. Eur J Neurol 2019;26(4):581–e543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schrag A, Sauerbier A, Chaudhuri KR. New clinical trials for non-motor manifestations of Parkinson’s disease. Mov Disord 2015;30 (11):1490–1504. [DOI] [PubMed] [Google Scholar]
- 12.Martinez-Martin P, Rodriguez-Blazquez C, Kurtis MM, Chaudhuri KR. The impact of non-motor symptoms on health-related quality of life of patients with Parkinson’s disease. Mov Disord 2011;26(3):399–406. [DOI] [PubMed] [Google Scholar]
- 13.Marras C, Chaudhuri KR. Nonmotor features of Parkinson’s disease subtypes. Mov Disord 2016;31(8):1095–1102. [DOI] [PubMed] [Google Scholar]
- 14.Sauerbier A, Rosa-Grilo M, Qamar MA, Chaudhuri KR. Nonmotor Subtyping in Parkinson’s Disease. Int Rev Neurobiol 2017;133: 447–478. [DOI] [PubMed] [Google Scholar]
- 15.Martinez-Martin P, Schrag A, Weintraub D, Rizos A, Rodriguez-Blazquez C, Chaudhuri KR. Pilot Study of the International Parkinson and Movement Disorder Society-sponsored Non-motor Rating Scale (MDS-NMS). Mov Disord Clin Pract 2019;6(3):227–234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Postuma RB, Berg D, Stern M, et al. MDS clinical diagnostic criteria for Parkinson’s disease. Mov Disord 2015;30(12):1591–1601. [DOI] [PubMed] [Google Scholar]
- 17.Nasreddine ZS, Phillips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005;53(4):695–699. [DOI] [PubMed] [Google Scholar]
- 18.Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology 2010;75(19):1717–1725. [DOI] [PubMed] [Google Scholar]
- 19.Beavers AS, Lounsbury JW, Richards JK, Huck SW, Skolits GJ, Esquivel SL. Practical Considerations for Using Exploratory Factor Analysis in Education Research. Pract Assess Res Eval 2013;18(6). [Google Scholar]
- 20.Terwee CB, Bot SD, de Boer MR, et al. Quality criteria were proposed for measurement properties of health status questionnaires. J Clin Epidemiol 2007;60(1):34–42. [DOI] [PubMed] [Google Scholar]
- 21.Hobart JC, Cano SJ, Warner TT, Thompson AJ. What sample sizes for reliability and validity studies in neurology? J Neurol 2012;259 (12):2681–2694. [DOI] [PubMed] [Google Scholar]
- 22.Schmitt E, Krack P, Castrioto A, et al. The Neuropsychiatric Fluctuations Scale for Parkinson’s Disease: A Pilot Study. Mov Disord Clin Pract 2018;5(3):265–272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chaudhuri KR, Martinez-Martin P, Brown RG, et al. The metric properties of a novel non-motor symptoms scale for Parkinson’s disease: Results from an international pilot study. Mov Disord 2007;22 (13):1901–1911. [DOI] [PubMed] [Google Scholar]
- 24.Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society-sponsored revision of the Unified Parkinson’s Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord 2008;23(15):2129–2170. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Martin P, Rodriguez-Blazquez C, Forjaz MJ, de Pedro J. The Clinical Impression of Severity Index for Parkinson’s Disease: international validation study. Mov Disord 2009;24(2):211–217. [DOI] [PubMed] [Google Scholar]
- 26.Forkmann T, Scherer A, Boecker M, Pawelzik M, Jostes R, Gauggel SJBP. The clinical global impression scale and the influence of patient or staff perspective on outcome. BMC Psychiatry 2011;11(1):83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ambrosio L, Portillo MC, Rodriguez-Blazquez C, et al. Satisfaction with Life Scale (SLS-6): First validation study in Parkinson’s disease population. Parkinsonism Relat Disord 2016;25:52–57. [DOI] [PubMed] [Google Scholar]
- 28.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson’s disease. Mov Disord 2010;25(15):2649–2653. [DOI] [PubMed] [Google Scholar]
- 29.Grimes DA, Hubacher D, Nanda K, Schulz KF, Moher D, Altman DG. The Good Clinical Practice guideline: a bronze standard for clinical research. Lancet 2005;366(9480):172–174. [DOI] [PubMed] [Google Scholar]
- 30.Smith S, Lamping D, Banerjee S, Harwood R, Foley B. Measurement of health-related quality of life for people with dementia: development of a new instrument (DEMQOL) and an evaluation of current methodology. Health Technol Assess 2005;2005(10). [DOI] [PubMed] [Google Scholar]
- 31.McHorney CA, Tarlov AR. Individual-patient monitoring in clinical practice: are available health status surveys adequate? Qual Life Res 1995;4(4):293–307. [DOI] [PubMed] [Google Scholar]
- 32.Hays RD, Anderson R, Revicki D. Psychometric considerations in evaluating health-related quality of life measures. Qual Life Res 1993;2(6):441–449. [DOI] [PubMed] [Google Scholar]
- 33.RL P Inter-item correlations In: Michalos AC. Encyclopedia of Quality of Life and Well-Being Research. Dordrecht: Springer Netherlands; 2014. [Google Scholar]
- 34.Clark LA, Watson D. Constructing validity: Basic issues in objective scale development. Psychol Assess 1995;7(3):309–319. [Google Scholar]
- 35.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33(1):159–174. [PubMed] [Google Scholar]
- 36.Feeny D, Farris K, Cote I, Johnson JA, Tsuyuki RT, Eng K. A cohort study found the RAND-12 and Health Utilities Index Mark 3 demonstrated construct validity in high-risk primary care patients. J Clin Epidemiol 2005;58(2):138–141. [DOI] [PubMed] [Google Scholar]
- 37.Chaudhuri KR, Jenner P, Antonini A. Should there be less emphasis on levodopa-induced dyskinesia in Parkinson’s disease? Mov Disord 2019;34(6):816–819. [DOI] [PubMed] [Google Scholar]
- 38.Seppi K, Ray Chaudhuri K, Coelho M, et al. Update on treatments for nonmotor symptoms of Parkinson’s disease-an evidence-based medicine review. Mov Disord 2019;34(2):180–198. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


