Abstract
Unusual nucleic acid structures are salient triggers of endogenous repair and can occur in sequence-specific contexts. Peptide nucleic acids (PNAs) rely on these principles to achieve non-enzymatic gene editing. By forming high-affinity heterotriplex structures within the genome, PNAs have been used to correct multiple human disease-relevant mutations with low off-target effects. Advances in molecular design, chemical modification, and delivery have enabled systemic in vivo application of PNAs resulting in detectable editing in preclinical mouse models. In a model of β-thalassemia, treated animals demonstrated clinically relevant protein restoration and disease phenotype amelioration, suggesting a potential for curative therapeutic application of PNAs to monogenic disorders. This review discusses the rationale and advances of PNA technologies and their application to gene editing with an emphasis on structural biochemistry and repair.
Keywords: peptide nucleic acids, PNA, triplex, gene editing, structure, recombination, repair, nanoparticles, β-thalassemia, cystic fibrosis
1. Introduction
Nucleic acid molecules are capable of forming a broad range of structures due to their backbone flexibility, hydrogen bonding combinations, and sequential monomeric building blocks. While canonically DNA is known to prefer a right-handed double helical structure, or B-DNA, a multitude of alternative nucleic acid structures have been reported since Watson and Crick first described the double helix in 1953 [1]. Left-handed double helical DNA (Z-DNA), triplex DNA (H-DNA), tetraplex DNA (e.g., G-quadruplex), and DNA:RNA looping hybrids (R-loops) are a few examples of the diverse formations these molecules adopt in nature, often in sequence-specific contexts [2]. The consequences of these curious structures have been under investigation for decades revealing critical roles in gene regulation, telomere protection, and recombination processes amongst others. However, while unusual structures expand and nuance the genomic toolbox, these sites have been shown to be associated with genomic instability in some contexts [3]. Consequentially, a suite of dedicated helicases, polymerases, and repair networks evolved in tandem to recognize and resolve these transient structures faithfully and maintain genomic integrity [4]. Unsurprisingly, owing to the inherent sequence-specific, recombinogenic properties of non-B-DNA structures and their ability to elicit endogenous repair, harnessing these molecular configurations as an approach to targeted gene modification has attracted considerable interest [2].
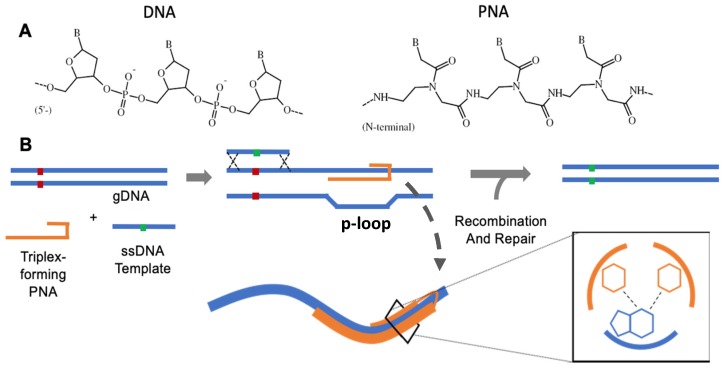
The field of structure-mediated gene targeting developed rapidly as investigators initially pursued the application of triplex-forming oligonucleotides (TFOs)—single-stranded DNA molecules that associate with the major groove of a DNA helix at polypurine stretches and stabilize via Hoogsteen hydrogen bonding (H-bonding) [5]. These sequence-targeted molecules were used to deliver mutagens to induce gene knockout and eventually were applied with short DNA templates to achieve homologous recombination [6,7]. While DNA TFO-induced gene editing demonstrated a modest ability to accomplish targeted sequence modification, the field took a major leap forward with the advent and application of a new class of synthetic DNA prototype, peptide nucleic acids (or PNAs), which are the focus of this review. First described by Nielsen and colleagues in 1991, PNAs are synthetic DNA analogues that feature a polyamide (i.e., protein-like) backbone, as opposed to a conventional phosphodiester backbone (Figure 1A, [8]). This key chemical modification endows PNAs with advantageous characteristics that make them especially amenable to targeted structure-induced recombination. Firstly, as a result of a neutrally charged polyamide backbone, and by minimizing repulsive negative forces between polymer backbones, PNAs are capable of forming remarkably high-affinity base-paired structures with DNA [9]. This effect is even more pronounced when two PNA strands coordinate with a single strand of DNA, resulting in an exceptionally stable heterotriplex structure relative to DNA:DNA:DNA homotriplexes (e.g., TFO) [10]. In contrast to TFOs that bind along the exposed major groove of a target helix, PNAs are capable of invading helices to coordinate around a single strand of DNA while displacing the opposite strand (termed a p-loop, Figure 1B, [5,11]). Further, due to their novel structure, PNAs are resistant to nuclease and protease-mediated degradation and are thus very stable in living cells [12]. Taken together, these attributes allow investigators to wield PNAs as a potent means to generate salient sequence-specific structures with genomic DNA (gDNA). The resulting bulky helix-distorting p-loop robustly recruits endogenous repair and, when provided a co-delivered ssDNA template specifying a sequence change, mediates the incorporation of a persistent genomic edit (Figure 1B, [13,14,15]).
Figure 1.
(A) Phosphodiester and polyamide backbone structures of DNA and PNA polymers, (B) Simplified schematic of triplex-forming PNA-mediated gene editing.
An exception in a field dominated by enzymatic approaches, PNAs are a powerful gene editing technology that utilizes modifiable molecules to generate recombinogenic structures. Decades of advances in PNA chemistries, systems of delivery, and their applications to gene editing have effectively developed this technology into a promising candidate for genetic therapy for systemic monogenetic human disease. Work from our laboratories at Yale University has shown that PNAs can be co-delivered with a ssDNA template using biodegradable polymeric nanoparticles and administered to animals directly in vivo [16,17,18,19,20]. To date, nanoparticle-delivered PNAs have been used to correct disease-implicated mutation in multiple tissues, across multiple disease models in mice—ex vivo, in vivo, and in utero [16,17,18,19,20,21,22]. This review explores advances in PNA gene editing as they relate to PNA chemistry, structural biology, delivery, and repair and recombination before summarizing on-going work and outlooks for this exciting developing field.
2. Pre-PNA: TFOs and Structure-Induced Recombination
Early on, investigators identified the potential use of TFOs as a means to recognize unique genomic sequences and to elicit a site-directed effect. Initial experiments from our group (1993) used TFOs to deliver conjugated mutagens, such as psoralen, to induce gene knockout [23]. That same year Kohwi and Panchenko made the fascinating observation that triplex-forming sequences were associated with recombination events in an orientation and length-dependent manner using plasmid-based assays in E. coli [24]. Three years later, Faruqi et al. (1996) [25] went on to demonstrate recombination induced by triplex formation via TFO, both with and without psoralen conjugates, in mammalian cells. This advance set the stage for using triplex technologies to induce specified sequence modifications.
In 1999, Chan et al. demonstrated editing at a single base via template-directed recombination for the first time using short ssDNA donor molecules tethered to a TFO targeting an SV40 vector. Editing frequencies occurred in the range of ~0.1% [7]. Later, work from Datta et al. using cell-free extracts showed that the covalent linkage between donor and TFO was not a requirement for editing to occur [26]. Importantly, this result suggested that TFO binding and triplex formation was the key event leading to repair and recombination. This led the authors to believe that strand breaks secondary to triplex repair could be responsible for forming recombination intermediates.
Although triplex editing via TFOs proved modestly effective, a few key limitations hindered overall efficiency. First, early work demonstrated that TFO binding affinity for its target is highly correlated with intracellular activity. Recombination experiments in cell-free extracts revealed that generally TFOs with a KD (equilibrium dissociation constant) less than or in the range of 10−7 M were necessary for measurable activity [27]. KD was also shown to correlate closely with TFO length, often requiring up to 30 nucleotides for activity, restricting the number of possible genomic targets. Further, because TFOs are composed of DNA they are subject to nuclease-mediated degradation and only transiently remain within the cell intact [12]. Various strategies have been tried in order to surmount some of these restrictions (summarized in sections of [28]), but none have progressed the technology close to therapeutically meaningful efficiencies. While work using TFOs demonstrated a proof-of-principle for using site-specific structure formation to induce recombination, their feasibility as a potentially therapeutic biotechnology fell short. Ideally such a technology needs to demonstrate superior triplex binding kinetics, intracellular stability, and broader versatility.
3. PNA Chemistry
The unique features of PNA oligomers satisfy the aforementioned criteria. For example, relatively short (10 mer) PNAs bind with nanomolar affinity (KD ~ 10−9) to complementary DNA targets, with apposite modifications (vide infra) able to improve affinity several orders of magnitude to high femtomolar (KD ~ 10−12) [29]. Further, the bifurcated character of PNAs—whereby the same compound is part nucleic acid, part peptide, but never entirely either—imparts stealth, since no known endogenous nucleases and/or proteases recognize PNAs as substrates [12]. Also, the ease of synthesis, based largely on established protocols for peptide syntheses, and availability of synthetically amenable sites in the backbone have allowed variations in PNA design and conformation to optimize DNA binding efficacy [30].
Although the superiority of appropriately designed PNA oligomers over TFOs for DNA binding is now well established in the literature, these trends are only intuitive in retrospect. In fact, the seminal work introducing PNAs for DNA recognition presented them as ligands for the accessible major groove of purine-rich DNA targets (like TFOs), where they could bind using the same Hoogsteen H-bonding interactions that stabilize TFO-based triplexes [8]. However, this study led to the surprising finding that pyrimidine-rich PNAs formed stable PNA2-DNA triplexes on the bound DNA strand by simultaneous displacement of the homologous region of the non-target (pyrimidine-rich) DNA strand [8]. Evidently, the PNA ligand was sufficiently homomorphous to DNA and possessed the binding affinity to invade an otherwise stable duplex structure harboring a complementary sequence, resulting in a complex where two PNA molecules engaged the Watson–Crick (WC) and Hoogsteen (HN) faces, respectively, of the same target strand (Figure 2A [8]). Confirmatory evidence [31] for this binding mode has since incentivized synthetic strategies to optimize the binding efficacy of PNA oligomers for duplex DNA targets, since invasion of B-form DNA at near-physiologic osmolality and acidity has been recognized, then [32] and now [33], as a major challenge for PNA binding. Indeed, it is the evolution of the PNA compound in pursuit of more favorable binding that has largely enabled strategies for PNA-induced gene editing [34].
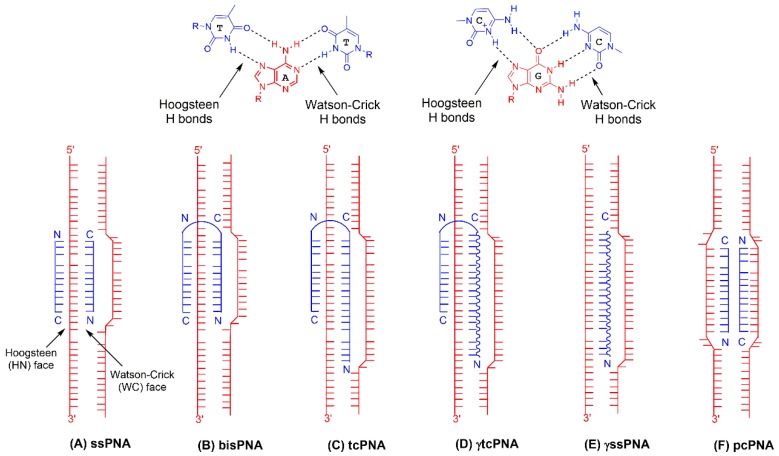
Figure 2.
PNA structural variations to drive exergonic strand invasion. (A) single-stranded (monomeric) PNA; (B) bis (dimeric) PNA; (C) tcPNA; (D) γtcPNA; (E) γssPNA; (F) pcPNA.
4. Triplex-Forming PNAs for Gene Editing
4.1. Bis-PNAs and Early Applications
Because the PNA-DNA binding reaction, like every molecular reaction, is driven by the standard free energy relationship
| (1) |
the synthetic strategies to enhance DNA recognition have focused on modulating both terms of the equation ( and , for entropy and enthalpy changes, respectively) to achieve exergonic () DNA binding reactions. For example, the realization that two polypyrimidine PNA molecules dimerized on the target DNA strand upon binding [31] led to the initial modification of tethering single PNA strands to create dimeric (bis) PNAs (Figure 2B [35,36]). This strategy effectively converted the binding reaction from a trimolecular process, where two PNA molecules dimerize on the bound DNA, to a bimolecular process, where tethered PNA domains on the same PNA molecule form H-bonds with nucleobase H-bond donors and acceptors on both faces of the target DNA, thus decreasing the translation entropy change () required for DNA binding [35,36].
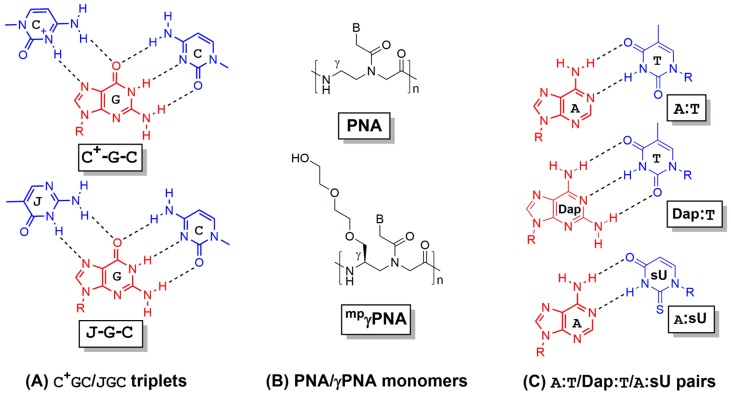
This new construct was also informed by ancillary studies establishing the orientational preferences for PNA binding to the WC and HN faces of the target strand [37]. Initial reports demonstrated the efficacy of this modification for duplex DNA invasion, with one report showing the EC50, PNA concentration necessary for binding 50% of duplex targets, to be > 500-fold higher for a bisPNA relative to the corresponding single PNA [35]. Further, because Hoogsteen base pairing requires the incoming cytosine (C) residues to provide the H-bond donors (Figure 3A), DNA strand invasion by single or bisPNAs, or indeed any PNA variation requiring Hoogsteen pairing, shows a strict pH dependence [38], since pKa < pH for C under physiologic conditions [39], requiring acidic conditions for strand invasion. Therefore, the pseudoisocytosine (J) residue, a structural isomer of C that mimics its protonated form (Figure 3A), was introduced to facilitate pH-independent binding [36]. Importantly, this modification allowed detection of DNA strand invasion at bisPNA concentrations three-fold lower than those observed for unmodified bisPNAs [36].
Figure 3.
Structural modifications in PNA backbone and nucleobases to enhance strand invasion. (A) Hydrogen bonding of C+GC and JGC triplets (B) PNA and gamma(γ) modified PNA monomers (C) A:T, Dap:T, and A:sU hydrogen binding pairs
Triplex formation on the bound DNA strand is also accelerated for bisPNAs relative to single pyrimidine PNAs, since hybridization on the WC face of the target, proposed to be the nucleation step for triplex formation, increases the effective concentration of the second, tethered PNA strand for subsequent base pairing on the HN face. This binding model is supported by thermal denaturation data which show reduced hysteresis—variance between the transition profiles recorded for denaturation (melting) and renaturation (annealing), due to slow association kinetics—for bisPNA-DNA complexes relative to those with single PNAs [36]. Because of the inverse relationship between and association rate constant ,
| (2) |
and because of the direct relationship between and free energy change,
| (3) |
accelerated binding kinetics as enabled by bisPNAs improve the binding efficacy of the ligand for DNA strand invasion by lowering .
With highly stable triplex formation capability, optimized nucleobase isomers, and dramatically improved binding kinetics, bisPNAs were the first PNAs successfully used for gene editing applications. In 2002, Rogers et al., showed targeted recombination with ssDNA donors using a supFG1 plasmid reporter system in human cell-free extracts [15]. A single base template-directed base conversion (G to C) in this plasmid system, followed by transformation into bacteria and plating in the presence of β-galactosidase, allowed for the ability to detect rare recombination events. bisPNAs tethered to short DNA templates and unconjugated bisPNAs were tested in this system. Unconjugated bisPNA and donors stimulated recombination at a frequency of 0.08%, an effect fivefold more active that the introduction of donor DNA alone [15].
Chin et al. (2008) went on to demonstrate the effectiveness of bisPNA mediated gene editing in mammalian cell culture using a disease-relevant reporter model [40]. bisPNAs were used to edit a single base β-thalassemia splicing mutation in a human β-globin intron (IVS2-1G→A) placed within a single copy of the GFP gene. This reporter cassette was placed within the genome of Chinese hamster ovary (CHO) cells. Thus, following a targeted editing event and splicing restoration, appropriately edited cells were able to express full-length GFP mRNA transcripts and fluoresce. In this study, bisPNAs designed to target a homopurine region 193 bp downstream from the target edit and a 60 mer ssDNA donor were co-nucleofected into CHO reporter cells and restored GFP expression in 0.2% of cells [40]. Importantly, GFP expression was maintained in culture for a month after nucleofection suggesting that editing events were persistent, heritable, and occurred as a result of genomic sequence change. Beyond an artificial reporter construct in CHO cells, Chin et al. went on to demonstrate bisPNA-mediated editing in the β-globin IVS2 introns in human K562 cell lines, mouse bone marrow containing a human β-globin locus, and human CD34+ progenitor cells [40]. Notably, after continued culturing, edited CD34+ lines differentiated into myeloid and erythroid lineages with persistent detectable sequence modification. These results highlighted PNA-mediated editing as a feasible translational technology. No longer a conceptual practice in plasmid assays, PNAs were used to genetically manipulate human hematopoietic stem cells (HSCs) ex vivo. This approach, followed by stem cell transplantation, is a viable therapeutic means to treat monogenetic hematologic disorders.
Two additional studies demonstrated the ability of bisPNAs to edit human CD34+ cells in culture. McNeer et al. (2011) employed a novel delivery approach to target CD34+ cells and edit the β-globin IVS2 intron using bisPNAs [41]. By encapsulating bisPNAs and ssDNA donors in spherical poly(lactic-co-glycolic acid) (PLGA) nanoparticles (NP) they showed superior reagent delivery and editing efficiencies approaching 1%. Moreover, using PLGA NPs showed no detectable reduction in cell viability and outperformed nucleofection delivery by 60-fold [41]. Developments in NP strategies for delivery are discussed in a dedicated section below in more detail. Finally, Chin et al. (2013) used bisPNAs and 100 mer ssDNA donors to target the promoter of the human γ-globin gene in CD34+ cells [42]. While inactive in adults, specific mutations in the γ-globin promoter alter transcription factor binding and reduce silencing. Inducing such mutations are a strategy to alleviate the burden of hemoglobinopathies such as β-thalassemia and sickle cell disease. By nucleofection, Chin et al. demonstrated up to 1.63% editing using bisPNAs to introduce a -117 G→A γ-globin promoter mutation in cultured CD34+ cells [42]. Bolstered by improved nanoparticle-mediated delivery, bisPNA approaches substantially progressed PNA technologies towards viability as a potential therapeutic genetic technology. Still, rational chemical improvement of PNAs has the potential to optimize binding kinetics and enhance recombinogenic potency even further.
4.2. tcPNAs and First In Vivo Studies
To modulate the enthalpic component () of PNA-DNA binding by strand invasion, tail-clamp (tc) PNA oligomers were designed by extending the PNA recognition domain on the WC face of the target DNA (Figure 2C [43,44]). This modification contributes to the binding enthalpy by increasing the number of base pairs (and H-bonds) annealing the PNA strand to its target [43,44]. For example, Bentin et al. showed that a 10-mer extension on the PNA C-terminus (which binds the DNA WC face) increased the thermal stability of the PNA-DNA complex ~ 40 °C and improves strand invasion into duplex DNA by up to 100-fold [43]. Interestingly, the improvement in binding efficacy for tcPNAs over bisPNAs is driven more by decelerated dissociation than by accelerated association, as demonstrated by data from independent groups showing tcPNAs to have two- to three-fold lower for strand invasion while also possessing much lower (> 250-fold) dissociation rate constants () relative to bisPNAs [43,44]. In this case, the direct relationship between and (eqn 2), and the direct relationship between and (eqn 3), have the cumulative effect of improving the strand invasion reaction by decreasing .
Equipped with the ability to form an overall more stable triplex, tcPNAs were first applied to gene editing by Schleifman et al. (2011) to introduce a stop codon into the CCR5 gene in human THP-1 cell lines and CD34+ cells [45]. As rationale behind this approach, naturally occurring knockout mutations of CCR5, a chemokine receptor required for HIV-1 entry into T-cells, imparts resistance to HIV infection. Nucleofected tcPNA reagents successfully modified 2.46% of THP-1 cells, a notable advantage over the 0.54% frequency achieved using bisPNAs in the same system. Modified cells demonstrated resistance to R5-tropic HIV-1 infection and were continually cultured for 98 days to demonstrate persistence. Five edited clones were maintained for 13 months with demonstrable heritable modification [45].
Schleifman et al. (2013) took this approach a step further marking the first ex vivo editing and engraftment studies utilizing PNA technologies encapsulated into PLGA NPs. In this study, PNA treated peripheral blood mononuclear cells (PBMCs) in culture were edited up to frequencies of 0.97% as determined by deep sequencing analysis [21]. The treated cell population was then engrafted into immune-deficient NOD-scid IL2rγnull mice. Flow cytometry analyses confirmed the presence of similar frequencies of engrafted human leukocyte and T-cell subsets in spleens 4 weeks post-transplantation for both treated and untreated PBMCs, indicating treatment had no effect on the ability of progenitor populations to engraft the animals. Finally, human PBMC engrafted mice were challenged with CCR5-tropic HIV-1BaL virus by intraperitoneal injection. Engrafted mice treated with PNAs maintained higher overall CD4+ T-cell counts that rose to levels similar to uninfected mice with concordant viral RNA levels approaching undetected levels [21]. These experiments highlighted the combined advantages of improved tcPNA chemistry with an elongated WC-binding tail and optimized NP-mediated delivery. These basic features, with minor modifications, remain the standard for the most potent PNA editing approaches used today.
In vivo applications with optimized tcPNA and NP reagents emerged shortly after these developments were introduced. PLGA NPs were previously used for systemic delivery of drugs in FDA-approved applications. Thus, barriers to direct systemic administration were low as the same materials used in in vitro and ex vivo approaches can be safely introduced directly into the bloodstream of animals. The first in vivo application of PNA-mediated gene editing was described by McNeer et al. (2013) using tcPNAs encapsulated in various NP formulations. NOD-scid IL2rγnull mice engrafted with human CD34+ HSCs were treated by systemic injection with NP-delivered tcPNAs and donor DNA targeted to the CCR5 and β-globin loci [16]. Engrafted mice treated intravenously with tcPNA particles demonstrated detectable targeted CCR5 editing in bone marrow, spleen, thymus, gut, and lung. Deep sequencing of whole spleen revealed 0.43% total editing with possibly much higher editing (>14%) in some isolated human progenitor-derived colonies. To further demonstrate the ability of tcPNA to modify HSCs, the same authors transplanted marrow from tcPNA/DNA treated mice into untreated NOD-scid IL2rγnull mice [16]. CCR5 modification was noted in recipient mice 10 weeks after serial transplantation. Finally, this same approach was demonstrated using a different tcPNA targeted to a β-globin intron within a GFP fluorescent reporter in human HSCs engrafted mice. These results showed, for the first time, that PNA gene editing reagents can be delivered systemically to modify relevant targets in vivo.
NP delivered tcPNAs have successfully targeted airway epithelium in vivo. Fields et al. (2013) employed an optimized NP formulation for intranasal delivery of tcPNAs to target the previously described β-globin intron/GFP reporter in the lungs of mice [18]. The authors successfully edited lung epithelium as well as alveolar macrophages and alveolar epithelial cells. McNeer and Anandalingham et al. (2015) used this same approach to intranasally deliver tcPNA/DNA donors to target and correct the F508del mutation of the cystic fibrosis transmembrane conductance regulator (CFTR) gene in a mouse model [19]. This particular mutation is a prevalent pathologic cystic fibrosis mutation seen in patients. Corrective editing in intranasally treated mice was assayed by deep sequencing and using a functional nasal potential difference assay (NPD) that reports functional chloride efflux in vivo. Mice treated with tcPNA/DNA showed significant reductions in NPD readouts consistent with wild-type voltages and 5.7% correction of CFTR F508del mutation in lung epithelium [19]. The above studies signify an expansion of the target repertoire and means of delivery for in vivo application of therapeutic PNA gene editing. Again, rational design of PNAs and delivery reagents proved capable of improving potency and progressing the diverse applications of the system.
4.3. PNA Gamma(γ) Modification and Further In Vivo and In Utero Application
Recognizing the enhancements in strand invasion efficacy imparted to the PNA by extension of the WC binding domain (as in tcPNAs), groups have incorporated gamma(γ)-PNA residues (Figure 3B [29,46]) into this segment of a tcPNA to derive γtcPNA (Figure 2D [20]). Although this review will mostly emphasize the utility and superiority of this modification for gene editing applications [20,22], this strategy was based on much earlier work showing γ moieties improve recognition of single- and double-strand DNA targets, at least in the context of WC pairing by single, monomeric PNAs [47,48]. γPNAs possess a synthetically installed stereogenic center at the γ position of the PNA backbone (Figure 3B), and this modification induces global conformational selection in the entire PNA oligomer, resulting in helical preorganization in a manner determined by the stereochemistry of the γ-position [29,46]. With the appropriate γ-configuration, itself determined by selection of the appropriate amino acid enantiomer at the start of monomer synthesis, the PNA oligomer can be engineered to match the helicity of the DNA target [49].
Helical homology confers several intuitive and empirical improvements to the binding reaction. For example, γ-modification accelerates association and decelerates dissociation in the context of DNA binding [29], both of which converge to lower relative to unmodified PNA as shown by eqn 2. As predicted by eqn 3, the improved affinity (lower ) manifests as a stronger exergonic reaction for γPNA-DNA binding compared to corresponding reactions with PNA (−ΔΔG = 5 kJ/mol [29]). Also, the improvement in binding kinetics, particularly in the association phase, is especially salient in the context of strand invasion, since earlier reports have shown that, once formed, even hybrid complexes containing sub-optimal PNA designs and/or PNA-DNA combinations remain stable for extended periods [50], highlighting a critical role for an efficient initiation/nucleation step.
The free energy gain (ΔΔG) conferred by γ modification provides an opportunity to simplify the design criteria for PNA oligomers effective for strand invasion. Accordingly, Ly and coworkers have shown that strategic implementation of γ-modification can render clamp formation, as in bis- and tcPNAs, expendable in the context of strand invasion [47,48]. Single, monomeric PNAs of appropriate length (≥15 mer) have been modified with γ residues and shown to be effective for sequence-specific strand invasion, with corresponding displacement of the homologous region on the non-target strand [47,48]. There are two merits of this design simplification: (1) by decreasing the length of PNAs necessary for strand invasion, γPNA modifications engender significant reductions in synthetic costs and effort necessary to obtain useful reagents; (2) because the enthalpic component of the binding reaction does not require significant (or any) H-bonding on the HN face of a polypurine DNA target, but is instead provided by enhanced H-bonding on the WC face of even mixed targets, γPNAs expand the genomic targeting range beyond sites with pronounced asymmetry in the strand distribution of purines and pyrimidines. This latter consideration is especially salient for gene editing, which shows a systematic dependence of modification efficiency on triplex-target site separation, at least in the context of TFO-induced triplexes [51]. Appropriately modified monomeric γPNAs targeting mixed-sequence genomic sites therefore provide an opportunity to modulate the hybrid (PNA-DNA)-target site separation in a systematic, predictable manner to elicit gene editing (Figure 2E [17]).
The enhanced invasion ability and helical pre-organization of γPNAs have generated the most potent editing reagents to date enabling robust in vivo application. Bahal et al. (2014) used γ modified tcPNA co-encapsulated with ssDNA donors in NPs to edit mouse primary bone marrow cells ex vivo and in vivo and demonstrated their superiority to unmodified tcPNA reagents [17]. Using a similar β-thalassemia intron IVS2/eGFP reporter to previous studies, the authors demonstrated approximately fourfold higher editing frequencies when using γtcPNA over unmodified tcPNAs with identical sequences.
Bahal et al. (2016) followed-up these promising experiments by applying these same modified reagents to a β-thalassemia disease mouse model [20]. In this model, mice contain no alleles for murine β-globin and only a single copy of human β-globin containing a thalassemia-associated splicing mutation in the IVS2 intron (position 654 - T→C). Consequentially, mice demonstrate a marked β-thalassemia disease phenotype including microcytic anemia and splenomegaly. γtcPNA/ssDNA NPs designed to target the pathologic mutation were administered to animals systemically by intravenous injection. Treated animals demonstrated appreciable editing in target tissues such as bone marrow and γ modified tcPNA reagents outperformed both unmodified tcPNA and non-triplex-forming γ modified ssPNAs. Thus, these studies again demonstrate the advantageous properties of PNAs conferred by tail-clamp triplex forming moieties and γ modification. Moreover, the authors described impressive disease phenotype amelioration after NP administration. Treated animals demonstrated dramatic reductions in spleen size, reduced reticulocytosis, as well as a persistent, to at least 140 days, elevations in hemoglobin to wild-type ranges. Notably, intravenous treatments with NPs were not associated with any measurable increase in inflammatory cytokines [20].
Recent work demonstrated the proof-of-concept of in utero gene editing in a mammalian mouse model using NPs containing γtcPNA and donor DNA [22]. Stemming from the observation that hematopoietic stem and progenitor cells may be edited at a higher efficiency than already differentiated cells [16,20], Ricciardi et al. endeavored to achieve gene editing during fetal development, when an organism contains a higher proportion of dividing and expanding stem and progenitor cell populations [22]. Using a mouse model of β-thalassemia, the authors administered NPs containing γtcPNA and donor DNA to mice in utero via two administration routes: intraamniotic and intravenous via the vitelline vein. The procedure was safe: PLGA NP administration did not affect fetal plasma cytokine levels relative to controls. Moreover, PLGA NPs did not affect survival rates, weight gain, or gross anatomy of mice that had been treated in utero and successfully weaned. As intravenous delivery resulted in the greatest amount of NP accumulation in the fetal liver, the site of fetal hematopoiesis, the authors assessed phenotypic amelioration following intravenous NP administration. Hallmarks of disease pathology were reversed including underlying anemia, reticulocytosis, splenomegaly, and associated extramedullary hematopoiesis. Treated mice had 100% survival 500 days after in utero NP treatment [22].
Notably, Ricciardi et al. achieved an editing frequency of ~6% in total bone marrow and ~10% in hematopoietic progenitor cells after a single in utero NP treatment. Additionally, the in utero results were attained with a fraction of the dose of NPs used postnatally (185 μg versus 8 mg) [20,22]. The biology of a fetal stem cell may lend itself more readily to gene editing. RNA sequencing experiments suggest that fetal liver HSCs, in general, possess higher expression of DNA repair pathway-related genes than adult bone marrow HSCs. More specifically, KEGG pathway analysis found that genes related to mismatch repair, homologous recombination, nucleotide excision repair, and base excision repair pathways were more highly expressed in fetal liver HSCs when compared to adult HSCs [52].
In addition to a potentially favorable transcriptome, the population of fetal liver HSCs is rapidly expanding, with approximately 100% of HSCs cycling every 24 h, which is in stark contrast to the adult bone marrow HSCs that are 90–95% quiescent (reviewed in [53]). The ability to target or access this rapidly dividing stem cell population within the fetal liver with PNA/DNA NPs may account for the higher levels of gene editing observed prenatally and could represent an important therapeutic opportunity for site-specific gene editing of hematopoietic disorders before birth.
4.4. Ongoing Work and Democratization
Active research in the area of triplex PNA editing continues. Recent data shared by Piotrowski-Daspit et al. [54,55], Ricciardi et al. [56], and Quijano et al. [57] point to further in utero and in vivo applications of PNA technologies to models of cystic fibrosis, β-thalassemia, and sickle cell disease. Bolstered by novel approaches to NP delivery [54,55] and new insight into mechanistic underpinnings [58], PNA editing has strong potentially to develop even further in the near future. Meanwhile, using a similar approach to triplex editing, recent work by Fèlix et al. demonstrated the use of a new type of DNA-based triplex-forming molecule consisting of an elongated Watson–Crick binding region with a clamping anti-parallel Hoogsteen binding region [59]. These DNA oligos contrast single-stranded TFOs and are more similar in design to tcPNAs. Further, Watson–Crick binding DNA stretches featured the intended sequence modifications within the clamping molecule and thus did not require the introduction of a separate ssDNA donor. Authors described corrections of single point mutations in the adenosyl phosphoribosyl transferase (aprt) gene in CHO cells [59]. Newly emerging studies like these indicate exciting new directions for triplex editing as more groups begin to apply this conceptual approach.
Notably, while PNA technologies have proven impressively effective, a limited number of groups are actively using these reagents for the purposes of gene editing. Required expertise in synthetic chemistry and novel design limit widespread use and ultimately the rate of development and improvement. Reminiscent of the slow uptake of initial nuclease-mediated gene editors, such as zinc-finger nucleases, it was not until the advent of TALENs, CRISPR, and democratized access to these technologies that use and understanding in the field began to accelerate. In the case of PNAs, resources are beginning to emerge that will enable their widespread adoption. A web tool designed to identify polypurine, and thus triplex-accommodating, regions within gene targets was developed by the Vazquez group allowing easy identifications of PNA-triplex targeting sites across human and mouse genomes (http://utw10685.utweb.utexas.edu/tfo/) [60]. Further, the advancement and adoption of automated peptide synthesizer platforms has begun to simplify and expedite a PNA synthesis process that previously required extensive and time-intensive chemistry protocols and expertise. Additionally, described below in Table 1, this review contains list of convenient guidelines and recommendations for the design and application of triplex-forming PNAs for gene editing. The development of PNAs into a clinically applicable and curative therapeutic tool depends on the ability of these novel molecules to be easily adopted and wielded by investigators. Reducing barriers to access and encouraging the adoption of PNAs for gene editing has promise to further accelerate advancement towards the ultimate goal of widespread therapeutic application in humans.
Table 1.
Guidelines for Triplex PNA Design for Gene Editing
| Guidelines for Triplex PNA Design for Gene Editing |
|---|
|
|
|
|
|
|
5. Delivery
As described above, employing various delivery strategies for PNA reagents rapidly accelerated the development of the technology for gene editing applications. Although PNAs hold significant therapeutic potential, on their own their biological application is limited by an inability to passively diffuse across cellular membranes. Using liposomes as a model for this hydrophobic barrier, Wittung et al. (1995) initially demonstrated that short PNAs (10 bp) had efflux times of 5.5 and 11 days, which was comparable to a control DNA [61]. With slow inherent uptake kinetics, adoption of successful delivery methods is pivotal to the in vitro and in vivo use of PNAs.
5.1. Peptide-Mediated Delivery of Peptide Nucleic Acids
Inspired by the uptake of TAT peptides [62,63,64], a class of well-known cell-penetrating peptide (CPP), PNAs have been directly modified with arginine residues to enhance their cellular uptake [29,65]. Zhou et al. synthesized PNAs bearing arginine sidechains at the alpha position and demonstrated enhanced perinuclear cellular uptake comparable to a fluorescently-labeled TAT peptide [66]. When this modification was moved to the gamma position, these uptake properties were retained, with the added benefit of preorganizing the PNA into a right-handed helix [67].
In addition to direct modifications, PNAs have also been conjugated to CPPs to enhance their cellular uptake [66,67,68]. CPPs are short, cationic peptides that vary in length, typically ranging from nine to thirty amino acids [62]. While several CPPs have been investigated to date, the most successful of these for delivery of PNAs has been penetratin, a sixteen residue peptide derived from the Drosophila Antennapedia gene [69,70,71]. Using PNAs targeted against the HIV trans-activation response (TAR) element (an RNA stem-loop), Turner et al. demonstrated that PNAs conjugated to penetratin could potently inhibit Tat-dependent trans-activation in HeLa cells [70]. Interestingly, when coupled to a TAT peptide, the PNAs were completely inactive, unless accompanied by the addition of chloroquine, which was presumed to facilitate endosomal escape [70].
Similarly, Rogers et al. (2012) have previously employed penetratin to deliver PNAs in vitro and in vivo [72]. Using PNAs targeted against a chromosomally integrated supFG1 reporter gene in mouse cells, they showed that penetratin-PNA conjugates achieved higher levels of uptake and targeted mutagenesis than passive uptake of the PNA alone. Importantly, these penetratin-PNA conjugates demonstrated enhanced biodistribution and targeted genome modification in vivo in several somatic tissues and compartments of the hematopoietic system [72].
In addition to traditional CPPs, recent work has described a strategy to deliver PNAs using a peptide which preferentially accumulates in acidic microenvironments [73,74]. Under physiologic pH, pHLIP (pH (low) insertion peptide)), weakly associates with the cellular membrane [75]. As pH is reduced, pHLIP becomes protonated, resulting in a transmembrane α-helix with its C-terminus inserted across the cellular membrane [76]. Using this peptide, Cheng et al. (2015) delivered PNAs against oncomiR-155, resulting in significant delays in tumor growth in vivo [77].
While peptide-mediated delivery of PNAs remains a promising approach for in vitro and in vivo applications, the need for excessively high and repeated dosing to achieve therapeutic benefit (10–50 mg/kg) reduces the potential for clinical translation. The broad effectiveness of CPPs specifically as delivery agents is also limited by their endosomal entrapment [78], requiring the use of lysosomotropic agents such as chloroquine or Ca2+ to enhance effectiveness [70,79]. pHLIP, though useful for targeting acidic microenvironments, is not a viable strategy for delivering PNAs to non-acidic tissues. Moreover, we have recently shown that pHLIP may be less effective at delivering PNAs greater than 25 nucleotides in length [75]. Finally, while peptides may serve as effective delivery vehicles for PNAs, the need for co-delivery of donor DNA for gene editing would require additional conjugation strategies to deliver both molecules. To overcome these limitations, recent work with PNAs has focused on the development of polymeric nanoparticles (NPs) to efficiently entrap and deliver PNA as well as donor DNA molecules to cells in vitro and in vivo.
5.2. PLGA Nanoparticle-Mediated Delivery of PNAs
As an alternative to peptide-mediated delivery of PNAs, several groups have investigated the use of NPs. While many of these delivery systems have been reviewed elsewhere [80], efforts have focused on developing NPs from poly(lactic-co-glycolic acid) (PLGA), a biocompatible and biodegradable polymer, well-known to the FDA [81]. In addition to their favorable safety profiles [82,83], drug release from PLGA NPs is highly tunable by adjusting polymer molecular weight and ratio of lactic to glycolic acid [84]. Recently, Mandl et al. showed that NP size can be tuned to alter biodistribution and accumulation of NPs in sites of interest, such bone marrow or lung [85].
In 2011, McNeer et al. first demonstrated that PNA and donor DNA molecules could be formulated into PLGA NPs using a double-emulsion solvent evaporation technique. In this process, the PNA and donor DNA encapsulants are first mixed and subsequently added to the polymer under vortex, forming the first water-in-oil emulsion. Following sonication, the PLGA NPs are stabilized through dropwise addition into a second aqueous solution containing a surfactant. After a final sonication step, the NPs are flash frozen and lyophilized prior to use. By co-encapsulating these molecules into NPs, this approach was used to mediate recombination in human CD34+ cells, resulting in site-specific modification of the β-globin locus [41]. While the level of modification was relatively modest (0.5–1%), the improvements in cell viability were significant compared to standard electroporation, which reduced cell viability to ~20% after 24 h. This observation was in sharp contrast to cells treated with blank or PNA/DNA NPs, which demonstrated 100% cell viability [41]. The use of PLGA NPs loaded with PNA and donor DNA resulted in a 60-fold increase in editing compared to cells electroporated with these reagents.
While robust in its delivery of PNA and donor DNA molecules in vitro, a major benefit of using PLGA NPs is their ability to effectively deliver these molecules in vivo [86]. Based on previously discussed work modifying CCR5 [41], McNeer, Schleifman et. al, used NPs with surface coated with TAT or penetratin peptides to treat mice engrafted with human CD34+ cells [16]. While prior work had demonstrated that TAT conjugated PNAs were not effective in the absence of chloroquine [63], TAT conjugated NPs consistently produced higher levels of gene editing in vitro in human CD34+ cells [16]. When administered systemically, TAT modified NPs achieved CCR5 modification in multiple tissues including disease relevant CD4+ and CD34+ human cells [16]. Importantly, these NPs were non-toxic to bone marrow or spleen progenitors as demonstrated by colony forming assays. Beyond these initial experiments PLGA NPs have been extensively used to deliver PNA and donor DNA. As discussed in above sections, PLGA NP delivery has been successfully applied to multiple in vivo studies and demonstrated the ability to targeted native HSCs in mice with therapeutic effects in disease models. Notably, and consistent with prior work, Bahal et al. (2016) and Ricciardi et al. (2018) found PNA/DNA PLGA NPs did not induce inflammatory cytokine secretion in treated animals [20,22].
5.3. PBAE/PLGA/MPG Nanoparticle-Mediated Delivery of PNAs
While the use of PLGA has been extensively explored for the delivery of PNA and donor DNA, new materials are being investigated, including polymer blends of PLGA with poly(β-amino esters) (PBAE) [18,19,87]. In particular, NPs made from blends of PLGA and PBAE have shown exceptional promise for the delivery of PNA and donor DNA to lungs following intranasal administration [18,29]. In developing these NPs, Fields et al. carefully explored the blending of PLGA with PBAE to enhance NP transfection and loading of DNA [88,89], while attenuating the observed toxicity of this cationic polymer [87]. To further enhance uptake, these NPs were surface-coated with a DSPE-PEG lipids conjugated to CPPs. With these modifications, cellular uptake of NPs in vitro was substantially improved [87] and subsequently shown to enhance NP uptake in mice following intranasal administration [18]. In particular, NPs were found to associate with approximately 30% of all lung cells, in contrast to unmodified NPs, which demonstrated little to no cellular associated with lung [18]. Though levels of editing were modest (0.6% in alveolar macrophages and 0.3% in alveolar epithelial cells), this early work provided proof that this approach could be used to edit genes in the lung following intranasal NP administration. Further, as with PLGA NPs, treatment with PLGA/PBAE/MPG NPs did not increase inflammatory cytokine production following in vivo application [19].
6. Mechanisms of PNA-Mediated Gene Editing
6.1. PNA Triplex Repair and Recombination
PNA-mediated gene editing relies on the ability of endogenous cellular factors to detect anomalous PNA/DNA structures within the genome. Structure repair ultimately enables a recombination event with a nearby homologous ssDNA donor molecule to incorporate a permanent sequence modification. The mechanisms by which PNA detection and repair can instigate recombination are not yet fully elucidated. Thus far, PNA editing has been shown to depend, in part, on nucleotide excision repair (NER) [15,40]. NER is a non-mutagenic repair pathway responsible for the removal of bulky, helix-distorting lesions within DNA—an effect known to be imparted by PNA invasion and structure formation [8,88]. NER relies on a network of factors including context-specific recognition factors (XPC, CSA/CSB, XPA/RPA), specialized helicases (XPD, XPB), and endonucleases that generate single strand nicks (XPG, XPF). Xeroderma pigmentosum group A (XPA), a central NER factor responsible for lesion detection and factor assembly, has been shown to be important for PNA editing to occur. Experiments in XPA-depleted cell-free HeLa extracts by Rogers et al. (2002) showed marked reductions in PNA repair and recombination [15]. Chin et al. (2008) made a similar observation when XPA was knocked down by siRNA in a human K562 cell line [40]. Notably, no other targeted gene editing technology is known to utilize non-mutagenic NER pathways. Rather, nucleases such as Cas9, TALENs, and zinc-finger nucleases are known to feature homology directed repair (HDR) or mutagenic non-homologous end joining (NHEJ) pathways at double strand breaks with mechanistically unknown contributions from Fanconi Anemia repair factors [89,90]. Base Editor and deaminase approaches, meanwhile, predominantly rely on enzymatic deamination of nucleobases, base excision repair (BER), and mismatch repair (MMR) to achieve single base modifications [91].
6.2. TFO Triplex Repair and Recombination
While the repair and recombination mechanisms specific to PNA-mediated editing remain under investigation, earlier work on TFO-mediated recombination offers insights into other structure-induced recombination processes and how PNA editing may occur. Similar to PNA triplexes, NER seems to play an important role in TFO triplex repair and recombination. Experiments in human XPA-immunodepleted cell-free extracts [26] and in XPA knockout human fibroblasts [14] both show marked reductions in TFO repair and recombination. Plasmid-based experiments in mammalian cells by Faruqi et al. (1999) showed reduced TFO-mediated recombination in XPA, XPG, and XPF deficient cells—an effect that was rescued partially by XPA cDNA complementation. Interestingly, knockdown of MMR factors MSH2 and MLH1 did not have an effect on overall recombination efficiency [14].
6.3. Endogenous Triplex Repair and Recombination
Insights from how cells repair endogenous triplex structures reveal more potential mechanisms of triplex repair and resolution. In work recently published by the Vasquez group (2018) they describe replication-dependent and replication-independent pathways for the repair of DNA triplexes (H-DNA) that form spontaneously between gDNA strands [3]. While replication-independent repair and cleavage rely on NER factors XPG and XPF, flap endonuclease 1 (FEN1) is a key factor that binds and cleaves endogenous H-DNA during replication [3]. The role of FEN1 in PNA or TFO-mediated recombination has yet to be explored.
6.4. ssDNA Donor Recombination and Rad51
Rad51 recombinase, an HDR factor that binds ssDNA overhangs for homology search and invasion, appears to play a role in TFO-mediated recombination. In the case of TFOs tethered to ssDNA templates, Rad51 overexpression increases targeted recombination with an episomal vector [26]. Conversely, experiments with whole-cell extracts immunodepleted of Rad51 showed reduced recombination frequencies [26].
In the broader field of gene editing, however, the exact role Rad51 plays in recombination with ssDNA donors remains unclear. Interestingly, work from the Corn group (2018) in CRISPR/Cas9 systems shows that while Rad51 siRNA knockdown significantly reduced recombination frequencies when using dsDNA donors (plasmid), no such effect existed when using ssDNA donor molecules [90]. Complicating the picture further, work from Davis and Maizels demonstrated that when using single strand nickase mutants of Cas9 and a ssDNA donor, thus leaving one DNA strand intact, knockdown of Rad51 improved recombination frequencies eightfold [92]. The same effect was observed when BRCA2, an HDR factor directly upstream of Rad51, was knocked down as well [92]. The authors go on to suggest that a distinct HDR sub-pathway called single strand annealing (SSA) and factors such as Rad52 may be responsible for ssDNA incorporation in the presence of single stranded nick intermediates [92,93].
In summary, Rad51 may promote, antagonize, or have little effect on ssDNA donor incorporation, depending on the context and approach. These diverging results are likely explained by how ssDNA oligonucleotides are able to interact with the intermediate recombinogenic structures or breaks induced by gene editing technologies. The ecosystem of often competing repair factors associated with structures at these genomic locations dictate preferred pathways of repair and recombination. Ultimately, future experiments will be needed in order to clarify the role Rad51 and strand search HDR pathways play in gene editing with ssDNA donors. Rad51 contribution to PNA-mediated editing is currently under study.
7. Other PNAs for Gene Editing
7.1. pcPNAs for Gene Editing
An alternative strategy to improve the exergonicity of strand invasion using monomeric PNAs has been explored by designing PNA pairs to hybridize both strands of the target region (Figure 2F [94]). This strategy relies largely on reaction enthalpy to drive hybridization, by effectively doubling the number of base pairs in the thermodynamic product of binding relative to the starting duplex but imposes a significant penalty in translational entropy since strand invasion in this context is a tetramolecular reaction. However, successful strand invasion with the appropriate combination of monomeric PNAs [94] is clear evidence that upon DNA hybridization, enthalpic gain compensates for entropic loss (), as is often observed for DNA-ligand interactions [95]. Crucial for the effectiveness of this strategy is nucleobase modification to increase enthalpic contribution while preventing self-quenching of the PNA pair, which would otherwise be WC complementary. Specifically, the diaminopurine (Dap) nucleobase has been substituted for adenine (A) to provide an additional H bond donor for pairing with thymine (T) (Figure 3C [94]). The more stable Dap:T pair relative to A:T pair, coupled with the expanded surface area of Dap that stabilizes base pair stacking interactions, drives exergonicity by lowering . Furthermore, to prevent quenching of the PNA pair, thiouracil (sU) has been substituted for T (Figure 3C [94]), a modification which, although inducing mild destabilization of the PNA-DNA hybrid, abrogates any pairing between the modified nucleobases (Dap:sU) due to increased electron density (and repulsion) relative to Dap:T and A:sU (Figure 3C).
Applying these principles, so-called pseudo-complementary PNAs (pcPNAs) were successfully applied to gene editing in 2009 by Lonkar et al. using a cell culture model [96]. CHO cells containing the GFP-IVS2-1 β-thalassemia reporter, described previously in this review, were electroporated with a pair of opposite strand-binding pcPNAs and ssDNA donor. FACS analysis of treated conditions demonstrated up to 0.78% correctly edited, GFP+ cells [96]. Thus, pcPNAs are capable of inducing targeted recombination with a ssDNA template, likely in a manner distinct from triplex-forming PNAs. Few studies have followed up this approach to editing since this study. Future work with these reagents, however, may provide further insights into the breadth of structures capable of inducing recombination for gene editing. While there is some evidence that NER pathways may also contribute to pcPNA-mediated editing [96], how this overall mechanism compares to triplex forming PNAs remains uninvestigated.
7.2. ssPNAs for Gene Editing
Another approach, reported by Bertoni and colleagues, features the use of a single stranded WC-binding PNA (ssPNA) without a ssDNA donor [97,98]. This strategy uses ssPNAs designed to bind a mutation target within the genome forming a PNA/DNA heteroduplex opposite a displaced gDNA strand. ssPNAs invade gDNA helices to target a single strand of DNA directly by WC pairing, and thus have no sequence restriction and do not need to accommodate Hoogsteen binding. These molecules are designed as ~25 mers homologous to a coding or template strand target with the exception of the intended modified base. Initial work by Kayali et al. in 2014 used ssPNAs to correct a splicing mutation in the dystrophin gene characteristic of the disorder Duchenne muscular dystrophy (DMD) [97]. Transfection of anti-template strand and anti-coding strand 18 mer ssPNAs via Lipofectamine™ 2000 into myoblasts derived from a DMD mouse model resulted in ~3% and ~7% editing respectively. Authors also described dystrophin-positive muscle fibers in DMD mice after direct injection of free ssPNAs directly into the tibialis anterior muscle. Two weeks after treatment, qPCR methods determined 2.8% of template strand and 3.3% of coding strand targeted alleles to be edited. Dystrophin-positive edited fibers were detected in vivo four months after treatment [97]. Further studies from the same group determined these reagents were capable of editing satellite cells ex vivo from the DMD mouse model and that modified cells were capable of self-renewal and differentiation [98]. Engrafted satellite cells in DMD mice differentiated and persisted at least 24 weeks. Notably, the frequency of dystrophin-positive muscle fibers increased with time and this effect correlated with improved muscle morphology in the tibialis anterior of mice [98].
Studies featuring the use of ssPNAs are of considerable interest not only because they expand the potential therapeutic repertoire of PNA technologies but because of their presumably distinct mechanisms of action. Taken together, these results suggest the exciting possibility that polymerases may be able to use PNAs as a viable substrate to introduce deoxynucleotides opposite PNA nucleobases. In this particular case, one could imagine MMR and BER pathways of repair implicated in processing single base mismatches within novel PNA/DNA duplex structures. Similar to PNA triplex-mediated editing, however, more work concerning the repair mechanisms by which these structures are processed is needed to further understand and rationally improve this approach.
8. Perspectives and Limitations
PNA reagents are a conceptually distinct approach to gene editing. Rather than relying on the catalytic activity of enzymes on genomic DNA substrates, PNAs non-enzymatically generate unusual nucleic acid structures within the genome to promote recombination. As a result of this novel approach, preliminary studies suggest the repair mechanisms by which PNAs accomplish gene editing is also distinct. In fact, PNA editing may utilize inherently non-mutagenic pathways such as nucleotide excision repair (NER), offering a powerful advantage over technologies like CRISPR/Cas9 that are characteristically plagued by mutagenic repair outcomes [15,40,89].
PNA-mediated gene editing has progressed rapidly over the past two decades. Advances in optimized PNA chemistry and binding kinetics as well as sophisticated non-toxic means of delivery are responsible for propelling the applications of PNA editing reagents forward. Since initial observations of induced recombination using plasmid systems in cell-free extracts, PNAs have evolved into potent tools capable of ex vivo, in vivo, and in utero application in mice with therapeutically consequential effects [16,17,18,19,20,21,22]. Despite impressive progress informed by the chemical and biomedical engineering aspects of PNA technologies, however, approaches to advancement driven by biological, mechanistic insight remain mostly untapped.
Understanding the mechanisms of PNA structure recognition, repair, and recombination hold enormous promise for the future of the technology and more broadly in the rapidly developing field of gene editing. While improvements to PNA chemistry have substantially progressed editing potency over time, a detailed understanding of how these modifications are preferentially detected within the genome and lead to or improve recombination with ssDNA donors remains elusive. Ultimately, a truly rational approach to reagent design must be informed by the mechanistic underpinnings of PNA-induced repair and recombination. This information has the capacity to further inform PNA reagent chemistry and design as well as provide opportunities to further improve potency by biasing relevant pathways of repair. Moreover, investigating the likely novel means by which structure-specific repair interfaces with homologous recombination may reveal pathways to non-mutagenic gene editing not previously considered.
Author Contributions
N.G.E. prepared the first draft. The final publication was prepared with contribution from all authors. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the NIGMS Medical Scientist Training Program (T32GM07205 to N.G.E., A.S.R. and E.Q.), the National Heart, Lung and Blood Institute (1F30HL149185 to N.G.E., F30HL134252 to A.S.R., and R01HL125892 to W.M.S. and P.M.G.), and NIGMS predoctoral training grant (5T32GM007223 to E.Q.).
Conflicts of Interest
N.G.E., A.S.R., E.Q., W.M.S., and P.M.G. are inventors on patents pertaining to gene editing that are assigned to Yale University. P.M.G. and W.M.S. are consultants for and have equity interests in Trucode Gene Repair, Inc.
Footnotes
Sample Availability: Samples of the compounds are not available.
References
- 1.Watson J.D., Crick F.H.C. Molecular Structure of Nucleic Acids: A Structure for Deoxyribose Nucleic Acid. Nature. 1953;171:737–738. doi: 10.1038/171737a0. [DOI] [PubMed] [Google Scholar]
- 2.Choi J., Majima T. Conformational changes of non-B DNA. Chem. Soc. Rev. 2011;40:5893–5909. doi: 10.1039/c1cs15153c. [DOI] [PubMed] [Google Scholar]
- 3.Zhao J., Wang G., Del Mundo I.M., McKinney J.A., Lu X., Bacolla A., Boulware S.B., Zhang C., Zhang H., Ren P., et al. Distinct Mechanisms of Nuclease-Directed DNA-Structure-Induced Genetic Instability in Cancer Genomes. Cell Rep. 2018;22:1200–1210. doi: 10.1016/j.celrep.2018.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Boyer A.-S., Grgurevic S., Cazaux C., Hoffmann J.-S. The Human Specialized DNA Polymerases and Non-B DNA: Vital Relationships to Preserve Genome Integrity. J. Mol. Biol. 2013;425:4767–4781. doi: 10.1016/j.jmb.2013.09.022. [DOI] [PubMed] [Google Scholar]
- 5.Frank-Kamenetskii M.D., Mirkin S.M. Triplex Dna Structures. Annu. Rev. Biochem. 1995;64:65–95. doi: 10.1146/annurev.bi.64.070195.000433. [DOI] [PubMed] [Google Scholar]
- 6.Havre P.A., Gunther E.J., Gasparro F.P., Glazer P.M. Targeted mutagenesis of DNA using triple helix-forming oligonucleotides linked to psoralen. Proc. Natl. Acad. Sci. USA. 1993;90:7879–7883. doi: 10.1073/pnas.90.16.7879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chan P.P., Lin M., Faruqi A.F., Powell J., Seidman M.M., Glazer P.M. Targeted correction of an episomal gene in mammalian cells by a short DNA fragment tethered to a triplex-forming oligonucleotide. J. Biol. Chem. 1999;274:11541–11548. doi: 10.1074/jbc.274.17.11541. [DOI] [PubMed] [Google Scholar]
- 8.Nielsen P.E., Egholm M., Berg R.H., Buchardt O. Sequence-selective recognition of DNA by strand displacement with a thymine-substituted polyamide. Science. 1991;254:1497–1500. doi: 10.1126/science.1962210. [DOI] [PubMed] [Google Scholar]
- 9.Egholm M., Buchardt O., Nielsen P.E., Berg R.H. Peptide nucleic acids (PNA). Oligonucleotide analogs with an achiral peptide backbone. J. Am. Chem. Soc. 1992;114:1895–1897. doi: 10.1021/ja00031a062. [DOI] [Google Scholar]
- 10.Kim S.K., Nielsen P.E., Egholm M., Buchardt O., Berg R.H., Norden B. Right-handed triplex formed between peptide nucleic acid PNA-T8 and poly(dA) shown by linear and circular dichroism spectroscopy. J. Am. Chem. Soc. 1993;115:6477–6481. doi: 10.1021/ja00068a001. [DOI] [Google Scholar]
- 11.Egholm M., Buchardt O., Christensen L., Behrens C., Freier S.M., Driver D.A., Berg R.H., Kim S.K., Norden B., Nielsen P.E. PNA hybridizes to complementary oligonucleotides obeying the Watson-Crick hydrogen-bonding rules. Nature. 1993;365:566–568. doi: 10.1038/365566a0. [DOI] [PubMed] [Google Scholar]
- 12.Demidov V.V., Potaman V.N., Frank-Kamenetskil M.D., Egholm M., Buchard O., Sönnichsen S.H., Nlelsen P.E. Stability of peptide nucleic acids in human serum and cellular extracts. Biochem. Pharmacol. 1994;48:1310–1313. doi: 10.1016/0006-2952(94)90171-6. [DOI] [PubMed] [Google Scholar]
- 13.Wang G., Seidman M.M., Glazer P.M. Mutagenesis in mammalian cells induced by triple helix formation and transcription-coupled repair. Science. 1996;271:802–805. doi: 10.1126/science.271.5250.802. [DOI] [PubMed] [Google Scholar]
- 14.Faruqi A.F., Datta H.J., Carroll D., Seidman M.M., Glazer P.M. Triple-Helix Formation Induces Recombination in Mammalian Cells via a Nucleotide Excision Repair-Dependent Pathway. Mol. Cell. Biol. 2000;20:990–1000. doi: 10.1128/MCB.20.3.990-1000.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rogers F.A., Vasquez K.M., Egholm M., Glazer P.M. Site-directed recombination via bifunctional PNA–DNA conjugates. Proc. Natl. Acad. Sci. USA. 2002;99:16695–16700. doi: 10.1073/pnas.262556899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McNeer N.A., Schleifman E.B., Cuthbert A., Brehm M., Jackson A., Cheng C., Anandalingam K., Kumar P., Shultz L.D., Greiner D.L., et al. Systemic delivery of triplex-forming PNA and donor DNA by nanoparticles mediates site-specific genome editing of human hematopoietic cells in vivo. Gene. 2013;20:658–669. doi: 10.1038/gt.2012.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bahal R., Quijano E., McNeer N.A., Liu Y., Bhunia D.C., López-Giráldez F., Fields R.J., Saltzman W.M., Ly D.H., Glazer P.M. Single-Stranded γPNAs for In Vivo Site-Specific Genome Editing via Watson-Crick Recognition. Curr. Gene. 2014;14:331–342. doi: 10.2174/1566523214666140825154158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fields R.J., Quijano E., McNeer N.A., Caputo C., Bahal R., Anandalingam K., Egan M.E., Glazer P.M., Saltzman W.M. Modified Poly(lactic-co-glycolic acid) Nanoparticles for Enhanced Cellular Uptake and Gene Editing in the Lung. Adv. Healthc. Mater. 2015;4:361–366. doi: 10.1002/adhm.201400355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McNeer N.A., Anandalingam K., Fields R.J., Caputo C., Kopic S., Gupta A., Quijano E., Polikoff L., Kong Y., Bahal R., et al. Correction of F508del CFTR in airway epithelium using nanoparticles delivering triplex-forming PNAs. Nat. Commun. 2015;6:6952. doi: 10.1038/ncomms7952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bahal R., Ali McNeer N., Quijano E., Liu Y., Sulkowski P., Turchick A., Lu Y.-C., Bhunia D.C., Manna A., Greiner D.L., et al. In vivo correction of anaemia in β-thalassemic mice by γPNA-mediated gene editing with nanoparticle delivery. Nat. Commun. 2016;7:1–14. doi: 10.1038/ncomms13304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Schleifman E.B., McNeer N.A., Jackson A., Yamtich J., Brehm M.A., Shultz L.D., Greiner D.L., Kumar P., Saltzman W.M., Glazer P.M. Site-specific Genome Editing in PBMCs With PLGA Nanoparticle-delivered PNAs Confers HIV-1 Resistance in Humanized Mice. Mol. Nucleic Acids. 2013;2:e135. doi: 10.1038/mtna.2013.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ricciardi A.S., Bahal R., Farrelly J.S., Quijano E., Bianchi A.H., Luks V.L., Putman R., López-Giráldez F., Coşkun S., Song E., et al. In utero nanoparticle delivery for site-specific genome editing. Nat. Commun. 2018;9:1–11. doi: 10.1038/s41467-018-04894-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Havre P.A., Glazer P.M. Targeted mutagenesis of simian virus 40 DNA mediated by a triple helix-forming oligonucleotide. J. Virol. 1993;67:7324–7331. doi: 10.1128/JVI.67.12.7324-7331.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kohwi Y., Panchenko Y. Transcription-dependent recombination induced by triple-helix formation. Genes Dev. 1993;7:1766–1778. doi: 10.1101/gad.7.9.1766. [DOI] [PubMed] [Google Scholar]
- 25.Faruqi A.F., Seidman M.M., Segal D.J., Carroll D., Glazer P.M. Recombination induced by triple-helix-targeted DNA damage in mammalian cells. Mol. Cell. Biol. 1996;16:6820–6828. doi: 10.1128/MCB.16.12.6820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Datta H.J., Chan P.P., Vasquez K.M., Gupta R.C., Glazer P.M. Triplex-induced recombination in human cell-free extracts. Dependence on XPA and HsRad51. J. Biol. Chem. 2001;276:18018–18023. doi: 10.1074/jbc.M011646200. [DOI] [PubMed] [Google Scholar]
- 27.Wang G., Levy D.D., Seidman M.M., Glazer P.M. Targeted mutagenesis in mammalian cells mediated by intracellular triple helix formation. Mol. Cell. Biol. 1995;15:1759–1768. doi: 10.1128/MCB.15.3.1759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chin J.Y., Glazer P.M. Repair of DNA lesions associated with triplex-forming oligonucleotides. Mol. Carcinog. 2009;48:389–399. doi: 10.1002/mc.20501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sahu B., Sacui I., Rapireddy S., Zanotti K.J., Bahal R., Armitage B.A., Ly D.H. Synthesis and characterization of conformationally preorganized, (R)-diethylene glycol-containing γ-peptide nucleic acids with superior hybridization properties and water solubility. J. Org. Chem. 2011;76:5614–5627. doi: 10.1021/jo200482d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nielsen P.E. Peptide Nucleic Acids: Protocols and Applications. Garland Science; New York, NY, USA: 2004. [Google Scholar]
- 31.Nielsen P.E., Egholm M., Buchardt O. Evidence for (PNA)2/DNA triplex structure upon binding of PNA to dsDNA by strand displacement. J. Mol. Recognit. 1994;7:165–170. doi: 10.1002/jmr.300070303. [DOI] [PubMed] [Google Scholar]
- 32.Peffer N.J., Hanvey J.C., Bisi J.E., Thomson S.A., Hassman C.F., Noble S.A., Babiss L.E. Strand-invasion of duplex DNA by peptide nucleic acid oligomers. Proc. Natl. Acad. Sci. USA. 1993;90:10648–10652. doi: 10.1073/pnas.90.22.10648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ananthanawat C., Hoven V.P., Vilaivan T., Su X. Surface plasmon resonance study of PNA interactions with double-stranded DNA. Biosens. Bioelectron. 2011;26:1918–1923. doi: 10.1016/j.bios.2010.05.027. [DOI] [PubMed] [Google Scholar]
- 34.Ricciardi A.S., Quijano E., Putman R., Saltzman W.M., Glazer P.M. Peptide Nucleic Acids as a Tool for Site-Specific Gene Editing. Molecules. 2018;23:632. doi: 10.3390/molecules23030632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Griffith M.C., Risen L.M., Greig M.J., Lesnik E.A., Sprankle K.G., Griffey R.H., Kiely J.S., Freier S.M. Single and Bis Peptide Nucleic Acids as Triplexing Agents: Binding and Stoichiometry. J. Am. Chem. Soc. 1995;117:831–832. doi: 10.1021/ja00107a033. [DOI] [Google Scholar]
- 36.Egholm M., Christensen L., Dueholm K.L., Buchardt O., Coull J., Nielsen P.E. Efficient pH-independent sequence-specific DNA binding by pseudoisocytosine-containing bis-PNA. Nucleic Acids Res. 1995;23:217–222. doi: 10.1093/nar/23.2.217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Betts L., Josey J.A., Veal J.M., Jordan S.R. A nucleic acid triple helix formed by a peptide nucleic acid-DNA complex. Science. 1995;270:1838–1841. doi: 10.1126/science.270.5243.1838. [DOI] [PubMed] [Google Scholar]
- 38.Hansen G.I., Bentin T., Larsen H.J., Nielsen P.E. Structural isomers of bis-PNA bound to a target in duplex DNA11Edited by I. Tinoco. J. Mol. Biol. 2001;307:67–74. doi: 10.1006/jmbi.2000.4487. [DOI] [PubMed] [Google Scholar]
- 39.Pack G.R., Wong L., Lamm G. PKa of cytosine on the third strand of triplex DNA: Preliminary Poisson–Boltzmann calculations. Int. J. Quantum Chem. 1998;70:1177–1184. doi: 10.1002/(SICI)1097-461X(1998)70:6<1177::AID-QUA7>3.0.CO;2-X. [DOI] [Google Scholar]
- 40.Chin J.Y., Kuan J.Y., Lonkar P.S., Krause D.S., Seidman M.M., Peterson K.R., Nielsen P.E., Kole R., Glazer P.M. Correction of a splice-site mutation in the beta-globin gene stimulated by triplex-forming peptide nucleic acids. Proc. Natl. Acad. Sci. USA. 2008;105:13514–13519. doi: 10.1073/pnas.0711793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McNeer N.A., Chin J.Y., Schleifman E.B., Fields R.J., Glazer P.M., Saltzman W.M. Nanoparticles deliver triplex-forming PNAs for site-specific genomic recombination in CD34+ human hematopoietic progenitors. Mol. Ther. 2011;19:172–180. doi: 10.1038/mt.2010.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chin J.Y., Reza F., Glazer P.M. Triplex-forming Peptide Nucleic Acids Induce Heritable Elevations in Gamma-globin Expression in Hematopoietic Progenitor Cells. Mol. Ther. 2013;21:580–587. doi: 10.1038/mt.2012.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bentin T., Larsen H.J., Nielsen P.E. Combined Triplex/Duplex Invasion of Double-Stranded DNA by “Tail-Clamp” Peptide Nucleic Acid. Biochemistry. 2003;42:13987–13995. doi: 10.1021/bi0351918. [DOI] [PubMed] [Google Scholar]
- 44.Kaihatsu K., Shah R.H., Zhao X., Corey D.R. Extending recognition by peptide nucleic acids (PNAs): Binding to duplex DNA and inhibition of transcription by tail-clamp PNA-peptide conjugates. Biochemistry. 2003;42:13996–14003. doi: 10.1021/bi035194k. [DOI] [PubMed] [Google Scholar]
- 45.Schleifman E.B., Bindra R., Leif J., Del Campo J., Rogers F.A., Uchil P., Kutsch O., Shultz L.D., Kumar P., Greiner D.L., et al. Targeted disruption of the CCR5 gene in human hematopoietic stem cells stimulated by peptide nucleic acids. Chem. Biol. 2011;18:1189–1198. doi: 10.1016/j.chembiol.2011.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dragulescu-Andrasi A., Rapireddy S., Frezza B.M., Gayathri C., Gil R.R., Ly D.H. A Simple γ-Backbone Modification Preorganizes Peptide Nucleic Acid into a Helical Structure. J. Am. Chem. Soc. 2006;128:10258–10267. doi: 10.1021/ja0625576. [DOI] [PubMed] [Google Scholar]
- 47.He G., Rapireddy S., Bahal R., Sahu B., Ly D.H. Strand invasion of extended, mixed-sequence B-DNA by gammaPNAs. J. Am. Chem. Soc. 2009;131:12088–12090. doi: 10.1021/ja900228j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bahal R., Sahu B., Rapireddy S., Lee C.-M., Ly D.H. Sequence-unrestricted, Watson-Crick recognition of double helical B-DNA by (R)-miniPEG-γPNAs. Chembiochem. 2012;13:56–60. doi: 10.1002/cbic.201100646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yeh J.I., Shivachev B., Rapireddy S., Crawford M.J., Gil R.R., Du S., Madrid M., Ly D.H. Crystal structure of chiral gammaPNA with complementary DNA strand: Insights into the stability and specificity of recognition and conformational preorganization. J. Am. Chem. Soc. 2010;132:10717–10727. doi: 10.1021/ja907225d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Demidov V.V., Yavnilovich M.V., Belotserkovskii B.P., Frank-Kamenetskii M.D., Nielsen P.E. Kinetics and mechanism of polyamide (“peptide”) nucleic acid binding to duplex DNA. Proc. Natl. Acad. Sci. USA. 1995;92:2637–2641. doi: 10.1073/pnas.92.7.2637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Knauert M.P., Lloyd J.A., Rogers F.A., Datta H.J., Bennett M.L., Weeks D.L., Glazer P.M. Distance and Affinity Dependence of Triplex-Induced Recombination. Biochemistry. 2005;44:3856–3864. doi: 10.1021/bi0481040. [DOI] [PubMed] [Google Scholar]
- 52.Manesia J.K., Xu Z., Broekaert D., Boon R., Van Vliet A., Eelen G., Vanwelden T., Stegen S., Van Gastel N., Pascual-Montano A., et al. Highly proliferative primitive fetal liver hematopoietic stem cells are fueled by oxidative metabolic pathways. Stem Cell Res. 2015;15:715–721. doi: 10.1016/j.scr.2015.11.001. [DOI] [PubMed] [Google Scholar]
- 53.Pietras E.M., Warr M.R., Passegué E. Cell cycle regulation in hematopoietic stem cells. J. Cell Biol. 2011;195:709–720. doi: 10.1083/jcb.201102131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Piotrowski-Daspit A.S., Barone C., Kauffman A.C., Lin C.Y., Nguyen R., Gupta A., Glazer P.M., Saltzman W.M., Egan M.E. Gene Correction of Cystic Fibrosis Mutations In Vitro and In Vivo Mediated by PNA Nanoparticles; Proceedings of the American Society of Gene and Cell Therapy 22nd Annual Meeting; Washington, DC, USA. 29 April–2 May 2019; Abstract Number 81. [Google Scholar]
- 55.Piotrowski-Daspit A.S., Kauffman A.C., Lin C.Y., Liu Y., Glazer P.M., Saltzman W.M. Gene Correction of Beta-Thalassemia Ex Vivo and In Vivo Mediated by PNA Nanoparticles; Proceedings of the American Society of Gene and Cell Therapy 22nd Annual Meeting; Washington, DC, USA. 29 April–2 May 2019; Abstract Number 976. [Google Scholar]
- 56.Ricciardi A., Barone C., Putnam R., Quijano E., Nguyen R., Gupta A., Mandl H., Freedman-Weiss M., Lopez-Giraldez F., Stitelman D., et al. Systemic in Utero Gene Editing as a Treatment for Cystic Fibrosis; Proceedings of the American Society of Gene and Cell Therapy 22nd Annual Meeting; Washington, DC, USA. 29 April–2 May 2019; Abstract Number 83. [Google Scholar]
- 57.Quijano E., Bahal R., Liu Y., Gupta A., Oyaghire S., Ricciardi A.S., Mandl H.K., Suh H.W., Lezon-Geyda K., Economos N., et al. Nanoparticle-Mediated Correction of the Sickle Cell Mutation; Proceedings of the American Society of Gene and Cell Therapy 22nd Annual Meeting; Washington, DC, USA. 29 April–2 May 2019; Abstract Number 594. [Google Scholar]
- 58.Economos N., Bahal R., Quijano E., Saltzman W.M., Glazer P. Mechanisms of Non-Enzymatic PNA-Mediated Gene Editing; Proceedings of the American Society of Gene and Cell Therapy 22nd Annual Meeting; Washington, DC, USA. 29 April–2 May 2019; Abstract Number 631. [Google Scholar]
- 59.Félix A.J., Ciudad C.J., Noé V. Correction of the aprt Gene Using Repair-Polypurine Reverse Hoogsteen Hairpins in Mammalian Cells. Mol. Ther. Nucleic Acids. 2020;19:683–695. doi: 10.1016/j.omtn.2019.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gaddis S.S., Wu Q., Thames H.D., DiGiovanni J., Walborg E.F., MacLeod M.C., Vasquez K.M. A web-based search engine for triplex-forming oligonucleotide target sequences. Oligonucleotides. 2006;16:196–201. doi: 10.1089/oli.2006.16.196. [DOI] [PubMed] [Google Scholar]
- 61.Wittung P., Kajanus J., Edwards K., Haaima G., Nielsen P.E., Nordén B., Malmström B.G. Phospholipid membrane permeability of peptide nucleic acid. FEBS Lett. 1995;375:27–29. doi: 10.1016/0014-5793(95)00409-3. [DOI] [PubMed] [Google Scholar]
- 62.Fonseca S.B., Pereira M.P., Kelley S.O. Recent advances in the use of cell-penetrating peptides for medical and biological applications. Adv. Drug Deliv. Rev. 2009;61:953–964. doi: 10.1016/j.addr.2009.06.001. [DOI] [PubMed] [Google Scholar]
- 63.Zhang X.-Y., Dinh A., Cronin J., Li S.-C., Reiser J. Cellular uptake and lysosomal delivery of galactocerebrosidase tagged with the HIV Tat protein transduction domain. J. Neurochem. 2008;104:1055–1064. doi: 10.1111/j.1471-4159.2007.05030.x. [DOI] [PubMed] [Google Scholar]
- 64.Wender P.A., Mitchell D.J., Pattabiraman K., Pelkey E.T., Steinman L., Rothbard J.B. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. USA. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Zhou P., Wang M., Du L., Fisher G.W., Waggoner A., Ly D.H. Novel Binding and Efficient Cellular Uptake of Guanidine-Based Peptide Nucleic Acids (GPNA) J. Am. Chem. Soc. 2003;125:6878–6879. doi: 10.1021/ja029665m. [DOI] [PubMed] [Google Scholar]
- 66.Fabani M.M., Gait M.J. miR-122 targeting with LNA/2′-O-methyl oligonucleotide mixmers, peptide nucleic acids (PNA), and PNA–peptide conjugates. RNA. 2008;14:336–346. doi: 10.1261/rna.844108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Aldrian-Herrada G., Desarménien M.G., Orcel H., Boissin-Agasse L., Méry J., Brugidou J., Rabié A. A peptide nucleic acid (PNA) is more rapidly internalized in cultured neurons when coupled to a retro-inverso delivery peptide. The antisense activity depresses the target mRNA and protein in magnocellular oxytocin neurons. Nucleic Acids Res. 1998;26:4910–4916. doi: 10.1093/nar/26.21.4910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Pooga M., Soomets U., Hällbrink M., Valkna A., Saar K., Rezaei K., Kahl U., Hao J.X., Xu X.J., Wiesenfeld-Hallin Z., et al. Cell penetrating PNA constructs regulate galanin receptor levels and modify pain transmission in vivo. Nat. Biotechnol. 1998;16:857–861. doi: 10.1038/nbt0998-857. [DOI] [PubMed] [Google Scholar]
- 69.Shiraishi T., Nielsen P.E. Cellular delivery of peptide nucleic acids (PNAs) Methods Mol. Biol. 2014;1050:193–205. doi: 10.1007/978-1-62703-553-8_16. [DOI] [PubMed] [Google Scholar]
- 70.Turner J.J., Ivanova G.D., Verbeure B., Williams D., Arzumanov A.A., Abes S., Lebleu B., Gait M.J. Cell-penetrating peptide conjugates of peptide nucleic acids (PNA) as inhibitors of HIV-1 Tat-dependent trans-activation in cells. Nucleic Acids Res. 2005;33:6837–6849. doi: 10.1093/nar/gki991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Simmons C.G., Pitts A.E., Mayfield L.D., Shay J.W., Corey D.R. Synthesis and membrane permeability of PNA-peptide conjugates. Bioorganic Med. Chem. Lett. 1997;7:3001–3006. doi: 10.1016/S0960-894X(97)10136-6. [DOI] [Google Scholar]
- 72.Rogers F.A., Lin S.S., Hegan D.C., Krause D.S., Glazer P.M. Targeted gene modification of hematopoietic progenitor cells in mice following systemic administration of a PNA-peptide conjugate. Mol. Ther. 2012;20:109–118. doi: 10.1038/mt.2011.163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Adochite R.-C., Moshnikova A., Golijanin J., Andreev O.A., Katenka N.V., Reshetnyak Y.K. Comparative Study of Tumor Targeting and Biodistribution of pH (Low) Insertion Peptides (pHLIP(®) Peptides) Conjugated with Different Fluorescent Dyes. Mol. Imaging Biol. 2016;18:686–696. doi: 10.1007/s11307-016-0949-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Svoronos A.A., Bahal R., Pereira M.C., Barrera F.N., Deacon J.C., Bosenberg M., DiMaio D., Glazer P.M., Engelman D.M. Tumor-Targeted, Cytoplasmic Delivery of Large, Polar Molecules Using a pH-Low Insertion Peptide. Mol. Pharm. 2020 doi: 10.1021/acs.molpharmaceut.9b00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Reshetnyak Y.K., Andreev O.A., Lehnert U., Engelman D.M. Translocation of molecules into cells by pH-dependent insertion of a transmembrane helix. Proc. Natl. Acad. Sci. USA. 2006;103:6460–6465. doi: 10.1073/pnas.0601463103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Reshetnyak Y.K., Segala M., Andreev O.A., Engelman D.M. A Monomeric Membrane Peptide that Lives in Three Worlds: In Solution, Attached to, and Inserted across Lipid Bilayers. Biophys. J. 2007;93:2363–2372. doi: 10.1529/biophysj.107.109967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Cheng C.J., Bahal R., Babar I.A., Pincus Z., Barrera F., Liu C., Svoronos A., Braddock D.T., Glazer P.M., Engelman D.M., et al. MicroRNA silencing for cancer therapy targeted to the tumour microenvironment. Nature. 2015;518:107–110. doi: 10.1038/nature13905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Erazo-Oliveras A., Muthukrishnan N., Baker R., Wang T.-Y., Pellois J.-P. Improving the endosomal escape of cell-penetrating peptides and their cargos: Strategies and challenges. Pharmaceuticals. 2012;5:1177–1209. doi: 10.3390/ph5111177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shiraishi T., Pankratova S., Nielsen P.E. Calcium ions effectively enhance the effect of antisense peptide nucleic acids conjugated to cationic tat and oligoarginine peptides. Chem. Biol. 2005;12:923–929. doi: 10.1016/j.chembiol.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 80.Gupta A., Bahal R., Gupta M., Glazer P.M., Saltzman W.M. Nanotechnology for delivery of peptide nucleic acids (PNAs) J. Control. Release. 2016;240:302–311. doi: 10.1016/j.jconrel.2016.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Schoubben A., Ricci M., Giovagnoli S. Meeting the unmet: From traditional to cutting-edge techniques for poly lactide and poly lactide-co-glycolide microparticle manufacturing. J. Pharm. Investig. 2019;49:381–404. doi: 10.1007/s40005-019-00446-y. [DOI] [Google Scholar]
- 82.Semete B., Booysen L., Lemmer Y., Kalombo L., Katata L., Verschoor J., Swai H.S. In vivo evaluation of the biodistribution and safety of PLGA nanoparticles as drug delivery systems. Nanomedicine. 2010;6:662–671. doi: 10.1016/j.nano.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 83.Woodrow K.A., Cu Y., Booth C.J., Saucier-Sawyer J.K., Wood M.J., Saltzman W.M. Intravaginal gene silencing using biodegradable polymer nanoparticles densely loaded with small-interfering RNA. Nat. Mater. 2009;8:526–533. doi: 10.1038/nmat2444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Swider E., Koshkina O., Tel J., Cruz L.J., De Vries I.J.M., Srinivas M. Customizing poly(lactic-co-glycolic acid) particles for biomedical applications. Acta Biomater. 2018;73:38–51. doi: 10.1016/j.actbio.2018.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Mandl H.K., Quijano E., Suh H.W., Sparago E., Oeck S., Grun M., Glazer P.M., Saltzman W.M. Optimizing biodegradable nanoparticle size for tissue-specific delivery. J. Control. Release. 2019;314:92–101. doi: 10.1016/j.jconrel.2019.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.McNeer N.A., Schleifman E.B., Glazer P.M., Saltzman W.M. Polymer delivery systems for site-specific genome editing. J. Control. Release. 2011;155:312–316. doi: 10.1016/j.jconrel.2011.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Fields R.J., Cheng C.J., Quijano E., Weller C., Kristofik N., Duong N., Hoimes C., Egan M.E., Saltzman W.M. Surface modified poly(β amino ester)-containing nanoparticles for plasmid DNA delivery. J. Control. Release. 2012;164:41–48. doi: 10.1016/j.jconrel.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Petit C., Sancar A. Nucleotide excision repair: From E. coli to man. Biochimie. 1999;81:15–25. doi: 10.1016/S0300-9084(99)80034-0. [DOI] [PubMed] [Google Scholar]
- 89.Cubbon A., Ivancic-Bace I., Bolt E.L. CRISPR-Cas immunity, DNA repair and genome stability. Biosci. Rep. 2018;38 doi: 10.1042/BSR20180457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Richardson C.D., Kazane K.R., Feng S.J., Zelin E., Bray N.L., Schäfer A.J., Floor S.N., Corn J.E. CRISPR–Cas9 genome editing in human cells occurs via the Fanconi anemia pathway. Nat. Genet. 2018;50:1132–1139. doi: 10.1038/s41588-018-0174-0. [DOI] [PubMed] [Google Scholar]
- 91.Rees H.A., Liu D.R. Base editing: Precision chemistry on the genome and transcriptome of living cells. Nat. Rev. Genet. 2018;19:770–788. doi: 10.1038/s41576-018-0059-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Davis L., Maizels N. Homology-directed repair of DNA nicks via pathways distinct from canonical double-strand break repair. Proc. Natl. Acad. Sci. USA. 2014;111:E924–E932. doi: 10.1073/pnas.1400236111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Davis L., Maizels N. Two Distinct Pathways Support Gene Correction by Single-Stranded Donors at DNA Nicks. Cell Rep. 2016;17:1872–1881. doi: 10.1016/j.celrep.2016.10.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Lohse J., Dahl O., Nielsen P.E. Double duplex invasion by peptide nucleic acid: A general principle for sequence-specific targeting of double-stranded DNA. Proc. Natl. Acad. Sci. USA. 1999;96:11804–11808. doi: 10.1073/pnas.96.21.11804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Breslauer K.J., Remeta D.P., Chou W.Y., Ferrante R., Curry J., Zaunczkowski D., Snyder J.G., Marky L.A. Enthalpy-entropy compensations in drug-DNA binding studies. Proc. Natl. Acad. Sci. USA. 1987;84:8922–8926. doi: 10.1073/pnas.84.24.8922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Lonkar P., Kim K.-H., Kuan J.Y., Chin J.Y., Rogers F.A., Knauert M.P., Kole R., Nielsen P.E., Glazer P.M. Targeted correction of a thalassemia-associated beta-globin mutation induced by pseudo-complementary peptide nucleic acids. Nucleic Acids Res. 2009;37:3635–3644. doi: 10.1093/nar/gkp217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Kayali R., Bury F., Ballard M., Bertoni C. Site-directed gene repair of the dystrophin gene mediated by PNA-ssODNs. Hum. Mol. Genet. 2010;19:3266–3281. doi: 10.1093/hmg/ddq235. [DOI] [PubMed] [Google Scholar]
- 98.Nik-Ahd F., Bertoni C. Ex vivo gene editing of the dystrophin gene in muscle stem cells mediated by peptide nucleic acid single stranded oligodeoxynucleotides induces stable expression of dystrophin in a mouse model for Duchenne muscular dystrophy. Stem Cells. 2014;32:1817–1830. doi: 10.1002/stem.1668. [DOI] [PubMed] [Google Scholar]