Swarming surface motility is a complex adaptation leading to multidrug antibiotic resistance and virulence factor production in Pseudomonas aeruginosa. Here, we expanded previous studies to demonstrate that under swarming conditions, P. aeruginosa PA14 is more resistant to multiple antibiotics, including aminoglycosides, β-lactams, chloramphenicol, ciprofloxacin, tetracycline, trimethoprim, and macrolides, than swimming cells, but is not more resistant to polymyxin B.
KEYWORDS: Pseudomonas aeruginosa, RNA-Seq, antibiotic resistance, swarming motility, tobramycin
ABSTRACT
Swarming surface motility is a complex adaptation leading to multidrug antibiotic resistance and virulence factor production in Pseudomonas aeruginosa. Here, we expanded previous studies to demonstrate that under swarming conditions, P. aeruginosa PA14 is more resistant to multiple antibiotics, including aminoglycosides, β-lactams, chloramphenicol, ciprofloxacin, tetracycline, trimethoprim, and macrolides, than swimming cells, but is not more resistant to polymyxin B. We investigated the mechanism(s) of swarming-mediated antibiotic resistance by examining the transcriptomes of swarming cells and swarming cells treated with tobramycin by transcriptomics (RNA-Seq) and reverse transcriptase quantitative PCR (qRT-PCR). RNA-Seq of swarming cells (versus swimming) revealed 1,581 dysregulated genes, including 104 transcriptional regulators, two-component systems, and sigma factors, numerous upregulated virulence and iron acquisition factors, and downregulated ribosomal genes. Strain PA14 mutants in resistome genes that were dysregulated under swarming conditions were tested for their ability to swarm in the presence of tobramycin. In total, 41 mutants in genes dysregulated under swarming conditions were shown to be more resistant to tobramycin under swarming conditions, indicating that swarming-mediated tobramycin resistance was multideterminant. Focusing on two genes downregulated under swarming conditions, both prtN and wbpW mutants were more resistant to tobramycin, while the prtN mutant was additionally resistant to trimethoprim under swarming conditions; complementation of these mutants restored susceptibility. RNA-Seq of swarming cells treated with subinhibitory concentrations of tobramycin revealed the upregulation of the multidrug efflux pump MexXY and downregulation of virulence factors.
INTRODUCTION
Pseudomonas aeruginosa is a Gram-negative opportunistic pathogen. Ubiquitous in the environment, it infects a wide range of hosts, from plants to humans. In humans, P. aeruginosa has been implicated in a variety of diseases ranging from nosocomial pneumonia and bacterial keratitis to chronic cystic fibrosis (CF). Indeed P. aeruginosa is one of the most common CF pathogens, and up to 60% of patients are colonized by Pseudomonas by age 30 (1–4). Despite the aggressive use of antibiotics, P. aeruginosa infections in CF are difficult to clear and almost always become chronic (2, 3).
P. aeruginosa is also an important nosocomial pathogen, accounting for 11% to 14% of all nosocomial infections (5). Therapy for P. aeruginosa infections is difficult due to its resistance to many antibiotics. Combined with the fact that few novel antibiotics are under development, multidrug resistance represents a significant challenge to the health care system.
Antibiotic resistance can develop in a number of different ways, including intrinsic, acquired, and adaptive resistance. P. aeruginosa tends to be intrinsically resistant to antibiotics as a result of its low outer membrane permeability coupled to secondary mechanisms such as multidrug efflux and enzymatic hydrolysis, e.g., by β-lactamases (6, 7). Furthermore, P. aeruginosa can become more resistant through the horizontal acquisition of resistance genes on genetic elements such as plasmids or through mutation of its hundreds of resistance genes that are collectively termed the resistome (8–13). Another resistance mechanism that is important in P. aeruginosa is adaptive antibiotic resistance, whereby gene expression is altered due to its growth state or environmental conditions, including exposure to stresses. When the conditions that trigger adaptive resistance no longer exist, bacteria revert to a susceptible state. For example, adaptive resistance can be induced by exposure to stresses such as subinhibitory concentrations of antimicrobial agents (14), defined growth states such as biofilm formation, or environmental conditions such as anaerobiosis, altered temperatures, or nutrient limitation. Additionally, subinhibitory levels of polymyxins and cationic peptides or low divalent cation concentrations can activate two-component regulatory systems that trigger the increased expression of enzymes that modify lipopolysaccharide (LPS) to a less negatively charged or less fluid form, resulting in adaptive peptide resistance (14). Overexpression of β-lactamase in response to certain β-lactams is another common adaptive resistance mechanism. Adaptive resistance is also triggered when P. aeruginosa undergoes swarming motility (15, 16), but this has only been investigated in a limited fashion to date.
Swarming motility in P. aeruginosa is a rapid, coordinated surface-associated movement that occurs under semiviscous nitrogen-limiting conditions (15). Importantly, these conditions mimic the mucosal surfaces of the human lung (17); therefore, understanding how swarming in P. aeruginosa leads to multiple-antibiotic adaptive resistance is relevant to our understanding of lung infections and the limitations of antibiotic therapy in this situation. Features of the lung environment that are likely to support swarming include increased glucose levels in the diseased lung (18, 19), amino acids as the main nitrogen source, sufficient levels of magnesium (20), and a humid and viscous environment. Swarming is thought to have clinical relevance particularly in the acute or initial infection of lungs, since strains isolated from chronic infections tend to lose motility over time (21). Thus, swarming can allow for rapid colonization in the lung and the establishment of infection and is also important in the initial formation of biofilms (22), which are a common problem in infections due to their ability to persist and resist antimicrobial treatment.
In the swarm state, P. aeruginosa conditionally increases its resistance to several antibiotics (15, 16). This resistance is not dependent on prior antibiotic exposure and also occurs in other swarming species such as Salmonella sp. (23), Escherichia coli, and Bacillus subtilis (16).
Little is known about the genetic mechanisms that result in adaptive antibiotic resistance in P. aeruginosa swarming motility. Therefore, after confirming and extending the observation that P. aeruginosa exhibits resistance in the swarm state, transcriptomics (RNA-Seq) and reverse transcriptase quantitative PCR (qRT-PCR) were performed on swarming cells and swarming cells treated with tobramycin, and mutants in genes dysregulated under swarming conditions were selected and tested for altered antibiotic susceptibility under swarming conditions.
RESULTS
Swarming cells were resistant to multiple antibiotic classes.
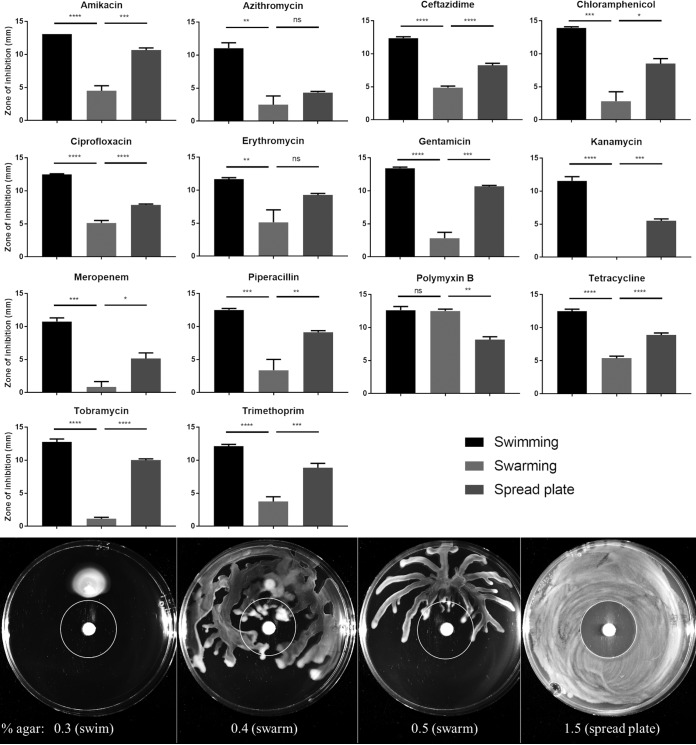
To confirm and extend the observation that P. aeruginosa exhibits resistance in the swarming state (15, 16), BM2 glucose agar plates solidified with various concentrations of agar (allowing for different modes of growth) were inoculated with mid-log-phase P. aeruginosa PA14 (Fig. 1; see also Fig. S1 in the supplemental material). After an overnight incubation, the zones of inhibition around antibiotic discs (i.e., the closest approach of motile cells to the antibiotic disc) were measured as an indicator of resistance. PA14 swarming cells on 0.4% agar were significantly more resistant to aminoglycosides (amikacin, gentamicin, kanamycin, and tobramycin) and β-lactams (ceftazidime, meropenem, and piperacillin) than those on the control swim and spread plates (Fig. 1, top). Swarming cells were also significantly more resistant to chloramphenicol, ciprofloxacin, tetracycline, and trimethoprim. For the macrolides, erythromycin and azithromycin, swarming cells were significantly more resistant than swimming cells, but not bacteria on 1.5% agar spread plates. Swarming cells were not resistant to polymyxin B (Fig. 1, top). Resistance of swarming cells was more readily observable at 0.4% agar, since this condition permitted better swarming (Fig. 1). However, similar trends were observed for swarming at 0.5% agar (Fig. S1).
FIG 1.
Swarming bacteria exhibited heightened resistance to most antibiotic classes. (Top) P. aeruginosa PA14 grown to mid-log phase was used to inoculate BM2 agar plates (0.3% agar for swimming, 0.4% for swarming, and 1.5% for spread plates) with antibiotic discs. After overnight incubation, the distances between the antibiotic discs and nearest visible growth were measured as the zones of inhibition. Averages ± standard errors are depicted. Statistically significant differences were determined by analysis of variance (ANOVA) using GraphPad Prism; n ≥ 3. (Bottom) Zone of inhibition assay for PA14 WT using tobramycin discs. *, P ≤ 0.05; **, P ≤ 0.01; ***, P ≤ 0.001; ****, P ≤ 0.0001; ns, not significant.
To confirm these results by a different method, cells were harvested from antibiotic-free swimming and swarming plates and subjected to tobramycin treatment. Swarming cells were killed more slowly, showing approximately 100-fold better survival than swimming cells after 300 min (Fig. 2).
FIG 2.
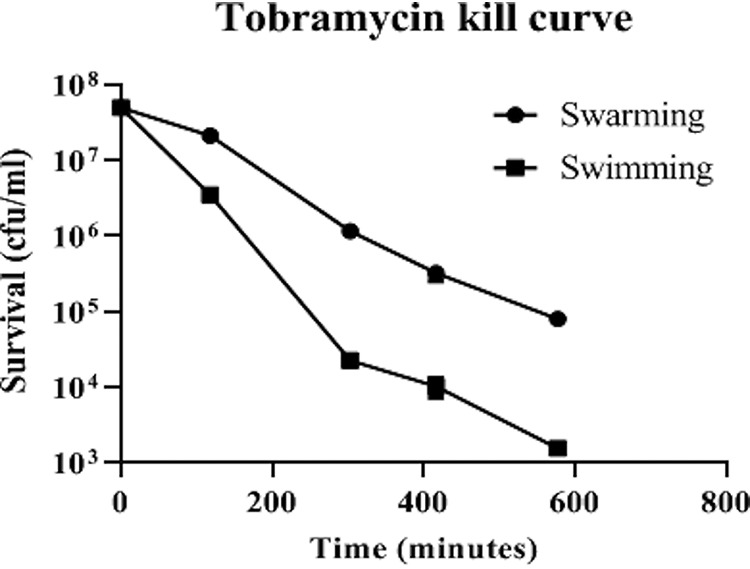
Tobramycin kill curve showing that swarming cells survived better than swimming cells in the presence of tobramycin. Averages ± standard errors are depicted; n = 3.
Swarming motility is a complex adaptation accompanied by many changes in resistome genes.
Due to the complexity of these resistance data and to enable an understanding of the global changes accompanying swarming motility, we first characterized the global gene expression changes accompanying swarming motility. Previous studies analyzing global gene expression changes were performed using microarrays with planktonic (broth culture-grown) cells as a control; this identified the dysregulation of 417 genes, including 18 regulators (15). Here, we improved this analysis by comparing swarming and swimming cells taken from plate cultures (varied only by the agar concentrations) and utilizing the more accurate method of RNA-Seq.
The comparison of swarming versus swimming by RNA-Seq revealed the differential expression of 1,581 genes (753 downregulated and 828 upregulated) (see Table S1). This was a substantial portion, 28%, of the P. aeruginosa genome, showing that swarming is a distinct and complex adaptation.
The dysregulated genes included 104 transcriptional regulators, two-component systems, and sigma factors (Table 1). There were several regulators of nitrogen metabolism, such as nirQ, nirG, nosR, and hutR. Other interesting regulators included BfiS, a two-component sensor involved in biofilm formation (24), PchR, a regulator of the ferripyochelin receptor gene (25), VqsR, a global regulator of quorum sensing and virulence (26), and AlgR, which is involved in coordinating alginate and rhamnolipid production and swarming and twitching motilities (27). The dysregulated sigma factors included hasI, femI, fiuI, foxI, fpvI, pvdS, rpoS, vreI, PA1351, PA2050, PA2093, and PA4896.
TABLE 1.
Selected results from swarm versus swim RNA-Seq comparisonsa
| Locus tag | PAO1 | Name | Product name | Padj | FCb |
|---|---|---|---|---|---|
| Transcriptional regulators, two-component systems and sigma factors | |||||
| PA14_00600 | PA0048 | Transcriptional regulator | 9.1E−06 | 1.7 | |
| PA14_00680 | PA0056 | LysR family transcriptional regulator | 2.0E−03 | 1.5 | |
| PA14_02250 | PA0178 | Two-component sensor | 5.2E−09 | 1.6 | |
| PA14_02260 | PA0179 | Two-component response regulator | 4.4E−07 | 1.5 | |
| PA14_02390 | PA0191 | Transcriptional regulator | 3.4E−08 | −2.0 | |
| PA14_02870 | PA0233 | Transcriptional regulator | 1.9E−08 | 1.5 | |
| PA14_03070 | PA0248 | Transcriptional regulator | 9.0E−06 | 1.6 | |
| PA14_03580 | PA0275 | Transcriptional regulator | 9.8E−07 | 1.5 | |
| PA14_04820 | PA0367 | laoR | TetR family transcriptional regulator | 4.4E−14 | 1.6 |
| PA14_06170 | PA0471 | fiuR | Transmembrane sensor | 1.0E−04 | 1.7 |
| PA14_06180 | PA0472 | fiuI | RNA polymerase sigma factor | 3.0E−05 | 1.5 |
| PA14_06690 | PA0513 | nirG | Transcriptional regulator | 1.8E−13 | −3.2 |
| PA14_06710 | PA0515 | Transcriptional regulator | 5.4E−13 | −3.4 | |
| PA14_06770 | PA0520 | nirQ | Regulatory protein | 4.6E−21 | −2.9 |
| PA14_06970 | PA0535 | Cro/CI family transcriptional regulator | 2.5E−09 | −1.6 | |
| PA14_07110 | PA0547 | ArsR family transcriptional regulator | 8.7E−27 | 1.7 | |
| PA14_09260 | PA4227 | pchR | Transcriptional regulator | 5.6E−28 | 3.8 |
| PA14_09680 | PA4197 | bfiS | Two-component sensor | 5.4E−09 | −1.7 |
| PA14_09790 | PA4182 | Transcriptional regulator | 1.3E−13 | −1.5 | |
| PA14_10530 | PA4132 | GntR family transcriptional regulator | 2.0E−28 | −1.5 | |
| PA14_10660 | PA4120 | Transcriptional regulator | 7.9E−05 | 1.9 | |
| PA14_10940 | PA4094 | AraC family transcriptional regulator | 2.0E−06 | 1.6 | |
| PA14_11120 | PA4080 | Response regulator | 6.9E−08 | 1.5 | |
| PA14_12140 | PA3995 | Transcriptional regulator | 1.3E−09 | 1.7 | |
| PA14_13000 | PA3932 | Transcriptional regulator | 1.2E−14 | −2.7 | |
| PA14_13150 | PA3921 | Transcriptional regulator | 1.6E−14 | 1.5 | |
| PA14_15240 | PA3776 | LysR family transcriptional regulator | 1.3E−03 | −1.6 | |
| PA14_15290 | PA3771 | Transcriptional regulator | 7.9E−06 | 1.9 | |
| PA14_15830 | PA3757 | nagR | GntR family transcriptional regulator | 2.9E−05 | −1.5 |
| PA14_16790 | PA3678 | mexL | TetR family transcriptional regulator | 6.8E−15 | 1.5 |
| PA14_17380 | PA3630 | gfnR | Glutathione-dependent formaldehyde neutralization regulator | 7.1E−11 | 1.8 |
| PA14_17480 | PA3622 | rpoS | RNA polymerase sigma factor | 1.9E−11 | 1.6 |
| PA14_17540 | PA3616 | Recombination regulator RecX | 6.3E−10 | −1.6 | |
| PA14_19380 | PA3458 | Transcriptional regulator | 3.8E−08 | 1.8 | |
| PA14_19990 | PA3410 | hasI | RNA polymerase ECF-subfamily sigma-70 factor | 3.6E−06 | −2.0 |
| PA14_20230 | PA3391 | nosR | Regulatory protein | 2.1E−37 | −17.2 |
| PA14_20780 | PA3346 | hsbR | Two-component response regulator | 2.6E−20 | 1.6 |
| PA14_23190 | PA3174 | hutR | Transcriptional regulator | 1.1E−12 | −2.2 |
| PA14_23590 | PA3133 | sawR | Transcriptional regulator | 5.2E−11 | −2.1 |
| PA14_24710 | PA3045 | rocA2 | Two-component response regulator | 9.1E−11 | −2.5 |
| PA14_24720 | PA3044 | rocsS2 | Two-component sensor | 5.2E−14 | −2.1 |
| PA14_25800 | PA2957 | TetR family transcriptional regulator | 3.5E−13 | −1.5 | |
| PA14_26330 | PA2917 | AraC family transcriptional regulator | 4.4E−18 | −2.3 | |
| PA14_26860 | PA2879 | LysR family transcriptional regulator | 3.2E−19 | 2.0 | |
| PA14_30580 | PA2591 | vqsR | LuxR family transcriptional regulator | 2.5E−21 | 1.6 |
| PA14_30840 | PA2571 | Signal transduction histidine kinase | 7.2E−09 | 1.5 | |
| PA14_32060 | PA2519 | xylS | Transcriptional regulator | 3.6E−08 | 1.8 |
| PA14_32460 | PA2488 | Transcriptional regulator | 7.7E−04 | 1.5 | |
| PA14_32710 | PA2468 | foxI | ECF subfamily RNA polymerase sigma-70 factor | 5.8E−05 | 1.8 |
| PA14_32720 | PA2467 | foxR | Transmembrane sensor | 1.7E−04 | 1.5 |
| PA14_33260 | PA2426 | pvdS | Extracytoplasmic-function sigma-70 factor | 9.7E−07 | 2.0 |
| PA14_33440 | PA2417 | LysR family transcriptional regulator | 3.5E−09 | 1.6 | |
| PA14_33800 | PA2387 | fpvI | RNA polymerase sigma factor | 1.8E−33 | 2.2 |
| PA14_33840 | PA2383 | Transcriptional regulator | 4.3E−17 | 2.9 | |
| PA14_34440 | PA2337 | mtlR | Transcriptional regulator | 1.7E−04 | 1.5 |
| PA14_34660 | PA2320 | gntR | Transcriptional regulator | 1.3E−19 | 1.6 |
| PA14_34730 | PA2312 | XRE family transcriptional regulator | 3.5E−15 | −3.0 | |
| PA14_34820 | PA2304 | ambC | Regulatory protein | 2.7E−20 | 3.5 |
| PA14_34830 | PA2303 | ambD | Regulatory protein | 1.2E−30 | 3.6 |
| PA14_34880 | PA2299 | GntR family transcriptional regulator | 7.2E−14 | 2.0 | |
| PA14_35250 | PA2267 | LysR family transcriptional regulator | 1.5E−06 | 1.6 | |
| PA14_35370 | PA2259 | ptxS | Transcriptional regulator | 1.3E−08 | −2.1 |
| PA14_35380 | PA2258 | ptxR | Transcriptional regulator | 1.7E−13 | 2.4 |
| PA14_36300 | PA2196 | TetR family transcriptional regulator | 5.0E−10 | 1.6 | |
| PA14_36420 | PA2177 | Sensor/response regulator hybrid | 3.7E−18 | 2.3 | |
| PA14_36990 | PA2133 | Cyclic-guanylate-specific phosphodiesterase | 4.8E−03 | −1.9 | |
| PA14_37140 | PA2121 | LysR family transcriptional regulator | 4.0E−03 | 1.6 | |
| PA14_37420 | PA2094 | Transmembrane sensor protein | 7.9E−13 | 3.7 | |
| PA14_37430 | PA2093 | RNA polymerase sigma factor | 2.4E−07 | 2.5 | |
| PA14_37580 | PA2082 | kynR | Leucine-responsive regulatory protein | 1.6E−04 | 1.5 |
| PA14_37980 | PA2051 | Fe2+ dicitrate sensor, membrane protein | 5.8E−08 | −3.3 | |
| PA14_37990 | PA2050 | RNA polymerase sigma factor | 1.5E−13 | −4.5 | |
| PA14_38250 | PA2032 | Transcriptional regulator | 8.7E−18 | 1.6 | |
| PA14_39800 | PA1912 | femI | ECF subfamily RNA polymerase sigma-70 factor | 2.6E−03 | −1.6 |
| PA14_39980 | PA1898 | qscR | Transcriptional regulator | 2.7E−07 | 1.7 |
| PA14_42390 | PA1713 | exsA | Transcriptional regulator | 1.3E−24 | 2.7 |
| PA14_45250 | PA1484 | Transcriptional regulator | 5.6E−05 | 1.5 | |
| PA14_45950 | PA1431 | rsaL | Regulatory protein | 2.1E−74 | 3.0 |
| PA14_46290 | PA1403 | TetR family transcriptional regulator | 4.2E−07 | 2.0 | |
| PA14_46810 | PA1351 | RNA polymerase ECF-subfamily sigma-70 factor | 1.7E−10 | 1.9 | |
| PA14_47390 | PA1301 | Transmembrane sensor | 2.4E−03 | 1.6 | |
| PA14_48160 | PA1243 | Sensor/response regulator hybrid | 1.4E−40 | 3.2 | |
| PA14_48830 | PA1196 | ddaR | Transcriptional regulator | 2.7E−07 | 1.6 |
| PA14_49170 | PA1180 | phoQ | Two-component sensor | 4.7E−16 | −2.0 |
| PA14_49180 | PA1179 | phoP | Two-component response regulator | 5.5E−14 | −1.8 |
| PA14_49790 | PA1128 | Transcriptional regulator | 1.9E−05 | 1.7 | |
| PA14_53410 | PA0839 | Transcriptional regulator | 2.7E−04 | −1.5 | |
| PA14_53720 | PA0816 | Transcriptional regulator | 2.1E−10 | 1.8 | |
| PA14_55160 | PA0707 | toxR | Transcriptional regulator | 2.4E−17 | 5.5 |
| PA14_55550 | PA0675 | vreI | ECF subfamily RNA polymerase sigma-70 factor | 8.4E−10 | −2.6 |
| PA14_55780 | PA4293 | pprA | Two-component sensor | 8.6E−18 | 2.5 |
| PA14_57140 | PA4396 | Two-component response regulator | 1.5E−17 | 1.6 | |
| PA14_58380 | PA4499 | psdR | Transcriptional regulator | 7.2E−10 | −1.6 |
| PA14_58510 | PA4508 | AsnC family transcriptional regulator | 7.7E−06 | −1.6 | |
| PA14_61620 | PA4659 | MerR family transcriptional regulator | 2.0E−11 | −1.9 | |
| PA14_63280 | PA4787 | Transcriptional regulator | 1.6E−13 | 1.6 | |
| PA14_64050 | PA4843 | gcbA | Two-component response regulator | 2.1E−48 | −1.9 |
| PA14_64500 | PA4878 | brlR | Transcriptional regulator | 1.6E−13 | −1.8 |
| PA14_64690 | PA4895 | Transmembrane sensor | 3.3E−06 | −1.8 | |
| PA14_64700 | PA4896 | RNA polymerase sigma factor | 1.3E−05 | −1.9 | |
| PA14_66850 | PA5059 | TetR family transcriptional regulator | 1.1E−09 | 1.7 | |
| PA14_69470 | PA5261 | algR | Alginate biosynthesis regulatory protein | 2.3E−12 | 1.6 |
| PA14_71170 | PA5389 | cdhR | AraC family transcriptional regulator | 1.1E−03 | 1.6 |
| PA14_71750 | PA5437 | LysR family transcriptional regulator | 2.5E−79 | −3.3 | |
| PA14_72380 | PA5483 | algB | Two-component response regulator | 2.3E−11 | 1.9 |
| PA14_72390 | PA5484 | kinB | Two-component sensor | 1.4E−16 | 1.9 |
| Multidrug efflux and β-lactamases | |||||
| PA14_01940 | PA0156 | triA | RND efflux membrane fusion protein | 6.6E−12 | 1.6 |
| PA14_09500 | PA4208 | opmD | Outer membrane protein | 1.3E−22 | 1.8 |
| PA14_09520 | PA4207 | mexI | RND efflux transporter | 2.0E−23 | 1.6 |
| PA14_18760 | PA3523 | mexP | RND efflux membrane fusion protein | 3.1E−10 | −4.0 |
| PA14_18780 | PA3522 | mexQ | RND efflux transporter | 1.5E−31 | −3.4 |
| PA14_18790 | PA3521 | opmE | Outer membrane efflux protein | 1.2E−12 | −3.3 |
| PA14_32390 | PA2494 | mexF | RND multidrug efflux transporter | 3.6E−14 | −2.0 |
| PA14_32400 | PA2493 | mexE | RND multidrug efflux membrane fusion protein | 4.2E−11 | −1.9 |
| PA14_38395 | PA2019 | mexX | Periplasmic multidrug efflux lipoprotein | 3.4E−07 | −1.8 |
| PA14_38410 | PA2018 | mexY | Multidrug efflux protein | 2.1E−06 | −1.6 |
| PA14_41280 | PA1797 | β-Lactamase | 2.8E−11 | 2.0 | |
| PA14_44520 | PA1541 | Drug efflux transporter | 1.5E−10 | −5.6 | |
| PA14_44530 | PA1540 | Multidrug efflux system protein MdtI | 6.7E−05 | −2.4 | |
| PA14_45910 | PA1435 | RND efflux membrane fusion protein | 1.1E−02 | −1.6 | |
| PA14_48240 | PA1238 | Outer membrane component of multidrug efflux pump | 4.3E−02 | 1.7 | |
| PA14_54700 | PA0740 | sdsA1 | β-Lactamase | 1.5E−03 | −1.5 |
These revealed 104 dysregulated transcriptional regulators and dysregulated efflux and β-lactamase genes.
FC, fold change.
RNA-Seq of swarm versus swim cells also revealed the downregulation of 55 ribosomal genes and other related translation factors (see Table S2). This was of interest, since the ribosome is the target of tobramycin. Interestingly, these genes included fusA1 and rplU, which are involved in tobramycin resistance in CF clinical isolates (28). The ribosome modulation factor rmf, which induces the dimerization of 70S subunits into an inactive form (29), was upregulated 1.8-fold.
A search was made for multidrug efflux transporters and β-lactamases, and 16 genes were found, both up- and downregulated (Table 1). Since the upregulated genes were not strongly induced, it seems unlikely that efflux or β-lactamase production is a major mechanism of the resistance intrinsic to swarm cells, although we show below that multidrug efflux could be induced upon tobramycin treatment.
RNA-Seq also revealed the upregulation of several pilus-related genes and rhamnosyltransferase 2 (rhlC) (Table S2). This is consistent with the requirement of pili and rhamnolipids for swarming motility in P. aeruginosa and the observation that a mutant in rcpA is unable to swarm (17).
Swarm cells also upregulated numerous genes in the types I, II, and III secretion systems (Table S2), including genes encoding virulence factors such as exotoxin A, exoenzyme S and Y, phospholipase PlcB, and elastases LasA and LasB. Many type VI secretion systems (T6SS) genes were also upregulated, although certain T6SS genes were downregulated (hcpC, vgrG4a, and vgrG4b) (Table S2). Lastly, many pyoverdine, pyochelin, and phenazine genes were also upregulated under swarming conditions (Table S2). This confirms previous studies indicating that swarming cells exhibit broad enhancement of virulence potential (15).
Multiple factors contributed to swarming-mediated antibiotic resistance.
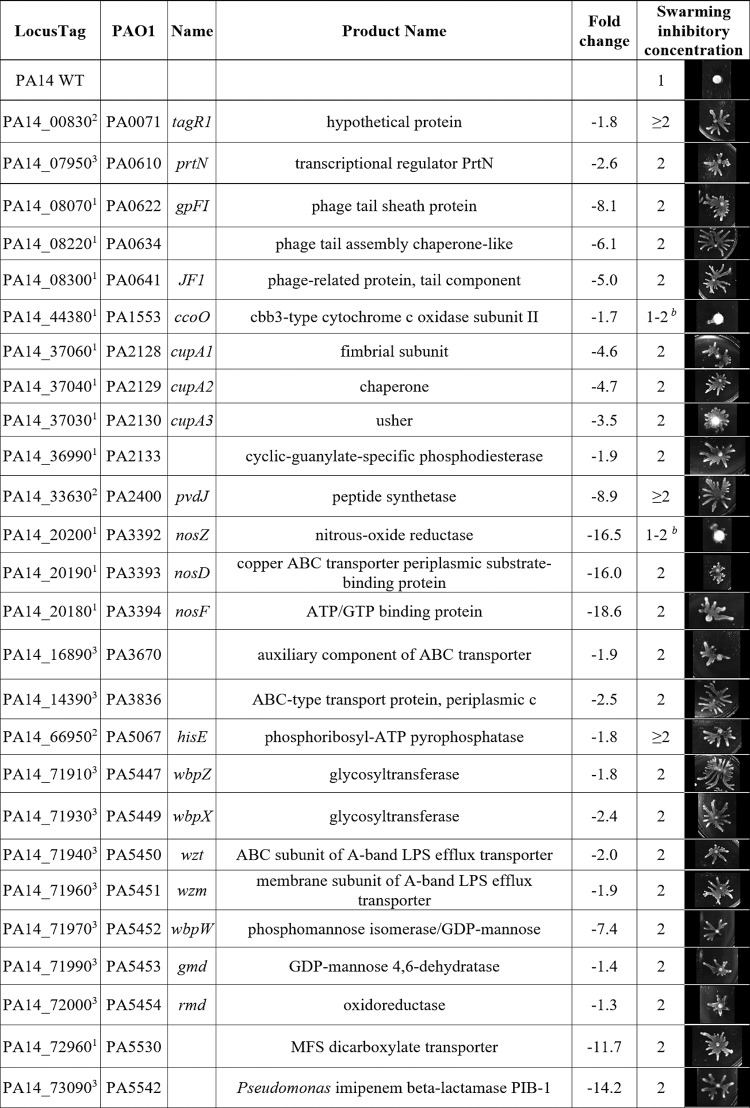
Having confirmed that swarming cells were resistant to multiple antibiotics, we sought to elucidate the mechanism(s) of swarming-mediated antibiotic resistance by testing mutants for swarming in the presence of antibiotic. Tobramycin was selected as the antibiotic of interest, since swarming cells were strongly resistant (Fig. 1). RNA-Seq data were analyzed to identify genes corresponding to the resistome (i.e., those genes that affect antibiotic resistance) (8–13) that were dysregulated under swarming conditions. Mutants in these genes, as well as some in operons of interest that did not initially appear in the list of dysregulated genes, were screened for altered tobramycin susceptibility under swarming conditions using the agar dilution method. A gene was considered to potentially contribute to swarming-mediated antibiotic resistance if it was downregulated and the corresponding mutant was resistant under swarming conditions (i.e., decreased expression of the gene in question led to resistance). Conversely, if the gene was upregulated and the corresponding mutant was supersusceptible under swarming conditions, this might also indicate a role in adaptive resistance, but this did not occur here (Table 2; see also Table S3). Mutants showing deficiencies in swarming motility in the absence of antibiotic were excluded from testing, as they would appear supersusceptible due to their lack of swarming ability rather than to true susceptibility.
TABLE 2.
Genes dysregulated under swarming conditions that matched the known resistome revealed 26 tobramycin resistance mutantsa
See references 8–13. PA14 transposon mutants in selected genes were tested for altered tobramycin susceptibility under swarming conditions using the agar dilution method (inhibitory concentrations shown in micrograms per milliliter tobramycin) along with images of swarming colonies at 1 μg/ml. Evidence of dysregulation came from swarm versus swim RNA-Seq (1) or tobramycin RNA-Seq (2). Selected genes were also confirmed by qRT-PCR from reference 15 (3). Additional mutants in genes showing no evidence of dysregulation (gmd and rmd) but belonging to operons containing dysregulated genes were also tested. Seventeen additional mutants are described in Table S3 in the supplemental material.
Two mutants showed minimal swarming at tobramycin 1 μg/ml but grew better than WT at 2 μg/ml.
A comprehensive description of the resistome under planktonic conditions has been published, with mutations in 135 genes leading to adaptive resistance to tobramycin (13). Interestingly, there was a moderate number of overlaps with the genes identified here as being likely involved in adaptive resistance, prominently including genes involved in membrane energization (ccoO [cytochrome c oxidase]), LPS biosynthesis (wbpW and its operon wbpZ-rmd), and nitrous oxide metabolism (where the nosZDF genes were 16.0- to 18.6-fold downregulated) and the gene for a major facilitator superfamily transporter, PA5530, which mediates α-ketoglutarate transport (30). In addition to these, there were some novel resistome genes previously described as being involved in susceptibility/resistance to other antibiotics, including a large phage/pyocin operon (PA0613-PA0641), which has been implicated in susceptibility to ciprofloxacin (31). Similarly, a cup fimbriae biosynthesis operon (cupA1-3) was identified that includes a cyclic-GMP phosphodiesterase previously implicated in the regulation of flagella, chemotaxis, and type III secretion as well as a TolC-like efflux protein (PA2133/fcsR [32]). CupA1 (implicated in ceftazidime susceptibility) was identified from the resistome study (11); whereas CupA3 (β-lactam and ciprofloxacin resistance) was identified in other studies (9). Other genes included PA3670, a component of an ABC transport system that was implicated in susceptibility to β-lactams, levofloxacin, and trimethoprim-sulfamethoxazole (identified from reference 9). PA3836, another ABC transport protein involved in ciprofloxacin resistance was identified in reference 10. Lastly, a carbapenemase-expressing gene (PA5542 [33]), for which the mutant was supersusceptible to β-lactams, was identified from reference 12. Thus, resistance under swarming conditions involved both canonical/known tobramycin resistance genes and novel genes.
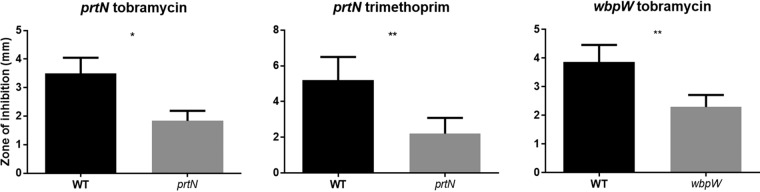
Two mutants, prtN and wbpW, were selected for study in greater detail, since they represented larger gene groups (the pyocins and LPS biosynthetic operon) with a uniform direction of regulation and phenotype. PrtN was of particular interest, since it is a regulator and could potentially affect the expression of many genes. The antibiotic susceptibility phenotypes of prtN and wbpW mutants were confirmed using both the disc diffusion and agar dilution methods (Fig. 3 and 4), and their appropriate dysregulation of gene expression under swarming conditions was confirmed using qRT-PCR (i.e., prtN and wbpW were downregulated by 2.6 ± 0.8 and 7.4 ± 2.8 [fold change ± standard error], respectively).
FIG 3.
Antibiotic susceptibility of PA14 mutants under swarming conditions using the disc diffusion method and 0.5% agar. Means ± standard errors are depicted. Statistically significant differences were determined by paired t tests using GraphPad Prism; n ≥ 4. *, P ≤ 0.05; **, P ≤ 0.01.
FIG 4.
Agar dilution method for determining the swarming inhibitory concentration (IC) of PA14 mutants on 0.5% agar. (A) tobramycin swarming IC = 1 μg/ml; (B) trimethoprim IC = 10 μg/ml; (C) tobramycin IC = 1 μg/ml; n ≥ 3.
A mutant in wbpW was resistant to tobramycin and had decreased membrane permeability.
Under swarming conditions, a mutant in wbpW, encoding an enzyme involved in A-band LPS synthesis (GDP-mannose pyrophosphorylase), was 2-fold more resistant to tobramycin (Fig. 3 and 4; Tables 2 and S4). The mutant in wbpW was complemented to normal susceptibility by reintroducing wbpW in a low-copy-number plasmid (Fig. 5B). We previously showed in P. aeruginosa that polycationic aminoglycosides, such as tobramycin, are taken up across the outer membrane via the self-promoted uptake system (34). The concept of self-promoted uptake is that polycationic antibiotics interact with divalent-cation binding sites on outer membrane surface LPS, causing disruption of these sites (since they are more bulky than the native divalent cations Mg2+ and Ca2+) and thus promote the uptake of the polycationic antibiotic. This membrane disruption can be probed using the fluorophore 1-N-phenyl-napthylamine (NPN). NPN is a dye that is normally excluded by wild-type P. aeruginosa and is weakly fluorescent in aqueous media but fluoresces strongly when it enters the hydrophobic interior of the outer membrane (13); thus, its uptake into bacterial membranes is an indicator of membrane permeabilization by tobramycin. In the NPN assay, after addition of tobramycin, the wbpW mutant had decreased membrane permeabilization, consistent with its reduced susceptibility, and this was complemented in the wbpW+ strain (Fig. 6) and is thus the likely cause of tobramycin resistance.
FIG 5.
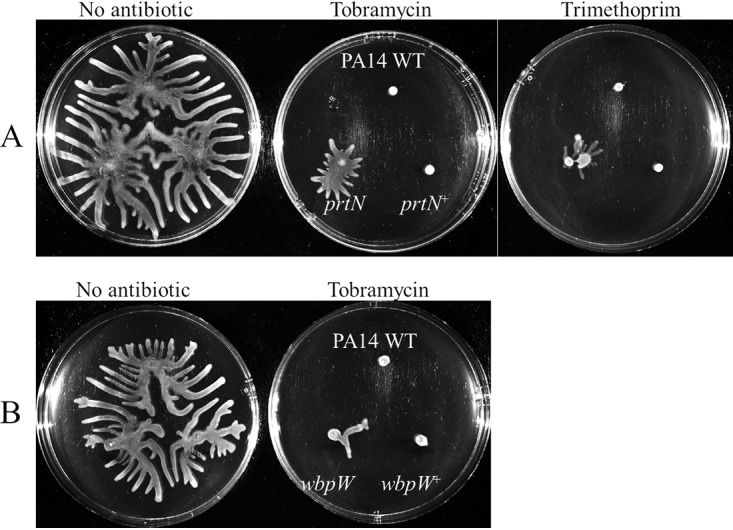
Complementation of swarming antibiotic susceptibility phenotypes for prtN (A) and wbpW (B) mutants. All strains were transformed with either the respective empty vector (WT and mutants) or a vector with insert (complemented “+” strains); n ≥ 3.
FIG 6.
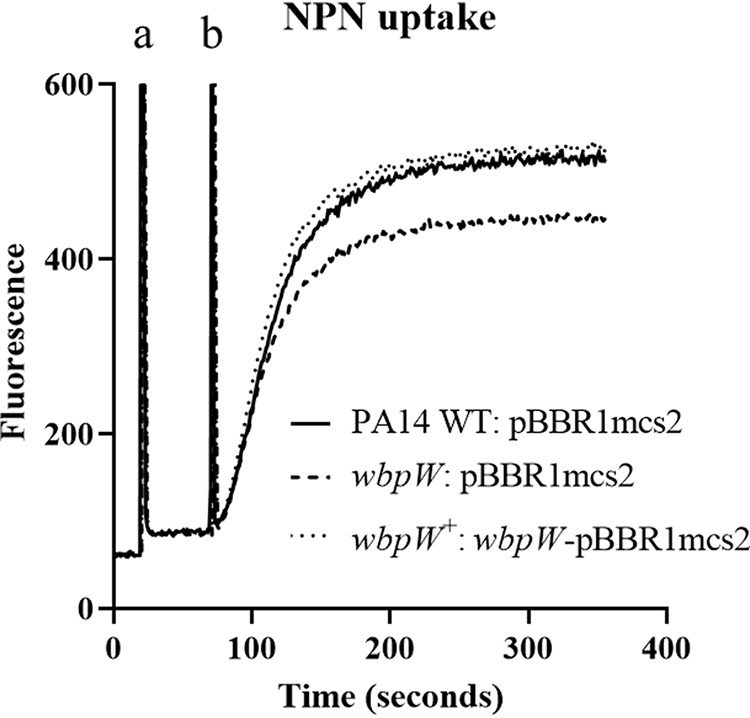
A wbpW mutant had reduced membrane permeabilization. Swarm cells were harvested and treated where indicated with NPN (a) and tobramycin (b); n = 3.
A prtN mutant induced supersusceptibility to tobramycin and trimethoprim.
The mutant in the gene prtN, the positive regulator of pyocin, showed partial resistance to both tobramycin and trimethoprim under swarming conditions (Fig. 3 and 4; Tables 2 and S4). Antibiotic susceptibility was restored by reintroducing prtN in an arabinose-inducible construct (pHERD20T) (Fig. 5A). Success with other vectors was limited due to lack of plasmid stability when prtN was constitutively expressed (data not shown). These results indicated that swarming cells might increase antibiotic resistance by downregulating a process regulated by PrtN, such as the biologically costly production of pyocins, also termed genotoxic stress (35). Consistent with the latter explanation, mutants in several genes downstream of and regulated by PrtN, namely, PA0613 to PA0641, were also tested for antibiotic susceptibility under swarming conditions and found to be resistant to tobramycin; although, unlike that for prtN, no significant differences were observed in trimethoprim susceptibility (Tables 2 and S3 and data not shown).
Antibiotic susceptibility was affected by growth conditions.
The mutants were also assayed under standard broth dilution MIC conditions in the rich medium LB and the minimal medium BM2 glucose (see Table S5). In contrast to their effect on susceptibility under swarming conditions, the mutants showed little difference in MICs compared to that of the wild type, with the exception of the prtN mutant, which was 2-fold more resistant to trimethoprim in LB. This indicated that growth conditions had an important effect on antibiotic resistance and suggests that standard screening methods might miss such phenotypes.
The tobramycin resistance phenotype was much more pronounced under swarming conditions (Table 2; Fig. 3 and 4) than for standard MICs (Table S5). When tested in a standard MIC, the prtN and wbpW mutants showed no increase in MIC (Table S5). Other representative mutants were also tested but showed no difference under standard MIC conditions (Table S6). Nevertheless, some of the genes listed in Table 2 likely do play a role beyond the swarming condition, since they were previously shown to be part of the tobramycin resistome (including ccoO, wbpW and its operon wbpZ-rmd, nosZDF, and PA5530) (13). It seems possible that some genes may confer resistance specifically under swarming conditions, whereas others may confer resistance under multiple conditions.
Subinhibitory tobramycin treatment under swarming conditions.
Swarming bacteria treated with the subinhibitory tobramycin dose of 0.5 μg/ml were compared to untreated swarm cells by RNA-Seq, since this was relevant to the increased resistance of cells swarming in the presence of tobramycin. Differential expression analysis revealed 224 genes, 186 of which were downregulated (Table S7). The downregulated genes included many virulence factors, particularly T3SS and pyoverdine genes (Table S7), indicating a secondary benefit of tobramycin treatment, even in the absence of killing. Among the upregulated genes was the efflux pump mexXY, involved in a known mechanism of aminoglycoside resistance (Table 3) (36). Eight ribosomal proteins and translation factors were also downregulated (efp, infA, infC, rplU, rpmB, rpsG, rpsS, and PA5492), as well as four genes in an LPS biosynthetic operon (wzz, wbpA, wbpI, and wbpL) (Table 3). Since pyocins were already implicated in swarming-mediated antibiotic resistance, it was interesting that the genes tolA and tolR, which are involved in the uptake of pyocin AR41, were downregulated (Table 3) (37).
TABLE 3.
Selected genes that were differentially expressed upon tobramycin treatment under swarming conditions
| Locus tag | PAO1 | Name | Product name | Padj | FCa |
|---|---|---|---|---|---|
| PA14_08810 | PA4267 | rpsG | 30S ribosomal protein S7 | 2.1E−02 | −1.7 |
| PA14_08890 | PA4259 | rpsS | 30S ribosomal protein S19 | 2.8E−02 | −1.8 |
| PA14_23360 | PA3160 | wzz | O antigen chain length regulator | 6.9E−03 | −1.8 |
| PA14_23370 | PA3148 | wbpI | Putative UDP-N-acetylglucosamine 2-epimerase | 2.6E−02 | −1.7 |
| PA14_23380 | PA3159 | wbpA | UDP-N-acetyl-d-mannosaminuronate dehydrogenase | 1.8E−02 | −2.1 |
| PA14_23460 | PA3145 | wbpL | Putative group 4 glycosyl transferase | 2.7E−02 | −1.8 |
| PA14_27210 | PA2851 | efp | Elongation factor P | 2.0E−02 | −1.6 |
| PA14_28660 | PA2743 | infC | Translation initiation factor IF-3 | 1.3E−02 | −1.9 |
| PA14_30240 | PA2619 | infA | Translation initiation factor IF-1 | 3.5E−03 | −1.5 |
| PA14_38380 | PA2020 | mexZ | Putative transcriptional regulator | 2.8E−02 | 1.6 |
| PA14_38395 | PA2019 | mexX | Periplasmic multidrug efflux lipoprotein precursor | 3.5E−22 | 8.5 |
| PA14_38410 | PA2018 | mexY | Multidrug efflux protein | 1.3E−36 | 8.0 |
| PA14_38430 | PA2016 | liuR | Regulatory gene of gnyRDBHAL cluster, GnyR | 7.8E−03 | −2.3 |
| PA14_41575 | PA1776 | sigX | RNA polymerase sigma factor | 1.4E−02 | −1.6 |
| PA14_42390 | PA1713 | exsA | Transcriptional regulator | 1.5E−07 | −2.4 |
| PA14_42460 | PA1707 | pcrH | Regulatory protein | 2.7E−02 | −1.8 |
| PA14_45950 | PA1431 | rsaL | Regulatory protein | 1.7E−02 | −1.8 |
| PA14_51730 | PA0971 | tolA | Membrane transport protein | 2.4E−03 | −1.6 |
| PA14_51740 | PA0970 | tolR | Membrane transport protein | 1.8E−02 | −1.5 |
| PA14_52570 | PA0905 | rsmA | Carbon storage regulator | 8.8E−03 | −1.9 |
| PA14_55160 | PA0707 | toxR | Transcriptional regulator | 5.8E−05 | −4.4 |
| PA14_56070 | PA4315 | mvaT | Transcriptional regulator, P16 subunit | 2.6E−02 | −1.5 |
| PA14_60460 | PA4568 | rplU | 50S ribosomal protein L21 | 4.0E−02 | −1.6 |
| PA14_70190 | PA5316 | rpmB | 50S ribosomal protein L28 | 1.6E−02 | −1.6 |
| PA14_72210 | PA5471 | armZ | Hypothetical protein | 2.3E−19 | 3.4 |
| PA14_72480 | PA5492 | Ribosome biogenesis GTP-binding protein YsxC | 3.9E−02 | −1.6 |
FC, fold change.
Dysregulated transcriptional regulators included two regulators of virulence factor production (exsA and toxR), mexZ, the repressor of mexXY (38), liuR, a regulator of the leucine/isovalerate utilization pathway (39), sigX, an extracytoplasmic function sigma factor involved in the regulation of the major porin OprF, antibiotic resistance, T3SS, swarming motility, biofilm formation, and carbon catabolite repression (40–42), rsaL, a repressor of virulence gene expression and quorum sensing (43, 44), mvaT, a global regulator of quorum sensing, virulence, and swarming motility (45), and rsmA, a posttranscriptional regulator of multidrug efflux, motility, quorum sensing, T6SS, and virulence (46–48) (Table 3). Interestingly, the mexXY operon has been shown to be upregulated in a SigX mutant, along with the repressor mexZ, providing more evidence that upregulation of mexXY can be independent of mexZ (42).
Resistome mutants were also tested from the tobramycin versus untreated RNA-Seq. Although some mutants could not be tested due to deficiencies in swarming motility, it was shown that mutants in tagR1 (a type VI secretion protein [HSI-I] with a sulfatase-modifying domain associated with the outer membrane [49, 50]), pvdJ (peptide synthetase of pyoverdine), and hisE (involved in histidine biosynthesis [49]) were resistant to tobramycin under swarming conditions and downregulated upon tobramycin treatment (Table 2). Interestingly, PvdQ, an acylase of the quorum sensing molecule N-(3-oxododecanoyl)-l-homo serine lactone (3-oxo-C12-HSL), also involved in pyoverdine synthesis, has already been shown to play a role in the antibiotic resistance of swarming cells (51). The mechanism of PvdQ-mediated resistance is via decreased membrane permeability (51).
DISCUSSION
In this study, 41 mutants were characterized as tobramycin resistant under swarming conditions. This lends support to a previous study that showed that the tobramycin resistome is quite large (135 genes), with many mutants (including 57 in energy metabolism) showing low-level resistance (13). Not all genes overlapped between this prior study and the current investigation, indicating that there may be distinct mechanisms of resistance in swarm cells and in broth-grown cells.
One mutant demonstrating complementable tobramycin and trimethoprim resistance and equivalent downregulation in the wild type under swarming conditions was in the prtN gene, which encodes a positive regulator of pyocin production under the control of the prtR repressor. Under UV stress, RecA causes the autocleavage of PrtR, leading to the expression of prtN and production of pyocins (52). A lysis cassette is also induced, including holin-like and lysozyme-like genes that cause cell lysis and the release of pyocin (35). Interestingly, a mutation inactivating the catalytic activity of PrtR resulted in increased resistance to aminoglycosides, ciprofloxacin, and UV stress (35), which was attributed to effects on genotoxic stress, while mutants in the phage tail-like bacteriocins regulated by prtRN were resistant to ciprofloxacin (10, 31). In other studies, a mutant in prtN was found to be resistant to piperacillin, cefotaxime, and trimethoprim-sulfamethoxazole (9).
Overall, the downregulation of the phage tail-like bacteriocins in the cluster of genes from PA0613 to PA0641 was a striking feature observed in the transcriptome of swarming cells (15 and the RNA-Seq study reported here). Mutating the positive regulator for these genes, prtN, resulted in resistance to both tobramycin and trimethoprim under swarming conditions, an effect consistent with the multiple resistance of the catalytically inactive (autocleavage resistant) repressor prtR mutant (35). Though pyocins play a role in intra- and interspecies competition, activation of these genes clearly incurs negative consequences for the cell. Such genes are likely maintained due to selective pressures in mixed bacterial communities. Additionally, self-killing activity in a portion of the community with damaged DNA may be beneficial to the population as a whole (53).
Trimethoprim inhibits dihydrofolate reductase, depleting the cell of tetrahydrofolate, a one-carbon donor for a number of important metabolites in the cell, including the nucleotide thymidylate (54). Therefore, as a consequence of trimethoprim treatment, DNA synthesis is inhibited and the DNA damage response is induced (54). Since the DNA damage response also leads to the activation of PrtN and induction of pyocin genes (35, 52), it would seem reasonable that a mutant in prtN is resistant to trimethoprim. An association between aminoglycoside resistance and the regulation of pyocins was previously shown (35), although the mechanism for this is less clear. It is also possible that other genes regulated by prtN might be responsible.
Another interesting mutant was in the gene encoding WbpW, which catalyzes the conversion of mannose-1-phosphate to GDP-d-mannose (55). Interestingly, GDP-d-mannose can be used in the synthesis of alginate but is also utilized by Rmd for the synthesis of A-band LPS (55). Mutants in genes involved in the synthesis of LPS were previously shown to lead to tobramycin resistance, due to the reduced ability of tobramycin to cross the outer membrane via self-promoted uptake (13). The resistance of the wbpW mutant was complemented by reintroducing wbpW in a low-copy-number plasmid (Fig. 5B); furthermore, the wbpW mutant had reduced membrane permeability (Fig. 6).
While wbpW was downregulated, low levels of expression could allow for a reduced amount of A-band LPS rather than a complete deficiency. Mutants in wbpW also have reduced but not completely absent A-band LPS due to the presence of two wbpW homologs that are bifunctional enzymes (algA and pslB) (56). Over time, strains unable to produce O antigen come to predominate in the lungs of cystic fibrosis patients (57), suggesting that LPS alterations might be one mechanism by which P. aeruginosa evades host recognition and adaptive immune responses. In addition, it has been shown that lipid A modifications are common and constitute a mechanism of immune evasion (58). In another study, the loss of B antigen was associated with increased T3SS activity (facilitating acute infections), while loss of O antigen resulted in increased lung damage in vivo (59). Together, these studies suggest that a reduction in A-band LPS might be beneficial for P. aeruginosa persistence in vivo.
The two RNA-Seq experiments (swarm versus swim and tobramycin versus untreated) also showed that there is an important distinction between the inherent resistance of swarm cells and the inducible response of swarm cells to the antibiotic tobramycin. In the absence of antibiotic, more than 1,500 genes were dysregulated, and antibiotic resistance is likely a cumulative effect of many different genes. However, multidrug efflux did not appear to play a major role, as found in another study (16). In contrast, in the presence of tobramycin, fewer genes were dysregulated, but an obvious mechanism of tobramycin resistance emerged in the overexpression of mexXY. Thus, it appears there are genetic factors that enable swarming cells to resist antibiotics in the native swarming state but also specific and distinct mechanisms of resistance that are induced upon antibiotic exposure. Perhaps a key role of swarming-mediated resistance genes is to allow sufficient time for more established mechanisms, such as multidrug efflux, to take effect.
Upon tobramycin treatment, downregulation of the genes wzz, wbpA, wbpI, and wbpL (Table 3) might also contribute to tobramycin resistance, although these genes were not identified in resistome studies (13). As these genes, like wbpW, are involved in LPS biosynthesis, the resulting alterations in LPS could potentially result in tobramycin resistance. However, the mutant in wzz was not resistant to tobramycin, and mutants in wbpAIL were either not available or deficient for swarming motility (data not shown); therefore, firm conclusions could not be drawn.
In both the absence and presence of tobramycin, ribosomal proteins and translation factors were downregulated under swarming conditions (Tables 1 and 3). Since aminoglycosides, as well as many other antibiotic classes, including macrolides, chloramphenicol, and tetracycline, target the bacterial ribosome, a decrease in translational activity could confer some level of resistance. However, since ribosomal proteins are essential genes, this hypothesis is difficult to test.
During swarming motility, many genes are dysregulated, resulting in a hardy multiple-antibiotic-resistant phenotype associated with increased virulence factor production and iron scavenging. Downregulation of pyocin genes could allow swarming cells to circumvent harmful agents, leading to greater resilience in the face of antibiotic treatment. Downregulation of wbpW could result in reduced uptake of tobramycin. Upon treatment with tobramycin, the multidrug efflux pump MexXY was strongly upregulated. The combination of these factors, plus other as-yet-undetermined resistance factors, results in a state of (reversible) multiple-antibiotic resistance during swarming motility in P. aeruginosa.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
P. aeruginosa strain UCBPP-PA14 and transposon mutants from the PA14 Harvard library (60) were routinely grown in Luria-Bertani broth and BM2 glucose minimal medium [62 mM potassium phosphate buffer (pH 7), 7 mM (NH4)2SO4, 0.5 mM MgSO4, 10 μM FeSO4, 0.4% (wt/vol) glucose and 0.1% (wt/vol) Casamino Acids]. For motility assays, (NH4)2SO4 was omitted from BM2. Gentamicin at 30 μg/ml was included in streak plates for PA14 transposon mutants. LB overnight cultures were diluted 1/50 and grown to mid-log phase (optical density at 600 nm [OD600] of 0.3 to 0.6) to initiate motility studies.
Motility assays.
The concentration of agar in BM2 glucose was varied to allow for different kinds of motility. Swimming was assayed at 0.25% and 0.3% agar and swarming at 0.4% and 0.5% agar, and spread plates were made with 1.5% agar. For consistency between conditions, all plates were composed of the same medium, excepting agar concentration, and 25 ml of medium was poured per plate and dried for 1 h. All plates were stab (swim) or spot (swarm) inoculated with 1.5 μl of mid-log-phase bacteria grown in BM2 glucose, except for spread plates, which were spread with 106 CFU per plate. Bacteria were always inoculated at the same distance from the disc or another spot inoculum by using a stencil pattern drawn on the bottom of all plates. After inoculation, plates were incubated 15 to 18 h at 37°C and imaged on the ChemiDoc Touch imaging system (Bio-Rad).
Disc diffusion assay.
Discs were impregnated with the amounts of antibiotics indicated in Table S8 in the supplemental material and allowed to dry briefly before being placed in the center of a BM2 glucose agar plate with 0.3%, 0.4%, 0.5%, or 1.5% agar. For swarming and swimming, bacteria were spot inoculated a distance of 19 mm from the edge of the disc; for the 1.5% agar plates, bacteria were spread onto the surface of the plate prior to adding the disc. After overnight incubation, the zone of inhibition, representing the closest distance between the edge of the disc and visible bacterial growth, was measured using a ruler.
RNA isolation and preparation.
Swarming motility on 0.5% agar was compared to swimming in 0.25% agar by harvesting the edge of the swarm front with a blunt toothpick. Swim colonies were harvested from within the agar using a Q-tip. To inhibit swarming in the swim plates, 1.4 mM (NH4)2SO4 was included in the medium. Harvested bacteria were transferred to RNAprotect bacterial reagent (Qiagen), pelleted, and stored at −80°C. For the swim samples, most of the agar was removed from the pellets by pipetting. Pellets were lysed by resuspension in 6 mg/ml lysozyme dissolved in Tris-EDTA (TE) buffer (pH 8.0; Thermo Fisher) supplemented with 5 U β-agarase I (NEB) to digest remaining agar in the swim pellets. RNA isolation then proceeded according to the manufacturer’s instructions using the RNeasy minikit (Qiagen). Eluted RNA was further purified with the Turbo DNA-free kit (Thermo Fisher). Two independent runs of RNA-Seq were performed with a total of 5 biological replicates for swarming and 6 biological replicates for swimming.
For experiments assessing the effects of subinhibitory tobramycin, the swarm fronts from PA14 wild type (WT) grown on BM2 glucose swarm plates (0.5% agar) prepared with and without tobramycin 0.5 μg/ml were harvested, and RNA was isolated as described above but omitting the use of agarase. Three each of untreated and tobramycin-treated samples were sequenced on the same run. The quality and quantity of RNA were confirmed using the Bioanalyzer instrument.
Library preparation and RNA-Seq analysis.
Swarm versus swim RNA samples were depleted of rRNA using the RiboZero bacterial kit (Illumina), and libraries were prepared using the KAPA Stranded Total RNA kit (KAPA Biosystems). Sequencing was performed on an Illumina HiSeq 2500 by the University of British Columbia Sequencing and Bioinformatics Consortium. Sequence quality was determined using FastQC v0.11.8 and MultiQC v1.7. Reads were mapped to the P. aeruginosa UCBPP-PA14 reference genome obtained from the Pseudomonas Genome Database (www.pseudomonas.com) using the alignment program STAR v2.6.1a. Counts were generated using v0.11.2 of the HTSeq count function.
Library sizes after removal of low-read-count genes had a median of 5,894,065, minimum of 2,961,650, and maximum of 9,286,814 uniquely mapped reads. The experiment date was included in the design formula to control for any potential batch effects. Differentially expressed genes between swarming and swimming were determined using the package DESeq2 v1.20.0 in R v3.5.3, with thresholds of an adjusted P value (Padj) of ≤0.05 and absolute fold change of ≥1.5. The complete list of differentially expressed genes is available in Table S7.
For the experiment involving subinhibitory tobramycin, the same procedure as described above was followed. Library sizes, after removal of low-read-count genes, had a median of 1,642,204 uniquely mapped reads (minimum of 551,582; maximum of 4,585,572). In addition to the 3 untreated swarming controls specifically obtained for this experiment, the swarming samples from the “swarm versus swim” experiment described herein were also included when performing all downstream analyses for the subinhibitory tobramycin experiment, bringing the total number of samples for this experiment to 11: 8 untreated swarming controls and 3 tobramycin-treated swarming samples. The experiment date was incorporated into the design formula to account for any potential batch effects. Differentially expressed genes between tobramycin-treated swarming samples and untreated swarming controls were determined with an adjusted P value threshold of ≤0.05 and absolute fold change of ≥1.5. The full list of differentially expressed genes processed by the package DESeq2 v1.20.0 in R v3.5.3 is available in Table S7.
Quantitative reverse transcriptase PCR.
Swarming motility was compared to that of planktonic cells as described previously (15), except strain PA14 was used and swarm plates were harvested at 16 h to prevent overgrowth. RNA was isolated and DNase digested as described above, but without the use of agarase, and quantified on a NanoDrop spectrophotometer ND-1000. RNA was then diluted to 1 ng/μl, and 5 μl was used in a total reaction volume of 25 μl. The qScript One-Step SYBR green RT-qPCR kit (Quantabio) was used and samples were run on a LightCycler 96 (Roche). Cycle threshold (CT) values were normalized to the housekeeping gene rpoD using the ΔΔCT method. Primers used for qRT-PCR are described in Table S9.
Swarming inhibitory concentration determination by the agar dilution method.
Antibiotics were incorporated into BM2 glucose swarming agar (0.5% agar) at various concentrations. After overnight incubation, the minimal concentration that completely inhibited swarming tendril formation was reported as the swarming inhibitory concentration (IC).
MIC assay.
Bacteria were seeded at 5 × 105 CFU/ml in a 2-fold concentration gradient of antibiotic in LB or BM2 glucose without (NH4)2SO4 at 100 μl/well in 96-well polystyrene round-bottom plates. After 24 h of incubation at 37°C, the minimum concentration to inhibit visible bacterial growth was reported as the MIC.
Outer membrane permeabilization assay.
Outer membrane permeability was assessed using the fluorescent dye N-phenyl-1-naphthylamine (NPN) as described previously (13) with minor modifications. Briefly, cells were harvested from antibiotic-free BM2 glucose swarm plates (0.5% agar), resuspended in 5 mM HEPES (pH 7.0) supplemented with 5 μM carbonyl cyanide m-chlorophenyl hydrazone, and then diluted to an OD600 of 0.5. Fluorescence was monitored in the PerkinElmer fluorescence spectrometer LS 55 at an excitation wavelength of 350 nm and emission wavelength of 420 nm. NPN was added at a final concentration of 10 μM, and then tobramycin was added at a final concentration of 40 μg/ml.
Kill curves.
Bacteria were harvested from antibiotic-free swim (0.3% agar) and swarm (0.5% agar) plates, resuspended in 62 mM potassium phosphate buffer (pH 7.0), and diluted to a final OD600 of 0.025 in 10 ml 62 mM potassium phosphate buffer. Cells were then treated with 20 μg/ml tobramycin with aeration at room temperature, and aliquots were periodically taken for serial dilution in phosphate-buffered saline (PBS; pH 7.4) for colony enumeration on LB plates.
Construction of complementation plasmids.
PA14 WT genomic DNA (gDNA) was isolated as specified in the Qiagen DNeasy Blood and Tissue kit protocol. Eighty-four nanograms was PCR amplified using the primers for prtN (forward [F], GGATCCATGCAGCCAACCATCGCC; reverse [R], TCTAGATCAGGATGCGATGCTGTCC) or wbpW (F, GGATCCATGCTGATTCCCGTGGTGC; R, TCTAGATCAGACCACCCTGCCGTA). PCR products were gel extracted with the GeneJet gel extraction kit (Thermo Fisher) and TOPO cloned (Invitrogen). Next, the TOPO reaction was transformed into TOP10 E. coli and selected with kanamycin (50 μg/ml). Plasmids were subsequently isolated according to the Thermo Fisher kit and digested with restriction endonucleases. wbpW was digested with BamHI and XbaI and cloned into pBBR1mcs2, and prtN was digested with SacI and XbaI and cloned into pHERD20T. After gel extraction, the fragments were ligated into a similarly digested vector with T4 DNA ligase (Thermo Scientific) and transformed into TOP10 E. coli. pBBR1mcs2 was selected with 50 μg/ml kanamycin and pHERD20T with 100 μg/ml ampicillin. Plasmid sequences were confirmed by Sanger sequencing at the Sequencing and Bioinformatics Consortium at UBC.
Transformation of P. aeruginosa.
Electrocompetent P. aeruginosa cells were transformed with both empty vector and vector with insert according to Choi et al. (61). The wild type was transformed with empty vectors pBBR1mcs2 and pHERD20T, the prtN mutant was transformed with the empty vector pHERD20T, and the wbpW mutant was transformed with the empty vector pBBR1mcs2. Complemented strains were generated by transforming the prtN mutant with the construct prtN-pHERD20T and the wbpW mutant with wbpW-pBBR1mcs2 (indicated with “+” in Fig. 5). Transformants were selected with 300 μg/ml carbenicillin for pHERD20T and 250 μg/ml kanamycin for pBBR1mcs2 and confirmed to carry the correct plasmid. For complementation with pHERD20T, expression from the vector was induced by adding 0.3% (wt/vol) arabinose.
Data availability.
Fastq and “count” files for all RNA-Seq samples are available on the Gene Expression Omnibus (GEO) under accession numbers GSE121504 (swarm versus swim) and GSE137676 (tobramycin versus untreated).
Supplementary Material
ACKNOWLEDGMENTS
Research reported in this publication was supported by a grant from the Canadian Institutes for Health Research (FDN-154287) and the Cystic Fibrosis (CF) Canada (award number 3177). The content is solely the responsibility of the authors and does not necessarily represent the official views of the Canadian Institutes for Health Research. S.R.C. is the recipient of CIHR Frederick Banting and Charles Best Canada Graduate Scholarship master’s (CGS-M) and doctoral (CGS-D) awards and a four-year fellowship for PhD students from UBC.
R.E.W.H. holds a Canada Research Chair in Health and Genomics and a UBC Killam Professorship. We thank Amy Lee for insightful advice.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Surette MG. 2014. The cystic fibrosis lung microbiome. Ann Am Thorac Soc 11:S61–S65. doi: 10.1513/AnnalsATS.201306-159MG. [DOI] [PubMed] [Google Scholar]
- 2.Davies JC. 2002. Pseudomonas aeruginosa in cystic fibrosis: pathogenesis and persistence. Paediatr Respir Rev 3:128–134. doi: 10.1016/s1526-0550(02)00003-3. [DOI] [PubMed] [Google Scholar]
- 3.Murray TS, Egan M, Kazmierczak BI. 2007. Pseudomonas aeruginosa chronic colonization in cystic fibrosis patients. Curr Opin Pediatr 19:83–88. doi: 10.1097/MOP.0b013e3280123a5d. [DOI] [PubMed] [Google Scholar]
- 4.Cystic Fibrosis Foundation. 2016. 2016 Patient registry annual data report. Cystic Fibrosis Foundation, Bethesda, MD. [Google Scholar]
- 5.Moore NM, Flaws ML. 2011. Introduction: Pseudomonas aeruginosa. Clin Lab Sci 24:41–42. doi: 10.29074/ascls.24.1.41. [DOI] [PubMed] [Google Scholar]
- 6.Fernández L, Hancock R. 2012. Adaptive and mutational resistance: role of porins and efflux pumps in drug resistance. Clin Microbiol Rev 25:661–681. doi: 10.1128/CMR.00043-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jo JTH, Brinkman FSL, Hancock R. 2003. Aminoglycoside efflux in Pseudomonas aeruginosa: involvement of novel outer membrane proteins. Antimicrob Agents Chemother 47:1101–1111. doi: 10.1128/aac.47.3.1101-1111.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brazas MD, Breidenstein EBM, Overhage J, Hancock R. 2007. Role of Lon, an ATP-dependent protease homolog, in resistance of Pseudomonas aeruginosa to ciprofloxacin. Antimicrob Agents Chemother 51:4276–4283. doi: 10.1128/AAC.00830-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dötsch A, Becker T, Pommerenke C, Magnowska Z, Jänsch L, Häussler S. 2009. Genomewide identification of genetic determinants of antimicrobial drug resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 53:2522–2531. doi: 10.1128/AAC.00035-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Breidenstein EBM, Khaira BK, Wiegand I, Overhage J, Hancock R. 2008. Complex ciprofloxacin resistome revealed by screening a Pseudomonas aeruginosa mutant library for altered susceptibility. Antimicrob Agents Chemother 52:4486–4491. doi: 10.1128/AAC.00222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alvarez-Ortega C, Wiegand I, Olivares J, Hancock REW, Martínez JL. 2010. Genetic determinants involved in the susceptibility of Pseudomonas aeruginosa to β-lactam antibiotics. Antimicrob Agents Chemother 54:4159–4167. doi: 10.1128/AAC.00257-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fajardo A, Martínez-Martín N, Mercadillo M, Galán JC, Ghysels B, Matthijs S, Cornelis P, Wiehlmann L, Tümmler B, Baquero F, Martínez JL. 2008. The neglected intrinsic resistome of bacterial pathogens. PLoS One 3:e1619. doi: 10.1371/journal.pone.0001619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schurek KN, Marr AK, Taylor PK, Wiegand I, Semenec L, Khaira BK, Hancock R. 2008. Novel genetic determinants of low-level aminoglycoside resistance in Pseudomonas aeruginosa. Antimicrob Agents Chemother 52:4213–4219. doi: 10.1128/AAC.00507-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fernández L, Jenssen H, Bains M, Wiegand I, Gooderham WJ, Hancock R. 2012. The two-component system CprRS senses cationic peptides and triggers adaptive resistance in Pseudomonas aeruginosa independently of ParRS. Antimicrob Agents Chemother 56:6212–6222. doi: 10.1128/AAC.01530-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Overhage J, Bains M, Brazas MD, Hancock R. 2008. Swarming of Pseudomonas aeruginosa is a complex adaptation leading to increased production of virulence factors and antibiotic resistance. J Bacteriol 190:2671–2679. doi: 10.1128/JB.01659-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lai S, Tremblay J, Déziel E. 2009. Swarming motility: a multicellular behaviour conferring antimicrobial resistance. Environ Microbiol 11:126–136. doi: 10.1111/j.1462-2920.2008.01747.x. [DOI] [PubMed] [Google Scholar]
- 17.Yeung ATY, Torfs ECW, Jamshidi F, Bains M, Wiegand I, Hancock REW, Overhage J. 2009. Swarming of Pseudomonas aeruginosa is controlled by a broad spectrum of transcriptional regulators, including MetR. J Bacteriol 191:5592–5602. doi: 10.1128/JB.00157-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gill SK, Hui K, Farne H, Garnett JP, Baines DL, Moore LSP, Holmes AH, Filloux A, Tregoning JS. 2016. Increased airway glucose increases airway bacterial load in hyperglycaemia. Sci Rep 6:27636–27610. doi: 10.1038/srep27636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baker EH, Clark N, Brennan AL, Fisher DA, Gyi KM, Hodson ME, Philips BJ, Baines DL, Wood DM. 2007. Hyperglycemia and cystic fibrosis alter respiratory fluid glucose concentrations estimated by breath condensate analysis. J Appl Physiol (1985) 102:1969–1975. doi: 10.1152/japplphysiol.01425.2006. [DOI] [PubMed] [Google Scholar]
- 20.Palmer KL, Aye LM, Whiteley M. 2007. Nutritional cues control Pseudomonas aeruginosa multicellular behavior in cystic fibrosis sputum. J Bacteriol 189:8079–8087. doi: 10.1128/JB.01138-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Winstanley C, O'Brien S, Brockhurst MA. 2016. Pseudomonas aeruginosa evolutionary adaptation and diversification in cystic fibrosis chronic lung infections. Trends Microbiol 24:327–337. doi: 10.1016/j.tim.2016.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.O'May C, Tufenkji N. 2011. The swarming motility of Pseudomonas aeruginosa is blocked by cranberry proanthocyanidins and other tannin-containing materials. Appl Environ Microbiol 77:3061–3067. doi: 10.1128/AEM.02677-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Butler MT, Wang Q, Harshey RM. 2010. Cell density and mobility protect swarming bacteria against antibiotics. Proc Natl Acad Sci U S A 107:3776–3781. doi: 10.1073/pnas.0910934107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Petrova OE, Sauer K. 2010. The novel two-component regulatory system BfiSR regulates biofilm development by controlling the small RNA rsmZ through CafA. J Bacteriol 192:5275–5288. doi: 10.1128/JB.00387-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Heinrichs DE, Poole K. 1996. PchR, a regulator of ferripyochelin receptor gene (fptA) expression in Pseudomonas aeruginosa, functions both as an activator and as a repressor. J Bacteriol 178:2586–2592. doi: 10.1128/jb.178.9.2586-2592.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liang H, Deng X, Ji Q, Sun F, Shen T, He C. 2012. The Pseudomonas aeruginosa global regulator VqsR directly inhibits QscR to control quorum-sensing and virulence gene expression. J Bacteriol 194:3098–3108. doi: 10.1128/JB.06679-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okkotsu Y, Tieku P, Fitzsimmons LF, Churchill ME, Schurr MJ. 2013. Pseudomonas aeruginosa AlgR phosphorylation modulates rhamnolipid production and motility. J Bacteriol 195:5499–5515. doi: 10.1128/JB.00726-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.López-Causapé C, Rubio R, Cabot G, Oliver A. 2018. Evolution of the Pseudomonas aeruginosa aminoglycoside mutational resistome in vitro and in the cystic fibrosis setting. Antimicrob Agents Chemother 62:e02583-17. doi: 10.1128/AAC.02583-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Izutsu K, Wada A, Wada C. 2001. Expression of ribosome modulation factor (RMF) in Escherichia coli requires ppGpp. Genes Cells 6:665–676. doi: 10.1046/j.1365-2443.2001.00457.x. [DOI] [PubMed] [Google Scholar]
- 30.Lundgren BR, Villegas-Peñaranda LR, Harris JR, Mottern AM, Dunn DM, Boddy CN, Nomura CT. 2014. Genetic analysis of the assimilation of C5-dicarboxylic acids in Pseudomonas aeruginosa PAO1. J Bacteriol 196:2543–2551. doi: 10.1128/JB.01615-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brazas MD, Hancock R. 2005. Ciprofloxacin induction of a susceptibility determinant in Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:3222–3227. doi: 10.1128/AAC.49.8.3222-3227.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rossello J, Lima A, Gil M, Duarte JR, Correa A, Carvalho PC, Kierbel A, Durán R. 2017. The EAL-domain protein FcsR regulates flagella, chemotaxis and type III secretion system in Pseudomonas aeruginosa by a phosphodiesterase independent mechanism. Sci Rep 7:10281. doi: 10.1038/s41598-017-09926-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fajardo A, Hernando-Amado S, Oliver A, Ball G, Filloux A, Martinez JL. 2014. Characterization of a novel Zn2+-dependent intrinsic imipenemase from Pseudomonas aeruginosa. J Antimicrob Chemother 69:2972–2978. doi: 10.1093/jac/dku267. [DOI] [PubMed] [Google Scholar]
- 34.Hancock REW, Raffle VJ, Nicas TI. 1981. Involvement of the outer membrane in gentamicin and streptomycin uptake and killing in Pseudomonas aeruginosa. Antimicrob Agents Chemother 19:777–785. doi: 10.1128/aac.19.5.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Penterman J, Singh PK, Walker GC. 2014. Biological cost of pyocin production during the SOS response in Pseudomonas aeruginosa. J Bacteriol 196:3351–3359. doi: 10.1128/JB.01889-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aires JR, Köhler T, Nikaido H, Plésiat P. 1999. Involvement of an active efflux system in the natural resistance of Pseudomonas aeruginosa to aminoglycosides. Antimicrob Agents Chemother 43:2624–2628. doi: 10.1128/AAC.43.11.2624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dennis JJ, Lafontaine ER, Sokol PA. 1996. Identification and characterization of the tolQRA genes of Pseudomonas aeruginosa. J Bacteriol 178:7059–7068. doi: 10.1128/jb.178.24.7059-7068.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Matsuo Y, Eda S, Gotoh N, Yoshihara E, Nakae T. 2004. MexZ-mediated regulation of mexXY multidrug efflux pump expression in Pseudomonas aeruginosa by binding on the mexZ-mexX intergenic DNA. FEMS Microbiol Lett 238:23–28. doi: 10.1016/j.femsle.2004.07.010. [DOI] [PubMed] [Google Scholar]
- 39.Díaz-Pérez AL, Núñez C, Meza Carmen V, Campos-García J. 2018. The expression of the genes involved in leucine catabolism of Pseudomonas aeruginosa is controlled by the transcriptional regulator LiuR and by the CbrAB/Crc system. Res Microbiol 169:324–334. doi: 10.1016/j.resmic.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 40.Gicquel G, Bouffartigues E, Bains M, Oxaran V, Rosay T, Lesouhaitier O, Connil N, Bazire A, Maillot O, Bénard M, Cornelis P, Hancock REW, Dufour A, Feuilloley MGJ, Orange N, Déziel E, Chevalier S. 2013. The extra-cytoplasmic function sigma factor SigX modulates biofilm and virulence-related properties in Pseudomonas aeruginosa. PLoS One 8:e80407. doi: 10.1371/journal.pone.0080407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Fléchard M, Duchesne R, Tahrioui A, Bouffartigues E, Depayras S, Hardouin J, Lagy C, Maillot O, Tortuel D, Azuama CO, Clamens T, Duclairoir-Poc C, Catel-Ferreira M, Gicquel G, Feuilloley MGJ, Lesouhaitier O, Heipieper HJ, Groleau MC, Déziel É, Cornelis P, Chevalier S. 2018. The absence of SigX results in impaired carbon metabolism and membrane fluidity in Pseudomonas aeruginosa. Sci Rep 8:1–13. doi: 10.1038/s41598-018-35503-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Blanka A, Schulz S, Eckweiler D, Franke R, Bielecka A, Nicolai T, Casilag F, Düvel J, Abraham WR, Kaever V, Häussler S. 2014. Identification of the alternative sigma factor SigX regulon and its implications for Pseudomonas aeruginosa pathogenicity. J Bacteriol 196:345–356. doi: 10.1128/JB.01034-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.De Kievit T, Seed PC, Nezezon J, Passador L, Iglewski BH. 1999. RsaL, a novel repressor of virulence gene expression in Pseudomonas aeruginosa. J Bacteriol 181:2175–2184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Rampioni G, Bertani I, Zennaro E, Polticelli F, Venturi V, Leoni L. 2006. The quorum-sensing negative regulator RsaL of Pseudomonas aeruginosa binds to the lasI promoter. J Bacteriol 188:815–819. doi: 10.1128/JB.188.2.815-819.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Diggle SP, Winzer K, Lazdunski A, Williams P, Cámara M. 2002. Advancing the quorum in Pseudomonas aeruginosa: MvaT and the regulation of N-acylhomoserine lactone production and virulence gene expression. J Bacteriol 184:2576–2586. doi: 10.1128/jb.184.10.2576-2586.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mulcahy H, O'Callaghan J, O'Grady EP, Maciá MD, Borrell N, Gómez C, Casey PG, Hill C, Adams C, Gahan CGM, Oliver A, O'Gara F. 2008. Pseudomonas aeruginosa RsmA plays an important role during murine infection by influencing colonization, virulence, persistence, and pulmonary inflammation. Infect Immun 76:632–638. doi: 10.1128/IAI.01132-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Allsopp LP, Wood TE, Howard SA, Maggiorelli F, Nolan LM, Wettstadt S, Filloux A. 2017. RsmA and AmrZ orchestrate the assembly of all three type VI secretion systems in Pseudomonas aeruginosa. Proc Natl Acad Sci U S A 114:7707–7712. doi: 10.1073/pnas.1700286114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Burrowes E, Baysse C, Adams C, O'Gara F. 2006. Influence of the regulatory protein RsmA on cellular functions in Pseudomonas aeruginosa PAO1, as revealed by transcriptome analysis. Microbiology 152:405–418. doi: 10.1099/mic.0.28324-0. [DOI] [PubMed] [Google Scholar]
- 49.Winsor GL, Lam DKW, Fleming L, Lo R, Whiteside MD, Yu NY, Hancock REW, Brinkman F. 2011. Pseudomonas genome database: improved comparative analysis and population genomics capability for Pseudomonas genomes. Nucleic Acids Res 39:D596–D600. doi: 10.1093/nar/gkq869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Casabona MG, Silverman JM, Sall KM, Boyer F, Couté Y, Poirel J, Grunwald D, Mougous JD, Elsen S, Attree I. 2013. An ABC transporter and an outer membrane lipoprotein participate in posttranslational activation of type VI secretion in Pseudomonas aeruginosa. Environ Microbiol 15:471–486. doi: 10.1111/j.1462-2920.2012.02816.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang L, Zhang C, Gong F, Li H, Xie X, Xia C, Chen J, Song Y, Shen A, Song J. 2013. Influence of Pseudomonas aeruginosa pvdQ gene on altering antibiotic susceptibility under swarming conditions. Curr Microbiol 66:152–161. doi: 10.1007/s00284-012-0217-1. [DOI] [PubMed] [Google Scholar]
- 52.Matsui H, Sano Y, Ishihara H, Shinomiya T. 1993. Regulation of pyocin genes in Pseudomonas aeruginosa by positive (prtN) and negative (prtR) regulatory genes. J Bacteriol 175:1257–1263. doi: 10.1128/jb.175.5.1257-1263.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Chang W, Small DA, Toghrol F, Bentley WE. 2005. Microarray analysis of Pseudomonas aeruginosa reveals induction of pyocin genes in response to hydrogen peroxide. BMC Genomics 6:115. doi: 10.1186/1471-2164-6-115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sangurdekar DP, Zhang Z, Khodursky AB. 2011. The association of DNA damage response and nucleotide level modulation with the antibacterial mechanism of the anti-folate drug trimethoprim. BMC Genomics 12:583. doi: 10.1186/1471-2164-12-583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Byrd MS, Sadovskaya I, Vinogradov E, Lu H, Sprinkle AB, Richardson SH, Ma L, Ralston B, Parsek MR, Anderson EM, Lam JS, Wozniak DJ. 2009. Genetic and biochemical analyses of the Pseudomonas aeruginosa Psl exopolysaccharide reveal overlapping roles for polysaccharide synthesis enzymes in Psl and LPS production. Mol Microbiol 73:622–638. doi: 10.1111/j.1365-2958.2009.06795.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.King JD, Kocíncová D, Westman EL, Lam JS. 2009. Lipopolysaccharide biosynthesis in Pseudomonas aeruginosa. Innate Immun 15:261–312. doi: 10.1177/1753425909106436. [DOI] [PubMed] [Google Scholar]
- 57.Hancock REW, Mutharia LM, Chan L, Darveau RP, Speert DP, Pier GB. 1983. Pseudomonas aeruginosa isolates from patients with cystic fibrosis: a class of serum-sensitive, nontypable strains deficient in lipopolysaccharide O side chains. Infect Immun 42:170–177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Cigana C, Curcuru L, Leone MR, Ierano T, Lore NI, Bianconi I, Silipo A, Cozzolino F, Lanzetta R, Molinaro A, Bernardini ML, Bragonzi A. 2009. Pseudomonas aeruginosa exploits lipid A and muropeptides modification as a strategy to lower innate immunity during cystic fibrosis lung infection. PLoS One 4:e8439. doi: 10.1371/journal.pone.0008439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Augustin DK, Song Y, Baek MS, Sawa Y, Singh G, Taylor B, Rubio-Mills A, Flanagan JL, Wiener-Kronish JP, Lynch SV. 2007. Presence or absence of lipopolysaccharide O antigens affects type III secretion by Pseudomonas aeruginosa. J Bacteriol 189:2203–2209. doi: 10.1128/JB.01839-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Liberati NT, Urbach JM, Miyata S, Lee DG, Drenkard E, Wu G, Villanueva J, Wei T, Ausubel FM. 2006. An ordered, nonredundant library of Pseudomonas aeruginosa strain PA14 transposon insertion mutants. Proc Natl Acad Sci U S A 103:2833–2838. doi: 10.1073/pnas.0511100103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Choi K, Kumar A, Schweizer HP. 2006. A 10-min method for preparation of highly electrocompetent Pseudomonas aeruginosa cells: application for DNA fragment transfer between chromosomes and plasmid transformation. J Microbiol Methods 64:391–397. doi: 10.1016/j.mimet.2005.06.001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Fastq and “count” files for all RNA-Seq samples are available on the Gene Expression Omnibus (GEO) under accession numbers GSE121504 (swarm versus swim) and GSE137676 (tobramycin versus untreated).