The antifungal pharmacopeia is critically small, particularly in light of the recent emergence of multidrug-resistant pathogens, such as Candida auris. Here, we report that derivatives of the antimalarial drug mefloquine have broad-spectrum antifungal activity against pathogenic yeasts and molds.
KEYWORDS: antifungal, mefloquine, repurposing, Candida, Cryptococcus, Aspergillus, Candida auris, Cryptococcus neoformans, antifungal agents, mefloquine
ABSTRACT
The antifungal pharmacopeia is critically small, particularly in light of the recent emergence of multidrug-resistant pathogens, such as Candida auris. Here, we report that derivatives of the antimalarial drug mefloquine have broad-spectrum antifungal activity against pathogenic yeasts and molds. In addition, the mefloquine derivatives have activity against clinical isolates that are resistant to one or more of the three classes of antifungal drugs currently used to treat invasive fungal infections, indicating that they have a novel mechanism of action. Importantly, the in vitro toxicity profiles obtained using human cell lines indicated that the toxicity profiles of the mefloquine derivatives are very similar to those of the parent mefloquine, despite being up to 64-fold more active against fungal cells. In addition to direct antifungal activity, subinhibitory concentrations of the mefloquine derivatives inhibited the expression of virulence traits, including filamentation in Candida albicans and capsule formation/melanization in Cryptococcus neoformans. Mode/mechanism-of-action experiments indicated that the mefloquine derivatives interfere with both mitochondrial and vacuolar function as part of a multitarget mechanism of action. The broad-spectrum scope of activity, blood-brain barrier penetration, and large number of previously synthesized analogs available combine to support the further optimization and development of the antifungal activity of this general class of drug-like molecules.
INTRODUCTION
The need for novel classes of antifungal drugs has never been more pressing (1). The numbers of patients who are at risk for developing invasive fungal infections continues to increase as therapies that modulate the immune system are introduced into clinical practice. In addition, antifungal drug resistance has been increasing with resistance to all classes of antifungal drugs now well recognized in pathogenic molds and yeast. Indeed, the recent emergence of the multidrug-resistant fungus Candida auris emphasizes the impact of this trend (2). Because we have only 3 classes of antifungal drugs, as opposed to at least 10 classes of antibacterials, the options for treating fungal infections are extremely limited (1). As such, the path toward an untreatable fungal infection is extremely short. Although recent progress has been made, with novel antifungal compounds moving into early-phase clinical trials, the pace of antifungal drug discovery must increase to ensure a robust pipeline of new drugs. Accordingly, novel approaches to identify antifungal drug candidates are needed (3).
In many areas of medicine, the repurposing of existing drugs for new indications has been pursued as an expedited approach to developing new therapies (4). This type of search most commonly begins with a high-throughput screen (HTS) of a library of FDA-approved drugs. We and others have identified antifungal small molecules using this approach, and two repurposed drugs have been evaluated as adjuvants in the treatment of cryptococcal meningoencephalitis (5, 6). Campaigns such as these have almost exclusively focused on identifying drugs that are candidates for direct translation to clinical use. However, a second type of repurposing approach involves the use of the identified molecule as a starting point for further optimization of the new activity. This has been termed selective optimization of side activities (SOSA) (7). The rationale for this approach is based on the fact that it is quite difficult to predict whether or not a novel chemical scaffold will have drug-like properties or undesirable toxicities. Therefore, optimizing a desired activity within a drug-like scaffold with well-characterized pharmacological properties and toxicities may lead to a clinically useful molecule more quickly than if development begins with a completely novel chemical entity. To our knowledge, this approach has not been widely applied to antifungal drug development.
We took a novel approach to identify a scaffold for SOSA-based optimization. Specifically, we performed manual literature searches for clinically used drugs that had been studied using genome-wide, yeast-based chemical genetic experiments to characterize their mechanisms of action (8). Many of the drugs evaluated in the large-scale yeast chemical genetic screens had relatively low levels of antifungal activity that would not normally be sufficient to emerge from a high-throughput screen. Consequently, this approach would allow us to access novel antifungal chemical space that could be amenable to further optimization. In addition, the chemical genetic profiles of the drugs provide hypothesis-generating data regarding the mechanism and mode of action.
Because most invasive fungal infections can affect the central nervous system (CNS) in some manner (9), we narrowed our search to molecules that were known to cross the blood-brain barrier. A drug that had been the focus of two yeast-based chemical genetic screens and that fit our criteria was the antimalarial mefloquine (MEF). MEF, previously WR 142490, is an orally available 4-quinoline-methanol (Fig. 1A) discovered by the Experimental Therapeutics Division of the Walter Reed Army Institute of Research during the early 1970s to treat malaria and was approved by FDA in 1989 (10). An attractive feature of MEF was the availability of structural analogs of MEF through the National Cancer Institute (NCI) Developmental Therapeutics Program.
FIG 1.
Chemical structures of mefloquine (A) and mefloquine derivatives obtained from the National Cancer Institute Chemical Repository (B).
MEF has been studied as a repurposing candidate against other human parasites, including those causing schistosomiasis, toxoplasmosis, and echinococcosis (11–17). MEF has also been shown to have antibacterial activity against Streptococcus pneumoniae and Mycobacterium tuberculosis (18, 19). Additionally, repurposing efforts have found that it has antiviral activity against Ebola, dengue, and Zika viruses (20, 21). Most importantly, a small set of MEF derivatives was shown to have activity against Cryptococcus neoformans and Candida albicans (22). Despite these promising findings, no additional characterization of the antifungal activity of the MEF derivatives has been reported. Here, we show that MEF derivatives have broad-spectrum antifungal activity against both drug-susceptible and drug-resistant fungi, show synergy with existing antifungal drugs, modulate the expression of virulence traits in both C. albicans and C. neoformans, and interfere with the functions and interactions of mitochondria and vacuoles in fungi.
RESULTS
Mefloquine derivatives have in vitro antifungal activity against a variety of human fungal pathogens.
To explore the antifungal activity of the MEF scaffold, we first tested the activity of MEF against a set of reference strains of pathogenic fungi, including C. albicans, Candida glabrata, C. auris, C. neoformans, and Aspergillus fumigatus, as well as the model yeast Saccharomyces cerevisiae (Table 1). Consistent with previous reports, MEF had minimal antifungal activity, with C. neoformans being the most susceptible pathogenic species. As discussed above, Kunin and Ellis (22) had reported that some MEF derivatives had improved antifungal activity relative to that of the parent drug. We therefore obtained 13 MEF derivatives from the NCI Chemical Repository and screened them for antifungal activity. To our delight, four derivatives showed improved antifungal activity relative to that of MEF (Fig. 1B; Table 1). The most active derivative was 4377, which had MIC values below 4 μg/ml for all species tested; 4377 was not previously examined by Kunin and Ellis (22).
TABLE 1.
MICs of mefloquine derivatives against susceptible fungal reference strains
| Species | Strain | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEF | 2450 | 4377 | 13480 | 305758 | AmB | FLC | CAS | VOR | ||
| C. albicans | SC5314 | >128 | 8 | 4 | 4–8 | 8 | 0.25 | 0.25 | 0.125–0.25 | |
| C. glabrata | BG2 | 128 | 8 | 4 | 2–4 | 8 | 1–2 | 8 | 0.5 | |
| C. auris | 0381 | 128 | 8 | 2 | 4 | 4 | 0.38a | 4a | 0.125a | |
| C. neoformans | H99 | 32 | 4 | 1 | 1 | 2 | 0.5–1 | 2–4 | ||
| S. cerevisiae | BY4741 | >16 | 8 | 4 | >16 | 16 | >16 | 0.03 | ||
| S. cerevisiae | BY4742 | >16 | 8 | 4 | 16 | 8 | >16 | 0.03 | ||
| S. cerevisiae | BY4743 | >16 | 8 | 4 | 16 | 8 | >16 | 0.03 | ||
| A. fumigatus | AF293 | > 64 | 8 | 2 | 4 | 4 | 1 | |||
| A. fumigatus | CEA10 | >64 | 8 | 2 | 4 | 8 | 0.5 | |||
| A. fumigatus | SPF93 | >64 | 8 | 2 | 4 | 8 | 0.5 | |||
MICs reported by the Centers for Disease Control and Prevention.
To confirm the antifungal activity of the MEF derivatives, we tested them for their activities against a variety of isolates with various susceptibilities to clinically used antifungal drugs, including fluconazole- and echinocandin-resistant Candida spp. and voriconazole (VOR)-resistant A. fumigatus. As shown in Table 2, the MICs of the MEF derivatives did not vary more than 2-fold among this diverse set of clinical fungal isolates. Most importantly, the MEF derivatives were active against strains with increased multidrug efflux activity, such as C. albicans strain TWO7243 #17 (23), indicating that the MEF derivatives were not susceptible to efflux pumps that are active against azoles. The MEF derivatives were also uniformly active against C. auris isolates with decreased susceptibility to fluconazole (FLC), caspofungin (CAS), and amphotericin B (AmB) (Table 2). These data firmly establish that this scaffold has broad-spectrum antifungal activity.
TABLE 2.
MICs of mefloquine derivatives against antifungal-resistant clinical isolates
| Species | Clinical isolate identifier | MIC (μg/ml) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| MEF | 2450 | 4377 | 13480 | 305758 | AmB | FLC | CAS | VOR | ||
| C. albicans | 1010 | >128 | 8 | 4 | 8 | 8 | 0.5 | >256 | 4 | |
| O-788 | >128 | 8 | 4 | 8 | 8 | 0.5 | >256 | 2 | ||
| TWO7243 #17 | >64 | 8 | 4 | 8 | 16 | 1 | >128 | 0.25 | ||
| C. glabrata | 1 | 128 | 8 | 2 | 2 | 4 | 0.5 | 2 | 2–4 | |
| 102 | >64 | 8 | 4 | 2–4 | 8 | 0.5 | ≥64 | 64 | ||
| 999 | >128 | 8 | 4 | 4 | 16 | 0.5 | 128 | 64 | ||
| 4720 | 128 | 8–16 | 2–4 | 4 | 8 | 0.25–0.5 | 128 | 64 | ||
| C. auris | 0383 | 128 | 16 | 4 | 4 | 8 | 0.38a | 128a | 0.25a | |
| 0385 | 128 | 8 | 4 | 4 | 8 | 0.5a | >256a | 0.5a | ||
| 0387 | 128 | 8 | 2 | 8 | 8 | 0.75a | 8a | 0.25a | ||
| 0388 | 128 | 8 | 4 | 8 | 8 | 1.5a | >256a | 1a | ||
| 0390 | 128 | 16 | 4 | 8 | 8 | 4a | >256a | 0.5a | ||
| C. neoformans | 101.14 | 64 | 4 | 2 | 2 | 4 | 1 | 16 | ||
| 103.98 | 64 | 4 | 2 | 2 | 4 | 1 | 4 | |||
| 111.00 | 32 | 2 | 1 | 1 | 2 | 1–2 | 2 | |||
| 118.00 | 64 | 4–8 | 2 | 2–4 | 4 | 0.5–1 | >16 | |||
| 120.04 | 32 | 4–8 | 2 | 2 | 4–8 | 0.5–1 | 4 | |||
| 127.01 | 32 | 4 | 1 | 2 | 2 | 1–2 | 1 | |||
| 138.97 | 32 | 4 | 1 | 1 | 2 | 1 | 4–8 | |||
| 153.02 | 32 | 4 | 1 | 2 | 2 | 2 | 4 | |||
| 158.03 | 32 | 4 | 1 | 2 | 2 | 2 | >16 | |||
| 226.03 | 64 | 4 | 2 | 2 | 4 | 2 | 16 | |||
| A. fumigatus | SPF98 | >64 | 8 | 2 | 4 | 8 | 4 | |||
MICs reported by the Centers for Disease Control and Prevention.
Finally, we attempted to isolate strains that were resistant to the MEF derivatives by serial passage of the strains through increasing concentrations of the compounds. Although some initially resistant colonies were identified, none were stably resistant, and the susceptibility of all strains reverted to the susceptibility of the parental strain following passage on compound-free medium. As such, the rates of development of spontaneous or selected resistance to the MEF derivatives were low. Overall, these derivatives of MEF had broad-spectrum activity against medically important fungi and were not affected by mechanisms of resistance against currently used antifungal drugs.
Combinations of mefloquine or mefloquine derivatives are fungicidal.
Combination antifungal therapy is of increasing interest as a means to modulate antifungal resistance and/or improve antifungal efficacy (24). To assess the interactions between currently used antifungal drugs and MEF derivatives, we performed checkerboard microdilution assays and analyzed the data using the fractional inhibitory concentration index (FICI). FICIs were determined for C. albicans (strain SC5314), C. glabrata (strain BG2), C. neoformans (strain H99), and C. glabrata (strain 102), the last of which is a clinical isolate resistant to both FLC and CAS. Analysis of the interactions between the MEF derivatives and either AmB, FLC, or CAS showed that the activity of all but one combination was additive (0.5 < FICI ≤ 4.0) (see Table S1 in the supplemental material). The combination of MEF derivative 2450 and CAS resulted in a synergistic interaction (FICI < 0.5). Interestingly, MEF derivative 4377 at one-half the MIC reduced the concentration of CAS required to inhibit growth by >64-fold in C. glabrata clinical isolate 102. Although this is formally an additive interaction because it was observed at only one-half the MIC of 4377, it indicates that the susceptibility of the strain to CAS was dramatically modulated by MEF derivative 4377.
A second type of clinically relevant interaction between two drugs is the conversion of drugs that are fungistatic by themselves to a combination that is fungicidal. This type of interaction is particularly relevant to the treatment of cryptococcal meningitis because fungicidal activity has been shown to improve outcomes (25). Specifically, outcomes for patients treated with the fungistatic agent FLC are much worse than those for patients treated with the fungicidal agent AmB (26). Accordingly, the identification of molecules that lead to fungicidal combinations with FLC has been pursued as an approach to improving the treatment of cryptococcal meningitis (27). Therefore, we investigated the killing kinetics of MEF and MEF derivatives combined with FLC against C. neoformans using time-kill analysis. As shown in Fig. 2A, the combination of fungistatic concentrations of MEF with FLC was fungicidal and sterilized the culture. A similar behavior was observed with the MEF derivatives, with all but derivative 13480 showing >2-log10 reductions in the number of CFU by 24 h (Fig. 2B). These data indicate that the combination of MEF derivatives with FLC may lead to improved efficacy relative to that of FLC alone against C. neoformans.
FIG 2.
The combination of mefloquine and mefloquine derivatives with fluconazole is fungicidal against Cryptococcus neoformans. (A) The combination of fungistatic concentrations of mefloquine (at one-quarter the MIC) and fluconazole (at one-half the MIC) is fungicidal for C. neoformans strain H99 incubated in YPD at 37°C. (B) All of the mefloquine derivatives except for 13480 showed similar fungicidal activity in combination with fluconazole under the conditions described above. Samples of each culture were plated on YPD at the indicated time points, and the numbers of CFU per milliliter were quantified using serial dilutions after incubation at 30°C. The curves represent the results of single experiments representative of those of at least two independent experiments. Error bars indicate the standard deviations from two technical replicates.
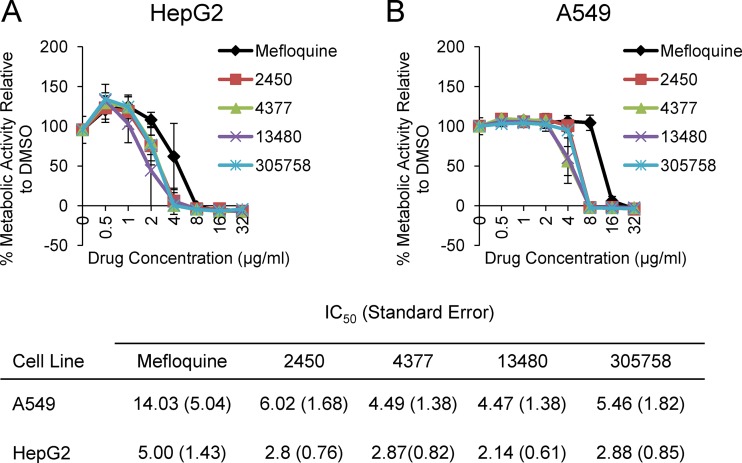
The in vitro toxicity of mefloquine derivatives against human cell lines is similar to that of mefloquine.
Mefloquine is an FDA-approved drug for the treatment of malaria, and its toxicity has been characterized extensively. In vitro, MEF is more toxic against immortalized human cell lines than it is against primary cell lines (28). For example, serum and brain concentrations of MEF range from 10 μM (∼4 μg/ml) to 50 μM (∼19 μg/ml), which is at or well above the half-maximal (50%) inhibitory concentration (IC50) of MEF against HepG2 cells (29). Therefore, MEF is much better tolerated in humans than what in vitro toxicity studies using standard immortalized human cell lines would indicate. It is not feasible to screen the toxicity of new derivatives in whole animals. Therefore, we compared the in vitro data for the new derivatives to those for MEF to provide a measure of their relative toxicity to the clinically used MEF. Specifically, we determined the IC50 of MEF and its derivatives against both HepG2 and A549 cells using metabolic activity assays. As shown in Fig. 3A and B, the IC50 of none of the derivatives differed from that of mefloquine by more than 2-fold. The antifungal activity for the derivatives improved by 8- to >64-fold. Therefore, the improved antifungal activity for the derivatives could not be attributed to a general increase in eukaryotic cell toxicity. Although MEF has relatively poor antifungal activity, it achieves very high tissue concentrations near its MIC against organisms such as Cryptococcus (30). The relatively small changes in toxicity associated with the increased antifungal activity are very encouraging and suggest that the scaffold may be amenable to in vivo therapy.
FIG 3.
The in vitro toxicities of mefloquine and mefloquine derivatives against two cell lines are similar. The indicated cell lines, HepG2 (A) and A549 (B), were treated with DMSO or the indicated mefloquine derivative. The metabolic activity of the cells was determined by measuring the reduction of a metabolically active dye (XTT) and compared to that of the untreated controls over the indicated range of drug concentrations. The curves are representative of those from three independent experiments, and the error bars indicate the standard deviations from three technical replicates. The table below panels A and B lists the half-maximal (50%) inhibitory concentrations (IC50) against the indicated human cell lines.
Mefloquine and mefloquine derivatives modulate fungal virulence factors.
Fungal virulence traits have long been studied for their contribution to disease pathogenesis, and recently, interest in targeting these processes for the development of new therapies has increased (31). Among the C. albicans virulence traits, hyphal morphogenesis has been one of the most extensively studied in terms of both pathogenesis and molecular targeting (32). Indeed, another quinolone antimalarial, quinacrine, inhibits C. albicans filamentation (33). We therefore examined the effect of MEF and its derivatives on C. albicans morphogenesis.
To investigate if MEF and the MEF derivatives affected the ability of C. albicans to form hyphae, the C. albicans reference strain SC5314 was exposed to sublethal concentrations of the molecules under conditions that induce filamentation (1% fetal bovine serum [FBS] in yeast extract-peptone-dextrose [YPD] medium at 37°C). At concentrations of one-half the MIC, MEF and its derivatives all suppressed filamentation; almost all treated cells remained in the yeast form but were still viable by propidium iodide (PI) staining (Fig. 4A). MEF showed dose-dependent inhibition of filamentation (Fig. 4C), while all the MEF derivatives completely inhibited filamentation at one-half the MIC (Fig. 4B). Taken together with the previously published data for quinacrine, this finding indicates that the quinolone class appears to be a general inhibitor of C. albicans filamentation (34).
FIG 4.
Mefloquine and mefloquine derivatives inhibit C. albicans filamentation at sub-growth-inhibitory concentrations. Candida albicans strain SC5314 was inoculated into YPD containing drug or DMSO and incubated for 1 h at 37°C with shaking. The cultures were supplemented with 1% fetal bovine serum and grown at 37°C with shaking for 1 h to induce filamentation. Samples of the cultures were stained with propidium iodide, harvested, and examined by microscopy. (A) Bright-field images of live C. albicans cells show that mefloquine and the mefloquine derivatives prevent filamentation. (B) Quantitative analysis was performed by visually inspecting the microscopy images of live cells in the yeast, pseudohyphal, and hyphal forms. (C) Quantitative analysis of live C. albicans cells indicates that filamentation is affected by mefloquine in a dose-dependent manner.
Two of the most important virulence characteristics of C. neoformans are polysaccharide capsule formation and melanization (35, 36). The polysaccharide capsule is unique among fungi and protects the cells against a variety of stresses, including host defense (35, 37). Importantly, C. neoformans strains that are not able to form a capsule are avirulent in mouse models (38). Melanization is also thought to protect cells against oxidative stress, such as macrophage-generated reactive oxygen species (ROS). Recent patient-derived data have shown that the treatment outcome correlates with the extent of melanin formation (39). Thus, a drug that prevents capsule and melanin formation may be useful as an adjuvant, even if it is not sufficiently active to directly kill C. neoformans (38).
C. neoformans H99 was exposed to subinhibitory concentrations of MEF and the MEF derivatives under capsule-inducing conditions, and capsule formation was evaluated at 48 h by India ink staining. Treated cells were stained with PI before imaging to confirm that they were still viable. All MEF derivatives caused a reduction in the number of cells with capsule and a decrease in the size of the capsule compared to the results obtained with dimethyl sulfoxide (DMSO) (Fig. 5A and B). Similarly, the MEF-related compounds reduced the melanization of C. neoformans cells incubated on l-3,4-dihydroxyphenylalanine (l-DOPA) plates (Fig. 5C). Taken together, these data indicate that MEF derivatives modulate the expression of key virulence properties of pathogenic fungi and directly kill the organisms.
FIG 5.
Mefloquine and mefloquine derivatives suppress the expression of C. neoformans virulence traits. (A and B) Cryptococcus neoformans strain H99 was incubated in DMEM at 37°C in 5% CO2 in the presence of DMSO or the indicated concentration of the mefloquine derivative (at one-half to one-quarter the MIC). The cells were harvested and stained with India ink. The cells were photographed (A), and the number of cells with or without a capsule was counted (n > 100 cells per experiment) and converted into a percentage. The bars in panel B indicate the means from two or three independent experiments, and the error bars are the standard deviations. (C) A series of 10-fold dilutions of H99 was spotted onto plates containing l-DOPA medium as well as the indicated concentration of mefloquine or the mefloquine derivative, incubated at 37°C for 48 h, and photographed. The photograph is representative of the photographs from two independent replicates.
Mode and mechanism of the antifungal activity of the mefloquine scaffold.
As alluded to in the introduction, the mechanism of action of MEF as an antimalarial drug has remained enigmatic, despite its many years of use in the clinical setting. Recent proposals for its mechanism of action either as an antimalarial drug or as part of repurposing to other indications include the following: a lysosomal/vacuole inhibitor (40), a disruptor of the mitochondrial proton motive force (41), a protein translation inhibitor (42), and a purine nucleoside phosphorylase (PNP) inhibitor (43). The last two proposals involve specific target proteins, a subunit of the 80S ribosome and the PNP enzyme. The yeast PNP enzyme is not essential, and, thus, this target was eliminated from further consideration. We tested the effect of MEF and the MEF derivatives on yeast protein translation but found no evidence of inhibition (data not shown). Soon after we completed these experiments, Sheridan et al. reported that they also did not observed any effect of MEF on malarial parasite cell protein translation (44).
MEF was identified to be a potential adjuvant therapy for chronic myeloid leukemia, and as part of a multidisciplinary effort to understand its mechanism of anticancer activity, a yeast-based chemical haploinsufficiency screen was performed to identify S. cerevisiae gene deletions that are hypersusceptible to MEF (40). The set of genes that emerged from this screen was enriched for genes involved in yeast vacuole and protein secretion processes. The results of follow-up cell biological studies using human cells were consistent with this potential mechanism; however, no experiments involving yeast were reported.
To investigate the effect of MEF derivatives on yeast vacuoles, log-phase S. cerevisiae (strain BY4741) cells were exposed to subinhibitory concentrations of compound or DMSO for 2 h, stained with FM4-64, and imaged. All MEF derivatives caused gross changes in vacuole morphology (Fig. 6); similar results were observed with C. albicans (data not shown). The changes began with the segmentation and invagination of a single vacuole and led to many cells having an increased number of fragmented vacuoles. Many cells showed a single large vacuole surrounded by various numbers of smaller vacuoles that resembled those of class F vacuolar protein-sorting mutants (45), which included mutants with VPS1 and VPS26 deletions. To directly compare phenotypes, we stained S. cerevisiae vps1Δ (strain BY4741 background) alongside DMSO or drug-treated wild-type S. cerevisiae (BY4741) cells, and indeed, many cells showed vacuoles with a morphology similar to that of the vps1Δ mutant (Fig. 6B).
FIG 6.
Mefloquine derivatives disrupt vacuolar morphology. Exponential-phase cultures of S. cerevisiae reference strain BY4741 were exposed to DMSO or the indicated drug concentrations in YPD at 30°C for 2 h. Concurrently, a culture of S. cerevisiae with a vps1Δ mutation (BY4741 background) was also grown in YPD at 30°C for 2 h without drug or DMSO. All cells were harvested and stained with FM4-64 to visualize the vacuole and photographed. The vacuolar morphology of the treated cultures was similar to the pattern reported for the vps1Δ strain. Propidium iodide staining of the same cultures showed that >95% of cells were viable at the time of processing.
Although the morphological effects of the MEF derivatives were not as uniform as the effects observed with the vps1Δ mutant, it seemed possible that Vps1 could be a target. Many VPS mutants mislocalized the vacuolar protein carboxypeptidase Y (CPY) to the extracellular space. However, we found that exposure of BY4741 to the MEF derivatives did not lead to the increased secretion of CPY by colony blot assays (data not shown). The vps1Δ mutant has a strong CPY secretion phenotype. Although this assay is not as sensitive as radiolabeling assays, it seems likely that the MEF derivatives do not have a profound effect on CPY mislocalization. Additionally, none of the MEF derivatives inhibited recombinant Vps1 in vitro (data not shown). Therefore, while MEF and its antifungal derivatives clearly affect the vacuole morphology, it seems unlikely that this effect is the sole mode of antifungal action.
Mefloquine and the mefloquine derivatives dissipate mitochondrial membrane potential and induce petite mutant formation.
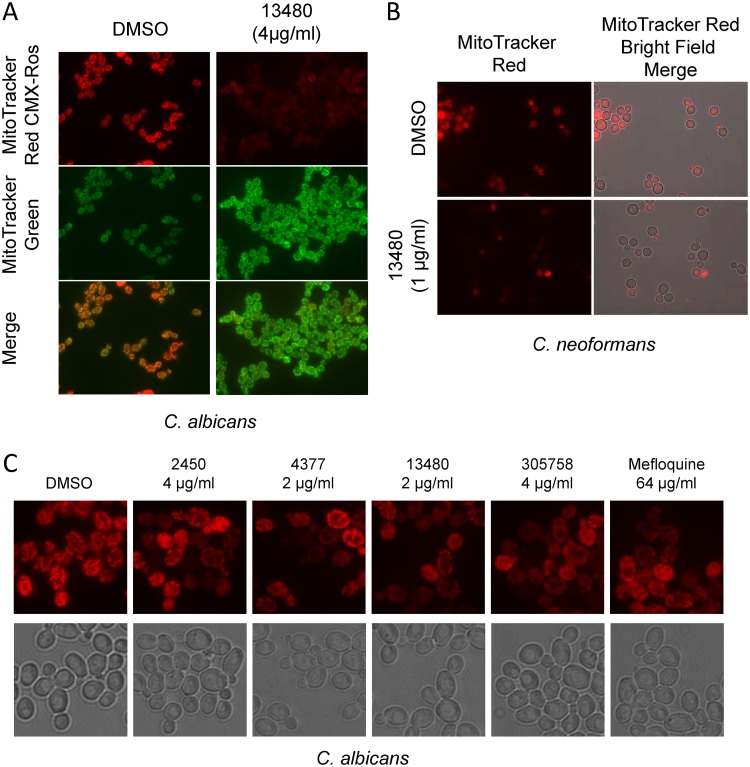
MEF has been shown to interfere with mitochondrial function in a variety of eukaryotic systems. Feng et al. found that MEF is among a large set of clinically used drugs that dissipate the proton motive force across the mitochondrial membrane of eukaryotic cells, including S. cerevisiae cells (41). The antimalarial activity of MEF has been attributed to mitochondrial dysregulation and the production of reactive oxygen species (ROS) (46). A similar mitochondrial effect has been reported in cervical cancer cell lines and results in a decrease in ATP levels as well as increased levels of cellular ROS that lead to cell death (47). To determine if MEF derivatives affect the mitochondrial proton motive force in pathogenic yeast, log-phase C. albicans (strain SC5314) cells were exposed to subinhibitory concentrations of drug or DMSO for 2 h, stained with MitoTracker Red CMXRos and MitoTracker Green, and imaged. MitoTracker Red uptake is dependent on the mitochondrial membrane potential, while MitoTracker Green uptake is not. As shown for the MEF derivative 13480, MitoTracker Red uptake was nearly abolished, while the morphology of the mitochondria was unchanged (Fig. 7A). The proton motive force was also decreased in C. neoformans (Fig. 7B). Additionally, similar results were obtained for the MEF derivatives 2450 (4 μg/ml), 4377 (2 μg/ml), and 305758 (4 μg/ml) and for MEF (64 μg/ml), indicating that these drugs dissipate the mitochondrial proton motive force as part of their antifungal activity (Fig. 7C).
FIG 7.
Mefloquine derivatives dissipate the proton motive force across the mitochondrial membrane. (A) Candida albicans strain SC5314 cells were treated with the indicated concentration of MEF derivative 13480 or DMSO for 2 h in YPD at 37°C. The cells were harvested, stained with either MitoTracker Red CMXRos or MitoTracker Green, and imaged. Identical exposure settings were used for both samples. Propidium iodide staining indicated that >95% of the cells were viable at the time of imaging. (B) Cryptococcus neoformans strain H99 cells were processed as described in the legend to panel A but for MitoTracker Red staining only. (C) Treatment with the other mefloquine derivatives showed a decreased uptake of MitoTracker Red by Candida albicans strain SC5314.
Two recently identified antifungal small molecules that directly target the mitochondria, T-2307 (48) and Inz-1 (49), have increased activity in nonfermentable carbon sources, such as glycerol, compared to that in standard glucose media. If mitochondrial disruption is the process driving the majority of MEF derivative antifungal activity, we reasoned that the MICs of the MEF derivatives should be much lower in the presence of glycerol. However, when using yeast extract-peptone (YP)-glycerol medium instead of YP-dextrose medium, the MICs of the MEF derivatives were decreased modestly (e.g., 2-fold; data not shown) for some derivatives or were unchanged. In addition, we compared the activities of the MEF derivatives against C. glabrata KK2001 and a respiration-deficient rho-negative (rho−) derivative of KK2001, which has no functioning mitochondrion. The MICs were identical for the parent KK2001 strain and the rho− KK2001 strain for MEF derivatives 2450 and 4377. If disruption of mitochondrial function was the sole activity of the MEF scaffold, then rho− strains should be resistant. Therefore, these data further suggest that the mitochondrion is targeted by the MEF scaffold and that such activity is not solely responsible for its antifungal properties.
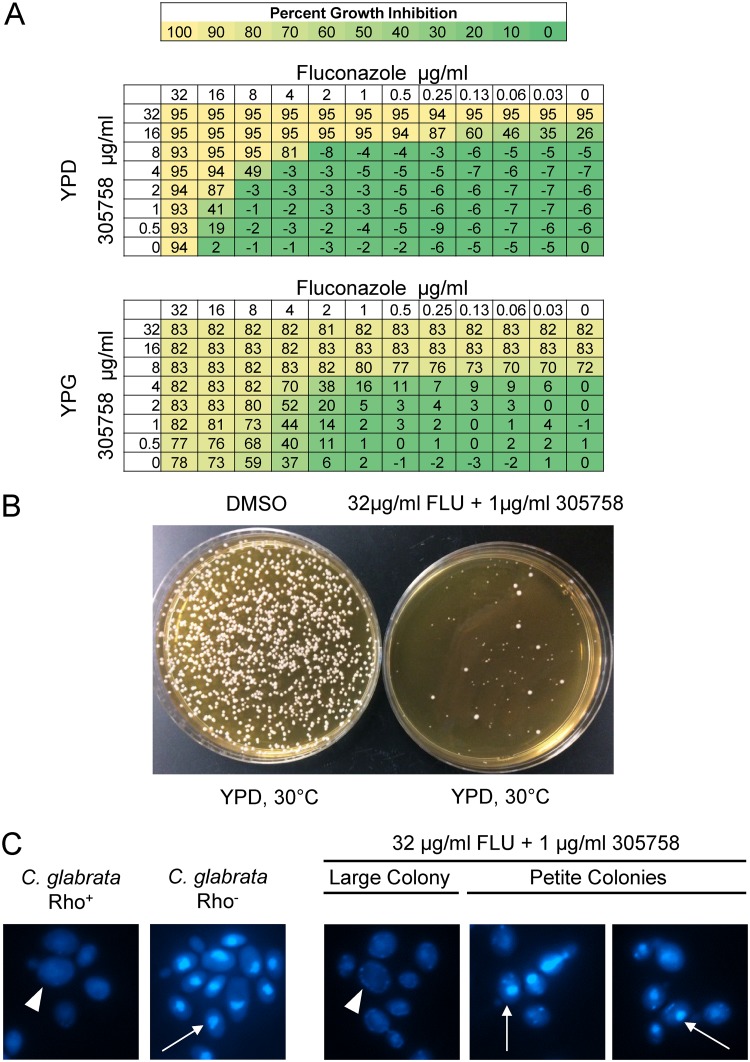
Interestingly, incubation of the parent KK2001 strain in the presence of an MEF analog for 48 h led to some growth in the presence of concentrations beyond the initial MIC observed at 24 h, indicating a possible Eagle effect or the development of tolerance (50). To investigate possible explanations for the supra-MIC growth, cells from wells containing MEF derivative 305758 (8 μg/ml) and DMSO only were plated in 10-fold dilutions on YPD plates and incubated at 30°C for 24 h. After 24 h, the cells treated with MEF derivative 305758 grew as a mixture of normal-sized and petite mutant colonies (Fig. 8A), while plates of the DMSO-exposed cells had normal-sized colonies only (data not shown). We suspected that these cells may be respiratory-deficient, petite mutant colonies. To confirm the petite phenotype, 15 petite mutant colonies and 4 controls (2 wild-type colonies that had been treated with DMSO only and 2 normal-sized colonies that had been treated with 305758 at 8 μg/ml) were patched onto YP–2% glycerol (YPG) plates and incubated at 30°C for 24 h. None of the 15 petite mutant colonies grew on the YPG plates, while all control colonies showed the expected amount of growth (Fig. 8B). This observation suggests that the MEF derivatives also affected mitochondrial genome stability.
FIG 8.
Mefloquine derivatives induce mitochondrial DNA instability in C. glabrata. (A) Candida glabrata strain KK2001 was incubated with MEF derivative 305758 overnight in YPD medium in an MIC plate with a 2-fold sequential dilution format. The MIC was read at 24 h (8 μg/ml). After 48 h, the surviving cells were plated on YPD, incubated at 30°C, and photographed. The black arrows indicate small or petite mutant colonies, while the white arrows indicate normal-sized colonies. (B) Small and normal-sized colonies were isolated under the conditions described above, patched on YP–2% glycerol (YPG) medium, and incubated at 30°C for 2 days and then imaged.
Petite mutants are resistant to FLC, primarily through the upregulation of efflux pumps (51). We therefore repeated the fractional inhibitory concentration (FIC) assays, combining FLC with the MEF derivative 4377 in YPD instead of the standard RPMI medium to facilitate the growth of the petite strains; we also carried out the FIC assay in YPG to suppress the formation of respiration-deficient strains. In addition, we measured cell density rather than use a visual readout to allow for a more nuanced assessment of the activity of the combinations. Both 4377 and FLC were modestly more active in YPG than in YPD (Fig. 9A); to our knowledge, this effect of glycerol medium on FLC has not been reported previously. Glycerol medium also potentiated the activity of the combination, as evidenced by a smaller area of minimal inhibition for the YPG experiment; the same effect was observed for all other MEF derivatives tested. The contents of the wells containing the combination with approximately 50% inhibition were plated on YPD and incubated at 30°C. The majority of the cells isolated from these plates had the morphology of petite mutants (Fig. 9B), and 20/20 representative petite mutant colonies were unable to grow on glycerol medium (data not shown). DAPI (4′,6-diamidino-2-phenylindole) staining of the respiration-deficient cells showed decreased mitochondrial DNA staining, confirming that these strains had alterations in the DNA content of their mitochondria (Fig. 9C). Thus, the MEF derivatives induce petite mutant formation which, in turn, appears to modulate the activity of FLC-MEF analog combinations. Importantly, we did not observe this phenomenon with C. neoformans or C. albicans, most likely because these strains are so-called petite mutant negative (data not shown) and cannot grow if their mitochondrial DNA has been lost (52). Taken together, these experiments strongly support the notion that MEF derivatives interfere with mitochondrial function but, like the vacuolar effects, that the mitochondrion is not the sole target driving the antifungal activity. Rather, the activity of MEF and its derivatives is best explained by a combination of effects on the mitochondria and vacuole.
FIG 9.
C. glabrata petite mutant formation modulates the activity of mefloquine derivative-fluconazole combinations. (A) Checkerboard assays with Candida glabrata strain BG2 were performed with MEF derivative 305758 and fluconazole in YPD and YPG media at 37°C. After 24 h of incubation, the optical density of each well was measured and compared to that of the DMSO-only well. The percentage of growth inhibition was calculated and is shown both numerically and in heat map form. (B) The surviving cells from a well with approximately 50% growth inhibition (32 μg/ml FLC plus 1 μg/ml 305758) and cells from the DMSO-only well were plated on YPD and incubated for 2 days at 30°C. Drug-treated cells produced a majority of petite mutant colonies, with few normal-sized colonies being present. (C) A Candida glabrata rho-positive (rho+) strain KK2001 and a rho-negative (rho−) derivative were stained with DAPI. The rho+ strains show a diffuse staining pattern indicative of both mitochondrial and nuclear DNA staining (indicated by the large arrowhead), while the rho− strains show a focal nuclear pattern indicative of nuclear staining only (indicated by the thin arrow). From the FIC well containing 32 μg/ml FLC plus 1 μg/ml 305758, normal-sized colony cells from panel B stain in a pattern consistent with rho+, while the petite mutant colony cells from panel B show the focal pattern consistent with rho−.
DISCUSSION
The small number of drugs and drug classes that can be used to treat invasive, life-threatening fungal infections necessitates an expansion of the types of approaches used to find new drugs as well as the types of molecular classes that are considered for further development. Drug repurposing has emerged as an attractive approach to expediting the drug development process. In general, such repurposing efforts have focused on identifying a specific drug that can be directly transitioned to a new clinical use without modification of the structure or formulation. Here, we adopted a repurposing approach to achieve a different goal: to identify a currently used drug with antifungal properties as a point of departure for further development and optimization.
One of the most compelling reasons to pursue repurposing approaches to identifying new drug candidates is that the molecules and scaffolds have a known pharmacology. The pharmacological properties of a new chemical entity are very difficult to predict a priori, and it is frequently difficult to redress serious metabolic or toxicologic liabilities by reengineering the scaffold. Accordingly, we chose to explore the antifungal properties of MEF further because its pharmacology has a number of features useful for the treatment of fungal infections (53). First, it is well absorbed and establishes high serum and tissue levels when taken orally. Second, it penetrates the blood-brain barrier and achieves high neural concentrations relative to those in plasma (29). Although new therapies for infections caused by the neurotropic pathogen C. neoformans represent one of the most important unmet clinical needs in medical mycology (25), it is important to recognize that almost all invasive fungal infections can lead to central nervous system (CNS) disease. Third, the use of fungal prophylaxis for high-risk patients has become established practice for many predisposing conditions (3). MEF has a long half-life and, thus, could be used in dosing regimens that would be quite amenable to prophylaxis. It is important to note that MEF has well-documented neurological/psychiatric side effects in a minority of patients (54). These are generally associated with long-term use in the setting of malaria prophylaxis. If molecules of this class ultimately progress through preclinical development, then it will be important to carefully consider the risk-benefit ratio of their use. However, the in vitro activity and pharmacological properties of the class seem worthy of consideration at this time.
The MEF scaffold had been known to have antifungal properties for over 20 years, so our project represents something of a rediscovery (22); however, no in-depth characterization of the antifungal properties of MEF or its derivatives has been undertaken. As others have shown, MEF has poor-to-modest in vitro antifungal activity against Candida spp., Cryptococcus, and Aspergillus. In contrast, the MEF derivatives that we obtained from the NCI Chemical Repository were much more active (up to 64-fold more active) against a broad spectrum of pathogens, including Candida spp., C. neoformans, and A. fumigatus. Overall, NCI MEF derivative 4377 was the most active analog, with its MICs being between 1 and 4 μg/ml for the species tested, though all four derivatives were similarly active, with MICs ranging from 2 to 8 μg/ml for the species tested.
Despite the significant increase in the antifungal activity of the MEF derivatives relative to that of MEF, the relative in vitro toxicity of the MEF derivatives against human cells was only minimally increased. This differential increase in antifungal activity is particularly important because it indicates that the MEF derivatives are not simply more active generalized eukaryotic cell poisons. As discussed above, primary cell and whole-animal toxicity studies will be needed to prioritize these molecules for further development because of the relative discordance between the in vitro and in vivo toxicity of this class of molecules. Indeed, Dow et al. found that both the NCI MEF derivative 2450 and the NCI MEF derivative 305758 are less neurotoxic than the parent MEF derivative (29). Large numbers of MEF derivatives and alkylamino-quinolines in general have been synthesized and evaluated as antimalarial molecules which might be exploited for an expedient structure-activity relationship-based optimization of the antifungal activity of this scaffold (55).
The recent emergence of drug-resistant and multidrug-resistant fungi has generated well-founded concerns in the face of the limited antifungal repertoire (2). Thus, the activity of the MEF derivatives against multidrug-resistant C. auris and C. glabrata, two of the most important drug-resistant organisms, is very promising. This feature indicates that the class is not susceptible to the mechanisms of resistance, such as increased efflux pump expression, that affect azole drugs. These observations also suggest that the mechanism and/or mode of action of the MEF class is distinct from that of the antifungal drugs currently in use. Based on these characteristics, further medicinal chemistry optimization of the antifungal activity appears to be warranted.
The ability of MEF and its derivatives to achieve high concentrations in the CNS makes them attractive candidates for use in the treatment of cryptococcal meningitis (29). Currently, the gold standard therapy for cryptococcal meningitis is AmB combined with flucytosine. As has been well documented, this regimen is not available in many resource-limited regions of the world with high burdens of cryptococcal meningitis (26). FLC is far less effective because of its fungistatic mode of action but is much easier to obtain and administer. The development of adjuvants to or combinations with FLC has been of interest to the field. Ideally, an orally available drug could be combined with FLC to yield a fungicidal cocktail with clinical efficacy similar to that of the AmB and flucytosine combination. Our in vitro time-kill studies indicate that MEF and its derivatives are fungicidal when combined with FLC and, thus, are candidates for combination therapy.
The exact mechanism of action for the antimalarial activity of MEF has remained ill-defined, despite its long-standing use. Although our studies on its mechanism of action as an antifungal have not identified a single protein target, new insights into the cellular effects and mode/mechanism of action have emerged. First, our data are most consistent with the idea that the antifungal activity of MEF and its derivatives is due to effects on more than one target. This conclusion is supported by the fact that we could not isolate stable resistant mutants by in vitro selection.
Second, we propose that at least part of the antifungal activity of the MEF class is due to the ability of agents within this class to interfere with the function of both the mitochondria and the vacuole. Other antifungal molecules that appear to more specifically target the mitochondria have been identified; however, these molecules typically show increased activity in the presence of nonfermentable carbon sources, such as glycerol, relative to that of fermentable carbon sources, such as glucose. The MEF derivatives do not show this effect, despite disrupting the proton motive force across the mitochondrial membrane and interfering with mitochondrial DNA stability. Thus, the mitochondria cannot be the sole target. In keeping with our first conclusion, other effects, such as those manifested by vacuolar disruption or secretory pathway blockade, are likely to combine with mitochondrial disruption. This proposal is also supported by previous chemical genetic data indicating that strains with mutations in vacuolar and secretory proteins (e.g., vps16Δ, vps33Δ, vma6Δ, vph1Δ, and vph2Δ strains) are hypersusceptible to MEF (40). Finally, we also assert that the lack of a defined, specific target explaining the antimalarial activity of MEF may be because, as with its antifungal activity, it has multiple effects and targets.
In summary, the broad-spectrum activity, the known and favorable pharmacological properties, and the novel, multitarget mechanism of action of MEF strongly support the development and optimization of the antifungal activity of the MEF scaffold.
MATERIALS AND METHODS
Yeast strains and general microbiological methods.
Medium was prepared using standard recipes (56). All fungal strain glycerol stocks were stored at −80°C and were propagated on yeast extract-peptone-dextrose (YPD) agar plates (1% [wt/vol] yeast extract, 2% [wt/vol] peptone, 2% [wt/vol] dextrose, 2% [wt/vol] agar). All strains were used within 10 days of streaking. C. albicans strain SC5314 was obtained from Gus Haidaris (University of Rochester). C. albicans and C. glabrata clinical isolates were obtained from Cornelius Clancy (University of Pittsburgh) and Theodore White (University of Missouri—Kansas City). C. auris clinical isolates were obtained from Daniel Diekema (University of Iowa). C. neoformans clinical isolates were obtained from John Perfect (Duke University). Unless otherwise indicated, experiments began with an initial culture of cells that were grown overnight (∼16 h) in YPD liquid medium with shaking at 30°C. All drugs were solubilized in DMSO.
In vitro antifungal susceptibility.
Antifungal susceptibility testing was performed using the protocols described in the CLSI M27-A3 document (57). The reported MIC values represent the highest value from at least three independent biological replicates performed in at least technical duplicate. Checkerboard fractional inhibitory concentration (FIC) assays were performed as previously described using the same medium and conditions used for the CLSI M27-A3 protocol (58). MIC and FIC results were read by visual inspection and/or from the readings of the cell optical density at an absorbance of 600 nm (OD600).
Time-kill assay.
Overnight cultures of C. neoformans (strain H99) were diluted in fresh YPD to an OD600 of 0.1 to 0.2 and grown at 30°C with shaking until the OD600 was ∼0.4. Once that OD600 was reached, the cultures were incubated at 37°C with shaking with vehicle or the concentration of drug indicated above (final DMSO concentration, 0.4%) for 24 h. A sample was taken at the 0-h time point before the addition of drug, and subsequent samples were taken every 4 h after the addition of drug. At each time point, serial dilutions of samples were plated on YPD agar plates and incubated at 30°C, and counts were obtained after 48 h. Experiments were done with at least two independent biological replicates and in technical duplicate.
In vitro toxicity assays.
Cell lines were obtained from ATCC and included A549 cells, human alveolar cells, and HepG2 human liver cells. The cells were cultured with 10% FBS, 1% penicillin-streptomycin, and 1% GlutaMAX in Dulbecco modified Eagle medium (DMEM)–Ham’s F-12 medium, 15 mM HEPES (A549 cells), or DMEM (HepG2 cells). Toxicity and viability were measured using a 2,3-bis-(2-methoxy-4-nitro-5-sulfophenyl)-2H-tetrazolium-5-carboxanilide salt (XTT) reduction assay to determine metabolic function. Cells were seeded and incubated at 37°C in 5% CO2 until the cells were ∼70% confluent (24 to 48 h). The medium was aspirated and replenished in combination with DMSO or serial dilutions of drug. Cells were incubated in drug or DMSO under the same conditions described above for an additional 24 h. A CyQUANT XTT cell viability assay kit (catalog number X12223; Thermo Fisher Scientific) was used, the assay was developed, and the results were measured according to the assay protocol.
C. albicans filamentation.
Overnight cultures of C. albicans (strain SC5314) were diluted 1:100 and incubated with drug or DMSO (final DMSO concentration in the culture, 0.5%) in YPD for 1 h with shaking at 37°C. After 1 h, the cultures were induced with 1% FBS and incubated for an additional hour with shaking at 37°C. Samples were centrifuged, resuspended in YPD, stained with propidium iodide at 10 μg/ml, incubated in the dark for 30 min, washed once with Dulbecco phosphate-buffered saline (DPBS), resuspended with DPBS, and imaged. Images were captured using a Nikon ES80 epifluorescence microscope and visualized with a CoolSnap charge-coupled-device camera and NIS-Elements software. Quantitative analysis was performed by visually inspecting the images, and the hyphae were measured using NIS-Elements software. The data represent the means from at least two biological replicates in which at least 100 cells were counted. Error bars indicate standard deviations.
C. neoformans melanization and capsule formation.
Overnight cultures of C. neoformans (strain H99) were washed in DPBS and resuspended to an OD600 of 1.0. Dilutions of 1:10 were made in sterile UltraPure DNase/RNase-free distilled water. Of those dilutions, 10 μl was spotted onto l-DOPA plates that contained either DMSO or drug in duplicate with at least two biological replicates. The plates were incubated at 37°C for 48 h and imaged.
Vacuole morphology.
Cells were grown overnight in YPD with shaking at 30°C. Cells were back diluted in fresh YPD to an OD600 of 0.2 to 0.3 and grown at 30°C with shaking until the cells reached log-phase growth (OD600 = 0.5 to 0.7). The cells were then exposed to DMSO or drug and incubated at 30°C for 2 h with shaking. After 2 h, samples were centrifuged, resuspended in YPD with 40 μM FM4-64, and incubated in the dark with shaking at 30°C for 15 min. The cells were centrifuged, resuspended in fresh YPD in a dark tube, and incubated with shaking at 30°C for 1 h. The cells were then centrifuged, resuspended in DPBS, mounted, and imaged on the aforementioned epifluorescence microscope.
Mitochondrial assays.
Cells were grown overnight in YPD with shaking at 30°C. On the next day, the overnight culture was back diluted into fresh YPD to an OD600 to 0.2 to 0.3 and grown at 30°C with shaking until the OD600 was 0.5 to 0.7. Once that OD600 was reached, the cells were exposed to DMSO or drug for 2 h for C. albicans (strain SC5314) or were exposed for 30 min for C. neoformans (strain H99) at 30°C with shaking. MitoTracker Red CMXRos (catalog number M7512; Thermo Fisher Scientific) and/or MitoTracker Green FM (catalog number M7514; Thermo Fisher Scientific) was prepared in warm YPD to a final concentration of 1 mM. Once drug incubation was complete, the cells were centrifuged and resuspended with the YPD and MitoTracker mixture and then incubated in the dark at 30°C for 50 min. The cells were centrifuged, washed with fresh YPD, resuspended with phosphate-buffered saline, mounted onto a slide, and imaged.
Identification and validation of petite mutants.
MICs for wild-type C. glabrata KK2001 were determined as described above, and the plates were read at 24 h and 48 h. At 48 h, the contents of the wells in which the cells grew in the presence of concentrations past the recorded 24-h MIC and control wells were diluted, spotted, and plated on YPD and YPG plates. The plates were incubated at 30°C overnight, and on the next day, petite mutants and larger control colonies from the YPD plates were patched onto fresh YPG plates and incubated at 30°C overnight to confirm the petite mutant phenotype. Glycerol stocks were made for future DAPI staining experiments. The same procedure was done for at least two separate C. glabrata BG2 FIC experiments of the mefloquine derivatives and fluconazole. Percent growth inhibition was determined for YPD and YPG FIC assay plates using the following formula: percent growth inhibition = [1 − (OD600 of treated cells/OD600 of untreated cells)] × 100. Petite mutants were verified via a secondary assay with DAPI staining. Briefly, petite mutant and control glycerol stocks were streaked onto YPD plates and grown for 48 h at 30°C. Overnight cultures were grown at 30°C for 16 h, and on the following day, the cells were washed in synthetic complete medium, back diluted in synthetic complete medium to an OD600 of 0.2 to 0.3, and grown to log phase at 30°C with shaking until the OD600 was 0.4 to 0.6. Once that OD was reached, the cells were stained with 2.5 μg/ml DAPI and grown for 30 min at 30°C with shaking. The cells were then centrifuged, resuspended in fixing reagent containing 4% formaldehyde in synthetic complete medium, and incubated at room temperature for 15 min. Then, the cells were washed with DPBS, resuspended in DPBS, mounted onto slides, and imaged on the epifluorescence microscope. The cells were always imaged on the same day that they were fixed and not after storage.
Supplementary Material
ACKNOWLEDGMENTS
We thank Scott Moye-Rowley (Iowa) for helpful discussion and the C. glabrata KK2001 strain, Theodore White (University of Missouri—Kansas City) for Candida clinical isolates, Cornelius Clancy (University of Pittsburgh) for Candida clinical isolates, and John Perfect (Duke University) for Cryptococcus clinical isolates.
This work was supported in part by a grant from the Burroughs Wellcome Fund (grant BWF1014095 to M.C.M.), National Institutes of Health grants TL1TR002000, and UL1TR002001 (to M.C.M.), and NIAID grant 1R21AI125094 (to D.J.K.).
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Roemer T, Krysan DJ. 2014. Antifungal drug development: challenges, unmet clinical needs, and new approaches. Cold Spring Harb Perspect Med 4:a019703. doi: 10.1101/cshperspect.a019703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Montoya MC, Moye-Rowley WS, Krysan DJ. 2019. Candida auris: the canary in the mine of antifungal drug resistance. ACS Infect Dis 5:1487–1492. doi: 10.1021/acsinfecdis.9b00239. [DOI] [PubMed] [Google Scholar]
- 3.Chen Y, Farrer RA, Giamberardino C, Sakthikumar S, Jones A, Yang T, Tenor JL, Wagih O, Van Wyk M, Govender NP, Mitchell TG, Litvintseva AP, Cuomo CA, Perfect JR. 2017. Microevolution of serial clinical isolates of Cryptococcus neoformans var. grubii and C. gattii. mBio 8:e00166-17. doi: 10.1128/mBio.00166-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pushpakom S, Iorio F, Eyers PA, Escott KJ, Hopper S, Wells A, Doig A, Guilliams T, Latimer J, McNamee C, Norris A, Sanseau P, Cavalla D, Pirmohamed M. 2019. Drug repurposing: progress, challenges and recommendations. Nat Rev Drug Discov 18:41–58. doi: 10.1038/nrd.2018.168. [DOI] [PubMed] [Google Scholar]
- 5.Rhein J, Huppler Hullsiek K, Tugume L, Nuwagira E, Mpoza E, Evans EE, Kiggundu R, Pastick KA, Ssebambulidde K, Akampurira A, Williams DA, Bangdiwala AS, Abassi M, Musubire AK, Nicol MR, Muzoora C, Meya DB, Boulware DR, ASTRO-CM Team. 2019. Adjunctive sertraline for HIV-associated cryptococcal meningitis: a randomised, placebo-controlled, double-blind phase 3 trial. Lancet Infect Dis 19:843–851. doi: 10.1016/S1473-3099(19)30127-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ngan NTT, Mai NTH, Tung NLN, Lan NPH, Tai LTH, Phu NH, Chau NVV, Binh TQ, Hung LQ, Beardsley J, White N, Lalloo D, Krysan D, Hope W, Geskus R, Wolbers M, Nhat LTH, Thwaites G, Kestelyn E, Day J. 2019. A randomized open label trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis. Wellcome Open Res 4:8. doi: 10.12688/wellcomeopenres.15010.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wermuth CG. 2006. Selective optimization of side activities: the SOSA approach. Drug Discov Today 11:160–164. doi: 10.1016/S1359-6446(05)03686-X. [DOI] [PubMed] [Google Scholar]
- 8.Lee AY, St Onge RP, Proctor MJ, Wallace IM, Nile AH, Spagnuolo PA, Jitkova Y, Gronda M, Wu Y, Kim MK, Cheung-Ong K, Torres NP, Spear ED, Han MK, Schlecht U, Suresh S, Duby G, Heisler LE, Surendra A, Fung E, Urbanus ML, Gebbia M, Lissina E, Miranda M, Chiang JH, Aparicio AM, Zeghouf M, Davis RW, Cherfils J, Boutry M, Kaiser CA, Cummins CL, Trimble WS, Brown GW, Schimmer AD, Bankaitis VA, Nislow C, Bader GD, Giaever G. 2014. Mapping the cellular response to small molecules using chemogenomic fitness signatures. Science 344:208–211. doi: 10.1126/science.1250217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schwartz S, Kontoyiannis DP, Harrison T, Ruhnke M. 2018. Advances in the diagnosis and treatment of fungal infections of the CNS. Lancet Neurol 17:362–372. doi: 10.1016/S1474-4422(18)30030-9. [DOI] [PubMed] [Google Scholar]
- 10.Croft AM. 2007. A lesson learnt: the rise and fall of Lariam and Halfan. J R Soc Med 100:170–174. doi: 10.1177/014107680710011411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panic G, Duthaler U, Speich B, Keiser J. 2014. Repurposing drugs for the treatment and control of helminth infections. Int J Parasitol Drugs Drug Resist 4:185–200. doi: 10.1016/j.ijpddr.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Keiser J, Utzinger J. 2012. Antimalarials in the treatment of schistosomiasis. Curr Pharm Des 18:3531–3538. [PubMed] [Google Scholar]
- 13.Keiser J, Chollet J, Xiao SH, Mei JY, Jiao PY, Utzinger J, Tanner M. 2009. Mefloquine—an aminoalcohol with promising antischistosomal properties in mice. PLoS Negl Trop Dis 3:e350. doi: 10.1371/journal.pntd.0000350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xiao SH, Mei JY, Jiao PY. 2011. Effect of mefloquine administered orally at single, multiple, or combined with artemether, artesunate, or praziquantel in treatment of mice infected with Schistosoma japonicum. Parasitol Res 108:399–406. doi: 10.1007/s00436-010-2080-y. [DOI] [PubMed] [Google Scholar]
- 15.Zhang X, Zhang T, Liu J, Li M, Fu Y, Xu J, Liu Q. 2017. Functional characterization of a unique cytochrome P450 in Toxoplasma gondii. Oncotarget 8:115079–115088. doi: 10.18632/oncotarget.23023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rufener R, Ritler D, Zielinski J, Dick L, da Silva ET, da Silva Araujo A, Joekel DE, Czock D, Goepfert C, Moraes AM, de Souza MVN, Müller J, Mevissen M, Hemphill A, Lundström-Stadelmann B. 2018. Activity of mefloquine and mefloquine derivatives against Echinococcus multilocularis. Int J Parasitol Drugs Drug Resist 8:331–340. doi: 10.1016/j.ijpddr.2018.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holfels E, McAuley J, Mack D, Milhous WK, McLeod R. 1994. In vitro effects of artemisinin ether, cycloguanil hydrochloride (alone and in combination with sulfadiazine), quinine sulfate, mefloquine, primaquine phosphate, trifluoperazine hydrochloride, and verapamil on Toxoplasma gondii. Antimicrob Agents Chemother 38:1392–1396. doi: 10.1128/aac.38.6.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Martin-Galiano AJ, Gorgojo B, Kunin CM, de la Campa AG. 2002. Mefloquine and new related compounds target the F(0) complex of the F(0)F(1) H(+)-ATPase of Streptococcus pneumoniae. Antimicrob Agents Chemother 46:1680–1687. doi: 10.1128/aac.46.6.1680-1687.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rodrigues-Junior VS, Villela AD, Gonçalves RSB, Abbadi BL, Trindade RV, López-Gavín A, Tudó G, González-Martín J, Basso LA, de Souza MVN, Campos MM, Santos DS. 2016. Mefloquine and its oxazolidine derivative compound are active against drug-resistant Mycobacterium tuberculosis strains and in a murine model of tuberculosis infection. Int J Antimicrob Agents 48:203–207. doi: 10.1016/j.ijantimicag.2016.04.029. [DOI] [PubMed] [Google Scholar]
- 20.Sun W, He S, Martinez-Romero C, Kouznetsova J, Tawa G, Xu M, Shinn P, Fisher E, Long Y, Motabar O, Yang S, Sanderson PE, Williamson PR, Garcia-Sastre A, Qiu X, Zheng W. 2017. Synergistic drug combination effectively blocks Ebola virus infection. Antiviral Res 137:165–172. doi: 10.1016/j.antiviral.2016.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Balasubramanian A, Teramoto T, Kulkarni AA, Bhattacharjee AK, Padmanabhan R. 2017. Antiviral activities of selected antimalarials against dengue virus type 2 and Zika virus. Antiviral Res 137:141–150. doi: 10.1016/j.antiviral.2016.11.015. [DOI] [PubMed] [Google Scholar]
- 22.Kunin CM, Ellis WY. 2000. Antimicrobial activities of mefloquine and a series of related compounds. Antimicrob Agents Chemother 44:848–852. doi: 10.1128/aac.44.4.848-852.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.White TC. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob Agents Chemother 41:1482–1487. doi: 10.1128/AAC.41.7.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang YL, Yu SJ, Heitman J, Wellington M, Chen YL. 2017. New facets of antifungal therapy. Virulence 8:222–236. doi: 10.1080/21505594.2016.1257457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Krysan DJ. 2016. Challenges in the development of novel anticryptococcal agents. Future Med Chem 8:1375–1377. doi: 10.4155/fmc-2016-0123. [DOI] [PubMed] [Google Scholar]
- 26.Lofgren S, Abassi M, Rhein J, Boulware DR. 2017. Recent advances in AIDS-related cryptococcal meningitis treatment with an emphasis on resource limited settings. Expert Rev Anti Infect Ther 15:331–340. doi: 10.1080/14787210.2017.1285697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Krysan DJ. 2015. Toward improved anti-cryptococcal drugs: novel molecules and repurposed drugs. Fungal Genet Biol 78:93–98. doi: 10.1016/j.fgb.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 28.Das S, Dielschneider R, Chanas-LaRue A, Johnston JB, Gibson SB. 2018. Antimalarial drugs trigger lysosome-mediated cell death in chronic lymphocytic leukemia (CLL) cells. Leuk Res 70:79–86. doi: 10.1016/j.leukres.2018.06.005. [DOI] [PubMed] [Google Scholar]
- 29.Dow GS, Koenig ML, Wolf L, Gerena L, Lopez-Sanchez M, Hudson TH, Bhattacharjee AK. 2004. The antimalarial potential of 4-quinolinecarbinolamines may be limited due to neurotoxicity and cross-resistance in mefloquine-resistant Plasmodium falciparum strains. Antimicrob Agents Chemother 48:2624–2632. doi: 10.1128/AAC.48.7.2624-2632.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kollaritsch H, Karbwang J, Wiedermann G, Mikolasek A, Na-Bangchang K, Wernsdorfer WH. 2000. Mefloquine concentration profiles during prophylactic dose regimens. Wien Klin Wochenschr 112:441–447. [PubMed] [Google Scholar]
- 31.Bahn YS. 2015. Exploiting fungal virulence-regulating transcription factors as novel antifungal drug targets. PLoS Pathog 11:e1004936. doi: 10.1371/journal.ppat.1004936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romo JA, Pierce CG, Chaturvedi AK, Lazzell AL, McHardy SF, Saville SP, Lopez-Ribot JL. 2017. Development of anti-virulence approaches for candidiasis via a novel series of small-molecule inhibitors of Candida albicans filamentation. mBio 8:e01991-17. doi: 10.1128/mBio.01991-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kulkarny VV, Chavez-Dozal A, Rane HS, Jahng M, Bernardo SM, Parra KJ, Lee SA. 2014. Quinacrine inhibits Candida albicans growth and filamentation at neutral pH. Antimicrob Agents Chemother 58:7501–7509. doi: 10.1128/AAC.03083-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bar-Yosef H, Vivanco Gonzalez N, Ben-Aroya S, Kron SJ, Kornitzer D. 2017. Chemical inhibitors of Candida albicans hyphal morphogenesis target endocytosis. Sci Rep 7:5692. doi: 10.1038/s41598-017-05741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zaragoza O. 2019. Basic principles of the virulence of Cryptococcus. Virulence 10:490–501. doi: 10.1080/21505594.2019.1614383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alspaugh JA. 2015. Virulence mechanisms and Cryptococcus neoformans pathogenesis. Fungal Genet Biol 78:55–58. doi: 10.1016/j.fgb.2014.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O'Meara TR, Alspaugh JA. 2012. The Cryptococcus neoformans capsule: a sword and a shield. Clin Microbiol Rev 25:387–408. doi: 10.1128/CMR.00001-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fromtling RA, Shadomy HJ, Jacobson ES. 1982. Decreased virulence in stable, acapsular mutants of Cryptococcus neoformans. Mycopathologia 79:23–29. doi: 10.1007/bf00636177. [DOI] [PubMed] [Google Scholar]
- 39.Beale MA, Sabiiti W, Robertson EJ, Fuentes-Cabrejo KM, O'Hanlon SJ, Jarvis JN, Loyse A, Meintjes G, Harrison TS, May RC, Fisher MC, Bicanic T. 2015. Genotypic diversity is associated with clinical outcome and phenotype in cryptococcal meningitis across southern Africa. PLoS Negl Trop Dis 9:e0003847. doi: 10.1371/journal.pntd.0003847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sukhai MA, Prabha S, Hurren R, Rutledge AC, Lee AY, Sriskanthadevan S, Sun H, Wang X, Skrtic M, Seneviratne A, Cusimano M, Jhas B, Gronda M, MacLean N, Cho EE, Spagnuolo PA, Sharmeen S, Gebbia M, Urbanus M, Eppert K, Dissanayake D, Jonet A, Dassonville-Klimpt A, Li X, Datti A, Ohashi PS, Wrana J, Rogers I, Sonnet P, Ellis WY, Corey SJ, Eaves C, Minden MD, Wang JC, Dick JE, Nislow C, Giaever G, Schimmer AD. 2013. Lysosomal disruption preferentially targets acute myeloid leukemia cells and progenitors. J Clin Invest 123:315–328. doi: 10.1172/JCI64180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Feng X, Zhu W, Schurig-Briccio LA, Lindert S, Shoen C, Hitchings R, Li J, Wang Y, Baig N, Zhou T, Kim BK, Crick DC, Cynamon M, McCammon JA, Gennis RB, Oldfield E. 2015. Antiinfectives targeting enzymes and the proton motive force. Proc Natl Acad Sci U S A 112:E7073–E7082. doi: 10.1073/pnas.1521988112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wong W, Bai XC, Sleebs BE, Triglia T, Brown A, Thompson JK, Jackson KE, Hanssen E, Marapana DS, Fernandez IS, Ralph SA, Cowman AF, Scheres SHW, Baum J. 2017. Mefloquine targets the Plasmodium falciparum 80S ribosome to inhibit protein synthesis. Nat Microbiol 2:17031. doi: 10.1038/nmicrobiol.2017.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dziekan JM, Yu H, Chen D, Dai L, Wirjanata G, Larsson A, Prabhu N, Sobota RM, Bozdech Z, Nordlund P. 2019. Identifying purine nucleoside phosphorylase as the target of quinine using cellular thermal shift assay. Sci Transl Med 11:eaau3174. doi: 10.1126/scitranslmed.aau3174. [DOI] [PubMed] [Google Scholar]
- 44.Sheridan CM, Garcia VE, Ahyong V, DeRisi JL. 2018. The Plasmodium falciparum cytoplasmic translation apparatus: a promising therapeutic target not yet exploited by clinically approved anti-malarials. Malar J 17:465. doi: 10.1186/s12936-018-2616-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Raymond CK, Howald-Stevenson I, Vater CA, Stevens TH. 1992. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol Biol Cell 3:1389–1402. doi: 10.1091/mbc.3.12.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Gunjan S, Singh SK, Sharma T, Dwivedi H, Chauhan BS, Imran Siddiqi M, Tripathi R. 2016. Mefloquine induces ROS mediated programmed cell death in malaria parasite: Plasmodium. Apoptosis 21:955–964. doi: 10.1007/s10495-016-1265-y. [DOI] [PubMed] [Google Scholar]
- 47.Li H, Jiao S, Li X, Banu H, Hamal S, Wang X. 2017. Therapeutic effects of antibiotic drug mefloquine against cervical cancer through impairing mitochondrial function and inhibiting mTOR pathway. Can J Physiol Pharmacol 95:43–50. doi: 10.1139/cjpp-2016-0124. [DOI] [PubMed] [Google Scholar]
- 48.Shibata T, Takahashi T, Yamada E, Kimura A, Nishikawa H, Hayakawa H, Nomura N, Mitsuyama J. 2012. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vincent BM, Langlois JB, Srinivas R, Lancaster AK, Scherz-Shouval R, Whitesell L, Tidor B, Buchwald SL, Lindquist S. 2016. A fungal-selective cytochrome bc1 inhibitor impairs virulence and prevents the evolution of drug resistance. Cell Chem Biol 23:978–991. doi: 10.1016/j.chembiol.2016.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rosenberg A, Ene IV, Bibi M, Zakin S, Segal ES, Ziv N, Dahan AM, Colombo AL, Bennett RJ, Berman J. 2018. Antifungal tolerance is a subpopulation effect distinct from resistance and is associated with persistent candidemia. Nat Commun 9:2470. doi: 10.1038/s41467-018-04926-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tsai HF, Krol AA, Sarti KE, Bennett JE. 2006. Candida glabrata PDR1, a transcriptional regulator of a pleiotropic drug resistance network, mediates azole resistance in clinical isolates and petite mutants. Antimicrob Agents Chemother 50:1384–1392. doi: 10.1128/AAC.50.4.1384-1392.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Shingu-Vazquez M, Traven A. 2011. Mitochondria and fungal pathogenesis: drug tolerance, virulence, and potential for antifungal therapy. Eukaryot Cell 10:1376–1383. doi: 10.1128/EC.05184-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Karbwang J, Harinasuta T. 1992. Overview: clinical pharmacology of antimalarials. Southeast Asian J Trop Med Public Health 23(Suppl 4):95–109. [PubMed] [Google Scholar]
- 54.Toovey S. 2009. Mefloquine neurotoxicity: a literature review. Travel Med Infect Dis 7:2–6. doi: 10.1016/j.tmaid.2008.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Korotchenko V, Sathunuru R, Gerena L, Caridha D, Li Q, Kreishman-Deitrick M, Smith PL, Lin AJ. 2015. Antimalarial activity of 4-amidinoquinoline and 10-amidinobenzonaphthyridine derivatives. J Med Chem 58:3411–3431. doi: 10.1021/jm501809x. [DOI] [PubMed] [Google Scholar]
- 56.Sherman F. 2002. Getting started with yeast. Methods Enzymol 350:3–41. doi: 10.1016/s0076-6879(02)50954-x. [DOI] [PubMed] [Google Scholar]
- 57.Clinical and Laboratory Standards Institute. 2008. Reference methods for broth dilution antifungal susceptibility testing of yeasts; third informational supplement. Document M27-A3 Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 58.Butts A, Koselny K, Chabrier-Rosello Y, Semighini CP, Brown JC, Wang X, Annadurai S, DiDone L, Tabroff J, Childers WE Jr, Abou-Gharbia M, Wellington M, Cardenas ME, Madhani HD, Heitman J, Krysan DJ. 2014. Estrogen receptor antagonists are anti-cryptococcal agents that directly bind EF hand proteins and synergize with fluconazole in vivo. mBio 5:e00765-13. doi: 10.1128/mBio.00765-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.