Methicillin-resistant Staphylococcus aureus (MRSA) opportunistic infections are a major health burden. Decolonization of hospitalized patients with mupirocin (MUP) has reduced the incidence of infection but has led to MUP resistance. DIBI is a developmental-stage anti-infective agent that sequesters bacterial iron and bolsters innate host iron-withdrawal defenses. Clinical isolates possessing low, high, or no MUP resistance all had similarly high susceptibilities to DIBI.
KEYWORDS: MRSA, iron sequestration, mupirocin resistance, nares decolonization
ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA) opportunistic infections are a major health burden. Decolonization of hospitalized patients with mupirocin (MUP) has reduced the incidence of infection but has led to MUP resistance. DIBI is a developmental-stage anti-infective agent that sequesters bacterial iron and bolsters innate host iron-withdrawal defenses. Clinical isolates possessing low, high, or no MUP resistance all had similarly high susceptibilities to DIBI. Intranasal DIBI reduced nares bacterial burdens in mice to the same extent as MUP. No resistance was found after exposure to DIBI.
INTRODUCTION
Staphylococcus aureus isolates colonize the nares of 30% of the human population (1, 2) and cause infections, including ventilator-associated pneumonias (3) and perioperative surgical-site infections (4, 5), ranking second at 40% of all hospital-acquired infections (HAI) (6). With all-patient HAI incidence at 4% (6), opportunistic S. aureus infections, including methicillin-resistant S. aureus (MRSA) are a major problem and health care burden.
Prophylactic nares decolonization, most often with mupirocin (MUP) has demonstrated reduced incidence of infection (5), but broader MUP use has led to increased incidence of resistance (7). Thus, it would be desirable to effect nares clearance of staphylococci with alternative agents, avoiding dependence on MUP. Potential alternatives, including bacitracin, fusidic acid, and bacteriophage, have not yet provided new therapeutics (8); and MUP adjuncts, including neomycin (9), propolis (8), and RnpA inhibitors (10), have not led to new treatments. New therapeutics with anti-infective activity for MUP-resistant isolates or that can substitute for MUP or work with MUP to extend its efficacy would have broad potential.
DIBI, the lead member of a new chemical class of purpose-designed anti-infective iron-sequestering polymers (11), is nontoxic to animals and bolsters innate host iron sequestration defenses (12, 13). This study investigated DIBI’s potential for nares decolonization of S. aureus isolates as an MUP alternative and an MUP adjunct.
Various S. aureus clinical isolates were tested for sensitivity to DIBI compared with MUP (Table 1). Maintenance, cultivation of the isolates, and testing were done with our previously established procedures, employing sufficient yet low-iron RPMI medium to ensure host-relevant iron levels (12–14). Isolates included ATCC 43300-MP01, a spontaneous MUP-resistant clone of ATCC 43300 isolated in our laboratory and various MUP-resistant isolates obtained from the U.S. Centers for Disease Control and Prevention (CDC); and ATCC BAA1708. MUP-sensitive plasmidless clones of ATCC BAA1708 and CDC0563, isolated in our laboratory after growth at 43°C to induce plasmid loss (15), were also tested.
TABLE 1.
Tested Staphylococcus aureus isolates and sensitivities to MUP and DIBI
| Isolate | Characteristica | MIC for: |
|||
|---|---|---|---|---|---|
| MUP in: |
DIBI in: |
||||
| μg/ml | μM | μg/ml | μM | ||
| ATCC 43300 | MUP-S, MRSA reference strain | ≤0.06 | ≤0.12 | 8 | 0.88 |
| ATCC 43300-MP01 | LLR, spontaneous mutant | 8 | 16 | 8 | 0.88 |
| ATCC BAA1708 | HLR, mupA+ plasmid, MRSA | 512 | 999 | 8 | 0.88 |
| ATCC BAA1708 without MupA | MUP-S, plasmid-less clone | 0.03 | 0.06 | 4 | 0.44 |
| CDC0563 | HLR, mupA+ plasmid | 512 | 999 | 4 | 0.44 |
| CDC0563 without MupA | MUP-S, plasmid-less clone | 0.03 | 0.06 | 2 | 0.22 |
| CDC0224 | HLR, mupA+ plasmid | 512 | 999 | 2 | 0.22 |
MIC cutoffs for MUP: sensitive (MUP-S), <8 μg/ml; low-level resistance (LLR), 8 to 256 μg/ml; high-level resistance (HLR), ≥512 μg/ml, as previously reported (16).
All isolates had a relatively high susceptibility to DIBI, with MICs between 2 and 8 μg/ml, i.e., equivalent to 0.22 to 0.88 μM DIBI (Table 1). MICs are reported in molarity and weight units to provide a proper weight-adjusted comparison of the 18-times-larger DIBI (9 kDa) versus MUP (0.5 kDa).
Importantly, DIBI sensitivity did not correlate with MUP sensitivity or resistance. The MUP-resistant MP01 clone retained its DIBI sensitivity, and plasmidless (without MupA) MUP-sensitive clones of ATCC BAA1708 and CDC0563 displayed similar DIBI sensitivities to their parental strains (Table 1). Our results suggest that DIBI targets functions different from those targeted by MUP.
Possible development of resistance to DIBI on prolonged exposure was tested using strain ATCC BAA1708 by its repeated passage in Mueller-Hinton broth (MHB) containing DIBI. For this, MHB was first partially relieved of its excessive iron content as described previously (12), and this medium still permitted repeated luxurious rapid overnight growth to a high Ymax (maximum population density in a culture) optical density (OD) (Table 2). Addition of DIBI resulted in iron-restricted growth with a substantially reduced Ymax, and this growth pattern was repeatable over successive subcultures. Ten individual clones isolated after five repeat subcultures in DIBI had DIBI sensitivities as low as or slightly lower than the initial culture (Table 2). These results suggest a low likelihood for development of resistance to DIBI consistent with the irreplaceable requirement for iron and the multitude of iron-dependent targets in this and other bacteria. In contrast, when strain ATCC 43300 was similarly subcultured in the presence of subinhibitory MUP, clone MP01 was isolated and found to have an elevated MIC for MUP (Table 1), exhibiting spontaneous low-level resistance, indicating that resistance to MUP can develop readily.
TABLE 2.
Effects of repeated subculturing of S. aureus ATCC BAA1708 in DIBI
| Growth medium |
Ymax (OD600) for subculture: |
DIBI MICc in: |
||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | Average | μg/ml | μM | |
| MHBa | 3.8 | 3.7 | ND | 2.8 | 3.2 | 3.40 | ||
| MHB plus DIBIb | 0.8 | 0.7 | 0.9 | 1.1 | 0.8 | 0.88 | 4 (±0) | 0.44 (±0) |
MHB partially deferrated. ND, not determined.
Deferrated MHB plus 10 μg/ml DIBI.
Average for 10 clones after 5 subcultures in MHB plus DIBI.
We have built on our earlier findings that DIBI reduces nares carriage of ATCC 43300 (12). Bacterial inoculum, mice, and animal procedures were as previously described, with all animal experiments approved by the Institutional Ethics Committee of Jubilant Biosys, Ltd., in full accordance with the Committee for the Purpose of Control and Supervision of Experiments on Animals (CPCSEA; India) guidelines (12). The previously established carriage model includes an initial establishment phase with bacterial inoculation (intranasally 2 days before and on day 0 of infection) followed by sustained carriage (>5 days postinfection [dpi]) (17). Bacterial burdens were determined at 5 dpi for all treatment groups (n = 6 mice), and results were analyzed using one-way analysis of variance with Tukey’s multiple comparisons using GraphPad Prism and P values of <0.01 or <0.001 (results shown are for a typical replicate experiment).
Our rationale was to compare DIBI activity with that of MUP using an established intranasal administration protocol that had been validated for MUP (17). Thus, only one or two treatments were utilized ≥2 days before sacrifice for comparative bacterial burden determinations, as opposed to multiple treatments over several days in an attempt to fully eradicate carriage. Both untreated and sham-treated (intranasal phosphate-buffered saline [PBS] vehicle) control and MUP-treated control (5 mg/kg [10 μmol/kg] in PBS once at 2 dpi) bacterial burdens were similar to those previously established (17) (Fig. 1).
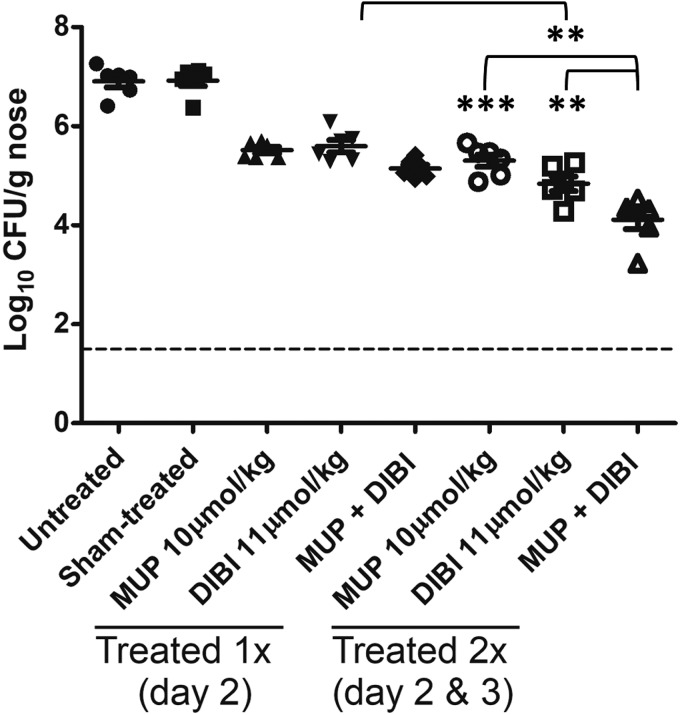
FIG 1.
DIBI reduces S. aureus nares carriage in mice. Groups of 6 mice each were infected intranasally with MRSA strain ATCC 43300 and, at 2 dpi, were treated once intranasally with PBS (sham), MUP (10 μmol/kg), DIBI (11 μmol/kg), or MUP plus DIBI. Additional groups were treated at both 2 and 3 dpi. All groups were sacrificed at 5 dpi, and nares bacterial burdens were enumerated by plate counting. All treatments provided significant (P < 0.001) burden reductions over sham controls (significance not shown). Administration twice of DIBI provided a significant reduction compared with a single treatment, and administration twice of MUP plus DIBI provided a significant reduction compared with 2 administrations of MUP or DIBI alone. Dotted line indicates limit of detection. **, P < 0.01; ***, P < 0.001.
We investigated the comparative treatment efficacies of MUP and DIBI alone and combined to assess how DIBI efficacy compared with that of MUP and whether DIBI displayed any antagonistic (reduced) or synergistic (enhanced) efficacy when combined with MUP. A single treatment with DIBI alone (100 mg/kg [11μmol/kg] in PBS) or MUP alone (10 μmol/kg in PBS) at 2 dpi provided similar bacterial burden reductions, both of which were significantly reduced compared with that in untreated controls, indicating that DIBI efficacy was similar to MUP efficacy (Fig. 1). When DIBI was administered alone twice after carriage was established (at 2 and 3 dpi), it reduced carriage better than MUP administered twice (Fig. 1). Separate testing of the influence of timing of the DIBI treatment was also studied during the establishment of infection (i.e., DIBI administered 2 days before and/or on day 0 of infection), and we observed a reduced bacterial burden at 5 dpi (>1 log reduction; data not shown).
Importantly, a single combination treatment of MUP with DIBI did not display antagonistic activity, which suggests that they are chemically and mechanistically compatible. Although this single combined treatment was slightly indicative of possible additive effects, the resulting reduction of bacterial burden did not differ significantly from that with individual treatments. Bacterial burdens were significantly lower in mice treated twice with DIBI plus MUP (2 and 3 dpi) than in those treated twice with MUP alone or DIBI alone (Fig. 1). Overall bacterial reductions in mice treated with MUP plus DIBI were >2.5 log higher than those in sham-treated mice. Testing with MUP, DIBI, or MUP plus DIBI using three treatments (2, 3, and 4 dpi) provided further burden reductions, but they were not significantly lower than those obtained with two treatments (data not shown).
Other studies have shown that staphylococcal growth and turnover are high during both human and murine nares carriage with active bacterial replication in the nose (18). DIBI restricts iron supply to S. aureus isolates impairing their growth (12), and DIBI’s iron sequestration activity in the nares is supported by the reported upregulated bacterial expression of IsdA, a cell wall component indicative of iron-limited conditions (18), as well as the overall upregulation of iron acquisition systems during nares carriage (19, 20). Our results suggest that iron supply to S. aureus isolates within the nares is a key determinant for establishment and maintenance of carriage and that DIBI appears to aid natural host iron-withdrawal mechanisms to suppress carriage.
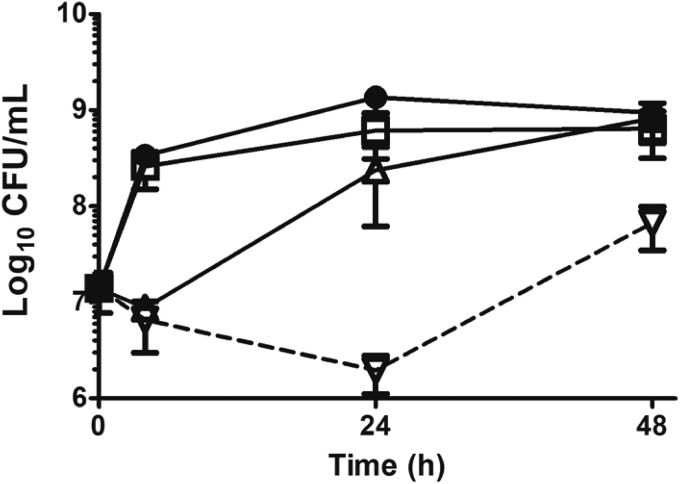
We further assessed interactions of MUP with DIBI in vitro using time-kill assays of strain ATCC 43300 with our previously reported procedure (12, 13). MUP 0.12 μg/ml caused slight initial killing, consistent with its primarily bacteriostatic activity (17), followed by strong recovery growth by 24 h (Fig. 2); whereas DIBI 5 μg/ml showed little apparent growth inhibition, as expected for the low concentration utilized, as assessed by CFU count. However, the combination of MUP plus DIBI provided continued killing over 24 h and prevented full recovery growth by 48 h. We reported previously that a relatively low concentration of DIBI as utilized in similar time-kill assays induced an iron-restricted bacterial physiology that predisposes both S. aureus (12) and Acinetobacter baumannii (13) isolates to enhanced killing by various discrete antibiotics. Our finding that DIBI’s killing enhancement extends to MUP has implications for providing a possible MUP-enhancing adjunct and addressing MUP resistance.
FIG 2.
Influence of DIBI on MUP killing in vitro. S. aureus strain ATCC 43300 was inoculated at ∼107 CFU/ml into RPMI or RPMI containing DIBI, MUP, or DIBI plus MUP and grown at 35°C. CFU/ml were determined at intervals over 48 h. ●, untreated control; □, DIBI 5 μg/ml; Δ, MUP 0.12 μg/ml; ▽, DIBI 5 μg/ml plus MUP 0.12 μg/ml. Data points represent means ± SEM from three independent experiments.
Despite the acknowledgment of MUP resistance as a consequence of its use and the evaluation of alternatives, MUP is still considered the most effective agent for presurgical nares decolonization (21). The findings presented here establish a proof in principle using an experimental in vivo model that DIBI has the potential to provide nasal decolonization as an MUP alternative or adjunct. Future testing to assess DIBI activity against other nares bacterial isolates, including streptococci, would be warranted. We have not tested streptococcal isolates from humans (e.g., S. pneumoniae or S. pyogenes). However, streptococci of other animal origin (e.g., S. agalactiae, S. dysgalactiae from bovine mastitis) have been found to be sensitive to DIBI in vitro (our unpublished data). Further testing of MRSA and other nares isolates would strengthen the case for eventual clinical trials with DIBI.
ACKNOWLEDGMENT
B.E.H. has a beneficial interest in and the other authors were employees of Chelation Partners, Inc., who sponsored this work.
REFERENCES
- 1.Liu GY. 2009. Molecular pathogenesis of Staphylococcus aureus infection. Pediatr Res 65:71R–77R. doi: 10.1203/PDR.0b013e31819dc44d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Abad CL, Pulia MS, Safdar N. 2013. Does the nose know? An update on MRSA decolonization strategies. Curr Infect Dis Rep 15:455–464. doi: 10.1007/s11908-013-0364-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luyt C-E, Hekimian G, Koulenti D, Chastre D. 2018. Microbial cause of ICU-acquired pneumonia: hospital-acquired pneumonia versus ventilator-associated pneumonia. Curr Opin Crit Care 24:332–338. doi: 10.1097/MCC.0000000000000526. [DOI] [PubMed] [Google Scholar]
- 4.Tong SYC, Davis JS, Eichenberger E, Holland TL, Fowler VG. 2015. Staphylococcus aureus infections: epidemiology, pathophysiology, clinical manifestations, and management. Clin Microbiol Rev 28:603–661. doi: 10.1128/CMR.00134-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Humphreys H, Becker K, Dohmen PM, Petrosillo N, Spencer M, van Rijen M, Wechsler-Fordos A, Pujol M, Dubouix A, Garau J. 2016. Staphylococcus aureus and surgical site infections: benefits of screening and decolonization before surgery. J Hosp Infect 94:295–304. doi: 10.1016/j.jhin.2016.06.011. [DOI] [PubMed] [Google Scholar]
- 6.Magill SS, Edwards JR, Bamberg W, Beldavs ZG, Dumyati G, Kainer MA, Lynfield R, Maloney M, McAllister-Hollod L, Nadle J, Ray SM, Thompson DL, Wilson LE, Fridkin SK, Emerging Infections Program Healthcare-Associated Infections and Antimicrobial Use Prevalence Survey Team. 2014. Multistate point-prevalence survey of health care-associated infections. N Engl J Med 370:1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Antonov NK, Garzon MC, Morel KD, Whittier S, Planet PJ, Lauren CT. 2015. High prevalence of mupirocin resistance in Staphylococcus aureus isolates from a pediatric population. Antimicrob Agents Chemother 59:3350–3356. doi: 10.1128/AAC.00079-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Poovelikunnel T, Gethin G, Humphreys H. 2015. Mupirocin resistance: clinical implications and potential alternatives for the eradication of MRSA. J Antimicrob Chemother 70:2681–2692. doi: 10.1093/jac/dkv169. [DOI] [PubMed] [Google Scholar]
- 9.Blanchard C, Brooks L, Beckley A, Colquhoun J, Dewhurst S, Dunman PM. 2016. Neomycin sulfate improves the antimicrobial activity of mupirocin-based antibacterial ointments. Antimicrob Agents Chemother 60:862–872. doi: 10.1128/AAC.02083-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lounsbury N, Eidem T, Colquhoun J, Mateo G, Abou-Gharbia M, Dunman PM, Childers WE. 2018. Novel inhibitors of Staphylococcus aureus RnpA that synergize with mupirocin. Bioorg Med Chem Lett 28:1127–1131. doi: 10.1016/j.bmcl.2018.01.022. [DOI] [PubMed] [Google Scholar]
- 11.Ang MTC, Gumbau-Brisa R, Allan DS, McDonald R, Ferguson MJ, Holbein BE, Bierenstiel M. 2018. DIBI, a 3-hydroxypyridin-4-one chelator iron-binding polymer with enhanced antimicrobial activity. Medchemcomm 9:1206–1212. doi: 10.1039/c8md00192h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Parquet MDC, Savage KA, Allan DS, Davidson RJ, Holbein BE. 2018. Novel iron-chelator DIBI inhibits Staphylococcus aureus growth, suppresses experimental MRSA infection in mice and enhances the activities of diverse antibiotics in vitro. Front Microbiol 9:1811. doi: 10.3389/fmicb.2018.01811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Parquet MDC, Savage KA, Allan DS, Ang MTC, Chen W, Logan SM, Holbein BE. 2019. Antibiotic-resistant Acinetobacter baumannii is susceptible to the novel iron-sequestering anti-infective DIBI in vitro and in experimental pneumonia in mice. Antimicrob Agents Chemother 63:e00855-19. doi: 10.1128/AAC.00855-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Savage KA, Parquet MDC, Allan DS, Davidson RJ, Holbein BE, Lilly EA, Fidel PL Jr. 2018. Iron restriction to clinical isolates of Candida albicans by the novel chelator DIBI inhibits growth and increases sensitivity to azoles in vitro and in vivo in a murine model of experimental vaginitis. Antimicrob Agents Chemother 62:e02576-17. doi: 10.1128/AAC.02576-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Trevors JT. 1986. Plasmid curing in bacteria. FEMS Microbiol Rev 1:149–157. doi: 10.1111/j.1574-6968.1986.tb01189.x. [DOI] [Google Scholar]
- 16.Hetem DJ, Bonten M. 2013. Clinical relevance of mupirocin resistance in Staphylococcus aureus. J Hosp Infect 85:249–256. doi: 10.1016/j.jhin.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Chhibber S, Gupta P, Kaur S. 2014. Bacteriophage as effective decolonising agent for elimination of MRSA from anterior nares of BALB/c mice. BMC Microbiol 14:212. doi: 10.1186/s12866-014-0212-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Burian M, Wolz C, Goerke C. 2010. Regulatory adaptation of Staphylococcus aureus during nasal colonization of humans. PLoS One 5:e10040. doi: 10.1371/journal.pone.0010040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bacconi M, Haag AF, Chiarot E, Donato P, Bagnoli F, Delany I, Bensi G. 2017. In vivo analysis of Staphylococcus aureus-infected mice reveals differential temporal and spatial expression patterns of fhuD2. Infect Immun 85:e00270-17. doi: 10.1128/IAI.00270-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chaves-Moreno D, Wos-Oxley ML, Jáuregui R, Medina E, Oxley APA, Pieper DH. 2016. Exploring the transcriptome of Staphylococcus aureus in its natural niche. Sci Rep 6:33174. doi: 10.1038/srep33174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Septimus EJ. 2019. What antimicrobials are most effective prior to surgery? Am J Infect Control 47:A53–A57. doi: 10.1016/j.ajic.2019.02.028. [DOI] [PubMed] [Google Scholar]