Many antibiotics carry caution stickers that warn against alcohol consumption. Data regarding concurrent use are sparse. An awareness of data that address this common clinical scenario is important so health care professionals can make informed clinical decisions and address questions in an evidence-based manner. The purpose of this systematic review was to determine the evidence behind alcohol warnings issued for many common antimicrobials.
KEYWORDS: adverse drug effects, antimicrobial agents, antimicrobial safety, drug interactions
ABSTRACT
Many antibiotics carry caution stickers that warn against alcohol consumption. Data regarding concurrent use are sparse. An awareness of data that address this common clinical scenario is important so health care professionals can make informed clinical decisions and address questions in an evidence-based manner. The purpose of this systematic review was to determine the evidence behind alcohol warnings issued for many common antimicrobials. The search was conducted from inception of each database to 2018 using PubMed, Medline via Ovid, and Embase. It included studies that involved interactions, effects on efficacy, and toxicity/adverse drug reactions (ADR) due to concomitant alcohol consumption and antimicrobials. All interactions were considered in terms of three components: (i) alteration in pharmacokinetics/pharmacodynamics (PK/PD) of antimicrobials and/or alcohol, (ii) change in antimicrobial efficacy, and (iii) development of toxicity/ADR. Available data support that oral penicillins, cefdinir, cefpodoxime, fluoroquinolones, azithromycin, tetracycline, nitrofurantoin, secnidazole, tinidazole, and fluconazole can be safely used with concomitant alcohol consumption. Data are equivocal for trimethoprim-sulfamethoxazole. Erythromycin may have reduced efficacy with alcohol consumption, and doxycycline may have reduced efficacy in chronic alcoholism. Alcohol low in tyramine may be consumed with oxazolidinones. The disulfiram-like reaction, though classically associated with metronidazole, occurs with uncertain frequency and with varied severity. Cephalosporins with a methylthiotetrazole (MTT) side chain or a methylthiodioxotriazine (MTDT) ring, ketoconazole, and griseofulvin have an increased risk of a disulfiram-like reaction. Alcohol and antimicrobial interactions are often lacking evidence. This review questions common beliefs due to poor, often conflicting data and identifies important knowledge gaps.
INTRODUCTION
“Can I drink alcohol with my antibiotic?” is a frequent query to health care professionals. Many prescription bottles come labeled with a sticker that warns against alcohol use with the antimicrobial (1). Understanding the evidence behind this warning is important, given the commonality of prescribing and the diverse classes and various properties of antimicrobials (2). The Centers for Disease Control and Prevention (CDC) reported that approximately 270 million antibiotics were prescribed for outpatients in 2016 (3). In its report on harmful interactions with alcohol, the National Institutes of Health (NIH) listed nitrofurantoin, metronidazole, griseofulvin, ketoconazole, isoniazid, cycloserine, and azithromycin (4). The National Consumers League and the Food and Drug Administration (FDA) have also warned consumers to avoid alcohol with linezolid, metronidazole, griseofulvin, and antimycobacterials (5). Alcohol warnings between pharmacy chains also differ, potentially leading to confusion for both patients and providers (Table 1).
TABLE 1.
Comparison of alcohol warnings from various retail pharmaciesa
| Antimicrobial | Alcohol warning from: |
||
|---|---|---|---|
| Walgreen’s | Rite Aid | CVS | |
| Cefprozil | No warning | Limit alcoholic beverages. | No warning |
| Minocycline | No warning | Limit alcoholic beverages. | No warning |
| Tetracycline | No warning | Limit alcoholic beverages. | No warning |
| Metronidazole | Avoid alcohol and products that have alcohol or propylene glycol in them while taking this drug and for at least 72 h after your last dose. Drinking alcohol may cause cramps, upset stomach, headaches, and flushing. | Avoid alcoholic beverages and products containing propylene glycol while taking this medication and for at least 3 days after finishing this medicine because severe stomach upset/cramps, nausea, vomiting, headache, and flushing may occur. | Do not take this medication with alcohol or any product that contains alcohol. Metronidazole can cause an unpleasant reaction when taken with alcohol. The reaction includes flushing, headache, nausea, vomiting, sweating, and increased thirst. The reaction can last from 30 min to several hours. |
| Tinidazole | Avoid alcohol and products that have alcohol or propylene glycol in them while taking this drug and for at least 72 h after your last dose. Drinking alcohol may cause cramps, upset stomach, headaches, and flushing. | Avoid alcoholic beverages while taking this medication and for at least 3 days after finishing this medicine because severe stomach upset/cramps, nausea, vomiting, headache, and flushing may occur. | Do not take this medication with alcohol or any product that contains alcohol. Avoid alcoholic drinks while you are taking this medicine and for 3 days afterward. Alcohol may make you feel dizzy, sick, or flushed. |
| Secnidazole | No data | No data | No data |
| Trimethoprim-sulfamethoxazole | Talk with your doctor before you drink alcohol. | No warning | Before taking this medication, tell your healthcare provider if you frequently drink alcohol-containing drinks. |
| Linezolid | No warning | Limit alcoholic beverages. | No warning |
| Tedizolid | No warning | No warning | No data |
| Ciprofloxacin | No warning | Limit alcoholic beverages. | No warning |
| Levofloxacin | No warning | Limit alcoholic beverages. | No data |
| Moxifloxacin | No warning | Limit alcoholic beverages. | No warning |
| Fluconazole | Talk with your doctor before you drink alcohol. | Limit alcoholic beverages. | Alcohol can increase possible damage to your liver. Avoid alcoholic drinks. |
| Ketoconazole | Avoid drinking alcohol while taking this drug. | Do not drink alcoholic beverages while taking ketoconazole because alcohol increases the risk of serious liver problems. Avoiding alcoholic beverages will also decrease the risk of a rare reaction with ketoconazole that may result in flushing, headache, and nausea. | This medication may interact with alcohol or any product that contains alcohol. Avoid alcohol while taking this medication. Alcohol can increase the risk of liver damage. |
| Griseofulvin | Talk with your doctor before you drink alcohol. | Avoid alcoholic beverages. Drinking alcohol during treatment with this drug could result in a fast heartbeat and flushing of the skin. | No warning |
| Rifampin | Talk with your doctor before you drink alcohol. | Limit alcoholic beverages. | Before taking this medication, your healthcare provider needs to know if you often drink alcohol. |
| Isoniazid | Talk with your doctor before you drink alcohol. | The risk of liver disease is increased in people who are 35 years and older, who use alcohol or illegal injection drugs, or who currently have long-term liver problems. Alcohol may increase the risk of liver disease. Avoid alcoholic beverages while using this medication. | This medication may interact with alcohol. Alcohol may interfere with the effect of this medication. Avoid alcoholic drinks. |
| Ethambutol | No warning | Alcohol may increase the risk of liver disease. Avoid alcoholic beverages while using this medication. | No warning |
| Ethionamide | Talk with your doctor before you drink alcohol. | Alcohol may increase the risk of liver disease or mental/mood changes. Avoid alcoholic beverages while using this medication. | Alcohol may interact with this medicine. Alcohol may interfere with the effect of this medication. Avoid alcoholic drinks. |
| Pyrazinamide | Talk with your doctor before you drink alcohol. | Alcohol may increase the risk of liver disease. Avoid alcoholic beverages while using this medication. | Before taking this medication, your healthcare provider needs to know if you frequently drink alcohol-containing drinks. |
| Cycloserine | Not listed | Avoid alcoholic beverages. | Alcohol may interact with this medication. Alcohol may interfere with the effect of this medication. Avoid alcoholic drinks. |
Antibiotics for which there are no warnings from Walgreen’s, Rite Aid, or CVS: cephalexin, cefdinir, doxycycline, nitrofurantoin, azithromycin, erythromycin, penicillin, and amoxicillin.
The potential interactions of antimicrobials with alcohol are best considered in three categories, all of which have patient implications: (i) alterations in pharmacokinetics and pharmacodynamics (PK/PD) of the antimicrobial and/or alcohol, (ii) changes in antimicrobial efficacy, and (iii) development of toxicity. PK/PD were considered together to describe the effect of drug and alcohol on absorption, distribution, metabolism, and excretion (PK) and the resultant effect of this interaction on the host (PD).
Concomitant use of alcohol with antimicrobials is believed to either decrease efficacy or lead to toxicity/ADR (6, 7). The classic example of a feared medication interaction with alcohol is the “disulfiram-like” reaction. Symptoms may include facial flushing, nausea, headache, vomiting, chest pain, vertigo, sweating, thirst, blurred vision, weakness, confusion, and hypotension (8).
Furthermore, alcohol can cause hepatic stress or injury with or without the use of potentially hepatotoxic medications. These concerns may be responsible for alcohol warnings that accompany many antimicrobials, but what are the data and strength of support for these warnings? The goal of this review was to summarize existing data, which in turn generates insights into the origin of these warnings. This review may also be helpful in assessing a patient who presents with an adverse drug effect which may or may not have been due to an alcohol and antibiotic interaction. Although we do not want to encourage alcohol use, it is important for health care professionals to be informed on this common clinical scenario, ensuring that patients can be educated and questions can be addressed in an evidence-based manner. If a reaction does occur, this review provides mechanisms and symptom complexes potentially allowing for a more efficient diagnosis.
SEARCH STRATEGY AND SELECTION CRITERIA
The articles were chosen after a search of published English language medical literature. A secondary search was performed via review of references found from the initial search. The search was conducted from inception to 2018 using PubMed, Medline via Ovid, and Embase and included systematic reviews, randomized controlled trials, observational studies, and case series/reports that involved drug interactions between alcohol and use of antibiotics. Search terms included “ethanol,” “alcohol,” “antibiotics,” “interactions,” and “toxicity.” Antibiotics included in the search were ciprofloxacin, moxifloxacin, levofloxacin, quinolone, fluoroquinolone, macrolide, azithromycin, clarithromycin, erythromycin, sulfonamide, trimethoprim-sulfamethoxazole, β-lactam, penicillin, amoxicillin and clavulanate, ampicillin, amoxicillin, cephalosporin, cefdinir, cefpodoxime, cephalexin, oxazolidinone, linezolid, tedizolid, nitroimidazole, metronidazole, tinidazole, lincosamide, lincomycin, nitrofurantoin, tetracycline, minocycline, doxycycline, antifungal, fluconazole, griseofulvin, antimycobacterials, rifampin, isoniazid, ethambutol, pyrazinamide, ethionamide, and cycloserine. All randomized controlled trials and results from smaller, nonrandomized, open-label studies were included, provided that the studies had adequate methodology as judged by the authors. For drugs with limited information, case reports/series were included. Online drug information centers for Walgreen’s, Rite Aid, and CVS pharmacies were queried for each antimicrobial for concurrent alcohol use warnings (Table 1). Tables are provided for ease of reference with overall recommendations for use with alcohol (Table 2), recommendations with moderate strength of evidence (Table 3), and recommendations with poor strength of evidence (Table 4).
TABLE 2.
Overall recommendations for use with alcohol
| Antimicrobial | Recommendations for use with alcohol |
|---|---|
| Penicillins | Alcohol may be consumed with penicillins.a |
| Cephalosporins | Avoid alcohol use with cefamandole, cefmetazole, cefoperazone, and cefotetan. Avoid alcohol use with ceftriaxone (i.v./i.m.)b due to the potential for a disulfiram-like reaction, which may be severe. Cefpodoxime and cefdinir are safe to be used with alcohol |
| Ciprofloxacin | Alcohol may be consumed with ciprofloxacin.a |
| Levofloxacin | Alcohol may be consumed with levofloxacin.a |
| Moxifloxacin | Alcohol may be consumed with moxifloxacin.a |
| Azithromycin | Alcohol may be consumed with azithromycin.a |
| Erythromycin | Alcohol should be avoided with erythromycin due to potential for delayed onset of action, decreased efficacy, and risk of toxicity. |
| Tetracycline | Alcohol may be consumed with tetracycline.a |
| Doxycycline | Alcohol may be consumed with doxycycline. Chronic alcoholics may require twice daily dosing of doxycycline. |
| Minocycline | Alcohol may be consumed with minocycline.a |
| Nitrofurantoin | Alcohol may be consumed with nitrofurantoin.a |
| Metronidazole | Data are controversial regarding risk of a disulfiram-like reaction. |
| Tinidazole | Alcohol may be consumed with tinidazole. |
| Secnidazole | Alcohol may be consumed with secnidazole.a |
| Trimethoprim-sulfamethoxazole | Alcohol may be consumed with trimethoprim-sulfamethoxazole, with minimal risk of adverse reactions. |
| Linezolid | Alcohol may be consumed with linezolid in moderation. Patients with high blood pressure should take caution to avoid excessive consumption of alcoholic beverages high in tyramine. |
| Tedizolid | Alcohol may be consumed with tedizolid in moderation. Patients with high blood pressure should take caution to avoid excessive consumption of alcoholic beverages high in tyramine. |
| Fluconazole | Alcohol may be consumed with fluconazole.a |
| Ketoconazole | Alcohol consumption should be avoided with ketoconazole due to risk of additive hepatotoxicity (from ketoconazole and alcohol) and adverse reactions. |
| Griseofulvin | Alcohol consumption should be avoided with griseofulvin due to risk of additive hepatotoxicity (from griseofulvin and alcohol) and adverse reactions. |
| Rifamycins | Rifamycins may be used in alcoholics without preexisting LFT elevations when appropriate monitoring can be performed. |
| Isoniazid | Alcohol consumption should be avoided with isoniazid due to risk of additive hepatotoxicity (from isoniazid and alcohol) and risk of adverse reactions. |
| Ethambutol | Unclear risk associated with alcohol consumption, as it is usually used in combination with other antituberculosis agents. |
| Ethionamide | Unclear risk associated with alcohol consumption; however, mild liver disease and alcohol use are not a contraindication for use of ethionamide if appropriate monitoring is performed. |
| Pyrazinamide | Possible risk of hepatotoxicity. Close monitoring and avoidance of alcohol are prudent. |
| Cycloserine | Unclear risk; package insert warns of seizure risk with alcohol consumption, but the basis for this warning was not identified. |
Caution should be exercised, as robust data are lacking.
i.v., intravenous; i.m., intramuscular.
TABLE 3.
Summary of alcohol and antimicrobial interactions with a moderate strength of evidence
| Antimicrobiala | PK/PD | Efficacy | Toxicity/adverse drug reaction |
|---|---|---|---|
| Penicillins | No effect on PK/PD of penicillin; no difference in peak concentrations, absorption, or elimination (10). Amoxicillin has delayed absorption (increased Tmax, increased half-life); no significant difference in Cmax or area under the curve (11). Alcohol has influence on the rate but not on the extent of amoxicillin absorption (11). | No data | No data |
| Cephalosporins | No effect on ceftriaxone PK/PD in an animal model (13). Biliary excretion of cefadroxil was increased with acute alcohol consumption in an animal model (14). Urinary excretion of cephalexin was lowered with acute alcohol consumption in an animal model (14). Decreased cephalexin and cefadroxil absorption with acute alcohol consumption in an animal model (14). No difference in biliary excretion, urinary excretion, or absorption of cephalexin and cefadroxil with chronic alcohol consumption in an animal model (14). | No effect on treatment of pneumonia in animal models (13). | Cephalosporins with an MTT side chain or an MTDT ring have an increased risk of a disulfiram-like reaction (15–22). |
| Doxycycline | Half-life shorter in alcoholics (34). Decreased absorption with “cheap wine”; most effect in alcoholics (35). PK/PD may not be affected by whiskey (35). | Decreases efficacy of treatment of Brucella melitensis in animal models (36). | No data |
| Nitrofurantoina | No data | No data | Previous reports of disulfiram-like reaction have been disproven (43, 44). |
| Metronidazolea | No data | No data | Disulfiram-like reaction (46). No disulfiram-like reaction (48, 52–54, 56, 57, 59, 61). Modification or alteration of the taste of alcohol (46). |
| Linezolid | No data | No data | Increased systolic blood pressure of ≥30 mm Hg with consumption of high-tyramine-containing food or beverages (including tap beers and red wines) (83). ≥100 mg tyramine is required to increase systolic blood pressure by at least 30 mm Hg (81). |
| Tedizolid | No data | No data | Increased systolic blood pressure (≥30 mm Hg) with consumption of 325 mg tyramine (86). |
| Tetracycline | Enhanced absorption (increased Cmax and area under the curve) (33). No effect on elimination (33, 34). | No data | No data |
Carries a risk of hepatoxicity.
TABLE 4.
Summary of alcohol and antimicrobial interactions with a poor strength of evidence
| Antimicrobial | PK/PD | Efficacy | Toxicity/adverse drug reaction |
|---|---|---|---|
| Azithromycina | PK and efficacy unaffected (13). | No data | No data |
| Erythromycina | Delayed onset of action (29). | Potentially decreased efficacy as a result of increased rate of elimination (29). | Unclear; data are contrasting—possible risk of increased intoxication (30, 31). |
| Minocyclinea | No data | No data | No human data. Animal data suggest that minocycline reduces alcohol intake and alcohol-induced neurotoxicity in the developing brain (37, 38).b |
| Ciprofloxacina | No data | No data | One case of erythema multiforme reported (25). |
| Levofloxacina | Generally unaffected (24). | Generally unaffected (24). | No data |
| Moxifloxacin | Generally unaffected (24). | Generally unaffected (24). | No data |
| Fluconazolea | No data | No data | No data |
| Ketoconazolea | No data | No data | Disulfiram-like reaction (89, 90). Tachycardia, nausea, vomiting, flushing, or liver damage (4). Rash (90). |
| Griseofulvina | No data | No data | Disulfiram-like reaction (94–96). Potentiates alcohol’s effects; flushing and tachycardia (123). Reaction can be severe (97). |
| Tinidazole | No data | No data | Potential concern for disulfiram-like reaction due to chemical similarity to metronidazole (72). |
| Secnidazole | No effect on aldehyde dehydrogenase activity or ethanol metabolism (73). | No data | No data |
| Trimethoprim-sulfamethoxazolea | No data | No data | Disulfiram-like reaction, including flushing, heart palpitations, headache, and nausea in one case report (75). |
| Rifamycinsa | No data | No data | Concomitant use does not appear to increase risk of hepatotoxicity (101, 102, 117). Fatal and nonfatal overdose and toxicity have been reported in patients with current or past alcohol abuse (104). |
| Isoniazida | No data | No data | Hepatitis, peripheral neuropathy (124). Tachycardia, nausea, vomiting, headache, changes in blood pressure, flushing, or liver damage (4). Although data are not optimal, it is reasonable to advise avoidance of alcohol consumption in patients taking isoniazid. A disulfiram-like reaction and a possible increased risk of hepatitis are potential concerns. |
| Ethambutola | No data | No data | No data |
| Ethionamidea | No data | No data | Possible increased risk of hepatotoxicity (114). Mild liver impairment in the setting of alcohol abuse is not a contraindication to therapy (114). |
| Pyrazinamidea | No data | No data | Possible increased risk of hepatotoxicity (114). |
| Cycloserine | Decreased cravings for alcohol via enhancement of glutaminergic activity via N-methyl-d-aspartate (NDMA) receptors (119–121). | No data | Package insert warns of an increased risk of seizure, but the basis for this warning was not identified (122). |
Carries a risk of hepatoxicity.
Caution should be exercised as robust data are lacking.
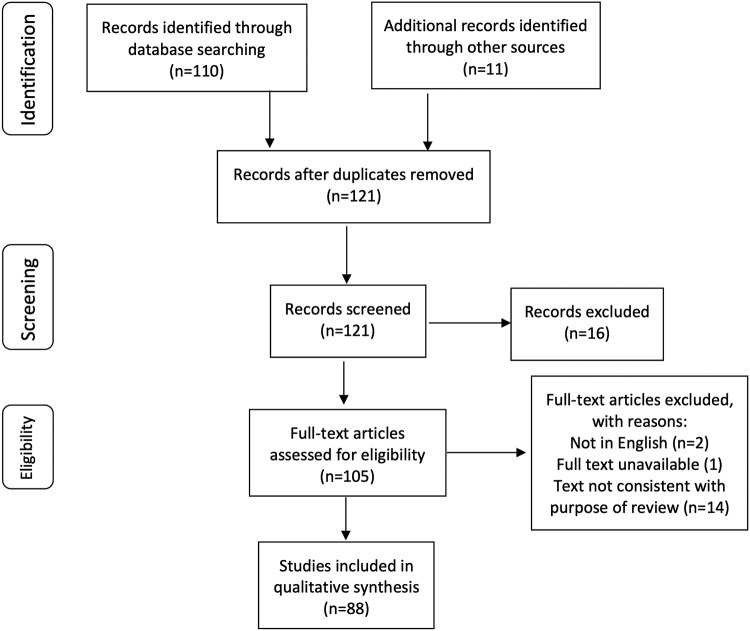
A total of 87 studies are included in this review, after many were excluded due to duplications or not being relevant to the review (Fig. 1).
FIG 1.
Flowchart of identification, inclusion and exclusions of studies.
BETA-LACTAMS
Beta-lactams are widely used and well-tolerated agents (9). Penicillins and cephalosporins are the major beta-lactam antibiotics and have an expansive range of clinical applications.
Penicillins.
(i) PK/PD. Studies have analyzed the PK of penicillin and amoxicillin when administered with alcohol. One study found that the PK of penicillin was not influenced by concomitant consumption of alcohol (10). When 2 million units of penicillin with alcohol (n = 3) was compared to 2 million units without alcohol (n = 3), there was no difference in elimination or absorption, as evidenced by peak drug concentrations.
The effect of the PK of the amoxicillin-alcohol interaction was studied in eight healthy volunteers receiving, on three separate occasions, amoxicillin (500 mg) with water or alcohol. The absorption of amoxicillin, when combined with alcohol, was delayed compared to its absorption with administration with water (11). This was demonstrated by an increased lag time, time to maximal concentration (Tmax), and half-life. Delay in absorption was postulated to be due to alcohol’s inhibition of gastric emptying and the lower solubility of amoxicillin in alcohol than in water. There were no differences in the rate of elimination of amoxicillin. However, the maximal concentration (Cmax) and the area under the curve (AUC) were not significantly modified, so alcohol has an effect on the rate of absorption but the extent of amoxicillin absorption is unchanged (11).
Amoxicillin and amoxicillin-clavulanic acid may lead to decreased alcohol consumption. Both of these beta-lactams have the ability to upregulate glutamate transporter-1 and phosphorylated-AKT levels, which are responsible for mediating the brain reward center for alcohol intake (12).
(ii) Efficacy/toxicity and ADR. To our knowledge, there are no data available on the efficacy/toxicity or ADR of penicillins.
Cephalosporins.
(i) PK/PD. Alcohol did not impact the PK of ceftriaxone in a rat pneumonia model (13). The effects of acute alcohol exposure (6% [vol/vol] ethanol) and chronic alcohol exposure (15% [vol/vol] ethanol for 60 days after 10 days of adaption with 5% and 10% [vol/vol] ethanol) on the PK of cephalexin and cefadroxil were examined in rats (14). Acute alcohol exposure increased the biliary excretion of cefadroxil and decreased the urinary excretion and absorption of cephalexin. Chronic alcohol exposure had no significant effect on absorption kinetics or biliary or urinary excretion for either of these antibiotics (14).
(ii) Efficacy. Alcohol did not affect the efficacy of ceftriaxone in a pneumonia model; survival rates were similar in alcohol-fed and control rats (13).
(iii) Toxicity/ADR. Several cephalosporins have been reported to cause disulfiram-like reactions, particularly those that possess a methylthiotetrazole (MTT) substituent (i.e., cefotetan, cefoperazone, cefamandole, cefmetazole) (15–22). The MTT structure resembles part of the disulfiram molecule. Ceftriaxone, which is commonly given as outpatient parenteral therapy, possesses a related methylthiodioxotriazine (MTDT) ring instead of an MTT side chain but has also been reported to cause disulfiram-like reactions (18, 19, 21, 22). The MTDT ring increases its elimination half-life and serum protein binding (19). A retrospective review conducted in China evaluated 78 cephalosporin-induced disulfiram-like reactions where drug hypersensitivity reactions were excluded via cephalosporin skin testing prior to intravenous cephalosporin receipt (18). Twenty (25.6%) of the reactions occurred in patients receiving ceftriaxone. Five patients died after consumption of alcohol after failed resuscitation attempts. Sweating was experienced by 63%, palpitations by 78%, dizziness by 56%, hypotension in 24%, tachycardia in 76%, premature atrial beat in 4%, and premature ventricular beat in 3% (18).
Summary of human data.
Alcohol influences the rate but not the extent of amoxicillin absorption. Cephalosporins with an MTT side chain or an MTDT ring have an increased risk of a disulfiram-like reaction with alcohol. Fatalities have been reported in a study conducted in China. Cephalosporins lacking these side chains appear safe to consume with alcohol. Commonly used cephalosporins, including cefdinir and cefpodoxime, do not possess the aforementioned side chains and are considered safe to use with alcohol.
FLUOROQUINOLONES
Fluoroquinolones (FQs) are a class of antibiotics that are approved for a variety of infections (23).
PK/PD.
The effects of alcohol on levofloxacin, moxifloxacin, and trovafloxacin were studied in a rat pneumonia model (24). The mean serum Cmax and AUC were higher in the ethanol-fed group for all FQs, with a statistically significant difference in the moxifloxacin group. The ethanol-fed group was found to have decreased protein binding and an increased free fraction of antibiotics.
Efficacy.
Levofloxacin, moxifloxacin, and trovafloxacin had improved efficacy in alcohol-fed rats compared to alcohol-free rats (24). All antibiotics were equally effective at improving survival and had improved efficacy in alcohol-fed rats. Moxifloxacin demonstrated dose-dependent survival in rats who did not receive alcohol, further supporting increased efficacy in the presence of alcohol, as the lower dose was equally as effective in alcohol-fed rats. Control groups had higher mortality rates that were thought to be due to higher protein binding in the absence of alcohol.
Toxicity/ADR.
Data are limited regarding the adverse effects of concomitant use of FQs and alcohol. One case report documents a 46-year-old male who developed erythema multiforme while receiving ciprofloxacin after consuming alcohol (25). The reaction resolved with continued ciprofloxacin use and abstention from alcohol.
Summary of human data.
Toxicity data are limited to a single case that cannot be clearly attributed to the combination of ciprofloxacin and alcohol.
MACROLIDES
Macrolide antibiotics are used for respiratory tract and mycobacterial infections (26–28).
PK/PD.
Erythromycin with alcohol consumption led to significantly prolonged lag time and a reduction of AUC in a small study (n = 8) (29). It was postulated that alcohol causes a delay in gastric emptying, resulting in a delay in absorption, lower peak concentrations, and faster elimination. In contrast, studies with erythromycin concluded that the PK of azithromycin was not affected by alcohol use in rats (13).
Erythromycin may impact blood alcohol concentrations (30, 31). Peak blood alcohol concentrations were, on average, 40% higher and the AUC was 14% higher with intravenous erythromycin (n = 10) than with placebo. In contrast, no significant difference in the PK/PD properties of alcohol were noted in comparison to those of placebo when alcohol was administered with oral erythromycin (n = 8) (30, 31).
Efficacy.
The efficacy of azithromycin was unaffected by alcohol, as survival rates were similar in both alcohol-fed rats and control groups in the pneumococcal pneumonia rat model (13).
Toxicity/ADR.
Azithromycin is listed in an NIH report on harmful interactions with alcohol (4). The basis for this recommendation is unclear, as published findings do not identify an interaction.
Summary of human data.
Alcohol may adversely affect the PK of erythromycin and may increase blood alcohol levels.
TETRACYCLINE DERIVATIVES
Tetracyclines have activity against Gram-positive and Gram-negative bacteria, species of Rickettsia, Chlamydia, and Mycoplasma, and some protozoa (32).
Tetracycline.
(i) PK/PD. The ingestion of 150 ml of alcohol with tetracycline (500 mg) increased the Cmax (from 9.3 to 12.4 μg/ml) and the AUC (from 62.7 to 94.3 μg/ml·h) compared to those values when water was ingested (33). No significant changes were found with elimination, suggesting that tetracycline has greater absorption in the presence of alcohol. The half-life of tetracycline with long-term alcohol consumption (3 to 15 years with drinking habits of 100 to 200 g of ethanol daily for the previous 1 to 6 months, n = 6) compared to controls (n = 6) was unaffected (8.7 ± 0.5 h versus 8.7 ± 0.6 h) (34).
(ii) Efficacy/toxicity and ADR. To our knowledge, there are no data available of the efficacy/toxicity or ADR of tetracycline.
Doxycycline.
(i) PK/PD. Doxycycline’s half-life was significantly shorter in alcoholics (n = 6) than in controls (n = 6): 10.5 ± 0.3 h versus 14.7 ± 0.7 h (P < 0.001) (34). In some patients with long-term alcohol consumption, the serum concentration of doxycycline decreased to below the minimum therapeutic concentration when dosed once daily, leading to the conclusion that twice daily dosing may be indicated in alcoholics (34).
In a randomized crossover trial, the effects of whiskey and red wine on the PK of doxycycline for six students was studied (35). Whiskey did not significantly modify the absorption of 200 mg of oral doxycycline. Drinking “poor-quality” red wine with a clear taste of acetic acid substantially delayed the absorption of doxycycline, which was postulated to be due to acetic acid’s irritating gastric mucosa and impeding gastric emptying. This did not affect doxycycline’s therapeutic levels (35). Gastric emptying was not measured (35). Acute intake of alcoholic beverages does not interfere with the PK of doxycycline to an extent that would affect its therapeutic levels.
(ii) Efficacy. Alcohol intake has been reported to diminish the antimicrobial effect of doxycycline. In a rat model, long-term alcohol ingestion (a 15-day liquid diet with alcohol accounting for ≥ 42.2% total calories) reduced the efficacy of doxycycline plus rifampin for the treatment of Brucella; the cure rate was 64.7% in alcohol-fed rats compared to 100% in controls (liquid diet without alcohol) (36).
(iii) Toxicity/ADR. To our knowledge, there are no data available on the toxicity/ADR of doxycycline.
Minocycline.
(i) PK/PD. Minocycline may affect alcohol intake. One study found that minocycline led to a modest reduction of alcohol intake in mice (37).
(ii) Efficacy. To our knowledge, there are no data available on the efficacy of minocycline.
(iii) Toxicity/ADR. Minocycline may attenuate alcohol-mediated toxicity in pregnant mice. Minocycline treatment in the third trimester protected against alcohol-induced neurotoxicity in the developing brain (38). No human data are available.
Summary of human data.
Consuming alcohol with tetracycline appears to increase the Cmax and AUC of tetracycline but does not affect its elimination, suggesting that tetracycline has greater absorption in the presence of alcohol, a potentially beneficial effect. While acute intake of alcohol is unlikely to impact therapeutic levels of doxycycline, alcoholics may have reduced doxycycline half-lives leading to subtherapeutic concentrations if dosed once daily.
NITROFURANTOIN
Nitrofurantoin is used to treat acute uncomplicated cystitis (39).
PK/PD and efficacy.
To our knowledge, there are no data available on the PK/PD or efficacy of nitrofurantoin.
Toxicity/ADR.
Historical studies have suggested that alcohol use with nitrofurantoin resulted in a disulfiram-like reaction (40–42). Newer reports have shown this reaction to be erroneous (43). Likewise, a study found that alcohol did not cause a disulfiram-like reaction with nitrofurantoin in volunteers (44).
Summary of human data.
Recent data do not support that nitrofurantoin and alcohol causes a disulfiram-like reaction or other adverse reactions/toxicities.
NITROIMIDAZOLES
Nitroimidazoles are used for parasitic or anaerobic infections (45). The most common example is metronidazole; however, tinidazole and secnidazole are also members of this class.
Metronidazole.
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of metronidazole.
(ii) Toxicity/ADR. The package labeling recommends against the use of metronidazole and alcohol within 48 h due to the risk of a disulfiram-like reaction (46). Although it is commonly believed that metronidazole mediates disulfiram reactions, data are contradictory. In 1964, a study stated that metronidazole may be effective for alcoholism based on 53 patients who had reduced desires to drink and lower tolerances and reported disulfiram-like reactions (47). Several early studies seemed to show benefit; however, these were uncontrolled, with limited patient numbers and follow-up (47–51). The majority of controlled studies failed to find benefit of metronidazole in the treatment of alcoholism (52–64). Multiple authors reported no disulfiram-like reactions (48, 52–54, 56, 57, 59, 61). Two authors observed higher rates of side effects in patients treated with metronidazole than with placebo (64, 65). Other authors described different degrees of reactions attributed to a disulfiram-like effect within the study populations (50, 58, 60, 62, 66).
Early in vitro studies suggested that metronidazole or its metabolites inhibited liver alcohol dehydrogenase (67–69). A more recent rat study found that metronidazole and alcohol increased intracolonic acetaldehyde levels, without altering blood levels (70). A double-blind, placebo-controlled study in 12 humans given oral metronidazole (200 mg) three times daily or matching placebo for 5 days prior to receipt of alcohol (0.4 g/kg) found no difference in aldehyde dehydrogenase levels between groups and no subjective symptoms of a disulfiram-like reaction (71). Alcohol and acetaldehyde levels were measured every 20 min over a 4-h period.
Tinidazole.
Package labeling recommends avoiding alcohol within 72 h of tinidazole due to its chemical similarity to metronidazole, but data to support this concern were not identified (72).
Secnidazole.
Per package labeling, alcohol did not affect alcohol dehydrogenase in in vitro studies and may be concurrently consumed with secnidazole (73).
Summary in humans.
Despite the widespread belief that metronidazole is contraindicated with alcohol consumption, the literature raises doubt. Interaction occurs with unclear frequency, and, when it occurs, it ensues with varying severity. The tinidazole package insert recommends avoiding alcohol. Secnidazole has no alcohol limitations.
SULFA ANTIBIOTICS
TMP-SMX.
Trimethoprim-sulfamethoxazole (TMP-SMX) is used for urinary tract infections and pneumocystis infections (74).
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of TMP-SMX.
(ii) Toxicity/ADR. Two young healthy hospital workers receiving 3 days of prophylactic TMP-SMX appeared to have a disulfiram-like reaction following alcohol consumption (75). One subject had recurrent symptoms with alcohol consumption on the following day, and the other had had multiple alcoholic beverages the previous day without incident.
First-generation sulfonylureas have been reported to cause facial flushing when administered with alcohol, via inhibition of acetaldehyde metabolism (76–79). As these compounds are chemically related, the occurrence of a disulfiram-like reaction with TMP-SMX seems mechanistically plausible.
(iii) Summary of human data. Adverse reaction/toxicity data are limited to possible disulfiram-like reactions in two individuals, but the reported reaction cannot be clearly attributed to the combination of TMP-SMX and alcohol.
OXAZOLIDINONES
The oxazolidinone class of antibiotics, including linezolid and tedizolid, are typically used in the treatment of resistant Gram-positive infections (80).
PK/PD and efficacy.
To our knowledge, there are no data available on the PK/PD or efficacy of oxazolidinone.
Toxicity/ADR.
Linezolid is a weak, nonspecific inhibitor of monoamine oxidase (MAO) enzymes (81). Studies have shown positive pressor responses in comparison with placebo with tyramine administration (81, 82). One patient developed heart block after taking linezolid and 7 mg of tyramine (81, 82). Per prescribing information, large quantities of beverages with a high tyramine content, including red wine and tap beers, should be avoided and limited to less than 100 mg of tyramine daily (83). Given linezolid’s weaker affinity and reversible MAO enzyme inhibition relative to that of other MAO inhibitors (MAOIs), other authors have investigated the need for a tyramine-restricted diet (81, 84). The pressor responses to an oral tyramine challenge were compared in subjects receiving linezolid or placebo (81). The smallest dose of tyramine resulting in a systolic blood pressure (SBP) increase of at least 30 mm Hg was 100 mg in the linezolid arm (81). Generally, the tyramine concentration in a high-tyramine meal ranged from 10 to 36 mg (85).
In vitro testing found that tedizolid reversibly inhibited MAO enzymes similarly to linezolid (80). A randomized, double-blind crossover trial of 30 healthy subjects assessed the pressor response to an oral tyramine challenge in patients treated with 200 mg of tedizolid. Seven subjects had a positive response of a 30 mm Hg increase in SBP during both placebo and treatment phases, suggesting that the result may have been related to other factors. The median tyramine dose required to produce a 30 mm Hg increase in SBP was 325 mg in the tedizolid group. Package labeling for tedizolid reflects no specific dietary limitations for tyramine-containing foods (86).
Summary of human data.
Alcohol may be safely consumed, in limited amounts, with linezolid. The amounts of tyramine in alcohol required to raise the SBP by 30 mm Hg, i.e., >100 mg for linezolid and >300 mg for tedizolid, exceed typical amounts present in a high-tyramine meal (10 to 36 mg) (85), 12 oz of tap beer (38 mg), bottled beer (1.5 mg), or wine (0.6 mg) (87).
AZOLES
Azoles are antifungals approved for the treatment of fungal infections (88).
PK/PD and efficacy.
To our knowledge, there are no data available on the PK/PD or efficacy of azoles.
Toxicity/ADR.
Case studies suggest that the concomitant use of ketoconazole and alcohol may precipitate a disulfiram-like reaction (89, 90). The NIH warned of nausea, vomiting, flushing, and liver damage when ketoconazole and alcohol are concomitantly used (4). In one study, of 12 patients receiving ketoconazole, one patient developed a disulfiram-like reaction believed to be due to alcohol consumption (91). Consumption of alcohol while on ketoconazole may also be associated with development of a rash—a woman developed a sunburn-like rash following social drinking on separate occasions throughout treatment with ketoconazole (90). The patient reported drinking a minimum of “part of a beer” and a maximum of 3 to 4 glasses of wine.
Although package labeling warns of potential hepatotoxicity from the use of fluconazole, an assertion not supported by the literature (89), there is no specific recommendation to avoid concomitant alcohol use (92). It should also be noted that the FDA and the NIH do not list fluconazole as having an interaction with alcohol (4, 5).
Summary of human data.
Concurrent use of alcohol precipitated disulfiram reactions in two patients treated with ketoconazole. Concurrent ketoconazole and alcohol consumption warrants caution. Alcohol may be consumed with fluconazole.
GRISEOFULVIN
Griseofulvin is an oral antifungal approved for the treatment of various ringworm infections due to Microsporum, Epidermophyton, and Trichophyton (93).
PK/PD and efficacy.
To our knowledge, there are no data available on the PK/PD or efficacy of griseofulvin.
Toxicity/ADR.
The use of alcohol with griseofulvin is not recommended by the National Consumers League, the FDA, or the NIH (4, 5). Package labeling warns that griseofulvin may potentiate the effects of alcohol, resulting in tachycardia and flushing (93). Disulfiram-like reactions have been reported in the literature (94–96). Reactions can range in severity; one patient required admission to the intensive care unit (97). This patient reportedly experienced symptoms following consumption of 500 mg of griseofulvin and a single can of beer.
Summary of human data.
Use of alcohol with griseofulvin should be avoided due to risk of a disulfiram-like reaction, which may be severe.
ANTIMYCOBACTERIALS
First-line treatment of pulmonary tuberculosis (TB) involves an initial phase of four agents (isoniazid, pyrazinamide, ethambutol, and rifampin) (98). Treatment is prolonged, with agents known to be hepatotoxic (98). Multidrug-resistant tuberculosis has necessitated the use of second-line agents, which can result in adverse neurological reactions, making concomitant use with alcohol undesirable (99).
Rifamycins.
Rifampin is part of first-line therapy for tuberculosis (98). Rifabutin and rifapentine are additional rifamycin agents that can be used in place of rifampin (100).
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of rifamycins.
(ii) Toxicity/ADR. A retrospective study of 531 patients treated for tuberculosis with rifampin and isoniazid found no difference in hepatotoxicity between alcoholics and nonalcoholics (101). This suggests that, in the absence of preexisting clinically significant derangement in liver function tests (LFTs), the use of rifampin and isoniazid is not contraindicated in alcoholics. In a retrospective review of 752 patients treated with either a three-drug (rifampin, isoniazid, and ethambutol) or four-drug (rifampin, isoniazid, ethambutol, and pyrazinamide) regimen, alcohol use was not associated with an increased risk of hepatotoxicity in 255 alcoholics (102). Further, alcohol was not significantly associated with an increased risk of hepatotoxicity in patients receiving a regimen consisting of isoniazid, rifampin, and pyrazinamide (103). In most cases of liver injury, including those with excessive alcohol consumption, therapy was able to be continued or successfully reintroduced. Cases of possible fatal and nonfatal hepatotoxicity have been reported, and the package insert recommends caution in patients with alcohol abuse (104).
(iii) Summary of human data. Rifamycins may cause hepatotoxicity; however, this does not preclude use with alcohol without preexisting LFT elevations when appropriate monitoring can be performed.
Isoniazid.
Isoniazid is used for the treatment of tuberculosis and nontuberculosis mycobacterial infections (98).
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of isoniazid.
(ii) Toxicity/ADR. A surveillance study of 13,838 patients on isoniazid by 21 health departments found that consuming at least one drink daily appeared to increase the risk of developing hepatitis (105). Probable isoniazid-induced hepatitis was twice as common in alcoholics than in nondrinkers and four times more likely if they consumed alcohol daily (105). Conversely, a smaller retrospective study of patients on isoniazid, rifampin, and pyrazinamide found that alcohol intake did not significantly impact hepatotoxicity (103).
Isoniazid is often listed as an agent that can cause a disulfiram-like reaction with alcohol due to its inhibition of aldehyde dehydrogenase (106–108). Symptoms include headache, palpitations, sweating, flushing, and hypotension (109, 110). It has also been postulated that such a reaction may be due to isoniazid’s inhibition of monoamine oxidase, as symptoms have been reported after consumption of wine (109). A study in rats suggested that the disulfiram-like reaction is not due to the inhibition of aldehyde dehydrogenase but rather the result of a biochemical or physiological change, including induction of cytochrome P450 2E1, hastening the elimination of acetaldehyde (111). Isoniazid was found to alter central monoaminergic neurotransmission (111). Although aldehyde dehydrogenase was inhibited with coadministration, blood acetaldehyde levels were not increased.
(iii) Summary of human data. Although data are not optimal, it is reasonable to advise avoidance of alcohol consumption in patients taking isoniazid. A disulfiram-like reaction and a possible increased risk of hepatitis are potential concerns.
Ethambutol.
Ethambutol is used in combination with other antimycobacterials as first-line therapy for treatment of tuberculosis (112).
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of ethambutol.
(ii) Toxicity/ADR. Although the FDA warns against the concomitant use of alcohol and antimycobacterials such as ethambutol, data are limited (5). One retrospective study of 752 patients evaluated a three-drug regimen (rifampin, isoniazid, and ethambutol) and a four-drug regimen (rifampin, isoniazid, ethambutol, and pyrazinamide) and found that consumption of alcohol was not associated with an increased risk of hepatotoxicity (102). Ethambutol has not been evaluated independently regarding its interaction with alcohol.
(iii) Summary of human data. Despite an FDA warning, we were unable to identify published data that demonstrate an increased risk with concomitant ethambutol and alcohol use. Caution and close monitoring may be warranted.
Ethionamide.
Ethionamide is used in the treatment of TB that is resistant to first-line agents (113).
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of ethionamide.
(ii) Toxicity/ADR. Ethionamide package labeling recommends against excessive alcohol consumption (113). Data to support this recommendation are limited. Ethionamide, often used in combination with pyrazinamide, is known to cause hepatotoxicity (113). An observational study of 55 alcoholics with TB found that mild liver impairment in alcoholics is not a contraindication for treatment with ethionamide (114). Of the 55 patients in that study, 30 received ethionamide and three patients developed parenchymal liver damage. Two of these cases may have been related to the concurrent use of pyrazinamide (114).
One case report details a severe psychiatric reaction requiring hospital admission in a patient with heavy alcohol consumption on combination therapy with isoniazid, streptomycin, and ethionamide (115). Ethionamide was deemed the likely pharmacologic culprit. Alcohol may have contributed, given the improvement following discontinuation of ethionamide and a 2-week cessation in alcohol intake.
(iii) Summary of human data. Historically, ethionamide was believed to cause hepatotoxicity with alcohol consumption. Though the literature is limited, mild liver disease and alcohol use are not an absolute contraindication, with appropriate monitoring.
Pyrazinamide.
Pyrazinamide is another agent used in combination therapy for the treatment of TB (116).
(i) PK/PD and efficacy. To our knowledge, there are no data available on the PK/PD or efficacy of pyrazinamide.
(ii) Toxicity/ADR. The risk of hepatotoxicity with the concomitant use of pyrazinamide and alcohol is controversial. Many believe that alcohol consumption increases the hepatotoxic effects of pyrazinamide (114). An observational study of 55 alcoholics receiving TB treatment found that most cases of hepatoxicity were associated with pyrazinamide (114). Lee et al., however, conducted a retrospective review of 148 patients treated with a 2-month regimen of rifampin and pyrazinamide for latent tuberculosis and concluded no hepatotoxicity risk with alcohol (117). In multivariate analysis, hepatotoxicity was associated with female gender and recent infection but not with alcohol use. Another retrospective study of patients receiving isoniazid, rifampin, and pyrazinamide found that alcohol intake did not significantly impact hepatotoxicity (103).
(iii) Summary of human data. The risk of hepatotoxicity with pyrazinamide is unclear. One study suggested an increased risk of hepatoxicity (114). However, others did not support this association (103, 117). Given the biologic plausibility, it would be prudent to avoid alcohol with pyrazinamide.
Cycloserine.
Cycloserine is a second-line agent in the treatment of tuberculosis and nontuberculosis mycobacterial diseases (98, 118).
(i) PK/PD. Studies suggest that cycloserine, specifically d-cycloserine, decreased cue-elicited craving for alcohol via enhancement of glutaminergic activity via N-methyl-d-aspartate (NDMA) receptors (119–121). The effect was lasting: participants reported a decrease in the number of drinks per day, percent drinking days, and percent heavy-drinking days (120). The results were statistically significant at the conclusion of the study but not at the 3-month follow-up.
(ii) Efficacy. To our knowledge, there are no data available on the efficacy of cycloserine.
(iii) Toxicity/ADR. Alcohol is contraindicated per the package labeling due to a speculative, but not established, increased risk of seizures (122).
(iv) Summary of human data. Cycloserine may decrease alcohol craving, and the package inset warns of seizures with concomitant alcohol use, but data to support this warning were not identified.
CONCLUSIONS
Antibiotics are among the most commonly prescribed medications in the outpatient setting (2). Warnings may vary depending on the pharmacy that dispense the prescription. Patients commonly are counseled or their prescription bottles carry a warning sticker to avoid consumption of alcohol while receiving antibiotics (1) (Table 1). However, warnings are pharmacy dependent. It is a common belief that concomitant use of alcohol with antibiotics either will cause toxicity/ADR or will decrease efficacy (6, 7). The evidence behind these beliefs is poor and controversial (Tables 2 to 4). The purpose of this review was to present the available literature on clinically significant interactions between oral antibiotics and alcohol to help guide prescribing and patient education in this area. A large number of knowledge gaps are also identified.
There are several limitations, primarily a lack of trials with high-quality evidence for many of the proposed interactions. Many of the studies were conducted in animals, or the literature was limited to case reports, making a specific attribution and generalizability difficult. Most of the studies were retrospective. In many studies, the amount of alcohol use was qualitative and self-reported and thus subject to recollection bias and an inability to determine a possible dose effect. Further, patients could have been concurrently consuming a multitude of drugs, which can confound hepatoxicity risk. Finally, many studies were conducted in alcoholics, leaving a gap of knowledge for the social drinker who may be taking antimicrobials. An awareness of these limitations can be used to inform future, higher-quality studies.
Despite these limitations, the findings of this review bring into question many of the conventionally accepted alcohol-antimicrobial interactions. While alcohol use should still be avoided with certain antimicrobials, use with select others appears to be acceptable.
ACKNOWLEDGMENTS
This review was unfunded. This material is the result of work supported with resources and the use of facilities at the Veterans Affairs Western New York Healthcare System. The contents of this manuscript are not intended to represent the views of the Department of Veterans Affairs or the United States government. We report no conflicts of interest.
REFERENCES
- 1.Lwanga J, Bingham JS, Bradbeer CS. 2008. Do antibiotics and alcohol mix? The beliefs of genitourinary clinic attendees. Br Med J 337:a2885. doi: 10.1136/bmj.a2885. [DOI] [Google Scholar]
- 2.Kantor ED, Rehm CD, Haas JS, Chan AT, Giovannucci EL. 2015. Trends in prescription drug use among adults in the United States from 1999–2012. JAMA 314:1818–1831. doi: 10.1001/jama.2015.13766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention. 2016. Outpatient antibiotic prescriptions—United States, 2016. Centers for Disease Control and Prevention, Atlanta, GA. [Google Scholar]
- 4.National Institute on Alcohol Abuse and Alcoholism. 2014. Harmful interactions mixing alcohol with medicines. NIH publication no. 13-5329. National Institute on Alcohol Abuse and Alcoholism, Bethesda, MD. [Google Scholar]
- 5.U.S. Food and Drug Administration. 2013. Avoid food-drug interactions. U.S. Food and Drug Administration, White Oak, MD.
- 6.Weathermon R, Crabb DW. 1999. Alcohol and medication interactions. Alcohol Res Health 23:40–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Moore AA, Whiteman EJ, Ward KT. 2007. Risks of combined alcohol-medication use in older adults. Am J Geriatr Pharmacother 5:64–74. doi: 10.1016/j.amjopharm.2007.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Barth KS, Malcolm RJ. 2010. Disulfiram: an old therapeutic with new applications. CNS Neurol Disord Drug Targets 9:5–12. doi: 10.2174/187152710790966678. [DOI] [PubMed] [Google Scholar]
- 9.Whittle J, Fine MJ, Joyce DZ, Lave JR, Young WW, Hough LJ, Kapoor WN. 1997. Community-acquired pneumonia: can it be defined with claims data? Am J Med Qual 12:187–193. doi: 10.1177/0885713X9701200404. [DOI] [PubMed] [Google Scholar]
- 10.Lindberg RL, Huupponen RK, Viljanen S, Pihlajamaki KK. 1987. Ethanol and the absorption of oral penicillin in man. Int J Clin Pharmacol Ther Toxicol 25:536–538. [PubMed] [Google Scholar]
- 11.Morasso MI, Hip A, Marquez M, Gonzalez C, Arancibia A. 1988. Amoxicillin kinetics and ethanol ingestion. Int J Clin Pharmacol Ther Toxicol 26:428–431. [PubMed] [Google Scholar]
- 12.Goodwani S, Rao PS, Bell RL, Sari Y. 2015. Amoxicillin and amoxicillin/clavulanate reduce ethanol intake and increase GLT-1 expression as well as AKT phosphorylation in mesocorticolimbic regions. Brain Res 1622:397–408. doi: 10.1016/j.brainres.2015.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Preheim LC, Olsen KM, Yue M, Snitily MU, Gentry MJ. 1999. Ethanol feeding does not affect the efficacy or pharmacokinetics of azithromycin, trovafloxacin, or ceftriaxone in a rat model of pneumococcal pneumonia. Alcohol Clin Exp Res 23:842–849. doi: 10.1111/j.1530-0277.1999.tb04192.x. [DOI] [PubMed] [Google Scholar]
- 14.Barrio Lera JP, Alvarez AI, Prieto JG. 1991. Effects of ethanol on the pharmacokinetics of cephalexin and cefadroxil in the rat. J Pharm Sci 80:511–516. doi: 10.1002/jps.2600800602. [DOI] [PubMed] [Google Scholar]
- 15.Dong H, Zhang J, Ren L, Liu Q, Zhu S. 2013. Unexpected death due to cefuroxime-induced disulfiram-like reaction. Indian J Pharmacol 45:399–400. doi: 10.4103/0253-7613.114991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kline SS, Mauro VF, Forney RB Jr, Freimer EH, Somani P. 1987. Cefotetan-induced disulfiram-type reactions and hypoprothrombinemia. Antimicrob Agents Chemother 31:1328–1331. doi: 10.1128/aac.31.9.1328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Reeves DS, Davies AJ. 1980. Antabuse effect with cephalosporins. Lancet 2:540. doi: 10.1016/s0140-6736(80)91868-1. [DOI] [PubMed] [Google Scholar]
- 18.Ren S, Cao Y, Zhang X, Jiao S, Qian S, Liu P. 2014. Cephalosporin induced disulfiram-like reaction: a retrospective review of 78 cases. Int Surg 99:142–146. doi: 10.9738/INTSURG-D-13-00086.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Small SM, Bacher RS, Jost SA. 2018. Disulfiram-like reaction involving ceftriaxone in a pediatric patient. J Pediatr Pharmacol Ther 23:168–171. doi: 10.5863/1551-6776-23.2.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Uri JV, Parks DB. 1983. Disulfiram-like reaction to certain cephalosporins. Ther Drug Monit 5:219–224. doi: 10.1097/00007691-198306000-00013. [DOI] [PubMed] [Google Scholar]
- 21.Billstein SA, Sudol TE. 1992. Disulfiram-like reactions rare with ceftriaxone. Geriatrics 47:70. [PubMed] [Google Scholar]
- 22.Moskovitz BL. 1984. Clinical adverse effects during ceftriaxone therapy. Am J Med 77:84–88. [PubMed] [Google Scholar]
- 23.Emmerson AM, Jones AM. 2003. The quinolones: decades of development and use. J Antimicrob Chemother 51(Suppl 1):13–20. doi: 10.1093/jac/dkg208. [DOI] [PubMed] [Google Scholar]
- 24.Olsen KM, Gentry-Nielsen M, Yue M, Snitily MU, Preheim LC. 2006. Effect of ethanol on fluoroquinolone efficacy in a rat model of pneumococcal pneumonia. Antimicrob Agents Chemother 50:210–219. doi: 10.1128/AAC.50.1.210-219.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lagoudianakis E, Pappas A, Koronakis N, Dallianoudis I, Kotzadimitriou K, Chrysikos J, Koukoutsis I, Antonakis P, Keramidaris D, Manouras A. 2009. Recurrent erythema multiforme after alcohol ingestion in a patient receiving ciprofloxacin: a case report. Cases J 2:7787. doi: 10.4076/1757-1626-2-7787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rapp RP, McCraney SA, Goodman NL, Shaddick DJ. 1994. New macrolide antibiotics: usefulness in infections caused by mycobacteria other than Mycobacterium tuberculosis. Ann Pharmacother 28:1255–1263. doi: 10.1177/106002809402801109. [DOI] [PubMed] [Google Scholar]
- 27.Mandell LA, Wunderink RG, Anzueto A, Bartlett JG, Campbell GD, Dean NC, Dowell SF, File TM Jr, Musher DM, Niederman MS, Torres A, Whitney CG, Infectious Diseases Society of America, American Thoracic Society. 2007. Infectious Diseases Society of America/American Thoracic Society consensus guidelines on the management of community-acquired pneumonia in adults. Clin Infect Dis 44(Suppl 2):S27–S72. doi: 10.1086/511159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sturgill MG, Rapp RP. 1992. Clarithromycin: review of a new macrolide antibiotic with improved microbiologic spectrum and favorable pharmacokinetic and adverse effect profiles. Ann Pharmacother 26:1099–1108. doi: 10.1177/106002809202600912. [DOI] [PubMed] [Google Scholar]
- 29.Morasso MI, Chavez J, Gai MN, Arancibia A. 1990. Influence of alcohol consumption on erythromycin ethylsuccinate kinetics. Int J Clin Pharmacol Ther Toxicol 28:426–429. [PubMed] [Google Scholar]
- 30.Edelbroek MA, Horowitz M, Wishart JM, Akkermans LM. 1993. Effects of erythromycin on gastric emptying, alcohol absorption and small intestinal transit in normal subjects. J Nucl Med 34:582–588. [PubMed] [Google Scholar]
- 31.Min DI, Noormohamed SE, Flanigan MJ. 1995. Effect of erythromycin on ethanol’s pharmacokinetics and perception of intoxication. Pharmacotherapy 15:164–169. [PubMed] [Google Scholar]
- 32.Lim WS, Macfarlane JT, Colthorpe CL. 2001. Pneumonia and pregnancy. Thorax 56:398–405. doi: 10.1136/thorax.56.5.398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seitz C, Garcia P, Arancibia A. 1995. Influence of ethanol ingestion on tetracycline kinetics. Int J Clin Pharmacol Ther 33:462–464. [PubMed] [Google Scholar]
- 34.Neuvonen PJ, Penttila O, Roos M, Tirkkonen J. 1976. Effect of long-term alcohol consumption on the half-life of tetracycline and doxycycline in man. Int J Clin Pharmacol Biopharm 14:303–307. [PubMed] [Google Scholar]
- 35.Mattila MJ, Laisi U, Linnoila M, Salonen R. 1982. Effect of alcoholic beverages on the pharmacokinetics of doxycycline in man. Acta Pharmacol Toxicol (Copenh) 50:370–373. doi: 10.1111/j.1600-0773.1982.tb00989.x. [DOI] [PubMed] [Google Scholar]
- 36.Yumuk Z, Dundar V. 2005. The effect of long-term ethanol feeding on efficacy of doxycycline plus rifampicin in the treatment of experimental brucellosis caused by Brucella melitensis in rats. J Chemother 17:509–513. doi: 10.1179/joc.2005.17.5.509. [DOI] [PubMed] [Google Scholar]
- 37.Agrawal RG, Hewetson A, George CM, Syapin PJ, Bergeson SE. 2011. Minocycline reduces ethanol drinking. Brain Behav Immun 25(Suppl 1):S165–S169. doi: 10.1016/j.bbi.2011.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wang X, Zhang K, Yang F, Ren Z, Xu M, Frank JA, Ke ZJ, Luo J. 2018. Minocycline protects developing brain against ethanol-induced damage. Neuropharmacology 129:84–99. doi: 10.1016/j.neuropharm.2017.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Procter and Gamble Pharmaceuticals. 2009. Macrobid-nitrofurantoin package insert. https://wwwaccessdatafdagov/drugsatfda_docs/label/2009/020064s019lblpdf.
- 40.Azarnoff DL, Hurwitz A. 1970. Drug interactions. Pharmacol Physicians 4:1–7. [PubMed] [Google Scholar]
- 41.Petrie JDD, Howie J. 1975. Drug interaction in general practice In Cluff LE, Petrie JC (ed), Clinical effects of interaction between drugs. Excerpta Medica, Amsterdam, Netherlands. [Google Scholar]
- 42.D’arcy PM. 1980. Food and drug interaction: influence of food on drug bioavailability and toxicity. Pharm Int I:238–244. [Google Scholar]
- 43.Rowles B, Worthen DB. 1982. Clinical drug information: a case of misinformation. N Engl J Med 306:113–114. [PubMed] [Google Scholar]
- 44.Miura K, Reckendorf HK. 1967. The nitrofurans. Prog Med Chem 5:320–381. doi: 10.1016/s0079-6468(08)70446-6. [DOI] [PubMed] [Google Scholar]
- 45.Edwards DI. 1980. Mechanisms of selective toxicity of metronidazole and other nitroimidazole drugs. Br J Vener Dis 56:285–290. doi: 10.1136/sti.56.5.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Pfizer. January 2018. Flagyl (metronidazole) (prescribing information). Pfizer, New York, NY. [Google Scholar]
- 47.Taylor JA. 1964. Metronidazole—a new agent for combined somatic and psychic therapy of alcoholism. a case study and preliminary report. Bull Los Angel Neuro Soc 29:158–162. [PubMed] [Google Scholar]
- 48.Semer JM, Friedland P, Vaisberg M, Greenberg A. 1966. The use of metronidazole in the treatment of alcoholism: a pilot study. Am J Psychiatry 123:722–724. doi: 10.1176/ajp.123.6.722. [DOI] [PubMed] [Google Scholar]
- 49.Friedland P, Vaisberg M. 1968. The use of metronidazole in the treatment of alchoholism. (A further study). Dis Nerv Syst 29:326–327. [PubMed] [Google Scholar]
- 50.Sansoy OM. 1970. Evaluation of metronidazole in the treatment of alcoholism. A comprehensive three-year study comprising 60 cases. Rocky Mt Med J 67:43–47. [PubMed] [Google Scholar]
- 51.Lehmann HE, Ban TA. 1967. Chemical reduction of the compulsion to drink with metronidazole: a new treatment modality in the therapeutic program of the alcoholic. Curr Ther Res Clin Exp 9:419–428. [PubMed] [Google Scholar]
- 52.Egan WP, Goetz R. 1968. Effect of metronidazole on drinking by alcoholics. Q J Stud Alcohol 29:899–902. [PubMed] [Google Scholar]
- 53.Gallant DM, Bishop MP, Camp E, Tisdale C. 1968. A six-month controlled evaluation of metronidazole (Flagyl) in chronic alcoholic patients. Curr Ther Res Clin Exp 10:82–87. [PubMed] [Google Scholar]
- 54.Gelder MG, Edwards G. 1968. Metronidazole in the treatment of alcohol addiction. A controlled trial. Br J Psychiatry 114:473–475. doi: 10.1192/bjp.114.509.473. [DOI] [PubMed] [Google Scholar]
- 55.Goodwin DW. 1967. Metronidazole in the treatment of alcoholism: a negative report. Am J Psychiatry 123:1276–1278. doi: 10.1176/ajp.123.10.1276. [DOI] [PubMed] [Google Scholar]
- 56.Goodwin DW, Reinhard J. 1972. Disulfiramlike effects of trichomonacidal drugs. A review and double-blind study. Q J Stud Alcohol 33:734–740. [PubMed] [Google Scholar]
- 57.Kaplan R, Blume S, Rosenberg S, Pitrelli J, Turner WJ. 1972. Phenytoin, metronidazole and multivitamins in the treatment of alcoholism. Q J Stud Alcohol 33:94–104. [PubMed] [Google Scholar]
- 58.Lal S. 1969. Metronidazole in the treatment of alcoholism. A clinical trial and review of the literature. Q J Stud Alcohol 30:140–151. [PubMed] [Google Scholar]
- 59.Linton PH, Hain JD. 1967. Metronidazole in the treatment of alcoholism. Q J Stud Alcohol 28:544–546. [PubMed] [Google Scholar]
- 60.Lysloff GO. 1972. Anti-addictive chemotherapy—metronidazole and alcohol aversion. Br J Addict Alcohol Other Drugs 67:239–244. doi: 10.1111/j.1360-0443.1972.tb01202.x. [DOI] [PubMed] [Google Scholar]
- 61.Merry J, Whitehead A. 1968. Metronidazole and alcoholism. Br J Psychiatry 114:859–861. doi: 10.1192/bjp.114.512.859. [DOI] [PubMed] [Google Scholar]
- 62.Penick SB, Carrier RN, Sheldon JB. 1969. Metronidazole in the treatment of alcoholism. Am J Psychiatry 125:1063–1066. doi: 10.1176/ajp.125.8.1063. [DOI] [PubMed] [Google Scholar]
- 63.Penick SB, Sheldon JB, Templer DI, Carrier RN. 1971. Four year follow-up of metronidazole treatment program for alcoholism. IMS Ind Med Surg 40:30–32. [PubMed] [Google Scholar]
- 64.Strassman HD, Adams B, Pearson AW. 1970. Metronidazole effect on social drinkers. Q J Stud Alcohol 31:394–398. [PubMed] [Google Scholar]
- 65.Seinson RP. 1971. Long term trial of metronidazole in male alcoholics. Br J Psychiatry 119:85–89. doi: 10.1192/bjp.119.548.85. [DOI] [PubMed] [Google Scholar]
- 66.Tyndel M, Fraser JG, Hartleib CJ. 1969. Metronidazole as an adjuvant in the treatment of alcoholism. Br J Addict Alcohol Other Drugs 64:57–61. doi: 10.1111/j.1360-0443.1969.tb01109.x. [DOI] [PubMed] [Google Scholar]
- 67.Edwards JA, Price J. 1967. Metronidazole and human alcohol dehydrogenase. Nature 214:190–191. doi: 10.1038/214190b0. [DOI] [PubMed] [Google Scholar]
- 68.Fried R, Fried LW. 1968. Inhibition of oxidizing enzymes by metronidazole. Experientia 24:56–57. doi: 10.1007/bf02136791. [DOI] [PubMed] [Google Scholar]
- 69.Gupta NK, Woodley CL, Fried R. 1970. Effect of metronidazole on liver alcohol dehydrogenase. Biochem Pharmacol 19:2805–2808. doi: 10.1016/0006-2952(70)90108-5. [DOI] [PubMed] [Google Scholar]
- 70.Tillonen J, Vakevainen S, Salaspuro V, Zhang Y, Rautio M, Jousimies-Somer H, Lindros K, Salaspuro M. 2000. Metronidazole increases intracolonic but not peripheral blood acetaldehyde in chronic ethanol-treated rats. Alcoholism Clin Exp Res 24:570–575. doi: 10.1111/j.1530-0277.2000.tb02026.x. [DOI] [PubMed] [Google Scholar]
- 71.Visapaa JP, Tillonen JS, Kaihovaara PS, Salaspuro MP. 2002. Lack of disulfiram-like reaction with metronidazole and ethanol. Ann Pharmacother 36:971–974. doi: 10.1345/aph.1A066. [DOI] [PubMed] [Google Scholar]
- 72.Mission Pharmacal Company. August 2007. Tindamax (tinidazole) (prescribing information). Mission Pharmacal Company, San Antonio, TX. [Google Scholar]
- 73.Lupin Pharmaceuticals. October 2017. Solosec (secnidazole) (prescribing information). Lupin Pharmaceuticals, Baltimore, MD. [Google Scholar]
- 74.Hoffmann-La Roche, Inc. June 2013. Bactrim (sulfamethoxazole and trimethoprim) (prescribing information). Hoffmann-La Roche, Inc., Philadelphia, PA: https://www.accessdata.fda.gov/drugsatfda_docs/label/2013/017377s068s073lbl.pdf. [Google Scholar]
- 75.Heelon MW, White M. 1998. Disulfiram-cotrimoxazole reaction. Pharmacotherapy 18:869–870. [PubMed] [Google Scholar]
- 76.Leslie RD, Pyke DA. 1978. Chlorpropamide-alcohol flushing: a dominantly inherited trait associated with diabetes. Br Med J 2:1519–1521. doi: 10.1136/bmj.2.6151.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hillson RM, Hockaday TD. 1984. Chlorpropamide-alcohol flush: a critical reappraisal. Diabetologia 26:6–11. doi: 10.1007/bf00252254. [DOI] [PubMed] [Google Scholar]
- 78.Groop L, Eriksson CJ, Huupponen R, Ylikahri R, Pelkonen R. 1984. Roles of chlorpropamide, alcohol and acetaldehyde in determining the chlorpropamide-alcohol flush. Diabetologia 26:34–38. doi: 10.1007/bf00252260. [DOI] [PubMed] [Google Scholar]
- 79.Carulli N, Manenti F, Gallo M, Salvioli GF. 1971. Alcohol-drugs interaction in man: alcohol and tolbutamide. Eur J Clin Invest 1:421–424. doi: 10.1111/j.1365-2362.1971.tb00552.x. [DOI] [PubMed] [Google Scholar]
- 80.Flanagan S, Bartizal K, Minassian SL, Fang E, Prokocimer P. 2013. In vitro, in vivo, and clinical studies of tedizolid to assess the potential for peripheral or central monoamine oxidase interactions. Antimicrob Agents Chemother 57:3060–3066. doi: 10.1128/AAC.00431-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Antal EJ, Hendershot PE, Batts DH, Sheu WP, Hopkins NK, Donaldson KM. 2001. Linezolid, a novel oxazolidinone antibiotic: assessment of monoamine oxidase inhibition using pressor response to oral tyramine. J Clin Pharmacol 41:552–562. doi: 10.1177/00912700122010294. [DOI] [PubMed] [Google Scholar]
- 82.Cantarini MV, Painter CJ, Gilmore EM, Bolger C, Watkins CL, Hughes AM. 2004. Effect of oral linezolid on the pressor response to intravenous tyramine. Br J Clin Pharmacol 58:470–475. doi: 10.1111/j.1365-2125.2004.02186.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Pharmacia and Upjohn. July 2018. Zyvox (linezolid) (prescribing information). Pharmacia and Upjohn, New York, NY. [Google Scholar]
- 84.Rumore MM, Roth M, Orfanos A. 2010. Dietary tyramine restriction for hospitalized patients on linezolid: an update. Nutr Clin Pract 25:265–269. doi: 10.1177/0884533610368711. [DOI] [PubMed] [Google Scholar]
- 85.Da Prada M, Zurcher G, Wuthrich I, Haefely WE. 1988. On tyramine, food, beverages and the reversible MAO inhibitor moclobemide. J Neural Transm Suppl 26:31–56. [PubMed] [Google Scholar]
- 86.Merck. October 2016. Sivextro (tedizolid) (prescribing information). Merck, Whitehouse Station, NJ. [Google Scholar]
- 87.Holden K. 2006. Meal ideas and menus: avoiding high-tyramine foods made easy. Teva Neuroscience, Inc, Kansas City, MO: https://www.mc.vanderbilt.edu/documents/neurology/files/Tyramine%20Menu%20Book%2006227101.pdf. [Google Scholar]
- 88.Albengres E, Le Louet H, Tillement JP. 1998. Systemic antifungal agents. Drug interactions of clinical significance. Drug Saf 18:83–97. doi: 10.2165/00002018-199818020-00001. [DOI] [PubMed] [Google Scholar]
- 89.Katz HI, Gupta AK. 1997. Oral antifungal drug interactions. Dermatol Clin 15:535–544. doi: 10.1016/s0733-8635(05)70460-5. [DOI] [PubMed] [Google Scholar]
- 90.Magnasco AJ, Magnasco LD. 1986. Interaction of ketoconazole and ethanol. Clin Pharm 5:522–523. [PubMed] [Google Scholar]
- 91.Fazio RA, Wickremesinghe PC, Arsura EL. 1983. Ketoconazole treatment of Candida esophagitis—a prospective study of 12 cases. Am J Gastroenterol 78:261–264. [PubMed] [Google Scholar]
- 92.Pfizer. 2010. Fluconazole (prescribing information). Pfizer, New York, NY. [Google Scholar]
- 93.Ortho Dermatologics. 2011. Grifulvin V (prescribing information). https://www.accessdata.fda.gov/drugsatfda_docs/label/2014/062279s022lbl.pdf.
- 94.Gilman AR, Nies A. 1990. Goodman and Gilman’s the pharmacological basis of therapeutics, vol 8 Pergamon Press, New York, NY. [Google Scholar]
- 95.Robinson MM. 1959. Griseofulvin therapy of superficial mycoses. Antibiot Annu 7:680–686. [PubMed] [Google Scholar]
- 96.Simon HR. 1961. Untoward reactions to antimicrobial agents. Griseofulvin. Annu Rev Med 12:119–120. [Google Scholar]
- 97.Fett DL, Vukov LF. 1994. An unusual case of severe griseofulvin-alcohol interaction. Ann Emerg Med 24:95–97. doi: 10.1016/s0196-0644(94)70167-9. [DOI] [PubMed] [Google Scholar]
- 98.Nahid P, Dorman SE, Alipanah N, Barry PM, Brozek JL, Cattamanchi A, Chaisson LH, Chaisson RE, Daley CL, Grzemska M, Higashi JM, Ho CS, Hopewell PC, Keshavjee SA, Lienhardt C, Menzies R, Merrifield C, Narita M, O’Brien R, Peloquin CA, Raftery A, Saukkonen J, Schaaf HS, Sotgiu G, Starke JR, Migliori GB, Vernon A. 2016. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America clinical practice guidelines: treatment of drug-susceptible tuberculosis. Clin Infect Dis 63:e147–e195. doi: 10.1093/cid/ciw376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Yew WW, Wong CF, Wong PC, Lee J, Chau CH. 1993. Adverse neurological reactions in patients with multidrug-resistant pulmonary tuberculosis after coadministration of cycloserine and ofloxacin. Clin Infect Dis 17:288–289. doi: 10.1093/clinids/17.2.288. [DOI] [PubMed] [Google Scholar]
- 100.Rothstein DM. 2016. Rifamycins, alone and in combination. Cold Spring Harb Perspect Med 6:a027011. doi: 10.1101/cshperspect.a027011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Cross FS, Long MW, Banner AS, Snider DE Jr. 1980. Rifampin-isoniazid therapy of alcoholic and nonalcoholic tuberculous patients in a U.S. Public Health Service Cooperative Therapy Trial. Am Rev Respir Dis 122:349–353. [DOI] [PubMed] [Google Scholar]
- 102.Dossing M, Wilcke JT, Askgaard DS, Nybo B. 1996. Liver injury during antituberculosis treatment: an 11-year study. Tuber Lung Dis 77:335–340. doi: 10.1016/s0962-8479(96)90098-2. [DOI] [PubMed] [Google Scholar]
- 103.Bouazzi OE, Hammi S, Bourkadi JE, Tebaa A, Tanani DS, Soulaymani-Bencheikh R, Badrane N, Bengueddour R. 2016. First line anti-tuberculosis induced hepatotoxicity: incidence and risk factors. Pan Afr Med J 25:167. doi: 10.11604/pamj.2016.25.167.10060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Sanofi-Aventis. 2019. Rifadin (rifampin) (prescribing information). Sanofi-Aventis, Bridgewater, NJ: http://products.sanofi.us/rifadin/Rifadin.pdf. [Google Scholar]
- 105.Kopanoff DE, Snider DE Jr, Caras GJ. 1978. Isoniazid-related hepatitis: a U.S. Public Health Service cooperative surveillance study. Am Rev Respir Dis 117:991–1001. [DOI] [PubMed] [Google Scholar]
- 106.Vasiliou V, Malamas M, Marselos M. 1986. The mechanism of alcohol intolerance produced by various therapeutic agents. Acta Pharmacol Toxicol (Copenh) 58:305–310. doi: 10.1111/j.1600-0773.1986.tb00114.x. [DOI] [PubMed] [Google Scholar]
- 107.Baciewicz AM, Self TH. 1985. Isoniazid interactions. South Med J 78:714–718. doi: 10.1097/00007611-198506000-00025. [DOI] [PubMed] [Google Scholar]
- 108.Noureldin M, Kj JL, Tran M. 2010. Drug-alcohol interactions: a review of three therapeutic classes. US Pharmacist 35:29–33. [Google Scholar]
- 109.Hauser MJ, Baier H. 1982. Interactions of isoniazid with foods. Drug Intell Clin Pharm 16:617–618. doi: 10.1177/106002808201600718. [DOI] [PubMed] [Google Scholar]
- 110.Kaneko T, Ishigatsubo Y. 2005. Isoniazid and food interactions: –fish, cheese, and wine. Intern Med 44:1120–1121. doi: 10.2169/internalmedicine.44.1120. [DOI] [PubMed] [Google Scholar]
- 111.Karamanakos PN, Pappas P, Boumba V, Vougiouklakis T, Marselos M. 2016. The alcohol intolerance produced by isoniazid is not due to a disulfiram-like reaction despite aldehyde dehydrogenase inhibition. Pharmacology 98:267–271. doi: 10.1159/000448759. [DOI] [PubMed] [Google Scholar]
- 112.Patheon Inc. January 2007. Myambutol (ethambutol) (prescribing information). Patheon Inc, Toronto, Canada: https://www.accessdata.fda.gov/drugsatfda_docs/label/2008/016320s063lbl.pdf. [Google Scholar]
- 113.Wyeth Pharmaceuticals. April 2018. Trecator (ethionamide) (prescribing information). Wyeth Pharmaceuticals Inc, Philadelphia, PA: https://www.pfizermedicalinformation.com/en-us/trecator. [Google Scholar]
- 114.Gryminski J, Lyczewska J, Styszewska H, Walczak J. 1970. Evaluation of the hepatotoxic effect of antituberculous drugs in tuberculosis patients abusing alcohol. Pol Med J 9:635–644. [PubMed] [Google Scholar]
- 115.Lansdown FS, Beran M, Litwak T. 1967. Psychotoxic reaction during ethionamide therapy. Am Rev Respir Dis 95:1053–1055. doi: 10.1164/arrd.1967.95.6.1053. [DOI] [PubMed] [Google Scholar]
- 116.Par Pharmaceutical. July 2018. Pyrazinamide (prescribing information). Par Pharmaceutical, Columbus, Ohio. [Google Scholar]
- 117.Lee AM, Mennone JZ, Jones RC, Paul WS. 2002. Risk factors for hepatotoxicity associated with rifampin and pyrazinamide for the treatment of latent tuberculosis infection: experience from three public health tuberculosis clinics. Int J Tuber Lung Dis 6:995–1000. [PubMed] [Google Scholar]
- 118.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, Iseman M, Olivier K, Ruoss S, von Reyn CF, Wallace RJ Jr, Winthrop K, Subcommittee A, American Thoracic Society, Infectious Disease Society of America. 2007. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 119.Kiefer F, Kirsch M, Bach P, Hoffmann S, Reinhard I, Jorde A, von der Goltz C, Spanagel R, Mann K, Loeber S, Vollstadt-Klein S. 2015. Effects of d-cycloserine on extinction of mesolimbic cue reactivity in alcoholism: a randomized placebo-controlled trial. Psychopharmacology (Berl) 232:2353–2362. doi: 10.1007/s00213-015-3882-5. [DOI] [PubMed] [Google Scholar]
- 120.MacKillop J, Few LR, Stojek MK, Murphy CM, Malutinok SF, Johnson FT, Hofmann SG, McGeary JE, Swift RM, Monti PM. 2015. d-Cycloserine to enhance extinction of cue-elicited craving for alcohol: a translational approach. Transl Psychiatry 5:e544. doi: 10.1038/tp.2015.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kamboj SK, Massey-Chase R, Rodney L, Das R, Almahdi B, Curran HV, Morgan CJ. 2011. Changes in cue reactivity and attentional bias following experimental cue exposure and response prevention: a laboratory study of the effects of d-cycloserine in heavy drinkers. Psychopharmacology (Berl) 217:25–37. doi: 10.1007/s00213-011-2254-z. [DOI] [PubMed] [Google Scholar]
- 122.Lilly. April 2005. Seromycin (cycloserine) (prescribing information). Lilly, Indianapolis, IN. [Google Scholar]
- 123.Valeant Pharmaceuticals. April 2016. Gris-PEG (griseofluvin) (prescribing information). Valeant Pharmaceuticals, Bridgewater, NJ. [Google Scholar]
- 124.Epic Pharma. 2016. Isoniazid prescribing information. Epic Pharma, Laurelton, NY. [Google Scholar]