Higher chloroquine doses can effectively treat up to 93 to 96% of malaria infections caused by Plasmodium falciparum carrying the resistance-conferring chloroquine resistance transporter (pfcrt) 76T allele. The tolerability of 50 (double the standard dose) and 70 mg/kg total chloroquine doses were assessed in this study. Fifteen 4- to 8-year-old children with uncomplicated malaria were given 10 mg/kg of chloroquine twice daily for 2 days and 5 mg/kg twice daily on the third day.
KEYWORDS: Guinea-Bissau, Plasmodium falciparum, cardiac safety, chloroquine, pfcrt, pharmacokinetics, tolerability, treatment
ABSTRACT
Higher chloroquine doses can effectively treat up to 93 to 96% of malaria infections caused by Plasmodium falciparum carrying the resistance-conferring chloroquine resistance transporter (pfcrt) 76T allele. The tolerability of 50 (double the standard dose) and 70 mg/kg total chloroquine doses were assessed in this study. Fifteen 4- to 8-year-old children with uncomplicated malaria were given 10 mg/kg of chloroquine twice daily for 2 days and 5 mg/kg twice daily on the third day. Fifteen additional children were given 5 mg/kg twice daily for 2 more days. Chloroquine concentrations, blood pressure, electrocardiograms (ECGs), parasite density, and adverse events were assessed until day 28. Both dosages were well tolerated, and symptoms resolved by day 3 in parallel with increasing chloroquine concentrations. The median corrected QT (QTc) interval was 12 to 26 ms higher at expected peak concentrations than at day 0 (P < 0.001). Pfcrt 76T was associated with delayed parasite clearance. Day 28 clinical and parasitological responses against P. falciparum with pfcrt 76T were 57% (4/7) and 67% (4/6) after treatment with 50 and 70 mg/kg, respectively. Dosages were well tolerated, and no severe cardiac adverse events occurred. The QTc interval increase was similar to that found in adults taking 25 mg/kg of chloroquine. (This study has been registered at ClinicalTrials.gov under identifier NCT01814423.)
INTRODUCTION
The recommended treatment for uncomplicated Plasmodium falciparum malaria is artemisinin-based combination therapy (1). New treatment options are needed due to the development of resistance (2). In vivo studies have shown that the response to chloroquine is concentration dependent and that chloroquine can achieve 93 to 96% treatment success against chloroquine-resistant P. falciparum (3, 4). Supporting in vitro data show that the efflux pump causing chloroquine resistance can be saturated (5, 6). However, the chloroquine concentration needed to eliminate chloroquine-resistant P. falciparum, the duration it needs to be maintained, and the possible toxicity of these concentrations are unknown.
Acute chloroquine toxicity is most likely associated with high peak concentrations, and splitting larger total doses into several smaller doses appears to be safe (3, 7). Four previous randomized clinical trials found that double-standard-dose chloroquine divided into two daily doses for 3 days was well tolerated in 536 children aged <15 years (3, 8–10). Furthermore, a triple standard dose of chloroquine split into 2 to 3 smaller daily doses for 5 days was well tolerated when routinely used for decades in Guinea-Bissau (7). Nevertheless, the potential toxicity of higher chloroquine doses needs to be studied. Of particular concern are the potential risk of cardiac arrhythmias due to prolongation of cardiac repolarization times (QT interval) and hypotension due to arteriolar and venodilation (11). This study assessed the safety and tolerability (including electrocardiogram [ECG] changes and blood pressure) of double and nearly triple the total standard dose of chloroquine used for treatment of children with uncomplicated P. falciparum malaria. Detailed pharmacokinetic and efficacy data will be presented in a subsequent publication.
RESULTS
Fifteen children were included in each study arm and followed until day 28. Median age, body weight, hemoglobin concentration, axillary temperature, parasite density, and sex distribution upon inclusion are shown in Table 1 . In addition, one 8-year-old child entered the study but was withdrawn 1.5 h after the first dose due to convulsions. The child was given rescue treatment and recovered, and data were not used in any analyses.
TABLE 1.
Baseline characteristics of study population
| Characteristic | Data for group given a chloroquine dose of: |
|
|---|---|---|
| 50 mg | 70 mg | |
| Number | 15 | 15 |
| Ratio, male to female | 6:9 | 8:7 |
| Age (years) (median [IQR]) | 6 (5–7) | 6 (5–8) |
| Body weight (kg) (median [IQR]) | 17 (15–20) | 17 (16–22) |
| P. falciparum (count/μl) (median [IQR]) | 19,800 (1,120–71,429) | 21,739 (1,760–85,106) |
| Hemoglobin (g/dl) (median [IQR]) | 112 (108–116) | 112 (100–130) |
| Temperature (°C) (median [IQR]) | 38 (37.4–39.1) | 38 (36.5–39.4) |
Tolerability and total doses administered.
Chloroquine doses were repeated due to vomiting after dose 1, 2, 3, and 4 in 3% (1/30), 10% (3/30), 7% (2/30), and 3% (1/30) of children, respectively. Chloroquine concentrations measured at the time of the dose given after the dosing when vomiting occurred (i.e., 8 to 15 h later) were within or very similar to the 95% confidence intervals (CIs) of the median in children who did not vomit, with one exception in which a child had a high, 6,099-nmol/liter concentration compared to the median of 3,375 (95% CI, 2,850 to 3,900) nmol/liter. Prior to study entry, 60% (18/30) of children vomited at home. Seventeen percent (5/30), 40% (12/30), and 27% (8/12) of children vomited at home between doses 1 to 2, 2 to 3, and 3 to 4, respectively. There was no significant difference between treatment arms. Vomiting did not occur after the fourth dose in either treatment arm.
The median (range) total chloroquine doses administered were 50 (50 to 53) mg/kg base and 70 (69 to 71) mg/kg base. In addition to chloroquine, 20 children were prescribed paracetamol, 1 trimethoprim-sulfamethoxazole (for infection with chloroquine-sensitive P. falciparum), and 3 amoxicillin.
Adverse events and symptom resolution.
Two patients reported having convulsions, and one had severe itching prior to study entry. No patient reported itching or development of a rash during the study, and the only reported convulsion occurred 1.5 h after the first dose, as described above.
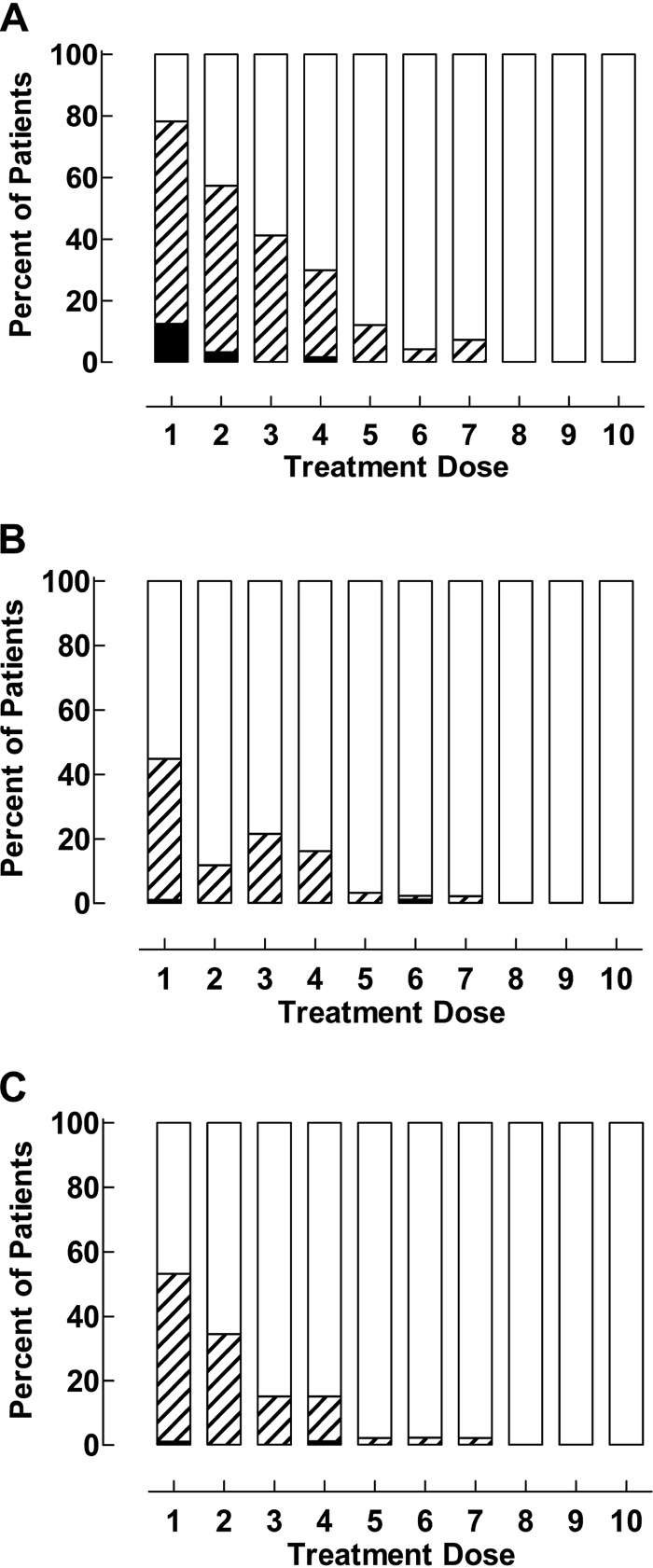
Fig. 1 shows the frequency of pooled symptoms of feeling unwell or having fever or joint pains or headache (Fig. 1A), vomiting or diarrhea or stomach pain (Fig. 1B), and sleeping or eating or drinking poorly (Fig. 1C). The symptoms were pooled to reflect general malaise, gastrointestinal symptoms, and survival functions, respectively. Resolution of all these symptoms (except convulsions and drinking poorly, which were not reported as occurring during treatment, and diarrhea, which only occurred in one patient) was associated with increasing chloroquine concentrations, irrespective of whether they were analyzed as individual parameters (P < 0.01) or pooled (P < 0.001). Symptom resolution was not affected by P. falciparum genotype, sex, age, parasite density, or parasite positivity versus negativity during follow-up.
FIG 1.
Symptom resolution during treatment with 50 and 70 mg/kg of chloroquine. (A) Feeling unwell, feverish, or having joint pains or headache. (B) Vomiting, diarrhea, or stomach pain. (C) Sleeping, eating, and drinking, White, no symptoms; stripes, moderate symptoms; black, severe symptoms.
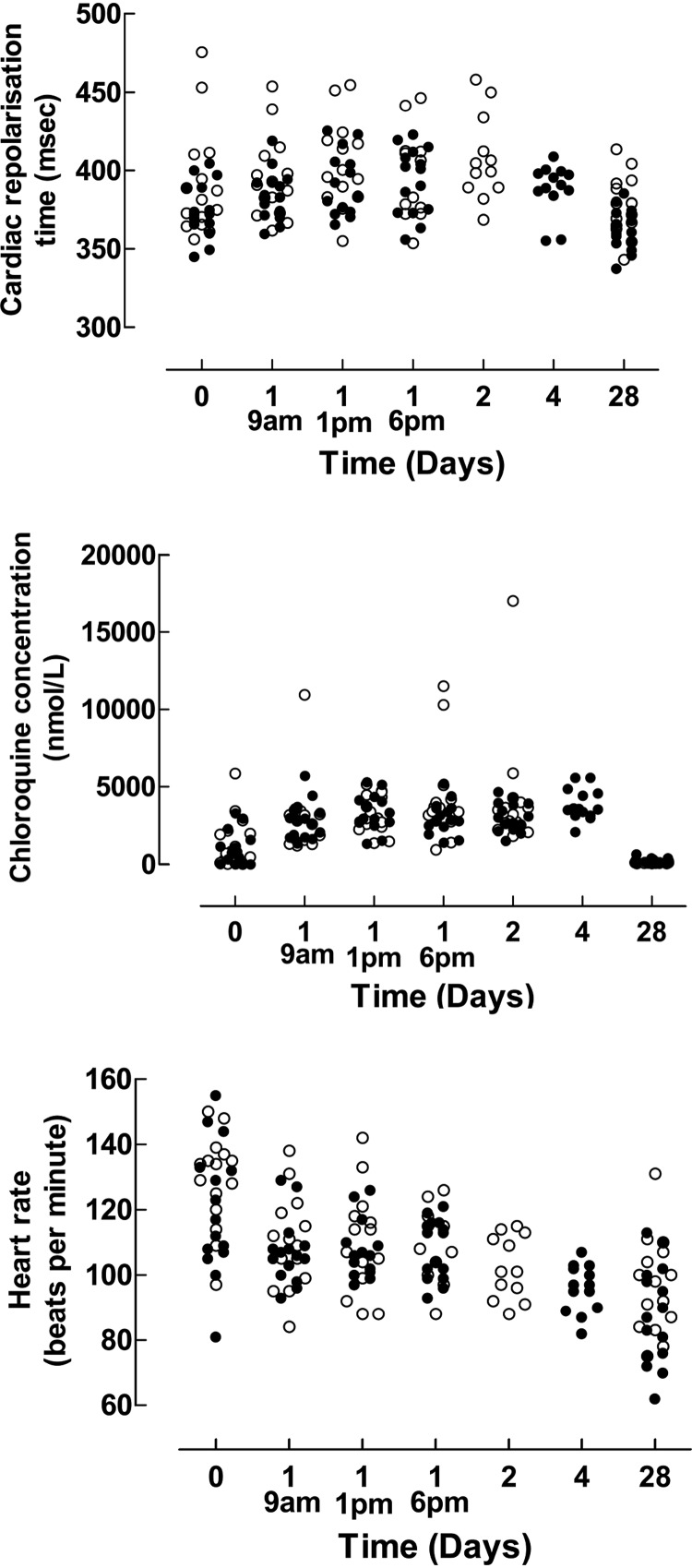
Chloroquine concentrations and cardiac parameters.
Median capillary blood chloroquine concentrations; corrected QT (QTc)0.4 (QTc0.4 = QT/RR0.4); QTcB (Bazett correction); heart rate; and systolic, diastolic, and mean arterial blood pressure before, during, and after treatment are shown in Table 2 and Fig. 2. QTc0.4 was used because it is proposed to be the most accurate correction factor for children and adolescents (11–14), and because the Bazett-corrected QT-interval was associated with heart rate (P < 0.005), whereas QTc0.4 was not in this study. QTc0.4 was significantly longer during treatment than at predose and day 28 (P < 0.001). There was no significant change in QTc0.4 when predose and day 28 were compared. Chloroquine concentrations were positively associated with QTc0.4 with a coefficient of 0.006 (95% CI, 0.004 to 0.009) ms per nM chloroquine increase (P < 0.001) and an R2 value of 0.1. Heart rate decreased significantly during treatment (P = 0.003) as well as over the whole study period (P < 0.001). Study duration (P < 0.001) and temperature (P = 0.02) were significantly associated with decreasing heart frequency, whereas chloroquine concentrations were not. No other cardiac parameter changed significantly during treatment.
TABLE 2.
QTc interval, heart rate, and blood pressure before, during, and after treatment with 50 and 70 mg/kg of chloroquine
| Measurement | Data for day (time): |
||||||
|---|---|---|---|---|---|---|---|
| 0a | 1 (9 a.m.) | 1 (1 p.m.) | 1 (6 p.m.) | 2b | 4c | 28 | |
| Chloroquine concentrations (nmol/L) (95% CI) | 815 (325–1,307) | 2,903 (2,246–3,560) | 2,968 (2,359–3,579) | 3,375 (2,850–3,900) | 2,734 (2,179–3,290) | 3,562 (2,996–4,129) | 122 (87–157) |
| QTc0.4 (ms) (95% CI)d | 375 (363–386) | 387 (379–395) | 396 (384–408) | 401 (383–418) | 399 (387–411) | 391 (382–400) | 369 (363–376) |
| QTc Bazett (ms) (95% CI) | 400 (390–420) | 410 (400–420) | 420 (410–430) | 420 (400–440) | 420 (410–440) | 410 (400–430) | 390 (380–400) |
| ΔQTc0.4 compared to day 0 (median [range]) | 7 (−56 to 52) | 15 (−58 to 64) | 13 (−34 to 59) | 12 (−26 to 46) | 14 (−33 to 38) | −11 (−84 to 23) | |
| Heart rate (bpm) (95% CI) | 128 (118–138) | 107 (104–110) | 107 (101–113) | 113 (104–122) | 101 (92–110) | 97 (90–104) | 92 (83–101) |
| Systolic blood pressure (95% CI)e | 99 (96–101) | 95 (91–98) | 94 (91–97) | 95 (92–97) | 95 (92–98) | 101 (96–106) | 98 (94–101) |
| Diastolic blood pressure (95% CI) | 56 (50–61) | 56 (53–59) | 56 (54–58) | 57 (55–58) | 58 (52–63) | 60 (49–71) | 59 (54–63) |
| Mean arterial pressure (95% CI) | 66 (62–70) | 66 (63–68) | 65 (63–67) | 65 (63–67) | 67 (62–71) | 70 (61–79) | 68 (64–71) |
Day 0 values are those determined before/at treatment start.
50 mg/kg group only.
70 mg/kg group only.
QTc0.4 increases of 52, 64, 59, and 47 ms on day 1 (9 a.m., 1 p.m., and 6 p.m.) and day 2, respectively, occurred in the same child. In parallel, the chloroquine concentrations were 1,556, 2,253, 2,809, and 2,225 nmol/liter, respectively, and the heart rate decreased from 135 upon inclusion to 111, 104, 104, and 101 on day 1 (9 a.m., 1 p.m., and 6 p.m.) and day 2, respectively.
Blood pressure was measured as mm/Hg.
FIG 2.
Cardiac repolarization time, chloroquine concentrations, and pulse rates before, during, and after treatment. The open and black circles represent the 50 mg/kg and 70 mg/kg total chloroquine doses, respectively.
There were no instances of torsade de pointes, sudden death, ventricular tachycardia, ventricular fibrillation, and flutter or syncope.
Parasite and fever clearance.
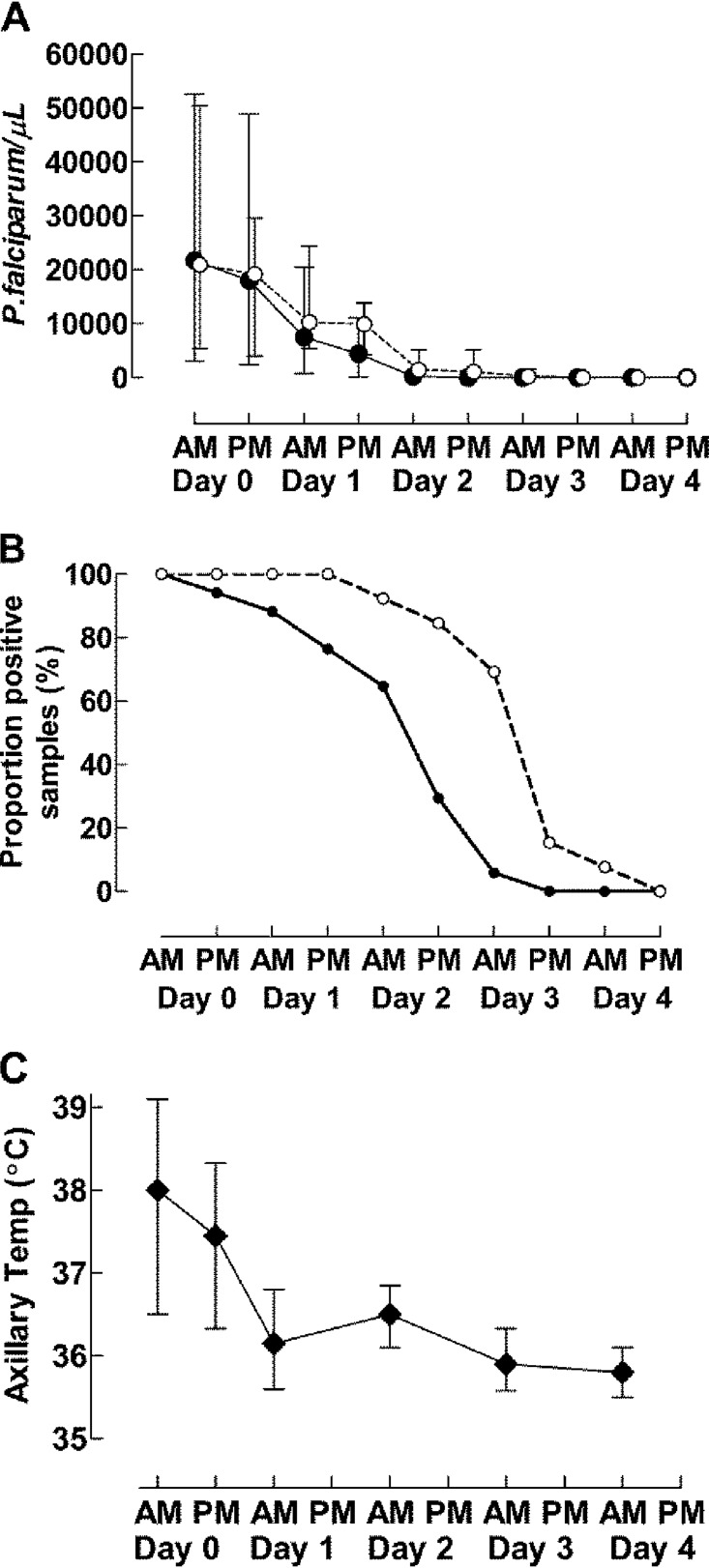
Parasite density (Fig. 3A) decreased with the number of doses taken (P < 0.001) but was not significantly associated with chloroquine concentrations or the P. falciparum chloroquine resistance (P. falciparum chloroquine resistance transporter [pfcrt] K76T) genotype. However, slide positivity (Fig. 3B) was linked with higher chloroquine concentrations (P < 0.001), the pfcrt 76T genotype (P < 0.001), and higher temperature (P < 0.001). Temperature (Fig. 3C) was not significantly associated with age, sex, chloroquine concentration, pfcrt K76T genotype, or parasite density.
FIG 3.
Parasite and fever clearance. (A) Median and interquartile range parasite density with the pfcrt K76T allele. (B) Proportion of children with P. falciparum parasitemia with the pfcrt K76T allele. (C) Median temperature and interquartile range. Black and open circles represent the chloroquine-sensitive pfcrt K76 and chloroquine-resistant pfcrt 76T alleles, respectively, in panels A and B.
Hemoglobin.
Pooled median hemoglobin concentrations (in grams per liter) were as follows: 112 (95% CI, 107 to 117) on day 0, 99 (95% CI, 92 to 106) on day 3, 100 (95% CI, 93 to 107) on day 14, and 109 (95% CI, 106 to 122) on day 28. Median hemoglobin decreased significantly between days 0 and 3 (P = 0.004), did not change significantly between days 3 and 14 or days 0 and 28, and increased significantly between days 3 and 28 (P = 0.005). Hemoglobin changes were not affected by age, sex, total chloroquine dose, treatment outcome, or parasite density.
Pfcrt K76T and pfmdr1 N86Y allele frequencies.
Pfcrt 76T was found in 13/30 (43%) samples, and pfcrt K76 was found in 17/30 (57%) samples. Multidrug resistance gene (Pfmdr1) N86 was found in 27/30 (90%) and pfmdr1 86Y in 3/30 (10%) samples. Haplotype frequencies were 16/30 for KN (53%), 1/30 for KY (3%), 1/1 for TN (37%), and 2/30 for TY (7%). Haplotype frequencies among recrudescent P. falciparum were 4/5 for TN and 1/5 for TY. The pfcrt 76T allele, but not the pfcrt 76 pfmdr1 86 haplotype TN, was associated with recrudescence (P = 0.009).
Treatment outcome.
Day 28 adequate clinical and parasitological response (ACPR) was 80% (12/15) and 87% (13/15) after treatment with 50 and 70 mg/kg, respectively. All treatment failures occurred in children infected with pfcrt 76T carrying P. falciparum. There was one early treatment failure (ETF) (day 3) and two late clinical failures (LCFs) (days 14 and 28) in the 50 mg/kg group and one LPF (day 21) and one LCF (day 21) in the 70 mg/kg group. Day 28 ACPRs against P. falciparum with pfcrt 76T were 57% (4/7) and 67% (4/6) after treatment with total doses of 50 and 70 mg/kg, respectively. Children with recrudescing P. falciparum were significantly more likely to have a positive slide on day 3 (4/5) than successfully treated children (6/25; P = 0.03, Fisher’s exact test). Total chloroquine dose, age, sex, and parasite density at enrollment were not associated with treatment failure.
DISCUSSION
The concentration-dependent response of chloroquine-resistant infections and the efficacy of up to 96% of higher chloroquine doses for treatment of P. falciparum resistant to standard-dose chloroquine has been established in vivo and in vitro (3, 4, 6). Previous data also showed that at least triple-standard-dose chloroquine split into 2 to 3 daily doses for 5 days was routinely prescribed and taken in Guinea-Bissau (7). Patients thus appeared to adhere to the prolonged treatment. Furthermore, malaria prevalence and incidence decreased concurrently with routine use of the 5-day triple-total-standard-dose treatment regimen, indicating that it was as effective as nonchloroquine-based regimens associated with decreasing malaria indices in other parts of Africa (15, 16). Higher chloroquine doses therefore have the potential to be used in the treatment of multidrug-resistant P. falciparum malaria. More data on the safety and tolerability of higher doses are needed and are partly addressed in this study.
Overall, the dosing schedules and attained chloroquine concentrations were well tolerated, and no drug-related serious adverse events were detected during treatment. Vomiting, a common symptom of malaria, was reported in 60% prior to study entry. Following entry, vomiting frequency decreased over time and was not reported after the 4th dose but appeared to be more common (although not significant) between doses 2 and 3 compared to doses 1 and 2. Vomiting was most probably a symptom of general illness rather than a concentration-dependent adverse event, and the increase between doses 2 and 3 is probably due to longer observation time than that between doses 1 and 2. In line with this, vomiting occurred with similar frequencies in a previous study comparing 50 mg/kg of chloroquine with standard-dose artemether-lumefantrine (9). Chloroquine is also very bitter, and the dose was repeated due to vomiting in 3 to 10% of children after doses 1 to 4. Repeated intake of a dose due to vomiting possibly resulted in a higher but still well-tolerated chloroquine concentration in 1 out of 7 children. No children vomited after dose 4, suggesting that general malaise was the main factor causing vomiting. The tolerability was in line with previous studies and decades of experience in Guinea-Bissau as well as a study on Giardia lamblia in which children were treated with 10 mg/kg twice daily for 5 days (7–10, 17).
One child was excluded due to convulsions 1.5 h after intake of the first 10-mg/kg dose. The chloroquine dose taken by this child was the same as that used in the very well-tolerated standard treatment regimen. Convulsions were therefore most probably the result of malaria and not due to chloroquine toxicity.
Quinoline drugs can prolong cardiac repolarization time, causing QT prolongation that can be associated with an increased risk of arrhythmia and sudden death, as seen with halofantrine (11, 18). Correlating QT prolongations with drug concentrations is challenging, as the QT interval is affected by many factors, including heart rate, age, sex, autonomic tone, and electrolyte concentrations, all of which change during malaria treatment (11). Nevertheless, two studies in healthy volunteers showed that chloroquine prolongs the QTc interval in a dose-dependent manner. The mean QTcF (Fridericia correction) increased by 6.1 ms in a Thai study after intake of 600 mg chloroquine and by 16 and 28 ms (Bazett correction) after intake of 600 mg and 1,200 mg chloroquine, respectively, in an American study. In both studies, QTc peaked when chloroquine concentrations peaked (19, 20). Thereafter, QTc gradually decreased with declining chloroquine concentrations. In line with this, the mean QTcF (Fridericia correction) increase was 11 ms on day 2 in malaria-infected adults taking standard doses of chloroquine (21). The 12-ms median QTc0.4 increase from baseline to before the third dose and the 26-ms increase corresponding to the peak concentration after the 3rd dose seen in the current study is thus in line with previous data. The lower chloroquine doses given on days 2 to 4 maintained approximately the same chloroquine concentrations as those attained on day 1, and in line with that, QTc0.4 did not change significantly between days 1 to 4. The chloroquine doses used in this study are thus as unlikely as standard-dose chloroquine to cause cardiac arrhythmias due to prolonged QTc intervals.
No patient had QTc0.4 exceeding 500 ms; however, one patient (3%) had up to 64 ms QTc0.4 prolongation on day 1 compared to day 0. The increase could be due to chloroquine but may be a normal daily variation that is reported to be up to 75 to 100 ms (22, 23). To further support this normal daily variation, QTc0.4 decreased by up to 58 ms on day 1 in other patients. In other studies, the maximum QTc (Bazett correction) prolongation was 63 ms in one healthy volunteer after intake of 1,200 mg chloroquine base and more than 60 ms (Fridericia correction) in five (3.2% of patients) between day 0 and day 2 after intake of standard-dose chloroquine for malaria treatment (19, 21). This again suggests that the doses used in this study are as unlikely as standard-dose chloroquine to cause cardiac arrhythmias.
Chloroquine has a negative inotropic effect at micromolar concentrations and causes hypotension due to arteriolar and venodilation when overdosed (11). Finding no significant change in blood pressure and decreasing heart rate corresponding to defervescence and clinical improvement indicates that the chloroquine doses used in this study do not have a significant negative inotropic effect. Furthermore, median steady-state chloroquine concentrations were 2,000 to 2,500 nmol/liter when used for the treatment of rheumatoid arthritis (24). These concentrations are only slightly lower than those reported here, and chloroquine is generally considered to be well tolerated when used for the treatment of rheumatoid arthritis, further supporting the tolerability of the concentrations found.
Hemoglobin decreased between day 0 and day 3 and then gradually increased, in line with a recent efficacy study with artemether-lumefantrine and dihydroartemisinin-piperaquine in Guinea-Bissau (25). The lower median hemoglobin value on day 3 was no doubt the result of malaria infection rather than any toxic effect of higher chloroquine doses. The rapid recovery also argues against a suppressive effect of the higher doses on red blood cell production in the bone marrow. Furthermore, the rapid recovery of hemoglobin despite chloroquine’s slower parasiticidal effect compared to artemisinin-based combination therapy and despite a 43% prevalence of P. falciparum resistant to standard-dose chloroquine is encouraging.
Fever and the decrease of parasite biomass were not affected by the chloroquine resistance genotype, indicating that the chloroquine concentrations attained were high enough to clear P. falciparum that is resistant to treatment with standard-dose chloroquine. This is in line with the previously shown concentration-dependent response of chloroquine-resistant infections (3, 5). However, pfcrt 76T was linked to prolonged slide positivity, probably indicating that the genotype enabled a subset of P. falciparum parasites to survive longer despite being exposed to chloroquine concentrations that were sufficient to clear the majority of infecting pfcrt 76T-carrying P. falciparum. Treatment efficacies against pfcrt 76T-carrying P. falciparum were therefore only 57% and 67% after taking 50 and 70 mg/kg, respectively, compared to 75% and 84% ACPR seen previously in children of ages <5 and 5 to 10 years, respectively, treated with 50 mg/kg (3). As attained chloroquine concentrations were capable of eliminating pfcrt 76T-carrying P. falciparum, a probable reason for the high rate of treatment failure was that the concentrations were not maintained for a sufficiently long period. The numbers are small, but a possible mechanism is that certain P. falciparum parasites enter a dormant stage when exposed to chloroquine, as described with artemisinins, atovaquone, and quinine (26–28). This hypothesis is supported by unpublished in vitro data showing that chloroquine induces dormancy and that elimination of chloroquine-resistant and -sensitive P. falciparum is time dependent as long as parasiticidal concentrations are maintained. As the parasiticidal concentrations during treatment were similar to those found during chronic treatment of rheumatoid arthritis, it is probable that maintaining sufficiently high concentrations for long enough is safe (24). A slow-release preparation taken once daily could possibly provide the means for doing this. Moreover, if the chloroquine concentrations attained during treatment of rheumatoid arthritis are sufficient to kill chloroquine-resistant malaria, the dosage regimen used for rheumatoid arthritis could possibly work as malaria prophylaxis.
Limitations of this study include the small number of patients. The limited age span makes it difficult to generalize the findings to other age groups, and it is improbable that rare adverse events would be detected. Moreover, the QT interval is affected by numerous variables, and QT correction is fraught with potential errors that must be considered but are difficult to avoid. On the other hand, the QT prolongation seen is that expected to be seen outside a clinical trial in which children with uncomplicated malaria suffer from vomiting, dehydration, electrolyte disturbances, stress, and numerous other factors that all affect the QT interval.
To conclude, treatment with 50 and 70 mg/kg of chloroquine as split doses for 3 and 5 days, respectively, was well tolerated. The dosages did not affect blood pressure but probably caused a QTc interval increase of similar magnitude to that seen when adults take standard-dose chloroquine (25 mg/kg), suggesting that arrhythmias are unlikely to occur with these dosages.
MATERIALS AND METHODS
Ethics and registration.
The study was conducted in accordance with the Declaration of Helsinki and Good Clinical Practice. Written informed consent was obtained from all parents or legal guardians. Ethical approval was obtained from Ministério da Saúde Pública in Guinea-Bissau (011/CNES/INASA/2013). The regional ethics committee in Stockholm, Sweden, approved the molecular analyses (2011/832-32/2). The study was registered at ClinicalTrials.gov (NCT01814423).
Setting and participants.
The study was conducted at the Bandim Health Center at the Bandim Health and Demographic Surveillance Site in suburban Bissau, Guinea-Bissau, between September 2013 and January 2014. The health center is equipped with microscopes and rapid diagnostic tests for malaria and usually has electricity but no other more advanced equipment. A portable ECG machine (Philips ECG monitor X2) was borrowed for the duration of the study. Inclusion criteria were monoinfection with P. falciparum without signs of severe malaria or danger signs, parasite density of 800 to 200,000 parasites/μl, axillary temperature >37.5°C or a history of fever during the previous 24 h, age of 4 to 8 years, hemoglobin >50 g/liter, no other significant illness, no reported intake of antimalarial drugs during the past week, and residence within the study area.
Previous data showed that attained chloroquine concentrations are age dependent with weight-based dosing (29). The 4- to 8-year age range was chosen to minimize the age-dependent chloroquine concentration range attained to include children who are underdosed with standard-dose chloroquine and to include the youngest children for whom it was feasible to do this study at our study site.
Study design.
This was a single-center, open-label, nonrandomized study in which 15 children took a total dose of 50 mg/kg of chloroquine over 3 days and 15 children took a total dose of 70 mg/kg of chloroquine over 5 days.
Procedures.
The study schedule is outlined in Table 3. Children attending the health center on Mondays were asked to participate. The first 10 volunteers were enrolled in the 50-mg/kg arm, the following 10 in the 70-mg/kg arm, the following 5 in 50-mg/kg arm, and the final 5 in 70-mg/kg arm. Children taking 50 mg/kg were seen twice daily on days 0 and 2 and once on day 3. Children taking 70 mg/kg were seen twice daily on days 0, 2, 3, and 4. All children were admitted between 9 a.m. and 6 p.m. on day 1; seen once daily on days 7, 14, 21, and 28; and assessed anytime during follow-up if the child was unwell.
TABLE 3.
Timetable of study proceduresa
| Procedure | Data for day: |
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 7 | 14 | 21 | 28 | |
| Total dose, 50 mg/kg chloroquine base | |||||||||
| Chloroquine dose (mg/kg) | 10 + 10 | 10 + 10 | 5 + 5 | ||||||
| Daily visitsb | 2 | Admitted from 9 a.m. to 6 p.m. | 2 | 1 | 1 | 1 | 1 | 1 | |
| ECG, BP, HRc | Yes | 9 a.m., 1 p.m., 6 p.m. | Yes | Yes | |||||
| Blood sampling for microscopy | Twice | Twice | Twice | Once | Once | Once | Once | Once | |
| Axillary temperature taken | Twice | Once | Once | Once | Once | Once | Once | Once | |
| Total dose, 70 mg/kg chloroquine base | |||||||||
| Chloroquine dose (mg/kg) | 10 + 10 | 10 + 10 | 5 + 5 | 5 + 5 | 5 + 5 | ||||
| Daily visitsb | 2 | Admitted from 9 a.m. to 6 p.m. | 2 | 2 | 2 | 1 | 1 | 1 | 1 |
| ECG, BP, HRc | Yes | 9 a.m., 1 p.m., 6 p.m. | Yes | Yes | |||||
| Blood sampling for microscopy | Twice | Twice | Twice | Twice | Twice | Once | Once | Once | Once |
| Axillary temperature taken | Twice | Once | Once | Once | Once | Once | Once | Once | Once |
Hemoglobin was measured on days 0, 3, 14, and 28. Blood samples for genotyping were taken on days 0, 7, 14, 21, and 28.
Blood samples for chloroquine concentrations were drawn prior to each dose and once daily whenever a child was visited, except for day 1 when samples were taken hourly.
ECGs, blood pressure (BP), and heart rates (HR) were determined from recumbent children who had rested for approximately 15 min. The parameters were assessed prior to treatment on day 0 and in conjunction with dose 6 in children taking 50 mg/kg and dose 10 in children taking 70 mg/kg.
Tablets with 160 mg chloroquine-phosphate (equivalent to 100 mg chloroquine base) were donated by Recip, Solna, Sweden. Chloroquine intake was observed. Food intake was not controlled. Treatment was repeated if vomiting occurred within 30 min. If vomiting recurred within the next 30 min, quinine was given intramuscularly and the child was withdrawn. Concomitantly prescribed drugs were administered and recorded.
Prior to each chloroquine dose, children and/or caregivers were asked if the child had had the following symptoms since the previous chloroquine dose: fever; convulsions; joint pains; diarrhea; stomach pain; itch; rash; palpitations; inability to eat, sleep, and drink; their general condition; and any other symptoms. Symptoms were graded as not present, moderate, or severe. Additionally, the attending nurse assessed the patient’s general condition as well, ill, or very ill based on her clinical judgment. Axillary temperatures were measured on admission, prior to the second dose on day 0, and during each morning during treatment.
On days 7, 14, 21, and 28 and on unscheduled visits, the clinical condition was assessed, temperature was measured, and caregivers were questioned about their child’s general condition, the occurrence of fever, any symptoms, hospital admission, or intake of any drugs.
Single six-lead digital ECGs were done with children in the recumbent position for approximately 15 min. ECGs were done at study entry, 9 a.m., 1 p.m., and 6 p.m. on days 1 and 28 in both study arms and during the mornings of days 2 and 4 in the 50- and 70-mg/kg arms, respectively (Table 3). Days 0 and 28 recordings were chosen to provide before and after baseline data. Day 1 recordings were chosen because we expected peak concentrations after the morning dose to occur at approximately 1 p.m., and we would therefore get QT times before, during, and after the peak concentration. Days 2 and 4 were chosen because they represented the practical time point corresponding to intake of the largest total dose. ECGs were run at 25 mm/sec and stored electronically. QT intervals were determined in lead II whenever possible and otherwise in the lead with the clearest ECG trace. An on-site investigator assessed QT intervals during the study, and three investigators calculated QT intervals separately by hand at a later time point. The mean of the three calculations is presented. All QT intervals were corrected for heart rate using the formula QTc=QT/RR0.4, as this performed better than the commonly used Fridericia and Bazett corrections.
Rescue treatment.
Quinine was given if a child developed severe malaria. P. falciparum detected after day 7 was treated with artemether-lumefantrine.
Laboratory methods.
Children with symptoms suggestive of malaria were screened using rapid diagnostic tests (First Response) and Giemsa-stained thick and thin smears. Species were identified and parasite densities quantified (per 200 white blood cells) using a microscope with ×1,000 magnification. A slide was considered negative after examination of 100 high-power fields. Thick and thin films were examined as outlined in Table 3 and whenever a child sought medical attention due to symptoms compatible with malaria.
Hemoglobin was measured using a HaemoCue (Ängelholm, Sweden).
Exactly 100 μl of whole blood was put onto filter papers (Whatman 3MM), dried, and then stored in individual sealed plastic bags with a desiccant (MiniPax; Sigma-Aldrich) for chloroquine concentration analyses and genotyping as outlined in Table 3 and whenever P. falciparum was detected after treatment.
Chloroquine and desethylchloroquine concentrations were determined at the Department of Clinical Pharmacology, Mahidol-Oxford Tropical Medicine Research Unit, Bangkok, Thailand, by a validated method using solid-phase extraction and liquid chromatography with tandem mass spectrometry (J. Ursing, unpublished data). The linear reportable ranges for quantification were 1.46 to 1,785 ng/ml and 2.36 to 1,785 ng/ml for chloroquine and desethylchloroquine, respectively. Three replicates of quality control samples at low, middle, and high concentrations were included in the analysis to ensure precision and accuracy. The observed total assay coefficient of variation was <9.0% in all quality control samples in accordance with US Food and Drug Administration requirements (30).
DNA was extracted from two 3-mm punches of blood-soaked filter paper using a Chelex-based extraction method. Previously described PCR-RFLP (restriction fragment length polymorphism) methods were used to detect K76T and N86Y alleles in P. falciparum chloroquine resistance transporter (pfcrt) and multidrug resistance genes (pfmdr1), respectively (31). New and recrudescent infections were distinguished by PCR analysis of merozoite surface proteins 1 and 2 and P. falciparum glutamate-rich protein in matching day 0 and day of recurring P. falciparum samples according to WHO recommendations (32). PCR products were resolved on agarose gels (Amresco, Solon, OH), stained with ethidium bromide, and visualized under UV transillumination (GelDoc; Bio-Rad, Hercules, CA, USA).
Treatment outcomes.
WHO criteria were used to define early treatment failures (ETFs), late clinical failures (LCFs), late parasitological failures (LPFs), and adequate clinical and parasitological response (ACPR) (33).
Endpoint.
The primary endpoint was adverse events by day 28. Secondary outcomes included parasite clearance rate, PCR-adjusted ACPR, and hemoglobin recovery.
Statistical analysis.
Chloroquine concentrations, parasite density, body temperature, QTc, heart rate, blood pressure, pulse rate, hemoglobin values, and age were analyzed as continuous variables. Symptoms were classified as present or not, as the frequency of children with severe symptoms was very low. The presence of parasites as identified by microscopy was also dichotomized. The presence of symptoms, presence of parasites, sex, pfcrt K76T, and pfmdr1 N86Y genotypes were analyzed as categorical variables. Medians, 95% CIs, and interquartile ranges (IQRs) of continuous variables were estimated using quantile regression with bootstrapping (100 repeats). The correlation between chloroquine concentrations and the continuous and categorical variables listed above were evaluated using univariate quantile regressions with bootstrapping (100 repeats). The correlations between QTc, heart rate, blood pressure, parasite density, temperature, and chloroquine concentrations were similarly evaluated using quantile regression but also using fixed-effects linear regression. In addition, conditional logistic regression was used when correlating the frequency of discrete and pooled symptoms with chloroquine concentrations. As the results obtained with the fixed effects and conditional logistic regressions were essentially the same as the results of the quantile regression, only data from the quantile regressions are presented. Fisher’s exact test was used to evaluate the correlation between categorical variables.
ACKNOWLEDGMENTS
We thank all children and families and all the staff in Guinea-Bissau involved in the study.
This work was supported by an award from the Anthony Cerami and Ann Dunne Foundation for World Health (http://acadforworldhealth.org/) award 20102011, the regional agreement on medical training and clinical research (ALF) project 20150224, between Stockholm County Council and Karolinska Institutet for Staffan Eksborg and Stockholm County Council (clinical researcher position to J.U.; award number 20160597). J.T. is supported by the Wellcome Trust of Great Britain and the Bill and Melinda Gates Foundation.
REFERENCES
- 1.World Health Organization. 2015. Guidelines for the treatment of malaria, 5th ed World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241549127/en/. [Google Scholar]
- 2.Dondorp AM, Nosten F, Yi P, Das D, Phyo AP, Tarning J, Lwin KM, Ariey F, Hanpithakpong W, Lee SJ, Ringwald P, Silamut K, Imwong M, Chotivanich K, Lim P, Herdman T, An SS, Yeung S, Singhasivanon P, Day NP, Lindegardh N, Socheat D, White NJ. 2009. Artemisinin resistance in Plasmodium falciparum malaria. N Engl J Med 361:455–467. doi: 10.1056/NEJMoa0808859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ursing J, Rombo L, Bergqvist Y, Rodrigues A, Kofoed PE. 2016. High-dose chloroquine for treatment of chloroquine-resistant Plasmodium falciparum malaria. J Infect Dis 213:1315–1321. doi: 10.1093/infdis/jiv590. [DOI] [PubMed] [Google Scholar]
- 4.Ursing J, Kofoed PE, Rodrigues A, Rombo L, Gil JP. 2007. Plasmodium falciparum genotypes associated with chloroquine and amodiaquine resistance in Guinea-Bissau. Am J Trop Med Hyg 76:844–848. doi: 10.4269/ajtmh.2007.76.844. [DOI] [PubMed] [Google Scholar]
- 5.Summers RL, Dave A, Dolstra TJ, Bellanca S, Marchetti RV, Nash MN, Richards SN, Goh V, Schenk RL, Stein WD, Kirk K, Sanchez CP, Lanzer M, Martin RE. 2014. Diverse mutational pathways converge on saturable chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Proc Natl Acad Sci U S A 111:E1759-67. doi: 10.1073/pnas.1322965111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin RE, Marchetti RV, Cowan AI, Howitt SM, Broer S, Kirk K. 2009. Chloroquine transport via the malaria parasite’s chloroquine resistance transporter. Science 325:1680–1682. doi: 10.1126/science.1175667. [DOI] [PubMed] [Google Scholar]
- 7.Ursing J, Kofoed PE, Rodrigues A, Bergqvist Y, Rombo L. 2009. Chloroquine is grossly overdosed and overused but well tolerated in Guinea-Bissau. Antimicrob Agents Chemother 53:180–185. doi: 10.1128/AAC.01111-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kofoed PE, Ursing J, Poulsen A, Rodrigues A, Bergquist Y, Aaby P, Rombo L. 2007. Different doses of amodiaquine and chloroquine for treatment of uncomplicated malaria in children in Guinea-Bissau: implications for future treatment recommendations. Trans R Soc Trop Med Hyg 101:231–238. doi: 10.1016/j.trstmh.2006.05.008. [DOI] [PubMed] [Google Scholar]
- 9.Ursing J, Kofoed P-E, Rodrigues A, Blessborn D, Thoft-Nielsen R, Björkman A, Rombo L. 2011. Similar efficacy and tolerability of double-dose chloroquine and artemether-lumefantrine for treatment of Plasmodium falciparum infection in Guinea-Bissau: a randomized trial. J Infect Dis 203:109–116. doi: 10.1093/infdis/jiq001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kofoed PE, Lopez F, Johansson P, Sandstrom A, Hedegaard K, Aaby P, Rombo L. 2002. Treatment of children with Plasmodium falciparum malaria with chloroquine in Guinea-Bissau. Am J Trop Med Hyg 67:28–31. doi: 10.4269/ajtmh.2002.67.28. [DOI] [PubMed] [Google Scholar]
- 11.White NJ. 2007. Cardiotoxicity of antimalarial drugs. Lancet Infect Dis 7:549–558. doi: 10.1016/S1473-3099(07)70187-1. [DOI] [PubMed] [Google Scholar]
- 12.Wernicke JF, Faries D, Breitung R, Girod D. 2005. QT correction methods in children and adolescents. J Cardiovasc Electrophysiol 16:76–81. doi: 10.1046/j.1540-8167.2005.03520.x. [DOI] [PubMed] [Google Scholar]
- 13.Price RN, Nosten F, White NJ. 1998. Prolongation of the QTc interval in African children treated for falciparum malaria. Am J Trop Med Hyg 59:503. doi: 10.4269/ajtmh.1998.59.503. [DOI] [PubMed] [Google Scholar]
- 14.vn Seidlein L, Jaffar S, Greenwood B. 1997. Prolongation of the QTc interval in African children treated for falciparum malaria. Am J Trop Med Hyg 56:494–497. doi: 10.4269/ajtmh.1997.56.494. [DOI] [PubMed] [Google Scholar]
- 15.Ursing J, Rombo L, Rodrigues A, Aaby P, Kofoed PE. 2014. Malaria transmission in Bissau, Guinea-Bissau between 1995 and 2012: malaria resurgence did not negatively affect mortality. PLoS One 9:e101167. doi: 10.1371/journal.pone.0101167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ceesay SJ, Casals-Pascual C, Erskine J, Anya SE, Duah NO, Fulford AJ, Sesay SS, Abubakar I, Dunyo S, Sey O, Palmer A, Fofana M, Corrah T, Bojang KA, Whittle HC, Greenwood BM, Conway DJ. 2008. Changes in malaria indices between 1999 and 2007 in The Gambia: a retrospective analysis. Lancet 372:1545–1554. doi: 10.1016/S0140-6736(08)61654-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Canete R, Rivas DE, Escobedo AA, Gonzalez ME, Almirall P, Brito K. 2010. A randomized, controlled, open-label trial evaluating the efficacy and safety of chloroquine in the treatment of giardiasis in children. West Indian Med J 59:607–611. [PubMed] [Google Scholar]
- 18.Nosten F, ter Kuile FO, Luxemburger C, Woodrow C, Kyle DE, Chongsuphajaisiddhi T, White NJ. 1993. Cardiac effects of antimalarial treatment with halofantrine. Lancet 341:1054–1056. doi: 10.1016/0140-6736(93)92412-m. [DOI] [PubMed] [Google Scholar]
- 19.Mzayek F, Deng H, Mather FJ, Wasilevich EC, Liu H, Hadi CM, Chansolme DH, Murphy HA, Melek BH, Tenaglia AN, Mushatt DM, Dreisbach AW, Lertora JJ, Krogstad DJ. 2007. Randomized dose-ranging controlled trial of AQ-13, a candidate antimalarial, and chloroquine in healthy volunteers. PLoS Clin Trials 2:e6. doi: 10.1371/journal.pctr.0020006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pukrittayakamee S, Tarning J, Jittamala P, Charunwatthana P, Lawpoolsri S, Lee SJ, Hanpithakpong W, Hanboonkunupakarn B, Day NP, Ashley EA, White NJ. 2014. Pharmacokinetic interactions between primaquine and chloroquine. Antimicrob Agents Chemother 58:3354–3359. doi: 10.1128/AAC.02794-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valecha N, Savargaonkar D, Srivastava B, Rao BH, Tripathi SK, Gogtay N, Kochar SK, Kumar NB, Rajadhyaksha GC, Lakhani JD, Solanki BB, Jalali RK, Arora S, Roy A, Saha N, Iyer SS, Sharma P, Anvikar AR. 2016. Comparison of the safety and efficacy of fixed-dose combination of arterolane maleate and piperaquine phosphate with chloroquine in acute, uncomplicated Plasmodium vivax malaria: a phase III, multicentric, open-label study. Malar J 15:42. doi: 10.1186/s12936-016-1084-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yap YG, Camm AJ. 2003. Drug induced QT prolongation and torsades de pointes. Heart 89:1363–1372. doi: 10.1136/heart.89.11.1363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mytton OT, Ashley EA, Peto L, Price RN, La Y, Hae R, Singhasivanon P, White NJ, Nosten F. 2007. Electrocardiographic safety evaluation of dihydroartemisinin piperaquine in the treatment of uncomplicated falciparum malaria. Am J Trop Med Hyg 77:447–450. doi: 10.4269/ajtmh.2007.77.447. [DOI] [PubMed] [Google Scholar]
- 24.Augustijns P, Geusens P, Verbeke N. 1992. Chloroquine levels in blood during chronic treatment of patients with rheumatoid arthritis. Eur J Clin Pharmacol 42:429–433. doi: 10.1007/bf00280130. [DOI] [PubMed] [Google Scholar]
- 25.Ursing J, Rombo L, Rodrigues A, Kofoed PE. 2016. Artemether-lumefantrine versus dihydroartemisinin-piperaquine for treatment of uncomplicated Plasmodium falciparum malaria in children aged less than 15 years in Guinea-Bissau - an open-label non-inferiority randomised clinical trial. PLoS One 11:e0161495. doi: 10.1371/journal.pone.0161495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Teuscher F, Gatton ML, Chen N, Peters J, Kyle DE, Cheng Q. 2010. Artemisinin-induced dormancy in plasmodium falciparum: duration, recovery rates, and implications in treatment failure. J Infect Dis 202:1362–1368. doi: 10.1086/656476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thapar MM, Gil JP, Bjorkman A. 2005. In vitro recrudescence of Plasmodium falciparum parasites suppressed to dormant state by atovaquone alone and in combination with proguanil. Trans R Soc Trop Med Hyg 99:62–70. doi: 10.1016/j.trstmh.2004.01.016. [DOI] [PubMed] [Google Scholar]
- 28.Mapaba E, Hellgren U, Landberg-Lindgren A, Rombo L. 1995. Susceptibility of Plasmodium falciparum to quinine in vitro: effects of drug concentrations and time of exposure. Trans R Soc Trop Med Hyg 89:85–89. doi: 10.1016/0035-9203(95)90671-1. [DOI] [PubMed] [Google Scholar]
- 29.Ursing J, Eksborg S, Rombo L, Bergqvist Y, Blessborn D, Rodrigues A, Kofoed PE. 2014. Chloroquine is grossly under dosed in young children with malaria: implications for drug resistance. PLoS One 9:e86801. doi: 10.1371/journal.pone.0086801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Food and Drug Administration. 2001. Bioanalytical method validation: guidance for industry. U.S. Department of Health and Human Services, Rockville, Maryland. [Google Scholar]
- 31.Djimde A, Doumbo OK, Cortese JF, Kayentao K, Doumbo S, Diourte Y, Dicko A, Su XZ, Nomura T, Fidock DA, Wellems TE, Plowe CV, Coulibaly D. 2001. A molecular marker for chloroquine-resistant falciparum malaria. N Engl J Med 344:257–263. doi: 10.1056/NEJM200101253440403. [DOI] [PubMed] [Google Scholar]
- 32.World Health Organization. 2007. Methods and techniques for clinical trials on antimalarial drug efficacy: genotyping to identify parasite populations. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 33.World Health Organization. 2009. Methods for surveillance of antimalarial drug efficacy. World Health Organization, Geneva, Switzerland: http://www.who.int/malaria/publications/atoz/9789241597531/en/. [Google Scholar]