Toxoplasma gondii is an obligate intracellular protozoan parasite and a successful parasitic pathogen in diverse organisms and host cell types. Hydroxylamine (HYD) and carboxymethoxylamine (CAR) have been reported as inhibitors of aspartate aminotransferases (AATs) and interfere with the proliferation in Plasmodium falciparum. Therefore, AATs are suggested as drug targets against Plasmodium. The T. gondii genome encodes only one predicted AAT in both T. gondii type I strain RH and type II strain PLK.
KEYWORDS: Toxoplasma gondii, toxoplasmosis, hydroxylamine, carboxymethoxylamine, aspartate aminotransferase
ABSTRACT
Toxoplasma gondii is an obligate intracellular protozoan parasite and a successful parasitic pathogen in diverse organisms and host cell types. Hydroxylamine (HYD) and carboxymethoxylamine (CAR) have been reported as inhibitors of aspartate aminotransferases (AATs) and interfere with the proliferation in Plasmodium falciparum. Therefore, AATs are suggested as drug targets against Plasmodium. The T. gondii genome encodes only one predicted AAT in both T. gondii type I strain RH and type II strain PLK. However, the effects of HYD and CAR, as well as their relationship with AAT, on T. gondii remain unclear. In this study, we found that HYD and CAR impaired the lytic cycle of T. gondii in vitro, including the inhibition of invasion or reinvasion, intracellular replication, and egress. Importantly, HYD and CAR could control acute toxoplasmosis in vivo. Further studies showed that HYD and CAR could inhibit the transamination activity of rTgAAT in vitro. However, our results confirmed that deficiency of AAT in both RH and PLK did not reduce the virulence in mice, although the growth ability of the parasites was affected in vitro. HYD and CAR could still inhibit the growth of AAT-deficient parasites. These findings indicated that HYD and CAR inhibition of T. gondii growth and control of toxoplasmosis can occur in an AAT-independent pathway. Overall, further studies focusing on the elucidation of the mechanism of inhibition are warranted. Our study hints at new substrates of HYD and CAR as potential drug targets to inhibit T. gondii growth.
INTRODUCTION
The phylum Apicomplexa comprises a grand group of intracellular parasites that have been implicated in many important human and veterinary diseases. Plasmodium spp. and Toxoplasma gondii are the most representative and best studied members of this large phylum (1). T. gondii is an obligate intracellular protozoan parasite which can infect warm-blooded animals, including humans (2). The majority of the isolates from North America and Europe belong to one of three distinct lineages based on the laboratory mouse model: acutely virulent type I, intermediate type II, and avirulent type III strains (3–5). T. gondii causes toxoplasmosis, and one-third of the world’s population is estimated to be infected with this parasite (6), but the infection is usually asymptomatic in immunocompetent individuals, whereas immunocompromised people may present with acute toxoplasmosis or even with severe and even fatal complications. Unfortunately, although the gold-standard treatment of toxoplasmosis uses a combination of sulfonamide and pyrimethamine drugs (7), treatments for T. gondii infections are suboptimal (8, 9), since synergistic activity could only be observed against tachyzoites and not against bradyzoites, and severe side effects and adverse drug reaction have been reported.
The T. gondii lytic cycle develops beginning with extracellular energy-dependent tachyzoite invasion into host cells, followed by rapid intracellular tachyzoite replication, egress, and reinvasion of neighboring cells (10). In these stages, not only glucose but also glutamine can enter the mitochondria as a carbon source (10–14), where glutamine can be utilized as carbon skeletons to the tricarboxylic acid (TCA) cycle via either the conversion of intermediate glutamate to α-ketoglutarate or the γ-aminobutyric acid (GABA) shunt (13). The T. gondii genome contains enzymes allowing speculation on a possible architecture of α-ketoglutarate pathway (1), which includes an aspartate aminotransferase (AAT). AAT, as metabolic enzyme, catalyzes the reversible conversion of oxaloacetate and glutamate into aspartate and α-ketoglutarate and subsequently α-ketoglutarate as an intermediate can enter the TCA cycle used for T. gondii proliferation (1). Metabolic enzymes play crucial roles not only in parasite proliferation but also in pathogenicity, which contributes to the virulence of parasites in mouse models and used as potential drug targets (13, 15–18).
Previous studies demonstrated that AAT of Plasmodium falciparum catalyzes the reversible reaction of aspartate and α-ketoglutarate to glutamate (1, 19, 20); these are important intermediates for developing carbon metabolism and the amino acid cycle for parasite survival. Hydroxylamine (HYD) and carboxymethoxylamine (CAR) are inhibitors of AATs, abolish the transamination activity of PfAAT, and interfere with the proliferation of the parasite (19–22). Therefore, PfAAT is believed to be a promising drug target.
Here, we analyzed AAT of T. gondii and evaluated the anti-T. gondii potential of HYD and CAR in vitro and in vivo. Our data indicated that HYD and CAR may be useful for treating toxoplasmosis and even acute T. gondii infection through an AAT-independent pathway.
RESULTS
HYD and CAR inhibit T. gondii growth in vitro.
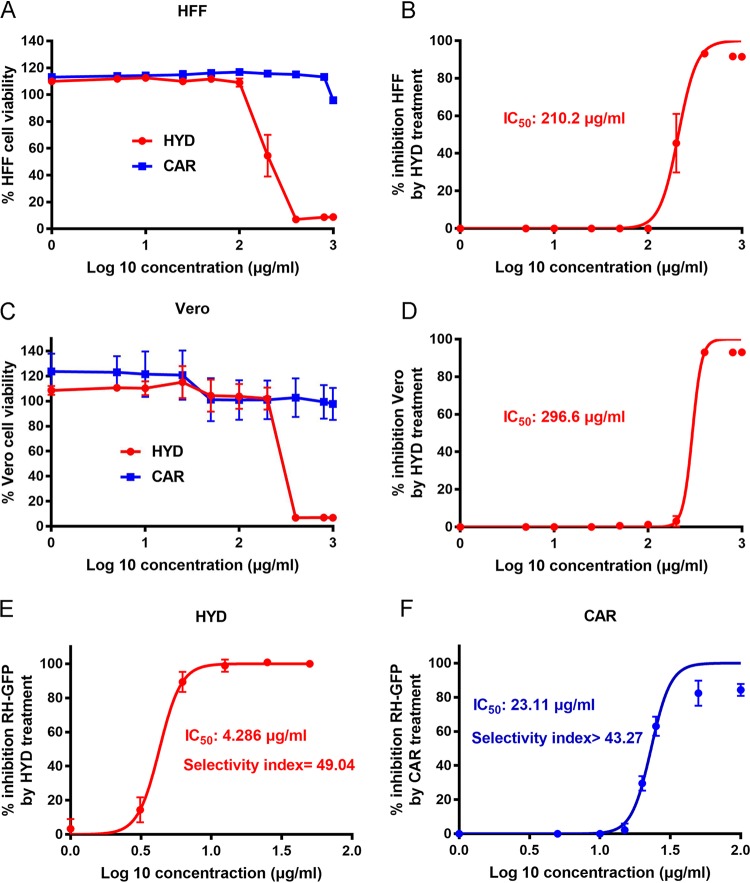
First, we analyzed the toxicity of HYD and CAR on host cells in vitro using a CCK-8 cell counting kit. When human foreskin fibroblast (HFF) cells were exposed to 100 μg/ml HYD for 24 h, the cell proliferation rate was 109.02%; when the cells were exposed to 200 μg/ml HYD, the proliferation rate fell to 54.51% (Fig. 1A). The cytotoxic 50% inhibitory concentration (IC50) value was determined to be 210.2 μg/ml (Fig. 1B). However, when HFF cells were treated with CAR, even at high concentrations of 1,000 μg/ml, the proliferation rate was >95.89% (Fig. 1A). Therefore, the safe concentrations of compounds for HFF cells were considered to be <100 μg/ml for HYD and 1,000 μg/ml for CAR in this study. For monkey kidney adherent epithelial (Vero) cells exposed to 200 μg/ml HYD the proliferation rate was 101.11%, while for Vero cells exposed to 400 μg/ml the proliferation rate was only 6.94% (Fig. 1C). The IC50 value of HYD against Vero cells was 296.6 μg/ml (Fig. 1D). A proliferation rate of 97.78% was noted on Vero cells treated with 1,000 μg/ml CAR (Fig. 1C). Therefore, the safe concentrations of compounds for Vero cells were considered to be <200 μg/ml HYD and 1,000 μg/ml CAR in this study.
FIG 1.
HYD and CAR were able to inhibit parasite growth. (A) HFF cell viability upon treatment with HYD and CAR. (B) Inhibition of HFF growth with HYD treatment. The 50% inhibitory concentration (IC50) was examined. (C) Vero cell viability upon treatment with HYD and CAR. (D) Inhibition of Vero cell growth by HYD treatment. (E and F) Inhibition of T. gondii type I parasites (RH-GFP) after HYD (E) and CAR (F) treatment. The IC50 and selectivity index values were determined.
To evaluate the potential of anti-Toxoplasma of compounds in vitro, we examined the fluorescence intensity of RH-GFP on HFF cells after treatment with HYD, CAR, dimethyl sulfoxide (DMSO; negative control), and sulfadiazine (positive control). RH-GFP parasite growth was inhibited by 50 μg/ml CAR with little fluorescence (see Fig. S1 in the supplemental material), while the green fluorescent protein (GFP) signal was still detected, even at the highest concentration of sulfadiazine (1 mg/ml). Both compounds were able to inhibit parasite growth, with IC50 values of 4.286 μg/ml for HYD and 23.11 μg/ml for CAR on RH-GFP (Fig. 1E and F). Meanwhile, the selectivity indices for HYD and CAR for RH-GFP on HFF cells were 49.04 and >43.27, respectively.
The T. gondii lytic cycle is impaired by HYD and CAR in vitro.
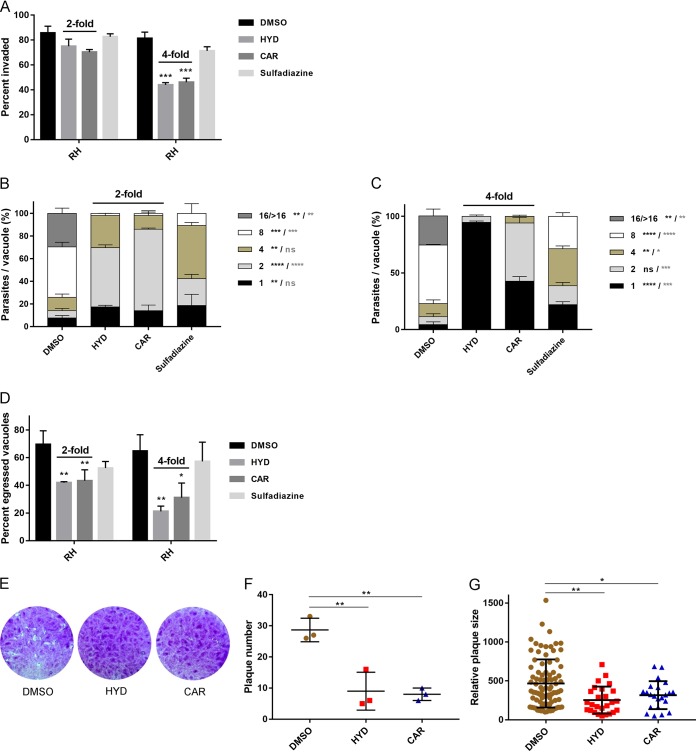
T. gondii lytic cycle starts from the energy-dependent extracellular tachyzoite invasion into host cells, followed by rapid intracellular tachyzoite replication, egress, and reinvasion of neighboring cells (10). Studies on plaque formation can result the complete rounds of T. gondii lytic cycle in vitro (23, 24). Therefore, to analyze the effects of HYD and CAR on the lytic cycle of T. gondii, 2- or 4-fold IC50 values for either of the compounds were used to perform attachment and invasion, replication, and egress assays on Vero cells; also, 2-fold IC50 values for compounds were used to conduct plaque formation assays on HFF cells.
For parasite invasion, 2-fold IC50 values for HYD or CAR decreased the percentages of invaded parasites, although not significantly. Remarkably, when treated by 4-fold IC50 values, the invasion was significantly reduced by 46.03% for HYD and 43.36% for CAR, whereas only a 12.41% reduction in tachyzoite invasion was determined upon treatment with even a high concentration of sulfadiazine (1 mg/ml) (Fig. 2A).
FIG 2.
T. gondii lytic cycle is impaired by HYD and CAR in vitro. (A) Invasion assay. Purified tachyzoites were pretreated with either of the two compounds of 2- or 4-fold IC50 values, sulfadiazine (1 mg/ml), or DMSO for 1 h at 37°C, followed by invasion for 2 h, and dual staining was used to determine the percentages of invaded parasites. (B and C) Intracellular replication assay. HYD and CAR 2-fold (B) or 4-fold (C) IC50 values were allowed to treat infected RH parasites for 24 h, and the numbers of parasites in 100 random vacuoles were counted and plotted. (D) Egress assay. Infected cells were treated with compounds at 2- and 4-fold IC50s for 10 min before incubation with 3 μM A23187. After incubation, mouse anti-SAG1 and rabbit anti-GRA7 were used to measure the percentage of staining egressed PVs. At least 300 vacuoles were counted per slip. (E) Plaque formation assay. A total of 150 fresh RH strain tachyzoites were used to infect the HFF cell monolayer and allowed to grow for 8 days with HYD or CAR at an 2-fold IC50 value, and then 0.1% crystal violet was used for staining. (F and G) Relative plaque numbers (F) and plaque sizes (G) from panel D. The data are presented as the means ± the SEM of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001 compared to DMSO treatment, determined by chi-square test (invasion and egress assay), Tukey’s multiple-comparison test (replication assay, a heavy asterisk represents HYD versus vehicle, and a light asterisk represents CAR versus vehicle), and one-way ANOVA plus Tukey-Kramer post hoc analysis (plaque assay).
To determine the effects of HYD and CAR on intracellular replication in vitro, Vero cells were infected with fresh tachyzoites for 24 h in the presence of 2- or 4-fold IC50 values for either of the compounds and 1 mg/ml sulfadiazine. Parasite replication was impaired upon treatment with 2-fold IC50 values of HYD or CAR, and replication was almost completely abolished by treatment with 4-fold IC50 values of these compounds (Fig. 2B and C). Notably, HYD showed a stronger inhibitory effect than did CAR. For the egress assay, both HYD and CAR pretreatment significantly decreased the egress rate compared to DMSO pretreatment (Fig. 2D), suggesting that tachyzoite egress from host cells was impaired.
We conducted a plaque assay to observe the complete round of the lytic cycle (Fig. 2E). In the presence of HYD or CAR, parasites formed smaller plaques with lower plaque numbers than those in DMSO treatment experiments (Fig. 2F and G). These results suggest that the T. gondii lytic cycle is impaired by HYD and CAR in vitro.
Treatment with HYD and CAR controls acute toxoplasmosis in mice.
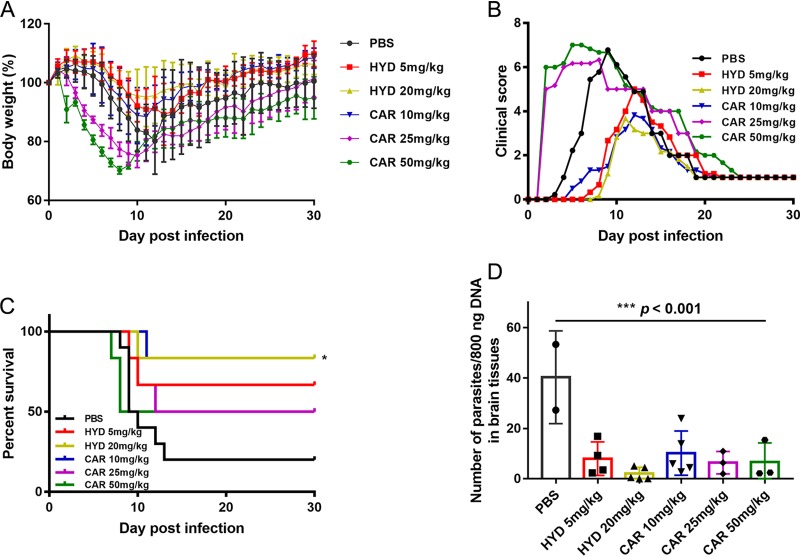
To evaluate the effects of compounds on T. gondii in vivo, female BALB/c mice were infected intraperitoneally (i.p.) with an acute dose of 5 × 104 type II PLK strain tachyzoites and treated by i.p. with 5 or 20 mg/kg (body weight) HYD; 10, 25, or 50 mg/kg (body weight) CAR; or phosphate-buffered saline (PBS) once daily from days 1 to 7 postinfection. Body weight, morbidity, mortality, and clinical signs were recorded. As expected, treatment with 20 mg/kg HYD and 10 mg/kg CAR significantly increased the survival rate during acute infection, with slight body weight reduction and lower clinical scores, compared to PBS treatment (Fig. 3A to C). However, the higher clinical scores were seen in the 25- and 50-mg/kg CAR-injected mice beginning 2 days postinfection (dpi). Moreover, 50 mg/kg CAR-treated mice died at 7 and 8 dpi, earlier than PBS-treated mice, suggesting that although CAR can control acute toxoplasmosis, high concentrations can be toxic to mice. On the other hand, the parasite burdens in surviving mouse brain tissues were examined by quantitative PCR (qPCR), and we observed significantly lower loads in HYD- and CAR-treated mouse brains than in PBS-treated mice (Fig. 3D). Collectively, HYD and CAR treatment controlled acute Toxoplasma infection in the mouse model, although some parasites were detected in the brain samples.
FIG 3.
HYD and CAR control acute Toxoplasma infection in mice. Female BALB/c mice were infected i.p. with an acute dose of 50,000 PLK tachyzoites and treated i.p. from day 1 to day 7 postinfection with 5 or 20 mg/kg HYD; 10, 25, or 50 mg/kg CAR; or PBS once daily. The body weight, morbidity, mortality, and clinical signs were noted. (A) Body weight (%). (B) Mean clinical scores. The scores varied from 0 (no signs) to 10 (all signs). (C) Survival rates. *, P < 0.05, log-rank (Mantel-Cox) test. (D) Parasite burdens of survival mice brains. At day 30 postinfection, the brains were collected, and DNA was extracted, 800 ng of DNA was used to detect the number of parasites. ***, P < 0.001, one-way ANOVA plus Tukey-Kramer post hoc analysis.
HYD and CAR inhibit the catalytic reaction of rTgAAT.
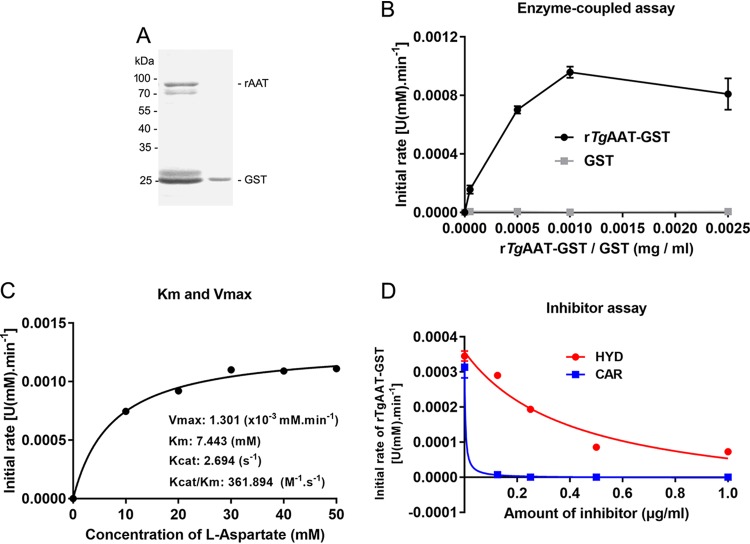
To analyze whether also HYD and CAR can inhibit enzymatic transamination activity of rTgAAT protein as in P. falciparum (19), we cloned and sequenced the gene encoding TgAAT from the T. gondii type I RH and type II PLK strains, indicating TgAATs coding sequences were 1,794 bp long, which is consistent with the predicted sequence (ToxoDB TGGT1_248600 and TGME49_248600), and soluble rTgRHAAT was expressed (Fig. 4A) and used to perform an enzyme-coupled assay, as described previously (19). Soluble rTgAAT (1 μg) was used to conduct enzyme reaction with the specific substrate l-aspartate. We demonstrated that l-aspartate is the preferred substrate of rTgAAT, with a specific activity of 0.078 ± 0.12 μmol min−1 mg−1 (Fig. 4B and C). As expected, the reaction of 1 μg of rTgAAT was inhibited by HYD and CAR treatment, with IC50s of 0.4158 and 0.0035 μg/ml, respectively (Fig. 4D).
FIG 4.
HYD and CAR impair rTgAAT catalytic activity. (A) Soluble rTgRHAAT expression. The concentration of soluble rTgAAT-fused GST was determined. (B) Generation of an enzyme-coupled assay. Expressed GST was used as a control. (C) Enzyme reaction. The enzyme activities of catalysis aspartate and α-ketoglutarate into glutamate were determined using 1 μg of rTgAAT. (D) Inhibitor assay. The enzyme reaction was inhibited with increasing amounts of HYD and CAR.
Knockout TgAAT on RH and PLK strains.
First, for the TgAAT sequence, the amino acid sequence alignment was analyzed, and the phylogenetic tree was constructed with AATs from other species, including Homo sapiens (AAC28622.1), Mus musculus (NP_034454.2), Plasmodium falciparum (KNG76684.1), Eimeria tenella (XP_013230025.1), Trypanosoma brucei (AAK73815.1), Giardia intestinalis (AAK73817.1), and Escherichia coli (AHG10759.1) (see Fig. S2A in the supplemental material). It revealed that TgAATs were distantly related to the predicted P. falciparum, with 33% identity. Western blot analysis revealed that the expression levels of native AAT proteins in RH and PLK strains were not significantly different (Fig. S2B and C), suggesting that TgAAT may play a similar role in both strains.
To investigate further the HYD and CAR target of AAT in T. gondii, we proceeded to generate two knockout strains (ΔRHaat and ΔPLKaat) using pDF-Cas9-sgAAT plasmid (Fig. S2D) and two complemented strains (ComRHAAT and ComPLKAAT strains) using pB-synoRHAAT or pB-synoPLKAAT plasmids (Fig. S2E) containing codons synonymous to TgAATs by CRISPR/Cas9-mediated genome editing. Diagnostic PCRs were used to screen single clones and to confirm gene deletions and complements. The AAT and uracil phosphoribosyl transferase (UPRT) genomic fragments were not amplified in knockout (Fig. S2F) and complemented (Fig. S2G) parasites, whereas the different fragments within plasmids were amplified. After selection, ΔRHaat, ΔPLKaat, ComRHAAT, ComPLKAAT, and wild-type strains were all verified by Western blotting (Fig. S2H). Protein bands were observed in the lysates of RH/PLK and ComRH/PLKAAT lines using a specific anti-TgAAT antibody, but not in the knockout strains. An indirect fluorescent antibody test (IFAT) demonstrated that TgAAT was expressed in the wild-type and complemented parasites but not in the mutant strains (Fig. S2I). These data confirmed the loss of AAT in both RH and PLK strains and the homologous integration and insertion of synoAAT at the UPRT site in representative complement.
Disruption of TgAAT on RH and PLK strains slows parasite growth in vitro.
To estimate the impact of AAT disruption on parasite growth in vitro, all strains were used to determine the production of plaques on Vero cell monolayers. The results showed that all strains are able to form plaques (Fig. 5A and C). Significantly smaller of plaque sizes were present in ΔPLKaat parasites compared to PLK parasites, whereas the plaques of ΔRHaat and RH parasites showed no significant differences (Fig. 5B and D).
FIG 5.
AAT deficiency slows down RH and PLK parasite growth in vitro. (A) Plaque assay. The growth of 150 Δaat or ComAAT tachyzoites in vitro was compared to that of parental RH strains. Plaques were visualized by staining with 0.1% crystal violet. (B) Relative size of the plaques in panel A. The data are presented as means ± the SEM of three independent experiments. (C) Plaque formation of the ΔPLKaat tachyzoites in vitro. Vero cells were infected by 300 tachyzoites and cultured for 12 days. (D) Relative size of plaques in panel C. The data are presented as means ± the SEM of three independent experiments. ***, P < 0.001, one-way ANOVA plus Tukey-Kramer post hoc analysis. (E and F) Invasion, replication, and egress assays of mutants compared to parental and complemented strains in RH (E) and PLK (F) lines. The data are presented as means ± the SEM of at least three independent experiments. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001, determined by chi-square test (invasion and egress assay) and Tukey’s multiple-comparison test (replication assay).
To better define the phenotype associated with the loss of AAT, the effect in invasion, intracellular replication, and egress was evaluated in vitro and compared to parental parasite growth under standard tachyzoite growth conditions. As shown in Fig. 5E, the deletion of AAT in the RH strain significantly inhibited the parasite’s invasion, replication, and egress abilities in vitro. Similar results were evident in the loss of AAT in PLK (Fig. 5F). These results show that AAT is dispensable for the intracellular growth of parasites but that the ability of parasites to spread in vitro is significantly impaired after the deletion of AAT in both RH and PLK strains.
Treatment with α-ketoglutarate rescues the growth defect in AAT deletion mutants.
TgAAT catalyzes glutamate into α-ketoglutarate, an important intermediate of carbon metabolism in mitochondria. To investigate whether α-ketoglutarate supplementation can rescue the growth defect in AAT deletion parasites, the levels of intracellular replication under different culture conditions were compared. The results showed that α-ketoglutarate supplementation (400 μM) significantly increased the 24-h replication rates in AAT-deficient parasites of the RH strain (Fig. 6A) and the PLK strain (Fig. 6B). A lack of more profound improvement by α-ketoglutarate may be due to poor uptake of this nutrient. Indeed, we also examined plaque formation, which resulted in the AAT deletion PLK mutant forming significantly larger plaques in the presence of exogenous α-ketoglutarate (Fig. 6C and D). Interestingly, the high concentration of α-ketoglutarate (2 mM) led to faster intracellular replication of AAT-deficient parasites compared to parental PLK in vitro (Fig. 6E). Taken together, the rescue of AAT-deleted mutants by α-ketoglutarate suggests that AAT plays an important role during T. gondii nutrient uptake.
FIG 6.
Supplementation with α-ketoglutarate (α-Keto) rescues growth defects in Δaat tachyzoites. (A) Supplementation assay with α-ketoglutarate (400 μM) on type 1 strain RH. Tachyzoites were grown with or without α-ketoglutarate 24 h postinfection, and the numbers of parasites in each PV were then determined. (B) PLK replication rescued by α-ketoglutarate (400 μM). Infected tachyzoites were cultured in Vero cells with or without α-ketoglutarate for 24 h, and then the numbers of parasites in each PV were determined. (C) Plaque formation of ΔPLKaat tachyzoites under α-ketoglutarate supplementation conditions. (D) Relative size of plaques in panel C. (E) The high concentration of α-ketoglutarate (2 mM) led to faster replication of AAT-deficient parasites compared to parental PLK in vitro. The data are presented as means ± the SEM of three independent experiments. –, no treatment with α-ketoglutarate; +, treatment by a low concentration (400 μM); ++, treatment by high a concentration (2 mM). *, P < 0.05; **, P < 0.01; ***, P < 0.001, one-way ANOVA plus Dunnett’s multiple-comparison test (replication assay) and one-way ANOVA plus Tukey-Kramer post hoc analysis (plaque assay).
Loss of TgAAT in PLK strain does not abolish the virulence of parasites in vivo.
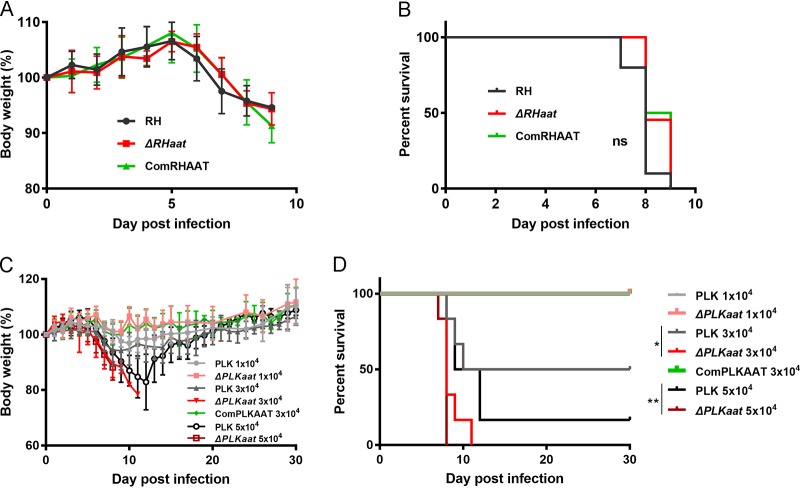
To assess the degree of virulence of Δaat mutants, mice were infected with increasing doses of tachyzoites (10,000, 30,000, or 50,000 parasites per mouse [PLK group] and 100 parasites per mouse [RH group]). At an infection dose of 100 tachyzoites per mouse, RH, ΔRHaat, and ComRHAAT parasites caused all 100% mortality within 9 dpi in animals with similar body weights (%) (Fig. 7A and B) and no differently acute symptoms (data not show). An infection dose of 1 × 104 PLK group tachyzoites was not fatal, whereas a dose of 3 × 104 or 5 × 104 ΔPLKaat parasites caused 100% mortality with a significant reduction in body weight (Fig. 7C and D). This virulence was even greater than that observed for wild-type PLK and ComPLKAAT parasites.
FIG 7.
Virulence tests of Δaat tachyzoites in mice. (A and B) Body weight (%) (A) and survival rate (B) during ΔRHaat infection. Mice were infected by i.p. injection with 100 RH (n = 11), ΔRHaat (n = 11), or ComRHAAT (n = 6) tachyzoites. (C) Body weight during ΔPLKaat infection. Six mice were infected with 10,000, 30,000, and 50,000 tachyzoites by i.p. injection. (D) Survival rate of PLK AAT deficiency parasite infection in mice. Survival curves of mice infected with ΔPLKaat tachyzoites were noted until day 30. *, P < 0.05; **, P < 0.01, log-rank (Mantel-Cox) test.
HYD and CAR inhibit T. gondii growth by an AAT-independent pathway.
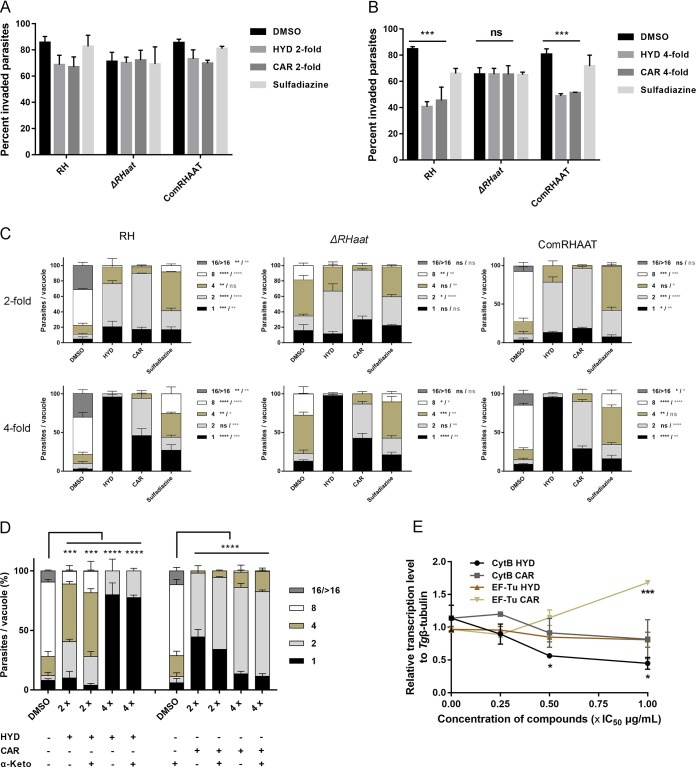
The aforementioned results show that HYD and CAR control acute T. gondii infection in mice; however, AAT deficiency does not abolish the virulence of PLK or RH in vivo. Therefore, HYD and CAR might inhibit T. gondii growth through AAT-independent pathway. To evaluate this possibility, AAT deletion parasites were treated with either HYD or CAR to determine the effects on extracellular invasion and intracellular replication. The results showed that treatment with as high as 4-fold IC50 values of HYD and CAR yielded invasion rates of mutant parasites that were not significantly different among the HYD, CAR, and DMSO treatment groups compared to the wild-type and complemented invasion rates (Fig. 8A and B). Interestingly, as presumed above, intracellular replication of AAT-deficient parasites was also slowed down by both 2- and 4-fold IC50s of HYD and CAR compared to parental parasites (Fig. 8C). On the other hand, the results showed that α-ketoglutarate supplementation significantly rescued defects due to loss of AAT, so we also sought to determine whether HYD- or CAR-impaired growth can be rescued by α-ketoglutarate. Our experiments revealed that the presence of α-ketoglutarate in HYD- or CAR-treated RH-infected cells slightly increased parasite replication (Fig. 8D). Altogether, HYD and CAR inhibit T. gondii growth in a way that is largely independent of the AAT pathway. Further, the use of a 0.25-, 0.5-, or 1-fold IC50 of HYD and CAR to treat RH parasite growth for 5 days showed that the relative transcription levels of mitochondrial genome (CytB gene) were reduced by increasing the compound concentrations (Fig. 8E), suggesting that compounds impairing the parasite lytic cycle may be related to mitochondrial function.
FIG 8.
HYD and CAR inhibit T. gondii growth through an AAT-independent pathway. (A and B) Effects of AAT-deficient parasite invasion treated with HYD and CAR compared to parental and complemented. The data show the means ± the SEM of three independent experiments. ***, P < 0.001, determined by chi-square test. (C) Effects of HYD and CAR treatment for AAT-deficient parasite replication. The concentration of the 2- or 4-fold IC50 values of HYD and CAR were used to treat parasite replication by three strains. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ****, P < 0.0001. A heavy asterisk stands for HYD versus vehicle, and a light asterisk stands for CAR versus vehicle, determined by Tukey’s multiple-comparison test. (D) α-Ketoglutarate (α-Keto) assay. α-Ketoglutarate was supplemented to the HYD and CAR treatment medium, and then replication was determined at 24 h postinfection. ***, P < 0.001; ****, P < 0.0001, determined by Tukey’s multiple-comparison test. (E) Effects of HYD and CAR on mitochondrial genome and apicoplast genome. A total of 2 × 107 tachyzoites were treated by 0.25-, 0.5-, and 1-fold IC50 values of HYD, CAR, or sulfadiazine (1 mg/ml) for 5 days to investigate the expression of CytB and the EF-Tu gene by qPCR. *, P < 0.05; ***, P < 0.001, one-way ANOVA plus Dunnett’s multiple-comparison test.
DISCUSSION
Our studies reveal that HYD and CAR inhibited T. gondii growth by impairing the rounds of the lytic cycle, including inactivated extracellular invasion or reinvasion, slowed intracellular replication, and inhibited egress resulting in unformed plaques. Importantly, HYD and CAR also showed anti-Toxoplasma activity in vivo. As for the inhibition of asexual stages, it is not surprising that acute infection of T. gondii was controlled by both compounds in mice.
A previous study reported that the parasitic IC50 value of sulfadiazine anti-T. gondii type I parasites (RH strain) on HFF cells was 70 μg/ml (25), whereas in the present study lower IC50 values of HYD and CAR on type I RH-GFP strain in HFF cells (4.286 and 23.11 μg/ml, respectively) were obtained. Regarding pyrimethamine, we did not test the effects in this study due to the currently used RH-GFP parasites, which have the pyrimethamine-resistant gene (DHFR) (26–28). However, a published study reveals that an IC50 value of pyrimethamine against T. gondii of 0.84 μg/ml and a selectivity index (SI) value of >11.9 (29). The obtained IC50s of HYD and CAR on type I strain (RH-GFP) are between the values for sulfadiazine and pyrimethamine, and the current results examined the high selectivity indices of 49.04 and >43.27 against T. gondii, confirming that HYD and CAR should be safe and efficient at inhibiting the growth of T. gondii.
It has been reported that HYD and CAR inhibited AAT activity in P. falciparum, which is responsible for the reversible reaction of aspartate and α-ketoglutarate into oxaloacetate and glutamate (19, 22). In the present study, although the reaction of transamination of rTgAAT, which catalyzes aspartate and α-ketoglutarate to glutamate, was inhibited by HYD and CAR treatments, >10-fold differences in the IC50 values of HYD or CAR against cultured type I T. gondii parasites versus rTgRHAAT enzyme were recorded. This weakens the argument that these compounds inhibited Toxoplasma parasites by targeting AAT. Our findings are in contrast to a previous study on P. falciparum which determined a parasitic IC50 value in the same range that of HYD for the rPfAAT enzyme in vitro (19). TgAATs are distantly related to the predicted P. falciparum AATs, with a relatively low 33% identity; thus, it is possible that the AATs in two apicomplexan parasites have different functions, and different sites of drug action may occur. In a previous study, Berger et al. (22) found that AATs also play a role in the final step of methionine regeneration from methylthioadenosine in parasitic protozoa, but since this reaction is dependent on transamination, it was not investigated here.
As expected, our results confirm that deficiency of AAT both in RH and in PLK inhibited the parasite’s ability to invade, replicate, egress, and form plaques in vitro, consistent with the inhibition of the T. gondii lytic cycle by HYD and CAR, although the loss of AAT did not completely abolish parasite growth. However, the intracellular replication of AAT-deficient parasites was inhibited by HYD or CAR similar to parental parasites. This suggests that another pathway might be the target of these compounds. HYD and CAR treatment did not significantly slow down the invasion rates of mutant parasites compared to parental parasites, which may imply that compounds have slight activity to inhibit invasion by AAT reaction, consistent with the current catalytic inhibitor assay. Collectively, HYD and CAR inhibit parasite growth largely through other substrates in T. gondii.
On the other hand, the growth defect due to loss of AAT was significantly rescued by treatment with α-ketoglutarate (400 μM). Interestingly, a high concentration (2 mM) of α-ketoglutarate led to faster intracellular replication of AAT-deficient parasites compared to parental PLK in vitro. This indicates that a loss of AAT in T. gondii impairs α-ketoglutarate homeostasis, which is related to parasite growth in vitro. This finding may also explain why a deficiency of AAT in PLK caused parasites to proliferate faster in mice (Fig. S3), since mice have higher metabolic α-ketoglutarate levels. High parasite burdens led to unexpected upregulation of cytokines and a lethal outcome, which is attributed to the enhanced virulence of PLK in vivo in accordance with previous investigations (30, 31). All of these findings indicate that although AAT is not essential for parasite growth under standard culture conditions, it plays a central role in the complex metabolic balance of α-ketoglutarate metabolism, which is largely involved in the carbon skeleton via glutamine metabolism, as in P. falciparum (1, 13, 19, 22, 32).
However, our data show that the impaired replication caused by HYD and CAR treatment was not rescued through α-ketoglutarate supplementation. These observations show that the inhibition activity of HYD and CAR may not be due to the association of the α-ketoglutarate mechanism to AAT or other metabolic enzymes of the α-ketoglutarate pathway (1, 13). In addition, this impairment of α-ketoglutarate homeostasis in T. gondii by a loss of AAT enhanced PLK parasitic virulence in vivo. Therefore, we doubt whether α-ketoglutarate reuptake affects the virulence of type II PLK parasites in vivo, and we assume that the metabolic enzymes directly related to α-ketoglutarate uptake may not be effective drug targets against T. gondii. Overall, our data suggest that the control of parasite growth by HYD and CAR is not a result of the inhibition of α-ketoglutarate pathway on T. gondii in vitro and in vivo.
In this study, the data showed that HYD and CAR control acute toxoplasmosis and inhibit T. gondii growth in mice but that AAT deficiency does not reduce the virulence of T. gondii in vivo. Hence, HYD and CAR might be acting on T. gondii through a manner independent of the AAT pathway in vivo. Interestingly, these two compounds exhibited similar inhibitory activities in different tests, which may be because of the hydroxylamine unit in the structure of CAR. Hydroxylamine and its compounds have the potential to inhibit the activity of some enzymes in viruses, bacteria, fungi, and protozoa (33, 34). HYD and CAR impairment of the parasite lytic cycle may be associated with the inhibition of the mitochondrial function of the α-ketoglutarate-independent pathway (35, 36). However, further studies focusing on the elucidation of the mechanism of inhibition are warranted. Our study hints at new substrates of HYD and CAR as potential drug targets to inhibit T. gondii growth.
MATERIALS AND METHODS
Animals.
Six-week-old female BALB/c mice were purchased from Clea Japan (Tokyo, Japan) for the preparation of polyclonal antibodies against T. gondii-specific proteins and for survival assays. Mice were housed in the animal facility of National Research Center for Protozoan Diseases, Obihiro University of Agriculture and Veterinary Medicine, with an adequate temperature (25 ± 2°C) and luminosity (12-h light, 12-h dark) under specific-pathogen-free conditions, with free access to food and water. All animal experiments started 1 week after habituation. The recommendations in the Guide for the Care and Use of Laboratory Animals of Obihiro University of Agriculture and Veterinary Medicine, Obihiro, Japan, were strictly followed. The protocol of the present study was approved by the Committee on the Ethics of Animal Experiments of Obihiro University of Agriculture and Veterinary Medicine (permissions 201711 and 2018728).
Parasite cultures.
T. gondii RH strain (type I) with hypoxanthine-xanthine-guanine phosphoribosyl transferase (HXGPRT) deficiency, RH-GFP (a green fluorescent protein expressing-RH strain) (26), and PLK strain (type II) were used in this study. The parasites were maintained in monkey kidney adherent epithelial (Vero) cells cultured in Eagle minimum essential medium (EMEM; Sigma) supplemented with 8% fetal bovine serum (FBS; Biowest, Japan), as well as 100 U ml−1 penicillin and 100 mg ml−1 streptomycin (Sigma), at 37°C and 5% CO2. Parasites were cultured in HFF cells maintained in Dulbecco modified Eagle medium (Sigma) supplemented with 10% FBS, 100 U ml−1 penicillin, and 100 mg ml−1 streptomycin at 37°C in a 5% CO2 atmosphere. For the purification of tachyzoites, the parasites and host cells were washed with sterile PBS and peeled from the plate with a cell scraper (BD Bioscience). The final pellet was passed through a 27-gauge needle syringe three times, filtered through a 5.0-μm-pore filter (Millipore), and counted.
Chemicals.
Hydroxylamine (HYD) and carboxymethoxylamine hemihydrochloride (CAR) were purchased from Sigma (catalog numbers 7803-49-8 and 86-08-8). The inhibitors were dissolved in double-distilled water and DMSO, respectively, and stored at –30°C until use.
Production of recombinant proteins and polyclonal antibodies.
TgAAT genes of the RH strain (Toxoplasma Genomics Resource TGGT1_248600) and the PLK strain (Toxoplasma Genomics Resource TGME49_248600) were amplified by PCR from T. gondii RH and PLK parasite cDNAs, respectively. The primers used included a SalI site (underlined) in the forward primer 5′-ACGC GTCGAC ATG TTT CCA ACT CTT AGT GAG AAC C-3′ and a NotI site (underlined) in the reverse primer 5′-ATAAGAAT GCGGCCGC TTA GCT TGC AGG AAC TGC CCG CAC CA-3′. PCR products digested with SalI and NotI were inserted into a pGEX-4T-1 plasmid vector treated with the same restriction enzymes (Roche, Switzerland). The fragments were sequenced using a BigDye terminator cycle sequencing kit (Applied Biosystems) and an ABI Prism 3100 genetic analyzer (Applied Biosystems). pGEX-4T-3-SAG1 (37) and pGEX-4T-3-GRA7 (38) plasmids from previous studies were used to produce recombinant TgSAG1-GST and TgGRA7-GST proteins. Recombinant pGEX-4T-1-AAT, pGEX-4T-3-SAG1, and pGEX-4T-3-GRA7 were expressed as glutathione S-transferase (GST) fusion protein in Escherichia coli BL21(DE3) (New England BioLabs, Inc.) and purified with glutathione-Sepharose 4B beads (GE Healthcare Life Sciences) according to the manufacturer’s instructions. Purified recombinant protein TgAAT-GST (100 μg) and TgSAG1-GST (100 μg) emulsified in Freund complete adjuvant (Sigma) were i.p. injected into BALB/c mice to produce mouse anti-TgAAT and anti-TgSAG1 polyclonal antibodies. Purified recombinant proteins TgSAG1-GST (1 mg) and TgGRA7-GST (1 mg) were subcutaneously injected into female Japanese white rabbits to produce rabbit anti-TgSAG1 and anti-TgGRA7 polyclonal antibodies.
Cytotoxicity analysis of HYD and CAR on HFF and Vero cells.
Cytotoxicity of the two compounds were determined both on HFF and Vero cells using a Cell Counting Kit-8 (CCK-8; Dojindo Molecular Technologies, Inc., Japan) according to the manufacturer’s instructions. Briefly, the cells were plated in 96-well plates at densities of 1 × 104 (HFFs) or 1 × 105 (Vero cells) per well and then incubated for 48 h (HFF cells) or 24 h (Vero cells) at 37°C in a 5% CO2 atmosphere. The cells were then exposed to the compounds at final concentrations of 1, 5, 10, 25, 50, 100, 200, 400, 800, or 1,000 μg/ml for 24 h, with CCK-8 reagent added and reacted for 4 h. The absorbance of the supernatant was measured at 450 nm using an MTP-120 microplate reader (Corona Electric, Ibaraki, Japan). Cell viability (%) was calculated in three independent experiments.
Inhibition assay of HYD and CAR on Toxoplasma in vitro.
To evaluate anti-Toxoplasma potential in vitro, a growth inhibition assay was performed with the two inhibitors and sulfadiazine (Sigma-Aldrich). Purified RH-GFP tachyzoites at 5 × 104/well were used and added to 96-well plates, where HFF cells were seeded (1 × 104 cells/well) and cultured for 48 h. After 4 h, the extracellular parasites were washed out. The compounds—at final concentrations of 1, 3.125, 6.25, 12.5, 25, or 50 μg/ml in HYD cultures or 1, 5, 10, 15, 20, 25, 50, or 100 μg/ml in CAR cultures—were added. Medium and sulfadiazine (1 mg/ml) were used as negative and positive controls, respectively. The fluorescence intensity of RH-GFP was measured using a microplate reader (SH-900; Corona Electric Co., Ltd., Ibaraki, Japan) after 72 h of incubation. Each concentration was determined in three independent experiments together to calculate the half-maximal inhibitory concentration (IC50) values on T. gondii using Prism 7 software (GraphPad Software, Inc., La Jolla, CA).
Growth assays on extracellular and intracellular T. gondii in vitro.
To examine the effects of compounds on parasite growth, 2- or 4-fold final concentrations (IC50) of compounds, DMSO, and sulfadiazine (1 mg/ml) were used to perform plaque formation, extracellular invasion, intracellular replication, and egress assays on RH and PLK strain parasites as follows. All assays were conducted in triplicate and repeated at least three times.
For the plaque assays, fresh purified tachyzoites (150 of RH and 300 of PLK per well) were inoculated onto monolayers of Vero cells or HFFs in 12-well plate and grown for 8 days for RH parasites and 12 days for PLK parasites at 37°C in 5% CO2. Subsequently, the samples were fixed with 4% paraformaldehyde, stained with 0.1% crystal violet, and imaged on a scanner to analyze the number and relative sizes of plaques, as described previously (24). For the compound assay, at 2 h after parasite infection, the old medium was replaced by the new medium with 2- or 4-fold IC50s of compounds and DMSO, followed by culture for 8 days.
The invasion assay was performed using the indirect fluorescent antibody test (IFAT) (39). Vero cells (1 × 105 per well) were used to plate on 12-well cultured for 24 h. Fresh purified tachyzoites (2 × 105 per well) were inoculated onto cell monolayers for 2 h infection under normal growth conditions. For the compound assay, tachyzoites (2 × 105) were pretreated with either of the two compounds at 2- or 4-fold IC50s, sulfadiazine (1 mg/ml), or DMSO for 1 h at 37°C and then cultured for 2 h. The coverslips were washed with PBS six times to remove extracellular parasites and fixed. Mouse anti-SAG1 polyclonal antibody diluted 1:500 and Alexa Fluor 594-conjugated goat anti-mouse IgG (Sigma) diluted 1:1,000 were then used to count the numbers of attached parasites. After permeabilization with 0.3% Triton X-100/PBS, rabbit anti-SAG1 polyclonal antibody diluted 1:500 and Alexa Fluor 488-conjugated goat anti-rabbit IgG (Sigma) were used to count the numbers of invading parasites, as described previously (3). Samples were examined using an All-in-One fluorescence microscope (BZ-900; Keyence, Japan). Cells that were stained both red and green were scored as attached parasites, whereas those that were stained only in green were scored as invaded parasites. Ten fields were randomly counted for each coverslip.
For the intracellular replication assay, the intracellular replication rate was evaluated by counting the numbers of parasites per vacuole, as previously described (39). Vero cells (1 × 105 per well) were used to plate on 12-well plates cultured for 24 h. Fresh purified tachyzoites (2 × 105 per well) were inoculated onto cell monolayers for 2 h of infection under normal growth conditions. Every well was washed with PBS six times to remove extracellular parasites and then cultured for 24 or 32 h. To examine compound effects on replication, at 2 h postinfection under standard growth conditions the new medium with a 2- or a 4-fold IC50 of compounds was added to cell well, followed by culture for 24 h. Tachyzoites in vacuoles were marked with mouse anti-SAG1 by IFAT at 24 or 32 h postinfection as described above and counted in at least 100 vacuoles.
For the egress assay, Vero cells (1 × 105 per well) were used to plate on 12-well cultured for 24 h. Fresh purified tachyzoites (2 × 105 per well) were inoculated onto cell monolayers for 32 h of infection under normal growth conditions, as previously described (3). The wells were washed with PBS five times to remove extracellular parasites and then incubated with 3 μM A23187 (Sigma). For the HYD and CAR assays, infected cells were treated by 2- and 4-fold IC50s of compounds for 10 min before incubation with 3 μM A23187. After incubation, the cells were fixed, and the IFAT was performed with mouse anti-SAG1 and rabbit anti-GRA7 to measure the percentages of egressed parasitophorous vacuoles (PVs). At least 300 vacuoles were counted per slip.
Virulence tests in mouse model.
Fresh tachyzoites were purified and counted as described above and then used to inject 7-week-old female BALB/c mice by i.p. injection. For treatment in the HYD and CAR assays, at 24 h postinfection, acutely infected mice were i.p. injected with the compounds (5 or 20 mg/kg HYD; 10, 25, and 50 mg/kg CAR; or PBS) for 7 days. Daily observations, such as body weight, morbidity, mortality, and clinical signs, were noted, as were the clinical scores (7). Surviving mice were monitored for 30 days, and blood was drawn at day 30 to confirm infection using an enzyme-linked immunosorbent assay; tissues were collected to determine parasite burdens through targeting of the TgB1 gene by qPCR. GraphPad Prism 7 was used to graph cumulative mortality as Kaplan-Meier survival plots, and the results were analyzed.
Enzyme activity assay.
Enzyme-coupled assays were performed as described previously (19). The concentration of soluble rTgAAT-fused GST was determined to be 1 μg, which was used to perform an enzyme-coupled assay with the specific substrates l-aspartate and α-ketoglutarate (Sigma). The basic reaction principle is that l-aspartate and α-ketoglutarate were catalyzed to glutamate by rTgAAT, and then glutamate with 3-acetylpyridine adenine dinucleotide (APAD; Sigma) are converted to APADH by glutamate dehydrogenase (Sigma); the absorbance of the supernatant was determined at 363 nm. Two reported specific enzymatic inhibitors, including HYD and CAR (Sigma), were used to perform the inhibitor assay (19). All experiments were tested three times independently.
Generation of possible HYD and CAR targeting gene AAT-knockout and complemented lines on RH and PLK strains.
All the primers used in this study are listed in Table S1 in the supplemental material. The T. gondii aspartate aminotransferase-targeting guide RNA (gRNA) sequence was designed using E-CRISP (E-Crisp.org). The TgAAT-specific CRISPR knockout plasmids (pDF-Cas9-sgRH/PLKAAT, Fig. S2D) containing Cas9, GFP, guide RNA, and dihydrofolate reductase DHFR expression cassettes were generated by replacing the original targeting gRNA in pDF-PLK-GRA9 plasmid (3). RH and PLK parasites (107 tachyzoites) were transfected with 10 μg of the pDF-Cas9-sgRH/PLKAAT plasmids, respectively, as previously described (40). Selection of stable transformants based on pyrimethamine (1 μM) was performed as described previously (41, 42). Stable knockout lines (ΔRHaat and ΔPLKaat strains) were isolated by limiting dilution in 96-well plates and confirmed by PCR, Western blotting (WB), and IFAT.
The full TgAAT fragment containing synonymous codons mutated at target sites for gRNA was amplified from RH and PLK cDNA by overlap PCR to generate the complemented AAT plasmids (pB-synoRHAAT and pB-synoPLKAAT), respectively. The ΔRHaat and ΔPLKaat parasites (107 tachyzoites) were transfected with pHX-UPRT and linearized pB-synoRHAAT or pB-synoPLKAAT by SacI (mass ratio, 1:5; Roche, Switzerland) to generate complemented strains (ComRHAAT and ComPLKAAT strains) by insertion at the UPRT locus, as described previously (3). Parasites were screened with 10 μM 5-fluorouracil (Sigma). Positive clones were confirmed as described above. All of the plasmids were verified by DNA sequencing before use.
Examination of parasite growth during AAT deficiency in vitro and in vivo.
To determine the effect on parasite growth after loss of AAT on RH and PLK strains, all strain parasites, including wild-type, Δaat, and ComAAT strains, were used to perform plaque formation, extracellular invasion, intracellular replication, and egress assays in vitro, as well as a virulence assay in vivo, as described above. All experiments were conducted in triplicate and repeated at least three times.
Metabolic treatment assay of Δaat strains in vitro.
For the metabolic treatment assay, α-ketoglutarate is dissolved in EMEM supplemented with 8% FBS, 100 U ml−1 penicillin, and 100 mg ml−1 streptomycin to a final concentration of 400 μM or 2 mM. The parasites were maintained in Vero cells in EMEM containing α-ketoglutarate or normal EMEM to perform an intracellular replication assay at 24 or 32 h postinfection and a plaque assay as described above, respectively. Subsequently, different phenotypes were recorded and analyzed.
Growth examination of treatment with HYD and CAR on extracellular and intracellular AAT-deficient parasites in vitro.
To determine whether HYD and CAR specifically targets Toxoplasma AAT protein, 2- or 4-fold IC50 values of the compounds and sulfadiazine were used to develop extracellular invasion and intracellular replication assays for RH, ΔRHaat, and ComRHAAT strains, as described above. In addition, parasite replication was examined during exposure to 2- or 4-fold of IC50s of two compounds and 400 μM α-ketoglutarate.
DNA isolation and quantitative PCR detection of T. gondii.
DNA was extracted from the brains of HYD-, CAR-, or PBS-treated surviving mice on day 30 by using a DNeasy Blood & Tissue kit (Qiagen, Germany) according to the manufacturer’s instructions. The brain DNA was then amplified with specific primers targeting to the T. gondii B1 gene (forward primer 5′-AAC GGG CGA GTA GCA CCT GAG GAG-3′ and reverse primer 5′-TGG GTC TAC GTC GAT GGC ATG ACA AC-3′) by qPCR. A standard curve was constructed using 10-fold serial dilutions of T. gondii DNA extracted from 105 parasites; thus, the curve ranged from 0.01 to 10,000 parasites. The parasite number was calculated from the standard curve. Likewise, DNA was extracted from 2 × 107 tachyzoites treated with 0.25-, 0.5-, or 1-fold IC50 values of HYD and CAR or sulfadiazine (1 mg/ml). DNA from each tachyzoite sample (30 ng) treated with 1/4, 1/2, and IC50 concentrations of HYD and CAR were used to amplify fragments of Toxoplasma mitochondrial genome cytochrome b (CytB) (forward primer 5′-TAC CGC TTG GAT GTC TGG TT-3′ and reverse primer 5′-AAC CTC CAA GTA GCC AAG GT-3′) and apicoplast genome elongation factor Tu (EF-Tu; using forward primer 5′-TGT GCT CCT GAA GAA ATA GC-3′ and reverse primer 5′-CAT TGG CCC ATC TAC AGC AG-3′), respectively, as previously described (33, 34). The expression levels of target genes were normalized to Tg β-tubulin (forward primer 5′-CAC TGG TAC ACG GGT GAA GGT-3′ and reverse primer 5′-ATT CTC CCT CTT CCT CTG CG-3′) mRNA levels using the 2ΔΔCT method.
The amplification was performed with DNA, 1× PowerUp SYBR Green Master Mix (Thermo Fisher Scientific, Inc., Waltham, MA), and 500 nM concentrations of gene-specific primers in a 10-μl total reaction volume using a standard protocol recommended by the manufacturer (2 min at 50°C, 10 min at 95°C, and then 40 cycles of 95°C for 15 s and 60°C for 1 min). Amplification, data acquisition, and data analysis were carried out using the ABI Prism 7900HT sequence detection system (Applied Biosystems), and the cycle threshold values (CT) were calculated as described previously (43).
Statistical analysis.
To graph and analyze the data, we used Prism 7 software. Statistical analyses were performed with an unpaired Student t test, the Tukey’s multiple-comparison test, a chi-square test, and one-way analysis of variance (ANOVA) plus Tukey-Kramer post hoc analysis. The data represent means ± standard errors of mean (SEM). Survival curves were generated using the Kaplan-Meier method, and statistical comparisons were made by the log-rank method. A P value of <0.05 was considered statistically significant.
Supplementary Material
ACKNOWLEDGMENTS
This study was supported by grants from Japan Society for the Promotion of Science Core-to-Core Program.
We declare that we have no competing interests.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Jacot D, Waller RF, Soldati-Favre D, MacPherson DA, MacRae JI. 2016. Apicomplexan energy metabolism: carbon source promiscuity and the quiescence hyperbole. Trends Parasitol 32:56–70. doi: 10.1016/j.pt.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 2.Weiss LM, Dubey JP. 2009. Toxoplasmosis: a history of clinical observations. Int J Parasitol 9:895–901. doi: 10.1016/j.ijpara.2009.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Guo H, Gao Y, Jia H, Moumouni PFA, Masatani T, Liu M, Lee SH, Galon EM, Li J, Li Y, Tumwebaze MA, Benedicto B, Xuan X. 2019. Characterization of strain-specific phenotypes associated with knockout of dense granule protein 9 in Toxoplasma gondii. Mol Biochem Parasitol 229:53–61. doi: 10.1016/j.molbiopara.2019.01.003. [DOI] [PubMed] [Google Scholar]
- 4.Howe DK, Sibley LD. 1995. Toxoplasma gondii comprises three clonal lineages: correlation of parasite genotype with human disease. J Infect Dis 172:1561–1566. doi: 10.1093/infdis/172.6.1561. [DOI] [PubMed] [Google Scholar]
- 5.Sibley LD, Ajioka JW. 2008. Population structure of Toxoplasma gondii: clonal expansion driven by infrequent recombination and selective sweeps. Annu Rev Microbiol 62:329–351. doi: 10.1146/annurev.micro.62.081307.162925. [DOI] [PubMed] [Google Scholar]
- 6.Halonen SK, Weiss LM. 2013. Toxoplasmosis. Handb Clin Neurol 114:125–145. doi: 10.1016/B978-0-444-53490-3.00008-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Leesombun A, Boonmasawai S, Shimoda N, Nishikawa Y. 2016. Effects of extracts from Thai Piperaceae plants against infection with Toxoplasma gondii. PLoS One 11:e0156116. doi: 10.1371/journal.pone.0156116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wei HX, Wei SS, Lindsay DS, Peng HJ. 2015. A systematic review and meta-analysis of the efficacy of anti-Toxoplasma gondii medicines in humans. PLoS One 10:e0138204. doi: 10.1371/journal.pone.0138204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scheele S, Geiger JA, DeRocher AE, Choi R, Smith TR, Hulverson MA, Vidadala RSR, Barrett LK, Maly DJ, Merritt EA, Ojo KK, Van Voorhis WC, Parsons M. 2018. Toxoplasma calcium-dependent protein kinase 1 inhibitors: probing activity and resistance using cellular thermal shift assays. Antimicrob Agents Chemother 62:e00051-18. doi: 10.1128/AAC.00051-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nitzsche R, Zagoriy V, Lucius R, Gupta N. 2016. Metabolic cooperation of glucose and glutamine is essential for the lytic cycle of obligate intracellular parasite Toxoplasma gondii. J Biol Chem 291:126–141. doi: 10.1074/jbc.M114.624619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fleige T, Fischer K, Ferguson DJ, Gross U, Bohne W. 2007. Carbohydrate metabolism in the Toxoplasma gondii apicoplast: localization of three glycolytic isoenzymes, the single pyruvate dehydrogenase complex, and a plastid phosphate translocator. Eukaryot Cell 6:984–996. doi: 10.1128/EC.00061-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fleige T, Pfaff N, Gross U, Bohne W. 2008. Localization of gluconeogenesis and tricarboxylic acid (TCA) cycle enzymes and first functional analysis of the TCA cycle in Toxoplasma gondii. Int J Parasitol 38:1121–1132. doi: 10.1016/j.ijpara.2008.01.007. [DOI] [PubMed] [Google Scholar]
- 13.MacRae JI, Sheiner L, Nahid A, Tonkin C, Striepen B, McConville MJ. 2012. Mitochondrial metabolism of glucose and glutamine is required for intracellular growth of Toxoplasma gondii. Cell Host Microbe 12:682–692. doi: 10.1016/j.chom.2012.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Blume M, Nitzsche R, Sternberg U, Gerlic M, Masters SL, Gupta N, McConville MJ. 2015. A Toxoplasma gondii gluconeogenic enzyme contributes to robust central carbon metabolism and is essential for replication and virulence. Cell Host Microbe 18:210–220. doi: 10.1016/j.chom.2015.07.008. [DOI] [PubMed] [Google Scholar]
- 15.Oppenheim RD, Creek DJ, Macrae JI, Modrzynska KK, Pino P, Limenitakis J, Polonais V, Seeber F, Barrett MP, Billker O, McConville MJ, Soldati-Favre D. 2014. BCKDH: the missing link in apicomplexan mitochondrial metabolism is required for full virulence of Toxoplasma gondii and Plasmodium berghei. PLoS Pathog 10:e1004263. doi: 10.1371/journal.ppat.1004263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng J, Jia H, Zheng Y. 2015. Knockout of leucine aminopeptidase in Toxoplasma gondii using CRISPR/Cas9. Int J Parasitol 45:141–148. doi: 10.1016/j.ijpara.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 17.Xia N, Yang J, Ye S, Zhang L, Zhou Y, Zhao J, David Sibley L, Shen B. 2018. Functional analysis of Toxoplasma lactate dehydrogenases suggests critical roles of lactate fermentation for parasite growth in vivo. Cell Microbiol 20:e12794. doi: 10.1111/cmi.12794. [DOI] [PubMed] [Google Scholar]
- 18.Xia N, Ye S, Liang X, Chen P, Zhou Y, Fang R, Zhao J, Gupta N, Yang S, Yuan J, Shen B. 2019. Pyruvate homeostasis as a determinant of parasite growth and metabolic plasticity in Toxoplasma gondii. mBio 10:e00898-19. doi: 10.1128/mBio.00898-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wrenger C, Müller IB, Schifferdecker AJ, Jain R, Jordanova R, Groves MR. 2011. Specific inhibition of the aspartate aminotransferase of Plasmodium falciparum. J Mol Biol 405:956–971. doi: 10.1016/j.jmb.2010.11.018. [DOI] [PubMed] [Google Scholar]
- 20.Wrenger C, Muller IB, Silber AM, Jordanova R, Lamzin VS, Groves MR. 2012. Aspartate aminotransferase: bridging carbohydrate and energy metabolism in Plasmodium falciparum. Curr Drug Metab 13:332–336. doi: 10.2174/138920012799320400. [DOI] [PubMed] [Google Scholar]
- 21.Marković-Housley Z, Schirmer T, Hohenester E, Khomutov AR, Khomutov RM, Karpeisky MY, Sandmeier E, Christen P, Jansonius JN. 1996. Crystal structures and solution studies of oxime adducts of mitochondrial aspartate aminotransferase. Eur J Biochem 236:1025–1032. doi: 10.1111/j.1432-1033.1996.01025.x. [DOI] [PubMed] [Google Scholar]
- 22.Berger LC, Wilson J, Wood P, Berger BJ. 2001. Methionine regeneration and aspartate aminotransferase in parasitic protozoa. J Bacteriol 183:4421–4434. doi: 10.1128/JB.183.15.4421-4434.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lourido S, Tang K, Sibley LD. 2012. Distinct signaling pathways control Toxoplasma egress and host-cell invasion. EMBO J 31:4524–4534. doi: 10.1038/emboj.2012.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shen B, Sibley LD. 2014. Toxoplasma aldolase is required for metabolism but dispensable for host-cell invasion. Proc Natl Acad Sci U S A 111:3567–3572. doi: 10.1073/pnas.1315156111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.de Oliveira TC, Silva DA, Rostkowska C, Béla SR, Ferro EA, Magalhães PM, Mineo JR. 2009. Toxoplasma gondii: effects of Artemisia annua L. on susceptibility to infection in experimental models in vitro and in vivo. Exp Parasitol 122:233–241. doi: 10.1016/j.exppara.2009.04.010. [DOI] [PubMed] [Google Scholar]
- 26.Nishikawa Y, Xuenan X, Makala L, Vielemeyer O, Joiner KA, Nagasawa H. 2003. Characterization of Toxoplasma gondii engineered to express mouse interferon-gamma. Int J Parasitol 33:1525–1535. doi: 10.1016/s0020-7519(03)00204-2. [DOI] [PubMed] [Google Scholar]
- 27.Nishikawa Y, Zhang H, Ibrahim HM, Ui F, Ogiso A, Xuan X. 2008. Construction of Toxoplasma gondii bradyzoite expressing the green fluorescent protein. Parasitol Int 57:219–222. doi: 10.1016/j.parint.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 28.Leesombun A, Iijima M, Umeda K, Kondoh D, Pagmadulam B, Abdou AM, Suzuki Y, Ohba SI, Isshiki K, Kimura T, Kubota Y, Sawa R, Nihei CI, Nishikawa Y. 2019. Metacytofilin is a potent therapeutic drug candidate for toxoplasmosis. J Infect Dis 2019:jiz501. [DOI] [PubMed] [Google Scholar]
- 29.Abugri DA, Witola WH, Russell AE, Troy RM. 2018. In vitro activity of the interaction between taxifolin (dihydroquercetin) and pyrimethamine against Toxoplasma gondii. Chem Biol Drug Des 91:194–201. doi: 10.1111/cbdd.13070. [DOI] [PubMed] [Google Scholar]
- 30.Suzuki Y, Orellana MA, Schreiber RD, Remington JS. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516–518. doi: 10.1126/science.3128869. [DOI] [PubMed] [Google Scholar]
- 31.Mordue DG, Monroy F, La Regina M, Dinarello CA, Sibley LD. 2001. Acute toxoplasmosis leads to lethal overproduction of Th1 cytokines. J Immunol 167:4574–4584. doi: 10.4049/jimmunol.167.8.4574. [DOI] [PubMed] [Google Scholar]
- 32.Jain R, Jordanova R, Müller IB, Wrenger C, Groves MR. 2010. Purification, crystallization, and preliminary X-ray analysis of the aspartate aminotransferase of Plasmodium falciparum. Acta Crystallogr Sect F Struct Biol Cryst Commun 66:409–412. doi: 10.1107/S1744309110003933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gross P. 1985. Biologic activity of hydroxylamine: a review. Crit Rev Toxicol 14:87–99. doi: 10.3109/10408448509023765. [DOI] [PubMed] [Google Scholar]
- 34.Sarojini G, Oliver DJ. 1985. Inhibition of glycine oxidation by carboxymethoxylamine, methoxylamine, and acethydrazide. Plant Physiol 77:786–789. doi: 10.1104/pp.77.3.786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uddin T, McFadden GI, Goodman CD, Uddin T, McFadden GI, Goodman CD. 2018. Validation of putative apicoplast-targeting drugs using a chemical supplementation assay in cultured human malaria parasites. Antimicrob Agents Chemother 62:e01161-17. doi: 10.1128/AAC.01161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Korsinczky M, Chen N, Kotecka B, Saul A, Rieckmann K, Cheng Q. 2000. Mutations in Plasmodium falciparum cytochrome b that are associated with atovaquone resistance are located at a putative drug-binding site. Antimicrob Agents Chemother 44:2100–2108. doi: 10.1128/aac.44.8.2100-2108.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kimbita EN, Xuan X, Huang X, Miyazawa T, Fukumoto S, Mishima M, Suzuki H, Sugimoto C, Nagasawa H, Fujisaki K, Suzuki N, Mikami T, Igarashi I. 2001. Serodiagnosis of Toxoplasma gondii infection in cats by enzyme-linked immunosorbent assay using recombinant SAG1. Vet Parasitol 102:35–44. doi: 10.1016/s0304-4017(01)00522-2. [DOI] [PubMed] [Google Scholar]
- 38.Masatani T, Matsuo T, Tanaka T, Terkawi MA, Lee EG, Goo YK, Aboge GO, Yamagishi J, Hayashi K, Kameyama K, Cao S, Nishikawa Y, Xuan X. 2013. TgGRA23, a novel Toxoplasma gondii dense granule protein associated with the parasitophorous vacuole membrane and intravacuolar network. Parasitol Int 62:372–379. doi: 10.1016/j.parint.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 39.Wang M, Cao S, Du N, Fu J, Li Z, Jia H, Song M. 2017. The moving junction protein RON4, although not critical, facilitates host cell invasion and stabilizes MJ members. Parasitology 144:1490–1497. doi: 10.1017/S0031182017000968. [DOI] [PubMed] [Google Scholar]
- 40.Soldati D, Boothroyd JC. 1993. Transient transfection and expression in the obligate intracellular parasite Toxoplasma gondii. Science 260:349–352. doi: 10.1126/science.8469986. [DOI] [PubMed] [Google Scholar]
- 41.Donald RG, Roos DS. 1998. Gene knock-outs and allelic replacements in Toxoplasma gondii: HXGPRT as a selectable marker for hit-and-run mutagenesis. Mol Biochem Parasitol 91:295–305. doi: 10.1016/s0166-6851(97)00210-7. [DOI] [PubMed] [Google Scholar]
- 42.Reynolds MG, Oh J, Roos DS. 2001. In vitro generation of novel pyrimethamine resistance mutations in the Toxoplasma gondii dihydrofolate reductase. Antimicrob Agents Chemother 45:1271–1277. doi: 10.1128/AAC.45.4.1271-1277.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mahmoud ME, Ui F, Salman D, Nishimura M, Nishikawa Y. 2015. Mechanisms of interferon-beta-induced inhibition of Toxoplasma gondii growth in murine macrophages and embryonic fibroblasts: role of immunity-related GTPase M1. Cell Microbiol 17:1069–1083. doi: 10.1111/cmi.12423. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.