The percentage of the time that the free drug concentration remains above a concentration threshold (%fT > concentration threshold) has frequently been identified to be the optimal pharmacokinetic (PK)-pharmacodynamic (PD) target of interest for tazobactam using in vitro infection models.
KEYWORDS: tazobactam, critical care, pharmacokinetics, pharmacodynamics, β-lactamase inhibitor
ABSTRACT
The percentage of the time that the free drug concentration remains above a concentration threshold (%fT > concentration threshold) has frequently been identified to be the optimal pharmacokinetic (PK)-pharmacodynamic (PD) target of interest for tazobactam using in vitro infection models. Similar in vitro models suggested that an 85% fT > concentration threshold of 2 μg/ml for tazobactam is required to demonstrate a 2-log10-unit decrease in the number of CFU per milliliter from that at the baseline at 24 h for high-level β-lactamase-producing Escherichia coli strains. The objective of this study was to characterize the tazobactam concentrations in a cohort of critically ill patients with Gram-negative bacterial infections, determine if traditional dosing regimens achieve a prespecified PK/PD target of an 80% fT > concentration threshold of 2 μg/ml, and propose alternative dosing regimens. Hospitalized critically ill adult patients receiving piperacillin-tazobactam (TZP) for a culture-positive Gram-negative bacterial infection were eligible to consent for study inclusion. Two blood samples were drawn, one during the midpoint of the dosing interval and one at the time of the trough concentration once the patient achieved PK steady state. A population PK model was developed using Phoenix NLME (v8.1) software to characterize the observed concentration-time profile of tazobactam, explore potential covariates to explain the variability in the clearance and volume parameters, and to simulate potential dosing regimens that would achieve the PK/PD target. The PK of tazobactam were adequately described by a one-compartment model with first-order elimination in 18 patients who provided consent. The final model incorporated creatinine clearance as a covariate on clearance. Simulations demonstrated target attainments of less than 50% for tazobactam using traditional dosing regimens (4/0.5 g over 30 min every 6 h). Target attainments of greater than 75% were achieved when using extended infusion times of 4 to 6 h or when administering TZP as a continuous infusion (16/2 g over 24 h). Traditional tazobactam dosing regimens fail to achieve conservative PK/PD targets in critically ill patients. Increases in the tazobactam dose or prolongation of the infusion rate may be warranted to achieve activity against β-lactamase-producing Gram-negative bacteria.
TEXT
There has been considerable interest in optimizing the pharmacokinetics (PK) and pharmacodynamics (PD) of β-lactams by leveraging the time-dependent killing activity of these compounds. However, far less is known about the PK/PD characteristics of β-lactamase inhibitors when given in combination with β-lactams (1). The increase in the incidence of bacteria producing extended-spectrum β-lactamases (ESBLs) creates significant clinical challenges that consequently lead to the increased use of carbapenems and carbapenem resistance (1, 2). A recent randomized controlled trial evaluated piperacillin-tazobactam (TZP) compared to meropenem for the definitive treatment of bloodstream infections caused by ceftriaxone-nonsusceptible Escherichia coli and Klebsiella pneumoniae strains, the majority of which (86%) were confirmed to be ESBL producers. Piperacillin-tazobactam did not meet the predefined noninferiority margin compared to meropenem, with 30-day mortality occurring in 12.3% of patients randomized to piperacillin-tazobactam but only 3.7% of patients in the meropenem group (3). Despite these findings, there is still interest in identifying noncarbapenem treatment options for infections caused by ESBL-producing bacteria, and optimization of the PK/PD of β-lactam–β-lactamase inhibitors (BLBLIs) may keep these drugs a viable option. Over the past decade, the use of extended- or continuous-infusion techniques to optimize β-lactam therapeutics has been investigated, but the impact on β-lactamase inhibitor activity is relatively unknown (3). Furthermore, BLBLI combination products are available only as fixed-dose combination products, making dose adjustments based on the β-lactamase component even more difficult.
Tazobactam is a suicide β-lactamase inhibitor that possesses inhibitory activity against Ambler class A enzymes, including ESBL variants (4). In vitro studies have demonstrated that the optimal PK/PD index for tazobactam, the percentage of the time that the free drug concentration remains above a concentration threshold (%fT > concentration threshold) varies, depending on the level of β-lactamase expression. An in vitro model with CTX-M-15-producing E. coli identified that the tazobactam threshold concentrations required when tazobactam is used in combination with piperacillin against strains with low, moderate, and high levels of β-lactamase expression were 0.25, 0.5, and 2 mg/liter, respectively (5). For isolates with high levels of CTX-M-15 expression, a 44.9%, 62.9%, and 84.9% fT > concentration threshold of 2 mg/liter of tazobactam was required for net bacterial stasis, a 1-log10-unit decrease in the number of CFU per milliliter at 24 h from that at the baseline, and a 2-log10-unit decrease in the number of CFU per milliliter at 24 h from that at the baseline, respectively. The optimal PK/PD index associated with tazobactam was also found to be the %fT > concentration threshold when tazobactam is used in combination with ceftolozane. Compared to the values for piperacillin, the %fT > concentration threshold values were lower when tazobactam was used with ceftolozane (35%, 50%, and 70% for net bacterial stasis, a 1-log10-unit decrease in the number of CFU per milliliter at 24 h from that at the baseline, and a 2-log10-unit decrease in the number of CFU per milliliter at 24 h from that at the baseline) (6). It is hypothesized that differences in β-lactamase binding and stability could result in differences in %fT > concentration threshold values.
When using average PK parameters associated with tazobactam, the standard 0.5 g administered every 6 h as a 30-min infusion may not reliably meet this target (1, 5, 6). Given these challenges, the objective of this study was to characterize tazobactam PK in a cohort of critically ill patients with Gram-negative bacterial infections using a population PK modeling approach. Currently, a population model that characterizes the pharmacokinetics of tazobactam in critically ill patients positive for Gram-negative bacterial infections is not reported in the literature. Population PK models have the potential to provide platforms for predicting concentration-time profiles in individual patients, accounting for subject-specific prognostic factors (covariates), thus offering clinicians tools to guide dosing decisions. In addition, the PK model was used to perform simulations to evaluate the impact of various infusion times on the %fT > concentration threshold for tazobactam.
RESULTS
Eighteen critically ill adult patients with culture-positive Gram-negative bacterial infections were consented for study inclusion. Baseline demographics and dosing history information are summarized in Table 1. An infusion duration of 30 min (traditional dosing) was used to administer TZP to all patients. Seven out of the 18 patients exhibited moderate to severe renal impairment (creatinine clearance [CLCR], <40 ml/min) at the time of sample collection. The tazobactam concentrations in plasma taken at the dosing interval midpoint ranged from 1.97 to 42.6 μg/ml. Trough concentrations were below the limit of quantification (<0.9 μg/ml) for 22% of the patients (4/18) and uninterpretable due to the assay in 16% of the patients (3/18). Seven out of 18 patients (39%) had an observed concentration of less than 2.85 μg/ml (equivalent to a free tazobactam concentration of 2 μg/ml, assuming an unbound fraction of 70%). Positive clinical outcomes, defined as the completion of an antibiotic treatment course without a change or addition of antibiotic therapy and the commencement of no additional antibiotics within 48 h of treatment cessation, were exhibited in 78% (14/18) of the patients. Out of the 4 patients with a negative outcome, only 2 patients had tazobactam concentrations of less than 2.85 μg/ml.
TABLE 1.
Baseline characteristicsa
| Patient characteristic (n = 18) | Value |
|---|---|
| Mean (SD) age (yr) | 63 (14.9) |
| No. (%) of male patients | 11 (61) |
| No. (%) of patients in the following location: | |
| Medical intensive care unit | 8 (44) |
| Neurosurgical intensive care unit | 5 (28) |
| Surgical intensive care unit | 4 (22) |
| Cardiac surgery intensive care unit | 1 (6) |
| Mean (SD) value for: | |
| Wt (kg) | 77.5 (21.6) |
| Serum creatinine concn (mg/dl) | 1.29 (1.15) |
| CLCRb (ml/min) | 78.2 (40.7) |
| APACHE II score | 24.2 (5.2) |
| SOFA score | 9.4 (4.1) |
| No. (%) of patients with infection at the following site: | |
| Respiratory | 9 (50) |
| Blood | 5 (28) |
| Urine | 2 (11) |
| Intra-abdominal | 1 (5.5) |
| Wound | 1 (5.5) |
| No. (%) of patients infected with the following organism: | |
| Pseudomonas aeruginosa | 7 (39) |
| Klebsiella pneumoniae | 3 (17) |
| E. coli | 2 (11) |
| Enterobacter spp. | 2 (11) |
| Serratia spp. | 1 (5.5) |
| Klebsiella oxytoca | 1 (5.5) |
| Shewanella putrefaciens | 1 (5.5) |
| Proteus spp. | 1 (5.5) |
| No. (%) of patients receiving the following piperacillin-tazobactam dose at the indicated interval: | |
| 4.5 g i.v. over 30 min q6h | 6 (33) |
| 4.5 g i.v. over 30 min q8h | 1 (6) |
| 3.375 g i.v. over 30 min q6h | 8 (44) |
| 2.25 g i.v. over 30 min q6h | 3 (17) |
APACHE II, Acute Physiology and Chronic Health Evaluation II; SOFA, Sequential Organ Failure Assessment; i.v., intravenous; q6h, every 6 h; q8h, every 8 h.
CLCR was calculated according to the Cockcroft-Gault equation.
Population pharmacokinetic analysis.
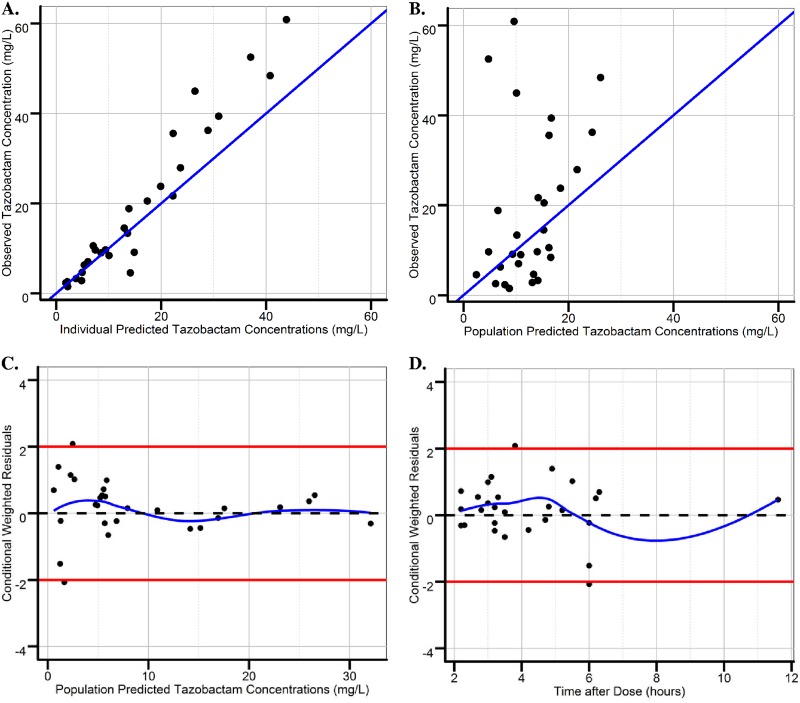
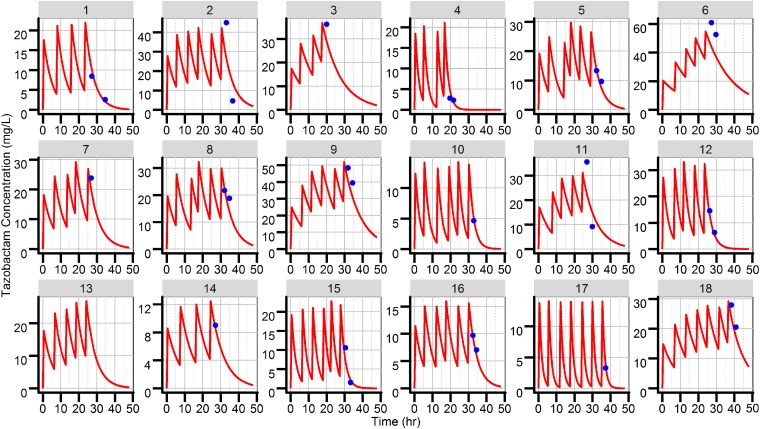
A total of 29 total tazobactam plasma concentrations from 18 different critically ill patients were used to develop the pharmacokinetic model. The PK of tazobactam were adequately described by a one-compartment model with first-order elimination. Due to the sparse nature of the data, the volume of distribution (V) was fixed to 20 liters (not estimated), based on previous pharmacokinetic studies (7–13). Creatinine clearance was found to be a statistically significant covariate (decreasing the objective function value [OFV] by 21 points) on clearance and decreased the between-subject variability on clearance by 15%. The inclusion of CLCR as a covariate on clearance is also clinically justified, as 68% of an administered dose is reported to be excreted as unchanged drug in the urine (5). Patients with normal renal function (CLCR, 120 ml/min) exhibited total drug clearance of 5.27 liters/h, whereas for patients with moderate renal function (CLCR, 60 ml/min), total drug clearance was 4.19 liters/h. Although total body weight was not found to be a statistically significant covariate (no change in OFV or a significant decrease in between-subject variability), the large range in total body weight found in this study (45 to 100 kg) justified the use of allometric scaling of V (14). Final parameter estimates along with nonparametric bootstrap-derived confidence intervals are provided in Table 2. Median parameter estimates from 500 bootstrap replications were similar to the population mean estimates from the final model. Goodness-of-fit plots demonstrated an adequate fit of the final model with minimal bias (Fig. 1). The individual predicted and observed tazobactam concentration-versus-time plots obtained using each patient’s complete dosing history are provided in Fig. 2.
TABLE 2.
Final population PK model parameter estimatesc
| Population parameter | Mean estimate | % RSE | % BSV | Shrinkage | Bootstrap median (n = 500) | Bootstrap 95% CI |
|---|---|---|---|---|---|---|
| CL (liters/h)a | 5.27 | 31.6 | 68 | 0.101 | 4.33 | 2.22–9.06 |
| V (liters)b | 20.0 | |||||
| CLCR effect on CL | 0.336 | 74.1 | 0.179 | 0.051–0.712 | ||
| RUV proportional error (%) | 44.1 | 30.2 | 0.299 | 45.6 | 16.6–71.5 |
CL = 5.27 · (CLCR/120)0.33.
V = 20 · (TBW/70)1.0, where TBW is total body weight.
CL, clearance; V, volume of distribution; CLCR, creatinine clearance; BSV, between-subject variability; RUV, residual unexplained variability; RSE: relative standard error; CI, confidence interval.
FIG 1.
Diagnostic plots of the final one-compartment model. Diagnostic plots of the final one-compartment model for observed versus individual predicted tazobactam concentrations (A), observed versus population predicted tazobactam concentrations (B), conditional weighted residuals versus population predicted tazobactam concentrations (C), and conditional weighted residuals versus the time after the dose (D). The blue lines in panels A and B represent lines of identity. The blue lines in panels C and D represent smoothed regression lines. The red lines in panels C and D represent outlier margins of y = −2 and y = 2.
FIG 2.
Individual goodness-of-fit plots. Blue dots represent the observed individual tazobactam concentrations. Red lines represent individual the predicted tazobactam concentrations.
Simulations for dose selection.
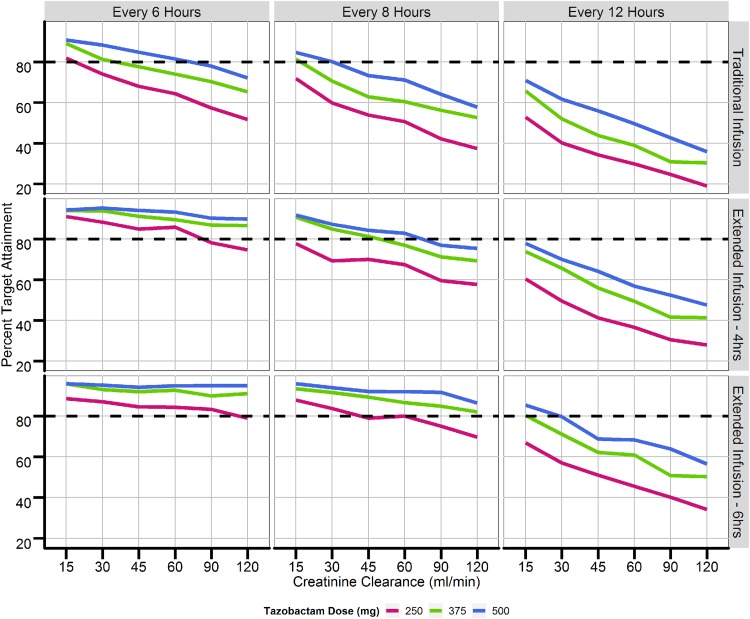
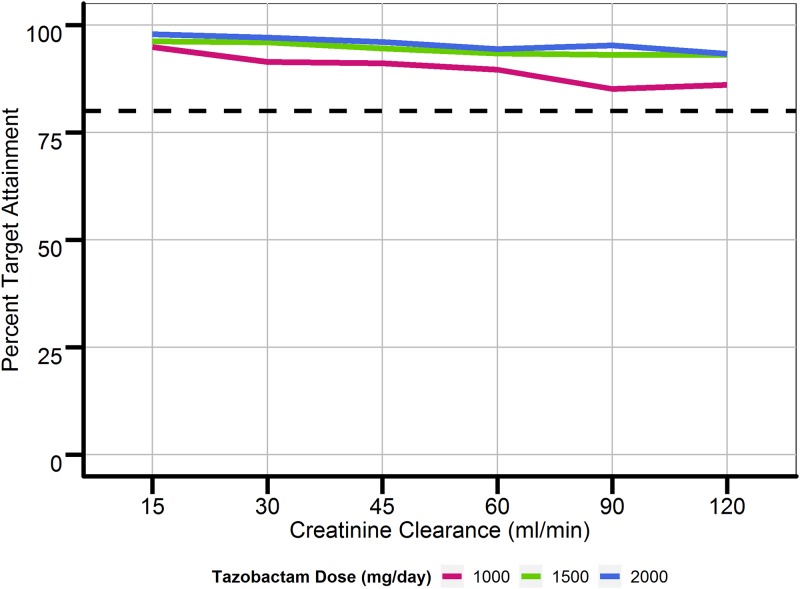
Figure 3 provides a summary of the simulations performed using data for 1,000 patients with CLCRs ranging from 15 to 120 ml/min and traditional dosing regimens (30-min infusions every 6, 8, and 12 h) versus extended-infusion dosing regimens (4-h infusions every 6, 8, and 12 h). Percent target attainments (a %fT > concentration threshold of a 2-μg/ml free tazobactam plasma concentration) for patients with renal function consisting of a CLCR of greater than 60 ml/min were less than 80% when using traditional dosing regimens for all doses of tazobactam. When using 4-h and 6-h extended infusions (similar to giving a continuous infusion if the dosing interval is equal to 6 h), percent target attainments were increased across all tazobactam doses and ranges of renal function. Under the assumption that one could quantify concentrations above 0.25 μg/ml using a bioanalytical assay with a limit of quantification lower than the one used in this study, the percent target attainment for strains producing low and moderate levels of β-lactamases could also be simulated. For strains with low to moderate levels of β-lactamase expression with targets of %fT > concentration thresholds of 0.25-μg/ml and a 0.5-μg/ml free tazobactam plasma concentrations, the simulations indicated that current traditional infusion regimens of TZP given every 6 h over 30 min achieve a percent target attainment of greater than 85% in patients with a CLCR of 120 ml/min. Increasing the dose interval to every 8 and 12 h led to a further decrease in percent target attainment. Figure 4 displays the highest percent target attainments achieved, based on the simulations, using 24-h continuous infusions at tazobactam doses of 1, 1.5, and 2 g (the maximum recommended dose of 16/2 g of TZP per day). Similar results were also observed when simulating a loading infusion dose (250 mg, 375 mg, or 500 mg) over 30 min, followed by a continuous infusion over 24 h, versus administering only a continuous infusion over 24 h. Results based on simulations using a threshold for strains producing high levels of a β-lactamase can also be extrapolated to results for strains producing low and moderate levels, given that a continuous 2-g infusion would lead to concentrations greater than 2 μg/ml over 24 h (%fT > concentration thresholds of 0.25-μg/ml and 0.5-μg/ml free tazobactam plasma concentrations for strains producing low and moderate levels, respectively).
FIG 3.
Percent target attainment for traditional and extended infusions of tazobactam. Target attainment was defined as achievement of tazobactam concentrations above 2.85 μg/ml (a 2-μg/ml free tazobactam concentration) for at least 85% of the specified dosing interval. The dashed lines represent 50% target attainment.
FIG 4.
Percent target attainment for continuous infusions of tazobactam. Target attainment was defined as achievement of tazobactam concentrations above 2.85 μg/ml (a 2-μg/ml free tazobactam concentration) for at least 85% of the specified dosing interval (the dosing interval for continuous infusion was defined as every 24 h). The dashed line represents 50% target attainment.
DISCUSSION
Although the PK/PD of β-lactams have been well characterized, limited research on the PK/PD properties of β-lactamase inhibitors is available to inform dosing regimens in the critically ill population. With the rapid increase in antibiotic resistance, there is an urgent need to optimize available BLBLI combination products to target multidrug-resistant organisms. Previous in vitro infection models have indicated that the %fT > threshold concentration was the PK/PD index that most correlated with tazobactam efficacy (4). Due to the heterogeneity in β-lactamase transcription by multidrug-resistant bacteria, a range of threshold concentrations of between 0.25 and 2 μg/ml was proposed in the prior in vitro studies for strains producing low to high levels of β-lactamases when tazobactam was used in combination with piperacillin. Therapeutic drug monitoring has been part of routine clinical practice for vancomycin and aminoglycoside therapy, unlike for BLBLI therapy. However, with limited pharmacokinetic experience, alternative dosing regimens, such as extended and continuous infusions, have been proposed to improve therapeutic outcomes in patients receiving β-lactam therapy.
The highlights of the present work include the characterization of tazobactam pharmacokinetics in a cohort of critically ill patients and the evaluation of different dosing regimens to improve therapeutic outcomes based on the targets identified in vitro. The one-compartment model with CLCR as a significant covariate on drug clearance developed in the present study adequately captured the observed tazobactam concentrations. The high between-subject variability on pharmacokinetic parameters could potentially be attributed to critical illness due to sepsis, multiorgan failure, and trauma (8). Prognostic factors, such as total body weight, lean body weight, gender, and age, were systematically analyzed during covariate selection but were not found to be statistically significant predictors of tazobactam pharmacokinetics. The individual trough concentrations observed in samples taken between 0.5 and 2 h prior to the next dose indicated that the current traditional infusion duration of 30 min does not achieve at least the 2-μg/ml threshold concentration required to target high-level-β-lactamase-producing strains. Limitations regarding the quantification limit of tazobactam (concentration below the limit of quantification, <0.9 μg/ml) do not allow for evaluating whether the target thresholds are met for strains producing low (0.25 μg/ml) and moderate (0.5 μg/ml) levels of β-lactamases.
In order to maximize the time-dependent killing pharmacodynamic property of β-lactams (exposure time above the MIC threshold), extended and continuous infusions have been proposed as dosing strategies that may be used as alternatives to conventional intermittent application. Simulations of different tazobactam infusion regimens using the final population PK model demonstrated that extended infusions achieve a higher percent target attainment than the traditional 30-min infusion. Percent target attainment increased when the infusion duration was extended from 4 to 6 h. These findings are similar to those of studies comparing extended infusions and intermittent dosing of various β-lactam antibiotics (12, 15, 16). Since a 6-h infusion every 6 h was effective in maintaining concentrations above the required target, continuous tazobactam infusion regimens of 1, 1.5, and 2 g over 24 h (equivalent to total daily doses of 250, 375, and 500 mg, respectively, when administered every 6 h) were simulated. The results of the simulations agreed with those of previous studies that suggested that the rates of target attainment with continuous-infusion dosing with the combined product, piperacillin, were higher (15, 16). Continuous infusions of TZP would yield a percent target attainment for tazobactam of at least 75% when administering 16/2 g over 24 h. The implementation of extended- or continuous-infusion dosing regimens in clinical practice has been complicated by Y-site compatibility issues when administered with vancomycin, commonly used with TZP for empirical broad-spectrum antibiotic therapy (17). Furthermore, the ability to dedicate a separate intravenous line for TZP in critically ill patients can be difficult due to the use of numerous other intravenous drugs.
A potential limitation to the characterization of tazobactam pharmacokinetics is the sparse sampling scheme used to measure tazobactam concentrations over time. Extensive prior information on the pharmacokinetics of tazobactam was used to complement the data at hand to develop an adequate population pharmacokinetic model. A one-compartment model was chosen a priori based on the lack of pharmacokinetic samples collected during the distribution phase. The one-compartment-based model structure may allow for a more precise estimation of clearance. Because no pharmacokinetic samples were taken at the end of infusion, an accurate estimation of the typical V and the corresponding between-subject variability may not be possible. Therefore, V was fixed to 20 liters based on the average V observed from patients receiving TZP or ceftolozane-tazobactam (C/T). The limited sample size of this study influenced the use of physiologically based covariates instead of statistically significant covariates during model development. V can best be characterized when pharmacokinetic information is available right at the end of the infusion. However, due to the limited number of samples collected, between-subject variability on V was not estimated. However, because of the large range of total body weights observed in this trial, V was instead allometrically scaled. Another limitation of this study is the lack of free tazobactam concentration data. Dynamic changes and concentration-dependent protein binding could impact tazobactam concentrations at the site of action. Critically ill patients are more susceptible to changes in protein binding due to the underlying disease state and drug interactions. Further studies that quantify free tazobactam concentrations should be considered to adequately predict the dosing regimens that optimize the target attainment of interest.
The typical clearance for tazobactam in this critically ill population was estimated to be lower (5.3 liters/h) than what was observed in healthy subjects or infected patients receiving TZP or C/T. Potential differences in clearance could be explained by multiorgan dysfunction, changes in protein binding, and drug interactions subsequent to polypharmacy frequently observed in the critically ill population (18). Previous studies conducted in healthy subjects receiving TZP indicated that the total tazobactam clearance ranged from 7.4 to 8.3 liters/h, whereas a higher tazobactam clearance was estimated in studies conducted in hospitalized patients with complicated intra-abdominal infections (10.7 liters/h), colorectal surgery patients (11.3 liters/h), and critically ill trauma patients (16.5 liters/h) (7, 8, 10, 13, 19). Both piperacillin and tazobactam are eliminated via glomerular filtration and renal tubular secretion (6). Several reports indicate competitive inhibition of tubular secretion, which could impact the total clearance of tazobactam (20). In contrast, current literature suggests that the clearance of tazobactam when used in combination with ceftolozane is markedly increased. The estimated tazobactam clearances achieved when tazobactam was given with ceftolozane in studies conducted in hospitalized patients with nosocomial pneumonia and intra-abdominal infections were 18.0 liters/h and 20.8 liters/h, respectively (9, 11). Pharmacokinetic studies in healthy patients indicate that ceftolozane is primarily eliminated via glomerular filtration. The higher clearance of tazobactam when it is given with ceftolozane than when it is given with piperacillin could be explained because of the lack of competitive tubular secretion (21).
Although 7 out of 18 patients were estimated to have a %fT < concentration threshold of 2 μg/ml of unbound tazobactam, only 4 patients exhibited negative clinical outcomes. Furthermore, the target threshold concentrations used to calculate percent target attainment were based on an in vitro study evaluating different strains of ESBL-producing E. coli. However, 16 out of 18 patients infected with bacterial strains other than E. coli were enrolled in this study, and several were infected with multidrug-resistant pathogens. Further studies are needed to identify the similarity of the threshold concentrations of other infections treated with TZP. Additional clinical studies are also needed to correlate the proposed tazobactam PK/PD target index with clinical outcomes. In summary, in a cohort of critically ill patients, traditional dosing of TZP did not achieve the PK/PD target goal for tazobactam in most patients. The population PK model developed adequately described tazobactam concentrations over time. Simulations of alternative dosing regimens suggest that extended or continuous infusions may achieve the target threshold concentrations found from in vitro studies. The ability to individualize β-lactamase inhibitors may justify the need for a wider range of fixed-dose combinations of BLBLIs or separate β-lactamase inhibitor products.
MATERIALS AND METHODS
Study population.
This prospective, observational cohort study was conducted in a large academic medical center where all subjects provided written informed consent. Patients were eligible for inclusion if they met the following criteria: (i) they were hospitalized adult patients (≥18 years of age) in an intensive care unit, (ii) they were receiving piperacillin-tazobactam (TZP), and (iii) they had a culture-positive Gram-negative bacterial infection. Patients were excluded if they underwent renal replacement therapy. Descriptive statistics, including the mean and standard deviation (SD), were used to describe patient baseline demographics and dosing history.
Study protocol.
The study protocol was approved by the Institutional Review Board of the University of Maryland, Baltimore. Written or witnessed verbal informed consent was obtained from all patients or their legally designated representative prior to study enrollment. TZP was dosed at the discretion of the patient’s primary care team. The Vitek 2 system (bioMérieux, Durham, NC) was used for organism identification and determination of the susceptibility of Enterobacteriaceae species, and the Kirby-Bauer disc diffusion method was used for determination of the susceptibility of non-lactose-fermenting Gram-negative organisms, with MICs being determined via Etest (bioMérieux, Durham, NC). The results are available on request. For patients infected with a non-lactose-fermenting Gram-negative organism for which the MIC was unavailable, the highest MIC for susceptible bacteria based on Clinical and Laboratory Standard Institute breakpoints for the antibiotic was assumed to represent a worst-case scenario of bacterial susceptibility (22). Two samples for determination of antibiotic levels were drawn, one at the midpoint (the middle of the dosing interval) and one at the time of the trough concentration (0.5 to 2 h prior to the next dose), once the patient was at steady state (at least 4 doses of TZP had been given). The serum creatinine level was measured with morning clinical labs during the day of pharmacokinetic sampling and was used to estimate creatinine clearance. The samples were frozen and batched for analysis at the Center for Anti-Infective Research and Development by validated chromatographic methods (lower limit of quantification, 0.9 μg/ml) (19).
Population pharmacokinetic analysis.
A population pharmacokinetic model was developed to describe the tazobactam concentration over time using Phoenix NLME (v8.1) software (Pharsight Corporation, Cary, NC, USA) and the first-order conditional estimation, extended-least-squares method. Based on previously reported literature and the reported sampling scheme (a maximum of two pharmacokinetic samples per patient), the concentration-time data from the patients were fitted to a one-compartment PK model (7, 8). Due to the availability of one or two concentrations above the limit of quantification per patient, prior information on clearance from published PK studies was used as the initial estimates for the population PK modeling analysis (7–13). A lack of concentration data at the end of infusion can lead to an incorrect estimation of the volume of distribution (V). Therefore, V was fixed to the average volume observed from current literature (20.0 liters) (7–13). Model selection was based on model diagnostic plots (individual and population predicted-versus-observed concentration plots, conditional weight residuals-versus-time plots), individual goodness-of-fit plots, the Akaike information criterion, and the precision of the parameter estimates. Between-subject random effects on clearance and volume parameters were assumed to follow a lognormal distribution, as illustrated in equation 1:
| (1) |
where θi is the value of the pharmacokinetic parameter for the ith individual, θpop is the population mean value of the pharmacokinetic parameter, and ηi represents the between-subject random effect for the ith individual and is assumed to be normally distributed with a mean of 0 and a variance of ω2. Proportional and additive/proportional combined residual error models were evaluated to account for within-subject variability. Covariate selection was performed by plotting individual random effects for each parameter against each covariate. The effects of prognostic factors, such as total body weight, gender, age, and creatinine clearance (CLCR), were evaluated. The effects of continuous and categorical covariates on pharmacokinetic parameters were evaluated using equations 2 and 3, respectively:
| (2) |
| (3) |
where COV is the covariate value for each subject (COV is equal to 1 or 0 for categorical variables), and θCOV is the covariate effect. Covariates were included in the model based on physiological relevance if the objective function value (OFV) decreased by 3.84 points according to the likelihood ratio test (χ2 test, P < 0.05; degrees of freedom [df] = 1) and if its inclusion decreased the between-subject variability of the parameter estimates. Individual pharmacokinetic parameter estimates were identified using the post hoc empirical Bayes estimates. Based on the estimated values of the individual pharmacokinetic parameters, individual prognostic factor values, and the final model’s structure, individual predicted tazobactam concentration-time profiles were obtained and evaluated graphically. Nonparametric bootstrap methods were used to quantify the uncertainty in the mean parameter estimates (500 bootstrap replicates with replacement). The M3 method was used to handle missing data attributed to concentrations below the reported lower limit of quantification (23).
Simulations for dose selection.
For optimal dose selection, 1,000 patients with a range of CLCRs (15 to 120 ml/min) were simulated using the final population PK model. Patients received commercially available doses of tazobactam (250, 375, and 500 mg) in combination with piperacillin as an intravenous infusion for the traditional infusion duration of 30 min, as extended infusions of 4 or 6 h, or as a continuous infusion (2 g over 24 h). Using a conservative approach, target attainment was defined as the achievement of free tazobactam threshold concentrations of 0.25, 0.5, and 2 μg/ml (corresponding to total tazobactam concentrations of 0.35, 0.72, and 2.87 μg/ml, respectively, assuming a 70% free fraction) at a %fT > concentration threshold of 85% for a 2-log10 unit decrease in the number of CFU per milliliter from that at the baseline (24).
ACKNOWLEDGMENTS
We thank David Nicolau for his assistance in specimen handling and processing and bioanalysis of the tazobactam concentration data.
No specific funding was received for the presented work.
We declare no conflicts of interest.
REFERENCES
- 1.Crass RL, Pai MP. 2019. Pharmacokinetics and pharmacodynamics of beta-lactamase inhibitors. Pharmacotherapy 39:182–195. doi: 10.1002/phar.2210. [DOI] [PubMed] [Google Scholar]
- 2.Harris PNA, Tambyah PA, Paterson DL. 2015. β-Lactam and β-lactamase inhibitor combinations in the treatment of extended-spectrum β-lactamase producing Enterobacteriaceae: time for a reappraisal in the era of few antibiotic options? Lancet Infect Dis 15:475–485. doi: 10.1016/S1473-3099(14)70950-8. [DOI] [PubMed] [Google Scholar]
- 3.Harris PNA, Tambyah PA, Lye DC, Mo Y, Lee TH, Yilmaz M, Alenazi TH, Arabi Y, Falcone M, Bassetti M, Righi E, Rogers BA, Kanj S, Bhally H, Iredell J, Mendelson M, Boyles TH, Looke D, Miyakis S, Walls G, Al Khamis M, Zikri A, Crowe A, Ingram P, Daneman N, Griffin P, Athan E, Lorenc P, Baker P, Roberts L, Beatson SA, Peleg AY, Harris-Brown T, Paterson DL, MERINO Trial Investigators and the Australasian Society for the Infectious Disease Clinical Research Network (ASID-CRN). 2018. Effect of piperacillin-tazobactam vs meropenem on 30-day mortality for patients with E coli or Klebsiella pneumoniae bloodstream infection and ceftriaxone resistance: a randomized clinical trial. JAMA 320:984–994. doi: 10.1001/jama.2018.12163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bush K, Macalintal C, Rasmussen BA, Lee VJ, Yang Y. 1993. Kinetic interactions of tazobactam with beta-lactamases from all major structural classes. Antimicrob Agents Chemother 37:851–858. doi: 10.1128/aac.37.4.851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nicasio AM, VanScoy BD, Mendes RE, Castanheira M, Bulik CC, Okusanya OO, Bhavnani SM, Forrest A, Jones RN, Friedrich LV, Steenbergen JN, Ambrose PG. 2016. Pharmacokinetics-pharmacodynamics of tazobactam in combination with piperacillin in an in vitro infection model. Antimicrob Agents Chemother 60:2075–2080. doi: 10.1128/AAC.02747-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wyeth Pharmaceuticals Inc. 2012. Zosyn package insert. Wyeth Pharmaceuticals Inc, Philadelphia, PA. [Google Scholar]
- 7.Silinskie KM, Domonoske BD, Cocanour CS. 2018. A pharmacokinetic analysis of continuously infused piperacillin/tazobactam in critically ill trauma patients. Trauma Cases Rev 4:1–8. doi: 10.23937/2469-5777/1510065. [DOI] [Google Scholar]
- 8.Kim MK, Xuan D, Quintiliani R, Nightingale CH, Nicolau DP. 2001. Pharmacokinetic and pharmacodynamic profile of high dose extended interval piperacillin-tazobactam. J Antimicrob Chemother 48:259–267. doi: 10.1093/jac/48.2.259. [DOI] [PubMed] [Google Scholar]
- 9.Kakara M, Larson K, Feng H-P, Shiomi M, Yoshitsugu H, Rizk ML. 2019. Population pharmacokinetics of tazobactam/ceftolozane in Japanese patients with complicated urinary tract infection and complicated intra-abdominal infection. J Infect Chemother 25:182–191. doi: 10.1016/j.jiac.2018.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Wise R, Logan M, Cooper M, Andrews JM. 1991. Pharmacokinetics and tissue penetration of tazobactam administered alone and with piperacillin. Antimicrob Agents Chemother 35:1081–1084. doi: 10.1128/aac.35.6.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Xiao AJ, Miller BW, Huntington JA, Nicolau DP. 2016. Ceftolozane/tazobactam pharmacokinetic/pharmacodynamic derived dose justification for phase 3 studies in patients with nosocomial pneumonia. J Clin Pharmacol 56:56–66. doi: 10.1002/jcph.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buck C, Bertram N, Ackermann T, Sauerbruch T, Derendorf H, Paar WD. 2005. Pharmacokinetics of piperacillin-tazobactam: intermittent dosing versus continuous infusion. Int J Antimicrob Agents 25:62–67. doi: 10.1016/j.ijantimicag.2004.08.012. [DOI] [PubMed] [Google Scholar]
- 13.Kinzig M, Sorgel F, Brismar B, Nord CE. 1992. Pharmacokinetic and tissue penetration of tazobactam and piperacillin in patients undergoing colorectal surgery. Antimicrob Agents Chemother 36:1997–2004. doi: 10.1128/aac.36.9.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boxenbaum H. 1982. Interspecies scaling, allometry, physiological time and ground plan of pharmacokinetics. J Pharmacokinet Biopharm 10:201–227. doi: 10.1007/bf01062336. [DOI] [PubMed] [Google Scholar]
- 15.Kim A, Sutherland CA, Kuti JL, Nicolau DP. 2007. Optimal dosing of piperacillin-tazobactam for the treatment of Pseudomonas aeruginosa infections: prolonged or continuous infusions? Pharmacotherapy 24:1490–1497. doi: 10.1592/phco.27.11.1490. [DOI] [PubMed] [Google Scholar]
- 16.Roberts JA, Abdul-Aziz MH, Davis JS, Dulhunty JM, Cotta MO, Myburgh J, Bellomo R, Lipman J. 2016. Continuous versus intermittent β-lactam infusion in severe sepsis: a meta-analysis of individual patient data from randomized trials. Am J Respir Crit Care Med 194:681–691. doi: 10.1164/rccm.201601-0024OC. [DOI] [PubMed] [Google Scholar]
- 17.Kufel WD, Miller CD, Johnson PR, Reid K, Zahra JJ, Seabury RW. 2017. Y-site incompatibility between premix concentrations of vancomycin and piperacillin-tazobactam: do current compatibility testing methodologies tell the whole story? Hosp Pharm 52:132–137. doi: 10.1310/hpj5202-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith BS, Yogaratnam D, Levasseur-Franklin KE, Forni A, Fong J. 2012. Introduction to drug pharmacokinetics in the critically ill patient. Chest 141:1327–1336. doi: 10.1378/chest.11-1396. [DOI] [PubMed] [Google Scholar]
- 19.Li C, Kuti JL, Nightingale CH, Mansfield DL, Dana A, Nicolau DP. 2005. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intra-abdominal infection. J Antimicrob Chemother 56:388–395. doi: 10.1093/jac/dki243. [DOI] [PubMed] [Google Scholar]
- 20.Komuro M, Maeda T, Kakuo H, Matsushita H, Shimada J. 1994. Inhibition of the renal excretion of tazobactam by piperacillin. J Antimicrob Chemother 34:555–564. doi: 10.1093/jac/34.4.555. [DOI] [PubMed] [Google Scholar]
- 21.Miller B, Hershberger E, Benziger D, Trinh M, Friedland L. 2012. Pharmacokinetics and safety of intravenous ceftolozane-tazobactam in healthy adult subjects following single and multiple ascending doses. Antimicrob Agents Chemother 56:3086–3091. doi: 10.1128/AAC.06349-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Clinical and Laboratory Standards Institute. 2018. M100—performance standards for antimicrobial susceptibility testing, 28th ed Clinical and Laboratory Standards Institute, Wayne, PA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 24.Wong G, Briscoe S, Adnan S, McWhinney B, Ungerer J, Lipman J, Roberts JA. 2013. Protein binding of β-lactam antibiotics in critically ill patients: can we successfully predict unbound concentrations? Antimicrob Agents Chemother 57:6165–6170. doi: 10.1128/AAC.00951-13. [DOI] [PMC free article] [PubMed] [Google Scholar]