Ibrexafungerp (SCY-078) is a novel first-in-class antifungal agent targeting glucan synthase. Candida auris is an emerging multidrug-resistant species that has caused outbreaks on five continents. We investigated the in vitro activity of ibrexafungerp against C. auris by applying EUCAST E.Def 7.3.1 methodology. C. albicans and C. glabrata, as well as anidulafungin, micafungin, amphotericin B, fluconazole, voriconazole, and isavuconazole, were included as comparators.
KEYWORDS: ibrexafungerp, C. auris, antifungal susceptibility, echinocandin resistance, EUCAST, SCY078, antifungal susceptibility testing, fks mutation
ABSTRACT
Ibrexafungerp (SCY-078) is a novel first-in-class antifungal agent targeting glucan synthase. Candida auris is an emerging multidrug-resistant species that has caused outbreaks on five continents. We investigated the in vitro activity of ibrexafungerp against C. auris by applying EUCAST E.Def 7.3.1 methodology. C. albicans and C. glabrata, as well as anidulafungin, micafungin, amphotericin B, fluconazole, voriconazole, and isavuconazole, were included as comparators. Three C. auris reference strains (CBS12372, CBS12373, and CBS10913) and 122 C. auris, 16 C. albicans, and 16 C. glabrata isolates were evaluated. C. albicans ATCC 64548, C. parapsilosis ATCC 22019, and C. krusei ATCC 6258 served as quality control strains. Echinocandin-resistant isolates were fks sequenced. MIC ranges and modal MIC and MIC50 values were determined. Wild-type upper limits (the upper MIC value where the wild-type distribution ends) were determined according to EUCAST principles for setting ECOFFs. Nine repetitions of three QC strains and MICs for C. albicans and C. glabrata yielded narrow MIC ranges with modal MICs in agreement with established EUCAST modal MICs, confirming a robust test performance. The ibrexafungerp MICs against C. auris isolates displayed a Gaussian distribution with a modal MIC (range) of 0.5 mg/liter (0.06 to 2 mg/liter), suggesting uniform susceptibility. Of 122 isolates, 8 were echinocandin resistant and harbored the S639F Fks1 alteration. All but one were fluconazole resistant, and the MIC distributions for voriconazole and isavuconazole were multimodal confirming variable susceptibility. Ibrexafungerp demonstrated promising activity against C. auris, including isolates resistant to echinocandins and/or other agents. The MICs were similar to those reported for the Clinical and Laboratory Standards Institute method, suggesting that a common clinical breakpoint may be appropriate.
INTRODUCTION
Ibrexafungerp (formerly SCY-078) is a novel first-in-class antifungal agent with in vitro activity against yeast, molds, and pneumocystis. It is currently in two phase 2 open-label studies to evaluate its efficacy and safety in patients with candidiasis caused by Candida auris (CARES) and in patients with refractory or intolerant fungal diseases (FURI), respectively. In addition, a phase 3, multicenter, randomized, double-blind, placebo-controlled study is under way to evaluate the efficacy and safety in subjects with acute vulvovaginal candidiasis (VANISH). The drug target is glucan synthase, but unlike the echinocandins ibrexafungerp is administered orally and retains activity against some fks mutant Candida species isolates (1–4).
C. auris is a recently recognized emerging yeast species associated with outbreaks in health care settings (5). It is considered a major threat to intensive care unit patients with a reported crude in-hospital mortality rate ranging between 30 and 72% (6–9). Knowledge of antifungal susceptibility of C. auris is of primary concern since C. auris almost consistently exhibits high fluconazole MICs (except in Colombian isolates [10]) and variable susceptibility to the other azoles, echinocandins and amphotericin B. Thus, treatment options in patients with invasive disease due to C. auris may become limited. A recent study from the Centers for Disease Control and Prevention (Atlanta, GA) reported that 93% of investigated C. auris isolates were resistant to fluconazole, 35% were resistant to amphotericin B, and 7% were resistant to echinocandins when susceptibility tested according to Clinical and Laboratory Standards Institute (CLSI) methodology (11). New antifungal agents, including ibrexafungerp, fosmanogepix, and VT-1578, have displayed in vitro activity against C. auris (1, 12–15). Although the in vitro activity of ibrexafungerp against C. auris has been investigated by the CLSI method M27-A3, MIC data obtained using the EUCAST methodology are scarce (16). Because MICs may differ among different susceptibility tests, the objective of the present study was to evaluate the in vitro activity of ibrexafungerp by the EUCAST E.Def 7.3.1 reference microdilution method. A large panel of well-identified nonduplicate clinical C. auris isolates, including both highly fluconazole- and echinocandin-resistant isolates and those in the susceptible range, were included, and the in vitro activity was compared to that of six comparator antifungal agents and to that against C. albicans and C. glabrata.
RESULTS
Nine repetitive tests of three recommended AFST quality control (QC) strains (C. albicans ATCC 64548, C. krusei ATCC 6258, and C. parapsilosis ATCC 22019) generated MICs that fell within one to three dilutions, suggesting a robust susceptibility test performance. The modal MICs were as follows: C. albicans ATCC 64548, 0.06 mg/liter; C. krusei ATCC 6258, 0.5 mg/liter; and C. parapsilosis ATCC 22019, 0.25 mg/liter (Table 1).
TABLE 1.
In vitro activity of ibrexafungerp and comparators against control isolates, as determined by EUCAST E.Def 7.3.1
| EUCAST-recommended QC strains | No. of repetitions | MIC (mg/liter) |
Range (mg/liter) | Tentative QC MIC target (range) in mg/liter | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | ||||
| C. albicans ATCC 64548 | 9 | 9 | 0.06 | 0.06 (0.03–0.125) | ||||||
| C. krusei ATCC 6258 | 9 | 8 | 1 | 0.5–1 | 0.5 (0.25–1) | |||||
| C. parapsilosis ATCC 22019 | 9 | 3 | 5 | 1 | 0.125–0.5 | 0.25 (0.125–0.5) | ||||
| C. auris | ||||||||||
| CBS10913 | 1 | 1 | ||||||||
| CBS12372 | 1 | 1 | ||||||||
| CBS12373 | 1 | 1 | ||||||||
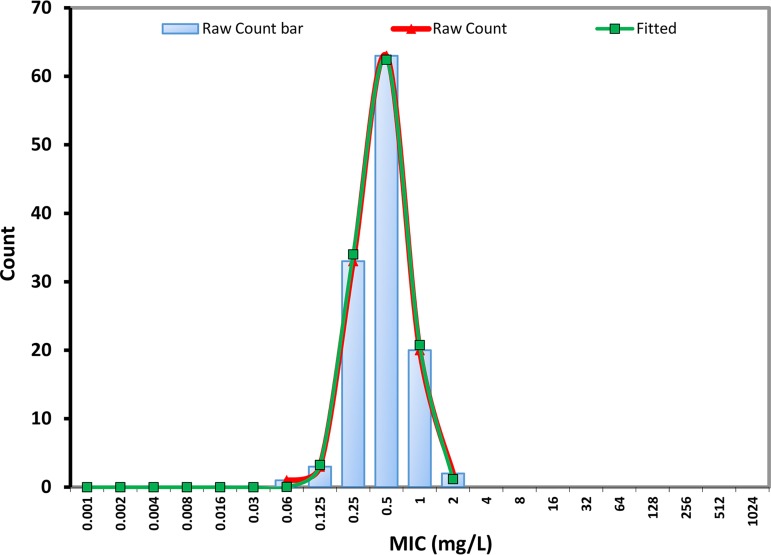
The EUCAST MICs of ibrexafungerp against the 122 C. auris isolates displayed a Gaussian distribution with a modal MIC and an MIC50 of 0.5 mg/liter and a range of 0.06 to 2 mg/liter (Table 2). The wild-type upper limit was 1 mg/liter determined visually, as well as by using the ECOFFinder program with 95, 97.5, or 99% of the modeled population included. The statistically fitted wild-type MIC curve was almost identical to the raw MIC curve (Fig. 1), confirming a robust susceptibility testing method and a population without isolates with acquired resistance mechanisms to ibrexafungerp. The MIC ranges for anidulafungin and micafungin were notably wider (0.016 to >32 mg/liter and 0.03 to >32 mg/liter, respectively) with eight isolates displaying high MICs (anidulafungin [4 to 32 mg/liter] and micafungin [32 mg/liter]) (Table 2). These isolates harbored the following fks alterations: S639F (n = 8) and all displayed wild-type susceptibility to ibrexafungerp (MICs of 0.25 mg/liter (n = 3) or 0.5 mg/liter (n = 5). All but one C. auris isolates were highly fluconazole resistant, and the MIC distributions for voriconazole and posaconazole were bi- and trimodal, suggesting variable susceptibility to these azoles. Finally, all isolates would be categorized as amphotericin B susceptible if adopting the breakpoint of 1 mg/liter; however, the MICs of 108/122 (88.5%) of the isolates fell at 1 mg/liter which are three, two, and one dilution greater than the EUCAST amphotericin B modal MIC for C. albicans, C. glabrata, and C. krusei, respectively (17). Thus, on a mg/liter basis ibrexafungerp was more active than amphotericin B and fluconazole and less active than the other four comparators when tested by EUCAST.
TABLE 2.
In vitro activity of ibrexafungerp (IBX) and comparators against C. auris and selected C. albicans and C. glabrata isolates, as determined by EUCAST E.Def 7.3.1a
| Strain and agent | MIC (mg/liter) |
MIC range(mg/liter) | Modal MIC (mg/liter) | MIC50 (mg/liter) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.004 | 0.008 | 0.016 | 0.03 | 0.06 | 0.125 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | ≥64 | ||||
| C. auris (n = 122) | ||||||||||||||||||
| IBX | 1 | 3 | 33 | 63 | 20 | 2 | 0.06–2 | 0.5 | 0.5 | |||||||||
| ANF* | 1 | 11 | 35 | 30 | 12 | 12 | 11 | 2 | 1 | 7 | 0.016–>32 | 0.06 | 0.125 | |||||
| MCF* | 5 | 30 | 70 | 9 | 8 | 0.03–>32 | 0.125 | 0.125 | ||||||||||
| AMB* | 14 | 108 | 0.5–1 | 1 | 1 | |||||||||||||
| FLU* | 1 | 2 | 10 | 109 | 0.5–≥64 | ≥64 | ≥64 | |||||||||||
| VOR* | 1 | 1 | 1 | 16 | 13 | 34 | 38 | 13 | 5 | ≤0.004–4 | Bimodal | 0.5 | ||||||
| ISA* | 20 | 1 | 1 | 19 | 9 | 19 | 21 | 21 | 6 | 5 | ≤0.004–2 | Trimodal | 0.125 | |||||
| C. albicans (n = 16) | ||||||||||||||||||
| IBX | 5 | 10 | 1 | 0.03–0.125 | 0.06 | 0.06 | ||||||||||||
| ANF | 10 | 6 | ≤0.004–0.008 | ≤0.004 | ≤0.004 | |||||||||||||
| MCF | 4 | 10 | 2 | 0.008–0.03 | 0.016 | 0.016 | ||||||||||||
| AMB | 1 | 6 | 9 | 0.06–0.25 | 0.25 | 0.25 | ||||||||||||
| FLU | 10 | 6 | 0.125–0.25 | 0.125 | 0.125 | |||||||||||||
| VOR | 12 | 4 | ≤0.004–0.008 | ≤0.004 | ≤0.004 | |||||||||||||
| ISA | 14 | 2 | ≤0.004–0.008 | ≤0.004 | ≤0.004 | |||||||||||||
| C. glabrata (n = 16) | ||||||||||||||||||
| IBX | 10 | 6 | 0.25–0.5 | 0.25 | 0.25 | |||||||||||||
| ANF | 4 | 12 | 0.016–0.03 | 0.03 | 0.03 | |||||||||||||
| MCF | 8 | 8 | 0.016-0.03 | 0.016/0.03 | 0.016 | |||||||||||||
| AMB | 1 | 1 | 11 | 3 | 0.03–0.5 | 0.25 | 0.25 | |||||||||||
| FLU | 6 | 10 | 2–4 | 4 | 4 | |||||||||||||
| VOR | 1 | 13 | 2 | 0.03–0.125 | 0.06 | 0.06 | ||||||||||||
| ISA | 1 | 3 | 6 | 6 | 0.016–0.125 | 0.06/0.125 | 0.06 | |||||||||||
Gray-shaded areas indicate concentrations not tested for that particular compound. An underlined value indicates a modal MIC for unimodal distributions but the lowest MIC peak for multimodal distributions, thus illustrating the modal MIC of the presumed wild-type distribution. The MIC distributions for comparator antifungals against C. auris indicated by an asterisk (*) are compiled from reference 1 except that isolates above the tested MIC range in that publication were retested using extended concentration ranges.
FIG 1.
EUCAST MIC distribution for ibrexafungerp against 122 clinical C. auris isolates. Raw counts are presented as bars and a red curve, whereas the fitted curve was determined by the ECOFF finder program (v2.0) that iteratively fits each subset of the data from left to right.
Finally, the ibrexafungerp MICs were determined for 16 C. albicans and 16 C. glabrata isolates (Table 2). The MIC ranges were narrow spanning three and two 2-fold dilutions, respectively, again suggesting a robust test performance, with modal MICs of 0.06 mg/liter (as for the repetitive testing of the C. albicans QC strain) and 0.25 mg/liter, respectively. The modal MICs for the comparator compounds were in agreement within ± one 2-fold dilution of the aggregated MIC data used for EUCAST breakpoint setting (EUCAST modal MICs for C. albicans/C. glabrata as follows: anidulafungin, 0.004/0.016; micafungin, 0.008/0.008; amphotericin B, 0.125/0.25; fluconazole, 0.25/8; and voriconazole, 0.008/0.125 mg/liter), again suggesting a robust performance of the susceptibility testing in this study (18).
DISCUSSION
Ibrexafungerp displayed uniform and potent activity against the 122 C. auris strains, including 8 that were anidulafungin and micafungin resistant. Activity against echinocandin-resistant C. auris isolates has previously been reported when tested by the CLSI method, though without information regarding the underlying molecular resistance mechanisms (1). Moreover, it has been demonstrated that ibrexafungerp retains activity against some fks mutants of C. glabrata (2–4, 19) and against a limited number of fks mutants of C. albicans, C. dubliniensis, C. tropicalis, and C. krusei isolates (4). C. auris is phylogenetically related to C. glabrata. The ibrexafungerp in vitro activity was recently investigated against 79 C. glabrata harboring 29 different hot spots alterations recognized to cause MIC elevations for the echinocandins. Elevated ibrexafungerp MICs were found against C. glabrata isolates with 3 out of 11 investigated fks1 alterations (625S, D632G, and D632Y) and against 5 of 18 fks2 alterations (F659del, F659S, F659V, L662W, and S663P) (20). Of note, ibrexafungerp MICs were not elevated against four included isolates harboring the S663F alteration in Fks2, which corresponds to the S639F alteration found in Fks1 of the eight highly echinocandin-resistant C. auris isolates included in this study.
The modal MIC obtained by EUCAST against C. auris was equal to or one 2-fold dilution lower than the modal MIC values previously obtained by the CLSI methodology (1, 12). Similarly, the modal MIC against C. albicans and C. glabrata were identical to those obtained by Marcos-Zambrano et al. using EUCAST and to those obtained by Schell et al. using CLSI and finally one step lower and one step higher, respectively, than those obtained by Pfaller et al. and Marcos-Zambrano et al. using CLSI methods (2, 4, 16). Taken together, these data suggest an excellent agreement between EUCAST and CLSI testing for C. auris, C. albicans, and C. glabrata, which may allow a single species-specific clinical breakpoint to be set for each of the organisms that will apply for both EUCAST and CLSI testing. This has obvious advantages. The EUCAST and CLSI MICs for anidulafungin and micafungin were not comparable, and as a result method-specific clinical breakpoints have been established (for example, the EUCAST susceptibility breakpoint is 0.03 mg/liter for C. albicans compared to 0.25 mg/liter for CLSI). Such method disagreement complicates MIC interpretation and the development of commercial susceptibility tests that correctly categorizes isolates as susceptible or resistant according to both standards.
In summary, our data confirm that ibrexafungerp appears to be a promising future agent against C. auris infections, including those involving acquired echinocandin or azole resistance. We demonstrate that EUCAST MIC testing of ibrexafungerp is robust against C. auris, as well as C. albicans and C. glabrata, and that EUCAST MICs mirror those obtained by CLSI testing, suggesting that mutual breakpoints for the two methods can be established. In light of the multiple-drug-resistant and highly transmissible potential for C. auris, these findings are a welcome step forward. Nevertheless, further studies, including studies of C. auris isolates from other parts of the world, are warranted, since differential susceptibilities to licensed compounds have been found among different C. auris clades.
MATERIALS AND METHODS
Isolates.
Three control C. auris strains (CBS12372, CBS12373, and CBS10913) and a total of 122 clinical isolates of C. auris collected from individual patients in six tertiary care hospitals in India from 2010 to 2015 were included. The clinical isolates were mainly from patients with candidemia (blood; n = 100), and other specimens (n = 22) from invasive Candida infections included tissue, pleural fluid, and a single isolate from pus. Species identification was performed using sequencing of the internal transcribed spacer region of the ribosomal subunit and confirmed by using a Bruker MALDI TOF MS apparatus before use. Eight were anidulafungin and echinocandin resistant and were fks sequenced as previously described (21). To confirm assay performance, an additional 16 drug-susceptible clinical isolates of C. albicans and C. glabrata from Danish patients were included. Finally, nine repetitions for the CLSI and EUCAST QC strains C. albicans ATCC 64548, C. parapsilosis ATCC 22019, and C. krusei ATCC 6258 were performed.
Susceptibility testing.
EUCAST MICs were determined following E.Def 7.3.1 methodology (22). Ibrexafungerp (SCY-078; Scynexis, Inc., Jersey City, NJ) pure substance was stored in aliquots at −80°C, and stock solutions were prepared in dimethyl sulfoxide (5,000 mg/liter; Sigma-Aldrich, Brøndby, Denmark). The final drug concentration ranges studied were 0.008 to 4 mg/liter. The following comparator compounds were also investigated (source of compound with the final concentration ranges in parentheses): anidulafungin (Pfizer A/S, Ballerup, Denmark; 0.004 to 4 mg/liter for C. albicans and C. glabrata isolates and 0.03 to 32 mg/liter for C. auris), micafungin (Astellas Pharma, Inc., Tokyo, Japan; 0.004 to 4 mg/liter for C. albicans and C. glabrata isolates and 0.03 to 32 mg/liter for C. auris), amphotericin B (Sigma-Aldrich; 0.004 to 4 mg/liter), fluconazole (Sigma-Aldrich; 0.03 to 32 mg/liter for bloodstream isolates and 0.5 to 256 mg/liter for C. auris), isavuconazole (Basilea Pharmaceutica, Ltd., Basel, Switzerland; 0.004 to 4 mg/liter), and voriconazole (Pfizer A/S, Ballerup, Denmark; 0.004 to 4 mg/liter) (23). Cell culture-treated samples (Nunc MicroWell 96-well microplates; Thermo Fisher Scientific, catalog no. 167008) were used throughout. Microtiter plates with 2-fold dilutions were prepared and frozen at −80°C prior to use.
Data management.
MIC ranges, modal MIC (the most common MIC), and MIC50 (i.e., the MIC that includes 50% of the isolates) values were calculated. Wild-type upper limits (WT-ULs), defined as the upper MIC value where the wild-type distribution ends, were determined following principles for setting EUCAST ECOFFs. However, since the values reported here are not formally accepted EUCAST ibrexafungerp ECOFFs, we use the term “WT-UL” to avoid confusion. The conventional method for determining an ECOFF is a visual inspection of histograms of the MICs for single species (“the eyeball method”) (24). In addition, WT-ULs were determined statistically using the EUCAST ECOFFinder program (24).
ACKNOWLEDGMENTS
We thank research technician Birgit Brandt for excellent technical assistance.
This study was supported by an unrestricted grant from Scynexis, Inc. The funder was involved in the study design and reviewed the manuscript but had no influence on the analysis of the results or the interpretation hereof.
M.C.A. has received personal speaker’s honoraria the past 5 years from Astellas, Basilea, Gilead, MSD, Pfizer, T2Candida, and Novartis. She has received research grants and payment for contract work paid to the Statens Serum Institute from Astellas, Basilea, Gilead, MSD, Novabiotics, T2Candida, F2G, Cidara, and Amplyx. K.M.J. has received a meeting grant from MSD and travel grants from F2G and Amplyx. R.K.H. has received meeting grants from MSD, Pfizer, Gilead, and Astellas and a research grant from Gilead.
REFERENCES
- 1.Berkow EL, Angulo D, Lockhart SR. 2017. In vitro activity of a novel glucan synthase inhibitor, SCY-078, against clinical isolates of Candida auris. Antimicrob Agents Chemother 61:e00435-17. doi: 10.1128/AAC.00435-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schell WA, Jones AM, Borroto-Esoda K, Alexander BD. 2017. Antifungal activity of SCY-078 and standard antifungal agents against 178 clinical isolates of resistant and susceptible Candida species. Antimicrob Agents Chemother 61:e01102-17. doi: 10.1128/AAC.01102-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wiederhold NP, Najvar LK, Jaramillo R, Olivo M, Pizzini J, Catano G, Patterson TF. 2018. Oral glucan synthase inhibitor SCY-078 is effective in an experimental murine model of invasive candidiasis caused by WT and echinocandin-resistant Candida glabrata. J Antimicrob Chemother 73:448–451. doi: 10.1093/jac/dkx422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pfaller MA, Messer SA, Rhomberg PR, Borroto-Esoda K, Castanheira M. 2017. Differential activity of the oral glucan synthase inhibitor SCY-078 against wild-type and echinocandin-resistant strains of Candida species. Antimicrob Agents Chemother 61:e00161-17. doi: 10.1128/AAC.00161-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nett JE. 2019. Candida auris: an emerging pathogen “incognito”? PLoS Pathog 15:e1007638. doi: 10.1371/journal.ppat.1007638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Khan Z, Ahmad S, Benwan K, Purohit P, Al-Obaid I, Bafna R, Emara M, Mokaddas E, Abdullah AA, Al-Obaid K, Joseph L. 2018. Invasive Candida auris infections in Kuwait hospitals: epidemiology, antifungal treatment, and outcome. Infection 46:641–650. doi: 10.1007/s15010-018-1164-y. [DOI] [PubMed] [Google Scholar]
- 7.Kohlenberg A, Struelens MJ, Monnet DL, Plachouras D, The Candida auris Survey Collaborative Group. 2018. Candida auris: epidemiological situation, laboratory capacity, and preparedness in European Union and European Economic Area countries, 2013 to 2017. Euro Surveill 23:pii=18-00136. doi: 10.2807/1560-7917.ES.2018.23.13.18-00136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290-20. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cortegiani A, Misseri G, Fasciana T, Giammanco A, Giarratano A, Chowdhary A. 2018. Epidemiology, clinical characteristics, resistance, and treatment of infections by Candida auris. j Intensive Care 6:1–13. doi: 10.1186/s40560-018-0342-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Escandón P, Chow NA, Caceres DH, Gade L, Berkow EL, Armstrong P, Rivera S, Misas E, Duarte C, Moulton-Meissner H, Welsh RM, Parra C, Pescador LA, Villalobos N, Salcedo S, Berrio I, Varón C, Espinosa-Bode A, Lockhart SR, Jackson BR, Litvintseva AP, Beltran M, Chiller TM. 2019. Molecular epidemiology of Candida auris in Colombia reveals a highly related, countrywide colonization with regional patterns in amphotericin B resistance. Clin Infect Dis 68:15–21. doi: 10.1093/cid/ciy411. [DOI] [PubMed] [Google Scholar]
- 11.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on three continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Larkin E, Hager C, Chandra J, Mukherjee PK, Retuerto M, Salem I, Long L, Isham N, Kovanda L, Borroto-Esoda K, Wring S, Angulo D, Ghannoum M. 2017. The emerging pathogen Candida auris: growth phenotype, virulence factors, activity of antifungals, and effect of SCY-078, a novel glucan synthesis inhibitor, on growth morphology and biofilm formation. Antimicrob Agents Chemother 61:e2396-16. doi: 10.1128/AAC.02396-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arendrup MC, Chowdhary A, Astvad KMT, Jørgensen KM, Arendrup MC, Chowdhary A, Astvad KMT, Jørgensen KM. 2018. APX001A in vitro activity against contemporary blood isolates and Candida auris determined by the EUCAST reference method. Antimicrob Agents Chemother 62:e01225-18. doi: 10.1128/AAC.01225-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hager CL, Larkin EL, Long L, Zohra Abidi F, Shaw KJ, Ghannoum MA. 2018. In vitro and in vivo evaluation of the antifungal activity of APX001A/APX001 against Candida auris. Antimicrob Agents Chemother 62:e02319-17. doi: 10.1128/AAC.02319-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wiederhold NP, Lockhart SR, Najvar LK, Berkow EL, Jaramillo R, Olivo M, Garvey EP, Yates CM, Schotzinger RJ, Catano G, Patterson TF. 2018. The fungal Cyp51-specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother 63:e02233-18. doi: 10.1128/AAC.02233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marcos-Zambrano LJ, Gómez-Perosanz M, Escribano P, Bouza E, Guinea J. 2017. The novel oral glucan synthase inhibitor SCY-078 shows in vitro activity against sessile and planktonic Candida spp. J Antimicrob Chemother 72:1969–1976. doi: 10.1093/jac/dkx010. [DOI] [PubMed] [Google Scholar]
- 17.European Committee on Antimicrobial Susceptibility Testing. 2010. Amphotericin B: rationale for the clinical breakpoints, version 1.0. EUCAST, Stockholm, Sweden: http://www.eucast.org/fileadmin/src/media/PDFs/EUCAST_files/Rationale_documents/AmphotericinB_rationale_20110429.pdf. [Google Scholar]
- 18.European Committee on Antimicrobial Susceptibility Testing. 2019. Rationale documents for antifungal agents. EUCAST, Stockholm, Sweden: http://www.eucast.org/astoffungi/rationale_documents_for_antifungals/. [Google Scholar]
- 19.Jiménez-Ortigosa C, Perez WB, Angulo D, Borroto-Esoda K, Perlin DS. 2017. De novo acquisition of resistance to SCY-078 in Candida glabrata involves FKS mutations that both overlap and are distinct from those conferring echinocandin resistance. Antimicrob Agents Chemother 61:e00833-17. doi: 10.1128/AAC.00833-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Barat S, Borroto-Esoda K, Angulo D. 2018. Ibrexafungerp (formerly SCY-078) displays potent in vitro activity against C. glabrata isolates with mutations in fks genes. ESCMID/ASM Conference on Drug Development to Meet the Challenge of Antimicrobial Resistance, Lisbon, Portugal. [Google Scholar]
- 21.Biagi MJ, Wiederhold NP, Gibas C, Wickes BL, Lozano V, Bleasdale SC, Danziger L. 2019. Development of high-level echinocandin resistance in a patient with recurrent candida auris candidemia secondary to chronic candiduria. Open Forum Infect Dis 6:ofz262. doi: 10.1093/ofid/ofz262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Arendrup MC, Meletiadis J, Mouton JW, Guinea J, Cuenca-Estrella M, Lagrou K, Howard SJ. 2016. EUCAST technical note on isavuconazole breakpoints for Aspergillus, itraconazole breakpoints for Candida, and updates for the antifungal susceptibility testing method documents. Clin Microbiol Infect 22:571.e1–4. doi: 10.1016/j.cmi.2016.01.017. [DOI] [PubMed] [Google Scholar]
- 23.Arendrup MC, Prakash A, Meletiadis J, Sharma C, Chowdhary A. 2017. Comparison of EUCAST and CLSI reference microdilution MICs of eight antifungal compounds for Candida auris and associated tentative epidemiological cutoff values. Antimicrob Agents Chemother 61:e00485-17. doi: 10.1128/AAC.00485-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Turnidge J, Kahlmeter G, Kronvall G. 2006. Statistical characterization of bacterial wild-type MIC value distributions and the determination of epidemiological cutoff values. Clin Microbiol Infect 12:418–425. doi: 10.1111/j.1469-0691.2006.01377.x. [DOI] [PubMed] [Google Scholar]