We report a case of a 62-year-old man treated for Streptococcus pneumoniae meningitis by ceftriaxone and dexamethasone. After neurological improvement, neurological degradation by vasculitis occurred, despite effective concentrations of ceftriaxone in the serum and cerebrospinal fluid (CSF). S. pneumoniae with increased MICs to third-generation-cephalosporins (3GC) was isolated from the ventricular fluid 10 days after the isolation of the first strain. Isolate analysis showed that a mutation in the penicillin-binding protein 2X (PBP2X) has occurred under treatment.
KEYWORDS: Streptococcus pneumoniae, penicillin-binding protein, 3GC resistance, meningitis, treatment failure, meningitis-related delayed cerebral injury
ABSTRACT
We report a case of a 62-year-old man treated for Streptococcus pneumoniae meningitis by ceftriaxone and dexamethasone. After neurological improvement, neurological degradation by vasculitis occurred, despite effective concentrations of ceftriaxone in the serum and cerebrospinal fluid (CSF). S. pneumoniae with increased MICs to third-generation-cephalosporins (3GC) was isolated from the ventricular fluid 10 days after the isolation of the first strain. Isolate analysis showed that a mutation in the penicillin-binding protein 2X (PBP2X) has occurred under treatment.
INTRODUCTION
Streptococcus pneumoniae-related meningitis is associated with high morbidity even in optimal therapeutic settings, with a case fatality rate close to 30% (1) and long-term neurological sequelae in a significant proportion of survivors (2). Penicillin was the first-line drug of choice for treating pneumococcal infections for many years until increasing global resistance to penicillin. Currently, in France, the first-line treatment of pneumococcal meningitis is based on third-generation cephalosporin (3GC) use (3). However, resistance to penicillin in clinical isolates of Streptococcus pneumoniae is related to the development of high-molecular-weight penicillin-binding proteins (PBP) that have greatly decreased affinity for the antibiotic (4). Resistance to 3GC involves only alterations of PBP2X and PBP1A, and this resistance can be transferred into a susceptible strain in a single round of transformation (5). Treatment failure with use of a 3GC in case of penicillin-resistant pneumococcal meningitis has been reported (6, 7). Mutations in PBP2X leading to 3GC resistance despite well-conducted antibiotic treatment have never been reported in vivo.
CASE PRESENTATION
A 62-year-old man was treated for 5 days with ear drops of ciprofloxacin and corticosteroids for left acute otitis media. Due to the occurrence of rhinorrhea with coughing and the persistence of the ear pain, he received paracetamol-tramadol. He was admitted 2 days later to the emergency department of Saint-Joseph Hospital in Paris, France, for clinical signs evocating meningoencephalitis, including fever (39.5°C), headache, stiff neck, and confusion. His blood pressure was 159/79 mm Hg, and his heart rate was 125 beats/min. His medical history was the recent finding of diabetes mellitus. Lumbar puncture and blood cultures were performed before delivery of ceftriaxone (3 g) and dexamethasone (10 mg every 6 h). A computed tomography (CT) scan ruled out a subarachnoid hemorrhage or an abscess. The cerebrospinal fluid (CSF) was cloudy, with an elevated protein level (7.1 g/liter), low glucose level (CSF/serum glucose ratio, 0.19), and leukocyte count of 940 × 106/liter with 90% neutrophils. Gram stain from the CSF using a Cytospin centrifuge showed Gram-positive cocci that suggest Streptococcus pneumoniae. The patient was admitted to the intensive care unit (ICU) (defined as day 1). S. pneumoniae was isolated both from CSF and blood cultures. It was resistant to penicillin G (MIC, 2 mg/liter), amoxicillin (MIC, 2 mg/liter), and cefixime (MIC, 16 mg/liter), with reduced susceptibility to cefotaxime and ceftriaxone (MICs, 0.75 mg/liter). MICs were determined using the Etest method (bioMérieux, Craponne, France). Treatment with ceftriaxone at 3 g/day was maintained, allowing neurological improvement at day 3. At day 7, deterioration in neurological status associated with decompensation of diabetes mellitus was noted and required mechanical ventilation. An overdose of ceftriaxone occurred at day 2, with a residual serum level of 154 mg/liter, which normalized at day 6, with a serum level of 47.2 mg/liter. An efficiency inhibitory quotient (IQ) was obtained between day 4 (IQ, 8) and day 9 (IQ, 10) with CSF levels of ceftriaxone of 2.2 mg/liter, 6 mg/liter, and 7.6 mg/liter at days 1, 4, and 9, respectively.
At day 10, the Glasgow Coma Scale score was 3, with a 40°C fever. A transcranial Doppler showed accelerated velocities in favor of a narrowing of the arterial calibers by vasculitis. Areactive bilateral mydriasis was observed, leading to immediate osmotherapy and placement of an external ventricular shunt. Then, digital subtraction angiography confirmed a very severe and diffuse narrowing of every intracranial artery (Fig. 1). At day 10, the concentration of ceftriaxone in the ventricular fluid obtained by external derivation was 1.2 mg/liter. This low level could be explained by weak diffusion in part by the extended vasculitis. S. pneumoniae was isolated from culture of the CSF, with susceptibility to penicillin G (MIC, 0.2 mg/liter), amoxicillin (MIC, 0.2 mg/liter), and cefixime (MIC, 0.75 mg/liter) but resistance to cefotaxime and ceftriaxone (MIC, 2 mg/liter) (Table 1).
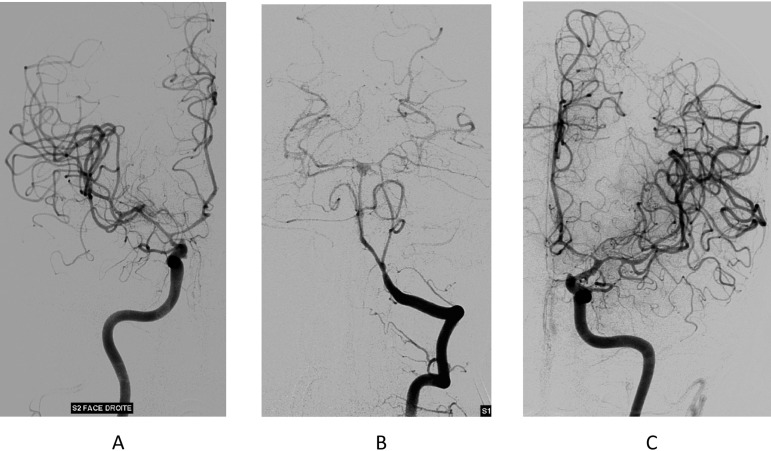
FIG 1.
Digital subtraction angiography, day 10. (A) Right internal carotid. (B) Left vertebral artery. (C) Left internal carotid. All the arteries of the circle of Willis are affected by an extremely extensive and severe narrowing, disclosing postinfectious arteritis.
TABLE 1.
Synthesis of microbiological data timing of antibiotic exposure and drug monitoring
| Data typea | Result for dayb: |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 17 | |
| Microbiological sample | ||||||||||||
| CSF | + | − | − | + | ||||||||
| Blood culture | + | − | + | − | − | − | − | − | ||||
| MIC (mg/liter) | ||||||||||||
| Penicillin G | 2 | 0.2 | ||||||||||
| Amoxicillin | 2 | 0.2 | ||||||||||
| Cefixime | 16 | 0.75 | ||||||||||
| Cefotaxime | 0.75 | 2 | ||||||||||
| Antibiotic exposure | ||||||||||||
| Ceftriaxone | x | x | x | x | x | x | x | x | x | x | x | |
| Intrathecal vancomycin | x | x | ||||||||||
| Rifampin | x | x | ||||||||||
| Ceftriaxone therapeutic drug monitoring concn (mg/liter) | ||||||||||||
| Serum | 154.1 | 47.2 | ||||||||||
| CSF | 2.2 | 6 | 7.6 | 1.2 | ||||||||
CSF, cerebrospinal fluid.
+, S. pneumoniae found in culture; −, sterile; x, positive for exposure.
CHALLENGE QUESTION
At day 10, the patient is now worsening on the septic and neurological level. S. pneumoniae was again isolated from CSF. The MIC of ceftriaxone was increased (2 mg/liter), but the MICs of penicillin G and amoxicillin decreased (0.2 mg/liter). Antibiotic treatment needs to be modified.
Which antimicrobial(s) would be best for the patient and why?
-
A.
Vancomycin plus rifampin
-
B.
High dosage of amoxicillin
-
C.
High dosage of cefotaxime
-
D.
Ceftobiprole
-
E.
Linezolid
TREATMENT AND OUTCOME
At day 10, antibiotic therapy was then modified for intravenous cefotaxime at 16 g/day plus rifampin at 1.2 g/day with intrathecal vancomycin (50 mg). Cerebral magnetic resonance imaging (MRI) at day 14 identified extensive ischemia of the cerebellum, protuberance, mesencephalon, cerebral peduncles, and bilateral basal ganglia (Fig. 2). Worsening of the neurological state led to the patient’s death on day 17. A coinfection due to two different strains was ruled out since S. pneumoniae isolates (day 1 and day 10) were characterized as serotype 14 and multilocus sequence type (MLST) 557. In addition, susceptibilities to various antibiotics (except β-lactams) were identical. Sequencing of the pbp1a, pbp2b, and pbp2x genes showed identical sequences for pbp1a and pbp2b and a single-nucleotide substitution in pbp2x (A→G) which led to Thr550→Ala in PBP2X (GenBank accession numbers MN025493 and MN025494). Regarding antibiotic administration, 3 g of ceftriaxone was given to the patient by the emergency department for clinical meningitis before any bacterial documentation. Due to a rapid improved clinical condition (day 3) and an overdose of ceftriaxone at day 2 (residual serum level, 154 mg/liter), we chose not to modify the ceftriaxone dosage according to the guidelines since they are not based on individual pharmacological monitoring (3, 8). Finally, it should be noted that the secondary neurological deterioration of this patient fell within the scope of a meningitis-related delayed cerebral injury (DCI). Late achievement of an efficiency inhibitory quotient at day 9 combined with the early administration of corticoids could have contributed to the occurrence of this DCI, especially since Streptococcus pneumoniae was involved (9, 10). Despite intensive care and therapeutic developments, Streptococcus pneumoniae is still responsible for a high rate of mortality (1, 2).
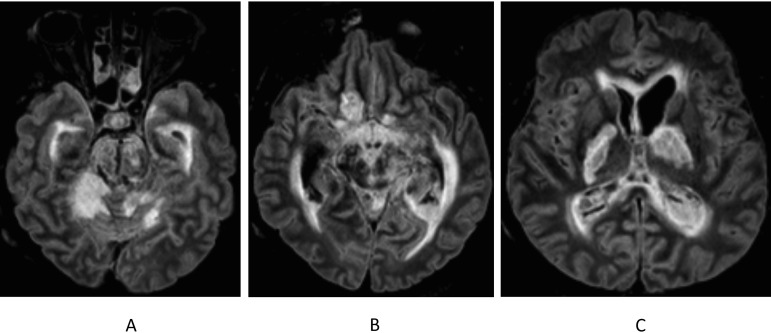
FIG 2.
Cerebral magnetic resonance imaging (fluid-attenuated inversion recovery), day 14. Extensive ischemia of cerebellum, protuberance, mesencephalon, cerebral peduncles, and bilateral basal ganglia. Periventricular hypersignal in relation to hydrocephalus and ventriculitis.
ACKNOWLEDGMENT
We thank Emmanuelle Varon (CNR Pneumocoques) for serotyping determination.
This Journal section presents a real, challenging case involving a multidrug-resistant organism. The case authors present the rationale for their therapeutic strategy and discuss the impact of mechanisms of resistance on clinical outcome. An expert clinician then provides a commentary on the case.
Footnotes
For the case commentary, see https://doi.org/10.1128/AAC.02251-19.
REFERENCES
- 1.Weisfelt M, van de Beek D, Spanjaard L, Reitsma JB, de Gans J. 2006. Clinical features, complications, and outcome in adults with pneumococcal meningitis: a prospective case series. Lancet Neurol 5:123–129. doi: 10.1016/S1474-4422(05)70288-X. [DOI] [PubMed] [Google Scholar]
- 2.Gouveia EL, Reis JN, Flannery B, Cordeiro SM, Lima JBT, Pinheiro RM, Salgado K, Mascarenhas AV, Carvalho MG, Beall BW, Reis MG, Ko AI. 2011. Clinical outcome of pneumococcal meningitis during the emergence of pencillin [sic]-resistant Streptococcus pneumoniae: an observational study. BMC Infect Dis 11:323. doi: 10.1186/1471-2334-11-323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.SPILF. 2018. Prise en charge des méningites bactériennes aiguës communautaires (à l’exclusion du nouveau-né): Actualisation 2017 de la Conférence de Consensus 2008. Societe de Pathologie Infectieuse de Langue Française, Paris, France: https://www.infectiologie.com/UserFiles/File/formation/desc/2017/seminaire-avril-2017/cours-mercredi-12.04/recommandations-meningites-bacteriennes-xduval.pdf. [Google Scholar]
- 4.Hakenbeck R, Tarpay M, Tomasz A. 1980. Multiple changes of penicillin-binding proteins in penicillin-resistant clinical isolates of Streptococcus pneumoniae. Antimicrob Agents Chemother 17:364–371. doi: 10.1128/aac.17.3.364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Muñoz R, Dowson CG, Daniels M, Coffey TJ, Martin C, Hakenbeck R, Spratt BG. 1992. Genetics of resistance to third-generation cephalosporins in clinical isolates of Streptococcus pneumoniae. Mol Microbiol 6:2461–2465. doi: 10.1111/j.1365-2958.1992.tb01422.x. [DOI] [PubMed] [Google Scholar]
- 6.John CC. 1994. Treatment failure with use of a third-generation cephalosporin for penicillin-resistant pneumococcal meningitis: case report and review. Clin Infect Dis 18:188–193. doi: 10.1093/clinids/18.2.188. [DOI] [PubMed] [Google Scholar]
- 7.Kaplan SL, Mason EO. 1998. Management of infections due to antibiotic-resistant Streptococcus pneumoniae. Clin Microbiol Rev 11:628–644. doi: 10.1128/CMR.11.4.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van de Beek D, Cabellos C, Dzupova O, Esposito S, Klein M, Kloek AT, Leib SL, Mourvillier B, Ostergaard C, Pagliano P, Pfister HW, Read RC, Sipahi OR, Brouwer MC, ESCMID Study Group for Infections of the Brain (ESGIB) . 2016. ESCMID guideline: diagnosis and treatment of acute bacterial meningitis. Clin Microbiol Infect 22(Suppl 3):S37–S62. doi: 10.1016/j.cmi.2016.01.007. [DOI] [PubMed] [Google Scholar]
- 9.Gallegos C, Tobolowsky F, Nigo M, Hasbun R. 2018. Delayed cerebral injury in adults with bacterial meningitis: a novel complication of adjunctive steroids? Crit Care Med 46:e811–e814. doi: 10.1097/CCM.0000000000003220. [DOI] [PubMed] [Google Scholar]
- 10.Tunkel AR, Hasbun R, Bhimraj A, Byers K, Kaplan SL, Scheld WM, van de Beek D, Bleck TP, Garton HJL, Zunt JR. 2017. 2017 Infectious Diseases Society of America’s clinical practice guidelines for healthcare-associated ventriculitis and meningitis. Clin Infect Dis 64:e34–e65. doi: 10.1093/cid/ciw861. [DOI] [PMC free article] [PubMed] [Google Scholar]