Methicillin-resistant Staphylococcus aureus (MRSA) has become a significant acute and chronic respiratory pathogen. While vancomycin is effective against MRSA, its relatively poor penetration into lung secretions and dose-limiting renal toxicity make it less effective in the respiratory setting. As inhaled administration of vancomycin would overcome these limitations, we developed a dry powder formulation suitable for inhalation (AeroVanc).
KEYWORDS: vancomycin, inhaled, MRSA, cystic fibrosis
ABSTRACT
Methicillin-resistant Staphylococcus aureus (MRSA) has become a significant acute and chronic respiratory pathogen. While vancomycin is effective against MRSA, its relatively poor penetration into lung secretions and dose-limiting renal toxicity make it less effective in the respiratory setting. As inhaled administration of vancomycin would overcome these limitations, we developed a dry powder formulation suitable for inhalation (AeroVanc). Here, we report a phase I, single-dose, dose-escalating study aimed at demonstrating safety and tolerability of AeroVanc. In part I, 18 healthy subjects received a single dose of 16 mg, 32 mg, or 80 mg of AeroVanc. Two subjects also received a 250-mg dose of intravenous vancomycin. In part 2 of the study, 32 mg and 80 mg AeroVanc were administered to subjects with cystic fibrosis as single doses. There were no serious side effects. A small drop in forced expiratory volume in 1 s (FEV1) was observed in 3 subjects with cystic fibrosis, one of whom required salbutamol. Vancomycin was rapidly absorbed after inhalation. Peak and mean plasma concentrations of vancomycin were dose proportional. The average minimum concentration of vancomycin in sputum remained above the usual MIC values for MRSA for up to 24 h (minimum sputum concentration [Cmin], 32-mg dose = 3.05 μg/ml, 80-mg dose = 8.0 μg/ml). In conclusion, AeroVanc was well tolerated and achieved high levels in sputum with a mean systemic absorption of 49%, making it a potential therapeutic strategy for respiratory infection with MRSA.
INTRODUCTION
Inhaled antibiotics have been delivered off label via nebulizer to patients with cystic fibrosis (CF) and non-cystic fibrosis bronchiectasis (BE) for more than 40 years (1). Tobramycin and aztreonam now have approved formulations for inhaled use in CF, and phase III clinical trials are under way with colistin and amikacin. Recent phase III trials have also been reported for two different formulations of ciprofloxacin as inhaled therapy in BE (2–4). A common factor among these antibiotics is that they principally target Gram-negative pathogens.
Methicillin-resistant Staphylococcus aureus (MRSA) has become a major respiratory pathogen in both acute infections like nosocomial pneumonia (5) as well as in chronic infections in the setting of CF (6) and BE (7, 8). In the setting of CF in particular, infection with MRSA is associated with significantly worse clinical outcomes (9, 10). While intravenous vancomycin is standard therapy for MRSA, in the respiratory setting, efficacy is reduced by relatively poor penetration into lung secretions and dose-limiting nephrotoxicity (11).
Inhaled antibiotic delivery offers the potential to achieve high concentrations of drug in the lungs while minimizing the risk of systemic side effects. Off-label use of inhaled vancomycin by nebulization of the intravenous formulation has been reported for treatment of MRSA in the setting of CF, including one small randomized controlled trial demonstrating a reduction in colonies but not eradication (12). Here, we report the development of a dry-powder formulation of vancomycin for inhaled delivery.
RESULTS
Subject characteristics.
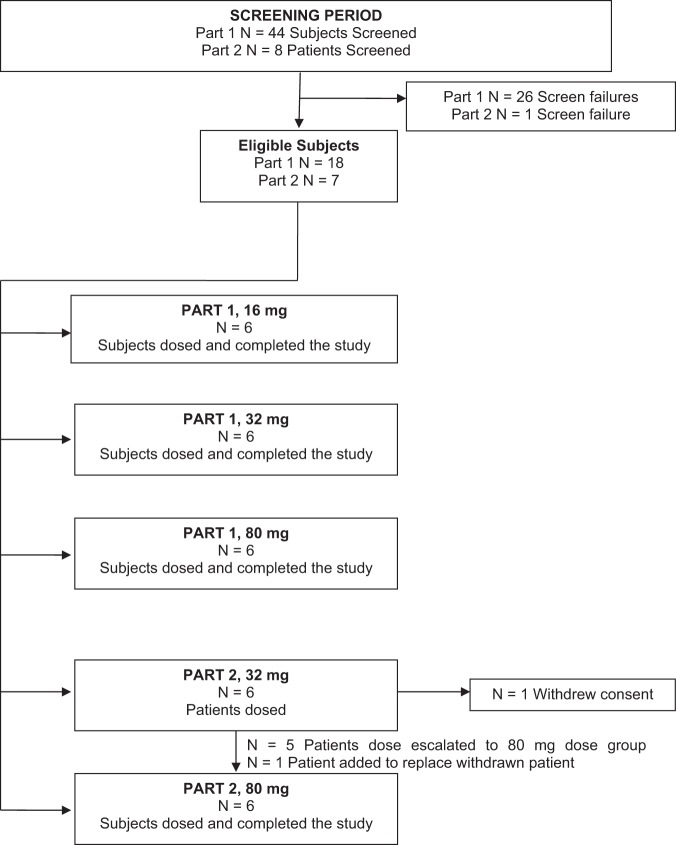
Eighteen subjects completed part I. Six patients with CF were enrolled in part II, and 5 completed both the 32-mg and 80-mg dosing protocols. One subject from part 2 withdrew informed consent to participate in the study after completing the 32-mg dose and was replaced by another subject who completed dosing with 80 mg only.
Subject disposition is shown in Fig. 1, and subject characteristics are summarized in Table 1.
FIG 1.
Disposition of subjects.
TABLE 1.
Summary of demographic data
| Demographic | Part 1 |
Part 2a |
|||
|---|---|---|---|---|---|
| 16 mg (n = 6) | 32 mg (n = 6) | 80 mg (n = 6) | 32 mg (n = 6) | 80 mg (n = 6) | |
| Mean age in years (SD) | 28.2 (10.5) | 24.7 (3.7) | 22.2 (2.8) | 28.5 (7.8) | 30.8 (7.9) |
| Sex | |||||
| No. male (%) | 6 (100.0) | 6 (100.0) | 6 (100.0) | 4 (66.7) | 3 (50.0) |
| No. female (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 2 (33.3) | 3 (50.0) |
| Race | |||||
| No. white (%) | 6 (100.0) | 5 (83.3) | 6 (100.0) | 6 (100.0) | 6 (100.0) |
| No. other (%) | 0 (0.0) | 1 (16.7) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Mean weight (kg) (SD) | 80.80 (9.45) | 76.23 (8.62) | 76.80 (4.04) | 60.50 (4.64) | 63.86 (4.96) |
| Mean height (cm) (SD) | 183.8 (5.3) | 181.7 (7.0) | 178.8 (4.6) | 168.7 (6.3) | 168.6 (4.2) |
| Mean body mass index (kg/m²) (SD) | 23.87 (2.14) | 23.13 (2.54) | 24.10 (2.26) | 21.32 (1.72) | 22.50 (1.85) |
One subject withdrew after the 32-mg dose and was replaced for the 80-mg dose by an alternative subject.
Pharmacokinetic data.
In part 1 of the study, 16 mg, 32 mg, and 80 mg AeroVanc were administered to healthy volunteers as single doses and compared to intravenous (i.v.) vancomycin. A relatively slow absorption phase, with time to reach the maximum plasma concentration (Tmax) around 2 h, was followed by a slightly longer AeroVanc elimination phase compared to that of i.v. vancomycin (Fig. 2). The Tmax decreased slightly as the dose increased. Maximum sputum concentration (Cmax) and area under the plasma concentration-time curve (AUC) were proportional to the dose delivered (Table 2). The average absolute bioavailability of vancomycin following administration of AeroVanc was 49% ± 8%.
FIG 2.
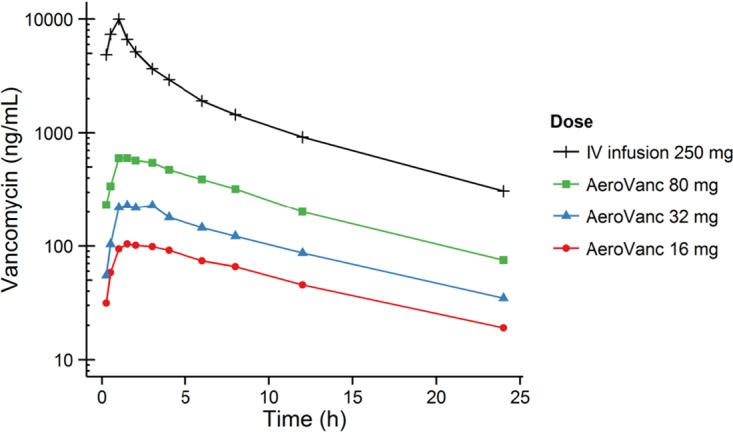
Summary plot (semi-log) of average vancomycin plasma concentration versus time.
TABLE 2.
Summary of mean plasma vancomycin pharmacokinetic parameters
| Parameter | Treatment group |
|||
|---|---|---|---|---|
| Mean (SD) for 16 mg | Mean (SD) for 32 mg | Mean (SD) for 80 mg | Mean (SD) for i.v. vancomycin 250 mg | |
| t1/2 (h) | 8.454 (2.017) | 8.648 (0.530) | 8.044 (1.296) | 7.226 (1.128) |
| Tmax (h) | 2.083 (0.801) | 1.833 (0.606) | 1.333 (0.408) | 0.917 (0.204) |
| Cmax (ng/ml) | 108.82 (33.16) | 231.50 (89.64) | 617.83 (230.03) | 10,028.33 (1,767.69) |
| AUCt (h·ng/ml) | 1,209.644 (237.696) | 2,379.790 (975.410) | 6,257.858 (1,506.939) | 41,027.792 (2,696.013) |
| AUCinf (h·ng/ml) | 1,461.407 (257.189) | 3,051.12 (959.14) | 7,135.735 (1,457.921) | 44,356.356 (3,623.420) |
| Mean absorption (%)a | 52.0 | 55.0 | 42.5 | |
Mean absorption calculated only from subjects who had both inhaled and intravenous vancomycin. Two subjects had both routes of administration in the 16-mg and 80-mg group, and only one subject had both routes in the 32-mg group.
In part 2 of the study, 32 mg and 80 mg AeroVanc were administered to patients with CF as single doses. Cmax in sputum occurred at the first time point measured (which was at 1 h) and increased with dose in a dose-proportional manner (Fig. 3). Minimum sputum concentration (Cmin) on average remained above the usual current MIC values of vancomycin for MRSA (13) for up to 24 h.
FIG 3.
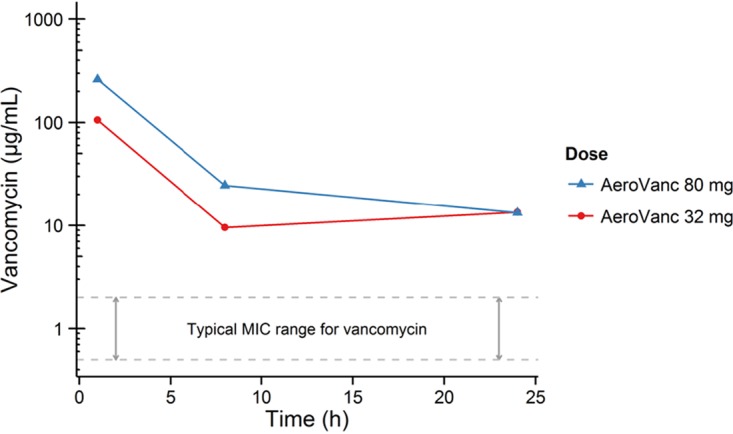
Mean vancomycin sputum concentration versus time.
Safety data.
The adverse event (AE) reporting profile is shown in Table 3. The most common events were cough, nausea, and a feeling of respiratory congestion. As shown in Table 3, the vast majority of symptoms were mild.
TABLE 3.
Summary of adverse events by severity
| Severity of adverse event | Part 1 |
Part 2 |
|||
|---|---|---|---|---|---|
| No. of subjects with AE (%) for the 16-mg group (n = 6) | No. of subjects with AE (%) for the 32-mg group (n = 6) | No. of subjects with AE (%) for the 80-mg group (n = 6) | No. of subjects with AE (%) for the 32-mg group (n = 6) | No. of subjects with AE (%) for the 80-mg group (n = 6) | |
| Mild | 2 (33.3) | 4 (66.7) | 2 (33.3) | 4 (66.7) | 5 (83.3) |
| Moderate | 0 (0.0) | 1 (16.7) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
| Severe | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (16.7) | 0 (0.0) |
With respect to the risk of bronchospasm, in part 1 of the study, there was no change in lung function over time (Table 4). Three subjects experienced a small reduction in the post-dose forced expiratory volume in 1 s (FEV1) percentage (7 to 11%), but only one of the subjects reported symptoms indicative of bronchoconstriction. None of the subjects required bronchodilator treatment, and the changes were considered by the data safety monitoring board (DSMB) to be clinically nonsignificant. There was no change in FEV1 following administration of i.v. vancomycin.
TABLE 4.
FEV1 by treatment group
| Visit | Mean (SD) of FEV1 (liters) |
||||
|---|---|---|---|---|---|
| Part 1 (healthy subjects) |
Part 2 (cystic fibrosis subjects) |
||||
| 16 mg (n = 6) | 32 mg (n = 6) | 80 mg (n = 6) | 32 mg (n = 6) | 80 mg (n = 6) | |
| Screening | 4.605 (0.639) | 4.518 (0.466) | 4.693 (0.213) | 1.967 (0.350) | 2.280 |
| Predose | 4.503 (0.637) | 4.418 (0.414) | 4.587 (0.268) | 1.872 (0.370) | 1.843 (0.354) |
| 30 min | 4.543 (0.685) | 4.362 (0.410) | 4.337 (0.303) | ||
| 1 h | 1.720 (0.449) | 1.620 (0.476) | |||
| 8 h | 1.687 (0.436) | 1.648 (0.419) | |||
| 24 h | 1.913 (0.455) | 1.750 (0.398) | |||
| Follow-up | 4.725 (0.843) | 4.370 (0.502) | 4.500 (0.393) | 2.220 () | 1.838 (0.473) |
In part 2 of the study, a relative decrease in FEV1 of 15% or more was observed in three of the seven subjects, in two subjects after both doses and in one subject after the high dose only. There was a greater reduction in mean FEV1 with the higher dose, although a dose relationship was not observed in all patients. Salbutamol treatment was used in three patients following AeroVanc administration. In two patients, salbutamol was used to reverse a reduced FEV1, whereas in a third patient, it was used to relieve mild symptoms of chest tightness.
DISCUSSION
In this study, we evaluated the pharmacokinetics and safety of a novel dry-powder formulation of vancomycin. We have shown that AeroVanc was well tolerated, and the pharmacokinetic properties are favorable for a therapeutic effect against MRSA.
Recent global surveillance data show vancomycin activity against S. aureus remains stable with an MIC90 of 1 mg/liter and 100% susceptibility in 2016 and no increase over time in isolates with a vancomycin MIC of >1 mg/liter (13). Our data show that AeroVanc produced sputum vancomycin levels significantly greater than 1 mg/liter over a 24-h period. While MIC levels do not necessarily equate to clinical effect, particularly in the setting of inhaled therapy where drug distribution may be unequal and secretions may further impair drug delivery, the high levels seen with AeroVanc are consistent with a possible therapeutic effect.
From a safety perspective, adverse effects were mild and infrequent, with the most common symptom being cough as expected. The most significant adverse effect was reduction in FEV1 or chest tightness with requirement for salbutamol in three of the subjects with CF. None of the subjects experienced significant distress, and all symptoms resolved following a single dose of salbutamol. Some bronchoconstriction is common in patients with CF following any dry-powder inhalation and usually can be prevented by prior bronchodilator therapy.
At the maximum 80-mg dose of inhaled vancomycin, with an estimated 50% bioavailability, this is well below the threshold for any likely renal toxicity even for patients with severe chronic renal failure. Achievement of steady-state levels many magnitudes above normal MIC levels is considered an important component of reducing the development of antibiotic resistance (14), even more so with chronic use such as in BE or CF. We did not test cumulative dosing in this study, so we were not able to determine what the range of steady-state concentrations would be in plasma or sputum. However, higher steady-state concentrations are likely to be achieved after cumulative use.
In summary, AeroVanc was well-tolerated and achieved vancomycin levels in sputum that warrant its further development as a potential therapeutic agent for respiratory infection with MRSA. Further studies are warranted to further assess the efficacy and safety of cumulative dosing of AeroVanc.
MATERIALS AND METHODS
Study design.
This was a phase I study with two parts.
Part 1 was an open-label, reference-controlled, single-dose escalating study to examine the pharmacokinetics, safety, and tolerability of 16 mg, 32 mg, and 80 mg of AeroVanc in healthy volunteers. Two subjects from each cohort also received a reference dose of intravenous (i.v.) vancomycin following a washout period.
Part 2 was an open-label, single-dose escalation study to examine the pulmonary pharmacokinetics, safety, and tolerability of 32 mg and 80 mg AeroVanc in patients with CF.
Subjects.
Part 1: Healthy male volunteers, aged 18 to 50 years old inclusive; able to communicate with site personnel and willingly provide consent; able to comply with the protocol; with a body mass index (BMI) of 20 to 30 kg/m2 and weight of 60 to 90 kg; no clinically significant abnormalities at screening; negative for human immunodeficiency virus, hepatitis B, and hepatitis C at screening; forced expiratory volume in 1 s (FEV1) greater than 75% of predicted age-adjusted value; were considered eligible for this study.
Part 2: Subjects with a confirmed diagnosis of CF; able to communicate with site personnel and willingly provide consent; able to comply with the protocol; aged over 18 years; FEV1, >40% of predicted; able to perform techniques to measure lung function; no liver enzymes greater than two times the upper limit of normal; able to spontaneously produce bronchial sputum daily; were considered eligible for this study.
Subjects with evidence of chronic organ failure, prior allergy to vancomycin, and current smokers were excluded. Subjects were also excluded if they had a history of recent respiratory infection or any other condition the investigator considered may interfere with the study or put the subject at risk.
In addition to the above exclusion criteria, subjects with CF were not eligible for part 2 of the study if they were receiving oral corticosteroids in doses exceeding 10 mg/day or 16 mg every other day; had a history of sputum culture or throat swab culture yielding Burkholderia Cepacia in the previous 2 years; had a history of positive MRSA culture or sputum culture positive for MRSA at screening; required daily continuous oxygen supplementation or requirement for more than 2 liters/min at night; had a history of previous allergies or sensitivity to vancomycin or other component(s) of the study drug; had changes in antimicrobial, bronchodilator, anti-inflammatory, or corticosteroid medications within 7 days prior to screening; had changes in physiotherapy technique or schedule within 7 days prior to screening; had a history of lung transplantation; had a chest X-ray at screening or within 90 days of screening, with abnormalities indicating a significant acute finding (e.g., lobar infiltrate and atelectasis, pneumothorax, or pleural effusion); had a positive pregnancy test; were a female of childbearing potential who was lactating or was not practicing an acceptable method of birth control (e.g., hormonal or barrier methods or intrauterine device); had findings at screening that, in the investigator’s opinion, would compromise the safety of the subject or the quality of the study data; had a history of severe cough/bronchospasm upon inhalation of dry-powder inhalation product; were considered “terminally ill” or eligible for lung transplantation; had a significant episode of hemoptysis (>60 ml) in the 3 months prior to enrollment.
Intervention.
AeroVanc is a novel dry-powder inhaler product using a reloadable single-dose powder inhaler (Monodose; Plastiape, Italy), each capsule containing 16 mg of vancomycin. The doses used in this study included 16 mg (one capsule), 32 mg (two capsules), and 80 mg (five capsules). The doses were administered by oral inhalation.
For those in part I receiving intravenous vancomycin, 250 mg was administered in 50 ml of 0.9% NaCl over 60 min.
Measurements.
Safety evaluation included adverse events, review of vital signs (temperature, heart rate, blood pressure, respiratory rate), electrocardiogram (ECG), laboratory safety testing, and spirometry (FEV1) at each time point.
Part 1 included analysis of vancomycin blood concentrations at predose, 0.25, 0.5, 1, 1.5, 2, 3, 4, 6, 8, 12, and 24 h after the beginning of AeroVanc administration.
The following parameters were calculated: maximum plasma concentration (Cmax); time to reach the maximum plasma concentration (Tmax); elimination half-life (t1/2) associated with the terminal slope (Kel) of the semilogarithmic drug concentration-time curve, calculated as 0.693/Kel, and area under the curve during a dosage interval at steady-state (AUC0–t, nominally 0 to 72 h, to be calculated using the linear trapezoidal rule), area under the plasma concentration-time curve from time zero to infinite time (AUC0-inf), calculated as the sum of AUClast and Clast/Kel in which Clast is the last observed quantifiable concentration; and absolute bioavailability of AeroVanc.
Part 2 included analysis of vancomycin sputum concentrations at 1, 8, and 24 h postdose as follows: maximum sputum concentration (Cmax) and minimum sputum concentrations (Cmin).
Safety definitions.
Severity of adverse events was graded as follows: mild, did not interfere with the subject’s usual function (i.e., there was awareness of symptoms or signs, but they were easily tolerated with no specific medical intervention required); moderate, interfered to some extent with the subject’s usual function (i.e., enough discomfort to interfere with usual activity and minimal intervention required); and severe, interfered significantly with the subject’s usual function (i.e., incapacity to work or to do usual activities and hospitalization or invasive intervention required).
A data safety monitoring board (DSMB) provided safety oversight of the study.
Statistical analysis.
All safety parameters were summarized using descriptive statistics and presented by time point and cohort/dose level.
All pharmacokinetic parameters were summarized using descriptive statistics and presented by time point and cohort/dose.
ACKNOWLEDGMENTS
John Lord and Taneli Jouhikainen are employees of Savara, Inc. and hold shares in the company. Thomas Hofmann has been a paid consultant to Savara, Inc. Grant Waterer has been paid consultancy fees by Savara, Inc. and holds shares in the company.
REFERENCES
- 1.Hodson ME, Penketh AR, Batten JC. 1981. Aerosol carbenicillin and gentamicin treatment of Pseudomonas aeruginosa infection in patients with cystic fibrosis. Lancet 2:1137–1139. doi: 10.1016/s0140-6736(81)90588-2. [DOI] [PubMed] [Google Scholar]
- 2.Haworth CS, Bilton D, Chalmers JD, Davis AM, Froehlich J, Gonda I, Thompson B, Wanner A, O'Donnell AE. 2019. Inhaled liposomal ciprofloxacin in patients with non-cystic fibrosis bronchiectasis and chronic lung infection with Pseudomonas aeruginosa (ORBIT-3 and ORBIT-4): two phase 3, randomised controlled trials. Lancet Respir Med 7:213–226. doi: 10.1016/S2213-2600(18)30427-2. [DOI] [PubMed] [Google Scholar]
- 3.Aksamit T, De Soyza A, Bandel TJ, Criollo M, Elborn JS, Operschall E, Polverino E, Roth K, Winthrop KL, Wilson R. 2018. RESPIRE 2: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 51:1702053. doi: 10.1183/13993003.02053-2017. [DOI] [PubMed] [Google Scholar]
- 4.De Soyza A, Aksamit T, Bandel TJ, Criollo M, Elborn JS, Operschall E, Polverino E, Roth K, Winthrop KL, Wilson R. 2018. RESPIRE 1: a phase III placebo-controlled randomised trial of ciprofloxacin dry powder for inhalation in non-cystic fibrosis bronchiectasis. Eur Respir J 51:1702052. doi: 10.1183/13993003.02052-2017. [DOI] [PubMed] [Google Scholar]
- 5.Kalil AC, Metersky ML, Klompas M, Muscedere J, Sweeney DA, Palmer LB, Napolitano LM, O'Grady NP, Bartlett JG, Carratalà J, El Solh AA, Ewig S, Fey PD, File TM, Restrepo MI, Roberts JA, Waterer GW, Cruse P, Knight SL, Brozek JL. 2016. Management of adults with hospital-acquired and ventilator-associated pneumonia: 2016 clinical practice guidelines by the Infectious Diseases Society of America and the American Thoracic Society. Clin Infect Dis 63:e61–e111. doi: 10.1093/cid/ciw353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Goss CH, Muhlebach MS. 2011. Review: Staphylococcus aureus and MRSA in cystic fibrosis. J Cyst Fibros 10:298–306. doi: 10.1016/j.jcf.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 7.Metersky ML, Aksamit TR, Barker A, Choate R, Daley CL, Daniels LA, DiMango A, Eden E, Griffith D, Johnson M, Knowles M, O'Donnell AE, Olivier K, Salathe M, Thomashow B, Tino G, Turino G, Winthrop KL, Mannino D. 2018. The prevalence and significance of Staphylococcus aureus in patients with non-cystic fibrosis bronchiectasis. Ann Am Thorac Soc 15:365–370. doi: 10.1513/AnnalsATS.201706-426OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Menéndez R, Méndez R, Polverino E, Rosales-Mayor E, Amara-Elori I, Reyes S, Sahuquillo-Arce JM, Fernández-Barat L, Alcaraz V, Torres A. 2017. Risk factors for multidrug-resistant pathogens in bronchiectasis exacerbations. BMC Infect Dis 17:659. doi: 10.1186/s12879-017-2754-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dasenbrook EC, Checkley W, Merlo CA, Konstan MW, Lechtzin N, Boyle MP. 2010. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA 303:2386–2392. doi: 10.1001/jama.2010.791. [DOI] [PubMed] [Google Scholar]
- 10.Vanderhelst E, De Meirleir L, Verbanck S, Pierard D, Vincken W, Malfroot A. 2012. Prevalence and impact on FEV1 decline of chronic methicillin-resistant Staphylococcus aureus (MRSA) colonization in patients with cystic fibrosis. A single-center, case control study of 165 patients. J Cyst Fibros 11:2–7. doi: 10.1016/j.jcf.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 11.Kollef MH. 2007. Limitations of vancomycin in the management of resistant staphylococcal infections. Clin Infect Dis 45(Suppl):S191–S195. doi: 10.1086/519470. [DOI] [PubMed] [Google Scholar]
- 12.Dezube R, Jennings MT, Rykiel M, Diener-West M, Boyle MP, Chmiel JF, Dasenbrook EC. 2019. Eradication of persistent methicillin-resistant Staphylococcus aureus infection in cystic fibrosis. J Cyst Fibros 18:357–363. doi: 10.1016/j.jcf.2018.07.005. [DOI] [PubMed] [Google Scholar]
- 13.Diekema DJ, Pfaller MA, Shortridge D, Zervos M, Jones RN. 2019. Twenty-year trends in antimicrobial susceptibilities among Staphylococcus aureus from the SENTRY antimicrobial surveillance program. Open Forum Infect Dis 6:S47–S53. doi: 10.1093/ofid/ofy270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bassetti M, Luyt CE, Nicolau DP, Pugin J. 2016. Characteristics of an ideal nebulized antibiotic for the treatment of pneumonia in the intubated patient. Ann Intensive Care 6:35. doi: 10.1186/s13613-016-0140-x. [DOI] [PMC free article] [PubMed] [Google Scholar]