Tuberculosis is an important cause of maternal morbidity, but little is known about the effects of pregnancy on antituberculosis drug concentrations. We developed population pharmacokinetic models to describe drug dispositions of isoniazid, pyrazinamide, and ethambutol in pregnant women with tuberculosis and HIV. HIV-positive pregnant women with tuberculosis receiving standard first-line tuberculosis treatment and participating in Tshepiso, a prospective cohort study in Soweto, South Africa, underwent sparse pharmacokinetic sampling at >36 weeks of gestation and 7 weeks postpartum.
KEYWORDS: NAT2, pregnancy, NONMEM, pharmacometrics, modeling, simulation
ABSTRACT
Tuberculosis is an important cause of maternal morbidity, but little is known about the effects of pregnancy on antituberculosis drug concentrations. We developed population pharmacokinetic models to describe drug dispositions of isoniazid, pyrazinamide, and ethambutol in pregnant women with tuberculosis and HIV. HIV-positive pregnant women with tuberculosis receiving standard first-line tuberculosis treatment and participating in Tshepiso, a prospective cohort study in Soweto, South Africa, underwent sparse pharmacokinetic sampling at >36 weeks of gestation and 7 weeks postpartum. The effects of pregnancy on the pharmacokinetics of isoniazid, pyrazinamide, and ethambutol were investigated via population pharmacokinetic modeling. Isoniazid, pyrazinamide, and ethambutol concentrations were available for 29, 18, and 18 women, respectively. Their median weight was 66 kg while pregnant and 64 kg postpartum. No significant differences were observed in drug clearance, volume of distribution, or bioavailability during and after pregnancy. The model-estimated isoniazid, pyrazinamide, and ethambutol area under the concentration-time curve from 0 to 24 h (AUC0–24) medians were, respectively, 6.88, 419, and 16.5 mg · h/liter during pregnancy versus 5.01, 407, and 19.0 mg · h/liter postpartum. The model-estimated maximum concentration (Cmax) medians for isoniazid, pyrazinamide, and ethambutol were, respectively, 1.39, 35.9, and 1.82 mg/liter during pregnancy versus 1.43, 34.5, and 2.11 mg/liter postpartum. A posteriori power calculations determined that our analysis was powered 91.8%, 59.2%, and 90.1% at a P of <0.01 to detect a 40% decrease in the AUCs of isoniazid, pyrazinamide, and ethambutol, respectively. Pregnancy does not appear to cause relevant changes in the exposure to isoniazid, pyrazinamide, and ethambutol. Additional studies of antituberculosis drugs in pregnancy are needed.
TEXT
Tuberculosis (TB) is an important cause of maternal mortality and morbidity globally; the immune changes of pregnancy increase the risk of progressing from latent TB infection to active TB disease (1, 2). Furthermore, tuberculosis in pregnancy carries high risks for the mother and the fetus, especially for those living with HIV, who have a 37-fold-increased risk of dying from tuberculosis and increased rates of obstetric complications (3–6). Similarly, infants born to women with HIV and tuberculosis have a significant risk of low birth weight, tuberculosis, and early mortality (6). Thus, optimizing the care of pregnant women with HIV-associated TB is critically important.
Physiological changes during pregnancy may alter pharmacokinetic parameters and therefore impact drug exposure, thus complicating dosing for many medications. These changes include reduced gastrointestinal motility and altered stomach pH, which impact the absorption and bioavailability of a drug. Additionally, reduced serum albumin may increase the unbound fraction of highly protein-bound drugs, thus increasing both the volume of distribution (V) and total clearance (CL), while maintaining comparable levels of unbound-drug concentrations. Finally, the altered activities of metabolizing isoenzymes (e.g., from the cytochrome P450 [CYP450] and UGT families), together with altered total and renal blood flow, also affect drug clearance. How all of these changes independently and synergistically affect drug concentration is highly dependent on the physicochemical and metabolic characteristics of the drug. Predictions are not always straightforward (7, 8).
Current World Health Organization tuberculosis treatment guidelines recommend the use of the same regimens and dosages for pregnant and nonpregnant woman: an intensive phase of rifampin (8 to 12 mg/kg of body weight), isoniazid (4 to 6 mg/kg), pyrazinamide (20 to 30 mg/kg), and ethambutol (15 to 25 mg/kg) daily for 2 months, followed by a continuation phase of 4 months with rifampin (8 to 12 mg/kg) and isoniazid (4 to 6 mg/kg), although some guidelines recommend that pyrazinamide be withheld in pregnancy (9). Previously, we reported that rifampin exposure was only marginally increased during pregnancy, supporting the use of standard doses (10). However, there are no published pharmacokinetic data for the other first-line tuberculosis drugs.
The aim of this study was to evaluate the changes in the pharmacokinetics of isoniazid, pyrazinamide, and ethambutol among women with tuberculosis and treated with standard tuberculosis treatment regimens, from pregnancy through the postpartum period.
RESULTS
Study data.
Data were available for 29 women taking isoniazid, 18 of whom also had concentrations for pyrazinamide and ethambutol. These patients underwent pharmacokinetic sampling for the drugs at a median (interquartile range) of 2.71 (1.29 to 3.57) weeks before delivery (18 were sampled during pregnancy and 3 were sampled during labor), and then 8 patients were sampled postpartum 6.64 (4.96 to 7.18) weeks after delivery. For pyrazinamide and ethambutol, 13 patients were sampled during pregnancy, 2 were sampled during labor, and 3 were sampled postpartum. The median weight of the patients was 66 kg during pregnancy and 63.5 kg postpartum. The demographics of the study participants are summarized in Table 1.
TABLE 1.
Demographics for patients included in the population pharmacokinetic analyses for each of the drugsa
| Characteristic | Median no. (interquartile range) or no. (%) of subjects given: |
|
|---|---|---|
| Isoniazid (n = 29) | Pyrazinamide and ethambutol (n = 18) | |
| Age (yr) (range) | 28.1 (25.2–29.9) | 26.7 (24.8–29.5) |
| Wt (kg) (range): | ||
| Prepartum | 66.0 (60.0–80.0) | 66 (58–77) |
| Postpartum | 63.5 (57.3–72.8) | 66 (59–72) |
| Fat-free mass (kg) (range)b: | ||
| Prepartum | 41.4 (37.4–46.2) | 41.4 (36.8–45.1) |
| Postpartum | 40.0 (36.9–44.6) | 40.8 (39.2–45.1) |
| No. (%) of subjects on: | ||
| EFV- based HAART | 24 (83) | 13 (72) |
| LPV/r-based HAART | 1 (3) | 1 (6) |
| No ART | 1 (3) | 1 (6) |
| No. (%) of subjects with NAT2 metabolizer status: | ||
| Slow | 11 (38) | |
| Intermediate | 10 (34) | |
| Rapid | 3 (10) | |
| Unknown | 5 (17) | |
ART, antiretroviral therapy; EFV, efavirenz; HAART, highly active antiretroviral therapy; LPV/r, lopinavir-ritonavir; NAT, N-acetyltransferase 2.
The number of prepartum women given isoniazid or pyrazinamide-ethambutol was 21 or 15, respectively, and the number of postpartum women given isoniazid or pyrazinamide-ethambutol was 8 or 3, respectively.
Three patients had undetectable predose concentrations for all measured drugs (rifampin, isoniazid, pyrazinamide, and ethambutol), but the concentrations following the observed dose in the clinic were in line with those of all the other subjects, so this was considered nonadherence and the dosing records from the previous days were disregarded.
The final data for isoniazid included a total of 37 pharmacokinetic profiles from 29 patients (8 paired), based on 141 plasma concentration measurements (77 during pregnancy and 64 postpartum). For isoniazid, 46 samples, mostly predose samples, were below the limit of quantification (BLQ). For pyrazinamide and ethambutol, 19 pharmacokinetic profiles were obtained from 18 patients (1 paired), based on 66 plasma concentration measurements (54 during pregnancy and 12 postpartum), and no data were BLQ.
Isoniazid pharmacokinetics.
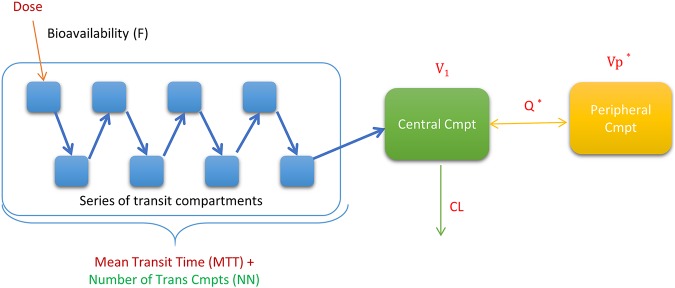
The pharmacokinetics of isoniazid is best described by a two-compartment disposition model with first-order elimination and transit compartments absorption. The model structure is depicted in Fig. 1. The model supported estimation of between-subject variability in clearance and between-occasion variability in mean transit time (MTT) and the absorption rate constant (Ka). N-Acetyltransferase 2 (NAT2) acetylator status was a significant covariate upon clearance (the change in the objective function value [OFV] was −29.9 points, with 2 degrees of freedom [P < 0.001]), and the estimated typical values of clearance were 97.1, 75.7, and 29.0 liters/h in fast, intermediate, and slow metabolizers, respectively. Finally, the effects of pregnancy and concomitant use of efavirenz on exposure parameters (clearance and bioavailability) were explored, and no statistically significant difference was observed.
FIG 1.
Structural model for isoniazid, pyrazinamide, and ethambutol. The dose of each drug is assumed to go through a series of transit compartments (Trans Cmpts) before being absorbed into the central compartment. It is then eliminated from the central compartment with first-order kinetics. An asterisk (*) indicates the peripheral compartment, which applies only to isoniazid and ethambutol. NN, number of transit compartments; V1, central volume of distribution; Vp, peripheral volume of distribution; Q, intercompartmental clearance; CL, central clearance.
The impact of the chosen method to handle BLQ values (M6 method) on the parameter estimates was tested by repeating the analysis after excluding these points, and no significant change was observed. Final parameter estimates are summarized in Table 2, and model-estimated secondary pharmacokinetic parameters are summarized in Table 3 and Fig. 2. A visual predictive check showing adequate fit is displayed in Fig. 3.
TABLE 2.
Final population pharmacokinetic model parameter estimates for isoniazid, pyrazinamide, and ethambutol
| Parameter description | Typical value (95% CI) fora: |
||
|---|---|---|---|
| Isoniazid | Pyrazinamide | Ethambutol | |
| CL for rapid NAT2 acetylators (liters/h)b | 97.1 (68.6–144) | ||
| CL for intermediate NAT2 acetylators (liters/h)b | 75.7 (59.4–95.8) | 3.39 (2.96–3.87) | 60.2 (53.7–68.5) |
| CL for slow NAT2 acetylators (liters/h)b | 29.0 (24.3–34.8) | ||
| V1 (liters)b,c | 130 (106-162) | 43.8 (39.7–47.2) | 268 (154–419) |
| Q (liters/h)b,c | 12.4 (5.64–31.3) | 174 (71.7–385) | |
| Vp (liters)b,c | 28.5 (10.8–50.1) | 334 (217–490) | |
| MTT (h)c | 1.21 (0.953–1.51) | 0.934 (0.565–1.17) | 2.15 (1.84–2.51) |
| NNc | 8.01 (3.95–14.9) | 3.78 (2.24–7.71) | 6.23 (3.21–11.6) |
| Bioavailability (F) | 1 fixed | 1 fixed | 1 fixed |
| Proportional error (%) | 22.2 (15.2–30.4) | 9.19 (6.99–14.4) | 23.3 (17.8–33) |
| Additive error (mg/liter) | 0.045 (0.035–0.062) | 0.011 (0.002–0.019) | 0.03e fixed |
| BSV of clearance (%CV)d | 12.7 (1.37–30.8) | 25.4 (17.1–40.3) | 4.69 (0.379–20.4) |
| BOV of mean transit time (%CV)d | 56.7 (43.3–78.0) | 71.9 (41.2–125) | 24.1 (15.9–36.0) |
| BOV of bioavailability (%CV)d | 36.7 (27.0–48.7) | 13.6 (5.81–18.7) | 20.1 (10.6–32.2) |
95% confidence intervals were obtained by the sampling importance resampling technique using PsN software.
Allometric scaling was used for the clearance (CL) by fat-free mass (FFM), intercompartmental clearance (Q), central volume of distribution (V1), and peripheral volume of distribution (Vp) (by weight); typical values are reported for the median FFM and median weight as reported in Table 1.
Prior values (uncertainty) were added to the parameter estimates, as follows: for isoniazid, the Q (16.1 liters/h [50%]), Vp (16.5 liters [50%]), absorption mean transit time (MTT) (0.924 h [50%]), and number of transit compartments (NN) (2.73 [50%]); for pyrazinamide, the MTT (0.84 h [30%]) and NN (2.6 [50%]); and for ethambutol, the V1 (266 liters [50%]), Vp (687 liters [50%]), Q (109 liters/h [50%]), MTT (2.54 h [50%]), and NN (11.1 [50%]).
Between-subject variability (BSV) and between-occasion variability (BOV) were assumed to be log-normally distributed and are reported as approximate %CVs.
The estimate of the additive error was not statistically significant from its lower bound (LLOQ/2); thus, it was fixed to that value.
TABLE 3.
Model-estimated secondary pharmacokinetic parametersa
| Time period |
Cmax (mg/liter) for: |
AUC0–24 (mg · h/liter) for: |
||||
|---|---|---|---|---|---|---|
| Isoniazid | Pyrazinamide | Ethambutol | Isoniazid | Pyrazinamide | Ethambutol | |
| Prepartum | 1.39 (1.13–1.60) | 35.9 (32.7–38.1) | 1.82 (1.61–2.14) | 6.88 (3.63–10.40) | 419 (370–541) | 16.5 (14.3–20.6) |
| Postpartum | 1.43 (1.09–1.86) | 34.5 (29.9–41.3) | 2.11 (1.85–2.46) | 5.01 (2.89–8.03) | 407 (336–514) | 19.0 (16.5–21.6) |
Data are given as medians (interquartile ranges). For INH, there were 21 (3 at birth) prepartum and 16 postpartum women; for PZA and EMB, there were 15 (2 at birth) prepartum and 4 postpartum women.
FIG 2.
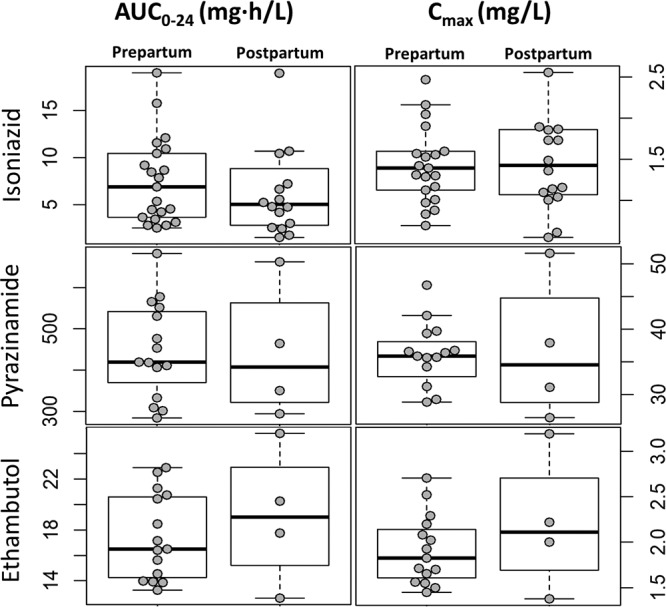
Box and whisker plots showing the AUC0–24 and Cmax values for the three drugs stratified by pre- and postpartum conditions. The dots represent individual values. Whiskers show the 2.5th and 97.5th percentiles.
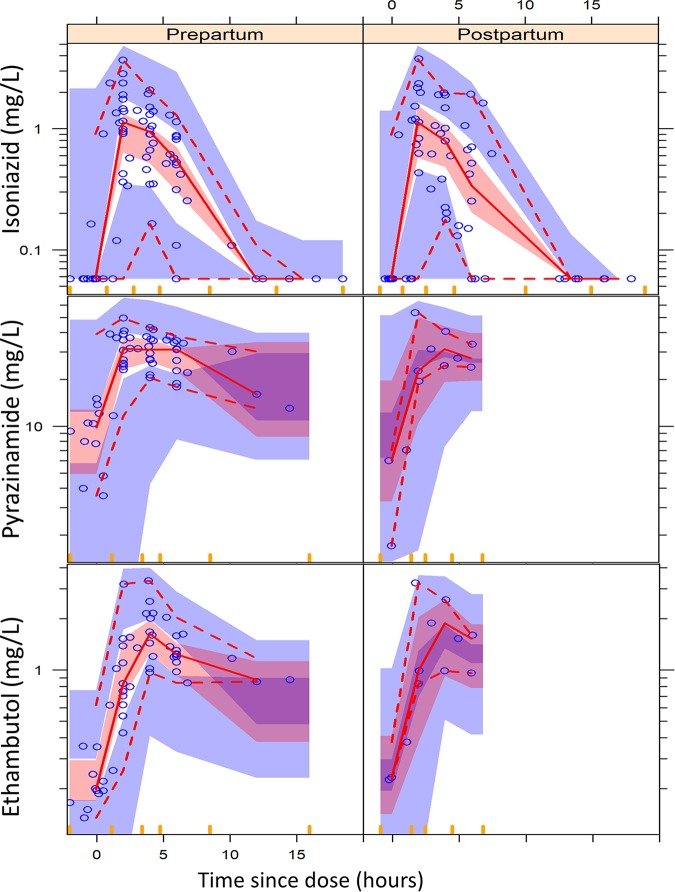
FIG 3.
Visual predictive check (VPC) for isoniazid, pyrazinamide, and ethambutol concentration versus time (time since the dose), stratified by pregnancy. The circles represent the original data, the dashed and solid lines are the 5th, 50th, and 95th percentiles of the original data, and the shaded areas are the corresponding 95% confidence intervals for the same percentiles, as predicted by the model. An appropriate model is expected to have most observed percentiles within the simulated confidence intervals.
Pyrazinamide pharmacokinetics.
The data were best fitted by a one-compartment disposition model with first-order elimination and transit compartment absorption (the structural model shown in Fig. 1); final parameter estimates are summarized in Table 2, and a visual predictive check appears in Fig. 3. The model estimated a clearance value of 3.39 liters/h for the typical patient. The effects of pregnancy and efavirenz on the exposure of the drug were investigated, and the model did not detect statistically significant effects.
Ethambutol pharmacokinetics.
The chosen structural model was a two-compartment disposition model with transit compartment absorption, as it gave the best fit of our data (depicted in Fig. 1). Pregnancy and efavirenz effects on clearance and bioavailability were investigated, and no statistically significant effects were detected. The typical value for clearance was 60.2 liters/h.
A posteriori parametric power estimation and design evaluation.
The stochastic simulation/reestimation procedure revealed that our study data and design (same dosing and sampling times, patient covariates, and variability in our study) had 91.8%, 59.2%, and 90.1% powers (P < 0.01) to detect a simulated 40% exposure difference for isoniazid, pyrazinamide, and ethambutol, respectively. The large difference in power between pyrazinamide and ethambutol, in spite of both drugs having only a few samples points in the postpartum arm, is owing to the low between-subject variability in clearance for ethambutol compared to that of pyrazinamide.
DISCUSSION
In general, pharmacokinetic data on the impact of pregnancy on drug exposures are scarce (11). Our findings suggest that there is no clinically significant pregnancy effect on exposure for the three drugs. Although the study design and sample size were limited and prevented robust estimation of all pharmacokinetic parameters without relying on prior information, the a posteriori power estimation suggests that, if large pregnancy effects (>40% reduction of exposure) were present between the 3rd trimester and postpartum, our analysis would have been powered to detect it at a P of <0.01, with powers of 91.8%, 59.2%, and 90.1% for isoniazid, pyrazinamide, and ethambutol, respectively.
The observed exposure values in our cohort for isoniazid were low compared with those announced in two conference proceedings on the pharmacokinetics of isoniazid in pregnancy. The first one (12) reported median maximum concentration (Cmax) values of 4.1 and 4.0 mg/liter for the 3rd trimester and postpartum, respectively, while the observed median Cmax values in our cohort in the 3rd trimester were 1.39 and 1.43 mg/liter postpartum. The second proceeding (13), which studied pregnant women with HIV coinfection, reported isoniazid clearance values of 72.3, 38.5, and 14.5 liters/h for fast, intermediate, and slow NAT2 acetylators, respectively. Median Cmax values during pregnancy and postpartum were 2.89 and 3.69 mg/liter, respectively, and area under the concentration-time curve from 0 to 24 h (AUC0–24) values during pregnancy were 8.05 and 11.1 mg · h/liter postpartum. In our cohort, the observed clearance values were 97.1, 75.7, and 29.0 liters/h for fast, intermediate, and slow metabolizers, with observed median AUC0–24s of 6.88 mg · h/liter during pregnancy and 5.01 mg · h/liter postpartum.
Compared to a previous report of a nonpregnant population (14), our exposure values for isoniazid are low. Median Cmaxs are reported to range from 3.0 to 6.5 mg/liter (10, 15–20), while the median values observed in our study were 1.39 and 1.43 mg/liter for prepartum and postpartum women, respectively. For AUC0–24s, the median reported values are between 10.0 and 22.5 mg · h/liter, while ours were 6.88 mg · h/liter prepartum and 5.01 mg · h/liter postpartum. These large discrepancies between our values and those in the literature for nonpregnant individuals may be attributed to several factors, including different distributions of the NAT2 genotype in different populations (21) (which was not accounted for in some of the reported values) and large variability in exposures possibly caused by sample handling and the instability of isoniazid at room temperature (22). On the other hand, it may be that isoniazid concentrations are lower both during pregnancy and early after pregnancy, with the pregnancy effect disappearing only a few months after delivery. So the lack of a pregnancy effect in our analysis might be explained by the choice of the postpartum condition as the comparator, as pointed out in references 23 and 24). It will be important in future studies to evaluate this further, given that isoniazid Cmax values of less than 3 mg/liter are considered to be subtherapeutic.
The observed pyrazinamide exposure in our cohort is in line with previously reported values in the literature dealing with HIV-positive tuberculosis-infected patients. The reported median Cmax (15–20, 25) ranged from 30 to 55 mg/liter, compared to a median value in our cohort of 35.9 mg/liter for prepartum and 34.5 mg/liter for postpartum women. Reported AUC0–24s (18, 19, 25) range between 344 and 420 mg · h/liter, and the median value observed in our cohort was 419 and 407 mg · h/liter for prepartum and postpartum women, respectively. This is reassuring; even if our analysis was poorly powered to detect a pregnancy effect for pyrazinamide, the values are very well aligned with those of a nonpregnant population, so pregnant women are not at risk of over- or underexposure to this anti-infective agent.
Ethambutol exposure values in our study were slightly lower than the reported results. Historical median Cmaxs range from 2.11 to 5.0 mg/liter (15, 17, 18, 25–27), and AUC0–24s range between 20.0 and 23.0 mg · h/liter (18, 25–27); our median values for Cmax were 1.82 mg/liter for prepartum and 2.1 mg/liter for postpartum women, and our AUC0–24s were 16.5 for prepartum and 19.0 mg · h/liter for postpartum women. The lower concentrations in our study may once again be due to the choice of the postpartum condition as the comparator, but the overall difference from historical values for nonpregnant women is very moderate, so our findings are reassuring that, for ethambutol as well, pregnant women are not at risk of inadequate exposure.
Our study has several limitations. First, the timing of the doses and adherence was not always accurately captured, as this was an opportunistic substudy within the Tshepiso Study among women who had study visits late in pregnancy or during labor and postpartum. Second, no HIV-negative women were included in the analysis, so we could not assess the effects of HIV coinfection with antiretroviral treatment on the pharmacology of tuberculosis drugs in the participants. Another limitation of the study is represented by the very few pharmacokinetic profiles and sample points of pyrazinamide and ethambutol available postpartum, since a majority of the patients were already in the continuation phase of their tuberculosis treatment at the time of pharmacokinetic sampling.
While this study was small, it is nevertheless unique, and the results are reassuring: pregnancy did not appear to meaningfully affect isoniazid, pyrazinamide, and ethambutol concentrations in women with HIV and tuberculosis, as previously reported for rifampin (10). The results of this study support the use of standard treatment without dose adjustment for tuberculosis treatment in pregnancy. Pregnant women are at high risk of progression from latent TB infection to active TB disease and to suffer adverse maternal and fetal outcomes related to tuberculosis. It is therefore imperative to provide data-supported, rational dosing for both the prophylaxis and the treatment of TB in this vulnerable population. Additional studies are needed across different geographic populations and with both first- and second-line anti-TB drugs.
MATERIALS AND METHODS
Study population.
The patients included in this analysis were enrolled in the Tshepiso Study, a prospective cohort study evaluating the effects of tuberculosis on maternal and infant outcomes in women with HIV infection. The clinical outcomes and pharmacokinetic analysis of rifampin and efavirenz have been published previously (10, 28), as have the main study results (6). The participants included in the current analysis were enrolled in the Tshepiso Study’s pharmacokinetic substudy (10).
Briefly, the participants were recruited from antenatal clinics and obstetrics wards at Chris Hani Baragwanath Hospital, Soweto, South Africa, between January 2011 and January 2013; they were pregnant women aged ≥18 years (with a gestational age of >13 weeks at the time of enrollment) and HIV positive. Cases had tuberculosis, and matched controls did not. Antiretroviral and tuberculosis treatment were dispensed by local public-sector clinics in accordance with South African national guidelines, and all treatment was self-administered.
Fixed-dose combination tablets provided by the national tuberculosis program were used in weight band-based doses; the number of tablets depended on patient weight (Table 4). For the intensive phase, Rifafour (Sanofi Aventis) tablets (150 mg rifampin, 75 mg isoniazid, 400 mg pyrazinamide, and 275 mg ethambutol) were administered, and for the continuation phase, Rifinah-150/75 or Rifinah-300/150 (Sanofi Aventis) were administered (each tablet contains 150 or 300 mg rifampin and 75 or 150 mg isoniazid). This study was approved by the institutional review boards of the Johns Hopkins School of Medicine and the universities of the Witwatersrand and Cape Town. Participants provided written informed consent.
TABLE 4.
Summary of weight band-based dosesa
| Pretreatment body wt (kg) | No. of tablets of RHZE (150, 75, 400, 275) in intensive phase (7 days a wk for 2 mo) | No. of tablets in continuation phase (7 days a wk for 4 mo) of: |
|
|---|---|---|---|
| RH (75, 150) | RH (300, 150) | ||
| 30–37 | 2 | 2 | |
| 38–54 | 3 | 3 | |
| 55–70 | 4 | 2 | |
| >70 | 5 | 2 | |
RHZE, rifampin, isoniazid, pyrazinamide, and ethambutol. Concentrations (in milligrams) of the respective drugs are given in parentheses.
Study protocol.
Women receiving standard first-line tuberculosis treatment underwent pharmacokinetic sampling at either 36 weeks of gestation or at delivery and then again at 6 weeks postpartum. Samples were collected predose and then 2, 4, and 6 to 8 h postdose. For women taking their medications prior to arrival in the clinic or in the evening and for women presenting in labor, opportunistic sampling was performed. In the latter group, samples were collected at 3-h intervals from presentation until delivery (maximum of four samples). In all patients, the timing of any doses prior to presentation to the clinic was recorded based on self-reporting.
We previously reported the methods used to determine N-acetyltransferase 2 (NAT2) genotype using known functional polymorphisms (28); 191G→A (rs1801279, *14), 341 T→C (rs1801280, *5), 590G→A (rs1799930, *6), and 857G→A (rs1799931, *7). NAT2 genotypes were categorized as follows: rapid (no variant allele), intermediate (1 variant allele), and slow (heterozygous for 2 different polymorphisms or homozygous for 1 polymorphism).
Drug concentration analysis.
Drug plasma concentrations were determined by liquid chromatography-tandem mass spectrometry (LC/MS/MS) performed in the Division of Clinical Pharmacology, University of Cape Town. For isoniazid, the calibration range was 0.195 (the lower limit of quantification [LLOQ]) to 25.0 mg/liter. The interday accuracy of the isoniazid assay ranged from 99.1% to 101.5%, and the percent coefficient of variation (%CV) of the precision ranged from 2.6% to 3.2%. For pyrazinamide, the calibration range was 0.200 (LLOQ) to 80.0 mg/liter. The interday accuracy of the pyrazinamide assay ranged from 99.7% to 102.3%, and the %CV of the precision ranged from 0.7% to 2.8%. For ethambutol, the calibration range was 0.084 (LLOQ) to 5.40 mg/liter. The interday accuracy of the ethambutol assay ranged from 103.3% to 105.8%, and the %CV of the precision ranged from 4.0% to 8.1%.
Pharmacokinetic analyses.
Population pharmacokinetic models of the drug concentration data for isoniazid, pyrazinamide, and ethambutol were analyzed using a nonlinear mixed-effects model in NONMEM version 7.4.2 with first-order conditional estimation with eta-epsilon interaction (29). Piraña, Perl-speaks-NONMEM (PsN) version 4.9.0, and Xpose4 were used to aid the modeling process and to prepare model diagnostics (30). One- and two-compartment disposition models with first-order elimination and first-order absorption (with or without a lag time or transit compartments [31]) were tested. Between-subject and -occasion random effects were included with the pharmacokinetic parameters with an assumption of log-normal distribution; a combined additive and proportional structure described the residual unexplained variability, with the additive component of the error constrained to be least 20% of the LLOQ. Values below the LLOQ were imputed to be the LLOQ divided by 2, except for consecutive values during the elimination phase of the pharmacokinetic profiles, which were excluded from the model fit according to the M6 method (29) but included in diagnostic plots. Allometric scaling (32) was used to adjust for the effect of body size on the disposition parameters, with testing of both total body weight and fat-free mass as body size descriptors. The allometric exponents were fixed to 0.75 and 1 for clearance and volume parameters, respectively. After the inclusion of allometric scaling, the following covariate effects were tested on the pharmacokinetic parameters: pregnancy, age, efavirenz versus lopinavir-ritonavir-based antiretroviral treatment, and, for isoniazid, formulation/phase of treatment (Rifafour in the intensive phase versus Rifinah during the continuation phase) and NAT2 acetylator genotype.
A missing NAT2 acetylator genotype was imputed using a mixture model based on the observed concentrations and a fixed relative proportion of each genotype in the overall population based on the parent Tshepiso Study (rapid NAT2 metabolizers constituted 13% of the population; 44% were classified as intermediate, and 43% were classified as slow metabolizers), as described in reference 33.
The development of the model and the inclusion of covariates were based on physiological plausibility, inspection of diagnostic plots, including visual predictive checks (34), and decreases in the objective function value (OFV), which was assumed to follow a χ2 distribution. The statistically significance cutoff for an additional degree of freedom (inclusion of one additional parameter) was an OFV drop of at least 3.84 points, corresponding to a P of <0.05. Covariates were added in a stepwise fashion in order of importance determined by the largest significant drop in the OFV. Weakly informative priors (35) were added as needed to stabilize the model and improve parameter estimates for the three drugs with ∼50% uncertainty. The typical values were obtained from previously published models for the three drugs in nonpregnant women with tuberculosis (25) after allometrically scaling the values to adjust for different body sizes between the two populations. Sampling importance resampling (SIR) (36) was used to assess the robustness of the final parameter estimates and to obtain the 95% confidence interval (CI).
A post hoc power analysis was performed on the final models to estimate the power to detect a clinically significant effect that was brought on by pregnancy using stochastic simulation and estimation (SSE), a feature of PsN (37) based on the algorithm published in reference 38. For the three drugs, we simulated a 70% clearance increase in pregnancy (−40% of the AUC). The simulated data sets (n = 200) were fitted with the full model (with the pregnancy effect being estimated) and a reduced model (no effect of pregnancy), and then we generated power curves using a chi-square significant level of 0.01.
ACKNOWLEDGMENTS
The study team is very grateful to the women who participated in the Tshepiso Study. We appreciate the contributions of the following individuals: Saba Shembe, Lala Maseko, Joyce Netshivhale, Abram Maubane, Agnes Shilubane, Matabogo Letutu, Yudesh Baliram, and Glenda Gray from the Perinatal HIV Research Unit; Eckhart Buchman, Sthe Velaphi, Sanjay Lala, and Khakhu Mathivha from the Chris Hani Baragwanath Academic Hospital; Jennifer Hoffmann from the Johns Hopkins University; and Gary Maartens, Jennifer Norman, Marilyn Solomons, Jose Francis, Roeland Wasemann, and Noha Abdelgawad from the University of Cape Town.
The Division of Clinical Pharmacology at the University of Cape Town gratefully acknowledges Novartis Pharma for their support of the development of pharmacometric skills in Africa.
Conceived and designed the experiments: K. E. Dooley, N. Martinson, and R. E. Chaisson. Performed the experiments: N. Martinson, Z. Waja, and M. Letutu. Performed the pharmacokinetics sample analysis: L. Wiesner. Analyzed the data: M. T. Abdelwahab, R. Leisegang, and P. Denti. Wrote the draft of the paper: M. T. Abdelwahab and P. Denti. All authors contributed to interpretation of the results, made comments on drafts, and approved the final version.
This work was supported by a grant from the National Institute of Child Health and Human Development of the National Institutes of Health (R01HD064354 to R. E. Chaisson). Other sources of support included NIH grants K23AI080842 (K. E. Dooley) and P30AI094189 (CFAR to R. E. Chaisson). H. McIlleron is supported by The Wellcome Trust (grant 206379/Z/17/Z). The National Institute of Allergy and Infectious Diseases (NIAID) provided funding to L. Wiesner under grant UM1 AI106701 and to J. S. Mathad under grant K23 AI129854; R. Leisegang was funded by an NIH Fogarty training fellowship under NIH/FIC grant 1 D43 TW010340-01 2017. The National Research Foundation provided funding to H. McIlleron (grant 90729) and P. Denti (grant 109056). M. T. Abdelwahab was supported by the Swedish Foundation for International Cooperation in Research and Higher Education (STINT) jointly with the South African National Research Council, National Research Foundation (NRF) (NRF grant 101575). The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
REFERENCES
- 1.Hoffmann CJ, Variava E, Rakgokong M, Masonoke K, van der Watt M, Chaisson RE, Martinson NA. 2013. High prevalence of pulmonary tuberculosis but low sensitivity of symptom screening among HIV-infected pregnant women in South Africa. PLoS One 8:e62211. doi: 10.1371/journal.pone.0062211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mathad JS, Gupta A. 2012. Tuberculosis in pregnant and postpartum women: epidemiology, management, and research gaps. Clin Infect Dis 55:1532–1549. doi: 10.1093/cid/cis732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mofenson LM, Laughon BE. 2007. Human immunodeficiency virus, mycobacterium tuberculosis, and pregnancy: a deadly combination. Clin Infect Dis 45:250–253. doi: 10.1086/518975. [DOI] [PubMed] [Google Scholar]
- 4.Pillay T, Sturm AW, Khan M, Adhikari M, Moodley J, Connolly C, Moodley D, Padayatchi N, Ramjee A, Coovadia HM, Sullivan JL. 2004. Vertical transmission of Mycobacterium tuberculosis in KwaZulu Natal: impact of HIV-1 co-infection. Int J Tuber Lung Dis 8:59–69. [PubMed] [Google Scholar]
- 5.Khan M, Pillay T, Moodley JM, Connolly CA, Durban Perinatal TB HIV-1 Study Group . 2001. Maternal mortality associated with tuberculosis-HIV-1 co-infection in Durban, South Africa. AIDS 15:1857–1863. doi: 10.1097/00002030-200109280-00016. [DOI] [PubMed] [Google Scholar]
- 6.Salazar-Austin N, Hoffmann J, Cohn S, Mashabela F, Waja Z, Lala S, Hoffmann C, Dooley KE, Chaisson RE, Martinson N. 2018. Poor obstetric and infant outcomes in human immunodeficiency virus-infected pregnant women with tuberculosis in South Africa: the Tshepiso Study. Clin Infect Dis 66:921–929. doi: 10.1093/cid/cix851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Anderson GD. 2005. Pregnancy-induced changes in pharmacokinetics: a mechanistic-based approach. Clin Pharmacokinet 44:989–1008. doi: 10.2165/00003088-200544100-00001. [DOI] [PubMed] [Google Scholar]
- 8.Wadelius M, Darj E, Frenne G, Rane A. 1997. Induction of CYP2D6 in pregnancy. Clin Pharmacol Ther 62:400–407. doi: 10.1016/S0009-9236(97)90118-1. [DOI] [PubMed] [Google Scholar]
- 9.American Thoracic Society, CDC, Infectious Diseases Society of America. 2003. Treatment of tuberculosis. MMWR Recomm Rep 52(RR-11):1–77. [PubMed] [Google Scholar]
- 10.Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, Msandiwa R, Castel S, Wiesner L, Chaisson RE, McIlleron H, Dooley KE. 2015. Population pharmacokinetics of rifampin in pregnant women with tuberculosis and HIV coinfection in Soweto, South Africa. Antimicrob Agents Chemother 60:1234–1241. doi: 10.1128/AAC.02051-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCormack SA, Best BM. 2014. Obstetric pharmacokinetic dosing studies are urgently needed. Front Pediatr 2:9. doi: 10.3389/fped.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schalkwyk M Van, Bekker A, Decloedt E, Theron GB, Cotton MF, Best B, Capparelli E, Stek A, Wang J, Mirochnick M, Team for the IPP . 2017. Pharmacokinetics of rifampin and isoniazid during pregnancy and postpartum in South African women. Presented at 9th International AIDS Society (IAS), Paris, France, 23 to 26 July 2017. [Google Scholar]
- 13.Gausi K, Weisner L, Norman J, Wallis CL, Onyango-Makumbi C, Chipato T, Theron G, Haas DW, Sterling TR, Browning R, Chakhtoura N, Weinberg A. 2019. Pharmacokinetics of isoniazid preventive therapy among HIV-infected pregnant women in high tuberculosis incidence settings. Presented at the 28th Annual Meeting of the Population Approach Group Europe (PAGE), Stockholm, Sweden, 11 to 14 June 2019. [Google Scholar]
- 14.Daskapan A, Idrus LR, Postma MJ, Wilffert B, Kosterink JGW, Stienstra Y, Touw DJ, Andersen AB, Bekker A, Denti P, Hemanth Kumar AK, Jeremiah K, Kwara A, McIlleron H, Meintjes G, van Oosterhout JJ, Ramachandran G, Rockwood N, Wilkinson RJ, van der Werf TS, Alffenaar J-W. 2019. A systematic review on the effect of HIV infection on the pharmacokinetics of first-line tuberculosis drugs. Clin Pharmacokinet 58:747–766. doi: 10.1007/s40262-018-0716-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chideya S, Winston CA, Peloquin CA, Bradford WZ, Hopewell PC, Wells CD, Reingold AL, Kenyon TA, Moeti TL, Tappero JW. 2009. Isoniazid, rifampin, ethambutol, and pyrazinamide pharmacokinetics and treatment outcomes among a predominantly HIV‐infected cohort of adults with tuberculosis from Botswana. Clin Infect Dis 48:1685–1694. doi: 10.1086/599040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Choudhri SH, Hawken M, Gathua S, Minyiri GO, Watkins W, Sahai J, Sitar DS, Aoki FY, Long R. 1997. Pharmacokinetics of antimycobacterial drugs in patients with tuberculosis, AIDS, and diarrhea. Clin Infect Dis 25:104–111. doi: 10.1086/514513. [DOI] [PubMed] [Google Scholar]
- 17.McIlleron H, Wash P, Burger A, Norman J, Folb PI, Smith P. 2006. Determinants of rifampin, isoniazid, pyrazinamide, and ethambutol pharmacokinetics in a cohort of tuberculosis patients. Antimicrob Agents Chemother 50:1170–1177. doi: 10.1128/AAC.50.4.1170-1177.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.van Oosterhout JJ, Dzinjalamala FK, Dimba A, Waterhouse D, Davies G, Zijlstra EE, Molyneux ME, Molyneux EM, Ward S. 2015. Pharmacokinetics of antituberculosis drugs in HIV-positive and HIV-negative adults in Malawi. Antimicrob Agents Chemother 59:6175–6180. doi: 10.1128/AAC.01193-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rockwood N, Meintjes G, Chirehwa M, Wiesner L, McIlleron H, Wilkinson RJ, Denti P. 2016. HIV-1 coinfection does not reduce exposure to rifampin, isoniazid, and pyrazinamide in South African tuberculosis outpatients. Antimicrob Agents Chemother 60:6050–6059. doi: 10.1128/AAC.00480-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Taylor B, Smith PJ. 1998. Does AIDS impair the absorption of antituberculosis agents? Int J Tuberc Lung Dis 2:670–675. [PubMed] [Google Scholar]
- 21.Sabbagh A, Darlu P, Crouau-Roy B, Poloni ES. 2011. Arylamine N-acetyltransferase 2 (NAT2) genetic diversity and traditional subsistence: a worldwide population survey. PLoS One 6:e18507. doi: 10.1371/journal.pone.0018507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poole NF, Meyer AE. 1960. Stability of isoniazid in aqueous solutions and plasma. Proc Soc Exp Biol Med 104:560–562. doi: 10.3181/00379727-104-25907. [DOI] [PubMed] [Google Scholar]
- 23.Salman S, Davis T. 2017. Regarding “Lactation status and studies of pyrimethamine pharmacokinetics in pregnancy.” CPT Pharmacometrics Syst Pharmacol 6:730. doi: 10.1002/psp4.12257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.de Kock M, Tarning J, Barnes KI, Denti P. 2017. Response to “Lactation status and studies of pyrimethamine pharmacokinetics in pregnancy.” CPT Pharmacometrics Syst Pharmacol 6:731. doi: 10.1002/psp4.12256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Denti P, Jeremiah K, Chigutsa E, Faurholt-Jepsen D, PrayGod G, Range N, Castel S, Wiesner L, Hagen CM, Christiansen M, Changalucha J, McIlleron H, Friis H, Andersen AB. 2015. Pharmacokinetics of isoniazid, pyrazinamide, and ethambutol in newly diagnosed pulmonary TB patients in Tanzania. PLoS One 10:e0141002. doi: 10.1371/journal.pone.0141002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tappero JW, Bradford WZ, Agerton TB, Hopewell P, Reingold AL, Lockman S, Oyewo A, Talbot EA, Kenyon TA, Moeti TL, Moffat HJ, Peloquin CA. 2005. Serum concentrations of antimycobacterial drugs in patients with pulmonary tuberculosis in Botswana. Clin Infect Dis 41:461–469. doi: 10.1086/431984. [DOI] [PubMed] [Google Scholar]
- 27.Tostmann A, Mtabho CM, Semvua HH, van den Boogaard J, Kibiki GS, Boeree MJ, Aarnoutse RE. 2013. Pharmacokinetics of first-line tuberculosis drugs in Tanzanian patients. Antimicrob Agents Chemother 57:3208–3213. doi: 10.1128/AAC.02599-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dooley KE, Denti P, Martinson N, Cohn S, Mashabela F, Hoffmann J, Haas DW, Hull J, Msandiwa R, Castel S, Wiesner L, Chaisson RE, McIlleron H. 2015. Pharmacokinetics of efavirenz and treatment of HIV-1 among pregnant women with and without tuberculosis coinfection. J Infect Dis 211:197–205. doi: 10.1093/infdis/jiu429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Beal SL. 2001. Ways to fit a PK model with some data below the quantification limit. J Pharmacokinet Pharmacodyn 28:481–504. doi: 10.1023/a:1012299115260. [DOI] [PubMed] [Google Scholar]
- 30.Keizer RJ, Karlsson MO, Hooker A. 2013. Modeling and simulation workbench for NONMEM: tutorial on Pirana, PsN, and Xpose. CPT Pharmacometrics Syst Pharmacol 2:e50. doi: 10.1038/psp.2013.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Savic RM, Jonker DM, Kerbusch T, Karlsson MO. 2007. Implementation of a transit compartment model for describing drug absorption in pharmacokinetic studies. J Pharmacokinet Pharmacodyn 34:711–726. doi: 10.1007/s10928-007-9066-0. [DOI] [PubMed] [Google Scholar]
- 32.Anderson BJ, Holford NH. 2008. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol 48:303–332. 2007/10/05. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- 33.Keizer RJ, Zandvliet AS, Beijnen JH, Schellens JHM, Huitema A. 2012. Performance of methods for handling missing categorical covariate data in population pharmacokinetic analyses. AAPS J 14:601–611. doi: 10.1208/s12248-012-9373-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Holfrod N. 2005. The visual predictive check: superiority to standard diagnostic (Rorschach) plot. Presented at the 14th Annual Meeting Population Approach Group Europe (PAGE), Pamplona, Spain, 16 to 17 June 2005. [Google Scholar]
- 35.Gisleskog PO, Karlsson MO, Beal SL. 2002. Use of prior information to stabilize a population data analysis. J Pharmacokinet Pharmacodyn 29:473–505. doi: 10.1023/a:1022972420004. [DOI] [PubMed] [Google Scholar]
- 36.Dosne A-G, Bergstrand M, Karlsson MO. 2017. An automated sampling importance resampling procedure for estimating parameter uncertainty. J Pharmacokinet Pharmacodyn 44:509–520. doi: 10.1007/s10928-017-9542-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lindbom L, Ribbing J, Jonsson EN. 2004. Perl-speaks-NONMEM (PsN)—a Perl module for NONMEM related programming. Comput Methods Programs Biomed 75:85–94. doi: 10.1016/j.cmpb.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 38.Ueckert S, Karlsson MO, Hooker AC. 2016. Accelerating Monte Carlo power studies through parametric power estimation. J Pharmacokinet Pharmacodyn 43:223–234. doi: 10.1007/s10928-016-9468-y. [DOI] [PMC free article] [PubMed] [Google Scholar]