This study aimed to evaluate the in vitro antimicrobial activity, heteroresistance emergence, and resistance mechanism of omadacycline (OMC) in clinical Enterococcus faecalis isolates from China. A total of 276 isolates were collected retrospectively in China from 2011 to 2015. The MICs of OMC, doxycycline (DOX), and minocycline (MIN) against E. faecalis were determined by broth microdilution. Tetracycline (TET)-specific resistance genes and multilocus sequence typing (MLST) of the isolates were investigated using PCR.
KEYWORDS: omadacycline, Enterococcus faecalis, heteroresistance, multilocus sequence typing, tetracycline-specific resistance genes
ABSTRACT
This study aimed to evaluate the in vitro antimicrobial activity, heteroresistance emergence, and resistance mechanism of omadacycline (OMC) in clinical Enterococcus faecalis isolates from China. A total of 276 isolates were collected retrospectively in China from 2011 to 2015. The MICs of OMC, doxycycline (DOX), and minocycline (MIN) against E. faecalis were determined by broth microdilution. Tetracycline (TET)-specific resistance genes and multilocus sequence typing (MLST) of the isolates were investigated using PCR. The detection frequency of OMC heteroresistance in E. faecalis was evaluated with population analysis profiling (PAP). The mechanism of OMC heteroresistance and resistance in E. faecalis was examined by amplifying 30S ribosomal subunit genes, RNA sequencing (RNA-Seq), and in vitro recombination experiments. The OMC MICs of clinical E. faecalis isolates ranged from ≤0.06 to 1.0 mg/liter, and 42% of the E. faecalis isolates with an OMC MIC of 1.0 mg/liter were found to be sequence type 16 (ST16). Six OMC-heteroresistant isolates with MIC values of ≤0.5 mg/liter were detected among 238 E. faecalis isolates. The resistant subpopulations of heteroresistant isolates showed OMC MICs in the range of 2 to 4 mg/liter and were found without 30S ribosomal subunit gene mutations. Moreover, RNA sequencing and in vitro recombination experiments demonstrated that overexpression of a bone morphogenetic protein (BMP) family ATP-binding cassette (ABC) transporter substrate-binding protein, OG1RF_RS00630, facilitated OMC heteroresistance in E. faecalis. In conclusion, OMC exhibited better activity against clinical E. faecalis isolates from China than that of DOX or MIN, and overexpression of OG1RF_RS00630 in E. faecalis facilitated the development of OMC heteroresistance.
INTRODUCTION
Enterococcus is a clinically significant pathogen of opportunistic infections, which include urinary tract infections, cholecystitis, endocarditis, bacteremia, and other infections of surgical sites and wounds. Approximately 80% of enterococcal infections are caused by Enterococcus faecalis, and the number of E. faecalis infections appears to be increasing in recent years (1–3). E. faecalis often exhibits resistance to several common antibiotics, such as cephalosporin, aminoglycosides, and sulfamethoxazole, through natural or acquired resistance mechanisms (4, 5). Recently, several reports have shown that the increased incidence of multidrug-resistant enterococci, including vancomycin (VAN)-resistant and linezolid (LZD)-resistant strains, has limited our treatment choices, and controlling multidrug-resistant E. faecalis infections has become a critical need in clinics (4–6).
Omadacycline (OMC) has been recently developed and is a first-in-class aminomethylcycline antibiotic with broad-spectrum activity against Gram-positive and Gram-negative aerobic, anaerobic, and atypical pathogens, including Staphylococcus, Enterococcus, Legionella, and Chlamydia species (7–10). Oral and intravenous OMC formulas have been evaluated in phase III clinical trials and have shown excellent efficacy in the treatment of acute skin and soft tissue infections and community-acquired pneumonia (10–13). OMC differs from earlier tetracycline (TET) derivatives—including doxycycline (DOX), minocycline (MIN), and expanded-spectrum glycylcycline antibiotics such as tigecycline (TGC)—owing to two major structural modifications. Recent reports have demonstrated the in vitro active antibacterial potency of OMC with MICs of ≤0.25 mg/liter against various Gram-positive microbes, including enterococci, methicillin-resistant Staphylococcus aureus, and multidrug-resistant Streptococcus pneumoniae (12–14). However, the antimicrobial activity of OMC against clinical isolates of E. faecalis from China has not been established.
Both OMC and TGC represent new derivatives of the TET class of antimicrobial drugs. These drugs are regarded as last-resort antimicrobial treatments for infections by difficult-to-treat bacteria, such as multidrug-resistant E. faecalis. TGC resistance has been linked to genetic mutations that affect the 30S ribosomal subunit of TET binding sites—including mutations that affect the genes encoding 16SrRNA (four copies) and ribosomal protein S10—as well as the overexpression of genes that encode efflux pump regulators, such as soxS, marA, ramA, and robA (15–18). Moreover, overexpression of tet(M) and tet(K) has been shown to increase TGC MIC values and favor the evolution of resistance in Enterococcus faecium (19).
Heteroresistance, which refers to the presence of subpopulations of bacterial cells with higher levels of antibiotic resistance than those of the surrounding populations in the same culture, can lead to the evolution of antibiotic resistance and treatment failure (20). Subpopulation analysis of OMC heteroresistance in E. faecalis can provide information about the risk of antibacterial resistance under antibiotic pressure. The incidence of OMC heteroresistance and its mechanisms in E. faecalis remain unclear. The extent to which TET/TGC resistance mechanisms may contribute to OMC heteroresistance and resistance evolution needs to be determined.
In the present study, we examined the in vitro antimicrobial activity of OMC against clinical E. faecalis isolates collected from patients in China. The clonality and OMC susceptibility with respect to the sequence type (ST) were analyzed. Furthermore, population analysis profiling (PAP), molecular sequencing techniques, and in vitro functional tests were performed to explore the incidence and underlying mechanism of OMC heteroresistance in clinical E. faecalis isolates. Particular attention was given to mutations that affect the 30S ribosome unit in E. faecalis strains with OMC-induced resistance.
RESULTS
In vitro antimicrobial activity of OMC against clinical E. faecalis isolates.
The clinical E. faecalis strains were isolated from various infective sample sources, including urine (48.6%), wound secretions (17.0%), blood (11.2%), and bile (7.3%), among others (Fig. S1). Moreover, the OMC MIC data of those isolates were obtained and are summarized in Table 1. Briefly, the data indicated that OMC had robust in vitro antimicrobial activity against E. faecalis, and the clinical E. faecalis isolates exhibited a high frequency of resistance to TET, gentamicin (GEN), and erythromycin (ERY). The range of OMC MIC values against E. faecalis was 0.06 to 1.0 mg/liter. We obtained lower MIC values (MIC50/90 = 0.5/1.0 mg/liter) for OMC than for DOX and MIN (MIC50/90 of 16/32 mg/liter). Notably, 64 LZD-nonsusceptible E. faecalis isolates, including 51 LZD-intermediate and 13 LZD-resistant isolates, and two VAN-intermediate strains were identified. In addition, OMC exhibited excellent antimicrobial activity (MIC ≤ 1 mg/liter) against all LZD- or VAN-nonsusceptible strains (Table 1).
TABLE 1.
Comparison of in vitro antimicrobial activity of OMC and various common antibiotics against E. faecalis
| Antibiotic | Resistance rate (%) | MIC breakpoint (μg/ml) | No. of isolates | MIC distribution and statistics for: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OMC |
DOX |
MIN |
||||||||||||||||
| No. of isolates with MIC (mg/liter) of: |
Range (mg/liter) | MIC50/90 (mg/liter) | No. of isolates with MIC (mg/liter) of: |
Range (mg/liter) | MIC50/90 (mg/liter) | No. of isolates with MIC (mg/liter) of: |
Range (mg/liter) | MIC50/90 (mg/liter) | ||||||||||
| ≤0.25 | 0.5 | ≥1 | ≤4 | 8 | ≥16 | ≤4 | 8 | ≥16 | ||||||||||
| Total (276) | 72 | 166 | 38 | ≤0.125–1 | 0.5/1 | 39 | 18 | 219 | 0.125–32 | 16/32 | 42 | 30 | 204 | 0.125–32 | 16/32 | |||
| Tetracycline | 83.7 | ≤4 | 39 | 17 | 21 | 1 | 0.125–1 | 0.5/0.5 | 35 | 4 | 0 | 0.125–8 | 0.25/4 | 37 | 1 | 1 | 0.125–16 | 0.125/4 |
| 8 | 6 | 2 | 4 | 0 | 0.125–0.5 | 0.5/0.5 | 2 | 0 | 4 | 4–16 | 4/16 | 2 | 1 | 3 | 4–16 | 8/16 | ||
| ≥16 | 231 | 53 | 141 | 37 | ≤0.125–1 | 0.5/1 | 2 | 14 | 215 | 4–32 | 16/32 | 3 | 28 | 200 | 0.25–32 | 16/32 | ||
| Linezolid | 4.7 | ≤2 | 212 | 59 | 124 | 29 | ≤0.125–1 | 0.5/1 | 33 | 16 | 163 | 0.125–32 | 16/32 | 36 | 25 | 151 | 0.125–32 | 16/32 |
| 4 | 51 | 10 | 32 | 9 | ≤0.125–1 | 0.5/1 | 5 | 1 | 45 | 0.125–32 | 16/32 | 5 | 2 | 44 | 0.125–32 | 16/32 | ||
| ≥8 | 13 | 3 | 10 | 0 | 0.125–0.5 | 0.5/0.5 | 1 | 1 | 11 | 0.5–32 | 16/32 | 1 | 3 | 9 | 0.125–32 | 16/16 | ||
| Vancomycin | 0 | ≤4 | 274 | 71 | 165 | 38 | ≤0.125–1 | 0.5/1 | 38 | 18 | 218 | 0.125–32 | 16/32 | 41 | 30 | 203 | 0.125–32 | 16/32 |
| 8–16 | 2 | 1 | 1 | 0 | 0.125–0.5 | 1 | 0 | 1 | 0.25–16 | 1 | 0 | 1 | 0.5–16 | |||||
| Ampicillin | 0.4 | ≤8 | 275 | 71 | 166 | 38 | ≤0.125–1 | 0.5/1 | 38 | 18 | 219 | 0.125–32 | 16/32 | 41 | 30 | 204 | 0.125–32 | 16/32 |
| ≥16 | 1 | 1 | 0 | 0 | 0.125 | 1 | 0 | 0 | 0.25 | 1 | 0 | 0 | 0.5 | |||||
| Gentamycin | 68.5 | ≤4 | 40 | 19 | 18 | 3 | ≤0.125–1 | 0.5/0.5 | 13 | 3 | 24 | 0.125–32 | 16/32 | 12 | 5 | 23 | 0.125–32 | 16/16 |
| 8 | 47 | 14 | 27 | 6 | ≤0.125–1 | 0.5/1 | 10 | 4 | 33 | 0.125–32 | 16/32 | 12 | 4 | 31 | 0.125–32 | 16/32 | ||
| ≥16 | 189 | 39 | 121 | 29 | ≤0.125–1 | 0.5/1 | 16 | 11 | 162 | 0.125–32 | 16/32 | 18 | 21 | 150 | 0.125–32 | 16/32 | ||
| Erythromycin | 76.8 | ≤0.5 | 8 | 1 | 6 | 1 | 0.25–1 | 0.5/1 | 5 | 1 | 2 | 0.125–16 | 0.25/16 | 5 | 1 | 2 | 0.125–16 | 0.125/16 |
| 1–4 | 56 | 21 | 29 | 6 | 0.25–1 | 0.5/1 | 18 | 2 | 36 | 0.125–32 | 16/32 | 20 | 4 | 32 | 0.125–32 | 16/32 | ||
| ≥8 | 212 | 50 | 131 | 31 | ≤0.125–1 | 0.5/1 | 16 | 15 | 181 | 0.125–32 | 16/32 | 17 | 25 | 170 | 0.125–32 | 16/32 | ||
| Ciprofloxacin | 26.1 | ≤1 | 168 | 48 | 98 | 22 | ≤0.125–1 | 0.5/1 | 22 | 14 | 132 | 0.125–32 | 16/32 | 23 | 17 | 128 | 0.125–32 | 16/32 |
| 2 | 36 | 7 | 23 | 6 | 0.125–1 | 0.5/1 | 10 | 1 | 25 | 0.125–32 | 16/32 | 10 | 1 | 25 | 0.125–32 | 16/32 | ||
| ≥4 | 72 | 17 | 45 | 10 | ≤0.125–1 | 0.5/1 | 7 | 3 | 62 | 0.125–32 | 16/32 | 9 | 12 | 51 | 0.125–32 | 16/32 | ||
| Nitrofurantoin | 0.7 | ≤32 | 270 | 71 | 162 | 37 | ≤0.125–1 | 0.5/1 | 38 | 17 | 215 | 0.125–32 | 16/32 | 41 | 29 | 200 | 0.125–32 | 16/32 |
| 64 | 4 | 1 | 2 | 1 | 0.25–1 | 0.5/1 | 0 | 0 | 4 | 16–32 | 16/32 | 0 | 0 | 4 | 16–32 | 16/32 | ||
| ≥128 | 2 | 0 | 2 | 0 | 0.5 | 1 | 1 | 0 | 4–8 | 1 | 1 | 0 | 4–8 | |||||
| Trimethoprim-sulfamethoxazole | 14.1 | ≤2/38 | 237 | 66 | 140 | 31 | ≤0.125–1 | 0.5/1 | 35 | 16 | 186 | 0.125–32 | 16/32 | 39 | 22 | 176 | 0.125–32 | 16/32 |
| ≥4/76 | 39 | 6 | 26 | 7 | 0.25–1 | 0.5/1 | 4 | 2 | 33 | 0.25–32 | 16/32 | 3 | 8 | 28 | 0.125–32 | 16/32 | ||
The distribution of TET-specific resistance genes in clinical E. faecalis isolates is shown in Table 2. The OMC MIC50/90 values of 0.5/1 mg/liter were obtained in E. faecalis isolates with at least one of the TET-specific resistance genes, which included tet(M), tet(K), and tet(L), suggesting that the presence of these genes did not affect OMC sensitivity in E. faecalis. In contrast, DOX and MIN MICs in E. faecalis isolates harboring tet(M), tet(K), or tet(L) genes were 16- to 32-fold higher than those of strains without these genes. Four TET-specific resistance genes, namely tet(O), tet(S), tet(W), and tet(U), were not found in any E. faecalis isolates (Table 2).
TABLE 2.
In vitro antimicrobial activity of OMC against E. faecalis with TET-specific resistance genes
| TET resistance gene(s) | Total no. of isolates | MIC distribution and statistics for: |
||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OMC |
DOX |
MIN |
||||||||||||||
| No. of isolates with MIC (mg/liter) of: |
MIC range (mg/liter) | MIC50/90 (mg/liter) | No. of isolates with MIC (mg/liter) of: |
MIC range (mg/liter) | MIC50/90 (mg/liter) | No. of isolates with MIC (mg/liter) of: |
MIC range (mg/liter) | MIC50/90 (mg/liter) | ||||||||
| ≤0.25 | 0.5 | ≥1 | ≤4 | 8 | ≥16 | ≤4 | 8 | ≥16 | ||||||||
| tet(M) | 162 | 40 | 104 | 18 | ≤0.125–1 | 0.5/1 | 5 | 14 | 143 | 0.25–32 | 16/32 | 5 | 23 | 134 | 0.5–32 | 16/32 |
| tet(L) | 5 | 2 | 1 | 2 | 0.125–1 | 0.5/1 | 2 | 0 | 3 | 0.125–32 | 16/32 | 2 | 0 | 3 | 0.125–32 | 16/32 |
| tet(M), tet(L) | 60 | 16 | 31 | 13 | ≤0.125–1 | 0.5/1 | 2 | 1 | 57 | 4–32 | 32/32 | 4 | 3 | 53 | 0.25–32 | 16/32 |
| tet(M), tet(K) | 4 | 0 | 2 | 2 | 0.5–1 | 0.5/1 | 0 | 0 | 4 | 16 | 16/16 | 0 | 2 | 2 | 8–16 | 8/16 |
| tet(M), tet(L), tet(K) | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 32 | 0 | 0 | 1 | 16 | |||
| None detected | 44 | 14 | 28 | 2 | 0.125–1 | 0.5/0.5 | 30 | 3 | 11 | 0.125–32 | 0.5/16 | 31 | 3 | 10 | 0.125–32 | 0.25/16 |
Clonality of OMC MIC distribution.
A total of 41 STs were identified among the E. faecalis isolates in this study. The predominant STs were ST16 (79/276; 28.6%) and ST179 (71/276; 25.7%) (Table 3 and Fig. S2). With respect to the relationship between ST and the OMC MIC, 20.2% of ST16 strains and only 8.4% of ST179 strains had an OMC MIC level of 1 mg/liter. Conversely, a large proportion (16/38; 42.1%) of the isolates with an OMC MIC level of 1 mg/liter were ST16 strains, demonstrating clonal clustering toward the ST16 genotype (Table 3).
TABLE 3.
The relationship among OMC, DOX, and MIN MIC distributions with ST16 and ST179 genotypes in E. faecalis
| Genotype | No. (%) of isolates | No. (%) of isolates with MIC (mg/liter) for: |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OMC |
DOX |
MIN |
||||||||
| ≤0.25 | 0.5 | ≥1 | ≤4 | 8 | ≥16 | ≤4 | 8 | ≥16 | ||
| ST16 | 79 (28.6) | 10 (12.7) | 53 (67.1) | 16 (20.2) | 0 | 4 (5.1) | 75 (94.9) | 1 (1.3) | 9 (11.4) | 69 (87.3) |
| ST179 | 71 (25.7) | 26 (36.7) | 39 (54.9) | 6 (8.4) | 1 (1.4) | 7 (9.9) | 63 (88.7) | 1 (1.4) | 8 (11.3) | 62 (87.3) |
OMC heteroresistance.
PAP was performed as a standard method to determine OMC heteroresistance. Overall, six OMC-heteroresistant isolates (EF16C361, EF16C291, EF16C3, EF16C28, EF16C350, and EF16C185) were detected among 238 clinical E. faecalis isolates with MIC values of ≤0.5 mg/liter. Their resistant subpopulations (EF16C361-RS, EF16C291-RS, EF16C3-RS, EF16C28-RS, EF16C350-RS, and EF16C185-RS) were selected from the 1 mg/liter OMC concentrations of the PAP tests. The OMC and TGC MICs of resistant subpopulations were 4- to 8-fold higher than those of the parental isolates, indicating similar elevations of MIC values across both antibiotics (Table 4). The OMC MICs of resistant subpopulations were decreased by 4- to 8-fold after 10 passages on antibiotic-free medium, which suggested that these subpopulations lacked stable mutations conferring OMC resistance and could be reversed to the susceptible phenotype after the removal of antibiotic pressure (Table S1). Moreover, the OMC MICs for these resistant subpopulations could be significantly reduced to ≤0.03 mg/liter and 0.5 mg/liter with the addition of the efflux pump inhibitors (EPIs) carbonyl cyanide m-chlorophenylhydrazone (CCCP) and Phe-Arg-β-naphthylamide (PaβN), respectively. This indicates that EPIs could potentiate OMC activity in E. faecalis (Table 4).
TABLE 4.
Antibiotic susceptibility and resistance mechanisms of the heteroresistant parental isolates, their resistant subpopulations, and strains with OMC-induced resistance
| Strain | ST | MIC (mg/liter) for:c |
Mutation(s)d |
|||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OMC plus CCCPa | OMC plus PAβN | OMC | TGC | RR1 | RR2 | RR3 | RR4 | S10 | ||
| EF16C361 | 179 | – | – | 0.5 | 0.25 | W | W | W | W | W |
| EF16C361-RSb | ≤0.03 | 0.5 | 4 | 2 | W | W | W | W | W | |
| EF16C361-IR | – | – | 8 | 8 | A1024C | T1088G/A1461G | A89G/C1265T/C1191T | W | W | |
| EF16C291 | 16 | – | – | 0.5 | 0.5 | W | W | W | W | W |
| EF16C291-RS | ≤0.03 | 0.5 | 4 | 2 | W | W | W | W | W | |
| EF16C291-IR1 | – | – | 8 | 4 | C1261T | G272A/A1461G | C1265T/C1191T | W | W | |
| EF16C291-IR2 | – | – | 16 | 4 | C988A/C1261T | G272A/G809A | C1265T/C1191T | W | W | |
| EF16C3 | 16 | – | – | 0.5 | 0.5 | W | W | W | W | W |
| EF16C3-RS | ≤0.03 | 0.5 | 2 | 2 | W | W | W | W | W | |
| EF16C3-IR1 | – | – | 16 | 16 | W | W/A1461G | C1265T/C1191T | W | W | |
| EF16C3-IR2 | – | – | 16 | 16 | W | W/A1461G | C1265T/C1191T | W | W | |
| EF16C28 | 179 | – | – | 0.5 | 0.25 | W | W | W | W | W |
| EF16C28-RS | ≤0.03 | 0.5 | 4 | 2 | W | W | W | W | W | |
| EF16C28-IR | – | – | 32 | 16 | W | T1041A/A1461G | C1265T/C1191T | W | W | |
| EF16C350 | 480 | – | – | 0.5 | 0.5 | W | W | W | W | W |
| EF16C350-RS | ≤0.03 | 0.5 | 4 | 2 | W | W | W | W | W | |
| EF16C350-IR | – | – | 16 | 4 | W | G878A | C1265T/C1191T | G811A/A1148G | W | |
| EF16C185 (EF16) | 16 | – | – | 0.5 | 0.5 | W | W | W | W | W |
| EF16C185-RS (EF16-O2) | ≤0.03 | 0.5 | 4 | 2 | W | W | W | W | W | |
| EF16C185-IR | – | – | 8 | 4 | C1261T/C743T | W | C860T/G1191A/G1265A/C1191T | W | W | |
| OG1RF | – | – | 0.125 | 0.125 | W | W | W | W | W | |
| OG1RF-IR1 | – | – | 32 | 32 | W | A1461G | C1265T/C1191T | C1416T | LYS57ARG | |
| OG1RF-IR2 | – | – | 32 | 32 | W | C914G/ATGA921-924TGAC/A1461 | C1265T/C1191T | W | HIS56TYR, LYS57ARG | |
CCCP, carbonyl cyanide m-chlorophenylhydrazone; PaβN, Phe-Arg-β-naphthylamide.
RS, OMC-resistant subpopulations; IR, isolates with OMC-induced resistance.
–, not determined.
In individual copies of 16S rRNA (RR1 to RR4) and the 30S ribosomal protein S10. W, wild-type.
Detection of 30S ribosomal unit mutations in OMC heteroresistant and resistant E. faecalis.
Ten strains with OMC-induced resistance (EF16C361-IR, EF16C291-IR1, EF16C291-IR2, EF16C3-IR1, EF16C3-IR2, EF16C28-IR, EF16C350-IR, EF16C185-IR, OG1RF-IR1, and OG1RF-IR2) were derived from the in vitro OMC induction of six heteroresistant parental isolates and the OG1RF strain. The genetic mutations of 30S ribosomal units were then determined in OMC-heteroresistant parental isolates, their resistant subpopulations, and strains with OMC-induced resistance. No genetic mutations of the 30S ribosomal subunits (four 16S rRNA copies and 30S ribosomal protein S10) were found in any of the OMC-heteroresistant parental isolates and their resistant subpopulations. In contrast, mutations in the 16S rRNA genes (C1261T polymorphism in RR1; A1461G polymorphism in RR2; C1265T and C1191T polymorphisms in RR3) were detected in strains with OMC-induced resistance. Furthermore, 30S ribosomal protein S10 mutations were found in only two strains with OMC-induced resistance (OG1RF-IR1 and OG1RF-IR2) (Table 4).
Differentially expressed genes between the heteroresistant parental isolate and its resistant subpopulation.
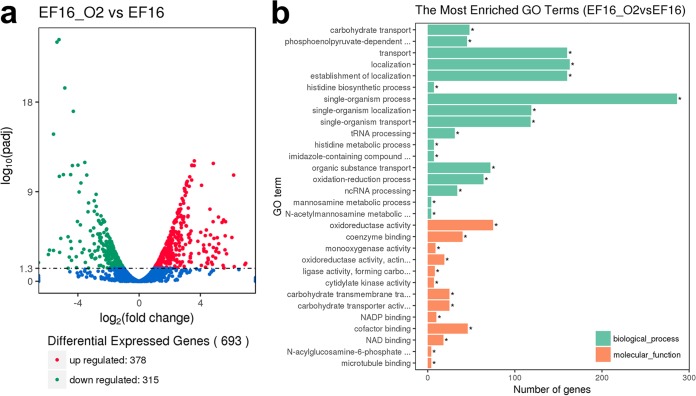
Because the heteroresistance mechanism in this study was elusive, we performed RNA sequencing (RNA-Seq) to compare the transcription of unique genes between the heteroresistant parental strain EF16C185 (EF16) and its resistant subpopulation EF16C185-RS (EF16-O2). Before RNA sequencing, the growth curves of EF16 and EF16-O2 were determined, and the growth rates of these two strains showed no significant difference (Fig. S3). A total of 693 differentially expressed genes (DEGs), including 378 upregulated and 315 downregulated genes, were identified in EF16-O2 compared to EF16 in RNA sequencing analysis (Fig. 1a and Tables S2 and S3). Mapping of these DEGs using gene ontology (GO) analysis showed that the most frequent pathways were related to carbohydrate metabolism (31 genes), cationic antimicrobial peptide resistance (21 genes), fructose/mannose metabolism (31 genes), the phosphotransferase system (38 genes), and terpenoid backbone biosynthesis (9 genes) (Fig. 1b).
FIG 1.
Differentially expressed genes (DEGs) between heteroresistant parental strain EF16 and its resistant subpopulation EF16-O2. (a) Distribution of DEGs. Red, green, and blue colors represent upregulated and downregulated genes and those genes not differentially expressed, respectively. The x axis represents fold change and the y axis represents the P value of DEGs. (b) Functional analysis of DEGs between the EF16-O2 and EF16 strains.
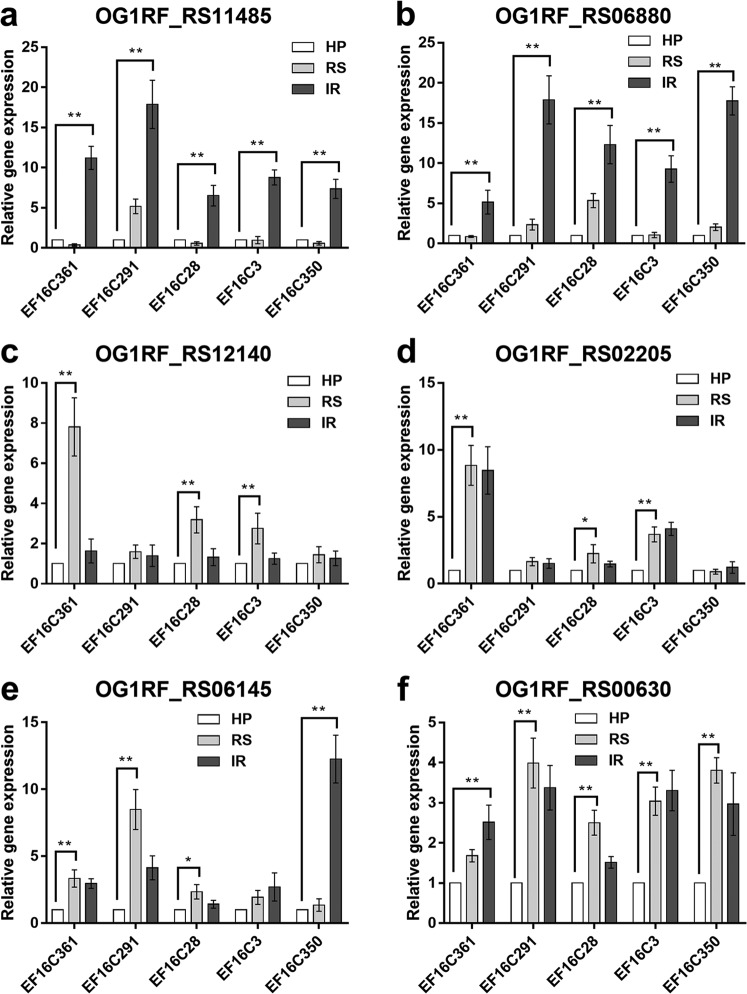
The OMC MICs in resistant subpopulations of heteroresistant isolates could be reversed to sensitive levels by adding an EPI, supporting the notion that membrane proteins or efflux pumps may participate in the initial upregulation of OMC MIC values during the progression of heteroresistance. Twelve upregulated DEGs, which showed potential associations with heteroresistance, were validated by quantitative real-time (qRT-PCR) in EF16 and EF16-O2 (Table 5). To further validate their impact on OMC susceptibility, the expression levels of those 12 DEGs were investigated in the remaining five heteroresistant parental isolates, their resistant subpopulations, and strains with OMC-induced resistance (Fig. 2 and Fig. S4). Overexpression of six candidate genes, namely, OG1RF_RS00630, OG1RF_RS12140, OG1RF_RS02205, OG1RF_RS06145, OG1RF_RS06880, and OG1RF_RS11485, was correlated with the occurrence of OMC heteroresistance or resistance (Fig. 2).
TABLE 5.
Transcriptional expression levels of 12 candidate differentially expressed genes between EF16-O2 and EF16b
| Gene identifier | Description or predicted function | Expression ratio (EF16-O2/ EF16) according to: |
|
|---|---|---|---|
| RNA-Seq (P value)a | qRT-PCRc | ||
| OG1RF_RS11300 | ABC transporter substrate-binding protein | 2.48 (0.0013) | 1.28 ± 0.17 |
| OG1RF_RS11485 | Ribose transporter RbsU | 2.62 (0.0006) | 1.40 ± 0.11 |
| OG1RF_RS10660 | ABC-F type ribosomal protection protein Lsa(A) | 2.77 (0.011) | 2.16 ± 0.14 |
| OG1RF_RS06880 | CoA-binding protein | 3.37 (0.0003) | 2.67 ± 0.35 |
| OG1RF_RS12140 | ABC transporter ATP-binding protein | 3.75 (<0.0001) | 15.70 ± 2.48 |
| OG1RF_RS02205 | ABC transporter ATP-binding protein | 3.84 (<0.0001) | 3.22 ± 0.49 |
| OG1RF_RS05865 | Sulfate ABC transporter ATP-binding protein | 4.21 (<0.0001) | 8.54 ± 1.24 |
| OG1RF_RS06145 | Molybdate ABC transporter substrate-binding protein | 5.25 (<0.0001) | 21.47 ± 4.53 |
| OG1RF_RS09080 | Sugar ABC transporter substrate-binding protein | 5.40 (<0.0001) | 39.61 ± 5.46 |
| OG1RF_RS12590 | ABC transporter permease | 6.60 (<0.0001) | 6.41 ± 0.79 |
| OG1RF_RS00350 | Alkaline shock response membrane anchor protein AmaP | 6.72 (<0.0001) | 2.23 ± 0.21 |
| OG1RF_RS00630 | BMP family ABC transporter substrate-binding protein | 7.73 (<0.0001) | 4.49 ± 0.64 |
The DEGs of RNA sequencing were defined by a change ratio of ≥2 and a P value of <0.05.
According to RNA sequencing and qRT-PCR.
qRT-PCR data are given as means ± standard deviations of results from three independent experiments.
FIG 2.
Relative transcriptional analysis of differentially expressed genes (DEGs) used in overexpression experiments. Relative expressions of OG1RF_RS11485 (a), OG1RF_RS06880 (b), OG1RF_RS12140 (c), OG1RF_RS02205 (d), OG1RF_RS06145 (e), and OG1RF_RS00630 (f) were determined by qRT-PCR analysis. The housekeeping gene recA was used as the endogenous reference gene. The heteroresistant parental strain was used as the reference strain (expression = 1.0). All qRT-PCRs were carried out in triplicate with three independent RNA samples. HP, OMC-heteroresistant parental strains; RS, OMC-resistant subpopulations; IR, strains with OMC-induced resistance. **, P < 0.01; *, P < 0.05.
Overexpression of OG1RF_RS00630 in E. faecalis contributes to the occurrence of OMC heteroresistance.
To confirm the roles of the candidate genes (OG1RF_RS12140, OG1RF_RS02205, OG1RF_RS06145, OG1RF_RS11485, OG1RF_RS06880, and OG1RF_RS00630) in OMC heteroresistance and resistance in E. faecalis, the overexpression of these genes in OMC-sensitive isolates was conducted. The overexpression plasmids pRS12140, pRS02205, pRS06145, pRS11485, pRS06880, and pRS00630 were constructed and transformed into OMC-sensitive isolates (three per gene), showing low expression level of the target gene (Table S4 and Table S5). Stable overexpression of the candidate genes in the transformed strains was confirmed by qRT-PCR (Fig. S5). Overexpression of the six candidate genes did not induce OMC MIC elevation in the absence of antibiotic pressure. However, PAP showed that overexpression of OG1RF_RS00630 led to the occurrence of the OMC-heteroresistant phenotype in all three OMC-sensitive E. faecalis isolates, compared with negative findings in the parental isolate controls (Table 6). Phylogenetic and homology analyses showed that E. faecalis OG1RF_RS00630 encodes a bone morphogenetic protein (BMP) family ATP-binding cassette (ABC) transporter substrate-binding protein that is ubiquitous in a variety of bacterial species and shares high identity (over 60%) with those of other Gram-positive bacteria (Fig. S6 and Table S6). Our data indicate that the product of this gene may contribute to OMC heteroresistance in E. faecalis.
TABLE 6.
OMC MICs in E. faecalis derivatives with overexpressing candidate genes and their influence on population analysis profiling
| Transformed plasmid | Isolate | OMC MIC (mg/liter) for: |
PAP test results for: |
||||
|---|---|---|---|---|---|---|---|
| Parental straina | Vector control strainb | Derivative strainc | Parental strain | Vector control strain | Derivative strain | ||
| pRS00630 | EF16C2 | 0.5 | 0.5 | 0.5 | Negative | Negative | Positive |
| EF16C105 | 0.5 | 0.5 | 0.5 | Negative | Negative | Positive | |
| EF16C284 | 0.25 | 0.25 | 0.125 | Negative | Negative | Positive | |
| pRS12140 | EF16C105 | 0.5 | 0.5 | 0.5 | Negative | Negative | Positive |
| EF16C39 | 0.5 | 0.25 | 0.25 | Negative | Negative | Negative | |
| EF16C40 | 0.25 | 0.25 | 0.25 | Negative | Negative | Negative | |
| pRS02205 | EF16C2 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative |
| EF16C105 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative | |
| EF16C39 | 0.5 | 0.25 | 0.5 | Negative | Negative | Negative | |
| pRS06145 | EF16C2 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative |
| EF16C105 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative | |
| EF16C283 | 0.5 | 0.5 | 0.25 | Negative | Negative | Positive | |
| pRS06880 | EF16C2 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative |
| EF16C105 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative | |
| EF16C284 | 0.25 | 0.25 | 0.25 | Negative | Negative | Negative | |
| pRS11485 | EF16C2 | 0.5 | 0.25 | 0.25 | Negative | Negative | Negative |
| EF16C105 | 0.5 | 0.5 | 0.5 | Negative | Negative | Negative | |
| EF16C39 | 0.5 | 0.5 | 0.25 | Negative | Negative | Negative | |
The parental strain was an OMC-sensitive E. faecalis isolate without an OMC-heteroresistant phenotype according to the PAP test.
The vector control strain was the parental strain transformed with the pIB166 vector.
The derivative strain was the parental strain transformed with each overexpression plasmid.
DISCUSSION
In the present study, OMC exhibited excellent in vitro antimicrobial activity against LZD-nonsusceptible E. faecalis, indicating potential application of OMC in the treatment of multidrug-resistant E. faecalis infections. E. faecalis maintained low OMC MICs in TET-resistant strains even as DOX and MIN MICs increased. The present findings support the efficacy of OMC against clinically infective E. faecalis in China (17, 18). The MIC50/90 of OMC in 276 E. faecalis isolates was 0.5/1.0 mg/liter in the present study, which was higher than that reported in previous studies (≤0.125/0.125 mg/liter) (7, 9, 13). This difference suggests that susceptibility of E. faecalis to OMC varies across geographical regions, which may lead to regional differences in clinical perspectives. The main STs observed in our sample were ST16 and ST179, which is consistent with prior reports (21, 22). Our data indicate the clonality of E. faecalis, with clustering of the 1 mg/liter OMC MIC level in the ST16 genotype. This underscores the risk of nosocomial transmission of E. faecalis strains with high OMC MICs.
The most common TET resistance mechanisms in Gram-positive and Gram-negative pathogens include ribosomal protection and efflux pump proteins. Notably, the dissemination of these TET-specific resistance factors has limited the clinical utility of TET and its derivatives, including DOX and MIN (17, 18). Antimicrobial susceptibility of new TET drugs, such as TGC and eravacycline, might be slightly influenced by these two mechanisms. The overexpression of some TET-specific resistance genes, including the genes that encode ribosomal protection and efflux pump proteins, have been reported to reduce TGC susceptibility (19). Our data showed that the ribosomal protection protein gene, tet(M), and the efflux pump genes, tet(K) and tet(L), did not affect OMC susceptibility. This finding indicates that OMC might have overcome these two common resistance mechanisms.
Although OMC retained low MIC values against clinical E. faecalis isolates in this study, monitoring the incidence of heteroresistance can provide information about the potential risk of OMC resistance in the future (23, 24). Six OMC-heteroresistant isolates were detected among 238 clinical E. faecalis isolates with MIC values of ≤0.5 mg/liter according to PAPs. The OMC and TGC MIC values were elevated synchronously in OMC-resistant subpopulations, pointing to a cross-resistance risk for these two antibiotics. Therefore, recognition of the development of OMC resistance in E. faecalis may be facilitated by monitoring OMC heteroresistance in clinical isolates. Furthermore, the OMC MICs of resistant subpopulations could be reversed after 10 passages on antibiotic-free medium, while those of isolates with OMC-induced resistance remained stable. This indicates that the resistant subpopulations were reversible and not the result of a stable mutation, which is consistent with the findings of previous research (25).
X-ray crystallography of TET in complex with the 30S ribosomal subunit of Thermus thermophilus revealed two TET binding sites on the 30S subunit (26, 27). As TGC and OMC are new TET derivatives, they are thought to target similar binding sites as TET. However, competition studies with radiolabeled TET indicate that OMC can inhibit in vitro translation at half the concentration required for TET (17), suggesting that OMC may have a 2-fold stronger affinity than TET for the ribosome. Mutations affecting TET binding sites of the 30S ribosomal subunit have been shown to confer TET and TGC resistance in several bacterial species (15–17). The mutational characteristics of OMC-induced resistance in E. faecalis in this study were consistent with the prior identification of various target-interaction related mutations in individual copies of 16S rRNA in bacteria with TGC-induced resistance (16). We detected mutations in all four 16S rRNA gene copies in E. faecalis under OMC pressure. Furthermore, strains having a greater number of genetic mutations in 16S rRNA copies tended to have greater OMC and TGC resistance. Mutations in the gene encoding the 30S ribosomal protein S10 occurred at a relatively low frequency (2/10; 20%) in our strains with OMC-induced resistance, compared to prior findings in multiple bacterial species under TGC pressure (15), indicating that the role of S10 in the evolution of OMC resistance needs to be further studied.
The lack of genetic mutations in 30S ribosomal subunits of OMC-heteroresistant parental isolates and their resistant subpopulations indicates that the MIC elevation observed with the emergence of OMC heteroresistance cannot be explained by such mutations. The TGC resistance and heteroresistance in several Gram-negative bacterial species have been linked to the overexpression of several efflux pump proteins (e.g., SoxS, MarA, RamA, and RobA) (28, 29). Our data showed that the addition of an EPI could restore sensitivity to OMC in resistant subpopulations of OMC-heteroresistant isolates, indicating that efflux pumps may be involved in OMC heteroresistance under antibiotic pressure. Following both RNA sequencing and qRT-PCR analyses, 12 upregulated DEGs encoding efflux pump proteins or membrane proteins were observed in the resistant subpopulations compared with the parental strains. Furthermore, in vitro recombination experiments indicated that overexpression of the candidate gene OG1RF_RS00630 favored OMC heteroresistance in E. faecalis but did not affect OMC and TGC MICs in the absence of antibiotic pressure. Phylogenetic and homology analyses indicated that the efflux protein encoded by OG1RF_RS00630 is an ATP-binding cassette family protein expressed mainly in the cellular membrane. A previous study showed that the ortholog of OG1RF_RS00630 (EF0177) from E. faecalis V583 is involved in ABC transporter-mediated ribonucleoside uptake (30). Our data demonstrate for the first time that OG1RF_RS00630 participates in the development of OMC heteroresistance in E. faecalis.
In conclusion, OMC exhibited robust antimicrobial effects against clinical E. faecalis isolates from China, with lower MIC50/90 values than those of DOX or MIN. Furthermore, OMC showed excellent in vitro antimicrobial activity to LZD-nonsusceptible clinical E. faecalis isolates. Isolates with OMC MICs of 1 mg/liter showed ST16 clonality. Mutations in 30S ribosome units were associated with OMC resistance, and overexpression of a BMP family ABC transporter substrate-binding protein (OG1RF_RS00630) in E. faecalis appeared to favor the occurrence of OMC heteroresistance. Moreover, the emergence of heteroresistance among clinical E. faecalis isolates in China, especially those with high OMC MICs, is noteworthy as a possible harbinger of future resistance development.
MATERIALS AND METHODS
Bacterial isolates, growth conditions, and chemicals.
A total of 276 nonduplicate clinical E. faecalis strains were collected retrospectively from inpatients at Shenzhen Nanshan People’s Hospital (a tertiary-care teaching center hospital in China with 1,200 beds) from 1 January 2011 to 31 December 2015. Bacterial species were identified by standard methods using a Vitek 2 compact system (bioMérieux, Marcy l’Etoile, France). E. faecalis ATCC 29212 was used as the quality control strain. All procedures performed were approved by the ethical standards of Shenzhen Nanshan People’s Hospital. The E. faecalis strains were cultured in tryptic soy broth (TSB; Oxoid, Basingstoke, UK) at 37°C with shaking at 220 rpm. The aminomethylcycline antibiotic OMC was purchased from MedChem Express (Princeton, NJ). DOX, MIN, and TGC were purchased from Aladdin (Shanghai, China).
Antibiotic susceptibility testing.
Antimicrobial susceptibility of several common antibiotics, including ciprofloxacin (CIP), ampicillin (AMP), nitrofurantoin (NIT), trimethoprim-sulfamethoxazole (SXT), GEN, ERY, LZD, VAN, and TET, were determined by broth microdilution using the Vitek 2 compact system (bioMérieux, Marcy l’Etoile, France). The MICs of OMC, DOX, MIN, and TGC were determined by the broth microdilution method according to Clinical and Laboratory Standards Institute (CLSI) guidelines (CLSI-M100-S26). As CLSI E. faecalis MIC breakpoints for OMC have not yet been established, we employed three MIC levels for OMC, ≤0.25 mg/liter (susceptible), 0.5 mg/liter (intermediate), and ≥1 mg/liter (resistant), based on the FDA recommendations (https://www.fda.gov/drugs/development-resources/omadacycline-injection-and-oral-products).
Population analysis profiles.
Population analysis profiling (PAP) was used as a reference method to investigate OMC heteroresistance among 238 clinical E. faecalis isolates with OMC MIC values of ≤0.5 mg/liter as described previously (23). Briefly, 50-μl aliquots of cell suspension (corresponding to a 0.5 McFarland standard for Enterococcus cultures grown on blood agar plates for 24 h at 37°C; approximately 1 × 108 CFU/ml) were spread onto Mueller-Hinton agar plates, with or without various concentrations of OMC (0.5, 1, 2, 3, 4, and 5 mg/liter). Plates were then incubated at 37°C and colonies were counted after 24 h. According to the FDA breakpoint of OMC MIC values for E. faecalis, OMC heteroresistance was defined as an OMC-susceptible isolate (MIC ≤0.5 mg/liter) with subpopulations growing in the presence of ≥1 mg/liter OMC, with a detection limit of 20 CFU/ml. Three colonies, categorized as the resistant subpopulations of each OMC-heteroresistant isolate, were selected from the 1 mg/liter OMC concentration of the PAP test, and the OMC MICs were reassessed after serial passaging on antibiotic-free medium to evaluate the stability of the heteroresistant phenotype. The OMC-resistant subpopulations or their parental strains were cultured in Mueller-Hinton broth (MHB) either supplemented with 1 mg/liter OMC or without OMC, respectively, for the subsequent experiments.
In vitro induction of OMC-resistance in E. faecalis under OMC pressure.
Six OMC-heteroresistant parental isolates and the OG1RF strain were used to induce OMC-resistant isolates. These isolates were subcultured serially in MHB containing gradually increasing concentrations of OMC, with the initial concentration being MIC values followed by successive increases to 2×, 4×, 8×, and 16× MIC (31). Strains were cultured for four passages before their entry into the next concentration. Isolates from the passages of each concentration were stored at −80°C in MHB containing 40% glycerol, until further determination of genetic mutations and subsequent MIC assays.
PCR for multilocus sequence typing and detection of TET resistance and 30S ribosomal subunit genes.
Total DNA samples were extracted from E. faecalis isolates in lysis buffer for microorganisms and submitted to direct PCR using the PCR mastermix (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer’s instructions. The multilocus sequence typing (MLST) was determined by PCR and sequence alignment as previously described (21, 32). We also used PCR to detect TET-specific resistance genes, including tet(K) and tet(L), which encode efflux pump proteins; tet(M), tet(S), tet(O), and tet(W), which encode ribosomal protection proteins; and tet(U), a putative TET resistance determinant, as previously described (22). Genetic mutations in 30S ribosomal subunits, including four 16S rRNA gene copies and the 30S ribosomal protein S10, were detected by PCR amplification and sequence alignment. The primers used in this study are listed in Tables S7 to S9.
Efflux pump inhibition.
The role of the efflux pump in the development of OMC heteroresistance was evaluated using the EPIs Phe-Arg-β-naphthylamide (PaβN; Sigma, St. Louis, MO) and carbonyl cyanide m-chlorophenylhydrazone (CCCP; Sigma). The OMC MICs of the resistant subpopulations of heteroresistant isolates were determined by agar dilution in the presence and absence of PAβN (50 mg/liter) or CCCP (50 mg/liter), as previously described (28). Efflux pump inhibition of antimicrobial susceptibility was considered significant if the magnitude of the MIC value was decreased at least 4-fold in the presence of EPIs, which is consistent with the methods of previous investigations (24).
Measurement of bacterial growth curves.
Overnight bacterial cultures of EF16C185 (EF16) or EF16C185-RS (EF16-O2) were diluted 1:200 into 1 ml of fresh MHB or into MHB containing 1 mg/liter OMC, respectively, of which 300 μl was added into each well of a 96-well plate. Three parallel wells were used for each sample. The plates were placed in a Bioscreen C MBR (Oy Growth Curves Ab Ltd., Helsinki, Finland), and the bacteria were grown at 37°C with shaking at 220 rpm. Growth curves of the strains were determined by measuring the optical density at 600 nm (OD600) at 30-min intervals over a period of 16 h.
RNA sequencing.
The heteroresistant parental strain EF16 or its resistant subpopulation EF16-O2 was grown overnight in 20 ml of antibiotic-free MHB or MHB containing 1 mg/liter OMC, respectively. The overnight cultures were diluted 1:100 into 50 ml of MHB under the same conditions and grown to the mid-log phase (4 h) at 37°C. The bacteria were harvested by centrifugation, and total RNA of EF16 and EF16-O2 was isolated using an RNeasy minikit (Qiagen, Hilden, Germany) according to the manufacturer’s instructions (three biological replicates for each strain). The RNA quality and quantity were determined by 1.0% formaldehyde denaturing agarose gel electrophoresis and spectrophotometry in a NanoDrop ND-1000 machine, respectively. RNA sequencing was performed as previously described (33). Briefly, rRNA was removed from total RNA with a Ribo-Zero rRNA removal kit for Gram-positive bacteria (Illumina, San Diego, CA). Fragmented RNA was used as a template for PCR with random primers. To build the cDNA libraries, cDNA fragments were purified using an AMPure XP system (Beckman Coulter, Beverly, USA) to select fragments of ∼150 to 200 bp in length. The PCR was then performed using Phusion high-fidelity DNA polymerase (Thermo Fisher Scientific, Waltham, MA), and the library quality was assessed on an Agilent Bioanalyzer 2100 system (Agilent Technologies, CA). The library preparations were then sequenced on an Illumina HiSeq X Ten platform, and reads of paired-end 150 bp (PE150) were generated. Clean data were obtained by removing rRNA reads, sequencing adapters, short fragment reads, and other low-quality reads from the raw data. The remaining reads were mapped to the reference genome of OG1RF on the NCBI website with Bowtie 2 software. When the reads were aligned, one mismatch with the reference sequence was allowed. The alignments were further processed using BEDTools software to determine the transcript expression levels and their differential expression between each two of the three samples. Differential expression of all transcripts was quantified using DEGseq software, and the fold change values were recorded. Genes with adjusted P values (Benjamini-Hochberg method) of <0.05 and at least a 2-fold difference in expression were considered DEGs.
Quantitative real-time PCR analysis.
Transcriptional levels of OMC heteroresistant candidate genes were determined by qRT-PCR with the primers listed in Table S10, according to previously described methods (24). Briefly, overnight cultures of the bacterial strains were diluted 1:100 into 10 ml of MHB (cultures of the resistant subpopulations supplemented with 1 mg/liter OMC) and grown for 4 h at 37°C. Total bacterial RNA was extracted using an RNeasy minikit (Qiagen, Hilden, Germany), and the extracted RNA was reverse transcribed into cDNA using a PrimeScript real-time (RT) reagent kit (TaKaRa Bio, Inc., Shiga, Japan). qRT-PCR was performed with the SYBR Premix Ex Taq II kit (TaKaRa Bio, Inc., Shiga, Japan) in a Mastercycler ep realplex system (Eppendorf, Hamburg, Germany). The internal control gene recA was used to normalize the expression of each candidate gene. The threshold cycle (CT) numbers were determined by the detection system software, and the data were analyzed based on the 2−ΔΔCT method. Expression levels of the target genes were compared with those of the E. faecalis OG1RF strain or the heteroresistant parental strain (expression = 1). All qRT-PCRs were carried out in triplicate with three independent RNA samples.
Gene overexpression.
Full-length candidate genes, which included OG1RF_RS12140, OG1RF_RS02205, OG1RF_RS06145, OG1RF_RS11485, OG1RF_RS06880, and OG1RF_RS00630, were amplified from the total DNA extracted from OG1RF isolates and then integrated separately into the pIB166 vector (the inserted gene was controlled by a P23 promoter for overexpression) (34). The positive clones were screened by chloramphenicol and verified by PCR and sequencing. The overexpression plasmids (pRS12140, pRS02205, pRS06145, pRS11485, pRS06880, and pRS00630) were then transformed separately into three OMC-sensitive isolates (Table S5) and further identified by PCR and sequencing. The pIB166 vector was transferred into the same isolates as a control. Subsequently, overnight cultures of the derivative and parental strains were diluted 1:100 into antibiotic-free MHB and grown for 4 h at 37°C, and transcriptional levels of candidate genes were measured by qRT-PCR as described above. The OMC MIC values for these derivatives were determined, and heteroresistance was evaluated by PAP determination under OMC pressure as described above. The primers for plasmid construction are listed in Table S11.
Statistical analysis.
Continuous data were analyzed with the Student’s t test and one-way factorial analysis of variance (ANOVAs) in the SPSS software package (version 17.0; Chicago, IL). P values of <0.05 were regarded as statistically significant.
Data availability.
The RNA sequencing data were deposited in the NCBI database under BioProject no. PRJNA505112 and BioSample no. SAMN10411100, SAMN10411101, SAMN10411102, SAMN10411103, SAMN10411104, and SAMN10411105.
Supplementary Material
ACKNOWLEDGMENTS
We thank Jingren Zhang (Center for Infectious Disease Research, School of Medicine, Tsinghua University, Beijing, China) for generously providing plasmid pIB166.
This work was supported by the National Natural Science Foundation of China (grants 81902033 and 81601797); the Science, Technology and Innovation Commission of Shenzhen Municipality basic research funds (grants JCYJ20180302144403714 and JCYJ20180302144721183) and key funds (grant JCYJ20170412143551332 and JCYJ 20180508162403996); the Sanming Project of Medicine in Shenzhen (grant SMGC201705029); and the science funds of the Nanshan District Government (grant 2018021).
Zhiwei Lin participated in the design of the study and drafted the manuscript. Zhiwei Lin, Zhangya Pu, and Guangjian Xu were involved in RNA-sequencing analysis, qRT-PCR assays, and overexpression studies, and performed the antibiotic susceptibility tests. Bing Bai, Zhong Chen, Xiang Sun, Jinxin Zheng, and Peiyu Li performed the PAP tests and PCR detections for MLST, TET resistance genes, and 30S ribosomal subunit genes. Qiwen Deng, Di Qu, and Zhijian Yu designed the study and revised the manuscript.
We declare that we have no conflicts of interest.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Comerlato CB, de Resende MCC, Caierão J, d’Azevedo PA. 2013. Presence of virulence factors in Enterococcus faecalis and Enterococcus faecium susceptible and resistant to vancomycin. Mem Inst Oswaldo Cruz 108:590–595. doi: 10.1590/s0074-02762013000500009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou Y, Chen H, He H, Du Y, Hu J, Li Y, Li Y, Zhou Y, Wang H, Chen Y, Nie Y. 2016. Increased Enterococcus faecalis infection is associated with clinically active Crohn disease. Medicine (Baltimore, MD) 95:e5019. doi: 10.1097/MD.0000000000005019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arias CA, Murray BE. 2012. The rise of the Enterococcus: beyond vancomycin resistance. Nat Rev Microbiol 10:266–278. doi: 10.1038/nrmicro2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mercuro NJ, Davis SL, Zervos MJ, Herc ES. 2018. Combatting resistant enterococcal infections: a pharmacotherapy review. Expert Opin Pharmacother 19:979–992. doi: 10.1080/14656566.2018.1479397. [DOI] [PubMed] [Google Scholar]
- 5.Breuer RJ, Hirt H, Dunny GM, Breuer RJ, Hirt H, Dunny GM. 2018. Mechanistic features of the enterococcal pCF10 sex pheromone response and the biology of Enterococcus faecalis in its natural habitat. J Bacteriol 200:e00733-17. doi: 10.1128/JB.00733-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hashemian SMR, Farhadi T, Ganjparvar M. 2018. Linezolid: a review of its properties, function, and use in critical care. Drug Des Devel Ther 12:1759–1767. doi: 10.2147/DDDT.S164515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Honeyman L, Ismail M, Nelson ML, Bhatia B, Bowser TE, Chen J, Mechiche R, Ohemeng K, Verma AK, Cannon EP, Macone A, Tanaka SK, Levy S. 2015. Structure-activity relationship of the aminomethylcyclines and the discovery of omadacycline. Antimicrob Agents Chemother 59:7044–7053. doi: 10.1128/AAC.01536-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Heidrich CG, Mitova S, Schedlbauer A, Connell SR, Fucini P, Steenbergen JN, Berens C, Heidrich C, Mitova S, Schedlbauer A, Connell S, Fucini P, Steenbergen J, Berens C. 2016. The novel aminomethylcycline omadacycline has high specificity for the primary tetracycline-binding site on the bacterial ribosome. Antibiotics (Basel) 5:32. doi: 10.3390/antibiotics5040032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Villano S, Steenbergen J, Loh E. 2016. Omadacycline: development of a novel aminomethylcycline antibiotic for treating drug-resistant bacterial infections. Future Microbiol 11:1421–1434. doi: 10.2217/fmb-2016-0100. [DOI] [PubMed] [Google Scholar]
- 10.Draper MP, Weir S, Macone A, Donatelli J, Trieber CA, Tanaka SK, Levy SB. 2014. Mechanism of action of the novel aminomethylcycline antibiotic omadacycline. Antimicrob Agents Chemother 58:1279–1283. doi: 10.1128/AAC.01066-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin W, Flarakos J, Du Y, Hu W, He H, Mangold J, Tanaka SK, Villano S. 2017. Pharmacokinetics, distribution, metabolism, and excretion of omadacycline following a single intravenous or oral dose of 14C-omadacycline in rats. Antimicrob Agents Chemother 61:e01784-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tanaka SK, Steenbergen J, Villano S. 2016. Discovery, pharmacology, and clinical profile of omadacycline, a novel aminomethylcycline antibiotic. Bioorg Med Chem 24:6409–6419. doi: 10.1016/j.bmc.2016.07.029. [DOI] [PubMed] [Google Scholar]
- 13.Pfaller MA, Huband MD, Rhomberg PR, Flamm RK. 2017. Surveillance of omadacycline activity against clinical isolates from a global collection (North America, Europe, Latin America, Asia-Western Pacific), 2010–2011. Antimicrob Agents Chemother 61:e00018-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pfaller MA, Rhomberg PR, Huband MD, Flamm RK. 2017. Activities of omadacycline and comparator agents against Staphylococcus aureus isolates from a surveillance program conducted in North America and Europe. Antimicrob Agents Chemother 61:e02411-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Beabout K, Hammerstrom TG, Perez AM, Magalhaes BF, Prater AG, Clements TP, Arias CA, Saxer G, Shamoo Y. 2015. The ribosomal S10 protein is a general target for decreased tigecycline susceptibility. Antimicrob Agents Chemother 59:5561–5566. doi: 10.1128/AAC.00547-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lupien A, Gingras H, Leprohon P, Ouellette M. 2015. Induced tigecycline resistance in Streptococcus pneumoniae mutants reveals mutations in ribosomal proteins and rRNA. J Antimicrob Chemother 70:2973–2980. doi: 10.1093/jac/dkv211. [DOI] [PubMed] [Google Scholar]
- 17.Nguyen F, Starosta AL, Arenz S, Sohmen D, Donhofer A, Wilson DN. 2014. Tetracycline antibiotics and resistance mechanisms. Biol Chem 395:559–575. doi: 10.1515/hsz-2013-0292. [DOI] [PubMed] [Google Scholar]
- 18.Grossman TH. 2016. Tetracycline Antibiotics and Resistance. Cold Spring Harb Perspect Med 6:a025387. doi: 10.1101/cshperspect.a025387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fiedler S, Bender JK, Klare I, Halbedel S, Grohmann E, Szewzyk U, Werner G. 2016. Tigecycline resistance in clinical isolates of Enterococcus faecium is mediated by an upregulation of plasmid-encoded tetracycline determinants tet(L) and tet(M). J Antimicrob Chemother 71:871–881. doi: 10.1093/jac/dkv420. [DOI] [PubMed] [Google Scholar]
- 20.El-Halfawy OM, Valvano MA. 2015. Antimicrobial heteroresistance: an emerging field in need of clarity. Clin Microbiol Rev 28:191–207. doi: 10.1128/CMR.00058-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zheng JX, Wu Y, Lin ZW, Pu ZY, Yao WM, Chen Z, Li DY, Deng QW, Qu D, Yu ZJ. 2017. Characteristics of and virulence factors associated with biofilm formation in clinical Enterococcus faecalis isolates in China. Front Microbiol 8:2338. doi: 10.3389/fmicb.2017.02338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bai B, Hu K, Li H, Yao W, Li D, Chen Z, Cheng H, Zheng J, Pan W, Deng M, Liu X, Lin Z, Deng Q, Yu Z. 2018. Effect of tedizolid on clinical Enterococcus isolates: in vitro activity, distribution of virulence factor, resistance genes and multilocus sequence typing. FEMS Microbiol Lett 365:fnx284. doi: 10.1093/femsle/fnx284. [DOI] [PubMed] [Google Scholar]
- 23.Halaby T, Kucukkose E, Janssen AB, Rogers MR, Doorduijn DJ, van der Zanden AG, Al Naiemi N, Vandenbroucke-Grauls CM, van Schaik W. 2016. Genomic characterization of colistin heteroresistance in Klebsiella pneumoniae during a nosocomial outbreak. Antimicrob Agents Chemother 60:6837–6843. doi: 10.1128/AAC.01344-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng JX, Lin ZW, Sun X, Lin WH, Chen Z, Wu Y, Qi GB, Deng QW, Qu D, Yu ZJ. 2018. Overexpression of OqxAB and MacAB efflux pumps contributes to eravacycline resistance and heteroresistance in clinical isolates of Klebsiella pneumoniae. Emerg Microbes Infect 7:139. doi: 10.1038/s41426-018-0141-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Band VI, Crispell EK, Napier BA, Herrera CM, Tharp GK, Vavikolanu K, Pohl J, Read TD, Bosinger SE, Trent MS, Burd EM, Weiss DS. 2016. Antibiotic failure mediated by a resistant subpopulation in Enterobacter cloacae. Nat Microbiol 1:16053. doi: 10.1038/nmicrobiol.2016.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brodersen DE, Clemons WM Jr, Carter AP, Morgan-Warren RJ, Wimberly BT, Ramakrishnan V. 2000. The structural basis for the action of the antibiotics tetracycline, pactamycin, and hygromycin B on the 30S ribosomal subunit. Cell 103:1143–1154. doi: 10.1016/S0092-8674(00)00216-6. [DOI] [PubMed] [Google Scholar]
- 27.Pioletti M, Schlunzen F, Harms J, Zarivach R, Gluhmann M, Avila H, Bashan A, Bartels H, Auerbach T, Jacobi C, Hartsch T, Yonath A, Franceschi F. 2001. Crystal structures of complexes of the small ribosomal subunit with tetracycline, edeine and IF3. EMBO J 20:1829–1839. doi: 10.1093/emboj/20.8.1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhong X, Xu H, Chen D, Zhou H, Hu X, Cheng G. 2014. First emergence of acrAB and oqxAB mediated tigecycline resistance in clinical isolates of Klebsiella pneumoniae pre-dating the use of tigecycline in a Chinese hospital. PLoS One 9:e115185. doi: 10.1371/journal.pone.0115185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Li R, Han Y, Zhou Y, Du Z, Wu H, Wang J, Chen Y. 2017. Tigecycline susceptibility and molecular resistance mechanisms among clinical Klebsiella pneumoniae strains isolated during non-tigecycline treatment. Microb Drug Resist 23:139–146. doi: 10.1089/mdr.2015.0258. [DOI] [PubMed] [Google Scholar]
- 30.Karlskas IL, Saleihan Z, Holo H, Mathiesen G, Eijsink VG. 2015. EF0176 and EF0177 from Enterococcus faecalis V583 are substrate-binding lipoproteins involved in ABC transporter mediated ribonucleoside uptake. Microbiology 161:754–764. doi: 10.1099/mic.0.000045. [DOI] [PubMed] [Google Scholar]
- 31.Yao W, Xu G, Bai B, Wang H, Deng M, Zheng J, Li D, Deng X, Liu X, Lin Z, Chen Z, Li G, Deng Q, Yu Z. 2018. In vitro-induced erythromycin resistance facilitates cross-resistance to the novel fluoroketolide, solithromycin, in Staphylococcus aureus. FEMS Microbiol Lett 365:fny116. doi: 10.1093/femsle/fny116. [DOI] [PubMed] [Google Scholar]
- 32.Ruiz-Garbajosa P, Bonten MJ, Robinson DA, Top J, Nallapareddy SR, Torres C, Coque TM, Canton R, Baquero F, Murray BE, del Campo R, Willems RJ. 2006. Multilocus sequence typing scheme for Enterococcus faecalis reveals hospital-adapted genetic complexes in a background of high rates of recombination. J Clin Microbiol 44:2220–2228. doi: 10.1128/JCM.02596-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wang X, Han H, Lv Z, Lin Z, Shang Y, Xu T, Wu Y, Zhang Y, Qu D. 2017. PhoU2 but not PhoU1 as an important regulator of biofilm formation and tolerance to multiple stresses by participating in various fundamental metabolic processes in Staphylococcus epidermidis. J Bacteriol 199:e00219-17. doi: 10.1128/JB.00219-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Biswas I, Jha JK, Fromm N. 2008. Shuttle expression plasmids for genetic studies in Streptococcus mutans. Microbiology 154:2275–2282. doi: 10.1099/mic.0.2008/019265-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The RNA sequencing data were deposited in the NCBI database under BioProject no. PRJNA505112 and BioSample no. SAMN10411100, SAMN10411101, SAMN10411102, SAMN10411103, SAMN10411104, and SAMN10411105.