Polymyxin B-based combinations are increasingly prescribed as a last-line option against extensively drug-resistant (XDR) Acinetobacter baumannii. It is unknown if such combinations can result in the development of nondividing persister cells in XDR A. baumannii. We investigated persister development upon exposure of XDR A. baumannii to polymyxin B-based antibiotic combinations using flow cytometry.
KEYWORDS: antibiotic persistence, carbapenem-resistant A. baumannii, time-kill study, tolerance
ABSTRACT
Polymyxin B-based combinations are increasingly prescribed as a last-line option against extensively drug-resistant (XDR) Acinetobacter baumannii. It is unknown if such combinations can result in the development of nondividing persister cells in XDR A. baumannii. We investigated persister development upon exposure of XDR A. baumannii to polymyxin B-based antibiotic combinations using flow cytometry. Time-kill studies (TKSs) were conducted in three nonclonal XDR A. baumannii strains with 5 log10 CFU/ml bacteria against polymyxin B alone and polymyxin B-based two-drug combinations over 24 h. At different time points, samples were obtained and enumerated by viable plating and flow cytometry. Propidium iodide and carboxyfluorescein succinimidyl ester dyes were used to differentiate between live and dead cells and between dividing and nondividing cells, respectively, at the single-cell level, and nondividing live cells were resuscitated and characterized phenotypically. Our results from viable plating showed that polymyxin B plus meropenem and polymyxin B plus rifampin were each bactericidal (>99.9% kill compared to the initial inoculum) against 2/3 XDR A. baumannii strains at 24 h. By flow cytometry, however, none of the combinations were bactericidal against XDR A. baumannii at 24 h. Further analysis using cellular dyes in flow cytometry revealed that upon exposure to polymyxin B-based combinations, XDR A. baumannii entered a viable but nondividing persister state. These bacterial cells reinitiated division upon the removal of antibiotic pressure and did not have a growth deficit compared to the parent strain. We conclude that persister cells develop in XDR A. baumannii upon exposure to polymyxin B-based combinations and that nonplating methods appear to complement viable-plating methods in describing the killing activity of polymyxin B-based combinations against XDR A. baumannii.
INTRODUCTION
Acinetobacter baumannii is a major nosocomial pathogen implicated in various life-threatening infections, including ventilator-associated pneumonia and bacteremia (1, 2). Extensive drug resistance in A. baumannii infections has typically been cited as the main culprit for therapy unresponsiveness in patients infected with A. baumannii, and to date, much has been elucidated about the species’ molecular mechanisms of resistance (2). However, recent studies have suggested that the presence of tolerant and/or persister cells also is an important factor in the development of recalcitrant A. baumannii infections (3–5). Persisters are a subset of cells within a population of antibiotic-sensitive bacterial cells that can survive lethal doses of antibiotics due to a nongrowing dormant state (i.e., tolerance) (6, 7). The formation of these cells has been shown to be induced by exposure to antibiotics or other stressors (6, 8). Persister cells are distinct from resistant cells as they do not actively divide in the presence of antibiotics and do not demonstrate any change in MICs upon regrowth (6, 9). In addition, unlike antibiotic resistance, which can be readily screened using standard clinical diagnostic methods, persistence and tolerance are currently undetectable by standard culture-based practices (6, 9).
Presently, antibiotic combination therapy, particularly polymyxin-based combinations, is considered one of the mainstay therapeutic options against infections caused by extensively drug-resistant (XDR) A. baumannii (10). The primary focus of combination therapy against XDR A. baumannii infections has been centered on overcoming antibiotic resistance (10, 11). To select an antibiotic(s) for combination with polymyxin B, time-kill studies (TKSs), where the number of live bacterial cells after exposure to different antibiotic combinations is determined by viable plate counts, are the most commonly used methods (12). Unfortunately, such culture-dependent assays cannot detect the presence of persisters, which may be capable of regrowth once the antibiotic pressure is removed (6, 9). Currently, it is unknown if tolerance or persistence develops in XDR A. baumannii upon exposure to polymyxin-based antibiotic combinations or if different polymyxin-based combinations have an effect on the degree of persister development.
In this study, we investigated the development of XDR A. baumannii persisters upon exposure to polymyxin B monotherapy and polymyxin B-based antibiotic combinations in TKSs using the cell division marker carboxyfluorescein succinimidyl ester (CFSE) in flow cytometry and compared it to the results obtained by classical viable plating (13). We further describe the fitness costs and the phenotypic and genotypic changes (if any) of the persisters upon resuscitation in nutrient medium.
RESULTS
Bacterial strains.
The MICs of the A. baumannii isolates are shown in Table 1. As shown, all three A. baumannii strains were nonsusceptible to all tested antibiotics except polymyxin B (14). The polymyxin B MICs for AB 8879, AB 112, and AB 9780 were 0.5 mg/liter, 1 mg/liter, and 0.5 mg/liter, respectively. There are no CLSI susceptibility breakpoints for rifampin against A. baumannii (14). The MICs of rifampin ranged from 2 to ≥16 mg/liter.
TABLE 1.
MICs for the three XDR A. baumannii strains
| Antibiotic | MIC (mg/liter) for strain: |
||
|---|---|---|---|
| AB 112 | AB 8879 | AB 9780 | |
| Ampicillin-sulbactam | 16/8 | 64/32 | ≥128/64 |
| Trimethoprim-sulfamethoxazole | ≥4/76 | ≥4/76 | ≥4/76 |
| Ciprofloxacin | ≥16 | ≥16 | ≥16 |
| Gentamicin | ≥64 | ≥64 | ≥64 |
| Rifampin | 4 | 2 | ≥16 |
| Polymyxin B | 1 | 0.5 | 0.5 |
| Tigecycline | 4 | 2 | 8 |
| Cefepime | ≥64 | ≥64 | ≥64 |
| Meropenem | ≥32 | ≥32 | ≥32 |
| Imipenem | ≥32 | ≥32 | ≥32 |
| Aztreonam | ≥64 | ≥64 | ≥64 |
| Piperacillin-tazobactam | ≥128/4 | ≥128/4 | ≥128/4 |
Calibration curve.
The relationship between log10 CFU per milliliter as determined by viable plating and log10 bacteria per milliliter as determined by flow cytometry is shown in Fig. S1 in the supplemental material. As shown, a linear relationship is demonstrated from the operating range of approximately 3 log10 CFU/ml to 7 log10 CFU/ml (r2 = 0.99 to 1.00). The lower limit of detection for flow cytometry is approximately 3 log10 CFU/ml; below this, the live bacterial count is indistinguishable from the background noise present in sterile Mueller-Hinton broth (MHB).
Bacterial counts in the TKS.
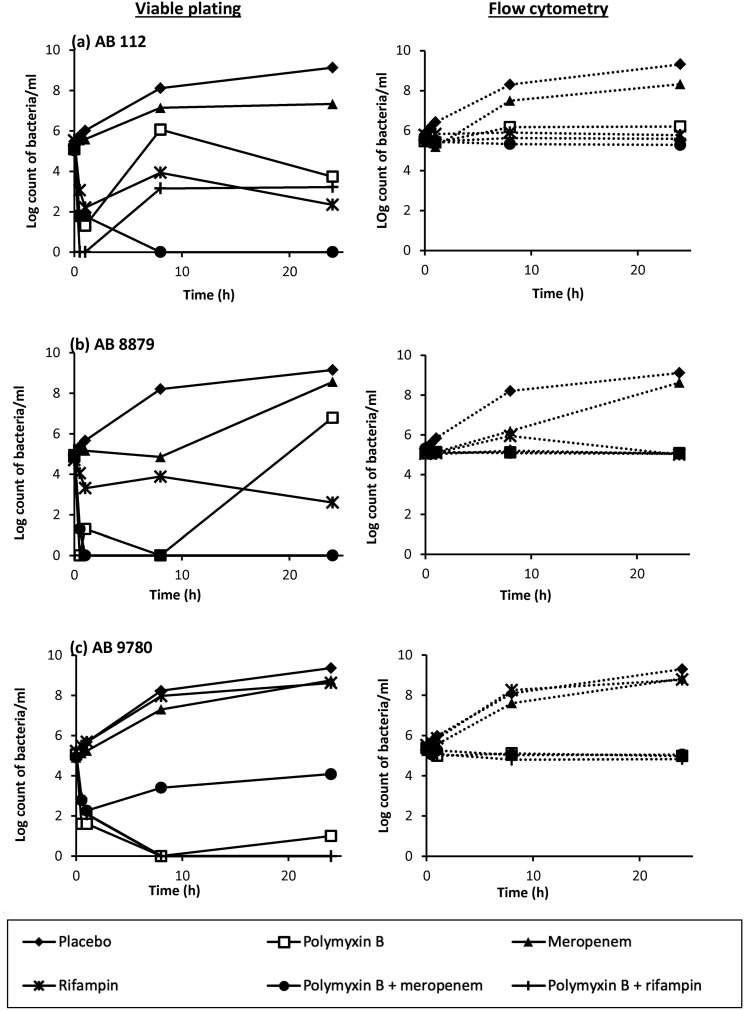
The viable bacterial counts by viable plating and flow cytometric analysis over the 24-h TKS for the three XDR A. baumannii strains are shown in Fig. 1, while the bacterial counts at 24 h are summarized in Table 2.
FIG 1.
Bacterial burdens in the 24-h TKS, as determined by viable plating and flow cytometry, for AB 112 (a), AB 8879 (b), and AB 9780 (c). In several of the antibiotic combinations where viable plating demonstrated bactericidal activity, bacterial counts remained at approximately 5 log10 CFU/ml to 6 log10 CFU/ml by flow cytometry.
TABLE 2.
Bacterial burdens at 24 h as determined by viable plating and flow cytometric analyses
| Antibiotic(s) | Log10 CFU/ml at 24 ha |
|||||
|---|---|---|---|---|---|---|
| AB 112 |
AB 8879 |
AB 9780 |
||||
| Viable plating | Flow cytometry | Viable plating | Flow cytometry | Viable plating | Flow cytometry | |
| No antibiotic | 9.13 | 9.31 | 9.15 | 9.11 | 9.36 | 9.30 |
| Polymyxin B | 3.73 | 6.20 | 6.79 | 5.07 | 1.60 | 4.98 |
| Rifampin | 2.34 | 5.77 | 2.60 | 5.00 | 8.62 | 8.77 |
| Meropenem | 7.33 | 8.32 | 8.56 | 8.62 | 8.75 | 8.83 |
| Polymyxin B + meropenem | 0.00 | 5.28 | 0.00 | 5.05 | 4.08 | 5.05 |
| Polymyxin B + rifampin | 3.22 | 5.58 | 0.00 | 5.00 | 0.00 | 4.83 |
Bactericidal combinations (≥3-log10 CFU/ml reduction in bacterial counts from the original inoculum) are in boldface type. The lower limit of detection for viable plating is 2.60 log CFU/ml.
(i) Viable plating. Meropenem alone was not bactericidal against all XDR A. baumannii isolates, resulting in an increase to approximately 9 log10 CFU/ml at 24 h. Polymyxin B alone resulted in bactericidal (a ≥3-log10 CFU/ml reduction in bacterial counts from the original inoculum) killing for all three isolates upon viable plating at 2 h; while this was sustained for AB 9780, regrowth was observed for AB 112 and AB 8879 at 24 h. The MICs of the regrowth bacteria upon monotherapy antibiotic exposure are shown in Table S1. As shown, the MICs of the regrowth bacteria upon polymyxin B monotherapy exposure remained similar to those of the parent isolate, but resistance amplification was observed in the regrowth bacteria upon rifampin monotherapy exposure. In antibiotic combination testing, polymyxin B plus meropenem and polymyxin B plus rifampin demonstrated bactericidal killing against 2/3 strains at 24 h upon viable plating.
(ii) Flow cytometry. The live bacterial counts obtained by flow cytometry appeared to be similar to those obtained by viable plating for all three XDR A. baumannii strains when no antibiotics were added. However, upon exposure to antibiotics, the live bacterial counts obtained by flow cytometry differed vastly (Table 2). Both polymyxin B plus meropenem and polymyxin B plus rifampin were not bactericidal against any of the XDR A. baumannii isolates at 24 h by flow cytometry; instead, live, nondividing bacterial counts remained at approximately 5 log10 cells/ml upon exposure to all polymyxin B-based antibiotic combinations.
Tolerant and persister cell formation upon antibiotic exposure.
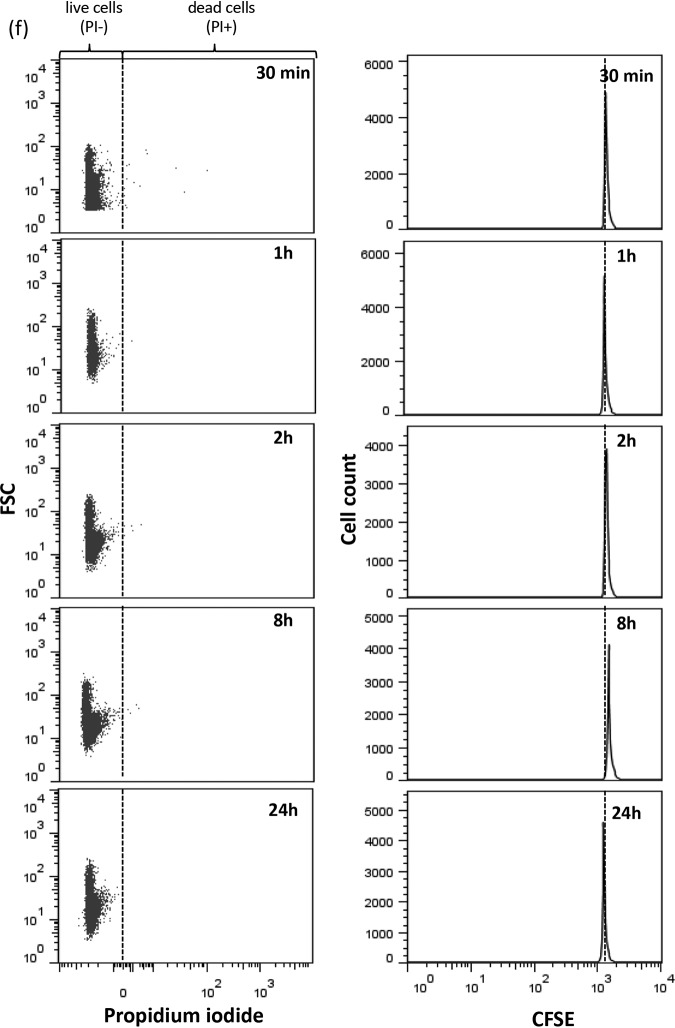
We further examined bacterial division over the 24-h TKS using CFSE dye in flow cytometry. The results for AB 112 are shown in Fig. 2. Cell division takes place as exponential bacterial growth takes place in the absence of antibiotics, resulting in a left shift of CFSE peaks as dye dilution continually occurs over time (Fig. 2a). The rate of CFSE dilution as estimated at the 30-min and 60-min time points appears to correspond to the known doubling time of A. baumannii (approximately 30 min). By 8 h, CFSE was diluted to levels only slightly above the unstained background levels, and no further division cycles could be observed. Upon exposure of bacteria to meropenem alone, we observed a significant decrease in the division rate of AB 112 compared to non-antibiotic-treated bacterial cells, as evident by the slowed CFSE dilution (Fig. 2b). There was a substantial percentage of killed bacterial cells (propidium iodide [PI]-positive cells) at each time point, suggesting continual bacterial killing upon exposure to meropenem across the 24-h period. Upon exposure to polymyxin B alone, AB 112 entered a nonreplicating state (no decrease in CFSE intensity over time) up to 2 h (Fig. 2c). For bacteria in this nonreplicating state, we observed minimal bacterial killing (most bacterial cells remained propidium iodide negative). Upon resumption of bacterial division at 8 h, bacterial killing was observed. Against rifampin alone and polymyxin B plus rifampin, AB 112 entered a nonreplicating state until 24 h, where some resumption of cell division was observed (Fig. 2d and e). Upon exposure to polymyxin B plus meropenem, AB 112 entered a nonreplicating state that persisted up to 24 h (Fig. 2f). Minimal bacterial killing was observed in this nonreplicating state, with live nondividing bacterial counts remaining at approximately 4 to 5 log10 cells/ml over the 24-h period.
FIG 2.
Live/dead cells (left) and CFSE dilution experiments (right) for AB 112 upon exposure to no antibiotics (a), meropenem (20 mg/liter) (b), polymyxin B (2 mg/liter) (c), rifampin (4 mg/liter) (d), polymyxin B (2 mg/liter) plus rifampin (4 mg/liter) (e), and polymyxin B (2 mg/liter) plus meropenem (20 mg/liter) (f) over a 24-h TKS. In the left dot plot, cells were classified into live and dead cells, on the basis of propidium iodide, against forward scatter (FSC), which is proportional to the diameter of the bacterial cells. In the right histograms, a reduction in the CFSE intensity over time indicated the presence of bacterial division.
Characterization of resuscitated cells.
We resuscitated the persister cells of AB 112 to polymyxin B plus meropenem, AB 8879 to polymyxin B plus meropenem and polymyxin B plus rifampin, and AB 9780 to polymyxin B plus rifampin and characterized the resuscitated cells. Flow cytometric analysis of the bacteria after the removal of antibiotics confirmed that resumption of division occurred approximately 24 to 36 h after the removal of the antibiotics (Fig. S2). The MICs, resistance mechanisms, and mean best-fit growth rate constant (Kg) values for the recovered isolates are shown in Table 3. As shown, the MICs of the recovered bacteria remained largely similar (within two 2-fold dilutions) to those of the parent isolate. Whole-genome sequencing showed that the resuscitated cells have resistomes that are similar to that of the parent strain, which is consistent with our phenotypic findings. The Kg values for AB 112, AB 8879, and AB 9780 were 1.69 h−1, 1.26 h−1, and 1.35 h−1, respectively. The growth rate of the resuscitated bacteria upon exposure to antibiotics was similar to that of the parent isolate, suggesting that no substantial biofitness deficit had developed.
TABLE 3.
Susceptibilities, resistance gene profiles, and growth rates of resuscitated XDR A. baumannii isolates after exposure to polymyxin B-based combinations
| Parameter | Value for organisma |
||||||
|---|---|---|---|---|---|---|---|
| AB 112 |
AB 8879 |
AB 9780 |
|||||
| Parent strain | Post-P+M | Parent strain | Post-P+M | Post-P+R | Parent strain | Post-P+R | |
| Mean Kg (h−1) | 1.69 | 1.43 | 1.26 | 1.28 | 1.33 | 1.35 | 1.33 |
| MIC (mg/liter) | |||||||
| Polymyxin B | 1 | 0.5 | 0.5 | 1 | 1 | 0.5 | 0.5 |
| Meropenem | ≥32 | ≥32 | ≥32 | 16 | ≥32 | ||
| Rifampin | 4 | 2 | 4 | ≥16 | ≥16 | ||
| Resistance gene profileb | |||||||
| Aminoglycosides | |||||||
| aac(3)-Ia | + | + | + | + | + | + | + |
| aadA1 | + | + | + | + | + | + | + |
| aph(3′)-VIa | + | + | + | + | + | + | + |
| aph(3′)-Ia | + | + | + | + | + | ||
| aph(6)-Id | + | + | + | ||||
| aph(3″)-Ib | + | + | + | ||||
| Beta-lactams | |||||||
| blaOXA-422 | + | + | + | ||||
| blaOXA-23 | + | + | + | + | + | + | + |
| blaOXA-507 | + | + | + | ||||
| blaADC-25 | + | + | + | + | + | + | + |
| blaOXA-66 | + | + | + | ||||
| blaOXA-51 | + | + | + | + | + | + | + |
| blaTEM-1D | + | + | + | + | + | ||
| blaOXA-69 | + | ||||||
| Phenicol | |||||||
| catA1 | + | + | |||||
| Sulfonamides | |||||||
| sul1 | + | + | + | + | + | + | + |
| sul2 | + | + | + | ||||
| Tetracycline | |||||||
| tet(B) | + | + | + | ||||
| tet(A) | + | + | |||||
P+M, polymyxin B plus meropenem; P+R, polymyxin B plus rifampin.
No resistance genes were found against colistin, fluoroquinolone, fosfomycin, fusidic acid, glycopeptides, macrolide, lincosamide, streptogramin B, nitroimidazole, oxazolidinone, rifampin, and trimethoprim.
DISCUSSION
We monitored the division of individual cells of XDR A. baumannii upon exposure to polymyxin B-based antibiotic combinations using flow cytometry, based on the dilution of CFSE upon cell division. Our results show that population-wide bacterial tolerance in XDR A. baumannii quickly occurs upon exposure to polymyxin B singly and in combination with multiple antibiotics. Notably, bacterial division was reinstated after 2 h upon exposure to polymyxin B alone but was maintained for 24 h upon exposure to polymyxin B-based antibiotic combinations. The nonreplicating cells, which were not detectable by conventional viable plating, were capable of reinitiating replication and growth when the antibiotic pressure was removed and did not appear to have any growth deficit compared to the parent strains.
The induction of slowed growth or dormancy in bacteria upon antibiotic exposure and other stressors was first described in Staphylococcus aureus by Joseph Bigger in 1944 and has been widely studied in the literature (8, 15–18). This survival strategy, known as “bet-hedging,” promotes long-term bacterial survival through the development of phenotypic variants that are poorly adapted to the present environment but preadapted for a future environment (6). As the cellular processes that are targeted by antibiotics are in a general “shutdown” state, dormant persister cells can tolerate antibiotics with different modes of action. These nondividing cells may be an important cause of antibiotic treatment failure and represent a probable reservoir in which further resistance can develop (6, 17). In a study by Van den Bergh et al., the authors showed that Escherichia coli persister cells readily emerge upon exposure to two to three cycles of daily amikacin at clinically relevant concentrations, reaching levels of up to 100% by the third treatment round (16). Further increasing amikacin concentrations did not appear to improve bacterial killing (16). In another study, by Levin-Reisman et al., the authors demonstrated the development of tolerance in E. coli when exposed to intermittent ampicillin and furthermore found that such tolerance preceded the emergence of resistance (17).
Flow cytometry measures bacterial cells at single-cell resolution and can be employed to identify interesting subpopulations with different physiological characteristics (19). As visualization of the bacteria is not based on growth on solid medium, nondividing dormant cells can be detected by flow cytometry (19). This is in contrast to conventional susceptibility testing, which cannot allow different subpopulations in a growing culture to be distinguished and fails to detect nondividing dormant or persister cells (19). Roostalu et al. employed the dilution of green fluorescent protein to monitor cell division and visualize nongrowing persisters in Escherichia coli (20). In another study, by Helaine et al., the replication dynamics of Salmonella enterica serovar Typhimurium was described using flow cytometry (21). In our study, we employed CFSE, a tracking dye commonly employed in lymphocyte proliferation studies, to visualize the replication dynamics of A. baumannii upon exposure to various polymyxin B-based combinations (22, 23). Labeling of the XDR A. baumannii cells with CFSE enabled us to monitor the presence of cell division in non-antibiotic-treated cells for up to 2 h, while double labeling with propidium iodide provided additional information to differentiate between dead cells and nongrowing live persister cells.
We observed an extended lag phase followed by a slowed division of AB 112 in the flow cytometric analysis in our study when the XDR A. baumannii strain was exposed to meropenem alone at clinically relevant concentrations. While continual bacterial killing was observed across the different time points in the flow cytometric analysis, the viable bacterial counts by viable plating appeared to be consistently lower than the live bacterial counts by flow cytometry at 2 h. Hence, we hypothesized that the reduction in CFU observed by viable plating over time was a combined effect of the slowed division and bacterial cell death. When bacteria were tested against single antibiotics, exposure to polymyxin B monotherapy and rifampin monotherapy resulted in an early nonreplicating state in AB 112, followed by a slow-dividing replicative state at 24 h, which was associated with bacterial regrowth in the presence of antibiotics. Interestingly, while regrowth isolates that occurred upon exposure to rifampin alone demonstrated an increase in rifampin MICs, we did not observe an increase in polymyxin B MICs in the regrowth isolates that emerged after exposure to polymyxin B alone. This suggested that the selection and amplification of resistance subpopulations may be only one of the various reasons for bacterial growth and survival in the presence of antibiotics; more studies will be required to examine this phenomenon.
Upon exposure to polymyxin B plus meropenem at clinically relevant concentrations, AB 112 entered a nonreplicating state, as evident by the lack of CFSE dilution. This nonreplicating state persisted up to 24 h, and no CFU were observed by viable plating during the nonreplicating state despite high viable cell counts (4 to 5 log10 CFU/ml) by flow cytometry. A similar phenomenon was described by Bulman et al., who observed a lack of growth by viable plating upon the exposure of an mcr-1-producing Escherichia coli isolate to polymyxin B plus aztreonam and demonstrated the presence of nonreplicative filamentous persisters by electron microscopy (24). In light of our findings, we surmise that conventional TKSs, which can enumerate only the actively replicating bacterial subset, may be insufficient to fully describe the bacterial killing effect of an antibiotic combination. Moving forward, we believe that there may be utility in employing additional methods (e.g., flow cytometry and microscopy) to complement the findings by conventional viable plating in TKSs to determine the full killing activity of antibiotic combinations.
An inherent characteristic of the persister phenomenon is the ability of bacterial persisters to resume growth under upon the removal of antibiotics (7). In our study, we observed that the XDR A. baumannii persister progeny were able to spontaneously restart cell division and growth after 24 h to 36 h upon the removal of antibiotic pressure and placement of the persister bacterial cells under favorable growth conditions. The prolonged lag time before the resumption of bacterial division, which has been reported in previous studies, may represent the work that a bacterial cell must do before returning to a condition that allows replication. While we did not establish the exact mechanisms that resulted in the resuscitation of the persister cells, we ruled out the possibility of regrowth due to the proliferation of a heteroresistant subpopulation or the proliferation of resistant mutants (25). Notably, the MICs of the exposed antibiotics for the “resuscitated” bacteria remained unchanged, and whole-genome sequencing did not show notable changes in the bacterial resistome. Upon head-to-head comparison of the fitness of the “resuscitated” bacteria with that of their parent strains by time-growth studies, we found no significant reduction in the growth rate. Our findings suggested that the conversion to the persister phenotype was not associated with a corresponding biological fitness cost upon regrowth; however, to confirm this, head-to-head virulence studies comparing the virulence of resuscitated cells to that of the parent phenotype will be needed.
Currently, few viable treatment options remain against XDR A. baumannii (2). We have shown in our study that polymyxin B-based antibiotic combinations, which have been widely considered last-line cornerstone therapies against XDR A. baumannii, resulted in the formation of nondividing persisters. Our findings worsened the already grim outlook on the battle against the ongoing antibiotic crisis and highlighted a potentially understudied cause of antibiotic treatment failure (26). Moving forward, the molecular process governing the development of antibiotic-induced persistence and the clinical impact of such antibiotic-tolerant cells will be further explored. Our study also shows that current culture-based plating methods are incapable of detecting persisters that develop upon antibiotic exposure. Methods that detect antibiotic tolerance and persistence, such as flow cytometry and microscopy, can be used to complement current viable plating methods when assessing the full killing activity of antibiotics and antibiotic combinations.
MATERIALS AND METHODS
Bacterial strains.
Three nonclonal clinical carbapenem-resistant A. baumannii isolates (AB 112, AB 8879, and AB 9780) collected in a nationwide surveillance study by the Network for Antimicrobial Resistance Surveillance (Singapore) were employed. A. baumannii ATCC 19606 was employed as a reference strain. MICs of multiple antibiotics were determined using custom-made broth microdilution panels (Trek Diagnostics, East Grinstead, UK) and interpreted according to CLSI standards (14). XDR was defined as nonsusceptibility to at least one agent in all but two or fewer antimicrobial categories (27). The molecular mechanisms of carbapenem resistance in the A. baumannii isolates and clonal relationship analyses were described previously (28). All three clinical carbapenem-resistant A. baumannii isolates were positive for the blaOXA-23-like and blaOXA-51-like carbapenemase genes, with ISAba1 upstream of the blaOXA-23-like gene (28). All bacterial strains were stored at −80°C in Cryobank storage vials, and fresh isolates were subcultured twice on 5% blood agar plates for 24 h at 35°C before each experiment.
Antimicrobial agents.
A total of three antibiotics were employed in the antibiotic killing assays. Meropenem, polymyxin B, and rifampin were purchased from Toronto Research Chemicals. All antibiotics except rifampin were prepared in sterile water and diluted to final concentrations with Mueller-Hinton II broth (Ca-MHB). Rifampin was reconstituted in dimethyl sulfoxide (DMSO) and serially diluted to the desired concentration in sterile water before being diluted to final concentrations with Ca-MHB. The final DMSO concentration (<1%, vol/vol) had no effect on bacterial growth (14).
Fluorochrome staining for flow cytometry.
We stained the bacterial cells with CFSE (Thermo Fisher Scientific, Singapore) prior to antibiotic exposure to examine cell division characteristics at the single-cell level. CFSE is a cell division marker that forms stable conjugates by binding irreversibly to aliphatic amines (13). Upon cell division, the fluorescence is halved, enabling individual cell division events in the population to be tracked (see Fig. S3 in the supplemental material) (13). This dye has been previously shown to be nontoxic to bacteria, even at high concentrations (8).
To stain the bacterial cells with CFSE, bacterial cultures grown overnight were prepared in Ca-MHB and incubated at 35°C until log phase was reached. The log-phase bacterial culture was then diluted in Ca-MHB according to the absorbance at 630 nm to an inoculum of approximately 6.6 log10 CFU/ml. One milliliter of bacteria was washed twice with phosphate-buffered saline (PBS) and stained with 150 μM CFSE in the presence of 0.02% Triton X-100 (Sigma-Aldrich, Singapore) for 30 min in a shaking incubator at 30°C (29). CFSE was then quenched with 10% fetal bovine serum (FBS) (Thermo Fisher Scientific, Singapore) in PBS, centrifuged at 1,800 × g at 4°C for 30 min, and resuspended in Ca-MHB. The resuspended bacteria were checked using a fluorescence microscope to ensure that cells were stained and viable.
To further differentiate between live nondividing and dead bacterial cells, we employed a combination of propidium iodide and SYTO-62 (Thermo Fisher Scientific, Singapore), applied directly before flow cytometry. SYTO-62 labels all bacteria, while propidium iodide enters and intercalates into cells with damaged membranes, leading to enhanced fluorescence, but is excluded by live bacteria with intact membranes. Propidium iodide and SYTO-62 were applied at 20 μM and 2.5 μM for 15 min before cells were acquired on a flow cytometer.
Calibration curves.
To demonstrate the relationship between bacterial counts obtained by viable counting and the number of live bacteria determined using flow cytometry, we plotted calibration curves of log10 CFU per milliliter versus log10 bacteria per milliliter in triplicate. Approximately 7 log10 CFU/ml of A. baumannii ATCC 19606 were suspended in Ca-MHB and incubated at 35°C until log-phase was reached. The inoculum was serially diluted to achieve concentrations of approximately 3 log10 CFU/ml to 7 log10 CFU/ml. Viable plate counts were performed by depositing serial 10-fold dilutions onto Mueller-Hinton agar (MHA) plates, incubating the plates at 35°C for 18 to 24 h, and enumerating the CFU visually. CountBright beads (20 μl) (Thermo Fisher Scientific, Singapore) were added prior to flow cytometric analysis to enumerate live bacteria.
TKS.
We performed a TKS for each antibiotic singly and in 2-antibiotic combinations with polymyxin B. The antibiotic concentrations chosen in the assays represent clinically achievable free or unbound serum or tissue concentrations (30–33). Polymyxin B was tested at 2 mg/liter, representing the steady-state free serum concentrations achievable with at least 30,000 U/kg of body weight of polymyxin B (30). The meropenem concentration at 20 mg/liter represents a steady-state unbound serum concentration at time above MIC of 40%, arising from a 2-g, 3-h infusion dose (31). Rifampin was tested at 4 mg/liter, representing the free peak concentration arising from a 600-mg intravenous dose (32).
Bacterial cultures grown overnight were prepared in Ca-MHB and incubated at 35°C until log phase was reached. The bacterial cells were then stained with CSFE as described above, the log-phase bacterial culture was then diluted in Ca-MHB according to the absorbance at 630 nm to a final baseline inoculum of approximately 5 log10 CFU/ml (1 × 105 CFU/ml to 5 × 105 CFU/ml), and 15 ml was transferred to sterile flasks, each containing 1 ml of the drug(s) at 16 times the target concentration, and placed into a shaking water bath at 35°C. Serial samples were obtained in duplicate at 0, 0.5, 1, 2, 8, and 24 h and measured by (i) viable plating, and (ii) flow cytometric analysis.
(i) Viable plating. Samples collected at each time point were centrifuged at 10,000 × g for 15 min and reconstituted with sterile normal saline to their original volume to minimize drug carryover effects. Total viable plate counts were performed by depositing serial 10-fold dilutions of the broth sample onto MHA plates, incubating the dilutions at 35°C for 18 to 24 h, and enumerating the cells visually. The lower limit of detection for the viable colony counts was 2.6 log10 CFU/ml.
(ii) Flow cytometric analysis. Samples collected at each time point were labeled with propidium iodide and SYTO-62 as described above. The stained bacteria were briefly sonicated and checked microscopically to ensure that the detected events were for single cells. CountBright beads (2 × 104 beads) that were added prior to flow cytometric analysis allowed the calculation of the volume of sample used in each run, for enumeration of live bacteria.
All samples were analyzed with a Cyan ADP instrument (Beckman Coulter, Brea, CA, USA). CFSE and propidium iodide were excited at 488 nm, and the fluorescence emission of each dye was detected in the fluorescein channel (530 ± 40 nm) and the PI channel (613 ± 20 nm), respectively. SYTO-62 was excited at 635 nm and detected in the allophycocyanin channel (665 ± 20 nm). A flow rate of 50 to 150 events per s was used to acquire the samples, and 10,000 SYTO-62-positive events were collected. All data were analyzed with FlowJo software 7.6.5 (FlowJo, LLC, Ashland, OR, USA).
Resuscitation of persister cells and characterization of resuscitated cells.
For samples that demonstrated regrowth by viable plating at 24 h in the TKS upon exposure to monotherapy antibiotics, we determined the MICs of the exposed antibiotics using the methods described above. For samples that demonstrated only live but nondividing bacterial cells (i.e., persisters) in the flow cytometric analysis at 24 h, we resuscitated the persister cells by centrifuging the sample at 10,000 × g for 5 min, resuspending the sample in fresh Ca-MHB, and incubating the sample in a shaking water bath at 35°C for 48 to 72 h until visible growth was observed. Samples were taken at regular intervals for microscopic and flow cytometric analyses to check for bacterial viability and the presence of cell division. At 72 h, the resuscitated bacteria were sampled and plated onto 5% blood agar plates for 24 h at 35°C. Determination of the MICs of the exposed antibiotic(s) was conducted for the recovered bacteria using commercial custom-made broth microdilution panels. For genomic analysis, library preparation was first conducted according to the protocol for rapid barcoding sequencing (catalog number SQK-RBK004) provided by Oxford Nanopore Technologies (Oxford, UK), with an initial DNA amount of 1 μg. Equal quantities of barcoded samples were pooled and concentrated using Agencourt AMPure XP beads (Beckman Coulter, CA, USA). The completed library was subsequently loaded onto R9.4 flow cells and sequenced on a MinION sequencer (Oxford Nanopore Technologies). Fast5 files were demultiplexed using Deepbinner and base called using Albacore. Barcoded reads agreed upon by Deepbinner and Albacore were used for further analysis. Processed reads were de novo assembled using the Canu assembler with default parameters. The draft genomes were polished using Nanopolish. The acquired antimicrobial resistance genes were identified using ResFinder v.3.0 (90% threshold and 60% minimum length). Growth kinetics of the recovered isolates were compared to those of the original isolates using in vitro time-growth studies using methods described previously (34). The exponential growth of the bacterial population over 24 h was analyzed using an adapted mathematical model (35), and the mean best-fit growth rate constant (Kg) was tabulated.
Supplementary Material
ACKNOWLEDGMENTS
This study was funded by National Medical Research Council Center grants NMRC/CG/M011/2017 and NMRC/CG/C005B/2017 and Singapore General Hospital (SGH) research grants SRG-NIG#03/2017 and OF04/2016. The funders have no role in study design, data collection and interpretation, or the decision to submit the work for publication.
We would like to acknowledge Rick Twee-Hee Ong and Cheng Yee Tang for their help with processing and assembly of MinION sequences.
We also thank the SGH Department of Clinical Research for assistance with flow cytometry work.
Footnotes
Supplemental material is available online only.
REFERENCES
- 1.Peleg AY, Seifert H, Paterson DL. 2008. Acinetobacter baumannii: emergence of a successful pathogen. Clin Microbiol Rev 21:538–582. doi: 10.1128/CMR.00058-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Doi Y, Murray GL, Peleg AY. 2015. Acinetobacter baumannii: evolution of antimicrobial resistance—treatment options. Semin Respir Crit Care Med 36:85–98. doi: 10.1055/s-0034-1398388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Barth VC Jr, Rodrigues BA, Bonatto GD, Gallo SW, Pagnussatti VE, Ferreira CA, de Oliveira SD. 2013. Heterogeneous persister cells formation in Acinetobacter baumannii. PLoS One 8:e84361. doi: 10.1371/journal.pone.0084361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chung ES, Wi YM, Ko KS. 2017. Variation in formation of persister cells against colistin in Acinetobacter baumannii isolates and its relationship with treatment failure. J Antimicrob Chemother 72:2133–2135. doi: 10.1093/jac/dkx102. [DOI] [PubMed] [Google Scholar]
- 5.Gallo SW, Donamore BK, Pagnussatti VE, Ferreira CA, de Oliveira SD. 2017. Effects of meropenem exposure in persister cells of Acinetobacter calcoaceticus-baumannii. Future Microbiol 12:131–140. doi: 10.2217/fmb-2016-0118. [DOI] [PubMed] [Google Scholar]
- 6.Van den Bergh B, Fauvart M, Michiels J. 2017. Formation, physiology, ecology, evolution and clinical importance of bacterial persisters. FEMS Microbiol Rev 41:219–251. doi: 10.1093/femsre/fux001. [DOI] [PubMed] [Google Scholar]
- 7.Balaban NQ, Helaine S, Lewis K, Ackermann M, Aldridge B, Andersson DI, Brynildsen MP, Bumann D, Camilli A, Collins JJ, Dehio C, Fortune S, Ghigo JM, Hardt WD, Harms A, Heinemann M, Hung DT, Jenal U, Levin BR, Michiels J, Storz G, Tan MW, Tenson T, Van Melderen L, Zinkernagel A. 2019. Definitions and guidelines for research on antibiotic persistence. Nat Rev Microbiol 17:441–448. doi: 10.1038/s41579-019-0207-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ueckert JE, Nebe von-Caron G, Bos AP, ter Steeg PF. 1997. Flow cytometric analysis of Lactobacillus plantarum to monitor lag times, cell division and injury. Lett Appl Microbiol 25:295–299. doi: 10.1046/j.1472-765x.1997.00225.x. [DOI] [PubMed] [Google Scholar]
- 9.Fisher RA, Gollan B, Helaine S. 2017. Persistent bacterial infections and persister cells. Nat Rev Microbiol 15:453–464. doi: 10.1038/nrmicro.2017.42. [DOI] [PubMed] [Google Scholar]
- 10.Zavascki AP, Bulitta JB, Landersdorfer CB. 2013. Combination therapy for carbapenem-resistant Gram-negative bacteria. Expert Rev Anti Infect Ther 11:1333–1353. doi: 10.1586/14787210.2013.845523. [DOI] [PubMed] [Google Scholar]
- 11.Fishbain J, Peleg AY. 2010. Treatment of Acinetobacter infections. Clin Infect Dis 51:79–84. doi: 10.1086/653120. [DOI] [PubMed] [Google Scholar]
- 12.Doern CD. 2014. When does 2 plus 2 equal 5? A review of antimicrobial synergy testing. J Clin Microbiol 52:4124–4128. doi: 10.1128/JCM.01121-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Banks HT, Sutton KL, Thompson WC, Bocharov G, Roose D, Schenkel T, Meyerhans A. 2011. Estimation of cell proliferation dynamics using CFSE data. Bull Math Biol 73:116–150. doi: 10.1007/s11538-010-9524-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.CLSI. 2017. Performance standards for antimicrobial susceptibility testing. CLSI, Wayne, PA. [Google Scholar]
- 15.Fridman O, Goldberg A, Ronin I, Shoresh N, Balaban NQ. 2014. Optimization of lag time underlies antibiotic tolerance in evolved bacterial populations. Nature 513:418–421. doi: 10.1038/nature13469. [DOI] [PubMed] [Google Scholar]
- 16.Van den Bergh B, Michiels JE, Wenseleers T, Windels EM, Boer PV, Kestemont D, De Meester L, Verstrepen KJ, Verstraeten N, Fauvart M, Michiels J. 2016. Frequency of antibiotic application drives rapid evolutionary adaptation of Escherichia coli persistence. Nat Microbiol 1:16020. doi: 10.1038/nmicrobiol.2016.20. [DOI] [PubMed] [Google Scholar]
- 17.Levin-Reisman I, Ronin I, Gefen O, Braniss I, Shoresh N, Balaban NQ. 2017. Antibiotic tolerance facilitates the evolution of resistance. Science 355:826–830. doi: 10.1126/science.aaj2191. [DOI] [PubMed] [Google Scholar]
- 18.Bigger JW. 1944. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet ii:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 19.Ambriz-Aviña V, Contreras-Garduño JA, Pedraza-Reyes M. 2014. Applications of flow cytometry to characterize bacterial physiological responses. Biomed Res Int 2014:461941. doi: 10.1155/2014/461941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roostalu J, Joers A, Luidalepp H, Kaldalu N, Tenson T. 2008. Cell division in Escherichia coli cultures monitored at single cell resolution. BMC Microbiol 8:68. doi: 10.1186/1471-2180-8-68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Helaine S, Thompson JA, Watson KG, Liu M, Boyle C, Holden DW. 2010. Dynamics of intracellular bacterial replication at the single cell level. Proc Natl Acad Sci U S A 107:3746–3751. doi: 10.1073/pnas.1000041107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hawkins ED, Hommel M, Turner ML, Battye FL, Markham JF, Hodgkin PD. 2007. Measuring lymphocyte proliferation, survival and differentiation using CFSE time-series data. Nat Protoc 2:2057–2067. doi: 10.1038/nprot.2007.297. [DOI] [PubMed] [Google Scholar]
- 23.Quah BJ, Parish CR. 2012. New and improved methods for measuring lymphocyte proliferation in vitro and in vivo using CFSE-like fluorescent dyes. J Immunol Methods 379:1–14. doi: 10.1016/j.jim.2012.02.012. [DOI] [PubMed] [Google Scholar]
- 24.Bulman ZP, Chen L, Walsh TJ, Satlin MJ, Qian Y, Bulitta JB, Peloquin CA, Holden PN, Nation RL, Li J, Kreiswirth BN, Tsuji BT. 2017. Polymyxin combinations combat Escherichia coli harboring mcr-1 and blaNDM-5: preparation for a postantibiotic era. mBio 8:e00540-17. doi: 10.1128/mBio.00540-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Li J, Rayner CR, Nation RL, Owen RJ, Spelman D, Tan KE, Liolios L. 2006. Heteroresistance to colistin in multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 50:2946–2950. doi: 10.1128/AAC.00103-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boucher HW, Talbot GH, Bradley JS, Edwards JE, Gilbert D, Rice LB, Scheld M, Spellberg B, Bartlett J. 2009. Bad bugs, no drugs: no ESKAPE! An update from the Infectious Diseases Society of America. Clin Infect Dis 48:1–12. doi: 10.1086/595011. [DOI] [PubMed] [Google Scholar]
- 27.Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, Giske CG, Harbarth S, Hindler JF, Kahlmeter G, Olsson-Liljequist B, Paterson DL, Rice LB, Stelling J, Struelens MJ, Vatopoulos A, Weber JT, Monnet DL. 2012. Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18:268–281. doi: 10.1111/j.1469-0691.2011.03570.x. [DOI] [PubMed] [Google Scholar]
- 28.Lim TP, Tan TY, Lee W, Sasikala S, Tan TT, Hsu LY, Kwa AL. 2011. In-vitro activity of polymyxin B, rifampicin, tigecycline alone and in combination against carbapenem-resistant Acinetobacter baumannii in Singapore. PLoS One 6:e18485. doi: 10.1371/journal.pone.0018485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Michel LV, Snyder J, Schmidt R, Milillo J, Grimaldi K, Kalmeta B, Khan MN, Sharma S, Wright LK, Pichichero ME. 2013. Dual orientation of the outer membrane lipoprotein P6 of nontypeable Haemophilus influenzae. J Bacteriol 195:3252–3259. doi: 10.1128/JB.00185-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 31.Tam VH, Schilling AN, Neshat S, Poole K, Melnick DA, Coyle EA. 2005. Optimization of meropenem minimum concentration/MIC ratio to suppress in vitro resistance of Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:4920–4927. doi: 10.1128/AAC.49.12.4920-4927.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Gumbo T, Louie A, Deziel MR, Liu W, Parsons LM, Salfinger M, Drusano GL. 2007. Concentration-dependent Mycobacterium tuberculosis killing and prevention of resistance by rifampin. Antimicrob Agents Chemother 51:3781–3788. doi: 10.1128/AAC.01533-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodvold KA, Gotfried MH, Cwik M, Korth-Bradley JM, Dukart G, Ellis-Grosse EJ. 2006. Serum, tissue and body fluid concentrations of tigecycline after a single 100 mg dose. J Antimicrob Chemother 58:1221–1229. doi: 10.1093/jac/dkl403. [DOI] [PubMed] [Google Scholar]
- 34.Cai Y, Lim TP, Teo J, Sasikala S, Lee W, Hong Y, Chan EC, Tan TY, Tan TT, Koh TH, Hsu LY, Kwa AL. 2016. In vitro activity of polymyxin B in combination with various antibiotics against extensively drug-resistant Enterobacter cloacae with decreased susceptibility to polymyxin B. Antimicrob Agents Chemother 60:5238–5246. doi: 10.1128/AAC.00270-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Tam VH, Schilling AN, Nikolaou M. 2005. Modelling time-kill studies to discern the pharmacodynamics of meropenem. J Antimicrob Chemother 55:699–706. doi: 10.1093/jac/dki086. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.