We evaluated the efficacy of escalating doses of exebacase administered with subtherapeutic daptomycin exposures against 8 Staphylococcus aureus isolates in a neutropenic murine thigh infection model. Daptomycin alone resulted in mean growth of 0.39 ± 1.19 log10 CFU/thigh.
KEYWORDS: lysin, Staphylococcus, methicillin-resistant S. aureus (MRSA), methicillin-susceptible S. aureus (MSSA), combination therapy
ABSTRACT
We evaluated the efficacy of escalating doses of exebacase administered with subtherapeutic daptomycin exposures against 8 Staphylococcus aureus isolates in a neutropenic murine thigh infection model. Daptomycin alone resulted in mean growth of 0.39 ± 1.19 log10 CFU/thigh. When administered with daptomycin, exebacase resulted in a mean log10 CFU/thigh reduction of −1.03 ± 0.72 (range, −0.77 ± 0.98 to −1.20 ± 0.59) across evaluated doses (15 to 90 mg/kg), indicative of potential in vivo synergy.
INTRODUCTION
Staphylococcus aureus is a highly opportunistic pathogen associated with increasing antimicrobial resistance, complicating medical treatment (1, 2). As a result, the development of alternative strategies to treat these infections is welcome. Exebacase (formerly, CF-301) is a novel bacteriophage-encoded lysin that achieves rapid bactericidal activity against S. aureus isolates via d-Ala-Gly endopeptidase activity, resulting in cell wall hydrolysis (3, 4). Lysins are of great interest because, in nature, as the lytic enzyme of bacteriophages (i.e., viruses that specifically infect bacteria), they have evolved to be pathogen specific without disturbing normal flora and have a low propensity for emergence of resistance (5, 6). Exebacase is manufactured as a purified protein and is being developed as an adjunctive agent to standard-of-care antibiotics to treat both methicillin-susceptible (MSSA) and methicillin-resistant (MRSA) S. aureus bacteremia and endocarditis (NCT03163446) (7). In parallel, robust preclinical testing of this compound in animal models is a valuable step in characterizing its antimicrobial activity. The present study was designed to determine the in vivo efficacy of exebacase alone and in combination with daptomycin against S. aureus isolates in a murine thigh infection model.
Eight S. aureus (1 MSSA, 7 MRSA) clinical isolates were utilized in the studies (Table 1). The MICs of daptomycin were determined in triplicate using CLSI broth microdilution methodology (8). The MICs of exebacase were determined in both 100% human and 100% ICR mouse (non-heat inactivated) serum for all isolates, as previously described (9). Notably, the impact of serum on exebacase activity has been observed to be similar with and without heat inactivation. Daptomycin MICs ranged from 0.25 to 0.5 μg/ml; all isolates demonstrated in vitro susceptibility according to CLSI breakpoints (8). Exebacase MICs of the isolates ranged from 0.5 to 2 μg/ml in human serum and from 16 to 128 μg/ml in mouse serum.
TABLE 1.
Phenotypic profiles of S. aureus isolates utilized in in vivo efficacy studies
| Organism | Strain | MIC (μg/ml) for: |
||
|---|---|---|---|---|
| Daptomycina | Exebacase in: |
|||
| Murine serumb | Human serumc | |||
| STA 513 | MRSA/LRSAd | 0.5 | 64 | 0.5 |
| STA 514 | MRSA | 0.5 | 16 | 0.5 |
| STA 516 | MRSA | 0.5 | 128 | 1 |
| STA 517 | MRSA | 0.5 | 32 | 2 |
| STA 518 | MRSA | 0.25 | 64 | 0.5 |
| STA 520 | MRSA | 0.5 | 128 | 0.5 |
| STA 521 | MRSA | 0.5 | 128 | 0.5 |
| STA 523 | MSSA | 0.5 | 128 | 0.5 |
Determined in Mueller-Hinton broth supplemented with calcium to a final concentration of 50 μg/ml.
Source: Envigo, pooled, female ICR murine serum (lot FLD170029).
Source: Sigma-Aldrich, pooled, male, type AB, from plasma (lot SLBS3701).
LRSA, linezolid-resistant Staphylococcus aureus.
The neutropenic murine thigh model study was conducted as previously published (10). Female ICR mice (Envigo RMS, Inc., Indianapolis, IN) were utilized in the study, and the protocol was approved by the Hartford Hospital Institutional Animal Care and Use Committee. Untreated control mice (3 per group) were sacrificed 2 h postinoculation to assess initial bacterial burden. Two hours postinoculation, treated mice (3 per group) received daptomycin alone, daptomycin plus 6 escalating doses of exebacase (15 to 90 mg/kg), or exebacase alone (90 mg/kg) subcutaneously. To ascertain the impact of dosing frequency on exebacase efficacy, dose fractionation studies were conducted using exebacase total daily doses of 75 and 90 mg/kg; doses were administered once (q24h) or three (q8h) times over a 24-h period. Control animals received the diluent vehicle (saline) with the same volume and schedule as those for the most frequently dosed regimen. Treated and 24-h control mice (3 per group) were sacrificed at the end of the study period, and thighs were harvested aseptically.
A previously developed human-simulated murine dosing regimen of daptomycin was utilized (10). This regimen in mice provided an area under the free drug concentration-time curve (AUC) over 24 h (fAUC0–24) similar to those achieved in healthy volunteers receiving daptomycin 6 mg/kg daily as described in the daptomycin package insert (11). In the current study, use of daptomycin alone resulted in significant bacterial reductions among all isolates given their susceptible MICs. To obtain a daptomycin regimen appropriate for evaluating synergy, daptomycin dose-ranging studies were performed to obtain a regimen that yielded stasis or growth of the isolates at 24 h. The relationship between daptomycin fAUC0-24/MIC and efficacy was determined for each isolate using the sigmoidal maximum effect (Emax) inhibitory model (WinNonlin version 5.0.1; Pharsight Corp., Mountain View, CA). The final daptomycin regimen provided a fAUC0–24 equivalent to 5% to 8% of that achieved in humans receiving daptomycin 6 mg/kg daily and was administered alone or in combination with exebacase in subsequent studies. Efficacy was quantified by the change in bacterial density in log10 CFU/thigh obtained in the mice after 24 h relative to that in 0-h control mice. Student's t test was used to compare antimicrobial efficacy between regimens, and a P value of ≤0.05 was defined as statistically significant.
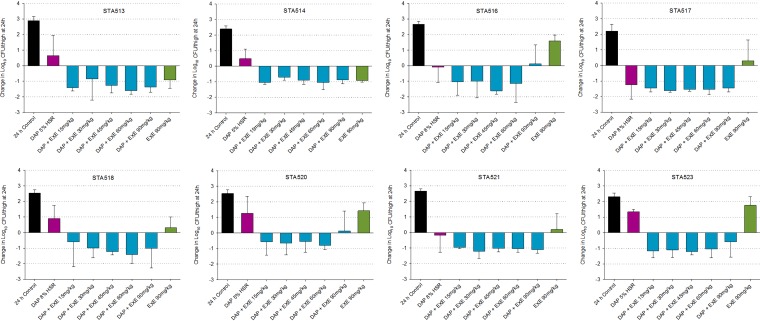
In vivo, 0-h control mice displayed a mean log10 CFU/thigh ± standard deviation of 5.77 ± 0.25, which increased to 8.28 ± 0.47 log10 CFU/thigh at 24 h, across all 8 isolates examined. Relative to that in 0-h controls, the mean bacterial growth at 24 h across all isolates in the daptomycin monotherapy treatment group was 0.39 ± 1.19 log10 CFU/thigh. Exebacase 15 mg/kg alone resulted in a mean growth of 0.76 ± 1.24 log10 CFU/thigh, whereas exebacase 90 mg/kg alone resulted in a −0.26 ± 1.25-log10 CFU/thigh reduction. The addition of exebacase 15 mg/kg to daptomycin resulted in a −1.03 ± 0.75-log10 CFU/thigh reduction, and higher exebacase doses did not yield further killing, with a mean log10 CFU/thigh reduction of −1.03 ± 0.72 (range, −0.77 ± 0.98 to −1.20 ± 0.59 log10 CFU/thigh reduction) across the range of doses. Bacterial density results for each isolate are depicted in Fig. 1. Overall, varying the exebacase dosing frequency had no impact on efficacy, as shown in Table 2, consistent with AUC/MIC being the pharmacodynamic driver of exebacase efficacy (12).
FIG 1.
Mean change in log10 CFU/thigh ± SD at 24 h relative to that of 0-h controls with daptomycin alone (DAP), exebacase alone (EXE), and escalating exebacase doses plus daptomycin.
TABLE 2.
Efficacy of fractionated doses of exebacase plus subtherapeutic daptomycin humanized exposure at 24 h versus that in 0-h controls in infected mice
| Isolate | Change (mean log10 CFU/thigh ± SD) with exebacase dose of: |
Change (mean log10 CFU/thigh ± SD) with exebacase dose of: |
||||
|---|---|---|---|---|---|---|
| 25 mg/kg q8h | 75 mg/kg q24h | P value | 30 mg/kg q8h | 90 mg/kg q24h | P value | |
| STA 513 | −1.69 ± 0.44 | −1.49 ± 0.3 | 0.379 | −1.72 ± 0.36 | −1.37 ± 0.34 | 0.114 |
| STA 514 | −1.12 ± 0.34 | −1.12 ± 0.13 | 1.000 | −1.04 ± 0.14 | −0.87 ± 0.26 | 0.189 |
| STA 516 | −1.17 ± 0.72 | −0.57 ± 1.39 | 0.370 | −1.51 ± 0.35 | 0.13 ± 1.2 | 0.009a |
| STA 517 | −1.5 ± 0.08 | −1.23 ± 0.7 | 0.370 | −1.27 ± 0.24 | −1.45 ± 0.24 | 0.223 |
| STA 518 | −0.59 ± 1.03 | −1.13 ± 0.27 | 0.242 | −1.02 ± 1.2 | −1.01 ± 1.25 | 0.989 |
| STA 520 | −0.26 ± 1.41 | −0.95 ± 0.15 | 0.261 | 0.6 ± 1.17 | 0.13 ± 1.28 | 0.522 |
| STA 521 | −1.09 ± 0.39 | −0.84 ± 0.15 | 0.174 | −1.16 ± 0.24 | −1.1 ± 0.22 | 0.661 |
| STA 523 | −0.93 ± 0.92 | −0.7 ± 0.97 | 0.682 | −0.4 ± 1.13 | −0.59 ± 0.98 | 0.762 |
Statistically significant.
The results of our in vivo study support reports demonstrating exebacase synergistic activity (13–15). In a MRSA septicemia model in which a high bacterial inoculum was chosen to render doses of standard-of-care antibiotics minimally efficacious, mice were inoculated intraperitoneally with 1 × 109 CFU and treated 2 h later with exebacase, daptomycin, or a combination of the drugs. At the 72-h endpoint, treatment with exebacase alone resulted in 17% to 50% survival, whereas treatment with daptomycin alone resulted in 7% to 31% survival. In contrast to these monotherapies, the addition of exebacase to daptomycin improved survival to 82% to 90% (14). In a recently completed checkerboard assay, exebacase demonstrated synergy when added to either daptomycin or vancomycin against isolates utilized in the current study (15).
Exebacase activity has been found to be potentiated by host serum factors, resulting in more enhanced bactericidal activity in some species (i.e., humans and rabbits) than in others (i.e., mice and rats), and may reflect a complex interaction of lysin and host albumin, lysozyme, and lipids (16). Indeed, exebacase exhibited substantially greater in vitro potency (32- to ≥100-fold) in human, rabbit, and dog serum than with conventional Mueller-Hinton broth and rodent serum (16). For in vivo validation, Indiani et al. (16) demonstrated that >100-fold-higher doses (exebacase 10 mg/kg) were required in the rat infective endocarditis model than in the rabbit model (exebacase 0.09 to 0.18 mg/kg) to demonstrate comparable efficacy, consistent with doses utilized in the current murine study. Studies are under way to further elucidate this novel synergistic mechanism among species.
Our findings are significant because S. aureus remains a primary cause of life-threatening infections (2). Exebacase, a potential first-in-class antimicrobial agent, used in combination with daptomycin was synergistic against S. aureus in a murine thigh infection model. These data support a role for adjunctive treatment in the management of S. aureus infections.
ACKNOWLEDGMENTS
We thank Christina Sutherland, Deborah Santini, Jennifer Tabor-Rennie, Sara Giovagnoli, Elizabeth Cyr, Kimelyn Greenwood, Alissa Padgett, Janice Cunningham, Michelle Insignares, Lauren McLellan, Elias Mullane, Sean Stainton, Safa Abuhussain, Lindsay Avery, and James Kidd from the Center for Anti-Infective Research and Development, Hartford, CT, for assistance with conducting the study.
This study was funded by ContraFect Corporation, Yonkers, NY.
D.P.N. has received research grants from ContraFect Corporation. T.C. and R.S. are employees of ContraFect Corporation. All other authors have no conflicts of interest to declare.
REFERENCES
- 1.Noskin GA, Rubin RJ, Schentag JJ, Kluytmans J, Hedblom EC, Smulders M, Lapetina E, Gemmen E. 2005. The burden of Staphylococcus aureus infections on hospitals in the United States. Arch Intern Med 165:1756. doi: 10.1001/archinte.165.15.1756. [DOI] [PubMed] [Google Scholar]
- 2.Liu C, Bayer A, Cosgrove SE, Daum RS, Fridkin SK, Gorwitz RJ, Kaplan SL, Karchmer AW, Levine DP, Murray BE, Rybak MJ, Talan DA, Chambers HF. 2011. Clinical practice guidelines by the Infectious Diseases Society of America for the treatment of methicillin-resistant Staphylococcus aureus infections in adults and children. Clin Infect Dis 52:e18–e55. doi: 10.1093/cid/ciq146. [DOI] [PubMed] [Google Scholar]
- 3.Wittekind M, Schuch R. 2016. Cell wall hydrolases and antibiotics: exploiting synergy to create efficacious new antimicrobial treatments. Curr Opin Microbiol 33:18–24. doi: 10.1016/j.mib.2016.05.006. [DOI] [PubMed] [Google Scholar]
- 4.Lood R, Molina H, Fischetti VA. 2017. Determining bacteriophage endopeptidase activity using either fluorophore-quencher labeled peptides combined with liquid chromatography-mass spectrometry (LC-MS) or Forster resonance energy transfer (FRET) assays. PLoS One 12:e0173919. doi: 10.1371/journal.pone.0173919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fischetti VA. 2008. Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400. doi: 10.1016/j.mib.2008.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fenton M, Ross P, McAuliffe O, O'Mahony J, Coffey A. 2010. Recombinant bacteriophage lysins as antibacterials. Bioeng Bugs 1:9–16. doi: 10.4161/bbug.1.1.9818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.ContraFect. 2017. Safety, efficacy and pharmacokinetics of CF-301 vs. placebo in addition to antibacterial therapy for treatment of S. aureus bacteremia. https://clinicaltrials.gov/ct2/show/NCT03163446.
- 8.Clinical and Laboratory Standards Institute. 2019. Performance standards for antimicrobial susceptibility testing; 29th ed. CLSI document M100. Clinical and Laboratory Standards Institute, Wayne, PA. [Google Scholar]
- 9.Oh JT, Cassino C, Schuch R. 2019. Postantibiotic and sub-MIC effects of exebacase (lysin CF-301) enhance antimicrobial activity against Staphylococcus aureus. Antimicrob Agents Chemother 63:e02616-18. doi: 10.1128/AAC.02616-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kidd JM, Abdelraouf K, Asempa TE, Humphries RM, Nicolau DP, Kidd JM, Abdelraouf K, Asempa TE, Humphries RM, Nicolau DP. 2018. Pharmacodynamics of daptomycin against Enterococcus faecium and Enterococcus faecalis in the murine thigh infection model. Antimicrob Agents Chemother 62:e00506-18. doi: 10.1128/AAC.00506-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Merck. 2017. Cubicin (daptomycin for injection) package insert. Merck, Kenilworth, NJ. [Google Scholar]
- 12.Ghahramani P, Chiu J, Asempa T, Abdelraouf K, Nicolau D, Abdelhady W, Xiong Y, Bayer A, Carabeo T, Cassino C, Schuch R, Lehoux D. 2019. PK-PD relationship and PK driver of efficacy of the novel antibacterial lysin exebacase (CF-301) in pre-clinical models. ASM Microbe 2019, San Francisco, CA. [Google Scholar]
- 13.Sauve K, Watson A, Yellin T, Jandourek A, Cassino C, Schuch R. 2017. Lysin CF-301 demonstrates in vitro synergy with conventional antibiotics against Staphylococcus aureus, abstr 1212. Abstr ID Week 2017, San Diego, CA. Infectious Diseases Society of America, Arlington, VA. [Google Scholar]
- 14.Schuch R, Lee HM, Schneider BC, Sauve KL, Law C, Khan BK, Rotolo JA, Horiuchi Y, Couto DE, Raz A, Fischetti VA, Huang DB, Nowinski RC, Wittekind M. 2014. Combination therapy with lysin CF-301 and antibiotic is superior to antibiotic alone for treating methicillin-resistant Staphylococcus aureus-induced murine bacteremia. J Infect Dis 209:1469–1478. doi: 10.1093/infdis/jit637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watson A, Sauve K, Cassino C, Schuch R. 2019. Exebacase demonstrates in vitro synergy with a broad range of antibiotics against both methicillin-resistant and methicillin-susceptible Staphylococcus aureus. Antimicrob Agents Chemother doi: 10.1128/AAC.01885-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R, Indiani C, Sauve K, Raz A, Abdelhady W, Xiong YQ, Cassino C, Bayer AS, Schuch R. 2019. The antistaphylococcal lysin, CF-301, activates key host factors in human blood to potentiate methicillin-resistant Staphylococcus aureus bacteriolysis. Antimicrob Agents Chemother 63:e02291-18. doi: 10.1128/AAC.02291-18. [DOI] [PMC free article] [PubMed] [Google Scholar]