The in vitro and in vivo activity of the arylamidine T-2307 against Candida auris was evaluated. T-2307 demonstrated in vitro activity (MIC ranges ≤ 0.008 to 0.015 μg/ml at 50% inhibition; 0.125 to >4 μg/ml at 100% inhibition).
KEYWORDS: T-2307, Candida auris, invasive candidiasis, murine model, in vitro susceptibility, caspofungin, fluconazole
ABSTRACT
The in vitro and in vivo activity of the arylamidine T-2307 against Candida auris was evaluated. T-2307 demonstrated in vitro activity (MIC ranges ≤ 0.008 to 0.015 μg/ml at 50% inhibition; 0.125 to >4 μg/ml at 100% inhibition). Treatment with T-2307 (3 mg/kg subcutaneous [SC] once daily) also significantly improved survival (70% at 21 days postinfection) and reduced kidney fungal burden (5.06 log10 CFU/g) compared to control (0% survival and 7.09 log10 CFU/g) (P < 0.01).
INTRODUCTION
Candida auris is an emerging fungal pathogen that has now been detected in institutions on multiple continents (1, 2). Invasive infections caused by this species are associated with high mortality rates, up to 59% in one retrospective study (1). Unfortunately, treatment options are limited as C. auris isolates are often resistant to antifungals, including fluconazole and other azoles (1, 3). Up to one third of isolates may be resistant to amphotericin B, with echinocandin resistance also being reported (3–5). T-2307 is an investigational arylamidine that is similar in structure to pentamidine and causes the collapse of fungal mitochondrial membrane potential (6, 7). This agent has shown to have potent in vitro and in vivo activity against Candida species, including isolates that are resistant to the azoles and echinocandins (8, 9). In vitro and in vivo activity has also been demonstrated against Cryptococcus, Aspergillus, and Fusarium species (6, 10, 11). Our objective was to evaluate the in vitro and in vivo activity of T-2307 against C. auris.
In vitro susceptibility testing was performed according to the Clinical and Laboratory Standards Institute (CLSI) M27-A3 standard against 10 isolates available from the FDA CDC Antibiotic Resistance (AR) Bank and 13 clinical isolates that were received for testing by the Fungus Testing Laboratory at the University of Texas Health Science Center at San Antonio (UTHSCSA), representing isolates from the South Asian and South American clades (12). The MIC of T-2307 was measured as the lowest concentration that inhibited both 50% and 100% of growth compared to the drug-free control after 24 h of incubation at 35°C, while the MICs of fluconazole and caspofungin were measured at 50% growth inhibition. Male ICR mice were rendered neutropenic with a single dose of 5-fluorouracil (5 mg/mouse) administered 24 h prior to inoculation, and a clinical isolate of C. auris (DI 17-46) was used to infect mice via the lateral tail vein as previously described (13, 14). Treatment with vehicle control, T-2307 (0.75, 1.5, or 3 mg/kg SC once daily), fluconazole (20 mg/kg orally [p.o.] once daily), or caspofungin (10 mg/kg intraperitoneally [i.p.] once daily) began 1 day postinoculation and continued for 7 days. Ten mice were included in each group in each study arm. In the fungal burden arm, mice were humanely euthanized on day 8 postinoculation, and kidneys and brains were collected, weighed, and homogenized for analysis of CFU (CFU/g). In the survival arm, mice were followed off therapy for 14 days, until day 21 postinoculation. Fungal burden was also assessed in the survival arm on day 21 or on the day the mice succumbed to infection. Differences in survival were assessed by Kaplan-Meier analysis with the log-rank test. ANOVA with Tukey’s post-hoc test for multiple comparisons were used to assess for differences in fungal burden and geometric mean (GM) MIC values.
T-2307 demonstrated in vitro activity against C. auris (Table 1). The MIC range using the 50% inhibition endpoint was < 0.008 to 0.015 μg/ml, but was markedly higher when measured using the 100% inhibition endpoint (0.25 to >4 μg/ml). Overall, the MICs for T-2307 using the 50% inhibition endpoint were lower than those for fluconazole (range 0.5 to >64 μg/ml) and caspofungin (< 0.015 to >8 μg/ml), and the GM MIC for T-2307 (0.011 μg/ml) was significantly lower than that observed for both fluconazole and caspofungin (14.6 and 0.24 μg/ml, respectively; P < 0.0001). The MICs for T-2307, fluconazole, and caspofungin against the infecting isolate were ≤ 0.008, >64, and 0.25 μg/ml, respectively. Additional in vitro testing of T-2307 is warranted against a larger set of C. auris isolates, including those from other clades.
TABLE 1.
In vitro activity of T-3207, fluconazole, and caspofungin against 23 C. auris isolatesa
| Activity level | T-2307 at 50% inhibition (μg/ml) | T-2307 at 100% inhibition (μg/ml) | Fluconazole at 50% inhibition (μg/ml) | Caspofungin at 50% inhibition (μg/ml) |
|---|---|---|---|---|
| MIC range | ≤0.008–0.015 | 0.125–>4 | 0.5–>64 | ≤0.015–>8 |
| MIC50 | 0.015 | >4 | 4 | 0.25 |
| MIC90 | 0.015 | >4 | >64 | 0.5 |
| GM MIC | 0.011 | 2.189 | 14.6 | 0.24 |
MICs read after 24 h of incubation at 35°C. MIC50 and MIC90, lowest concentrations that inhibited 50% and 90%, respectively, of the isolates tested; GM MIC, geometric mean MIC.
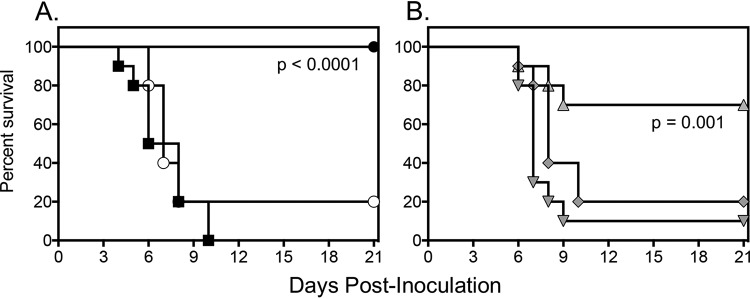
The in vitro activity of T-2307 did translate into in vivo efficacy, as the highest dose of T-2307 (3 mg/kg) resulted in significant improvements in median and percent survival (>21 days and 70%, respectively) compared to control (5 days and 0%, respectively) (P < 0.01) (Fig. 1). Similar improvements in survival were also observed in mice treated with high-dose caspofungin (>21 days and 100%, respectively) (P < 0.001). In contrast, neither the lower doses of T-2307 nor fluconazole improved survival.
FIG 1.
Survival curves in mice inoculated intravenously with C. auris and treated with vehicle control, fluconazole 20 mg/kg orally (p.o.) once a day (QD), or caspofungin 10 mg/kg intraperitoneally (i.p.) QD (A) or T-2307 at doses of 0.75 mg/kg, 1.5 mg/kg, or 3 mg/kg subcutenously QD (B). Treatment started 1 day postinoculation and continued for 7 days. Mice were then followed off therapy until day 21 postinoculation (14 days after therapy stopped). Black square, vehicle (untreated) control; white circle, fluconazole 20 mg/kg; black circle, caspofungin 10 mg/kg; inverted gray triangle, T-2307 0.75 mg/kg; gray rectangle, T-2307 1.5 mg/kg; gray triangle, T-2307 3 mg/kg. n = 10 mice per group.
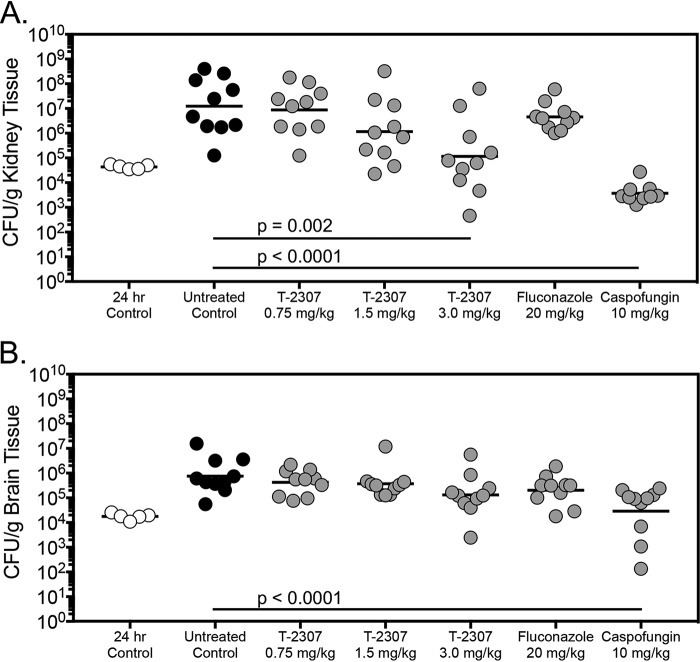
In the fungal burden arm, significant reductions in kidney CFU were also observed in mice treated with T-2307 at 3 mg/kg (mean 5.06 log10 CFU/g) and caspofungin (3.21 log10 CFU/g) compared to untreated control (7.09 log10 CFU/g) (P < 0.01) (Fig. 2). The activity of T-2307 was static in nature, as the fungal burden in the 3 mg/kg group was similar to that observed in the 24 h group measured just prior to the start of therapy. Reductions in kidney fungal burden were not observed in mice treated with the lower doses of T-2307 or fluconazole. Brain fungal burden observed in the caspofungin group (4.45 log10 CFU/g) was significantly lower than in the untreated control (5.88 log10 CFU/g) (P < 0.001) on day 8 postinoculation, but not in mice treated with T-2307 or fluconazole.
FIG 2.
Kidney (A) and brain (B) fungal burden (CFU/g) in mice with invasive candidiasis secondary to C. auris in the fungal burden arm. CFU/g were measured on day 8 postinoculation after 7 days of therapy. n = 10 mice in the vehicle control and treatment groups; n = 5 mice in the 24 h control group.
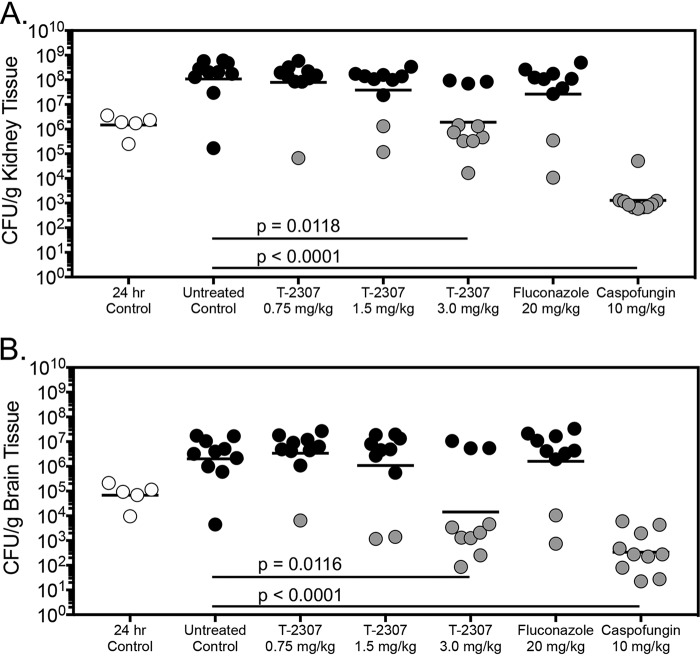
In the survival arm, kidney fungal burden was significantly lower in the T-2307 3 mg/kg (6.28 log10 CFU/g) and caspofungin groups (3.11 log10 CFU/g) compared to untreated control (8.04 log10 CFU/g) (P ≤ 0.01) (Fig. 3). Interestingly, brain fungal burden was significantly reduced in mice treated with T-2307 at 3 mg/kg (4.16 log10 CFU/g) and caspofungin (2.51 log10 CFU/g) versus untreated control (6.31 log10 CFU/g) (P ≤ 0.01). Previous studies have demonstrated reductions in brain and ocular tissue fungal burden in mice infected with Cryptococcus gattii and Candida albicans, respectively, and treated with T-2307 (10, 11). In the current study, there was also a clear relationship between fungal burden and survival, as treated mice that survived to the day 21 endpoint had lower kidney and brain fungal burden compared to those that succumbed to infection. The survival and fungal burden results for the control, fluconazole, and caspofungin groups are consistent with those we have previously reported demonstrating the overall reproducibility of this model (13, 14).
FIG 3.
Kidney (A) and brain (B) fungal burden (CFU/g) in mice with invasive candidiasis secondary to C. auris in the survival arm. CFU/g were measured on day 8 postinoculation after 7 days of therapy. n = 10 mice in the vehicle control and treatment groups; n = 5 mice in the 24 h control group. Black circles represent mice that succumbed to infection prior to day 21; gray circles represent mice that survived to the survival endpoint.
These results demonstrate that T-2307 may be effective against invasive infections caused by C. auris, as both in vitro and in vivo activity were observed against this emerging pathogen. The reductions in fungal burden observed in this study were less than those previously observed by our group against echinocandin-resistant C. albicans in immunocompetent mice (9), but were similar to those observed against echinocandin-resistant C. glabrata infections in neutropenic mice (8). This suggests that the in vivo efficacy of T-2307 may be influenced by host immune status, similar to what has been reported for other antifungals in murine models of infection (15, 16). It is unknown if efficacy could be improved with higher doses or more frequent administration. Additional studies are warranted, including pharmacokinetic/pharmacodynamic assessments.
ACKNOWLEDGMENTS
This project utilized preclinical services funded by the National Institute of Allergy and Infectious Diseases (NIAID), National Institutes of Health, Department of Health and Human Services under contracts no. HHS272201100018I and HHSN272201700039I - Task Orders A28 and A01, respectively, to the University of Texas Health Science Center at San Antonio.
Transparency declarations: N.P.W. has received research support to the UT Health San Antonio from Astellas, bioMérieux, Cidara, F2G, Merck, Pfizer, and Viamet, and has served on advisory boards for Astellas and Mayne Pharma, and as a speaker for Gilead. T.F.P. has received research grants to UT Health San Antonio from Cidara, and has served as a consultant for Astellas, Basilea, Gilead, Merck, Pfizer, Toyama, Viamet, and Scynexis. Y.F. and J.M. are employees of Fujifilm Toyama Chemical Co., Ltd.
REFERENCES
- 1.Lockhart SR, Etienne KA, Vallabhaneni S, Farooqi J, Chowdhary A, Govender NP, Colombo AL, Calvo B, Cuomo CA, Desjardins CA, Berkow EL, Castanheira M, Magobo RE, Jabeen K, Asghar RJ, Meis JF, Jackson B, Chiller T, Litvintseva AP. 2017. Simultaneous emergence of multidrug-resistant Candida auris on 3 continents confirmed by whole-genome sequencing and epidemiological analyses. Clin Infect Dis 64:134–140. doi: 10.1093/cid/ciw691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Jeffery-Smith A, Taori SK, Schelenz S, Jeffery K, Johnson EM, Borman A, Candida auris Incident Management Team, Manuel R, Brown CS. 2018. Candida auris: a review of the literature. Clin Microbiol Rev 31:e00029-17. doi: 10.1128/CMR.00029-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chowdhary A, Sharma C, Meis JF. 2017. Candida auris: a rapidly emerging cause of hospital-acquired multidrug-resistant fungal infections globally. PLoS Pathog 13:e1006290. doi: 10.1371/journal.ppat.1006290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sharma C, Kumar N, Pandey R, Meis JF, Chowdhary A. 2016. Whole genome sequencing of emerging multidrug resistant Candida auris isolates in India demonstrates low genetic variation. New Microbes New Infect 13:77–82. doi: 10.1016/j.nmni.2016.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vallabhaneni S, MSD, Kallen A, Tsay S, Chow N, Welsh R, Kerins J, Kemble SK, Pacilli M, Black SR, Landon E, Ridgway J, Palmore TN, Zelzany A, Adams EH, Quinn M, Chaturvedi S, Greenko J, Fernandez R, Southwick K, Furuya EY, Calfee DP, Hamula C, Patel G, Barrett P, Lafaro P, Berkow EL, Moulton-Meissner H, Noble-Wang J, Fagan RP, Jackson BR, Lockhart SR, Litvintseva AP, Chiller TM. 2016. Investigation of the first seven reported cases of Candida auris, a globally emerging invasive, multidrug-resistant fungus - United States, May 2013-August 2016. MMWR Morb Mortal Wkly Rep 65:1234–1237. doi: 10.15585/mmwr.mm6544e1. [DOI] [PubMed] [Google Scholar]
- 6.Mitsuyama J, Nomura N, Hashimoto K, Yamada E, Nishikawa H, Kaeriyama M, Kimura A, Todo Y, Narita H. 2008. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine. Antimicrob Agents Chemother 52:1318–1324. doi: 10.1128/AAC.01159-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shibata T, Takahashi T, Yamada E, Kimura A, Nishikawa H, Hayakawa H, Nomura N, Mitsuyama J. 2012. T-2307 causes collapse of mitochondrial membrane potential in yeast. Antimicrob Agents Chemother 56:5892–5897. doi: 10.1128/AAC.05954-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wiederhold NP, Najvar LK, Fothergill AW, Bocanegra R, Olivo M, McCarthy DI, Fukuda Y, Mitsuyama J, Patterson TF. 2016. The novel arylamidine T-2307 demonstrates in vitro and in vivo activity against echinocandin-resistant Candida glabrata. J Antimicrob Chemother 71:692–695. doi: 10.1093/jac/dkv398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wiederhold NP, Najvar LK, Fothergill AW, Bocanegra R, Olivo M, McCarthy DI, Kirkpatrick WR, Fukuda Y, Mitsuyama J, Patterson TF. 2015. The novel arylamidine T-2307 maintains in vitro and in vivo activity against echinocandin-resistant Candida albicans. Antimicrob Agents Chemother 59:1341–1343. doi: 10.1128/AAC.04228-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abe M, Nakamura S, Kinjo Y, Masuyama Y, Mitsuyama J, Kaku M, Miyazaki Y. 2019. Efficacy of T-2307, a novel arylamidine, against ocular complications of disseminated candidiasis in mice. J Antimicrob Chemother 74:1327–1332. doi: 10.1093/jac/dkz020. [DOI] [PubMed] [Google Scholar]
- 11.Nishikawa H, Fukuda Y, Mitsuyama J, Tashiro M, Tanaka A, Takazono T, Saijo T, Yamamoto K, Nakamura S, Imamura Y, Miyazaki T, Kakeya H, Yamamoto Y, Yanagihara K, Mukae H, Kohno S, Izumikawa K. 2017. In vitro and in vivo antifungal activities of T-2307, a novel arylamidine, against Cryptococcus gattii: an emerging fungal pathogen. J Antimicrob Chemother 72:1709–1713. doi: 10.1093/jac/dkx020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Clinical and Laboratory Standards Institute. 2008. Reference method for broth dilution antifungal susceptibility testing of filamentous fungi: approved standard 2nd ed Clinical and Laboratory Standards Institute, Wayne, Pennsylvania. [Google Scholar]
- 13.Wiederhold NP, Lockhart SR, Najvar LK, Berkow EL, Jaramillo R, Olivo M, Garvey EP, Yates CM, Schotzinger RJ, Catano G, Patterson TF. 2019. The fungal Cyp51-specific inhibitor VT-1598 demonstrates in vitro and in vivo activity against Candida auris. Antimicrob Agents Chemother 63:e02233-18. doi: 10.1128/AAC.02233-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wiederhold NP, Najvar LK, Shaw KJ, Jaramillo R, Patterson H, Olivo M, Catano G, Patterson TF. 2019. Efficacy of delayed therapy with fosmanogepix (APX001) in a murine model of Candida auris invasive candidiasis. Antimicrob Agents Chemother 63:e01120-19. doi: 10.1128/AAC.01120-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gumbo T, Drusano GL, Liu W, Ma L, Deziel MR, Drusano MF, Louie A. 2006. Anidulafungin pharmacokinetics and microbial response in neutropenic mice with disseminated candidiasis. Antimicrob Agents Chemother 50:3695–3700. doi: 10.1128/AAC.00507-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wiederhold NP, Najvar LK, Bocanegra R, Kirkpatrick WR, Patterson TF. 2012. Comparison of anidulafungin’s and fluconazole’s in vivo activity in neutropenic and non-neutropenic models of invasive candidiasis. Clin Microbiol Infect 18:E20–3. doi: 10.1111/j.1469-0691.2011.03712.x. [DOI] [PubMed] [Google Scholar]