Abstract
Background
The incidence and prognosis of coronary slow-flow (CSF) and no-reflow phenomenon (NRP) in patients with coronary chronic total occlusion (CTO) who underwent percutaneous coronary intervention (PCI) remain unclear.
Methods
This single-center prospective study aimed to investigate the incidence of CSF/NRP during CTO interventional therapy, determine predictors of CSF/NRP, and evaluate its effect on patient outcomes.
Results
In this study, 552 patients with CTO who underwent PCI were included. CSF/NRP occurred in 16.1% of them. They had higher incidences of diabetes mellitus (53.9% vs 36.3%, p=0.002) and hypertension (50.6% vs 37.1%, p=0.018) and a lower incidence of retrograde filling grade >2 (34.8% vs 47.1%, p=0.036). Patients with CSF/NRP had a higher neutrophil ratio (55.6±19.4 vs 52.4±18.3, p=0.038) and levels of low-density lipoprotein (LDL; 3.0±0.8 vs 2.8±0.6, p=0.029), fasting glucose (FG; 8.3±1.3 vs 6.8±1.1, p=0.005), uric acid (332.6±82.9 vs 308.2±62.8, p=0.045), and high-sensitivity C-reactive protein (Hs-CRP; 9.8±4.8 vs 7.3±3.9, p=0.036). A multivariate logistic regression analysis revealed that diabetes mellitus (odds ratio [OR], 1.962; 95% confidence interval [CI]: 1.198–2.721; p=0.042), mean platelet volume (MPV; OR,1.284; 95% CI, 1.108–1.895; p=0.046), LDL cholesterol (LDL-C; OR, 1.383; 95% CI, 1.105–2.491; p=0.036), FG (OR, 2.095; 95% CI, 1.495–2.899; p=0.018), Hs-CRP(OR, 2.218; 95% CI, 1.556–3.519; p=0.029), and retrograde filling of grade >2 (OR, 0.822; 95% CI, 0.622–0.907; p=0.037) were independent predictors of CSF/NRP in CTO patients who underwent PCI. Kaplan-Meier analysis revealed that the patients in the CSF/NRP group had a significantly lower cumulative major cardiac and cerebrovascular events (MACCE)-free survival than those in the non-CSF/NRP group (p<0.0001).
Conclusion
Of the patients with CTO who underwent PCI, 16.1% developed CSF/NRP and had a significantly lower cumulative MACCE-free survival rate. Diabetes mellitus; higher levels of MPV, LDL-C, FG, and Hs-CRP; and a lower incidence of retrograde filling grade >2 were independent predictors of CSF/NRP in CTO patients who underwent PCI. Thus, they can be used for risk stratification.
Keywords: coronary slow-flow, no-reflow phenomenon, coronary chronic total occlusion, PCI, prognosis
Introduction
Percutaneous coronary intervention (PCI) plays a crucial role in the management of coronary artery disease, especially in the setting of acute ST-elevation myocardial infarction (STEMI). However, even if complete revascularization is achieved, adequate reperfusion in the myocardium as demonstrated on angiography is not necessarily maintained.1,2 Previous studies showed that the prevalence of CSF/NRP in all patients who underwent PCI was 2–3.2%.3–5 Studies also confirmed that CSF/NRP occurred in a significant proportion of patients with STEMI during emergency PCI with an incidence of 30–40%.6–8 Increasing evidence has suggested that CSF/NRP is associated with PCI-related myocardial infarction, in-hospital mortality, and a poor prognosis.4,9,10
CTO is defined as thrombolysis in myocardial infarction (TIMI) grade 0 flow with a 3-month duration documented angiographically or clinically defined.11 Previous studies showed that approximately 20% of patients with coronary heart disease have at least one vascular CTO lesion.11 Accumulating evidence has confirmed that successful revascularization can effectively improve myocardial ischemia and relieve angina,12 improve left ventricular function,13 and improve clinical outcomes14,15 in patients with CTO. However, a higher risk of CTO-PCI, longer procedure duration, and larger number of implanted stents made it different from routine interventional therapy, and the incidence of CSF/NRP and its effects on prognosis remain unclear.
This study sought to investigate the incidence of CSF/NRP in patients with CTO who underwent PCI, determine the predictors of CSF/NRP, and assess its effects on patient outcomes.
Methods
Study Design and Patients
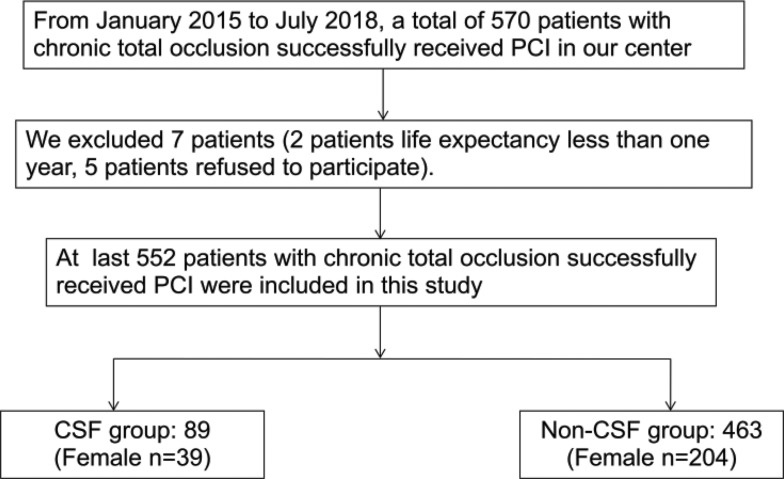
In this study, patients with a confirmed diagnosis of CTO were consecutively selected between January 2015 and May 2018 at our hospital. The exclusion criteria were as follows: current pregnancy; known allergy to aspirin, clopidogrel, or ticagrelor; refusal to accept interventional therapy or no stent implantation; life expectancy <1 year; and refusal to participate in the trial. CSF/NRP was defined as TIMI grade ≤2 by two experienced cardiologists (Ai-jie Hou and Bo Luan).A total of 552 patients were enrolled in the study, including 89 with CSF/NRP and 463 with normal blood flow (Figure 1).
Figure 1.
Study profile.
Abbreviations: PCI, percutaneous coronary intervention; CSF, coronary slow-flow.
The radial artery was the routine access point, but the physician determined the access as needed. During the procedure, a standard dose of unfractionated heparin 100 IU/kg was used as anticoagulant therapy, and unfractionated heparin 2000 IU was added every hour. The use of glycoprotein IIb/IIIa inhibitor (GPI) was left to the physician’s discretion. The study was approved by the Institutional Review Board of the People’s Hospital of Liaoning Province and complied with the Declaration of Helsinki. All patients signed informed consent before participation.
Study end points
The primary study end point was a composite of major cardiac and cerebrovascular events (MACCEs), including cardiac death, target vessel revascularization (TVR), stent thrombosis, nonfatal myocardial infarction (MI), PCI-associated MI (during the hospitalization period), and nonfatal stroke within 1 year of follow-up. MI was defined as the presence of pathological Q waves in at least two consecutive leads, or without a pathological Q wave, the markers of myocardial injury increased to more than twice the upper limit of normal but was not related to PCI or bypass.16 PCI-related MI was defined as an elevated cardiac troponin T (cTn) value to more than five times the 99th percentile of cTn level in patients with normal baseline values.16
Statistical Analysis
The Statistical Package for the Social Sciences for Windows version 20 (IBM SPSS Inc., Chicago, IL, USA) was used for the statistical analysis. Normally distributed continuous data are described as mean±standard deviation, while non-normally distributed data are shown as median (range). Categorical variables are shown as number and percentage. To compare continuous variables, Student’s t test or the Mann–Whitney U-test was used. To compare categorical variables, the chi-square test and Fisher’s exact test were used in the case of sparse data. Kaplan-Meier graphs were used to assess survival without MACCEs. All tests were two-sided, and p values <0.05 were considered significant.
Results
A total of 552 CTO patients who underwent PCI were included in this study (Figure 1). CSF/NRP occurred in 16.1% of the patients. The patients with CSF/NRP had higher incidences of diabetes mellitus (53.9% vs 36.3%, p=0.002) and hypertension (50.6% vs 37.1%, p=0.018) and a lower rate of retrograde filling of grade >2 (34.8% vs 47.1%, p=0.036; Tables 1 and 2). The patients with CSF/NRP had a higher neutrophil ratio (55.6±19.4 vs 52.4±18.3, p=0.038) and higher levels of low-density lipoprotein (LDL; 3.0±0.8 vs 2.8±0.6, p=0.029), FG (8.3± 1.3 vs 6.8 ±1.1, p=0.005), uric acid (332.6±82.9 vs 308.2±62.8, p=0.045), and high-sensitivity C-reactive protein (Hs-CRP; 9.8±4.8 vs 7.3±3.9, p=0.036; Table 2).
Table 1.
Clinical Characteristics of the Study Population
| Variable | CSF/NRP Group (n=89) | Non- CSF/NRP Group (n=463) | Pvalue |
|---|---|---|---|
| Age(years) | 64.5±7.9 | 65.2±8.1 | 0.854 |
| Sex (female), n(%) | 39(43.8%) | 204(44.1%) | 1 |
| Body mass index (kg/m2) | 22.8±3.5 | 23.1±3.7 | 0.628 |
| Diabetes mellitus, n(%) | 48(53.9%) | 168(36.3%) | 0.002 |
| Hypertension, n(%) | 45(50.6%) | 172(37.1%) | 0.018 |
| Current smoker, n(%) | 32(36.0%) | 142(30.7%) | 0.383 |
| Previous MI, n(%) | 41(46.1%) | 212(45.8%) | 1 |
| Previous ischemic CVA, n(%) | 9(10.1%) | 41(8.9%) | 0.840 |
| LVEF(%) | 40.8±6.2 | 40.2±6.4 | 0.328 |
| NYHA 2–3 on admission, n(%) | 62(69.7%) | 362(78.2%) | 0.099 |
| eGFR, mL/min/1.73 mm2 | 60.5±22.8 | 61.1±23.6 | 0.462 |
| ACEIs/ARBs, n(%) | 42(47.2%) | 231(49.9%) | 0.646 |
| Beta-blockers, n(%) | 40(44.9%) | 229(49.5%) | 0.488 |
| Statin, n(%) | 86(96.6%) | 454(98.1%) | 0.421 |
| PPI, n(%) | 84(94.4%) | 449(97.0%) | 0.210 |
| WBC count (109/L) | 6.8±1.9 | 6.7±1.7 | 0.764 |
| MPV(fL) | 10.1±0.8 | 10.4±0.9 | 0.195 |
| Neutrophil ratio (%) | 55.6±19.4 | 52.4±18.3 | 0.038 |
| PDW (%) | 11.9±1.8 | 11.2±1.5 | 0.214 |
| HDL-C (mmol/L) | 1.2±0.4 | 1.1±0.3 | 0.29 |
| LDL-C (mmol/L) | 3.0±0.8 | 2.8±0.6 | 0.029 |
| TG (mmol/L) | 1.6±0.9 | 1.5±0.8 | 0.682 |
| TC (mmol/L) | 4.6±0.9 | 4.4±0.8 | 0.692 |
| FG (mmol/L) | 8.3± 1.3 | 6.8±1.1 | 0.005 |
| Creatinine (mg/dL) | 58.4±11.9 | 57.9±11.6 | 0.328 |
| UA (mmol/L) | 332.6±82.9 | 308.2±62.8 | 0.045 |
| Hs-CRP (mmol/L) | 9.8±4.8 | 7.3±3.9 | 0.037 |
Note: Bold values are statistically significant (p< 0.05).
Abbreviations: MI, myocardial infarction; CVA, cerebrovascular accident; GI, gastrointestinal; LVEF, left ventricular ejection fraction; NYHA, New York Heart Association; eGFR, estimated glomerular filtration rate; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin receptor blockers; PPI, proton pump inhibitor; MPV,mean platelet volume; PDW, platelet distribution width; HDL, high-density lipoprotein; LDL, low-density lipoprotein; TG, triglyceride; TC, total cholesterol; FG, fasting glucose; UA, uric acid; Hs-CRP,high-sensitivity C-reactive protein.
Table 2.
Procedure-Related Characteristics of the Studied Patients
| Variables | CSF/NRP Group (n=89) | Non- CSF/NRP Group (n=463) | Pvalue |
|---|---|---|---|
| Procedural access | |||
| Radial | 32(36.0%) | 146(31.5%) | 0.242 |
| Femoral | 22(24.7%) | 118(25.5%) | 0.896 |
| Radial and femoral | 35(39.3%) | 199(43.0%) | 0.559 |
| Total amount of contrast(mL) | 245.8±123.6 | 239.4±119.7 | 0.681 |
| Total time of procedure (min) | 118.4±56.7 | 116.5±55.8 | 0.395 |
| LAD occlusion, n (%) | 23(25.8%) | 126(27.2%) | 0.797 |
| LCX occlusion, n (%) | 22(24.7%) | 118(25.5%) | 0.896 |
| RCA occlusion, n (%) | 47(52.8%) | 225(48.6%) | 0.489 |
| Occlusion lesion length, mm | 56.8±24.7 | 57.4±23.6 | 0.628 |
| Retrograde filling > grade 2 | 31(34.8%) | 218(47.1%) | 0.036 |
| Reverse wire technique | 38(42.7%) | 186(40.2%) | 0.724 |
| IABP, n(%) | 6(6.7%) | 36(7.8%) | 0.813 |
| IVUS, n(%) | 17(19.1%) | 102(22.0%) | 0.577 |
| Number of stents per patient | 2.6±1.8 | 2.7±1.9 | 0.684 |
| Stent length (mean, mm) | 62.8±22.5 | 63.4±24.3 | 0.892 |
| Glycoprotein IIb/IIIa receptor inhibitor, n(%) | 42(47.2%) | 252(54.4%) | 0.246 |
| LMWH (%) | 29(32.6%) | 135(29.2%) | 0.528 |
Abbreviations: LAD, left anterior descending artery; LCX, left circumflex coronary artery; RCA, right coronary artery; IABP, intra-aortic balloon pump; IVUS, intravascular ultrasonography; TIMI, thrombolysis in myocardial infarction; LMWH, low-molecular-weight heparin.
Age, female sex, smoking, body mass index, diabetes mellitus, hypertension, mean platelet volume (MPV), neutrophil ratio, platelet distribution width (PDW), LDL cholesterol (LDL-C), fasting glucose (FG), uric acid, Hs-CRP, retrograde filling of grade >2, and GPI were analyzed in univariate analysis. Diabetes mellitus, hypertension, MPV, neutrophil ratio, PDW, LDL-C, FG, Hs-CRP, and retrograde filling of grade >2 were included in the multivariate logistic regression analysis. Diabetes mellitus (odds ratio [OR], 1.962; 95% confidence interval [CI], 1.198–2.721; p=0.042), MPV(OR, 1.284; 95% CI, 1.108–1.895, p = 0.046), LDL-C (OR, 1.383; 95% CI, 1.105–2.491, p = 0.036), FG(OR, 2.095; 95% CI, 1.495–2.899, p = 0.018), Hs-CRP (OR, 2.218; 95% CI, 1.556–3.519; p = 0.029), and retrograde filling of grade >2 (OR, 0.822; 95% CI, 0.622–0.907; p = 0.037) were independent predictors of CSF/NRP in patients with CTO treated with PCI (Table 3).
Table 3.
Univariate and Stepwise Multivariate Logistic Regression Analysis of Risk Factors of CSF/NRP
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P value | OR | 95% CI | P value | |
| Age | 1.000 | 0.729–1.213 | 0.782 | – | – | – |
| Female sex | 0.892 | 0.658–1.869 | 0.692 | – | – | – |
| Current smoking | 1.124 | 0.732–1.685 | 0.358 | – | – | – |
| Body mass index | 1.097 | 0.912–2.289 | 0.529 | – | – | – |
| Diabetes mellitus | 1.895 | 1.271–2.826 | 0.035 | 1.962 | 1.198–2.721 | 0.042 |
| Hypertension | 1.684 | 1.118–1.959 | 0.029 | 1.375 | 1.122–1.899 | 0.182 |
| PDW | 1.321 | 1.09–1.59 | 0.029 | 1.204 | 1.181–1.688 | 0.089 |
| Neutrophil ratio | 1.294 | 1.112–1.985 | 0.034 | 1.182 | 1.091–1.979 | 0.132 |
| MPV | 1.307 | 1.092–1.995 | 0.026 | 1.284 | 1.108–1.895 | 0.046 |
| LDL-C | 1.422 | 1.166–2.521 | 0.019 | 1.383 | 1.105–2.491 | 0.036 |
| FG | 2.114 | 1.562–3.095 | 0.028 | 2.095 | 1.495–2.899 | 0.018 |
| UA | 1.186 | 0.897–1.568 | 0.624 | – | – | – |
| Hs-CRP | 2.323 | 1.629–3.592 | 0.012 | 2.218 | 1.556–3.519 | 0.029 |
| Retrograde filling > grade 2 | 0.782 | 0.556–0.925 | 0.025 | 0.822 | 0.622–0.907 | 0.037 |
| GPI | 1.195 | 0.893–1.688 | 0.329 | – | – | – |
Note: Bold values are statistically significant (p< 0.05).
Abbreviations: MPV, mean platelet volume; PDW, platelet distribution width; LDL, low-density lipoprotein; FG, fasting glucose; UA, uric acid; Hs-CRP, high-sensitivity C-reactive protein; GPI,glycoprotein IIb/IIIa receptor inhibitor.
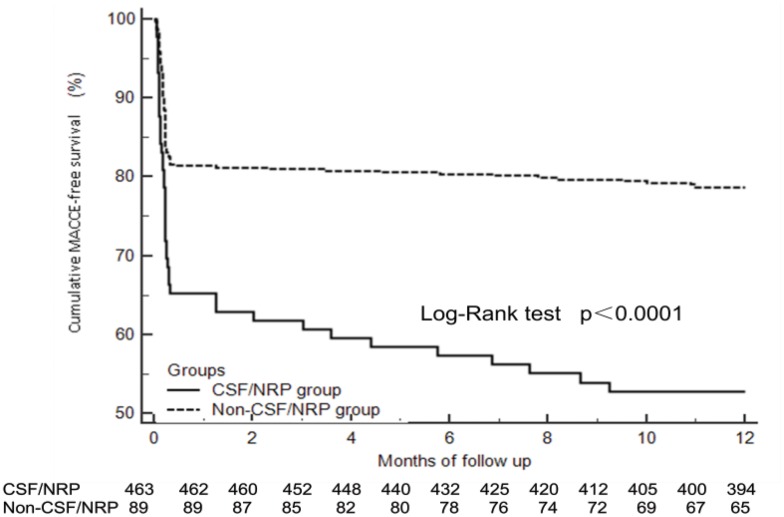
During the hospitalization period, the incidence of PCI-related MI was significantly higher in the CSF/NRP group than in the non-CSF/NRP group (32.6% vs 14.9%, p<0.001), and the incidence of MACCEs was also significantly higher in the CSF/NRP group than in the non-CSF/NRP group (37.1% vs 18.6%, p<0.001). At 1-year follow-up, the incidences of TVR (22.0% vs 5.8%, p<0.001) and MACCE (47.2% vs 21.4%, p<0.001) were significantly higher in the CSF/NRP group than in the non-CSF/NRP group (Table 4). Kaplan-Meier analysis revealed that the cumulative MACCE-free survival was significantly lower in the CSF/NRP group than in the non-CSF/NRP group (p<0.0001; Figure 2).
Table 4.
MACCE During Hospital Stay and at 12-Monthfollow-Up
| Variable | CSF/NRP Group (n=89) | Non- CSF/NRP Group (n=463) | Pvalue |
|---|---|---|---|
| MACCE during hospitalization | |||
| Cardiogenic death, n(%) | 1(1.1%) | 4(0.9%) | 1 |
| AMI, n(%) | 1(1.1%) | 6(1.3%) | 1 |
| PCI-relatedMI, n(%) | 29(32.6%) | 69(14.9%) | <0.001 |
| Stent thrombosis, n(%) | 1(1.1%) | 7(1.5%) | 1 |
| Stroke, n(%) | 0 | 2(0.4%) | 1 |
| TVR, n(%) | 3(3.4%) | 13(2.8%) | 0.731 |
| Overall MACCE, n(%) | 33(37.1%) | 86(18.6%) | <0.001 |
| MACCE at12-month follow-up | |||
| Cardiogenic death, n(%) | 1(1.1%) | 6(1.3%) | 1 |
| AMI, n(%) | 2(2.2%) | 16(3.5%) | 0.751 |
| Stent thrombosis, n(%) | 6(6.7%) | 22(4.8%) | 0.430 |
| Stroke, n(%) | 1(1.1%) | 3(0.6%) | 0.506 |
| TVR, n(%) | 11(22.0%) | 27(5.8%) | <0.001 |
| Overall MACCE, n(%) | 42(47.2%) | 99(21.4%) | <0.001 |
Note: Bold values are statistically significant (p< 0.05).
Abbreviations: MACCE, major adverse cardiac and cerebral events; PCI, percutaneous coronary intervention; TVR, target vessel revascularization; AMI, acute myocardial infarction.
Figure 2.
Cumulative MACCE-free survival.
Discussion
This study was the first to investigate the incidence, predictive factors, and prognosis of CSF/NRP inpatients with CTO who successfully underwent PCI. The study confirmed that16.1% of patients with CTO who underwent PCI developed CSF, which had a significantly lower cumulative MACCE-free survival rate. Diabetes mellitus; higher MPV and levels of LDL-C, FG, and Hs-CRP; and a lower retrograde filling level (grade >2) were independent predictors of CSF/NRP in patients with CTO who underwent PCI. Thus, these factors can be used for risk stratification.
The etiology of CSF/NRP is complex and still not fully understood. Its possible mechanisms include endothelial dysfunction, microvascular ischemia and edema, embolization, and reperfusion injury.17 Currently, the incidence of CSF/NRP varies widely among different study populations. Most of these studies were in STEMI patients. Some recent studies demonstrated that the proportion of NRP in all PCI patients was2–3.2%.3–5 Studies also confirmed that CSF/NRP occurred at a prevalence of 30–40% in STEMI patients during emergency PCI.6–8 The present study showed that the incidence of CSF/NRP in such patients was as high as 16.1%. We speculated that the guide wire may penetrate the proximal or distal fibrous cap during antegrade interventional therapy or the retrograde wire technique, causing the release of lipids from atherosclerotic plaques that may lead to the occlusion of distal blood vessels, especially micro vessels.
Although many researchers have proposed several predictors of CSF/NRP,18 their predictive value in different study populations requires further investigation. Studies have confirmed that >50% of patients with STEMI have abnormal glucose metabolism. Although early revascularization can achieve significant clinical results, patients with abnormal glucose metabolism have poorer microvascular perfusion and a higher risk of developing CSF/NRP.19 The present study also found that diabetes mellitus and a high fasting blood glucose level are independent predictors of CSF/NRP as shown in previous studies. The present study demonstrated that a high LDL-C level is associated with CSF/NRP. Previous studies confirmed that hypercholesterolemia is associated with endothelial dysfunction because it reduces the bioavailability of vascular nitric oxide.20 Pretreatment with statins effectively prevents CSF/NRP,21 reduces the incidence of postoperative MI,22 and improves myocardial perfusion after PCI.23 These findings also indirectly confirm that hyperlipidemia is involved in the pathogenesis of CSF/NRP.
Studies have shown that a higher inflammation status plays an important role in the development of CSF/NRP during emergency PCI for STEMI.24 Oduncuet al25 showed that patients with NRP have a higher baseline Hs-CRP level. The independent relationship between Hs-CRP and NRP development, which was discovered in this study, has also been reported previously.26 The present study confirmed that MPV was significantly elevated in the CSF/NRP group and is an independent predictor of CSF/NRP. We suggest that MPV is an indicator of platelet activation that plays an important role in the pathophysiology of cardiovascular disease; previous studies also confirmed its association with CSF/NRP.18 We also found that patients with better collateral circulation had a lower incidence of CSF/NRP, presumably because the collateral circulation provides better myocardial perfusion to occluded vessels and its endothelial and microvascular functions are relatively less damaged; thus, the incidence of CSF/NRP is lower because reperfusion-related damage is less severe.
CSF/NRP is clinically important, as increasing evidence has suggested that it is associated with PCI-related MI, in-hospital mortality, and poor prognosis.4,9,10 In addition, Butler et al reported that transient CSF/NRP is even more common than persistent CSF/NRP and results in significantly increased 30-day morbidity and mortality rates.27 Our study found that CTO-PCI patients with CSF/NRPs had significantly increased incidences of perioperative MI, MACCEs during hospitalization and the 1-year follow-up period, and TVRat1-year follow-up. Their MACCE-free survival rate was significantly lower than that of non-CSF/NRP patients.
The CSF/NRP condition of CTO-PCI patients warrants additional attention due to its unique pathophysiology and complex interventional procedures. We recommend that these conditions be identified separately from acute MI. As this was a small-sample single-center study, some bias exists. In the future, a large-sample multicenter prospective randomized controlled trial is needed to validate our conclusions.
Conclusion
Of the patients with CTO who underwent PCI, 16.1% developed CSF/NRP and had a significantly lower cumulative MACCE-free survival rate. Diabetes mellitus; higher MPV, LDL-C, FG, and Hs-CRP levels; and a lower incidence of retrograde filling grade >2 were independent predictors of CSF/NRP in CTO patients who underwent PCI. Thus, these factors can be used for risk stratification.
Abbreviations
CSF, coronary slow-flow; NRP, no-reflow phenomenon; TIMI, thrombolysis in myocardial infarction; CTO, coronary chronic total occlusion; PCI, percutaneous coronary intervention; MACCE, major adverse cardiac and cerebral events; TVR, target vessel revascularization; GPI, glycoprotein IIb/IIIa inhibitor.
Ethics Approval and Informed Consent
The study had already been approved by Ethics Committee of the People’s Hospital of China Medical University and all subjects provided their informed, written consent before participation.
Data Sharing Statement
The data supporting the results in the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Stone GW, Cox D, Garcia E, et al. Normal flow (TIMI-3) before mechanical reperfusion therapy is an independent determinant of survival in acute myocardial infarction: analysis from the primary angioplasty in myocardial infarction trials. Circulation. 2001;104(6):636–641. doi: 10.1161/hc3101.093701 [DOI] [PubMed] [Google Scholar]
- 2.Ramjane K, Han L, Jin C. The diagnosis and treatment of the no-reflow phenomenon in patients with myocardial infarction undergoing percutaneous coronary intervention. Exp Clin Cardiol. 2008;13(3):121–128. [PMC free article] [PubMed] [Google Scholar]
- 3.Piana RN, Paik GY, Moscucci M, et al. Incidence and treatment of ‘no-reflow’ after percutaneous coronary intervention. Circulation. 1994;89(6):2514–2518. doi: 10.1161/01.CIR.89.6.2514 [DOI] [PubMed] [Google Scholar]
- 4.Resnic FS, Wainstein M, Lee MK, et al. No-reflow is an independent predictor of death and myocardial infarction after percutaneous coronary intervention. Am Heart J. 2003;145(1):42–46. doi: 10.1067/mhj.2003.36 [DOI] [PubMed] [Google Scholar]
- 5.Cenko E, Ricci B, Kedev S, et al. The no-reflow phenomenon in the young and in the elderly. Int J Cardiol. 2016;222:1122–1128. doi: 10.1016/j.ijcard.2016.07.209 [DOI] [PubMed] [Google Scholar]
- 6.Ito H, Maruyama A, Iwakura K, et al. Clinical implications of the ‘no reflow’ phenomenon: a predictor of complications and left ventricular remodeling in reperfused anterior wall myocardial infarction. Circulation. 1996;93(2):223–228. doi: 10.1161/01.CIR.93.2.223 [DOI] [PubMed] [Google Scholar]
- 7.Ito H, Okamura A, Iwakura K, et al. Myocardial perfusion patterns related to thrombolysis in myocardial infarction perfusion grades after coronary angioplasty in patients with acute anterior wall myocardial infarction. Circulation. 1996;93(11):1993–1999. doi: 10.1161/01.CIR.93.11.1993 [DOI] [PubMed] [Google Scholar]
- 8.Iwakura K, Ito H, Takiuchi S, et al. Alternation in the coronary blood flow velocity pattern in patients with no reflow and reperfused acute myocardial infarction. Circulation. 1996;94(6):1269–1275. doi: 10.1161/01.CIR.94.6.1269 [DOI] [PubMed] [Google Scholar]
- 9.Ramírez-Moreno A, Cardenal R, Pera C, et al. Predictors and prognostic value of myocardial injury following stent implantation. Int J Cardiol. 2004;97(2):193–198. doi: 10.1016/j.ijcard.2003.07.031 [DOI] [PubMed] [Google Scholar]
- 10.Yip HK, Chen MC, Chang HW, et al. Angiographic morphologic features of infarctrelated arteries and timely reperfusion in acute myocardial infarction: predictors of slow-flow and no-reflow phenomenon. Chest. 2002;122(4):1322–1332. doi: 10.1378/chest.122.4.1322 [DOI] [PubMed] [Google Scholar]
- 11.Stone GW, Kandzari DE, Mehran R, et al. Percutaneous recanalization of chronically occluded coronary arteries: a consensus document: part I. Circulation. 2005;112(15):2364–2372. doi: 10.1161/CIRCULATIONAHA.104.481283 [DOI] [PubMed] [Google Scholar]
- 12.Rossello X, Pujadas S, Serra A, et al. Assessment of inducible myocardial ischemia, quality of life, and functional status after successful percutaneous revascularization in patients with chronic total coronary occlusion. Am J Cardiol. 2016;117(5):720–726. doi: 10.1016/j.amjcard.2015.12.001 [DOI] [PubMed] [Google Scholar]
- 13.Hoebers LP, Claessen BE, Elias J, Dangas GD, Mehran R, Henriques JP. Meta-analysis on the impact of percutaneous coronary intervention of chronic total occlusions on left ventricular function and clinical outcome. Int J Cardiol. 2015;187:90–96. doi: 10.1016/j.ijcard.2015.03.164 [DOI] [PubMed] [Google Scholar]
- 14.Tomasello SD, Boukhris M, Giubilato S, et al. Management strategies in patients affected by chronic total occlusions: results from the Italian registry of chronic total occlusions. Eur Heart J. 2015;36(45):3189–3198. doi: 10.1093/eurheartj/ehv450 [DOI] [PubMed] [Google Scholar]
- 15.Hoye A, van Domburg RT, Sonnenschein K, Serruys PW. Percutaneous coronary intervention for chronic total occlusions: the thoraxcenter experience 1992–2002. Eur Heart J. 2005;26(24):2630–2636. doi: 10.1093/eurheartj/ehi498 [DOI] [PubMed] [Google Scholar]
- 16.Thygesen K, Alpert JS, White HD, et al. Universal definition of myocardial infarction. Circulation. 2007;116:2634–2653. doi: 10.1161/CIRCULATIONAHA.107.187397 [DOI] [PubMed] [Google Scholar]
- 17.Butler MJ, Chan W, Taylor AJ, Dart AM, Duffy SJ. Management of the no-reflow phenomenon. Pharmacol Ther. 2011;132(1):72–85. [DOI] [PubMed] [Google Scholar]
- 18.Wong DT, Puri R, Richardson JD, Worthley MI, Worthley SG. Myocardial ‘no-reflow’— diagnosis, pathophysiology and treatment. Int J Cardiol. 2013;167(5):1798–1806. doi: 10.1016/j.ijcard.2012.12.049 [DOI] [PubMed] [Google Scholar]
- 19.Collet JP, Montalescot G. The acute reperfusion management of STEMI in patients with impaired glucose tolerance and type 2 diabetes. Diab Vasc Dis Res. 2005;2(3):136–143. doi: 10.3132/dvdr.2005.021 [DOI] [PubMed] [Google Scholar]
- 20.Goodwill AG, Stapleton PA, James ME, D’Audiffret AC, Frisbee JC. Increased arachidonic acid-induced thromboxane generation impairs skeletal muscle arteriolar dilation with genetic dyslipidemia. Microcirculation. 2008;15(7):621–631. doi: 10.1080/10739680802308334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Iwakura K, Ito H, Kawano S, et al. Chronic pre-treatment of statins is associated with the reduction of the no-reflow phenomenon in the patients with reperfused acute myocardial infarction. Eur Heart J. 2006;27(5):534–539. doi: 10.1093/eurheartj/ehi715 [DOI] [PubMed] [Google Scholar]
- 22.Winchester DE, Wen X, Xie L, Bavry AA. Evidence of pre-procedural statin therapy a meta-analysis of randomized trials. J Am Coll Cardiol. 2010;56(14):1099–1109. doi: 10.1016/j.jacc.2010.04.023 [DOI] [PubMed] [Google Scholar]
- 23.Manfrini O, Pizzi C, Morgagni G, Fontana F, Bugiardini R. Effect of pravastatin on myocardial perfusion after percutaneous transluminal coronary angioplasty, Am. J Cardiol. 2004;93(11):1391–1393. doi: 10.1016/j.amjcard.2004.02.037 [DOI] [PubMed] [Google Scholar]
- 24.Kurtul A, Murat SN, Yarlioglues M, Duran M, Celik IE, Kilic A. Mild to moderate renal impairment is associated with no-refowphenomenonafter primary percutaneous coronary intervention in acute myocardial infarction. Angiology. 2015;66(7):644–651. doi: 10.1177/0003319714546738 [DOI] [PubMed] [Google Scholar]
- 25.Oduncu V, Tanalp AC, Erkol A, et al. Impact of chronic pre-treatment of statins on the level of systemic infammation and myocardial perfusion in patients undergoing primary angioplasty. Am J Cardiol. 2011;107(2):179–185. doi: 10.1016/j.amjcard.2010.09.008 [DOI] [PubMed] [Google Scholar]
- 26.Zhao Y, Yang J, Ji Y, et al. Usefulness of fibrinogen-to-albumin ratio to predict no-reflow and short-term prognosis in patients with ST-segment elevation myocardial infarction undergoing primary percutaneous coronary intervention. Heart Vessels. 2019;34(10):1600–1607. doi: 10.1007/s00380-019-01399-w [DOI] [PubMed] [Google Scholar]
- 27.Butler, M, Ajani, A, Andrianopoulos, N, et al. Predictors and outcomes of the no-reflow phenomenon. Heart Lung Circ. 17(S3):S176. [Google Scholar]