Abstract
Objective(s):
Cirrhotic cardiomyopathy is a complication of uncured cirrhosis which is associated with hyporesponsiveness of the heart to sympathetic stimulation. The enhancement of portal pressure, nitric oxide (NO) level, pro-inflammatory mediators and down-regulation of Suppressor of Cytokine Signaling 1 (SOCS1) are involved in this situations. The present study seeks to examine the beneficial effect of thalidomide on cirrhotic cardiomyopathy.
Materials and Methods:
The male rats were grouped as: Sham/saline, Sham/Thalidomide, Bile Duct Ligation (BDL)/saline and BDL/Thalidomide. BDL model of cirrhosis was used. In the treatment groups, thalidomide (200 mg/kg/day) was administrated by intragastrial gavage for 28 consecutive days, the chronotropic response was assessed in isolated atria by isoproterenol stimulation. Serum levels of NO, IL-6 and TNF-α hepatic level were evaluated. The intrasplenic pulp pressure (ISPP) as the portal pressure and histopathologic assessment were assessed. Real time RT-PCR was used for the evaluation of SOCS1 gene expression.
Results:
Our results showed that thalidomide administration could significantly increase the atrial chronotropic response in BDL animals. The increased level of portal pressure decreased by thalidomide in BDL animals. Thalidomide could ameliorate the histopathological conditions of BDL rats. Furthermore, the chronic treatment by this drug diminished the elevated levels of NO, TNF-α and IL-6 in BDL animals. On the other hand, hepatic SOCS1 expression was up-regulated by thalidomide treatment in this group.
Conclusion:
Thalidomide improves the chronotropic hyporesponsiveness of isolated atria in BDL. This effect is probably mediated by the inhibiting NO, TNF-α and IL-6 production, reducing portal pressure and increasing the expression of SOCS1.
Key Words: Cirrhotic cardiomyopathy, IL-6, SOCS1, Thalidomide, TNF-α
Introduction
Liver cirrhosis is defined by the end-stage of extended fibrosis, regenerative nodules and widespread inflammation (1). Cirrhotic cardiomyopathy is a cirrhosis complication which is characterized by impaired inotropic and chronotropic responsiveness to sympathetic stimulation (2). One of the main causes of cirrhotic cardiomyopathy is the hyperdynamic circulatory syndrome (HC) which is associated with portal hypertension (PTH), systemic and splanchnic NO overproduction. PTH in cirrhosis is caused by increased intrahepatic resistance (IHR). In response to this event, the over release of splanchnic NO and overall hypotension occur. To compensate for this condition, cardiac chronotropic increases that in turn induces the decline of heart function and myocardium contraction in the long term (3). On the other hand, the intensification and progression of liver fibrosis is related to the enhancement of TNF-α and IL-6 levels in the liver (4, 5). Furthermore, TNF-α plays a pivotal role in increasing NO in cirrhosis (6) and also in NO overproduction (7). Suppressor of Cytokine Signaling 1 (SOCS1) is a suppressor of cytokine signaling that has a role in the modulation of cytokine-mediated immune responses (8). Several studies have indicated that during hepatic chronic diseases and cirrhosis, the expression of SOCS1 decreases (9-11) and this expression diminishes along with cirrhosis progression (11). Isoproterenol is a potent agonist of β-adrenoceptors that has positive inotropic and chronotropic effects on the myocardium. These effects of β1-adrenoceptors are applied through G-protein coupled receptor and the rise of cAMP (12). Previous studies have exhibited that responsiveness to isoproterenol reduces in cirrhosis (3). Thalidomide (N-α-phthalimidoglutarimid) is an old drug with immunomodulatory and anti-inflammatory effects that are used in the treatment of diseases with TNF-α involvement (13). One of the main effects of thalidomide in cirrhosis is mediated by the reduction of hepatic fibro- inflammation. This effect is probably exerted by the inhibition of collagen deposition and also anti-inflammatory effects that are mediated by the prevention of TNF-α and IL-6 production (14). Chong et al. reported that thalidomide could decrease liver fibrosis by the inhibition of TNF-α, and activate hepatic stellate cells in dimethylnitrosamine-intoxicated rats (14). In another study, Yang et al. noted that thalidomide could decrease portal vein pressure (PVP) through the reduction of TNF-α level in BDL rats (15). Regarding all of the above-mentioned evidence and given the insufficient number of studies concerning the effects of thalidomide in cirrhotic cardiac disorders, we decided to examine the possible mechanism of action as well as the effect of thalidomide on the chronotropic hyporesponsiveness of isolated atria from BDL rat to β-adrenergic stimulation.
Materials and Methods
All materials were purchased from Sigma (Pool, UK), unless indicated in the text, otherwise. All animal procedures were in agreement with “Guide for the Care and Use of Laboratory Animals” (NIH US publication No 85-23, revised1985) recommendations.
Animals
Male Wistar albino rats (body weight around 250–280 g) were used for this study. Animals were obtained from the Department of Pharmacology (Tehran University of Medical Sciences). Animals were housed in a normal temperature (around 22 °C), controlled humidity and light-dark cycle of 12 hr. Food and tap water provided ad libitum too.
The animals were randomly distributed into four groups, namely; sham-operated +saline group (sham/Saline), bile duct ligated cirrhotic+ saline group (BDL/Saline), sham-operated + Thalidomide group (sham/Thl), bile duct ligated+ Thalidomide (BDL/Thl). Thalidomide was administrated at dose 200 mg/kg/day by intragastrically gavage route for 28 days, beginning from the day of surgery. Each group consisted of 7 rats. All studies related to animals were done in agreement with the standards guidelines of animal care and the Council Directive of Laboratory Animals, Tehran University of Medical Sciences, Iran.
Cirrhosis induction
For the general anaesthesia, ketamine 100 mg/kg and xylazine 8 mg/kg used intraperitoneally. Cirrhosis was induced via double ligation-section of the common bile duct, consistent with the previously described method (16) . The sham procedure involved a similar operation but without ligation or cutting of the bile duct. All animals were anesthetized and sacrificed at day 28 post operation when cirrhosis had established.
Preparations of isolated atria
For the study of cardiac chronotropic response to adrenergic stimulation, isolated beating left and right atria were used. Hence, after general anaesthesia by ketamine 100 mg/kg and xylazine 0.05 mg/kg, intraperitoneally, the rat atria were isolated in cold oxygenated physiological salt solution; it has been suspended in a 20 ml organ bath chamber under isometric tension of 1 g force. The composition of physiological solution in millimolars was as follows: NaCl, 112; KCl, 5; CaCl2, 1.8; MgCl2, 1; NaH2PO4, 0.5; KH2PO4, 0.5; NaHCO3, 25; glucose, 10; and EDTA, 0.004 and pH 7.35-7.45 that aerated with 95% O2 and 5% CO2.The right atrium was used for recording the spontaneous atrial beating, that was stimulated by cumulative concentrations of isoproterenol from 10-10 to 10-6mol/l. Tensions caused by atria beating, converted to electrical signals by Power Lab system (ADInstruments Pty Ltd. Australia). Recording, analysis, and display of electronic data, did by LabChart v8.1.11 software (ADInstruments Pty Ltd).
Assessment of plasma nitrite/nitrate concentrations:
Plasma nitrate and nitrite levels were measured as indicators of NO production The measurements were performed according to the method described previously (17). Plasma samples were deproteinized by centrifugation through a 30-kDa molecular weight filter (Centricon Millipore) at 14,000 rpm, for 1.5–3 hr at 4 °C. After filling the plate with samples (100 μl), adding of a saturated solution of VCl3 (100 μl) to each well was quickly followed by addition of the Griess reagents (50 μl each). Sulfanilamide and naphthylethylenediamine dihydrochloride were used for the preparation of Griess reagents. The plate was incubated at 37 °C for 30 min and then absorbance at 540 nm was calculated using a standard plate reader (800™ TS Absorbance Reader, BioTek Instruments). Fresh standard solutions of nitrate were included in each test.
Pathological examination
Hepatic and serum level of TNF-α, and hepatic IL-6, levels were quantified with ELISA kits following the manufacturer’s directions (Quantikine Elisa) Hepatic amount of TNF-α was calculated per 1 g of wet tissue in 5 ml of phosphate buffer solution (PBS) and expressed as pg/g of liver tissue. The minimum detectable concentration of TNF-α is 1 pg/ml.
Measurements of IL-6, TNF- α
Hepatic and serum level of TNF-α, and hepatic IL-6, were quantified with ELISA kits following the manufacturer’s directions hepatic amount of TNF-α was calculated per 1 g of wet tissue in 5 ml of phosphate buffer solution (PBS) and expressed as pg/g of liver tissue. The minimum detectable concentration of TNF-α is 1 pg/ml.
Assessment of intrasplenic pulp pressure (ISPP)
As an index of portal vein pressure, ISPP was evaluated according to previously described techniques (18). Concisely, under general anesthesia (ketamine 100 mg/kg and xylazine 8 mg/kg IP), the spleen was exposed after the abdominal cavity opening; then a 20 gauge needle insert into the splenic parenchyma and intrasplenic pressure was evaluated (18). The needle was related to a pressure transducer that was attached to a Power Lab system (ADInstruments Pty Ltd.Australia). The external zero reference point was placed at the middle part of animal. The pressure reading was accepted when a stable recording was attained. Splenic pulp pressure calibration was performed using an upright tube filled with saline solution and is expressed in mm H2O.
Measurement of SOCS1 expression
Rat liver samples were removed and quickly dipped in liquid nitrogen. Total RNA was isolated using RNeasy Fibrous Tissue Mini Kit (QIAGEN) in accordance to manufacturer’s guidelines. Genomic DNA was removed with DNase (Promega). First strand cDNA was created with deoxyribonuclease (DNase)-treated RNA, random hexamer primer (p (dN) 6) and ribonuclease-free water, and then warmed at 70 °C for 5 min and placed on ice. Ribonuclease inhibitor, reverse transcriptase, and deoxynucleoside triphosphates were added and incubated at 42 °C for 1 hr. The primer sequences for the genes and internal standard were as follows (19):Rat SOCS1 (product size: 160): forward 5´-AGCTGTGTCGCCAGCGCATC-3´; SOCS1: reverse 5´-CAGAAGTGGGAGGCATCTCA-3´(20); Rat β-actin, forward: 5’-TCCTGGGTATGGAATCCTG-3’’, reverse: 5’- CTTCTGCATCCTGTCAGCAA-3’( product size: 190 bp).For real-time PCR we used of 1.0 µl of cDNA template, 100 nM each of forward and reverse oligonucleotide primers, and 10.0 µl of optimized PCR Mastermix in a total reaction volume of 20.0 µl, with a Rotor-Gene QRT-PCR cycler, followed by 38 cycles of the annealing step (45 s at 60 °C) and an extension–elongation step (1 min at 72 °C). For analyzing the data, we used the competitive critical threshold (ΔΔct) method, in which the SOCS1 RNA was adjusted to β-Actin.
Statistical analysis
Statistical analyses were carried out using GraphPad Prism 5.0 software (GraphPad Inc., SanDiego, CA, USA). The results are presented as mean±SEM. One-way ANOVA along with Bonferroni post-test was performed for multiple comparison where appropriate. Moreover, differences between non-normally distributed variables were examined by Kruskal–Wallis and Dunn’s test post hoc. P-values less than 0.05 were considered statistically significant.
Results
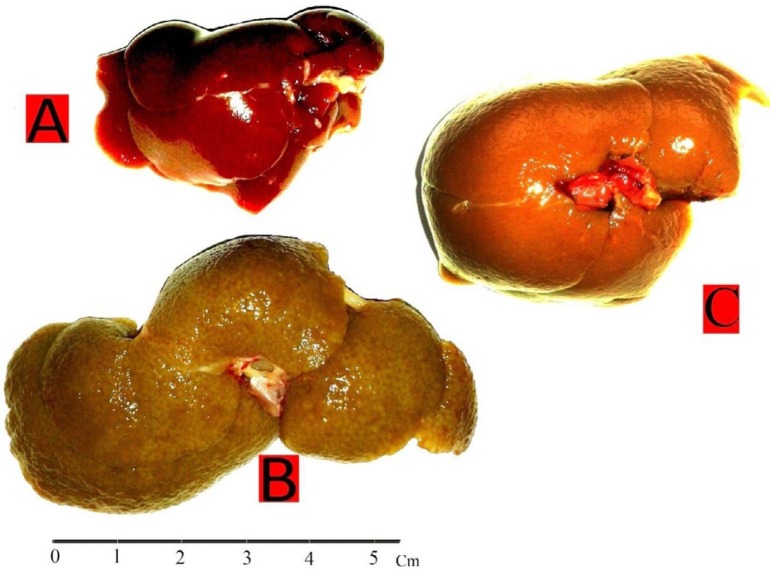
BDL rats shown signs of biliary cirrhosis such as jaundice: dark urine, steatorrhea and ascites from the third post-surgery. In BDL group the stiffness and liver size was more than control group. It also had a nodular appearance with pale colour (dark cream) in the BDL group compared to the control group (Figure 1). Therefore, we visually inspected the cirrhotic appearance of the liver in all experimental animals to confirm the progression of cirrhosis.
Figure 1.
Macroscopic view of the rat liver: Control group (A); BDL group (B) with hepatomegaly, yellow pigmentation of bilirubin, macronodulation, grainy & pale surface; BDL/Thl group (C) improvement of cirrhotic conditions than BDL group
BDL: Bile Duct Ligated; Thl: Thalidomide
In vitro study
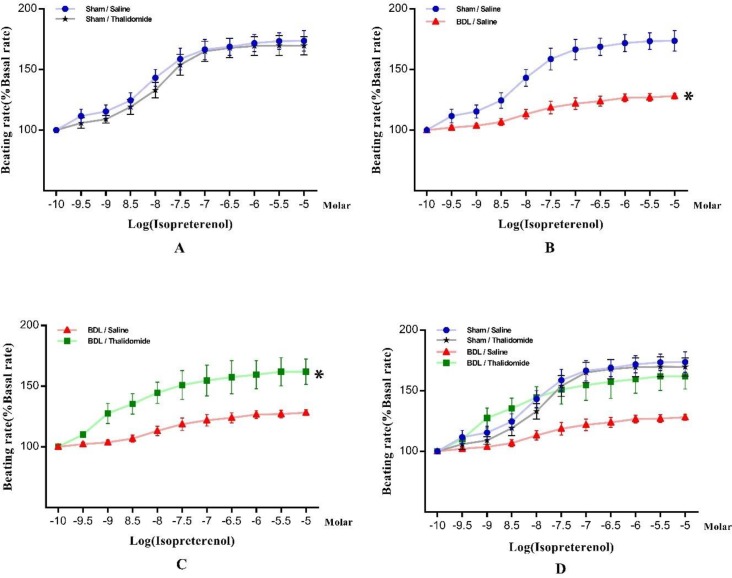
In this part of the experiments, we aimed to measure the rate of atrium responsiveness in the study groups to isoproterenol stimulant. Therefore, normalized graph of heart rate than increasing isoproterenol and ANOVA one-way test were used for comparison between study groups. In more details, our data showed that thalidomide administration has no effect in sham group on chronotropic response (Figure 2A). besides, chronotropic responses to isoproterenol were impaired significantly in isolated atria following chronic bile duct ligation (FBDL vs sham =1.083, P<0.05, Figure 2B). However, the maximum response (Rmax) to isoproterenol was lower in BDL/ Saline group in comparison with Sham/Saline group but this difference was not significance (Table 1). Furthermore, there was no significant difference in isoproterenol EC50 between those groups (Data not be shown). Thalidomide could increase chronotropic responses to isoproterenol in BDL rats, significantly (FBDL vs BDL/ Thl = 2.041, P<0.05, Figure 2C). Although Rmax was noticeably increased after thalidomide treatment in BDL/ Thalidomide rats (Table 1) it’s not significant, EC50 of isoproterenol did not show a significant difference between animals in BDL/ Saline than BDL/ Thalidomide groups (log EC50 = -7.550 ±0.23 and -8.450± 0.27, respectively).
Figure 2.
Chronotropic concentration-dependent responsiveness to isoproterenol stimulation that were obtained on spontaneous beating isolated, after 28 day, in all study groups (A), Sham- operated in comparison with Sham-operated treated with thalidomide (B), bile duct ligated than Sham-operated (C) bile duct ligated treated with thalidomide versus bile duct ligated. (D) All above mentioned groups. Data are expressed as % increase in basal beating rate and shown as mean±SEM, *P<0.05, (One-way ANOVA, bonferroni post-test) (n=7)
Table 1.
The median and range of atrial maximum chronotropic response to β-adrenergic stimulation (Rmax) in study groups. (non-parametric Dunn’s test) (n=7)
| Group | Rmax Median(Range) |
|---|---|
| Sham / Saline | 156(145-195) |
| Sham / Thalidomide | 152 (130-169) |
| BDL / Saline | 137 (118-153) |
| BDL / Thalidomide | 150 (138-154) |
BDL: Bile duct ligated
Intrasplenic pulp pressure
Intrasplenic pulp pressure (ISPP) was measured and used in place of a portal venous pressure (PVP) index. In BDL/ Saline rats as shown in Figure 3, PVP increased significantly 4 weeks after bile duct ligation (P<0.0001). Our data has shown that thalidomide can decrease PVP in BDL rats significantly (P<0. 05).
Figure 3.
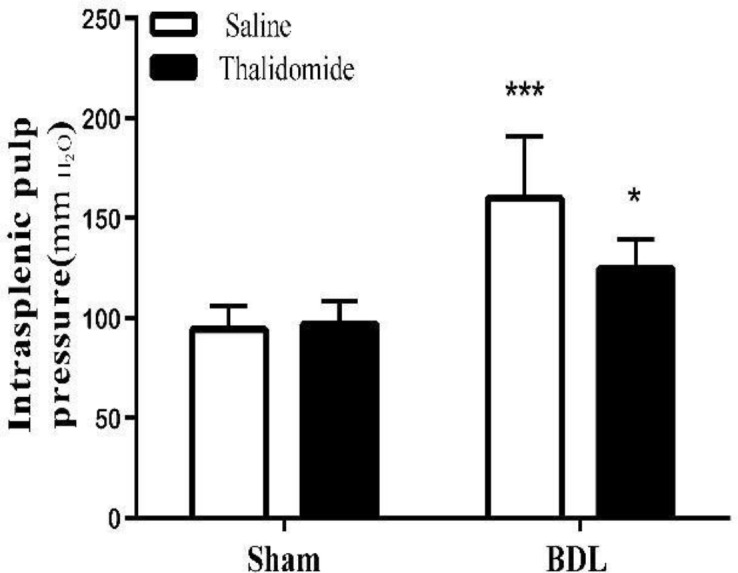
Increasing intrasplenic pulp pressure in sham- operated than bile duct ligated rats (***P<0.001) and its diminishing with thalidomide treatment (*P<0.05), mean±SEM. (One- way ANOVA, bonferroni post-test) (n=7)
Plasma nitrite/nitrate concentrations
Assessment of serum nitrite/nitrate concentration used to NO systemic index; therefore, NO was significantly higher in the BDL/ Saline than Sham/ Saline group (P<0.001). Furthermore, it lessened in BDL/ Saline group in comparison with the BDL/ Thalidomide group significantly (Table 2).
Table 2.
Hepatic and serum TNF-α, hepatic IL-6 and serum NO concentrations in experimental groups (a P <0.001, b P<0.01compared with sham/Saline) (c P<0.05 compared with BDL/Saline, mean±SEM. (One -way ANOVA, bonferroni post-test) (n=7)
| Group | Hepatic TNF-α(Pg/gProtein) |
Hepatic IL-6(Pg/gProtein) |
Serum TNF-α(Pg/ml) |
Serum NO(µM/L) |
|---|---|---|---|---|
| Sham/Saline | 149.2±3.7 | 13.75±0.6 | 4.555±1.1 | 20.50±4.1 |
| Sham/Thl | 140.1±0.7 | 15.37±0.5 | 5.082±0.8 | 23.99±2.3 |
| BDL/Saline | 392.6±0.9a | 22.05±1.9 b | 36.91±1.1a | 54.92±2.864b |
| BDL/Thl | 352.6±1.5c | 19.24±0.9c | 29.60±1.7c | 31.35±1.374c |
TNF- α , I L-6 levels
Assessment of TNF-α and IL-6 levels in the study group by ELISA method has shown that chronic bile duct ligation can increase noted cytokines in liver significantly (PTNF-α <0.001, PIL-6 <0.01). Additionally, hepatic levels of these cytokines, in treated BDL rats with thalidomide, decreased significantly (P<0.05). Also TNF-α serum levels increased in BDL rats compared with sham-operated animals; moreover, TNF-α levels were significantly decreased in BDL/ Thalidomide rat livers (Table 2).
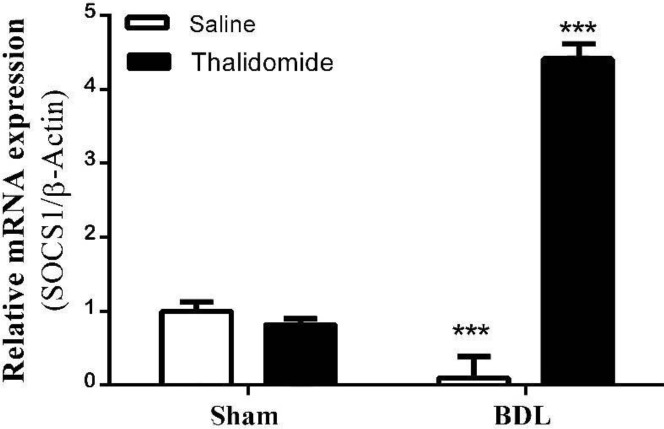
Quantitative measurement of hepatic SOCS1mRNA expression
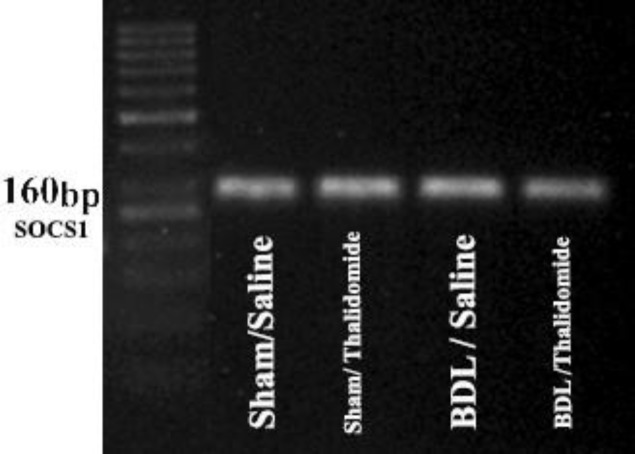
We used RT-PCR to assess the expression of SOCS1 in rat liver and observed that a single band was visible in all of the study groups after amplification of cDNA using a specific primer for SOCS1 (Figure 4). Our data showed that (Figure 5), relative SOCS1 expression decreased significantly in bile duct ligated group than the sham-operated group (P<0.001). Additionally, its expression was notable and significantly increase in BDL/Thl group compared with BDL/saline (P<0.001).
Figure 4.
The expression of SOCS1 c-DNA obtained from RT-PCR in livers of study groups that appear in single band (n=7)
SOCS1: Suppressor of Cytokine Signaling 1
Figure 5.
The relative SOCS1 mRNA expression in experimental groups; SOCS1 expression reduced in BDL rats than sham and increased in BDL/Thl rats than BDL (***P<0.001), mean±SEM. (One -way ANOVA, bonferroni post-test) (n=7)
BDL: Bile Duct Ligated; Thl: Thalidomide; SOCS1: Suppressor of Cytokine Signaling 1
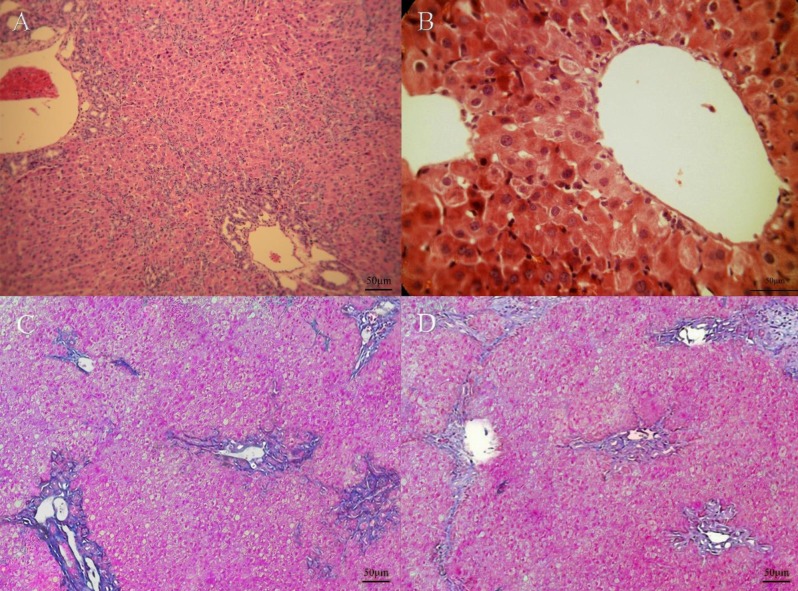
Histopathology
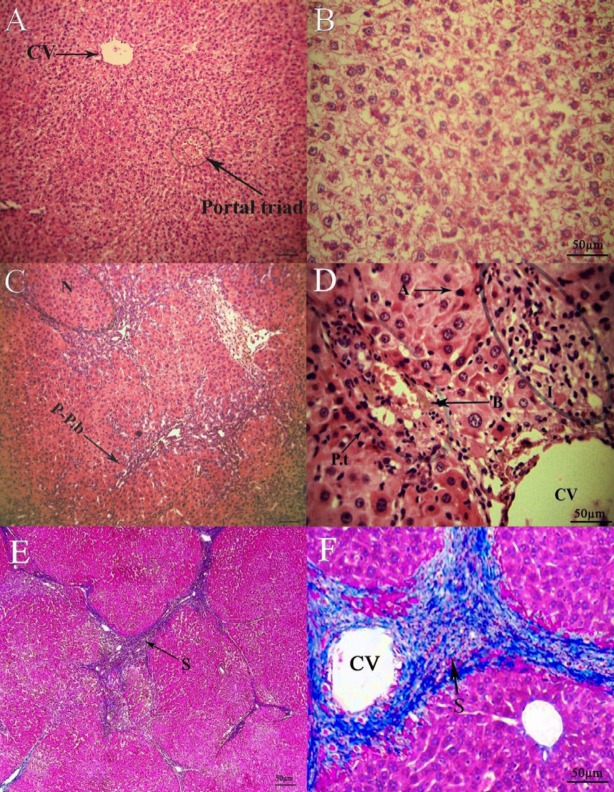
The hepatic lobules architecture in the Sham/Saline group was of high significance, and there was no fibroplasia and inflammatory cell infiltration (Fig 6, A-B). Nevertheless, in the rats of BDL/ Saline group, the lobules of the liver were separated and encompassed by periportal and perivenular collagen deposition. Wide bridging fibrosis (portal- portal and portal- central connection with fibrotic bands) led to apparent pseudo-lobules and micronodules. Moreover, there was severity of hepatocytes that ultimately ends up in apoptosis and necrosis. Furthermore, a widespread inflammatory cell infiltration proliferating bile ductules (Fig 6C-F) was observed. Thalidomide treatment in bile duct ligated rats resulted in the improvement of necro-inflammation and lymphocytes infiltration. Additionally, thalidomide could shorten and disconnect septa in hepatic tissue that reduce the micronodular appearance and recover liver homogeneity (Fig 7A-D).
Figure 6.
Hematoxylin-eosin staining of liver tissue; A: sham (100x), B: sham (200x), C: BDL (100x) N, micronodulation;P-P.b, portal-portal bridge; D: BDL(200x) P.t, portal triad; B, bilirubin pigments; A, apoptotic cells; I, lymphocytic infiltration. Masson-trichrome staining of liver tissue; E: BDL (100x), F: BDL (200x), S,fibrous septa
BDL: Bile duct ligated
Figure 7.
Hematoxylin-eosin staining of liver tissue; A: BDL/Thl (100x), B: BDL/Thl (200x). Masson-trichrome staining of liver tissue; C-D: BDL/Thl (100x). micronoulation,liver heterogeneity, bile ductule proliferation, collagen deposition and fibrotic septa bands decrease in cirrhotic rats by Thalidomide treatment
BDL: Bile Duct Ligated; Thl: Thalidomide
Discussion
Cirrhotic cardiomyopathy is a complication of cirrhosis wherein physical or pharmacological stimulation cannot induce an adequate increase in heart rate (2). This condition is related to the development of a hemodynamic disorder which is called “hyperdynamic circulatory syndrome” (HC), and characterizes both portal hypertension (PTH) and NO overproduction (3). Moreover, it was shown that TNF-α and IL-6 levels are elevated in cirrhosis, and these enhancements are associated with HC intensification (4, 5, 21). Furthermore, the expression of SOCS1 as a modulator of hepatic inflammation decreases in cirrhosis condition (11). As an immunomodulatory and anti-inflammatory drug, thalidomide decreases the TNF-α and IL-6 levels (22). Although previous studies have indicated that thalidomide inhibits fibrosis and inflammation progression in cirrhosis, there are not enough studies concerning its role in cirrhotic cardiomyopathy and also the involvement of SOCS1 in this effect. Hence, in the present study we examined the effect of chronic thalidomide administration in BDL rats on atrial chronotropic hyporesponsiveness to β-adrenergic stimulation. Our results revealed that thalidomide could improve atrial response to β-adrenergic stimulation in cirrhotic rats. The histopathology finding of the present report indicated that bile duct proliferation, collagen deposition, and inflammatory cell infiltration could increase in the liver after cirrhosis. Moreover, this research indicated that thalidomide treatment could improve the histopathological conditions of cirrhotic liver. This is consistent with the findings presented by Napoli et al. in their investigations on 28-day BDL rats (23). The present results revealed that TNF-α and IL-6 levels could increase in cirrhotic rat livers compared to sham-operated animals, and chronic thalidomide administration could significantly diminish the abovementioned cytokine in the BDL group. It was previously indicated that the elevated levels of hepatic TNF-α and IL-6 in BDL rats were decreased by thalidomide administration in BDL animals (24). Following the ligation of the common bile duct, the hepatic stellate cell is activated by TNF-α and IL-6. These cytokines caused collagen deposition, fibrotic septa band formation, and the deterioration of microvasculature (25, 26). Hence, thalidomide is possibly able to improve liver function and histopathological conditions in BDL rats. However, further studies are required for more clarification. Additionally, the findings of the present study indicated that NO and TNF-α levels in serum could be augmented in the BDL group compared to the sham-operated one. Plasma-elevated NO was well demonstrated by Mani et al. in BDL rats (27), and plasma raised TNF-α concentration was adequately indicated by Fernandez-Martınez et al. in the BDL rats (1). Furthermore, it was exhibited that NO overproduction could be mediated by TNF-α (28). In accordance with previous studies, we showed that portal vein pressure (PVP) could significantly rise along with the establishment of HC in BDL rats (18, 29). The findings of the present study indicated that SOCS1 mRNA expression was lower in bile duct ligated rats compared to sham-operated ones. This phenomenon was reported by Yoshida et al. in the case of dimethylnitrosamine-induced cirrhosis in mice (11). It was determined that the deficiency of SOCS1 could induce severe hepatic disorders (8, 10, 30). It has also been shown that SOCS1 gene is silenced by hypermethylation in cirrhosis (31, 32). The chronic administration of thalidomide in this study could ameliorate collagen deposition, inflammatory cell infiltration, and an overall liver uniformity. In agreement with the results, Ta-Sen Yeh et al. indicated that thalidomide could inhibit hepatic necroinflammation in cirrhotic rats (33). Therewith, thalidomide reduced hepatic TNF-α and IL-6 levels in cirrhotic rats, and reversed amplified systemic NO and PTH in those rats. Consistent with this finding, Ying-Ying Yang et al. reported that thalidomide could diminish the hepatic levels of TNF-α, IL-6 and increase PTH, in cirrhotic rats (33). Moreover, it was previously displayed that excessive NO and PVP could be reduced by thalidomide therapy in the model of portal hypertension. It seems that all of the above-mentioned actions of thalidomide were mediated by TNF-α inhibition. Furthermore, the inhibition of IL-6 by thalidomide is partly responsible for the reduction of collagen deposition and fibrosis. Surprisingly, this study is the first research indicating that thalidomide could significantly increase SOCS1 mRNA levels in cirrhotic rats. It was shown that thalidomide analog could upregulate the SOCS1 expression through the demethylation of its silenced promoter region (34). Our results revealed that with chronic common bile duct ligation, the responsiveness of isolated atria chronotropic to β-adrenergic stimulation, is impaired. This finding was clearly noted by Mani et al. and Jazaeri et al. concerning BDL rats (27, 35). By HC progression and effective vasodilation, a systemic hypotension occurs. To compensate, tachycardia and cardiac overstrain take place that result in insufficient myocardium response to stress in long term. On the basis of our results, the chronic thalidomide administration in cirrhotic rats removes the hyporesponsiveness of isolated atria to the chronotropic effect of isoproterenol stimulation.
Conclusion
The possible mechanism of action of thalidomide for the improvement of cardiac function in cirrhotic rats is mediated by the amelioration of disturbed liver architecture, the decrease of IHR, PTH, and the augmentation of NO systemic in cirrhosis.
Conflicts of Interest
None of the authors of this paper has a financial or personal relationship with other people or organizations that could inappropriately influence or bias the content of the paper.
Acknowledgment
The study was supported by a grant from Experimental Medicine Research Center, Tehran University of Medical Sciences, Iran (Grant No: 93-02-30-25972) and a grant (96002757) from Iran National Science Foundation (INSF).
References
- 1.Fernandez-Martinez E, Perez-Alvarez V, Tsutsumi V, Shibayama M, Muriel P. Chronic bile duct obstruction induces changes in plasma and hepatic levels of cytokines and nitric oxide in the rat. Exp Toxicol Pathol. 2006;58:49–58. doi: 10.1016/j.etp.2006.03.002. [DOI] [PubMed] [Google Scholar]
- 2.Bernardi M. Cirrhotic cardiomyopathy. Clin Liver Dis. 2013;2:99–101. doi: 10.1002/cld.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Møller S, Henriksen J H. Cirrhotic cardiomyopathy. J Hepatol. 2010;53:179–190. doi: 10.1016/j.jhep.2010.02.023. [DOI] [PubMed] [Google Scholar]
- 4.Choi I, Kang H S, Yang Y, Pyun K H. IL-6 induces hepatic inflammation and collagen synthesis in vivo. Clin Exp Immunol. 1994;95:530–535. doi: 10.1111/j.1365-2249.1994.tb07031.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sudo K, Yamada Y, Moriwaki H, Saito K, Seishima M. Lack of tumor necrosis factor receptor type 1 inhibits liver fibrosis induced by carbon tetrachloride in mice. Cytokine. 2005;29:236–244. doi: 10.1016/j.cyto.2004.11.001. [DOI] [PubMed] [Google Scholar]
- 6.Hu L S, George J, Wang J H. Current concepts on the role of nitric oxide in portal hypertension. World J Gastroenterol. 2013;19:1707–1717. doi: 10.3748/wjg.v19.i11.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kilbourn R G, Gross S S, Jubran A, Adams J, Griffith O W, Levi R, et al. NG-methyl-L-arginine inhibits tumor necrosis factor-induced hypotension: implications for the involvement of nitric oxide. Proc Natl Acad Sci USA. 1990;87:3629–3632. doi: 10.1073/pnas.87.9.3629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alexander W S, Starr R, Fenner J E, Scott C L, Handman E, Sprigg N S, et al. SOCS1 is a critical inhibitor of interferon gamma signaling and prevents the potentially fatal neonatal actions of this cytokine. Cell. 1999;98:597–608. doi: 10.1016/s0092-8674(00)80047-1. [DOI] [PubMed] [Google Scholar]
- 9.Hong F, Jaruga B, Kim W H, Radaeva S, El-Assal O N, Tian Z, et al. Opposing roles of STAT1 and STAT3 in T cell-mediated hepatitis: regulation by SOCS. J Clin Invest. 2002;110:1503–1513. doi: 10.1172/JCI15841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Naka T, Tsutsui H, Fujimoto M, Kawazoe Y, Kohzaki H, Morita Y, et al. SOCS-1/SSI-1-deficient NKT cells participate in severe hepatitis through dysregulated cross-talk inhibition of IFN-gamma and IL-4 signaling in vivo. Immunity. 2001;14:535–545. doi: 10.1016/s1074-7613(01)00132-7. [DOI] [PubMed] [Google Scholar]
- 11.Yoshida T, Ogata H, Kamio M, Joo A, Shiraishi H, Tokunaga Y, et al. SOCS1 is a suppressor of liver fibrosis and hepatitis-induced carcinogenesis. J Exp Med. 2004;199:1701–1707. doi: 10.1084/jem.20031675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Brunton L, Chabner B A, Knollman B. IGoodman & Gilman’s the Pharmacological Basis of Therapeutics. 2011;12 [Google Scholar]
- 13.Shanbhag PS, Viswanath V, Torsekar R. Thalidomide: current status. Indian J Dermatol Venereol Leprol. 2006;72:75. doi: 10.4103/0378-6323.19732. [DOI] [PubMed] [Google Scholar]
- 14.Chong L-W, Hsu Y-C, Chiu Y-T, Yang K-C, Huang Y-T. Anti-fibrotic effects of thalidomide on hepatic stellate cells and dimethylnitrosamine-intoxicated rats. J Biomed Sc. 2006;13:403–418. doi: 10.1007/s11373-006-9079-5. [DOI] [PubMed] [Google Scholar]
- 15.Yang Y Y, Lee K C, Huang Y T, Lee F Y, Chau G Y, Loong C C, et al. Inhibition of hepatic tumour necrosis factor-α attenuates the anandamide-induced vasoconstrictive response in cirrhotic rat livers. Liver Int. 2009;29:678–685. doi: 10.1111/j.1478-3231.2009.01983.x. [DOI] [PubMed] [Google Scholar]
- 16.Bashiri H, Hosseini-Chegeni H, Alsadat Sharifi K, Sahebgharani M, Salari A-A. Activation of TRPV1 receptors affects memory function and hippocampal TRPV1 and CREB mRNA expression in a rat model of biliary cirrhosis. Neurol Res. 2018;40:938–947. doi: 10.1080/01616412.2018.1504158. [DOI] [PubMed] [Google Scholar]
- 17.Ebrahimkhani M R, Sadeghipour H, Dehghani M, Kiani S, Payabvash S, Riazi K, et al. Homocysteine alterations in experimental cholestasis and its subsequent cirrhosis. Life Sci. 2005;76:2497–2512. doi: 10.1016/j.lfs.2004.12.009. [DOI] [PubMed] [Google Scholar]
- 18.Ackerman Z, Karmeli F, Amir G, Rachmilewitz D. Renal vasoactive mediator generation in portal hypertensive and bile duct ligated rats. J Hepatol. 1996;24:478–486. doi: 10.1016/s0168-8278(96)80169-3. [DOI] [PubMed] [Google Scholar]
- 19.Raoofian R, Noori-Daloii M R, Saee-Rad S, Modarresi M H, Ghaffari S H, Mojarrad M, et al. Differential expression of human homeodomain TGIFLX in brain tumor cell lines. Acta Med Iran. 2013:834–841. [PubMed] [Google Scholar]
- 20.Bagnyukova T V, Tryndyak V P, Muskhelishvili L, Ross S A, Beland F A, Pogribny I P. Epigenetic down-regulation of the suppressor of cytokine signaling 1 (Socs1) gene is associated with the STAT3 activation and development of hepatocellular carcinoma induced by methyl-deficiency in rats. Cell Cycle. 2008;7:3202–3210. doi: 10.4161/cc.7.20.6816. [DOI] [PubMed] [Google Scholar]
- 21.Lopez-Talavera J C, Cadelina G, Olchowski J, Merrill W, Groszmann R J. Thalidomide inhibits tumor necrosis factor α, decreases nitric oxide synthesis, and ameliorates the hyperdynamic circulatory syndrome in portal-hypertensive rats. Hepatology. 1996;23:1616–1621. doi: 10.1002/hep.510230644. [DOI] [PubMed] [Google Scholar]
- 22.Franks M E, Macpherson G R, Figg W D. Thalidomide. The Lancet. 2004;363:1802–1811. doi: 10.1016/S0140-6736(04)16308-3. [DOI] [PubMed] [Google Scholar]
- 23.Napoli J, Prentice D, Niinami C, Bishop G A, Desmond P, McCaughan G W. Sequential increases in the intrahepatic expression of epidermal growth factor, basic fibroblast growth factor, and transforming growth factor β in a bile duct ligated rat model of cirrhosis. Hepatology. 1997;26:624–633. doi: 10.1002/hep.510260314. [DOI] [PubMed] [Google Scholar]
- 24.Wnendt S, Finkam M, Winter W, Ossig J, Raabe G, Zwingenberger K. Enantioselective inhibition of TNF-α release by thalidomide and thalidomide-analogues. Chirality. 1996;8:390–396. doi: 10.1002/(SICI)1520-636X(1996)8:5<390::AID-CHIR6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 25.Das S, Santra A, Lahiri S, Mazumder D G. Implications of oxidative stress and hepatic cytokine (TNF-α and IL-6) response in the pathogenesis of hepatic collagenesis in chronic arsenic toxicity. Toxicol Appl Pharmacol. 2005;204:18–26. doi: 10.1016/j.taap.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 26.Reeves H L, Friedman S L. Activation of hepatic stellate cells—a key issue in liver fibrosis. Front Biosci. 2002;7:808–826. doi: 10.2741/reeves. [DOI] [PubMed] [Google Scholar]
- 27.Mani A R, Ippolito S, Ollosson R, Moore K P. Nitration of cardiac proteins is associated with abnormal cardiac chronotropic responses in rats with biliary cirrhosis. Hepatology. 2006;43:847–856. doi: 10.1002/hep.21115. [DOI] [PubMed] [Google Scholar]
- 28.Wiest R, Groszmann R J. The paradox of nitric oxide in cirrhosis and portal hypertension: too much, not enough. Hepatology. 2002;35:478–491. doi: 10.1053/jhep.2002.31432. [DOI] [PubMed] [Google Scholar]
- 29.Yokoyama Y, Xu H, Kresge N, Keller S, Sarmadi A H, Baveja R, et al. Role of thromboxane A2 in early BDL-induced portal hypertension. Am J Physiol Gastrointest Liver Physiol. 2003;284:G453–G460. doi: 10.1152/ajpgi.00315.2002. [DOI] [PubMed] [Google Scholar]
- 30.Torisu T, Nakaya M, Watanabe S, Hashimoto M, Yoshida H, Chinen T, et al. Suppressor of cytokine signaling 1 protects mice against concanavalin A–induced hepatitis by inhibiting apoptosis. Hepatology. 2008;47:1644–1654. doi: 10.1002/hep.22214. [DOI] [PubMed] [Google Scholar]
- 31.Ammerpohl O, Pratschke J, Schafmayer C, Haake A, Faber W, von Kampen O, et al. Distinct DNA methylation patterns in cirrhotic liver and hepatocellular carcinoma. Liver Int. 2012;130:1319–1328. doi: 10.1002/ijc.26136. [DOI] [PubMed] [Google Scholar]
- 32.Okochi O, Hibi K, Sakai M, Inoue S, Takeda S, Kaneko T, et al. Methylation-mediated silencing of SOCS-1 gene in hepatocellular carcinoma derived from cirrhosis. Clin Cancer Res. 2003;9:5295–5298. [PubMed] [Google Scholar]
- 33.Yeh T-S, Ho Y-P, Huang S-F, Yeh J-N, Jan Y-Y, Chen M-F. Thalidomide salvages lethal hepatic necroinflammation and accelerates recovery from cirrhosis in rats. J Hepatol. 2004;41:606–612. doi: 10.1016/j.jhep.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 34.Görgün G, Calabrese E, Soydan E, Hideshima T, Perrone G, Bandi M, et al. Immunomodulatory effects of lenalidomide and pomalidomide on interaction of tumor and bone marrow accessory cells in multiple myeloma. Blood. 2010;116:3227–3237. doi: 10.1182/blood-2010-04-279893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jazaeri F, Tavangar S M, Ghazi-Khansari M, Khorramizadeh M R, Mani A R, Dehpour A R. Cirrhosis is associated with development of tolerance to cardiac chronotropic effect of endotoxin in rats. Liver Int. 2013;33:368–374. doi: 10.1111/liv.12039. [DOI] [PubMed] [Google Scholar]