Abstract
Objective
Hepatitis B virus (HBV) has a worldwide distribution and remains a leading public health problem in China.
Method
Automated chemiluminescence microparticle immunoassay was used to test all five markers of HBV serology in serum samples among 696,048 patients, pregnant women, and normal subjects in Beijing from 2008 to 2018.
Results
The overall prevalence of subjects categorized as previous/ occult HBV infection, inactive HBsAg carrier, active HBV infection, HBsAg, HBV susceptible, and immune via vaccination was 29.4%, 4.8%, 1.4%, 6.4%, 33.9% and 30.3%, respectively; men had a significantly higher prevalence of HBV infection than women. The prevalence of HBsAg was around 0.5% in subjects ≤ 10 years of age, increased dramatically to 3.7% in subjects between 11 and 20 years of age, reached the highest level of 7.9% in subjects between 41 and 50 years of age, and finally decreased to 2.8% in subjects ≥ 81 years of age. During the 10 years from 2008 to 2018, the prevalence of HBsAg was stabilized at about 6.0%, and indicators of HBV susceptibility, previous/ occult HBV infection, and immunity via vaccination were not further improved, despite the constant implementation of HBV vaccination since 1992. All four age groups (21 − 30y, 31 − 40y, 41 − 50y and 51 − 60y) of the normal adult population were found to have a significantly lower prevalence of HBsAg and HBV susceptibility but significantly higher prevalence of immunity via vaccination compared with corresponding age groups of the sub-total population.
Conclusions
Although high coverage has been established among infants and young children, their vaccination alone could not reduce HBV infection in the adult Chinese population quickly. Adult populations with more vaccinated individuals are found to have fewer individuals with HBsAg. Vaccination in adults or at least in high-risk adults is an urgent need to decrease horizontal HBV transmission in China.
Keywords: Hepatitis B virus (HBV), Hepatitis B surface antigen (HBsAg), Anti-HBs, Catch-up vaccination, Chemiluminescence microparticle immunoassay (CMIA)
1. Introduction
Hepatitis B virus (HBV) has a worldwide distribution and remains as a leading public health problem. The World Health Organization (WHO) estimated that 257 million persons had chronic HBV infection globally in 2015 with approximately 887,000 deaths annually related to two major consequences of HBV infection, cirrhosis and hepatocellular carcinoma [1]. Serologic prevalence of the hepatitis B surface antigen (HBsAg) is usually used to define the endemicity of active HBV infection in various geographic areas worldwide. A prevalence of HBsAg of ≥ 8%, 5%−7%, 2%−4%, and < 2% are defined as highly endemic, high intermediate, low intermediate, and low endemic areas, respectively [2], [3]. In China, a universal HBV vaccination was implemented in infants in 1992 [4], and HBV vaccination was integrated into the national immunization program in 2002 [5]. Prior to the implementation of national HBV vaccination in 1992, the prevalence of HBsAg was 9.8% in Chinese children ≤ 4 years of age in a large sample size survey [6]. Since then, the prevalence of HBsAg has decreased dramatically to 0.3% in children between 1 and 4 years of age in 2014 [7]. However, the data for Chinese adults or the general population may not be as optimistic as for the children. The prevalence of HBsAg in the population aged between 1 and 59 years dropped from 9.8% in 1992 to 7.2% in 2006 [3], but 10 years later it remained as high as 7.7% in Eastern China in 2015 [8], 7.07% in pregnant women in Northwest China in 2018 [9], and 10.98% and 7.43% in patients visiting a general hospital in the South China in 2018 [10] and in Northeast China in 2019 [11], respectively. Furthermore, the prevalence of serum HBsAg/HBeAg was found to be 10.5% among 1.9 million couples preparing for pregnancy in rural China in 2017 [12]. Therefore, these areas remain a high intermediate endemic of HBV infection.
In this study population, there were 696,048 patients, pregnant women, and healthy subjects who visited Peking Union Medical College Hospital (PUMCH), Beijing for routine medical purposes or health examinations from 2008 to 2018, and they were tested for all five markers of HBV serology, including HBsAg, anti-HBs, hepatitis B virus e-antigen (HBeAg), anti-HBe, and anti-hepatitis B virus core antigen (anti-HBc). The aim of the study was to analyze the data of different stages of HBV infection as defined by HBV serology, including HBV susceptibility, HBV immunity via vaccination, previous/ occult HBV infection, inactive HBsAg carrier, and active HBV infection among this study population within various age groups during the 10-year time span. We focused on the current situation of HBV infection and challenges to our current vaccination policy with the main objective of eliminating HBV by 2030.
2. Materials and methods
2.1. Ethics statement
Our study was approved by the Ethics Committee (reference no. S-K735) of PUMCH, the Institution Review Board (IRB) of Peking Union Medical College Hospital, and was waived for requirement of informed consent as the blood samples were collected and tested for routine medical purposes. The results of HBV serologic markers had been reported to the patients and doctors before they were analyzed for this study. The patients’ identifications were also removed before the analysis.
2.2. Study samples
A total of 696,048 blood samples from patients, pregnant women, and healthy subjects visiting the PUMCH for routine medical purposes or health examinations were collected and tested for all five markers of HBV serology, including HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc from May 2008 to December 2018. Our hospital has 1800 beds and attended to 3.79 million outpatients and 109,000 hospitalized patients in 2018. Half of patients in our hospital were Beijing residents, and rests were from other parts of China, mainly Northern China. Most pregnant women and healthy subjects were Beijing residents.
Inclusion criteria were the availability of blood samples from patients, pregnant women, or healthy subjects who: were Chinese; had demographic information; and had laboratory results for all five markers of HBV serology.
Exclusion criteria were the lack of availability of blood samples from patients, pregnant women, or healthy subjects who: were foreigners (as the study was designed only for Chinese subjects); had no demographic information; had no laboratory results; or had laboratory results but less than five markers of HBV serology. More than one blood sample per person within 1 year were considered to be repeated samples, and only the first sample was recruited and the rest excluded. Samples for more than one year apart were treated as new samples.
2.3. Laboratory testing
Blood samples were collected using sterile vacutainers and all sera were separated by centrifugation. The serum samples were tested for HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc using automated chemiluminescence microparticle immunoassay (CMIA, Abbott Architect i2000 SR, Abbott Laboratories, Wiesbaden, Germany). According the manufacturer’s instruction, when the signal-to-cutoff ratio (S/CO) of a serum specimen was equal to or greater than 1.0, a positive reaction was reported, whereas a ratio of <1.0 reported a negative reaction for testing of HBsAg, HBeAg, anti-HBe, and anti-HBc. For testing of anti-HBs, the samples with a concentration of anti-HBs equal to or more than 10mIU/mL were reported as positive reactions and were immune via vaccination.
2.4. Statistical analysis
The results of the tests for HBV serological markers were stored in a server installed with our laboratory information system (LIS) after the results were reported. The data were exported to Microsoft Excel 2007 (Microsoft Corp., New York, NY), where they were sorted and preliminarily calculated. Statistical analysis was performed using IBM SPSS software (version 21.0). P-values < 0.05 were considered statistically significant. The chi-squared test with continuity correction was used to compare the prevalence of HBV infection.
3. Results
3.1. The demographic characteristics and definition of HBV infection
There were a total of 696,048 blood samples that were tested for HBsAg, anti-HBs, HBeAg, anti-HBe, and anti-HBc from 2008 to 2018, in which 263,591 (37.9%) samples were from men and 432,457 (62.1%) were from women. Among 696,048 blood samples, 133,491 (19.2%) blood samples were from the healthy population who underwent health examinations, 11,535 (1.7%) from patients visiting the hepatitis & infectious disease clinics, 35,468 (5.1%) from pregnant women undergoing prenatal care, and 51,5554 (74.1%) from other patients for various medical reasons including surgery, invasive operation, blood transfusion, etc. 6,752 duplicated samples for more than one year apart were included. The demographic characteristics of patients, pregnant women and the healthy subjects visiting PUMCH is shown in Table 1.
Table 1.
Demographic characteristics of patients, pregnant women, and the healthy population visiting PUMCH, Beijing, 2008–2018 (n = 696,048).
| Variable | Mean age (yr.) | No. of patients /participants | % # | |
|---|---|---|---|---|
| Sex | Male | 46.2 | 263,591 | 37.9 |
| Female | 42.7 | 432,457 | 62.1 | |
| Department | Health medicine## | 41.8 | 133,491 | 19.2 |
| Male | 42.3 | 74,263 | ||
| Female | 41.0 | 59,228 | ||
| Hepatitis & infectious disease clinics | 39.9 | 11,535 | 1.7 | |
| Male | 38.9 | 6896 | ||
| Female | 41.3 | 4639 | ||
| Pregnant women | 31.8 | 35,468 | 5.1 | |
| Others | 45.5 | 515,554 | 74.1 | |
| Age | 0–2 | N/A | 1,581 | 0.23 |
| 3–5 | N/A | 2,234 | 0.32 | |
| 6–10 | N/A | 3,893 | 0.56 | |
| 11–20 | N/A | 20,394 | 2.9 | |
| 21–30 | N/A | 118,376 | 17.0 | |
| 31–40 | N/A | 175,118 | 25.2 | |
| 41–50 | N/A | 143,229 | 20.6 | |
| 51–60 | N/A | 115,690 | 16.6 | |
| 61–70 | N/A | 71,971 | 10.3 | |
| 71–80 | N/A | 35,799 | 5.1 | |
| ≥ 81 | N/A | 7,763 | 1.1 | |
| Year | 2008 | 42.0 | 44,019 | 6.3 |
| 2009 | 43.0 | 88,147 | 12.7 | |
| 2010 | 44.7 | 61,912 | 8.9 | |
| 2011 | 44.6 | 49,991 | 7.2 | |
| 2012 | 44.0 | 55,588 | 8.0 | |
| 2013 | 43.6 | 57,135 | 8.2 | |
| 2014 | 43.7 | 62,286 | 8.9 | |
| 2015 | 44.3 | 61,783 | 8.9 | |
| 2016 | 44.5 | 66,633 | 9.6 | |
| 2017 | 44.5 | 73,142 | 10.5 | |
| 2018 | 44.8 | 75,412 | 10.8 |
% value indicates the number of each category that were divided by the total number of study population (n = 696,048).
The healthy population consisted of employees from companies, schools, hospitals, or government agencies in Beijing who underwent annual health examinations.
On the basis of HBV serological markers, different stages of HBV infection, including HBV susceptible, HBV immune via vaccination, previous/occult HBV infection, inactive HBsAg carrier and active HBV infection are defined in Table 2 [13], [14]. The definition of immune via vaccination in our study is a positive reaction of anti-HBs with concentration ≥ 10mIU/mL (HBV immunity).
Table 2.
The definition of different stages of HBV infection on the basis of serological markers.
| HBsAg | Anti-HBs | HBeAg | Anti-HBe | Anti-HBc | |
|---|---|---|---|---|---|
| Susceptible | − | − | − | − | − |
| Immune via vaccination | − | + | − | − | − |
| Previous /occult infection | − | +/− | − | +/− | + |
| Inactive HBsAg carrier | + | − | − | + | + |
| Active infection | + | − | + | − | + |
3.2. Prevalence of HBV infection
As shown in Table 3, the overall prevalence of previous/occult HBV infection, inactive HBsAg carrier, active HBV infection, and HBsAg among 696,048 samples were 29.4%, 4.8%, 1.4%, and 6.4%, respectively; men had a significantly higher prevalence than women (χ2 = 2,077.7–5,145.3, P < 0.0001). Furthermore, the overall prevalence of HBV susceptible and immune via vaccination among 696,048 samples was 33.9% and 30.3%, respectively; women had a significantly higher prevalence than men (χ2 = 1,161.9–3,498.5, P < 0.0001).
Table 3.
Prevalence of HBV infection in men and women visiting PUMCH, Beijing, 2008–2018 (n = 696,048).
| Men Pos no. (%) |
Women Pos no. (%) |
Total Pos no. (%) |
χ2-value | P-value | |
|---|---|---|---|---|---|
| Susceptible | 82,756 (31.4) | 153,013 (35.4) | 235,769 (33.9) | 1,161.9 | <0.0001## |
| Immune via vaccination # | 68,868 (26.1) | 142,036 (32.8) | 210,904 (30.3) | 3,498.5 | <0.0001 |
| Previous /occult infection | 87,803 (33.3) | 116,586 (27.0) | 204,406 (29.4) | 3,184.8 | <0.0001 |
| Inactive HBsAg carrier | 17,246 (6.5) | 16,162 (3.7) | 33,408 (4.8) | 2,820.3 | <0.0001 |
| Active infection | 5,906 (2.2) | 3,937(0.91) | 9,843 (1.4) | 2,077.7 | <0.0001 |
| HBsAg | 24,067 (9.1) | 2,0683 (4.8) | 44,750 (6.4) | 5,145.3 | <0.0001 |
Immune via vaccination, only positive reaction of anti-HBs with concentration ≥ 10mIU/mL (HBV immunity).
Comparison of prevalence of HBV susceptibility between overall men and overall women; the same patterns were used for other comparisons in Table 3.
3.3. Age-related prevalence of HBV infection
Age-related prevalence of HBV infection is shown in Table 4. The prevalence of HBsAg was around 0.5% in subjects ≤ 10 years of age. However, the prevalence of HBsAg showed a dramatic increase to 3.7% in subjects between 11 and 20 years of age, an increase to 7.9% in subjects between 41 and 50 years of age, and a decrease to 2.8% with increasing age in subjects ≥ 81 years of age. The prevalence of previous/ occult HBV infection increased steadily with age to 56.0% in subjects ≥ 81 years of ages. The prevalence of HBV immunity via vaccination decreased with age, from the highest level of 75.5% in subjects less than 2 years of age to 10.5% in subjects ≥ 81 years of ages. The prevalence of HBV susceptibility was almost more than 30% in all age groups of the total study population.
Table 4.
Prevalence of HBV infection in different age groups of the population visiting PUMCH, Beijing, 2008–2018 (n = 696,048).
| Age (yr.) | Susceptible Pos no. (%) |
Immune via vaccination Pos no. (%) |
Previous/ occult infection Pos no. (%) |
Inactive HBsAg carrier Pos no. (%) |
Active infection Pos no. (%) |
HBsAg Pos no. (%) |
|---|---|---|---|---|---|---|
| 0–2 | 212 (13.4) | 1,193 (75.5) | 163 (10.3) | 0 (0) | 3 (0.19) | 7 (0.44) |
| 3–5 | 724 (32.4) | 1,466 (65.6) | 33 (1.5) | 2 (0.09) | 9 (0.40) | 11 (0.49) |
| 6–10 | 1,742 (44.7) | 2,060 (52.9) | 69 (1.8) | 5 (0.13) | 16 (0.41) | 22 (0.56) |
| 11–20 | 7,696 (37.7) | 10,772 (52.8) | 1,168 (5.7) | 259 (1.3) | 476(2.3) | 747 (3.7) |
| 21–30 | 31,680 (26.8) | 62,133 (52.5) | 18,056 (15.3) | 3,808 (3.2) | 2,436 (2.1) | 6,470 (5.5) |
| 31–40 | 53,264 (30.4) | 67,938 (38.8) | 42,453 (24.2) | 8,377 (4.8) | 2,636 (1.5) | 11,422 (6.5) |
| 41–50 | 52,967 (37.0) | 32,845 (22.9) | 46,055 (32.2) | 8,810 (6.2) | 2,132 (1.5) | 11,318 (7.9) |
| 51–60 | 45,254 (39.1) | 19,582 (16.9) | 42,231 (36.5) | 6,827 (5.9) | 1,405 (1.2) | 8,521 (7.4) |
| 61–70 | 27,656 (38.4) | 8,487 (11.8) | 31,366 (43.5) | 3,711 (5.2) | 567 (0.79) | 4,418 (6.1) |
| 71–80 | 12,055 (33.7) | 3,756 (10.5) | 18,365 (51.3) | 1,423 (4.0) | 141 (0.39) | 1,596 (4.5) |
| ≥81 | 2,519 (32.4) | 672 (8.7) | 4,349 (56.0) | 186 (2.4) | 22 (0.28) | 218 (2.8) |
| Total | 235,769 (33.9) | 210,904 (30.3) | 204,406 (29.4) | 33,408 (4.8) | 9,843 (1.4) | 44,750 (6.4) |
3.4. Yearly-related prevalence of HBV infection
The yearly-related prevalence of HBV infection is shown in Table 5, the prevalence of HBsAg was relatively consistent throughout each year for the entire study period, from 5.7% in 2008–2009 to 6.1% in 2018. The prevalence of HBV susceptibility, immunity via vaccination and previous/ occult HBV infections were also stable at around 30% annually from 2008 to 2018.
Table 5.
Yearly-related prevalence of HBV infection in the population visiting PUMCH, Beijing, 2008–2018 (n = 696,048).
| Year | Susceptible Pos no. (%) |
Immune via vaccination Pos no. (%) |
Previous/occult infection Pos no. (%) |
Inactive HBsAg carrier Pos no. (%) |
Active infection Pos no. (%) |
HBsAg Pos no. (%) |
|---|---|---|---|---|---|---|
| 2008 | 13,724 (31.2) | 14,755 (33.5) | 12,994 (29.5) | 1,917 (4.4) | 567 (1.3) | 2,526 (5.7) |
| 2009 | 28,395 (32.2) | 28,009 (31.8) | 26,707 (30.3) | 3,776 (4.3) | 1,100 (1.2) | 5,008 (5.7) |
| 2010 | 20,710 (33.5) | 17,628 (28.5) | 19,513 (31.5) | 2,990 (4.8) | 944 (1.5) | 4,039 (6.5) |
| 2011 | 17,455 (34.9) | 12,959 (25.9) | 16,116 (32.2) | 2,514 (5.0) | 836 (1.7) | 3,437 (6.9) |
| 2012 | 18,255 (32.8) | 16,045 (28.9) | 17,397 (31.3) | 2,891 (5.2) | 849 (1.5) | 3,859 (6.9) |
| 2013 | 19,313 (33.8) | 16,716 (29.3) | 17,184 (30.0) | 2,864 (5.0) | 875 (1.5) | 3,886 (6.8) |
| 2014 | 21,033 (33.8) | 18,762 (30.1) | 18,277 (29.3) | 3,130 (5.0) | 917 (1.5) | 4,197 (6.7) |
| 2015 | 21,736 (35.2) | 17,944 (29.0) | 17,976 (29.1) | 3,066 (5.0) | 918 (1.5) | 4,115 (6.7) |
| 2016 | 23,296 (35.0) | 20,329 (30.5) | 18,611 (27.9) | 3,276 (4.9) | 950 (1.4) | 4,382 (6.6) |
| 2017 | 25,687 (35.1) | 22,906 (31.3) | 19,802 (27.1) | 3,565 (4.9) | 940 (1.3) | 4,731 (6.5) |
| 2018 | 26,165 (34.7) | 24,851 (33.0) | 19,786 (26.3) | 3,419 (4.5) | 947 (1.3) | 4,570 (6.1) |
| Total | 235,769 (33.9) | 210,904 (30.3) | 204,406 (29.4) | 33,408 (4.8) | 9,843 (1.4) | 44,750 (6.4) |
3.5. Prevalence of HBV infection among the sub-total population
The prevalence of HBsAg was 49.9% (5,757/11,535) among 11,535 patients visiting the hepatitis and infectious disease clinic [52.7% (3,637/6,896) for male patients and 45.7% for female patients (2,120/4,639)]. As nearly 50% of the 11,535 patients visiting the hepatitis and infectious disease clinic had chronic HBV infection, and in order to make our study population (696,048) closer to community population, data of these 11,535 patients were removed from the total population and Table 6 was prepared with the rest (termed sub-total population, n = 68, 4513) of the total population. The results or trends of the sub-total shown in Tables 6 were similar to results or trends of total population shown in Table 3.
Table 6.
Prevalence of HBV infection in men and women in a sub-total population visiting PUMCH, Beijing, 2008–2018 (n = 68, 4513).
| Men Pos no. (%) |
Women Pos no. (%) |
Total Pos no. (%) |
χ2-value | P-value | |
|---|---|---|---|---|---|
| Susceptible | 81,789 (31.8) | 152,199 (35.6) | 233,988 (34.2) | 983.1 | <0.0001# |
| Immune via vaccination | 67,823 (26.4) | 141,272 (33.0) | 209,095 (30.5) | 3,293.6 | <0.0001 |
| Previous/occult infection | 86,534 (33.7) | 115,648 (27.0) | 202,182 (29.5) | 3,438.1 | <0.0001 |
| Inactive HBsAg carrier | 15,123 (5.9) | 14,814 (3.5) | 29,937 (4.4) | 2,262.2 | <0.0001 |
| Active infection | 4,550 (1.8) | 3,234 (0.76) | 7,784 (1.1) | 1,473.9 | <0.0001 |
| HBsAg | 2,0430 (8.0) | 18,563 (4.3) | 38,993 (5.7) | 3,912.7 | <0.0001 |
Sub-total population: the total populations of 696,048 were subtracted by 11,535 patients visiting the hepatitis & infectious disease clinic.
Comparison of prevalence of HBV susceptibility between men and women; the same pattern was used for other comparisons in Table 6.
3.6. Prevalence of HBV infection among the healthy population
There were 133,491 healthy subjects from the health medicine department who underwent annual health examinations during the 10 year study period, all of whom were employees from companies, schools, hospitals, or government agencies in Beijing. The healthy subjects were tested for all five marker of HBV serology and the results are shown in Table 7. The prevalence of HBsAg was 4.3% (5, 695/ 133, 491) among the healthy population, 5.2% (3,869/74,263) for men and 3.1% (1,826/59,228) for women. Men also had a significantly higher prevalence of previous / occult HBV infection, inactive HBsAg carrier, active HBV infection, and HBsAg than women (χ2 = 81.9–414.6, P < 0.0001). Women had a significantly higher prevalence of immunity via vaccination than men (χ2 = 518.1, P < 0.0001).
Table 7.
Prevalence of HBV infection in men and women of healthy populations, PUMCH, Beijing, 2008–2018 (n = 133,491).
| Men Pos no. (%) |
Women Pos no. (%) |
Total Pos no. (%) |
χ2-value | P-value | |
|---|---|---|---|---|---|
| Susceptible | 19,043 (25.6) | 15,760 (26.6) | 34,803 (26.1) | ||
| Immune via vaccination | 29,129 (39.2) | 26,898 (45.4) | 56,027 (42.0) | 518.1 | <0.0001# |
| Previous /occult infection | 22,214 (29.9) | 14,743 (24.9) | 36,957 (27.7) | 414.6 | <0.0001 |
| Inactive HBsAg carrier | 3,199 (4.3) | 1,566 (2.6) | 4,765 (3.6) | 264.5 | <0.0001 |
| Active infection | 580 (0.78) | 232 (0.29) | 812 (0.61) | 81.9 | <0.0001 |
| HBsAg | 3,869 (5.2) | 1,826 (3.1) | 5,695 (4.3) | 364.4 | <0.0001 |
Comparison of prevalence of HBV immunity via vaccination between men and women of the healthy population; the same pattern was used for other comparisons in Table 7.
3.7. Comparison of prevalence of HBV infection among adult age groups
Comparison of prevalence of HBV infection among four age groups between the adult healthy population and adult sub-total population are shown in Table 8. The prevalence of HBV susceptibility and HBsAg (including inactive HBsAg carrier and HBV active infection) were significantly lower in four age groups (21–30 y, 31–40 y, 41–50 y and 51–60 y) among the healthy population than the sub-total population (χ2 = 90.7–857.2, P < 0.0001). In contrast, the prevalence of HBV immunity via vaccination was significantly higher in the four age groups among the healthy population than the sub-total population (χ2 = 567.8–2,024.1, P < 0.0001).
Table 8.
Comparison of prevalence of HBV infection among four age groups between the healthy and sub-total populations visiting PUMCH, Beijing, 2008–2018.
| Age (yr.) | Susceptible Pos no. (%) |
Immune via vaccination Pos no. (%) |
Previous/occult infection Pos no. (%) |
Inactive HBsAg carrier Pos no. (%) |
Active infection Pos no. (%) |
HBsAg Pos no. (%) |
|---|---|---|---|---|---|---|
| 21–30# | 5,778 (20.7) | 17,245 (61.9) | 4,163 (14.9) | 461 (1.6) | 209 (0.75) | 681 (2.4) |
| 21–30## | 31,272 (27.1) | 61,351 (53.1) | 17,728 (15.4) | 3,096(2.7) | 1,738 (1.5) | 4,999(4.3) |
| χ2 | 474.7### | 686.8 | 209.9 | |||
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| 31–40 | 9,838 (24.6) | 18,242 (45.7) | 10,090 (25.3) | 1,445(3.6) | 264 (0.66) | 1,743(4.4) |
| 31–40 | 52,857 (30.7) | 67,414 (39.2) | 41,935 (24.4) | 7,318(4.3) | 2,056 (1.2) | 9,713(5.6) |
| χ2 | 576.5 | 567.8 | 103.8 | |||
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| 41–50 | 9,592 (28.7) | 11,228 (33.6) | 10,636 (31.9) | 1,668 (5.0) | 217 (0.65) | 1,926 (5.8) |
| 41–50 | 52,577(37.3) | 32,669 (23.2) | 45,573 (32.3) | 8,102 (5.7) | 1,793(1.3) | 10,223(7.2) |
| χ2 | 857.2 | 1572.7 | 90.7 | |||
| P-value | <0.0001 | <0.0001 | <0.0001 | |||
| 51–60 | 6,070 (29.4) | 6,283 (30.5) | 7,214 (35.0) | 854 (4.1) | 84 (0.41) | 962 (4.7) |
| 51–60 | 44,939 (39.4) | 19,463 (17.1) | 41,856 (36.7) | 6,255 (5.5) | 1,210 (1.1) | 7,725 (6.7) |
| χ2 | 739.8 | 2,024.1 | 128.7 | |||
| P-value | <0.0001 | <0.0001 | <0.0001 |
Sub-total population (n = 68, 4513): the total populations of 696,048 were subtracted by 11,535 patients visiting the hepatitis & infectious disease clinic.
Age group located at upper line represents the age group from normal population; age groups located at other upper lines in Table 8 were also from normal population.
Age group located at down line represents the age group from sub-total population; age groups located at other down lines in Table 8 were also from sub-total population.
Comparison of prevalence of HBsAg between 21 and 30 age group of normal population and 21–30 age group of sub-total population, same pattern was used for other comparisons in Table 8.
3.8. Prevalence of HBV infection in subjects born during and after 1992
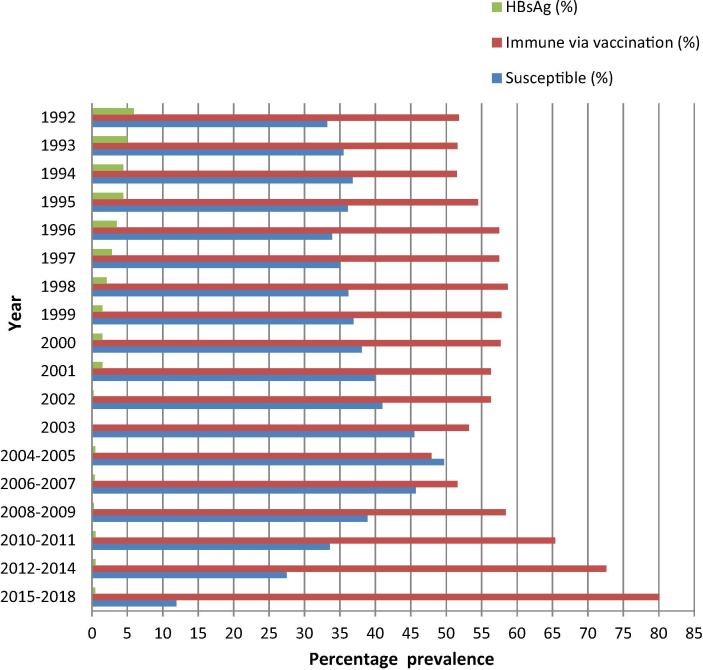
The prevalence of HBsAg, HBV susceptibility, and immunity via vaccination in subjects born in 1992 and later are shown in Fig. 1. The prevalence of HBsAg in the population born in 1992 was 5.9% and decreased annually, to 1.5% and less than 1% for those born in 2001 and 2002, respectively. The prevalence of immunity via vaccination increased steadily, and the prevalence of HBV susceptibility decreased in populations born in 2004–2005 and later.
Fig. 1.
The prevalence of HBsAg, HBV susceptibility, and immunity via vaccination in the population born during and after 1992.
4. Discussion
The prevalence of HBsAg in 2015 among randomized the resident population aged 0–59 in Hangzhou, Eastern China was 6.19% [15] and 6.1% among the general Chinese population aged 1–59 years [16]. As shown in Table 3, the overall prevalence of HBsAg was 6.4% among the total 696,048 samples from patients, pregnant women and healthy subjects following routine examinations. The first limitation of our study was that our samples were from a single center, therefore the prevalence of HBV infection may not be representative of the general population. However, due to the large sample size and since half the patients were from outside of Beijing, our results may represent a slightly wider geographical range within China. The second limitation of our study was that the subjects were not actually asked whether they had been vaccinated. So the percentage of population with anti-HBs at the concentration ≥ 10mIU/mL in our study was lower than the percentage of population who were actually immunized with HBV vaccine because there were many people who lost antibody over time or had no responses to the vaccination.
Similar to other reports [8], [16], [17]; men always had significantly higher prevalence of HBsAg than women in our study (Table 6). This sex difference occurred not only in the ill population but also among healthy subjects undergoing routine examinations, and in patients visiting the hepatitis & infectious disease clinic. Furthermore, men were also found to have a significantly higher prevalence of previous / occult HBV infection and significantly lower prevalence of immunity via vaccination than women in the total study population, in the sub-total population (Table 6) and in the healthy population (Table 7).
As shown in Fig. 1, it took 10 years to reduce the prevalence of HBsAg from 5.9% among populations born in 1992 to less than 1% among those born in 2002 due to the gradual increase of vaccination coverage since its implementation in 1992. There was a retrogression of HBV vaccination campaigns among subjects born between the years 2000 to 2004/5 because of a serial decrease in vaccine-induced immunity with a slight increase in participants from the susceptible population. The turning point was 2004–2005 when subjects born then and after were found to have steadily increasing incidence of immunity via vaccination and decreased incidence of HBV susceptibility.
The prevalence of HBsAg was around 0.5% in subjects ≤ 10 years of age (Table 4). This finding, those shown in Fig. 1, and the findings of other studies [7], [8], [18], [19], [20] indicate that a HBV immunization program with high coverage has been established among infants and young children in China. However, the prevalence of HBsAg among populations aged 11–20 y increased to 3.7% and 7.9% among the age group 41–50 y. This is due to a decrease in immunity via vaccination from nearly 50% to 20% and the susceptibility rate remained more than 30% (Table 4), causing a circulation (horizontal transmission) of HBV infection among youths and adults, independent from immunized infants and young children. Furthermore, this horizontal HBV circulation (indicated as previous / occult HBV infection) continued with ages and went to population over 81 years old. Interestingly, the prevalence of HBsAg decreased in populations ≥ 51 years old, because the number of deaths of patients with HBsAg exceeds the number of newly emerged patients with HBsAg due to horizontal HBV infection.
Unfortunately, during the 10 years from 2008 to 2018 as shown in Table 5, the prevalence of susceptibility, previous / occult HBV infection, inactive HBsAg carrier, active HBV infection, and HBsAg, did not decline and prevalence of HBV immunity via vaccination was not found to increase despite the consistent administration of HBV vaccinations since 1992. These results confirm that HBV vaccination among infants and young children alone could not reduce the prevalence of HBV infection in the adult population rapidly, otherwise we have to wait 60 years to see the decrease of HBV infection when these immunized infants and young children reach old age. Vaccination in adults is urgently needed to reduce HBV infection in China [21] and the catch-up vaccination can yield a good immune response in adults [22], [23]. Also, 90% of anti-HBs seroconversion can be achieved in the standard schedule of vaccination in healthy adults [24].
Adult subjects with low prevalence of susceptibility and high prevalence of immunity via vaccination were found to have a low prevalence of HBsAg. As shown in Table 8, all four age groups (21 − 30y, 31 − 40y, 41 − 50y and 51 − 60y) of the healthy adult population were found to have a significantly lower prevalence of HBV susceptibility and higher prevalence of immunity via vaccination than the corresponding four age groups of the sub-total population (χ2 = 90.7–857.2, P < 0.0001). Meanwhile, all four age groups of the healthy adult population were also found to have a significantly lower prevalence of inactive HBsAg carrier, active HBV infection, and HBsAg than the four groups of sub-total population (χ2 = 567.8–2.024.1, P < 0.0001). Therefore, the comparison between the adult healthy and adult sub-total populations, the latter were defined as total population subtracted by patients visiting the hepatitis and infectious disease clinic, have indicated that HBV vaccination in the adult population will cause a low incidence of HBV susceptibility and subsequently reduce the incidence of HBsAg.
The World Health Assembly approved the WHO global health sector strategy on viral hepatitis 2016–2021 to eliminate hepatitis B disease by 2030 with a target of reducing new infections by 90% and mortality by 65% [25]. Two Chinese studies [26], [27] suggested giving priority to diagnosis and treatment of HBV infected patients in order to reduce mortality by 65%. But reducing new infections by 90% by 2030 will be a challenge in China. Based on our survey, most of new HBV infections were coming from unimmunized youths and adults (Table 4). HBV vaccination in adults or catch-up vaccinations are currently neither an urgent need nor a priority in China, although it is the most economical and effective way to control HBV infection in adults. The procedure of implementation of HBV catch-up vaccination seems simple; youths and adults without documentation of previous HBV infection or vaccination should be tested for anti-HBs and persons without anti-HBs should be vaccinated. The universal screen of anti-HBs and HBV vaccination in adults above is the most effective way to reduce HBV infection in adults but it may be difficult to implement due to huge population in China. An alternative way may start at high risk population such as MSM, intravenous drug users, dialysis recipients etc. This alternative way may combine with upcoming HCV eradication strategy in some countries [28], [29], in which both anti-HBs and anti-HCV are screened to find HBV susceptible individuals and HCV infected patients among the high risk populations. HBV vaccination will be implemented in the former and direct-acting antiviral (DAA) therapy followed in the latter. However, potential unwillingness to be immunized among HBV susceptible adults could be problem, even among Chinese health care workers [30].
5. Conclusions
Hepatitis B virus (HBV) has a worldwide distribution and remains a leading public health problem in China. We used automated chemiluminescence microparticle immunoassay to test all five markers of HBV serology in serum samples among 696,048 patients, pregnant women, and normal subjects in Beijing from 2008 to 2018. During the 10 years, the prevalence of HBsAg was around 0.5% in subjects ≤ 10 years of age, but total prevalence of HBsAg was stabilized at about 6.0%, and indicators of HBV susceptibility, previous / occult HBV infection, and immunity via vaccination were not further improved despite the constant implementation of HBV vaccination since 1992. Although high coverage has been established among infants and young children, their vaccination alone could not reduce HBV infection in the adult Chinese population quickly. Adult populations with more vaccinated individuals are found to have fewer individuals with HBsAg. Vaccination in adults or at least in high-risk adults is an urgent need to decrease horizontal HBV transmission in China.
Author contributions
Shaoxia Xu, Weihong Zhang, Qiaofeng Wang, and Anping Ni conceived and designed the experiments. Shaoxia Xu, Weihong Zhang, Qiaofeng Wang, Jingtao Cui, Wenjuan Yan, and Hongjie Xie performed the experiments. Shaoxia Xu, Qiaofeng Wang and Anping Ni analyzed the data. Shaoxia Xu, Weihong Zhang, and Anping Ni wrote the manuscript. All authors have reviewed and approved the final manuscript.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for –profit sectors.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We thank Jun Wang and Wenhang Yang at our institution for providing excellent technical assistance. We would like to thank Editage (www.editage.com) for their English language editing
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jvacx.2020.100057.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
References
- 1.Global Hepatitis Report, World Health Organization, Geneva, 2017. Available at htpp:/apps.who.int/iris/bitstream/10665/255016/9789241565455_eng,pdf?ua=a, accessed May 2017.
- 2.Guideline for the prevention, care and treatment of persons with chronic hepatitis B infection, World Health Organization, Geneva, 2015. Available at htpp:/apps.who.int/iris/bitstream/10665/154590/1/9789241549059_eng,pdf;sequence=1, accessed April, 2017. [PubMed]
- 3.Schweitzer Aparna, Horn Johannes, Mikolajczyk Rafael T, Krause Gérard, Ott Jördis J. Estimations of worldwide prevalence of chronic hepatitis B virus infection: a systematic review of data published between 1965 and 2013. Lancet. 2015;386(10003):1546–1555. doi: 10.1016/S0140-6736(15)61412-X. [DOI] [PubMed] [Google Scholar]
- 4.Liang X., Bi S., Yang W., Wang L., Cui G., Cui F. Epidemiological serosurvey of hepatitis B in China—declining HBV prevalence due to hepatitis B vaccination. Vaccine. 2009;27:6550–6557. doi: 10.1016/j.vaccine.2009.08.048. [DOI] [PubMed] [Google Scholar]
- 5.Liang X., Cui F., Hadler S., Wang X., Luo H., Chen Y. Origins, design and implementation of the China GAVI project. Vaccine. 2013;31(Suppl 9):J8–J14. doi: 10.1016/j.vaccine.2012.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Xia GL, Liu CB, Cao HL, et al. Prevalence of hepatitis B and C virus infections in the general Chinese population. Results from a nationwide cross-sectional seroepidemiologic study of hepatitis A, B, C, and E virus infections in China, 1992. Int Hepatol Commun. 1996, 5:62-73.
- 7.Cui F., Shen L., Li L., Wang H., Wang F., Bi S. Prevention of Chronic Hepatitis B after 3 Decades of Escalating Vaccination Policy. China. Emerg Infect Dis. 2017;23:765–772. doi: 10.3201/eid2305.161477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Huang P., Zhu L.G., Zhu Y.F., Yue M., Su J., Zhu F.C. Seroepidemiology of hepatitis B virus infection and impact of vaccination. World J Gastroenterol. 2015;21:7842–7850. doi: 10.3748/wjg.v21.i25.7842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen T.J. Epidemiological data from Shaanxi, China. Medicine (Baltimore) 2018;97(27) doi: 10.1097/MD.0000000000011406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Meng Jing, Xu Hongqin, Sui Dongming, Jiang Jing, Li Jie, Gao Yanhang, Niu Junqi. A retrospective serological survey of hepatitis B virus infection in Northeast China. BMC Infect Dis. 2019;19(1) doi: 10.1186/s12879-019-4091-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cao W.W., Zhou R.R., Ou X., Shi L.X., Xiao C.Q., Chen T.Y. Prevalence of hepatitis B virus, hepatitis C virus, human immunodeficiency virus and Treponema pallidum infections in hospitalized patients before transfusion in Xiangya hospital Central South University, China from 2011 to 2016. BMC Infect Dis. 2018 Apr 2;18(1):145. doi: 10.1186/s12879-018-3051-7. PMID:29606088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Liu J., Zhang S., Wang Q., Shen H., Zhang M., Zhang Y. Prevalence of HBsAg/HBeAg amongst 1 936 801 couples preparing for pregnancy in rural China: An observational study. J Viral Hepat. 2017 Aug;24(8):679–686. doi: 10.1111/jvh.12693. Epub 2017 Mar 27 PMID: 28199770. [DOI] [PubMed] [Google Scholar]
- 13.Taylor Ryan, Horvat Rebecca T. 11th ed. American Society of Microbiology; 2015. Manual of Clinical Microbiology; pp. 1841–1858. [Google Scholar]
- 14.Raimondo G., Locarnini S., Pollicino T., Levrero M., Zoulim F., Lok A.S. Taormina Workshop on Occult HBV Infection Faculty Members. Update of the statements on biology and clinical impact of occult hepatitis b virus infection. J Hepatol. 2019;71(2):397–408. doi: 10.1016/j.jhep.2019.03.034. [DOI] [PubMed] [Google Scholar]
- 15.Du J., Xu Y., Wang J., Liu S., Liu Y., Zhang X. 24 year outcomes of hepatitis B vaccination in Hangzhou. China. Hum Vaccin Immunother. 2015;11(8):2051–2060. doi: 10.1080/21645515.2015.1008873. PMID: 25714188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Weilu, Ji Zhaohua, Wang Ling, Xiao Dan, Yan Yongping. A meta-analysis of HBsAg-positive rate among general Chinese populations aged 1–-59 years. Infectious Diseases. 2015;47(12):878–888. doi: 10.3109/23744235.2015.1064541. [DOI] [PubMed] [Google Scholar]
- 17.Viral C.D.C. hepatitis—statistics and surveillance. Atlanta, GA: US Department of Health and. Human Services, CDC. 2017 [Google Scholar]
- 18.Lin X., Yang J., Lu H., Zhou Y., Zhou G., Wu H. Minimization of hepatitis B infection among children in Jiangsu, China, 12years after integration of hepatitis B vaccine into the expanded program on immunization. Vaccine. 2016 Dec 12;34(51):6458–6463. doi: 10.1016/j.vaccine.2016.11.022. Epub 2016 Nov 17 PMID:27866767. [DOI] [PubMed] [Google Scholar]
- 19.Zhu Q., Shao X., Chen S., Li D., Chen X., Liu W. Epidemiological serosurvey of hepatitis virus among children aged 1–14 years in Guangdong Province. China. Int J Infect Dis. 2018 Jun;71:25–29. doi: 10.1016/j.ijid.2018.01.027. Epub 2018 Feb 9 PMID:29408358. [DOI] [PubMed] [Google Scholar]
- 20.Zeng Fangfang, Guo Pi, Huang Yun, Xin Wei, Du Zhicheng, Zhu Shuming, Deng Yu, Zhang Dingmei, Hao Yuantao. Epidemiology of hepatitis B virus infection: results from a community-based study of 0.15 million residents in South China. Sci Rep. 2016;6(1) doi: 10.1038/srep36186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qiu Y., Ren J., Yao J. Healthy adult vaccination: An urgent need to prevent hepatitis B in China. Hum Vaccin Immunother. 2016;12(3):773–778. doi: 10.1080/21645515.2015.1086519. PMID:26337328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lu J., Yan B., Liu J., Wu W., Feng Y., Xu A. Comparison of anti-HBs persistence after hepatitis B vaccination on two-dose schedule and three-dose schedule among adults: results from a 12-year follow up study in China. Hum Vaccin Immunother. 2019;15(5):1171–1176. doi: 10.1080/21645515.2018.1554972. Epub 2019 Jan 30 PMID:3049975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J., Yao J., Shan H., Chen Y., Jiang Z.G., Ren J.J. Comparison of the effect of two different doses of recombinant hepatitis B vaccine on immunogenicity in healthy adults. Hum Vaccin Immunother. 2015;11(5):1108–1113. doi: 10.4161/21645515.2014.988547. PMID:25607773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X, Wang J, Chen X, Yu M, Yu S, Sun Y, et al. Short-term immunogenicity of standard and accelerated hepatitis B virus vaccination schedules in healthy adults: a comparative field study in China. Biosci Rep. 2018 Oct 31; 38(5): BSR20180846. Prepublished online 2018 Sep 10. Published online 2018 Oct 15. doi: 10.1042/BSR20180846. PMCID: PMC6435458. [DOI] [PMC free article] [PubMed]
- 25.Global health sector strategy on viral hepatitis 2016–2021. Geneva: World Health Organization; 2016. Available from: http://www.who.int/hepatitis/strategy2016-2021/ghss-hep/en/.
- 26.Liu J., Liang W., Jing W., Liu M. Countdown to 2030: eliminating hepatitis B disease, China. Bull World Health Organ. 2019;97(3):230–238. doi: 10.2471/BLT.18.219469. Epub 2019 Jan 28 PMID:30992636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang S, Wang F, Zhang Z. Current advances in the elimination of hepatitis B in China by 2030. Front Med. 2017 Dec;11(4):490-501. doi: 10.1007/s11684-017-0598-4. Epub 2017 Nov 23. Review. PMID:29170919. [DOI] [PubMed]
- 28.Turner B.J., Rochat A., Lill S., Bobadilla R., Hernandez L., Choi A. Hepatitis C Virus Screening and Care: Complexity of Implementation in Primary Care Practices Serving Disadvantaged Populations. Ann Intern Med. 2019 doi: 10.7326/M18-3573. PMID:31791065. [DOI] [PubMed] [Google Scholar]
- 29.Wasitthankasem R, Vichaiwattana P, Siripon N, Posuwan N, Auphimai C, Klinfueng S, et al. Liver disease burden and required treatment expenditures for hepatitis C virus (HCV) infection in Thailand: Implications for HCV elimination in the new therapeutic era, a population-based study. PLoS One. 2018 Apr 24;13(4):e0196301. doi: 10.1371/journal.pone.0196301. eCollection 2018. PMID:29689073. [DOI] [PMC free article] [PubMed]
- 30.Yuan Q, Wang F, Zheng H, Zhang G, Miao N, Sun X, et al. Hepatitis B vaccination coverage among health care workers in China. PLoS One. 2019 May 7;14(5):e0216598. doi: 10.1371/journal.pone.0216598. eCollection 2019. PMID:31063488. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.