Summary
Core components of plastid protein import and the principle of using N-terminal targeting sequences are conserved across the Archaeplastida, but lineage-specific differences exist. Here we compare, in light of plastid protein import, the response to high-light stress from representatives of the three archaeplastidal groups. Similar to land plants, Chlamydomonas reinhardtii displays a broad response to high-light stress, not observed to the same degree in the glaucophyte Cyanophora paradoxa or the rhodophyte Porphyridium purpureum. We find that only the Chloroplastida encode both Toc75 and Oep80 in parallel and suggest that elaborate high-light stress response is supported by changes in plastid protein import. We propose the origin of a phenylalanine-independent import pathway via Toc75 allowed higher import rates to rapidly service high-light stress, but with the cost of reduced specificity. Changes in plastid protein import define the origin of the green lineage, whose greatest evolutionary success was arguably the colonization of land.
Subject Areas: Biological Sciences, Plant Biology, Plant Evolution
Graphical Abstract
Highlights
-
•
Chloroplastida evolved a dual system, Toc75/Oep80, for high throughput protein import
-
•
Loss of F-based targeting led to dual organelle targeting using a single ambiguous NTS
-
•
Relaxation of functional constraints allowed a wider Toc/Tic modification
-
•
A broad response to high-light stress appears unique to Chloroplastida
Biological Sciences; Plant Biology; Plant Evolution
Introduction
Mitochondria and plastids are of endosymbiotic origin and compartments surrounded by a double membrane (Zimorski et al., 2014, Archibald, 2015). Most possess their own genomes, but the bulk of their former coding capacity was either lost or integrated into the nuclear genome (Timmis et al., 2004, Martin and Herrmann, 1998). As a consequence, most of their proteins are post-translationally imported. Guiding of precursor proteins to the mitochondrial matrix or plastid stroma typically relies on N-terminal targeting sequences (NTS) (Schleiff and Becker, 2011, Dudek et al., 2013, Paila et al., 2015), although some exceptions are known (Goldberg et al., 2008, Hamilton et al., 2014, Garg et al., 2015). Archaeplastidal plastids have a monophyletic origin (Rodríguez-Ezpeleta et al., 2005, Jackson and Reyes-Prieto, 2014, Sánchez-Baracaldo et al., 2017), which is also evident from the conserved nature of plastid import components, a reliable indicator for the monophyly of organelles (Cavalier-Smith, 1999, Kalanon and McFadden, 2008, Gould et al., 2015).
Although they share a single origin, the plastids of the three algal lineages have evolved considerable differences since their divergence more than a billion years ago (Gibson et al., 2017, de Vries et al., 2016). These include, but are not limited to, (1) the thickness of a remaining peptidoglycan layer (Pfanzagl et al., 1996, Hirano et al., 2016), (2) the localization of starch deposits (Suzuki and Suzuki, 2013), (3) the coding capacity of their genomes (Timmis et al., 2004, Allen et al., 2012), (4) pigment composition and the types of antenna complexes used (Tomitani et al., 1999), (5) the absence or presence of a xanthophyll cycle (Goss and Jakob, 2010), and (6) the composition of the protein import machinery (Day et al., 2014, Kikuchi et al., 2013). It raises the question to what degree the two—critical changes in protein import and changes in plastid biology—are connected, and whether one of the two conditioned or enabled the other. Although most information about plastid protein targeting stems from the green lineage (Köhler et al., 2015), several remarkable differences between the protein import in plastids of the three algal groups (Glaucophyta, Rhodopyhta, and Chloroplastida) are known.
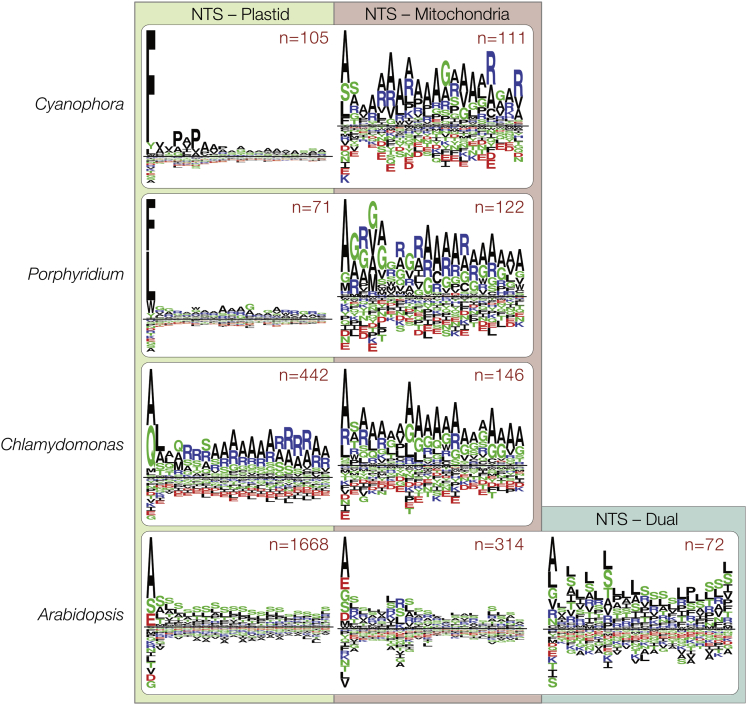
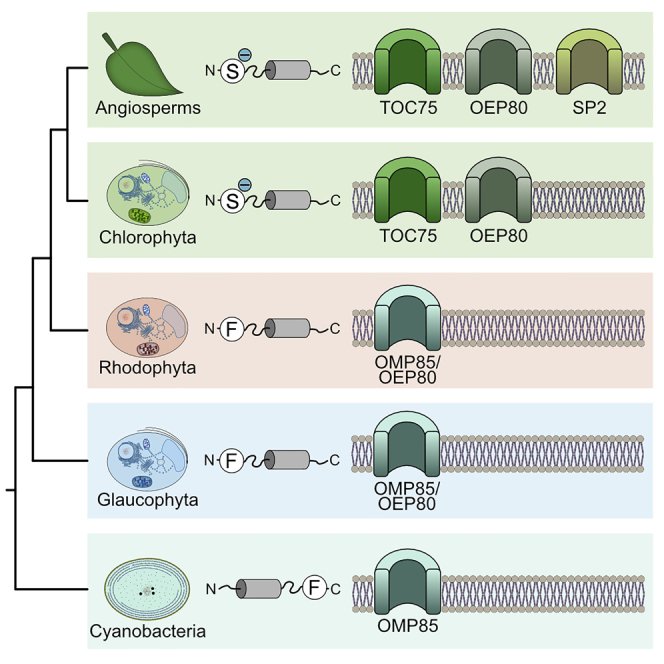
One important difference concerns the NTS that targets proteins to the stroma. Rhodophytes and glaucophytes employ a single amino-acid-based motif to target proteins to their plastids (Gould et al., 2015, Köhler et al., 2015, Steiner et al., 2005, Wunder et al., 2007). In most cases this amino acid is a phenylalanine, less frequently other bulky aromatic amino acids (Köhler et al., 2015, Gruber et al., 2007). The F-based motif is found at the very N-terminus of the NTS (Figure 1) and even retained in organisms with secondary plastids of red algal origin, such as the cryptophyteGuillardia theta, the diatom Phaeodactylum tricornumutm, and the parasite Toxoplasma gondii (Patron and Waller, 2007). It is uncertain why the F-based motif was lost in Chloroplastida, but it came with several changes such as a rise in phosphorylatable serine residues that might help in avoiding erroneous targeting to the mitochondria (Garg and Gould, 2016, Lee et al., 2006).
Figure 1.
Targeting Motifs of Organelle Targeted Proteins
NTSs of plastid- or mitochondria-targeted proteins of C. paradoxa, P. purpureum, C. reinhardtii and A. thaliana. The four species showcase the NTS for plastid- or mitochondria-targeting in the Glaucophyta, Rhodophyta, Chlorophyta and Streptophyta, respectively. Although an F-based plastid targeting motif is evident in Glaucophyta and Rhodophyta, it was lost in the green lineage.
Despite a tendency toward organelle specificity, eukaryotes also target many proteins simultaneously to two different compartments, a process known as dual-targeting. Dual-targeting can affect different combinations of compartments (Karniely and Pines, 2005, Carrie and Small, 2013), in plants also the two organelles of endosymbiotic origin. About 100 proteins are dually targeted to the mitochondria and plastids of Arabidopsis thaliana after their translation (Carrie and Small, 2013, Carrie et al., 2009). This large number is a consequence of the similarity between the two import mechanisms performed by Tom/Tim (translocator of the outer and inner mitochondrial membrane) and Toc/Tic (translocator of the outer and inner chloroplast membrane) (Schleiff and Becker, 2011, Garg and Gould, 2016). In A. thaliana, a duplicate of the Toc64 receptor localizes to the outer mitochondrial membrane and now functions in mitochondrial import (Chew et al., 2004). Both Arabidopsis organelles also use the same targeting-associated PURPLE ACID PHOSPHATASE2 (AtPAP2) at their outer membranes (Sun et al., 2012, Law et al., 2015). The extent of dual-targeting in non-chloroplastidal species remains largely unexplored.
To investigate plastid targeting in a comparative approach across the three main algal lineages, we generated RNA-Seq, pigment profile, and trans-electron microscopy data from three different conditions (with high-light stress as the stimulus) for the chlorophyte Chlamydomonas reinhardtii, the rhodophyte Porphyridium purpureum, and the glaucophyte Cyanophora paradoxa. The data were compared and evaluated in light of evolutionary changes regarding protein import. Our analysis connects the loss of F-based targeting and the emergence of new critical import proteins in the ancestor of the Chloroplastida, with a series of major changes connected to the origin of the green lineage.
Results
Adaptive Changes of Common Photosynthetic Pigments upon High-Light Stress
Plants react in particular to changes in light intensity (Lichtenthaler et al., 1981, Zhu, 2016). To analyze the differences that high-light stress has on the three algae, representing the three major groups (Table 1), we set out to perform comparative studies. The algae were adapted to growing at 50 μmol photons m−2s−1 under a 12/12-h day-night cycle and at 20°C. Through rapid light curves we assessed that at 600 μmol photons m−2s−1, a saturation of the photosystems was reached in all three species (Figure S1). For the high-light stress treatment, the algae were therefore exposed to 600 μmol photons m−2s−1 for 1 h. For comparison we determined the pigment profiles from cultures that were either 6 h into the night or 6 h into the day phase.
Table 1.
Major Differences among the Three Primary Algae Lineages and Land Plants, concerning Their Coding Capacity, Composition of the Photosynthetic Apparatus, and Carbon Storage Properties
| Organism | Protein-Coding Genes |
Antenna Proteins | Chlorophylls | Antenna Pigments | Thylakoid Organization | Starch & Storage | ||
|---|---|---|---|---|---|---|---|---|
| Nucleus | Plastid | Mitochondrion | ||||||
| Arabidopsis thaliana (Streptophyte plant) | 35,176 | 88 | 122 | LHC protein complex | a,b | Beta-Carotin, lutein, neoxanthin, violaxanthin, antheraxanthin, zeaxanthin | Stacked, Grana | Starch |
| Chara braunii (Streptophyte algae) | 23,546 | 105 | 46 | LHC protein complex | a,b | Beta-Carotin, lutein, neoxanthin, violaxanthin, antheraxanthin, zeaxanthin | Stacked | Starch |
| Chlamydomonas reinhardtii (Chlorophyte algae) | 14,411 | 69 | 8 | LHC protein complex | a,b | Beta-Carotin, lutein, neoxanthin, violaxanthin, antheraxanthin, zeaxanthin | Stacked | Starch |
| Porphyridium purpureum (Rhodophyte) | 8,355 | 199 | ND | Phycobilisomes | a | Beta-Carotin, zeaxanthin | Unstacked, equidistant and single | Glycogen, floridean starch |
| Cyanophora paradoxa (Glaucophyte) | 27,921 (25,831) | 136 | 44 | Phycobilisomes | a | Beta-Carotin, zeaxanthin | Unstacked, equidistant and single | Floridean starch |
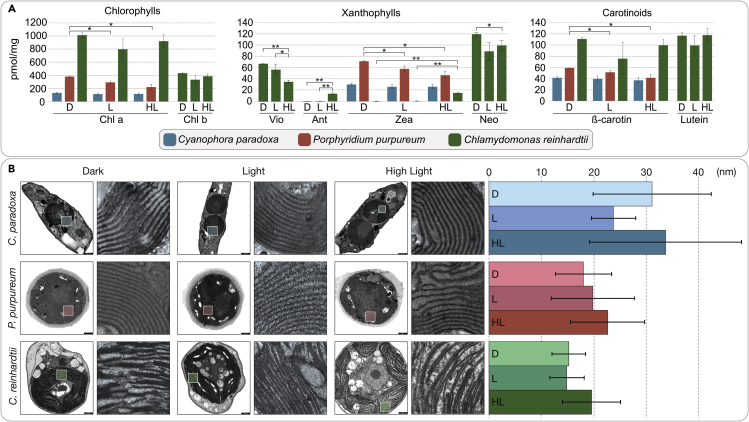
The glaucophyte C. paradoxa showed no significant change in pigment concentration or composition, neither at night nor after light stress (Figure 2A). For the red alga P. purpureum we observed only very marginal changes and the concentration of pigments for the samples collected at night was the highest. Pigment concentrations seemed to slowly decrease during the day and even further under high-light stress. This was observed for all three major pigment groups at a similar rate (Figure 2A). Only in the green alga C. reinhardtii the pigment composition changed significantly, especially upon high-light stress (Figure 2A). Here in particular the xanthophyll cycle—the enzyme-driven and reversible conversion of violaxanthin into zeaxanthin—was evident, a component of non-photochemical quenching thought to be absent in glauco- and rhodophytes (Goss and Jakob, 2010). Concentrations of chlorophylls and carotenoids actually increased under high-light stress in C. reinhardtii, demonstrating their rapid de novo synthesis.
Figure 2.
Pigment Profiles and Analysis of Thylakoid Stack Distance during High-Light Stress
(A) Pigments were extracted by homogenization with acetone and their concentrations determined by an HPLC analysis. In both the glaucophyte and rhodophyte the pigment concentrations remain rather stable, and only a slight decrease in the overall pigment concentration is observed during the day and even more so during high-light stress. On the contrary, in C. reinhardtii all three types of pigment change their concentration significantly and e.g., the stepwise reduction of violaxanthin (Vio) to antheraxanthin (Ant) and zeaxanthin (zea) is evident.
(B) Cells from the three different conditions were fixed and analyzed using transmission electron microscopy and distances between the thylakoids were measured using Fiji. A statistically significant increase in thylakoid distance upon high-light stress is only observed in C. reinhardtii, although a similar but less significant trend is observed in the red alga P. purpureum. (*<0.05 ** < 0.001 *** < 0.0001). pmol/mg, picomol/milligram of dry weight. Scale bars equal 1000 nm.
The thylakoid stacks (grana) of land plants relax under high-light stress in order for the repair mechanism of the photosystems to properly function (Khatoon et al., 2009). This concerns in particular the degradation of the D1 protein through the membrane-bound protease FtsH, whose dimerized size is too large for the space where two thylakoid stacks align (Yoshioka-Nishimura and Yamamoto, 2014). Algae form different types of thylakoid stacks (Bertrand, 2010, Tsekos et al., 1996) but no grana-like structures. We performed trans-electron microscopy (TEM)-based analysis of the cells from the three different conditions and determined the distance between neighboring thylakoid stacks. The differences we observed were in all cases marginal, but only in the case of C. reinhardtii did we observe a statistically significant increase in spacing upon high-light stress (Figure 2B).
The Transcriptional Response to High-Light Stress Is Most Pronounced in the Chlorophyte
We also generated RNA-Seq data on all samples. They reveal stark differences among the three species in terms of overall transcriptional regulation (Figure 3). In the chlorophyte, the response to high-light stress was the most pronounced among the three algae, both regarding the number of differentially expressed genes as well as the number of upregulated genes during high-light conditions. For each condition a clear separation was observed, and a specific gene set was found to be upregulated relative to the average expression of each gene over all conditions (Figure 3).
Figure 3.
Differentially Expressed Genes of C. reinhardtii, C. paradoxa, and P. purpureum
Visualization of all differentially expressed genes of C. reinhardtii, C. paradoxa, and P. purpureum, colored according to the logarithmic fold change relative to the average expression across all conditions, mean centered and color coded in log2 space. For C. reinhardtii and C. paradoxa, the fold change's significance of all visualized transcripts is at least 0.001. For P. purpureum, the significance cutoff was lowered to 0.05, because the original cutoff revealed only 90 differentially expressed genes. C. reinhardtii shows distinct sets of genes, each tailored toward one of the tested light conditions. C. paradoxa and P. purpureum, on the other hand, do not show such an adaptation to altering light conditions, especially not to high-light (p = 0.001). C. paradoxa does not change much of its gene expression between daylight and high-light conditions, showing its lack of adaptation. Although P. purpureum expresses a set of genes only during high-light conditions, their differential expression was only detectable by lowering the significance cutoff. Even if all differentially expressed genes of P. purpureum are considered, its transcriptional changes during high-light remain minor.
Under high-light conditions the chlorophyte upregulates the expression of photosynthesis machinery components as well as proteins that promote photoprotection. A total of 418 transcripts were found to be differentially expressed (of a total of 2,810 differential expressed transcripts between any conditions), 274 values of which were significantly upregulated compared with daylight conditions (Table S3). The upregulated photoprotective proteins include stress-related chlorophyll-binding proteins 1 and 3 involved in energy-dependent quenching to dissipate excess energy (Bonente et al., 2011), members of the early-light inducible protein family (Elip), and ancestral homologs of the non-photochemical quenching-associated PSBS/LHCSR3 family (Hutin et al., 2003, Engelken et al., 2010), a CPD photolyase class II that reverses the formation of pyrimidine dimers that result from the exposure to strong UV radiation (Carell et al., 2001), and chlorophyll b reductases and beta-carotene hydroxylases that prevent over-excitation of the photosystem and protect the cells from high-light intensities (Sato et al., 2015, Davison et al., 2002). Next to these photoprotective proteins, photosynthesis house-keeping genes such as PSII Pbs27, Rieske protein, PSII subunit 28, and several proteins of the LHC superfamily were upregulated as well as a few stress-response proteins such as the plastidal homolog of DnaJ and other members of the HSP70 protein family that together form a multichaperone complex (Willmund et al., 2008) (Table S3).
In the glaucophyte C. paradoxa, most of the 1,463 differentially expressed transcripts were found upregulated during darkness in correspondence to nightly proliferation (Figure 3). The overall difference between day and night was far more pronounced than day versus high-light and the difference between light and high-light conditions smaller than in Chlamydomonas (Table S3). In comparison to the green alga, fewer proteins involved in photosynthesis regulation and photoprotection were found to be upregulated during high-light stress, but they also included several Elip proteins.
The identification of differentially expressed genes in P. purpureum proved more difficult. Only 90 transcripts were initially identified, but by lowering the significance cut-off according to the standard edgeR protocol we were able to detect another 980. The expression profile matches that of C. paradoxa, although the number of regulated genes is far smaller (when compared with the same cutoff of p = 0.001). For the transition from daylight to high-light, a total of 38 transcripts were identified (Table S3). The most notable genes that were upregulated during high-light stress were a high-light inducible protein (Hlip) involved in non-photochemical quenching (Komenda and Sobotka, 2016) and several heat shock proteins (HSP70). The response to light stress was far weaker than in the other two algae (p = 0.001), but P. purpureum might regulate its RNA levels through the extensive use of miRNAs (Gao et al., 2016), which could contribute to the lower levels of differentially expressed genes identified.
Comparisons of the most highly upregulated proteins of each of the three algae among all conditions revealed additional differences in light-dependent differential gene expression. Although C. reinhardtii upregulated the synthesis of several photosynthesis and plastid-related proteins during light and high-light conditions, C. paradoxa and P. purpureum upregulated only a few. In the case of C. paradoxa, the biggest notable difference was the focus on protein biosynthesis during darkness/night. The 50 most highly upregulated proteins during the night were ribosomal proteins (approximately 90%), indicating an increase in overall protein biosynthesis and proliferation (Table S4). We observed photosynthesis machinery as well as photoprotection components to be among the most upregulated proteins in combination only in C. reinhardtii, illustrating the chlorophyte's more elaborate and immediate ability to adapt to changing light conditions compared with the other two screened algae.
The Red Toc75 Is an Oep80 and Toc75 Unique to Chloroplastida
Most members of the ArabidopsisToc75 family have been characterized. This includes the main import channel of the outer membrane, Toc75 (Kessler et al., 1994) (TOC75-III, TAIR: At3g46740), as well as Oep80 (TOC75-V, TAIR: At5g19620) whose exact function remains unresolved while the protein is essential for plant viability (Hsu et al., 2008, Patel et al., 2008), and the most recently characterized SP2 (TAIR: At3g44160), which serves protein export for chloroplast-associated protein degradation (Ling et al., 2019). The situation in rhodophytes and glaucophytes differs. They do not seem to encode the same number of Toc75 homologs (Day et al., 2014, Töpel et al., 2012).
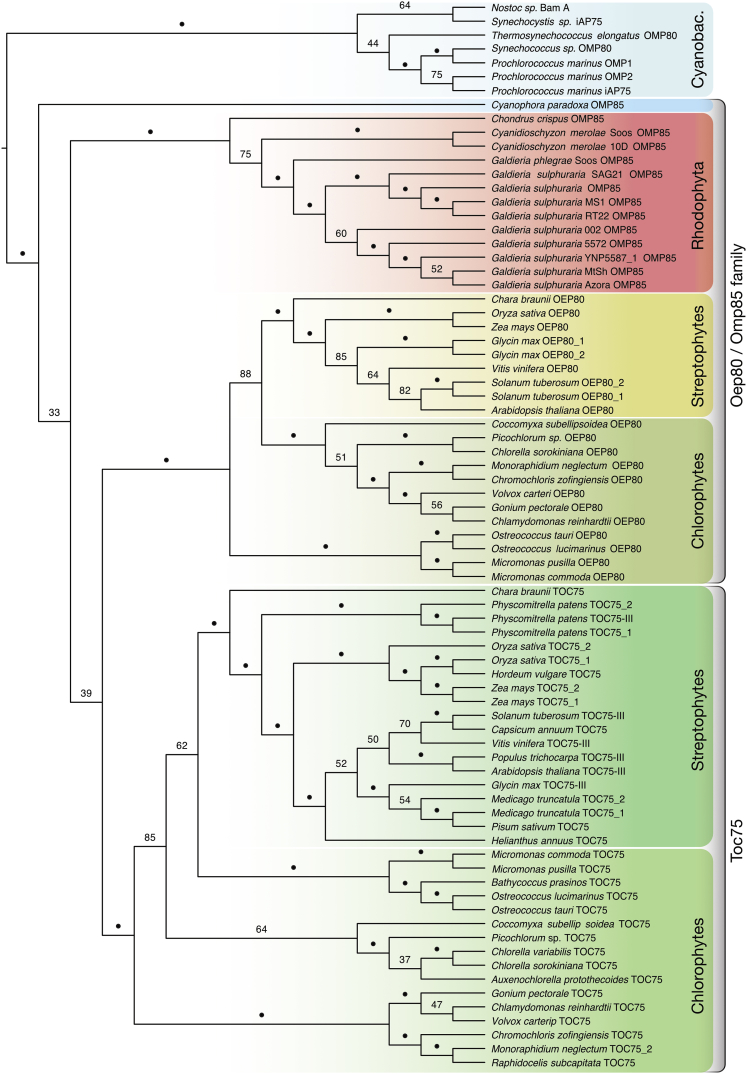
We collected 77 eukaryotic proteins of the Toc75 and Oep80 family from 44 eukaryotic species and rooted them against their cyanobacterial homologs for the construction of a phylogenetic tree. The single glaucophyte sequence sits basal to all others, whereas the rhodophyte sequences form a well-supported group that is sister to all chloroplastidal sequences (Figure 4). The sequences of green algae and plants fall into two distinct and again well-supported clusters: one comprises a group of proteins including the AtOep80, the other a group containing the main import pore AtToc75. Within these two groups separating the Oep80 from the Toc75 proteins, the separation between the chloro- and streptophytes is observed, as well as the basal branching of the streptophyte alga Chara braunii related to the ancestor of land plants (Wickett et al., 2014, Nishiyama et al., 2018) (Figure 4).
Figure 4.
Phylogenetic Analysis of Oep80 and Toc75 Homologs
A total of 77 amino acid sequences of Oep80/Toc75 homologs from members of the chlorophytes, rhodophytes, and glaucophytes were used for phylogeny reconstruction via RAxML (PROTCATWAGF) with 100 bootstraps. The tree was rooted on the split between the monophyletic cyanobacteria and the eukaryotic sequences. The cyanobacteria as well as all three algal groups form monophyletic groups. Within the green lineage, the Toc75 and Oep80 sequences form separate clusters, indicating the emergence of Toc75 within the green lineage. Bootstrap values > 90 are represented by dots.
Discussion
If one measures evolutionary success by species diversity, the green lineage is the most successful. About 16,000 green algal, 5,000 rhodophyte, and 13 glaucophyte species have been recognized (with >100,000, 500–1,000 and about a dozen that remain to be described, respectively) (Andersen, 1992). Another 400,000 land plant species (Govaerts, 2001) evolved since the conquering of land some 480 million years ago (Kenrick et al., 2012, Delwiche and Cooper, 2015). We argue that the evolutionary origin and success of the green lineage hinges upon early changes in plastid protein targeting.
Algae and plant cells target more than a thousand proteins specifically to each of their two compartments of endosymbiotic origin. Plastid targeting evolved in a cell that had already established mitochondrial targeting, yet both import machineries share similarities and both rely on specific NTSs for matrix and stroma targeting (Schleiff and Becker, 2011). The origin of the mitochondrial NTS is uncertain, but its positive charge was an early requirement to overcome the bioenergetic inner mitochondrial membrane (Garg et al., 2015). The most N-terminal domain carries the charged residues critical for distinguishing between mitochondrial- and plastid-targeting (Figure 1), whereas the C-terminus is exchangeable (Lee et al., 2019). Because the plastid is younger and because the photosynthetic organelle evolved in a eukaryotic cell instead of contributing to its actual origin, we understand more about the origin of the plastid NTS.
On the Origin of the N-terminal Targeting Sequence
It has been speculated that N-terminal targeting sequences evolved from antimicrobial peptides (AMPs) (Wollman, 2016), as both share similarities in terms of charged amino acid residues, the ability to form amphiphilic α-helices, and because they are frequently identified in host-endosymbiont relationships (Mergaert et al., 2017). One example regarding the latter is Paulinella chromatophora, whose chromatophore origin is independent from that of the Archaeplastida and much younger (Nowack, 2014). Two types of NTSs were identified that target nuclear-encoded proteins to the chromatophore, but both are not related to the simultaneously identified AMPs (Singer et al., 2017), which argues against an AMP-origin of the NTS in Paulinella. The concept is also not compatible with the origin of phenylalanine-based plastid targeting and Toc75.
The components of the Toc and Tic machinery share a mixed pro- and eukaryotic ancestry (Jarvis and Soll, 2001, Day and Theg, 2018). Toc75, the β-barrel import pore in the outer membrane, is of prokaryotic origin and a member of the Omp85 superfamily (Day et al., 2014). Some bacterial Omp85's recognize their substrates through a C-terminal phenylalanine (Robert et al., 2006) and evidence is emerging that the POTRA domains of Toc75 act as binding sites for the NTS (O'Neil et al., 2017). If we recall that the phenylalanine-based motif is retained in rhodophytes and glaucophytes (Wunder et al., 2007), we can conclude that the pNTS did not evolve from AMPs but rather adapted in evolution and traces back to a recognition signal for the cyanobacterial Omp85 that evolved into Toc75 (Sommer et al., 2011). The ancestral character of phenylalanine-based plastid-targeting was lost with the origin of the Chloroplastida and we suggest simultaneously to the expansion of the Toc75 family, with significant consequences for the green lineage.
Dual-Targeting Using a Single Ambiguous Signal as a Result of Losing the F-based Motif
The use of an F-based motif offered an elegant solution to the archaeplastidal ancestor. It utilized an existing translocons-substrate recognition mechanism and allowed to distinguish cytosolically translated mitochondrial from plastid proteins through a single amino-acid-based motif. With the emergence of the green Toc75 and loss of the F-based motif, false targeting likely increased. One counter-measure was the increase in phosphorylation sites in the NTS, which adds negative charge and hampers import of the substrate by mitochondria (Garg et al., 2015, Lee et al., 2006, Law et al., 2015). Many proteins, however, remain dual targeted in Arabidopsis (Carrie and Small, 2013), and we predict this is restricted to the green lineage. Dual-targeting to mitochondrion and plastid does occur in algae with a red plastid, but through alternative transcription/translation initiation and not through the use of a single ambiguous NTS (Gile et al., 2015).
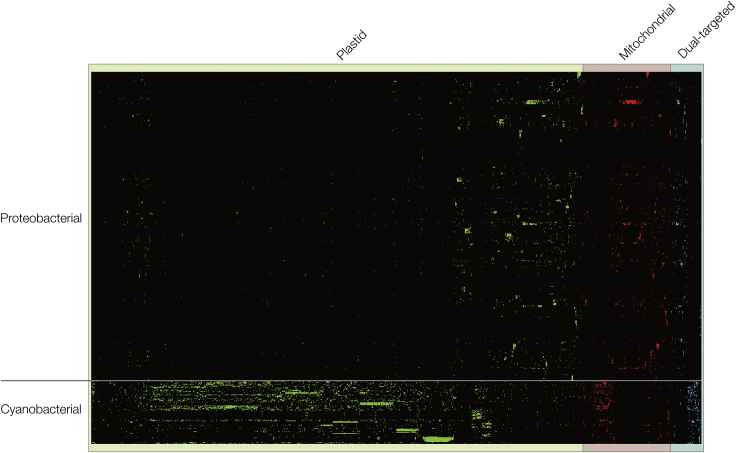
Evolution is blind. Dual-targeting evolves from falsely targeted proteins that initially might not offer a direct benefit but are also not detrimental to the cell's viability. This can re-localize or establish entire new pathways (Martin, 2010). Our phylogenetic analysis (Figure 5) shows there is no apparent preference regarding the evolutionary origin (cyanobacterial vs. proteobacterial) and flow of dual-targeted proteins: as much proteins of cyanobacterial origin are targeted to the mitochondria as there are vice versa (Figure 5).
Figure 5.
Phylogenetic Origin of Plastid- and Mitochondria-Targeted Proteins of Arabidopsis
Binary presence and absence pattern of homologs of plastid- (green), mitochondria- (red), and dual-targeted (blue) proteins of A. thaliana within 94 cyanobacterial and 460 alphaproteobacterial proteomes. Organisms are sorted according to previously constructed group-specific phylogenies, whereas genes are sorted by hierarchical clustering. Most homologs of plastid-targeted genes were identified in cyanobacteria, but for more than one-fifth (22%) of the plastid-targeted genes the majority of homologs were identified in alphaproteobacteria. In the case of mitochondria-targeted genes, for almost one-third (35%) of the genes most homologs were identified in cyanobacteria instead of alphaproteobacteria. The phylogenetic signal of the dual-targeted genes is more evenly distributed among cyanobacteria and alphaproteobacteria, with one-half (45%) showing a cyanobacterial origin and the other half (55%) showing an alphaproteobacterial origin.
Dual-targeted proteins are largely part of the transcription and translation machinery (Carrie and Small, 2013), which might include the plastid-associated polymerases whose dual-targeting in Chloroplastida might be an ancestral trait of the lineage (Nishiyama et al., 2018). Both the mitochondrion and plastid have a genome and as such information processing proteins suit a dual-targeting route well. A simultaneous control over the transcription and translation of both organelles might allow for a faster and accurate response or simply easier house-keeping. Dual-targeting reinforces the simultaneous addressing of the two organelles of endosymbiotic origin, which we speculate offers an evolutionary advantage to cells carrying dozens of mitochondria and plastids simultaneously such as the cells of land plants but not all algae.
An Oep80-Derived Toc75 Is Unique to the Green Lineage
One of the earliest descriptions of Toc75 was for a protein isolated from pea (Schnell et al., 1994). Conserved homologs across all Chloroplastida were quickly identified (Kalanon and McFadden, 2008, Shi and Theg, 2013) but required more effort across the diversity of the Archaeplastida. Through the identification of an Omp85 homolog in algae with secondary red plastids, it became evident that all phototrophic eukaryotes harbor beta-barrel-forming proteins of an extended Omp85 family that form the import pore in the outer plastid membrane (Bullmann et al., 2010), but with a decisive difference regarding the number of encoded homologs.
Our phylogenetic analysis of Toc75 and Oep80 supports previous analyses without the need of any sequence trimming. It demonstrates the clear-cut, likely also functional, separation between the Toc75 and Oep80 proteins of Chloroplastida (Day et al., 2014). The red sequences are closer to their prokaryotic homologs, and the green Toc75 is further derived. From the perspective of phylogeny, there is little doubt that Toc75 is unique to the green lineage and originated from the duplication of a green lineage-specific Oep80/Omp85. This suggests a division of labor at the outer chloroplast membrane not found in rhodoplasts or cyanelles, the benefits of which are plenty. Glauco- and rhodophytes work with a single import pore, whereas Arabidopsis and its green relatives encode a single full-length Toc75 and a single full-length Oep80. Both of the latter are expressed at high levels in a conserved ratio and in the different tissues according to the gene expression atlas of the TAIR database (Berardini et al., 2015). Their presence is needed simultaneously and appears synchronized.
We speculate that the duplication of Omp85/Oep80 allows for a more efficient, faster, and versatile protein import. It might be a prerequisite for the elaborate response to high-light stress, which our data support (Figures 2 and 3). A response to high-light stress is evident in all three lineages (Figure 3), but differs in quantity and detail. Chlamydomonas not only alters its gene expression network the most upon high-light stress but also focuses more on photosynthesis maintenance and protection (Table S3), reacts less stressed, and rapidly synthesizes pigments denovo (Figure 2). The upregulation of Elips that are of cyanobacterial origin occurs in all three lineages, but they were only expanded and diversified in the green lineage (Heddad and Adamska, 2002). Retrograde signaling (a critical part of the response to high-light stress) is limited by the plastid's import capacity (Wu et al., 2019), highlighting the direct dependence.
If Oep80's main duty is indeed the integration of beta-barrel proteins (and maybe other delicate substrates of unknown nature), then it releases Toc75 from this job. This would then mirror the situation in mitochondria. Here, Tom40 acts as the main import channel, whereas Sam50 receives beta-barrel proteins with a complicated topology from Tom40 for their integration into the outer mitochondrial membrane (Wiedemann et al., 2003). The division of labor appears more effective than the simple increase in number of a single import gateway. This could have allowed the endosymbiotic gene transfer of the small subunit of RubisCo to the nucleus, a trademark of the green lineage (Broglie et al., 1983, Coen et al., 1977). The sheer amount of RbcS protein required to be imported might simply overstrain the Omp85/Oep80 of rhodo- and glaucophytes, and its gene transfer from the plastid to the nucleus is hence selected against. These patterns allow to speculate on the sequence of evolutionary events.
Initially a duplication of the ancestral green Omp85 occurred and both paralogs might have performed the same duty early on. Mutations in one of the two copies led to an independence of F-based targeting, alternative substrate recognition, the emergence of NTS phosphorylation (Garg and Gould, 2016), and a cytosolic 14-3-3/Hsp70-based guidance complex (May and Soll, 2000) that we predict is unique to the green lineage, too. The plastid-encoded Tic214(YCF1)/YCF2/FtsHi complex emerged early in chlorophyte evolution, too, maybe through the duplication of an early Tic20-like protein (Wunder et al., 2007, Kikuchi et al., 2018). The components of this complex are highly diverse, except for a C-terminal motif, and were entirely lost in grasses without impacting protein import (de Vries et al., 2015, de Vries et al., 2017). Other components were added such as Tic40 that further increases import efficiency (Chou et al., 2003) and which is absent from rhodo- and glaucophytes (Kalanon and McFadden, 2008). Ever more plastid proteins went via the Toc75 route, apart from maybe some slow folding proteins of the outer-membrane that continued to be integrated via Oep80. Noteworthy, however, both import pathways remain linked in their function (Day et al., 2019). A more recent extension was the emergence of the CHLORAD pathway (chloroplast-associated protein degradation). Its central component, SP2, is an Omp85 paralog, too, which however lacks the POTRA domains (Ling et al., 2019). It likely emerged in angiosperms and might facilitate the remodeling of plastids (e.g., of a chloroplast to a chromoplast), a feature that in its entirety is unique to the evolutionary more recent land plants and their embryoplast (de Vries et al., 2016). Therefore, the implementation of yet another plastid protein transport pathway based on an Omp85/Oep80 duplication coincided with yet another major step in land plant evolution.
Conclusion
Plastid endosymbiosis introduced phototrophs to the eukaryotic Tree of Life. A critical step was the evolution of a basicToc/Tic protein import machinery that is conserved across all algae and plants. It is evident that major modifications of the Tic/Toc machinery and changes in the targeting sequences occurred early in the origin of the Chloroplastida. This concerns especially (1) the loss of phenylalanine-based targeting and (2) the emergence of new import machinery components including Tic40, a plastid-encoded Tic214, and a Toc75 that evolved from the duplication of the ancestral green Omp85. We suggest that the former resulted in the emergence of dual organelle (plastid and mitochondrion) targeting using a single ambiguous targeting sequence and that the latter introduced an import pathway for nuclear-encoded proteins that permitted higher import rates at the expense of targeting specificity. The main import pore of the green plastid, Toc75, was freed from dealing with slow-folding proteins of the outer membrane and permitting rapid import of proteins required to cope with high-light stress. Whatever the details regarding the substrates imported by Oep80, the Chloroplastida make use of two Omp85 homologs, Oep80 and Toc75, where rhodophytes and glaucophytes use only one. The transition to life on land was a transition to high-light stress. Responses to high-light require the efficient and immediate import of over a hundred nuclear-encoded plastid proteins simultaneously after retrograde plastid signaling. This, we speculate, was realized by the implementation of an efficient plastid import pathway that enabled the evolutionary success of the Chloroplastida.
Limitations of the Study
Transcriptome assemblies profit immensely from reference genomes during assembly. Since current reference genomes of P. purpureum and C. paradoxa still lack quality, we assembled the trancriptomes de novo. By updating reference genomes and improving their quality, we might get a better insight into light-stress-induced gene regulation within the three major algal lineages. Many components of protein targeting to the mitochondrion and/or plastid have been identified but for a few their exact function remains unclear. Furthermore, direct protein import kinetics via proteomic studies are still limited by reliable plastid isolation protocols for all three species.
Methods
All methods can be found in the accompanying Transparent Methods supplemental file.
Acknowledgments
We thank Matheus Sanita Lima for discussing dual-targeting and Prof. Peter Jahns for providing access to the HPLC and help in analyzing the pigment profiles. This work was supported through the DFG (267205415– SFB 1208) and the VolkswagenStiftung (Life).
Author Contributions
SBG together with SGG designed the study and drafted the manuscript. MH cultured the algae, did microscopy, light stress experiments, and early stages of transcriptome assembly. MRK did the phylogenomic analysis of Omp85/Oep80 homologs and dual-, mitochondria-, or plastid-targeted proteins as well as the identification of differentially expressed genes during the three tested light conditions.
Declaration of Interests
The authors declare no competing interests.
Published: March 27, 2020
Footnotes
Supplemental Information can be found online at https://doi.org/10.1016/j.isci.2020.100896.
Data and Code Availability
Transcriptomes are available via the Sequence Read Archive of NCBI (https://www.ncbi.nlm.nih.gov/sra) with the accession number PRJNA509798.
Supplemental Information
Organisms are provided with name, genome assembly accession and taxonomy. The order of organisms along the Yaxis in the presence/absence plot is according to the order in this table.
Only transcripts with logarithmic fold-changes of at least 2 were considered (p = 0.001). Annotation via BLASTp and InterProScan providing the IPR-, Pfam and GO accessions if available.
Annotation via BLASTp and InterProScan providing the IPR-, Pfam and GO accessions if available. Only transcripts with logarithmic fold-changes of at least 2 were considered (p = 0.001 if not stated otherwise).
References
- Allen D.K., Laclair R.W., Ohlrogge J.B., Shachar-Hill Y. Isotope labelling of Rubisco subunits provides in vivo information on subcellular biosynthesis and exchange of amino acids between compartments. Plant Cell Environ. 2012;35:1232–1244. doi: 10.1111/j.1365-3040.2012.02485.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen R.A. Diversity of eukaryotic algae. Biodivers.Conserv. 1992;1:267–292. [Google Scholar]
- Archibald J.M. Endosymbiosis and eukaryotic cell evolution. Curr. Biol. 2015;25:R911–R921. doi: 10.1016/j.cub.2015.07.055. [DOI] [PubMed] [Google Scholar]
- Berardini T.Z., Reiser L., Li D., Mezheritsky Y., Muller R., Strait E., Huala E. The arabidopsis information resource: making and mining the “gold standard” annotated reference plant genome. Genesis. 2015;53:474–485. doi: 10.1002/dvg.22877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand M. Carotenoid biosynthesis in diatoms. Photosynthesis Res. 2010;106:89–102. doi: 10.1007/s11120-010-9589-x. [DOI] [PubMed] [Google Scholar]
- Bonente G., Ballottari M., Truong T.B., Morosinotto T., Ahn T.K., Fleming G.R., Niyogi K.K., Bassi R. Analysis of LHcSR3, a protein essential for feedback de-excitation in the green alga Chlamydomonas reinhardtii. PLoS Biol. 2011;9:e1000577. doi: 10.1371/journal.pbio.1000577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Broglie R., Coruzzi G., Lamppa G., Keith B., Chua N.H. Structural analysis of nuclear genes coding for the precursor to the small subunit of wheat ribulose-1,5-bisphosphate carboxylase. Biotechnology. 1983;1:55–61. [Google Scholar]
- Bullmann L., Haarmann R., Mirus O., Bredemeier R., Hempel F., Maier U.G., Schleiff E. Filling the gap, evolutionarily conserved Omp85 in plastids of chromalveolates. J. Biol. Chem. 2010;285:6848–6856. doi: 10.1074/jbc.M109.074807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carell T., Burgdorf L.T., Kundu L.M., Cichon M. The mechanism of action of DNA photolyases. Curr. Opin. Chem. Biol. 2001;5:491–498. doi: 10.1016/s1367-5931(00)00239-8. [DOI] [PubMed] [Google Scholar]
- Carrie C., Small I. A reevaluation of dual-targeting of proteins to mitochondria and chloroplasts. Biochim. Biophys. Acta. 2013;1833:253–259. doi: 10.1016/j.bbamcr.2012.05.029. [DOI] [PubMed] [Google Scholar]
- Carrie C., Kühn K., Murcha M.W., Duncan O., Small I.D., O’Toole N., Whelan J. Approaches to defining dual-targeted proteins in Arabidopsis. Plant J. 2009;57:1128–1139. doi: 10.1111/j.1365-313X.2008.03745.x. [DOI] [PubMed] [Google Scholar]
- Cavalier-Smith T. Principles of protein and lipid targeting in secondary symbiogenesis: euglenoid, dinoflagellate, and sporozoan plastid origins and the eukaryote family tree. J. Eukaryot. Microbiol. 1999;46:347–366. doi: 10.1111/j.1550-7408.1999.tb04614.x. [DOI] [PubMed] [Google Scholar]
- Chew O., Lister R., Qbadou S., Heazlewood J.L., Soll J., Schleiff E., Millar A.H., Whelan J. A plant outer mitochondrial membrane protein with high amino acid sequence identity to a chloroplast protein import receptor. FEBS Lett. 2004;557:109–114. doi: 10.1016/s0014-5793(03)01457-1. [DOI] [PubMed] [Google Scholar]
- Chou M.L., Fitzpatrick L.M., Tu S.L., Budziszewski G., Potter-Lewis S., Akita M., Levin J.Z., Keegstra K., Li H.M. Tic40, a membrane-anchored co-chaperone homolog in the chloroplast protein translocon. EMBO J. 2003;22:2970–2980. doi: 10.1093/emboj/cdg281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coen D.M., Bedbrook J.R., Bogorad L., Rich A. Maize chloroplast DNA fragment encoding the large subunit of ribulosebisphosphate carboxylase. Proc. Natl. Acad. Sci. U S A. 1977;74:5487–5491. doi: 10.1073/pnas.74.12.5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davison P.A., Hunter C.N., Horton P. Overexpression of β-carotene hydroxylase enhances stress tolerance in Arabidopsis. Nature. 2002;418:203–206. doi: 10.1038/nature00861. [DOI] [PubMed] [Google Scholar]
- Day P.M., Theg S.M. Evolution of protein transport to the chloroplast envelope membranes. Photosynthesis Res. 2018;138:315–326. doi: 10.1007/s11120-018-0540-x. [DOI] [PubMed] [Google Scholar]
- Day P.M., Inoue K., Theg S.M. Chloroplast outer membrane β-barrel proteins use components of the general import Apparatus. Plant Cell. 2019;31:1845–1855. doi: 10.1105/tpc.19.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day P.M., Potter D., Inoue K. Evolution and targeting of omp85 homologs in the chloroplast outer envelope membrane. Front. Plant Sci. 2014;5:1–18. doi: 10.3389/fpls.2014.00535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwiche C.F., Cooper E.D. .The evolutionary origin of a terrestrial flora. Curr. Biol. 2015;25:R899–R910. doi: 10.1016/j.cub.2015.08.029. [DOI] [PubMed] [Google Scholar]
- Dudek J., Rehling P., van der Laan M. Mitochondrial protein import: common principles and physiological networks. Biochim. Biophys. Acta. 2013;1833:274–285. doi: 10.1016/j.bbamcr.2012.05.028. [DOI] [PubMed] [Google Scholar]
- Engelken J., Brinkmann H., Adamska I. .Taxonomic distribution and origins of the extended LHC (light-harvesting complex) antenna protein superfamily. BMC Evol. Biol. 2010;10:233. doi: 10.1186/1471-2148-10-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gao F., Nan F., Feng J., Lv J., Liu Q., Xie S. Identification of conserved and novel microRNAs in Porphyridium purpureum via deep sequencing and bioinformatics. BMC Genomics. 2016;17:612. doi: 10.1186/s12864-016-2985-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg S.G., Gould S.B. Therole of charge in protein targeting evolution. Trends Cell Biol. 2016;26:894–905. doi: 10.1016/j.tcb.2016.07.001. [DOI] [PubMed] [Google Scholar]
- Garg S., Stölting J., Zimorski V., Rada P., Tachezy J., Martin W.F., Gould S.B. Conservation of transit peptide-Independent protein import into the mitochondrial and hydrogenosomal matrix. Genome Biol. Evol. 2015;7:2716–2726. doi: 10.1093/gbe/evv175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson T.M., Shih P.M., Cumming V.M., Fischer W.W., Crockford P.W., Hodgskiss M.S.W., Wörndle S., Creaser R.A., Rainbird R.H., Skulski T.M., Halverson G. Precise age of Bangiomorpha pubescens dates the origin of eukaryotic photosynthesis. Geology. 2017;46:135–138. [Google Scholar]
- Gile G.H., Moog D., Slamovits C.H., Maier U.G., Archibald J.M. Dual organellar targeting of aminoacyl-tRNA synthetases in diatoms and cryptophytes. Genome Biol. Evol. 2015;7:1728–1742. doi: 10.1093/gbe/evv095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldberg A.V., Molik S., Tsaousis A.D., Neumann K., Kuhnke G., Delbac F., Vivares C.P., Hirt R.P., Lill R., Embley T.M. Localization and functionality of microsporidian iron-sulphur cluster assembly proteins. Nature. 2008;452:624–628. doi: 10.1038/nature06606. [DOI] [PubMed] [Google Scholar]
- Goss R., Jakob T. Regulation and function of xanthophyll cycle-dependent photoprotection in algae. Photosynth. Res. 2010;106:103–122. doi: 10.1007/s11120-010-9536-x. [DOI] [PubMed] [Google Scholar]
- Gould S.B., Maier U.G., Martin W.F. Protein import and the origin of red complex plastids. Curr. Biol. 2015;25:R515–R521. doi: 10.1016/j.cub.2015.04.033. [DOI] [PubMed] [Google Scholar]
- Govaerts R. How many species of seed plants are there? Taxon. 2001;50:1085–1090. [Google Scholar]
- Gruber A., Vugrinec S., Hempel F., Gould S.B., Maier U.G., Kroth P.G. Protein targeting into complex diatom plastids: functional characterisation of a specific targeting motif. Plant Mol. Biol. 2007;64:519–530. doi: 10.1007/s11103-007-9171-x. [DOI] [PubMed] [Google Scholar]
- Hamilton V.N., Singha U.K., Smith J.T., Weems E., Chaudhuri M. Trypanosome alternative oxidase possesses both an N-terminal and internal mitochondrial targeting signal. Eukaryot. Cell. 2014;13:539–547. doi: 10.1128/EC.00312-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddad M., Adamska I. The evolution of light stress proteins in photosynthetic organisms. Comp. Funct. Genomics. 2002;3:504–510. doi: 10.1002/cfg.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano T., Tanidokoro K., Shimizu Y., Kawarabayasi Y., Ohshima T., Sato M., Tadano S., Ishikawa H., Takio S., Takechi K. Moss chloroplasts are surrounded by a peptidoglycan wall containing D-amino acids. Plant Cell. 2016;28:1521–1532. doi: 10.1105/tpc.16.00104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu S.C., Patel R., Bédard J., Jarvis P., Inoue K. Two distinct Omp85 paralogs in the chloroplast outer envelope membrane are essential for embryogenesis in Arabidopsis thaliana. Plant Signal. Behav. 2008;3:1134–1135. doi: 10.4161/psb.3.12.7095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutin C., Nussaume L., Moise N., Moya I., Kloppstech K., Havaux M. Early light-induced proteins protect Arabidopsis from photooxidative stress. Proc. Natl. Acad. Sci. U S A. 2003;100:4921–4926. doi: 10.1073/pnas.0736939100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C.J., Reyes-Prieto A. The mitochondrial genomes of the glaucophytes gloeochaete wittrockiana and cyanoptyche gloeocystis: multilocus phylogenetics suggests amonophyleticarchaeplastida. Genome Biol. Evol. 2014;6:2774–2785. doi: 10.1093/gbe/evu218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis P., Soll J. Toc, Tic, and chloroplast protein import. Biochim. Biophys. Acta. 2001;1541:64–79. doi: 10.1016/s0167-4889(01)00147-1. [DOI] [PubMed] [Google Scholar]
- Kalanon M., McFadden G.I. The chloroplast protein translocation complexes of Chlamydomonas reinhardtii: a bioinformatic comparison of Toc and Tic components in plants, green algae and red algae. Genetics. 2008;179:95–112. doi: 10.1534/genetics.107.085704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniely S., Pines O. Single translation-dual destination: mechanisms of dual protein targeting in eukaryotes. EMBO Rep. 2005;6:420–425. doi: 10.1038/sj.embor.7400394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kenrick P., Wellman C.H., Schneider H., Edgecombe G.D. A timeline for terrestrialization: consequences for the carbon cycle in the Palaeozoic. Philos. Trans. R. Soc. B Biol. Sci. 2012;367:519–536. doi: 10.1098/rstb.2011.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler F., Blobel G., Patel H.A., Schnell D.J. Identification of two GTP-binding proteins in the chloroplast protein import machinery. Science. 1994;266:1035–1039. doi: 10.1126/science.7973656. [DOI] [PubMed] [Google Scholar]
- Khatoon M., Inagawa K., Pospišil P., Yamashita A., Yoshioka M., Lundin B., Horie J., Morita N., Jajoo A., Yamamoto Y., Yamamoto Y. Quality control of photosystem II: thylakoid unstacking necessary to avoid further damage to the D1 protein and to facilitate D1 degradation under light stress in spinach thylakoids. J. Biol. Chem. 2009;284:25343–25352. doi: 10.1074/jbc.M109.007740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S., Asakura Y., Imai M., Nakahira Y., Kotani Y., Hashiguchi Y., Nakai Y., Takafuji K., Bédard J., Hirabayashi-Ishioka Y. A Ycf2-FtsHi heteromeric AAA-ATPase complex is required for chloroplast protein import. Plant Cell. 2018;30:2677–2703. doi: 10.1105/tpc.18.00357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kikuchi S., Bédard J., Hirano M., Hirabayashi Y., Oishi M., Imai M., Takase M., Ide T., Nakai M. Uncovering the protein translocon at the chloroplast inner envelope membrane. Science. 2013;339:571–574. doi: 10.1126/science.1229262. [DOI] [PubMed] [Google Scholar]
- Komenda J., Sobotka R. Cyanobacterial high-light-inducible proteins - protectors of chlorophyll-protein synthesis and assembly. Biochim. Biophys. Acta Bioenerg. 2016;1857:288–295. doi: 10.1016/j.bbabio.2015.08.011. [DOI] [PubMed] [Google Scholar]
- Köhler D., Dobritzsch D., Hoehenwarter W., Helm S., Steiner J.M., Baginsky S. Identification of protein N-termini in Cyanophora paradoxa cyanelles: transit peptide composition and sequence determinants for precursor maturation. Front. Plant Sci. 2015;6:1–11. doi: 10.3389/fpls.2015.00559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law Y.S., Zhang R., Guan X., Cheng S., Sun F., Duncan O., Murcha M.W., Whelan J., Lim B.L. Phosphorylation and dephosphorylation of the presequence of precursor MULTIPLE ORGANELLAR RNA EDITING FACTOR3 during import into mitochondria from arabidopsis. Plant Physiol. 2015;169:1344–1355. doi: 10.1104/pp.15.01115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee D.W., Lee S., Lee J., Woo S., Razzak M.A., Vitale A., Hwang I. Molecular mechanism of the specificity of protein import into chloroplasts and mitochondria in plant cells. Mol. Plant. 2019;12:951–966. doi: 10.1016/j.molp.2019.03.003. [DOI] [PubMed] [Google Scholar]
- Lee J., O’Neill R.C., Park M.W., Gravel M., Braun P.E. Mitochondrial localization of CNP2 is regulated by phosphorylation of the N-terminal targeting signal by PKC: Implications of a mitochondrial function for CNP2 in glial and non-glial cells. Mol. Cell. Neurosci. 2006;31:446–462. doi: 10.1016/j.mcn.2005.10.017. [DOI] [PubMed] [Google Scholar]
- Lichtenthaler H.K., Buschmann C., Doll M., Fietz H., Bach T., Kozel U., Meier D., Rahmsdorf U. Photosynthetic activity, chloroplast ultrastructure, and leaf characteristics of high-light and low-light plants and of sun and shade leaves. Photosynth. Res. 1981;2:115–141. doi: 10.1007/BF00028752. [DOI] [PubMed] [Google Scholar]
- Ling Q., Broad W., Trösch R., Töpel M., Demiral Sert T., Lymperopoulos P., Baldwin A., Jarvis R.P. Ubiquitin-dependent chloroplast-associated protein degradation in plants. Science. 2019;363:eaav4467. doi: 10.1126/science.aav4467. [DOI] [PubMed] [Google Scholar]
- Martin W., Herrmann R.G. Gene transfer from organelles to the nucleus: how much, what happens, and why? Plant Physiol. 1998;118:9–17. doi: 10.1104/pp.118.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin W. Evolutionary origins of metabolic compartmentalization in eukaryotes. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2010;365:847–855. doi: 10.1098/rstb.2009.0252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- May T., Soll J. 14-3-3 proteins form a guidance complex with chloroplast precursor proteins in plants. Plant Cell. 2000;12:53–63. doi: 10.1105/tpc.12.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mergaert P., Kikuchi Y., Shigenobu S., Nowack E.C.M. Metabolic integration of bacterial endosymbionts through antimicrobial peptides. Trends Microbiol. 2017;25:703–712. doi: 10.1016/j.tim.2017.04.007. [DOI] [PubMed] [Google Scholar]
- Nishiyama T., Sakayama H., de Vries J., Buschmann H., Saint-Marcoux D., Ullrich K.K., Haas F.B., Vanderstraeten L., Becker D., Lang D. The Chara genome: secondary complexity and implications for plant terrestrialization. Cell. 2018;174:448–464. doi: 10.1016/j.cell.2018.06.033. [DOI] [PubMed] [Google Scholar]
- Nowack E.C.M. Paulinella chromatophora - rethinking the transition from endosymbiont to organelle. Acta Societatis Botanicorum Poloniae. 2014;83:387–397. [Google Scholar]
- O’Neil P.K., Richardson L.G.L., Paila Y.D., Piszczek G., Chakravarthy S., Noinaj N., Schnell D. The POTRA domains of Toc75 exhibit chaperone-like function to facilitate import into chloroplasts. Proc. Natl. Acad. Sci. U S A. 2017;114:E4868–E4876. doi: 10.1073/pnas.1621179114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paila Y.D., Richardson L.G.L., Schnell D.J. New insights into the mechanism of chloroplast protein import and its integration with protein quality control, organelle biogenesis and development. J. Mol. Biol. 2015;427:1038–1060. doi: 10.1016/j.jmb.2014.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel R., Hsu S.C., Bédard J., Inoue K., Jarvis P. The Omp85-related chloroplast outer envelope protein OEP80 is essential for viability in Arabidopsis. Plant Physiol. 2008;148:235–245. doi: 10.1104/pp.108.122754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patron N.J., Waller R.F. Transit peptide diversity and divergence: a global analysis of plastid targeting signals. BioEssays. 2007;29:1048–1058. doi: 10.1002/bies.20638. [DOI] [PubMed] [Google Scholar]
- Pfanzagl B., Zenker A., Pittenauer E., Allmaier G., Martinez-Torrecuadrada J., Schmid E.R., De Pedro M.A., Löffelhardt W. Primary structure of cyanelle peptidoglycan of Cyanophora paradoxa: a prokaryotic cell wall as part of an organelle envelope. J. Bacteriol. 1996;178:332–339. doi: 10.1128/jb.178.2.332-339.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robert V., Volokhina E.B., Senf F., Bos M.P., Van Gelder P., Tommassen J. Assembly factor Omp85 recognizes its outer membrane protein substrates by a species-specific C-terminal motif. PLoS Biol. 2006;4:1984–1995. doi: 10.1371/journal.pbio.0040377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodríguez-Ezpeleta N., Brinkmann H., Burey S.C., Roure B., Burger G., Löffelhardt W., Bohnert H.J., Philippe H., Lang B.F. Monophyly of primary photosynthetic eukaryotes: green plants, red algae, and glaucophytes. Curr. Biol. 2005;15:1325–1330. doi: 10.1016/j.cub.2005.06.040. [DOI] [PubMed] [Google Scholar]
- Sato R., Ito H., Tanaka A. Chlorophyll b degradation by chlorophyll b reductase under high-light conditions. Photosynth. Res. 2015;126:249–259. doi: 10.1007/s11120-015-0145-6. [DOI] [PubMed] [Google Scholar]
- Schleiff E., Becker T. Common ground for protein translocation: access control for mitochondria and chloroplasts. Nat. Rev. Mol. Cell Biol. 2011;12:48–59. doi: 10.1038/nrm3027. [DOI] [PubMed] [Google Scholar]
- Schnell D.J., Kessler F., Blobel G. Isolation of components of the chloroplast protein import machinery. Science. 1994;266:1007–1012. doi: 10.1126/science.7973649. [DOI] [PubMed] [Google Scholar]
- Shi L.X., Theg S.M. The chloroplast protein import system: from algae to trees. Biochim. Biophys. Acta. 2013;1833:314–331. doi: 10.1016/j.bbamcr.2012.10.002. [DOI] [PubMed] [Google Scholar]
- Singer A., Poschmann G., Mühlich C., Valadez-Cano C., Hänsch S., Hüren V., Rensing S.A., Stühler K., Nowack E.C.M. Massive protein import into the early-evolutionary-stage photosynthetic organelle of the amoeba Paulinella chromatophora. Curr. Biol. 2017;27:2763–2773. doi: 10.1016/j.cub.2017.08.010. [DOI] [PubMed] [Google Scholar]
- Sommer M.S., Daum B., Gross L.E., Weis B.L.M., Mirus O., Abram L., Maier U.G., Kühlbrandt W., Schleiff E. Chloroplast Omp85 proteins change orientation during evolution. Proc. Natl. Acad. Sci. U S A. 2011;108:13841–13846. doi: 10.1073/pnas.1108626108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steiner J.M., Yusa F., Pompe J.A., Löffelhardt W. Homologous protein import machineries in chloroplasts and cyanelles. Plant J. 2005;44:646–652. doi: 10.1111/j.1365-313X.2005.02559.x. [DOI] [PubMed] [Google Scholar]
- Sun F., Carrie C., Law S., Murcha M.W., Zhang R., Law Y.S., Suen P.K., Whelan J., Lim B.L. AtPAP2 is a tail-anchored protein in the outer membrane of chloroplasts and mitochondria. Plant Signal. Behav. 2012;7:927–932. doi: 10.4161/psb.20769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki E., Suzuki R. Variation of storage polysaccharides in phototrophic microorganisms. J. Appl. Glycosci. 2013;60:21–27. [Google Scholar]
- Sánchez-Baracaldo P., Raven J.A., Pisani D., Knoll A.H. Early photosynthetic eukaryotes inhabited low-salinity habitats. Proc. Natl. Acad. Sci. U S A. 2017;114:E7737–E7745. doi: 10.1073/pnas.1620089114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timmis J.N., Ayliff M.A., Huang C.Y., Martin W. Endosymbiotic gene transfer: organelle genomes forge eukaryotic chromosomes. Nat. Rev. Genet. 2004;5:123–135. doi: 10.1038/nrg1271. [DOI] [PubMed] [Google Scholar]
- Tomitani A., Okada K., Miyashita H., Matthijs H.C.P., Ohno T., Tanaka A. Chlorophyll b and phycobilins in the common ancestor of cyanobacteria and chloroplasts. Nature. 1999;400:159–162. doi: 10.1038/22101. [DOI] [PubMed] [Google Scholar]
- Tsekos I., Reiss H.D., Orfanidis S., Orologas N. Ultrastructure and supramolecular organization of photosynthetic membranes of some marine red algae. New Phytol. 1996;133:543–551. [Google Scholar]
- Töpel M., Ling Q., Jarvis P. Neofunctionalization within the Omp85 protein superfamily during chloroplast evolution. Plant Signal. Behav. 2012;7:161–164. doi: 10.4161/psb.18677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J., Sousa F.L., Bölter B., Soll J., Gould S.B. YCF1: a green TIC? Plant Cell. 2015;27:1827–1833. doi: 10.1105/tpc.114.135541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Vries J., Stanton A., Archibald J.M., Gould S.B. Streptophyte terrestrialization in light of plastid evolution. Trends Plant Sci. 2016;21:467–476. doi: 10.1016/j.tplants.2016.01.021. [DOI] [PubMed] [Google Scholar]
- de Vries J., Archibald J.M., Gould S.B. The carboxy terminus of YCF1 contains a motif conserved throughout >500 million years of streptophyte evolution. Genome Biol. Evol. 2017;9:473–479. doi: 10.1093/gbe/evx013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickett N.J., Mirarab S., Nguyen N., Warnow T., Carpenter E., Matasci N., Ayyampalayam S., Barker M.S., Burleigh J.G., Gitzendanner M.A. Phylotranscriptomic analysis of the origin and early diversification of land plants. Proc. Natl. Acad. Sci. U S A. 2014;111:E4859–E4868. doi: 10.1073/pnas.1323926111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedemann N., Kozjak V., Chacinska A., Schönfisch B., Rospert S., Ryan M.T., Pfanner N., Meisinger C. Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature. 2003;424:565–571. doi: 10.1038/nature01753. [DOI] [PubMed] [Google Scholar]
- Willmund F., Dorn K.V., Schulz-Raffelt M., Schroda M. The chloroplast DnaJ homolog CDJ1 of Chlamydomonas reinhardtii is part of a multichaperone complex containing HSP70B, CGE1, and HSP90C. Plant Physiol. 2008;148:2070–2082. doi: 10.1104/pp.108.127944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollman F.A. An antimicrobial origin of transit peptides accounts for early endosymbiotic events. Traffic. 2016;17:1322–1328. doi: 10.1111/tra.12446. [DOI] [PubMed] [Google Scholar]
- Wu G.Z., Meyer E.H., Richter A.S., Schuster M., Ling Q., Schöttler M.A., Walther D., Zoschke R., Grimm B., Jarvis R.P., Böck R. Control of retrograde signalling by protein import and cytosolic folding stress. Nat. Plants. 2019;5:525–538. doi: 10.1038/s41477-019-0415-y. [DOI] [PubMed] [Google Scholar]
- Wunder T., Martin R., Löffelhardt W., Schleiff E., Steiner J.M. The invariant phenylalanine of precursor proteins discloses the importance of Omp85 for protein translocation into cyanelles. BMC Evol. Biol. 2007;7:236. doi: 10.1186/1471-2148-7-236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshioka-Nishimura M., Yamamoto Y. Quality control of Photosystem II: the molecular basis for the action of FtsH protease and the dynamics of the thylakoid membranes. J. Photochem. Photobiol. B Biol. 2014;137:100–106. doi: 10.1016/j.jphotobiol.2014.02.012. [DOI] [PubMed] [Google Scholar]
- Zhu J.K. Abiotic stress signaling and responses in plants. Cell. 2016;167:313–324. doi: 10.1016/j.cell.2016.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimorski V., Ku C., Martin W.F., Gould S.B. Endosymbiotic theory for organelle origins. Curr. Opin. Microbiol. 2014;22:38–48. doi: 10.1016/j.mib.2014.09.008. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Organisms are provided with name, genome assembly accession and taxonomy. The order of organisms along the Yaxis in the presence/absence plot is according to the order in this table.
Only transcripts with logarithmic fold-changes of at least 2 were considered (p = 0.001). Annotation via BLASTp and InterProScan providing the IPR-, Pfam and GO accessions if available.
Annotation via BLASTp and InterProScan providing the IPR-, Pfam and GO accessions if available. Only transcripts with logarithmic fold-changes of at least 2 were considered (p = 0.001 if not stated otherwise).
Data Availability Statement
Transcriptomes are available via the Sequence Read Archive of NCBI (https://www.ncbi.nlm.nih.gov/sra) with the accession number PRJNA509798.