Abstract
Objective: Strong cravings to smoke are an obstacle to cessation success. Unfortunately, cessation medication and counseling only modestly quell craving. This pilot study was designed to examine the feasibility of mobile games as a response strategy to craving and whether a fully powered trial is warranted.
Materials and Methods: Smokers interested in quitting (N = 30) were offered 4 weeks of nicotine patch plus counseling and randomized to quit with (games-on) versus without (games-off) access to 11 commercial mobile games. Outcomes included post-target quit day (TQD) game play, craving, smoking, and quitting. Almost all P's were >0.05; outcomes should be interpreted with caution due to the small N.
Results: Of games-on participants (n = 16), one played games ≥80% of days post-TQD (22/28 days); 38% played >1/3 of days; 25% did not play. Games-on participants reported games moderately helped them cope with cravings; M = 3.22 on a scale from 1 (not at all) to 5 (very much). Also, games-on participants showed a slight decrease in craving from baseline to 1-week post-TQD (2.35–2.25 on a 0–5 point-scale), whereas games-off participants showed an increase (2.01–2.53). Games-on participants showed greater decreases in craving after playing a game than after the passage of time (when an app imposed a 2-minute wait period following their game request), but there was little evidence games-on versus games-off participants differed in mean post-TQD cigarettes/day. Games-on participants reported modestly but not significantly higher continuous abstinence through day 28 (31.3% vs. 21.4%).
Conclusion: Feasibility results encourage a fully powered trial of this easily disseminable intervention. Clinical Trial Registration: ClinicalTrials.gov NCT02164383
Keywords: Smoking cessation treatment, Tobacco dependence, Craving, Mobile games, Smartphones
Introduction
Post-quit craving levels often predict smoking cessation failure1 and mediate the effects of cessation interventions on abstinence.2,3 Unfortunately, cessation medication and counseling only quell craving modestly. Developing supplemental interventions that address craving therefore appears to be a promising way to increase cessation success.2 Cessation apps, websites, and texts sometimes include games as distractions from cravings,4–9 but, as far as we know, no one has directly tested whether mobile games can help smokers quit.
Theory and research suggest craving processing occupies capacity-limited cognitive resources,10–13 and that occupation of such resources by nondrug processing (particularly by tasks with a visual component14; e.g., the game tetris15,16) may reduce craving processing and levels.17–19 Thus, in theory, games should interfere with the cognitive processing underlying craving. Of course, games could help alleviate craving through other mechanisms (e.g., shifting attention allocation, providing a coping strategy).
Mobile games may be effective as a craving response strategy because games are: (1) reinforcing so people should want and remember to play them; (2) nearly always accessible; and (3) adaptive to skill level, which helps induce flow (activity immersion, lack of self-consciousness, and losing track of time).20 Because this intervention was, as far as we know, untested with smokers, this pilot study explored whether smokers randomized to quit with, versus without, mobile games would play the games, report the games were helpful, demonstrate reduced craving, and smoke fewer cigarettes post-target quit day (TQD). This pilot study should inform the decision to proceed with a fully powered randomized controlled trial.
Materials and Methods
Participants
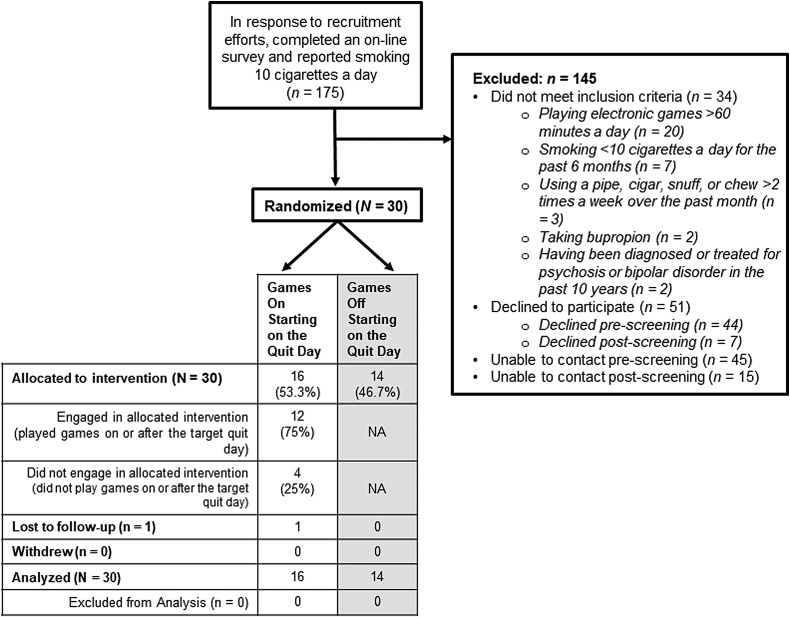
Participants were 30 adult smokers interested in quitting (see Table 1 for their baseline characteristics). The modest sample size was selected for convenience and feasibility. Participants were recruited through Facebook advertisements targeting smokers in the area. Clicking on the ad directed the person to a contact request form. Those who completed the form were contacted by study staff and phone screened. Inclusion criteria included: (1) if they used electronic cigarettes, agreeing to stop for the duration of the study, (2) agreeing to not play mobile games if randomized to the games-off condition, (3) blowing an expired carbon monoxide (CO) ≥6 ppm at the baseline visit, and (4) playing electronic games ≤60 minutes a day (in an effort to ensure playing mobile games would be a relatively novel experience not associated with smoking, and refraining from playing mobile games if randomized to the games-off condition would not be too challenging). See Figure 1 for exclusion reasons. Although many potential participants were excluded (145/175), the majority of those excluded (111) either declined to participate or we were unable to contact them pre- or postscreening. Recruitment through Facebook was relatively straightforward so the high exclusion rate did not affect feasibility. A University of Wisconsin Institutional Review Board approved this trial (reference number 2013-1606), and procedures followed the Helsinki Declaration of 1975, as revised in 2008; participants gave written informed consent.
Table 1.
Baseline Sample Characteristics
| Total sample (N = 30) | Randomized to games on (n = 16) | Randomized to games off (n = 14) | |
|---|---|---|---|
| Female (%) | 53.3 | 56.3 | 50.0 |
| White (%) | 90.0 | 87.5 | 92.9 |
| African American (%) | 3.3 | 0.0 | 7.1 |
| Multiracial (%) | 6.7 | 12.5 | 0.0 |
| Hispanic (%) | 3.3 | 6.25 | 0.0 |
| College diploma (%) | 30.0 | 31.3 | 28.6 |
| Mean age in years (SD) | 40.73 (12.03) | 38.06 (10.27) | 43.79 (13.50) |
| Mean cigarettes per day (SD) | 15.53 (5.54) | 15.80 (6.23) | 15.23 (4.84) |
| Baseline carbon monoxide in ppm | 24.27 (9.35) | 23.75 (8.15) | 24.86 (10.85) |
| Did not use electronic cigarettes (e-cigarettes) in the past month (%) | 86.7 | 81.2 | 92.9 |
| Used e-cigarettes less than weekly or just once in the past month (%) | 13.4 | 18.8 | 7.1 |
| Mean minutes of electronic game play a day at baseline | 43.40 (88.26) | 54.50 (116.54) | 30.71 (37.15) |
| Reported not playing electronic games at all at baseline (%) | 30.0 | 25.0 | 35.7 |
| Reported owning a smartphone (%) | 90.0 | 81.3 | 100.0 |
We do not report statistical tests of baseline differences between the two conditions in part because such tests would be very underpowered.
SD, standard deviation.
FIG. 1.
CONSORT diagram.
Procedure
This study took place from October 2014 to May 2016 in south-central Wisconsin. Case managers enrolled and randomized participants to quit with (games-on) versus without (games-off) study game access for 4 weeks post-TQD. Randomization was determined using stratified randomization (through a computer random number generator) with four strata based on sex (male/female) and craving (low/high). The cut score for classifying craving as low or high was determined using the median score on four Wisconsin Inventory of Smoking Dependence Motives items in a prior sample.21 Case managers could view the allocation sequence for each of the four strata if they chose (i.e., allocation was not concealed); however, the sequence consisted of four numbers (so the type of condition was not spelled out for the case managers) and the order of the four conditions was unpredictable. Case managers were instructed to randomize participants to the next available number in the appropriate strata, and quality assurance checks indicated that they did so.
At Visit 1 (∼day −13 pre-TQD), everyone was issued an Android smartphone and instructed on how to play several of the games. On days −12 and −11, staff remotely blocked participants' games access and participants provided baseline craving ratings. On days −10 and −9, participants practiced playing the games. Phone counseling on day −9 encouraged participants to reduce their smoking by 50% from day −8 to −1, while every 2 days participants alternated having games access versus no access, to permit examination using the entire sample of 30 participants of whether craving (while smoking at reduced levels) was lower on days with games access versus without access (analyses for this exploratory question not reported in this study, in part, because of a high rate of missing data). Although case managers assigned participants to an experimental condition at Visit 1, participants only learned their randomized condition on day −1. Participants earned up to $70 for assessments.
Medication and counseling
Case managers offered participants 4 weeks of 21-mg nicotine patches plus five manualized counseling sessions (15 minutes by phone on days −9 and −1; 15 minutes in-person on day +1; 10 minutes by phone at week 2; and 5 minutes in-person at week 4). The day −9 counseling focused on reasons for quitting and smoking reduction strategies; on day −1 it focused on preparing for the quit attempt; and the remaining counseling sessions focused on how the quit attempt was progressing and problem solving as needed. Case managers encouraged participants randomized to games to play the games to help with urges to smoke.
The case managers were bachelor-level providers with experience conducting smoking cessation counseling; all were rated as proficient in this study's counseling protocol during role plays and were supervised by a licensed clinical psychologist who listened to recordings of some of the counseling. The percentage of participants attending the counseling for games-on versus games-off participants were: 81.3% versus 85.7% for day −9; 81.3% versus 92.9% for day −1; 68.8% versus 78.6% for day +1; 68.8% versus 71.4% for week 2; and 68.8% versus 78.6% for week 4.
The study app
The app was custom developed for this study using the Android Java Software Development Kit (SDK). Eleven commercial games (Supplementary Table S1) were selected for the study based on high staff and Google Play Store ratings. A variety of games were selected: arcade games (three games, including Ski Safari), puzzle games (three games, including Quell Memento+), one word game, one board game, one card game (Solitaire), one tower defense action game, and one running game (Temple Run). Clicking on any of these games triggered the app to display a pregame craving rating request which required a response. After participants entered their craving rating, the game continued to launch. The app recorded which game participants clicked on, participants' pregame craving ratings, the date and time the game launched (which occurred automatically immediately after participants responded to the pregame craving rating), what time participants pressed the home button or back arrow to close the game, and their postgame craving rating.
Study staff remotely blocked access to the games according to a predetermined schedule based on randomized condition (i.e., from day −8 to −1 pre-quit staff blocked games so that every 2 days participants alternated having games access or no access with the order counterbalanced, and staff blocked games post-quit for games-off participants). Staff blocked the games using the IBM MaaS360 mobile device management software. When the games were blocked, the games were still visible on the study phone's home screen. Participants could still click on a game and the app would display a craving rating request, but after participants entered their craving rating, the game would not continue to launch; instead, the phone would display an error message stating that the participant did not have permission to play the game. When games-off participants attended the TQD visit, study staff deleted the games from their study phone so the games were no longer visible on their home screen.
Assessments
Ecological momentary assessment
From 2 weeks pre- to 4 weeks post-TQD, participants received four online survey links a day on their study phone and were asked to complete them. Constructs assessed included craving, and, in the evening, cigarettes smoked that day.
Craving assessment
Craving was assessed with the question “How strong is your urge to smoke right now?” (0 = “No urge” to 5 = ”Extremely strong”; adapted from prior research22,23). This craving item was assessed in three contexts: (1) through notifications on the study phone asking participants to complete the ecological momentary assessment (EMA) surveys just described four times/day; (2) through notifications on the study phone whenever participants launched or ended a game; and (3) through notifications on the study phone before and after a 2-minute wait period that was triggered every fifth time participants tried to launch a game. This third context was designed to assess whether participants showed greater decreases in craving after playing a game than after the passage of time (i.e., than after a 2-minute wait period). Thus, every fifth time participants tried to launch a game, they were required to rate their craving and then this message would appear: “Just a moment… You can play games in 2 minutes.” After 2 minutes, participants received a notification requiring them to rerate their craving after which they were permitted to launch a game.
Smoking assessment
We had several sources of information on participants' smoking: (1) their cigarettes/day based on their EMA evening reports; (2) whether they reported smoking during one of the other three daily EMA reports; (3) their cigarettes/day collected through timeline follow-back24 at day −13 (i.e., at baseline, for the last 7 days), at day +1, and at weeks 2 and 4; and (4) their expired CO level at week 4.
We used all relevant data sources to calculate the following smoking outcome variables: (1) cigarettes/day through week 4; (2) continuous abstinence through week 4 (day 27); and (3) CO-confirmed point-prevalence abstinence at week 4. For all of these variables, the EMA evening report of cigarettes/day was the primary source (however any EMA-reported smoking during the other three daily EMA reports caused a given day to be classified as non-abstinent). Missing EMA evening reports of cigarettes/day were filled in using independently gathered timeline follow-back data on cigarettes/day. If timeline follow-back data were not available, any further missing cigarettes/day data were left as missing. For continuous and point-prevalence abstinence, we assumed missing timeline follow-back data equaled smoking. CO-confirmed point-prevalence abstinence required a CO ≤5 ppm at the week 4 visit, no reports of smoking through EMA over the past 7 days, and reporting 0 cigarettes/day for the past 7 days in timeline follow-back.
Qualitative interviews
At the end of the final visit at week 4, case managers completed a 10-minute qualitative interview with participants and took notes on their responses. The interview consisted of 12 questions designed to elicit suggestions for improving the smartphone game intervention. Questions included whether participants found playing mobile games helped them cope with urges to smoke, and whether they thought playing games helped them quit or could help them quit. Guided by Braun and Clarke's approach,25 we conducted a thematic analysis of the qualitative interviews.
Outcomes
The primary prespecified outcome was mean percentage change in cigarettes/day from baseline to across the first 4 weeks post-TQD. Although we designated a primary outcome, we intended all the feasibility and outcome data listed below to inform the decision about whether to proceed with a fully powered randomized controlled trial (while remaining cognizant of the outcome data's unreliability). Thus, planned analyses addressed whether smokers randomized to quit with, versus without, mobile games on average: (1) reported reduced EMA-assessed craving from baseline (days −12 and −11) to week 1, and (2) smoked fewer cigarettes post-TQD. We also planned to evaluate: (1) how much games-on participants played the study games (mean number of days played; mean minutes/day played as recorded by the app), (2) the extent they reported the games helped them cope with cravings on a questionnaire item at week 4, and (3) whether they showed greater decreases in craving after playing a game than after the passage of time. Finally, we collected data on abstinence (continuous abstinence through week 4 and point-prevalence abstinence at week 4) but recognize that the small sample size means these data have limited reliability.
Analysis
We used descriptive statistics to describe the game play and ratings of the extent that games helped participants cope with cravings. In exploratory analyses, we conducted a mixed analysis of variance (ANOVA) to examine changes in craving with a between-subjects group effect (games-on vs. games-off) and a within-subjects repeated measures time effect (baseline EMA craving vs. EMA craving in week 1). We used a paired-samples t-test to examine decreases in craving after playing a game versus after the passage of time. We conducted a univariate ANOVA to compare percentage change in cigarettes/day between the intervention and control groups, and used logistic regression to examine the abstinence outcomes.
Effect sizes were computed for most variables, typically based on means and standard deviation (SD) ratios but using odds ratios (ORs) for binary outcomes. Almost no effects were significant (P's > 0.05). Due to the small N, all outcomes and effect sizes have questionable reliability and should be interpreted with caution.
Results
Study game play
See Supplementary Data and Table 1 for information on cleaning data recorded by the study app, including the frequency games-on participants played specific games; see also participants' week 4 ratings of the extent specific games helped them cope with urges. At week 4, games-on participants completing the questionnaire (n = 9) reported that games moderately helped them cope with cravings; M = 3.22, SD = 1.20 on a scale from 1 (not at all) to 5 (very much; 3 participants said “a little,” 3 said “moderately,” 1 said “quite a bit,” and 2 said “very much”).
Only one games-on participant (6.3%) played at least one study game on ≥80% of days post-TQD (22 of 28 days); 37.5% played on >1/3 of days; and 25.0% did not play study games post-TQD. The games-on participants played a mean of 7.63 days (SD = 6.75; range 0–23 days) of 28 post-TQD, and across the 4 weeks they played for a mean of 14.36 minutes/day (SD = 26.71: M = 27.00 minutes/day in week 1).
Nonexperimental game play and other distractions
At week 4, 45.5% of games-off participants (5/11 respondents) and 50.0% of games-on participants (5/10 respondents) reported playing games on mobile devices other than their study smartphone in the last 2 weeks. Among those reporting playing nonexperimental games, games-off participants reported playing them a mean of 3.40 days/week (SD = 1.67) for 26.00 minutes/day (SD = 13.87), whereas games-on participants reported playing them a mean of 3.80 days/week (SD = 2.95) for 57.00 minutes/day [SD = 71.73; t(8) = −0.95, P = 0.37, d = 0.60 for minutes/day, confidence interval (95% CI) −0.67 to 1.87].
At week 4, 7/11 games-off participants (63.6%) reported using “online resources besides games (like Facebook, texting, or surfing the web) to cope with urges to smoke” in the last 4 weeks and reported doing this a mean of 4.79 days/week (SD = 1.96) for a mean of 50.29 minutes/day (SD = 43.50). A total of 6/10 games-on participants (60.0%) reported using such nongame online resources and reported doing this a mean of 6.33 days/week (SD = 1.63) for a mean of 108.33 minutes/day [SD = 86.35; t(11) = −1.57, P = 0.15, d = −0.87 for minutes/day, 95% CI −2.01 to 0.27].
Craving and smoking heaviness
Impact of games on craving
We conducted a mixed ANOVA with a between-subjects group effect (games-on vs. games-off) and a within-subjects repeated measures time effect (baseline EMA craving ratings on days −12 and −11 when nobody had games access vs. EMA craving during week 1 before the majority had relapsed). Games-on participants (n = 12) showed a slight decrease in mean craving from baseline to week 1 from 2.35 (SD = 0.86) to 2.25 (SD = 0.72), whereas games-off participants (n = 8) showed an increase from 2.01 (SD = 0.92) to 2.53 (SD = 1.23): group × time interaction = F(1, 18) = 1.43, P = 0.25, ηp2 = 0.07.
Postgame craving
Games-on participants (n = 10) showed greater decreases in craving after playing a game than after the passage of time (when the app imposed a 2-minute wait period following their game request). In a paired-samples t-test, craving ratings increased slightly from pre- to post-2-minute delay (Mpre-minus-post = −0.04, SD = 0.69) but decreased from pre- to post-playing a game [Mpre-minus-post = 0.34, SD = 0.54; t(9) = −1.38, P = 0.20, d = −0.87, 95% CI −2.17 to 0.43].
Smoking heaviness
Smokers in the two conditions showed similar decreases in cigarettes/day (the primary outcome) from baseline to the first 4 weeks post-TQD. Among all participants (abstinent or not; N = 30), mean percentage changes in cigarettes/day (from baseline to across the first 4 weeks post-TQD) were Mgames = −86.08 (SD = 22.06) versus Mno games = −84.26 percentage change (SD = 30.03); F(1, 28) = 0.04, P = 0.85, ηp2 = 0.001.
Abstinence
Examining the full sample (N = 30) in exploratory intent-to-treat analyses (coded 0 = smoking and 1 = abstinent with missing data assumed to mean smoking), those randomized to games-on versus games-off had a slightly higher week 4 CO-confirmed point-prevalence abstinence rate (25.0% vs. 21.4%; OR = 0.82, P = 0.82, 95% CI 0.15 to 4.51), and a modestly higher continuous self-reported abstinence rate through day 28 (31.3% vs. 21.4%; OR = 0.60, P = 0.55, 95% CI 0.11 to 3.15). Dividing games-on participants into those playing ≤ versus >6 minutes/day (the median) across the first 4 weeks showed 12.5% versus 37.5%, respectively, achieved CO-confirmed abstinence at 4 weeks and 12.5% versus 50% achieved self-reported continuous abstinence.
E-cigarette use did not appear to play a role in these findings as e-cigarette use was minimal. At week 4, the majority of participants reported not using e-cigarettes at all in the last 4 weeks (16/21; 76.2%), and the majority of those who did use them (4/5; 80%) reported only a single use in the last 4 weeks.
Qualitative interviews
We conducted a thematic analysis25 of the week 4 qualitative interviews with 10 games-on participants. In terms of games as a distraction tool, the majority reported that the games were absorbing and helped keep their minds off urges to smoke. There appeared to be general agreement that the games helped with urges to smoke long enough for the urges to pass.
Two participants who successfully quit described experiencing flow while using games to cope with urges to smoke. The first said of playing a specific game: “I could beat every single level so I would just kind of sit there and it would take time, and then all of a sudden I'd realize 10 minutes had gone by and then like maybe the craving had subsided.” The second participant said the games were a “big, big, big factor” in helping her quit and that the games kept “my hands and my mind busy” so there would be a “lot of stuff going on around me and I wouldn't even notice it” (i.e., flow). She also reported: “Because I was so used to taking a break and going outside and having a cigarette at the same time every day…. I take the phone and I go somewhere else in the building and sit down and take a break and play the games. I purposely set aside time for it.” Participants seemed to feel the games generally maintained their effectiveness over time. Some participants downloaded games to personal devices so they could continue using games to cope with cravings after the study ended.
Concerns included: the small size of the phone (expressed by older participants), needing to ensure the study phone was charged, situations such as driving and work interfering with being able to use games to cope, and some games becoming too difficult at the higher levels (expressed by four people). Several observed games needed to be low stress and challenging, but not too challenging.
Discussion
These findings encourage further exploration of games as a craving coping strategy. Of course, due to the small sample size, these pilot findings must be interpreted with caution. Nevertheless, based on the effect sizes and directions, games-on versus games-off participants seemed to show: (1) slight reductions in craving 1 week post-TQD (games-off showed an increase); (2) relatively large pre-to-post game-playing-reductions in craving relative to change over a 2-minute wait period; (3) modestly higher quit rates, especially for continuous abstinence; and (4) greater use of online resources and nonexperimental games. This last finding perhaps suggests games-on participants found games helpful and sought more opportunities to cope in this way (or were dissatisfied with the study games or their access to such games). Finally, the qualitative interviews tended to support games as a coping strategy. Games-on participants generally reported games were helpful, typically indicating the games suppressed craving long enough for the urge to smoke to pass.
Not all findings suggested that games were effective (e.g., there was no effect of games on post-TQD cigarettes/day). Perhaps the biggest negative finding was that the games were so little played. On average, games-on participants played games only 7–8 days of the 28 days post-TQD. While this suggests game play was not highly valued by participants, this finding may be misleading. First, heavy users of games were excluded from the study so the sample may overrepresent people who do not like games. Second, games-on participants played nonexperimental games at a relatively high rate, and all reported the 2-minute delay before every fifth game was frustrating. The qualitative data also suggest game playing was valued and that certain game features may lead to greater effectiveness (e.g., use of larger mobile devices, and better titration of difficulty level so the games induce flow but not frustration). It is notable that Solitaire, the most well-liked game and the game participants rated as most helpful in terms of coping with urges to smoke, they also rated as one of the least challenging games.
Research limitations include: the small N which precluded well-powered inferential tests; the nonrepresentativeness of the sample and the high rate of exclusion, limiting generalizability; the extent to which data were missing; the obstacles to game play (small phones, a 2-minute delay before every fifth game); app glitches (e.g., redundant craving rating requests); and relatively heavy nonexperimental game use by both games-on and games-off participants. Not offering the games-off participants access to study games pre-quit would likely help decrease their nonexperimental game play. If this change is made (and games are also not blocked every fifth time they are played—both design choices were efforts to compensate for the small sample size), the other study methods appear appropriate for a definitive trial. Furthermore, the finding that smokers generally found games a helpful distraction from cravings appears likely to generalize to a definitive trial.
In conclusion, despite this study's limitations, this experiment yielded enough promising evidence for games as a craving coping strategy to support additional research, including a well-powered definitive randomized controlled trial, evaluating this easily disseminable intervention. The sample size estimate for such a trial should be based on what would represent a clinically meaningful effect on abstinence rather than on the effects found in this study, which are unreliable due to the small sample size.26
Supplementary Material
Acknowledgments
The authors are grateful to the staff and students at the Center for Tobacco Research and Intervention in the University of Wisconsin School of Medicine and Public Health for their help with this research. They are particularly grateful to Elana Brubaker, Kathleen Cantu, Todd Hayes-Birchler, Douglas Jorenby, Paul Kohn, Madeline Oguss, Stevens Smith, and David Vogt for their help with this study.
Disclaimer
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. None of the funding sources had a role in the study design, collection, analysis, or interpretation of the data, writing the article, or the decision to submit the article for publication.
Author Disclosure Statement
No competing financial interests exist.
Funding Information
This work was supported by the National Cancer Institute (grants P50CA143188, P01CA180945, and K05CA139871) to the University of Wisconsin Center for Tobacco Research and Intervention, by the University of Wisconsin School of Medicine and Public Health Department of Family Medicine Small Grant Program, and by the Wisconsin Partnership Program. This work was carried out in part while T.R.S. was a Primary Care Research Fellow supported by a National Research Service Award (T32HP10010) from the Health Resources and Services Administration to the University of Wisconsin Department of Family Medicine. This research was also supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427.
Supplementary Material
References
- 1. Piper ME, Schlam TR, Cook JW, et al. Tobacco withdrawal components and their relations with cessation success. Psychopharmacology (Berl) 2011; 216:569–578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bolt DM, Piper ME, Theobald WE, Baker TB. Why two smoking cessation agents work better than one: Role of craving suppression. J Consult Clin Psychol 2012; 80:54–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Piper ME, Federman EB, McCarthy DE, et al. Using mediational models to explore the nature of tobacco motivation and tobacco treatment effects. J Abnorm Psychol 2008; 117:94–105 [DOI] [PubMed] [Google Scholar]
- 4. Abroms LC, Lee Westmaas J, Bontemps-Jones J, et al. A content analysis of popular smartphone apps for smoking cessation. Am J Prev Med 2013; 45:732–736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Abroms LC, Johnson PR, Leavitt LE, et al. A randomized trial of text messaging for smoking cessation in pregnant women. Am J Prev Med 2017; 53:781–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. DeLaughter KL, Sadasivam RS, Kamberi A, et al. Crave-Out: A distraction/motivation mobile game to assist in smoking cessation. JMIR Serious Games 2016; 26:e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Edwards EA, Caton H, Lumsden J, Rivas C. Creating a theoretically grounded, gamified health app: Lessons from developing the Cigbreak smoking cessation mobile phone game. JMIR Serious Games 2018; 6:e10252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ubhi HK, Michie S, Kotz D, et al. A mobile app to aid smoking cessation: Preliminary evaluation of SmokeFree28. J Med Internet Res 2015; 17:e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bindoff I, de Salas K, Peterson G, et al. Quittr: The design of a video game to support smoking cessation. JMIR Serious Games 2016; 4:e19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kavanagh DJ, Andrade J, May J. Imaginary relish and exquisite torture: The elaborated intrusion theory of desire. Psychol Rev 2005; 112:446–467 [DOI] [PubMed] [Google Scholar]
- 11. Curtin JJ, McCarthy DE, Piper ME, Baker TB. Implicit and explicit drug motivational processes: A model of boundary conditions. In: Weirs RW, Stacy AW, eds. Handbook of Implicit Cognition and Addiction. Thousand Oaks, CA: Sage; 2006:233–250 [Google Scholar]
- 12. Kemps E, Tiggemann M, Grigg M. Food cravings consume limited cognitive resources. J Exp Psychol Appl 2008;14:247–254 [DOI] [PubMed] [Google Scholar]
- 13. Tiffany ST. A cognitive model of drug urges and drug-use behavior: Role of automatic and nonautomatic processes. Psychol Rev 1990; 97:147–168 [DOI] [PubMed] [Google Scholar]
- 14. May J, Andrade J, Panabokke N, Kavanagh D. Visuospatial tasks suppress craving for cigarettes. Behav Res Ther 2010; 48:476–485 [DOI] [PubMed] [Google Scholar]
- 15. Skorka-Brown J, Andrade J, May J. Playing ‘Tetris’ reduces the strength, frequency and vividness of naturally occurring cravings. Appetite 2014; 76:161–165 [DOI] [PubMed] [Google Scholar]
- 16. Skorka-Brown J, Andrade J, Whalley B, May J. Playing Tetris decreases drug and other cravings in real world settings. Addict Behav 2015; 51:165–170 [DOI] [PubMed] [Google Scholar]
- 17. May J, Andrade J, Willoughby K, Brown C. An attentional control task reduces intrusive thoughts about smoking. Nicotine Tob Res 2012; 14:472–478 [DOI] [PubMed] [Google Scholar]
- 18. van Dillen LF, Andrade J. Derailing the streetcar named desire. Cognitive distractions reduce individual differences in cravings and unhealthy snacking in response to palatable food. Appetite 2016; 96:102–110 [DOI] [PubMed] [Google Scholar]
- 19. Hebert ET, Stevens EM, Frank SG, et al. An ecological momentary intervention for smoking cessation: The associations of just-in-time, tailored messages with lapse risk factors. Addict Behav 2018; 78:30–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Csikszentmihalyi M. Beyond Boredom and Anxiety: Experiencing Flow in Work and Play. San Francisco, CA: Jossey-Bass; 1975 [Google Scholar]
- 21. Piper ME, Smith SS, Schlam TR, et al. A randomized placebo-controlled clinical trial of 5 smoking cessation pharmacotherapies. Arch Gen Psychiatry 2009; 66:1253–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. West R, Hajek P. Evaluation of the mood and physical symptoms scale (MPSS) to assess cigarette withdrawal. Psychopharmacology (Berl) 2004; 177:195–199 [DOI] [PubMed] [Google Scholar]
- 23. Oh H, Taylor AH. Self-regulating smoking and snacking through physical activity. Health Psychol 2014; 33:349–359 [DOI] [PubMed] [Google Scholar]
- 24. Robinson SM, Sobell LC, Sobell MB, Leo GI. Reliability of the Timeline Followback for cocaine, cannabis, and cigarette use. Psychol Addict Behav 2014; 28:154–162 [DOI] [PubMed] [Google Scholar]
- 25. Braun V, Clarke V. Using thematic analysis in psychology. Qual Res Psychol 2006; 3:77–101 [Google Scholar]
- 26. Leon AC, Davis LL, Kraemer HC. The role and interpretation of pilot studies in clinical research. J Psychiatr Res 2011; 45:626–629 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.