Abstract
Objective
Understanding the heterogeneous pathology in Alzheimer’s disease (AD) and related tauopathies is one of the most urgent and fundamental challenges facing the discovery of novel disease modifying therapies. Through monitoring ensembles of toxic and non-toxic tau oligomers spontaneously formed in cells, our biosensor technology can identify tool compounds that modulate tau oligomer structure and toxicity, providing much needed insight into the nature and properties of toxic tau oligomers.
Background
Tauopathies are a group of neurodegenerative disorders characterized by pathological aggregation of the microtubule binding protein tau. Recent studies suggest that tau oligomers are the primary toxic species in tauopathies.
New/Updated Hypothesis
We hypothesize that tau biosensors capable of monitoring tau oligomer conformation are able to identify tool compounds that modulate the structure and conformation of these tau assemblies, providing key insight into the unique structural fingerprints of toxic tau oligomers. These fingerprints will provide gravely needed biomarker profiles to improve staging of early tauopathy pathology as well as generate lead compounds for potential new therapeutics. Our time-resolved FRET (TR-FRET) biosensors provide us an exquisitely sensitive technique to monitor minute structural changes in monomer and oligomer conformation. In this proof-of-concept study, we identified a novel tool compound, MK-886, which directly binds tau, perturbs the conformation of toxic tau oligomers, and rescues tau induced cytotoxicity. Furthermore, we show that MK-886 alters the conformation of tau monomer at the proline-rich and microtubule binding regions, stabilizing an on-pathway oligomer.
Major Challenges for the Hypothesis
Our approach monitors changes in the ensemble of assemblies that are spontaneously formed in cells but does not specifically isolate or enrich unique toxic tau species. However, TR-FRET does not provide high-resolution, atomic scale information, requiring additional experimental techniques to resolve the structural features stabilized by different tool compounds.
Linkage to Other Major Theories
Our biosensor technology is broadly applicable to other areas of tauopathy therapeutic development. These biosensors can be readily modified for different isoforms of tau, specific post-translational modifications, as well as familial AD associated mutations. We are eager to explore tau interactions with chaperone proteins, monitor cross-reactivity with other intrinsically disordered proteins, and target seeded oligomer pathology.
Keywords: Tau oligomerization, Heterogeneous tau oligomers, Conformational ensembles, Fibrillation kinetics, Small-molecule inhibitors, Time-resolved FRET
2. Objective
This paper emphasizes the need for targeting the heterogeneous ensemble of toxic tau oligomers in Alzheimer’s disease (AD) and other tauopathies based on emerging biosensor technology. We report on two novel cellular fluorescence resonance energy transfer (FRET) biosensors that monitor tau oligomer and monomer conformations and can be used as a high-throughput screening (HTS) platform to identify novel tool compounds that modulate tau oligomer conformations, thereby attenuating their toxicity. With our biosensors and HTS platform we are poised to (1) study the conformational ensemble of tau oligomers; (2) identify novel tool compounds capable of targeting tau species, both monomer and oligomer, to disrupt oligomer formation or stabilize different tau conformations; (3) develop a biophysical fingerprint that delineates toxic and non-toxic oligomers, allowing us to better stage tau pathology, improve biomarker development, and reduce the heterogeneity present in clinical trials; and (4) provide a novel therapeutic pipeline to identify lead compounds that target spontaneously formed, early-stage oligomers, instead of late-stage neurofibrillary tangles (NFTs).
3. Background
a. Historical evolution
Tauopathies, including AD, are a group of neurodegenerative disorders characterized by the presence of tau inclusions in affected brain regions [1]. Despite decades of rigorous and focused research, there are currently no significant disease modifying therapies for AD or related tauopathies [2]. Furthermore, there is a dearth of compounds that target tau, with only five out of the 105 small molecules currently in clinical trials being tau-focused [3,4]. Hence, there is desperate need for technologies that enable the identification of tau-focused disease-modifying therapies [4–6].
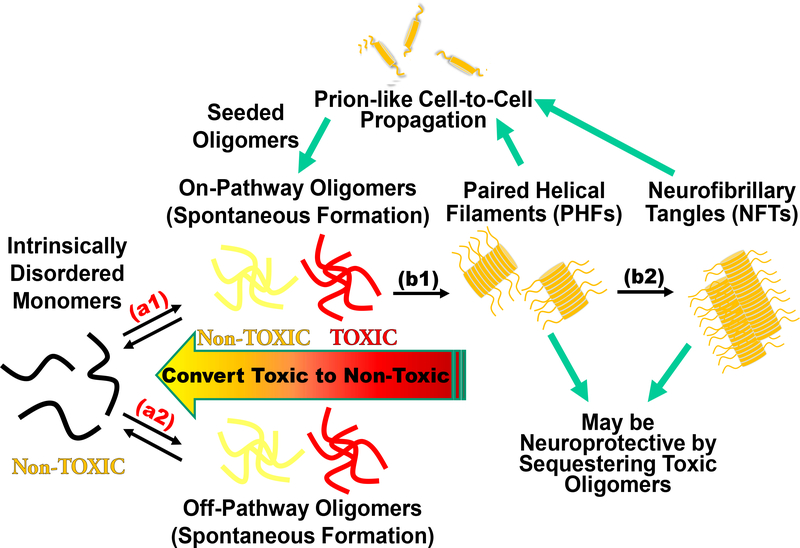
Tau is an intrinsically disordered protein (IDP) that plays an important role in the regulation of microtubule stability and axonal transport [7]. Under pathological conditions, tau is hyperphosphorylated and detaches from microtubules, accumulating in the cytosol [8]. These pathological conditions have been correlated with upstream mitochondrial dysfunctions in the Krebs cycle and/or the electron transport system, oxidative stress [9,10], as well as defects in neuron morphology and axonal transport [11]. Unbound tau can misfold, initiating the tau fibrillogenesis cascade with an initial formation of tau oligomers that subsequently nucleate into paired helical filaments (PHFs), and eventually intracellular NFTs (Fig. 1) [12]. NFTs have been the primary histopathological hallmark of tauopathies, with their presence in the brain showing significant correlation with the degree of cognitive impairment [13]. However, recent studies suggest that these large insoluble NFTs are not the principle toxic species, implicating soluble oligomeric tau—intermediate tau assemblies formed prior to PHFs—in the induction of neurodegeneration [14,15]. Tau oligomers promote cytotoxicity in vitro and are linked to neurodegeneration and cognitive phenotypes in vivo [15–21]. They exist as an ensemble of distinct assemblies which include both toxic and non-toxic, on- and off-pathway species along the fibrillogenesis cascade (Fig. 1) [22–28]. Critically, no specific toxic tau oligomer species has been isolated or identified to date [29–31].
Fig. 1. Tau fibrillogenesis cascade for tauopathies and Alzheimer’s disease (AD).
The intrinsically disordered tau monomer is capable of misfolding into spontaneously formed oligomers, producing toxic assemblies implicated in AD (arrows a1 and a2). While oligomers are metastable and difficult to monitor with high precision and accuracy, the large assemblies (paired helical filaments (PHFs) and neurofibrillary tangles (NFTs), arrows b1 and b2) form irreversibly with β-sheet structures. The fibrillar species can be excreted via exosomes leading to a prion-like cell-to-cell propagation of pathology and may induce seeded oligomerization. NFTs may be neuroprotective by sequestering toxic oligomers and disruption of NFTs may induce toxicity from elevated concentrations of toxic oligomers. Our cellular time-resolved fluorescence resonance energy transfer (FRET) biosensors target reversible spontaneous oligomerization (a1 and a2), while also monitoring seeded aggregation and downstream processes such as fibrillization (b1 and b2) with high sensitivity.
b. Rationale
Recent efforts to target toxic tau oligomers have yielded compounds with low micromolar IC50 [32–38]. Of these small molecules, only methylene blue advanced to phase III clinical trials, albeit with unsuccessful results [39]. One commonality among these molecules is that they were initially identified using in vitro purified protein assays [32–38]. These systems do not recapitulate the cellular environment, lacking the numerous chaperone proteins that may be required to produce the ensemble of tau oligomers that populate the fibrillogenesis cascade. Additionally, purified protein assays are only capable of identifying hits that directly perturb tau and are wholly naive against indirect mechanism of action (MOA). Furthermore, many of the small molecules discovered in purified protein assays disrupted both fibrils and tau oligomers, the former having recently been suggested to be potentially inert and neuroprotective [40]. Therapeutic development for tauopathies has thus begun to shift from targeting large fibrillar aggregates to inhibiting or disrupting the formation of these toxic tau oligomers [14,31,41,42]. The complex heterogeneity of tau oligomers likely requires the cellular environment (e.g. post-translational modifications (PTM) and chaperone proteins) to produce the ensemble of toxic and non-toxic tau assemblies. Hence, a cellular biosensor approach capable of monitoring this ensemble holds promise as a novel HTS platform to discover more effective therapeutics.
Building upon the groundbreaking biosensors developed by the Diamond group (which were engineered to detect pathogenic species in patient biofluids as a biomarker for AD diagnosis) [43], we have developed a technology platform that directly monitors spontaneous tau oligomerization in cells, enabling therapeutic targeting of early-stage tau pathology. Our robust assay can be easily modified to accommodate additional tauopathy cell models and new pathological phenotypes as they continue to be elucidated. Through our approach we will develop two unique classes of tool compounds, direct tau binders and indirect tau effectors (modifying tau oligomers through orthogonal pathways). The interplay between direct and indirect MOA and corresponding changes to tau oligomer conformations and toxicity will provide much needed insight into tau pathology.
We engineered two distinct FRET biosensors to monitor tau oligomerization. These biosensors were used for HTS of the NIH Clinical Collection (NCC) library using our state-of-the-art fluorescence lifetime plate reader (FLT-PR) [44]. Fluorescence lifetime (FLT) detection increases the precision of FRET-based screening by a factor of 30 compared with conventional fluorescence intensity detection [45], and provides exquisite sensitivity to resolve minute structural changes within protein ensembles. This sensitivity allows direct detection of conformational changes within an ensemble of oligomers (e.g. conversion from toxic to non-toxic oligomers), the dissociation of oligomers, or even changes in monomer conformations [46–48]. The FRET biosensors express full-length 2N4R wild-type (WT) tau and fluorescent protein fusion constructs in living cells, allowing us to monitor inter-molecular or intra-molecular tau interactions. Using full-length 2N4R WT tau ensures the targeting of spontaneously formed ensembles of tau oligomers, not fibrils, as the 2N4R isoform of WT tau does not fibrillize without seeding [49–52].
After first establishing that the new technology platform specifically monitors tau oligomer formation (using known tau aggregators), we identified a small molecule, MK-886, that directly binds tau and strongly attenuates FRET with an EC50 of 1.40 μM in our HTS HEK293 cells (and a FRET EC50 of 1.06 μM in SH-SY5Y cells). The compound rescues tau induced cell cytotoxicity with an IC50 of 0.523 μM. To elucidate the MOA, we used an advanced single-molecule FRET (smFRET) technique to show that MK-886 perturbs the folding of purified, monomeric tau in the proline-rich and microtubule-binding regions. This effect is recapitulated in our cellular intra-molecular FRET biosensor and indicates an unfolding of the two termini of tau.
To further explore MK-886’s MOA we employed a heparin induced thioflavin-T (ThT) aggregation assay with purified tau. MK-886 reduces the lag phase of tau fibrillization and is unable to nucleate tau fibrillization without the presence of heparin. It has been shown that overexpression of P301L tau does not induce fibril formation in SH-SY5Y cells [52], suggesting that P301L tau induced toxicity is due to toxic oligomer. Because MK-886 rescues tau induced cytotoxicity while not fully ablating tau oligomer associated FRET, we hypothesize that the rescue of P301L tau induced cytotoxicity by MK-886 is through conversion of toxic tau oligomers into non-toxic oligomers. Whether these new oligomers are on- or off-pathway species is difficult to determine in our cellular system, without the use of inducers. Thus, the rescue of P301L tau induced toxicity could be through an accelerated conversion of toxic tau oligomers into neuroprotective fibrils. Nevertheless, our new technology is well suited to identify novel compounds capable of remodeling tau oligomers and rescuing tau induced cytotoxicity.
4. New or updated hypothesis
Updated hypothesis
We hypothesize that the spontaneously formed ensemble of tau oligomers includes early-stage toxic tau species and by resolving conformational differences between toxic and non-toxic oligomers, we can target toxic tau assemblies, thereby rescuing tau induced pathology. Small-molecule modulation of tau conformations can be correlated with changes in the FRET signal as well as tau induced cytotoxicity. Lastly, through investigating tau oligomerization in the cellular environment, we include other protein machineries (e.g. chaperone proteins) which may play significant roles in tau pathogenesis.
a. Early experimental or observational data
Inter-molecular FRET biosensor directly monitors structural changes in tau oligomers in cells
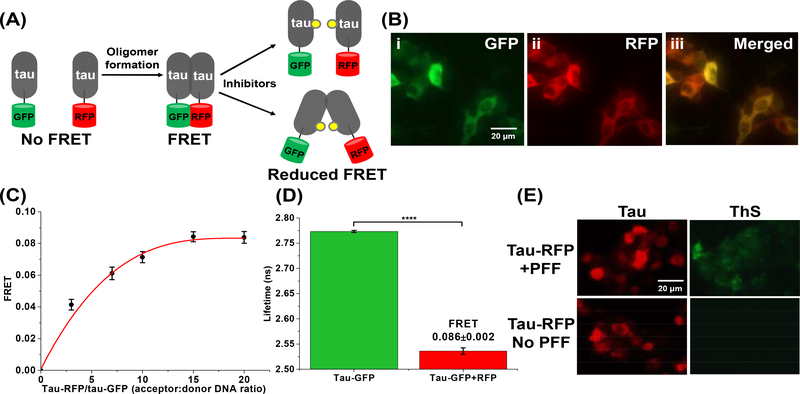
To develop an in-cell HTS platform that can detect small-molecule modulation of tau oligomerization and/or perturbation of tau conformational states, we engineered two cellular tau FRET biosensors. We used human embryonic kidney 293 cells (HEK293) expressing full-length 2N4R WT tau fused to green (GFP) or red (RFP) fluorescent proteins (tau-GFP/RFP or “tau FRET biosensor”) (Fig. 2A). Expression and homogeneity of the FRET biosensor were determined by fluorescence microscopy and immunoblotting. Fluorescence microscopy images showed that the tau proteins were evenly distributed in the cytosol of the cells, with no discernable puncta (which would have indicated more progressive aggregation, e.g. fibril formation) or other non-uniformities (Fig. 2B). Western blot analysis of the tau biosensor cell lysates confirmed the expression of fluorophore-tagged tau (Supplementary Fig. 1A).
Fig. 2: The tau inter-molecular FRET biosensor and fluorescence lifetime technology enable direct monitoring of tau oligomerization in cells.
(A) Schematic representation of live-cell based tau inter-molecular FRET biosensor. FRET signal is observed when tau oligomers form, which can be modulated by small-molecule inhibitors. Tau oligomer is drawn as a dimer for illustration but it can be any species more than a dimer (≥2-mers). (B) Fluorescence microscopy images of (i) GFP channel, (ii) RFP channel and (iii) merged channel of HEK293 cells expressing tau-GFP/RFP (tau FRET biosensor). (C) Titration of tau-RFP (acceptor) to tau-GFP (donor) illustrates that the FRET efficiency of the biosensor follows a hyperbolic dependence on acceptor concentration. (D) Fluorescence lifetime measurements of the tau biosensor show efficient FRET, indicating tau self-association. (E) Thioflavin-S (ThS) staining of HEK293 cells expressing tau-RFP (in same total DNA concentration as used in the FRET biosensor) in the presence and absence of the positive control of tau preformed fibrils (PFF). Data are means ± SD of three independent experiments. ****P < 0.0001 by two-tailed unpaired t test.
We next tested the functionality of the tau FRET biosensor by measuring FRET efficiency using the FLT-PR [44]. The value of FRET efficiency reflects the ensemble-averaged intermolecular proximity between tau molecules, which is derived from the distance between the donor and acceptor fluorophore fused to the tau proteins. FRET between tau-GFP (donor) and tau-RFP (acceptor) in live cells showed hyperbolic dependence on acceptor concentration (Fig. 2C), with a maximum energy transfer efficiency (E) of 0.086±0.002, illustrated through a substantial decrease in the donor FLT in the presence of the acceptor (Fig. 2D), indicating the formation of tau oligomers in cells. The kinetics of formation of the tau-tau assemblies was also measured by FRET, showing that the WT tau biosensor has an optimal FRET after 48 hours of expression (Supplementary Fig. 1B). We confirmed that the FRET observed from cells expressing tau biosensor arises from specific tau-tau interactions and not from non-specific interactions between the free fluorophores (Supplementary Fig. 1C–D). Furthermore, we showed that the FRET biosensor is sensitive to the addition of forskolin, a small molecule known to induce tau hyperphosphorylation and self-association, but not to gossypetin, a small molecule known to inhibit or remodel of tau fibrils (Supplementary Fig. 1E) [53]. To confirm that only oligomeric species of tau, but not fibrils, were present in the tau biosensor cells, we performed a thioflavin-S (ThS) assay in cells expressing tau-RFP at the same concentration of tau-GFP/RFP dual transfected cells (tau-GFP was not used as it will interfere with the ThS signal), with treatment of exogenous tau preformed fibril (PFF) as a positive control. Results from the ThS assay illustrate that only cells treated with PFF have a positive ThS signal (Fig. 2E) with a significant increase in the ThS intensity (Supplementary Fig. 1F), confirming that no fibrils (e.g. β-sheet tau assemblies) are present in the biosensor cells, and more importantly that the FRET signal is the result of tau oligomerization.
Identification of novel small molecules from HTS of the NCC library that perturb the conformational ensembles of tau oligomers
Using our cellular tau FRET biosensor, we performed a HTS of the NCC library (727 bioactive compounds) to identify compounds that perturb the conformational ensembles of tau oligomers. The NCC library is a collection of small molecules that have been previously tested in clinical trials, and have known safety profiles and details on potential MOA.
After an initial quality control check of the tau FRET biosensor on each day of screening (fluorescent waveform signal level and coefficient of variance), the cells were dispensed into drug plates and incubated with the compounds (10 μM) or DMSO control wells for 2 hours. FLT measurements were acquired with the FLT-PR. A single-exponential fit was used to determine the FLT from cells expressing the tau FRET biosensor (τDA) or expressing a tau-GFP donor-only control (τD) to determine FRET efficiency (Eq. 1). As FLT measurements are prone to interference from fluorescent compounds, a stringent fluorescent compound filter was used to flag 30 such compounds as potential false-positives [46,47]. FRET efficiency from all compounds that passed the fluorescent compound filter are plotted (Fig. 3A) and a histogram of the FRET distribution from these compounds was fit to a Gaussian curve to obtain a mean and standard deviation (SD) for the screen (Supplementary Fig. 2A).
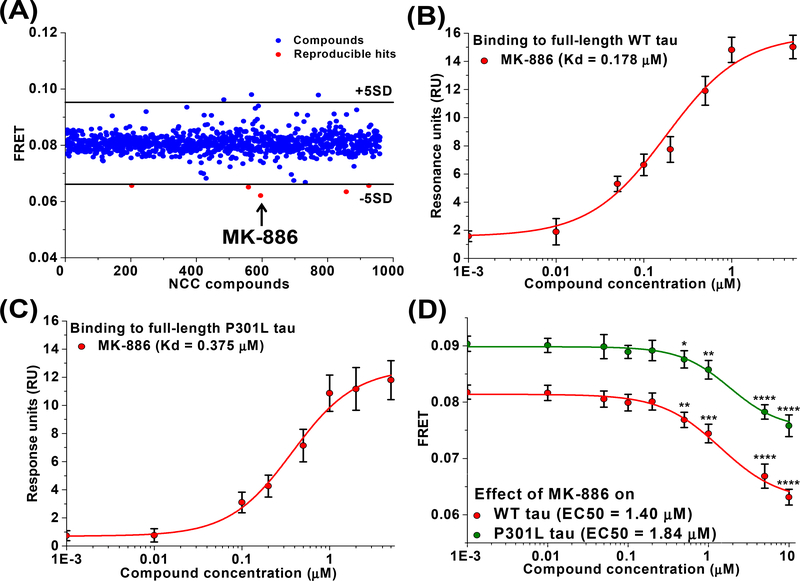
Fig. 3: Identification of MK-886 as a small molecule that directly perturbs conformational ensemble of tau oligomers.
(A) Representative pilot screening with NCC library containing 727 compounds. A FRET efficiency cutoff threshold was applied at a change in FRET efficiency of 5SD (black lines). Five reproducible hits decreased FRET by more than 5SD below the mean of all cells (red) and MK-886 induced the largest FRET change (arrow). Surface plasmon resonance (SPR) binding curve for MK-886 to purified recombinant full-length 2N4R (B) wild-type (WT) and (C) P301L tau proteins. (D) FRET analysis of the dose response of MK-886 in both WT and P301L tau inter-molecular biosensors indicates EC50 values of 1.40 and 1.84 μM respectively. Data are means ± SD of three independent experiments. *P < 0.05, **P < 0.01, ***P < 0.001 and ****P < 0.0001 by two-tailed unpaired t test.
Our initial goal was to discover compounds that alter the conformational ensembles of tau oligomers with the potential of disrupting tau-tau interactions, leading us to focus our search to compounds that reduce FRET (though other compounds that increase FRET could potentially remodel toxic oligomers and be of interest in future studies). Five reproducible hits from the library were shown to decrease FRET by more than 5SD below the mean of all wells (Fig. 3A, highlighted in red) while not appearing as hits in the donor-only control screen (Supplementary Fig. 2B–C).
Binding of hit compounds to purified tau proteins
We next used surface plasmon resonance (SPR) to determine if these five hit compounds bind tau, delineating a potential direct or indirect MOA with tau. Of the five hits that reduced FRET with our tau biosensor, MK-886 was the only hit to demonstrate dose-dependent binding to purified WT tau protein with Kd = 0.178 μM (Fig. 3B and Supplementary Fig. 2D). MK-886 also showed binding to purified P301L tau protein, a more aggregation prone mutant of tau [54], with Kd = 0.375 μM (Fig. 3C and Supplementary Fig. 2E). Interestingly, MK-886 also had the strongest change in FRET (Fig. 3A, highlighted in arrow). The other four hit compounds did not show direct binding to immobilized tau protein (Supplementary Fig. 2F) and therefore most likely attenuate tau FRET through an indirect MOA. All subsequent analysis in this study is focused on MK-866. The indirect MOA compounds, although outside the scope of this study, are potentially useful and are briefly discussed below.
FRET dose-response of MK-886 with cellular tau inter-molecular FRET biosensors
The relative effective concentration (EC50) of MK-886 was determined by in-cell FRET measurements using the WT tau biosensor. The compound decreased FRET efficiency in a dose-dependent manner with an EC50 value of 1.40 μM (Fig. 3D). We also performed a FRET dose response using P301L tau FRET biosensor. The FRET efficiency of this biosensor was found to be higher than that of WT tau biosensor (Supplementary Fig. 3A) which is consistent with the known tendency of P301L tau to be hyperphosphorylated and hence more oligomeric [54]. Similar to WT, we observed a dose-dependent decrease in the FRET efficiency of the P301L tau biosensor with MK-886 with an EC50 value of 1.84 μM (Fig. 3D), confirming that the hit compound also remodels tau oligomers in a disease-relevant model. Interestingly, we observed that MK-886 lowered the FRET level of P301L biosensor to the basal FRET level of WT tau biosensor. This may suggest that MK-886 disrupts the toxic oligomers of P301L tau and converts them to less toxic conformations that are similar to the conformations adopted by the WT tau. In addition, we confirmed that the small molecule was acting specifically on tau and was not acting on the cytosolic fluorophores (Supplementary Fig. 3B–D). Assay quality (Ź) was determined using MK-886 (Eq. 2). The Ź value of 0.72±0.02 indicates excellent assay quality, validating MK-886 as a positive control tool-compound for targeting tau oligomers.
We also expressed the P301L tau FRET biosensor in the SH-SY5Y neuronal cell model, and similar FRET was observed as in the HEK293 cells (Supplementary Fig. 4A). This FRET again reflects the formation of oligomers, as it is known that β-sheet fibrils do not spontaneously form in SH-SY5Y overexpressing P301L tau unless aggregation inducers are used [52]. MK-886 reduced FRET from P301L tau biosensor in the SH-SY5Y cells with an EC50 of 1.06 μM (Supplementary Fig. 4B). Importantly, as will now be shown, if toxicity is observed when P301L tau is overexpressed in SH-SY5Y cells, and the toxicity is rescued by MK-886, it will further support the conclusion that the small molecule converts toxic tau oligomers into a non-toxic conformational state.
MK-886 reduces tau induced cell cytotoxicity in SH-SY5Y cells with nanomolar potency
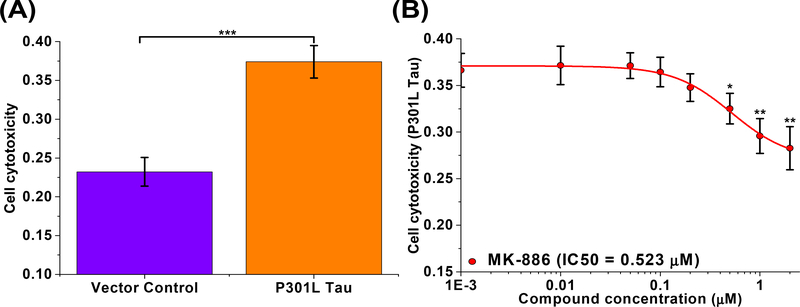
We next tested the effect of MK-886 on P301L tau induced cytotoxicity in the SH-SY5Y neuroblastoma cell model of tauopathy [20,32,55,56]. Overexpression of P301L tau showed significantly greater cell death (37%) when compared to the vector-only control (23%) after 96 hours of expression (Fig. 4A and Supplementary Fig. 5A). Treatment with MK-886 (1 nM to 2 μM) to cells overexpressing P301L tau showed significant rescue of P301L tau induced cytotoxicity in a dose-dependent manner, with an IC50 of 0.523 μM (Fig. 4B), the same order of magnitude as MK-886’s binding affinity for recombinant P301L tau protein. The two-fold difference in IC50 of MK-886 in the cell cytotoxicity assay (0.523 μM) and the EC50 from the FRET assay (1.06 μM) may be due to the different treatment conditions as well as the expression of unlabeled vs. fluorophore-tagged P301L tau in each assay respectively.
Fig. 4: Rescue of tau induced cell cytotoxicity in SH-SY5Y human neuroblastoma cells by MK-886.
(A) SH-SY5Y cells were transfected with vector control and P301L tau. Significant cell death is observed in cells transfected with P301L tau as compared to the vector control. (B) MK-886 rescued P301L tau induced cytotoxicity in SH-SY5Y cells with an IC50 of 0.523 μM. Data are means ± SD of three independent experiments. *P < 0.05, **P < 0.01, and ***P < 0.001 by two-tailed unpaired t test.
We note that MK-886—which was blindly identified in our HTS—has been shown to play a role in modulating AD-related amyloid and tau pathology through inhibition of 5-lipoxygenase (5-LOX)-activating protein (FLAP) [57], potentially altering the clearance and phosphorylation state of tau [58,59]. Our observations suggest that MK-886 rescues tau induced cytotoxicity through direct binding to tau protein and not by modulating FLAP, a previously undescribed mechanism of action. SH-SY5Y cells do not express 5-LOX or FLAP and therefore are a particularly well suited model to evaluate alternative MOA for MK-886 rescue of tau induced cytotoxicity [60]. We also confirmed that there were no changes in the relative levels of expressed tau (Supplementary Fig. 5B) or the phosphorylation state (Supplementary Fig. 5C) due to MK-886 treatment in the SH-SY5Y cell model, although it remains possible that MK-886 may be altering other PTMs such as acetylation or oxidation.
In addition, it has been shown that expression of P301L tau may also result in cell death through down-regulating the expression of survivin, inhibitor of apoptosis proteins (IAPs) or X-linked inhibitor of apoptosis proteins (XIAPs) [55]. Thus, we needed to rule out the possibility that MK-886 rescues P301L tau induced cell cytotoxicity by modulating the expression of these genes, rather than by directly altering tau conformations. To do so, we used two small molecules (YM-155 and UC-112) that are potent suppressors of the expression of survivin, IAPs and XIAPs, thus mimicking the effect of P301L tau on this pathway. This allowed us to test whether MK-886 can rescue cell cytotoxicity in the absence of P301L tau, simply by upregulating these survival genes [61]. Our results showed that MK-886 did not rescue the cell cytotoxicity induced by YM-155 (1 μM) or UC-112 (1 μM) (Supplementary Fig. 6A–B). Thus, these important control experiments strongly suggest that MK-886 rescues P301L tau induced cell cytotoxicity by directly perturbing the conformations of the toxic tau oligomers to form a non-toxic conformation.
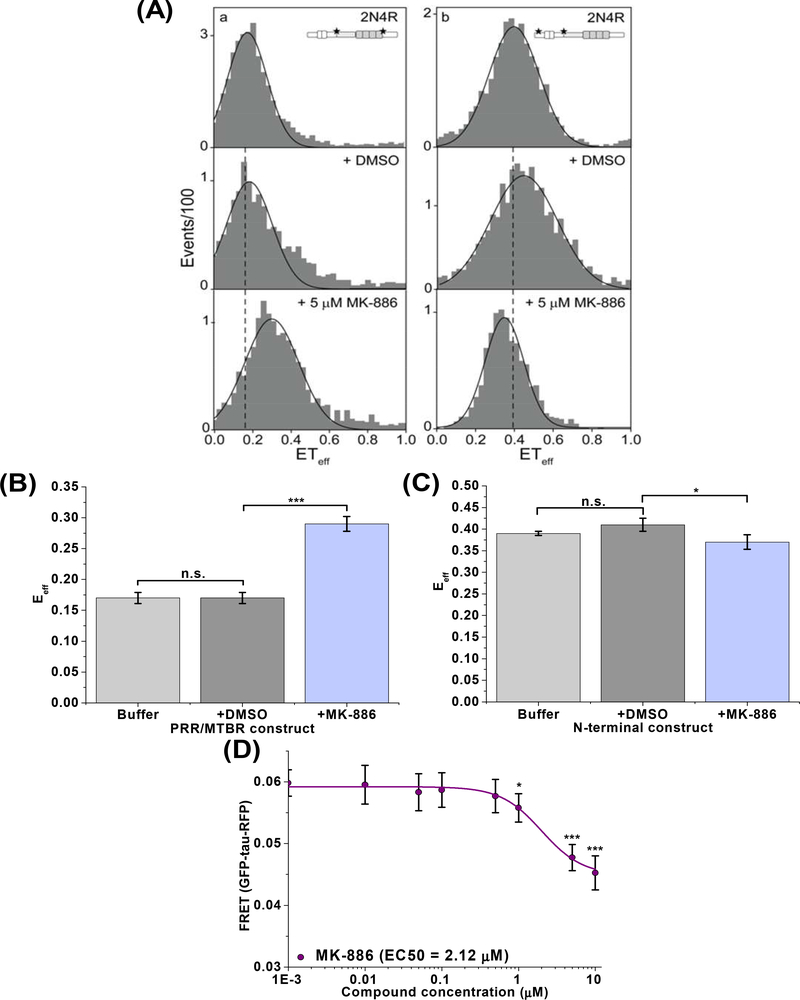
MK-886 specifically perturbs the PRR/MTBR of tau monomer and induces conformational changes of the cellular tau intra-molecular biosensor
To further investigate the MOA of MK-886, we used single-molecule FRET (smFRET) to examine the effect of MK-886 on monomeric tau. Using two different doubly fluorescent-labeled tau constructs (labeled at the proline rich region/microtubule binding region (PRR/MTBR) or at the N-terminal domain) [62], we monitored the conformation of two distinct regions of tau (Fig. 5A). The smFRET shows that MK-886 causes a substantial increase in FRET for the PRR/MTBR targeted construct (Fig. 5B) but only a minor decrease in FRET for the N-terminal domain construct (Fig. 5C). This suggests that MK-886 specifically binds and induces a conformational change in tau monomer at the PRR/MTBR region, resulting in a subsequent loss of interactions between the N-terminal domain and the PRR/MTBR. To determine whether MK-886 also perturbs the monomer conformation of tau in cells, we tested the compound with a cellular tau intra-molecular FRET biosensor (GFP-tau-RFP). The intra-molecular FRET biosensor has a basal 6% FRET signal (Supplementary Fig. 7), illustrating the intra-molecular interactions arising from the paper-clip monomeric structure in which the N- and C-terminus of tau are folded to close proximity [63]. Treatment with MK-886 reduced intra-molecular FRET with an EC50 of 2.12 μM, similar to that of oligomer modulation, suggesting that the change in conformational states of oligomers is due in part to perturbation of the tau monomer (Fig. 5D).
Fig. 5: MK-886 binds and perturbs tau monomer conformation.
(A) Single-molecule FRET (smFRET) measurements in the absence and presence of MK-886 with WT 2N4R tau double labeled at the proline-rich region/microtubule binding region (PRR/MTBR, left) or at the N-terminal domain (right). Tau schematic represents the labelling position for each construct. The black line is drawn from the peak of the histogram in buffer for comparison with DMSO and MK-886 samples. Representative histograms are shown. (B) Quantification of the smFRET measurements indicates that the PRR/MTBR becomes substantially more compact (increase in FRET) upon binding MK-886 (5 μM) (A, bottom left) when compared to tau in buffer (A, top left) or DMSO (A, middle left) while the N-terminal domain (C) shows only minor differences in the presence of MK-886 (5 μM) (A, right). (D) FRET analysis of the dose response of MK-886 in the cellular tau intra-molecular biosensor indicates an EC50 value of 2.12 μM, similar to that of oligomer modulation, suggesting that the change in conformational states of oligomers is due in part to conformational changes of tau monomer. Data are means ± SD of three independent experiments. *P < 0.05, ***P < 0.001 and n.s. indicates not significant by two-tailed unpaired t test.
It has been suggested that the folding over of tau’s two termini to form the classic “paper-clip” structure is due to electrostatic interactions that arise from the opposite net charges of the N-terminal and MTBR domains [63]. While this global folding is specific, it has been shown to be a rather weak interaction [63]. We speculate that the binding of MK-886 to the PRR/MTBR of tau may shield these interactions and lead to an opening of the two termini, resulting in the observed decrease in FRET of the intra-molecular FRET biosensor. From our previous observations with smFRET on tau constructs, this type of conformational change is often accompanied by the PRR/MTBR becoming substantially more compact (increase in FRET) in recombinant protein systems [62].
MK-886 stabilizes tau conformations that promote the formation of β-sheet-positive fibrils in the presence of aggregation inducer
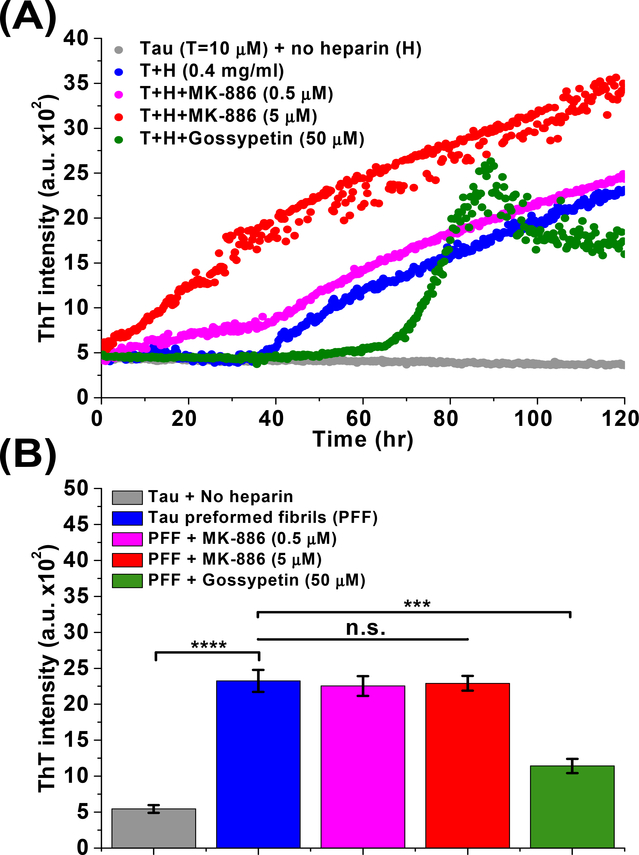
We have shown that MK-886 directly binds to immobilized tau, modulates tau oligomer and monomer conformation (both in cells and purified proteins), and rescues tau induced cytotoxicity. To further explore MK-886’s MOA and identify whether MK-886 targets on- or off-pathway oligomers, we performed a heparin induced thioflavin-T (ThT) aggregation assay in the absence and presence of MK-886. MK-886 shortens the lag phase of tau β-sheet fibril formation in a dose-dependent manner (Fig. 6A), suggesting that it induces or stabilizes on-pathway, early-stage species in the amyloidogenic cascade. We confirmed that MK-886 did not have a direct effect on ThT fluorescence (Supplementary Fig. 8A) and did not act to nucleate for fibril formation (Supplementary Fig. 8B). In addition, we tested if MK-886 disrupts tau preformed fibrils (PFF). Comparison of MK-886 to gossypetin (a known remodeler of tau fibrils) illustrates that MK-886 did not reduce the ThT signal from tau PFF, whereas gossypetin showed a significant decrease, indicating the disruption of β-sheet fibril structure (Fig. 6B). These results, in combination with the changes in FRET and reduction of tau induced cytotoxicity, suggest that MK-886 alters the conformational ensemble of tau oligomers favoring a subset of non-toxic, on-pathway oligomers that promote tau fibrillization.
Fig. 6: MK-886 alters the ensemble of conformational states of tau oligomers by stabilizing a fibrillization promoting conformation.
(A) Effect of MK-886 on the tau fibrillization cascade as characterizaed by thioflavin-T (ThT) assay with purified WT tau proteins. Fibrillization was induced by heparin (0.4 mg/ml) in the presence of DMSO control, MK-886 (0.5 and 5μM) and gossypetin (50 μM, a known small-molecule inhibitor or remodeler of tau fibrils as positive control). (B) Effect of MK-886 on tau preformed fibrils (PFF) with gossypetin as a positive control. All samples were treated with DTT (5 mM). Data are means ± SD of three independent experiments. ***P < 0.001, ****P < 0.0001 and n.s. indicates not significant by two-tailed unpaired t test.
b. Future experiments and validation studies
The identity of a specific, toxic tau oligomeric species remains elusive. Indeed, it is unlikely that a single, unique toxic conformation exists. It is far more likely that an ensemble of toxic oligomers (differing in size, conformation, and even molecular constituency) populates the fibrillogenesis cascade [22–28]. This heterogeneity in tau oligomer targets highlights the need for an ultra-sensitive screening platform capable of monitoring structural changes within the ensemble of tau assemblies. Our FRET-based platform for monitoring full-length tau oligomerization in cells is a new technology that is capable of doing this, as well as elucidating novel compounds which alter conformation and oligomerization, thereby providing a new pipeline of therapeutic discovery for tauopathies.
With this technology in hand, we and others are in a position to explore multiple important issues. First, screens will now be done of larger libraries and of libraries built specifically for targeting the central nervous system (CNS) (i.e. favorable blood-brain barrier permeability). These screens will dramatically increase the statistical sampling of small-molecule induced changes in time-resolved FRET (TR-FRET) signatures. With a larger sample, the high information content of TR-FRET can, when complemented with other structural tools (discussed below), be used to cluster compounds into distinct classes based on their myriad of structural effects on the targets [47]. To more adequately generalize the patho-physiological relevance of these clustered structural motifs, we will move to inducible cell lines [43], as well as alternate cell lines including eventually patient-derived induced pluripotent stem cells (iPSC) neurons [64] and iPSC-derived spheroids [65,66]. Additional extensions of this technology for small molecule discovery should include using different cellular models of tau pathology including, among others, modification of the oligomerization trigger through the addition of tau seeds (fibrils, oligomers, or monomer), upregulation of specific kinases or chaperone proteins (e.g. GSK3β or HSP70), and treatment with environmental toxins [14]. This will allow us to examine multiple proposed mechanisms of induced tauopathies, providing key insight into differences between on- and off-pathway oligomerization [67]. Each of these steps will be critical to building a more complete and useful correlation between structural heterogeneity and toxicity.
Additionally, as we have shown that full-length tau can be engineered as a cell-based, FRET-biosensor of oligomerization (here using the 2N4R isoform), we should now explore potential nuances in how oligomerization depends on tau isoform. Not only will we be able to explore the propensity of different isoforms to oligomerize, but should also test the likelihood that different isoforms co-mingle in heterogenous oligomers. It will be of great interest to ascertain whether lead compounds are isoform specific or can target multiple distinct isoforms. Broadly speaking, information on isoform-specific oligomerization could be useful in designing more effective, patient-stratified design of clinical trials [68]. The Diamond group pioneered the tau cellular biomarker field with their tau repeat domain (tau-RD) FRET biosensor cells [43]. Use of full-length tau thus expands on the existing technology and should facilitate additional stratification of potential biomarkers present in AD versus other tauopathies. Following Diamond, experiments that compare the sensitivity and relative reactivity of each biosensor to different tauopathy associated biofluids will provide new insight into heterogeneity in tau assemblies inherent in these distinct diseases.
Ultimately, determining and validating the specific MOA by which lead compounds act—be they through direct binding to tau monomers/oligomers or indirectly by altering cellular processes that lead to alterations in oligomers—will require comprehensive approaches to link biophysical experiments with cell biological observables. One set of immediately accessible questions is how the compounds’ impact on monomer folding and/or oligomerization relates to tau localization in cells, most obviously on microtubule binding. Here, future work will further probe post-translational modifications (including hyperphosphorylation, which we started here using forskolin) and acetylation, specifically testing how compounds alter the relationship between microtubule unbinding and tau folding/aggregation. As another example, tau has recently been shown to mislocalize to dendritic spines, disrupting synaptic transmission in primary neurons [69]. Whether these or numerous other mechanisms related to mislocalization can explain the cytoprotective effects of lead compounds will require additional experiments. For example, nano-imaging modalities like fluorescence-lifetime imaging microscopy (FLIM) and time-correlated single photon counting (TCSPC) can provide the necessary spatial resolution to correlate subcellular localization (e.g. microtubules, cytosol, mitochondria, etc.) and distinct tau conformations [70].
5. Major challenges for the hypothesis
The technology described here is based on the hypothesis that tau biosensors expressed in cells can be used to accomplish two complementary, but distinct goals: 1) they can be used to find small molecules that modulate tau toxicity; and 2) they can provide a direct, albeit low-resolution reporting of the structures and conformations of a heterogeneous ensemble of toxic and non-toxic states. For the first part of this hypothesis, namely modulation of toxicity, we have demonstrated the power of our approach with MK-886. The second part is more of a challenge. This broader and longer-term hypothesis is built on the idea that biophysical tools, such as TR-FRET, can provide key insight into the unique structural fingerprints of heterogeneous toxic tau oligomers, and that this detail can be exploited in drug discovery. This is a far more nuanced and difficult bar for the field to meet, but is nonetheless a goal that should and, we believe, can be tackled. Why is this difficult? On the one hand, unlike fibrils, oligomers are highly heterogeneous (number of monomers per aggregate, local or transient structural motifs/folds, molecular constituency, etc...), making it improbable that high-resolution structural biology tools are or ever will be applicable to their study (they simply lack well-defined secondary or tertiary structural elements). On the other hand, the field currently relies only on low-resolution techniques that provide little to no structural information (e.g. antibody recognition, protease protection, detergent resistance). Finding a middle ground requires a set of structural techniques that can adequately and accurately interrogate oligomers, but more crucially can stratify structural fingerprints at a quantitatively useful and reproducible resolution. Absent high-resolution structures, we should nonetheless be able to ask: what are the critical amino acids that dictate oligomer-prone monomer folds and what are the deleterious inter-monomeric amino acid motifs that dictate toxicity? As an example of how this can begin to happen, we complemented our TR-FRET with smFRET, and were able to isolate the region of tau impacted by MK-886 binding. But this is just a start, as far more detailed information should be obtainable using a set of creative and state-of-the-art experimental and computational approaches to interrogate these structures [71–73].
In theory, TR-FRET waveforms contain high-content information that can resolve relative species populations and protein-protein distance distributions [74]. Unfortunately, TR-FRET alone does not provide atomic structural resolution to uniquely identify specific species. The process of extracting this structural data from TR-FRET requires model fitting. The challenge is that the model must be constrained by information we do not yet have, including constraints on the stoichiometry of the tau oligomer. This highlights a current limitation in analyzing tau-tau TR-FRET as there are no well-defined structural states and the exact toxic species, including the number of interacting tau monomers, is unknown. To begin to make progress in this regard will require additional biophysical tools. These will include, among others, analytical ultracentrifugation (AUC) and analytical gel filtration for oligomer size. Despite the promise of these techniques for grouping oligomeric species into meaningful clusters, doing so in cells will be the greatest challenge moving forward. Higher resolution structural information for stratifying oligomeric species can be obtained in purified, in vitro assemblies using other spectroscopic techniques such as nuclear magnetic resonance (NMR) and electron paramagnetic resonance (EPR). However, because tau oligomers in cells likely consist of other molecular constituents and are folded with the help of chaperones, there is a real danger in relying too heavily on the folding of these assemblies outside of the native cellular environment. There have been recent advances that allow for the use of high-resolution techniques, such as NMR, in cells [75–77]. These and other advances in biophysical tools will be of critical importance in the coming years.
6. Linkage to other major theories
Tauopathies have a vast heterogeneity in their clinical presentations (e.g. AD, fronto-temporal dementia and movement disorders, amongst others) [78] which is one of the major challenges that plagues current clinical trials [79]. This heterogeneity may be explained in part by strong histopathologic differences and differential laminar and regional brain distributions. For therapeutic intervention, an equally important potential source of this heterogeneity is molecular variations such as isoform composition and post-translational modifications [78,80]. Hence, there is a need for robust tools and/or biomarkers to stage and delineate (particularly, at the molecular level) the numerous different tauopathies.
Although our biosensors and HTS technology are focused on the oligomer hypothesis of tauopathy, they are directly translatable into other areas of tauopathy research and therapeutic discovery. The presence of misfolded tau and the formation of the tau oligomers can be attributed to upstream dysfunctions in neurophysiology and axonal transport. For example, mitochondrial dysfunction and oxidative stress are believed to be a prominent early event in the pathogenesis of AD, contributing to tau phosphorylation and the formation of neurofibrillary tangles [81]. In particular, deterioration of mitochondrial functions such as impairments in the activity of Krebs cycle enzymes and electron transport machineries (e.g. cytochrome c oxidase (COX)) have been correlated with severity in the clinical state of tauopathies and AD [9,10]. Importantly, impaired COX activity can potentiate the generation of mitochondrial-derived reactive oxidative species (ROS), suggesting that defective mitochondrial bioenergetics and oxidative stress are coupled in a vicious cycle [10]. It has also been shown in that in familial AD, tau and amyloid-beta (Aβ) can augment the pathological deterioration of mitochondrial function [82]. In addition, there is compelling evidence that tau can play a role in controlling motor protein–driven vesicle transport along microtubules [83]. Furthermore, there is an age-dependent decline in axonal transport rates which correlates with increases in hyperphosphorylated tau [11]. Our FRET biosensors can be used to study these effects. For example, we can use the intra-molecular biosensor to study the global tau folding when it is bound to microtubules versus when it is detached from microtubules. Our inter-molecular biosensor can also be used to monitor the kinetics and the extent of free soluble tau that are detached from microtubules and start to form oligomers, providing important fundamental information in understanding early stage events underlying tau pathology.
Misfolded or oligomerized tau can be a symptom, as much as a cause, of an underlying pathology. Despite our focus on disrupting oligomers, using the cellular tau biosensors to modulate upstream effectors of tau dysfunction may actually hold the most promise. The TR-FRET screen in cells does not discriminate between compounds that act directly on tau folding/oligomerization and those that operate indirectly by binding to other upstream targets. Coupling secondary biophysical assays to the screen allows us to elucidate direct versus indirect MOA. Compounds that act through an indirect MOA—e.g. those that rescue dysfunctional autophagy or mitochondrial functions, endoplasmic reticulum or oxidative stress—can provide insight into specific pathways that are disrupted in tauopathy, giving rise to novel therapeutic targets and strategies. Upon inspection, this appears likely the case for several of the compounds we identified in our screen but whose MOA we have not yet elucidated. Two of these compounds (bumetanide and torsemide) are both loop diuretics which inhibit the sodium-potassium-chloride cotransporter (NKCC1) in vascular smooth muscle and have been shown to reduce the risk of AD dementia in both adults with normal cognition or with mild cognitive impairments [84]. The other two hits (benzbromarone and triclosan) have been shown to attenuate oxidative stress [85] and induce autophagy [86] (respectively), both known cellular dysfunctions in AD.
Our biosensors should be useful in a variety of other contexts being pursued aggressively in the field. One example is the prion-like propagation of tau pathology [43]. We plan to use the technology in an HTS campaign targeting the uptake and propagation of toxic tau species. We have already begun to see the power of the technology in this way, observing increased FRET due to uptake of WT and truncated tau protein by our biosensor (data not shown). A second example builds on mounting evidence of cross-reactivity and comorbidity amongst misfolded proteins in numerous neurodegenerative diseases. These players include other IDPs, Aβ and α-synuclein [87]. Our biosensors can directly probe these types of interactions via co-expression of these constructs into the tau biosensor cells, and/or treating the tau biosensor with Aβ or α-synuclein fibrils/oligomers and monitoring FRET, cytotoxicity, and uptake. Different donor and acceptor labelled proteins (e.g. tau-GFP/Aβ-RFP etc.) can also be developed and utilized to study potential direct protein-protein interactions. Our strategy, combining cellular fluorescent biosensor and TR-FRET measurement in a HTS platform, is broadly applicable to other drug discovery efforts targeting IDPs involved in numerous neurodegenerative diseases.
A straightforward improvement will be to express tau in a stable or inducible cell line as well as in human iPSCs for physiological relevance. This will further improve conditions for screening in a native environment. In addition, we will launch further HTS campaigns using the most optimized tau FRET biosensors to screen CNS-focused libraries such as the CNS-MPO or CNS-Set library to ensure small molecules have a high probability of crossing the blood-brain barrier. In conjunction with smFRET and other techniques such as AUC, the biosensors can be used to develop a clearer picture of how mutations (e.g. P301L), different tau isoforms and post-translation modifications alter the conformations of oligomers, and what determines toxicity. Ultimately, we will need to test the functional effect of the hit compounds in animal models with tau oligomer induced pathology to see if they rescue the pathology. If the compounds are functional in the animal models, they will be poised to be tested in clinical trials. As for biomarkers development, an immediate next step is to compare our biosensor with the existing tau-RD FRET biosensor developed by the Diamond group to test their sensitivity in cerebrospinal fluid (CSF) from AD patients. If our biosensor is sensitive to these patient samples, we can then further investigate how different groups of patient samples may have different effects on our FRET biosensor. This will act as an initial step in potentially stratifying the patients into different groups to be tested in clinical trials for more effective targeting and drug discovery.
Supplementary Material
Acknowledgements
We thank Nagamani Vunnam, Malaney C. Young and Breeanne M. Brand from the Sachs Group, Tory Schaaf, Samantha Yuen, Andrew Thompson, and Razvan Cornea from the Thomas Group and Benjamin Grant from Fluorescence Innovations, for technical support and discussions. We also thank Dr. David Odde from University of Minnesota for discussions. Compound dispensing and surface plasmon resonance (SPR) (S10 Shared Instrument Grant 1S10OD021539-01 funded by the Office of Research Infrastructure Programs (ORIP) / National Institutes of Health (NIH)) at the UMN Institute of Therapeutic Drug Discovery and Development (ITDD) High-Throughput Screening Laboratory, and spectroscopy at the UMN Biophysical Technology Center. This research uses technology patented by the University of Minnesota, with an exclusive commercial license to Photonic Pharma LLC. The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This study was supported by U.S. NIH grants to J.N.S. (R01AG053951) and D.D.T. (R37AG026160). C.H.L. was supported by a Doctoral Dissertation Fellowship from the University of Minnesota.
Appendix
Materials and Methods
Molecular biology
To generate tau-GFP and tau-RFP, cDNA encoding full-length 2N4R tau (441 amino acids) was fused to the N-terminus of EGFP and TagRFP vectors. The P301L mutation was introduced by QuikChange mutagenesis (Agilent Technologies, Santa Clara, CA) and sequenced for confirmation. The GFP-tau-RFP was generated by fusing the N-terminal of tau to the C-terminus of GFP and the C-terminus of tau to the N-terminus of RFP. All constructs contain the monomeric mutation A206K to prevent constitutive fluorophore clustering [88].
Cell culture and generation of stable cell lines
HEK293 and SH-SY5Y cells (ATCC) were cultured in phenol red-free Dulbecco’s Modified Eagle Medium (DMEM, Gibco) supplemented with 2 mM L-Glutamine (Invitrogen), heat-inactivated 10% fetal bovine serum (FBS HI, Gibco), 100 U/ml penicillin and 100 μg/ml streptomycin (Gibco). Cell cultures were maintained in an incubator with 5% CO2 (Forma Series II Water Jacket CO2 Incubator, Thermo Scientific) at 37 °C. Both the WT and P301L tau inter-molecular FRET biosensor were generated by transiently transfecting HEK293 cells using Lipofectamine 3000 (Invitrogen) with WT or P301L tau-GFP and tau-RFP (1:20 DNA plasmid concentration ratio). The effectiveness of HEK293 cells transfected with FRET constructs as a HTS platform has been demonstrated in our previous work [46,47]. P301L tau inter-molecular FRET biosensor was also transiently expressed in SH-SY5Y cells using Lipofectamine 3000 (Invitrogen). To generate stable cell lines expressing GFP-tau-RFP or tau-GFP only, HEK293 cells were transiently transfected using Lipofectamine 3000 with GFP-tau-RFP or tau-GFP DNA plasmids. Transiently transfected cells were treated with G418 (Enzo Life Sciences, Farmingdale, NY) to eliminate non-expressing cells. Stable cell lines expressing GFP-tau-RFP or tau-GFP with the largest population of expressing cells were selected by fluorescence microscopy. The GFP-linker-RFP (linker contains 32 amino acids, GFP-32AA-RFP) control stable cell line was generated as described previously [89]. The control cells expressing only free soluble fluorophores (GFP or RFP only) were generated by transiently transfecting HEK293 cells using Lipofectamine 3000 with plasmids containing GFP or RFP DNAs at the same plasmid concentration as the inter-molecular tau FRET biosensor.
Pilot screening with NIH clinical collection (NCC) library
The NIH Clinical Collection (NCC) library, containing 727 compounds, was purchased from Evotec (Hamburg, Germany), formatted into 96-well mother plates using an FX liquid dispenser, and formatted across three 384-well plates at 50 nL (10 μM final concentration/well) using an Echo liquid dispenser. DMSO (matching %v/v) was loaded as in-plate no-compound negative controls to make a total of 960 wells. The 384-well flat, black-bottom polypropylene plates (PN 781209, Greiner Bio-One) were selected as the assay plates for their low autofluorescence and low interwell cross talk. The plates were sealed and stored at −20 °C until use. Two days prior to screening, HEK293 cells were transfected using Lipofectamine 3000 with WT tau-GFP/RFP (WT tau FRET biosensor) in 15 × 100 mm plates (5 × 106 cells/plate) and the stable tau-GFP cell line (donor-only control) was expanded in five 225 cm2 flasks. On each day of screening, the compound plates were equilibrated to room temperature (25 °C). The cells were harvested from the 100 mm plates by incubating with TrypLE (Invitrogen) for 5 min, washed three times in PBS by centrifugation at 300 g and filtered using 70 μm cell strainers (BD Falcon). Cell viability, assessed using a trypan blue assay, was >95%. Cells were diluted to 1 million cells/ml using an automated cell counter (Countess, Invitrogen). Expression of tau-GFP and tau-GFP/RFP (tau FRET biosensor) was confirmed by fluorescence microscopy prior to each screen. After resuspension and dilution in PBS, the biosensor cells were constantly and gently stirred using a magnetic stir bar at room temperature, keeping the cells in suspension and evenly distributed to avoid clumping. During screening, cells (50 μl/well) were dispensed by a Multidrop Combi Reagent Dispenser (Thermo Fisher Scientific) into the 384-well assay plates containing the compounds and allowed to incubate at room temperature for 2 hours before readings were taken by the fluorescence lifetime plate reader (Fluorescence Innovations, Inc) as described previously [46,47].
HTS and fluorescence lifetime data analysis
As described previously [46,47], time-resolved fluorescence waveforms for each well were fit with single-exponential decays using least-squares minimization global analysis software to give donor-acceptor lifetime (τDA) and donor-only lifetime (τD). FRET efficiency (E) was then calculated based on Equation 1.
| Eq. 1 |
Assay quality was determined with the lead compound (MK-886) as positive control and DMSO as a negative control and calculated based on Equation 2 [90],
| Eq. 2 |
where σp and σn are the standard deviations (SD) of the observed τDA values, and μp and μn are the mean τDA values of the positive and negative controls. To make this metric less sensitive to strong outliers, we utilized the normalized median absolute deviation (1.4826*MAD) and median in place of the standard deviation and mean, respectively [91].
Fluorescent compounds were flagged as potential false positives due to interference from compound fluorescence by a set of stringent fluorescent compound filters based on analysis of the spectral waveforms of each well from the NCC screen [46,47]. After removal of fluorescent compounds, a histogram of the FRET distribution from all compounds in the screen was plotted and fit to a Gaussian curve to obtain the mean (μ) and standard deviation (σ, SD). A hit was defined as a compound that decreased the FRET efficiency by more than five times the standard deviation (5SD) relative to the mean μ. Five reproducible hits, MK-886 (Cayman Chemical), Benzbromarone (Millipore Sigma), Bumetanide (Millipore Sigma), Torsemide (Millipore Sigma) and Triclosan (Millipore Sigma) were purchased.
Protein purification
Full-length 2N4R WT tau proteins were purified from E. coli using previously published protocols [62]. Full-length tau was expressed with a cleavable His-tag. After elution from a nickel column, cleavage of the His-tag was achieved by incubation with tobacco etch virus (TEV) protease at room temperature for at least 4 hours, followed by passing through the His-tag column again to separate cleaved and uncleaved protein and remove the TEV. Final purification was performed by size-exclusion chromatography and the purity of the proteins was assessed by 4%–15% SDS-PAGE gels (Bio-Rad) under reducing conditions, followed by Coomassie staining. Fractions of pure proteins from the gels were pooled together and the protein stock concentrations were measured using the BCA assay (Thermo Fisher Scientific). Full-length 2N4R P301L tau protein was purchased (rPeptide).
Surface plasmon resonance (SPR) binding assay
Binding affinity between full-length 2N4R WT or P301L tau and the hit compounds were determined by SPR analysis using BIAcore S200. Recombinant tau proteins were immobilized on the CM5 sensor chip (Biacore, GE Healthcare) via amine coupling. Briefly, the dextran surface was activated with a 1:1 mixture of 0.4 M 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride and 0.1 M N-hydroxysuccinimide. WT or P301L tau proteins (20 μg/ml) in 10 mM sodium acetate at pH 3.5–4.0 were flowed past a working surface before blocking the remaining activated carboxymethyl groups with 1 M ethanolamine at pH 8.5 to achieve a level of 1200 RU suitable for binding analysis. The reference surface was activated and reacted with only ethanolamine.
For direct binding assays to the tau proteins, hit compounds at eight different concentrations (1 nM to 5 μM), as well as DMSO-only controls, were prepared in HEPES-EP containing a total of 2% DMSO. The samples were injected over both the reference and tau immobilized surfaces at 10 μl/min for 180 seconds and dissociated in glycine-HCl pH 2.5. All the samples, along with blanks from buffer and DMSO-only controls, were measured on a 96-well microplate (Biacore, GE Healthcare) at 25 °C. Reflectivity response data points were extracted from response curves at 5 seconds prior to the end of the injection to determine steady-state binding. All the data were double referenced with blanks using standard procedures with Biacore S200 Evaluation Software v1.0.
FRET dose-response assay
MK-886, which shows direct binding to tau proteins and the strongest change in FRET, was tested in a FRET dose-response assay. The compound was dissolved in DMSO to make a 10 mM stock solution, which was serially diluted in 96-well mother plates. MK-886 was screened at different concentrations (1 nM to 10 μM). Compound (1 μl) was transferred from the mother plates into assay plates using a Mosquito HV liquid handler (TTP Labtech Ltd, UK). Three days prior to conducting the assays, the stable GFP-tau-RFP cells and GFP-32AA-RFP control cells were expanded in two 225 cm2 flasks (Corning). The preparations for WT or P301L tau-GFP/RFP FRET biosensors and the soluble GFP/RFP controls cells were carried out similar as above.
Cell cytotoxicity assay
Cell cytotoxicity was measured using the CytoTox-Glo (Promega Corporation) luminescence assay kit. SH-SY5Y human neuroblastoma cells were plated at a density of 1 × 106 cells/well in a 6-well plate (Corning) and transfected with unlabeled full-length 2N4R P301L tau or equivalent vector-only control for 24 hours. The transfected cells were then plated at a density of 10000 cells/well in white solid 96-well plate (Corning) with a total volume of 100 μl, followed by treatment with MK-886 at eight different concentrations (1 nM to 2 μM), as well as DMSO-only controls, for another 72 hours. After incubation, 50 μl of CytoTox-Glo Cytotoxicity Assay Reagent was added to all wells followed by mixing by orbital shaking and incubation for 15 minutes at room temperature. The first luminescence reading was measured using a Cytation3 Cell Imaging Multi-Mode Reader luminometer (BioTek). 50 μl of Lysis Reagent with 1% Triton X-100 was then added, followed by incubation at room temperature for 15 min, and luminescence was measured again using the luminometer. Cell cytotoxicity was calculated following the manufacturer protocol. Effect of MK-886 on the suppressors of inhibitors of apoptosis (IAPs) (YM-155 and UC-112) was tested with untransfected SH-SY5Y cells plated in white solid 96-well plate with treatment of YM-155 (1 μM) or UC-112 (1 μM) in the absence or presence of MK-886 (0.5, 1 or 2 μM).
Western blot analysis
To test the expression of tau FRET biosensors, HEK293 cells were plated in a 100 mm plate at a density of 5 × 106 cells/plate and transfected with tau-GFP/RFP (tau FRET biosensor) plasmid. To test the clearance and phosphorylation state of tau in the cytotoxicity assay, SH-SY5Y cells were plated in a six-well plate at a density of 1 × 106 cells/well and transfected with unlabeled P301L tau plasmid for 24 hours followed by treatment of MK-886 (2 μM) for 72 hours. In both cases, cells were lysed for 30 minutes on ice with radioimmunoprecipitation assay (RIPA) lysis buffer (Pierce RIPA buffer, Thermo Fisher Scientific) containing 1% protease inhibitor (Clontech, Mountain View, CA) and 1% phosphatase inhibitors (Millipore Sigma), and centrifuged at 15,000 g at 4 °C for 15 min. The total protein concentration of lysates was determined by bicinchoninic acid (BCA) assay (Pierce), and equal amounts of total protein (60 μg) were mixed with 4× Bio-Rad sample buffer and loaded onto 4%–15% Trisglycine sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels (Bio-Rad, Hercules, CA). Proteins were transferred to polyvinylidene fluoride (PVDF) membrane (Immobilon-FL, EMD Millipore, Billerica, MA) and probed using antibodies against tau (Tau-5, Thermo Fisher Scientific) or antibody specific to Serine 396 of tau (Phospho-Tau S396, Thermo Fisher Scientific) with β-actin (ab8227, Abcam, Cambridge, MA) used as loading control. Blots were quantified on the Odyssey scanner (LI-COR Biosciences, Lincoln, NE).
Protein labelling and single-molecule FRET (smFRET) measurements
For site-specific labeling with maleimide-reactive fluorophores, cysteine residues were introduced using QuikChange Site-Directed Mutagenesis (Stratagene). Naturally occurring cysteines were mutated to serines. Labelling positions were selected to roughly mark the boundaries of the N-terminal domain or the proline-rich and microtubule-binding region of tau. Tau protein was purified as described above and labeled immediately following purification following published protocols [62]. Briefly, the protein (typically 200 μL of ~100 μM protein) was incubated with 1 mM DTT for 30 minutes at room temperature followed by exchange into labeling buffer (20 mM Tris pH 7.4, 50 mM NaCl, and 6 M guanidine HCl) to remove DTT. The protein was incubated with the donor fluorophore, Alexa Fluor 488 maleimide (Invitrogen), at a protein to dye ratio of 2:1 at room temperature for one hour with stirring. The acceptor dye, Alexa Fluor 594 maleimide (Invitrogen), was added at a 5-fold molar excess and incubated overnight at 4°C with stirring. Excess dye was then removed by buffer-exchanging the labeled solution into 20 mM Tris (pH 7.4) and 50 mM NaCl buffer using Amicon concentrators (Millipore) and then passed over two coupled HiTrap Desalting Columns (GE Life Sciences).
Single-molecule FRET measurements were carried out using ~30 pM of labelled tau in phosphate buffer (40 mM potassium phosphate, 50 mM KCl, pH 7.4) in 8-chambered Nunc coverslips (ThermoFisher) passivated with poly(ethylene glycol) poly(L-lysine) (PEG-PLL) to reduce protein adsorption to the chambers. Control measurements included DMSO to match the concentration in samples containing MK-886. Measurements were made on a MicroTime 200 time-resolved confocal microscope (Picoquant) in pulsed interleaved excitation FRET (PIE-FRET) mode. Laser power from 485 and 561 nm lasers, operated at 40 MHz pulse rate, was adjusted to ~30 μW before sample illumination. Fluorescence emission was collected through the objective and passed through a 150 μm diameter pinhole. Photons were separated by an HQ585LP dichroic in combination with ET525/50M and HQ600LP filters and detected by avalanche photodiodes. Photon traces were collected in 1 ms time bins for one hour. A cutoff of 25 counts/ms was applied to discriminate between bursts arising from fluorescently labeled protein and background noise. No bursts were identified in photon traces with DMSO only and MK-886 only when this criterion was applied. The FRET efficiency (ETeff) was calculated using SymphoTime 64 software. SmFRET histograms were fit with Gaussian distributions to determine the peak ETeff values. Alignment of instrument and analysis were verified using 10 base pair, 14 base pair and 18 base pair dsDNA standards.
Thioflavin-S (ThS) assay
HEK293 cells were transfected with tau-RFP (at equivalent DNA concentration as used in the tau FRET biosensor) for 48 hours prior to the addition of tau preformed fibrils (PFF). Tau-GFP was not used as it would interfere with the thioflavin-S (ThS) signal. To make the PFF, 100 μl of purified tau proteins (10 μM) with DTT (5 mM) and heparin (0.4mg/ml) were first incubated for 120 hours at 37°C and shook at 1000 rpm in a thermal shaker (Thermo Fisher Scientific). After incubation, the sample was subjected to ultracentrifugation at 80,000 rpm for 30 minutes. The pellet was collected and sonicated to break up the fibrils into smaller pieces. The concentration of the fibrils was then measured by BCA. The sonicated fibrils were then treated to the transfected cells at a concentration of 40 μg/ml for 24 hours before conducting the ThS assay. Thioflavin-S (ThS, Millipore Sigma, product no. T1892) was dissolved in PBS buffer and was filtered through a 0.2 μm syringe filter to make a stock solution of 2.5 mM. For the ThS assay, cells were fixed with 1 ml of 4% paraformaldehyde in TBS for 15 minutes followed by washing with 1 ml of TBS for 5 minutes twice. After fixing, cells were permeabilized with 1ml of 1% Triton in TBS for 5 minutes, followed by washing with 1ml of TBS for 5 minutes twice. After permeabilization, cells were then treated with 0.002% ThS in TBS and incubate in the dark for 20 minutes. Cells were then washed twice with 50% ethanol for 10 minutes each and finally washed twice with TBS for 5 minutes each. Cells were then imaged with a fluorescence microscope using EVOS-FL cell imaging systems at 20X magnification. Mean fluorescence intensity for each image was quantified using ImageJ and values were normalized to untransfected controls.
Thioflavin-T (ThT) assay
Thioflavin-T (ThT, Sigma, product no. T3516) was dissolved in PBS buffer and was filtered through a 0.2 μm syringe filter to make a stock solution of 2.5 mM. ThT was then diluted to 20 μM prior to addition to the tau proteins. The samples for ThT measurements were prepared by mixing 25 μl of 20 μM tau proteins with 25 μl of 20 μM of ThT, resulting in final concentrations of 10 μM tau proteins and 10 μM ThT. DTT (5 mM) and heparin (0.4 mg/ml) were then added to the samples; a control sample lacked addition of heparin. Lastly, the samples were treated with MK-886 (0.5 μM or 5 μM) and gossypetin (50 μM) with DMSO added to the no-compound controls. The ThT samples (50 μl each) were transferred to a black 96-well non-binding surface microplate with clear bottom (Corning product no. 3655) and incubated at 37°C with mild shaking (200 rpm) in the Cytation 3 plate reader. The ThT fluorescence was measured by the Cytation 3 plate reader through the bottom of the plate with excitation filter of 440 nm and emission filter of 480 nm. Readings were acquired every 20 minutes for a total of 120 hours.
Statistical analysis
Data are shown as mean ± standard deviation unless stated otherwise. Statistical analysis was performed by a two-tailed unpaired t test (Student’s t test) using GraphPad Software to determine statistical significance for all experiments. Values of P <0.05 were considered statistically significant. GraphPad style in using asterisks to denote P values in figures was used (*P < 0.05, **P < 0.01, ***P < 0.001, ****P < 0.0001 and n.s. indicates not significant).
Footnotes
Competing interests
David D. Thomas holds equity in and serves as executive officer for Photonic Pharma LLC, a company that owns intellectual property related to technology used in part of this project. These relationships have been reviewed and managed by the University of Minnesota in accordance with its conflict-of-interest polices.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Wood JG, Mirra SS, Pollock NJ, Binder LI. Neurofibrillary tangles of Alzheimer disease share antigenic determinants with the axonal microtubule-associated protein tau (tau). Proceedings of the National Academy of Sciences of the United States of America. 1986;83(11):4040–4043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Orr ME, Sullivan AC, Frost B. A Brief Overview of Tauopathy: Causes, Consequences, and Therapeutic Strategies. Trends in Pharmacological Sciences. 2017;38(7):637–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cummings J, Lee G, Ritter A, Zhong K. Alzheimer’s disease drug development pipeline: 2018. Alzheimer’s & Dementia: Translational Research & Clinical Interventions. 2018;4:195–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Medina M An Overview on the Clinical Development of Tau-Based Therapeutics. International Journal of Molecular Sciences. 2018;19(4):1160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brunden KR, Trojanowski JQ, Lee VMY. Advances in tau-focused drug discovery for Alzheimer’s disease and related tauopathies. Nature reviews Drug discovery. 2009;8(10):783–793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giacobini E, Gold G. Alzheimer disease therapy—moving from amyloid-β to tau. Nature Reviews Neurology. 2013;9:677. [DOI] [PubMed] [Google Scholar]
- 7.AVILA J, LUCAS JJ, PÉREZ M, HERNÁNDEZ F. Role of Tau Protein in Both Physiological and Pathological Conditions. Physiological Reviews. 2004;84(2):361–384. [DOI] [PubMed] [Google Scholar]
- 8.Bramblett GT, Goedert M, Jakes R, Merrick SE, Trojanowski JQ, Lee VMY. Abnormal tau phosphorylation at Ser396 in alzheimer’s disease recapitulates development and contributes to reduced microtubule binding. Neuron. 1993;10(6):1089–1099. [DOI] [PubMed] [Google Scholar]
- 9.Bubber P, Haroutunian V, Fisch G, Blass JP, Gibson GE. Mitochondrial abnormalities in Alzheimer brain: mechanistic implications. Annals of neurology. 2005;57(5):695–703. [DOI] [PubMed] [Google Scholar]
- 10.Sultana R, Butterfield DA. Oxidative modification of brain proteins in Alzheimer’s disease: perspective on future studies based on results of redox proteomics studies. Journal of Alzheimer’s disease : JAD. 2013;33 Suppl 1:S243–251. [DOI] [PubMed] [Google Scholar]
- 11.Majid T, Ali YO, Venkitaramani DV, Jang M-K, Lu H-C, Pautler RG. In vivo axonal transport deficits in a mouse model of fronto-temporal dementia. NeuroImage: Clinical. 2014;4:711–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sahara N, Maeda S, Takashima A. Tau Oligomerization: A Role for Tau Aggregation Intermediates Linked to Neurodegeneration. Current Alzheimer Research. 2008;5(6):591–598. [DOI] [PubMed] [Google Scholar]
- 13.Ballatore C, Lee VMY, Trojanowski JQ. Tau-mediated neurodegeneration in Alzheimer’s disease and related disorders. Nature Reviews Neuroscience. 2007;8:663. [DOI] [PubMed] [Google Scholar]
- 14.Gerson JE, Castillo-Carranza DL, Kayed R. Advances in Therapeutics for Neurodegenerative Tauopathies: Moving toward the Specific Targeting of the Most Toxic Tau Species. ACS Chemical Neuroscience. 2014;5(9):752–769. [DOI] [PubMed] [Google Scholar]
- 15.Maeda S, Sahara N, Saito Y, Murayama S, Ikai A, Takashima A. Increased levels of granular tau oligomers: An early sign of brain aging and Alzheimer’s disease. Neuroscience Research. 2006;54(3):197–201. [DOI] [PubMed] [Google Scholar]
- 16.Wittmann CW, Wszolek MF, Shulman JM, et al. Tauopathy in Drosophila: Neurodegeneration Without Neurofibrillary Tangles. Science. 2001;293(5530):711–714. [DOI] [PubMed] [Google Scholar]
- 17.Santacruz K, Lewis J, Spires T, et al. Tau suppression in a neurodegenerative mouse model improves memory function. Science. 2005;309(5733):476–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Berger Z, Roder H, Hanna A, et al. Accumulation of Pathological Tau Species and Memory Loss in a Conditional Model of Tauopathy. The Journal of Neuroscience. 2007;27(14):3650–3662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lasagna-Reeves CA, Castillo-Carranza DL, Sengupta U, et al. Identification of oligomers at early stages of tau aggregation in Alzheimer’s disease. The FASEB Journal. 2012;26(5):1946–1959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Flach K, Hilbrich I, Schiffmann A, et al. Tau oligomers impair artificial membrane integrity and cellular viability. Journal of Biological Chemistry. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ward SM, Himmelstein DS, Lancia JK, Binder LI. Tau oligomers and tau toxicity in neurodegenerative disease. Biochemical Society transactions. 2012;40(4):667–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nath A, Sammalkorpi M, DeWitt DC, et al. The conformational ensembles of α-synuclein and tau: combining single-molecule FRET and simulations. Biophysical journal. 2012;103(9):1940–1949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Akoury E, Gajda M, Pickhardt M, et al. Inhibition of Tau Filament Formation by Conformational Modulation. Journal of the American Chemical Society. 2013;135(7):2853–2862. [DOI] [PubMed] [Google Scholar]
- 24.Gerson JE, Mudher A, Kayed R. Potential mechanisms and implications for the formation of tau oligomeric strains. Critical Reviews in Biochemistry and Molecular Biology. 2016;51(6):482–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Weaver CL, Espinoza M, Kress Y, Davies P. Conformational change as one of the earliest alterations of tau in Alzheimer’s disease. Neurobiology of Aging. 2000;21(5):719–727. [DOI] [PubMed] [Google Scholar]
- 26.Sharma AM, Thomas TL, Woodard DR, Kashmer OM, Diamond MI. Tau monomer encodes strains. eLife. 2018;7:e37813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mirbaha H, Chen D, Morazova OA, et al. Inert and seed-competent tau monomers suggest structural origins of aggregation. eLife. 2018;7:e36584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Huang RY-C, Iacob RE, Sankaranarayanan S, et al. Probing Conformational Dynamics of Tau Protein by Hydrogen/Deuterium Exchange Mass Spectrometry. Journal of The American Society for Mass Spectrometry. 2018;29(1):174–182. [DOI] [PubMed] [Google Scholar]
- 29.Götz J, Xia D, Leinenga G, Chew YL, Nicholas H. What Renders TAU Toxic. Frontiers in neurology. 2013;4:72–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gendron TF, Petrucelli L. The role of tau in neurodegeneration. Molecular Neurodegeneration. 2009;4(1):13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kopeikina KJ, Hyman BT, Spires-Jones TL. Soluble forms of tau are toxic in Alzheimer’s disease. Translational neuroscience. 2012;3(3):223–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lo Cascio F, Kayed R. Azure C Targets and Modulates Toxic Tau Oligomers. ACS Chemical Neuroscience. 2018;9(6):1317–1326. [DOI] [PubMed] [Google Scholar]
- 33.Wischik CM, Edwards PC, Lai RY, Roth M, Harrington CR. Selective inhibition of Alzheimer disease-like tau aggregation by phenothiazines. Proceedings of the National Academy of Sciences of the United States of America. 1996;93(20):11213–11218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wobst HJ, Sharma A, Diamond MI, Wanker EE, Bieschke J. The green tea polyphenol (−)-epigallocatechin gallate prevents the aggregation of tau protein into toxic oligomers at substoichiometric ratios. FEBS Letters. 2015;589(1):77–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rane JS, Bhaumik P, Panda D. Curcumin Inhibits Tau Aggregation and Disintegrates Preformed Tau Filaments in vitro. Journal of Alzheimer’s Disease. 2017;60(3):999–1014. [DOI] [PubMed] [Google Scholar]
- 36.Wang P, Lo Cascio F, Gao J, Kayed R, Huang X. Binding and neurotoxicity mitigation of toxic tau oligomers by synthetic heparin like oligosaccharides. Chemical Communications. 2018;54(72):10120–10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taniguchi S, Suzuki N, Masuda M, et al. Inhibition of Heparin-induced Tau Filament Formation by Phenothiazines, Polyphenols, and Porphyrins. Journal of Biological Chemistry. 2005;280(9):7614–7623. [DOI] [PubMed] [Google Scholar]
- 38.Baggett DW, Nath A. The Rational Discovery of a Tau Aggregation Inhibitor. Biochemistry. 2018;57(42):6099–6107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gauthier S, Feldman HH, Schneider LS, et al. Efficacy and safety of tau-aggregation inhibitor therapy in patients with mild or moderate Alzheimer’s disease: a randomised, controlled, double-blind, parallel-arm, phase 3 trial. Lancet. 2016;388(10062):2873–2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cowan CM, Mudher A. Are tau aggregates toxic or protective in tauopathies? Frontiers in neurology. 2013;4:114–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Guzmán-Martinez L, Farías GA, Maccioni RB. Tau oligomers as potential targets for Alzheimer’s diagnosis and novel drugs. Frontiers in neurology. 2013;4:167–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Davidowitz E, Chatterjee I, Moe J. Targeting tau oligomers for therapeutic development for Alzheimer’s disease and tauopathies. Curr Topics Biotechnol. 2008;4:47–64. [Google Scholar]
- 43.Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J Biol Chem. 2012;287(23):19440–19451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Petersen KJ, Peterson KC, Muretta JM, Higgins SE, Gillispie GD, Thomas DD. Fluorescence lifetime plate reader: Resolution and precision meet high-throughput. The Review of Scientific Instruments. 2014;85(11):113101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Gruber SJ, Cornea RL, Li J, et al. Discovery of enzyme modulators via high-throughput time-resolved FRET in living cells. Journal of Biomolecular Screening. 2014;19(2):215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lo CH, Vunnam N, Lewis AK, et al. An Innovative High-Throughput Screening Approach for Discovery of Small Molecules That Inhibit TNF Receptors. SLAS DISCOVERY: Advancing Life Sciences R&D. 2017;22(8):950–961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Schaaf TM, Peterson KC, Grant BD, et al. High-Throughput Spectral and Lifetime-Based FRET Screening in Living Cells to Identify Small-Molecule Effectors of SERCA. SLAS DISCOVERY: Advancing Life Sciences R&D. 2017;22(3):262–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lo CH, Schaaf TM, Grant BD, et al. Non-competitive inhibition of TNF Receptor 1 through long-range perturbation of conformational states by small molecules. Science Signaling, Under Revision. 2019. [Google Scholar]
- 49.Kuret J, Congdon EE, Li G, Yin H, Yu X, Zhong Q. Evaluating triggers and enhancers of tau fibrillization. Microscopy Research and Technique. 2005;67(3–4):141–155. [DOI] [PubMed] [Google Scholar]
- 50.Ko L-w, Deture M, Sahara N, Chihab R, Yen S-H. Cellular Models for Tau Filament Assembly. Vol 192003. [DOI] [PubMed] [Google Scholar]
- 51.Chirita CN, Congdon EE, Yin H, Kuret J. Triggers of Full-Length Tau Aggregation: A Role for Partially Folded Intermediates. Biochemistry. 2005;44(15):5862–5872. [DOI] [PubMed] [Google Scholar]
- 52.Ferrari A, Hoerndli F, Baechi T, Nitsch RM, Götz J. β-Amyloid Induces Paired Helical Filament-like Tau Filaments in Tissue Culture. Journal of Biological Chemistry. 2003;278(41):40162–40168. [DOI] [PubMed] [Google Scholar]
- 53.Tak H, Haque MM, Kim MJ, et al. Bimolecular fluorescence complementation; lighting-up tau-tau interaction in living cells. PLoS One. 2013;8(12). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sahara N, Lewis J, DeTure M, et al. Assembly of tau in transgenic animals expressing P301L tau: alteration of phosphorylation and solubility. Journal of neurochemistry. 2002;83(6):1498–1508. [DOI] [PubMed] [Google Scholar]
- 55.Zhao Z, Ho L, Suh J, et al. A role of P301L tau mutant in anti-apoptotic gene expression, cell cycle and apoptosis. Molecular and Cellular Neuroscience. 2003;24(2):367–379. [DOI] [PubMed] [Google Scholar]
- 56.Schulz KL, Eckert A, Rhein V, et al. A New Link to Mitochondrial Impairment in Tauopathies. Molecular Neurobiology. 2012;46(1):205–216. [DOI] [PubMed] [Google Scholar]
- 57.Mancini JA, Prasit P, Coppolino MG, et al. 5-Lipoxygenase-activating protein is the target of a novel hybrid of two classes of leukotriene biosynthesis inhibitors. Molecular Pharmacology. 1992;41(2):267–272. [PubMed] [Google Scholar]
- 58.Chu J, Li J-G, Ceballos-Diaz C, Golde T, Praticò D. The influence of 5-lipoxygenase on Alzheimer’s disease-related tau pathology: in vivo and in vitro evidence. Biological psychiatry. 2013;74(5):321–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Valera E, Dargusch R, Maher PA, Schubert D. Modulation of 5-lipoxygenase in proteotoxicity and Alzheimer’s disease. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2013;33(25):10512–10525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Klegeris A, McGeer PL. Toxicity of human monocytic THP-1 cells and microglia toward SH-SY5Y neuroblastoma cells is reduced by inhibitors of 5-lipoxygenase and its activating protein FLAP. Journal of Leukocyte Biology. 2003;73(3):369–378. [DOI] [PubMed] [Google Scholar]
- 61.Xiao M, Li W. Recent Advances on Small-Molecule Survivin Inhibitors. Curr Med Chem. 2015;22(9):1136–1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Elbaum-Garfinkle S, Rhoades E. Identification of an aggregation-prone structure of tau. Journal of the American Chemical Society. 2012;134(40):16607–16613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jeganathan S, von Bergen M, Brutlach H, Steinhoff HJ, Mandelkow E. Global hairpin folding of tau in solution. Biochemistry. 2006;45(7):2283–2293. [DOI] [PubMed] [Google Scholar]
- 64.Verheyen A, Diels A, Dijkmans J, et al. Using Human iPSC-Derived Neurons to Model TAU Aggregation. PLOS ONE. 2016;10(12):e0146127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee H-K, Velazquez Sanchez C, Chen M, et al. Three Dimensional Human Neuro-Spheroid Model of Alzheimer’s Disease Based on Differentiated Induced Pluripotent Stem Cells. PloS one. 2016;11(9):e0163072– e0163072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Raja WK, Mungenast AE, Lin Y-T, et al. Self-Organizing 3D Human Neural Tissue Derived from Induced Pluripotent Stem Cells Recapitulate Alzheimer’s Disease Phenotypes. PloS one. 2016;11(9):e0161969– e0161969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Kjaergaard M, Dear AJ, Kundel F, et al. Oligomer Diversity during the Aggregation of the Repeat Region of Tau. ACS chemical neuroscience. 2018;9(12):3060–3071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Dujardin S, Bégard S, Caillierez R, et al. Different tau species lead to heterogeneous tau pathology propagation and misfolding. Acta Neuropathologica Communications. 2018;6(1):132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Zhao X, Kotilinek LA, Smith B, et al. Caspase-2 cleavage of tau reversibly impairs memory. Nature Medicine. 2016;22(11):1268–1276. [DOI] [PubMed] [Google Scholar]
- 70.Chen W, Young LJ, Lu M, et al. Fluorescence Self-Quenching from Reporter Dyes Informs on the Structural Properties of Amyloid Clusters Formed in Vitro and in Cells. Nano Letters. 2017;17(1):143–149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Romo TD, Lewis AK, Braun AR, Grossfield A, Sachs JN. Minimal Nucleation State of alpha-Synuclein Is Stabilized by Dynamic Threonine-Water Networks. ACS Chem Neurosci. 2017;8(9):1859–1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Tuttle MD, Comellas G, Nieuwkoop AJ, et al. Solid-state NMR structure of a pathogenic fibril of full-length human alpha-synuclein. Nature structural & molecular biology. 2016;23(5):409–415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rodriguez JA, Ivanova MI, Sawaya MR, et al. Structure of the toxic core of alpha-synuclein from invisible crystals. Nature. 2015;525(7570):486–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Muretta JM, Kyrychenko; Kast A; Gillispie ASLDJ; Thomas DD GD. High-performance time-resolved fluorescence by direct waveform recording. Review of Scientific Instruments. 2010;81(10):103101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zhang S, Wang C, Lu J, et al. In-Cell NMR Study of Tau and MARK2 Phosphorylated Tau. International Journal of Molecular Sciences. 2018;20(1):90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Theillet FX, Binolfi A, Bekei B, et al. Structural disorder of monomeric alpha-synuclein persists in mammalian cells. Nature. 2016;530(7588):45–50. [DOI] [PubMed] [Google Scholar]
- 77.Zigoneanu IG, Pielak GJ. Interaction of alpha-synuclein and a cell penetrating fusion peptide with higher eukaryotic cell membranes assessed by (1)(9)F NMR. Molecular pharmaceutics. 2012;9(4):1024–1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Kovacs GG. Invited review: Neuropathology of tauopathies: principles and practice. Neuropathology and Applied Neurobiology. 2015;41(1):3–23. [DOI] [PubMed] [Google Scholar]
- 79.Devi G, Scheltens P. Heterogeneity of Alzheimer’s disease: consequence for drug trials? Alzheimer’s Research & Therapy. 2018;10(1):122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Ghetti B, Oblak AL, Boeve BF, Johnson KA, Dickerson BC, Goedert M. Invited review: Frontotemporal dementia caused by microtubule-associated protein tau gene (MAPT) mutations: a chameleon for neuropathology and neuroimaging. Neuropathology and Applied Neurobiology. 2015;41(1):24–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Mondragon-Rodriguez S, Perry G, Zhu X, Moreira PI, Acevedo-Aquino MC, Williams S. Phosphorylation of tau protein as the link between oxidative stress, mitochondrial dysfunction, and connectivity failure: implications for Alzheimer’s disease. Oxid Med Cell Longev. 2013;2013:940603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Rhein V, Song X, Wiesner A, et al. Amyloid-beta and tau synergistically impair the oxidative phosphorylation system in triple transgenic Alzheimer’s disease mice. Proc Natl Acad Sci U S A. 2009;106(47):20057–20062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Ebneth A, Godemann R, Stamer K, Illenberger S, Trinczek B, Mandelkow E. Overexpression of tau protein inhibits kinesin-dependent trafficking of vesicles, mitochondria, and endoplasmic reticulum: implications for Alzheimer’s disease. The Journal of cell biology. 1998;143(3):777–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Yasar S, Xia J, Yao W, et al. Antihypertensive drugs decrease risk of Alzheimer disease: Ginkgo Evaluation of Memory Study. Neurology. 2013;81(10):896–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Muraya N, Kadowaki D, Miyamura S, et al. Benzbromarone Attenuates Oxidative Stress in Angiotensin II- and Salt-Induced Hypertensive Model Rats. Oxid Med Cell Longev. 2018;2018:7635274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wang C, Yu Z, Shi X, et al. Triclosan Enhances the Clearing of Pathogenic Intracellular Salmonella or Candida albicans but Disturbs the Intestinal Microbiota through mTOR-Independent Autophagy. Frontiers in cellular and infection microbiology. 2018;8:49–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Moussaud S, Jones DR, Moussaud-Lamodiere EL, Delenclos M, Ross OA, McLean PJ. Alpha-synuclein and tau: teammates in neurodegeneration? Mol Neurodegener. 2014;9:43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zacharias DA, Violin JD, Newton AC, Tsien RY. Partitioning of lipid-modified monomeric GFPs into membrane microdomains of live cells. Science. 2002;296(5569):913–916. [DOI] [PubMed] [Google Scholar]
- 89.Schaaf T, Li A, Grant B, et al. Red-Shifted FRET Biosensors for High-Throughput Fluorescence Lifetime Screening. Biosensors. 2018;8(4):99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Zhang JH, Chung TD, Oldenburg KR. A Simple Statistical Parameter for Use in Evaluation and Validation of High Throughput Screening Assays. Journal of biomolecular screening. 1999;4(2):67–73. [DOI] [PubMed] [Google Scholar]
- 91.Birmingham A, Selfors LM, Forster T, et al. Statistical methods for analysis of high-throughput RNA interference screens. Nature methods. 2009;6(8):569–575. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.