Introduction
Estimates show that the number of Mexican older adults will reach 32.4 million in 2050 [1]. Data from the Health and Aging Survey in Latin America revealed that at least 11% of the population older than 60 years has some degree of cognitive impairment [2]. In countries like Mexico, the general prevalence of cognitive impairment is approximately 7% [3].
A timely diagnosis is important to ensure adequate treatment and care for patients with dementia. In the United States, half of the patients with Alzheimer’s disease are properly diagnosed, whereas in countries like Mexico less than 25% of cases are identified [4,5]. In clinical settings, underdiagnosis of this condition may be explained by the lack of time for consultation, the absence of diagnostic tests easily applied and adapted to the population, and possibly, by underestimation of the importance of cognitive and functional changes [6,7]. Longitudinal population-based studies provide important information about the prevalence and risk factors for diseases in their natural environment, such as dementia, and can help identify symptoms at an early stage. Current treatment approaches focus on the planning of strategies that provide support and training to caregivers and the use of symptomatic drugs that could reduce or stabilize the progression of the disease.
Evidence of gait slowing during early stages of dementia has led investigators to propose the concept of Motoric Cognitive risk (MCR), a pre-dementia syndrome characterized by slow gait, subjective memory complaint, intact functional capacity and a lack of dementia diagnosis [8]. Analyses of 22 cohort studies from different countries showed that MCR was associated with a 1.5 to 2.7 risk of incident cognitive impairment in the individual cohorts and with a 2-fold increase risk of dementia in the pooled sample. The early identification of individuals at risk of developing dementia based on the detection of MCR constitutes a simple clinical approach. [9].
Therefore, the aim of this study was to determine the prevalence of MCR in a sample of Mexican adults over 60 years of age, describe associated risk factors and determine the risk of progression to cognitive impairment after three years of follow-up.
Materials and Methods
Data was obtained from the Mexican Health and Aging Study (MHAS), a large, national representative study of older Mexicans (age 50 or older) and their spouses. The aim and design of the MHAS has been previously published [10]. The study started in 2001 and has three fielded follow-ups in 2003, 2012 and 2015. Information from a subsample of subjects who participated in the 2012 wave was used for the present study. Data was assessed through performance tests, anthropometric measures and blood samples. Information from the 2012 and 2015 waves was used at baseline and follow-up, respectively. [11].
Figure 1 presents a flowchart of the process followed to select the sample. From a total of 2,086 subjects of the subsample, only those aged 60 years or older and with complete information on cognitive and MCR variables were selected. The resulting 860 subjects were further classified based on their cognitive scores in two groups; with normal or impaired cognitive functioning. The normal cognition group was the final sample at baseline (n=726), while the impaired group was excluded from the analysis (see MCR criteria below). The next step was to classify all cognitively normal subjects in those who met criteria for MCR (n=104) and those who didn’t (n=622). After 3 years of follow-up, 664 subjects were identified, while 62 (8.5%) had died, were lost or had incomplete information. Finally, we analyzed the follow-up sample to determine the change in cognitive functioning.
Figure 1.
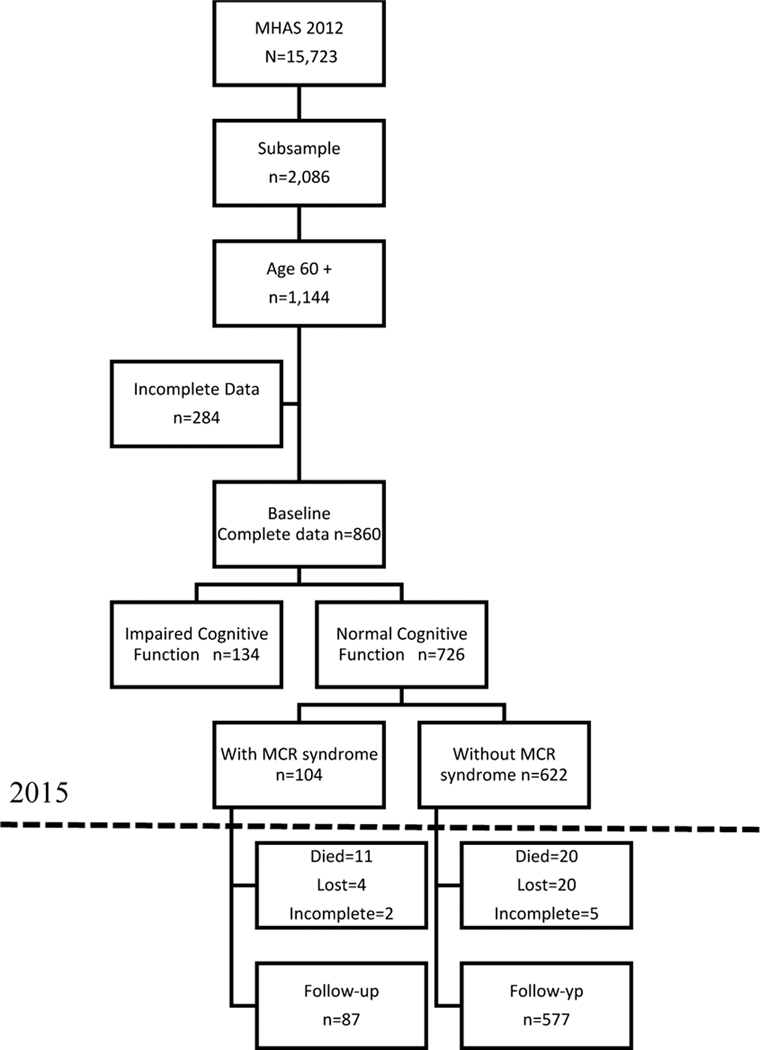
Flowchart of sample selection
Subjects
Subjects With MCR syndrome:
Based on the definition of MCR syndrome proposed by Verghese et al. [8,19] all subjects who met all the following criteria were considered with MCR:
Self-reported memory complaints. This variable was defined as the presence of memory complaint mentioned by the subject as “inadequate” or “poor” according to the the question: Compared to the last two years, would you say your memory is? The other possible answers were “better” and “more or less the same”.
The absence of cognitive impairment. Cognitive functioning in the MHAS was assessed with the use of the Cross-Cultural Cognitive Examination (CCCE), a screening tool that evaluates cognitive status and that has been previously standardized for Mexican population with Z scores adjusted by age and education [12,13]. The CCCE assesses cognitive function using several tasks that measure visuospatial abilities, visual memory, verbal learning memory, verbal recall, visual scanning, orientation, verbal fluency and numeracy. A composite score above −1.5 standard deviations was considered as normal cognitive function. Subjects who participated in the MHAS and could not answer the CCCE because of health or sensory limitations were assessed for cognitive function through a proxy using the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE). Subjects who had a score of 3.4 or more were classified as cognitively impaired [14]
Gait speed disturbances. Assessment of gait speed in MHAS is done through a performance measure. The subject is asked to follow the instruction: “Please walk to the end of the path with your usual speed as if walking in the street to go to the store”. Time in minutes and seconds to walk four meters in two different opportunities was averaged and registered. For this study, a value greater than 0.8 m/s in both men and women was considered as low gait speed; except for women with a height <1.45 m in which the cutoff value was >0.66 m/s [15].
Functional independence. Subjects were classified as independent if they did not have difficulty performing any instrumental activities of daily living (IADL) included in the MHAS; preparing a hot meal, shopping for groceries, managing money and taking medications [16].
Subjects without MCR:
Considering that subjects who didn’t met criteria for MCR could have one or two of negative symptoms, a situation that could generate confusion with the definition of a healthy status, we classified them in three groups: 1) subjects with memory complaint only, b) subjects with slow gait speed only, and c) subjects without any symptom (healthy group).
Confounding variables:
Sociodemographic variables: age, sex and years of education.
Health variables: We included self-reported measures of hypertension, diabetes mellitus, stroke, coronary artery disease and falls in the last two years. Depressive symptoms were defined based on a cut-point of five on the geriatric depression scale included in MHAS [17].
Outcome variable
Cognitive impairment. Subjects with a total score in the cognitive test (CCCE) equal or less than −1.5 standard deviations and an IQCODE greater than 3.4 points were classified as cognitively impaired in the 2015 wave.
Statistical Analysis
We present our descriptive results stratified by baseline status. Two-sample t-tests for continuous variables and χ2 tests or Fisher’s exact test for categorical variables were used as appropriate. To determine the risk of cognitive impairment in subjects who had MCR syndrome at baseline, we used a Cox model to compute the relative risk with 95% Confidence Interval (CI) adjusting for the confounding variables. The variable “time for the appearance of cognitive impairment” was defined as the mid-point between the date (day/month/year) of the first interview and the date of the second interview and was registered in years. Statistical analysis was performed using SPSS software for Windows (SPSS Inc., Chicago, IL version 22.0).
Results
MCR Global prevalence was 14.3%, (95% IC: 11.9–17.1), it was slightly higher in women vs men (15.2% vs 13.3) and there was an exponential increase with age, showing a growth that reached 42.3% (95% CI 31.9–53.4) among individuals aged 80 and older. Decreasing prevalence with increasing years of education was particularly apparent in older adults with 7 or more years of education. 10.7% (95% CI: 6.8–16.2)
Sociodemographic characteristics health status and cognitive performance of the sample at baseline are shown in Table I. Compared to the normal group, subjects with MCR were older (74.3 ± 8.3, p<0.001) and had less years of education (4.5 ± 3.0, p<0.05). There were no differences between men and women. Regarding comorbidities, diabetes mellitus was the only condition significantly higher in the MCR group (33% vs 23%, p=0.05), although a general pattern of higher comorbidities such as hypertension, falls, and depression was observed. Regarding the performance in the cognitive evaluation performed in baseline 2012 as expected, significant differences were observed between the RCM group and the Healthy group in all the cognitive domains except in attention (visual scanning). It was the group with only slow gait who showed significant differences in the cognitive domains of memory and visuospatial alterations abilities between the group RCM and Healthy group.
Table I.
Sociodemographic, health status and cognitive evaluation, at baseline characteristics by motoric cognitive risk, memory complaint and slow gait group, MHAS-2012
| All n=735 | Healthy (No MCR*) n=141 | Memory complaint n=418 | Slow Gait n=69 | MCR* n=107 | p value | |
|---|---|---|---|---|---|---|
| Age, years mean (SD) | 69.8 (7.6) | 67.6 (5.8) A** | 68 (6.9) | 74 (8.8) | 74 (8.3) | <.001 |
| Female (%) | 462 (54) | 77 (54) | 214 (51) | 41 (59.4) | 60 (56) | .503 |
| Education, years Mean (SD) | 5.35 (3.8) | 7.5 (5.5) A** | 5.1 (3.4) | 4.5 (3.1) | 5.5 (4) | <.001 |
| Diabetes n (%) | 209 (24) | 24 (17)A** | 98 (23) | 19 (28) | 35 (33) | <.003 |
| Hypertension n (%) | 442 (51) | 63 (45) | 210 (50) | 33 (48) | 61 (57) | .008 |
| Falls n (%) | 358 (42) | 47 (33)A** | 172 (41) | 33 (48) | 52 (49) | .010 |
| Stroke n (%) | 17 (2) | 2 (1.4%) | 9 (2.2%) | 0 (0%) | 3 (2.8%) | .336 |
| Coronary artery disease, (%) | 23 (3) | 3 (2.1%) | 8 (1.9%) | 2 (2.9%) | 5 (4.7%) | .148 |
| Chronic disease n (%) (range >2) | 177 (19) | 68 (52%) A** | 247 (59%) | 38 (55%) | 75 (70%) | .002 |
| Depression (%) | 350 (40) | 20 (21) A** | 177 (43) B** | 32 (46) C** | 52 (48) | <.001 |
| CCCE* | ||||||
| Orientation Mean (SD) | 2.5(0.5) | 2.6(0.7) A** | 2.3(0.4) | 2.2(0.3) | 1.9(0.3) | .005 |
| Verbal learning memory Mean (SD) | 4.84 (1.7) | 4.89 (1.8) A** | 4.81 (1.6) | 4.79 (1.8) C** | 4.78(1.6) | .007 |
| Verbal recall memory Mean (SD) | 4.33 (2) | 4.43(2) A** | 4.23 (1.9) | 4.18 (2) | 4.12(1.9) | .013 |
| Visual scanning | 27.8 (17.4) | 28.7 (18.8) | 27.3 (17.8) | 26.9 (17.5) | 26.8 (15.7) | .460 |
| Visuospatial abilities Mean (SD) | 5.52 (1.1) | 5.53 (1.2) | 5.51 (1.1) D** | 5.48 (1.2) C** | 5.32 (1.5) | .037 |
| Visual memory Mean (SD) | 4.84 (1.7) | 4.89 (1.8) | 4.81 (1.6) | 4.79 (1.8) | 4.78(1.6) | .077 |
| Verbal Fluency Mean (SD) | 15.4 (9.2) | 15.7 (10.1) | 15.3 (9.3) | 15.04 (9) | 15 (8) | .333 |
| Numeracy (SD) | 10.2(2.1) | 10.4(5.6) A** | 9.7(3.2.) | 9.8(3.1) C** | 9.4(3.1) | .007 |
MRC: Motoric cognitive risk. CCCE: Cross Cultural Cognitive Examination, Chronic disease: hypertension, diabetes, falls, coronary artery disease.
Post-hoc Bonferroni analysis A: MCR versus Healthy, p<.05, B: MCR versus self-reported cognitive complaint p <.05, C: MCR versus slow gait, p<.05, and D: Self-reported cognitive complaint versus slow gait, p <.05. ***p<.005.
After a mean follow-up of 2.9 years, 113 cases of incident cognitive impairment were found. As shown in Table 2 the risk of cognitive impairment was 2.52 times higher in older adults with MCR syndrome (HR: 2.5, 95% CI: 1.42 – 4.48, p=.002). After adjusting for sociodemographic and health covariates, the risk of cognitive impairment slightly changed and remained statistically significant (HR: 2.46, 95% CI: 1.25–4.84, p=.009). The risk of cognitive impairment in subjects only with memory complaints or with slow gait was not significant in the unadjusted or adjusted models.
Table 2.
MCR and risk of incident cognitive impairment
| Model | HR 95% CI | |
|---|---|---|
| Motoric Cognitive Risk | Unadjusted | 2.5 (1.42–4.48) |
| Adjusted** | 2.46 (1.25–4.84) | |
| Memory complaints | Unadjusted | 1.07 (0.63–1.81) |
| Adjusted** | 0.97 (0.56–1.68) | |
| Slow gait | Unadjusted | 1.76 (0.72–4.26) |
| Adjusted** | 1.32 (0.48–3.61) |
MCR: Motoric cognitive risk, HR: Hazard ratio
Adjusted for age, education, history of diabetes, hypertension, falls and depression
Discussion
Our study showed 14.3% prevalence of MCR in Mexican older adults similar to that reported by the Kurihara Project in Japan (11.1%) [18] and in the range of the prevalence rates in 6 low- or middle-income countries (5.3% to 15.6%) reported by Verghese et al [19] in a multicenter study. Other cohort studies have also found prevalence ranging from 2% in Australia to UK [20,21] to 15% in India [22].
The association with greater age in older adults with MCR found in this and other studies, has been explained as the result of primary and secondary motor area alterations (critical for gait regulation) as well as in other involved support structures (parietal lobes, the fronto-striatal circuits and corpus callosum), which during the aging process, hinder the execution of activities learned in early stages of life. In addition, the sensory alterations that accompany aging also contribute to alterations in gait [23, 24, 25].
The much lower education in MCR subjects has also been previously reported by Verghese and Doi [19,26]. Gonzales et al. [27] demonstrated changes in gait speed and performance of tasks that involve working memory in patients with low education, supporting the hypothesis of a relationship between education and MCR [28,29]. These findings suggest that working memory impairment is one of the earliest manifestations of cognitive decline in elders [30]
Regarding comorbidities, our study found an association between MCR and diabetes mellitus diagnosis, like that reported by Doi et al [26]. This association can be a consequence of cerebral changes produced by this disease; atrophy, alteration of white matter integrity, and vascular lesions that affect brain capacity, making it more vulnerable to pathological changes, considering that these changes can appear pre-clinically (particularly in cerebrovascular and Alzheimer’s diseases) [31]. For main geriatric problems, such as falls and depression, our study found that they were present in almost half of the subjects with MCR. These results are in line with other studies that have previously demonstrated this association [26]. Casillaya et al [32] suggests that MCR is a clinical syndrome that predicts adverse health outcomes such as falls in older adults. On the other hand, researchers have found that cognitive impairment, slow gait speed, and depressive symptoms occur simultaneously in older adults [33]. Although depressive symptoms and reduced mobility coexist in older adults [34], the relationship between depressive symptoms and MCR requires further research [26].
Our study shows that individuals with MCR have 2.5 times more risk of progressing to cognitive impairment. This result is similar to those reported in a meta-analysis by Kueper et al. [35] and in the study of Verghese et al. in 2014 [19] who showed hazard ratios that ranged between 1.65 (1.30–2.10) in a sample of Hispanic older adults living in the US to 3.54 (2.05–6.12) reported in an Italian cohort. These authors propose that MCR could be used to detect individuals at risk of developing early cognitive impairment whenever the symptom combination of subjective memory complaints and low gait speed are present.
The study has several limitations. First, incomplete information about critical variables due to the nature of an in-home study had an impact on the sample size as well as attrition with loss to follow-up over time. Second, the use of self-reported questions about comorbidities, could have underestimated their frequency, and because of the underdiagnosis of chronic degenerative diseases in the general population. Third, our analyses are based on the total score of a cognitive test, leaving out the possibility to consider specific cognitive domains that are more likely to deteriorate with MCR.
Nevertheless, there are several strengths worth mentioning. First, we worked with a subsample from a representative study of Mexican older adults where information concerning diverse variables was gathered. This allowed us to include other medical conditions and sociodemographic data with possible confounding effects. Second, as a longitudinal cohort study, it provided the possibility to follow the change in cognitive function and analyze the role of MCR as a risk factor. Third, the assessment of functional independence in our study was defined by the lack of difficulty with instrumental activities of daily living, which involve greater cognitive demand than the basic activities of daily living used in other studies [19] ensuring a normal cognitive function at baseline. Fourth, to our knowledge, this is the first study recognizing this syndrome in Mexican population.
In conclusion, our study suggests that MCR syndrome is a risk factor for cognitive impairment and a highly prevalent condition among Mexican older adults. Gait evaluation in individuals with subjective memory complaints could allow the early identification of persons at risk of developing cognitive impairment and possibly subsequent dementia. More longitudinal studies are needed to confirm this association.
Acknowledgments
Funding: This research did not receive any funding from agencies in the public, commercial, or not-for-profit sector
Footnotes
Ethical issues:
The study protocol complies with the current laws of Mexico.
Conflicts of Interest:
The authors declare that they have no conflicts of interest and have no sponsor or funding arrangements concerning their research.
References
- 1.Instituto Nacional de Estadística, Geografía e Informática (2017) Estadísticas a propósito del día internacional de las personas de la tercera edad. INEGI; http://www.inegi.org.mx/saladeprensa/aproposito/2014/adultos0.pdf Accessed 11 September 2017 [Google Scholar]
- 2.Organización Panamericana de la Salud. Encuesta Multicéntrica, Salud Bienestar y Envejecimiento (SABE) en América Latina y el Caribe (2001). http://envejecimiento.csic.es/documentos/documentos/paho-salud-01.pdf Accessed 11 September 2017
- 3.Mejía S, Miguel-Jaimes A, Villa A, Ruiz L, Gutiérrez LM (2007) Cognitive impairment and associated factors in older adults in Mexico. Salúd Publica Mex 49 suppl 4, 475–481 [DOI] [PubMed] [Google Scholar]
- 4.Solomon PR, Murphy CA (2005) Should we screen for Alzheimer’s disease? A review of the evidence for and against screening Alzheimer’s disease in primary care practice. Geriatrics 60:26–31 [PubMed] [Google Scholar]
- 5.Prince M, Acosta D, Ferri CP, et al. (2012) Dementia incidence and mortality in middle-income countries, and associations with indicators of cognitive reserve: a 10/66 Dementia Research Group population-based cohort study. Lancet 7;380(9836):50–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bush C, Kozak J, Elmslie T (1997) Screening for cognitive impairment in the elderly. Can Fam Physician 43:1763–8 [PMC free article] [PubMed] [Google Scholar]
- 7.Doraiswamy PM, Steffens DC, Pitchumoni S, et al. (1998) Early recognition of Alzheimer’s disease: what is consensual? What is controversial? What is practical? J Clin Psychiatry 59 Suppl 13:6–18 [PubMed] [Google Scholar]
- 8.Verghese J, Wang C, Lipton RB, et al. (2013) Motoric cognitive risk syndrome and the risk of dementia. J Gerontol A Biol Sci Med Sci 68:412–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Montero M, Bergman H, Phillips NA, et al. (2009) Dual-tasking and gait in people with mild cognitive impairment. The effect of working memory. BMC Geriatr 9:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wong R, Espinoza M, Palloni A (2007) Adultos mayores mexicanos en contexto socioeconómico amplio: salud y envejecimiento. Salud Pública Mex 49: s436–s447. [DOI] [PubMed] [Google Scholar]
- 11.Wong R, Michaels A, Palloni A (2017) Cohort Profile: The Mexican Health and Aging Study (MHAS). Int J Epidemiol 1;46(2): e2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wolfe N, Imai Y, Otani C, et al. (1992) Criterion validity of the cross-cultural cognitive examination in Japan. J Gerontol 47: 289–91 [DOI] [PubMed] [Google Scholar]
- 13.Mejía S, Wong R, Michaels A (2015) Normative and standardized data for cognitive measures in the Mexican Health and Aging Study. Salud Pública Mex 57: s90–s96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jorms AF, Scott R, Cullen JS, et al. (1991) Performance of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) as a screening test for dementia. Psychol Med 21(3):785–90 [DOI] [PubMed] [Google Scholar]
- 15.Pérez MU, Sánchez N, González M, et al. (2016) Sarcopenia prevalence using simple measurements and population-based cutoff values. J Lat Am Geriatr Med 2(1): 8–13 [PMC free article] [PubMed] [Google Scholar]
- 16.Lawton MP, Brody EM (1969) Assessment of older people: self-maintaining and instrumental activities of daily living. The Gerontologist 9: 179–86 [PubMed] [Google Scholar]
- 17.Dorantes G, Ávila JA, Mejía S, et al. (2001) Factores asociados con la dependencia funcional en los adultos mayores: un análisis secundario del Estudio Nacional sobre Salud y Envejecimiento en México. Rev Panam Salud Pública 22:1–11 [DOI] [PubMed] [Google Scholar]
- 18.Aguilar SG, Fuente A, Ávila JA, et al. (2007) Validez y confiabilidad del cuestionario del ENASEM para la depresión en adultos mayores. Salud Pública Mex 49:256–62 [DOI] [PubMed] [Google Scholar]
- 19.Kumai K, Meguro K, Kasai M, et al. (2016) Neuroepidemiologic and Neurobehavioral Characteristics of Motoric Cognitive Risk Syndrome in an Old-Old Population: The Kurihara Project. Dement Geriatr Cogn Disord Extra 6:176–82 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verghese J, Annweiler C, Ayers E, et al. (2014) Motoric cognitive risk syndrome: multicountry prevalence and dementia risk. Neurology 83:718–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lord S, Galna B, Verghese J, et al. (2013) Independent domains of gait in older adults and associated motor and nonmotor attributes: validation of a factor analysis approach. J Gerontol A Biol Sci Med Sci Jul; 68(7):820–7 [DOI] [PubMed] [Google Scholar]
- 22.Callisaya ML, Blizzard L, McGinley JL, et al. (2010) Sensorimotor factors affecting gait variability in older people- a population-based study. J Gerontol A Biol Sci Med Sci Apr; 65(4):386–92 [DOI] [PubMed] [Google Scholar]
- 23.Verghese J, Noone ML, Johnson B, et al. (2012) Picture-based memory impairment screen for dementia. J Am Geriatr Soc Nov; 60(11): 2116–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Goldman R, Grossman M (2008) Update on Apraxia. Curr Neurol Neurosci Rep 8(6):490–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sturman DA, Moghadamm B (2011) The Neurobiology of Adolescence: Changes in brain architecture, functional dynamics, and behavioral tendencies. Neurosci Biobehav Rev 35(8):1704–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Meyer JS, Barron DW (1960) Apraxia of gait: A clinico-physiological study. Brain 83(2):261–84 [Google Scholar]
- 27.Doi T, Verghese J, Shimada H, et al. (2015) Motoric Cognitive Risk Syndrome: Prevalence and Risk Factors in Japanese Seniors. J Am Med Dir Assoc 16: 1103e21–25 [DOI] [PubMed] [Google Scholar]
- 28.Gonzales JU, James CR, Yang HS, et al. (2016) Different cognitive functions discriminate gait performance in younger and older women: A pilot study. Gait Posture 50:89–95 [DOI] [PubMed] [Google Scholar]
- 29.Economou A, Papageorgiou S, Karageorgiou C (2006) Working-delayed memory difference detects mild cognitive impairment without being affected by age and education. J Clin Exp Neuropsychol 28:528–35 [DOI] [PubMed] [Google Scholar]
- 30.Albert MS, Moss MB, Tanzi R, Jones K (2001) Preclinical prediction of AD using neuropsychological tests. J Int Neuropsychol Soc Jul; 7(5):631–9 [DOI] [PubMed] [Google Scholar]
- 31.Biessels GJ, Strachan MW, Visseren FL, et al. (2014) Dementia and cognitive decline in type 2 diabetes and prediabetic stages: towards targeted interventions. Lancet Diabetes Endocrinol 2: 246–e255 [DOI] [PubMed] [Google Scholar]
- 32.Callisaya ML, Ayers E, Birzilai N, et al. (2016) Motoric Cognitive Risk Syndrome and Falls Risk: A Multi-Center Study. J Alzheimers Dis 53(3): 1043–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hajjar I, Yang F, Sorond F, et al. (2009) A novel aging phenotype of slow gait, impaired executive function, and depressive symptoms: relationship to blood pressure and other cardiovascular risks. J Gerontol A Biol Sci Med Sci 64(9): 994–1001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lampinen P, Heikkinen E (2003) Reduced mobility and physical activity as predictors of depressive symptoms among community-dwelling older adults: an eight-year follow-up study. Aging Clin Exp Res 15(3): 205–11 [DOI] [PubMed] [Google Scholar]
- 35.Kueper JK, Speechley M, Lingum NR, et al. (2017) Motor function and incident dementia: a systematic review and meta-analysis. Age Ageing 46:729–38 [DOI] [PubMed] [Google Scholar]


