Abstract
Background
Umbilical arterial catheters (UACs) are among the most commonly used monitoring methodologies in neonatal intensive care. There seems to be significant variance between neonatal intensive care units in exactly how these catheters are used. This variance involves heparin dosing, catheter materials and catheter design and positioning of the catheter.
Objectives
To determine whether the use of heparin in fluids infused through an umbilical arterial catheter in newborn infants influences the frequency of clinical ischemic events, catheter occlusion, aortic thrombosis, intraventricular hemorrhage, hypertension, death, or the duration of catheter usability.
Search methods
Randomized and quasi‐randomized controlled trials of umbilical catheterization use were obtained using the search methods of the Cochrane Neonatal Review Group. The Cochrane Library, MEDLINE (search via PubMed), CINAHL and EMBASE were searched from 1999 to 2009.
Selection criteria
Randomized trials in newborn infants of any birthweight or gestation. Comparison of heparinised to non heparinised infusion fluids, including comparison of heparin in the infusate to heparin just in the flush solution. Clinically important end points such as catheter occlusion or aortic thrombosis.
Data collection and analysis
There were five randomized controlled trials retrieved. All gave details of the incidence of catheter occlusion. Two also reported the incidence of aortic thrombosis. The intervention was reasonably consistent: heparin in the infusate at a concentration of 1 unit/mL was investigated in all trials except one which used a concentration of 0.25 units/mL. Studies generally included both term and preterm infants.
Main results
Heparinization of the infusate decreases the incidence of catheter occlusion but does not affect the frequency of aortic thrombosis. Heparinization of the flush solution is not an adequate alternative. There does not appear to be an effect on frequency of intraventricular hemorrhage, death or clinical ischemic phenomena.
Authors' conclusions
Heparinization of the fluid infused through an umbilical arterial catheter decreases the likelihood of umbilical arterial catheters occluding. The lowest concentration tested so far (0.25 units/mL) has been shown to be effective. Heparinization of flushes without heparinizing the infusate is ineffective. The frequency of aortic thrombosis has not been shown to be affected; however, the confidence intervals for this effect are very wide. The frequency of intraventricular hemorrhage has not been shown to be affected by heparinization of the infusate, but again the confidence intervals are very wide and even a major increase in the incidence of grade 3 and 4 intraventricular hemorrhage would not have been detected.
Plain language summary
Umbilical artery catheters in the newborn: effects of heparin
The umbilical artery catheters (tubes) (UACs) commonly used in neonatal intensive care to monitor babies can sometimes cause them problems. They can be placed in high or low positions, and come in different materials and designs. The blood anticoagulant, heparin, theoretically helps prevent blood clots forming (thromboses), but high doses could lead to haemorrhage (bleeding). This review found that low heparin doses are effective in preventing catheters becoming blocked and needing to be re‐inserted. There is not enough evidence to rule out the possibility of adverse effects. Heparin does not seem to lower the rate of blood clots in the major artery.
Background
Description of the condition
Umbilical arterial catheters (UACs) are among the most commonly used monitoring methodologies in neonatal intensive care. There seems to be significant variance between neonatal intensive care units in exactly how these catheters are used. This variance involves heparin dosing, catheter materials and catheter design and positioning of the catheter. Immediately after insertion of a UAC, local vascular compromise is frequently evident, usually in the form of blue or white toes, but occasionally with more severe and extensive ischemic manifestations. Aortic thrombi and renal ischemia have also been described. Some epidemiologic and case control studies have also shown that use of umbilical artery catheterisation is statistically associated with the later development of necrotizing enterocolitis. In order to minimize these morbidities some aspects of the use of UACs have been subjected to a small number of randomized control trials.
Description of the intervention
Most NICUs now add heparin to the fluid being infused through the UAC.
How the intervention might work
Several studies have examined whether this practice affects catheter occlusions or aortic thrombosis, and whether adding heparin to fluids used to intermittently flush the UAC, in place of adding heparin to the infusate, is adequate.
Heparin exposure at high dosage may be associated epidemiologically with an increased risk of intraventricular hemorrhage (Lesko 1986; Malloy 1995). It is therefore necessary to ensure that heparinization of fluids does indeed have a benefit as well as to determine whether clinically important adverse effects have been reliably assessed in newborn infants.
Why it is important to do this review
This review updates the existing review "Umbilical artery catheters in the newborn: effects of heparin" published in the Cochrane Database of Systematic Reviews (Barrington 1999).
Objectives
To determine whether the use of heparin in fluids infused through an umbilical arterial catheter in newborn infants influences the frequency of clinical ischemic events, catheter occlusion, aortic thrombosis, intraventricular hemorrhage, hypertension, death, or the duration of catheter usability.
Methods
Criteria for considering studies for this review
Types of studies
Randomized and quasi randomized clinical studies were selected. Clinically relevant outcomes were collated with survival and long term disability being given the greatest weight. Both term and preterm infants were included.
Types of participants
Newborn infants, both preterm and term. Entry criterion was usually simply the 'need' for an umbilical artery catheter, as defined by the attending medical staff.
Types of interventions
Random assignment to administration of heparin in the infusate or control without heparin. Random assignment to a group which received heparin in intermittent flush solution only was considered separately.
Types of outcome measures
Catheter occlusion;
Aortic thrombosis;
Death;
Intraventricular hemorrhage;
Hypertension;
Clinical ischemic events.
Search methods for identification of studies
The standard search method of the Cochrane Neonatal Review Group was used.
Electronic searches
Randomized and quasi randomized controlled trials of umbilical catheterization use were obtained from the following sources:1. MEDLINE Search using Melvyl Medline Plus and the keyword headings 'Umbilic#', 'Catheter#' and subject heading 'Infant, Newborn'.
The bibliography cited in each publication obtained was searched in order to identify additional relevant articles.
The original search was completed in November 1997. The search was updated in November 1998.
The search was updated in April 2009: The Cochrane Library, MEDLINE (search via PubMed), CINAHL and EMBASE were searched from 1999 to 2009. Search terms: umbilic* AND catheter. Limits: human, newborn infant and clinical trial. No language restrictions were applied.
Searching other resources
Randomized and quasi randomized controlled trials of umbilical catheterization use were obtained from searching the following other resources:
1. Effective Care of the Newborn Infant, edited by JC Sinclair and MB Bracken
2. Search of personal data files
Clinical trials registries were also searched for ongoing or recently completed trials (clinicaltrials.gov; controlled‐trials.com; and who.int/ictrp)
Data collection and analysis
The standard methods of the Cochrane Neonatal Review Group Guidelines were employed.
Selection of studies
Reports were first reviewed to determine whether there was a concurrent control group, and discarded if not. The method of assignment to control and intervention groups was then determined and if not random or quasi random, then the trial was discarded.
Data extraction and management
The review author extracted, assessed and coded all data for each study using a form that was designed specifically for this review. For each study, final data was entered into RevMan by the review author (KB).
Assessment of risk of bias in included studies
The standard method of the Cochrane Neonatal Review Group were employed. Each identified trial was assessed for methodological quality with respect to a) masking of allocation b) masking of intervention c) completeness of follow‐up d) masking of outcome assessment. This information is included in the Characteristics of Included Studies Table.
For the update in 2009, the risk of bias table was completed in order to address the following questions:
1. Sequence generation: Was the allocation sequence adequately generated?
2. Allocation concealment: Was allocation adequately concealed?
3. Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
4. Incomplete outcome data: Were incomplete outcome data adequately addressed?
5. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Measures of treatment effect
Statistical analyses was performed using Review Manager software. For categorical outcomes, estimates for relative risk and risk difference were calculated. For outcomes measured on a continuous scale, estimates for weighted mean difference were calculated. 95% confidence intervals were used.
Assessment of heterogeneity
Heterogeneity between trials was evaluated by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. A fixed effects model for meta‐analysis.
Data synthesis
If multiple trials were found and meta‐analysis was judged to be appropriate, the analysis would be done using Review Manager software (RevMan 5). All meta‐analyses were to be done using the fixed effect model.
Subgroup analysis and investigation of heterogeneity
If data was available, subgroup analysis based on gestational age or birth weight were planned.
Results
Description of studies
The original search strategy retrieved 849 articles. These were further reduced by restricting the search by use of the terms prospective, random#, control#, comparative or clinical. When studies of animal subjects and of epidural catheterisation were also removed, 233 reports remained. The abstract of each was read individually to determine if there was a possibility that the papers were reporting randomized prospective controlled trials. This final examination revealed six trials investigating heparin use, one of which was a trial published only as an abstract and reporting a study comparing heparinization of the infusate to full systemic heparinization (McDonald 1984). This trial was excluded from further analysis. All five remaining trials included both term and preterm infants. Several of these trials do not give information regarding the total dose of heparin administered to the infants. This would, of course, be highly relevant to the occurrence of hemorrhagic complications. Where such information is given it is noted below. At the time of the updated search in 1999 one further randomized controlled trial was added (Chang 1997).
Ankola 1993 performed a non‐masked study in 30 infants who required catheter placement for clinical indications. Heparin was administered at a lower dose than any of the other studies at 0.25 units/mL. Total heparin dose received was 25 to 50 units per kg per day. Maximum heparin infusion rate was 38 (SD 7) U/kg/d. Major outcome variables were duration of catheter usability, catheter occlusion, intraventricular hemorrhage, necrotizing enterocolitis, clinical ischemic phenomena (blue discoloration of toes) and sepsis.
Bosque 1986 randomised 47 infants to receive either heparin at a dose of 1 unit/mL added to the infusate and no heparin in the solution used to flush the catheter, or no heparin in the infusate and 1 unit/mL added to the flush solution. Infants were stratified by birth weight. The group with heparin in the infusate received 80 to 220 U/kg/d of heparin, with a mean of 120 U/kg/d. The group with heparin in the flush received 0.8 to 9.8 U/kg/d with a mean of 3.9 U/kg/d. The major outcome variables were catheter occlusion, ischemic complications, prothrombin time and partial thromboplastin time.
David 1981 studied 50 infants (one later removed from analysis) randomly assigned to either 1 unit heparin/mL, in both the infusate and the flush, to no heparin in either fluid. Total heparin doses were not clearly stated but the highest dosage ranged from 125 to 200 U/kg/d. The major outcome variable of the study was the occurrence of aortic thrombi detected by single shot aortogram performed at the time of catheter removal, if the catheter had been in place for more than 24 to 72 hours. Autopsies, if available, were also used to determine aortic clots. Clotting studies were also performed
Horgan 1987 performed a trial in 111 term and preterm infants. Infants were randomly allocated to one of four nurseries. In two nurseries heparinized fluids were used, in the other two heparin was not used. The text also states that allocation to a particular nursery was based on the availability of bed space. This study may therefore have had 'haphazard' rather than truly random allocation, and allocation was certainly not masked. Both high and low catheters were allowed according to physician preference. Total heparin doses ranged from 0.46 to 3.4 U/kg/hr, with a mean of 0.9 U/kg/hr. Outcome variables reported were aortic thrombi determined by ultrasound, catheter occlusion, intraventricular hemorrhage of grade 3 or 4, sustained hypertension, (systolic pressure above 100 mm Hg or mean >85 mm Hg in term infants and > 70 mm Hg in premature infants).
Rajani 1979 performed a well masked randomized study with the randomization code maintained by pharmacy until the end of the study. 62 infants, term and preterm, were randomized to either heparin or placebo added at 1 unit/ml to the catheter infusate. Fluids ran at a minimum of 5 mL/hr in all but one infant, giving a total heparin dose in excess of 5 units/hr for all except one infant in the treatment group. It was noted in the discussion that patients received between 100 and 200 U/kg/d. Outcome variables were duration of catheter 'life‐span', incidence of occlusion, clotting studies, and clinical ischemic phenomena defined as blue toes lasting for more than 8 hours.
Chang 1997 reported a prospective masked trial of heparin usage in umbilical catheters. This study assessed the effects of the use of heparin in both umbilical arterial and venous catheters, at a concentration of 1 unit/mL. The study only included preterm infants at risk for development of intraventricular hemorrhage by reason of a gestational age of less than 31 completed weeks gestational age. The study period was from birth to the fifth day of life, and heparin doses received by the infant averaged 4.3 U/kg/hr (no standard deviation given). The primary outcome variable was the occurrence of intraventricular hemorrhage, of any grade. Hemostatic variables were also determined and compared between groups. Mortality rates and severe intraventricular hemorrhage rates were reported and are included in the meta‐analysis.
Risk of bias in included studies
Ankola 1993 Masking of allocation: No method for masking allocation is described, no details of the randomization process are given. Masking of intervention: Not attempted Completeness of follow up: All patients are accounted for Masking of Outcome: Not attempted
Bosque 1986 Masking of allocation: Allocation was determined by a table of random numbers and "a separate pad of paper was used for each weight group". There does not appear to have been adequate allocation concealment. Masking of intervention: Not attempted. Completeness of follow up: All infants appear to have been accounted for. Masking of outcome: Not attempted. Other Comments: Hypothesis not described. Study was stopped when it was found that all of the occlusions were in the flush only group, and that the difference was statistically significant.
David 1981 Masking of allocation: Allocation clearly described: A pad containing randomly ordered group designations was used, the top page torn off for each patient, assignments not visible until torn off. Masking of intervention: Not attempted. Completeness of follow up: Yes. Masking of outcome: Not attempted for most outcomes, aortograms were read by a radiologist unaware of group assignment.
Horgan 1987 Masking of allocation: Not attempted. Masking of intervention: Not attempted. Completeness of follow up: All infants appear to have been accounted for. The numbers of infants who actually had ultrasounds is not stated, even though two infants had aortic thrombosis diagnosed by clinical symptoms alone. Masking of outcome: Not attempted. Other comments: Hypotheses stated, authors do not describe how they arrived at a sample size.
Rajani 1979 Masking of allocation: This appears to have been adequate. Masking of intervention: This was placebo controlled fully masked study. Completeness of follow up: All infants accounted for. Masking of outcome: Yes. Other comments: Hypothesis stated, No sample size determination is described. Life table analysis of catheters with and without heparin was performed.
Chang 1997 Masking of allocation: Randomization was revealed only to the pharmacist involved and therefore this appears to have been adequate. Masking of intervention: This was a placebo controlled masked study. Completeness of follow up: Outcomes are described for all except 5 infants deleted from analysis as they contravened exclusion criteria. Masking of outcome: It appears that the interpretation of the ultrasounds was performed by a single masked radiologist, and this was therefore adequate. Other comments: Both venous and arterial lines were studied in this trial.
Effects of interventions
Heparin in infusate compared to no heparin (Comparison 1):
Heparin in the infusate clearly decreases the incidence of catheter occlusion; (four trials 252 infants) typical Relative Risk 0.20; (95% CI 0.11, 0.35) (Outcome 1.1.1). This result was remarkably consistent between studies.
Using heparin solely in the flush solution and not in the infusate does not appear to be effective in preventing catheter occlusion (Bosque 1986).
A concentration of 0.25 units/mL, in comparison to no heparin, was effective in one trial (Ankola 1993), and appears to have a similar efficacy to higher concentrations but has not been directly compared to a higher concentration.
The duration of catheter usability was reported by several of the studies by the use of life table analysis. (David 1981; Rajani 1979; Ankola 1993). All showed a significant prolongation of the life of the catheter with heparinization.
Aortic thrombosis incidence, however, does not appear to be affected by heparin use. Neither of the two studies that examined this showed an effect (David 1981; Horgan 1987). The overall relative risk was 0.71; 95% CI 0.42, 1.22 (Outcome 1.2).
Coagulation studies were described in several reports (David 1981; Horgan 1987, Bosque 1985, Rajani 1979; Chang 1997). The timing of performance of the studies, methodologic details and detail of reporting of the results varied so greatly that a meta‐analysis was not performed. However, in general terms none of the studies showed a significant prolongation of prothrombin or partial thromboplastin times. Horgan 1987 and Chang 1997 did not show an effect on fibrinogen concentrations; David 1981 did not show an effect on thrombin or reptilase times; and Rajani 1979 did not show an effect on antithrombin 3 concentrations, whereas Chang 1997 showed a decrease in antithrombin 3 concentrations at the end of the study in the heparin group.
Hypertension was reported by only one of the studies (Horgan 1987) and was significantly reduced by the addition of heparin, relative risk 0.05; 95% CI 0.00, 0.78 (Outcome 1.3).
Grade 3 and 4 Intraventricular hemorrhage was not affected (Ankola 1993; Horgan 1987; Chang 1997), relative risk 1.07; 95% CI 0.58, 1.99 (3 trials, 254 infants) (Outcome 1.4).
Clinical ischemic phenomena were reported by three of the studies; none reported a significant effect of heparinization. The overall RR was 1.28; 95% CI 0.66, 2.48 (3 trials, 139 infants) (Outcome 1.5).
Mortality was reported in four studies (Ankola 1993; Horgan 1987; Rajani 1979; Chang 1997). Each failed to show a significant difference between heparinized and non‐heparinized fluids. The overall relative risk was 0.87; 95% CI 0.50, 1.49 (Outcome 1.6). David 1981 mentioned that mortality was not significantly different between groups, but did not give figures. (Ankola 1993 mentioned that one control patient died "during the period of this data collection").
Discussion
The one excluded study (McDonald 1984) compared full systemic heparinization using 20 ‐ 25 U/kg/hr of heparin to heparinization of the infusate (1 ‐ 2 U/mL). The first 19 infants in the study were reported in an abstract. The incidence of aortic thrombosis, determined by aortography, was reported as 1/11 with full heparinization vs. 4/8 with heparinized infusate. These proportions are not statistically significant by Fisher exact test. The abstract stated that the intervention was "without complications including ICH". We will attempt to obtain further information regarding this study. In particular, the incidence of intracranial hemorrhage will be sought.
Heparinization of the infusate appears to be effective in reducing the incidence of catheter occlusion. This leads to an increase in the usable lifespan of the catheter. There is not a statistically significant effect of heparinization on the frequency of aortic thrombi. However, the confidence intervals for this effect are wide, as only 134 infants have been evaluated for this common and important outcome. Thus it remains possible that a decrease in aortic thrombosis of as much as 50% could have been missed in the studies published to date. The lack of documented effect of heparin on aortic thrombosis, if confirmed in further studies, may suggest that mechanical factors may be more important, or that systemic anticoagulation may be necessary to prevent this complication. Clinically apparent ischemic phenomena were also unaffected by heparinization, again suggesting that mechanical factors may be most important in determining the frequency of blue or white toes.
There was no effect of routine use of heparin on mean values of coagulation tests or on analyses of clotting factors. The decrease in AT3 demonstrated by Chang 1997 is of questionable significance, in general heparin administration has been associated with an increase in AT3 levels, so a slight decrease, which remained within the accepted normal range is of uncertain significance. The wide range of administered heparin doses means that there remains a possibility of an effect of heparin on coagulation in that group of infants who receive greater volumes of heparinized fluids.
Adverse effects were not noted. The incidence of intraventricular hemorrhage was not different between groups, but it must be remembered that almost all of these studies included infants of all gestational ages, many of whom would be at very low risk for this complication. A relatively small number of infants have been evaluated for extensive intracranial hemorrhage (n = 254). The confidence intervals for effect on intracranial hemorrhage are very wide and still include the possibility that routine addition of heparin to umbilical artery catheters doubles the incidence of grade 3 and 4 hemorrhages! The actual total dose of heparin differed widely because of varying infusion rates, and was not standardized between groups. Infants who receive the highest doses of heparin may be at increased risk for intraventricular hemorrhage as suggested by two epidemiologic studies (Lesko 1986; Malloy 1995).
Mortality was also not significantly affected by heparinization (n evaluated = 316), but again the confidence intervals are very wide. There are no clear data on the frequency of necrotising enterocolitis which was an outcome variable in only one study (the incidence in both groups was zero, Ankola 1993).
Authors' conclusions
Implications for practice.
Heparinization of fluids administered via umbilical arterial catheter appears to decrease the incidence of catheter occlusion, so prolonging the life of the catheter. The effective concentration may be as low as 0.25 units/mL. Until further data are available it appears to be reasonable to use the lowest effective concentration of heparin to maintain catheter patency.
Implications for research.
Further research should evaluate the efficacy of using even lower concentrations of heparin. Other methods for reducing catheter occlusions or preventing aortic thrombosis, such as the use of heparin bonded catheters, should be compared to heparinization. Because of the imprecision of our estimates of the risk of heparinization of infusates on hemorrhagic complications and the effects on major thrombotic complications, further research must adequately assess the incidence of aortic thrombosis and of major intraventricular hemorrhage.
What's new
| Date | Event | Description |
|---|---|---|
| 4 August 2009 | New search has been performed | This review updates the existing review "Umbilical artery catheters in the newborn: effects of heparin" published in the Cochrane Database of Systematic Reviews (Barrington 1999). Updated search found no new trials. No changes to conclusions. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 1, 1999
| Date | Event | Description |
|---|---|---|
| 22 October 2008 | Amended | Converted to new review format. |
Acknowledgements
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Heparin in infusate compared to no heparin.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Catheter occlusion prior to removal | 5 | 299 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.19 [0.10, 0.33] |
| 1.1 Studies comparing heparin in the infusate to no heparin | 4 | 252 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.20 [0.11, 0.35] |
| 1.2 Studies comparing heparin in the infusate to heparin only in the flush | 1 | 47 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.09 [0.01, 1.52] |
| 2 Aortic thrombosis | 2 | 134 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.42, 1.22] |
| 3 Hypertension | 1 | 111 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.05 [0.00, 0.78] |
| 4 Intraventricular hemorrhage, grade 3 and 4 | 3 | 254 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.07 [0.58, 1.99] |
| 5 Clinical ischemic phenomena | 3 | 139 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.28 [0.66, 2.48] |
| 6 Death | 4 | 316 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.50, 1.49] |
1.1. Analysis.
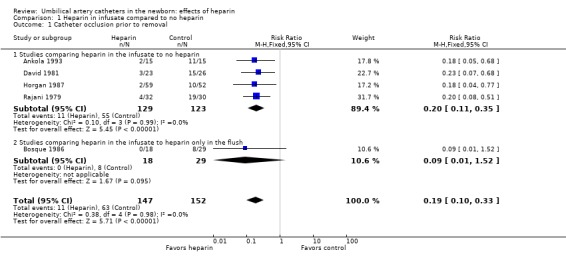
Comparison 1 Heparin in infusate compared to no heparin, Outcome 1 Catheter occlusion prior to removal.
1.2. Analysis.
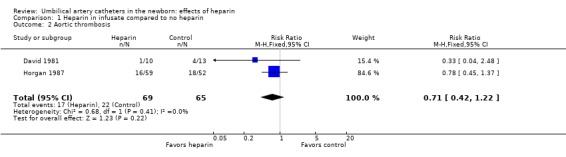
Comparison 1 Heparin in infusate compared to no heparin, Outcome 2 Aortic thrombosis.
1.3. Analysis.
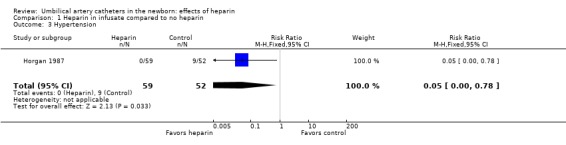
Comparison 1 Heparin in infusate compared to no heparin, Outcome 3 Hypertension.
1.4. Analysis.
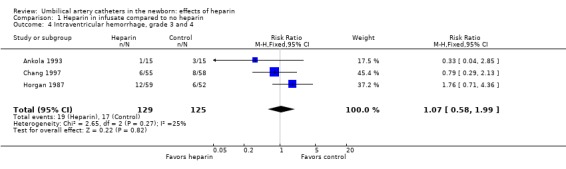
Comparison 1 Heparin in infusate compared to no heparin, Outcome 4 Intraventricular hemorrhage, grade 3 and 4.
1.5. Analysis.
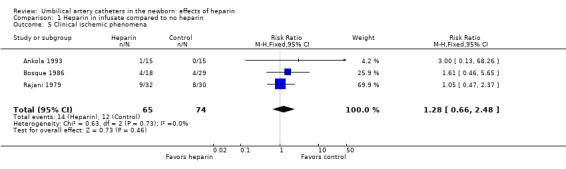
Comparison 1 Heparin in infusate compared to no heparin, Outcome 5 Clinical ischemic phenomena.
1.6. Analysis.
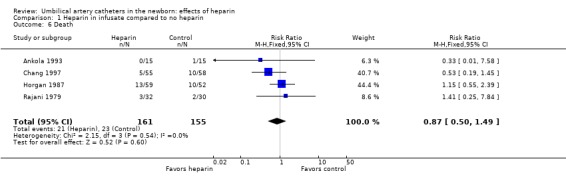
Comparison 1 Heparin in infusate compared to no heparin, Outcome 6 Death.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ankola 1993.
| Methods | Single center randomized study. Masking of allocation; not described. Masking of intervention; No. Completeness of follow up; Yes. Masking of outcome; No. | |
| Participants | 30 term and preterm infants. | |
| Interventions | Heparin at 0.25 units/mL, or no heparin, in the infusate. Flush solutions did not contain heparin. | |
| Outcomes | Catheter occlusion, intraventricular hemorrhage, PT, PTT were only measured in the heparin group. Blue toes. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Unclear risk | No details of the randomization process are given. |
| Allocation concealment? | Unclear risk | No method for masking allocation is described, no details of the randomization process are given. |
| Blinding? All outcomes | High risk | Masking of intervention: Not attempted. Masking of Outcome: Not attempted. |
| Incomplete outcome data addressed? All outcomes | Low risk | Completeness of follow‐up: All patients are accounted for. |
Bosque 1986.
| Methods | Randomised single center study. Masking of allocation; appears not to have been done. Masking of intervention; No. Completeness of follow up; Yes. masking of outcome; No. | |
| Participants | 47 preterm and term infants stratified by birth weight. | |
| Interventions | Infants received either heparin at a dose of 1 unit/mL added to the infusate and no heparin in the solution used to flush the catheter, or no heparin in the infusate and 1 unit/mL to the flush solution. | |
| Outcomes | Clotting studies (PT and PTT), incidence of catheter occlusion, duration of catheter usability. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation was determined by a table of random numbers and "a separate pad of paper was used for each weight group". |
| Allocation concealment? | High risk | There does not appear to have been adequate allocation concealment. |
| Blinding? All outcomes | High risk | Masking of Intervention: Not attempted. Masking of outcome: Not attempted. |
| Incomplete outcome data addressed? All outcomes | Low risk | Completeness of follow‐up: All infants appear to have been accounted for. |
| Free of other bias? | Unclear risk | Other Comments: Hypothesis not described. Study was stopped when it was found that all of the occlusions were in the flush only group, and that the difference was statistically significant. |
Chang 1997.
| Methods | Single center masked prospective controlled trial. Masking of allocation; yes. Masking of intervention; yes. Completeness of follow up; yes. Masking of outcome; yes. | |
| Participants | Preterm infants, <31 weeks gestational age, who had umbilical catheters placed. Either arterial or venous catheters or both were acceptable. | |
| Interventions | Addition of heparin 1 unit/mL to the infusate, compared to placebo. No heparin in the flush solution. | |
| Outcomes | Intraventricular hemorrhage diagnosed by a head ultrasound at 1 week of age was the primary outcome variable. Secondary outcomes included clotting times (PT, PTT) and coagulation factor assays (fibrinogen, AT3). | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Masking of allocation: Randomization was revealed only to the pharmacist involved and therefore this appears to have been adequate. |
| Blinding? All outcomes | Low risk | Masking of intervention: This was a placebo controlled masked study. Masking of outcome: It appears that the interpretation of the ultrasounds was performed by a single masked radiologist, and this was therefore adequate. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Completeness of follow‐up: Outcomes are described for all except 5 infants deleted from analysis as they contravened exclusion criteria. |
| Free of other bias? | Unclear risk | Other comments: Both venous and arterial lines were studied in this trial. |
David 1981.
| Methods | Single center randomized study. Masking of allocation; Yes. Masking of intervention; No. Completeness of follow up; Yes. Masking of outcome; No. | |
| Participants | 50 term and preterm infants requiring umbilical arterial catheterisation for clinical care. | |
| Interventions | Heparin at 1 unit/mL in the infusate and the flush compared to no heparin in either the infusate or the flush. | |
| Outcomes | Clinical ischemic phenomena, hematuria, clotting studies, Aortic thrombi on aortograms performed after 24 to 72 hours or at autopsy. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Allocation clearly described: A pad containing randomly ordered group designations was used, the top page torn off for each patient, assignments not visible until torn off. |
| Allocation concealment? | Low risk | Masking of allocation: As noted above. A pad containing randomly ordered group designations was used, the top page torn off for each patient, assignments not visible until torn off. |
| Blinding? All outcomes | High risk | Masking of intervention: Not attempted. Masking of outcome: Not attempted for most outcomes, aortograms were read by a radiologist unaware of group assignment. |
| Incomplete outcome data addressed? All outcomes | Low risk | Completeness of follow‐up: Yes. |
Horgan 1987.
| Methods | Single center randomized study. Masking of allocation; No. Masking of intervention; No. Completeness of follow up; Yes. Masking of Outcome; No. | |
| Participants | 111 infants "randomly" allocated to one of four nurseries, two of which used heparin and two of which didn't. | |
| Interventions | Heparin at 1 unit/mL in the infusate, running at 2 mL/hr. The flush solutions did not contain heparin. | |
| Outcomes | Frequency of catheter occlusion. Aortic thrombosis by ultrasound within 12 to 24 hours after catheter removal, PT and PTT. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | Masking of Allocation: Not attempted. |
| Blinding? All outcomes | High risk | Masking of Intervention: Not attempted. Masking of outcome: Not attempted. |
| Incomplete outcome data addressed? All outcomes | Unclear risk | Completeness of follow‐up: All infants appear to have been accounted for. The numbers of infants who actually had ultrasounds is not stated, even though two infants had aortic thrombosis diagnosed by clinical symptoms alone. |
| Free of other bias? | Unclear risk | Other comments: Hypotheses stated, authors do not describe how they arrived at a sample size. |
Rajani 1979.
| Methods | Single center randomized study. Masking of allocation; yes. Masking of intervention; Yes. Completeness of followup; Yes. Masking of outcome; Yes. | |
| Participants | 62 term and preterm infants requiring umbilical arterial catheterisation for clinical therapy. | |
| Interventions | Heparin at 1 unit/mL or placebo (5% dextrose) added to UAC fluid. No heparin was added to the flush solution. | |
| Outcomes | Clinical ischemic phenomena, catheter occlusion, PT and PTT. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | Masking of allocation: This appears to have been adequate. |
| Blinding? All outcomes | Low risk | Masking of intervention: This was placebo controlled fully masked study. Masking of outcome: Yes. |
| Incomplete outcome data addressed? All outcomes | Low risk | Completeness of follow‐up: All infants accounted for. |
| Free of other bias? | Unclear risk | Other comments: Hypothesis stated, No sample size determination is described. Life table analysis of catheters with and without heparin was performed. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| McDonald 1984 | Abstract only published; data not given in sufficient detail to analyze. This was a comparison of heparinization of the infusate to complete systemic heparinization of the infant. Reported as a "trial in progress"." |
Contributions of authors
Keith Barrington (KB) wrote the original review and updated the review in 1999. The 2009 update was conducted centrally by the Cochrane Neonatal Review Group staff (Yolanda Montagne, Roger Soll, Diane Haughton) and reviewed and approved by KB.
Declarations of interest
None
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ankola 1993 {published data only}
- Ankola PA, Atakent YS. Effect of adding heparin in very low concentration to the infusate to prolong the patency of umbilical artery catheters. American Journal of Perinatology 1993;10:229‐32. [DOI] [PubMed] [Google Scholar]
Bosque 1986 {published data only}
- Bosque E, Weaver L. Continuous versus intermittent heparin infusion of umbilical artery catheters in the newborn infant. Journal of Pediatrics 1986;108:141‐3. [DOI] [PubMed] [Google Scholar]
Chang 1997 {published data only}
- Chang GY, Lueder FL, DiMichele DM, et al. Heparin and the risk of intraventricular hemorrhage in premature infants. Journal of Pediatrics 1997;131:362‐6. [DOI] [PubMed] [Google Scholar]
David 1981 {published data only}
- David RJ, Merten DF, Anderson JC, Gross S. Prevention of umbilical artery catheter clots with heparinized infusates. Developmental Pharmacology and Therapeutics 1981;2:117‐26. [PubMed] [Google Scholar]
Horgan 1987 {published data only}
- Horgan MJ, Bartoletti A. Effect of heparin infusates in umbilical arterial catheters on frequency of thrombotic complications. Journal of Pediatrics 1987;111:774‐8. [DOI] [PubMed] [Google Scholar]
Rajani 1979 {published data only}
- Rajani K, Goetzman B, Wennberg RP, Turner E, Abildgaard C. Effect of heparinization of fluids infused through an umbilical artery catheter on catheter patency and frequency of complications. Pediatrics 1979;63:552‐6. [PubMed] [Google Scholar]
References to studies excluded from this review
McDonald 1984 {published data only}
- McDonald MM, Johnson ML, Rumack CM, Marlar R, Hathaway WE. Heparin prevention of catheter related thromboses. Pediatric Research 1984;18:335A. [Google Scholar]
Additional references
Lesko 1986
- Lesko SM, Mitchell AA, Epstein MF, Louik C, Giacoia GP, Shapiro S. Heparin use as a risk factor for intraventricular hemorrhage in low‐birth‐weight infants. New England Journal of Medicine 1986;314:1156‐60. [DOI] [PubMed] [Google Scholar]
Malloy 1995
- Malloy MH, Cutter GR. The association of heparin exposure with intraventricular hemorrhage among very low birth weight infants. Journal of Perinatology 1995;15:185‐91. [PubMed] [Google Scholar]
References to other published versions of this review
Barrington 1997
- Barrington KJ. Umbilical artery catheters: heparin usage (Cochrane Review). Cochrane Database of Systematic Reviews 1997, Issue 4. [DOI: 10.1002/14651858.CD000507] [DOI] [PMC free article] [PubMed] [Google Scholar]
Barrington 1999
- Barrington KJ. Umbilical artery catheters in the newborn: effects of heparin. Cochrane Database of Systematic Reviews 1999, Issue 1. [DOI: 10.1002/14651858.CD000507] [DOI] [PMC free article] [PubMed] [Google Scholar]


