Abstract
Background
Brain oedema is a major cause of early death after stroke. A 10% solution of glycerol is a hyperosmolar agent that is claimed to reduce brain oedema.
Objectives
To determine whether intravenous glycerol treatment in acute stroke, either ischaemic or haemorrhagic, influences death rates and functional outcome in the short or long term, and whether the treatment is safe.
Search methods
The Cochrane Stroke Group trials register was searched (January 2003), and some trialists were personally contacted.
Selection criteria
All completed, randomised and quasi‐randomised, controlled, published and unpublished comparisons, evaluating clinical outcome in which intravenous glycerol treatment was initiated within the first days after stroke onset.
Data collection and analysis
Two reviewers independently applied the inclusion criteria, assessed trial quality and extracted data and this was checked with all co‐reviewers. Death from all causes, functional outcome, and adverse effects were analysed.
Main results
Eleven completed, randomised trials comparing intravenous glycerol and control were considered. Analysis of death during the scheduled treatment period for acute ischaemic and/or haemorrhagic stroke was possible in 10 trials where 482 glycerol treated patients were compared with 463 control patients. Glycerol was associated with a non‐significant reduction in the odds of death within the scheduled treatment period (odds ratio (OR) 0.78, 95% confidence intervals (CI) 0.58 to 1.06). Among patients with definite or probable ischaemic stroke, glycerol was associated with a significant reduction in the odds of death during the scheduled treatment period (OR 0.65, 95% CI 0.44 to 0.97). However, at the end of the scheduled follow‐up period, there was no significant difference in the odds of death (OR 0.98, 95% CI 0.73 to 1.31). Functional outcome was reported in only two studies but there were non‐significantly more patients who had a good outcome at the end of scheduled follow up (OR 0.73, 95% CI 0.37 to 1.42). Haemolysis seems to be the only relevant adverse effect of glycerol treatment.
Authors' conclusions
This systematic review suggests a favourable effect of glycerol treatment on short term survival in patients with probable or definite ischaemic stroke but the confidence intervals were wide and the magnitude of the treatment effect may be only minimal. Due to the relatively small number of patients, and that the trials were performed in the pre‐CT era, the results must be interpreted cautiously. The lack of evidence of benefit in long term survival does not support the routine or selective use of glycerol treatment in patients with acute stroke.
Plain language summary
Glycerol for acute stroke
There is not enough evidence to show if glycerol can reduce the disabling effects of brain swelling due to acute stroke. Brain swelling (or oedema) is a major cause of early death and long‐term disability after stroke (a sudden catastrophe in the brain either because an artery to the brain blocks, or because an artery in or on the brain ruptures and bleeds). A 10% solution of glycerol might reduce brain swelling and therefore reduce the risk of death and long‐term disability after a stroke. The review found some evidence that glycerol improves the short term survival after stroke, but there was not enough evidence to decide whether glycerol helps avoid disability after stroke. Adverse effects of glycerol treatment did not happen often, but a small number of treated patients were found to have blood in their urine (this disappeared after the glycerol treatment was stopped). More research is needed.
Background
The 30‐day case fatality rate in prospective, community‐based studies of patients with first ever ischaemic stroke is 10% and with first ever haemorrhagic stroke about 52% (Bamford 1990). Patients admitted to hospital with acute stroke are also at high risk of dying, the observed one‐month case fatality rate in trials with placebo treatment varies from 21% to 50% (a' Rogvi‐Hansen 1992). Stroke‐related oedema is maximal two to four days after stroke onset and is a major cause of early neurological deterioration and death (Toni 1995). Glycerol is a hyperosmolar agent that is claimed to reduce infarct‐related oedema without rebound effect (Cantore 1964), to increase cerebral blood flow, and to improve cerebral metabolism by recoupling the uncoupled oxidative phosphorylation in the ischaemic brain tissue (Meyer 1972; Meyer 1975). Side effects of the treatment seem to be negligible (a' Rogvi‐Hansen 1992).
A striking difference in the use of anti‐oedema osmotic agents (OA) among the 36 countries which participated in the International Stroke Trial (IST 1997) has been described (Ricci 1998). Subgroup analyses of the main study for Italian patients showed that OA, mainly glycerol, had been given to a high proportion of patients: 48.3% (95% CI 46.6 to 50) of 3437 Italian patients (in 77 centres) compared to 29.6% (95% CI 26.4 to 32.9) of 759 patients in Poland, and less than 2% in the vast majority of the other countries where the remaining 15,239 patients were included. It was suggested that OA treatment did not change the prognosis, or even had a negative prognostic effect (Righetti 1994). The differences in the therapeutical approach reflect a substantial uncertainty in the clinical use of such an inexpensive and apparently safe drug in severe stroke patients.
In this updated review, data from haemorrhagic stroke patients were included since in the old studies, run in pre‐CT era, definite diagnosis of ischaemic stroke was not possible and moreover it enables the efficacy of glycerol on both pathological subtypes of stroke to be evaluated.
Objectives
The aim of this review is to determine the effectiveness and safety of early intravenous (I.V.) administration of glycerol in acute stroke. The review wishes to test the following hypotheses: (1) I.V. glycerol is effective in reducing case fatality in the acute phase of stroke; (2) I.V. glycerol is effective in reducing case fatality some months after stroke; (3) I.V. glycerol reduces the proportion of patients dead or dependent in activities of daily living some months after stroke; (4) I.V. glycerol treatment is safe.
Methods
Criteria for considering studies for this review
Types of studies
All completed, randomised and quasi‐randomised, controlled, unconfounded, published and unpublished comparisons evaluating the clinical outcome of I.V. glycerol treatment initiated within the first two days from stroke onset were included. In only one study was glycerol treatment initiated within the first four days.
Types of participants
The participants in the trials suffered from definite or presumed ischaemic strokes and/or definite haemorrhagic strokes. Cases of presumed ischaemic stroke reflect the fact that the majority of the studies were done before access to CT scanning was routine. However, efforts were made in each trial to differentiate intracranial haemorrhage by lumbar puncture or arteriography. Patients with subarachnoid haemorrhage were excluded from the analyses.
Types of interventions
Studies comparing glycerol treatment to a control group in the acute period of the stroke were included.
Types of outcome measures
The outcome measures were: (1) the number of deaths in each group, from whatever cause, during the scheduled treatment period; (2) the number of deaths in each group, from whatever cause, by the end of trial follow up (within six months of the stroke onset); (3) disability evaluated among survivors by modified Rankin Scale of 3 to 5 or by Barthel Index of < 19/20, or comparable scales of activity of daily living by the end of trial follow up (within six months of the stroke onset); (4) poor prognosis in each group, by the end of trial follow up considered as death or disability; (5) any registered adverse effect of the treatment according to the definitions given by the authors of each studies.
Wherever possible, results were determined by intention‐to‐treat analysis.
Search methods for identification of studies
See: 'Specialized register' section in Cochrane Stroke Group
Relevant trials were identified in the Cochrane Stroke Group trials register. The register was last searched by the Review Group Co‐ordinator for this review in January 2003. Further information was obtained from personal contact with some authors.
Data collection and analysis
Each trial was evaluated by two reviewers independently and a structured methodology and data collection form was completed for trials selected for inclusion. For trials reported in a language other than English, the form was sent by the Cochrane Stroke Group to a person familiar with the language for data extraction. Any disagreement between the two reviewers was cross‐checked and solved by discussion among all the reviewers either by formal meeting or by electronic mail.
The methodological quality of each trial has not been assessed by any scoring system, but the following details were noted: randomisation and type of allocation concealment, patient and assessor blinding, data evaluated with intention to treat analysis, number of patients who withdrew from treatment and/or were lost to follow up. The primary analysis included all trials independently of the method of randomisation, allocation concealment and intention to treat analysis.
A sensitivity analysis was performed to compare the overall results of all trials with those of the truly randomised trials. As glycerol might have a different level of efficacy in patients with ischaemic and haemorrhagic stroke, or in patients with different levels of consciousness, specified subgroups had been identified as definite haemorrhagic stroke and probable and definite ischaemic stroke.
Unconfounded trials comparing glycerol with control were subdivided by whether or not background anti‐oedema treatment (e.g. dexamethasone) had been given to both groups or not.
The statistical analysis was performed using a standard fixed‐effects model as described by Yusuf and Peto (Yusuf 1985).
Results
Description of studies
See 'Characteristics of Included Studies'. Eleven of the 17 trials identified fulfilled the entry criteria. Three trials compared glycerol in saline versus saline, two trials compared glycerol with saline/glucose versus saline/glucose, one trial compared glycerol in a non‐specified solution versus saline/glucose, one trial compared glycerol in glucose versus saline, one trial compared glycerol in 5% glucose versus 10% glucose, and one trial compared glycerol plus dexamethasone versus dexamethasone alone. One trial with glycerol in saline/glucose and one trial with glycerol in a non‐specified solution did not have a placebo group.
Risk of bias in included studies
(1) Randomisation ‐ all the trials included were randomised. (2) Type of allocation concealment ‐ in six trials allocation concealment was adequate (level A); in four trials there was uncertainty (level B); one trial was inadequate (level C). (3) Patient and assessor blinding ‐ in three trials placebo was not used, in one case the assessment was blind. (4) Intention to treat analysis ‐ eight trials were evaluated according to an intention to treat analysis.
Effects of interventions
Analysis of death within the scheduled treatment period for acute ischaemic and/or haemorrhagic stroke was possible in 10 trials where 482 glycerol‐treated patients were compared to 463 control patients. Glycerol was associated with a non‐significant reduction in odds of death (Odds Ratio (OR) 0.78), with wide confidence intervals (95% Confidence Intervals (CI) 0.58 to 1.06). There was no evidence of statistical heterogeneity between trials (chi square = 11.89; df = 9; p = 0.25).
Excluding the trial using dexamethasone in both groups (Albizzati 1979), given the possible negative interaction of the combination of glycerol with this drug, the results became marginally significant (OR 0.73, 95% CI 0.53 to 1.00). Sensitivity analysis including only truly randomised trials with adequate concealment did not substantially alter the result (OR 0.69, 95% CI 0.47 to 1.00). There is no evidence that the treatment effect of glycerol is different in patients with definite or probable ischaemic stroke and patients with haemorrhagic stroke (p = 0.4). Among patients randomised with definite or probably ischaemic stroke, glycerol was associated with a significant reduction in the odds of death during the scheduled treatment period (OR 0.65, 95% CI 0.44 to 0.97).
Analysis of the effects of glycerol on death at the end of scheduled follow up was possible in eight trials where 412 glycerol treated patients were compared to 389 control patients. There was no evidence of any effect of glycerol on this outcome (OR 0.98, 95% CI 0.73 to 1.31). Excluding the trial using dexamethasone in both groups (Albizzati 1979), the results did not change (OR 0.95, 95% CI 0.69 to 1.30) similarly, the results were unaltered after sensitivity analysis including only truly randomised trials with adequate concealment (OR 0.89, 95% CI 0.64 to 1.24) and in subgroup analyses of only definite or probable ischaemic stroke patients (OR 0.85, 95% CI 0.59 to 1.24). Death at the end of scheduled follow up was not analysed in haemorrhagic stroke patients as only one relatively small study was available.
Although many trials reported some results of functional outcome using disability scales, the functional outcome was comparable only in two studies (Azzimondi; Bayer 1987), using data analysable as dichotomous variables (modified Rankin Scale). Glycerol was associated with a non‐significant reduction in the odds of being dead or dependent at the end of scheduled follow up (OR 0.73, 95% CI 0.37 to 1.42) but these comparisons must be interpreted with caution due to the limited number of patients (127 versus 107).
Side effects of the active treatment were considered in six trials and not reported in five. One trial (Azzimondi) reported macro haematuria among 15 of 44 (34%) patients in the treated group (7/20 in low dose group and 8/24 in high dose group) and only one case of heart failure in the high dose group. No adverse events were reported in the control group. Fawer et al (Fawer 1978) reported a mean 5 mm Hg increase in systolic blood pressure during glycerol infusion. No changes were registered in the control group. Frei et al (Frei 1987) found biochemical, subclinical signs of haemolysis in 98% of the glycerol treated patients with a mean drop of 1.7 g% in haemoglobin during the trial for both glycerol and glycerol plus dextran treated patients. No adverse effects were reported in the control group. Friedli et al (Friedli 1979) did not find adverse effects. Yu (Yu 1992) observed gross haemoglobinuria leading to discontinuation of the glycerol among 9 of 107 treated patients (8%) and 2 of 109 of control patients (1.8%) (OR 4.9, 95% CI 0.98 to 47.5), whereas Yu (Yu 1993) found haemoglobinuria among 8 of 56 (14.3%) treated patients, but in none of the 57 controls .
Discussion
The number of identified trials of glycerol treatment for acute stroke and the total number of patients included were low. The method of randomisation and concealment were adequate in less than half of the trials.
Few patients had their stroke confirmed by CT‐scan, and therefore the diagnosis of haemorrhagic and of ischaemic stroke was not reliable in most of the studies. Furthermore, lacunar stroke patients (by definition a group at negligible risk of cerebral oedema) were overtly excluded only in three studies (Azzimondi; Fawer 1978; Yu 1993) but their clinical definition of lacunar stroke was not reported. In only one study (Azzimondi) was the clinical suspicion of cerebral oedema considered in the list of inclusion criteria.
In six of the trials, glucose was used as control, which may not be completely inert. In one study all patients were treated with steroids. To verify any possible interference of dexamethasone treatment we repeated the analysis without this study, but the results remained the same.
The results of the statistical review must be interpreted with circumspection. The analysis of the review indicated a decrease in case fatality rate in the acute phase of the disease. This effect was statistically significant when only patients with probable or definite ischaemic stroke were considered.
Furthermore, the only trial with a statistically significant favourable effect on death within the scheduled treatment period (Bayer 1987) did not undertake an intention‐to‐treat analysis (which might well have given a less promising result).
There was no apparent benefit from glycerol among definite haemorrhagic stroke patients, but the low number of cases does not allow for reliable conclusions.
Case fatality at the end of scheduled follow‐up was virtually the same in the two groups. There were not enough data to evaluate the effect of glycerol on long‐term disability. Thus, the implication that glycerol only delays death at the cost of increasing disability cannot be rejected. The only side effect reported in a small proportion of patients in the group treated with glycerol was haematuria, though this effect was not explicitly registered in all studies.
Authors' conclusions
Implications for practice.
This systematic review suggests that glycerol treatment has an effect on short‐term survival in a subgroup of patients with probable or definite ischaemic stroke. Because of the relatively small number of patients and the fact that most of the trials were performed in the pre‐CT era, the results must be interpreted cautiously. Furthermore, most patients included in the studies did not have impairment of consciousness. The lack of statistical significance of the long term effect does not support the wide use of glycerol treatment in acute stroke. Moreover, this review does not provide reliable evidence on the efficacy of the glycerol treatment in patients with established cerebral oedema.
Implications for research.
Glycerol treatment is inexpensive and appears to be safe. This review suggests that it may be associated with the reduction in the number of patients dying within the first few days of stroke onset but it was not possible to rule out a negative effect on long‐term disability. The treatment should therefore now be tested in a much larger scale randomised controlled trial comparing glycerol with non glycerol treatment, perhaps restricted to patients who have clinical evidence of cerebral oedema, in which the long term effects of treatment on disability, handicap and quality of life are reliably assessed.
What's new
| Date | Event | Description |
|---|---|---|
| 21 August 2008 | Amended | Converted to new review format. |
History
Protocol first published: Issue 4, 2000 Review first published: Issue 4, 2000
| Date | Event | Description |
|---|---|---|
| 13 June 2003 | New search has been performed | The former review was integrated with data from both haemorrhagic and ischaemic stroke patients, in order to evaluate the efficacy of glycerol on all strokes. Three further studies were added: Albizzati (1979), Azzimondi (personal communication) and Yu (1992). In one study (Mathew 1972) haemorrhagic patients were considered as well. For consistency, the same criteria were applied to all studies in the same way. Two reviewers blindly checked both the old and the new studies for eligibility and quality and repeated the data extraction. Any disagreement was discussed among all the co‐workers. The study by Gelmers (1975) was also not included in this second review but, unlike previously, was excluded owing to its inadequate method of randomisation. The study by Gagliardi (1991) was not included and is awaiting further assessment, because randomisation and inclusion and exclusion criteria were not clear. Some new analyses were added:
(1) subgroup analysis between ischaemic and haemorrhagic stroke;
(2) sensitivity analysis to truly randomised trials.
The study by Albizzati (1979) was included because it was unconfounded but analysis was further repeated without this study to evaluate any possible interaction between dexamethasone and glycerol. For this update (2003) a new trial has been identified (Kario 1995), but it has been excluded because it did not meet our inclusion criteria. |
Acknowledgements
We should like to thank the Cochrane Stroke Group for their collaboration and assistance. We also wish to acknowledge Dr G. Azzimondi, Dr M. Casmiro and Dr R. D'Alessandro for sending us their data and comments.
Data and analyses
Comparison 1. CI and/or PICH stroke: intravenous glycerol versus avoid glycerol.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death within the scheduled treatment period | 10 | 945 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.58, 1.06] |
| 2 Death at the end of scheduled follow‐up | 8 | 801 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.98 [0.73, 1.31] |
| 3 Dead or disabled at end of scheduled follow‐up | 2 | 234 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.73 [0.37, 1.42] |
| 4 Truly randomised studies, death within the scheduled treatment period. | 5 | 590 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.69 [0.47, 1.00] |
| 5 Truly randomised studies, death at the end of scheduled follow up | 5 | 614 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.89 [0.64, 1.24] |
1.1. Analysis.
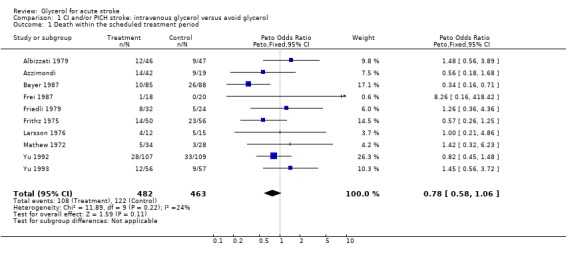
Comparison 1 CI and/or PICH stroke: intravenous glycerol versus avoid glycerol, Outcome 1 Death within the scheduled treatment period.
1.2. Analysis.
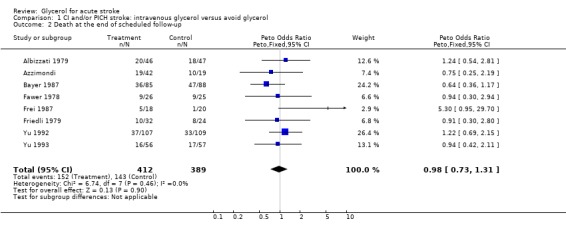
Comparison 1 CI and/or PICH stroke: intravenous glycerol versus avoid glycerol, Outcome 2 Death at the end of scheduled follow‐up.
1.3. Analysis.
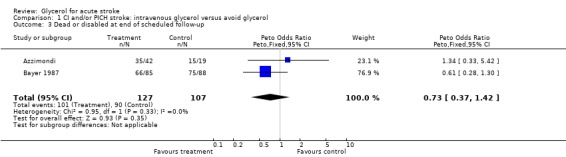
Comparison 1 CI and/or PICH stroke: intravenous glycerol versus avoid glycerol, Outcome 3 Dead or disabled at end of scheduled follow‐up.
1.4. Analysis.
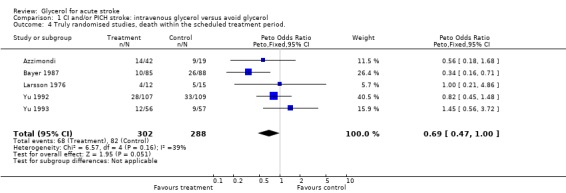
Comparison 1 CI and/or PICH stroke: intravenous glycerol versus avoid glycerol, Outcome 4 Truly randomised studies, death within the scheduled treatment period..
1.5. Analysis.
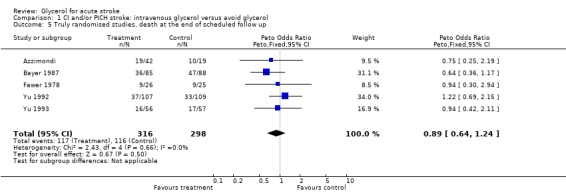
Comparison 1 CI and/or PICH stroke: intravenous glycerol versus avoid glycerol, Outcome 5 Truly randomised studies, death at the end of scheduled follow up.
Comparison 2. Intravenous glycerol versus avoid glycerol: results presented separately for CI and PICH.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death within the scheduled treatment period | 8 | 825 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.71 [0.51, 0.99] |
| 1.1 Ischaemic | 7 | 601 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.65 [0.44, 0.97] |
| 1.2 Haemorrhagic | 2 | 224 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.86 [0.48, 1.53] |
| 2 Death at the end of scheduled follow‐up | 6 | 492 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.59, 1.24] |
| 2.1 Ischaemic | 6 | 492 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.85 [0.59, 1.24] |
| 2.2 Haemorrhagic | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
2.1. Analysis.
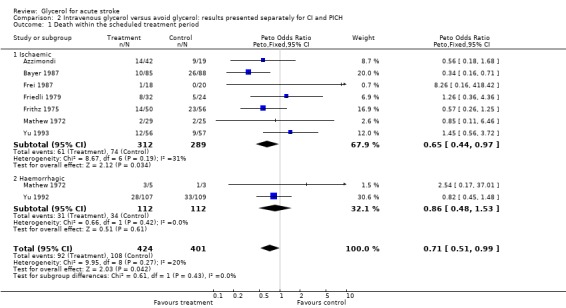
Comparison 2 Intravenous glycerol versus avoid glycerol: results presented separately for CI and PICH, Outcome 1 Death within the scheduled treatment period.
2.2. Analysis.
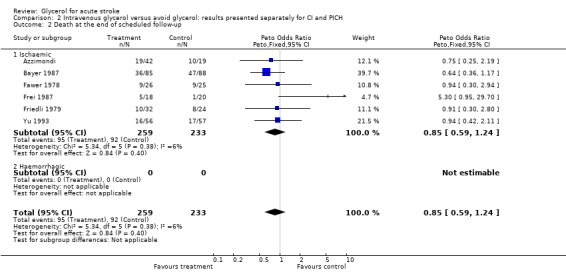
Comparison 2 Intravenous glycerol versus avoid glycerol: results presented separately for CI and PICH, Outcome 2 Death at the end of scheduled follow‐up.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Albizzati 1979.
| Methods | From 12/1975 to 6/1976. Randomised. Unknown allocation concealment. Not double blind. 30 excluded from the analysis. Non intention to treat. | |
| Participants | Any type of stroke: ischaemic, hemorrhagic, carotid and vertebrobasilar stroke. TIA and SAH excluded. 93 cases in the final analysis + 30 excluded from the analysis. F = 43, M = 50. Mean age 67.6 years. Any severity considered. Time entry window: 24 hours. | |
| Interventions | 50 g glycerol in 500 ml saline i.v. over 2 hours + 16 mg dexamethasone i.m. for 7 days. | |
| Outcomes | Mortality at 7, 30 days. Neurological evaluation, coma and disability status scale at 7 and 30 days. | |
| Notes | Steroids were administered in treatment and in control group. 24 patients out of 93 considered had no CT scan. 30 randomised were excluded from the analyses: 7 had a SAH; 14 in glycerol + dexamethasone group and 9 in dexamethasone group discontinued the treatment. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Azzimondi.
| Methods | From 07/1994 to 06/1997. Randomised. Adequate allocation concealment. Outcome assesment blind. 3 patients excluded from the analysis and 3 patients lost to follow‐up. Intention to treat. | |
| Participants | First ever ischaemic stroke in the carotid artery territory with impairment of conscioussness. Excluded: deep and depassè coma according to Bricolo's (1975) classification. Hemorrhagic, vertebrobasilar and lacunar stroke; impaired conscioussness due to causes other than stroke; renal hepatic and cardiac failure. 67 cases randomised, F = 38, M = 29. Mean age 75 years. Time entry window: 12 hours. | |
| Interventions | Glycerol 10% i.v. in 500 ml in over 4 hours vs glycerol 10% i.v. in 500 ml b.i.d. vs avoid glycerol. | |
| Outcomes | Death at 7, 30 days, 6 and 12 months. Disability (Barthel index) and disability/handicap (modified Rankin Scale) at 6 and 12 months. Side effects of treatment. | |
| Notes | Not published. No data available for 3 randomised patients. 1 patient in avoid glycerol, 2 patients in glycerol low dose lost at follow‐up. Low and high doses of glycerol considered together. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Bayer 1987.
| Methods | Randomised. Concealment: predetermined random order. Double‐blind. 19 patients exluded from the analysis. Non intention to treat. | |
| Participants | Probable ischaemic stroke in the carotid artery territory with hemiparesis. Excluded: probable brainstem stroke, TIA, residual deficit from previous strokes, other major neurological disorder and patients treatable with anticoagulants and other presumed to be effective. Renal, hepatic and cardiac failure. 192 cases randomised (19 excluded from the analysis) F = 107, M = 66. Range 67 to 96 years; mean age 78.4 years. Any severity considered but imminent death. Time entry window < 48hours. | |
| Interventions | Glycerol 10% i.v. in 500 ml saline vs 500 ml saline over 4 hours for 6 days. | |
| Outcomes | Death at 7 days and 3 months and 1 year. Disability/handicap scale (Rankin scale) at 3 months and 1 year. Length of hospital stay. | |
| Notes | 19 excluded from the analysis: 11 had exclusion criteria after randomisation, 2 out of time window and 6 treated with not considered drugs. Lumbar puncture ?%, Allen score in half of the trial, CT‐scan only when in doubt of diagnosis: 42 out 173. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Fawer 1978.
| Methods | Randomised. Concealment: open list of random numbers. Double‐blind. 13 excluded from the analysis. Non intention to treat. | |
| Participants | Probable ischaemic stroke in the middle cerebral artery territory. Excluded: signs of intracranial haemorrhage, TIA, lacunar stroke, cerebral tumour, heart failure. 51 patients considered in the analysis + 13 withdrawn Mean age: 71 years. ?Sex. Any severity considered. Time window entry < 48 hours. | |
| Interventions | Glycerol i.v. in 250 ml of saline and glucose 5% or 250 ml of NaCl 0.9% and glucose 5% over 2 hours q.i.d. for 6 days. | |
| Outcomes | Death at 4 months. Neurological and Global performances score system (original) at 7 days and 4 months. Side effects of treatment. | |
| Notes | 13 excluded from the analysis. 11 had exclusion criteria after randomisation, 1 treated with no considered drugs in the trial, 1 sudden deterioration. Lumbar puncture in all patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Frei 1987.
| Methods | From 5/1982 to 3/1984 Randomised. Unknown allocation concealment. Double blind 2 patients withdrawn. Intention‐to‐treat. | |
| Participants | Ischaemic stroke with hemiplegia. Excluded: > 80 years, TIA, previous deficits from stroke, cerebral haemorrhage, tumour, treatment with anticoagulants. Renal, hepatic and cardiac failure. 38 patients, F = 12, M = 26. Range 53 to 80 years; mean age 67 years. Patients in coma not considered. Time entry window: between 24 and 32 hours from onset. | |
| Interventions | Glycerol 10%i.v. in 500 ml followed by 500 ml placebo (glucose 2.5% plus NaCl 0.45%) vs 500 ml placebo + 500 ml placebo for 7 days. (Only patients treated with glycerol/placebo vs placebo/placebo included in the review). | |
| Outcomes | Death at 7 days and 6 months Neurological score (Modified Mathew scale) at 7 days and 3 and 6 months. Side effects of treatment. | |
| Notes | 3 treatment regimes were considered in the trial but only patients treated with glycerol/placebo vs placebo/placebo are included in the review. 23 patients in the glycerol 10% in 500 ml followed by 50 g dextran plus 25 g dextrose in 500 ml followed by placebo 500 ml group were excluded. CT scan in all patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Friedli 1979.
| Methods | From 1976 to 1978, Randomised. Unknown allocation concealment. Double‐blind. ?Intention to treat. | |
| Participants | Probable ischaemic stroke. Excluded: intracranial haemorrhages, TIA, uncontrolled diabetes mellitus and severe renal and hepatic failure. 56 patients, F = 22, M = 34. Median 71.7 years, range 40 to 91 years. Not specified level of severity. Time entry window: 24 hours. | |
| Interventions | Glycerol 10% i.v. in 500 ml vs 500 ml glucose 5% over 1 or 2 hours for 6 days. | |
| Outcomes | Death at 14 days and 6 months Neurological score (Modified Mathew scale) at 14 days and 6 months. Side effects of treatment. | |
| Notes | Lumbar puncture in all patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Frithz 1975.
| Methods | Ran for 14 months. Randomised. Concealment: even/uneven dates of birth. Not blind. Intention to treat. | |
| Participants | Probable ischaemic stroke. Excluded: cerebral haemorrhage, cerebral embolism, systematic diseases (SLE, leukaemia, cardiac and renal failure) and patients older than 80 years. 106 patients, F = 59, M = 47. Range 58 to 79 years; mean age 69.5 years. Any severity considered. Time entry window: 24 hours | |
| Interventions | Glycerol 10% i.v. in 500 ml glucose 5% in 25% saline over 6 hours for 6 days. | |
| Outcomes | Death at 6 days Neurological score (Modified Mathew scale) at 6 days. | |
| Notes | Lumbar puncture in all patients. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | High risk | C ‐ Inadequate |
Larsson 1976.
| Methods | Randomised. Concealment: coded containers controlled by hospital pharmacy. Double blind. Intention to treat. | |
| Participants | Any type of stroke. Excluded: probable MI, TIA, renal, hepatic and cardiac failure, diabetic coma, severe systemic infections, exogenous intoxications. 27 patients, ?Sex, ?Age. Any severity considered. Time entry window: 6 hours. | |
| Interventions | Glycerol 10% i.v. in 500 ml solution with dextrose 5% vs 500 ml solution with dextrose 10% over 6 hours for 6 days. | |
| Outcomes | Death at 10 days Neurological scoring system (original) at 10 days and 3 months but not available. | |
| Notes | Lumbar puncture in most patients, positive for blood in 2. 5 massive haemorrhages at necroscopy. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Mathew 1972.
| Methods | Randomised. Concealment: unknown. Double blind. Intention to treat. | |
| Participants | Ischaemic stroke or spontaneous hypertensive intracerebral haemorrhage Excluded: patients with acute MI, severe cardiac dysrythmia, cardiogenic shock, renal failure, severe systemic infections. 62 patients (54 ischaemic and 8 haemorrhagic strokes), F = 28, M = 34. Mean age 67.4 years. Any severity considered. Time entry window: 4 days. | |
| Interventions | 50 g glycerol i.v. in 500 ml of 5% glucose in 25% saline vs 500 ml of 5% glucose in 25% saline for 4 days (44 ischaemic patients + 8 haemorrhagic patients) or 6 days (10 patients). | |
| Outcomes | Death at 14 days Neurological system score (Mathew scale, original) at 14 days. | |
| Notes | Brain scan and lumbar puncture in all patients. Cerebral artheriography in doubt cases. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Unclear risk | B ‐ Unclear |
Yu 1992.
| Methods | Randomised. Concealment: according to stratified randomised block design. Double blind. 19 patients lost to follow up. Intention to treat. | |
| Participants | Spontaneous supratentorial heamorrhage. Excluded: previous history of stroke, posterior fossa haemorrhage or due to aneurysm or arteriovenous malformations or conditions warranting neurosurgery. Collagen vascular diseases, bleeding diathesis, anticoagulant therapy. 216 patients, F = 78, M = 138. Mean age 64 years. Any severity considered. Time entry window: within 45 hours from the onset. | |
| Interventions | Glycerol 10% i.v. in 500 ml saline vs 500 ml saline over 4hours for 6 days | |
| Outcomes | Death at 6 days and 6 months. Neurological score system (Scoring System of the Scandinavian Stroke Study group). Glasgow Coma Scale and Barthel Index (data not available for 17 glycerol and 13 control patients). Side effects of treatment. | |
| Notes | CT scan in all patients. 19 lost to follow up: 10 glycerol + 9 control group. 9 patients in glycerol and 2 in control group withdrawn treatment due to side effects, considered in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
Yu 1993.
| Methods | Randomised. Concealment: according to stratified randomised block design. Double blind. 11 lost to follow up. Intention to treat. | |
| Participants | Ischaemic stroke. Excluded: posterior fossa ischaemic lesions, lacunar infarct, collagen disease, cerebral haemorrhage. 113 patients; F = 51, M = 62. Mean age 67.9 years. Time entry window: 48 hours. | |
| Interventions | Glycerol 10% i.v. in 500 ml saline vs 500 ml saline over 4 hours for 6 days. | |
| Outcomes | Death at 6 days and 6 months. Neurological score system (Scoring System of the Scandinavian Stroke Study group). Glasgow Coma Scale and Barthel Index (data not available for all patients). Side effects of treatment. | |
| Notes | CT scan in all patients. 11 lost to follow up: 7 glycerol and 4 control group. 8 glycerol withdrawn treatment due to side effects, considered in the analysis. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment? | Low risk | A ‐ Adequate |
F: female M: male MI: myocardial infarction SAH: subarachnoid haemorrhage SLE: systemtic lupus erythematosus TIA: transient ischaemic attack
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Dekoninck 1978 | Comparison of vincamine + glycerol versus placebo + glycerol. |
| Gahlot 1982 | Glycerol compared to dexamethasone and not to placebo. |
| Gelmers 1975 | Non randomised: control patients were 'selected' to have comparable degrees and similar risk factors to treated patients. Assessors not blind. |
| Gilsanz 1975 | Glycerol treatment compared to dexamethasone and not to placebo. |
| Kario 1995 | Glycerol treatment was not randomised. |
| Kramer 1981 | Glycerol treatment compared to dextran treatment and not to placebo. |
Contributions of authors
Professor Boysen was the contact reviewer for the original review in this topic and commented on the updated version.
All reviewers were involved in planning the updated version and the expansion of the scope of the original review to include hemorrhage stroke as well as ischaemic stroke.
Dr Celani and Dr Cantisani independently checked the quality of all the studies and extracted the data. Dr Righetti, Dr Sterzi and Dr Ricci discussed with Dr Celani and Dr Cantisani any disagreements, and together contributed to the writing of the review.
Dr Righetti, the contact reviewer for this review, is the guarantor.
Declarations of interest
None known
Edited (no change to conclusions)
References
References to studies included in this review
Albizzati 1979 {published data only}
- Albizzati MG, Candelise L, Capitani E, Colombo A, Spinnler H. Association of glycerol to dexamethasone in treatment of stroke patients. Acta Neurologica Scandinavica 1979;60:77‐84. [DOI] [PubMed] [Google Scholar]
Azzimondi {unpublished data only}
- Azzimondi G, Casmiro M, D'Alessandro R, Fiorani L, Nonino F, Rinaldi R, Bassein L. Intravenous glycerol in acute ischemic stroke. A multicentre randomised placebo‐controlled trial. Unpublished.
Bayer 1987 {published data only}
- Bayer AJ, Pathy MS, Newcombe R. Double‐blind randomised trial of intravenous glycerol in acute stroke (plus letters). Lancet 1987;1:405‐8. [DOI] [PubMed] [Google Scholar]
Fawer 1978 {published data only}
- Fawer R, Justafre JC, Berger JP, Schelling JL. Intravenous glycerol in cerebral infarction: a controlled 4‐month trial. Stroke 1978;9:484‐6. [DOI] [PubMed] [Google Scholar]
Frei 1987 {published data only}
- Frei A, Cottier C, Wunderlich P, Ludin E. Glycerol and dextran combined in the therapy of acute stroke. A placebo‐controlled, double‐blind trial with a planned interim analysis. Stroke 1987;18:373‐9. [DOI] [PubMed] [Google Scholar]
Friedli 1979 {published data only}
- Friedli W, Imbach P, Ghisleni‐Steinegger S, Schwarz C, Maire P. Treatment with 10% glycerin in acute ischemic cerebral infarct. Double blind study. Schweizeriche Medizinische Wochenschrift 1979;109:737‐42. [PubMed] [Google Scholar]
Frithz 1975 {published data only}
- Frithz G, Werner I. The effect of glycerol infusion in acute cerebral infarction. Acta Medica Scandinavica 1975;198:287‐9. [DOI] [PubMed] [Google Scholar]
Larsson 1976 {published data only}
- Larsson O, Marinovich N, Barber K. Double‐blind trial of glycerol therapy in early stroke. Lancet 1976;1:832‐4. [DOI] [PubMed] [Google Scholar]
Mathew 1972 {published data only}
- Mathew NT, Rivera VM, Meyer JS, Charney JZ, Hartmann A. Double‐blind evaluation of glycerol therapy in acute cerebral infarction. Lancet 1972;2:1327‐9. [DOI] [PubMed] [Google Scholar]
Yu 1992 {published data only}
- Yu YL, Kumana CR, Lauder IJ, Cheung YK, Chan FL, Kou M, Chang CM, Cheung RTF, Fong KY. Treatment of acute cerebral haemorrhage with intravenous glycerol. A double‐blind, placebo‐controlled randomised trial. Stroke 1992;23:967‐71. [DOI] [PubMed] [Google Scholar]
Yu 1993 {published data only}
- Kumana CR, Chan GT, Yu YL, Lauder IJ, Chan TK, Kou M. Investigation of intravascular haemolysis during treatment of acute stroke with intravenous glycerol. British Journal of Clinical Pharmacology 1990;29:347‐53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu YL, Kumana CR, Lauder IJ, Cheung YK, Chan FL, Kou M, Fong KY, Cheung RTF, Chang CM. Treatment of acute cortical infarct with intravenous glycerol. A double‐blind randomised controlled trial. Stroke 1993;24:1119‐24. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Dekoninck 1978 {published data only}
- Dekoninck WJ, Jocquet P, Jacquy J, Henriet M. Comparative study of the clinical effects of vincamine + glycerol versus glycerol + placebo in the acute phase of stroke. Arzneimittelforschung 1978;28:1654‐7. [PubMed] [Google Scholar]
Gahlot 1982 {published data only}
- Gahlot SR, Goyal RK, Swaroop AK, Mathur RN. Intravenous glycerol vs dexamethasone therapy in the management of acute cerebral oedema in patients with acute cerebral infarction. Journal of the Association of Physicians of India 1982;30:575‐8. [PubMed] [Google Scholar]
Gelmers 1975 {published data only}
- Gelmers HJ. Effect of glycerol treatment on the natural history of acute cerebral infarction. Clinical Neurology and Neurosurgery 1975;78:277‐82. [DOI] [PubMed] [Google Scholar]
Gilsanz 1975 {published data only}
- Gilsanz V, Rebollar JL, Buencuerpo J, Chantres MT. Controlled trial of glycerol versus dexamethasone in the treatment of cerebral oedema in acute cerebral infarction. Lancet 1975;1:1049‐51. [DOI] [PubMed] [Google Scholar]
Kario 1995 {published data only}
- Kario K, Kodama K, Koide M, Matsuo T. Thrombin inhibition in the acute phase of ischaemic stroke using argatroban. Blood Coagulation and Fibrinolysis 1995;6:423‐7. [DOI] [PubMed] [Google Scholar]
Kramer 1981 {published data only}
- Kramer W, Rompel C, Umlauf B, Ulm K. Controlled, comparative blind study on the effect of glycerin infusions in recent ischemic crises. Die Medizinische Welt 1981;32:813‐6. [PubMed] [Google Scholar]
References to studies awaiting assessment
Gagliardi 1991 {published data only}
- Gagliardi JR, Lauretti da Silva Guedes M, Carvalho Neto RD. Evaluation of glycerol in the treatment of cerebral infarction [Avaliaçao do glicerol no tratamento de infarto cerebral]. Revista da Associação Médica Brasileira 1991;37:27‐35. [PubMed] [Google Scholar]
Additional references
Bamford 1990
- Bamford J, Dennis M, Sandercock P, Burn J, Warlow C. The frequency, causes and timing of death within 30 days of a first stroke: the Oxfordshire Community Stroke Project. Journal of Neurology Neurosurgery and Psychiatry 1990;53:824‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
Cantore 1964
- Cantore G, Guidetti B, Virno M. Oral glycerol for the reduction of intracranial pressure. Journal of Neurosurgery 1964;21:278‐83. [DOI] [PubMed] [Google Scholar]
IST 1997
- The International Stroke Trial (IST). A randomised trial of aspirin, subcutaneous heparin, both, or neither among 19435 patients with acute ischaemic stroke. International Stroke Trial Collaborative Group. Lancet 1997;349:1569‐81. [PubMed] [Google Scholar]
Meyer 1972
- Meyer JS, Fununchi Y, Shimazu K, Okuchi I, Ericsson HD. Effect of intravenous infusion of glycerol on hemisferic bloodflow and metabolism in patients with acute cerebral infarction. Stroke 1972;3:168‐80. [DOI] [PubMed] [Google Scholar]
Meyer 1975
- Meyer JS, Itoh Y, Okamako S, et al. Circulatory and metabolic effects of glycerol infusion in patients with acute cerebral infarction. Circulation 1975;51:701‐12. [DOI] [PubMed] [Google Scholar]
Ricci 1998
- Ricci S, Celani MG, Righetti E, Cantisani AT, Spizzichino L. Use of antioedema agents in patients with ischaemic stroke: a pharmacoepidemiological analysis. European Journal of Neurology 1998;5(Suppl 1):s8. [Google Scholar]
Righetti 1994
- Righetti E, Celani MG, Ricci S on behalf of IST Study Group. Pharmacological approach to ischaemic stroke in two European countries: the experience of IST [Approccio farmacologico all' ictus ischemico in due Paesi Europei: l' esperienza dello Studio IST]. Atti dell' VIII Convegno Nazionale di Neuroepidemiologia, Genova, 1‐3 Dicembre. 1994.
Toni 1995
- Toni D. Fiorelli M. Gentile M. Bastianello S. Sacchetti ML. Argentino C. Pozzilli C. Fieschi C. Progressing neurological deficit secondary to acute ischemic stroke: A study on predictability, pathogenesis, and prognosis. Archives of Neurology 1995;52(7):670‐5. [DOI] [PubMed] [Google Scholar]
Yusuf 1985
- Yusuf S, Peto R, Lewis J, Collins R, Sleight P. Beta blockade during and after myocardial infarction: An overview of the randomised trials. Progress in Cardiovascular Diseases 1985;27:335‐71. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
a' Rogvi‐Hansen 1992
- a' Rogvi‐Hansen B, Boysen G. Intravenous glycerol treatment of acute stroke. A statistical review. Cerebrovascular Diseases 1992;2:11‐3. [Google Scholar]


