Abstract
Background
Apnea of prematurity may lead to hypoxemia and bradycardia requiring resuscitative measures being instituted. Many treatments have been used in infants with apnea of prematurity including methylxanthines. Physical stimulation is often used to restart breathing and it is possible that repeated stimulation such as with an oscillating mattress or other kinesthetic stimulation, might also be used to treat infants with apnea and prevent its consequences.
Objectives
To determine if kinesthetic stimulation is more effective than a methylxanthine in preventing clinically important apnea in preterm infants with apnea.
Search methods
The standard search strategy of the Cochrane Neonatal Review Group was used. This included searches of the Cochrane Central Register of Controlled Trials (The Cochrane Library, Issue 4, 2009), the Oxford Database of Perinatal Trials, MEDLINE, PREMEDLINE, CINAHL and EMBASE in October 2009. Searches were performed of previous reviews including cross references, abstracts, conferences, symposia proceedings, expert informants, and journal handsearches mainly in the English language.
Selection criteria
All trials using random or quasi‐random patient allocation in which kinesthetic stimulation was compared to methylxanthine therapy for apnea of prematurity were eligible.
Data collection and analysis
Standard methods of the Cochrane Collaboration and its Neonatal Review Group were used with separate evaluation of trial quality, data extraction by both authors and synthesis of data using relative risk and weighted mean difference. Measures of severity of apnea as well as the response to treatment were consistent with an evaluation of 'clinical apnea' as defined by the American Academy of Pediatrics.
Main results
A single study of 20 infants (Saigal 1986) demonstrated a significant benefit to the infants receiving theophylline compared to those on an oscillating water bed (OWB) in terms of mean rates of clinically important apnea (apnea > 14 seconds associated with bradycardia < 100 or cyanosis or receiving stimulation). There were no significant differences in adverse effects (death, sleep states, the Albert Einstein Neurobehavioral Index, adverse neurological outcomes, and the Bayley Mental Development Index at six and 12 months) although the infants on the OWB had a higher psychomotor index at six but not 12 months.
Authors' conclusions
The results of this review should be treated with caution. Theophylline has been shown in one small study to be superior to kinesthetic stimulation at treating clinically important apnea of prematurity. There are currently no clear research questions regarding the comparison of methylxanthines and kinesthetic stimulation to treat apnea of prematurity.
Plain language summary
Kinesthetic stimulation versus theophylline for apnea in preterm infants
There is some evidence that theophylline may be more effective for apnea in preterm babies than kinesthetic stimulation, but more research is needed.
Apnea is a pause in breathing of greater than 20 seconds. It may occur repeatedly in preterm babies (born before 34 weeks). Immaturity alone can cause apnea, but so can infections. Apnea may be harmful to the developing brain or organs if it continues. Various methods have been tried to reduce apnea in premature babies including drugs, physical stimulation by nurses and kinesthetic stimulation (using an oscillating mattress which moves from side to side). The review of trials found some evidence that the drug theophylline may be more effective than kinesthetic stimulation for apnea but more research is needed.
Background
Description of the condition
Apnea in infants has been defined as a pause in breathing of greater than 20 seconds or an apneic event less than 20 seconds associated with bradycardia and/or cyanosis (Nelson 1978). Recurrent episodes of apnea are common in preterm infants and the incidence and severity increases at lower gestational ages. Although it can occur spontaneously and be attributed to prematurity alone, it can also be provoked or made more severe if there is some additional insult such as infection, hypoxemia or intracranial pathology (Henderson‐Smart 1995).
Description of the intervention
Various treatments for apnea in preterm infants have been used including physical stimulation by nursing staff, pharmacological stimulation including methylxanthines (HendersonSmart 2005a) and continuous positive airway pressure (CPAP) (HendersonSmart 2005b). Kinesthetic stimulation using various forms of oscillating mattress has been used in both prevention and treatment for apnea which are the subject of other Cochrane reviews (HendersonSmart 2005c; Osborn 2005). This review compares the effects of kinesthetic stimulation and methylxanthines for the treatment of preterm infants with apnea.
How the intervention might work
Physical stimulation by nursing staff is commonly used to arouse the apneic infant and so stimulate breathing. This raises the question of whether frequent physical stimuli might reduce the number of apneic events. Furthermore, some believe that the preterm infant is deprived of the frequent stimuli that would be felt in utero and that substituting these with an oscillating mattress to provide kinesthetic stimulation might improve growth and development.
Why it is important to do this review
If prolonged, apnea can lead to hypoxemia and reflex bradycardia which may require active resuscitative efforts to reverse. There are clinical concerns that these episodes might be harmful to the developing brain or cause dysfunction to the gut or developing organs, although there are no data to support this. Frequent episodes may be accompanied by respiratory failure of sufficient severity as to lead to intubation and the use of intermittent positive pressure ventilation (IPPV).
Objectives
To determine if kinesthetic stimulation is more effective than a methylxanthine in preventing clinically important apnea, the need for mechanical ventilation or continuous positive airways pressure support, and neurodevelopmental disability in preterm infants with apnea.
Methods
Criteria for considering studies for this review
Types of studies
All trials using random or quasi‐random patient allocation in which kinesthetic stimulation was compared to methylxanthine therapy for apnea of prematurity, were eligible.
Types of participants
Preterm infants with recurrent clinical apnea with or without associated bradycardia, cyanosis or hypoxia.
Types of interventions
Kinesthetic stimulation (various forms of oscillating mattresses or other repetitive stimulation involving moving the baby) compared with methylxanthine for the treatment of apnea.
Types of outcome measures
Primary outcomes
1) Persisting apneas with or without bradycardia (>4 and >10 episodes/day); 2) Greater than a 50% reduction in the daily rates of apnea with or without bradycardia;
3) Death before hospital discharge;
Secondary outcomes
4) Hypoxemic episodes associated with apnea; 5) Failure of treatment as indicated by use of additional measures such as use of mechanical ventilation (IPPV), CPAP, or doxapram; 6) Side effects (tachycardia, feed intolerance); 7) Rate of intraventricular haemorrhage; 8) Neurodevelopmental status at follow up.
Search methods for identification of studies
Electronic searches
The search was updated October 2009 including searches of CENTRAL (The Cochrane Library, Issue 4, 2009), EMBASE, MEDLINE, PREMEDLINE and CINAHL, supplemented by searches of abstracts of the Society for Pediatric Research hand searched for the years 2005 to 2009 inclusive and the Perinatal Society of Australia and New Zealand years 2000 to 2009.
An updated search was performed of MEDLINE and PREMEDLINE, EMBASE, CINAHL and CENTRAL (October 2009) using the search terms ['kinesthetic or (water bed) or (air bed) or oscillating or rocking or (vertical pulsating)] and [methylxamine or methylxantheine or theophylline or caffeine] and [infant or neonat*] and [apnoea or apnea].
Original search: The standard search strategy of the Cochrane Neonatal Review Group was used. This included searches of the Oxford Database of Perinatal Trials, Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library, Issue 4, 2004), MEDLINE (1966 to December 2004), EMBASE (1966 to December 2004) and CINAHL (1982 to December 2004) were searched. Abstracts of the Society for Pediatric Research were hand searched for the years 1996 to 2004 inclusive.
The Cochrane Controlled Trials Register was searched using search terms '(theophylline or methylxanthine) and (infant or preterm or neonate or newborn)', 'water bed', 'air bed', 'oscillating', '(apnea or apnoea) and (infant or preterm or neonate or newborn)', 'rocking', and 'vertical pulsating'.
MEDLINE was searched using Mesh headings 'apnea and infant‐premature', 'theophylline and infant‐premature', text words 'water bed', 'air bed', 'oscillating', '(apnea or apnoea) and (infant or premature or preterm or neonate or newborn)', '(theophylline) and (infant or preterm or neonate or newborn)' 'rocking', and 'vertical pulsating'.
The Oxford Database of Perinatal Trials was searched using search terms 'apnea' and 'methylxanthines'.
Searching other resources
Additional searches were performed of previous reviews including cross references, abstracts, conferences and symposia proceedings, expert informants, journal hand searching mainly in the English language.
Data collection and analysis
Standard methods of the Cochrane Collaboration and its Neonatal Review Group were used.
Selection of studies
Trial eligibility was assessed independently by both review authors. DIfferences were resolved through consensus.
Data extraction and management
Each review author extracted the data separately into a standard data table and then compared and resolved differences. Trial details were entered into table 'Characteristics of Included Studies' and data into 'data and analyses' using RevMan software.
Assessment of risk of bias in included studies
The methodological quality of each trial was reviewed by the second review author blinded to trial authors and institution(s). Each review author extracted the data separately, then compared and resolved differences. Studies were assessed for selection bias (blinding of randomisation), performance bias (blinding of intervention), attrition bias (complete follow‐up), and detection bias (blinding of outcome measurement). Each criterion were characterized as Yes, Can’t tell, No. This information was added to the table 'Characteristics of Included Studies'.
In addition, the following issues were evaluated and entered into the Risk of Bias Table:
1. Sequence generation: Was the allocation sequence adequately generated?
2. Allocation concealment: Was allocation adequately concealed?
3. Blinding of participants, personnel and outcome assessors: Was knowledge of the allocated intervention adequately prevented during the study? At study entry? At the time of outcome assessment?
4. Incomplete outcome data: Were incomplete outcome data adequately addressed?
5. Selective outcome reporting: Are reports of the study free of suggestion of selective outcome reporting?
6. Other sources of bias: Was the study apparently free of other problems that could put it at a high risk of bias?
Measures of treatment effect
The standard method of the Neonatal Review Group to synthesise data was followed. Statistical analyses were performed using Review Manager software. Categorical data were analyzed using relative risk (RR), risk difference (RD) and the number needed to treat (NNT). Continuous data were analyzed using weighted mean difference (WMD). The 95% Confidence interval (CI) was reported on all estimates.
Unit of analysis issues
The unit of randomisation was the intended unit of analysis.
Dealing with missing data
Authors of study with missing data were contacted.
Assessment of heterogeneity
If multiple studies were identified, we planned to examine heterogeneity between trials by inspecting the forest plots and quantifying the impact of heterogeneity using the I2 statistic. If we detected statistical heterogeneity, we planned to explore the possible causes (for example, differences in study quality, participants, intervention regimens or outcome assessments) using post hoc sub group analyses.
Data synthesis
If multiple studies were identified and meta‐analysis was judged to be appropriate, the analysis would have been performed using Review Manager software (RevMan 5, Cochrane Collaboration). For estimates of typical relative risk and risk difference, we planned to use the Mantel‐Haenszel method. For measured quantities, we planned to use the inverse variance method. All meta‐analyses were to be done using the fixed effect model.
Results
Description of studies
Results of the search
No new eligible studies were found in the searches to October 2009. Three ineligible studies were entered into the table 'Characteristics of Excluded Studies'.
Details of the included study (Saigal 1986) have been entered into the table 'Characteristics of Included Studies'.
Included studies
This study (Saigal 1986) randomized infants to either a regularly oscillating water bed (12 to 14 cycles/minute) or oral theophylline (or aminophylline) 6 mg/kg loading dose, 2 mg/kg 12 hourly maintenance dose (adjusted after 4 days to keep serum levels 6 to 12 micrograms/ml).
Preterm infants included in the study were of birth weight 750 to 1750 g, age one to 21 days, and > 5 apnea/bradycardia per 24 hrs. They were stratified by birth weight < 1000 g, 1000 to 1499 g and 1500 to 1750 g, and excluded if they had a major congenital abnormality, grade 3 or 4 IVH, IPPV > 48 hrs, or had secondary apnea. Of 111 admissions in the weight range, 43 were eligible, two refused, 21 conflicted with other research protocols, and 20 were randomized.
Apnea and bradycardia events were recorded by nursing staff (monitor alarms due to apnea > 14 seconds or bradycardia < 100 bpm) and cyanosis, need for stimulation and ventilation recorded. Polygraphic recording was also assessed for apnea > 14 seconds by a blinded observer. Death, use of IPPV, sleep states, Albert Einstein Neurobehavioral Scales at term equivalent age, auditory/cardiac habituation test at three months corrected, and Bayley Scales of Infant Development at six and 12 months corrected were assessed.
A power calculation was performed and 50 infants were estimated to be required but the study was performed as a feasibility study of 20 infants.
Excluded studies
The updated search October 2009 identified three studies that were entered into excluded studies table.
Risk of bias in included studies
Details of the methodological quality of the Saigal 1986 study are given in the table 'Characteristics of Included Studies'.
Allocation
Randomization was by use of random number table. Randomization was in three birth weight strata. Concealment of allocation was not specified.
Blinding
Due to the nature of the treatment, blinding of intervention was not possible.
The primary outcomes of nursing recorded apnea/bradycardia were not blindly assessed. Polygraphic assessment of apnea, assessment of sleep states, Albert Einstein Neurobehavioral Scales at term equivalent age, auditory/cardiac habituation test at three months corrected, and Bayley Scales of Infant Development at six and 12 months corrected were assessed blind to treatment allocation.
Incomplete outcome data
Follow‐up was complete for apnea, mortality and use of CPAP and IPPV. Neurodevelopmental follow‐up of surviving infants was complete except for two deaths in the oscillating water bed group and one death in theophylline group.
Selective reporting
None.
Other potential sources of bias
None apparent.
Effects of interventions
Saigal 1986 reported that infants in the theophylline group had slightly more respiratory distress syndrome at study entry than infants on the oscillating water bed (OWB). They also had different rates of baseline apnea (higher in the infants on the OWB). No significant differences were seen between the groups in demographic and other neonatal data.
Categorical data on apnea/bradycardic episodes could not be obtained as original data could not be located by the author. Data could not be extracted to determine: 1) persisting apneas with or without bradycardia (> 4 and > 10 episodes /day) and 2) greater than a 50% reduction in the daily rates of apnea with or without bradycardia. Daily rates of clinically important apnea (nursing observation) were available for episodes of apnea (> 14 seconds) with bradycardia (< 100 bpm) and cyanosis OR receiving stimulation, as well as for episodes of apnea (> 14 seconds) with bradycardia (< 100 bpm) and cyanosis AND receiving stimulation.
Saigal 1986 reported no significant difference in apnea defined as daily episodes of apnea associated with bradycardia and cyanosis and receiving stimulation (MD 3.36, 95%B CI ‐0.07, 6.79) (Outcome 1.1). However, infants on an OWB compared to those receiving theophylline had a significant increase in daily episodes of apnea defined as apnea and bradycardia and cyanosis or receiving stimulation (MD 4.88, 95% CI 0.33, 9.43) (Outcome 1.2). Blinded polygraphic recordings also supported the finding of a significant benefit of theophylline as compared to the OWB in mean frequencies of apnea and bradycardia. Saigal also reported blinded polygraphic recordings of apnea > 15 seconds and bradycardia < 80 bpm. Saigal found baseline differences in rates of apnea between the two groups. Using analysis of co‐variance to correct for these differences, Saigal reported a significantly lower incidence of both apnea > 15 seconds and bradycardia < 80 bpm in the theophylline group.
There was no significant difference in terms of sleep states, death, neurological abnormality, neurological abnormality or death, the Einstein Neurodevelopmental Scale at term corrected age, or the Bayley Mental Development Index at six or 12 months corrected age (Outcomes 1.3 ‐ 1.9). A difference in psychomotor performance was found with infants on the OWB performing better at six months, but not 12 months corrected age (Outcome 1.10).
Discussion
Summary of main results
The single trial in this review suggests that theophylline is significantly better than the oscillating water bed (OWB) at reducing clinically significant apnea without important adverse effects in terms of sleep states, neurological abnormality and abnormal neurodevelopment.
Although a previous review has suggested a lack of effect of prophylactic kinesthetic stimulation at preventing apnea of prematurity (HendersonSmart 2005c), this does not preclude a possible benefit of kinesthetic stimulation in the treatment of infants with established apnea of prematurity (Osborn 2005).
Overall completeness and applicability of evidence
The limitations of this review are the availability of a single study, the small sample size of the study, and the lack of dichotomous data for rates of clinically significant apnea.
Quality of the evidence
The single small study had methodological concerns including unclear allocation sequence, lack of blinding of treatment and nurse recorded events.
Potential biases in the review process
None apparent.
Authors' conclusions
Implications for practice.
The results of this review should be treated with caution. Theophylline has been shown in one small study to be superior to kinesthetic stimulation at treating clinically important apnea of prematurity.
Implications for research.
There are currently no clear research questions regarding the comparison of methylxanthines and kinesthetic stimulation to treat apnea of prematurity.
What's new
| Date | Event | Description |
|---|---|---|
| 15 October 2009 | New search has been performed | This updates the review "Kinesthetic stimulation versus theophylline for apnea in preterm infants" published in The Cochrane Library, Issue 2, 2002 (Osborn 2002). No new eligible studies found in an updated search October 2009. |
History
Protocol first published: Issue 4, 1997 Review first published: Issue 2, 1998
| Date | Event | Description |
|---|---|---|
| 16 May 2008 | Amended | Converted to new review format. |
| 1 December 2004 | New search has been performed | This review updates the existing review of "Kinesthetic stimulation versus theophylline for treating apnea in preterm infants" published in The Cochrane Library, Issue 2, 1998 and previously updated in Issye 2, 2002 (Henderson‐Smart 2002). No additional studies or data were obtained on the updated search to December 2004. |
| 18 February 1998 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Professor Saroj Saigal for her kind attempt at locating the raw data.
The Cochrane Neonatal Review Group has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
Data and analyses
Comparison 1. Oscillating water bed vs theophylline.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Daily rate apnea/ bradycardia/ cyanosis and stimulation | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 3.36 [‐0.07, 6.79] |
| 2 Daily rate apnea/ bradycardia/ cyanosis or stimulation | 1 | 20 | Mean Difference (IV, Fixed, 95% CI) | 4.88 [0.33, 9.43] |
| 3 Death | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 2.44 [0.26, 22.80] |
| 4 Use of IPPV or CPAP | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 8.4 [0.49, 144.04] |
| 5 Neurological abnormality in survivors. | 1 | 17 | Risk Ratio (M‐H, Fixed, 95% CI) | 4.13 [0.19, 88.71] |
| 6 Neurological abnormality in survivors or death | 1 | 20 | Risk Ratio (M‐H, Fixed, 95% CI) | 3.67 [0.46, 29.49] |
| 7 Bayley MDI at 6 months | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.00, 19.00] |
| 8 Bayley PDI at 6 months | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 15.0 [3.10, 26.90] |
| 9 Bayley MDI at 12 months | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 6.0 [‐5.90, 17.90] |
| 10 Bayley PDI at 12 months | 1 | 17 | Mean Difference (IV, Fixed, 95% CI) | 7.0 [‐5.17, 19.17] |
1.1. Analysis.
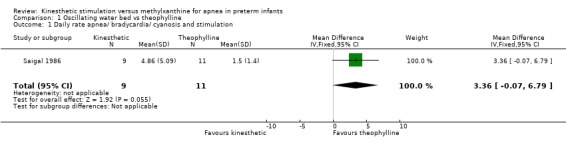
Comparison 1 Oscillating water bed vs theophylline, Outcome 1 Daily rate apnea/ bradycardia/ cyanosis and stimulation.
1.2. Analysis.
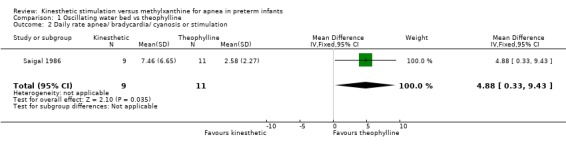
Comparison 1 Oscillating water bed vs theophylline, Outcome 2 Daily rate apnea/ bradycardia/ cyanosis or stimulation.
1.3. Analysis.
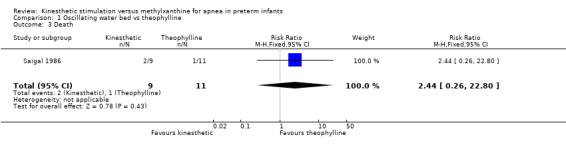
Comparison 1 Oscillating water bed vs theophylline, Outcome 3 Death.
1.4. Analysis.
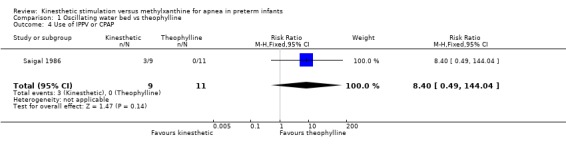
Comparison 1 Oscillating water bed vs theophylline, Outcome 4 Use of IPPV or CPAP.
1.5. Analysis.
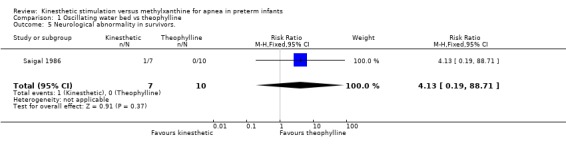
Comparison 1 Oscillating water bed vs theophylline, Outcome 5 Neurological abnormality in survivors..
1.6. Analysis.
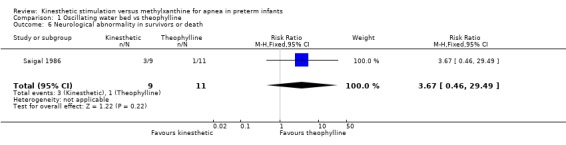
Comparison 1 Oscillating water bed vs theophylline, Outcome 6 Neurological abnormality in survivors or death.
1.7. Analysis.
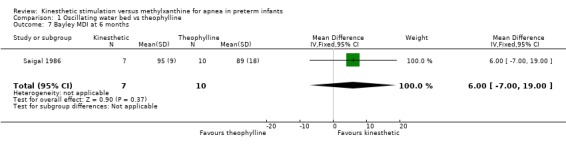
Comparison 1 Oscillating water bed vs theophylline, Outcome 7 Bayley MDI at 6 months.
1.8. Analysis.
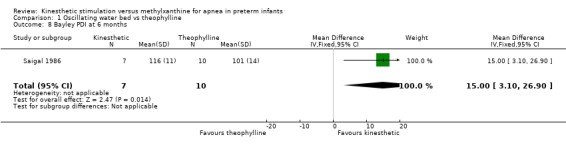
Comparison 1 Oscillating water bed vs theophylline, Outcome 8 Bayley PDI at 6 months.
1.9. Analysis.
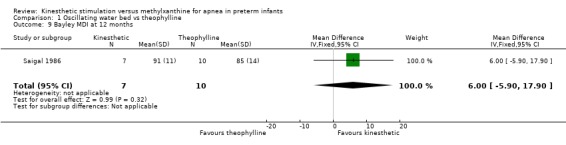
Comparison 1 Oscillating water bed vs theophylline, Outcome 9 Bayley MDI at 12 months.
1.10. Analysis.
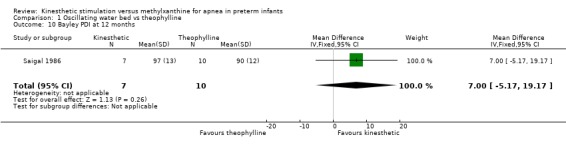
Comparison 1 Oscillating water bed vs theophylline, Outcome 10 Bayley PDI at 12 months.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Saigal 1986.
| Methods | Randomised controlled trial ‐ parrallel design. | |
| Participants | Preterm infants of birth weight 750 ‐ 1750 g, age 1 ‐ 21 days, > 5 apnea/bradycardia per 24 hrs. Stratified by birth weight < 1000 g, 1000 ‐ 1499 g and 1500 ‐ 1750 g. Excluded if; major congenital abnormality; grade 3 or 4 IVH; IPPV > 48 hrs; secondary apnea. Of 43 admissions in weight range, 43 eligible, 2 refused, 21 conflicted with other research protocols, 20 randomized. | |
| Interventions | Oscillating water bed 12 ‐ 14/min vs theophylline 6 mg/kg loading dose and 2 mg/kg 12 hrly (adjusted after 4 days to keep serum levels 6 ‐ 12 micrograms/ml) (or equivalent dose of aminophylline if not tolerating feeds). | |
| Outcomes | Clinical (nursing records) and polygraph recorded apnea/bradycardia; death; use of IPPV; sleep states; Albert Einstein Neurobehavioral Scales at term equivalent age; auditory/cardiac habituation test at 3 months corrected; Bayley Scales of Infant Development at 6 and 12 months corrected. | |
| Notes | Original data requested from author but not available. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Adequate sequence generation? | Low risk | Random number table. |
| Allocation concealment? | Unclear risk | Not specified. Unlikely to be concealed given that the treatments were pharmacological (theophylline) versus physical (kinesthetic). |
| Blinding? Short term outcomes | High risk | No for nursing recorded apnea/bradycardia. Yes for polygraphic recorded apnea/bradycardia. |
| Blinding? Long term outcomes | Low risk | Yes for long term follow up. |
| Blinding? Treatment | High risk | Due to the nature of the treatment, blinding of intervention was not possible. |
| Incomplete outcome data addressed? All outcomes | Low risk | All randomised infants reported. |
| Free of selective reporting? | Low risk | All randomised infants measured and reported similarly. |
| Free of other bias? | High risk | Power unsatisfactory as 50 infants estimated to be needed. Done as feasibility study of 20 infants. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Hayes 2007 | Cohort study of effect of methylxanthine treatment of apnea on infant arousals, wakefulness and movement. |
| Korner 1982 | Observational study of effect on apnea of an oscillating water bed in theophylline treated infants. |
| Svenningsen 1995 | Cohort study of theophylline and non‐theophylline treated infants treated with an oscillating air mattress for apnea. |
Differences between protocol and review
Updated to Revman 5 format. Methodology not changed.
Contributions of authors
DO and DHS performed all aspects of the review and review updates collaboratively. Eligibility, critical appraisal and data extraction were performed independently by both reviewer authors with differences resolved by consensus.
Sources of support
Internal sources
NSW Centre for Perinatal Health Services Research, University of Sydney, Australia.
Department of Neonatal Medicine, Royal Prince Alfred Hospital, Sydney, Australia.
External sources
No sources of support supplied
Declarations of interest
None.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Saigal 1986 {published data only}
- Saigal S, Campell D, Watts J, Ferguson S, Duffy A. Immediate and longterm outcomes of the use of an oscillating water bed or theophylline in preterm infants with apnea: a randomized clinical trial. Journal of Perinatology 1986;6:33‐8. [Google Scholar]
References to studies excluded from this review
Hayes 2007 {published data only}
- Hayes MJ, Akilesh MR, Fukumizu M, Gilles AA, Sallinen BA, Troese M, Paul JA. Apneic preterms and methylxanthines: arousal deficits, sleep fragmentation and suppressed spontaneous movements. Journal of Perinatology 2007;27:782‐9. [DOI] [PubMed] [Google Scholar]
Korner 1982 {published data only}
- Korner AF, Ruppel EM, Rho JM. Effects of water beds on the sleep and motility of theophylline‐treated preterm infants. Pediatrics 1982;70:864‐9. [PubMed] [Google Scholar]
Svenningsen 1995 {published data only}
- Svenningsen NW, Wittstrom C, Hellstrom‐Westas L. OSCILLO‐oscillating air mattress in neonatal care of very preterm babies. Technology and Health Care 1995;3:43‐6. [PubMed] [Google Scholar]
Additional references
Henderson‐Smart 1995
- Henderson‐Smart DJ. Recurrent apnoea. In: Yu VYH editor(s). Bailliere's Clinical Paediatrics. Vol. 3, No. 1 Pulmonary Problems in the Perinatal Period and their Sequelae, London: Bailliere Tindall, 1995:203‐22. [Google Scholar]
HendersonSmart 2005a
- Henderson‐Smart DJ, Steer P. Methylxanthine treatment for apnea in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD000140] [DOI] [Google Scholar]
HendersonSmart 2005b
- Henderson‐Smart DJ, Subramaniam P, Davis PG. Continuous positive airway pressure versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD001072] [DOI] [PubMed] [Google Scholar]
HendersonSmart 2005c
- Henderson‐Smart DJ, Osborn DA. Kinesthetic stimulation for preventing apnea in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD000373] [DOI] [PubMed] [Google Scholar]
Nelson 1978
- Nelson NM. Members of the Task Force on Prolonged Apnea of the American Academy of Pediatrics. Prolonged apnea. Pediatrics 1978;61:651‐2. [PubMed] [Google Scholar]
Osborn 2005
- Osborn DA, Henderson‐Smart DJ. Kinesthetic stimulation for treating apnea in preterm infants. Cochrane Database of Systematic Reviews 2005, Issue 2. [DOI: 10.1002/14651858.CD000499] [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
Osborn 1998
- Osborn DA, Henderson‐Smart DJ. Kinesthetic stimulation versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews 1998, Issue 2. [DOI: 10.1002/14651858.CD000502] [DOI] [PMC free article] [PubMed] [Google Scholar]
Osborn 2002
- Osborn DA, Henderson‐Smart DJ. Kinesthetic stimulation versus theophylline for apnea in preterm infants. Cochrane Database of Systematic Reviews 2002, Issue 2. [DOI: 10.1002/14651858.CD000502] [DOI] [PMC free article] [PubMed] [Google Scholar]


