Abstract
Background
Vitamin A is necessary for normal lung growth and the integrity of respiratory tract epithelial cells. Preterm infants have low vitamin A status at birth and this has been associated with an increased risk of developing chronic lung disease.
Objectives
To evaluate supplementation with vitamin A on the incidence of death or neonatal chronic lung disease and long‐term neurodevelopmental disability in very low birth weight (VLBW) infants compared with a control (placebo or no supplementation), and to consider the effect of the supplementation route, dose, and timing.
Search methods
For the original review and subsequent updates, we searched the Cochrane Central Register of Controlled Trials (CENTRAL, The Cochrane Library), MEDLINE, Science Citation Index, and the Oxford Database of Perinatal Trials. The reference lists of relevant trials, paediatric and nutrition journals, and conference abstracts and proceedings were handsearched up to 2010.
For the 2016 update, we used the standard search strategy of the Cochrane Neonatal Review group to search the Cochrane Central Register of Controlled Trials (CENTRAL 2016, Issue 4), MEDLINE via PubMed (1 May 2016), EMBASE (1 May 2016), and CINAHL (1 May 2016). We also searched clinical trials' databases, conference proceedings, and the reference lists of retrieved articles for randomised controlled trials and quasi‐randomised trials.
Selection criteria
Randomised controlled trials comparing vitamin A supplementation with a control (placebo or no supplementation) or other dosage regimens in VLBW infants (birth weight ≤ 1500 grams or less than 32 weeks' gestation).
Data collection and analysis
Two review authors screened the search results, extracted data, and assessed the trials for risk of bias. Results were reported as risk ratios (RR), risk differences (RD), and number needed to treat to benefit (NNTB), all with 95% confidence intervals (CI). Trialists were contacted for additional data.
Main results
Eleven trials met the inclusion criteria. Ten trials (1460 infants) compared vitamin A supplementation with a control and one (120 infants) compared different regimens of vitamin A supplementation. Compared to the control group, vitamin A appeared to have a small benefit in reducing the risk of death or oxygen requirement at one month of age (typical RR 0.93, 95% CI 0.88 to 0.99; typical RD −0.05, 95% CI −0.10 to −0.01; NNTB 20, 95% CI 10 to 100; 6 studies, 1165 infants) and the risk of chronic lung disease (oxygen requirement) at 36 weeks' postmenstrual age (typical RR 0.87, 95% CI 0.77 to 0.99; typical RD −0.07, 95% CI −0.13 to −0.01; NNTB 11, 95% CI 6 to 100; 5 studies, 986 infants) (moderate‐quality evidence). There was a marginal reduction of the combined outcome of death or chronic lung disease (typical RR 0.92, 95% CI 0.84 to 1.01; typical RD −0.05, 95% CI −0.11 to 0.01; 4 studies, 1089 infants). Neurodevelopmental assessment of 88% of the surviving infants in the largest trial showed no difference between the groups at 18 to 22 months of age, corrected for prematurity (low‐quality evidence). There is no evidence to support different vitamin A dosing regimens. No adverse effects of vitamin A supplementation were reported, but it was noted that intramuscular injections of vitamin A were painful.
Authors' conclusions
Whether clinicians decide to utilise repeat intramuscular doses of vitamin A to prevent chronic lung disease may depend upon the local incidence of this outcome and the value attached to achieving a modest reduction in the outcome balanced against the lack of other proven benefits and the acceptability of the treatment. Information on long‐term neurodevelopmental status suggests no evidence of either benefit or harm from the intervention.
Plain language summary
Vitamin A supplementation to prevent deaths and short‐ and long‐term illness in very low birth weight infants
Review question: Does supplementation with vitamin A prevent death, chronic lung injury and long‐term neurodevelopmental disability in very low birth weight infants compared with a control (placebo or no supplementation)?
Background: Vitamin A is a group of fat‐soluble compounds used by the body for regulation and promotion of growth and differentiation of many cells, including cells in the retina of the eye and the cells that line the air passages in the lungs. Preterm infants have low vitamin A levels at birth. This may contribute to an increased risk of developing chronic lung disease and hence a requirement for oxygen. It is possible that an additional vitamin A supplement may reduce complications of prematurity, including abnormal development of the retina (retinopathy), bleeding in the brain (intraventricular haemorrhage), and damage to the gut from inflammation (necrotising enterocolitis) as well as reducing respiratory infections. Too much vitamin A is potentially harmful as it can raise intracranial pressure and cause skin and mucous membrane changes (injury or lesions), and vomiting.
Study characteristics: Eleven trials were included in this review, ten comparing vitamin A with a control (placebo or no supplementation) and one comparing different vitamin A regimens. The search for eligible trials was updated in May 2016.
Results: Compared to the control group, supplementing very low birth weight infants with vitamin A appears to have a small benefit in reducing the risk of death or oxygen requirement at one month of age and the risk of chronic lung disease (oxygen requirement) at 36 weeks' postmenstrual age (moderate‐quality evidence). There was a marginal reduction of the combined outcome of death or chronic lung disease (moderate‐quality evidence). Although there is a statistical reduction in chronic lung disease, these findings are consistent with either a meaningful impact on chronic lung disease or a negligible impact. The one trial that investigated neurodevelopmental status at 18 to 22 months of age correcting for prematurity found no evidence of benefit or harm associated with vitamin A supplementation compared to control (low‐quality evidence). No adverse effects of vitamin A supplementation were reported, but it was noted that intramuscular injections of vitamin A were painful.
Conclusions: Whether clinicians decide to utilise repeat intramuscular doses of vitamin A to prevent chronic lung disease may depend upon the local incidence of this outcome and the value attached to achieving a modest reduction in the outcome balanced against the lack of other proven benefits and the acceptability of the treatment. Information on long‐term neurodevelopmental status suggests no evidence of either benefit or harm from the intervention.
Summary of findings
Summary of findings for the main comparison. Supplemental vitamin A compared to no supplementation in very low birth weight infants.
| Supplemental vitamin A compared to no supplementation in very low birth weight infants | ||||||
| Patient or population: very low birth weight infants Intervention: supplemental vitamin A Comparison: no supplementation | ||||||
| Outcomes | Illustrative comparative risks* (95% CI) | Relative effect (95% CI) | No of Participants (studies) | Quality of the evidence (GRADE) | Comments | |
| Assumed risk | Corresponding risk | |||||
| no supplementation | Supplemental vitamin A | |||||
| Neonatal death | 168 per 1000 | 144 per 1000 (111 to 186) | RR 0.86 (0.66 to 1.11) | 1165 (6 RCTs) | ⊕⊕⊕⊝ MODERATE 1 | Imprecision: 95% CI are wide and imprecise |
| Chronic lung disease (oxygen use at 28 days in survivors) | 725 per 1000 | 674 per 1000 (623 to 732) | RR 0.93 (0.86 to 1.01) | 1070 (7 RCTs) | ⊕⊕⊕⊕ MODERATE 2 | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Death or chronic lung disease (oxygen use at 28 days) | 798 per 1000 | 742 per 1000 (702 to 790) | RR 0.93 (0.88 to 0.99) | 1165 (6 RCTs) | ⊕⊕⊕⊕ MODERATE2 | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Death before 36 weeks' postmenstrual age | 168 per 1000 | 168 per 1000 (129 to 216) | RR 1.00 (0.77 to 1.29) | 1089 (4 RCTs) | ⊕⊕⊕⊝ MODERATE1 | Imprecision: 95% CI are wide and imprecise |
| Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors) | 546 per 1000 | 486 per 1000 (426 to 546) | RR 0.87 (0.77 to 0.99) | 986 (5 RCTs) | ⊕⊕⊕⊝ MODERATE2 | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Death or chronic lung disease (oxygen use at 36 weeks' postmenstrual age) | 622 per 1000 | 573 per 1000 (523 to 629) | RR 0.92 (0.84 to 1.01) | 1089 (4 RCTs) | ⊕⊕⊕⊝ MODERATE2 | Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect |
| Neurodevelopmental impairment at 18 to 22 months | 481 per 1000 | 428 per 1000 (356 to 520) | RR 0.89 (0.74 to 1.08) | 538 (1 RCT) | ⊕⊕⊝⊝ LOW 3 | Concerning imprecision: does not met the optimal information size criteria |
| *The basis for the assumed risk (e.g. the median control group risk across studies) is provided in footnotes. The corresponding risk (and its 95% confidence interval) is based on the assumed risk in the comparison group and the relative effect of the intervention (and its 95% CI). CI: Confidence interval; | ||||||
| GRADE Working Group grades of evidence High quality: Further research is very unlikely to change our confidence in the estimate of effect. Moderate quality: Further research is likely to have an important impact on our confidence in the estimate of effect and may change the estimate. Low quality: Further research is very likely to have an important impact on our confidence in the estimate of effect and is likely to change the estimate. Very low quality: We are very uncertain about the estimate. | ||||||
1Imprecision: 95% CI are wide and imprecise
2Imprecision: although the 95% CI is narrow, the result is consistent with minimal clnical effect
3Concerning imprecision; does not met the optimal information size criteria
Background
Description of the condition
Vitamin A is the generic name for a group of fat‐soluble compounds which have the biological activity of the primary alcohol retinol. Vitamin A is involved in the regulation and promotion of growth and differentiation of many cells and in maintaining the integrity of the epithelial cells of the respiratory tract. Vitamin A is also necessary for formation of the photosensitive visual pigment in the retina, reproductive functions, and immunocompetence. Carotenoids, dietary precursors of vitamin A, have antioxidant properties.
The foetus accumulates vitamin A in the third trimester. The transport mechanism of vitamin A across the placenta and its regulation are not fully established. Premature infants have reduced hepatic stores of retinyl ester. In the plasma, vitamin A is bound to a specific carrier protein, retinol‐binding protein (RBP), and the resulting complex is further complexed with transthyretin (formerly pre‐albumin) (Mactier 2005). Premature infants have lower concentrations of plasma RBP than term infants, and most preterm infants have low plasma vitamin A concentrations and low plasma retinol/RBP molar ratios, suggesting they are vitamin A deficient (Shenai 1993). Inadequate provision and delivery of vitamin A postnatally may exacerbate the problem.
Description of the intervention
Preterm infants who are unable to tolerate oral feeds are routinely fed parenterally with both an amino acid and dextrose mixture and a lipid emulsion. Multivitamin preparations containing retinol or an equivalent are commonly added to the amino acid and dextrose mixture and infused over 24 to 48 hours, but significant losses in delivered vitamin A have been shown to result from light degradation and from adsorption to the tubing. Alternatively, the multivitamins may be added to the lipid infusate (Greene 1987). Kennedy 1997 demonstrated improved serum retinol concentrations following intramuscular injections given three days per week. This route of administration has been adopted in several recent studies. In preterm infants who are able to tolerate enteral feeds, the absorption of enteral vitamin A by the immature gut may be poor.
An 'adequate' concentration of plasma vitamin A in very low birth weight (VLBW) infants is not known. Concentrations below 200 μg/L (0.70 μmol/L) have been considered deficient in premature infants, and concentrations below 100 μg/L (0.35 μmol/L) indicate severe deficiency and depleted liver stores. Both the plasma RBP response (Shenai 1990) and the relative rise in serum retinal concentration (Zachman 1996) following intramuscular vitamin A administration have been described as useful tests to assess functional vitamin A status. However, in a recent review Mactier 2005 concluded that the relationship between measures of vitamin A concentration and functional vitamin A status is not clear in preterm infants.
Vitamin A deficiency in laboratory animals produces a sequence of histopathological changes in the respiratory tract epithelium including necrotising tracheobronchiolitis and squamous metaplasia. These changes can be reversed by restoration of an adequate vitamin A status. Similar changes are observed in ventilated infants with chronic neonatal lung injury, leading to the suggestion that vitamin A deficiency may contribute to such injury and supplementation with vitamin A may facilitate healing and recovery (Chytil 1992; Shenai 1993). Two earlier studies reported that VLBW infants who developed chronic lung disease had lower concentrations of vitamin A than similar infants without chronic lung disease (Hustead 1984; Shenai 1985), although other studies from an era when all infants received more adequate supplementation have given conflicting results (Chabra 1994; Spears 2004).
How the intervention might work
In the 1920s, vitamin A was considered to be an anti‐infective agent. There is increasing evidence that vitamin A does have a role in immune function (Bates 1995). Several studies in areas of the world where there is generally poor nutritional status have suggested vitamin A supplementation in infancy may be associated with decreased mortality and morbidity. In infants in Indonesia, Humphrey 1996 reported that a single dose of 52 μmol (50,000 IU) given orally to term infants at birth reduced infant mortality and the prevalence of severe respiratory infections compared with placebo. A Cochrane review concluded that two oral doses of 200,000 IU in children under two years of age with measles was associated with a reduced risk of overall mortality and of pneumonia‐specific mortality (Huiming 2005).
Vitamin A has a role early in gestation in the development of the cardiovascular system (Mactier 2005). Animal models suggest higher vitamin A concentrations may accelerate postnatal constriction of the ductus arteriosus. The possibility that vitamin A supplementation may ameliorate other complications of prematurity, including retinopathy, intraventricular haemorrhage, and necrotising enterocolitis, has been suggested by a number of authors, although the basis for any effect is not clearly established.
Vitamin A is potentially toxic and raised intracranial pressure and vomiting have been described in infants receiving large doses. In children and adults, chronic hypervitaminosis A may include bone and joint pain, mucocutaneous lesions, and hepatic dysfunction, but the syndrome has not been recognised in preterm infants.
Why it is important to do this review
Although a role for vitamin A in neonatal chronic lung disease is not in doubt, uncertainty exists regarding the efficacy of supplementation and whether additional benefit may be obtained by achieving concentrations beyond sufficiency.
This is an update of previous versions of this Cochrane Review (Darlow 1998; Darlow 2000; Darlow 2002; Darlow 2007; Darlow 2011).
Objectives
To evaluate supplementation with vitamin A on the incidence of death or neonatal chronic lung disease and long‐term neurodevelopmental disability in very low birth weight (VLBW) infants compared with a control (placebo or no supplementation), and to consider the effect of the supplementation route, dose, and timing.
Methods
Criteria for considering studies for this review
Types of studies
Randomised and quasi‐randomised controlled trials.
Types of participants
Very low birth weight (VLBW) infants (defined as birth weight ≤ 1500 grams or less than 32 weeks' gestation).
Types of interventions
Vitamin A supplementation compared with control (placebo or no supplementation)
Different vitamin A supplementation dosage regimens
Types of outcome measures
Primary outcomes
Death (at 28 days and at hospital discharge)
Chronic lung disease (defined as oxygen use at 28 days or at 36 weeks' postmenstrual age)
Death or chronic lung disease (defined as oxygen use at 28 days or at 36 weeks' postmenstrual age)
Secondary outcomes
Vitamin A concentration (for studies comparing vitamin A dosage)
Nosocomial infection
Patent ductus arteriosus*
Necrotising enterocolitis*
Intraventricular haemorrhage (any intraventricular haemorrhage or severe intraventricular haemorrhage grades 3 and 4) (Papile 1978)*
Periventricular leukomalacia*
Retinopathy of prematurity (any, or requiring laser therapy)**
Neurodevelopment at 18 to 24 months
Death at 18 to 24 months**
Death or neurodevelopmental impairment at 18 to 24 months**
Adverse effects, including manifestations of hypervitaminosis A, particularly raised intracranial pressure and mucocutaneous lesions
*Additional secondary outcomes added in the 2010 update
** Additional secondary outcomes added in the 2016 update
Search methods for identification of studies
We used the criteria and standard methods of Cochrane and Cochrane Neonatal (see the Cochrane Neonatal Group search strategy for specialized register).
Electronic searches
We conducted a comprehensive search including: the Cochrane Central Register of Controlled Trials (CENTRAL) in The Cochrane Library, Issue 4, 2016; MEDLINE via PubMed (1966 to 1 May 2016); EMBASE (1980 to 1 May 2016); CINAHL (1982 to 1 May 2016). We used the following search terms: (vitamin A or retinol), plus database‐specific limiters for RCTs and neonates (see Appendix 1 for the full search strategies for each database).
We applied no language restrictions. We searched the reference lists of any articles selected for inclusion in this review in order to identify additional relevant articles.
Searching other resources
We handsearched issues of paediatric and nutritional journals and abstracts from the Pediatric Academic Societies' Annual Meeting from 2002 to 2010. We also searched for conference abstracts from Pediatric Academic Societies (PAS) and the European Society for Paediatric Research (ESPR). Searches were carried out in Abstracts to View (2000 to 2014) and Pediatric Reseach.
We searched clinical trials registries for ongoing or recently completed trials (clinicaltrials.gov; the World Health Organization’s International Trials Registry and Platform www.whoint/ictrp/search/en/; and the ISRCTN Registry) without date limits up to May 2016.
Data collection and analysis
Selection of studies
We included all trials fulfilling the review's selection criteria. We cited all identified articles and made the decision regarding inclusion or exclusion of the studies by consensus. In the event of disagreements, we sought the opinion of a third party (Figure 1).
1.
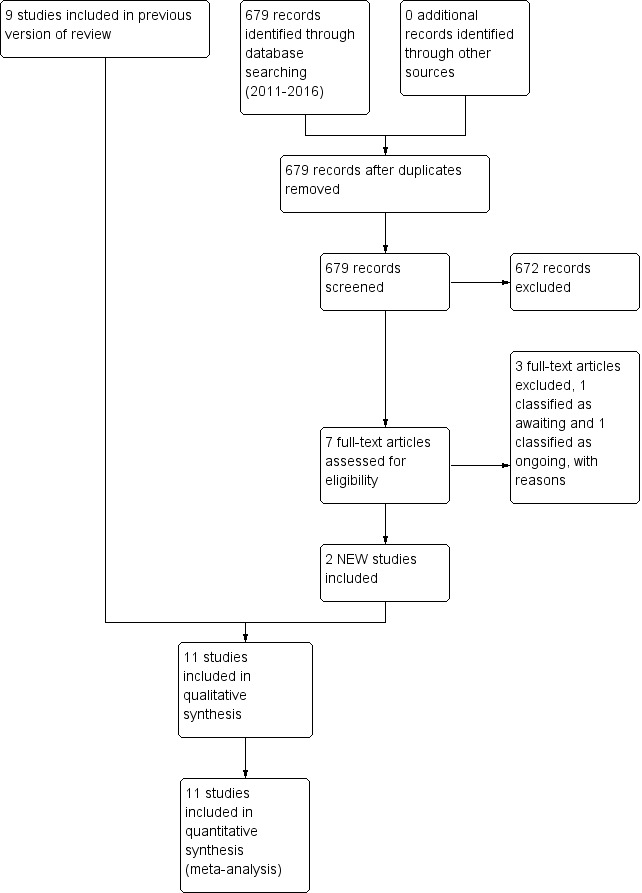
Study flow diagram: review update
Data extraction and management
For each included study, we collected information regarding the method of randomisation, blinding, drug intervention, stratification, trial location, and whether the trial was conducted at a single centre or multiple centres. We also collected information regarding inclusion criteria, including gestational age and postnatal age at the time of treatment. The list of data extraction fields for the trial interventions and outcomes are shown in Appendix 2.
We completed data collection sheets before comparing them and resolving any discrepancies by referring to the original sources. For each study, one review author entered the final and agreed data into Review Manager 5 and a second review author checked the data. We referred to a third party to resolve any disagreements.
Assessment of risk of bias in included studies
For the 2010 and 2016 updates, one author assessed the risk of bias in the included studies according to the methods in the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011). This built on the previous version's assessment of the methodological quality of the included studies. Any uncertainties were resolved through discussion with a second author.
We assessed the following factors and judged them as having a high, low, or unclear risk of bias using the criteria in Higgins 2011:
Sequence generation (checking for possible selection bias)
Allocation concealment (checking for possible selection bias)
Blinding (checking for possible performance bias and detection bias)
Incomplete outcome data (checking for possible attrition bias through withdrawals, dropouts, protocol deviations)
Selective outcome reporting (whether all of the study’s pre‐specified outcomes were reported (by checking the trial protocol if available) or whether the study failed to include results of a key outcome that would have been expected to have been reported)
Other biases (including source of funding and sample size calculation)
For each item, and where possible, we have provided a supporting quotation from the trial article.
Measures of treatment effect
We conducted separate analyses for each of the following outcomes: neonatal death; chronic lung disease; retinopathy of prematurity; sepsis (one or more defined episodes); and neurodevelopmental disability. Since there were only a small numbers of deaths, we conducted analyses using the composite endpoints of neonatal chronic lung disease or death, and neurodevelopmental disability or death. All analyses were conducted on an intention‐to‐treat basis. We stratified the analyses by route of vitamin A administration.
For dichotomous outcomes, we analysed the effect of vitamin A supplementation via both the risk ratio (RR) and the risk difference (RD) with 95% confidence intervals (CI). From 1/RD we calculated the number need to treat to benefit (NNTB) or the number needed to treat for an additional harmful outcome (NNTH). For vitamin A concentrations the intention was to focus the analysis on the mean difference between the supplemented and control groups. We combined continuous data using the standardised mean difference (SMD) and 95% CI.
Assessment of heterogeneity
We assessed between‐study heterogeneity using the standard Chi² test and the I² statistic.
Data synthesis
We performed the meta‐analysis using Review Manager 5. We used the Mantel‐Haenszel method for estimates of the pooled RR and RD. For measured quantities we used the inverse variance method. We used the fixed‐effect model for all meta‐analyses.
Pooled estimates of the effects and corresponding 95% confidence interval of the outcomes of neonatal death, chronic lung disease (oxygen use at 28 days in survivors), death or chronic lung disease (oxygen use at 28 days), death before 36 weeks' postmenstrual age, chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors) and neurodevelopmental disability at 18 to 22 months are included in the 'Summary of findings' tables in which the summary of the assessment of the evidence found for each outcome is presented.
Quality of evidence
For the 2016 update, we used the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach, as outlined in the GRADE Handbook (Schünemann 2013), to assess the quality of evidence for the following (clinically relevant) outcomes: neonatal death; chronic lung disease at 28 days and 36 weeks' postmenstrual age; death or chronic lung disease at 28 days and 36 weeks' postmenstrual age; and neurodevelopmental impairment at 18 to 22 months.
Two authors independently assessed the quality of the evidence for each of the outcomes above. We considered evidence from randomised controlled trials as high quality but downgraded the evidence one level for serious (or two levels for very serious) limitations based upon the following: design (risk of bias); consistency across studies; directness of the evidence; precision of estimates; and presence of publication bias. We used the GRADEpro‐GDT 2013 Guideline Development Tool to create a ‘Summary of findings’ table to report the quality of the evidence.
The GRADE approach results in an assessment of the quality of a body of evidence in one of four grades:
High: We are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: We are moderately confident in the effect estimate: the true effect is likely to be close to the estimate of the effect, but there is a possibility that it is substantially different.
Low: Our confidence in the effect estimate is limited: the true effect may be substantially different from the estimate of the effect.
Very low: We have very little confidence in the effect estimate: the true effect is likely to be substantially different from the estimate of effect.
Subgroup analysis and investigation of heterogeneity
If data were available, we planned subgroup analyses to address the effect of the route, dose, and timing of supplementation.
Sensitivity analysis
Where appropriate, we planned sensitivity analyses to explore the impact of the risk of bias assessments.
Results
Description of studies
Results of the search
We identified 21 potentially relevant trials of which 11 met the eligibility criteria (Shenai 1987; Papagaroufalis 1988; Pearson 1992; Bental 1994; Werkman 1994; Tyson 1999; Wardle 2001; Ambalavanan 2003; Ravishankar 2003; Mactier 2012; Kiatchoosakun 2014). Ten trials compared vitamin A with a control, while one trial compared three different vitamin A dosing regimens and had a primary outcome of serum retinol concentrations at 28 days (Ambalavanan 2003); see trial details in the 'Characteristics of included studies' table.
We excluded ten trials from the review (Aranda 1992; Landman 1992; Koo 1993; Robbins 1993; Rush 1994; Coutsoudis 1996; Coutsoudis 2000; Aurvag 2007; Longardt 2014; Schmiedchen 2016) (see 'Characteristics of excluded studies'). Robbins 1993 was not a randomised controlled trial. Coutsoudis 1996 and Coutsoudis 2000 enrolled low birth weight infants of less than 36 weeks' gestation and the primary outcome was the incidence of respiratory infections in the first year of life. Aurvag 2007 compared vitamin A supplementation using human milk fortifier with historical controls and did not report clinical outcomes. Four studies were studies of pharmacodynamics that reported vitamin A concentrations but not clinical outcomes (Aranda 1992; Landman 1992; Koo 1993; Rush 1994). The unpublished study by Aranda 1992 did not state the route of vitamin A supplementation and the reported vitamin A levels appeared to contain a decimal point error. Landman 1992 and Rush 1994 compared equivalent doses of vitamin A administered either enterally or intramuscularly. The data in Rush 1994 were only reported graphically. Koo 1993 compared three doses of vitamin A added to preterm formula, which were given for an unstated period of time.
Longardt 2014 was not a randomised controlled trial and reported on serum retinol, retinyl palmitate, total retinol‐binding protein 4 (RBP4), retinol‐unbound RBP4 (apo‐RBP4) and transthyretin concentrations. Clinically relevant outcomes were not reported.
Schmiedchen 2016 studied the impact of urinary retinol excretion on the serum retinol concentration of 63 VLBW infants treated with vitamin A (5000 IU intramuscular, 3 times/week for 4 weeks) compared to 38 untreated infants classified as control group. The groups were not randomised. See trial details in the Characteristics of excluded studies table.
Calisici 2014 is published only as an abstract and is awaiting further assessment. See trial details in the Characteristics of studies awaiting classification table.
Trial location and size
Most trials were conducted in the USA (Shenai 1987; Pearson 1992; Werkman 1994; Tyson 1999; Ambalavanan 2003; Ravishankar 2003). Three trials were conducted in Europe: in Greece (Papagaroufalis 1988); and the UK (Wardle 2001; Mactier 2012). One trial was conducted in South Africa (Bental 1994); and one trial was conducted in Thailand (Kiatchoosakun 2014).
Most trials included fewer than 100 infants (Shenai 1987; Papagaroufalis 1988; Pearson 1992; Bental 1994; Werkman 1994; Ravishankar 2003; Mactier 2012; Kiatchoosakun 2014). Two included between 100 and 200 participants (Wardle 2001; Ambalavanan 2003); and one trial included 807 infants (Tyson 1999).
Participants
Vitamin A versus control
The ten trials comparing vitamin A with a control reported outcomes for 1460 infants, 735 treated with vitamin A and 725 control infants.
Shenai 1987: Single centre randomised controlled trial. Enrolled 40 infants, birth weight 700 to 1300 grams and gestational age 26 to 30 weeks, requiring supplemental oxygen and mechanical ventilation for at least 72 hours in the first week; Caucasian infants only; birth weight appropriate for gestational age; no congenital abnormalities.
Papagaroufalis 1988: Single centre randomised controlled trial. Enrolled 55 infants with birth weight ≤ 1300 grams; gestation < 29 weeks; requirement for > 40% oxygen and mechanical ventilation for > 72 hours in first week.
Pearson 1992: Multicentre randomised controlled trial. Enrolled 49 infants with birth weight 700 to 1100 grams requiring supplemental oxygen and mechanical ventilation between 72 and 96 hours of age with previous cumulative duration of ventilation and oxygen > 48 hours. Infants with growth restriction (undefined), congenital or chromosomal abnormalities, hydrops fetalis, congenital infection, neonatal hepatitis or 'do not resuscitate' order were excluded.
Bental 1994: Single centre randomised controlled trial. Enrolled 60 infants with birth weight between 1000 and 1500 grams; gestational age of ≤ 34 weeks; infants were receiving supplemental oxygen and mechanical ventilation for at least 72 hours during the first week; no congenital anomalies or infection; black South African infants.
Werkman 1994: Single centre randomised controlled trial. Enrolled 86 infants with birth weight 725 to 1300 grams, age < 96 hours; no intraventricular or periventricular haemorrhage, history of maternal substance abuse, or sexually transmitted disease; no congenital anomalies.
Tyson 1999: Multicentre randomised controlled trial. Enrolled 807 infants with birth weight 401 to 1000 grams requiring supplemental oxygen or mechanical ventilation at 24 hours of age; and no major congenital anomalies, non‐bacterial infection, or terminal illness.
Wardle 2001: Multicentre randomised controlled trial. Enrolled 154 infants with birth weight < 1000 grams; no life‐threatening congenital abnormalities; consent before 24 hours of age.
Ravishankar 2003: Single centre randomised controlled trial. Enrolled 40 infants with birth weight 500 to 1500 grams; gestation < 32 weeks; having indwelling umbilical line; no major congenital malformations or chromosomal anomalies; and < 24 hours of age.
Mactier 2012: Double‐blind, randomised controlled trial conducted at three neonatal centres in Glasgow. Enrolled 89 infants (42 supplemented and 47 controls) < 32 weeks' gestation or < 1501 grams birth weight, or both.
Supplemented infants received additional intramuscular vitamin A 10,000 IU 3 times weekly from day 2 for a minimum of 2 weeks or until establishment of oral feeding.
Kiatchoosakun 2014: Randomised controlled trial to assess the effect of vitamin A supplementation for prevention of bronchopulmonary dysplasia in VLBW premature infants. Eighty premature infants weighing < 1500 grams who received mechanical ventilation or oxygen supplementation at 24 hours of age and admitted to Neonatal units of Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand.
Vitamin A dosing regimens
Ambalavanan 2003: Single centre randomised controlled trial evaluating vitamin A dosage. Enrolled 120 infants, birth weight between 401 and 1000 grams; requiring mechanical ventilation or supplemental oxygen at 24 hours of age; and no major congenital anomalies, non‐bacterial infection, or terminal illness. Ambalavanan 2003 used the same entry criteria as Tyson 1999.
Interventions
Vitamin A versus control
Ten trials compared vitamin A with a control. All but Werkman 1994 and Wardle 2001 gave supplemental vitamin A (water‐soluble retinyl palmitate) by intramuscular injection soon after birth, usually day four, and over the next 28 days. The trials varied in the vitamin A dose and regimen.
Shenai 1987: Vitamin A (water‐soluble retinyl palmitate) versus control
Supplemental 2000 IU vitamin A by intramuscular injection alternate days from day 4 for a total of 14 injections (n = 20) compared to normal saline placebo (n = 20)
Other: both control and study infants received vitamin A 400 IU/dL in parenteral nutrition and 240 to 550 IU/dL from milk plus 1500 IU supplements when fed orally
Papagaroufalis 1988: Vitamin A (water‐soluble retinyl palmitate) versus control
Supplemental 4000 IU vitamin A intramuscular injection from day 4 to 6 on alternate days until extubated (n = 27) compared to normal saline placebo (n = 28).
Pearson 1992: Vitamin A (water‐soluble retinyl palmitate) versus control
Supplemental 2000 IU vitamin A by intramuscular injection alternate days from day 4 for 14 doses (n = 27) compared to normal saline placebo or mock injection (n = 22)
Other: both control and study infants received 1200 to 1500 IU/d vitamin A in protein‐dextrose solution when on parenteral nutrition; when fed orally all infants received vitamin A 250 to 1030 IU/100 mL milk; antenatal steroids received by 26% infants in the vitamin A group and 41% in the control group; > 90% received an artificial surfactant
Bental 1994: Vitamin A (water‐soluble retinyl palmitate) versus control
Supplemental 4000 IU vitamin A intramuscular injection 3 times a week from day 4 for a total of 12 injections (n = 31); no supplementation (n = 29)
Other: some control and study infants received vitamin A 1500 to 3000 IU after 1 week of age when fed orally
Werkman 1994: Vitamin A (retinyl palmitate) versus control
Supplemental vitamin A 80,000 RE/L (giving about 1300 to 3300 IU/kg/d) in intravenous lipid infused over 16 h from randomisation (48 to 96 hours) and while receiving parenteral nutrition (n = 44) compared to no supplementation (n = 42)
Other: both control and study infants received vitamin A as multivitamin preparation added to protein‐dextrose solution (birth weight < 1000 grams 1.5 mL/d, 210 RE/d; > 1000 grams 3.4 mL/d, 476 RE/d) and oral supplements when fed orally
Tyson 1999: Vitamin A (water‐soluble retinyl palmitate) versus control
Supplemental 5000 IU vitamin A intramuscular injection 3 times a week for 4 weeks (n = 405) compared to sham injection (n = 402)
Other: control and study infants received similar intakes of vitamin A from non‐study enteral and parenteral sources but amounts not stated; antenatal steroids received by 76% of infants in the vitamin A group and 74% in the control group; > 80% received a natural surfactant
Wardle 2001: Vitamin A (form not stated) versus control
Supplemental 5000 IU vitamin A orally daily until day 28 (n = 77) compared to same volume look‐alike placebo liquid (n = 77)
Other: control and study infants received 23 IU/kg/d vitamin A (stated amount but probably 233 IU) in intralipid when on parenteral nutrition; when on full enteral feeds and more than 14 days of age all infants received 5000 IU/kg/d vitamin A orally; antenatal steroids received by 77% in the vitamin A group and 82% in the control group; all but 1 infant in the control group received an artificial surfactant
Ravishankar 2003: Vitamin A (water‐miscible preparation of vitamin A (Aquasol A)) versus control
Supplemental 1500 to 3000 IU vitamin A (based on weight) by intramuscular injection on days 1, 3 and 7 (n = 22) compared to sham injection (n = 18)
Other: most infants received parenteral nutrition including 466 IU/dL vitamin A and an additional 1000 IU supplement when fed orally; antenatal steroids received by 86% of infants in the vitamin A group and 72% in the control group
Mactier 2012: Supplemented infants received additional intramuscular vitamin A 10,000 IU 3 times weekly from day 2 for a minimum of 2 weeks or until establishment of oral feeding.
Kiatchoosakun 2014: Infants were assigned to receive either intramuscular vitamin A 5000 IU 3 times/week (treatment group) or sham procedure (control group) for four weeks.
Study and control infants were also administered standard vitamin A. However, the amount of vitamin A in standard therapy, and hence received by the control groups, varied between studies. When on parenteral nutrition infants in Shenai 1987 received vitamin A 400 IU/100 mL of protein‐dextrose infusion and usually less than 700 IU/kg/d from all sources. Infants in Pearson 1992 received 1200 to 1500 IU/d of vitamin A in the protein‐dextrose solution. In Bental 1994, infants on parenteral nutrition received no vitamin A but some received 1500 to 3000 IU/d after one week when fed orally. In Papagaroufalis 1988, the amount of standard vitamin A was not stated. In Werkman 1994, standard vitamin A was added to the protein‐dextrose solution, with infants less than 1000 grams receiving 700 IU/d and infants more than 1000 grams receiving 1580 IU/d. In Tyson 1999, infants received approximately 700 IU/kg/d in the first week, principally in protein‐dextrose solution, and closer to 1000 IU/kg/d in weeks two to four from all sources (data estimated from a graph). Infants in Wardle 2001 were stated to receive 23 IU/kg/d added to intralipid when on parenteral nutrition (however, the standard UK dose at that time was 233 IU/kg/d and it was probable that infants in this study received this dose) and 5000 IU/kg/day orally when on full enteral feeds from the 14th postnatal day. In Ravishankar 2003, most infants on parenteral nutrition received 466 IU vitamin A added to 100 mL of the protein‐dextrose solution, and 1500 IU/d orally when fully enterally fed. In Mactier 2012, infants on parenteral nutrition received routine fat soluble vitamins as part of their lipid infusion (Vitlipid N). Lipid infusion was stopped once the infants had achieved 75% of their expected enteral intake and all infants received oral supplementation of vitamin A on day 14 of life if tolerating enteral feeds.
Vitamin A dosing regimens
Ambalavanan 2003: Infants were randomised to receive a standard vitamin A supplement by 5000 IU intramuscular injection of vitamin A on three days per week for four weeks (considered as the control group) (n=40), a higher dose of 10,000 IU on three days per week for four weeks (n = 40), or a once‐per‐week dose of 15,000 IU for four weeks (n = 40).
Outcomes
We contacted several trial authors for further details about the trial outcomes and data. Bental 1994 gave complete data, published in an earlier abstract from 1990, and we sought and obtained further clarification from the trial authors concerning two outcomes: oxygen use at one month in survivors; and death or oxygen use at one month. We were able to update the data of Papagaroufalis 1988 with information provided by the trial author to Dr K Kennedy at the time of an earlier review (Kennedy 1997). We obtained information on the age of infants at death from the corresponding author of Ravishankar 2003. Further information on the numbers examined for retinopathy of prematurity and numbers with any retinopathy in Wardle 2001 were provided by the trial author. We also received further information on the timing of death, numbers of infants having serum retinol estimations at 28 days, and any retinopathy of prematurity from the author of Ambalavanan 2003.
Vitamin A versus control
Shenai 1987: Death before 31 days, bronchopulmonary dysplasia (oxygen requirement or ventilation at 31 days plus characteristic chest x‐ray), total days' oxygen, episodes of sepsis, retinopathy of prematurity, and vitamin A levels.
Papagaroufalis 1988: Death before 31 days, bronchopulmonary dysplasia (oxygen requirement and characteristic chest x‐ray on day 31) and vitamin A levels. Unpublished data of Papagaroufalis 1988 were available for 28, 29, 30 and 31 days.
Pearson 1992: Death before 31 days, bronchopulmonary dysplasia (oxygen requirement at 31 days and characteristic chest x‐ray), oxygen requirement at 34 weeks' post‐conceptual age, retinopathy of prematurity, plasma vitamin A levels and retinyl binding protein at various times.
Bental 1994: Death before 31 days, bronchopulmonary dysplasia (oxygen or ventilation on day 31 and characteristic chest x‐ray; full data obtained from trial authors), culture‐proven sepsis, vitamin A levels at various times.
Werkman 1994: Bronchopulmonary dysplasia (oxygen beyond 28 days and characteristic chest x‐ray, total days' oxygen requirement, vitamin A and retinol‐binding protein levels at various times.
Tyson 1999: Oxygen requirement at, or death before, 36 weeks' postmenstrual age; oxygen requirement at, or death before, 28 days of age; culture‐proven sepsis; grade 3 or 4 intracranial haemorrhage; periventricular leukomalacia; vitamin A on study day 28 in subset of infants and relative dose‐response to 2000 IU vitamin A intramuscular. Examination for potential toxicity weekly. Assessment of neurodevelopmental status at 18 to 22 months' corrected age is available in Ambalavanan 2005 (companion paper for Tyson 1999). Additional subgroup analysis of 'small for gestational age' infants.
Wardle 2001: Oxygen requirement at 28 days, death pre‐discharge, oxygen requirement at 36 weeks' postmenstrual age, retinopathy of prematurity requiring treatment. Wardle 2001 also reported on a patent ductus arteriosus requiring treatment with indomethacin or surgical closure, but the timing of this was uncertain as to whether some infants had died before this outcome; hence we did not include these data.
Ravishankar 2003: Failure of patent ductus arteriosus closure, defined as patent ductus arteriosus larger than trivial on day 14, indomethacin treatment, or surgical ligation. Ravishankar 2003 also reported deaths (and further information on the timing of these was obtained from the trial authors) and oxygen requirement at 36 weeks' postmenstrual age.
Mactier 2012: Hepatic stores were assessed by relative dose response (RDR). The primary outcome measure was cone‐corrected dark‐adapted retinal rod sensitivity measured by electroretinogram at 36 weeks' postmenstrual age (PMA). Complications of prematurity were reported including use of respiratory support, intraventricular haemorrhage, retinopathy of prematurity, supplemental oxygen at 36 weeks' postmenstrual age, and death
Kiatchoosakun 2014: The investigators reported on serum vitamin A levels, and complications of prematurity including supplemental oxygen at 36 weeks postmenstrual age, duration of intubation, days on oxygen therapy, and length of hospital stay.
Vitamin A dosing regimens
The primary outcome measure for the trial by Ambalavanan 2003 was serum retinol concentrations in μg/L at 28 days. Ambalavanan 2003 also reported on death before 36 weeks' postmenstrual age, oxygen requirement at or death before 36 weeks' postmenstrual age, and threshold retinopathy of prematurity. We obtained further information on timing of death, numbers of infants having serum retinol estimations, and any retinopathy of prematurity from the trial authors.
Risk of bias in included studies
Full details of the 'Risk of bias' assessment are available by trial in the 'Assessment of risk of bias in included studies' tables and are summarised in Figure 2.
2.
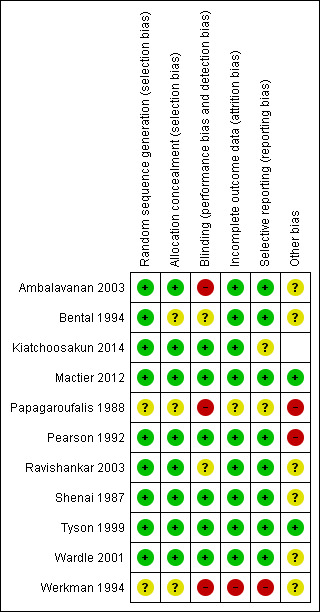
'Risk of bias' summary: review authors' judgements about each risk of bias item for each included study.
Allocation
Of the 11 included trials, eight used adequate methods to generate the allocation sequence and seven used adequate methods to conceal allocation (low risk of bias). Papagaroufalis 1988 and Werkman 1994 did not provide details to allow this risk of bias component to be assessed, and Bental 1994 provided details only for the sequence generation method (computer‐generated random numbers).
Ambalavanan 2003 used shuffled blocks of sealed opaque envelopes for randomisation and stratified randomisation by birth weight. The randomisation and allocation for Pearson 1992, Ravishankar 2003, Shenai 1987, Mactier 2012, Kiatchoosakun 2014 and Wardle 2001 were conducted by someone outside of the trial team and used sealed envelopes.
Blinding
Six trials blinded participants to the intervention and outcome assessors (Shenai 1987; Pearson 1992; Tyson 1999; Wardle 2001; Mactier 2012; Kiatchoosakun 2014). Pearson 1992, for example, used opaque tape to conceal the contents of the syringes and reported that the “staff responsible for patient care had no knowledge of group designation”.
Incomplete outcome data
All but Werkman 1994 and Papagaroufalis 1988 had complete follow‐up of participants. Werkman 1994 did not report outcomes on all infants entered into the study as they excluded 10 who transferred to another centre for surgery and two who died, for whom group assignment was not stated. For Papagaroufalis 1988, no information was available in the study abstract.
Selective reporting
Most trials were free of selective reporting in that it was clear that all of the trial’s pre‐specified outcome measures and all expected outcome measures of interest to the review were reported. This was unclear for Papagaroufalis 1988 because only a study abstract (from a conference proceedings) was available, while Werkman 1994 did not report all outcome measures of interest.
Other potential sources of bias
This item was evaluated in relation to information about trial funding (such as industry funding) and sample size calculation. Tyson 1999 appeared to be free of these sources of bias because the study protocol was described in detail, including the sample size calculation, and the trial was free of industry funding. Mactier 2012 was funded by the Chief Scientific Office, Scotland, and appeared to be free of other potential sources of bias. Papagaroufalis 1988 and Pearson 1992 were assessed as having a high risk of bias for this component: Papagaroufalis 1988 because little information was available in the abstract; and Pearson 1992 because few of the eligible infants were enrolled in the trial. Information about this item for the 'Risk of bias' assessment was unclear in the other studies.
Effects of interventions
See: Table 1
Comparison 1: Supplemental vitamin A versus no supplementation
Neonatal death (Outcome 1.1)
Six trials reported on neonatal death (Shenai 1987; Papagaroufalis 1988; Pearson 1992; Bental 1994; Tyson 1999; Wardle 2001); none showed a significant difference between the vitamin A and control groups. The meta‐analysis does not support a reduction of the risk of neonatal death associated with receiving vitamin A (typical RR 0.86, 95% CI 0.66 to 1.11; typical RD −0.02, 95% CI −0.06 to 0.02; 1165 infants) (Analysis 1.1; Figure 3).The quality of evidence for this outcome is moderate because of imprecision of estimates of effect (Table 1).
1.1. Analysis.
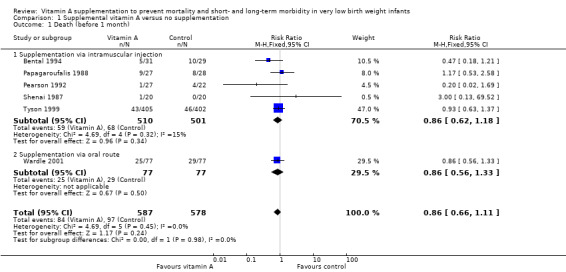
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 1 Death (before 1 month).
3.
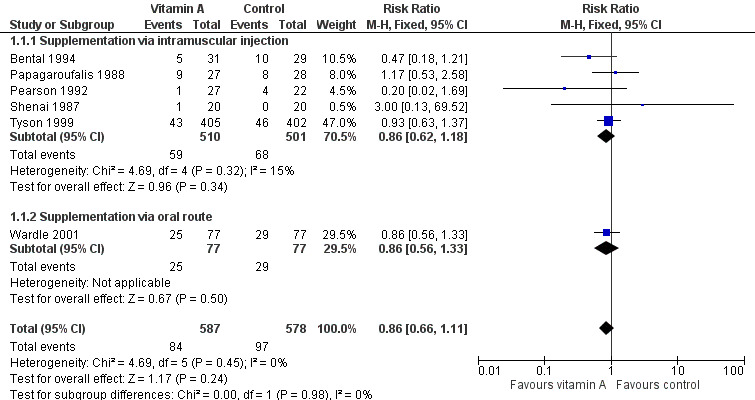
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.1 Death (before 1 month).
Chronic lung disease (oxygen use at 28 days in survivors) (Outcome 1.2)
Seven trials (the six trials above plus Werkman 1994) reported on chronic lung disease at 28 days. Only one study (Shenai 1987) reported a statistically significant difference between intervention groups. The largest trial showed an important trend to no differences between treatment arms (RR 0.97, 95% CI 0.89 to 1.06; 718 infants) (Tyson 1999). The meta‐analysis of these seven studies showed a trend towards a reduced risk of chronic lung disease between treatment arms (typical RR 0.93, 95% CI 0.86 to 1.01; typical RD −0.05, 95% CI −0.10 to 0.00; 1070 infants) (Analysis 1.2; Figure 4). The overall quality of the evidence for this outcome was judged as moderate; we downgraded the quality of evidence based upon imprecision because although the 95% CI for the typical RR is narrow the result is consistent with minimal clnical effect.
1.2. Analysis.
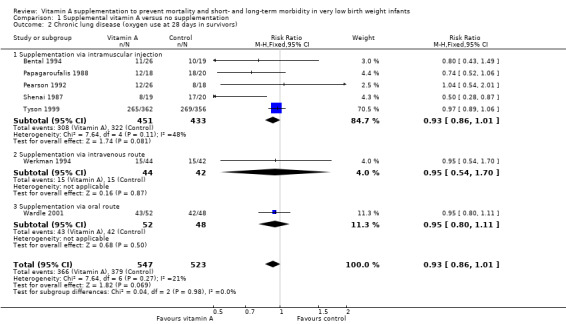
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 2 Chronic lung disease (oxygen use at 28 days in survivors).
4.
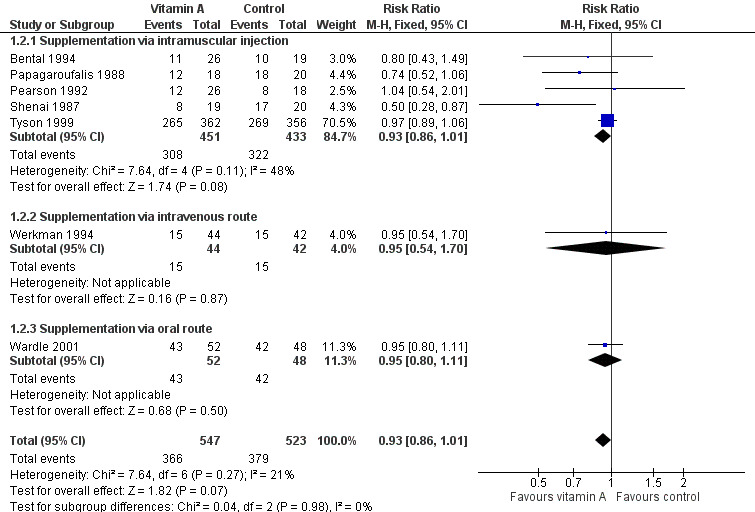
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.2 Chronic lung disease (oxygen use at 28 days in survivors).
Death or chronic lung disease (oxygen use at 28 days) (Outcome 1.3)
Data for the combined outcome of death or chronic lung disease at 28 days were available in six trials (Shenai 1987; Papagaroufalis 1988; Pearson 1992; Bental 1994; Tyson 1999; Wardle 2001). Only one study reported a statistically significant difference in this outcome (Shenai 1987). When the meta‐analysis was confined to the five trials reporting on supplementation via intramuscular injection (excluding Wardle 2001) there was a trend towards a reduction in death or oxygen use at one month that was of borderline statistical significance (typical RR 0.93, 95% CI 0.86 to 1.00; typical RD −0.06, 95% CI −0.11 to −0.00; NNTB 17, 95% CI 9 to 1000+; 1011 infants) (Analysis 1.3). When Wardle 2001 (in which oral vitamin A supplementation was given to treated infants) was included in the meta‐analysis the pooled data showed a significant reduction in this outcome (typical RR 0.93, 95% CI 0.88 to 0.99; typical RD −0.05, 95% CI −0.10 to −0.01; NNTB 20, 95% CI 10 to 100; 1165 infants, I² = 37%) (Analysis 1.3; Figure 5). The overall quality of the evidence for this outcome was judged as moderate; we downgraded the quality of evidence based upon imprecision because although the 95% CI for the typical RR is narrow the result is consistent with minimal clnical effect.
1.3. Analysis.
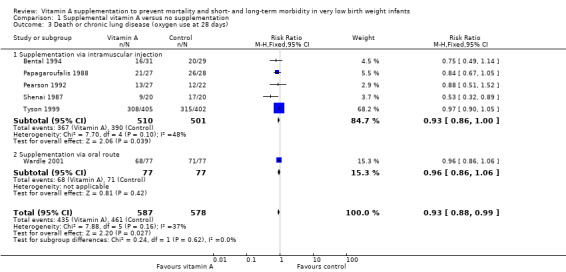
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 3 Death or chronic lung disease (oxygen use at 28 days).
5.
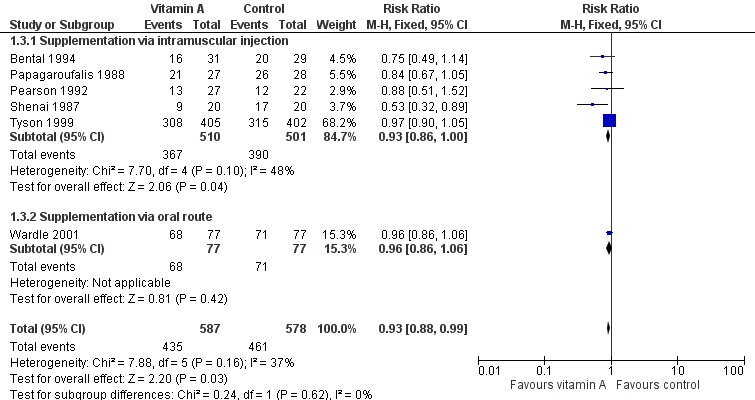
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.3 Death or chronic lung disease (oxygen use at 28 days).
Death (before 36 weeks' postmenstrual age) (Outcome 1.4)
Four trials reported on this outcome (Tyson 1999; Wardle 2001; Ravishankar 2003; Mactier 2012). Kiatchoosakun 2014 reported "death before discharge" (but not at 36 weeks' PMA) and therefore is not included in this analysis. The polled estimate of the effect showed no significant differences between the vitamin A and control groups (typical RR 1.00, 95% CI 0.77 to 1.29; 1089 infants) (Analysis 1.4; Figure 6). The overall quality of the evidence for this outcome is moderate because of imprecision in estimates.
1.4. Analysis.
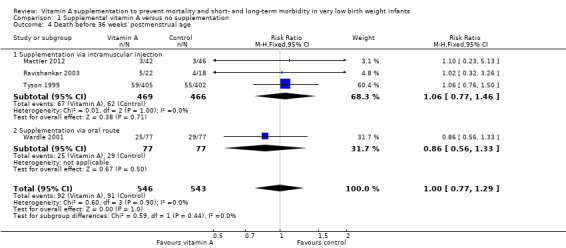
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 4 Death before 36 weeks' postmenstrual age.
6.
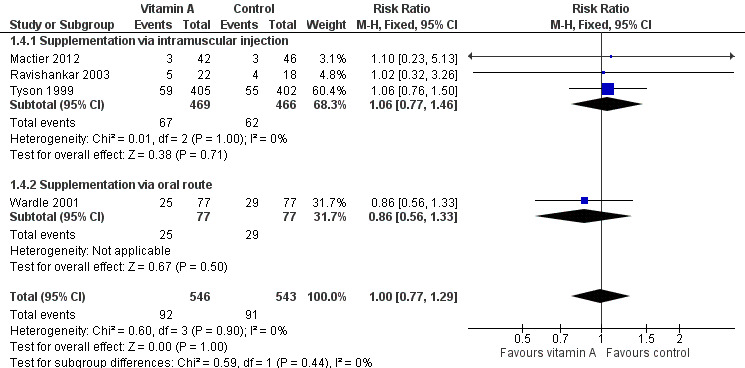
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.4 Death before 36 weeks' postmenstrual age.
Chronic lung disease (oxygen use at 36 weeks' postmenstrual age) (Outcome 1.5)
Five trials reported on this outcome (Tyson 1999; Wardle 2001; Ravishankar 2003; Mactier 2012; Kiatchoosakun 2014). Tyson 1999 reported a marginal reduction in oxygen use at 36 weeks postmenstrual age in the vitamin A group (RR 0.85, 95% CI 0.73 to 0.98; RD −0.09, 95% CI −0.16 to −0.01; NNTB 11, 95% CI 6 to 100; 693 infants).
Similar results are seen in the pooled analysis, with a small beneficial effect of vitamin A on the risk of chronic lung disease at 36 weeks' postmenstrual age (typical RR 0.87, 95% CI 0.77 to 0.99; typical RD −0.07, 95% CI −0.13 to −0.01; NNTB 14, 95% CI 8 to 100; 986 infants) (Analysis 1.5; Figure 7). The overall quality of the evidence for this outcome was judged as moderate; we downgraded the quality of evidence based upon imprecision because although the 95% CI for the typical RR is narrow the result is consistent with minimal clnical effect.
1.5. Analysis.
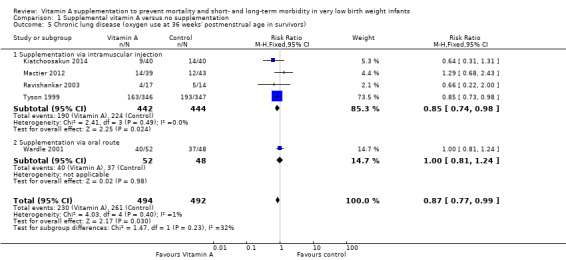
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 5 Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors).
7.
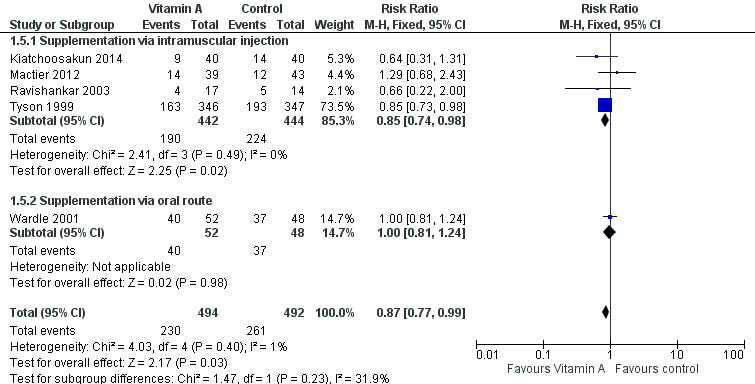
Forest plot of comparison: 1 Supplemental vitamin A versus no supplementation, outcome: 1.5 Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors).
Death or chronic lung disease (Oxygen use at 36 weeks' postmenstrual age) (Outcome 1.6)
Four trials reported on this outcome (Tyson 1999; Wardle 2001; Ravishankar 2003; Mactier 2012). Tyson 1999 showed a trend towards reduction in death or chronic lung disease in the vitamin A group that was of borderline statistical significance (RR 0.89, 95% CI 0.79 to 1.00; RD −0.07, 95% CI −0.14 to 0.00). Pooling the data from the four trials did not meaningfully alter this finding (typical RR 0.92, 95% CI 0.84 to 1.01; typical RD −0.05, 95% CI −0.11 to 0.01; 1089 infants) (Analysis 1.6). Including the outcome data at 34 weeks' postmenstrual age from Pearson 1992 in this analysis, there was essentially no difference to these results. The overall quality of the evidence for this outcome was judged as moderate; we downgraded the quality of evidence based upon imprecision because although the 95% CI for the typical RR is narrow the result is consistent with minimal clnical effect.
1.6. Analysis.
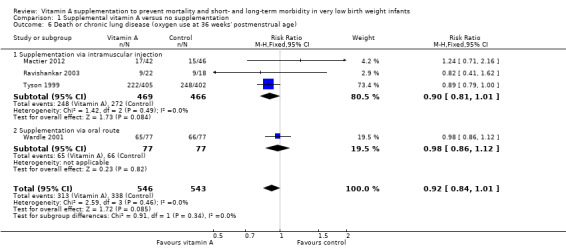
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 6 Death or chronic lung disease (oxygen use at 36 weeks' postmenstrual age).
Death (at 18 to 24 months) (Outcome 1.7)
Tyson 1999 reported on this outcome and found no significant difference between the vitamin A and control groups (RR 0.95, 95% CI 0.71 to 1.27; RD −0.01, 95% CI −0.06 to 0.04; 807 infants (Analysis 1.7).
1.7. Analysis.
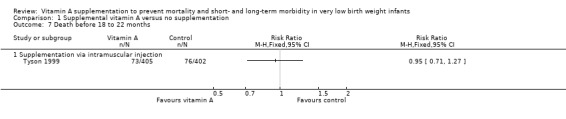
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 7 Death before 18 to 22 months.
Neurodevelopmental impairment at 18 to 24 months (Outcome 1.8)
Ambalavanan 2005 followed up 88% of the surviving infants who participated in the Tyson 1999 trial at 18 to 22 months corrected age. Forty‐one infants could not be assessed, leaving 290 infants in the intervention group and 289 in the control group (15% lost to follow‐up in both groups). There was no difference between the groups in either neurodevelopment impairment, defined as one or more of: Bayley II Mental Index less than 70, Psychomotor Index less than 70, any cerebral palsy, blind in both eyes, or bilateral hearing aids (RR 0.89, 95% CI 0.74 to 1.08; RD −0.05, 95% CI −0.04 to 0.03; 1538 infants) (Analysis 1.8). The overall quality of the evidence for this outcome is moderate because of imprecision in estimates.
1.8. Analysis.
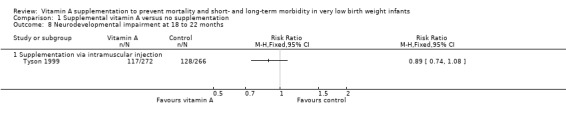
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 8 Neurodevelopmental impairment at 18 to 22 months.
Death or neurodevelopmental impairment at 18 to 24 months (Outcome 1.9)
Tyson 1999 reported on this outcome and found no significant differences between the vitamin A and control groups (RR 0.92, 95% CI 0.81 to 1.05; RD −0.05, 95% CI −0.12 to 0.03; 687 infants) (Analysis 1.9).
1.9. Analysis.
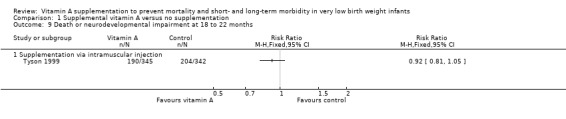
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 9 Death or neurodevelopmental impairment at 18 to 22 months.
Patent ductus arteriosus (Outcome 1.10)
Ravishankar 2003 found no significant difference in the failure of ductal closure or treatment by day 14 between the vitamin A and control groups (RR 0.98, 95% CI 0.56 to 1.72; RD −0.01, 95% CI −0.32 to 0.30; 40 infants) (Analysis 1.10).
1.10. Analysis.
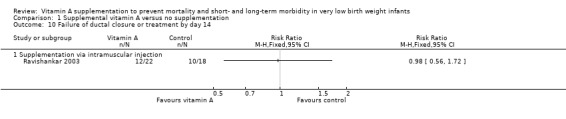
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 10 Failure of ductal closure or treatment by day 14.
Kiatchoosakun 2014 reported on any evidence of PDA diagnosed by echocardiography but did not report rates of treatment between the two groups.
Sepsis (Outcome 1.11)
Three trials reported on one or more episodes of culture‐proven nosocomial sepsis (Bental 1994; Tyson 1999; Kiatchoosakun 2014). The pooled data showed a non‐significant trend towards a reduction in sepsis in the vitamin A group (typical RR 0.89, 95% CI 0.76 to 1.04; typical RD −0.05, 95% CI −0.11 to 0.01; 947 infants) (Analysis 1.11).
1.11. Analysis.
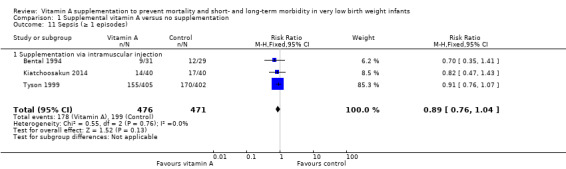
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 11 Sepsis (≥ 1 episodes).
Necrotising enterocolitis (Outcome 1.12)
Four trials reported on necrotising enterocolitis (Bental 1994; Tyson 1999; Wardle 2001; Kiatchoosakun 2014); and the pooled data showed no significant difference between the vitamin A and control groups (typical RR 0.92, 95% CI 0.67 to 1.27; typical RD −0.01, 95% CI −0.05 to 0.03; 1101 infants) (Analysis 1.12).
1.12. Analysis.
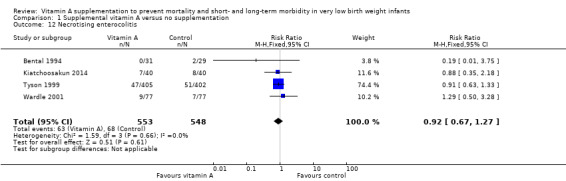
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 12 Necrotising enterocolitis.
Intraventricular haemorrhage (Outcome 1.13)
Three trials reported on any intraventricular haemorrhage (Bental 1994; Tyson 1999; Ravishankar 2003). The pooled data found no significant difference between the vitamin A and control groups (typical RR 0.95, 95% CI 0.82 to 1.10; typical RD −0.02, 95% CI −0.09 to 0.04; 907 infants) (Analysis 1.13).
1.13. Analysis.
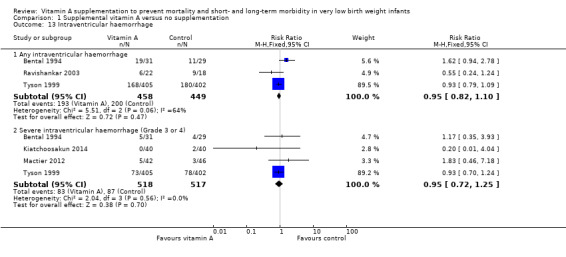
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 13 Intraventricular haemorrhage.
Four of the trials also reported on severe intraventricular haemorrhage (Bental 1994; Tyson 1999; Mactier 2012; Kiatchoosakun 2014). There was no significant difference between the vitamin A and control groups (typical RR 0.95, 95% CI 0.72 to 1.25; typical RD −0.01, 95% CI −0.05 to 0.04; 1035 infants) (Analysis 1.13).
Periventricular leukomalacia (Outcome 1.14)
Tyson 1999 reported on this outcome and found no significant differences in periventricular leukomalacia between the vitamin A and control groups (RR 0.74, 95% CI 0.44 to 1.25; RD −0.01, 95% CI −0.05 to 0.03; 646 infants) (Analysis 1.14).
1.14. Analysis.
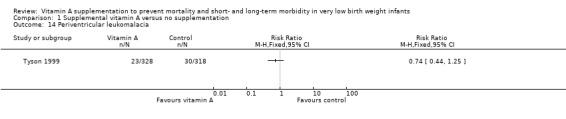
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 14 Periventricular leukomalacia.
Retinopathy of prematurity (Outcome 1.15)
Three trials reported on this outcome (Shenai 1987; Pearson 1992; Kiatchoosakun 2014). Additional data was obtained from the trial author of Wardle 2001. Shenai 1987 and Kiatchoosakun 2014 noted a trend to reduced incidence of retinopathy of prematurity in the vitamin A group; this was reflected in the pooled trial data (typical RR 0.81, 95% CI 0.65 to 1.01; typical RD −0.10, 95% CI −0.20 to 0.00; 255 infants)(Analysis 1.15).
1.15. Analysis.
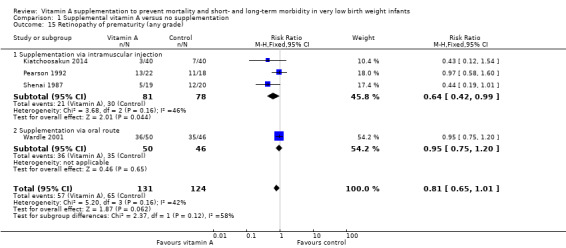
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 15 Retinopathy of prematurity (any grade).
Retinopathy of prematurity requiring laser therapy (Outcome 1.16)
Two trials reported on retinopathy of prematurity requiring laser therapy (Wardle 2001; Mactier 2012). No difference was seen in the risk of retinopathy of prematurity requiring laser therapy (typical RR 1.27, 95% CI 0.55, 2.94; 236 infants).
Adverse effects
The studies by Bental 1994, Papagaroufalis 1988, and Ravishankar 2003 make no comment on monitoring for adverse effects. Shenai 1987 noted that clinical monitoring for toxicity included periodic anterior fontanelle pressure assessment to detect raised intracranial pressure and reported no clinical or biochemical evidence of toxicity. Pearson 1992 reported that intramuscular injections of vitamin A were painful, and both this trial and Werkman 1994 found no evidence of biochemical toxicity. Wardle 2001 noted that the incidence of potential adverse effects, seizures, and persistent vomiting did not differ between the groups. Infants in Tyson 1999 were independently assessed for signs of toxicity on a weekly basis; a suspected or definite increase in fontanelle tension was slightly more frequent in control than supplemented infants (18% versus 15%, P = 0.26), and potential toxicity unexplained by other factors occurred in 0.8% controls and 1.0% supplemented infants.
Vitamin A dosing regimens
Comparison 2: Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 × week for 4 weeks
Comparison 3: Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks
Ambalavanan 2003 compared a 'standard' intramuscular regimen of supplemental vitamin A (5000 IU 3 × per week for 4 weeks) as used in Tyson 1999, with a higher dose (10,000 IU 3 × per week for 4 weeks) and with a once‐per‐week dose (15,000 IU weekly for 4 weeks). The vitamin A was given intramuscularly, and the trial's primary outcome was the serum retinol concentration at 28 days
Death before 36 weeks' postmenstrual age (Outcomes 2.1 and 3.1)
There was no significant difference for this outcome between the higher‐ and standard‐dose groups (80 infants) (Analysis 2.1), or between the once‐a‐week and standard regimens (80 infants) (Analysis 3.1).
2.1. Analysis.
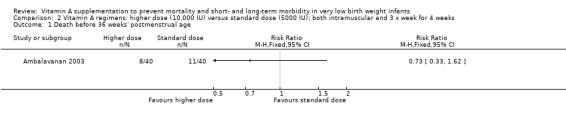
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 1 Death before 36 weeks' postmenstrual age.
3.1. Analysis.
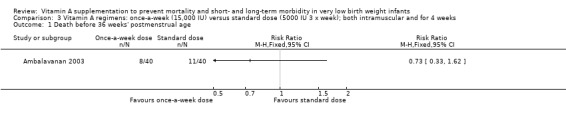
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 1 Death before 36 weeks' postmenstrual age.
Oxygen use at 36 weeks' postmenstrual age in survivors (Outcomes 2.2 and 3.2)
There was no significant difference for this outcome between the higher‐ and standard‐dose groups (61 infants) (Analysis 2.2), or between the once‐a‐week and standard regimens (61 infants, Analysis 3.2).
2.2. Analysis.
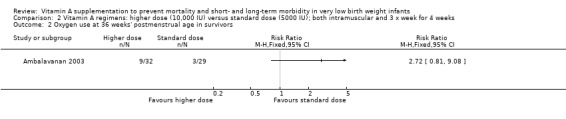
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 2 Oxygen use at 36 weeks' postmenstrual age in survivors.
3.2. Analysis.
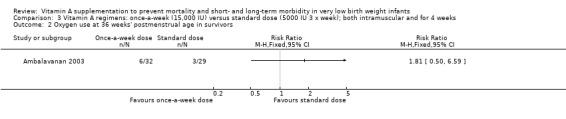
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 2 Oxygen use at 36 weeks' postmenstrual age in survivors.
Death or oxygen use at 36 weeks' postmenstrual age (Outcomes 2.3 and 3.3)
There was no significant difference for this outcome between the higher‐ and standard‐dose groups (80 infants) (Analysis 2.3), or between the once‐a‐week and standard regimens (80 infants, Analysis 3.3).
2.3. Analysis.
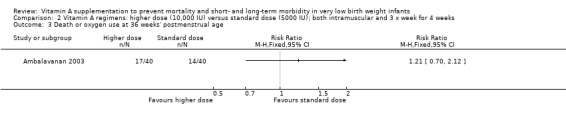
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 3 Death or oxygen use at 36 weeks' postmenstrual age.
3.3. Analysis.
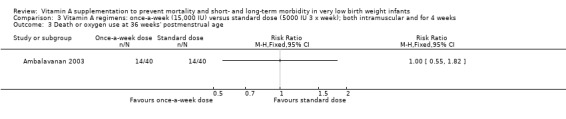
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 3 Death or oxygen use at 36 weeks' postmenstrual age.
Vitamin A concentration (retinol) (Outcomes 2.4, 2.5, 3.4 and 3.5)
There was no significant difference between the standard dose and higher dose for this outcome when measured as retinol (μg/L) on study day 28 (55 infants) (Analysis 2.4) or as retinol (< 200 μg/L) on day 28 (%) (55 infants) (Analysis 2.5).
2.4. Analysis.
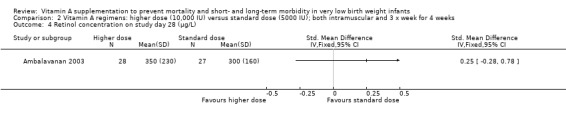
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 4 Retinol concentration on study day 28 (μg/L).
2.5. Analysis.
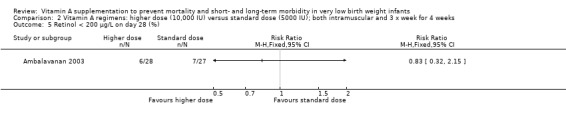
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 5 Retinol < 200 μg/L on day 28 (%).
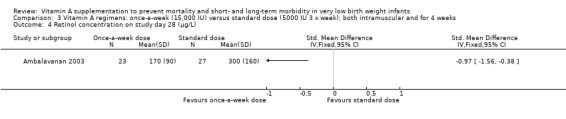
The once‐per‐week regimen resulted in significantly lower concentrations than the standard regimen on study day 28 (SMD −0.97, 95% CI ‐1.56 to −0.38; 50 infants (Analysis 3.4). The once‐per‐week dose regimen also resulted in a higher proportion of infants with 28‐day retinol concentrations below 200 μg/L (RR 2.52, 95% CI 1.24 to 5.09; 50 infants) (Analysis 3.5)
3.4. Analysis.
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 4 Retinol concentration on study day 28 (μg/L).
3.5. Analysis.
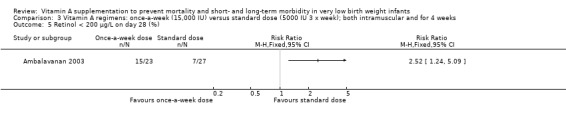
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 5 Retinol < 200 μg/L on day 28 (%).
Necrotising enterocolitis (Outcome 2.6 and 3.6)
There were no significant differences for this outcome between the higher‐ and standard‐dose groups (80 infants) (Analysis 2.6) or between the once‐a‐week and standard regimens (80 infants) (Analysis 3.6).
2.6. Analysis.
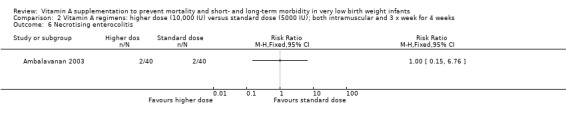
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 6 Necrotising enterocolitis.
3.6. Analysis.
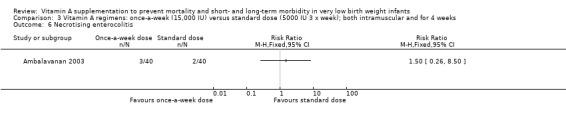
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 6 Necrotising enterocolitis.
Retinopathy of prematurity (Outcomes 2.7, 2.8, 3.7 and 3.8)
There was a trend to fewer infants with retinopathy of prematurity in the higher‐dose group compared to the standard‐dose group, for both any grade (63 infants)(Analysis 2.7) and threshold disease (63 infants)(Analysis 2.8).
2.7. Analysis.
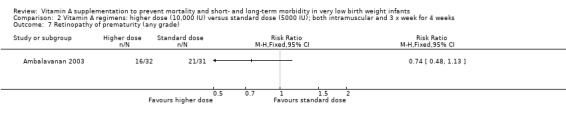
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 7 Retinopathy of prematurity (any grade).
2.8. Analysis.
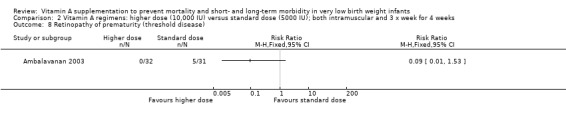
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 8 Retinopathy of prematurity (threshold disease).
When we compared the once‐a‐week regimen with the standard regimen there was a trend to fewer infants with retinopathy of prematurity for any grade (64 infants) (Analysis 3.7) but there was no significant difference between the groups when measured as threshold disease (64 infants) (Analysis 3.8).
3.7. Analysis.
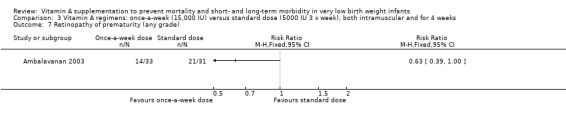
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 7 Retinopathy of prematurity (any grade).
3.8. Analysis.
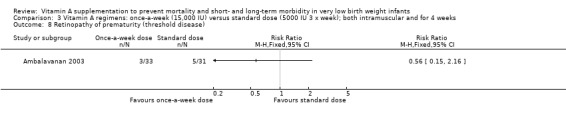
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 8 Retinopathy of prematurity (threshold disease).
For both analyses, only small numbers of infants were involved and the trial lacked power to assess this outcome.
Sepsis (Outcomes 2.9 and 3.9)
There were no significant differences in the number of episodes of sepsis between the higher‐ and standard‐dose groups (80 infants) (Analysis 2.9) or between the once‐a‐week and standard regimens (80 infants) (Analysis 3.9).
2.9. Analysis.
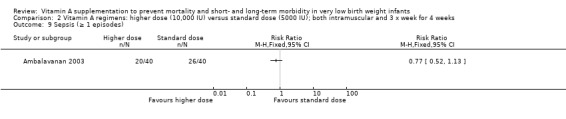
Comparison 2 Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks, Outcome 9 Sepsis (≥ 1 episodes).
3.9. Analysis.
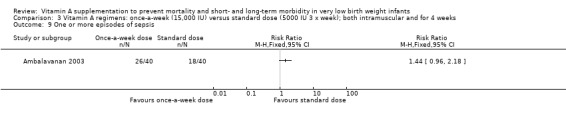
Comparison 3 Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks, Outcome 9 One or more episodes of sepsis.
Vitamin A concentration
The data did not allow a quantitative analysis of vitamin A concentrations among survivors.
Adverse effects
Ambalavanan 2003 monitored for potential adverse effects. One infant in the higher‐dose group was noted to have transient fullness of the anterior fontanelle without other causes and which resolved in 48 hours.
Discussion
This Cochrane review includes 11 randomised controlled trials, ten comparing vitamin A with placebo and one comparing different vitamin A regimens. Tyson 1999 is the largest trial included in the review with a sample size (807 infants) more than five times that of the next largest trial (154 infants, Wardle 2001).
The first version of this review reported a significant reduction in death or oxygen use at one month of age in vitamin A‐treated infants (RR 0.75, 95% CI 0.62 to 0.91) (Darlow 1998). The addition of Tyson 1999, which found no difference in outcome at one month, to the pooled data resulted in the meta‐analysis showing only a trend of borderline significance towards reduction in death or oxygen use at this time (RR 0.93, 95% CI 0.86 to 1.00) (Darlow 2000). Wardle 2001, the only trial to give supplemental vitamin A via the enteral route, had a moderate sample size (154) and found no significant benefit of supplementation; however, the further addition of data from this trial resulted in the meta‐analysis showing a small but statistically significant reduction in this outcome (RR 0.93, 95% CI 0.88 to 0.99; RD −0.05, 95% CI −0.10 to −0.01; NNTB 20, 95% CI 10 to 100).
Four trials which gave intramuscular vitamin A to supplemented infants reported outcomes at 36 weeks' postmenstrual age (Tyson 1999; Wardle 2001; Ravishankar 2003; Mactier 2012). The meta‐analysis of these trials supports a marginal reduction in oxygen use in vitamin A‐treated infants at 36 weeks' postmenstrual age (typical RR 0.89, 95% CI 0.78 to 1.00; typical RD −0.06, 95% CI −0.13 to 0.00; NNTB 16, 95% CI 8 to 1000+; 906 infants) as well as a marginal reduction in death or oxygen use at 36 weeks' postmenstrual age (typical RR 0.92, 95% CI 0.84 to 1.01; typical RD −0.05, 95% CI −0.11 to 0.01; 1089 infants). From the meta‐analysis of the combined data, the NNTB with regard to oxygen requirement at 36 weeks is 13 with wide 95% confidence intervals, ranging from 7 to more than 1000. It is also noteworthy that there was no difference in other outcomes, including days of ventilation and length of stay, and between vitamin A‐supplemented and control infants in Tyson 1999.
Some differences between the trials may be explained by the differences in patient populations (birth weight and ethnicity), the differences in both the route of vitamin A supplementation (intramuscular, intravenous in lipid emulsion, or enteral), and the dose given. Tyson 1999 and Wardle 2001 included somewhat lower birth weight infants (401 to 1000 grams and < 1000 grams respectively) than most other trials whereas other trials used various lower weight limits (500 grams for Ravishankar 2003; 700 grams for Pearson 1992 and Shenai 1987; 725 grams for Werkman 1994; and 1000 grams for Bental 1994). The incidence of supplemental oxygen requirement at one month of age in vitamin A‐supplemented infants was higher at 73% in Tyson 1999 and 83% in Wardle 2001, compared with a range of 34% to 67% in the other trials; this is consistent with their lower birth weight and gestational age. For the smallest infants the outcome at 36 weeks' postmenstrual age may be more clinically relevant.
In all the included trials there were higher vitamin A or retinol concentrations in the infants in the vitamin A group compared with the control group at most of the time periods studied. Kennedy 1997 reported that an intramuscular dose of 5000 IU vitamin A three times per week was required to achieve serum concentrations greater than 250 μg/L in most very low birth weight infants. This was the dose used by Tyson 1999 and was generally greater than the dose used in earlier studies; for example, Bental 1994 used 4000 IU three times weekly, Papagaroufalis 1988 used 4000 IU on alternate days while the infants were ventilated, Pearson 1992 and Shenai 1987 used 2000 IU on alternate days, and Ravishankar 2003 used between 1500 IU and 3000 IU for three doses only. Nevertheless, in Tyson 1999 25% of infants who received supplemental vitamin A and 54% of controls (data from the first 300 enrolled infants) had vitamin A concentrations below 200 μg/L on day 28. Similar percentages, 22% of those who received supplemental vitamin A and 45% of controls, had a relative dose response (change in the serum retinol concentration divided by the pre‐injection concentration) of more than 10% following an intramuscular dose of 2000 IU. Taken together, these data suggest that an even higher dose of vitamin A given intramuscularly may be required to achieve vitamin A sufficiency in very premature infants.
In Wardle 2001, infants received a much higher cumulative dose of supplemental vitamin A than in other studies (140,000 IU in 28 days compared with 60,000 IU in Tyson 1999) but by the enteral route. Vitamin A concentrations were only measured in the first 84 infants enrolled and the median concentration 24 hours after the first dose was significantly higher in supplemented infants (230 μg/L versus 150 μg/L). At 7 and 28 days of age, however, there were no significant differences in vitamin A concentrations between the groups and the median concentration in both groups was less than 200 μg/L at these times. Vitamin A absorption is less efficient using the enteral route. Rush 1994 compared the same dose of vitamin A (2000 IU/kg on alternate days) given by intramuscular injection or orally and reported that the former route gave higher plasma vitamin A concentrations after one week. Landman 1992 reported that enteral supplementation with 5000 IU vitamin A daily resulted in similar plasma concentrations at 32 days of age to those resulting from 2000 IU vitamin A on alternate days by the intramuscular route.
There were also quite marked differences in the vitamin A dose received by the control groups. This has previously been suggested to account for differences in outcome between the early studies (Lorch 1994). Infants in the control group in Pearson 1992 received higher doses of vitamin A and had mean vitamin A concentrations at weeks three and four of greater than 200 μg/L. This is higher than in infants in the control group in Shenai 1987 in which mean vitamin A concentrations were less than 150 μg/L at these times. One interpretation is that Shenai 1987 demonstrated a benefit of supplemental vitamin A in a population with vitamin deficiency, while Pearson 1992 showed a minimal benefit of additional supplementation in a population with more adequate vitamin status. However, Georgieff 1989 reported that postnatal steroids led to a near doubling of plasma vitamin A concentrations, and this finding was confirmed in Tyson 1999. Certainly variability in exposure to postnatal steroids complicates the interpretation of these data. Two trials included in this review reported the incidence of treatment with postnatal steroids (Pearson 1992, 46% in vitamin A group and 44% in controls; Wardle 2001, 39% and 34% respectively), while two others noted that steroids were used in some infants (Bental 1994; Tyson 1999).
Further information on the optimal dosage of intramuscular vitamin A for infants with birth weights 401 to 1000 grams is available from the Ambalavanan 2003 trial. Ambalavanan 2003 compared the dose regimen used by Tyson 1999 (5000 IU three times weekly for four weeks) with both a higher dose (10,000 IU three times weekly for four weeks) and a once‐a‐week dose (15,000 IU weekly for four weeks) in 120 infants. Only two infants received postnatal steroids between study days 21 and 28. Compared with the standard regimen, the higher dose regimen was not associated with a significantly higher mean retinol concentration at 28 days and there were no differences between the groups in the proportion of infants having concentrations of less than 200 μg/L at this time (26% versus 21%). The once‐a‐day regimen, however, was associated with significantly lower mean concentrations at 28 days and increased the risk of infants having concentrations of less than 200 μg/L at this time by a factor of 2.5 (26% versus 65%).
Many other variables will also affect the rate of chronic lung disease, which is known to vary considerably between centres. These factors include use of antenatal steroids (stated in four trials: Pearson 1992 where they were received by 26% of study infants and 41% controls; Tyson 1999 where the rates were 76% and 74%; Wardle 2001 where the rates were 77% and 82%; and Ravishankar 2003 where the rates were 86% and 72%); exogenous surfactant (stated in three trials: Pearson 1992 where > 90% received an artificial surfactant; Tyson 1999 where > 80% received a natural surfactant; and Wardle 2001 where all but one infant in the control group received an artificial surfactant); mode of ventilation including early nasal continuous positive airway pressure; postnatal steroids; and criteria for prescribing supplemental oxygen.
Despite the variations among trials discussed above, the quality assessment of the body of evidence found in this review for the critical neonatal outcomes was judged to be of moderate quality. The effect of vitamin A on 28‐day outcomes (chronic lung disease or death, or both), death before 36 weeks' postmenstrual age, and chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors) is of moderate quality; therefore the possibility exists that the true effect is different than that estimated here and further research could change the estimates of the effects on these last outcomes.
Less is known regarding follow‐up at 18 to 24 months. Ambalavanan 2003 assessed 85% of infants who participated in the study of Tyson 1999. Results regarding the long‐term effects that vitamin A could have on either neurodevelopment impairment (RR 0.89, 95% CI 0.74 to 1.08) or the combined outcome of death or neurodevelopmental impairment (RR 0.92, 95% CI 0.81 to 1.05) were inconclusive. The low quality of evidence for the neurodevelopment impairment makes evident the need for further research to define the long‐term effects of the supplementation of vitamin A in preterms. This trial was not powered appropriately to assess long‐term outcomes, and the benefit or harm from repeated intramuscular vitamin A following birth remains uncertain.
The studies did not generally report the frequency or severity of adverse events, although Pearson 1992 noted that repeat intramuscular injections may have been painful.
Authors' conclusions
Implications for practice.
Supplementing very low birth weight infants with vitamin A is associated with a small benefit in terms of reducing death or oxygen use at one month of age and a marginal reduction in oxygen use at 36 weeks' postmenstrual age. Supplementation with vitamin A may also reduce the incidence of retinopathy of prematurity and of nosocomial sepsis. The trials do not allow judgement as to the best route of supplementation, although the one trial that gave enteral vitamin A found no significant benefit for supplementation. One trial compared different intramuscular dosing regimens and the results suggest that, at least for infants with birth weight 401 to 1000 grams, the optimal dose appears to be 5000 IU three times weekly for four weeks, although on this regimen 26% of infants still had plasma vitamin A concentrations below 200 μg/L (0.70 μmol/L), which may indicate deficiency (Ambalavanan 2003).
The conclusion of a marginal benefit at 36 weeks' postmenstrual age is derived from the results of five trials in which most infants had a birth weight less than 1000 grams. An earlier version of this Cochrane Review stated that whether clinicians decide to utilise repeat intramuscular doses of vitamin A may well depend upon the incidence of supplemental oxygen requirements at 36 weeks' postmenstrual age in extremely low birth weight infants in their unit; and their own assessment in conjunction with the review's findings of the benefits of a modest reduction in this outcome balanced against a lack of other proven benefits and the acceptability of the treatment (Darlow 2002). Long‐term follow‐up data at 18 to 22 months' corrected age of the largest included trial has shown no evidence of either benefit or harm at this time (Tyson 1999). If a decision has been made not to treat for the early benefits the follow‐up study is unlikely to change that decision. On the other hand, if a decision has been made to treat for early benefits the follow‐up study is reassuring in that long‐term harmful effects are unlikely.
Implications for research.
Further investigations are warranted as to the relationship between biochemical and functional vitamin A status in very low birth weight infants. Also, the benefits in terms of vitamin A status, clinical outcomes, safety, and acceptability to caretakers and parents of delivering vitamin A in an intravenous lipid emulsion from the first days of life, compared with delivery by intramuscular injections, should be investigated in a randomised controlled trial.
There is an epidemic of retinopathy of prematurity in middle‐income and low‐income countries that affects many infants who are more mature at birth and have a greater birth weight than most infants who acquire severe retinopathy in more developed countries. In many of these countries the vitamin A status of both mothers and infants is particularly poor. The trends towards less retinopathy of prematurity associated with supplemental vitamin A and higher‐dose regimens seen in this review suggest that further trials should be undertaken in these countries to assess the possible contribution of poor perinatal vitamin A status to retinopathy of prematurity, and the need for intervention studies in these populations.
What's new
| Date | Event | Description |
|---|---|---|
| 1 May 2016 | New citation required but conclusions have not changed | Conclusions not changed. |
| 1 May 2016 | New search has been performed | This updates the review 'Vitamin A supplementation to prevent mortality and short and long‐term morbidity in very low birth weight infants' published in the Cochrane Database of Systematic Reviews (Darlow 2011). Search updated May 2016. Two new trials were identified (Mactier 2012; Kiatchoosakun 2014). Additional outcome of 'ROP requiring treatment' added post hoc. Summary of findings table added. |
History
Protocol first published: Issue 4, 1998 Review first published: Issue 2, 2000
| Date | Event | Description |
|---|---|---|
| 31 August 2011 | New citation required but conclusions have not changed | Additional secondary outcomes added. The methods have been updated to include the new risk of bias assessment and 'Summary of findings' tables. The risk of bias assessment has been completed for each trial and 'Summary of findings' tables added to the review. |
| 31 August 2011 | New search has been performed | This updates the review "Vitamin A supplementation to prevent mortality and short and long‐term morbidity in very low birthweight infants" published in the Cochrane Database of Systematic Reviews (Darlow 2007). Search updated August 2011. No new trials. |
| 12 June 2008 | Amended | Converted to new review format. |
| 15 July 2007 | New search has been performed | This review updates the existing review "Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants", published in the Cochrane Database of Systematic Reviews in 2002, Issue 2 (Darlow 2002). One further small trial has been identified and data from this trial added to the pooled data for the meta‐analysis. There are now data on the neurodevelopmental status at 18 to 22 months postmenstrual age for infants included in the largest vitamin A supplementation trial, and this information has been included in this review. In addition, data from one trial that compared three different intramuscular vitamin A dosing regimens have been included. The previous version of this review concluded that supplementing very low birthweight infants with vitamin A is associated with a reduction in oxygen requirement among survivors at 36 weeks postmenstrual age, as well as a reduction in death or oxygen requirement at one month of age. With data from the additional study, these conclusions remain the same. The information on follow‐up at 18 to 22 months postmenstrual age from the largest included trial showed no evidence or benefit or harm from the intervention. Based on biochemical data, the one study that investigated different intramuscular vitamin A dosing regimens suggested that the regimen used in the largest trial (5000 IU 3x weekly for four weeks) was optimal even though some infants still had poor vitamin A status. The conclusion remains unchanged: clinicians must decide whether to utilise repeat intramuscular doses of vitamin A based upon the incidence of supplemental oxygen requirement at 36 weeks postmenstrual age in extremely low birthweight infants in their unit and their own assessment, based upon the review, of the benefits of a modest reduction in this outcome balanced against lack of other proven benefits and the acceptability of treatment. The follow‐up data would support a decision either to treat or not to treat. |
| 15 July 2007 | New citation required but conclusions have not changed | Substantive amendment. |
Notes
None
Acknowledgements
We are grateful to Dr K Kennedy, University of Texas Southwestern Medical Center, Dallas, for supplying some original data and for helpful advice. We are also grateful to Drs Bengal, Wardle, Gelb (with regard to the Ravishankar 2003 trial), and Ambalavanan for supplying some original data for earlier versions of this Cochrane review.
Cochrane Neonatal has been funded in part with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN267200603418C.
The 2010 update was completed centrally by Roger Soll of Cochrane Neonatal with the approval of the authors.
The authors thank the Cochrane Editorial Unit and the CNRG for the support in preparing the 'Risk of bias' tables and the 'Summary of findings' tables for the 2016 update.
Appendices
Appendix 1. Standard search methodology
PubMed: ((infant, newborn[MeSH] OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or infan* or neonat*) AND (randomized controlled trial [pt] OR controlled clinical trial [pt] OR Clinical Trial[ptyp] OR randomized [tiab] OR placebo [tiab] OR clinical trials as topic [mesh: noexp] OR randomly [tiab] OR trial [ti]) NOT (animals [mh] NOT humans [mh]))
Embase: (infant, newborn or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW or Newborn or infan* or neonat*) AND (human not animal) AND (randomized controlled trial or controlled clinical trial or randomized or placebo or clinical trials as topic or randomly or trial or clinical trial)
CINAHL: (infant, newborn OR newborn OR neonate OR neonatal OR premature OR low birth weight OR VLBW OR LBW or Newborn or infan* or neonat*) AND (randomized controlled trial OR controlled clinical trial OR randomized OR placebo OR clinical trials as topic OR randomly OR trial OR PT clinical trial)
Cochrane Library: (infant or newborn or neonate or neonatal or premature or very low birth weight or low birth weight or VLBW or LBW)
Appendix 2. Data extraction fields for interventions and outcomes
The authors collected the following data from each included trial.
General information
Supplementation dose
Supplementation frequency
Time of initiation of supplementation
Vitamin A intake of control groups
Pre‐randomisation vitamin A concentrations of supplemented and control groups (if available)
Post‐randomisation vitamin A concentrations
Number of the following outcomes in the supplemented and control groups
Deaths
Reported as having a significant patent ductus arteriosus at 2 weeks of age
Developing neonatal chronic lung disease in the supplemented and control groups
Patent ductus arteriosus
Necrotising enterocolitis
Intraventricular haemorrhage
Periventricular leukomalacia
Retinopathy of prematurity
≥ 1 episodes of defined sepsis
Long‐term neurodevelopmental disability
Mean, standard deviation, and range of the following outcomes in the supplemented and control groups
Gestational age
Birth weight
Data and analyses
Comparison 1. Supplemental vitamin A versus no supplementation.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death (before 1 month) | 6 | 1165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.66, 1.11] |
| 1.1 Supplementation via intramuscular injection | 5 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.62, 1.18] |
| 1.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 2 Chronic lung disease (oxygen use at 28 days in survivors) | 7 | 1070 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 2.1 Supplementation via intramuscular injection | 5 | 884 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.01] |
| 2.2 Supplementation via intravenous route | 1 | 86 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.54, 1.70] |
| 2.3 Supplementation via oral route | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.80, 1.11] |
| 3 Death or chronic lung disease (oxygen use at 28 days) | 6 | 1165 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.88, 0.99] |
| 3.1 Supplementation via intramuscular injection | 5 | 1011 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.93 [0.86, 1.00] |
| 3.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.96 [0.86, 1.06] |
| 4 Death before 36 weeks' postmenstrual age | 4 | 1089 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.77, 1.29] |
| 4.1 Supplementation via intramuscular injection | 3 | 935 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.06 [0.77, 1.46] |
| 4.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.86 [0.56, 1.33] |
| 5 Chronic lung disease (oxygen use at 36 weeks' postmenstrual age in survivors) | 5 | 986 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.87 [0.77, 0.99] |
| 5.1 Supplementation via intramuscular injection | 4 | 886 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.85 [0.74, 0.98] |
| 5.2 Supplementation via oral route | 1 | 100 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.00 [0.81, 1.24] |
| 6 Death or chronic lung disease (oxygen use at 36 weeks' postmenstrual age) | 4 | 1089 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.84, 1.01] |
| 6.1 Supplementation via intramuscular injection | 3 | 935 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.81, 1.01] |
| 6.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.98 [0.86, 1.12] |
| 7 Death before 18 to 22 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 8 Neurodevelopmental impairment at 18 to 22 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 9 Death or neurodevelopmental impairment at 18 to 22 months | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 10 Failure of ductal closure or treatment by day 14 | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 10.1 Supplementation via intramuscular injection | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] | |
| 11 Sepsis (≥ 1 episodes) | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 11.1 Supplementation via intramuscular injection | 3 | 947 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.89 [0.76, 1.04] |
| 12 Necrotising enterocolitis | 4 | 1101 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.92 [0.67, 1.27] |
| 13 Intraventricular haemorrhage | 5 | Risk Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 13.1 Any intraventricular haemorrhage | 3 | 907 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.82, 1.10] |
| 13.2 Severe intraventricular haemorrhage (Grade 3 or 4) | 4 | 1035 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.72, 1.25] |
| 14 Periventricular leukomalacia | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 15 Retinopathy of prematurity (any grade) | 4 | 255 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.81 [0.65, 1.01] |
| 15.1 Supplementation via intramuscular injection | 3 | 159 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.64 [0.42, 0.99] |
| 15.2 Supplementation via oral route | 1 | 96 | Risk Ratio (M‐H, Fixed, 95% CI) | 0.95 [0.75, 1.20] |
| 16 Retinopathy of prematurity requiring laser therapy | 2 | 236 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.27 [0.55, 2.94] |
| 16.1 Supplementation via intramuscular injection | 1 | 82 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.84 [0.47, 7.19] |
| 16.2 Supplementation via oral route | 1 | 154 | Risk Ratio (M‐H, Fixed, 95% CI) | 1.0 [0.34, 2.96] |
1.16. Analysis.
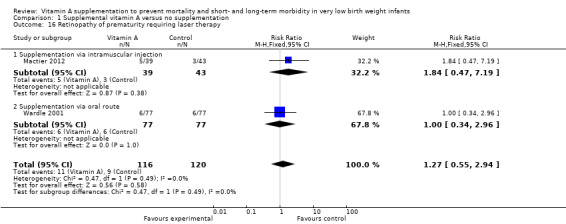
Comparison 1 Supplemental vitamin A versus no supplementation, Outcome 16 Retinopathy of prematurity requiring laser therapy.
Comparison 2. Vitamin A regimens: higher dose (10,000 IU) versus standard dose (5000 IU); both intramuscular and 3 x week for 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death before 36 weeks' postmenstrual age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Oxygen use at 36 weeks' postmenstrual age in survivors | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Death or oxygen use at 36 weeks' postmenstrual age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Retinol concentration on study day 28 (μg/L) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Retinol < 200 μg/L on day 28 (%) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Necrotising enterocolitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Retinopathy of prematurity (any grade) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Retinopathy of prematurity (threshold disease) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 Sepsis (≥ 1 episodes) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Comparison 3. Vitamin A regimens: once‐a‐week (15,000 IU) versus standard dose (5000 IU 3 x week); both intramuscular and for 4 weeks.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Death before 36 weeks' postmenstrual age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 2 Oxygen use at 36 weeks' postmenstrual age in survivors | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 3 Death or oxygen use at 36 weeks' postmenstrual age | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 4 Retinol concentration on study day 28 (μg/L) | 1 | Std. Mean Difference (IV, Fixed, 95% CI) | Totals not selected | |
| 5 Retinol < 200 μg/L on day 28 (%) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 6 Necrotising enterocolitis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 7 Retinopathy of prematurity (any grade) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 8 Retinopathy of prematurity (threshold disease) | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected | |
| 9 One or more episodes of sepsis | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Ambalavanan 2003.
| Methods | Single centre randomised controlled trial | |
| Participants | Number: 120 Description: birth weight between 401 and 1000 grams; requiring mechanical ventilation or supplemental oxygen at 24 hours of age; and no major congenital anomalies, non‐bacterial infection, or terminal illness |
|
| Interventions | Comparison of vitamin A (water‐soluble retinyl palmitate) regimens
Other: 2 infants received postnatal steroids between study days 21 and 28 |
|
| Outcomes |
|
|
| Notes | Location: University of Alabama at Birmingham (UAB), USA Trial author provided further information on the timing of death, numbers of infants having serum retinol estimations at 28 days, and any retinopathy of prematurity |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "random assignment stratified by birth weight (401‐750 grams, 751‐1000 grams)" (pg 657) |
| Allocation concealment (selection bias) | Low risk | Used "shuffled blocks (of sizes 3, 6, or 9) of sealed opaque envelopes" (pg 657) |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: interventions were not provided in the same way for the three groups Blinding of outcome measurements: yes ("Outcome evaluations were performed by one of the investigators, masked to treatment group assignment", pg 657) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants; intention‐to‐treat analysis was conducted |
| Selective reporting (reporting bias) | Low risk | Authors reported all pre‐specified outcomes |
| Other bias | Unclear risk | Source of funding not reported |
Bental 1994.
| Methods | Single centre randomised controlled trial | |
| Participants | Number: 60 Description: birth weight between 1000 and 1500 grams; gestational age of ≤ 34 weeks; infants were receiving supplemental oxygen and mechanical ventilation for at least 72 hours during the first week; no congenital anomalies or infection; black South African infants |
|
| Interventions | Vitamin A (water‐soluble retinyl palmitate) vs control
Other: Some control and study infants received vitamin A 1500 to 3000 IU after 1 week of age when fed orally |
|
| Outcomes |
|
|
| Notes | Location: Baragwanath Hospital, Soweto, South Africa Complete data published in an earlier abstract from 1990; we sought and obtained further clarification from the trial authors concerning 2 outcomes: oxygen use at 1 month in survivors; and death or oxygen use at 1 month |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers were computer‐generated in blocks of 10 (pg 142) |
| Allocation concealment (selection bias) | Unclear risk | No information provided |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: unclear because no information about the control group intervention Blinding of outcome measurement: 2 investigators administered the vitamin A and were not involved in the treatment of study infants; and the intensive care unit staff were unaware of the group allocation of infants (pg 142) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants |
| Selective reporting (reporting bias) | Low risk | All expected outcomes were reported |
| Other bias | Unclear risk | Poor reporting of study design; supported by a grant from the South African Medical Research Council |
Kiatchoosakun 2014.
| Methods | Randomised controlled trial to assess the effect of vitamin A supplementation for prevention of bronchopulmonary dysplasia in VLBW premature infants. | |
| Participants | Eighty premature infants weighing < 1500 grams who received mechanical ventilation or oxygen supplementation at 24 hours of age, admitted to Neonatal Units of Srinagarind Hospital, Khon Kaen University, Khon Kaen, Thailand. | |
| Interventions | Infants were assigned to receive either intramuscular vitamin A 5000 IU 3 times/week (treatment group) or sham procedure (control group) for four weeks. | |
| Outcomes | The investigators reported on serum vitamin A levels, and complications of prematurity including supplemental oxygen at 36 weeks' postmenstrual age, duration of intubation, days on oxygen therapy, and length of hospital stay. | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomised controlled trial to assess the effect of vitamin A supplementation for prevention of bronchopulmonary dysplasia in VLBW premature infants. Infants were assigned to vitamin A or control group "by a research nurse using a randomization list (with sealed envelopes containing the treatment assignments randomised by blocks of 4)". |
| Allocation concealment (selection bias) | Low risk | "..control infants received a sham procedure rather than a placebo injection. For each treatment, a screen was placed around the bed, a pacifier was used for non‐pharmacological pain management, and the injection site was covered with cotton and tape. The same covering was placed on the control infants". |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Attending neonatologist unaware of intervention |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Complete follow‐up of enrolled infants |
| Selective reporting (reporting bias) | Unclear risk | Incomplete reporting of death at 36 weeks' postmenstrual age |
Mactier 2012.
| Methods | double‐blind, randomised controlled trial conducted at three neonatal centres in Glasgow | |
| Participants | Number: 89 infants (42 supplemented and 47 controls) Description: infants < 32 weeks' gestation or < 1501 grams birth weight, or both |
|
| Interventions | Supplemented infants received additional intramuscular vitamin A 10,000 IU 3 times weekly from day 2 for a minimum of 2 weeks or until establishment of oral feeding | |
| Outcomes | Hepatic stores were assessed by relative dose response (RDR). The primary outcome measure was cone‐corrected dark‐adapted retinal rod sensitivity measured by electroretinogram at 36 weeks' postmenstrual age (PMA). Complications of prematurity were reported including use of respiratory support, intraventricular haemorrhage, retinopathy of prematurity, supplemental oxygen at 36 weeks postmenstrual age and death | |
| Notes | www.ClinicalTrials.gov NCT004174404 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation was undertaken by the pharmacy staff |
| Allocation concealment (selection bias) | Low risk | Randomisation was undertaken by the pharmacy staff and vitamin A or and empty box were delivered in a sealed bag to the infants bedside |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Clinical outcomes reported on all enrolled infants; some loss to follow‐up on retinol levels |
| Selective reporting (reporting bias) | Low risk | |
| Other bias | Low risk | |
Papagaroufalis 1988.
| Methods | Single centre randomised controlled trial | |
| Participants | Number: 55 Description: birth weight ≤ 1300 grams; gestation < 29 weeks; requirement for > 40% oxygen and mechanical ventilation for > 72 hours in first week |
|
| Interventions | Vitamin A (water‐soluble retinyl palmitate) vs control
|
|
| Outcomes |
Vitamin A levels at various times |
|
| Notes | Location: not stated; possibly Aghia Sophia Children's Hospital and Institute of Child Health, Athens, Greece (authors' affiliation) Amount of vitamin A in unsupplemented infants not stated Trial author provided Dr K Kennedy with additional data at the time of an earlier review (Kennedy 1997), and these data are included in this review |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomised, blind controlled study |
| Allocation concealment (selection bias) | Unclear risk | No information |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: no information provided Blinding of outcome measurement: no |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | No information provided in the conference proceeding |
| Selective reporting (reporting bias) | Unclear risk | Based on the conference proceeding, no relevant information is provided to make a judgement |
| Other bias | High risk | Report of the study is poor and only based on an abstract |
Pearson 1992.
| Methods | Multicentre randomised controlled trial | |
| Participants | Number: 49 Description: birth weight 700 to 1100 grams; requiring supplemental oxygen and mechanical ventilation between 72 and 96 hours of age with previous cumulative duration of ventilation and oxygen > 48 hours; no growth retardation (undefined), congenital, or chromosomal abnormalities, hydrops fetalis, congenital infection, neonatal hepatitis or 'do not resuscitate' order |
|
| Interventions | Vitamin A (water‐soluble retinyl palmitate) versus control
Other: both control and study infants received 1200 to 1500 IU/d vitamin A in protein‐dextrose solution when on parenteral nutrition; when fed orally all infants received vitamin A 250 to 1030 IU/100 mL milk; antenatal steroids received by 26% infants in the vitamin A group and 41% in the control group; > 90% received an artificial surfactant |
|
| Outcomes |
|
|
| Notes | Location: hospitals in North Carolina, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Multicentre randomised controlled trial; infants were stratified according to hospital, gender, and weight (pg 421) |
| Allocation concealment (selection bias) | Low risk | Randomisation was performed using sequential sealed envelopes; randomisation and drug preparation performed by someone not involved with patients (pg 421) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: "Opaque tape concealed the contents of syringes to ensure blinding" (pg 421) Blinding of outcome: "At all sites, staff responsible for patient care had no knowledge of group designation" (pg 421) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcomes reported |
| Other bias | High risk | Small study; sample size calculation performed based on high incidence rate of bronchopulmonary dysplasia; blinded interim analysis performed of the 49 patients enrolled (pg 423) 161 infants eligible, 74 of them met the inclusion criteria and 49 were enrolled |
Ravishankar 2003.
| Methods | Single centre randomised controlled trial | |
| Participants | Number: 40 Description: birth weight 500 to 1500 grams; gestation < 32 weeks; having indwelling umbilical line; no major congenital malformations or chromosomal anomalies; and < 24 hours of age |
|
| Interventions | Vitamin A (water‐miscible preparation of vitamin A (Aquasol A)) versus control
Other: most infants received parenteral nutrition including 466 IU/dL vitamin A and an additional 1000 IU supplement when fed orally; antenatal steroids received by 86% of infants in the vitamin A group and 72% in the control group |
|
| Outcomes |
|
|
| Notes | Location: Mount Sinai Medical Center, New York, New York, USA Patent ductus arteriosus closure primary outcome Corresponding author provided information on the age at death for infants |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomisation was performed with stratification by weight; the categories were 500 to 750 grams, 750 to 1000 grams, 1000 to 1250 grams, and 1250 to 1500 grams (pg 645) |
| Allocation concealment (selection bias) | Low risk | "Sealed envelopes containing cards with the group designation were maintained in the pharmacy department" (pg 645) |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding of intervention: none – infants in the intervention group received intramuscular injections and the placebo group did not receive these Blinding of outcome measurement: yes – "The staff responsible for the care of these infants had no knowledge of group assignment or outcome of study echocardiograms" (pg 645) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants |
| Selective reporting (reporting bias) | Low risk | Authors reported all pre‐specified outcomes |
| Other bias | Unclear risk | Small sample size; source of funding not reported |
Shenai 1987.
| Methods | Single centre randomised controlled trial | |
| Participants | Number: 40 Description: birth weight 700 to 1300 grams; gestation 26 to 30 week; requiring supplemental oxygen and mechanical ventilation for at least 72 hours in the first week; Caucasian infants only; birth weight appropriate for gestational age; no congenital abnormalities |
|
| Interventions | Vitamin A (water‐soluble retinyl palmitate) versus control
Other: both control and study infants received vitamin A 400 IU/dL in parenteral nutrition and 240 to 550 IU/dL from milk plus 1500 IU supplements when fed orally |
|
| Outcomes |
|
|
| Notes | Location: Vanderbilt Medical Centre, Nashville, Tennessee, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Single centre randomised controlled trial; sealed envelopes containing cards indicating group designation were used for randomisation (pg 270) |
| Allocation concealment (selection bias) | Low risk | Randomisation was carried out by pharmacists who had no knowledge of the clinical status of the infants (pg 270) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: control group received intramuscular saline solution exactly in the same manner of the intervention (pg 270–1) Blinding of outcome measurement: neonatal intensive care unit staff responsible for patient management had no knowledge of the group designation (pg 270) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants; all but one infant enrolled in the study completed the trial |
| Selective reporting (reporting bias) | Low risk | All clinically relevant outcomes reported |
| Other bias | Unclear risk | Sample size calculation performed based on high incidence rate of bronchopulmonary dysplasia |
Tyson 1999.
| Methods | Multicentre randomised controlled trial | |
| Participants | Number: 807 Description: birth weight 401 to 1000 grams; requiring supplemental oxygen or mechanical ventilation at 24 hours of age; and no major congenital anomalies, non‐bacterial infection, or terminal illness Conducted at hospitals in the NICHD Neonatal Network (USA) |
|
| Interventions | Vitamin A (water‐soluble retinyl palmitate) versus control
Other: control and study infants received similar intakes of vitamin A from non‐study enteral and parenteral sources but amounts not stated; antenatal steroids received by 76% of infants in the vitamin A group and 74% in the control group; > 80% received a natural surfactant |
|
| Outcomes |
|
|
| Notes | A subgroup analysis of 'small for gestational age' (SGA) infants from the original NICHD trial was performed to test the hypothesis that in infants requiring early respiratory support, vitamin A supplementation decreases the relative risk of BPD or death in premature SGA infants to a greater extent than in gestational age‐equivalent vitamin A‐treated 'appropriate for gestational age' (AGA) infants. Although vitamin A supplementation significantly increased serum retinol concentrations in AGA ELBW infants (median [5th percentile, 95th percentile]: 16.3 [−7.0, 68.8] versus 2.4 [−13.9, 55.1]; P < 0.001), no increases were noted in SGA ELBW infants. Given the limited power of this analysis due to a low number of SGA infants, these data did not provide evidence to support the hypothesis that vitamin A supplementation in preterm SGA infants requiring early respiratory support decreases the relative risk of BPD or death as compared with preterm AGA infants. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | "The infants were stratified according to center and birth weight (401 to 750 grams or 751 to 1000 grams) and assigned to the vitamin A or control group by a hospital pharmacist using a randomization list." (pg 1962) |
| Allocation concealment (selection bias) | Low risk | Sealed envelopes containing the treatment assignments were used (pg 1963) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: yes Blinding of outcome: outcome were assessed by research nurses that transmitted the data electronically to a biostatistical co‐ordinating centre (pg 1963) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants; intention‐to‐treat analysis was conducted |
| Selective reporting (reporting bias) | Low risk | Authors reported all pre‐specified outcomes |
| Other bias | Low risk | Study protocol described in detail, including sample size calculation Supported by cooperative agreements with the National Institute of Child Health and Human Development, and by General Clinical Research Center grants |
Wardle 2001.
| Methods | Multicentre randomised controlled trial | |
| Participants | Number: 154 Description: birth weight < 1000 grams; no life‐threatening congenital abnormalities; consent before 24 hours of age |
|
| Interventions | Vitamin A (form not stated) versus control
Other: control and study infants received 23 IU/kg/d vitamin A (stated amount but probably 233 IU) in intralipid when on parenteral nutrition; when on full enteral feeds and more than 14 days of age all infants received 5000 IU/kg/d vitamin A orally; antenatal steroids received by 77% in the vitamin A group and 82% in the control group; all but 1 infant in the control group received an artificial surfactant |
|
| Outcomes |
|
|
| Notes | Location: Liverpool Women’s Hospital, Liverpool, UK Trial author provided further information on the numbers examined for retinopathy of prematurity and numbers with any retinopathy |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation assigned using a computerised random number generator (pg F10) |
| Allocation concealment (selection bias) | Low risk | Sealed opaque numbered envelopes containing the treatment allocation (pg F10) |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding of intervention: "The control group received an equivalent volume of an inert placebo solution (which had been prepared in the hospital pharmacy to look identical with the vitamin A solution) given in the same way" (pg F10) Blinding of outcome: "Both the medical and nursing staff caring for the infants and administering the vitamin A and placebo solutions were unaware of the treatment allocation" (pg F10) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Detailed outcome information is provided for all randomised participants; intention‐to‐treat analysis was conducted |
| Selective reporting (reporting bias) | Low risk | Authors reported all pre‐specified outcomes |
| Other bias | Unclear risk | Study protocol described in detail, including sample size calculation; source of funding not reported |
Werkman 1994.
| Methods | Single centre randomised controlled trial | |
| Participants | Number: 86 Description: birth weight 725 to 1300 grams; < 96 hours of age; no intraventricular or periventricular haemorrhage, history of maternal substance abuse, or sexually transmitted disease; no congenital anomalies |
|
| Interventions | Vitamin A (retinyl palmitate) versus control
Other: both control and study infants received vitamin A as multivitamin preparation added to protein‐dextrose solution (birth weight < 1000 grams 1.5 mL/d, 210 RE/d; > 1000 grams 3.4 mL/d, 476 RE/d) and oral supplements when fed orally |
|
| Outcomes |
|
|
| Notes | Location: Newborn Centre, University of Tennessee, Tennessee, USA | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Eligible infants were randomly assigned; method of sequence generation not reported |
| Allocation concealment (selection bias) | Unclear risk | Method of allocation concealment not reported |
| Blinding (performance bias and detection bias) All outcomes | High risk | Blinding of intervention: details not reported Blinding of outcome: no |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 12/98 randomised participants left the study early (10 transferred to another hospital, 2 died from sepsis) and their data were not included in the analysis |
| Selective reporting (reporting bias) | High risk | Not all outcomes of interest were reported |
| Other bias | Unclear risk | Supported by a grant of the National Eye Institute and a gift from Ross Laboratories (pg 586) Not enough detail of protocol is provided |
h: hour IU: international units wk: week
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aranda 1992 | Route of supplementation not stated and reported vitamin A levels appear to contain a decimal point error; no clinical outcomes. Published as abstract only. |
| Aurvag 2007 | Compared vitamin A supplementation of human milk fortifier given to VLBW infants to historical controls; no clinical outcomes reported. |
| Coutsoudis 1996 | Randomised, double‐blind placebo‐controlled trial was designed to determine whether high‐dose (25,000 IU) enteral vitamin A, to correct deficiency, would be absorbed and well tolerated in low birth weight (LBW) neonates. Thirty‐five LBW infants (950 to 1700 grams; gestational age 27 to 36 weeks) were allocated to receive either placebo or vitamin A (25,000 IU) via nasogastric tube on the first day of the study (between 36 and 60 hours after delivery). The dose was repeated on study days 4 and 8. Serum retinol concentrations were determined pre‐ and post‐supplementation. Toxic effects of vitamin A were monitored by noting vomiting, drowsiness and irritability, and palpating for a bulging fontanelle. Included a broad population of preterm or low birth weight infants and did not report on relevant clinical outcomes. |
| Coutsoudis 2000 | Randomised, double‐blind, placebo‐controlled trial to investigate the effect of vitamin A supplementation on the incidence and severity of respiratory infections in LBW infants during their first year of life. The primary outcome was incidence of respiratory infections during the first year of life. One hundred and thirty LBW infants (gestational age < 36 weeks and birth weight 950 to 1700 grams) were enrolled in the study. The infants were randomly allocated to a vitamin A or placebo group. Infants in the vitamin A group received 25,000 IU of vitamin A (retinyl palmitate, Arovit drops, Roche, Basle, Switzerland) on study days 1, 4 and 8. Study day 1 was between 36 and 60 hours after delivery. Infants in the placebo group received a placebo (formulated by Roche) with a similar appearance and packed in the same dropper bottles as the vitamin A drops. Included a broad population of preterm or low birth weight infants and did not report on relevant clinical outcomes. |
| Koo 1993 | Formula‐fed infants with birth weights ≤ 1500 grams (n = 61) were randomly assigned to receive one of three preterm infant formulas. Experimental formula feedings were continued until infants weighed approximately 2 kg or until hospital discharge. Investigators measured vitamin A status as indicated by serum retinol and retinol‐binding protein (RBP) concentrations. Relevant clinical outcomes not reported. |
| Landman 1992 | Compared 2000 IU of intramuscular vitamin A administered on alternate days for 28 days to giving 5000 IU of vitamin A per day with the early introduction of feeds. The vitamin A status of 10 preterm infants (mean gestation 30.5 weeks) who received intramuscular vitamin supplementation was compared with that of nine infants (mean gestation 30.7 weeks) given enteral vitamin A. Vitamin A status was evaluated on the 32nd day of life using plasma retinol and retinol‐binding protein (RBP) concentrations and a modified relative dose response (RDR) test. Relevant clinical outcomes not reported. |
| Longardt 2014 | Non‐randomised trial. Prospective study of 69 preterm infants (median birth weight 995 grams, gestational age 28 weeks), in which 51 received 5000 IU vitamin A three times per week intramuscular (IM) for 4 weeks and 18 infants without IM vitamin A served as controls. Serum retinol, retinyl palmitate, total retinol‐binding protein 4 (RBP4), retinol‐unbound RBP4 (apo‐RBP4) and transthyretin concentrations were determined at days 3 (D3) and 28 (D28) of life. |
| Robbins 1993 | Non‐randomised trial. Evaluated two regimens of vitamin A supplementation on the incidence of BPD. In group I (n = 24), vitamin A levels were measured after 1 week of parenteral nutrition and, if low, the infant was given a supplement of 2000 IU intramuscularly three times a week, beginning on the 10th to 14th day of life. In group II (n = 24), the same supplementation was begun on the second to fourth day of life. In both groups, when enteral feedings reached 60 kcal/kg per day, 2500 IU/d vitamin A was given orally. Reported on the incidence of oxygen support at 28 days, BPD at 36 weeks' gestational age and length of neonatal intensive care unit stay. |
| Rush 1994 | Compared enteral with intramuscular administration of vitamin A; vitamin A concentrations only reported graphically; no relevant clinical outcomes reported. |
| Schmiedchen 2016 | Studied the impact of urinary retinol excretion on the serum retinol concentration of 63 VLBW infants treated with vitamin A (5000 IU intramuscular, 3 times/week for 4 weeks) compared to 38 untreated infants classified as control group. Groups not randomised. |
Characteristics of studies awaiting assessment [ordered by study ID]
Calisici 2014.
| Methods | Randomised controlled trial |
| Participants | Infants gestational age < 32 weeks and birth weight < 1250 grams, having an oxygen support of FiO₂ > 0.21 within the first 24 hours of life. |
| Interventions | Intervention: Oral Vitamin A was administered at a dose of 30,000 IU/kg for 6 weeks beginning in the first two days of life. |
| Outcomes | BPD and mortality rates were compared between control and therapy groups. The study enrolled 209 infants. There were 110 patients in Group 1 and 99 patients in Group 2. Prematurity‐related problems and diseases during postnatal period; RDS, late‐onset sepsis, PDA, pneumothorax, severe intracranial haemorrhage, ROP, BPD, and mortality were similar between the groups. |
| Notes | Presented at EAPS 2014 and published as an abstract; awaiting publication in peer‐reviewed literature |
Characteristics of ongoing studies [ordered by study ID]
Meyer 2013.
| Trial name or title | NeoVitaA Trial |
| Methods | Multicentre, double‐blind randomised controlled trial |
| Participants | ELBW neonates requiring mechanical ventilation, noninvasive ventilatory support or supplemental oxygen at 24 hours of age |
| Interventions | Postnatal high‐dose oral vitamin A supplementation (5000 IU vitamin A/kg/day versus placebo) for 28 days |
| Outcomes | The primary endpoint is the proportion of children who died before 36 weeks' gestational age or developed moderate or severe BPD. BPD is defined as the need for supplemental oxygen to maintain SaO₂ of ≥ 92% at rest at 36 weeks' postmenstrual age (PMA). Clinical secondary endpoints include the following: BPD (including mild form), intraventricular haemorrhage, periventricular leukomalacia, retinopathy of prematurity, necrotising enterocolitis, total number of days of mechanical ventilation and oxygen supplementation, and safety and tolerability of high‐dose vitamin A supplementation. |
| Starting date | |
| Contact information | |
| Notes | Meyer S, Gortner L, NeoVitaA Trial Investigators. Early Postnatal Additional High‐Dose Oral Vitamin A Supplementation versus Placebo for 28 Days for Preventing Bronchopulmonary Dysplasia or Death in Extremely Low Birth Weight Infants. Neonatology 2014;105(3):182‐8. [PubMed: 24434948] |
BPD: bronchopulmonary dysplasia ELBW: extremely low birth weight IU: international units
Differences between protocol and review
Differences in methods between protocol and review published in 1998
Addition of the outcome ‘retinopathy of prematurity’.
Dichotomous outcomes analysed via the relative risk, risk difference and number needed to treat (protocol only noted analysis using the risk difference).
Details about heterogeneity and the effect on the analysis (whether to pool and if so via fixed‐effect or random‐effects models); details of meta‐regression if substantial heterogeneity; and information about subgroup analyses by infant size, deleted from the review.
Differences in methods between review published 1998 and review published 2000
Addition of the outcome ‘nosocomial infection’.
Differences in methods between review published 2000 and review published 2007
Addition of the comparison ‘Vitamin A supplementation comparing dosage regimes’.
Addition of the outcome ‘neurodevelopmental disability’.
Differences in methods between review published 2007 and review published 2011
Addition of the outcomes ‘Patent ductus arteriosus’, ‘Necrotizing enterocolitis’, ‘Intraventricular haemorrhage’ and ‘Periventricular leukomalacia’.
Addition of searching clinical trials' registers.
Addition of 'Risk of bias' assessment of included studies using the methods of the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011).
Addition of fixed‐effect model for all meta‐analyses.
Change from mean difference to standardised mean difference.
Addition of quality of the evidence assessment using the GRADE methodology. 'Summary of findings' tables added with outcomes: death before one month, oxygen use at one month in survivors, death before 36 weeks PMA, oxygen use at 36 weeks PMA in survivors, death before 18 to 22 months, neurodevelopmental impairment at 18 to 22 months, failure of ductal closure or treatment by day 14, one or more episodes of sepsis, necrotizing enterocolitis, intraventricular haemorrhage (IVH) ‐ any IVH, retinopathy of prematurity (any grade).
Addition of subgroup analyses by route, dose, and timing of supplementation.
Addition of sensitivity analyses to assess the impact of risk of bias assessments.
Differences in methods between review published 2011 and review published 2016
Outcome changed from ‘Retinopathy of prematurity’ to ‘Retinopathy of prematurity (any, or requiring laser therapy)’.
Addition of the composite outcome ‘death or chronic lung disease at 28 days and 36 weeks postmenstrual age’ to the 'Summary of findings' table.
Deletion of the following outcomes from the 'Summary of findings' table: failure of ductal closure or treatment by day 14, one or more episodes of sepsis, necrotizing enterocolitis, intraventricular haemorrhage (IVH) ‐ any IVH, retinopathy of prematurity (any grade).
Addition of detail about how quality of the evidence would be assessed.
Differences between methods stated and methods conducted
In ‘Methods > Data collection and analysis > Assessment of risk of bias of included studies’, the assessment of detection bias (blinding of outcome assessors) has not been mentioned, but in the risk of bias assessments detection bias has been combined with performance bias.
Contributions of authors
The original review was conducted and written by Brian A Darlow and PJ Graham. The review update was facilitated by Roger Soll, Co‐ordinating Editor of Cochrane Neonatal and Maria Ximena Rojas, Associate Editor of Cochrane Neonatal and approved by the original authors.
Sources of support
Internal sources
Christchurch School of Medicine, University of Otago, New Zealand.
External sources
-
WHO, Switzerland.
A grant was provided by the WHO for an updated of this Cochrane Review.
-
Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA.
Editorial support of the Cochrane Neonatal Review Group has been funded with Federal funds from the Eunice Kennedy Shriver National Institute of Child Health and Human Development National Institutes of Health, Department of Health and Human Services, USA, under Contract No. HHSN275201100016C
Declarations of interest
None known.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
Ambalavanan 2003 {published and unpublished data}
- Ambalavanan N, Wu TJ, Tyson JE, Kennedy KA, Roane C, Carlo WA. A comparison of three vitamin A dosing regimens in extremely‐low‐birth‐weight infants. Journal of Pediatrics 2003;142(6):656‐61. [DOI] [PubMed] [Google Scholar]
Bental 1994 {published and unpublished data}
- Bental RY, Cooper PA, Cummins RR, Sandler DL, Wainer S, Rotschild A. Vitamin A therapy ‐ effects on the incidence of bronchopulmonary dysplasia. South African Journal of Food Science and Nutrition 1994;6(4):141‐5. [Google Scholar]
- Bental RY, Cooper PA, Sandler D, Wainer S. The effects of vitamin A therapy on the incidence of chronic lung disease in premature infants [abstract]. Pediatric Research 1990;25:296A. [Google Scholar]
Kiatchoosakun 2014 {published data only}
- Kiatchoosakun P, Jirapradittha J, Panthongviriyakul MC, Khampitak T, Yongvanit P, Boonsiri P. Vitamin A supplementation for prevention of bronchopulmonary dysplasia in very‐low‐birth‐weight premature Thai infants: a randomized trial. Journal of the Medical Association of Thailand 2014;97(Suppl 10):S82‐8. [PUBMED: 25816542] [PubMed] [Google Scholar]
Mactier 2012 {published data only}
- Mactier H, McCulloch DL, Hamilton R, Galloway P, Bradnam MS, Young D, et al. Vitamin A supplementation improves retinal function in infants at risk of retinopathy of prematurity. Journal of Pediatrics 2012;160(6):954‐9. [DOI] [PubMed] [Google Scholar]
Papagaroufalis 1988 {unpublished data only}
- Papagaroufalis C, Cairis M, Pantazatou E, Meghreli Ch, Xanthou M. A trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia [abstract]. Pediatric Research 1988;23:518A. [Google Scholar]
Pearson 1992 {published data only}
- Pearson E, Bose C, Snidow T, Ransom L, Young T, Bose G, et al. Trial of vitamin A supplementation in very low birth weight infants at risk for bronchopulmonary dysplasia. Journal of Pediatrics 1992;121(3):420‐7. [DOI] [PubMed] [Google Scholar]
Ravishankar 2003 {published and unpublished data}
- Nafday S, Ravishankar C, Green RS, Kamenir SA, Lorber R, Stacewicz‐Sapuntzakis M, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants [abstract]. Pediatric Research 2002;51:308A. [DOI] [PubMed] [Google Scholar]
- Ravishankar C, Nafday S, Green RS, Kamenir S, Lorber R, Stacewicz‐Sapuntzakis M, et al. A trial of vitamin A therapy to facilitate ductal closure in premature infants. Journal of Pediatrics 2003;143(5):644‐8. [DOI] [PubMed] [Google Scholar]
Shenai 1987 {published data only}
- Shenai JP, Kennedy KA, Chytil F, Stahlman MT. Clinical trial of vitamin A supplementation in infants susceptible to bronchopulmonary dysplasia. Journal of Pediatrics 1987;111(2):269‐77. [DOI] [PubMed] [Google Scholar]
Tyson 1999 {published data only}
- Ambalavanan N, Tyson JE, Kennedy KA, Hansen NI, Vohr BR, Wright LL, et al, National Institute of Child Health and Human Development Neonatal Research Network. Vitamin A supplementation for extremely low birth weight infants: outcome at 18 to 22 months. Pediatrics 2005;115(3):e249‐54. [DOI] [PubMed] [Google Scholar]
- Londhe VA, Nolen TL, Das A, Higgins RD, Tyson JE, Oh W, et al. Eunice Kennedy Shriver National Institute of Child Health and Human Development Neonatal Research Network. Vitamin A supplementation in extremely low‐birth‐weight infants: subgroup analysis in small‐for‐gestational‐age infants. American Journal of Perinatology 2013;30(9):771‐80. [DOI: 10.1055/s-0032-1333410] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyson JE, Ehrenkranz RA, Stoll BJ, Wright LL, Mele L, Kennedy KA, et al. Vitamin (Vit) A supplementation to increase survival without chronic lung disease (CLD) in extremely low birth weight (ELBW) infants: a 14‐center randomized trial [abstract]. Pediatric Research 1998;43:199A. [Google Scholar]
- Tyson JE, Wright LL, Oh W, Kennedy KA, Mele L, Ehrenkranz RA, et al. Vitamin A supplementation for extremely‐low‐birth‐weight infants. National Institute of Child Health and Human Development Neonatal Research Network. New England Journal of Medicine 1999;340(25):1962‐8. [DOI] [PubMed] [Google Scholar]
Wardle 2001 {published and unpublished data}
- Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation to prevent chronic lung disease [abstract]. Pediatric Research 1999;45:914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardle SP, Hughes A, Chen S, Shaw NJ. Randomised controlled trial of oral vitamin A supplementation in preterm infants to prevent chronic lung disease. Archives of Disease in Childhood Fetal and Neonatal Edition 2001;84(1):F9‐F13. [DOI] [PMC free article] [PubMed] [Google Scholar]
Werkman 1994 {published data only}
- Werkman SH, Peeples JM, Cooke RJ, Tolley EA, Carlson SE. Effect of vitamin A supplementation of intravenous lipids on early vitamin A intake and status of premature infants. American Journal of Clinical Nutriton 1994;59(3):586‐92. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aranda 1992 {unpublished data only}
- Aranda JV, Akramoff L, Beharry K, Ling E, Papageorgiou A. Plasma concentrations of vitamin A and E during supplemental therapy in premature infants [abstract]. Pediatric Research 1992;31:57A. [Google Scholar]
Aurvag 2007 {published data only}
- Aurvåg AK, Henriksen C, Drevon CA, Iversen PO, Nakstad B. Improved vitamin A supplementation regimen for breastfed very low birth weight infant. Acta Pædiatrica 2007;96(9):1296‐302. [DOI] [PubMed] [Google Scholar]
Coutsoudis 1996 {published data only}
- Coutsoudis A, Adhikari M, Pillay K, Coovadia HM. Absorption of high‐dose enteral vitamin A in low‐birth‐weight neonates. South African Medical Journal 1996;86(10 Suppl):1337‐9. [PubMed] [Google Scholar]
Coutsoudis 2000 {published data only}
- Coutsoudis A, Adhikari M, Pillay K, Kuhn L, Coovadia HM. Effect of vitamin A supplementation on morbidity of low‐birth‐weight neonates. South African Medical Journal 2000;90(7):730‐6. [PubMed] [Google Scholar]
Koo 1993 {unpublished data only}
- Koo W, Krug‐Wispe S, Tsang R. Effect of differential enteral vitamin A intakes in very low birth weight infants [abstract]. Pediatric Research 1993;33:306A. [Google Scholar]
- Koo WW, Krug‐Wispe S, Succop P, Tsang RC, Neylan M. Effect of different vitamin A intakes on very‐low‐birth‐weight infants. American Journal of Clinical Nutrition 1995;62(6):1216‐20. [DOI] [PubMed] [Google Scholar]
Landman 1992 {published data only}
- Landman J, Sive A, Heese HD, Van der Elst C, Sacks R. Comparison of enteral and intramuscular vitamin A supplementation in preterm infants. Early Human Development 1992;30(2):163‐70. [DOI] [PubMed] [Google Scholar]
Longardt 2014 {published data only}
- Longardt AC, Schmiedchen B, Raila J, Schweigert FJ, Obladen M, Bührer C, et al. Characterization of the vitamin A transport in preterm infants after repeated high‐dose vitamin A injections. European Journal of Clinical Nutrition 2014;68(12):1300‐4. [PUBMED: 25315494] [DOI] [PubMed] [Google Scholar]
Robbins 1993 {published data only}
- Robbins ST, Fletcher AB. Early vs delayed vitamin A supplementation in very‐low‐birth‐weight infants. Journal of Parenteral and Enteral Nutrition 1993;17(3):220‐5. [DOI] [PubMed] [Google Scholar]
Rush 1994 {published data only}
- Rush MG, Shenai JP, Parker RA, Chytil F. Intramuscular versus enteral vitamin A supplementation in very low birth weight neonates. Journal of Pediatrics 1994;125(3):458‐62. [DOI] [PubMed] [Google Scholar]
Schmiedchen 2016 {published data only}
- Schmiedchen B, Longardt AC, Loui A, Bührer C, Raila J, Schweigert FJ. Effect of vitamin A supplementation on the urinary retinol excretion in very low birth weight infants. European Journal of Pediatrics 2016;175(3):365‐72. [PUBMED: 26475348] [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
Calisici 2014 {published data only}
- Calisici E, Yarci E, Degirmencioglu H, Oncel MY, Oguz SS, Uras N, et al. PO‐0731 The effects of early oral vitamin a treatment on the prevention of bronchopulmonary dysplasia in the low birth weight infants. Archives of Disease in Childhood 2014;99(Suppl 2):A494. [DOI: 10.1136/archdischild-2014-307384.1371] [DOI] [Google Scholar]
References to ongoing studies
Meyer 2013 {published data only}
- Meyer S, Gortner L, Monz D, Maurer E, Nohr D, Biesalski HK, NeoVitaA Study Group. Vitamin A administration using nasogastric tubes. Early Human Development 2014;90(10):625‐6. [DOI] [PubMed] [Google Scholar]
- Meyer S, Gortner L, NeoVitaA Trial Investigators. Early postnatal additional high‐dose oral vitamin A supplementation versus placebo for 28 days for preventing bronchopulmonary dysplasia or death in extremely low birth weight infants. Neonatology 2014;105(3):182‐8. [PUBMED: 24434948] [DOI] [PubMed] [Google Scholar]
- Meyer S, Kronfeld K, Gräber S, Butzer R, Wahl H, Gortner L. Vitamin A to prevent bronchopulmonary dysplasia: the NeoVitaA trial. Journal of Maternal‐fetal & Neonatal Medicine 2013;26(5):544‐5. [DOI] [PubMed] [Google Scholar]
Additional references
Bates 1995
- Bates CJ. Vitamin A. Lancet 1995;345(8941):31‐5. [DOI] [PubMed] [Google Scholar]
Chabra 1994
- Chabra S, Arnold JD, Leslie GI, Bowen JR, Earl J, Wood F. Vitamin A status in preterm neonates with and without chronic lung disease. Journal of Paediatrics and Child Health 1994;30(5):432‐5. [DOI] [PubMed] [Google Scholar]
Chytil 1992
- Chytil F. The lungs and vitamin A. American Journal of Physiology 1992;262(5 Pt 1):L517‐27. [DOI] [PubMed] [Google Scholar]
Georgieff 1989
- Georgieff MK, Mammel MC, Mills MM, Gunter EW, Johnson DE, Thompson TR. Effect of postnatal steroid administration on serum vitamin A concentrations in newborn infants with respiratory compromise. Journal of Pediatrics 1989;114(2):301‐4. [DOI] [PubMed] [Google Scholar]
GRADEpro‐GDT 2013 [Computer program]
- Grading of Recommendations Assessment, Development and Evaluation (GRADE) Working Group. GRADEpro‐GDT. GRADE working group, 2013.
Greene 1987
- Greene HL, Phillips BL, Franck L, Fillmore CM, Said HM, Murrell JE, et al. Persistently low retinol levels during and after parenteral feeding of very low birthweight infants; examination of losses into intravenous administration sets and a method of prevention by addition to a lipid emulsion. Pediatrics 1987;79(6):894‐900. [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org,.
Huiming 2005
- Huiming Y, Chaomin W, Meng M. Vitamin A for treating measles in children. Cochrane Database of Systematic Reviews 2005, Issue 4. [DOI: 10.1002/14651858.CD001479.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
Humphrey 1996
- Humphrey JH, Agoestina T, Wu L, Usman A, Nurachim M, Subardja D, et al. Impact of neonatal vitamin A supplementation on infant morbidity and mortality. Journal of Pediatrics 1996;128(4):489‐96. [DOI] [PubMed] [Google Scholar]
Hustead 1984
- Hustead VA, Gutcher GR, Anderson SA, Zachman RD. Relationship of vitamin A (retinol) status to lung disease in the preterm infant. Journal of Pediatrics 1984;104(4):610‐5. [DOI] [PubMed] [Google Scholar]
Kennedy 1997
- Kennedy KA, Stoll BJ, Ehrenkranz RA, Oh W, Wright LL, Stevenson DK, et al. Vitamin A to prevent bronchopulmonary dysplasia in very‐low‐birth‐weight infants: has the dose been too low? The NICHD Neonatal Research Network. Early Human Development 1997;49(1):19‐31. [DOI] [PubMed] [Google Scholar]
Lorch 1994
- Lorch V, Gaylord MS. Vitamin A and bronchopulmonary dysplasia. Journal of Pediatrics 1994;124(2):328‐9. [DOI] [PubMed] [Google Scholar]
Mactier 2005
- Mactier H, Weaver LT. Vitamin A and preterm infants: what we know, what we don't know, and what we need to know. Archives of Disease in Childhood Fetal and Neonatal Edition 2005;90(2):F103‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
Papile 1978
- Papile LA, Burstein J, Burstein R, Koffler H. Incidence and evolution of subependymal and intraventricular hemorrhage: a study of infants with birth weights less than 1,500 gm. Journal of Pediatrics 1978;92(4):529‐34. [DOI] [PubMed] [Google Scholar]
Review Manager 5 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2008.
Schünemann 2013
- Schünemann H, Brożek J, Guyatt G, Oxman A, editors. The GRADE Working Group. GRADE handbook for grading quality of evidence and strength of recommendations [Updated October 2013]. Available from www.guidelinedevelopment.org/handbook.
Shenai 1985
- Shenai JP, Chytil F, Stahlman MT. Vitamin A status of neonates with bronchopulmonary dysplasia. Pediatric Research 1985;19(2):185‐8. [DOI] [PubMed] [Google Scholar]
Shenai 1990
- Shenai JP, Rush MG, Stahlman MT, Chytil F. Plasma retinol‐binding protein response to vitamin A administration in infants susceptible to bronchopulmonary dysplasia. Journal of Pediatrics 1990;116(4):607‐14. [DOI] [PubMed] [Google Scholar]
Shenai 1993
- Shenai JP. Vitamin A. In: Tsang RC, Lucas A, Uauy R, Zlotkin S editor(s). Nutritional Needs of the Preterm Infant: Scientific Basis and Practical Guidelines. Baltimore: Williams and Wilkins, 1993:87‐100. [Google Scholar]
Spears 2004
- Spears K, Cheney C, Zerzan J. Low plasma retinol concentrations increase the risk of developing bronchopulmonary dysplasia and long‐term respiratory disability in very‐low‐birth‐weight infants. American Journal of Clinical Nutrition 2004;80(6):1589‐94. [DOI] [PubMed] [Google Scholar]
Zachman 1996
- Zachman RD, Samuels DP, Brand JM, Winston JF, Pi JT. Use of the intramuscular relative‐dose‐response test to predict bronchopulmonary dysplasia in premature infants. American Journal of Clinical Nutrition 1996;63(1):123‐9. [DOI] [PubMed] [Google Scholar]
References to other published versions of this review
Darlow 1998
- Darlow BA, Graham PJ. Vitamin A supplementation in very low birthweight infants. Cochrane Database of Systematic Reviews 1998, Issue 4. [DOI: 10.1002/14651858.CD000501] [DOI] [PubMed] [Google Scholar]
Darlow 2000
- Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database of Systematic Reviews 2000, Issue 2. [DOI: 10.1002/14651858.CD000501] [DOI] [PubMed] [Google Scholar]
Darlow 2002
- Darlow BA, Graham PJ. Vitamin A supplementation for preventing morbidity and mortality in very low birthweight infants. Cochrane Database of Systematic Reviews 2002, Issue 4. [DOI: 10.1002/14651858.CD000501] [DOI] [PubMed] [Google Scholar]
Darlow 2007
- Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short and long‐term morbidity in very low birthweight infants. Cochrane Database of Systematic Reviews 2007, Issue 4. [DOI: 10.1002/14651858.CD000501.pub2] [DOI] [PubMed] [Google Scholar]
Darlow 2011
- Darlow BA, Graham PJ. Vitamin A supplementation to prevent mortality and short‐ and long‐term morbidity in very low birthweight infants. Cochrane Database of Systematic Reviews 2011, Issue 10. [DOI: 10.1002/14651858.CD000501.pub3] [DOI] [PubMed] [Google Scholar]


