Abstract
Background and Objectives:
The management of benign biliary stricture in patients with altered anatomy secondary to surgery is challenging. Percutaneous transhepatic biliary drainage (BD) represents the standard therapy for benign biliary stricture, but it is associated with nontrivial morbidity rates. Despite the increasing application of guided BD (EUS-BD) for the management of malignant obstruction, its role in patients with benign biliary stricture is limited. This retrospective study aimed to evaluate the feasibility, safety, and clinical effectiveness of EUS-BD with multiple transanastomotic plastic stent treatment in patients with benign biliary stricture.
Materials and Methods:
This study included consecutive patients who underwent EUS-BD for benign biliary stenosis at our center. EUS-BD with fully covered self-expandable metal stent placement was performed first. When feasible, the stricture was treated by balloon dilation with the placement of a transanastomotic double-pigtail plastic stent. Scheduled procedures were repeated to remove the metal stent and replace the plastic stent to treat the stenosis. Technical success and adverse events (AEs) were assessed.
Results:
Twelve patients underwent EUS-BD for benign biliary strictures. Procedural and clinical successes were achieved in all patients (100%). Multistenting treatment was performed in 10/12 patients (77%). The median number of stents inserted, maximum number of stents placed, and median time of retreatment were 2.4 (range: 1–4), 4, and 3.4 (range: 1–7), respectively. In total, 4/12 patients (33.3%) developed AEs that required endoscopic interventions (Clavien-Dindo Grade III).
Conclusions:
EUS-BD with the placement of multiple trans-stenosis plastic stents is a safe, feasible, and well-tolerated alternative for the management of benign biliary stricture in patients with surgery-altered anatomy. Long-term follow-up is necessary to support our results.
Keywords: Altered anatomy, benign biliary stricture, EUS-guided biliary drainage
INTRODUCTION
ERCP with balloon dilation, followed by placement of multiple plastic stents or a fully covered self-expandable metal stent (FC-SEMS), is widely considered the first-line treatment for benign biliary strictures.[1,2] However, ERCP may not be feasible in patients with surgery-altered anatomy, such as hepaticojejunal anastomosis after Billroth II reconstruction, Whipple procedure, Roux-en-Y limb, or Roux-en-Y gastric bypass, or in cases in which the papilla is not accessible due to severe duodenal inflammatory stricture. In all these cases, percutaneous transhepatic biliary drainage (PTBD) with balloon dilation and internal–external drainage placement represents the standard therapy. However, PTBD is associated with 2%–14% morbidity,[3,4] poor long-term outcomes, and recurrence of stenosis of up to 34% at 3 years.[5] Redo surgery is a rescue treatment, resulting in the highest long-term stricture resolution rate at 84%,[6] but this treatment is associated with 9%–67% morbidity and up to 3% mortality in the postoperative period.[7,8]
Recently, EUS-guided biliary drainage (EUS-BD) has been increasingly utilized as an alternative to PTBD for the treatment of malignant biliary strictures.[9,10] Drainage under EUS guidance can be achieved from the stomach to the left intrahepatic ducts (hepaticogastrostomy [HG]) or from the duodenal bulb to the common bile duct (CBD) (choledochoduodenostomy). Notably, HG has been proposed in cases of hilar strictures, in the presence of altered anatomy, or in cases of inaccessibility of the papilla.[11] A recent systemic review with meta-analysis comparing EUS-BD and PTBD in 483 patients with malignant biliary obstruction from nine studies[12] found that, despite similar technical success rates, EUS-BD was associated with better clinical success, fewer adverse events (AEs), and a lower reintervention rate and that this procedure was overall more cost-effective compared to PTBD. More recently, EUS-BD has even been proposed as a first-line therapy in cases of malignant biliary stenosis.[13]
Despite increasing evidence of the safety of EUS-BD for the management of malignant biliary obstruction, its utilization in patients with benign strictures and more generally benign biliary diseases is still limited.[14,15,16,17,18,19] Thus, the aim of this study was to retrospectively evaluate the feasibility, safety, and clinical effectiveness of EUS-BD with multiple transanastomotic plastic stent placement for the management of benign biliary strictures in patients with surgery-altered anatomy.
MATERIALS AND METHODS
All consecutive patients who underwent EUS-BD for benign biliary strictures at our institution between May 2016 and August 2018 were retrospectively retrieved from a prospectively collected database. The study was not registered as a clinical trial and was not considered for institutional review board approval. Indication for EUS-BD was the presence of benign biliary stricture in which ERCP was impossible due to surgery-altered anatomy. Since 2016, at our institution, EUS-BD has been considered the first-line or second-line treatment after failure of a previous PTBD at the discretion of the operators.
The primary outcome was procedural success defined as EUS-guided FC-SEMS placement from the left intrahepatic duct into the stomach or the jejunum. The secondary outcome was the ability to treat the biliary stenosis through repeated sessions of multiple plastic stents placement. Clinical effectiveness and AEs were also determined according to the Clavien-Dindo classification of surgical complications.[20] Each patient provided informed consent before undertaking the procedure. General anesthesia was inducted after endotracheal intubation. Procedures were performed with patients in the supine position under fluoroscopic control.
Two stages of surgical treatment were performed. The first procedure was EUS-BD with placement of a FC-SEMS, whose distal tip was placed in the left intrahepatic biliary tree and the proximal tip in the gastrointestinal (GI) tract. A linear-array therapeutic echoendoscope (EG38UTK, Pentax, Tokyo, Japan) was passed into the stomach or the jejunum to visualize the left lobe of the liver. Once intrahepatic biliary dilation was identified, a 19G access needle (EchoTip Ultra 19-A, Cook Medical, Bloomington, Indiana, USA) or a standard 19G needle (EchoTip Ultra 19, Cook Medical, Bloomington, Indiana, USA) was used to puncture the selected intrahepatic duct. Contrast injection confirmed the correct position of the needle inside the duct. After flushing the needle with 2 cc saline solution, a 0.035-inch 450-cm long guidewire (Acrobat 2, Cook Medical, Bloomington, Indiana, USA, or Jagwire, Boston Scientific, Natick, Massachusetts, USA) was pushed into the biliary tree through the needle. Diathermic fistulation of the access was performed using a 6-Fr cystostome (Endoflex Company, Voerde, Germany). An 80 mm × 10 mm FC-SEMS (Evolution, Cook Medical, Bloomington, Indiana, USA) was released between the intrahepatic biliary tract and the gastric or jejunal lumen. If the guidewire can easily be passed through the anastomosed site, before FC-SEMS placement, the stricture was dilated using an 8-mm balloon (Hurricane, Boston Scientific, Natick, Massachusetts, USA), and a transanastomotic double-pigtail plastic stent (Compass, Cook Medical, Bloomington, Indiana, USA) was inserted inside the FC-SEMS between the gastric or jejunal lumen and the duodenum. Otherwise, a double-pigtail plastic stent was left inside the FC-SEMS with the distal tip placed in the biliary duct to reduce the risk of FC-SEMS migration [Figure 1].
Figure 1.
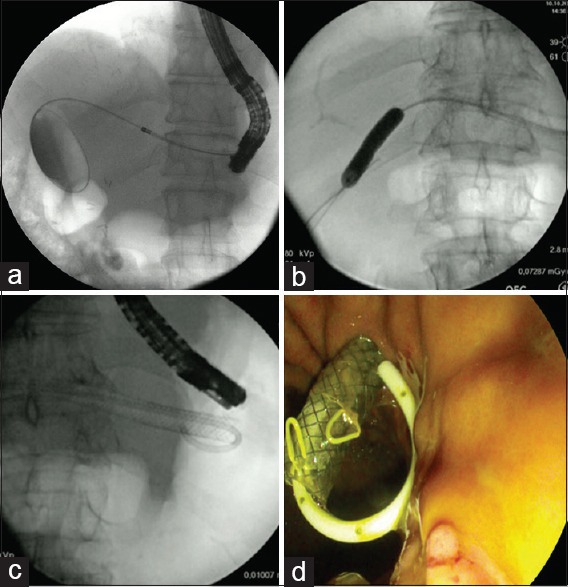
(a) Fluoroscopic image of the 6-Fr cystostome (Endoflex Company, Germany) making the diathermic fistula between the intrahepatic biliary tract and the gastric lumen, (b) 8-mm balloon dilation of the anastomotic stricture, (c) fully covered self-expandable metal stent placement with a double-pigtail plastic stent inside, (d) endoscopic image at the end of the procedure
After approximately 1 month, a second procedure was performed to remove the FC-SEMS, treat the stenosis by balloon dilation, and place one or more double-pigtail plastic stents through the anastomotic stricture. Endoscopic reevaluation was carried out every 3–6 months to perform plastic stent removal, cholangiography, balloon dilation, and stent placement.
All the values were expressed in means or medians. Given the small sample size, no statistical analysis was required.
RESULTS
Patient characteristics
A total of 12 patients (6 men and 6 women; median age: 63 [range: 35–85] years) underwent EUS-BD for benign biliary strictures in surgically altered anatomy between May 2016 and September 2018 at our tertiary referral center. All patients were admitted for acute cholangitis. In 11 cases, patients had stenosis of the hepaticojejunal anastomosis, while one patient had stenosis of the distal CBD after Roux-Y limb total gastrectomy. Eight patients (66.6%) underwent surgery for malignant diseases as follows: five Whipple surgeries for pancreatic adenocarcinoma (n = 2), cholangiocarcinoma (n = 1), intraductal papillary mucinous neoplasm (n = 1), and pancreatic neuroendocrine tumor (n = 1); one gastrectomy for gastric adenocarcinoma; one right hepatectomy for colorectal metastasis; and one surgery for gallbladder adenocarcinoma with a consequent rupture of the right branch of hepatic artery from a biloma. The other four patients (33.3%) underwent surgery for benign disease (leakage of the CBD during cholecystectomy for gallbladder lithiasis). The patient characteristics are reported in Table 1.
Table 1.
Patient characteristics
| Patient | Age | Sex | Indication for drainage | Previous PTBD | Indication for previous surgery |
|---|---|---|---|---|---|
| 1 | 74 | Female | Acute cholangitis | Yes | Malignant |
| 2 | 53 | Male | Acute cholangitis | No | Malignant |
| 3 | 78 | Female | Acute cholangitis | No | Malignant |
| 4 | 70 | Female | Acute cholangitis | Yes | Benign |
| 5 | 61 | Male | Acute cholangitis | Yes | Malignant |
| 6 | 60 | Female | Acute cholangitis | No | Benign |
| 7 | 85 | Male | Acute cholangitis | No | Benign |
| 8 | 72 | Female | Acute cholangitis | No | Malignant |
| 9 | 62 | Male | Acute cholangitis | Yes | Malignant |
| 10 | 35 | Female | Acute cholangitis | No | Malignant |
| 11 | 54 | Male | Acute cholangitis | No | Benign |
| 12 | 57 | Male | Acute cholangitis | No | Malignant |
PTBD: Percutaneous biliary drainage
First procedure
All the 12 patients (100%) achieved procedural and clinical success. Four patients (33.3%) underwent EUS-HG for stenosis relapse after a previous treatment with PTBD. In the other eight patients (66.7%), EUS drainage was chosen as the first-line treatment. Eleven patients underwent HG, and the patient with Roux-Y limb underwent hepaticojejunostomy (HJS). In six patients (50%), the guidewire has passed through the stenotic anastomosis during the first procedure, and after 8-mm balloon dilation of the stricture and FC-SEMS placement, a transanastomotic double-pigtail plastic stent was inserted. In the other six patients (50%), crossing the stenosis with the guidewire was not possible during the first procedure, and a double-pigtail plastic stent was left in place inside the FC-SEMS [Table 2].
Table 2.
Treatment details
| Patient | EUS-BD | Multistenting treatment | Maximum number of plastic stent | Number of treatment | Treatment length (months) | Treatment end | AEs | ||
|---|---|---|---|---|---|---|---|---|---|
| Type | FC-SEMS Type size (mm×mm) | Plastic stent Type size (cm×Fr) | |||||||
| 1 | HG | Evolution Cook 80×10 | Cook Compass 11×7 | Yes | 3 | 8 | 24 | Ongoing | Intrahepatic FC-SEMS migration |
| 2 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 2 | 6 | 12 | Yes | No |
| 3 | HG | Evolution Cook 80×10 | Cook Compass 10×8.5 | No | - | - | - | - | Intrahepatic FC-SEMS migration |
| 4 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 2 | 5 | 28 | Ongoing | No |
| 5 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 1 | 3 | 13 | Ongoing | No |
| 6 | HG | Evolution Cook 80×10 | Cook Compass 10×8.5 | No | - | - | - | - | No |
| 7 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 4 | 7 | 14 | Ongoing | No |
| 8 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 3 | 4 | 14 | Ongoing | No |
| 9 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 2 | 3 | 14 | Ongoing | Gastric bleeding after FC-SEMS placement |
| 10 | HG | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 3 | 3 | 5 | Ongoing | No |
| 11 | HG | Evolution Cook 80×10 | Cook Compass 15×7 | Yes | 2 | 2 | 2 | Ongoing | Cholangitis |
| 12 | HJ | Evolution Cook 80×10 | Cook Compass 10×7 | Yes | 2 | 2 | 2 | Ongoing | No |
AEs: Adverse events, BD: Biliary drainage, FC-SEMS: Fully covered self-expandable metallic stent, HG: Hepaticogastrostomy, HJ: Hepaticojejunostomy
Second procedure
All 12 patients underwent a second procedure with FC-SEMS removal after a median period of 42 (range: 29–90) days. During the second treatment in 11 cases (92%), the stenotic anastomosis was crossed, and after 8-mm balloon dilatation, a median of two double-pigtail plastic stents (range: 1–3) was inserted to treat the stenosis. In one case, we were unable to pass the guidewire through a severe anastomotic stricture, so we performed cholangioscopy pushing forward the new digital cholangioscope (SpyGlass DS Direct Visualization System; Boston Scientific, Natick, Massachusetts, USA) through the HG in the CBD and crossing the stenosis with the guidewire under direct vision. In this specific case, we also performed biopsies of the stricture using SpyBite Biopsy Forceps (Boston Scientific, Natick, Massachusetts, USA) to exclude malignancy.
Follow-up
Multistenting treatment of the anastomotic biliary stricture was performed in 10/12 patients (77%). In only one case (8%), we failed to pass the guidewire through the stenosis, and after 2 attempts, the patient was excluded from the EUS-BD treatment and underwent percutaneous drainage. Another patient, who presented with rupture of the right branch of the hepatic artery, developed ischemic stenosis of the right and left intrahepatic biliary ducts after 3 months and required percutaneous drainage of the right biliary tree; this case was therefore excluded from the study.
Of the ten patients who continued the treatment, nine (75%) are still undergoing treatment with a median period of 12.8 (range: 2–28) months, while one patient completed the treatment after 12 months, and this patient is asymptomatic after 17 months. The median number of stents inserted, maximum number of stents placed, and median time of retreatment were 2.4 (range: 1–4), 4, and 3.4 (range: 1–7) months, respectively.
Adverse events
In total, 4/12 patients (33.3%) developed complications after the procedure that required endoscopic interventions (Clavien-Dindo Grade III).[19] One patient developed relapse of cholangitis at 2 days after the first treatment and subsequently underwent computed tomography and a second procedure to replace the 10 mm × 80 mm FC-SEMS with a 10 mm × 60 mm FC-SEMS, which immediately resolved the symptoms. One patient had gastric bleeding after the first procedure at the entry site of the FC-SEMS, which was treated by coagulation and placement of two endoclips. Two patients had recurrent cholangitis at 5 and 22 days after the EUS-HG procedure due to intrahepatic migration of the SEMS. In the first patient, a 10 mm × 40 mm FC-SEMS was placed in the site of the previous FC-SEMS with a transanastomotic double-pigtail plastic stent. In the second patient, two transanastomotic double-pigtail plastic stents were placed after the metallic stent was removed.
DISCUSSION
This retrospective study evaluated the feasibility, safety, and clinical effectiveness of using endoscopic HG or HJS to treat benign stenosis of the CBD in patients with surgery-altered anatomy for which, as a consequence, ERCP is not an option. In these patients, two critical points are the main focus, i.e., the ability to drain the biliary duct and to treat the biliary stricture, which is usually caused by stenosis of the hepaticojejunal anastomosis in cases of altered anatomy.
For patients with altered anatomy and benign indication, PTBD is a well-known alternative with good technical results (75%–100%). Currently, the percutaneous approach consists of 6–12-mm balloon dilatation followed by placement of a large-bore catheter (12–18 Fr) for extended periods. However, this treatment requires multiple procedures, and the prolonged use of indwelling percutaneous catheters that cause catheter-related complications, patient discomfort, and reduction in the quality of life[21,22,23] makes this option less desirable. Moreover, the recurrence rate varied from 15% to 44%.[5] In cases with benign strictures, the use of permanent metal stents is not recommended, while fully-covered (FC) stents have been used with a 97%–100% technical success rate.[24,25] Even in the case of metal stent placement, drainage catheters are still required for prolonged periods, but there is a risk of stent migration, and retrieval procedures may be technically challenging.[26] Recently, Yun et al.[27] compared balloon dilation with the percutaneous placement of a temporarily covered stent designed for spontaneous migration and demonstrated a lower rate of recurrent strictures for the stent group (54.5% vs. 13.0%) and better 1- and 3-year primary patency rates (90.2% and 84.9% for the stent group and 75.1% and 52.8% for the balloon group).
Moreover, to obtain transpapillary BD, enteroscopy-assisted ERCP can be performed, with an overall technical success rate of approximately 76% (range: 58%–100%).[28,29,30] Nevertheless, this technique is time-consuming, and currently, there are reduced toolsets for therapeutic intervention. Recently, in a few case series, endoscopic enteral-enteral bypasses have been proposed to reach the papilla of Vater using the duodenoscope after the creation of an EUS-guided enteral anastomosis with the placement of a lumen-apposing metal stent.[31,32]
Despite the increasing application of EUS-BD for malignant biliary obstruction, data on EUS-BD for benign biliary strictures are limited.[15,16,17,18,19] Miranda-García et al.[15] described EUS-BD in seven patients using a two-step approach, i.e., EUS-HG followed by antegrade stricture dilation with or without antegrade stent placement. Technical success in passing the anastomotic stricture with balloon dilation and transanastomotic plastic stent placement was achieved in 4/7 patients, with a high rate of stent migration (57%), while clinical success was obtained in all patients (100%) as a consequence of the presence of HG. Recently, James et al. retrospective studied 20 patients with altered anatomy and benign indications who underwent EUS-BD.[16] Of these patients, 11 experienced biliary stenosis, and the technical success rate was 100%. In their series, the treatment of the stricture was not systematically reported.
In our study, we focused on two different points as follows: the ability to perform the EUS-BD with FC-SEMS placement and the capability to treat the biliary stricture with trans-stenosis plastic stenting. The first step is the management of acute cholangitis, overcoming the obstruction by creating a connection between the left intrahepatic tree and the GI tract. We used FC-SEMS to avoid biliary leakage and facilitate easy stent removal after 1 month. However, a drawback of using FC stent is the risk of intrahepatic migration, with consequent need for an urgent endoscopy to remove the stent. We experienced stent migration in two cases after 5 and 22 days. We did not remove the stent in the first case because 5 days is still too early, and we only added a 4-cm FC-SEMS to the distal part; in the second case, we replaced the stent with two double-pigtail plastic stents.
The second step consists of passing the guidewire through the stricture by balloon dilation and double-pigtail plastic stent placement. If the stenosis was easily passable, then we performed this step in the first procedure; otherwise, we tried this step during the second procedure. We considered treating the stricture as mandatory for the treatment.
Overall, the creation of EUS-BD was possible in all patients, without any periprocedural AEs, and acute cholangitis was resolved in all patients. The second task was performed in 11/12 patients, of which the stenosis was treated during the first procedure in six cases. We failed to pass the guidewire through the stricture in only one case, and we interrupted the treatment after two attempts. In one case, we performed cholangioscopy to pass through the biliary stenosis. The use of the digital cholangioscope (SpyGlass DS Direct Visualization System; Boston Scientific Natick, Massachusetts, USA) has been reported for the management of difficult biliary stenosis during EUS-guided hepaticoenterostomy.[17,33] Direct visualization can facilitate manipulation of the guidewire through the stricture, reducing the risk of failure. In case of severe stricture, we suggest performing cholangioscopy with SpyGlass that can even allow direct collection of biopsies and exclude, in selected cases, malignancy.
In this study, patients underwent regular therapy sessions every 3–6 months, during which we removed the transanastomotic plastic stents. We dilated the stricture and placed at least the same number or, when possible, an increasing number of trans-stenotic double-pigtail plastic stents.
Of the ten patients who continued the treatment after the first session, nine are still undergoing treatment. Only one patient had completed the therapy, with stent removal after 12 months, and this patient is still asymptomatic after 17 months of follow-up. Of the nine patients under treatment, seven had a treatment period shorter than 15 months and only two had a treatment period of more than 15 months (24 and 28 months). We did not remove the stents from patients with more than 12 months of treatment because they refused the procedure for fear of stricture recurrence. Moreover, the two patients with the longest treatment periods had experienced failed percutaneous drainage before the EUS-BD. In these cases, failed EUS-BD would mean that surgery is the last option. These data suggest that patients well tolerated EUS-BD multistenting treatment; unlike internal–external drainage which does not require patient care, the procedure is not painful and prevents inadvertent dislodgement of the stent which negatively affects the quality of life.
In our series, 8/12 patients had benign anastomotic strictures after surgery for malignant disease. In patients in whom we could not exclude the possibility of tumor relapse, EUS-HG has been a good and well-tolerated treatment.
Compared to malignant biliary obstruction, which is characterized by an important dilation of intrahepatic biliary ducts, benign strictures typically present with slight dilation of the intrahepatic branches, making the EUS approach more difficult. Given the complexity of the procedure, EUS-HG is performed only by expert endoscopists in the referral center.
Mukai et al. published a study of 37 cases of EUS-BD for benign biliary obstruction, of which 21 cases were anastomotic strictures.[17] The overall technical success of the creation of the hepatoenteric tract by EUS was 91.9%, with a clinical success rate of 91.9%. For the creation of the hepatoenteric fistula, a single-pigtail plastic stent dedicated for EUS-HG or EUS-HJS (8F diameter, 20 cm long; Gadelius Medical Co., Ltd., Tokyo, Japan) was used in 31 cases, and a partially covered SEMS (PC-SEMS) (8 mm or 10 mm diameter, 10 cm or 12 cm long; Taewoong Medical Co., Seoul, Korea) was used in the remaining three cases. The use of a dedicated EUS-HG or EUS-HJS plastic stent shaped to reduce the risk of biliary leakage and migration could become an alternative to the conventional FC-SEMS in a selective patient.[34] On the contrary, as PC-SEMS removal after 1 month is extremely challenging, we do not suggest the use of these stents in case of benign biliary obstruction, and a FC-SEMS is preferred even if it is associated with a higher risk of AEs. Placement of a double-pigtail plastic stent inside a FC-SEMS can decrease the risk of migration or maintain the hepato-gastric fistula in case of metallic stent displacement, making a second procedure easier. We experienced two cases of intrahepatic stent migration managed successfully by endoscopic procedure. Accurate fluoroscopic and endoscopic control of FC-SEMS position during stent release and leaving at least 2 cm of the proximal tip of the metallic stent in the stomach may avoid the risk of obstruction of intrahepatic biliary branches and reduce the risk of intrahepatic migration.
Our series has several limitations. First, we presented preliminary data, and as most of the patients are still undergoing treatment, we do not have information about stenosis relapse or long-term outcomes. Second, we did not establish a standardized treatment length, and we included patients that were still under treatment after more than 12 months. Moreover, our sample was small, and different types of benign strictures were included.
CONCLUSIONS
EUS-HG with placement of multiple transanastomotic plastic stents in patients with surgery-altered anatomy could become a safe, feasible, and well-tolerated alternative for the management of benign biliary stricture. Moreover, the use of digital SpyGlass cholangioscope in case of difficult biliary stenosis during EUS-HG could reduce the risk of failure in passing the stricture. However, long-term follow-up is necessary to provide stronger support for our results.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Costamagna G, Boškoski I. Current treatment of benign biliary strictures. Ann Gastroenterol. 2013;26:37–40. [PMC free article] [PubMed] [Google Scholar]
- 2.Khan MA, Baron TH, Kamal F, et al. Efficacy of self-expandable metal stents in management of benign biliary strictures and comparison with multiple plastic stents: A meta-analysis. Endoscopy. 2017;49:682–94. doi: 10.1055/s-0043-109865. [DOI] [PubMed] [Google Scholar]
- 3.Kühn JP, Busemann A, Lerch MM, et al. Percutaneous biliary drainage in patients with nondilated intrahepatic bile ducts compared with patients with dilated intrahepatic bile ducts. AJR Am J Roentgenol. 2010;195:851–7. doi: 10.2214/AJR.09.3461. [DOI] [PubMed] [Google Scholar]
- 4.Kucukay F, Okten RS, Yurdakul M, et al. Long-term results of percutaneous biliary balloon dilation treatment for benign hepaticojejunostomy strictures: Are repeated balloon dilations necessary? J Vasc Interv Radiol. 2012;23:1347–55. doi: 10.1016/j.jvir.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 5.Gwon DI, Ko GY, Ko HK, et al. Percutaneous transhepatic treatment using retrievable covered stents in patients with benign biliary strictures: Mid-term outcomes in 68 patients. Dig Dis Sci. 2013;58:3270–9. doi: 10.1007/s10620-013-2784-9. [DOI] [PubMed] [Google Scholar]
- 6.Huszár O, Kokas B, Mátrai P, et al. Meta-analysis of the long term success rate of different interventions in benign biliary strictures. PLoS One. 2017;12:e0169618. doi: 10.1371/journal.pone.0169618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Luu C, Lee B, Stabile BE. Choledochoduodenostomy as the biliary-enteric bypass of choice for benign and malignant distal common bile duct strictures. Am Surg. 2013;79:1054–7. [PubMed] [Google Scholar]
- 8.Khajanchee YS, Cassera MA, Hammill CW, et al. Outcomes following laparoscopic choledochoduodenostomy in the management of benign biliary obstruction. J Gastrointest Surg. 2012;16:801–5. doi: 10.1007/s11605-011-1768-3. [DOI] [PubMed] [Google Scholar]
- 9.Rimbaş M, Larghi A, Kunda R. EUS-guided biliary drainage: Is it ready for prime time? Endosc Ultrasound. 2017;6:S122–6. doi: 10.4103/eus.eus_78_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Boulay BR, Lo SK. Endoscopic ultrasound-guided biliary drainage. Gastrointest Endosc Clin N Am. 2018;28:171–85. doi: 10.1016/j.giec.2017.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Uemura RS, Khan MA, Otoch JP, et al. EUS-guided choledochoduodenostomy versus hepaticogastrostomy: A systematic review and meta-analysis. J Clin Gastroenterol. 2018;52:123–30. doi: 10.1097/MCG.0000000000000948. [DOI] [PubMed] [Google Scholar]
- 12.Sharaiha RZ, Khan MA, Kamal F, et al. Efficacy and safety of EUS-guided biliary drainage in comparison with percutaneous biliary drainage when ERCP fails: A systematic review and meta-analysis. Gastrointest Endosc. 2017;85:904–14. doi: 10.1016/j.gie.2016.12.023. [DOI] [PubMed] [Google Scholar]
- 13.Paik WH, Lee TH, Park DH, et al. EUS-guided biliary drainage versus ERCP for the primary palliation of malignant biliary obstruction: A multicenter randomized clinical trial. Am J Gastroenterol. 2018;113:987–97. doi: 10.1038/s41395-018-0122-8. [DOI] [PubMed] [Google Scholar]
- 14.Ogura T, Masuda D, Takeuchi T, et al. Visualization and removal of intrahepatic bile duct stones through EUS-guided hepaticogastrostomy. Gastrointest Endosc. 2016;84:531–2. doi: 10.1016/j.gie.2016.03.1475. [DOI] [PubMed] [Google Scholar]
- 15.Miranda-García P, Gonzalez JM, Tellechea JI, et al. EUS hepaticogastrostomy for bilioenteric anastomotic strictures: A permanent access for repeated ambulatory dilations? Results from a pilot study. Endosc Int Open. 2016;4:E461–5. doi: 10.1055/s-0042-103241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.James TW, Fan YC, Baron TH. EUS-guided hepaticoenterostomy as a portal to allow definitive antegrade treatment of benign biliary diseases in patients with surgically altered anatomy. Gastrointest Endosc. 2018;88:547–54. doi: 10.1016/j.gie.2018.04.2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukai S, Itoi T, Sofuni A, et al. EUS-guided antegrade intervention for benign biliary diseases in patients with surgically altered anatomy (with videos) Gastrointest Endosc. 2019;89:399–407. doi: 10.1016/j.gie.2018.07.030. [DOI] [PubMed] [Google Scholar]
- 18.Artifon EL, Safatle-Ribeiro AV, Ferreira FC, et al. EUS-guided antegrade transhepatic placement of a self-expandable metal stent in hepatico-jejunal anastomosis. JOP. 2011;12:610–3. [PubMed] [Google Scholar]
- 19.Park DH, Jang JW, Lee SS, et al. EUS-guided transhepatic antegrade balloon dilation for benign bilioenteric anastomotic strictures in a patient with hepaticojejunostomy. Gastrointest Endosc. 2012;75:692–3. doi: 10.1016/j.gie.2011.04.013. [DOI] [PubMed] [Google Scholar]
- 20.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: A new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–13. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nennstiel S, Weber A, Frick G, et al. Drainage-related complications in percutaneous transhepatic biliary drainage: An analysis over 10 years. J Clin Gastroenterol. 2015;49:764–70. doi: 10.1097/MCG.0000000000000275. [DOI] [PubMed] [Google Scholar]
- 22.Sharaiha RZ, Kumta NA, Desai AP, et al. Endoscopic ultrasound-guided biliary drainage versus percutaneous transhepatic biliary drainage: Predictors of successful outcome in patients who fail endoscopic retrograde cholangiopancreatography. Surg Endosc. 2016;30:5500–5. doi: 10.1007/s00464-016-4913-y. [DOI] [PubMed] [Google Scholar]
- 23.Oh HC, Lee SK, Lee TY, et al. Analysis of percutaneous transhepatic cholangioscopy-related complications and the risk factors for those complications. Endoscopy. 2007;39:731–6. doi: 10.1055/s-2007-966577. [DOI] [PubMed] [Google Scholar]
- 24.Gwon DI, Shim HJ, Kwak BK. Retrievable biliary stent-graft in the treatment of benign biliary strictures. J Vasc Interv Radiol. 2008;19:1328–35. doi: 10.1016/j.jvir.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 25.Kim J, Ko GY, Sung KB, et al. Percutaneously placed covered retrievable stents for the treatment of biliary anastomotic strictures following living donor liver transplantation. Liver Transpl. 2010;16:1410–20. doi: 10.1002/lt.22173. [DOI] [PubMed] [Google Scholar]
- 26.Kim JH, Gwon DI, Ko GY, et al. Temporary placement of retrievable fully covered metallic stents versus percutaneous balloon dilation in the treatment of benign biliary strictures. J Vasc Interv Radiol. 2011;22:893–9. doi: 10.1016/j.jvir.2011.02.009. [DOI] [PubMed] [Google Scholar]
- 27.Yun G, Yoon CJ, Seong NJ. Percutaneous treatment of benign bilioenteric anastomotic strictures: Temporary covered stent placement versus balloon dilatation. Eur Radiol. 2019;5):2690–97. doi: 10.1007/s00330-018-5776-5. [DOI] [PubMed] [Google Scholar]
- 28.Wang F, Xu B, Li Q, et al. Endoscopic retrograde cholangiopancreatography in patients with surgically altered anatomy: One single center's experience. Medicine (Baltimore) 2016;95:e5743. doi: 10.1097/MD.0000000000005743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mönkemüller K, Fry LC, Bellutti M, et al. ERCP with the double balloon enteroscope in patients with roux-en-Y anastomosis. Surg Endosc. 2009;23:1961–7. doi: 10.1007/s00464-008-0239-8. [DOI] [PubMed] [Google Scholar]
- 30.Itokawa F, Itoi T, Ishii K, et al. Single – And double-balloon enteroscopy-assisted endoscopic retrograde cholangiopancreatography in patients with roux-en-Y plus hepaticojejunostomy anastomosis and Whipple resection. Dig Endosc. 2014;26(Suppl 2):136–43. doi: 10.1111/den.12254. [DOI] [PubMed] [Google Scholar]
- 31.Urbach DR, Harnish JL, McIlroy JH, et al. A measure of quality of life after abdominal surgery. Qual Life Res. 2006;15:1053–61. doi: 10.1007/s11136-006-0047-3. [DOI] [PubMed] [Google Scholar]
- 32.Mutignani M, Manta R, Pugliese F, et al. Endoscopic ultrasound-guided duodenojejunal anastomosis to treat postsurgical roux-en-Y hepaticojejunostomy stricture: A dream or a reality? Endoscopy. 2015;47(Suppl 1):E350–1. doi: 10.1055/s-0034-1392424. [DOI] [PubMed] [Google Scholar]
- 33.Tonozuka R, Mukai S, Tsuchiya T, et al. Recanalization after biliojejunostomy by use of a new digital per-oral cholangioscope through the hepaticogastrostomy route. VideoGIE. 2016;1:63–5. doi: 10.1016/j.vgie.2016.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Umeda J, Itoi T, Tsuchiya T, et al. A newly designed plastic stent for EUS-guided hepaticogastrostomy: A prospective preliminary feasibility study (with videos) Gastrointest Endosc. 2015;82:390–6e2. doi: 10.1016/j.gie.2015.02.041. [DOI] [PubMed] [Google Scholar]


