Abstract
Purpose
Anlotinib, a newly developed oral small-molecule receptor tyrosine kinase inhibitor (TKI), has been shown to have encouraging activity against sarcoma. The purpose of this study was to retrospectively evaluate the safety and clinical efficacy of chemotherapy combined with anlotinib plus anlotinib maintenance in advanced/metastatic soft tissue sarcoma (STS) patients in a real-world setting in China.
Patients and Methods
We retrospectively collected the medical data of thirty-two patients with advanced/metastatic STS who received chemotherapy combined with anlotinib plus anlotinib maintenance therapy. The objective response rate (ORR) and disease control rate (DCR) were calculated according to the RECIST 1.1 criteria. The progression-free rates (PFRs) at three and six months, the progression-free survival (PFS) time, and adverse events were recorded.
Results
On the basis of investigator assessments, two patients (6%) achieved CR (complete response) and nine patients (28%) achieved PR (partial response), with an ORR of 34%. Eleven patients (34%) achieved SD (stable disease), and ten patients (31%) achieved PD (progression disease), with a DCR of 69%. The progression-free rates (PFRs) at three and six months were 81% and 69%, respectively. The median PFS time was 8.2 months. The hematologic and non-hematologic toxicities were manageable. The most common grade 3 and 4 adverse events were febrile neutropenia (9%), leukopenia (19%), thrombocytopenia (3%), anemia (6%), anorexia (6%), vomiting (3%), and hypertension (6%). The combination therapy was generally well tolerated.
Conclusion
Our study suggests that chemotherapy combined with anlotinib plus anlotinib maintenance therapy had good efficacy and resulted in more favorable survival with good tolerance among patients with advanced/metastatic STS.
Keywords: advanced/metastatic soft tissue sarcoma, anlotinib, chemotherapy, toxicity
Introduction
Soft tissue sarcomas (STSs) are a heterogeneous type of solid tumor that originates from mesenchymal tissue.1 STSs, which are divided into more than 50 types by The World Health Organization (WHO), account for approximately 1% of adult malignant tumors and 15% of pediatric malignant tumors.2 The main treatments for STS are surgery, radiotherapy and chemotherapy. Although STS has a low incidence, it is extremely harmful, and there is a high possibility of metastasis and recurrence.3 STS usually metastasizes to the lungs, and abdominal soft tissue sarcomas usually metastasize to the peritoneum or liver.4 For advanced soft tissue sarcoma patients who have lost the chance of surgery, chemotherapy is the main method of prolonging survival.5 The conventional first-line treatment for advanced STS is either doxorubicin as monotherapy or in combination with ifosfamide; the objective response rate is 25~30%, and the median overall survival time is approximately 12–17 months.6 Response rates increase with higher doses of chemotherapy drugs, however at the cost of a worse safety profile and no increase in OS. Because of the limited benefits seen from current treatments, there is an urgent clinical need for therapies with improved survival and safety.7
Tumor angiogenesis is an important process in tumorigenesis and metastasis for STS. Thus, anti-angiogenic treatments are important for the control of soft tissue sarcoma.8 Some new drugs for STS have been explored and used in preclinical studies and clinical trials.9–11 Anlotinib was developed by China as a new oral multi-targeted receptor TKI. Anlotinib inhibits vascular endothelial growth factor/vascular endothelial growth factor receptor (VEGF/VEGFR) signaling by targeting VEGFR1/2/3 and FGFR1/2/3/4 with high affinity. Furthermore, it suppresses the activity of platelet-derived growth factor receptor α and β, c-Kit and RET, resulting in significant inhibition of tumor proliferation in preclinical studies.12–14 Anlotinib has encouraging antitumor effects and acceptable toxicity in advanced lung adenocarcinoma, lung squamous cell carcinoma, STS, medullary thyroid cancer, small cell lung cancer and metastatic renal clear cell cancer.15–18 A Phase II clinical trial showed that a total of 166 relapsed advanced STS patients had a significant survival benefit after treatment with anlotinib, with a 12-week progression-free rate of 68.42% and an objective response rate of 12.65%. The PFS and OS were 5.63 and 12.33 months, respectively.10 The findings from a phase IIb, placebo-controlled trial including 233 patients with recurrent advanced STS showed that anlotinib compared with a placebo significantly prolonged progression-free survival by 4.8 months (6.27 months vs 1.47 months, P< 0.0001). In that study, the ORR (10.13% vs 1.33%, P=0.0145) and DCR (55.7% vs 22.67%, P < 0.0001) were also significantly improved, but the overall survival data have not been published.
Although these results are encouraging, the outcome is still not satisfactory. Therefore, it is appropriate to attempt adding anlotinib to chemotherapy to determine whether the combination can further improve the efficacy of the treatment; furthermore, the adverse reactions to anlotinib are mild, and the toxicities of the two therapies are different. Preclinical studies have shown that anti-angiogenic drugs combined with cytotoxic chemotherapy constitute an effective method of overcoming drug resistance to chemotherapy drugs and inhibiting tumor growth.19–21 Moreover, many clinical data also suggest that the addition of anti-angiogenic drugs to conventional chemotherapy may result in more effective treatment for advanced non-squamous NSCLC,22 colon cancer,23 renal cancer,24 ovarian cancer25 and STS.26
Therefore, a retrospective single-center analysis was conducted to evaluate the efficacy and safety and to describe the use of anlotinib combined with chemotherapy in clinical practice in patients with different histological subtypes of advanced STS.
Materials and Methods
Patient Characteristics
Data from advanced STS patients at the Affiliated Cancer Hospital of Zhengzhou University were analyzed in this retrospective study. Advanced STS patients who received anlotinib combined with chemotherapy were included. If the STS did not progress after 2–6 cycles of combination therapy, then the patient received maintenance therapy with anlotinib. Other inclusion criteria were an ECOG performance status of 0–2, histologically confirmed advanced/metastatic STS, age between 10 and 70 years and adequate bone marrow, liver and kidney functions. From April 2018 to September 2019, a total of 32 advanced/metastatic STS patients were included in the retrospective study.
The clinical variables collected were sex, age, history of surgery, history of radiotherapy, chemotherapy, median number of treatment cycles, treatment toxicities and dose reductions. Absolute neutrophil counts, white blood cell counts, platelet counts and hemoglobin levels were recorded before and after each cycle. We also recorded changes in total bilirubin, transaminase, creatinine, and triglyceride levels; proteinuria; thyroid function; and hypertension. Before the combination treatment was administered, written informed consent was obtained from all patients.
Treatment Methods
Advanced STS patients received anlotinib plus chemotherapy. If the STS did not progress after 2–6 cycles of combination therapy, the patient received maintenance therapy with anlotinib. Anlotinib was initially administered orally at a dosage of 12 mg or 10 mg daily for 14 days and then discontinued for 7 days (3-week cycle). The clinicians determined the initial drug dose of anlotinib depending on the patient’s condition.
Safety and Outcome Assessment
The therapeutic effect was evaluated according to the Response Evaluation Criteria in Solid Tumors (RECIST 1.1) criteria by computed tomography scans every two cycles. The disease control rate (DCR) was defined as the total percentage of patients with stable disease (SD), a partial response (PR) and a complete response (CR). PFS was defined from the beginning of combination treatment to tumor progression or death from any cause. In addition, treatment-related adverse events were assessed according to the Common Terminology Criteria for Adverse Events (CTCAE) version 4.03.
Statistical Analysis
Data were analyzed with SPSS software (version 14.0). The descriptive variables regarding treatment-related toxicities and patient characteristics were directly calculated from the database. The median PFS was calculated using the Kaplan-Meier method.
Ethics
This study was approved by the Ethics Committee of Henan Cancer Hospital. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the Helsinki declaration. The informed consent form was waived as this is a retrospective study. The patient data used in the study were confidential.
Results
Patient Characteristics
The baseline characteristics of the advanced STS patients, including sex, age, histological subtypes, ECOG performance status, radiotherapy history, surgery history, chemotherapy history, are summarized in Table 1. Seventeen patients were male, and fifteen were female, with an average age of 45 years (range: 15–69 years). The pathological types were liposarcoma (LPS), synovial sarcoma (SS), leiomyosarcoma (LMS), undifferentiated pleomorphic sarcoma (UPS), fibrosarcoma (FS), embryonal rhabdomyosarcoma (ERMS), primitive neuroectodermal tumor/Ewing sarcoma (PNET/ES) and angiosarcoma (AS). A total of 94% patients (30/32) had a surgical history, and 38% (12/32) patients with prior use of radiotherapy. A total of 32 STS patients underwent treatment with anlotinib combined with chemotherapy. There were 8 patients (25%) for whom this was the first-line treatment, 13 patients (41%) for whom this was a second-line treatment, and 11 patients (34%) for whom this was a third-line treatment. Twenty-nine patients (29/32.91%) received 12 mg of anlotinib as the initial dose, and 3 patients (3/32.9%) received 10 mg anlotinib as the starting dose for 14 days, followed by discontinuation of anlotinib administration for 7 days (3-week cycle). The chemotherapy regimens were based on anthracyclines and ifosfamide, and other drugs included dacarbazine, gemcitabine, docetaxel, temozolomide, vincristine, and cyclophosphamide every 21 days.
Table 1.
Patient Demographics and Clinical Characteristics
| Baseline Characteristics | Number of Patients (32) | Percentage (%) |
|---|---|---|
| Age (years) | ||
| Median | 45 | |
| Range | 15–69 | |
| Sex | ||
| Male | 17 | 53 |
| Female | 15 | 47 |
| ECOG performance status | ||
| 0 | 14 | 44 |
| 1 | 17 | 53 |
| 2 | 1 | 3 |
| Histology | ||
| UPS | 6 | 19 |
| LPS | 4 | 12 |
| LMS | 6 | 19 |
| SS | 4 | 12 |
| FS | 2 | 6 |
| ERMS | 5 | 16 |
| PNET/ES | 3 | 10 |
| AS | 2 | 6 |
| Treatment lines of anlotinib combination therapy | ||
| First line | 8 | 25 |
| Second line | 12 | 37.5 |
| Third line | 12 | 37.5 |
| Anlotinib dose | ||
| 12 mg | 29 | 91 |
| 10 mg | 3 | 9 |
| Radiotherapy history | ||
| Yes | 12 | 38 |
| No | 20 | 62 |
| Surgery history | ||
| Yes | 30 | 94 |
| No | 2 | 6 |
| Chemotherapy history | ||
| Yes | 26 | 81 |
| No | 6 | 19 |
Abbreviation: ECOG, Eastern Cooperative Oncology Group.
Among them, 30 non-progressive STS patients received anlotinib maintenance therapy after 2–6 cycles of combined treatment. AEs were collected for all patients.
Efficacy
The median number of cycles administered to the 32 advanced STS patients was four (range: 2–6). A total of two patients (6%) achieved CR and nine patients (28%) achieved PR with an ORR of 34%. Eleven patients (34%) archived SD, and ten patients (31%) achieved PD with a DCR of 69% (Table 2).
Table 2.
Overall Response to Treatment
| Tumor Response | Anlotinib plus chemotherapy (n =32) | |
|---|---|---|
| No. | % | |
| Complete response | 2 | 6 |
| Partial response | 9 | 28 |
| Stable disease | 11 | 34 |
| Progressive disease | 10 | 31 |
| RR | 11 | 34 |
| DCR | 22 | 69 |
| Median PFS | 8.2 months | |
| PFR 3 months | 81 | |
| PFR 6 months | 69 | |
Abbreviations: PR, partial response; SD, stable disease; PD, progressive disease; RR, response rate; DCR, disease control rate; PFS, progression free survival; PFR, progression-free rate.
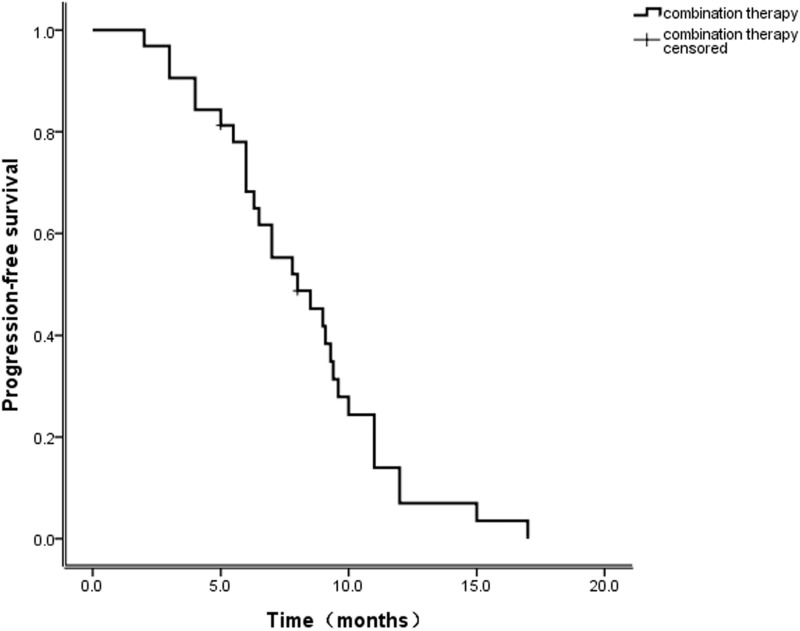
Among those receiving this combination therapy as a first-line treatment, the ORR was 50%, and the DCR was 75% (2 CR, 2 PR, 2 SD, and 2PD). Among those for whom it was the second-line treatment, the ORR was 33.3%, and the DCR was 75% (4 PR, 5 SD, and 3 PD). Among the patients for whom the combination therapy was the third-line treatment, the ORR was 25%, and the DCR was 58.3% (3 PR, 4 SD, and 5 PD). The median follow-up period was 14 months. The progression-free rates (PFRs) at three and six months were 81% and 69%, respectively (Table 2). The median PFS time for treated patients was 8.2 months (Figure 1; 95% CI: 5.4–10.6). The median OS was not achieved by the time of analysis.
Figure 1.
Progression-free survival.
As shown in Table 3, the histologic subtypes of the responders were as follows: undifferentiated pleomorphic sarcoma (n = 3, 50%), liposarcoma (n = 0, 0%), leiomyosarcoma (n = 3, 50%), synovial sarcoma (n = 1, 25%), fibrosarcoma (n = 0, 0%), embryonal rhabdomyosarcoma (n = 2, 40%), primitive neuroectodermal tumor/Ewing sarcoma (n = 2, 66.7%) and angiosarcoma (n = 0, 0%). The disease control rates for the histologic subtypes were as follows: undifferentiated pleomorphic sarcoma (n = 4, 66.7%), liposarcoma (n = 2, 50%), leiomyosarcoma (n = 5, 83.3%), synovial sarcoma (n = 3, 75%), fibrosarcoma (n = 1, 50%), embryonal rhabdomyosarcoma (n = 3, 60%), primitive neuroectodermal tumor/Ewing sarcoma (n = 3, 100%) and angiosarcoma (AS) (n = 1, 50%) (Table 3).
Table 3.
Efficacy Data by Histologic Category
| Histology Category | Total (n = 32) | |||
|---|---|---|---|---|
| CR No.(%) | PR No.(%) | SD No.(%) | PD No.(%) | |
| UPS | 1(16.7) | 2(33.3) | 1(16.7) | 2(33.3) |
| LPS | 0(0.0) | 0(0.0) | 2(50.0) | 2(50.0) |
| LMS | 1(16.7) | 2(33.3) | 2(33.3) | 1(16.7) |
| SS | 0(0.0) | 1(25.0) | 2(50.0) | 1(25.0) |
| FS | 0(0.0) | 0(0.0) | 1(50.0) | 1(50.0) |
| ERMS | 0(0.0) | 2(40.0) | 1(20.0) | 2(40.0) |
| PNET/ES | 0(0.0) | 2(66.7) | 1(33.3) | 0(0.0) |
| AS | 0(0.0) | 0(0.0) | 1(50.0) | 1(50.0) |
| Total | 2(6.2) | 9(28.1) | 11(34.4) | 10(31.3) |
Abbreviations: UPS, undifferentiated pleomorphic sarcoma; LPS, liposarcoma; LMS, leiomyosarcoma; SS, synovial sarcoma; FS, fibrosarcoma; ERMS, embryonal rhabdomyosarcoma; PNET/ES, primitive neuroectodermal tumor/Ewing sarcoma; AS, angiosarcoma.
Adverse Events
The common treatment-related adverse events are shown in Table 4. The most common grade 3 and 4 adverse events were febrile neutropenia (n = 3, 9%), leukopenia (n = 6, 19%), thrombocytopenia (n = 1, 3%), anemia (n = 2, 6%), anorexia (n = 2, 6%), vomiting (n = 1, 3%), and hypertension (n = 2, 6%) (Table 4). The hematologic and non-hematologic toxicities were manageable, and the incidence of toxicities was similar to that in a prior report of advanced STS chemotherapy.
Table 4.
Toxicity Profile
| Toxicity | Total No.(%) | I + II No.(%) | III+ IV No.(%) |
|---|---|---|---|
| Leukopenia | 22(69) | 16(50) | 6(19) |
| Febrile neutropenia | 9(28) | 6(19) | 3(9) |
| Thrombocytopenia | 4(12) | 3(9) | 1(3) |
| Anemia | 19(59) | 17(53) | 2(6) |
| Anorexia | 16(50) | 14(44) | 2(6) |
| Nausea/vomiting | 14(44) | 13(41) | 1(3) |
| Diarrhea | 3(9) | 3(9) | 0(0) |
| Hypertension | 8(25) | 6(19) | 2(6) |
| Fatigue | 12(38) | 12(38) | 0(0) |
| Hand-foot skin reaction | 2(6) | 2(6) | 0(0) |
| Triglyceride elevation | 8(25) | 8(25) | 0(0) |
| Proteinuria | 7(22) | 7(22) | 0(0) |
| ALT elevation | 9(28) | 9(28) | 0(0) |
| AST elevation | 8(25) | 8(25) | 0(0) |
| Hypothyroidism | 5(16) | 5(16) | 0(0) |
The most frequent adverse events of anlotinib maintenance therapy were hypertension, hand-foot skin reaction, triglyceride level elevation, hypothyroidism, proteinuria and fatigue. The toxicity profile was generally consistent with that in a prior report of anlotinib in a phase I/II study. No patients stopped treatment with anlotinib due to side effects during maintenance therapy. Moreover, no treatment-related deaths occurred. The combination was generally well tolerated.
Discussion
STSs in general are relatively insensitive to chemotherapy compared to tumors of epithelial origin. The prognosis of advanced STS patients is still poor, with a median OS in range of 12–17 months.6,27 When patients experience failure of first-line chemotherapy, there is currently no standard second-line treatment option in China, and there are few therapeutic drugs available.28 Anlotinib not only inhibits tumor cell proliferation but also inhibits tumor angiogenesis and has been shown to have antitumor activity in advanced STS.29 In addition, anlotinib has been approved as a second-line treatment for advanced/metastatic STS by the China Food and Drug Administration in 2019.
Preclinical studies have provided strong evidence that the use of anti-angiogenic agents can promote tumor vascular normalization, thereby improving drug delivery and efficacy in many solid tumors, including STS. Over the past decade, a number of clinical studies have shown that bevacizumab combined with chemotherapy significantly prolongs PFS and OS compared to chemotherapy alone for advanced NSCLC and colon, ovarian, and renal cancers.22 Verschraegen CF26 reported a Phase I trial that showed that bevacizumab combined with docetaxel and gemcitabine in 36 patients with advanced/metastatic STS had an ORR of 31%, including 4 CR, 6 PR, and 18 SD. In addition to the angiogenic pathway, factors in proliferative pathway, such as PDGF and c-Kit, are also likely to contribute to the high malignancy of STS.13 Reported by the Spanish sarcoma study group, a prospective randomized phase II study in 35 advanced STS patients received sorafenib combined with ifosfamide, which proved to be a promising therapy, with three-month and six-month PFS rates of 66% and 37%, a median PFS time of 4.8 months, and a OS time of 16.2 months.30 A prospective phase II clinical study is ongoing to evaluate the safety and effectiveness of anlotinib combined with epirubicin and ifosfamide in the treatment of metastatic or advanced soft tissue sarcomas. Therefore, the combination of chemotherapy with other antiangiogenic active drugs may lead to better efficacy for advanced STS.
Thus far, this is the first study of chemotherapy combined with anlotinib for advanced STS patients. In this study, a total of two patients (6%) achieved CR and nine patients (28%) achieved PR, with an ORR of 34%. Eleven patients (34%) achieved SD, and ten patients (31%) achieved PD, with a DCR of 69%. As a first-line treatment, the ORR was 50%, and the DCR was 75% (2 CR, 4 PR, 1 SD, and1 PD); as a second-line treatment, the ORR was 33.3%, and the DCR was 75% (5 PR, 5 SD, and2 PD); and as a third-line treatment, the ORR was 25%, and the DCR was 58.3% (2 PR, 6 SD, and 3 PD). The median PFS was 8.2 months, and the PFRs at three and six months were 81% and 69%, respectively. The median OS was not achieved. Therapy with the combined treatment plus anlotinib maintenance resulted in an overall good response. Our findings showed obvious improvement of the PFS with the addition of anlotinib. However, another phase II trial showed that in 106 metastatic/advanced leiomyosarcoma, gemcitabine combined with pazopanib as second-line therapy, followed by pazopanib maintenance failed to meet its primary objective and was not beneficial for these patients. Moreover, there were significant toxicity, especially haematological adverse events during the combined treatment with gemcitabine and pazopanib.31 This toxicity diminished during the pazopanib monotherapy. Another phase Ib/II study of gemcitabine and docetaxel in combination with pazopanib for the neoadjuvant treatment of soft tissue sarcomas resulted in significant toxicity; despite pathologic responses, no objective responses occurred. The trial was discontinued because of slow accrual after inclusion of five patients.32
Treatment tolerance and compliance are very critical issues. In our study, the most common grade 3 and 4 adverse events were febrile neutropenia (9%), leukopenia (19%), thrombocytopenia (3%), anemia (6%), anorexia (6%), vomiting (3%), and hypertension (6%). The hematologic and non-hematologic toxicities were manageable and acceptable, and the incidence of toxicities was similar to that in a prior report of advanced STS chemotherapy. Most toxicities of combination therapy were reversible with appropriate medical management or dose reduction. No patients stopped treatment due to side effects. The toxicity of anlotinib maintenance therapy was mild, and the lipid metabolism and thyroid dysfunction were reversible. The manageable toxicity may allow its combination with conventional chemotherapy, ultimately leading to further improvements in advanced STS. Overall, in the lack of prospective clinical trials, the study confirms to some extent the value of anlotinib combined with chemotherapy in patients with advanced STS.
On the other hand, we need to recognize some limitations of the research. The main limitation is that the study was retrospective. In addition, the incidence rate of STS is relatively low, and the histological types are heterogeneous. Finally, this was a small-sample study.
Conclusion
In summary, we found that chemotherapy plus anlotinib combined with anlotinib maintenance is reliable, safe and feasible for advanced STSs. Ultimately, we also need to conduct a randomized and prospective trial to demonstrate the clinical benefits of chemotherapy in combination with anlotinib.
Disclosure
The authors report no conflicts of interest related to this work.
References
- 1.Sinha S, Peach AH. Diagnosis and management of soft tissue sarcoma. BMJ. 2010;341:c7170. doi: 10.1136/bmj.c7170 [DOI] [PubMed] [Google Scholar]
- 2.Clark MA, Fisher C, Judson I, Thomas JM. Soft-tissue sarcomas in adults. N Engl J Med. 2005;353(7):701–711. doi: 10.1056/NEJMra041866 [DOI] [PubMed] [Google Scholar]
- 3.Cesne AL, Bauer S, Demetri GD, et al. Safety and efficacy of pazopanib in advanced soft tissue sarcoma: PALETTE (EORTC 62072) subgroup analyses. BMC Cancer. 2019;19(1):794. doi: 10.1186/s12885-019-5988-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.von Mehren M, Randall RL, Benjamin RS, et al. Soft tissue sarcoma, version 2.2016, NCCN clinical practice guidelines in oncology. J Natl Compr Canc Netw. 2016;14(6):758–786. doi: 10.6004/jnccn.2016.0078 [DOI] [PubMed] [Google Scholar]
- 5.Mark L, Miah AB, Khin T, Judson IR, Charlotte B. Systemic treatment of soft-tissue sarcoma-gold standard and novel therapies. Nat Rev Clin Oncol. 2014;11(4):187. doi: 10.1038/nrclinonc.2014.26 [DOI] [PubMed] [Google Scholar]
- 6.Tap WD, Papai Z, Van Tine BA, et al. Doxorubicin plus evofosfamide versus doxorubicin alone in locally advanced, unresectable or metastatic soft-tissue sarcoma (TH CR-406/SARC021): an international, multicentre, open-label, randomised Phase 3 trial. Lancet Oncol. 2017;18(8):1089–1103. S1470204517303819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sheng JY, Movva S. Systemic therapy for advanced soft tissue sarcoma. Surg Clin North Am. 2016;96(5):1141–1156. doi: 10.1016/j.suc.2016.06.006 [DOI] [PubMed] [Google Scholar]
- 8.Li F, Liao Z, Zhao J, et al. Efficacy and safety of apatinib in stage IV sarcomas: experience of a major sarcoma center in China. Oncotarget. 2017;8(38):64471–64480. doi: 10.18632/oncotarget.16293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van der Graaf WT, Blay JY, Chawla SP, et al. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet. 2012;379(9829):1879–1886. doi: 10.1016/S0140-6736(12)60651-5 [DOI] [PubMed] [Google Scholar]
- 10.Chi Y, Fang Z, Hong XN, Yao Y, Cai J. Safety and efficacy of anlotinib, a multikinase angiogenesis inhibitor, in patients with refractory metastatic soft tissue sarcoma. Clin Cancer Res. 2018;24(21):5233–5238. clincanres.3766.2017. [DOI] [PubMed] [Google Scholar]
- 11.Tawbi HA, Burgess M, Bolejack V, et al. Pembrolizumab in advanced soft-tissue sarcoma and bone sarcoma (SARC028): a multicentre, two-cohort, single-arm, open-label, Phase 2 trial. Lancet Oncol. 2017;18(11):1493–1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin B, Song X, Yang D, Bai D, Yao Y, Lu N. Anlotinib inhibits angiogenesis via suppressing the activation of VEGFR2, PDGFRβ and FGFR1. Gene. 2018;654:S0378111918301550. doi: 10.1016/j.gene.2018.02.026 [DOI] [PubMed] [Google Scholar]
- 13.Sun Y, Wei N, Feng D, et al. Safety, pharmacokinetics, and antitumor properties of anlotinib, an oral multi-target tyrosine kinase inhibitor, in patients with advanced refractory solid tumors. J Hematol Oncol. 2016;9(1):105. doi: 10.1186/s13045-016-0332-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xie C, Wan X, Quan H, et al. Preclinical characterization of anlotinib, a highly potent and selective vascular endothelial growth factor receptor‐2 inhibitor. Cancer Sci. 2018;109(4):1207–1219. doi: 10.1111/cas.2018.109.issue-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Yang S, Zhang Z, Wang Q. Emerging therapies for small cell lung cancer. J Hematol Oncol. 2019;12(1):47. doi: 10.1186/s13045-019-0736-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhou AP, Bai Y, Song Y, et al. Anlotinib versus sunitinib as first-line treatment for metastatic renal cell carcinoma: a randomized phase II clinical trial. Oncologist. 2019;24(8):e702–e708. doi: 10.1634/theoncologist.2018-0839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Han B, Li K, Zhao Y, et al. Anlotinib as a third-line therapy in patients with refractory advanced non-small-cell lung cancer: a multicentre, randomised phase II trial (ALTER0302). Br J Cancer. 2018;118(5):654–661. doi: 10.1038/bjc.2017.478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sun Y, Du F, Gao M, et al. Anlotinib for the treatment of patients with locally advanced or metastatic medullary thyroid cancer. Thyroid. 2018;28(11):1455–1461. doi: 10.1089/thy.2018.0022 [DOI] [PubMed] [Google Scholar]
- 19.Wenhong R, Borys K, Guy L, et al. Combined vascular endothelial growth factor receptor/epidermal growth factor receptor blockade with chemotherapy for treatment of local, uterine, and metastatic soft tissue sarcoma. Clin Cancer Res. 2008;14(17):5466. doi: 10.1158/1078-0432.CCR-08-0562 [DOI] [PubMed] [Google Scholar]
- 20.Sushil K, Reza Bayat M, Reihaneh S, et al. Metronomic oral topotecan with pazopanib is an active antiangiogenic regimen in mouse models of aggressive pediatric solid tumor. Clin Cancer Res. 2011;17(17):5656–5667. doi: 10.1158/1078-0432.CCR-11-0078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hamberg P, Steeghs N, Loos WJ, et al. Decreased exposure to sunitinib due to concomitant administration of ifosfamide: results of a phase I and pharmacokinetic study on the combination of sunitinib and ifosfamide in patients with advanced solid malignancies. Br J Cancer. 2010;102(12):1699. doi: 10.1038/sj.bjc.6605696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caicun Z, Yi-Long W, Gongyan C, et al. BEYOND: a randomized, double-blind, placebo-controlled, multicenter, Phase III Study of first-line carboplatin/paclitaxel plus bevacizumab or placebo in Chinese patients with advanced or recurrent nonsquamous non-small-cell lung cancer. J Clin Oncol. 2015;33(19):2197–2204. doi: 10.1200/JCO.2014.59.4424 [DOI] [PubMed] [Google Scholar]
- 23.Hurwitz H, Fehrenbacher L, Novotny W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335. doi: 10.1056/NEJMoa032691 [DOI] [PubMed] [Google Scholar]
- 24.Escudier B, Bellmunt J, Négrier S, et al. Phase III trial of bevacizumab plus interferon alfa-2a in patients with metastatic renal cell carcinoma (AVOREN): final analysis of overall survival. J Clin Oncol. 2010;28(13):2144–2150. doi: 10.1200/JCO.2009.26.7849 [DOI] [PubMed] [Google Scholar]
- 25.Oza AM, Cook AD, Pfisterer J, et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015;16(8):928–936. doi: 10.1016/S1470-2045(15)00086-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Verschraegen CF, Arias-Pulido H, Lee S-J, et al. Phase IB study of the combination of docetaxel, gemcitabine, and bevacizumab in patients with advanced or recurrent soft tissue sarcoma: the axtell regimen. Ann Oncol. 2012;23(3):785. doi: 10.1093/annonc/mdr299 [DOI] [PubMed] [Google Scholar]
- 27.Ryan CW, Merimsky O, Agulnik M, et al. PICASSO III: a Phase III, placebo-controlled study of doxorubicin with or without palifosfamide in patients with metastatic soft tissue sarcoma. J Clin Oncol. 2016;34:3898–3905. doi: 10.1200/JCO.2016.67.6684 [DOI] [PubMed] [Google Scholar]
- 28.Schoffski P, Cornillie J, Wozniak A, Li H, Hompes D. Soft tissue sarcoma: an update on systemic treatment options for patients with advanced disease. Oncol Res Treat. 2014;37(6):355–362. doi: 10.1159/000362631 [DOI] [PubMed] [Google Scholar]
- 29.Shen G, Zheng F, Ren D, et al. Anlotinib: a novel multi-targeting tyrosine kinase inhibitor in clinical development. J Hematol Oncol. 2018;11(1):120. doi: 10.1186/s13045-018-0664-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Muro XGD, Maurel J, Trufero JM, et al. Phase II trial of ifosfamide in combination with the VEGFR inhibitor sorafenib in advanced soft tissue sarcoma: a Spanish group for research on sarcomas (GEIS) study. Invest New Drugs. 2018;36(17):1–8. doi: 10.1007/s10637-017-0500-x [DOI] [PubMed] [Google Scholar]
- 31.Pautier P, Penel N, Ray-Coquard I, et al. A phase II of gemcitabine combined with pazopanib followed by pazopanib maintenance, as second-line treatment in patients with advanced leiomyosarcomas: a unicancer French Sarcoma Group study (LMS03 study). Eur j Cancer. 2020;125:31–37. [DOI] [PubMed] [Google Scholar]
- 32.Munhoz RR, D’Angelo SP, Gounder MM, et al. A Phase Ib/II Study of gemcitabine and docetaxel in combination with pazopanib for the neoadjuvant treatment of soft tissue sarcomas. Oncologist. 2015;20(11):1245–1246. doi: 10.1634/theoncologist.2015-0245 [DOI] [PMC free article] [PubMed] [Google Scholar]