Abstract
Purpose
Differentiated thyroid cancer (DTC) responds to VEGF receptor inhibitors. VEGF signals through RAS/RAF/ MEK signaling. We evaluated the safety and efficacy of the VEGF receptor inhibitor pazopanib and MEK inhibitor trametinib in advanced solid tumors and DTC.
Patients and Methods
Patients with advanced solid tumors were enrolled in a phase I, multicenter trial with a DTC expansion cohort. Patients received pazopanib 400–800 mg and trametinib 1–2 mg daily. Efficacy in the expansion cohort was assessed with objective response (OR) at 6 months of treatment.
Results
Twenty-six patients were enrolled in five dose levels. MTD was not reached; the recommended phase II dose was pazopanib 800 mg orally and trametinib 2 mg orally every day. There was one dose-limiting toxicity on dose level 1 with grade 3 fatigue and muscle weakness. Common grade 3 adverse events were elevated transaminases (19%), diarrhea (15%), hypertension (12%), and fatigue (8%). Thirteen patients were enrolled in the DTC cohort; OR was 33% (95% confidence interval, 9.9, 65.1%) and median progression-free survival was 10.7 months. The cohort was terminated after planned interim analysis suggested insufficiently increased activity against the historical control of pazopanib alone. Reduction in tumor diameter negatively correlated with p-ERK change in tumor (Spearman ρ = −0.71; P = 0.05). NRAS mutation was associated with response (Fisher exact P = 0.008).
Conclusions
Pazopanib + trametinib was tolerable at full single-agent doses with clinical activity in DTC but did not achieve the prespecified response rate target.
Introduction
The VEGF pathway is an important signaling cascade responsible for angiogenesis, with secondary effects that include increasing proliferation and metastasis of many cancers including differentiated thyroid cancer (DTC; refs. 1–3). VEGF binds to its ligand receptor, VEGF receptors (R) 1–3, which then activates a downstream signaling cascade leading to prosurvival signaling and transcription of genes involved in angiogenesis. Like many receptor tyrosine kinases, VEGFR1–3 can activate major prosurvival circuits including AKT/PI3 K signaling as well as well as RAS signaling. The RAF/MEK/ERK signaling pathway is involved in the regulation of normal cell proliferation, survival, and differentiation, and this pathway is frequently aberrantly upregulated in a wide number of cancers including DTC (4, 5).
DTC is a highly prevalent cancer with >600,000 patients living with the disease in the United States presently (6). The mainstay of therapy for the 90% of patients found with early or locally advanced disease is surgery and radioiodine therapy (7, 8). Mutations in BRAF and RAS genes, along with rearrangements in RET, TRK, and ALK genes and others have been found in the majority (70%) of patients (9). Two approved therapies for advanced, radioiodine-refractory DTC, lenvatinib and sorafenib, are multikinase inhibitors targeting VEGFR2 in addition to other kinases (10, 11). Pazopanib is an orally available multikinase inhibitor of VEGFR, platelet-derived growth factor (PDGF) receptor, KIT, fibroblast growth factor (FGF) receptor, and RAF which is approved for advanced renal cell carcinoma and advanced refractory soft-tissue sarcoma (12, 13). Phase II clinical trials of pazopanib in DTC have also shown activity, with 49% of patients having RECIST partial responses (PR; refs. 14, 15).
MEK inhibitors have been tested less extensively in DTC. Selumetinib, a selective MEK 1/2 inhibitor, has been tested as a potential redifferentiating agent prior to radioiodine therapy in a small study of 20 patients, with PRs in five patients and stable disease in three others (16).
Our group has previously reported that the combination of trametinib, an orally available highly specific inhibitor of MEK1 and MEK2, and pazopanib in thyroid cancer cell lines and xenograft models results in synergistic inhibition of tumor growth (17). We hypothesized that inhibition of receptor tyrosine kinases VEGFR and PDGFR with the addition of MEK inhibitor trametinib could improve clinical benefit for patients with DTC compared with pazopanib alone. We enrolled a phase I study of the combination of pazopanib and trametinib in all solid tumors with an expansion cohort in DTC at the MTD.
Patients and Methods
Study design
This was an open-label, multicenter [Sidney Kimmel Comprehensive Cancer Center (SKCCC) at John Hopkins University (JHU, Baltimore, MD) and The University of Texas MD Anderson Comprehensive Cancer Center (MDACC, Houston, TX)] trial funded by the National Comprehensive Cancer Network. Patients older than 18 years with advanced solid tumors that were refractory to standard-of-care treatment options were eligible for the dose-finding cohort of the study, and expansion cohorts for DTC, cholangiocarcinoma, and soft-tissue sarcoma were subsequently enrolled at the MTD; here, we report the dose-finding and DTC expansion cohorts. Subjects enrolling in the DTC expansion cohort must have had progressive disease within 6 months of enrolling in the study as assessed by successive imaging and disease that was also amenable to biopsy. Patients with DTC must have had radioiodine nonavid lesions or radioiodine avid lesions that have not responded to treatment with radioactive iodine [defined as ≥600 millicuries (mCi), last dose at least 6 months prior to enrollment]. Other eligibility criteria included the presence of RECIST criteria 1.1 measurable disease, an Eastern Cooperative Oncology Group performance status ≤1 (or Karnofsky performance status ≥60%) and adequate organ function as defined by absolute neutrophil count ≥1,500 cells/μL, platelet count ≥100,000 cells/μL, international normalized ratio ≤1.2 × upper limit of normal (unless stabilized with anticoagulation therapy and within the recommended range for the desired level of anticoagulation), total bilirubin ≤1.5 × upper limit of normal (or, in patients with Gilbert syndrome, total bilirubin >1.5 × as long as direct bilirubin is normal), and serum creatinine ≤1.5 × upper limit of normal or creatinine clearance ≥45 mL/minute and urine protein to creatinine ratio <1, or, if >1,24-hour urine protein <1 g (17).
Evaluation and treatment
The protocol was approved by the Institutional Review Board at both study sites, and complied with the International Ethical Guidelines for Biomedical Research Involving Human Subjects and the Declaration of Helsinki. Eligible patients were enrolled centrally at the SKCCC at JHU (Baltimore, MD). The trial was registered under ClinicalTrials.gov as . All patients provided written informed consent prior to enrollment. The study drugs (trametinib and pazopanib) were provided by GlaxoSmithKline (eventually Novartis).
For the dose-finding cohort, patients received escalating doses of pazopanib [dose levels (DL) 1:400 mg; DL 2: 600 mg; DL 3–5: 800 mg] and trametinib (DL 1–3: 1 mg; DL 4: 1.5 mg; DL5: 2 mg) orally daily every day of a 28-day cycle. Three patients would be treated per cohort in a standard 3+3 dose escalation design. If none of the first three patients experienced a dose-limiting toxicity (DLT), then the next three would be treated using the next higher dose. If one of the first three had a DLT, an additional three patients would be enrolled at that level. If only one of six developed a DLT, then the dose would be escalated for the next three patients. If two or more of the six had a DLT, then the dose would be deescalated to the previous dose (or dose level-1) where an additional three patients would be treated. The MTD was defined as the highest dose at which zero or one DLTs are observed in six patients. A total of six patients would be treated at the MTD. The expansion cohort patients were treated at the MTD established in our initial dose-finding cohort.
The treatment protocol allowed dose delays or reduction if patients experienced unacceptable side effects and adverse reactions related to study drug(s). Patients were evaluated every cycle for trial therapy compliance and monitoring of adverse events. The NCI Common Terminology Criteria for Adverse Events (CTCAE) version 4.0 was implemented for adverse event monitoring. Disease assessments (CT or MRI) were performed at baseline and then every other cycle. Response was evaluated according to the RECIST, version 1.1 (18). Upon progression of disease, patients were monitored for long-term adverse events and survival.
Study design
For the dose-finding cohort, the 3+3 dose escalation design had a target toxicity of less than 30%. For a dose level to be deemed tolerable, at least three patients must have been treated for 4 weeks without the development of a DLT prior to treating a new cohort at a higher dose level. In addition, all patients defining a dose level as tolerable (i.e., no DLT in cycle 1) must have received a minimum of 75% of the intended dose for each study drug during cycle 1.
For the expansion cohort in DTC, the primary endpoint was objective response (OR) rate by 6 months of treatment. At study entry, each patient was categorized into one of two subtype groups: papillary or follicular/Hurthle cell. On the basis of the phase II clinical trial with pazopanib, we expected that the papillary subtype would have a response rate of 33% and the follicular/Hurthle cell subtype would have a response rate of 75% (14, 15). An interim futility analysis would be conducted after response information was available on the first 12 patients in the expansion cohort. If the futility boundary was crossed, we would consider stopping the study and declaring that the combination was not showing sufficient activity to warrant further investigation (Supplementary Methods). The stopping boundary for futility was a guideline, however, because the sample size was small. Specifically, the stopping rule called for a review if there was a 75% probability that either the response rate in the papillary subtype was less than 25% or the response rate in the follicular/Hurthle call subtype was less than 67% (Supplementary Table S1).
Pharmacokinetic analysis
Pazopanib and trametinib pharmacokinetic (PK) analyses were performed in patients in the DTC expansion cohort. A full pazopanib PK profile was obtained after the first dose on cycle 1 day 1 through 24 hours. In addition, pazopanib and trametinib trough concentration were obtained on cycle 1 day 15 and at the time of second tumor biopsy (i.e., C2D1 ± 48 hours). Plasma levels of total trametinib and pazopanib were measured using a validated LC/MS-MS method. Briefly, trametinib was extracted from plasma using acetonitrile. Chromatographic separation was achieved with a Waters X-Terra C18 column (3.5 μm, 2.1 × 50 mm) and isocratic elution with an acetonitrile-water-formic acid (70:30:0.1, v/v/v) mobile phase over a 3-minute total analytical run time. An AB Sciex 5500 triple quadrupole mass spectrometer operated in positive electrospray ionization mode was used for the detection of trametinib. The assay range was 1–200 ng/mL. Briefly, pazopanib was extracted from plasma using acetonitrile. Chromatographic separation was achieved with a Waters X-Terra C18 column (3.5 μm, 2.1 × 50 mm) and isocratic elution with an ammonium acetate-acetonitrile containing 0.1% formic acid (40:60, v/v) mobile phase over a 3-minute total analytical run time. An AB Sciex 5500 triple quadrupole mass spectrometer operated in positive electrospray ionization mode was used for the detection of pazopanib. The assay range was 0.010–1 μg/mL, with a 1:100 dilution allowing for quantitation up to 100 μg/mL. Quality assurance samples were assayed with each analytic run and were within 15% of the nominal concentration for both assays. Individual pazopanib plasma concentrations were analyzed by standard noncompartmental PK methods using Phoenix WinNonlin v7.0 (Certara LP). Maximum plasma concentration (Cmax) was the observed maximum value. Area under the plasma concentration time curve (AUC) was calculated using the linear trapezoidal rule. Steady-state trough concentrations (Css,min) were calculated as an average of samples obtained on cycle 1 day 15 and at the time of pharmacodynamic (PD) studies.
PD analysis
Fresh tumor biopsies were obtained just prior to therapy and after one cycle of combination therapy in the DTC expansion cohort. Biopsies were processed using standard formalin fixation, paraffin embedding at the appropriate histology core facility for each institution and then stored at room temperature prior to analysis. IHC with pERK was performed using anti-phospho-p44/42 MAPK (ERK1/2; Thr202/Tyr204; 20G11; 4376S, Cell Signaling Technology) rabbit mAb. Staining was performed on an automated staining system (Leica-Bond, Leica Microsystem Inc.). Tissue sections (4–5 μm thick) from paraffin-embedded formalin-fixed tissue were deparaffinized and prepared for staining. Sections were incubated with primary antibody (1:400 dilution) for 15 minutes followed by incubation with a biotin-free HRP-conjugated secondary antibody. A brown signal was developed with DAB-detection according to the manufacturer’s instructions (Leica Microsystems Inc.). Staining was scored by pathologist (J.A. Bishop) who applied a HistoScore (H-score) calculated by the percentage of tumor cells staining multiplied by the intensity of staining (on a scale of 0–3; ref. 19). Only nuclear staining was regarded as positive.
Mutational analysis
DTC cohort patients had hotspot mutational sequencing on tumor DNA from fresh biopsy specimens or archival tissue (if fresh biopsy tissue was not available) performed by nextgeneration sequencing using AmpliSeq Cancer Hotspot Panel (v2) for targeted multigene amplification, as described previously (20, 21). Briefly, we used the Ion AmpliSeq Library Kit 2.0 for library preparation, Ion Personal Genome Machine Hi-Q OT2 Kit and Ion OneTouch 2 Instrument for emulsion PCR and template preparation, and the Ion Personal Genome Machine Hi-Q Sequencing Kit with the Ion 318 Chip and Personal Genome Machine as the sequencing platform (Life Technologies). The DNA input ranged from 1 ng to 30 ng, as measured by Qubit 20 Fluorometer (Life Technologies). Up to eight specimens were barcoded using Ion Xpress Barcode Adapters (Life Technologies) for each Ion 318 chip. One to three controls (a nontemplate control, a normal peripheral blood control from a male, and/or positive control specimens) were included in each chip. Positive controls were mixed DNA specimens from several cell lines with known mutations as reported previously.
Statistical methods
Proportions are reported with exact 95% binomial confidence intervals (CIs). Spearman correlation coefficient was used to assess the association between percent reduction in tumor diameter by RECIST and change in p-ERK expression. Binomial probabilities were compared with χ2 or Fisher exact tests as appropriate. Event time distributions for overall survival (OS) and progression-free survival (PFS) were estimated with the method of Kaplan and Meier and CIs calculated using the method of Brookmeyer and Crowley. PK parameters were summarized descriptively. Mann–Whitney U tests were used to assess correlations between drug exposure and toxicity. All P values reported are twosided, and the significance level was set at 0.05 for all analyses. Statistical analyses were performed using SAS version 9.4 (SAS Institute Inc) and R version 3.4.
Results
Patients
From November 2011 until April 2013, 26 patients with advanced solid tumors were enrolled [MD Anderson Cancer Center (Houston, TX; n = 14 pts) and Johns Hopkins University (Baltimore, MD; n = 12)]. From August 2013 until December 2014, 13 patients with DTC were enrolled at MD Anderson
Cancer Center (Houston, TX; n = 11 pts) and Johns Hopkins University (Baltimore, MD; n = 2) in this expansion cohort of pazopanib plus trametinib. The demographic and disease characteristics of the patients entered onto this study are shown in Table 1. All patients were treated according to the study protocol, and no patients remain on study at the time of this analysis. In the phase I cohort of the study, 69% of patients discontinued study therapy for disease progression, 12% for physician- or patient-initiated withdrawal, and 19% for toxicity. In the DTC cohort, those numbers were 62%, 31%, and 8%, respectively.
Table 1.
Baseline patient characteristics
| Phase I | Thyroid expansion | |
|---|---|---|
| Characteristic | (N = 26) | (N = 13) |
| Age | ||
| Median | 59 | 60 |
| Range | 33–82 | 32–74 |
| Sex (%) | ||
| Male | 14 (54) | 7 (54) |
| Female | 12 (46) | 6 (46) |
| Race (%) | ||
| White | 20 (77) | 11 (85) |
| Black | 2 (8) | 0 |
| Asian | 2 (8) | 1 (8) |
| Unknown or other | 2 (8) | 1 (8) |
| Previous regimens | ||
| Median | 4 | 1 |
| Range | 0–13 | 0–5 |
| Radiation-based therapy | 11 | 13 |
| Treatment site (%) | ||
| MD Anderson Cancer Center (Houston, TX) | 14 (54) | 11 (85) |
| Johns Hopkins University (Baltimore, MD) | 12 (46) | 2 (15) |
| ECOG performance status (%) | ||
| 0 | 10 (38) | 2 (15) |
| 1 | 16 (62) | 11 (85) |
| Tumor histology (%) | ||
| Thyroid subtype | ||
| Papillary | 2 (8) | 6 (46) |
| Follicular | 1 (4) | 5 (38) |
| Hurthle cell | 0 | 1 (8) |
| Poorly differentiated | 1 (4) | 1 (8) |
| Carcinoma | 1 (4) | |
| Basal cell carcinoma | 1 (4) | |
| Cholangiocarcinoma | 2 (8) | |
| Colorectal cancer | 2 (8) | |
| Endometrial cancer | 1 (4) | |
| Maxillary sinus cancer | 1 (4) | |
| Melanoma | 4 (15) | |
| Merkel cell carcinoma | 1 (4) | |
| Ovarian cancer | 3 (12) | |
| Pancreatic ductal adenocarcinoma | 1 (4) | |
| Pancreatic islet cell tumor | 1 (4) | |
| Sarcoma | 2 (8) | |
| Serous papillary cystadenocarcinoma | 1 (4) | |
| Squamous cell base of tongue cancer | 1 (4) |
Toxicity
Dose escalation cohort
Treatment-related adverse events are detailed in Table 2. One DLT of grade 3 fatigue possibly related to study treatment was noted and this dose level was expanded; seven patients were enrolled because of one patient being unevaluable for toxicity due to missing doses in the first cycle of study drug administration. There were no further DLTs in the remainder of the study and the trial enrolled 19 more patients on dose levels 2–5. Any patient who came off study in the first cycle of therapy not due to treatment-related toxicity was replaced as per protocol.
Table 2.
Treatment-related adverse events occurring two or more participants and all grade 3/4 treatment-related adverse events (all cycles)
| Grade 1 |
Grade 2 |
Grade 3 |
Grade 4 |
Any grade |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Toxicity | Dose escalation | Thyroid expansion | Dose escalation | Thyroid expansion | Dose escalation | Thyroid expansion | Dose escalation | Thyroid expansion | Dose escalation | Thyroid expansion |
| Abdominal pain | 3 | 0 | 2 | 0 | 2 | 1 | 0 | 0 | 7 (27%) | 1 (8%) |
| Alopecia | 1 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 1 (4%) | 3 (23%) |
| Anorexia | 8 | 3 | 3 | 3 | 0 | 0 | 0 | 0 | 11 (42%) | 6 (46%) |
| Arthralgia | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 2 (8%) | 0 |
| Blurry vision/vision changes | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (15%) |
| Constipation | 3 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 4 (15%) | 2 (15%) |
| Cracking skin | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (12%) | 0 |
| Diarrhea | 10 | 2 | 8 | 6 | 4 | 3 | 0 | 0 | 22 (85%) | 11 (85%) |
| Dizziness | 5 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 5 (19%) | 3 (23%) |
| Dry eyes/mouth | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (15%) | 0 |
| Dry skin | 4 | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (15%) | 2 (15%) |
| Edema | 5 | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 6 (23%) | 3 (23%) |
| Decreased EF | 0 | 0 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 2 (15%) |
| Epistaxis | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (12%) | 0 |
| Esophageal ulcer | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 1 (8%) |
| Fatigue | 8 | 6 | 6 | 2 | 2 | 1 | 0 | 0 | 16 (62%) | 9 (69%) |
| Flatulence | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (12%) | 0 |
| Headache | 4 | 0 | 1 | 0 | 1 | 0 | 0 | 0 | 6 (23%) | 0 |
| Heartburn | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 (12%) | 0 |
| Hypertension | 4 | 2 | 7 | 3 | 3 | 6 | 0 | 0 | 14 (54%) | 11 (85%) |
| Hypokalemia | 2 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (8%) | 0 |
| Hypomagnesemia | 3 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 3 (12%) | 1 (8%) |
| Hypopigmentation | 2 | 3 | 0 | 0 | 0 | 0 | 0 | 0 | 2 (8%) | 3 (23%) |
| Mucositis | 4 | 4 | 0 | 0 | 0 | 0 | 0 | 0 | 4 (15%) | 4 (31%) |
| Muscle cramping | 2 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 3 (12%) | 0 |
| Muscle weakness | 2 | 0 | 0 | 0 | 1 | 0 | 0 | 0 | 3 (12%) | 0 |
| Nausea/vomiting | 12 | 7 | 4 | 5 | 0 | 0 | 0 | 0 | 16 (62%) | 12 (92%) |
| Neutropenia | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 1 (4%) | 1 (8%) |
| Palmar-plantar erythrodysesthesia | 1 | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 1 (4%) | 2 (15%) |
| Proteinuria | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 0 | 2 (8%) | 1 (8%) |
| Pruritus | 2 | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 2 (8%) | 2 (15%) |
| Rash | 13 | 5 | 4 | 5 | 1 | 3 | 0 | 0 | 18 (69%) | 13 (100%) |
| Taste changes | 5 | 4 | 2 | 1 | 0 | 0 | 0 | 0 | 7 (27%) | 5 (38%) |
| Thrombocytopenia | 2 | 1 | 1 | 1 | 0 | 0 | 0 | 0 | 3 (12%) | 2 (15%) |
| Transaminitis | 3 | 1 | 0 | 2 | 5 | 1 | 0 | 0 | 8 (31%) | 4 (31%) |
| Weight loss | 3 | 2 | 1 | 0 | 0 | 1 | 0 | 0 | 4 (15%) | 1 (8%) |
There were no treatment-related deaths and no grade 4 toxicities; the most common grade 3 toxicities were transaminitis (19%), diarrhea (15%), and hypertension (12%). Common low-grade treatment-related toxicities were diarrhea, rash, hypertension, nausea/vomiting, fatigue, and anorexia. Rash was managed well with topical steroids and dosage holds as necessary; no patient was dose-reduced because of rash. Hypertension was also well managed with antihypertensive therapy.
Nine patients were dose-reduced; five had pazopanib reduced alone, one had trametinib reduced alone, and three patients had both agents reduced. Diarrhea was the reason for dose reduction of pazopanib 50% of the time, followed by fatigue and hypertension. One patient was diagnosed with glaucoma and trametinib was reduced alone for that subject. Median dose intensity for both agents was 100% (pazopanib range, 18%–100%; trametinib range, 18%–100%).
DTC expansion cohort
Further characterizing toxicity was a secondary endpoint of the expansion cohort in DTC. Generally, treatment-related adverse events were similar in the DTC expansion cohort compared with the dose escalation cohort. Nausea/ vomiting, hypertension, rash, and diarrhea were still the most common toxicities and usually low grade. Four patients discontinued study therapy due to nonprotocol-mandated adverse events and subsequent impact on quality of life at one to six cycles (median 5); low-grade diarrhea and fatigue were the common causes in these cases.
Efficacy
Dose escalation cohort
Twenty-five patients were evaluable for response. Three patients had PRs (12%) and 18 (72%) had stable disease for a disease control rate of 84% (Fig. 1). The three patients with PRs were DTC (confirmed), ovarian cancer (unconfirmed), and cholangiocarcinoma (unconfirmed). The only other patient with cholangiocarcinoma on the study had a best response of stable disease. Nine patients (35%) were on study >6 cycles (DTC: three patients; colorectal: two patients; cholangiocarcinoma, melanoma, synovial cell sarcoma, ovarian cancer: one patient each).
Figure 1.:
Best RECIST 1.1 criteria responses in dose escalation cohort. Twenty-five patients were available for response (24 patients represented in figure and patient 25 not represented because of clinical progression without imaging). Abbreviations: BCC, basal cell; CCA, cholangiocarcinoma, CRC, colorectal; EC, endometrial, FTC, follicular thyroid, MM, melanoma; MSC, maxillary sinus; OV, ovarian; PANC, pancreatic islet cell; PSC, papillary thyroid; SSC, squamous cell tongue; SS, synovial cell carcinoma; THY, thyroid; ULS, uterine leiomyosarcoma.
DTC expansion cohort
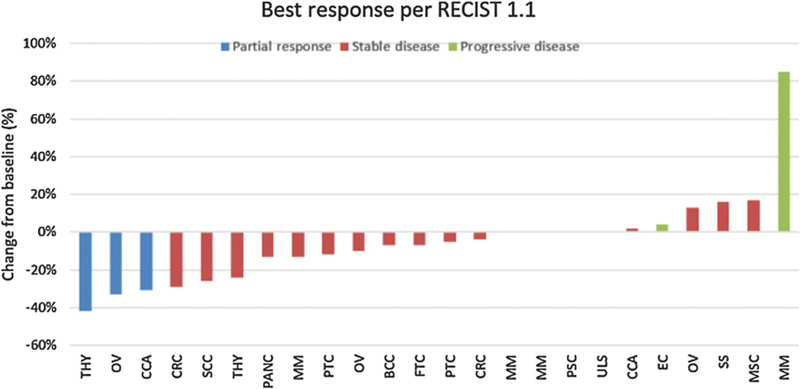
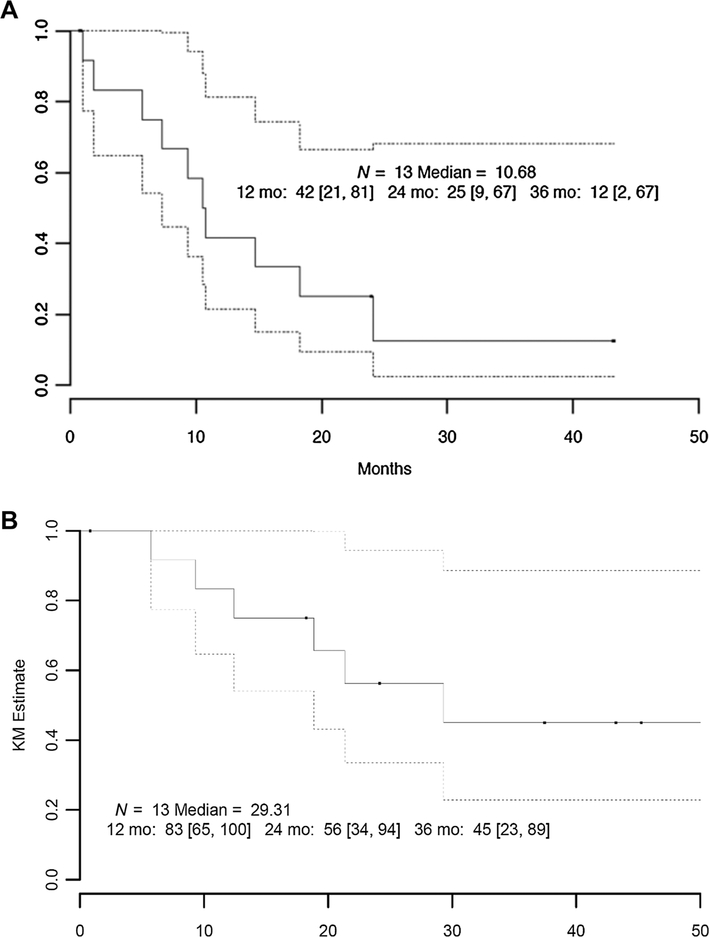
The primary endpoint being monitored for this expansion cohort was the 6-month objective response (OR) rate. Of the 13 patients enrolled on study, 12 (93%) were evaluable for disease response. Four patients had a RECIST criteria response (33%), while 50% had stable disease (Fig. 2). PFS and OS were secondary endpoints of the study. The median follow-up, calculated by the reverse Kaplan–Meier method, was 37 months. PFS and OS are shown in Fig. 3. The median PFS was 10.7 months (95% CI: 7.3, NA). The 2-year PFS was 25% (95% CI: 9%, 67%). Median survival was 29.3 months (95% CI: 18.9, NA).
Figure 2.:
Best RECIST 1.1 criteria responses in differentiated thyroid cohort. Eleven patients are represented in the figure, with the 12th evaluable patient not represented because of clinical progression without imaging.
* Represents new lesions resulting in RECIST criteria progression.
Figure 3.:
Kaplan–Meier estimate of PFS (A) and OS (B) in differentiated thyroid cohort. The dotted lines represent 95% CIs.
The interim analysis for this trial was performed after 12 patients were enrolled. At that time, the 6-month response information was available on the first 12 patients in the expansion cohort. The sample sizes in the papillary and follicular subtypes were six and six, respectively, and there had been two and two ORs in the papillary and follicular subtypes. The posterior probabilities of OR less than 25% in the papillary and less than 67% in the follicular groups were 0.287 and 0.86, respectively. Because there was an 86% probability that the response rate in the follicular group was less than 67%, the study was paused for a futility review, and ultimately halted.
Pharmacokinetics
PK data was obtained from the 13 patients on the expansion cohort of 800 mg of pazopanib and 2 mg of trametinib. For pazopanib, the Cmax, AUC0–24hr, and Css,min were 44.5 ± 31.8 μg/ mL 952.2 ± 680.0 μg*hour/mL, and 33.8 ± 17.3 μg/mL, respectively. The Css,min for trametinib was 18.7 ± 6.6 ng/mL. There were also no statistically significant correlations between the worst grade of toxicity for the most common toxicities during cycle 1 and during treatment and pazopanib or trametinib exposure (P > 0.05). There were also no statistically significant correlations between the responses and pazopanib or trametinib exposure (P > 0.05).
Pharmacodynamics
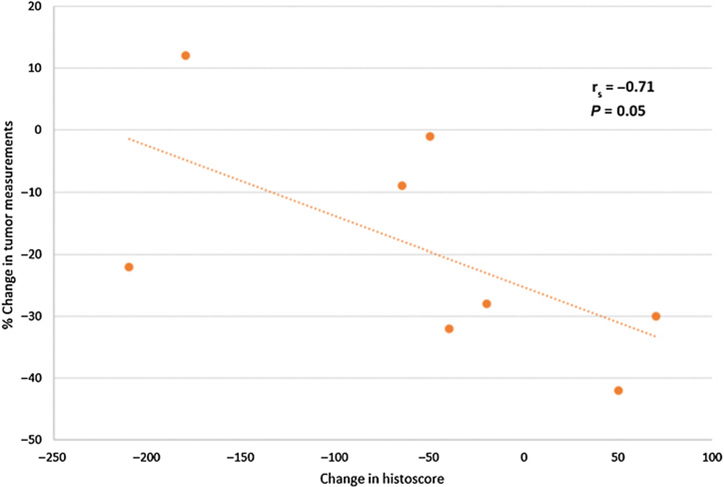
The correlation of change in p-ERK expression with best RECIST response was assessed. Of the 13 patients in the DTC cohort, four did not have tumor cells present in the posttreatment biopsy, and one patient did not have best response assessed, leaving eight patients available for this analysis. The percent reduction in tumor diameter by RECIST was negatively correlated with change in p-ERK expression posttreatment, Spearman correlation coefficient: −0.71, P = 0.05. Overall, the median change in p-ERK expression score was −50, with an interquartile range of −65 to −20 with seven of nine patients having reductions in p-ERK in posttreatment specimens (Fig. 4). There were also no statistically significant correlations between the change in p-ERK expression score and pazopanib or trametinib exposure.
Figure 4.:
Change in pERK in relation to change in tumor size. Pre- versus posttreatment biopsies were assessed for pERK. Changes in tumor size were assessed by RECIST 1.1 criteria.
Mutational testing
Eleven patients had tumor tissue available for sequencing, and 10 of these patients were evaluable for response. NRAS was present in three of three (100%) responders, and none of the seven patients with stable disease or progressive disease (Fisher exact P = 0.008). One responder did not have available tissue for sequencing. Other mutations included BRAF V600E or TP53 (two patients) and PIK3CA, SMO, CDKN2A, ALB1, and RB1 (one patient each).
Discussion
Targeting progrowth signal transduction circuits with combination molecularly targeted inhibition is a rational therapeutic strategy with notable successes in solid tumors, such as BRAF V600E–mutated metastatic melanoma and colorectal cancer (22–25). Inhibition at the signaling receptor level as well as downstream signaling proteins theoretically allows more potent inhibition of a given pathway and the potential to stymie compensatory feedback loops that result in resistance to molecularly targeted agents. We employed this strategy to target VEGF receptor signaling at the receptor level with pazopanib and downstream with the MEK inhibitor trametinib, both in tumor cells and endothelial cells. In preclinical studies in DTC, pazopanib treatment was associated with paradoxical upregulation of p-ERK, an effect that could be abrogated by concurrent trametinib (17).
We did see activity with this regimen in the expected tumor type of DTC, where we focused our primary expansion cohort, as well as cholangiocarcinoma, somewhat less expectedly. Bible and colleagues reported significant activity of single-agent pazopanib in progressive radioiodine refractory DTC, with an overall confirmed PR rate of 49% (14, 15). The median PFS in this phase II study was 360 days. Patients with follicular thyroid cancer had a 73% PR rate (8/11), whereas patients with papillary thyroid cancer had PR 33% of the time (5/11 patients). Mutational analysis and comparable PD studies are not available. This study applied a futility monitoring plan that would pause the study if there was a greater than 75% probability that the 6-month OR rate with the pazopanib/trametinib combination was not superior to pazopanib alone in the Bible study (less than 25% in the papillary subtype or less than 67% in the follicular/Hurthle cell subtype). Both the PR rate and the median PFS were not superior for the combination. Small sample size and patient selection differences in the two studies including prior treatment history could potentially skew this comparison.
MEK inhibitors have had modest activity in cholangiocarcinoma with response rates less than 10%, and have been mostly abandoned in that histology (26, 27). In addition, a single phase II study of bevacizumab and erlotinib in treatment-naÏve cholangiocarcinoma showed a response rate of 12%, suggesting the possible benefit of VEGF targeting in this rare tumor, although the relative contribution of VEGF inhibition versus EGFR inhibition is unknown for this nonrandomized trial (28). Our study showed benefit for both of the patients with cholangiocarcinoma who were enrolled (one progressive disease and one durable stable disease greater than 6 months), with the major caveat of a sample size of only two patients. Indeed, we added an expansion cohort of cholangiocarcinoma to this trial and previously reported a 4-month PFS of 40% (95% CI: 24.7%–64.6%) that showed a trend toward increased 4-month PFS as compared with the prespecified null hypothesized 4-month PFS of 25%, but this difference did not reach statistical significance (P = 0.063; ref. 29). A final expansion cohort in soft-tissue sarcoma did not show signs of compelling activity (30).
Our correlative studies yielded some interesting results in our exploratory analyses. Our initial hypothesis was that combination pazopanib and trametinib would result in significant inhibition of the MAPK signaling pathway, and that this would correlate with clinical response. Indeed, seven of nine patients with paired tumor biopsies had inhibition of p-ERK after treatment, although without single-agent comparators, we cannot determine whether it is more or less p-ERK inhibition compared with either agent alone. However, inhibition of p-ERK correlated with lack of tumor response to therapy, opposite of our initial hypothesis. Limitation of tissue and the small patient numbers did not allow us to understand the mechanism behind this exploratory finding. A possible explanation is that inhibition of the MAPK pathway may result in compensatory increases in other prosurvival pathways, resulting in resistance to this therapeutic strategy; this phenomenon was observed in a recent model of melanoma and low-grade glioma where MEK inhibition resulted in decreased p-ERK but resistance emerged with upregulation of PI3K/AKT (31, 32). A second possible explanation is that the higher levels of p-ERK are a direct effect of pazopanib treatment, comparable with effects observed in the cell culture and mouse models.
Arguably, our most interesting correlative study explored the effect of common hotspot mutations on the potential efficacy of the pazopanib/trametinib combination in our patients with DTC. Three of 11 patients with available tissue had NRAS mutations, slightly higher than expected possibly due to the patients enrolled being more advanced cases, as RAS mutations have been suggested to represent a more aggressive phenotype (8, 33). All three patients with NRAS mutations had PRs (with the final PR patient not having available tissue for sequencing). With the necessary caveats regarding the low number of patients in this analysis, the data pose an important question regarding whether NRAS-mutated tumors have a necessary addition to the MAPK signaling pathway making them uniquely sensitive to this approach. In the phase II study of the MEK inhibitor selumetinib in DTC, patients with NRAS-mutated disease had increased effectiveness of therapy in comparison with BRAF-mutated disease, although again with small numbers (16). Lenvatinib, another VEGFR/FGFR tyrosine kinase inhibitor, is approved in DTC, and a phase II study suggested that RAS-mutated patients had more benefit from lenvatinib than wild-type tumors, although this finding was not substantiated in the subsequent phase III study (34).
In conclusion, our data suggest that pazopanib and trametinib are tolerable and active in patients with advanced solid tumors, particularly in patients with DTC. The lack of single-agent comparator arms do not allow for assessment of the activity of the combination over either drug alone. However, the trial did not meet prespecified threshold for activity for the patients with follicular thyroid cancer, resulting in closing the trial for enrollment. Future study of the combination in NRAS-mutated DTC in a larger, randomized trial to truly validate the findings would be meaningful to assess whether this strategy has any role in the care of patients with DTC.
Supplementary Material
Translational Relevance.
Molecularly targeted agents have had meaningful efficacy as single agents in multiple tumor types, but have not resulted in durable benefit due to resistance coming often from increased feedback signaling. We previously demonstrated that combination targeted therapy of VEGF signaling by signal transduction inhibitors inhibiting at the receptor level and downstream using MAPK kinase (MEK) inhibition was more effective than either drug alone in preclinical models of differentiated thyroid cancer (DTC). We report a phase I clinical trial of VEGF receptor inhibitor pazopanib with MEK inhibitor trametinib with an expansion in DTC showing activity of the combination, with potential increased benefit in NRAS-mutated DTC. Our results support the hypothesis that combination-targeted therapy with pazopanib and trametinib should be further explored in this molecular subset of DTC.
Acknowledgments
We thank the patients and their families for participating in this study. This study was approved and funded by the National Comprehensive Cancer Network (NCCN) Oncology Research Program (ORP) from general research support provided by Novartis Pharmaceuticals Corporation (formerly GlaxoSmithKline, LLC). The project described was supported, in part, by the Analytical Pharmacology Core of the Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins [NIH grants P30 CA006973 and UL1 TR 001079, and the Shared Instrument Grant (S10RR026824)]. Grant Number UL1 TR 001079 is from the National Center for Advancing Translational Sciences (NCATS) a component of the NIH, and NIH Roadmap for Medical Research.
The costs of publication of this article were defrayed in part by the payment of page charges. This article must therefore be hereby marked advertisement in accordance with 18 U.S.C. Section 1734 solely to indicate this fact.
Disclosure of Potential Conflicts of Interest
R. Kurzrock is an employee of CureMatch, Inc.; reports receiving commercial research grants from Incyte, Genentech, Merck Serono, Pfizer, Sequenom, Foundation Medicine, Guardant Health, Grifols, Konica Minolta, and Omin-Seq; reports receiving speakers bureau honoraria from Roche; holds ownership interest (including patents) in IDbyDNA, CureMatch Inc., and Soluventis; and is a consultant/advisory board member for Gaido, LOXO, X-Biotech, Actuate Therapeutics, Roche and NeoMed, and Soluventis. V. Subbiah reports receiving other commercial research support from Novartis, Bayer, GlaxoSmithKline, Nanocarrier, Vegenics, Celgene, Northwestbiotherapeutics, Berghealth, Incyte, Fugifilm, Pharmamar, Inhibrx, D3, Pfizer, Multivir, Amgen, ABBVIE, Agensys, Boston Biomedical, Exelixis, Blueprint Medicines, LOXO Oncology, Takeda, and Roche. R. Sharma has immediate family members employed by NDB BIO LLC. No potential conflicts of interest were disclosed by the other authors.
Footnotes
Disclaimer
The contents of this article are solely the responsibility of the authors and do not necessarily represent the official view of the Johns Hopkins ICTR, NCATS or NIH.
Note: Supplementary data for this article are available at Clinical Cancer Research Online (http://clincancerres.aacrjournals.org/).
References
- 1.Arjaans M, Schröder CP, Oosting SF, Dafni U, Kleibeuker JE, de Vries EG. VEGF pathway targeting agents, vessel normalization and tumor drug uptake: from bench to bedside. Oncotarget 2016;7:21247–58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee SH, Jeong D, Han YS, Baek MJ. Pivotal role of vascular endothelial growth factor pathway in tumor angiogenesis. Ann Surg Treat Res 2015;89: 1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel-Rahman O Targeting vascular endothelial growth factor (VEGF) pathway in iodine-refractory differentiated thyroid carcinoma (DTC): from bench to bedside. Crit Rev Oncol Hematol 2015;94:45–54. [DOI] [PubMed] [Google Scholar]
- 4.Shah S, Brock EJ, Ji K, Mattingly RR. Ras and Rap1: a tale of two GTPases. Semin Cancer Biol 2019;54:29–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nikiforov YE. Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 2008;21:S37–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin 2018;68:7–30. [DOI] [PubMed] [Google Scholar]
- 7.Rusinek D, Chmielik E, Krajewska J, Jarzab M, Oczko-Wojciechowska M, Czarniecka A, et al. Current advances in thyroid cancer management. Are we ready for the epidemic rise of diagnoses? Int J Mol Sci 2017;18:pii: E1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Raue F, Frank-Raue K. Thyroid cancer: risk-stratified management and individualized therapy. Clin Cancer Res 2016;22:5012–21. [DOI] [PubMed] [Google Scholar]
- 9.Nikiforov YE. Thyroid cancer in 2015: molecular landscape of thyroid cancer continues to be deciphered. Nat Rev Endocrinol 2016;12:67–8. [DOI] [PubMed] [Google Scholar]
- 10.Naoum GE, Morkos M, Kim B, Arafat W. Novel targeted therapies and immunotherapy for advanced thyroid cancers. Mol Cancer 2018; 17:51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, et al. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 2015;372:621–30. [DOI] [PubMed] [Google Scholar]
- 12.van der Graaf WT, Blay JY, Chawla SP, Kim DW, Bui-Nguyen B, Casali PG, et al. EORTC Soft Tissue and Bone Sarcoma Group; PALETTE study group. Pazopanib for metastatic soft-tissue sarcoma (PALETTE): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet 2012;379:1879–86. [DOI] [PubMed] [Google Scholar]
- 13.Motzer RJ, Hutson TE, Cella D, Reeves J, Hawkins R, Guo J, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 2013;369: 722–31. [DOI] [PubMed] [Google Scholar]
- 14.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Endocrine Malignancies Disease Oriented Group, Mayo Clinic Cancer Center, and the Mayo Phase 2 Consortium. A multicenter phase 2 trial of pazopanib in metastatic and progressive medullary thyroid carcinoma: MC057H. J Clin Endocrinol Metab 2014;99:1687–93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bible KC, Suman VJ, Molina JR, Smallridge RC, Maples WJ, Menefee ME, et al. Efficacy of pazopanib in progressive, radioiodine-refractory, metastatic differentiated thyroid cancers: results of a phase 2 consortium study. Lancet Oncol 2010;11:962–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ho AL, Grewal RK, Leboeuf R, Sherman EJ, Pfister DG, Deandreis D, et al. Selumetinib-enhanced radioiodine uptake in advanced thyroid cancer. N Engl J Med 2013;368:623–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ball DW, Jin N, Xue P, Bhan S, Ahmed SR, Rosen DM, et al. Trametinib with and without pazopanib has potent preclinical activity in thyroid cancer. Oncol Rep 2015;34:2319–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schwartz LH, Litière S, de Vries E, Ford R, Gwyther S, Mandrekar S, et al. RECIST 1.1-update and clarification: from the RECIST committee. Eur J Cancer 2016;62:132–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maitra A, Ashfaq R, Gunn CR, Rahman A, Yeo CJ, Sohn TA, et al. Cyclooxygenase 2 expression in pancreatic adenocarcinoma and pancreatic intraepithelial neoplasia: an immunohistochemical analysis with automated cellular imaging. Am J Clin Pathol 2002;118:194–201. [DOI] [PubMed] [Google Scholar]
- 20.Zheng G, Tseng LH, Chen G, Haley L, Illei P, Gocke CD, et al. Clinical detection and categorization of uncommon and concomitant mutations involving BRAF. BMC Cancer 2015;15:779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Illei PB, Belchis D, Tseng LH, Nguyen D, De Marchi F, Haley L, et al. Clinical mutational profiling of 1006 lung cancers by next generation sequencing. Oncotarget 2017;8:96684–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hong DS, Morris VK, El Osta B, Sorokin AV, Janku F, Fu S, et al. Phase IB study of vemurafenib in combination with irinotecan and cetuximab in patients with metastatic colorectal cancer with BRAFV600E mutation. Cancer Discov 2016;6:1352–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Corcoran RB, Atreya CE, Falchook GS, Kwak EL, Ryan DP, Bendell JC, et al. Combined BRAF and MEK inhibition with dabrafenib and trametinib in BRAF V600-mutant colorectal cancer. J Clin Oncol 2015;33: 4023–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Long GV, Eroglu Z, Infante J, Patel S, Daud A, Johnson DB, et al. Long-term outcomes in patients with BRAF V600-mutant metastatic melanoma who received dabrafenib combined with trametinib. J Clin Oncol 2018;36: 667–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Robert C, Karaszewska B, Schachter J, Rutkowski P, Mackiewicz A, Stroiakovski D, et al. Improved overall survival in melanoma with combined dabrafenib and trametinib. N Engl J Med 2015;372:30–9. [DOI] [PubMed] [Google Scholar]
- 26.Finn RS, Javle MM, Tan BR, Weekes CD, Bendell JC, Patnaik A, et al. A phase I study of MEK inhibitor MEK162 (ARRY-438162) in patients with biliary tract cancer. J Clin Oncol 30:4s,2012(suppl; abstr TPS220). [Google Scholar]
- 27.Bekaii-Saab T, Phelps MA, Li X, Saji M, Goff L, Kauh JS, et al. Multiinstitutional phase II study of selumetinib in patients with metastatic biliary cancers. J Clin Oncol 2011;29:2357–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubner SJ, Mahoney MR, Kolesar JL, Loconte NK, Kim GP, Pitot HC, et al. Report of a multicenter phase II trial testing a combination of biweekly bevacizumab and daily erlotinib in patients with unresectable biliary cancer: a phase II Consortium study. J Clin Oncol 2010;28:3491–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Shroff RT, Yarchoan M, O’Connor A, Gallagher D, Zahurak ML, Rosner G, et al. The oral VEGF receptor tyrosine kinase inhibitor pazopanib in combination with the MEK inhibitor trametinib in advanced cholangiocarcinoma. Br J Cancer 2017;116:1402–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Subbiah V, Meyer C, Zinner R, Meric-Bernstam F,Zahurak ML, O’Connor A, et al. Phase Ib/II study of the safety and efficacy of combination therapy with multikinase VEGF inhibitor pazopanib and MEK inhibitor trametinib in advanced soft tissue sarcoma. Clin Cancer Res 2017;23:4027–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brighton HE, Angus SP, Bo T, Roques J, Tagliatela AC, Darr DB, et al. New mechanisms of resistance to MEK inhibitors in melanoma revealed by intravital imaging. Cancer Res 2018;78:542–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jain P, Silva A, Han HJ, Lang SS, Zhu Y, Boucher K, et al. Overcoming resistance to single-agent therapy for oncogenic BRAF gene fusions via combinatorial targeting of MAPK and PI3K/mTOR signaling pathways. Oncotarget 2017;8:84697–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Howell GM, Hodak SP, Yip L. RAS mutations in thyroid cancer. Oncologist 2013;18:926–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ball DW, Sherman SI, Jarzab B, Cabanillas ME, Martins R, Shah MH, et al. Lenvatinib treatment of advanced RAI-refractory differentiated thyroid cancer (DTC): cytokine and angiogenic factor (CAF) profiling in combination with tumor genetic analysis to identify markers associated with response. J Clin Oncol 30, 2012(suppl; abstr 5518). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.