Abstract
Goal:
To determine whether Excellent bowel cleansing is superior to Good for the detection of adenomas.
Background:
High quality colonoscopy requires Adequate bowel preparation. However, it is unknown whether adenoma detection differs between subcategories of Adequate cleansing.
Study:
We utilized a retrospective, cross-sectional study design to obtain data about patients undergoing colonoscopy at a single university center between August 31, 2011 and September 1, 2012. Primary outcome was adenoma detection rate (ADR), the percentage of patients with ≥ 1 adenoma. Secondary outcomes included adenomas per colonoscopy, adenoma distribution (proximal vs. distal), and detection of advanced adenomas, sessile serrated polyps (SSP), and cancer.
Results:
The electronic medical record of 5113 consecutive colonoscopies with Good or Excellent preparation was queried for preparation quality, colonoscopy indication, demographics, medical history, and history of adenoma and colon cancer. Exclusion criteria were age below 18 years, inflammatory bowel disease, or familial polyposis. Adenoma detection was not superior with Excellent cleansing as compared with Good for ADR [respectively, 26% vs. 29%, odds ratio 0.97 (0.85, 1.11), P = 0.618] or adenomas per colonoscopy [respectively, 0.437 vs. 0.499, incidence rate ratio (IRR) 0.98 (0.90, 1.07), P = 0.705]. Excellent cleansing demonstrated superior detection of SSPs [IRR 1.66 (1.14, 2.40), P = 0.008] and advanced adenomas [IRR 1.37 (1.09, 1.72), P = 0.007] but not colon cancer [odds ratio 0.286 (0.083, 0.985), P = 0.0474].
Conclusions:
ADR is not significantly different between the Adequate subcategories of Excellent and Good. However, Excellent cleansing is associated with superior detection of advanced adenomas and SSPs. If confirmed, achieving an Excellent preparation may improve colonoscopy performance in the proximal colon where SSPs primarily occur.
Keywords: colonoscopy, adenoma, colon cleansing, quality, scale
Current guidelines support the use of colonoscopy for colorectal cancer screening.1-4 Inadequate bowel preparation, occurring in as many as 20% of colonoscopies, negatively impacts a variety of colonoscopy quality measures.5-8 One such important measure, adenoma detection, is lower when colon cleansing is Inadequate.5,7,9
Whether differences within the category of Adequate cleansing are associated with clinically important differences in adenoma detection is unclear. For instance, when using the clinically popular Aronchick scale for evaluating colon cleansing, is a rating of Excellent associated with superior adenoma detection as compared with Good? One study not powered for this endpoint found better adenoma detection with Excellent cleansing.10 However, this study’s finding alone is insufficient to establish the advantage of an Excellent preparation over one that is Good.
If descriptors such as Good and Excellent have no significant impact on quality measures, their use may be counterproductive. A greater number of categories may make a grading scale unwieldy for clinicians. In addition, when the primary outcome of a study is establishing noninferiority of a purgative with respect to adequacy, secondary analyses that reveal statistically significant differences between comparator agents within the category of Adequate (proportion of Good vs. Excellent preparations) may suggest advantages that are not clinically relevant.
For grading preparation adequacy, we adopted a scale based upon the US Multi-Society Task Force on Colorectal Cancer definition that an Adequate preparation allows detection of a polyp >5 mm in any colon segment. 11 In addition, an Adequate preparation would not affect (ie, shorten) the endoscopist’s recommendation for interval colonoscopy. A preparation fulfilling both requirements with little or no flushing or suctioning was rated Excellent, and a preparation fulfilling both requirements but needing a lot of suctioning or flushing was rated Good (Table 1). Using this grading scale, we performed a retrospective analysis comparing adenoma detection with Good versus Excellent cleansing. The aim of this study was to determine whether adenoma detection is superior with an Excellent preparation.
TABLE 1.
Definition of Adequate and Inadequate Colon Preparations
| Preparation Quality |
Able to Identify ≥ 5mm Polyp Anywhere in Colon |
Would Not Shorten Surveillance Interval Due to Preparation |
Flushing and Suctioning |
|---|---|---|---|
| Adequate | |||
| Excellent | Yes | Yes | None/Minimal |
| Good | Yes | Yes | Large amount |
| Inadequate* | No or Yes | No or Yes | Not specified |
At least 1 answer of “No” defines preparation as Inadequate.
MATERIALS AND METHODS
This was a retrospective, observational study performed at a single university center. The study was approved by the Institutional Review Board at Thomas Jefferson University on July 25, 2012. Patients were excluded for the following: age below 18 years, diagnosis of inflammatory bowel disease, familial polyposis, incomplete colonoscopy, unlisted indication for colonoscopy, or missing demographic data (age, gender, or race).
An electronic endoscopic data base (Endoworks, Version 7.4.42.16, 2007, Olympus America Inc., Melville, NY) was retrospectively queried for the period August 31, 2011 to September 1, 2012 to identify colonoscopies with preparation designations of Good or Excellent. For these patients, the same data base was again queried to obtain procedure indication (screening, surveillance, symptom), personal and family history of adenoma or colorectal cancer, and the location, morphology, size, and number of polyps. Guidelines for coding polyps were developed before starting the study and reviewed with all investigators entering data to ensure consistency between investigators. A conservative approach was used so as not to overestimate polyp number. Unless a numerical description was used for polyp number, descriptions such as “few,” “several,” or “multiple” were interpreted as 2 polyps.
This group of colonoscopy patients was deidentified with respect to name. Using the medical record number, demographic and medical history was obtained by electronically querying the electronic medical record (JUPempower). This information included age, gender, race, body mass index (BMI), tobacco use, family history, and personal history of colorectal cancer. Family and personal history of adenoma was drawn only from the colonoscopy indication recorded in Endoworks, as the majority of patients did not have a history of adenoma data recorded in the electronic medical record.
For all patients in whom a biopsy or polypectomy was reported, investigators reviewed the pathology report located within our hospital’s inpatient electronic medical record. This manual review was used to classify histology (adenoma, cancer, hyperplasia, other) and other details, including sessile serrated polyp (SSP) phenotype, villous component, and degree of adenoma dysplasia (low grade vs. high grade).
About 5% of colonoscopies were performed by a Fellow together with an Attending, and the remainder by an Attending alone. Nearly all colonoscopies were performed with Olympus H180AL and CFH180 colonoscopes, although on rare occasions an Olympus CFQ160L model was used (Olympus America Inc.). The endoscopy laboratory was equipped with 3 different monitors: Olympus OEV2LIH high definition (Olympus Imaging America Inc., Center Valley, PA), National Display System SC-SX19-A1A11 LCD (NDS Surgical Imaging LCC, San Jose, CA), and Panasonic TH50PH10 plasma monitor (Panasonic System, Newark, NJ). The respective pixel resolution for each monitor was: Olympus 1920 × 1200, National Display Systems 1280 × 1024, and Panasonic 1366 × 768. The majority of colonoscopies used split dose MoviPrep (Salix Pharmaceuticals Inc., Raleigh, NC), and greater than 95% were out-patient procedures.
In March 2011, our institution adopted a new grading scale for defining Good and Excellent cleansing for colonoscopy that was utilized in this study. The grading scale has 3 categories: Excellent, Good, and Inadequate. Both Excellent and Good were considered Adequate. This scale required an adequately cleansed colon to (a) permit detection of polyps 5mm or greater in any segment of the colon and (b) not lead the physician to shorten their recommendation for interval colonoscopy. The second criterion was applied regardless of whether or not the patient was a candidate for surveillance colonoscopy. A preparation fulfilling both requirements with little or no flushing or suctioning was considered Excellent, and a preparation fulfilling both requirements but needing a lot of suctioning or flushing was considered Good (Table 1). If at least one of these 2 criteria were not fulfilled, the preparation was considered Inadequate. There are no subcategories of Inadequate in our scale. The determination of Adequate versus Inadequate is based on mucosal visualization achieved upon withdrawal. For differentiating Good from Excellent, the amount of suctioning and flushing required during the entire procedure was considered.
All physicians performing colonoscopy reviewed and agreed upon the aforementioned criteria for an Adequate preparation (including the subcategories of Good and Excellent) before the grading scale was adopted and instituted. Upon instituting this scale, the endoscopy reporting system (Endoworks) no longer defaulted to a preset cleansing assessment when creating a report, but instead required the physician to manually enter Excellent, Good, or Inadequate. The colonoscopies were performed by 29 board certified gastroenterologists (GI). We regularly audited physician compliance with respect to recording the colon cleansing assessment. Around the time of this study, 3446 examinations were audited, revealing a compliance rate of 85% over 6 months. The outliers within this audit were 2 low-volume colonoscopists whose compliance rates were 0% and 5%; together, these physicians accounted for 3.7% of the audited colonoscopies.
The primary outcome was adenoma detection rate (ADR), defined as the proportion of colonoscopies in which at least 1 adenoma was detected. ADR was compared for Excellent and Good preparations using logistic regression. We hypothesized the superiority of Excellent cleansing would be established if ADR were at least 15% higher as compared with Good. Assuming an overall ADR = 30%, with Adequate preparations comprised of 70% Good and 30% Excellent, ~4400 colonoscopies were necessary to find a 1.15 relative risk (RR) between groups with 82% power using a 2-sided α = 0.05.
Secondary outcomes included adenomas per colonoscopy (total number of adenomas/total number of colonoscopies), adenoma distribution throughout the colon, SSPs, advanced adenomas, and cancer. Advanced adenomas were defined as lesions having at least one of the following features: size ≥ 10 mm, high-grade dysplasia, or villous component. SSPs were identified based on histology rather than endoscopic appearance. At our institution, all endoscopy specimens are reviewed by dedicated GI pathologists who are experienced at identifying polyp subtypes including SSPs. Within the GI pathology group, it is a common practice to obtain a second opinion when pathologic interpretation is challenging and the name of the consulting pathologist is routinely included in the final report. We included SSPs in the advanced lesion category if ≥ 10mm in size or dysplastic.
Associations between bowel preparation classification (Good and Excellent) and clinical and demographic characteristics were summarized and tested using counts and percentages with χ2tests or mean ± SD and t tests, as appropriate. Presence of at least 1 adenoma, ADR, was analyzed as a dichotomous variable (yes or no) using multivariable logistic regression. Number of adenomas (range, 0 to 11) was analyzed using multivariable Poisson regression. Both multivariable models included the following covariates in addition to bowel preparation: age (in decades), gender (male or female), race (African American vs. non-African American), family history of colorectal cancer (yes or no), family history of adenoma (yes or no), personal history of colorectal cancer (yes or no), personal history of adenoma (yes or no), and colonoscopy indication (screening, surveillance, or symptoms). BMI data were unavailable for roughly 38% of the cohort. As such, it was not included in the multivariable analyses; however, as a sensitivity analysis, models of ADR and number of adenomas were rerun to include BMI (underweight, normal, overweight or obese) on the subset of patients (n = 3219) with BMI information available. The results of the sensitivity analyses (not shown) were nearly identical to the main results. Furthermore, <50% of all patients had data recorded in the electronic medical record under smoking status; therefore, tobacco use could not be evaluated as an independent variable between the groups.
Secondary outcomes were modeled using multivariable Poisson or logistic regression with the same covariates as the main outcome. A significance level of α = 0.05 was used for all tests. All analyses were performed in SAS 9.3 (SAS Institute Inc., Cary, NC).
RESULTS
From August 31, 2011 to September 1, 2012, 5637 patients undergoing colonoscopy with either a Good or Excellent preparation were identified. Of these, a total of 524 patients met the exclusion criteria: 453 with a diagnosis of inflammatory bowel disease, 35 due to age below 18 years, 22 because of missing data or incomplete colonoscopy, and 14 with a diagnosis of familial polyposis. A total of 5113 colonoscopies were analyzed, with 3112 having a Good preparation and 2001 having an Excellent preparation (Fig. 1). The Good and Excellent bowel preparation groups were similar with regards to gender, race, BMI, and smoking status. Differences between the study groups were observed for age, family history of polyps and colon cancer, personal history of adenoma and colon cancer, and indication for colonoscopy. Table 2 summarizes the characteristics of the 2 study groups.
FIGURE 1.
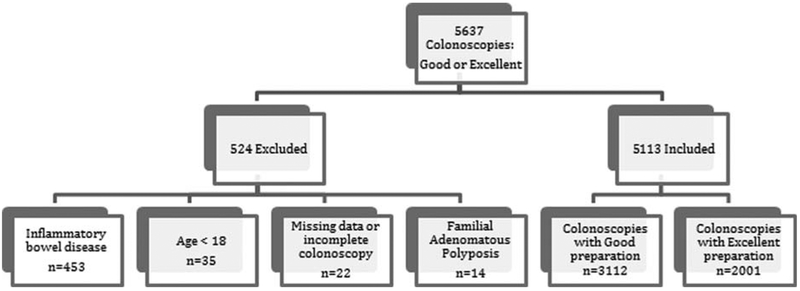
Patient flow diagram. Disposition of 5637 consecutive colonoscopies with Good or Excellent preparation.
TABLE 2.
Baseline Characteristics of Good and Excellent Groups
| Baseline Characteristics | Good Preparation (n = 3112) | Excellent Preparation (n = 2001) | P |
|---|---|---|---|
| Age (mean ± SD) | 59.6 ± 12.3 | 56.2 ± 12.9 | < 0.0001 |
| Gender [n (%)] | 0.2862 | ||
| Female | 1732 (56) | 1144 (57) | |
| Male | 1380 (44) | 857 (43) | |
| Race [n (%)] | 0.9282 | ||
| Non-African American | 2386 (77) | 1532 (77) | |
| African American | 726 (23) | 469 (23) | |
| Smoking status [n (%)] | 0.3958 | ||
| Never | 604 (51) | 415 (53) | |
| Previous | 363 (31) | 246 (32) | |
| Current | 208 (18) | 120 (15) | |
| Unknown | 1937 (62) | 1220 (61) | |
| Family history of colorectal cancer [n (%)] | 0.0001 | ||
| No | 2877 (92) | 1762 (88) | |
| Yes | 235 (8) | 239 (12) | |
| Family history of polyps [n (%)] | < 0.0001 | ||
| No | 3062 (98) | 1834 (92) | |
| Yes | 50 (2) | 167 (8) | |
| Personal history of colorectal cancer [n (%)] | 0.0001 | ||
| No | 3013 (97) | 1972 (99) | |
| Yes | 99 (3) | 29 (1) | |
| Personal history of polyp [n (%)] | < 0.0001 | ||
| No | 2389 (77) | 1674 (84) | |
| Yes | 723 (23) | 327 (16) | |
| Indication [n (%)] | < 0.0001 | ||
| Screening | 1008 (32) | 816 (41) | |
| Surveillance | 1116 (36) | 482 (24) | |
| Symptoms | 988 (32) | 703 (35) | |
| Body mass index (BMI) [n (%)] | 0.3865 | ||
| Underweight (< 18.5) | 34 (1) | 20 (1) | |
| Normal (18.5-25) | 544 (17) | 350 (17) | |
| Overweight (25-30) | 652 (21) | 444 (22) | |
| Obese (> 30) | 740 (24) | 435 (22) | |
| Unknown BMI | 1142 (37) | 752 (38) | |
Adenoma detection is summarized in Table 3. The ADR was not superior for preparations rated Excellent as compared with those rated Good (respectively, 26% vs. 29%; odds ratio for Excellent = 0.97 (0.85, 1.11), P = 0.618). Furthermore, the number of adenomas per colonoscopy was also not significantly different between Excellent and Good preparations [respectively, 0.437 vs. 0.499; incidence rate ratio (IRR) for Excellent = 0.98 (0.90, 1.07), P = 0.705]. ADR and adenomas per colonoscopy were also not significantly different between the study groups when analyzed for patients below 50 years of age and for patients 50 years of age and older.
TABLE 3.
Adenoma Detection Rate and Adenomas Per Colonoscopy
| Excellent (n = 2001) | Good (n = 3112) | Multivariable Poisson and Logistic Regression | |||
|---|---|---|---|---|---|
| OR*/IRR† | CI | P | |||
| Any adenoma [n (%)]‡ | |||||
| No | 1482 (74) | 2213 (71) | |||
| Yes | 519 (26) | 899 (29) | 0.97* | 0.85, 1.11 | 0.618 |
| No. adenomas [n (%)]§ | |||||
| 0 | 1482 (74) | 2213 (71) | |||
| 1 | 327 (16) | 557 (18) | |||
| ≥ 2 | 192 (9.6) | 342 (11) | |||
| Adenomas per colonoscopy (mean + SD)∥ | 0.437 ± 0.96 | 0.499 ± 1.04 | 0.98† | 0.90, 1.07 | 0.705 |
OR, odds ratio.
IRR, incidence rate ratio.
Any adenoma = patients with at least 1 adenoma per colonoscopy; logistic regression.
Number of adenomas = gross number of adenomas.
Adenomas per colonoscopy = number of adenomas/number of colonoscopies; multivariable Poisson.
CI indicates confidence interval; IRR, incidence rate ratio; OR, odds ratio.
Adenoma detection was not significantly different between Excellent and Good when analyzed by location within the colon. Neither proximal (proximal to splenic flexure) nor distal (splenic flexure to rectum) adenoma detection differed significantly between Excellent and Good preparations (Proximal, Excellent vs. Good: 0.272 ± 0.72 vs. 0.324 ± 0.80; IRR 0.958 (0.861, 1.065), P = 0.426) (Distal, Excellent vs. Good: 0.164 ± 0.48 vs. 0.174 ± 0.55; IRR 1.022 (0.888, 1.176), P = 0.764).
Across both groups, patient factors associated with significantly greater adenoma detection included advancing age (P < 0.001), male gender (P < 0.001), personal history of adenoma (P < 0.001), family history of polyps (P = 0.002), and colonoscopy indication of surveillance (P = 0.003). A personal history of colorectal cancer approached significance (P = 0.06).
Differences in detection of advanced adenomas and SSPs are presented in Table 4. Excellent, as compared with Good, colon cleansing was associated with superior detection of advanced adenomas (respectively, mean number advanced adenomas/colonoscopy = 0.076 vs. 0.056; IRR 1.368 (1.092, 1.715), P = 0.0065) and SSPs (respectively, mean number SSPs/colonoscopy = 0.029 vs. 0.019; IRR 1.656 (1.141, 2.403), P = 0.0079). When SSPs were excluded from the advanced adenoma analysis, the incidence of advanced adenomas between the 2 groups was no longer significantly different. Although 22 adenocarcinomas were observed overall, 3 were detected in patients with an Excellent preparation and 19 cases in patients whose preparation was Good (odds ratio for Excellent vs. Good = 0.286 (0.083, 0.985), P = 0.0474). One gastrointestinal stromal tumor and 1 carcinoid tumor were excluded from analysis.
TABLE 4.
Advanced Adenomas and Sessile Serrated
| MNP |
Total No. Polyps |
Multivariable Poisson |
|||||
|---|---|---|---|---|---|---|---|
| Type of Lesions | Excellent | Good | Excellent (n = 2001) | Good (n = 3112) | IRR* | CI | P |
| Advanced adenomas† | 0.076 ± 0.34 | 0.056 ± 0.32 | 151 | 174 | 1.368 | 1.092, 1.715 | 0.0065 |
| Sessile serrated polyps | 0.029 ± 0.29 | 0.019 ± 0.18 | 57 | 59 | 1.656 | 1.141, 2.403 | 0.0079 |
IRR, incidence rate ratio; multivariable Poisson regression.
Advanced adenomas = lesions with size ≥ 10 mm, high-grade dysplasia, or villous component.
CI indicates confidence interval; MNP, mean number of polyps.
DISCUSSION
Our study found that overall adenoma detection was not superior with Excellent colon cleansing as compared with Good. This finding was consistent whether adenoma detection was defined as number of patients having at least 1 adenoma or the number of adenomas per colonoscopy. Furthermore, Excellent cleansing was not superior for adenoma detection when considering location (proximal vs. distal colon) or age (below 50 years vs. 50 years and above). The definition of Adequate cleansing used for this study was built upon the definition supported by the US Multi-Society Task Force on Colorectal Cancer that an Adequate preparation allows detection of a polyp >5 mm in any colon segment.11 We expanded on this definition of Adequate by also requiring that the quality of bowel cleansing be good enough such that the colonoscopist would not shorten the recommendation for interval colonoscopy. This study focused on the subcategories of Adequate (Excellent and Good) defined by the amount of flushing and suctioning required to achieve an Adequate preparation.
We did find that advanced adenomas and SSPs were detected significantly more often in patients with Excellent cleansing. Advanced adenoma detection was not superior if SSPs were excluded from the analysis. Although not a primary outcome, the association between Excellent cleansing and SSPs may be important. SSPs occur more commonly in the proximal colon, are often flat, and have a mucous cap, which is an important identifying feature.12 SSPs may represent up to one-third of colon polyps with malignant potential,13-15 are difficult to identify, and their detection varies considerably between endoscopists.15,16 Failure to detect SSPs may contribute to the lower efficacy of colonoscopy for reducing proximal colon cancer mortality.17 Colon cleansing efficacy has historically been lower in the proximal colon,18 at least before guidelines recommending administering at least part of the purgative close to the time of colonoscopy.4 Although vigorous flushing can “upgrade” a preparation from Inadequate to Adequate, this may come at the cost of removing the identifying mucous cap from SSPs.
The ADR and incidence of SSPs and cancer observed in our study are within the range previously reported.16,19-26 We found that a higher incidence of cancer was detected with Good preparations over Excellent preparations. This is likely a type I error as the total number of cancers detected was low and the ability to adjust for potentially important confounding variables was limited. We suspect the overall detection of cancer would not be susceptible to the more subtle differences between Good and Excellent colon cleansing. Similar discrepancies in cancer detection have been found in prior studies.5,7 We cannot exclude that the superior detection of advanced lesions and SSPs with Excellent cleansing, a secondary outcome in this study, was also a result of a type 1 error.
To date, the evidence is tenuous that adenoma detection is superior with Excellent cleansing as compared with Good. One published study comprised of 107 patients compared the efficacy of 2 purgatives and, as a secondary outcome, evaluated the relationship between adenoma detection and Good and Excellent cleansing.10 Superior detection with Excellent cleansing led these investigators to conclude that an Excellent bowel preparation leads to improved adenoma detection compared with Good.10 In contrast, our study reported herein required review of 4400 colonoscopies to achieve 82% power to find a 1.15 RR in adenoma detection with an Excellent preparation as compared to Good. It is likely that the difference in outcomes stems from the published study’s smaller sample size.
Currently available validated grading scales for colon cleansing include the quantitative Ottawa and Boston scales and the more descriptive and qualitative Aronchick scale.27-29 The Ottawa and Boston scales assign a numerical score to each bowel segment based on mucosal cleanliness. The Ottawa scale also takes into account the amount of fluid in the colon. Increasingly used for purgative studies, these scales have not been widely adopted by clinicians. The Aronchick scale, or variants thereof, is widely used in clinical practice. This scale considers mucosal visibility and the amount of flushing and suctioning, and it grades cleansing as Poor/Inadequate, Fair, Good, or Excellent. For clinical studies, a score of Good or Excellent is generally considered Adequate, and Poor/Inadequate or Fair is classified as Inadequate.30 The Aronchick scale has fair interobserver agreement for most of the colon (intraclass correlation = 0.31 for the colon distal to the hepatic flexure) and has not been published as a full manuscript.29
Each iteration of the Multi-Society Task Force on Colorectal Cancer has suggested that an Adequate preparation is one in which a >5 mm polyp can be found in any colon segment.11,31,32 This is a practical and simple definition, taking into consideration that rarely are very small polyps malignant or have advanced histologic features. A large study by Butterly et al33 found that polyps <5 mm had a 1.7% risk for advanced histology, whereas polyps 5 to 10mm had a 10.1% risk of advanced histology. For our scale, we chose our cutoff for adequacy as ≥ 5 mm.
Recognizing that preparation quality influences the recommendation for recall,34,35 our scale considers the effect of preparation on the endoscopist’s recommendation for interval colonoscopy as a second criterion for establishing adequacy. Gastroenterologists progressively shorten the recommendation for interval colonoscopy as the colon preparation worsens.34 And, the likelihood of receiving a recommendation for a 10-year interval after screening colonoscopy is markedly greater when the preparation is Adequate.35 Because there is a high degree of noncompliance with guidelines for interval colonoscopy, our scale asks the grader to assess whether the quality of bowel cleansing would shorten their recommendation for recall and not whether the preparation quality would affect their compliance with published guidelines.36-38 For the purpose of grading the preparation, the second criterion is considered regardless of whether the patient is a candidate for interval colonoscopy. A bowel preparation meeting both criteria—ability to detect a ≥ 5mm polyp in any colon segment and no shortening of the endoscopist’s recommendation for interval colonoscopy— was considered Adequate.
There are some limitations to this study. First, this study was performed at a single, academic center, which may limit the generalizability of the results. Second, our grading scale, a modification of a nonvalidated grading scale endorsed by the Multi-Society Task Force on Colorectal Cancer, has itself not yet been validated. 11, 31, 32 One of the purposes for this study was to prepare for a validation study of our scoring system. Specifically, we sought to determine whether to validate Inadequate versus Adequate, or Inadequate versus Good and Excellent. Our primary outcome suggests a binary scale of Adequate versus Inadequate would be sufficient, whereas our secondary outcomes suggest an Excellent preparation may be advantageous and therefore, categorizing Adequate as Good or Excellent should be considered. Further confirmation of superior detection of SSPs and advanced adenomas with Excellent cleansing is warranted.
Our study is also subject to the inherent hurdles associated with a retrospective design. Because of the retrospective design, we chose as our primary outcome the ADR (the number of patients with at least 1 adenoma divided by the number of colonoscopies). Although conservative measures were used for counting multiple polyps placed in 1 specimen jar, the total number of adenomas detected in each study group is subject to a greater likelihood of miscounting than the ADR. Yet, by this measure as well (total adenomas/total colonoscopies), Excellent cleansing was not superior to Good. As our study was designed to show a RR of 1.15 for adenoma detection with Excellent cleansing, a <15% improvement in detection with Excellent cleansing cannot be excluded with this study.
As procedure duration (total, insertion, and withdrawal) was not available, we are unable to assess the effect of this variable on our findings. It is generally recognized that longer withdrawal time is associated with higher ADR.39 For SSPs, even longer withdrawal times seem to differentiate high from low detectors.40 Besides the time spent inspecting the mucosa, the effort required to achieve Adequate mucosal visualization likely influences procedure duration. We speculate that the additional flushing and/or suctioning differentiating Good from Excellent may have been associated with longer procedure times for preparations graded as Good.
Availability of the baseline patient characteristics of smoking and BMI was incomplete. Therefore, BMI and smoking status could not be analyzed as independent variables between groups. A sensitivity analysis was performed on the subset of patients with BMI data and no difference was found in the primary outcome of ADR. Furthermore, data regarding the specific colonoscope and monitor for each procedure were not captured, making us unable to evaluate the relationship between colonoscope or video monitor resolution and adenoma detection. A meta-analysis has demonstrated an adenoma detection advantage, albeit small at 3.5%, with use of a high-definition colonoscope.41 However, we believe failure to record these data had little impact on our results as nearly all colonoscopies were performed with high-definition colonoscopes, and all monitors had very good resolution.
At the time of our study, we did not have the capability to record individual Attendings’ ADRs electronically. As such, this field could not be queried for inclusion in the study. Therefore, although Excellent cleansing did not afford superior adenoma detection when all colonoscopies were considered, we cannot exclude differences within subgroups such as high and low adenoma detectors. Such differences may be important as a relationship between a doctor’s ADR and their evaluation of bowel cleansing has been observed.42 A large multicenter study by Thomas-Gibson et al42 in patients undergoing flexible sigmoidoscopy found doctors with low ADRs were more likely to grade as “Adequate” a bowel preparation that was “poor” using a standardly agreed upon definition. Of note, although this study focused on grading Adequate versus Inadequate, our study compared 2 subcategories of Adequate cleansing, Good and Excellent. For our study, such an effect may have affected the ratio of Good to Excellent between colonoscopists. Mitigating this possibility is that all endoscopists reviewed and agreed on the grading criteria prior our grading scale being adopted.
In summary, this study did not find superior adenoma detection during colonoscopy with preparations rated Excellent as compared with those that were Good. However, we did observe superior detection of both advanced adenomas and SSPs with Excellent cleansing. These data will serve as a foundation for a validation study of the grading scale utilized in this study.
Acknowledgments
D.M.K. has received grants for Thomas Jefferson University Hospitals from Salix Pharmaceutical in the past. The remaining authors declare that they have nothing to disclose.
REFERENCES
- 1.US Preventive Services Task Force. Screening for colorectal cancer: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2008;149:627–637. [DOI] [PubMed] [Google Scholar]
- 2.Whitlock EP, Lin JS, Liles E, et al. Screening for colorectal cancer: a targeted, updated systematic review for the US Preventive Services Task Force. Ann Intern Med. 2008; 149:638–658. [DOI] [PubMed] [Google Scholar]
- 3.Qaseem A, Denberg TD, Hopkins RH Jr, et al. Screening for colorectal cancer: a guidance statement from the American College of Physicians. Ann Intern Med. 2012;156:378–386. [DOI] [PubMed] [Google Scholar]
- 4.Rex DK, Johnson DA, Anderson JC, et al. American College of Gastroenterology guidelines for colorectal cancer screening 2009 [corrected]. Am J Gastroenterol. 2009;104:739–750. [DOI] [PubMed] [Google Scholar]
- 5.Harewood GC, Sharma VK, de Garmo P. Impact of colonoscopy preparation quality on detection of suspected colonic neoplasia. Gastrointest Endosc. 2003;58:76–79. [DOI] [PubMed] [Google Scholar]
- 6.Ness RM, Manam R, Hoen H, et al. Predictors of inadequate bowel preparation for colonoscopy. Am J Gastroenterol. 2001;96:1797–1802. [DOI] [PubMed] [Google Scholar]
- 7.Froehlich F, Wietlisbach V, Gonvers JJ, et al. Impact of colonic cleansing on quality and diagnostic yield of colonoscopy: the European panel of appropriateness of gastrointestinal endoscopy European multicenter study. Gastrointest Endosc. 2005;61:378–384. [DOI] [PubMed] [Google Scholar]
- 8.Sherer EA, Imler TD, Imperiale TF. The effect of colonoscopy preparation quality on adenoma detection rates. Gastrointest Endosc. 2012;75:545–553. [DOI] [PubMed] [Google Scholar]
- 9.Parra-Blanco A, Nicolas-Perez D, Gimeno-Garcia A, et al. The timing of bowel preparation before colonoscopy determines the quality of cleansing, and is a significant factor contributing to the detection of flat lesions: a randomized study. World J Gastroenterol. 2006;12:6161–6166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cohen LB, Sanyal SM, Von Althann C, et al. Clinical trial: 2-L polyethylene glycol-based lavage solutions for colonoscopy preparation—a randomized, single-blind study of two formulations. Aliment Pharmacol Ther. 2010;32:637–644. [DOI] [PubMed] [Google Scholar]
- 11.Rex DK, Bond JH, Winawer S, et al. Quality in the technical performance of colonoscopy and the continuous quality improvement process for colonoscopy: recommendations of the US Multi-Society Task Force on Colorectal Cancer. Am J Gastroenterol. 2002;97:1296–1308. [DOI] [PubMed] [Google Scholar]
- 12.Rex DK, Ahnen DJ, Baron JA, et al. Serrated lesions of the colorectum: review and recommendations from an expert panel. Am J Gastroenterol. 2012;107:1315–1329; quiz 1314, 1330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leggett B, Whitehall V. Role of the serrated pathway in colorectal cancer pathogenesis. Gastroenterology. 2010;138: 2088–2100. [DOI] [PubMed] [Google Scholar]
- 14.Boparai KS, Mathus-Vliegen EM, Koornstra JJ, et al. Increased colorectal cancer risk during follow-up in patients with hyperplastic polyposis syndrome: a multicentre cohort study. Gut. 2010;59:1094–1100. [DOI] [PubMed] [Google Scholar]
- 15.Kahi CJ, Hewett DG, Norton DL, et al. Prevalence and variable detection of proximal colon serrated polyps during screening colonoscopy. Clin Gastroenterol Hepatol. 2011;9:42–46. [DOI] [PubMed] [Google Scholar]
- 16.Hetzel JT, Huang CS, Coukos JA, et al. Variation in the detection of serrated polyps in an average risk colorectal cancer screening cohort. Am J Gastroenterol. 2010;105:2656–2664. [DOI] [PubMed] [Google Scholar]
- 17.Baxter NN, Goldwasser MA, Paszat LF, et al. Association of colonoscopy and death from colorectal cancer. Ann Intern Med. 2009;150:1–8. [DOI] [PubMed] [Google Scholar]
- 18.Frommer D Cleansing ability and tolerance of three bowel preparations for colonoscopy. Dis Colon Rectum. 1997;40: 100–104. [DOI] [PubMed] [Google Scholar]
- 19.Barclay RL, Vicari JJ, Doughty AS, et al. Colonoscopic withdrawal times and adenoma detection during screening colonoscopy. N Engl J Med. 2006;355:2533–2541. [DOI] [PubMed] [Google Scholar]
- 20.Buchner AM, Shahid MW, Heckman MG, et al. Trainee participation is associated with increased small adenoma detection. Gastrointest Endosc. 2011;73:1223–1231. [DOI] [PubMed] [Google Scholar]
- 21.de Jonge V, Sint Nicolaas J, Cahen DL, et al. Quality evaluation of colonoscopy reporting and colonoscopy performance in daily clinical practice. Gastrointest Endosc. 2012;75:98–106. [DOI] [PubMed] [Google Scholar]
- 22.Boursi B, Halak A, Umansky M, et al. Colonoscopic screening of an average-risk population for colorectal neoplasia. Endoscopy. 2009;41:516–521. [DOI] [PubMed] [Google Scholar]
- 23.Schoenfeld P, Cash B, Flood A, et al. Colonoscopic screening of average-risk women for colorectal neoplasia. N Engl J Med. 2005;352:2061–2068. [DOI] [PubMed] [Google Scholar]
- 24.Strul H, Kariv R, Leshno M, et al. The prevalence rate and anatomic location of colorectal adenoma and cancer detected by colonoscopy in average-risk individuals aged 40-80 years. Am J Gastroenterol. 2006;101:255–262. [DOI] [PubMed] [Google Scholar]
- 25.Leung WK, Tang V, Lui PC. Detection rates of proximal or large serrated polyps in Chinese patients undergoing screening colonoscopy. J Dig Dis. 2012;13:466–471. [DOI] [PubMed] [Google Scholar]
- 26.Gurudu SR, Heigh RI, De Petris G, et al. Sessile serrated adenomas: demographic, endoscopic and pathological characteristics. World J Gastroenterol. 2010;16:3402–3405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lai EJ, Calderwood AH, Doros G, et al. The Boston bowel preparation scale: a valid and reliable instrument for colonoscopy-oriented research. Gastrointest Endosc. 2009;69:620–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rostom A, Jolicoeur E. Validation of a new scale for the assessment of bowel preparation quality. Gastrointest Endosc. 2004;59:482–486. [DOI] [PubMed] [Google Scholar]
- 29.Aronchik CA, Lipshutz W, DuFrayne F, et al. Validation of an instrument to assess colon cleansing. Am J Gastroenterol. 1999;94:2667. [Google Scholar]
- 30.Kastenberg D, Chasen R, Choudhary C, et al. Efficacy and safety of sodium phosphate tablets compared with PEG solution in colon cleansing: two identically designed, randomized, controlled, parallel group, multicenter phase III trials. Gastrointest Endosc. 2001;54:705–713. [DOI] [PubMed] [Google Scholar]
- 31.Lieberman DA, Rex DK, Winawer SJ, et al. Guidelines for colonoscopy surveillance after screening and polypectomy: a consensus update by the US Multi-Society Task Force on Colorectal Cancer. Gastroenterology. 2012;143:844–857. [DOI] [PubMed] [Google Scholar]
- 32.Rex DK, Petrini JL, Baron TH, et al. Quality indicators for colonoscopy. Am J Gastroenterol. 2006;101:873–885. [DOI] [PubMed] [Google Scholar]
- 33.Butterly LF, Chase MP, Pohl H, et al. Prevalence of clinically important histology in small adenomas. Clin Gastroenterol Hepatol. 2006;4:343–348. [DOI] [PubMed] [Google Scholar]
- 34.Ben-Horin S, Bar-Meir S, Avidan B. The impact of colon cleanliness assessment on endoscopists’ recommendations for follow-up colonoscopy. Am J Gastroenterol. 2007;102:2680–2685. [DOI] [PubMed] [Google Scholar]
- 35.Shieh FK, Gunaratnam N, Mohamud SO, et al. MiraLAX-atorade bowel prep versus GoLytely before screening colonoscopy: an endoscopic database study in a community hospital. J Clin Gastroenterol. 2012;46:e96–e100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mysliwiec PA, Brown ML, Klabunde CN, et al. Are physicians doing too much colonoscopy? A national survey of colorectal surveillance after polypectomy. Ann Intern Med. 2004;141: 264–271. [DOI] [PubMed] [Google Scholar]
- 37.Boolchand V, Olds G, Singh J, et al. Colorectal screening after polypectomy: a national survey study of primary care physicians. Ann Intern Med. 2006;145:654–659. [DOI] [PubMed] [Google Scholar]
- 38.Chokshi RV, Hovis CE, Colditz GA, et al. Physician recommendations and patient adherence after inadequate bowel preparation on screening colonoscopy. Dig Dis Sci. 2013;58: 2151–2155. [DOI] [PubMed] [Google Scholar]
- 39.Butterly L, Robinson CM, Anderson JC, et al. Serrated and adenomatous polyp detection increases with longer withdrawal time: results from the New Hampshire Colonoscopy Registry. Am J Gastroenterol. 2014;109:417–426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.de Wijkerslooth TR, Stoop EM, Bossuyt PM, et al. Differences in proximal serrated polyp detection among endoscopists are associated with variability in withdrawal time. Gastrointest Endosc. 2013;77:617–623. [DOI] [PubMed] [Google Scholar]
- 41.Subramanian V, Mannath J, Hawkey CJ, et al. High definition colonoscopy vs. standard video endoscopy for the detection of colonic polyps: a meta-analysis. Endoscopy. 2011;43:499–505. [DOI] [PubMed] [Google Scholar]
- 42.Thomas-Gibson S, Rogers P, Cooper S, et al. Judgement of the quality of bowel preparation at screening flexible sigmoidoscopy is associated with variability in adenoma detection rates. Endoscopy. 2006;38:456–460. [DOI] [PubMed] [Google Scholar]


