Abstract
Background & objectives:
Stearoyl-CoA desaturase 1 (SCD1) is a key lipogenic enzyme responsible for endogenous synthesis of monounsaturated fatty acids (MUFA) and plays a key role in various pathophysiology, including fatty liver diseases. In this experimental study the impact of vitamin A deficiency was assessed on SCD1 regulation in relation to kidney biology, under high fructose (HFr) diet-fed condition in rats.
Methods:
Forty male weanling (21 day old) Wistar rats were divided into four groups control, vitamin A-deficient (VAD), HFr, VAD with HFr consisting of eight rats each, except 16 for the VAD group. The groups received one of the following diets: control, VAD, HFr and VAD with HFr for 16 wk, except half of the VAD diet-fed rats were shifted to HFr diet, after eight week period.
Results:
Feeding of VAD diet (alone or with HFr) significantly reduced the kidney retinol (0.51, 0.44 μg/g vs. 2.1 μg/g; P<0.05), while increased oleic (C18:1) and total MUFA levels (23.3, 22.2% and 27.3, 25.4% respectively vs. 14.7 and 16.6%; P<0.05) without affecting the SCD1, both at protein and mRNA levels, when compared with HFr. Comparable, immunohistological staining for SCD1 was observed in the distal convoluted tubules. Despite an increase in MUFA, morphology, triglyceride content and markers of kidney function were not affected by VAD diet feeding.
Interpretation & conclusions:
Feeding of VAD diet either alone or under HFr condition increased the kidney oleic acid (C18:1) levels and thus total MUFA, which corroborated with elevated SCD1 activity index, without affecting its expression status. However, these changes did not alter the kidney morphology and function. Thus, nutrient-gene regulation in kidney biology seems to be divergent.
Keywords: Desaturase, inflammation, injury, lipids, oleic acid, oxidative stress, steatosis, VAD
Vitamin A, the autacoid hormone, exerts a wide range of biological functions, and its role has been shown in the kidney development and function1,2,3,4,5. Previously, a study from our laboratory showed that feeding of vitamin A deficiency diet offered protection against high fructose (HFr)-induced liver steatosis through regulating the stearoyl-CoA desaturase 1 (SCD1)-catalyzed monounsaturated fatty acid (MUFA), docosahexaneonoic acid (DHA; C22:6, n-3) and its active metabolite; resolvin D1 levels6. SCD1, the microsomal enzyme converts the saturated fatty acids (SFA) into MUFA by incorporating ‘cis’ double bond at 9th position and thus known as delta-9 desaturase. Palmitic (C16:0) and stearic (C18:0) acids are the preferred substrates for SCD1 and it converts them into palmitoleic (C16:1) and oleic (C18:1) acids, respectively7.
The ratio of SFA to MUFA plays a critical role in cell membrane fluidity and function. Studies have demonstrated the key regulatory role of SCD1 in hepatic function and steatosis in HFr-induced experimental models8. In addition to well-established link between intake of HFr corn sugar-containing beverages and hepatic steatosis, epidemiological studies have found an association with renal diseases including increased blood pressure, hypertension and albuminuria in humans9,10,11,12. However, the impact of vitamin A deficiency on the regulation of SCD1 in the kidney under HFr condition has not been reported. Therefore, the present study was aimed at assessing the effect of chronic feeding of vitamin A-deficient (VAD) diet on kidney biology with special reference to SCD1 regulation in the HFr diet-fed rat model.
Material & Methods
Triglycerides, uric acid and creatinine assay kits were procured from BioSystems S.A. (Barcelona, Spain). Primary antibodies against cyclooxygenase 2 (COX2), inducible nitric oxide synthase (iNOS) and SCD1 were obtained from Santa Cruz Biotechnology, Dallas, TX, USA. Polyvinylidene fluoride membrane and enhanced chemiluminescent reagent used were from Pall Corporation (Portsmouth, UK) and Bio-Rad (Hercules, CA, USA), respectively. For quantitative real-time polymerase chain reaction analysis (qPCR), first strand cDNA synthesis kit (New England Biolabs, Ipswich, MA, USA) and VeriQuest Fast SYBR Green qPCR master mix (Affymetrix, Santa Clara, CA, USA) were used. Primary antibody for glyceraldehyde 3-phosphate dehydrogenase (GAPDH), fatty acid standards, retinol standards, secondary antibodies and Trizol reagents were from Sigma-Aldrich (St. Louis, MO, USA). Immunoprecipitation kit was of GE Healthcare Life Sciences (Marlborough, MA, USA). All the experimental diets were procured from Research Diets, Inc. New Brunswick, NJ, USA.
Experimental design: Forty male weanling (21 day old) Wistar rats were obtained from the National Centre for Laboratory Animal Sciences (now National Animal Resource Facility for Biomedical Research, NARFBR), National Institute of Nutrition, Hyderabad, India. They were broadly divided into four groups, provided isocaloric diets of AIN93G composition for eight weeks initially and designated as control, VAD, HFr and VAD with HFr (VADHFr), consisting of eight rats in each group, except VAD group, which had 16 rats. At the end of eight weeks, blood was drawn from overnight-fasted animals from retro-orbital sinus for plasma biochemical analyses; then, half of the VAD diet-fed rats were shifted to HFr diet (VAD(s)HFr) and the experiment was continued for another eight weeks with the same dietary regimen. The diet and fatty acid composition of oil used in the study were given in the Tables I and II, respectively. Animals were housed individually with an ambient temperature 22±1°C, relative humidity of 50-60 per cent, 12 h:12 h light-dark cycle. The study was approved by the Institutional Animal Ethics Committee of the National Institute of Nutrition (P07/IAEC/NIN/2012/04/SMJ/RATS WNIN M40). Weekly body weights and food intake were recorded. At the end of 16th wk, blood was collected from retro-orbital sinus, after overnight fasting and rats were sacrificed, various tissues were excised, weighed, rapidly frozen in liquid nitrogen and stored at −80°C, until further analysis.
Table I.
Composition of experimental diets
| Ingredient | Control diet | Vitamin A-deficient diet | High fructose diet | Vitamin A-deficient diet with high fructose | ||||
|---|---|---|---|---|---|---|---|---|
| g | kcal per cent | g | kcal per cent | g | kcal per cent | g | kcal per cent | |
| Casein | 200 | 800 | 200 | 800 | 200 | 800 | 200 | 800 |
| L-Cystine | 3 | 12 | 3 | 12 | 3 | 12 | 3 | 12 |
| Corn starch | 397.4 | 1590 | 397.4 | 1590 | - | - | - | - |
| Maltodextrin 10 | 132 | 528 | 132 | 528 | - | - | - | - |
| Sucrose | 100 | 400 | 100 | 400 | - | - | - | - |
| Fructose | - | - | - | - | 629.4 | 2518 | 629.4 | 2518 |
| Cellulose, BW200 | 50 | 0 | 50 | - | 50 | - | 50 | - |
| Soybean oil | 70 | 630 | 70 | 630 | 70 | 630 | 70 | 630 |
| t-Butylhydroquinone | 0.014 | - | 0.014 | - | 0.014 | - | 0.014 | - |
| Mineral mix S10022G | 35 | - | 35 | - | 35 | - | 35 | - |
| Vitamin mix V10037 Vitamin mix V13002 | 10 | 40 | - | - | 10 | 40 | - | - |
| No added vitamin A | - | - | 10 | 40 | - | - | 10 | 40 |
| Choline bitartrate | 2.5 | - | 2.5 | - | 2.5 | - | 2.5 | - |
| Total | 1000 | 4000 | 1000 | 4000 | 1000 | 4000 | 1000 | 4000 |
| Ingredient | Control diet | Vitamin A-deficient diet | High fructose diet | Vitamin A-deficient diet with high fructose | ||||
| g per cent | kcal per cent | g per cent | kcal per cent | g per cent | kcal per cent | g per cent | kcal per cent | |
| Protein | 20 | 20 | 20 | 20 | 20 | 20 | 20 | 20 |
| Carbohydrate | 64 | 64 | 64 | 64 | 64 | 64 | 64 | 64 |
| Fat | 7 | 16 | 7 | 16 | 7 | 16 | 7 | 16 |
| Total | 100 | 100 | 100 | 100 | ||||
| kcal/g | 4.0 | 4.0 | 4.0 | 4.0 | ||||
Various experimental isocaloric diets were formulated according to the AIN93G composition and supplied by Research Diets Inc., USA
Table II.
Fatty acid composition of soybean oil
| Major fatty acids | g/100 g |
|---|---|
| Myristic acid (C14:0) | 0.1 |
| Palmitic acid (C16:0) | 10.35 |
| Palmitoleic acid (C16:1) | 0.1 |
| Stearic acid (C18:0) | 3.85 |
| Oleic acid (C18:1) | 23.0 |
| Linoleic acid (C18:2) | 51.75 |
| α-linolenic acid (C18:3) | 7.4 |
| Arachidic acid (C20:0) | 0.35 |
| Paullinic acid (C20:1) | 0.25 |
| Behenic acid (C22:0) | 0.25 |
| Lignoceric acid (C24:0) | 0.15 |
Source: Research Diets Inc., USA
Biochemical analyses: Plasma uric acid and creatinine levels were measured according to the manufacturer's instruction. Retinol levels in the kidney were quantified by HPLC method as described earlier6. Kidney thiobarbituric acid reactive substances (TBARS); malondialdehyde (MDA) levels were measured according to the method of Mihara and Uchiyama13. Kidney triglyceride content, fatty acid composition and fatty acid desaturase activity indices [ratios of palmitoleic (C16:1) to palmitic (C16:0) acid and oleic (C18:1) to stearic (C18:0) acid], the indicators of SCD1 activity, were calculated as reported earlier6,14.
Kidney histology and immunohistological examination: Histological staining using haematoxylin and eosin (H and E) and immunohistological staining for SCD1 of the kidney were performed and analyzed as described earlier6.
Immunoprecipitation and immunoblotting: Kidney samples (100 mg) were homogenized in T-PER tissue protein extraction reagent (Thermo Scientific, Rockford, USA) supplemented with five per cent protease inhibitor and one per cent phosphatase inhibitor cocktails. After differential centrifugation, a constant amount of protein (10 μg) from the post-mitochondrial fraction was used for immunoprecipitation using SCD1 antibodies, according to the manufacturer's protocol and performed immunoblotting; crude homogenate of 40 μg protein was used to detect the COX2 and iNOS. Expression of GAPDH was used as a loading control and the blots were analyzed using Image J 1.46r software (National Institutes of Health, NY, USA) as reported earlier6.
Gene expression by quantitative real-time polymerase chain reaction (qPCR): Total RNA (1 μg) from the kidney was used for cDNA synthesis and quantitative PCR was performed with SYBR green fluorescent dye in LightCycler480 Real Time-PCR system (Roche Molecular Systems, Inc., CA, USA), using gene-specific primers (Integrated DNA Technologies Inc., Iowa, USA) for SCD1 (forward: 5'-AACGAGAGGGTTGGTTGT TG-3', reverse: 5'-CCCATGCCTCTGGTCTTTTA-3'). Endogenous expression of β-actin was carried out, using the following forward and reverse primers, respectively; 5'-CTTGCAGCTCCTCCGTCGCC-3’ and 5'-ACCCTGGTGCCTAGGGCGG-3', for normalization and relative expression levels were calculated as reported earlier6. The primers were synthesised from Integrated DNA Technologies, Inc., Iowa, USA.
Statistical analysis: Data were expressed as means±standard error of means. Statistical significance was determined by one-way ANOVA, with the post hoc Tukey's test. IBM SPSS statistics 19.0 software (IBM Corp., Armonk, NY, USA) was used for analyses.
Results
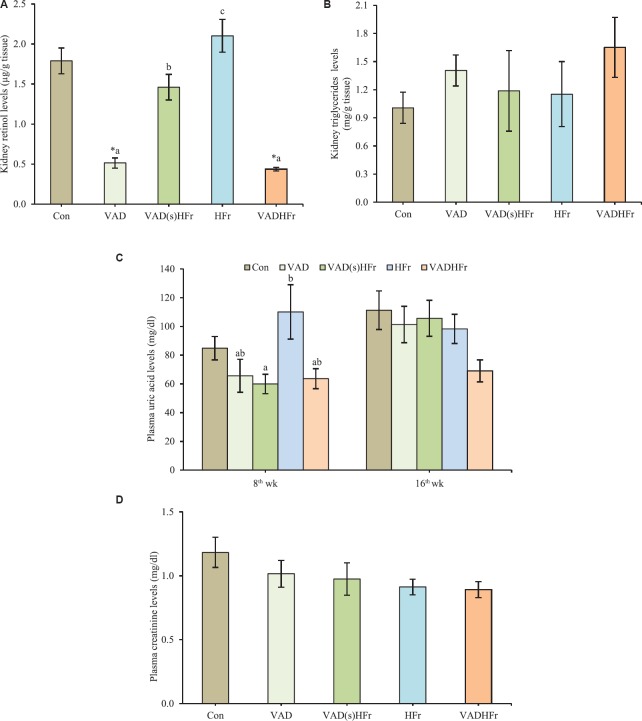
Impact of VAD on body weight, kidney biochemistry and histology: The chronic feeding of VAD diet (either alone or with HFr), displayed lower body weight and weight gain, when compared to the control and HFr-diet fed groups (Table III). However, the food intake was comparable among all the groups, except, VADHFr group, which had lower food intake6. Further, the feeding of VAD diet did not affect the kidney weight, however, found higher, when adjusted for 100 g body weight, compared to the control and HFr groups (Table III). Further, the feeding of diet that sans vitamin A [either alone (VAD) or with HFr (VADHFr)] significantly reduced the kidney retinol levels (P<0.05) and got elevated (P<0.05), when the animals were shifted from VAD to HFr diet (VAD(s)HFr), however, triglyceride contents of the kidney remained unaltered (Fig. 1A and B). In addition, no significant changes were observed with respect to uric acid levels both at 8th and 16th wk, particularly in the VAD diet-fed groups. The levels were significantly higher in the HFr diet-fed group, compared to the group that was shifted from VAD to HFr at 8th wk (Fig. 1C). The creatinine levels in plasma were comparable among all the groups (Fig. 1D).
Table III.
Impact of vitamin A-deficient diet on body weight and kidney weight
| Weight (g) | Experimental groups | ||||
|---|---|---|---|---|---|
| Control (n=8) | VAD (n=8) | VAD(s)HFr (n=8) | HFr (n=8) | VADHFr (n=7) | |
| Initial body weight | 40±1.10 | 40±0.84 | 41±1.39 | 40±1.10 | 40±1.04 |
| Final body weight | 393±16.8 | 304±15.5*,ab | 323±16.8*,ab | 356±16.2b | 282±7.3*,a |
| Kidney weight | 2.6±0.10 | 2.4±0.13 | 2.5±0.13 | 2.5±0.12 | 2.3±0.04 |
| Kidney weight for 100 g body weight | 0.66±0.20 | 0.77±0.37*,ab | 0.77±0.29*,ab | 0.71±0.15b | 0.82±0.21*,a |
Values are expressed as means±SEM and number of animals in each group is given in parenthesis. Data were analyzed by one-way ANOVA with the post hoc Tukey’s test. *P≤0.05, compared to control group and values bearing different superscripts are significantly different at P≤0.05 level. VAD, vitamin A-deficient diet; VAD(s)HFr, vitamin A-deficient diet shifted to high fructose diet; HFr, high fructose diet; VADHFr, vitamin A-deficient diet with high fructose; SEM, standard error of mean
Fig. 1.
Impact of vitamin A-deficient diet on kidney biology. (A) Kidney retinol levels, (B) kidney triglycerides levels, (C) plasma uric acid levels at 8th and 16th wk, and (D) plasma creatinine levels. Values are expressed as means±SEM of 7-8 rats from each group. Data were analyzed by one-way ANOVA with the post hoc Tukey's test. *P≤0.05, compared to control group and values bearing different superscripts are significantly different at P≤0.05 level. Con, control diet; VAD, vitamin A-deficient diet; VAD(s)HFr, vitamin A-deficient diet shifted to high fructose diet; HFr, high fructose diet; VADHFr, vitamin A-deficient diet with high fructose; SEM, standard error of mean.
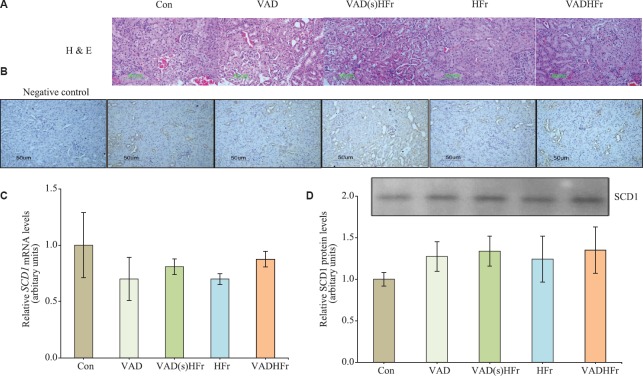
Histological examination of the kidney sections stained with H and E, revealed the normal morphological appearances without any degenerated tubules and focal fat deposits across the groups (Fig. 2A).
Fig. 2.
Impact of vitamin A-deficient diet on kidney stearoyl-CoA desaturase 1 (SCD1) expression. (A) Representative photomicrographs of histology sections of kidney stained with H and E, (B) immunohistological staining of kidney SCD1, and (C and D) expression levels of SCD1 for mRNA and protein (Representative immunoprecipitated-immunoblot) with densitometry analysis, respectively. Values are expressed as means±SEM of 4-5 rats from each group. Data were analyzed by one-way ANOVA with the post hoc Tukey's test. Photomicrographs were taken at ×20 using Nikon-Eclipse E800 microscope. Different diets as given in Fig. 1 legend.
Impact of VAD on kidney SCD1 expression and fatty acid composition: Immunohistological examination of the kidney SCD1, revealed that the staining was predominantly found in the distal convoluted tubules and comparable among groups, while the regions of proximal convoluted tubules and glomeruli stained negative for the SCD1 (Fig. 2B). Further, to confirm the expression status, kidney SCD1 at gene and protein levels were measured and found that neither vitamin A deficiency nor HFr diet had an effect on the expression levels of SCD1 (Fig. 2C and D).
Despite unaltered SCD1 expression levels, VAD diet-fed groups, displayed significant increase in oleic (C18:1) acid, with a concomitant reduction of stearic (C18:0) acid levels, as compared to that of HFr diet-fed group. Among PUFA, linoleic (C18:2, n-6), α-linolenic (C18:3, n-3) and dihomo-γ-linolenic (C20:3, n-6) acids showed an increase, while arachidonic (C20:4, n-6) and docosahexaenoic (C22:6, n-3) acids levels decreased in VAD diet-fed groups (VAD and VADHFr), compared to the group consuming HFr diet (Table IV).
Table IV.
Impact of vitamin A-deficient diet on kidney fatty acid composition
| Major fatty acids (nmol %) | Experimental groups | ||||
|---|---|---|---|---|---|
| Control | VAD | VAD(s)HFr | HFr | VADHFr | |
| Myristic acid (C14:0) | 0.85±0.19 | 1.22±0.17 | 1.06±0.21 | 1.06±0.20 | 1.20±0.18 |
| Myristoleic acid (C14:1) | 0.17±0.03 | 0.14±0.02 | 0.14±0.02 | 0.18±0.02 | 0.13±0.02 |
| Palmitic acid (C16:0) | 23.4±1.36 | 23.7±0.77 | 23.3±0.97 | 23.7±1.24 | 23.5±0.99 |
| Palmitoleic acid (C16:1) | 2.9±0.49 | 3.9±0.74 | 2.8±0.41 | 1.9±0.20 | 3.4±0.51 |
| Stearic acid (C18:0) | 13.1±1.56 | 9.9±1.37a | 11.0±1.34ab | 15.6±0.39b | 10.7±0.87a |
| Oleic acid (C18:1) | 19.4±2.52 | 23.3±1.22a | 22.0±2.16a | 14.7±0.79b | 21.9±0.85a |
| Linoleic acid (C18:2, n-6) | 18.2±0.95 | 23.6±0.67*,a | 21.6±1.59a | 16.5±0.87b | 22.0±0.60a |
| α-Linolenic acid (C18:3, n-3) | 1.0±0.23 | 1.4±0.13a | 1.1±0.17ab | 0.60±0.07b | 1.3±0.14a |
| Dihomo-γ-linolenic acid (C20:3, n-6) | 0.47±0.05 | 0.32±0.05a | 0.29±0.04a | 0.54±0.04b | 0.35±0.04a |
| Arachidonic acid (C20:4, n-6) | 18.9±2.07 | 11.5±1.10*,a | 15.4±2.52a | 23.3±1.07b | 14.3±1.04a |
| Docosapentaenoic acid (C22:5, n-3) | 0.27±0.09 | 0.13±0.04 | 0.18±0.06 | 0.29±0.08 | 0.18±0.05 |
| Docosahexaenoic acid (C22:6, n-3) | 1.4±0.23 | 0.81±0.05a | 1.1±0.19ab | 1.5±0.18b | 0.91±0.05a |
Values are expressed as means±SEM of 5 rats from each group. Data were analyzed by one-way ANOVA with the post hoc Tukey’s test. *P≤0.05 compared to control group and values bearing different superscripts are significantly different at P≤0.05 level.
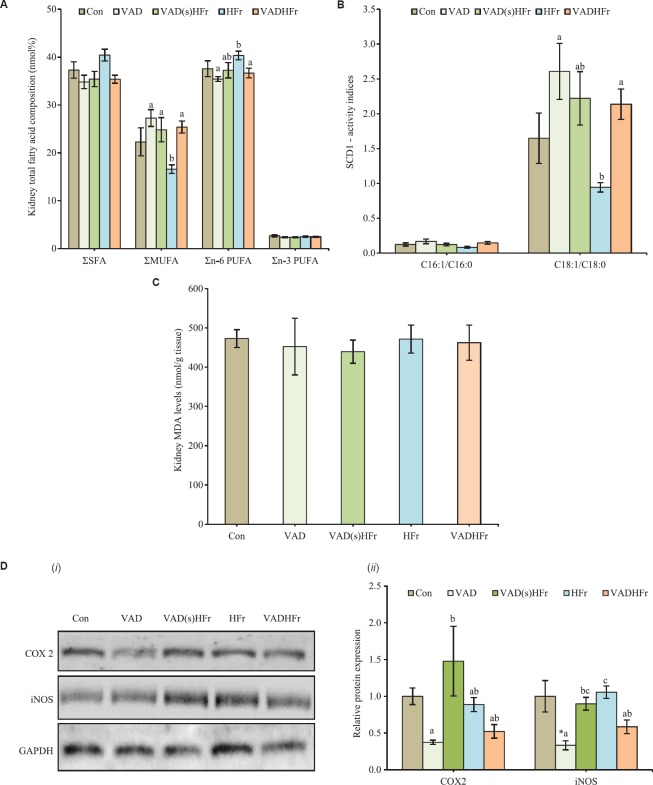
In terms of total fatty acids of various classes in the kidney, MUFA levels markedly elevated, while n-6 PUFA levels showed a reduction in response to VAD diet feeding (VAD and VADHFr) as compared with the groups that received HFr diet. However, the SFA and n-3 PUFA levels remained unaltered (Fig. 3A). The calculated fatty acid desaturase activity index for palmitoleic to palmitic (C16:1/C16:0) was comparable among groups. However, the fatty acid desaturase index for oleic to stearic (C18:1/C18:0) acids was found significantly high in the VAD diet fed groups, when compared with HFr diet-fed group (Fig. 3B).
Fig. 3.
Impact of vitamin A-deficient diet on kidney biochemistry. (A) Total fatty acids of various classes, (B) desaturase activity indices, (C) malondialdehyde (MDA) levels, and (D) representative (i) immunoblots, and (ii) densitometry analyses for cyclooxygenase 2 (COX2) and inducible nitric oxide synthase (iNOS). Expression of glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as loading control. Values are expressed as means±SEM of 5 rats from each group and 3-4 rats for immunoblot analysis. Data were analyzed by one-way ANOVA with the post hoc Tukey's test. *P≤0.05 compared to control group and values bearing different superscripts are significantly different at P≤0.05 level. Different diets as given in Fig. 1 legend. Σ, sum of saturated fatty acids (SFA) or monounsaturated fatty acids (MUFA) or n-6 polyunsaturated fatty acids (PUFA) or n-3 PUFA.
Impact of VAD on oxidative stress and inflammatory markers: One of the oxidative stress markers, MDA levels were found comparable among the groups (Fig. 3C). Although, the histological examination showed absence of inflammation, expression of proteins associated with inflammatory process, namely COX2 and iNOS were measured in the kidney. It was observed that the chronic VAD diet feeding (alone and with HFr), had no effect on the COX2, but significantly reduced the expression levels of iNOS, when compared to that of HFr diet. Notably, the COX2 protein levels markedly increased in the group that was shifted to HFr (VAD(s)HFr), as compared to that of VAD diet-fed group (Fig. 3D).
Discussion
The impact of VAD diet on kidney biology, specifically on lipid metabolism in relation to SCD1 regulation was studied in the present study. As reported earlier, though plasma and liver triglyceride levels were decreased by VAD diet feeding6; in the present study, VAD diet displayed no effect on either kidney triglyceride content or the expression status of SCD1, both at mRNA and protein levels. The MUFA:oleic (C18:1) acid levels markedly increased in VAD diet-fed group. Previously we reported that the feeding of VAD diet attenuated HFr-induced hypertriglyceridemia, hepatic triglyceride accumulation, which was partly through downregulation of liver SCD1 and the observed reduction in liver MUFA level was corroborated with decreased hepatic SCD1 levels6. Contrarily, in the kidney, despite no change in the expression levels of SCD1, the oleic acid (C18:1) levels increased in the VAD diet fed groups. In general, oleic acid (C18:1) is obtained either directly through the dietary fat source or by SCD1-mediated conversion of stearic acid (C18:0). As the dietary fat source was common for all the experimental diets, it implicated that the increased activity of SCD1, as reflected by increased fatty acid desaturase index for oleic to stearic acid (C18:1/C18;0), might be attributed for increased oleic acid (C18:1) levels. Although the specific activity of SCD1 was not measured in the present study, earlier studies from our laboratory demonstrated the positive correlation between MUFA and fatty acid desaturase activity indices, an indirect measure of SCD1 activity6,14.
Rezamand et al15 have reported the MUFA levels in various bovine tissues and its association with the mRNA and protein levels of SCD1. Although, they found a positive correlation between SCD1 expression and desaturases index of oleic to stearic (C18:1/C18:0) acid across the tissues, but failed to observe such correlation within some of the tissues studied and, concluded that association between MUFA and the abundance of SCD1 mRNA or protein appeared to be tissue specific. Similarly, our data from kidney suggested that association between SCD1 (mRNA and/or protein expression) and fatty acid desaturase activity index or MUFA levels was tissue specific.
Iwai et al16 reported that proximal tubular cells treated with MUFA displayed resistance to SFA-mediated apoptosis. In patients with diabetic nephropathy, overexpression of SCD1 has been found in the podocytes of glomeruli. Further, in the podocytes, overexpression of SCD1 was found to inhibit the SFA; palmitic acid-induced apoptosis and endoplasmic reticulum stress, and thus offering protection against free fatty acid-mediated lipotoxicity in diabetic condition17. The data suggest that increased MUFA offers protection against lipotoxicity and thus it can be speculated that under VAD condition, kidney biology undergoes adaptive changes to protect against and/or cope up with the metabolic insult.
In human mesangial cells, all trans-retinoic acid treatment has been shown to increase the expression of COX (2 and 1) and prostaglandin E2 (PGE2) and thus inflammation of kidney cells18. On the contrary, in rat glomerular mesangial cells, pre-treatment with retinoic acid has been shown to suppress the transforming growth factor-β-stimulated expression of pro-inflammatory molecule, COX2 and its catalyzed products; PGE2 and thromboxane A219. Although, the COX2 levels remained unaltered in the present study, the decreased expression of iNOS, particularly in the VAD diet fed rats along with lowered kidney retinol status indicated reduced pro-inflammatory status prevailing in the kidney. This affirmed the normal kidney function (as indicated by unaltered plasma uric acid and creatinine levels) and the absence of inflammation, as observed in the histological examinations. The present study lacked data on other parameters such as nephron mass, blood pressure and urine volume.
The observed no change in triglyceride levels was in line with the previous report of de Castro et al20, wherein HFr diet feeding did not affect the weight and lipid deposition of the kidney in younger rats of Fischer strain, while the authors observed changes at adult age and concluded age-dependant response to HFr diet. Previously, studies have reported increased oxidative stress and lipid peroxidation in liver and aorta of rats consuming VAD diet. However, the levels of MDA; the secondary product of lipid peroxidation was comparable among all the groups21,22. Over all, the data raised the concern that despite the HFr and VAD diet consumption, there were no phenotypic changes seen in the kidney. It requires further studies to understand the role of these nutrients in kidney biology. It could be possible that the duration of the study was limited or shorter to observe the phenotypic changes in the kidney, including injury and function.
In conclusion, the feeding of VAD diet showed a significant reduction in retinol levels, but elevated the kidney total MUFA, mainly due to increased oleic acid (C18:1) levels, possibly through increased SCD1 desaturase activity, as evident from the higher ratio of oleic (C18:1) to stearic (C18:0) acid, without affecting the SCD1 protein levels. Despite an increase in oleic acid (C18:1), the kidney triglyceride levels remained unaltered by VAD diet consumption, in addition to the morphology and functions. Thus, the data suggest that unlike liver, the interaction between vitamin A status and SCD1 in the kidney under HFr diet-fed condition seems to be divergent and therefore, deeper understanding of SCD1 in relation to kidney biology is required for targeting and/or treating the kidney diseases by dietary and pharmacological agents.
Acknowledgment
Shrimati K. Sharda is acknowledged for the help in sample processing for kidney histological examination. The first author (MRGR) acknowledges the Department of Science & Technology (DST), New Delhi, for the INSPIRE fellowship. Authors acknowledge Dr A. Vajreswari for critical reviewing of the manuscript.
Footnotes
Financial support & sponsorship: Authors acknowledge the Indian Council of Medical Research, New Delhi, for the financial support extended to the study (Ref no. 5/4/3-10/TF/2011/NCD-II).
Conflicts of Interest: None.
References
- 1.Jeyakumar SM, Vajreswari A. Vitamin A as a key regulator of obesity & its associated disorders: Evidences from an obese rat model. Indian J Med Res. 2015;141:275–84. doi: 10.4103/0971-5916.156554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Merlet-Bénichou C. Influence of fetal environment on kidney development. Int J Dev Biol. 1999;43:453–6. [PubMed] [Google Scholar]
- 3.Bhat PV, Manolescu DC. Role of vitamin A in determining nephron mass and possible relationship to hypertension. J Nutr. 2008;138:1407–10. doi: 10.1093/jn/138.8.1407. [DOI] [PubMed] [Google Scholar]
- 4.Goodyer P, Kurpad A, Rekha S, Muthayya S, Dwarkanath P, Iyengar A, et al. Effects of maternal vitamin A status on kidney development: A pilot study. Pediatr Nephrol. 2007;22:209–14. doi: 10.1007/s00467-006-0213-4. [DOI] [PubMed] [Google Scholar]
- 5.El-Khashab EK, Hamdy AM, Maher KM, Fouad MA, Abbas GZ. Effect of maternal vitamin A deficiency during pregnancy on neonatal kidney size. J Perinat Med. 2013;41:199–203. doi: 10.1515/jpm-2012-0026. [DOI] [PubMed] [Google Scholar]
- 6.Raja Gopal Reddy M, Pavan Kumar C, Mahesh M, Sravan Kumar M, Mullapudi Venkata S, Putcha UK, et al. Vitamin A deficiency suppresses high fructose-induced triglyceride synthesis and elevates resolvin D1 levels. Biochim Biophys Acta. 2016;1861:156–65. doi: 10.1016/j.bbalip.2015.11.005. [DOI] [PubMed] [Google Scholar]
- 7.Ntambi JM, Miyazaki M. Regulation of stearoyl-CoA desaturases and role in metabolism. Prog Lipid Res. 2004;43:91–104. doi: 10.1016/s0163-7827(03)00039-0. [DOI] [PubMed] [Google Scholar]
- 8.Flowers MT, Ntambi JM. Stearoyl-CoA desaturase and its relation to high-carbohydrate diets and obesity. Biochim Biophys Acta. 2009;1791:85–91. doi: 10.1016/j.bbalip.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chung M, Ma J, Patel K, Berger S, Lau J, Lichtenstein AH. Fructose, high-fructose corn syrup, sucrose, and nonalcoholic fatty liver disease or indexes of liver health: A systematic review and meta-analysis. Am J Clin Nutr. 2014;100:833–49. doi: 10.3945/ajcn.114.086314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shoham DA, Durazo-Arvizu R, Kramer H, Luke A, Vupputuri S, Kshirsagar A, et al. Sugary soda consumption and albuminuria: Results from the National Health and Nutrition Examination Survey, 1999-2004. PLoS One. 2008;3:e3431. doi: 10.1371/journal.pone.0003431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jalal DI, Smits G, Johnson RJ, Chonchol M. Increased fructose associates with elevated blood pressure. J Am Soc Nephrol. 2010;21:1543–9. doi: 10.1681/ASN.2009111111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Pozo SE, Schold J, Nakagawa T, Sánchez-Lozada LG, Johnson RJ, Lillo JL. Excessive fructose intake induces the features of metabolic syndrome in healthy adult men: Role of uric acid in the hypertensive response. Int J Obes (Lond) 2010;34:454–61. doi: 10.1038/ijo.2009.259. [DOI] [PubMed] [Google Scholar]
- 13.Mihara M, Uchiyama M. Determination of malonaldehyde precursor in tissues by thiobarbituric acid test. Anal Biochem. 1978;86:271–8. doi: 10.1016/0003-2697(78)90342-1. [DOI] [PubMed] [Google Scholar]
- 14.Jeyakumar SM, Lopamudra P, Padmini S, Balakrishna N, Giridharan NV, Vajreswari A. Fatty acid desaturation index correlates with body mass and adiposity indices of obesity in Wistar NIN obese mutant rat strains WNIN/Ob and WNIN/GR-Ob. Nutr Metab (Lond) 2009;6:27. doi: 10.1186/1743-7075-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rezamand P, Watts JS, Yavah KM, Mosley EE, Ma L, Corl BA, et al. Relationship between stearoyl-CoA desaturase 1 gene expression, relative protein abundance, and its fatty acid products in bovine tissues. J Dairy Res. 2014;81:333–9. doi: 10.1017/S0022029914000181. [DOI] [PubMed] [Google Scholar]
- 16.Iwai T, Kume S, Chin-Kanasaki M, Kuwagata S, Araki H, Takeda N, et al. Stearoyl-CoA desaturase-1 protects cells against lipotoxicity Mediated apoptosis in proximal tubular cells. Int J Mol Sci. 2016;17 doi: 10.3390/ijms17111868. pii E1868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sieber J, Weins A, Kampe K, Gruber S, Lindenmeyer MT, Cohen CD, et al. Susceptibility of podocytes to palmitic acid is regulated by stearoyl-CoA desaturases 1 and 2. Am J Pathol. 2013;183:735–44. doi: 10.1016/j.ajpath.2013.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alique M, Moreno V, Kitamura M, Xu Q, Lucio-Cazana FJ. Kinase-dependent, retinoic acid receptor-independent up-regulation of cyclooxygenase-2 by all-trans retinoic acid in human mesangial cells. Br J Pharmacol. 2006;149:215–25. doi: 10.1038/sj.bjp.0706842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Han J, Zhang L, Chen X, Yang B, Guo N, Fan Y. Effects of all-trans retinoic acid on signal pathway of cyclooxygenase-2 and Smad3 in transforming growth factor-β-stimulated glomerular mesangial cells. Exp Biol Med (Maywood) 2014;239:272–83. doi: 10.1177/1535370213519216. [DOI] [PubMed] [Google Scholar]
- 20.de Castro UG, dos Santos RA, Silva ME, de Lima WG, Campagnole-Santos MJ, Alzamora AC. Age-dependent effect of high-fructose and high-fat diets on lipid metabolism and lipid accumulation in liver and kidney of rats. Lipids Health Dis. 2013;12:136. doi: 10.1186/1476-511X-12-136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gatica L, Alvarez S, Gomez N, Zago MP, Oteiza P, Oliveros L, et al. Vitamin A deficiency induces prooxidant environment and inflammation in rat aorta. Free Radic Res. 2005;39:621–8. doi: 10.1080/10715760500072214. [DOI] [PubMed] [Google Scholar]
- 22.Arruda SF, Siqueira EM, de Valência FF. Vitamin A deficiency increases hepcidin expression and oxidative stress in rat. Nutrition. 2009;25:472–8. doi: 10.1016/j.nut.2008.11.030. [DOI] [PubMed] [Google Scholar]