Abstract
Significance: Four decades have passed since the first successful human embryo conceived from a fertilization in vitro. Despite all advances, success rates in assisted reproduction techniques still remain unsatisfactory and it is well established that oxidative stress can be one of the major factors causing failure in in vitro fertilization (IVF) techniques.
Recent Advances: In the past years, researchers have been shown details of the supportive role CCs play along oocyte maturation, development, and fertilization processes. Regarding redox metabolism, it is now evident that the synergism between gamete and somatic CCs is fundamental to further support a healthy embryo, since the oocyte lacks several defense mechanisms that are provided by the CCs.
Critical Issues: There are many sources of reactive oxygen species (ROS) in the female reproductive tract in vivo that can be exacerbated (or aggravated) by pathological features. While an imbalance between ROS and antioxidants can result in oxidative damage, physiological levels of ROS are essential for oocyte maturation, ovulation, and early embryonic growth where they act as signaling molecules. At the event of an assisted reproduction procedure, the cumulus/oophorus complex is exposed to additional sources of oxidative stress in vitro. The cumulus cells (CCs) play essential roles in protecting the oocytes from oxidative damage.
Future Directions: More studies are needed to elucidate redox biology in human CCs and oocyte. Also, randomized controlled trials will identify possible benefits of in vivo or in vitro administration of antioxidants for patients seeking IVF procedure.
Keywords: cumulus cells, redox, antioxidant, infertility, oocyte, IVF
Introduction
Cumulus cells (CCs) play essential roles in the oocyte's growth and maturation processes. For example, they protect the oocyte from oxidative stress damage (116) and cope with substrates that the oocyte is incapable of metabolizing (7). In the follicle, oocyte and CCs maintain an intense bidirectional communication by metabolite exchange in several biological processes. The direct communication via gap junctions allows the two cell types to exchange small molecules and ions (7,30,75). However, the CC-oocyte communication goes beyond gap junction transfer and involves oocyte-secreted factors that drive the paracrine signaling in CCs, regulating in a loop manner the CC metabolism (29). This bidirectional communication orchestrates the oocyte and follicle growth, maturation, and ovulation processes (29, 94, 95, 111). The CCs are the sensors to follicle and oocyte health, and are capable of modulating the microenvironment in response to specific demands (25).
Reactive oxygen species (ROS) are produced in the healthy follicle during physiological processes and are important for oocyte maturation (10, 32). Despite their essential role, an excessive production of ROS can be detrimental to the follicle, affecting its oocyte maturation (17, 51). As a result of oxidative stress, an exacerbated inflammatory reaction is generated in the oocyte and also in the CCs, inducing an imbalance in growth factor and cytokine production leading to a detrimental effect on reproduction (5). These insults can come from the external environment, provoked by patients' lifestyle, from the inner follicle microenvironment, originating from dysfunctional CCs, or from the oocyte itself, caused by aneuploidies or other defects of the gamete. Either way, those insults can impact on CC's redox metabolic functioning that can, therefore, act as a gatekeeper for oocytes' developmental potential. This review focuses on the supportive role of CCs, on the relationship between redox imbalance and oocyte quality, on the impact of female reproductive pathologies on oxidative stress in the cumulus/oocyte complex (COC), and suggests clinical applications of proposed biomarkers of redox activity. We also make suggestions of antioxidant management in the clinical environment. Oxygen (O2) radicals in reproduction (91), or oxidative stress in the female reproductive tract (2), on female infertility (4, 45), in the oocyte (26), in the oocyte during in vitro maturation (19, 59) and in the sperm (122) are or were earlier discussed elsewhere and are not addressed in this review.
The Intimate Relationship of CCs with Oocytes
In the mammalian ovary, oocytes are contained inside follicles, structures that when matured are composed of somatic granulosa cells and filled with follicular fluid (FF). In the antral follicle, the cumulus oophorus, a specialized subgroup of granulosa cells, are surrounding the gamete (Fig. 1) (22). CCs differentiate from mural granulosa cells by the action of oocyte-secreted factors and ovarian hormones (41). The most inner CC layers, called corona radiata, are in direct contact with the oocyte through transzonal projections. These extensions of the granulosa cells transgress the oocyte's zona pellucida and form specialized junctions with the oolemma (76).
FIG. 1.
Anatomy of the ovary. In the mature antral follicle, the oocyte is surrounded by specialized granulosa cells, named the cumulus cells, which are in contact with the follicular fluid inside the antrum. Color images are available online.
Together, CCs and oocyte form the COC, located inside the antral follicle in contact with FF, COCs are surviving in an avascular compartment. The FF is a plasma-like fluid, originating from the plasma (25) and constituting a source of COC metabolites, small signaling molecules, proteins, ROS, and antioxidants (38). The CCs are the gatekeepers for the oocyte with its surroundings. Thus, the CCs act as a biological barrier that selects and processes the metabolites that oocyte will receive.
Oxidative Phosphorylation Is the Source of Energy and Biosynthesis for the Oocyte
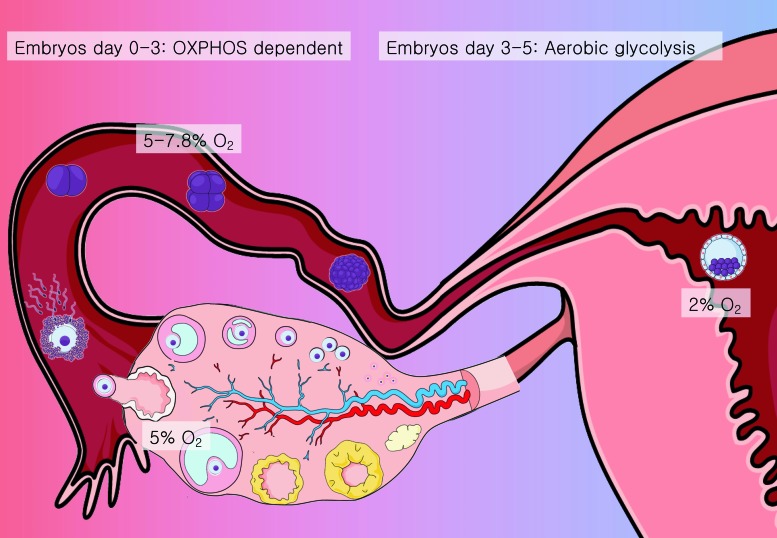
The female reproductive tract is considered a hypoxic environment, with O2 concentration variating between 2% and 8% (80) (Fig. 2), while the antral follicle is exposed to O2 concentrations between 1% and 4% (55).
FIG. 2.
O2 exposure in the female reproductive tract. The mammalian reproductive female tract is a hypoxic environment, with O2 pressure ranging between 2% and 8%. The ovaries receive around 5% O2 from the circulatory system, while the oocytes are limited to follicular fluid and cumulus cells for their supply. The COC and preimplantation embryo are adapted to this hypoxic environment. The oocyte, contained inside the antral follicle, and the embryo until day 3 stage are highly dependent on OXPHOS for energy production. From days 3 to 5, on its way across the fallopian tube to the uterus, the embryo experiences an O2 supply around 2%–5% O2 in mammals; it shifts its metabolism to aerobic glycolysis. COC, cumulus/oocyte complex; O2, oxygen; OXPHOS, oxidative phosphorylation. Color images are available online.
The oocyte does not metabolize glucose, but is highly dependent on oxidative phosphorylation (OXPHOS) to obtain energy. CCs can metabolize glucose captured in the follicle's microenvironment through glycolysis, producing pyruvate that is sent to the oocyte for further processing.
It was recently found by Dunning and colleagues (63) that mural cells and CCs have an abundant amount of hemoglobin, a molecule with high O2 affinity. This finding goes in accordance with the fact that although the oocyte relies on OXPHOS and electron transport chain reaction for energy generation, it is located inside the antral follicle, a hypoxic microenvironment with only 2% O2 available (55). The CC's proximity with oocyte and antrum allows hemoglobin to capture the slightest available O2 to be transferred to the oocyte for OXPHOS. Even though OXPHOS followed by electron transport chain reaction is the most efficient metabolic pathway for energy production in eukaryotic cells, it has a high price: it produces ROS as a by-product (47, 48). Proton leak from OXPHOS in mammalian oocytes can represent up to 37% of mitochondrial respiration, which might indicate a high response to ROS production (106). Although ROS are essential for some biological processes such as signaling molecules, they can react with biomolecules such as lipids and nucleic acids, causing cell damage and oxidative stress, harming the oocyte and lowering its quality (103).
The oocyte does not have the capacity on its own to mobilize all the necessary antioxidant defense mechanisms. This protection is provided by the surrounding CCs (102). Besides O2 rescuing, hemoglobin possesses other functions of extreme relevance in the COC: it functions as an antioxidant molecule, capable of protecting cells from oxidative stress via scavenging reactions with hydrogen peroxide (H2O2) and nitric oxide (NO). These features were discussed elsewhere (63).
The healthy (and young) female reproductive tract provides all the necessary conditions for follicle growth, oocyte maturation, and embryo development, a feature still not equally reproduced in vitro (39). While therapeutically effective, in vitro fertilization (IVF) techniques fail to replicate comparable rates of good-quality oocytes and embryos as observed in healthy individuals.
In Vivo and In Vitro Sources of Oxidative Stress in the COC
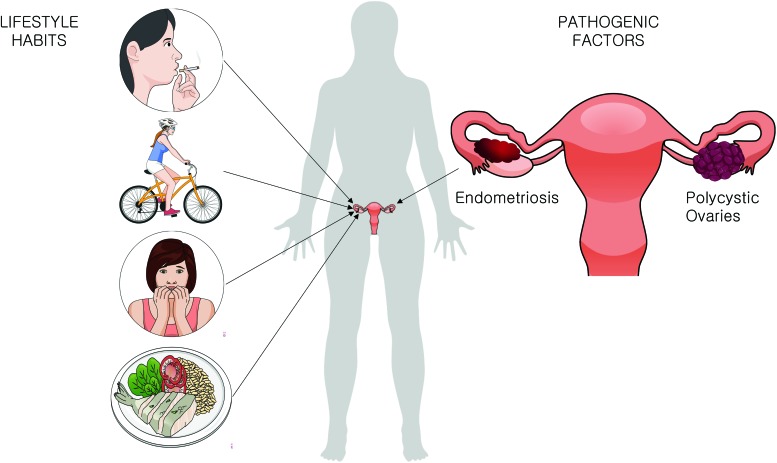
Oxidative stress and redox imbalance is known to play a significant role in infertility (8). There are several possible sources of reactive species that can impact on the COC's health, either in vivo, influenced by the women's lifestyle or physical conditions, or in vitro, during the IVF technique (88). The review by Agarwal et al. summarizes different sources of ROS in vivo and in vitro (3). Smoking (105), exercising regularly (23), the diet (56, 61), stress (86), body mass index (BMI) (43), and pathologies such as endometriosis (53) and polycystic ovary syndrome (PCOS) (97) lower the fertility capacity (2) and impact the functioning of CCs (Fig. 3).
FIG. 3.
In vivo sources of oxidative stress. There are many potential sources of ROS generation and possible oxidative stress in vivo. COCs are directly impacted by lifestyle habits such as smoking, exercising routine, stress, and nutritional habits. Besides, pathologies such as endometriosis and polycystic ovaries significantly impact on cumulus and oocyte health and functioning. ROS, reactive oxygen species. Color images are available online.
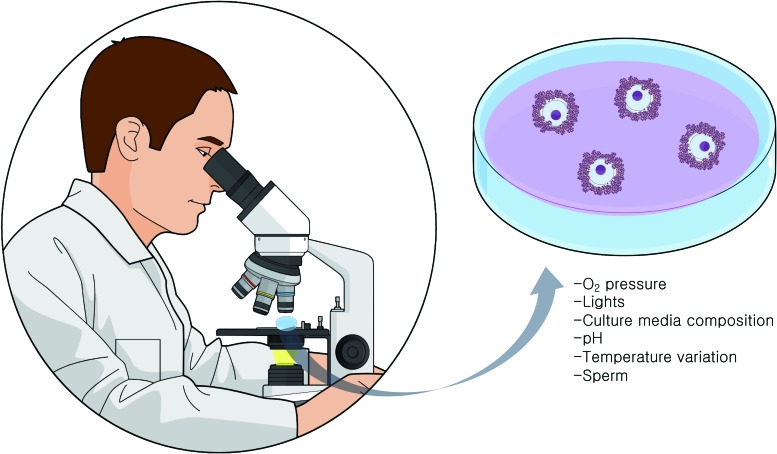
During IVF, the COCs are exposed to several potential sources of oxidative damage (3). In ovarian stimulation procedures, multiple follicles will be induced to mature through the use of gonadotropins. In this process, numerous genes related to inflammation are induced in the ovarian follicles (90). ROS originate from the inflammatory cells, which are attracted by the luteinizing hormone surge, also also by the activation of cytochrome P450 system in the steroidogenic cells of the follicle (133) (Fig. 4). Once COCs are obtained in the culture dish, variations in O2 pressure (45), exposure to visible light wavelengths (79, 107), pH, and different media compositions (68) can generate ROS. At insemination, sperm concentration and quality (96) are additional factors that directly impact on COC oxidative stress levels. It should be noted that the influences of CCs on oocyte metabolism change accordingly to environmental factors such as O2 tension (15). For example, expression levels of some antioxidant enzymes are regulated by hypoxia factors (14).
FIG. 4.
In vitro sources of oxidative stress. During in vitro fertilization techniques, the COC is exposed to several potential sources of oxidative stress. O2 pressure, visible lights, culture media composition, pH changes, temperature variations, and sperm concentrations can generate ROS, provoking an imbalance in redox potential and causing oxidative damage. Color images are available online.
CC Redox Biology
The FF in direct proximity of the COC can act like an antioxidant buffer, maintaining the redox balance in vivo (38). While some studies found significant correlations of ROS/antioxidant levels in FF and oocyte quality (21), others reported no correlation (21). Because of its origin, it might be possible that the FF composition reflects rather the plasma composition than the COC biological state.
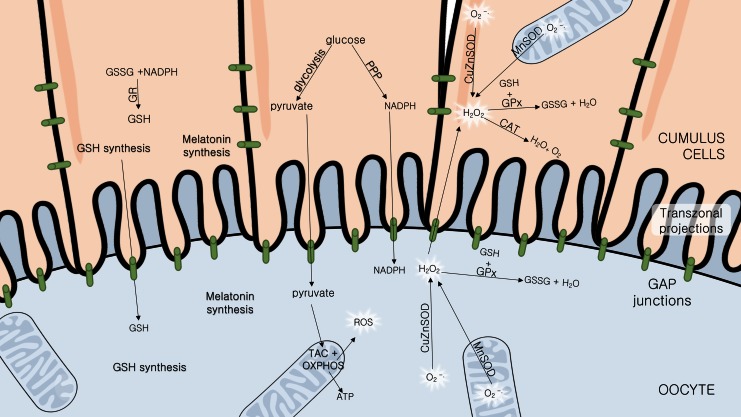
DNA damage caused by oxidative stress in granulosa cells is inversely correlated with fertilization and embryo quality rates (99). CCs are mainly responsible for the oocyte oxidative stress defense (116). When CC antioxidant capacity is low, oocyte quality might be affected (33, 77). The mature metaphase II (MII) oocyte being transcriptionally silent (135), the contribution of CC metabolite production (such as glutathione and melatonin) is even more important at this stage of maturation. Importantly, when metabolizing glucose, the CCs also generate reduced nicotinamide adenine dinucleotide phosphate (NADPH) for biosynthesis by the pentose phosphate pathway (PPP), contributing to the redox balance of the oocyte (46) (Fig. 5), deviating <3% of the already small portion of the glucose it can metabolize to PPP (124). NADPH is essential for reactive species metabolization, since it is necessary for reduced glutathione (GSH) recycling. A perfect functioning of both glycolysis and PPPs in CCs has been shown to be essential for oocyte health in mouse (62).
FIG. 5.
Cumulus cell defensive mechanisms against oxidative stress in the oocyte. The cumulus cells are connected between themselves and the oocyte through gap junctions present in the transzonal projections that permit the transfer of several molecules essential for oocyte survival. The cumulus cells capture glucose from the follicle microenvironment and process it through glycolysis, generating pyruvate that the oocyte will metabolize through the TAC+OXPHOS, producing biomolecules, energy (ATP), and ROS. ROS such as the anion superoxide (O2•−) can be detrimental. The cumulus also deviates glucose to the PPP, essential for amino acid production and NADPH recycling, a cofactor essential for antioxidant reactions. Essential defensive molecules such as GSH and NADPH are also supplied. Besides that, the cumulus is responsible for CAT production, an enzyme that metabolizes the reactive hydrogen peroxide and that is not expressed by the oocyte. The cumulus and oocyte also possess SOD, the enzyme responsible for metabolizing superoxide anion into peroxide, a less reactive form; GR promotes GSSG recycling back to the reduced form, GSH; and GPx metabolizes peroxide into water and O2, using GSH as an electron acceptor, and reduces lipid hydroperoxides. All these enzymes are involved in oxidative stress defense. SOD can be located at the cytoplasm as the copper and zinc variant (CuZnSOD), or in mitochondria as the manganese variant (MnSOD). Both cell types are capable of synthesizing melatonin, but the oocyte itself does not produce enough levels of antioxidant defenses, being dependent on the cumulus cells. CAT, catalase; CuZnSOD, copper/zinc superoxide dismutase; GPx, glutathione peroxidase; GR, glutathione reductase; GSH, reduced glutathione; GSSG, oxidized glutathione; MnSOD, manganese superoxide dismutase; NADPH, reduced nicotinamide adenine dinucleotide phosphate; PPP, pentose phosphate pathway; SOD, superoxide dismutase; TAC, tricarboxylic acid cycle. Color images are available online.
The tripeptide glutathione (GSH), the main intracellular antioxidant, plays an important protective role against oxidative damage of biomolecules and in detoxification processes (72). GSH concentrations have been related to the oocytes' meiotic spindle morphology and fertilization, and posteriorly, in early embryo development (66). It is produced by CCs (69) and oocyte (30), but optimal GSH levels in oocyte are dependent on CC synthesis (40) (Fig. 5). This was demonstrated experimentally; oocytes stripped from their CCs showed a higher cryotolerance when pretreated with an exogenous glutathione donor (121), suggesting a compensatory effect by GSH supplementation in the absence of CCs.
The CCs protect the oocyte also through the expression of several antioxidant enzymes (6). Besides low expression of phosphofructokinase (78), oocytes lack also other enzymes essential for their survival. Instead of being expressed by the gametes, these enzymes are provided by the CCs. Catalase (CAT), for example, is an antioxidant enzyme not expressed in oocytes (77). It metabolizes H2O2 into nonreactive molecules (H2O and O2) and its action is supplied by the CCs (Fig. 5). When exposed to H2O2, oocytes still inside the COC were protected from oxidative stress damage, while oocytes from which CCs have been stripped-off presented a major decrease in viability (33).
The expression of antioxidant enzymes in CCs is correlated with oocyte and embryo quality (43) and even with pregnancy rates and fetal development (70, 74). Glutathione-S-transferases (GSTs) form a large family of enzymes that protect cells from oxidative damage, lipid peroxidation of membranes caused by ROS and toxic compounds (52). Wathlet et al. correlated glutathione S-transferase alpha-3 and -4 and glutathione peroxidase (GPx) 3 in CC expression with pregnancy outcome (129). GPx3 is an extracellular, seleno-containing enzyme that catalyzes the reduction of H2O2 and lipid peroxides using GSH as cofactor (67). Therefore, it acts as an important antioxidant in reproductive biology.
Superoxide dismutase (SOD), the enzyme responsible for metabolizing the reactive superoxide anion (O2•−) into H2O2 and O2, is expressed in CCs and oocytes, in mitochondria (manganese superoxide dismutase [MnSOD]) and cytoplasm (Cu/ZnSOD). Importantly, its expression and activity level in CCs was positively correlated with successful pregnancy (70).
The inducible form of nitric oxide synthase enzyme generates nitric oxide (NO•). NO• has dual effects in the ovary. It is an essential nonpolar signaling molecule that freely diffuses through membranes. It is involved in oocyte maturation and ovulation, but in a dose-dependent matter can become a source of oxidative insult (120). CCs are the oocyte's NO• source, stimulating oocyte maturation (16). CCs from COCs that fertilize successfully synthesize less iNOS and HO-1, another redox-sensitive gene and important antioxidant enzyme, indicating that COCs with higher antioxidant activity might be reflecting a defense mechanism against an oxidative insult occurring in the follicle and related to a diminished biological capacity of the oocyte (13).
The differences in oxidative damage and ROS levels in oocytes fertilized using the classical IVF technique compared with intracytoplasmic sperm injection (ICSI) suggest that the CCs play a major role in protecting the oocyte from ROS (9). The ICSI technique consists of detaching the CCs from the oocyte to allow the injection of a single sperm through a needle inside the female germ cell (81). ICSI is mainly used in cases of severe male factor infertility, with low sperm fertilization capacity. Although ICSI represents a great advance in those cases, overcoming the difficulties of a natural fertilization, it requires the denudation of the oocyte, increasing significantly its exposure to the environment.
In classic IVF, the CCs are not removed and the oocyte fertilization by the sperm happens in a more natural way. When the biological shield of cumulus-corona is maintained, the oocyte retains a living barrier of antioxidants that protect it from external ROS sources, increased O2 tension, and exaggerated exposure to sperm (45). The improved antioxidant defenses are another element of the synergism between CCs and the gamete.
It is known that the patients' clinical characteristics such as age (70, 71, 117, 118), BMI (83, 93), infertility causes (101, 134), and stimulation protocol applied (82, 130) influence directly the CC biological characteristics. Gene and protein expression patterns, for example, change drastically according to the stimulation protocol applied, even though the success rates can remain similar (37, 49).
Pathological Patterns of Redox Metabolism in Human CCs
The important roles played by CCs to ensure oocyte's health suggest that changes in their structural or physiological composition could be related to infertility (70). CC gene expression and biochemical activity are directly influenced by the oocyte conditions, the follicular environment, and interactions with the ovarian environment. In that manner, the CC pattern might indirectly reflect the biological processes taking place in the oocyte (85).
Several studies analyzed redox compounds in the follicular environment and found significant differences between patient's pathophysiological profiles, such as endometriosis (24, 87), PCOS (125), advanced maternal age (131), and obesity (127). These differences may be a consequence of the patient's overall status/pathology, and not necessarily representative of the oocyte status; however, they need to be taken into account as potential confounders when searching for possible oocyte quality indicators. In PCOS patients, for example, the total antioxidant status in plasma is decreased (34, 126). It was also found that SOD activity in serum and FF was significantly lower; however, there was no correlation with oocyte fertilization capacity, embryo quality, or pregnancy rates (101). The significant alteration in enzyme activity can vary for each pathophysiological condition. In patients with ovarian dysfunction and endometriosis, the opposite pattern has been observed, with copper/zinc superoxide dismutase (CuZnSOD) activity being elevated (70).
Endometriosis is a pelvic inflammatory disease characterized by the occurrence of implants of endometrial tissue outside the uterine cavity, with high levels of oxidative damage in ovarian cells (98). In fact, DNA damage caused by oxidative stress in granulosa cells is higher in patients with endometriosis (99). Erratic patterns of antioxidant enzymes are known to occur in CCs from patients with PCOS and endometriosis (70).
PCOS is an endocrine and metabolic condition that causes a complex imbalance in ovarian function and ovulation process (42). In PCOS patients, CCs have shown to have a higher mitochondrial ROS production and a lower antioxidant capacity, with lower GSH/GSSG (oxidized glutathione), NADH/NAD+, and NADPH/NADP+ (oxidized nicotinamide adenine dinucleotide phosphate) ratios, suggesting enhanced oxidative stress (134). In those patients, a higher GPX3 gene expression in CCs is correlated with blastocyst formation (54).
It is clear that pathologies change the metabolic functioning and redox status of CCs. However, even though these cells are behaving differently than in the optimal physiological state, their gene expression pattern might be presenting an adaptive response to the external insults and not necessarily reflecting a poor oocyte quality. For example, CuZnSOD levels are significantly decreased in female patients with age-related infertility (67). Thus, Matos et al. correlated increased levels of SOD activity in this patient group with successful Assisted Reproduction Technique (ART) outcomes (70). This might indicate that CCs are increasing their defense mechanisms against insults that could harm their oocyte. The authors also observed, however, that in patients diagnosed with endometriosis or ovarian dysfunction, CC SOD activity is significantly increased (70).
Fertility starts to decline in an accelerated matter in women from the second half of the third decade of living (73). Aging of aerobic cells is directly related to oxidative damage caused by ROS (36). Aging is one of the most important factors in oocyte competence. It is known that the aging ovary suffers imbalances of redox metabolism and protein's carbonyl stress (117). Mature oocyte mitochondria are originated from few precursors since embryonic life. In this way, oocytes from women approaching their forty might present aged mitochondria, with higher levels of mitochondrial DNA damage and stress (26). In fact, oocytes from advanced maternal age present lower levels of messenger RNA stores and lower efficiency of DNA repair (50).
Granulosa cells of patients with advanced maternal age present an overexpression of up to 10 times higher of GST teta 1 levels (57, 58). The GST enzyme being of great importance in cellular xenobiotic detoxification, this could be indicative of a compensatory mechanism of CCs in an effort to maintain the oocyte's health. A lower expression of the genes encoding SODs (70) and catalase (119) in CCs and granulosa cells has been reported in relation to mitochondrial swelling and degeneration, as a reflection of the high levels of oxidative stress and mitochondrial dysfunctions (119). CCs from advanced maternal age patients also reveal differences both on messenger RNA and protein expressions involved in OXPHOS, mitochondrial function, and posttranscriptional splicing (71). Mitochondrial respiratory activity dependent on coenzyme Q10 is decreased in granulosa cells from older women (12). In accordance with these results, it was shown that CCs have significantly lower expression of genes involved in coenzyme Q synthesis (11).
These observations make us aware of the fact that different patient characteristics influence directly on CC functioning, with the cells adapting to potential insults. These particular effects must be taken into account in the biological functioning of the COC and in the therapeutic management in the clinics.
Targeting COC Redox Biology in the Clinical Scenario
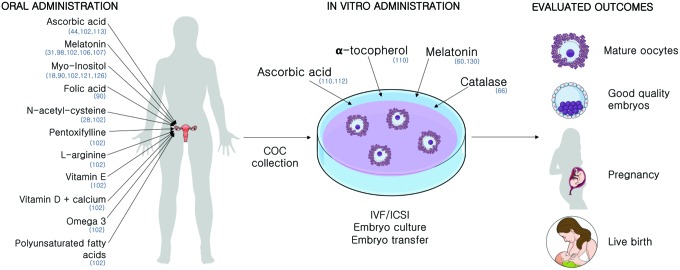
Two strategies can be adopted while using antioxidants for improving IVF outcomes: oral administration, to alleviate endogenous sources of oxidative stress such as pathologies, and the supplementation of antioxidants in the laboratory, to diminish the impact of oxidative stress caused by the in vitro environment (Fig. 6).
FIG. 6.
Antioxidant administration strategies for overcoming COC oxidative stress. Several approaches have been studied in humans. Different antioxidants have been administrated orally, in vivo, or after COC collection, in vitro. While different outcomes were evaluated, distinct time frames of administration, concentrations, patient groups, and in vitro conditions make it difficult to find comparable results. In parentheses are the referenced studies for the administration of each substance. Color images are available online.
Oral administration of antioxidants
Besides its action as a hormone driving nycthemeral rhythm by the pineal gland, melatonin turned out to possess several local properties (1). FF has higher levels of melatonin than plasma, indicating an important action in the ovary. Its role on preservation of gametes (20) and usage in ART is abundant and reviewed elsewhere (35). It is an extremely efficient antioxidant (35) produced by the COC (27) that plays a major role in the follicles' oxidative defense (89, 109, 110).
Melatonin's scavenger properties have been explored in ART (65). Several researchers have studied the effects of oral supplementation with different outcomes (100, 109). Melatonin administration improved the number of retrieved oocytes, oocyte maturation rates, and embryo development rates (31). It was also shown to improve fertilization rates from patients with previous failed IVF cycles (108).
The combined administration of melatonin and myo-inositol, a precursor of phosphoinosides, essential for oocyte maturation and embryo development, has shown positive results (128). Women with a previous failed IVF cycle received oral administration of melatonin+myo-inositol for 3 months before a new cycle showed improved oocyte maturation, fertilization, embryo number, and quality rates (123). Another study administrating myo-inositol+folic acid compared with myo-inositol+folic acid+melatonin, even though the treatment time frame was shorter, showed that melatonin improves the capability of myo-inositol to improve oocyte maturation and embryo development rates (92). Myo-inositol administration in PCOS patients was also shown to positively impact IVF outcomes (18).
The acetylated form of the amino acid l-cysteine, N-acetyl cysteine (NAC), is a powerful antioxidant and the precursor of GSH synthesis, a tripeptide of major importance in COC antioxidant defense. The oral supplementation of NAC significantly improved the number of good-quality embryos and clinical pregnancy rates and diminished granulosa cell apoptosis rates in patients (28) independently of the stimulation protocol used.
The administration of ascorbic acid, or vitamin C, in fertility treatments has been showing contradictory results. While some observed a detrimental effect (115), others have not shown any significant differences in implantation and pregnancy rates after its administration (44).
A large meta-analysis on antioxidant oral supplementation analyzing administration of combinations of antioxidants, pentoxifylline, NAC, melatonin, l-arginine, vitamin E, myo-inositol, vitamin C, vitamin D+calcium, and omega-3-polyunsaturated fatty acids against placebo administration included 28 trials involving 3548 women and showed that there is no evidence of improvement in fertility treatments using this approach (104). The authors stated that this may due to poor reporting of the outcomes and the small number of well-conducted studies. Hence, proper randomized-controlled trial (RCT) studies are required for conclusion whether oral administration of antioxidants has a positive effect on ART outcomes. Here, it is also of great importance to decide upon the most appropriate time frame as well as the optimal dosage for treatment with antioxidants.
In vitro administration of antioxidants
The administration of antioxidants on COCs in vitro has also shown some promising results. Supplementation with low concentrations of melatonin (10−2–102 nM) improved maturation rates of denuded oocytes (132). Also, the incubation of oocytes from PCOS patients with melatonin showed positive results in implantation rates (60). However, higher concentrations of melatonin medium supplementation (105–107 nM) were shown to be detrimental (132). The beneficial effects of antioxidant administration in vitro are dose dependent (113).
Besides its controversial effects in oral administration, the addition of ascorbate to IVF medium also did not improve embryo quality in cultures with 5% O2 (114).
Catalase administration decreased the rate of oxidation of COCs compared with nonsupplemented commercial media (68), implying that H2O2 is a major ROS generator in COC in vitro culture. Catalase is absent in oocytes, being physiologically supplied by CCs in vivo.
Even though the study of Tao et al. was not conducted on humans but in porcine, it brings interesting results about the contribution of CCs to the in vitro environment. While denuded porcine oocytes supplemented with α-tocopherol showed a greater rate of progression to the MII stage, the same was not observed supplementing COCs, since oocytes within the complex already have a high spontaneous MII maturation rate, reinforcing the role of CCs in oocyte maturation and protection against oxidative insult. Also, the administration of α-tocopherol and L-ascorbic acid prevented CC DNA fragmentation when cultured at 20% O2, but no effect was seen in cumulus-enclosed oocytes (112).
Just like the influence of pathologies on COC functioning in vivo, it is of major importance to consider the differences of the in vitro environment when analyzing the potential beneficial effects of antioxidant administration. In a study with bovine oocytes, the antioxidant benefits of melatonin administration were even higher when culture was conducted with exposure to a high O2 tension (84).
Conclusion
Alterations in CCs may have several causes and may be responsible for reproductive disadvantage, this being a direct cause, a reflection of a decline in the functional and structural qualities of the oocyte, or a consequence of a detrimental follicular environment that affects both the oocyte and the somatic cells. Either way, studying CCs and their relationship with oocyte quality could guide us toward valuable tools for improving routine IVF rates. Nevertheless, it is paramount to also take into consideration the patient-specific background: clinical characteristics such as age, BMI, pathological features, stimulation protocols, and in vitro environment characteristics such as O2 tension, media composition, and manipulation conditions. Many authors have indicated that all these parameters will cause distinct metabolic and gene expression patterns in COCs, directly affecting the functionality and health of the oocyte and its developmental fate.
Oxidative stress is one of the major causes of poor oocyte quality. In the healthy female tract, along with the physiological environment, the CCs provide the necessary antioxidant defenses the oocyte needs for an optimal development. In vitro conditions stand far from the ideal parameters found in vivo. More physiological culture conditions can be promoted in the clinical environment, by more sophisticated technological features. Minor parameter adjustments to optimize the cultures are within reach to obtain higher success rates in IVF. For example, FF provokes much lower levels of ROS when compared with several commonly used culture media (68). Developing a medium composition that mimics the FF in vivo, and its changes in relation to the temporal variations in hormone concentration would enable the cultured COCs to better support oocyte's metabolic needs. Critically important are also the physical characteristics of the in vivo environment, such as O2 tension and temperature, since both cell types have the ability to adapt their metabolic pathways according to those parameters. Submitting the COCs to suboptimal conditions might result in a major effort of the cells to survive, deviating their energy from biosynthetic routes to damage control process, impairing their potential for normal further development. “Ex ovo Omnia,” the potential of the oocyte in generating all life as expressed by the illustrious 17th century physician and scientist William Harvey, is still true. What has been learned over the last 50 years, thanks to the development of IVF and the access to the so far closed environment of the ovarian follicle, is that the oocyte's unique capacity to generate all cells is only effective by outsourcing critical functions to corona-CCs. The cumulus functions as a Praetorian Guard to preserve oocyte's integrity for further development; hence, these cells constitute an important target for future optimization of oocyte culture.
Future Directions
There is still a lot to unravel about the redox metabolism of CC: how it is regulated, how the external sources affect it, its responses to the environment, and how it modulates the oocyte's health. Our research addresses the redox metabolism pattern in the cumulus/corona/oocyte complexes under several culture conditions and different patient groups. The levels of redox enzymes and molecules are being correlated with embryo development and patients' characteristics. Such studies are relevant to better understand the biology of CCs, and to open new possibilities of future treatments and clinical approaches. For assessing the real effects of antioxidant administration in IVF, RCTs are indispensable.
Abbreviations Used
- ART
assisted reproduction techniques
- BMI
body mass index
- CCs
cumulus cells
- COC
cumulus/oocyte complex
- CuZnSOD
copper/zinc superoxide dismutase
- FF
follicular fluid
- GPx
glutathione peroxidase
- GSH
reduced glutathione
- GSSG
oxidized glutathione
- GST
glutathione-S-transferase
- H2O2
hydrogen peroxide
- ICSI
intracytoplasmic sperm injection
- IVF
in vitro fertilization
- MII
metaphase II
- NAC
N-acetyl cysteine
- NADPH
reduced nicotinamide adenine dinucleotide phosphate
- O2
oxygen
- OXPHOS
oxidative phosphorylation
- PCOS
polycystic ovary syndrome
- PPP
pentose phosphate pathway
- RCT
randomized-controlled trial
- ROS
reactive oxygen species
- SOD
superoxide dismutase
- TAC
tricarboxylic acid cycle
Funding Information
We thankfully appreciate the Brazilian Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) (INCT-TM/CNPq/FAPESP-465458/2014-9) funding, Brazilian Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES-PDSE 47/2017-Seleção 2018) and PRONEX/FAPERGS (16/2551-0000499-4) that made this research feasible. L.v.M. received a fellowship from CNPq and CAPES during this research and F.K. received a fellowship from MCT/CNPq (306439/2014-0).
References
- 1. Acuña-Castroviejo D, Escames G, Venegas C, Díaz-Casado ME, Lima-Cabello E, López LC, Rosales-Corral S, Tan DX, and Reiter RJ. Extrapineal melatonin: sources, regulation, and potential functions. Cell Mol Life Sci 71: 2997–3025, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Agarwal A, Aponte-Mellado A, Premkumar BJ, Shaman A, and Gupta S. The effects of oxidative stress on female reproduction: a review. Reprod Biol Endocrinol 10: 1–31, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Agarwal A, Durairajanayagam D, and du Plessis SS. Utility of antioxidants during assisted reproductive techniques: an evidence based review. Reprod Biol Endocrinol 12: 1–19, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Agarwal A, Gupta S, and Sharma R. Oxidative stress and its implications in female infertility—a clinician's perspective. Reprod Biomed Online 11: 641–650, 2005 [DOI] [PubMed] [Google Scholar]
- 5. Agarwal A, Gupta S, and Sikka S. The role of free radicals and antioxidants in reproduction. Curr Opin Obstet Gynecol 18: 325–332, 2006 [DOI] [PubMed] [Google Scholar]
- 6. Ali AA, Bilodeau JF, and Sirard MA. Antioxidant requirements for bovine oocytes varies during in vitro maturation, fertilization and development. Theriogenology 59: 939–949, 2003 [DOI] [PubMed] [Google Scholar]
- 7. Barbehenn EK, Wales RG, and Lowry OH. The explanation for the blockade of glycolysis in early mouse embryos. Proc Natl Acad Sci U S A 71: 1056–1060, 1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becatti M, Fucci R, Mannucci A, Barygina V, Mugnaini M, Criscuoli L, Giachini C, Bertocci F, Picone R, Emmi G, Evangelisti P, Rizzello F, Cozzi C, Taddei N, Fiorillo C, and Coccia ME. A biochemical approach to detect oxidative stress in infertile women undergoing assisted reproductive technology procedures. Int J Mol Sci 19: 1–15, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bedaiwy MA, Falcone T, Mohamed MS, Aleem AAN, Sharma RK, Worley SE, Thornton J, and Agarwal A. Differential growth of human embryos in vitro: role of reactive oxygen species. Fertil Steril 82: 593–600, 2004 [DOI] [PubMed] [Google Scholar]
- 10. Behrman HR, Kodaman PH, Preston SL, and Gao S. Oxidative stress and the ovary. J Soc Gynecol Investig 8: S40–S42, 2001 [DOI] [PubMed] [Google Scholar]
- 11. Ben-Meir A, Kim K, Mcquaid R, Esfandiari N, Bentov Y, Casper RF, and Jurisicova A. Co-enzyme Q10 supplementation rescues cumulus cells dysfunction in a maternal aging model. Antioxidants 8: 1–10, 2019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ben-Meir A, Yahalomi S, Moshe B, Shufaro Y, Reubinoff B, and Saada A. Coenzyme Q-dependent mitochondrial respiratory chain activity in granulosa cells is reduced with aging. Fertil Steril 104: 724–727, 2015 [DOI] [PubMed] [Google Scholar]
- 13. Bergandi L, Basso G, Evangelista F, Canosa S, Dalmasso P, Aldieri E, Revelli A, Benedetto C, and Ghigo D. Inducible nitric oxide synthase and heme oxygenase 1 are expressed in human cumulus cells and may be used as biomarkers of oocyte competence. Reprod Sci 21: 1370–1377, 2014 [DOI] [PubMed] [Google Scholar]
- 14. Bierl C, Voetsch B, Jin RC, Handy DE, and Loscalzo J. Determinants of human plasma glutathione peroxidase (GPx-3) expression. J Biol Chem 279: 26839–26845, 2004 [DOI] [PubMed] [Google Scholar]
- 15. Van Blerkom J, Davis P, and Thalhammer V. Regulation of mitochondrial polarity in mouse and human oocytes: the influence of cumulus derived nitric oxide. Mol Hum Reprod 14: 431–444, 2008 [DOI] [PubMed] [Google Scholar]
- 16. Bu S, Xia G, Tao Y, Lei L, and Zhou B. Dual effects of nitric oxide on meiotic maturation of mouse cumulus cell-enclosed oocytes in vitro. Mol Cell Endocrinol 207: 21–30, 2003 [DOI] [PubMed] [Google Scholar]
- 17. Cecconi S, Ciccarelli C, Barberi M, Macchiarelli G, and Canipari R. Granulosa cell-oocyte interactions. Eur J Obstet Gynecol Reprod Biol 115: 19–22, 2004 [DOI] [PubMed] [Google Scholar]
- 18. Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, and Gulino F. Effects of Myo-Inositol supplementation on oocyte's quality in PCOS patients: a double blind trial. Eur Rev Med Pharm Sci 15: 509–514, 2011 [PubMed] [Google Scholar]
- 19. Combelles CMH, Gupta S, and Agarwal A. Could oxidative stress influence the in-vitro maturation of oocytes? Reprod Biomed Online 18: 864–880, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cruz MHC, Leal CLV, da Cruz JF, Tan DX, and Reiter RJ. Role of melatonin on production and preservation of gametes and embryos: a brief review. Anim Reprod Sci 145: 150–160, 2014 [DOI] [PubMed] [Google Scholar]
- 21. Das S, Chattopadhyay R, Ghosh S, Ghosh S, Goswami SK, Chakravarty BN, and Chaudhury K. Reactive oxygen species level in follicular fluid—embryo quality marker in IVF? Hum Reprod 21: 2403–2407, 2006 [DOI] [PubMed] [Google Scholar]
- 22. Dekel N and Beers WH. Development of the rat oocyte in vitro: inhibition and induction of maturation in the presence or absence of the cumulus oophorus. Dev Biol 75: 247–254, 1980 [DOI] [PubMed] [Google Scholar]
- 23. Domar AD, Rooney KL, Milstein M, and Conboy L. Lifestyle habits of 12,800 IVF patients: prevalence of negative lifestyle behaviors, and impact of region and insurance coverage. Hum Fertil 18: 253–257, 2015 [DOI] [PubMed] [Google Scholar]
- 24. Donabela FC, Meola J, Padovan CC, De Paz CCP, and Navarro PA. Higher SOD1 gene expression in cumulus cells from infertile women with moderate and severe endometriosis. Reprod Sci 22: 1452–1460, 2015 [DOI] [PubMed] [Google Scholar]
- 25. Dumesic DA, Meldrum DR, Katz-Jaffe MG, Krisher RL, and Schoolcraft WB. Oocyte environment: follicular fluid and cumulus cells are critical for oocyte health. Fertil Steril 103: 303–316, 2015 [DOI] [PubMed] [Google Scholar]
- 26. Eichenlaub-Ritter U, Wieczorek M, Lüke S, and Seidel T. Age related changes in mitochondrial function and new approaches to study redox regulation in mammalian oocytes in response to age or maturation conditions. Mitochondrion 11: 783–796, 2011 [DOI] [PubMed] [Google Scholar]
- 27. El-Raey M, Geshi M, Somfai T, Kaneda M, Hirako M, Abdel-Ghaffar AE, Sosa GA, El-Roos MEAA, and Nagai T. Evidence of melatonin synthesis in the cumulus oocyte complexes and its role in enhancing oocyte maturation in vitro in cattle. Mol Reprod Dev 78: 250–262, 2011 [DOI] [PubMed] [Google Scholar]
- 28. Elgindy EA, El-Huseiny AM, Mostafa I, Gaballah AM, and Ahmed TA. N-acetyl cysteine: could it be an effective adjuvant therapy in ICSI cycles? A preliminary study. Reprod Biomed Online 20: 789–796, 2010 [DOI] [PubMed] [Google Scholar]
- 29. Eppig JJ. Intercommunication between mammalian oocytes and companion somatic cells. BioEssays 13: 569–574, 1991 [DOI] [PubMed] [Google Scholar]
- 30. Eppig JJ. Coordination of nuclear and cytoplasmic oocyte maturation in eutherian mammals. Reprod Fertil Dev 8: 485–489, 1996 [DOI] [PubMed] [Google Scholar]
- 31. Eryilmaz OG, Devran A, Sarikaya E, Aksakal FN, Mollamahmutoğlu L, and Cicek N. Melatonin improves the oocyte and the embryo in IVF patients with sleep disturbances, but does not improve the sleeping problems. J Assist Reprod Genet 28: 815–820, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Espey LL. Ovulation as an inflammatory reaction a hypothesis. Biol Reprod 22: 73–106, 1980 [DOI] [PubMed] [Google Scholar]
- 33. Fatehi AN, Roelen BAJ, Colenbrander B, Schoevers EJ, Gadella BM, Bevers MM, and Van Den Hurk R. Presence of cumulus cells during in vitro fertilization protects the bovine oocyte against oxidative stress and improves first cleavage but does not affect further development. Zygote 13: 177–185, 2005 [DOI] [PubMed] [Google Scholar]
- 34. Fenkci V, Fenkci S, Yilmazer M, and Serteser M. Decreased total antioxidant status and increased oxidative stress in women with polycystic ovary syndrome may contribute to the risk of cardiovascular disease. Fertil Steril 80: 123–127, 2003 [DOI] [PubMed] [Google Scholar]
- 35. Fernando S and Rombauts L. Melatonin: shedding light on infertility?—A review of the recent literature. J Ovarian Res 7: 1–14, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Finkel T and Holbrook NJ. Oxidants, oxidative stress and the biology of ageing. Nature 408: 239–247, 2000 [DOI] [PubMed] [Google Scholar]
- 37. Foster R, Segers I, Smart D, Adriaenssens T, Smitz J, Arce JC, and Princivalle M. A differential cytokine expression profile is induced by highly purified human menopausal gonadotropin and recombinant follicle-stimulating hormone in a pre- and postovulatory mouse follicle culture model. Fertil Steril 93: 1464–1476, 2010 [DOI] [PubMed] [Google Scholar]
- 38. Freitas C, Neto AC, Matos L, Silva E, Ribeiro Â, Silva-Carvalho JL, and Almeida H. Follicular fluid redox involvement for ovarian follicle growth. J Ovarian Res 10: 1–10, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Gad A, Besenfelder U, Rings F, Ghanem N, Hossain MM, Tesfaye D, Lonergan P, Becker A, Cinar U, Schellander K, Havlicek V, and Holker M. Effect of reproductive tract environment following controlled ovarian hyperstimulation treatment on embryo development and global transcriptome profile of blastocysts: implications for animal breeding and human assisted reproduction. Hum Reprod 26: 1693–1707, 2011 [DOI] [PubMed] [Google Scholar]
- 40. Geshi M, Takenouchi N, Yamauchi N, and Nagai T. Effects of sodium pyruvate in nonserum maturation medium on maturation, fertilization, and subsequent development of bovine oocytes with or without cumulus cells. Biol Reprod 63: 1730–1734, 2000 [DOI] [PubMed] [Google Scholar]
- 41. Gilchrist RB, Lane M, and Thompson JG. Oocyte-secreted factors: regulators of cumulus cell function and oocyte quality. Hum Reprod Update 14: 159–177, 2008 [DOI] [PubMed] [Google Scholar]
- 42. Goodman NF, Cobin RH, Futterweit W, Glueck JS, Legro RS, and Carmina E. American Association of Clinical Endocrinologists, American College of Endocrinology, and Androgen Excess and PCOS Society Disease State clinical review: guide to the best practices in the evaluation and treatment of polycystic ovary syndrome-part 2. Endocr Pract 21: 1415–1426, 2015 [DOI] [PubMed] [Google Scholar]
- 43. Gorshinova VK, Tsvirkun D V., Sukhanova IA, Tarasova N V., Volodina MA, Marey M V., Smolnikova VU, Vysokikh MY, and Sukhikh GT. Cumulus cell mitochondrial activity in relation to body mass index in women undergoing assisted reproductive therapy. BBA Clin 7: 141–146, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Griesinger G, Franke K, Kinast C, Kutzelnigg A, Kulin S, Kaali SG, and Feichtinger W. Ascorbic acid supplement during luteal phase in IVF. J Assist Reprod Genet 19: 164–168, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Gupta S, Malhotra N, Sharma D, Chandra A, and Ashok A. Oxidative stress and its role in female infertility and assisted reproduction: clinical implications. Int J Fertil Steril 2: 147–164, 2009 [Google Scholar]
- 46. Gutnisky C, Dalvit GC, Thompson JG, and Cetica PD. Pentose phosphate pathway activity: effect on in vitro maturation and oxidative status of bovine oocytes. Reprod Fertil Dev 25: 1026–1035, 2013 [DOI] [PubMed] [Google Scholar]
- 47. Halliwell B. Biochemistry of oxidative stress. Biochem Soc Trans 35: 1147–1150, 2007 [DOI] [PubMed] [Google Scholar]
- 48. Halliwell B and Gutteridge JMC. Measurement of reactive species. In: Free Radicals in Biology and Medicine, 4th ed., edited by Davies K. Oxford, United Kingdom: Clarendon Press, 2006, pp. 449–450 [Google Scholar]
- 49. Hamamah S, Matha V, Berthenet C, Anahory T, Loup V, Dechaud H, Hedon B, Fernandez A, and Lamb N. Comparative protein expression profiling in human cumulus cells in relation to oocyte fertilization and ovarian stimulation protocol. Reprod Biomed Online 13: 807–814, 2006 [DOI] [PubMed] [Google Scholar]
- 50. Hamatani T, Falco G, Carter MG, Akutsu H, Stagg CA, Sharov AA, Dudekula DB, VanBuren V, and Ko MSH. Age-associated alteration of gene expression patterns in mouse oocytes. Hum Mol Genet 13: 2263–2278, 2004 [DOI] [PubMed] [Google Scholar]
- 51. Harvey A, Kind KL, and Thompson JG. REDOX regulation of early embryo development. Reproduction 123: 479–486, 2002 [DOI] [PubMed] [Google Scholar]
- 52. Hayes JD and Pulford DJ. The glutathione S-transferase super- gene family regulation of GST* and the contribution of theisoenzymes to cancer chemoprotection and drug resistance. Crit Rev Biochem Mol Biol 30: 445–460, 1995 [DOI] [PubMed] [Google Scholar]
- 53. Hsu AL, Townsend PM, Oehninger S, and Castora FJ. Endometriosis may be associated with mitochondrial dysfunction in cumulus cells from subjects undergoing in vitro fertilization-intracytoplasmic sperm injection, as reflected by decreased adenosine triphosphate production. Fertil Steril 103: 347.e1–352.e1, 2015 [DOI] [PubMed] [Google Scholar]
- 54. Huang X, Hao C, Shen X, Zhang Y, and Liu X. RUNX2, GPX3 and PTX3 gene expression profiling in cumulus cells are reflective oocyte/embryo competence and potentially reliable predictors of embryo developmental competence in PCOS patients. Reprod Biol Endocrinol 11: 1–10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Huey S, Abuhamad A, Barroso G, Hsu MI, Kolm P, Mayer J, and Oehninger S. Perifollicular blood flow Doppler indices, but not follicular pO2, pCO2, or pH, predict oocyte developmental competence in in vitro fertilization. Fertil Steril 72: 707–712, 1999 [DOI] [PubMed] [Google Scholar]
- 56. Igosheva N, Abramov AY, Poston L, Eckert JJ, Fleming TP, Duchen MR, and McConnell J. Maternal diet-induced obesity alters mitochondrial activity and redox status in mouse oocytes and zygotes. PLoS One 5: 1–8, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Ito M, Imai M, Muraki M, Miyado K, Qin J, Kyuwa S, Yoshikawa Y, Hosoi Y, Saito H, and Takahashi Y. GSTT1 is upregulated by oxidative stress through p38-mk2 signaling pathway in human granulosa cells: possible association with mitochondrial activity. Aging (Albany NY) 3: 1213–1223, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Ito M, Muraki M, Takahashi Y, Imai M, Tsukui T, Yamakawa N, Nakagawa K, Ohgi S, Horikawa T, Iwasaki W, Iida A, Nishi Y, Yanase T, Nawata H, Miyado K, Kono T, Hosoi Y, and Saito H. Glutathione S-transferase theta 1 expressed in granulosa cells as a biomarker for oocyte quality in age-related infertility. Fertil Steril 90: 1026–1035, 2008 [DOI] [PubMed] [Google Scholar]
- 59. Khazaei M and Aghaz F. Reactive oxygen species generation and use of antioxidants during in vitro maturation of oocytes. Int J Fertil Steril 11: 63–70, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Kim MK, Park EA, Kim HJ, Choi WY, Cho JH, Lee WS, Cha KY, Kim YS, Lee DR, and Yoon TK. Does supplementation of in-vitro culture medium with melatonin improve IVF outcome in PCOS? Reprod Biomed Online 26: 22–29, 2013 [DOI] [PubMed] [Google Scholar]
- 61. Li J, Wang S, Wang B, Wei H, Liu X, Hao J, Duan Y, Hua J, Zheng X, Feng X, and Yan X. High-fat-diet impaired mitochondrial function of cumulus cells but improved the efficiency of parthenogenetic embryonic quality in mice. Animal Cells Syst (Seoul) 22: 243–252, 2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Li Q, Miao D-Q, Zhou P, Wu Y-G, Gao D, Wei D-L, Cui W, and Tan J-H. Glucose metabolism in mouse cumulus cells prevents oocyte aging by maintaining both energy supply and the intracellular redox potential. Biol Reprod 84: 1111–1118, 2011 [DOI] [PubMed] [Google Scholar]
- 63. Lim M, Brown HM, Kind KL, Thompson JG, and Dunning KR. Hemoglobin: potential roles in the oocyte and early embryo. Biol Reprod 101: 262–270, 2019 [DOI] [PubMed] [Google Scholar]
- 64. This reference has been deleted.
- 65. Loren P, Sánchez R, Arias ME, Felmer R, Risopatrón J, and Cheuquemán C. Melatonin scavenger properties against oxidative and nitrosative stress: impact on gamete handling and in vitro embryo production in humans and other mammals. Int J Mol Sci 18: 1–17, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Luberda Z. The role of glutathione in mammalian gametes. Reprod Biol 5: 5–17, 2005 [PubMed] [Google Scholar]
- 67. Maddipati KR and Marnett LJ. Characerization of the major hydroperoxide-reducing activity of human plasma. Purification and properties of a selenium-dependent glutathione peroxidase. J Biol Chem 262: 17398–17403, 1987 [PubMed] [Google Scholar]
- 68. Martín-Romero FJ, Miguel-Lasobras EM, Domínguez-Arroyo JA, González-Carrera E, and Álvarez IS. Contribution of culture media to oxidative stress and its effect on human oocytes. RBM Online 17: 652–661, 2008 [DOI] [PubMed] [Google Scholar]
- 69. de Matos DG, Furnus CC, and Moses DF. Glutathione synthesis during in vitro maturation of bovine oocytes: role of cumulus cells. Biol Reprod 57: 1420–1425, 1997 [DOI] [PubMed] [Google Scholar]
- 70. Matos L, Stevenson D, Gomes F, Silva-Carvalho JL, and Almeida H. Superoxide dismutase expression in human cumulus oophorus cells. Mol Hum Reprod 15: 411–419, 2009 [DOI] [PubMed] [Google Scholar]
- 71. McReynolds S, Dzieciatkowska M, McCallie BR, Mitchell SD, Stevens J, Hansen K, Schoolcraft WB, and Katz-Jaffe MG. Impact of maternal aging on the molecular signature of human cumulus cells. Fertil Steril 98: 1574.e5–1580.e5, 2012 [DOI] [PubMed] [Google Scholar]
- 72. Meister A. Selective modification of glutathione metabolism. Science (80-) 220: 472–477, 1983 [DOI] [PubMed] [Google Scholar]
- 73. Menken J, Trussell J, and Larsen U. Age and infertility. Science 233: 1389–1394, 1986 [DOI] [PubMed] [Google Scholar]
- 74. van Montfoort APA, Geraedts JPM, Dumoulin JCM, Stassen APM, Evers JLH, and Ayoubi TAY. Differential gene expression in cumulus cells as a prognostic indicator of embryo viability: a microarray analysis. Mol Hum Reprod 14: 157–168, 2008 [DOI] [PubMed] [Google Scholar]
- 75. Moor RM, Smith MW, and Dawson RMC. Measurement of intercellular coupling between oocytes and cumulus cells using intracellular markers. Exp Cell Res 126: 15–29, 1980 [DOI] [PubMed] [Google Scholar]
- 76. Motta PM, Makabe S, Naguro T, and Correr S. Oocyte follicle cells association during development of human ovarian follicle. A study by high resolution scanning and transmission electron microscopy. Arch Histol Cytol 57: 369–394, 2008 [DOI] [PubMed] [Google Scholar]
- 77. Mouatassim S El, Guérin P, and Ménézo Y. Expression of genes encoding antioxidant enzymes in human and mouse oocytes during the final stages of maturation. Mol Hum Reprod 5: 720–725, 1999 [DOI] [PubMed] [Google Scholar]
- 78. Mouatassim S El, Mazout A, Bellec V, and Menezo Y. Glucose metabolism during the final stage of human oocyte maturation: genetic expression of hexokinase, glucose phosphate isomerase and phosphofructokinase. Zygote 7: 45–50, 1999 [DOI] [PubMed] [Google Scholar]
- 79. Nakayama T, Noda Y, Goto Y, and Mori T. Effects of visible light and other environmental factors on the production of oxygen radicals by hamster embryos. Theriogenology 41: 499–510, 1994 [DOI] [PubMed] [Google Scholar]
- 80. Ng KYB, Mingels R, Morgan H, Macklon N, and Cheong Y. In vivo oxygen, temperature and pH dynamics in the female reproductive tract and their importance in human conception: a systematic review. Hum Reprod Update 24: 15–34, 2018 [DOI] [PubMed] [Google Scholar]
- 81. Palermo G, Joris H, Devroey P, and Van Steirteghem AC. Pregnancies after intracytoplasmic injection of single spermatozoon into an oocyte. Lancet 340: 17–18, 1992 [DOI] [PubMed] [Google Scholar]
- 82. Palini S, Benedetti S, Tagliamonte MC, De Stefani S, Primiterra M, Polli V, Rocchi P, Catalani S, Battistelli S, Canestrari F, and Bulletti C. Influence of ovarian stimulation for IVF/ICSI on the antioxidant defence system and relationship to outcome. Reprod Biomed Online 29: 65–71, 2014 [DOI] [PubMed] [Google Scholar]
- 83. Pantasri T and Norman RJ. The effects of being overweight and obese on female reproduction: a review. Gynecol Endocrinol 30: 90–94, 2014 [DOI] [PubMed] [Google Scholar]
- 84. Papis K, Poleszczuk O, Wenta-Muchalska E, and Modlinski JA. Melatonin effect on bovine embryo development in vitro in relation to oxygen concentration. J Pineal Res 43: 321–326, 2007 [DOI] [PubMed] [Google Scholar]
- 85. Patrizio P, Fragouli E, Bianchi V, Borini A, and Wells D. Molecular methods for selection of the ideal oocyte. Reprod Biomed Online 15: 346–353, 2007 [DOI] [PubMed] [Google Scholar]
- 86. Prasad S, Tiwari M, Pandey AN, Shrivastav TG, and Chaube SK. Impact of stress on oocyte quality and reproductive outcome. J Biomed Sci 23: 36, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Prieto L, Quesada JF, Cambero O, Pacheco A, Pellicer A, Codoceo R, and Garcia-Velasco JA. Analysis of follicular fluid and serum markers of oxidative stress in women with infertility related to endometriosis. Fertil Steril 98: 126–130, 2012 [DOI] [PubMed] [Google Scholar]
- 88. Rakhit M, Gokul SR, Agarwal A, and du Plessis SS. Antioxidant strategies to overcome OS in IVF-embryo transfer. In: Studies on Women's Health, 1st ed., edited by Agarwal A, Aziz N and Rizk B. Totowa, NJ: Humana Press, 2013, pp. 237–262 [Google Scholar]
- 89. Reiter RJ, Tan DX, Tamura H, Cruz MHC, and Fuentes-Broto L. Clinical relevance of melatonin in ovarian and placental physiology: a review. Gynecol Endocrinol 30: 83–89, 2014 [DOI] [PubMed] [Google Scholar]
- 90. Richards JS, Russell DL, Ochsner S, and Espey LL. Ovulation: new dimensions and new regulators of the inflammatory-like response. Annu Rev Physiol 64: 69–92, 2002 [DOI] [PubMed] [Google Scholar]
- 91. Riley JCM and Behrman HR. Oxygen radicals and reactive oxygen species in reproduction. Exp Biol Med 198: 781–791, 2013 [DOI] [PubMed] [Google Scholar]
- 92. Rizzo P, Raffone E, and Benedetto V. Effect of the treatment with myo-inositol plus folic acid plus melatonin in comparison with a treatment with myo-inositol plus folic acid on oocyte quality and. Eur Rev Med Pharmacol 14: 555–561, 2010 [PubMed] [Google Scholar]
- 93. Robker RL, Akison LK, Bennett BD, Thrupp PN, Chura LR, Russell DL, Lane M, and Norman RJ. Obese women exhibit differences in ovarian metabolites, hormones, and gene expression compared with moderate-weight women. J Clin Endocrinol Metab 94: 1533–1540, 2009 [DOI] [PubMed] [Google Scholar]
- 94. Russell DL, Gilchrist RB, Brown HM, and Thompson JG. Bidirectional communication between cumulus cells and the oocyte: old hands and new players? Theriogenology 86: 62–68, 2016 [DOI] [PubMed] [Google Scholar]
- 95. Russell DL and Robker RL. Molecular mechanisms of ovulation: co-ordination through the cumulus complex. Hum Reprod Update 13: 289–312, 2007 [DOI] [PubMed] [Google Scholar]
- 96. Saleh RA, Agarwal A, Nada EA, El-Tonsy MH, Sharma RK, Meyer A, Nelson DR, and Thomas AJ. Negative effects of increased sperm DNA damage in relation to seminal oxidative stress in men with idiopathic and male factor infertility. Fertil Steril 79: 1597–1605, 2003 [DOI] [PubMed] [Google Scholar]
- 97. Salehi E, Aflatoonian R, Moeini A, Yamini N, Asadi E, Khosravizadeh Z, Tarzjani MD, Harat ZN, and Abolhassani F. Apoptotic biomarkers in cumulus cells in relation to embryo quality in polycystic ovary syndrome. Arch Gynecol Obstet 296: 1219–1227, 2017 [DOI] [PubMed] [Google Scholar]
- 98. Scutiero G, Iannone P, Bernardi G, Bonaccorsi G, Spadaro S, Volta CA, Greco P, and Nappi L. Oxidative stress and endometriosis: a systematic review of the literature. Oxid Med Cell Longev 2017: 1–7, 2017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Seino T. Eight-hydroxy-2′-deoxyguanosine in granulosa cells is correlated with the quality of oocytes and embryos in an in vitro fertilization-embryo transfer program. Fertil Steril 77: 1184–1190, 2002 [DOI] [PubMed] [Google Scholar]
- 100. Seko LMD, Moroni RM, Leitao VMS, Teixeira DM, Nastri CO, and Martins WP. Melatonin supplementation during controlled ovarian stimulation for women undergoing assisted reproductive technology: systematic review and meta-analysis of randomized controlled trials. Fertil Steril 101: 154.e4–161.e4, 2014 [DOI] [PubMed] [Google Scholar]
- 101. Seleem AK, El Refaeey AA, Shaalan D, Sherbiny Y, and Badawy A. Superoxide dismutase in polycystic ovary syndrome patients undergoing intracytoplasmic sperm injection. J Assist Reprod Genet 31: 499–504, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Shaeib F, Khan SN, Ali I, Thakur M, Saed MG, Dai J, Awonuga AO, Banerjee J, and Abu-Soud HM. The defensive role of cumulus cells against reactive oxygen species insult in metaphase II mouse oocytes. Reprod Sci 23: 498–507, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Shkolnik K, Tadmor A, Ben-Dor S, Nevo N, Galiani D, and Dekel N. Reactive oxygen species are indispensable in ovulation. Proc Natl Acad Sci U S A 108: 1462–1467, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104. Showell MG, Brown J, Clarke J, and Hart RJ. Antioxidants for female subfertility. Cochrane database Syst Rev 8: CD007807, 2013 [DOI] [PubMed] [Google Scholar]
- 105. Sinkó I, Mórocz M, Zádori J, Kokavszky K, and Raskó I. Effect of cigarette smoking on DNA damage of human cumulus cells analyzed by comet assay. Reprod Toxicol 20: 65–71, 2005 [DOI] [PubMed] [Google Scholar]
- 106. Sugimura S, Matoba S, Hashiyada Y, Aikawa Y, Ohtake M, Matsuda H, Kobayashi S, Konishi K, and Imai K. Oxidative phosphorylation-linked respiration in individual bovine oocytes. J Reprod Dev 58: 636–641, 2012 [DOI] [PubMed] [Google Scholar]
- 107. Takenaka M, Horiuchi T, and Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci U S A 104: 14289–14293, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Tamura H, Takasaki A, Miwa I, Taniguchi K, Maekawa R, Asada H, Taketani T, Matsuoka A, Yamagata Y, Shimamura K, Morioka H, Ishikawa H, Reiter RJ, and Sugino N. Oxidative stress impairs oocyte quality and melatonin protects oocytes from free radical damage and improves fertilization rate. J Pineal Res 44: 280–287, 2008 [DOI] [PubMed] [Google Scholar]
- 109. Tamura H, Taketani T, Tanabe M, Kizuka F, Lee L, Tamura I, Maekawa R, Aasada H, Yamagata Y, Sugino N, and Takasaki A. The role of melatonin as an antioxidant in the follicle. J Ovarian Res 5: 1–9, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Tan DX, Manchester LC, Hardeland R, Lopez-Burillo S, Mayo JC, Sainz RM, and Reiter RJ. Melatonin: a hormone, a tissue factor, an autocoid, a paracoid, and an antioxidant vitamin. J Pineal Res 34: 75–78, 2003 [DOI] [PubMed] [Google Scholar]
- 111. Tanghe S, Van Soom A, Nauwynck H, Coryn M, and de Kruif A. Minireview: functions of the cumulus oophorus during oocyte maturation, ovulation, and fertilization. Mol Reprod Dev 61: 414–424, 2002 [DOI] [PubMed] [Google Scholar]
- 112. Tao Y, Chen H, Tian NN, Huo DT, Li G, Zhang YH, Liu Y, Fang FG, Ding JP, and Zhang XR. Effects of L-ascorbic acid, α-tocopherol and co-culture on in vitro developmental potential of porcine cumulus cells free oocytes. Reprod Domest Anim 45: 19–25, 2010 [DOI] [PubMed] [Google Scholar]
- 113. Tao Y, Zhou B, Xia G, Wang F, Wu Z, and Fu M. Exposure to L-ascorbic acid or α-tocopherol facilitates the development of porcine denuded oocytes from metaphase I to metaphase II and prevents cumulus cells from fragmentation. Reprod Domest Anim 39: 52–57, 2004 [DOI] [PubMed] [Google Scholar]
- 114. Tarin JJ, de los Santos MJ, de Oliveira MNM, Pellicer A, and Bonilla-Musoles F. Ascorbate-supplemented media in short-term cultures of human embryos. Hum Reprod 9: 1717–1722, 1994 [DOI] [PubMed] [Google Scholar]
- 115. Tarín JJ, Pérez-Albalá S, García-Pérez MA, and Cano A. Effect of dietary supplementation with a mixture of vitamins C and E on fertilization of tertiary butyl hydroperoxide-treated oocytes and parthenogenetic activation in the mouse. Theriogenology 57: 869–881, 2002 [DOI] [PubMed] [Google Scholar]
- 116. Tatemoto H, Sakurai N, and Muto N. Protection of porcine oocytes against apoptotic cell death caused by oxidative stress during In vitro maturation: role of cumulus cells. Biol Reprod 63: 805–810, 2000 [DOI] [PubMed] [Google Scholar]
- 117. Tatone C and Amicarelli F. The aging ovary—the poor granulosa cells. Fertil Steril 99: 12–17, 2013 [DOI] [PubMed] [Google Scholar]
- 118. Tatone C, Amicarelli F, Carbone MC, Monteleone P, Caserta D, Marci R, Artini PG, Piomboni P, and Focarelli R. Cellular and molecular aspects of ovarian follicle ageing. Hum Reprod Update 14: 131–142, 2008 [DOI] [PubMed] [Google Scholar]
- 119. Tatone C, Carbone MC, Falone S, Aimola P, Giardinelli A, Caserta D, Marci R, Pandolfi A, Ragnelli AM, and Amicarelli F. Age-dependent changes in the expression of superoxide dismutases and catalase are associated with ultrastructural modifications in human granulosa cells. Mol Hum Reprod 12: 655–660, 2006 [DOI] [PubMed] [Google Scholar]
- 120. Thaler C and Epel D. Nitric oxide in oocyte maturation, ovulation, fertilization, cleavage and implantation: a little dab'll do ya. Curr Pharm Des 9: 399–409, 2003 [DOI] [PubMed] [Google Scholar]
- 121. Trapphoff T, Heiligentag M, Simon J, Staubach N, Seidel T, Otte K, Fröhlich T, Arnold GJ, and Eichenlaub-Ritter U. Improved cryotolerance and developmental potential of in vitro and in vivo matured mouse oocytes by supplementing with a glutathione donor prior to vitrification. Mol Hum Reprod 22: 867–881, 2016 [DOI] [PubMed] [Google Scholar]
- 122. Tremellen K. Oxidative stress and male infertility: a clinical perspective. In: Studies on Men's Health and Fertility, edited by Agarwal A, Aitken R, and Alvarez J. Totowa, NJ: Humana Press, 2012, pp. 325–353 [Google Scholar]
- 123. Unfer V, Raffone E, Rizzo P, and Buffo S. Effect of a supplementation with myo-inositol plus melatonin on oocyte quality in women who failed to conceive in previous in vitro fertilization cycles for poor oocyte quality: a prospective, longitudinal, cohort study. Gynecol Endocrinol 27: 857–861, 2011 [DOI] [PubMed] [Google Scholar]
- 124. Urner F and Sakkas D. A Possible role for the pentose phosphate pathway of spermatozoa ingamete fusion in the mouse. Biol Reprod 60: 733–739, 2005 [DOI] [PubMed] [Google Scholar]
- 125. Velthut A, Zilmer M, Zilmer K, Kaart T, Karro H, and Salumets A. Elevated blood plasma antioxidant status is favourable for achieving IVF/ICSI pregnancy. Reprod Biomed Online 26: 345–352, 2013 [DOI] [PubMed] [Google Scholar]
- 126. Verit FF, Erel O, and Kocyigit A. Association of increased total antioxidant capacity and anovulation in nonobese infertile patients with clomiphene citrate-resistant polycystic ovary syndrome. Fertil Steril 88: 418–424, 2007 [DOI] [PubMed] [Google Scholar]
- 127. Vilser C, Hueller H, Nowicki M, Hmeidan FA, Blumenauer V, and Spanel-Borowski K. The variable expression of lectin-like oxidized low-density lipoprotein receptor (LOX-1) and signs of autophagy and apoptosis in freshly harvested human granulosa cells depend on gonadotropin dose, age, and body weight. Fertil Steril 93: 2706–2715, 2010 [DOI] [PubMed] [Google Scholar]
- 128. Vitale SG, Corrado F, Laganà AS, Rossetti P, Buscema M, Rapisarda AMC, Valenti G, Sapia F, La Vignera S, and Condorelli RA. How to achieve high-quality oocytes? The key role of Myo-inositol and melatonin. Int J Endocrinol 2016: 1–9, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129. Wathlet S, Adriaenssens T, Segers I, Verheyen G, van Landuyt L, Coucke W, Devroey P, and Smitz J. Pregnancy prediction in single embryo transfer cycles after ICSI using QPCR: validation in oocytes from the same cohort. PLoS One 8: 1–10, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130. Wathlet S, Adriaenssens T, Segers I, Verheyen G, Van De Velde H, Coucke W, Ron El R, Devroey P, and Smitz J. Cumulus cell gene expression predicts better cleavage-stage embryo or blastocyst development and pregnancy for ICSI patients. Hum Reprod 26: 1035–1051, 2011 [DOI] [PubMed] [Google Scholar]
- 131. Wdowiak A and Wdowiak A. Comparing antioxidant enzyme levels in follicular fluid in ICSI-treated patients. Gynecol Obstet Fertil 43: 515–521, 2015 [DOI] [PubMed] [Google Scholar]
- 132. Wei D, Zhang C, Xie J, Song X, Yin B, Liu Q, Hu L, Hao H, Geng J, and Wang P. Supplementation with low concentrations of melatonin improves nuclear maturation of human oocytes in vitro. J Assist Reprod Genet 30: 933–938, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133. Yoshioka S, Ochsner S, Russell DL, Ujioka T, Fujii S, Richards JS, and Espey LL. Expression of tumor necrosis factor-stimulated gene-6 in the rat ovary in response to an ovulatory dose of gonadotropin. Endocrinology 141: 4114–4119, 2000 [DOI] [PubMed] [Google Scholar]
- 134. Zhao H, Zhao Y, Li T, Li M, Li J, Li R, Liu P, Yu Y, and Qiao J. Metabolism alteration in follicular niche: the nexus among intermediary metabolism, mitochondrial function, and classic polycystic ovary syndrome. Free Radic Biol Med 86: 295–307, 2015 [DOI] [PubMed] [Google Scholar]
- 135. Zuccotti M, Bellone M, Longo F, Redi CA, and Garagna S. Fully-mature antral mouse oocytes are transcriptionally silent but their heterochromatin maintains a transcriptional permissive histone acetylation profile. J Assist Reprod Genet 28: 1193–1196, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]