Abstract
Low gestational weight gain (GWG) and low mid‐upper arm circumference (MUAC) are associated with adverse pregnancy outcomes. We aimed to assess the prevalence and determinants of low GWG and low MUAC among pregnant women in rural Zinder, Niger. A community‐based survey was conducted among 1,384 pregnant women in the catchment areas of 18 integrated health centers in the region of Zinder, Niger. Weight and MUAC were measured during an in‐home visit and again 1 month later, when haemoglobin concentration and micronutrient status were also assessed. The prevalence of low GWG was defined based on the 2009 United States Institute of Medicine (U.S. IOM) guidelines (<0.35 kg/week) and less than the third centile of the International Fetal and Newborn Growth Consortium for the 21st Century (INTERGROWTH‐21st) standards. Factors associated with GWG and MUAC were identified using logistic regression models adjusting for season, village, and gestational age. The median (interquartile range) age was 25.0 (20.7, 30.0) years, and 16.4% were ≤19 years. The prevalence of low GWG were 62.9% and 27.5% according to 2009 IOM and less than the third INTERGROWTH‐21st centile, respectively; 24.9% had low MUAC. Higher α‐1‐acid glycoprotein (OR = 1.7, 95% CI [1.1, 2.8]) and C‐reactive protein (OR = 1.2, 95% CI [1.02, 1.50]) increased the odds of low GWG. Adolescents (OR = 2.7, 95% CI [1.8, 4.0]), housewives (OR = 1.97, 95% CI [1.36, 2.86]), and those who reported recent food assistance (OR = 1.80, 95% CI [1.04, 3.11]) had higher odds of low MUAC. Prevalence of low GWG and low MUAC was high among pregnant women. Determinants of GWG and MUAC included socio‐economic, demographic, and biological factors, although only markers of inflammation were consistent predictors across different definitions of low GWG.
Keywords: low income countries, maternal nutrition, nutritional status, pregnancy, undernutrition, weight gain
Key messages.
GWG and MUAC are strong predictors of birth outcomes. In low‐income countries, information on GWG and MUAC is limited.
There was a high prevalence of low GWG and low MUAC among pregnant women in rural Zinder, Niger.
Determinants of GWG and MUAC included socio‐economic, demographic, and biological factors, particularly markers of inflammation.
Considering the importance of adequate GWG and MUAC for maternal health and pregnancy outcomes, effective and cost‐effective interventions to ensure adequate GWG and nutritional status are needed.
List of abbreviations
- AGP
α‐1‐acid glycoprotein (AGP)
- ANC
Antenatal care
- CRP
C‐reactive protein
- GA
Gestational age
- GEE
Generalized estimated equation
- GWG
Gestational Weight Gain
- HFIA
Household Food Insecurity Access
- HRP2
Histidine‐rich protein 2
- IHC
Integrated Health Center
- IUGR
Intrauterine growth restriction
- KAP
Knowledge Attitude and Practice
- LBW
Low birth weight
- MDD‐W
Minimum Dietary Diversity for Women
- MUAC
Mid‐Upper Arm Circumference
- NiMaNu
Niger Maternal Nutrition
- OR
Odds ratio
- pZn
Plasma zinc
- RBP
Retinol binding protein
- SES
Socio‐economic status
- SFH
Symphysis fundal height
- SGA
Small for gestational age
- sTfR
Soluble transferrin receptor
- U.S. IOM
United States Institute of Medicine
- WHO
World Health Organization
1. INTRODUCTION
Gestational weight gain (GWG) is a complex biological phenomenon that influences pregnancy outcomes (IOM and NRC, 2009). An appropriate GWG is essential for optimal pregnancy outcomes for both the mother and her infant (Asefa & Nemomsa, 2016). Low GWG is associated with adverse outcomes including low birth weight (LBW; Edwards, Hellerstedt, Alton, Story, & Himes, 1996; Hellerstedt, Himes, Story, Alton, & Edwards, 1997; Hickey, McNeal, Menefee, & Ivey, 1997; Schieve, Cogswell, & Scanlon, 1998), intrauterine growth restriction (Strauss & Dietz, 1999), small for gestational age (SGA; Hellerstedt et al., 1997; Hickey et al., 1997; Nielsen, Gittelsohn, Anliker, & O'Brien, 2006), and preterm delivery (Hickey, Cliver, Goldenberg, McNeal, & Hoffman, 1995; Stotland, Cheng, Hopkins, & Caughey, 2006). Excessive GWG is associated with an increased risk for macrosomia, gestational diabetes (Hedderson et al., 2006), cesarean section, and postpartum weight retention (Caulfield, Stoltzfus, & Witter, 1998; Hellerstedt et al., 1997; Viswanathan et al., 2008). Determinants of GWG include a range of maternal biological (e.g., age, parity, stature, and genetics), metabolic, and social factors (e.g., socio‐economic status [SES], education, physical activity, and diet; IOM and NRC, 2009).
In its 2009 guidelines, the U.S. Institute of Medicine (IOM) recommended a range for total GWG of 5 to 18 kg, with a GWG rate of 0.17 to 0.58 kg per week during the second and third trimesters (IOM and NRC, 2009). These guidelines of GWG are based on pre‐pregnancy body mass index (BMI; IOM and NRC, 2009) and may only be relevant for women in the United States and other high‐income countries. For low‐income countries across the world, there has been limited information on recommended GWG, and the World Health Organization (WHO) does not currently have recommendations for GWG. However, the International Fetal and Newborn Growth Consortium for the 21st Century Project (INTERGROWTH‐21st), which was implemented in eight countries, has recently published GWG standards based on gestational age (GA) in weeks (Cheikh Ismail et al., 2016). These standards describe the GWG patterns of normal weight women at low risk of adverse maternal and perinatal outcomes and are intended to be applied globally.
Mid‐upper arm circumference (MUAC) is also a strong indicator for predicting adverse birth outcomes in low‐resource settings (Ververs, Antierens, Sackl, Staderini, & Captier, 2013). Low‐maternal MUAC was shown to be associated with an increased risk of LBW (Assefa, Berhane, & Worku, 2012; Karim & Mascie‐Taylor, 1997; Lechtig, 1988; Mohanty et al., 2006; Rollins et al., 2007; Sebayang et al., 2012), preterm labour/birth (Begum, Buckshe, & Pande, 2003; Kalanda, Verhoeff, Chimsuku, Harper, & Brabin, 2006; Sebayang et al., 2012), disproportionate intrauterine growth (Kalanda et al., 2006), birth asphyxia (Lee et al., 2009), and SGA (Sebayang et al., 2012).
Due to the lack of WHO GWG recommendations, and a shortage of basic supplies, including scales, in many health centers in low‐income countries, GWG monitoring is not a common practice in many parts of the world. Moreover, it has been shown that health workers lack adequate knowledge and skills to effectively monitor GWG (Goiburu, Alfonzo, Aranda, Riveros, & Ughelli, 2006; Mowe et al., 2006; Mowe et al., 2008). In Niger, the preterm birth rate and LBW prevalence are high, with 9.4 preterm births per 100 live births (Blencowe et al., 2012) and 27% of infants born LBW (UNICEF, 2017). In addition, the fertility rate of 7.4 children per woman is the highest in the world (UNICEF, 2017). However, information on pregnant women's nutritional status and GWG are limited. Thus, the objectives of the present study were to estimate the prevalence and the determinants of low GWG and low MUAC among pregnant women in Zinder, Niger.
2. METHODS
2.1. Study design and participants
The present study was a community‐based survey conducted as part of the baseline assessment for the Niger Maternal Nutrition (NiMaNu) Project. This project was a programmatic intervention to improve antenatal care (ANC) services and compared pre‐ and post‐intervention cohorts of pregnant women. Methods for the baseline survey assessments have been described in detail elsewhere (K. Begum et al., 2018; Wessells et al., 2017). Briefly, the community‐based baseline survey was conducted from March 2014 to September 2015. We enrolled pregnant women in 68 rural villages belonging to 18 integrated health centers (IHCs) in the catchment area of two health districts (Mirriah and Zinder), in the Zinder region, Niger. The 18 IHCs were selected based on their accessibility and their distance to Zinder, the regional capital, and because of the limited number and scope of interventions that were being implemented in their catchment area. Within the catchment area of each IHC, the village containing the IHC was automatically included in the survey, as well as one additional randomly selected village among those with a health post. Among the remaining villages in the catchment area of each IHC, four villages ≤10 km and four villages >10 km were randomly selected and randomized to order of participation. Pregnant women from the first two selected villages (IHC‐village and health post village), and the first two villages from the subsequent randomization were enrolled, with a target of enrolling approximately 16–20 women per village and a sample size of approximately 77 women per IHC. When the target number of women was not met by the first four villages in each IHC, women were included from the remaining villages of each IHC following the order of the randomization list. Within each village, participants were identified using a random walk method (United Nations, 2008).
The enrolment of participants was implemented continuously over a period of 18 months with approximatively one new IHC surveyed each month. All identified pregnant women (regardless of gestational week) were eligible for study participation, if they had resided in a participating village for at least 6 months prior to enrolment and had no plans to move out of the study area within the next 2 months. A woman was excluded if she had a severe illness warranting immediate hospital referral or was unable to provide consent due to impaired decision‐making ability.
2.2. Data collection and outcomes
Each enrolled woman participated in two study visits. During the first contact (Visit 1), we obtained written informed consent and interviewed women using a structured questionnaire to collect information regarding SES, demographics, and knowledge, attitude, and practices relating to diet, health, pregnancy (current and previous), and ANC attendance. Pregnant women were weighed in light clothing in duplicate to 50‐g precision (SECA 874). Women's height (SECA 213, Seca, Hamburg, Germany), MUAC (ShorrTape© Measuring Tape), and symphysis‐fundal height (ShorrTape© Measuring Tape, Weigh and Measure, Olney, MD) were measured in duplicate to 0.1‐cm precision. A third measurement was performed, and the mean of the two closest measurements was calculated when the first two measurements were >0.2 kg (weight) or >0.5 cm apart (height, MUAC, and symphysis‐fundal height).
Approximatively 1 month later (Visit 2), each participating pregnant woman was invited to a follow‐up assessment. The structured interviews and anthropometric measurements were repeated. Capillary blood samples were drawn to assess haemoglobin concentration by HemoCue® Hb 201+ (Hemocue, Inc; Lake Forest, CA). As described elsewhere (Wessells et al., 2017), venous blood samples (7.5 ml) were collected in a subgroup of participants for the measurement of folate, vitamin B12, retinol binding protein, plasma ferritin, soluble transferrin receptor (sTfR), zinc, α‐1‐acid glycoprotein (AGP), C‐reactive protein (CRP), and histidine‐rich protein II (HRP2) concentrations.
GA was estimated as a weighted average of the following obtained information: reported last menstrual period (by estimated number of months, lunar cycles, and/or proximity to a religious or cultural event), time elapsed since quickening, and two fundal height measurements taken approximately 1 month apart (Hess & Ouedraogo, 2016). Three proxy indices (housing quality, household assets, and household livestock) were used to estimate the household SES, as previously described (K. Begum et al., 2018). Household food insecurity was assessed using the Household Food Insecurity Access categories (Coates, Swindale, & Bilinsky, 2007). Pregnant women's dietary practices were assessed using a list‐based food frequency questionnaire, and those who reported consuming at least five of 10 defined food groups in the previous 24 hr were considered to meet the Minimum Dietary Diversity for Women (FAO and FHI 360, 2016).
The outcomes of this study included GWG per week (in kilograms), low GWG, MUAC (in centimeters), and low MUAC. GWG per week was calculated by subtracting weight at Visit 1 from the weight recorded on Visit 2 divided by the number of elapsed days and multiplied by seven. Adequacy of GWG was assessed by comparing GWG of the study participants with the 2009 U.S. IOM guidelines for GWG and the INTERGROWTH‐21st standards, as described in more detail below. Low MUAC was defined as <23 cm (Ververs et al., 2013).
2.2.1. GWG compared with the 2009 U.S. IOM guidelines for GWG
The mean GWG recommended by the U.S. IOM is 0.45 kg/week, with ranges from 0.44 to 0.58 kg/week for underweight and 0.35 to 0.50 kg/week for normal weight women, respectively (IOM and NRC, 2009). These guidelines are based on pre‐pregnancy BMI, which are not known for the participants in the present study. Thus, considering that the majority of women in the present study were likely to be either underweight or of normal weight (Institut National de la Statistique & ICF International, 2007), a GWG of 0.35–0.58 kg/week was considered within the IOM guidelines. Less than 0.35 kg/week was considered as GWG below the IOM guidelines (or a proxy for low GWG) and >0.58 kg/week as GWG above the IOM guidelines (or a proxy of excessive GWG). Because the IOM guidelines apply to women in the second and third trimester of gestation, women in their first trimester of pregnancy were excluded from this classification. Considering that the majority of women had low or adequate GWG in the present study population, GWG was transformed in a dichotomous variable (i.e., GWG < 0.35 kg and GWG ≥ 0.35 kg) to assess factors associated with low GWG.
2.2.2. GWG compared with the INTERGROWTH‐21st standards
We calculated the observed GWG per week for each pregnant woman according to her estimated GA in weeks. Because the published INTERGROWTH‐21st standards represent cumulative GWG, they cannot be directly compared with the observed GWG per week. Rather, the observed GWG was compared with the GWG rate per week of women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH‐21st Project (personal communication). Observed GWG less than the third centile of the GWG by GA of the INTERGROWTH‐21st standards was considered to be GWG below the INTERGROWTH‐21st standards (or a proxy for low GWG). Observed GWG between the third and the 97th centile was considered GWG within the standards (or a proxy of normal weight gain), and observed GWG > 97th centile of expected GWG was considered GWG above the INTERGROWTH‐21st standards (or a proxy of excessive GWG). Observed GWG per week of the participants was also compared with the median of the INTERGROWTH‐21st standards and categorically defined as being above or below the INTERGROWTH‐21st median.
2.3. Sample size
The overall sample size for the NiMaNu project was specified to be able to detect with 80% power a difference of 10% in the prevalence of anaemia as the primary outcome of the programmatic intervention (Hess & Ouedraogo, 2016). Assuming an initial anaemia prevalence of 50%, a significance level of 0.05, power of 0.80, and a design effect of 2 to account for the cluster sampling design, a sample size of 768 was needed, which was then inflated by 17% for attrition, yielding a target sample size of 925 pregnant women for the baseline survey. However, for the impact assessment of the main trial described elsewhere (Hess & Ouedraogo, 2016), the baseline survey was extended for 6 months to allow statistical models to account for the potential seasonal effects of participants' enrolment on the outcome measures. Based on the same assumption as above, the additional sample size required for the baseline survey was estimated at 77 pregnant women per month for a total of 463 over 6 months. In total, 1,388 pregnant women were needed to be enrolled in the baseline survey to provide 80% power to detect a difference of 10% in the prevalence of anaemia in the primary impact assessment. Data were successfully obtained from 1,385 pregnant women. This sample size was adequate to estimate the prevalence of low GWG + 3.5% (95% CI), assuming a prevalence of 50%.
2.4. Statistical analysis
Data were double‐entered and compared using EpiData Entry version 3.1 (Odense, Denmark). All statistical analyses were performed using the SAS System version 9.4 (SAS Institute, Cary, NC, USA). We analysed available data from the baseline survey of the NiMaNu project. Data were examined using univariate analysis (graphical plotting) to look for outliers. Outliers that were clearly impossible or implausible values were corrected if possible, or trimmed when correction was not possible, which was the case for one GWG and one MUAC measurement. A detailed statistical analyses plan is available (Hess & Ouedraogo, 2016).
GWG per week and MUAC were assessed for conformance to the normal distribution. Predictors not normally distributed (i.e., ferritin, sTfR, CRP, AGP, and folate and vitamin B12) were natural log transformed. Descriptive analysis of initial characteristics of study participants was performed. Factors associated with low GWG and low MUAC, as well as GWG per week and MUAC as continuous variables, were identified using generalized estimating equation models, in SAS proc glimmix to permit adjusting for cluster effects by village. All models were minimally adjusted to include year, season, and village, and analyses were performed using robust standard errors. All predictors were run in individual models, and the minimally adjusted odds ratio (for low GWG and MUAC as binary outcomes) and the minimally adjusted mean difference (for GWG and MUAC as continuous outcomes) from each individual model were reported.
Potential predictors were identified based on a literature review and background knowledge and prespecified in the statistical analyses plan (Hess & Ouedraogo, 2016). These included maternal age, education, number of pregnancies, number of living children, height, occupation, SES, household food insecurity, reported increase or decrease in the number of meals per day and quantity of food consumed due to pregnancy, reported receipt of food assistance, adequate minimum dietary diversity, micronutrient status (plasma ferritin, zinc, and retinol binding protein adjusted for inflammation; Wessells et al., 2017; sTfR, vitamin B12, and folate), markers of inflammation (AGP and CRP), and malaria antigenemia (HRP2). To explore which predictors were consistently and significantly associated with GWG per week and low GWG, we ran five independent analyses including GWG per week and GWG < 0.35 kg/week adjusting for women's GA, and GWG per week, GWG less than the third centile and GWG < 50th centile INTERGROWTH‐21st standards not adjusting for GA following the methods of the respective standards (Cheikh Ismail et al., 2016; Hutcheon & Bodnar, 2018; IOM and NRC, 2009). If a predictor was associated with at least three GWG (GWG per week and/or different definitions of low GWG) or both of the MUAC outcomes (MUAC in centimeters or low MUAC), it was considered to be a consistent predictor of low GWG or undernutrition. A P value <.05 was considered as statistically significant for the all tests performed.
2.5. Ethics
This study was part of the NiMaNu Project, which was approved by the National Ethical Committee in Niamey (Niger) and the Institutional Review Boards of the University of California, Davis (USA). The study was registered at www.clinicaltrials.gov as NCT01832688. In the presence of a neutral witness, consent materials were presented in both written and oral formats. Informed consent was obtained and documented with a written signature or a fingerprint.
3. RESULTS
3.1. Characteristics of study population
A total of 1,385 pregnant women were enrolled during the baseline survey, and 67.9 % (n = 940) completed Visit 2 (Figure 1), with 81.9 % (n = 770) of these women assessed for micronutrient status. Attrition at Visit 2 was due to birth, relocation, consent withdrawal, stillbirth, and maternal death. The mean participants' age was 26.2 ± 6.4 years, and only 20.9% had attended any formal schooling. The majority of the participants considered themselves to be primarily housewives (83.2%), and 53.1% were in their third trimester of gestation (Table 1). Of the 1,385 pregnant women interviewed, 39.5% of women had attended at least four ANC during their last pregnancy as described in Table 1.
Figure 1.
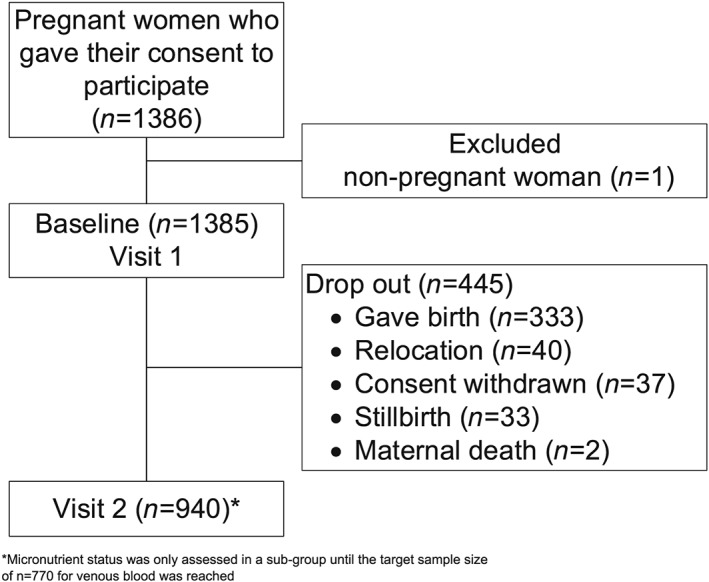
Flow chart for participants included in the present study
Table 1.
Characteristics of pregnant women who were enrolled in the baseline survey
| Variables | Value |
|---|---|
| N | 1,385 |
| Age, years (mean ± SD) | 26.2 ± 6.4 |
| Adolescent (≤19 years) | 221 (16.4%) |
| Adult (>19 years) | 1,131 (83.6%) |
| Ethnicity | |
| Hausa | 1,188 (85.8%) |
| Others | 196 (14.2%) |
| Education | |
| Any formal education | 289 (20.9%) |
| No formal education or literacy training only or koranic school | 1,095 (79.1%) |
| Principal occupation | |
| Housewife | 1,151 (83.2%) |
| Nonhousewife | 233 (16.8%) |
| Marital status | |
| Married | 1,363 (99.1%) |
| Separated/divorced or widow | 13 (0.9%) |
| Trimestera | |
| First | 33 (2.4%) |
| Second | 612 (44.5%) |
| Third | 730 (53.1%) |
| Obstetric history | |
| Age at first pregnancy, median (25th, 75th) | 16.0 (16.0–17.0) |
| (Min–max) | (12.0–35.0) |
| Gravidity | |
| Primigravida | 178 (12.9%) |
| Multigravida | 1,206 (87.1%) |
| Number of pregnancies, median (25th, 75th) | 5 (3–8) |
| Number of living children, median (25th, 75th) | 3 (2–5) |
| Outcome of previous pregnancy | |
| Child born alive, still living | 1,029 (85.3 %) |
| Child not born alive or born alive and had since died | 177 (14.7%) |
| Height (cm)b | 158.2 (157.9, 158.6) |
| Attended any ANC during last pregnancy | 1,108 (91.9%) |
| Attended at least four ANC during last pregnancy | 476 (39.5%) |
| Health facility delivery during last pregnancy | 419 (34.9%) |
| Reported food intake during the week before the interview | |
| Increased | 286 (20.7%) |
| Decreased | 797 (57.7%) |
| No change | 298 (21.6%) |
| Biochemistry assessmentc | |
| Haemoglobin concentration, g/dl | 9.6 (9.5, 9.7) |
| Plasma folate concentration, nmol/L | 11.2 (10.9, 11.5) |
| Plasma vitamin B12 concentration, pmol/L | 181.3 (176.3, 186.2) |
| Retinol binding protein concentration,d μmol/L | 1.08 (1.06, 1.10) |
| Plasma ferritin concentration,d μg/L | 42.8 (40.0, 45.5) |
| Soluble transferrin receptor concentration, mg/L | 8.69 (8.33, 9.04) |
| Plasma zinc concentration,d μg/dL | 52.2 (51.6, 52.9) |
| α‐1‐acid glycoprotein concentration, g/L | 0.43 (0.41, 0.45) |
| C‐reactive protein concentration, mg/L | 5.32 (4.56, 6.08) |
| Plasma histidine‐rich protein II concentration, ng/ml | 0.17 (0.14, 0.20) |
| Household level characteristics | |
| Household head's education level | |
| Any formal education | 261 (21.6%) |
| No formal education or literacy training only or koranic school | 950 (78.4%) |
| Principal occupation of the household head | |
| Farming related occupation | 578 (42.0%) |
| Nonfarming‐related occupation | 798 (58.0%) |
| Levels of household food insecurity | |
| Food secure | 432 (31.2%) |
| Mildly food insecure | 150 (10.9%) |
| Moderately food insecure | 345 (25.0%) |
| Severely food insecure | 454 (32.9%) |
| Season of enrolment | |
| Lean, rain (June–September) | 501 (36.2%) |
| Dry, postharvest (October–February) | 467 (33.7%) |
| Hot (March–May) | 416 (30.1%) |
Abbreviations: ANC, antenatal care.
Trimester at Visit 1.
Mean (95% CI).
Sample size varied by indicator: Haemoglobin, n = 919; Plasma folate, n = 739; Plasma vitamin B12, n = 744; Retinol binding protein, n = 769; Plasma ferritin, n = 769; Soluble transferrin receptor, n = 769; Plasma zinc, n = 723; α‐1‐acid glycoprotein, n = 769; C‐reactive protein, n = 769; Plasma histidine‐rich protein II, n = 769.
Adjusted for acute phase proteins
GWG analyses included all pregnant women who completed follow‐up Visit 2 and were in the second and third trimester of pregnancy (n = 917); 1,384 women enrolled at Visit 1 were included in the MUAC analyses. Using the U.S. IOM 2009 guidelines and the INTERGROWTH‐21st standards, the prevalence of low GWG was 62.9% and 27.5%, respectively (Table 2). In contrast, 13.1% and 2.0% of pregnant women were above the 2009 IOM guidelines and above the 97th centile of the INTERGROWTH‐21st standards, respectively, indicating excessive GWG (Figure S1, Table 2). The median MUAC was 24.1 (interquartile range 18.0, 36.9) cm, and 24.9% had low MUAC (Table 2).
Table 2.
Prevalence of low GWG, excessive GWG, and low MUAC among study participants
| Variables | Value |
|---|---|
| Participants (n)a | 1,385 |
| GWG per week, kg, in second trimesterb | 0.27 (−2.01, 3.05) |
| GWG per week, kg, in the third trimesterb | 0.20 (−2.29, 2.96) |
| Classification of GWG | |
| According to the IOM guidelines | |
| GWG below the IOM guideline for GWG | 574 (62.9%) |
| GWG within the IOM guideline for GWG | 218 (23.9%) |
| GWG above the IOM guidelines for GWG | 119 (13.1%) |
| According to the INTERGROWTH‐21st standards | |
| GWG less than the third centile | 252 (27.5%) |
| GWG <50th centile | 758 (82.7%) |
| GWG >97th centile | 18 (2.0%) |
| MUACb , c, cm | 24.1 (23.0, 26.0) |
| Low MUAC (<23 cm) | 344 (24.9%) |
Abbreviations: GWG, gestational weight gain; IOM, Institute of Medicine; MUAC, mid‐upper arm circumference.
Sample size per outcome: GWG, n = 917, (only women in their second and third trimester of gestation at Visit 1 and who completed both Visits 1 and 2 were considered).
GWG in the second trimester, n = 554; GWG in the third trimester, n = 348; MUAC, n = 1,384.
median (25th, 75th), or n (%) all such values.
3.2. Determinants of GWG per week and low GWG among pregnant women
Analyses performed using the minimally adjusted models indicated that two predictors were consistently and significantly associated with both GWG per week and low GWG, as defined by both U.S. IOM guidelines and INTERGROWTH‐21st standards (Table 3), and some predictors were significant in individual models.
Table 3.
| Variables | GWG (continuous) not adjusting for GA | GWG (continuous) adjusting for GA | GWG below IOM guidelines (<0.35 kg/week) adjusting for GA | GWG less than the third centile of INTERGROWTH‐21st standards not adjusting for GA | GWG <50th centile of INTERGROWTH‐21st standards not adjusting for GA |
|---|---|---|---|---|---|
| Adolescent | |||||
| Yes | −0.004 [−0.08, 0.07] | −0.01 [−0.09, 0.08] | 1.4 [0.9, 2.4] | 0.8 [0.5, 1.4] | 1.0 [0.5, 2.0] |
| No | Ref | Ref | Ref | Ref | Ref |
| Education | |||||
| Any formal education | 0.06 [−0.02, 0.14] | 0.06 [−0.02, 0.14] | 0.9 [0.5, 1.5] | 0.5 [0.3, 0.9]* | 1.1 [0.6, 1.9] |
| No education | Ref | Ref | Ref | Ref | Ref |
| Principal occupation | |||||
| Housewife | −0.06 [−0.15, 0.04] | −0.05 [−0.15, 0.04] | 1.4 [0.9, 2.1] | 1.0 [0.64, 1.57] | 1.0 [0.7, 1.7] |
| Nonhousewife | Ref | Ref | Ref | Ref | Ref |
| Number of pregnancies | −0.009 [−0.02, 0.002] | −0.009 [−0.02, 0.001] | 1.04 [0.97, 1.12] | 1.1 [0.97, 1.16] | 1.11 [1.03, 1.20]* |
| Number of living children | −0.0068[−0.02, 0.01] | −0.009 [−0.02, 0.006] | 1.02 [0.94, 1.11] | 1.1 [0.98, 1.20] | 1.0 [0.9, 1.1] |
| Height | 0.004 [−0.001,0.01] | 0.004 [−0.001, 0.01] | 0.96 [0.92, 0.98]* | 0.98 [0.95, 1.02] | 0.94 [0.91, 0.98]* |
| Household livestock index | |||||
| Above the median | 0.003 [−0.05, 0.06] | 0.001 [−0.06, 0.06] | 1.0 [0.7, 1.4] | 0.8 [0.6, 1.2] | 1.1 [0.8, 1.6] |
| At or below the median | Ref | Ref | Ref | Ref | Ref |
| Housing quality index | |||||
| Above the median | −0.02 [−0.10, 0.06] | −0.02 [−0.10, 0.06] | 1.1 [0.7, 1.6] | 1.1 [0.8, 1.6] | 1.1 [0.6, 1.8] |
| At or below the median | Ref | Ref | Ref | Ref | Ref |
| Household asset index | |||||
| Above the median | −0.02 [−0.08, 0.03] | −0.02 [−0.07, 0.04] | 1.1 [0.8, 1.5] | 1.1 [0.7, 1.7] | 1.2 [0.8, 1.8] |
| At or below the median | Ref | Ref | Ref | Ref | Ref |
| Reported food intake | |||||
| Increased | 0.004 [−0.10, 0.11] | 0.01 [−0.1, 0.12] | 0.9 [0.5, 1.6] | 1.1 [0.6, 2.2] | 1.0 [0.5, 2.0] |
| Decreased | −0.005 [−0.09, 0.08] | −0.004 [−0.09, 0.08] | 0.8 [0.4, 1.4] | 1.3 [0.7, 2.4] | 0.9 [0.4, 1.8] |
| No change | Ref | Ref | Ref | Ref | Ref |
| Number of meals | |||||
| Increased | −0.03 [−0.14, 0.08] | −0.03 [−0.13, 0.08] | 1.1 [0.5, 1.9] | 1.2 [0.7, 2.1] | 1.1 [0.5, 2.4] |
| Decreased | −0.02 [−0.10, 0.05] | −0.02 [−0.09, 0.05] | 0.8 [0.5, 1.4] | 1.2 [0.8, 2.0] | 1.1 [0.6, 1.9] |
| No change | Ref | Ref | Ref | Ref | Ref |
| Levels of household food insecurity | |||||
| Food secure | −0.06 [−0.17, 0.04] | −0.07 [−0.18, 0.04] | 1.8 [1.0, 3.4]* | 0.20 [0.65, 2.28] | 0.51 [0.84, 3.33] |
| Mildly food insecure | −0.03 [−0.15, 0.09] | −0.04 [−0.16, 0.09] | 1.7 [0.8, 3.4] | 0.10 [0.50, 2.60] | 0.15 [0.49, 2.74] |
| Moderately food insecure | −0.04 [−0.17, 0.09] | −0.04 [−0.17, 0.09] | 1.4 [0.7, 2.7] | 0.03 [0.50, 2.14] | 0.46 [0.79, 3.22] |
| Severely food insecure | Ref | Ref | Ref | Ref | Ref |
| Adequate minimum dietary diversity | 0.01 [−0.05, 0.07] | −0.01 [−0.08, 0.05] | 0.7 [0.5, 1.1] | 0.93 [0.64, 1.34] | 0.8 [0.5, 1.3] |
| Received food assistance | −0.008 [−0.10, 0.08] | −0.003 [−0.09, 0.09] | 1.6 [0.9, 3.0]** | 1.03 [0.57, 1.86] | 1.4 [0.6, 3.4] |
| Haemoglobinc, g/dl | 0.21 [0.02, 0.4]* | 0.2 [0.006, 0.40]* | 0.29 [0.10, 0.88]* | 0.4 [0.1, 1.6] | 0.9 [0.2, 4.2] |
| Plasma folate concentration, nmol/L | 0.04 [−0.07, 0.14] | 0.03 [−0.06, 0.13] | 0.8 [0.4, 1.6] | 0.97 [0.50, 1.90] | 0.8 [0.3, 2.0] |
| Plasma vitamin B12 concentration, pmol/L | −0.04 [−0.14, 0.07] | −0.06 [−0.17, 0.05] | 2.0 [0.99, 3.79]** | 1.2 [0.6, 2.3] | 1.4 [0.7, 2.9] |
| Retinol binding protein concentrationd , μmol/L | −0.07 [−0.17, 0.03] | −0.06 [−0.16, 0.04] | 1.6 [0.8, 3.1] | 0.97 [0.5, 2.0] | 1.6 [0.6, 4.0] |
| Plasma ferritin concentrationd, μg/L | −0.05 [−0.10, 0.006] | −0.05 [−0.11, 0.003]** | 1.22 [0.88, 1.71] | 1.5 [1.1, 2.0]* | 1.1 [0.8, 1.6] |
| Soluble transferrin receptor concentration, mg/L | −0.05 [−0.11, 0.02] | −0.04 [−0.11, 0.03] | 1.6 [1.04, 2.5]* | 1.4 [0.8, 2.2] | 1.0 [0.6, 1.5] |
| Plasma zinc concentrationd, μg/dl | −0.002 [0.006, 0.002] | −0.002 [−0.007, 0.002] | 1.0 [0.97, 1.03] | 1.0 [0.98, 1.03] | 1.03 [1.0, 1.07]** |
| α‐1‐acid glycoprotein concentration, g/L | −0.10 [−0.17, −0.02]* | −0.1 [−0.2, −0.02]* | 1.7 [1.1, 2.8]* | 1.9 [1,2, 3.0]* | 1.3 [0.7, 2.3] |
| C‐reactive protein concentration, mg/L | −0.002 [−0.006, −0.002]* | −0.03 [−0.06, −0.01]* | 1.14 [0.98, 1.32] | 1.2 [1.02, 1.50]* | 1.1 [1.0, 1.3] |
| Plasma histidine‐rich protein II concentration, ng/L | −0.06 [−0.13, 0.002]** | −0.06 [−0.13, 0.003]** | 1.72 [1.01, 2.92]* | 1.3 [0.8, 2.2] | 1.2 [0.6, 2.3] |
Sample size for GWG analysis, n = 918, (only women in their second and third trimester of gestation at Visit 1 and who completed both Visits 1 and 2 were considered) and sample size for biochemistry indicators, n = 770.
Minimally adjusted model: adjusted for year, season, and village because of the nature of the study design.
A 0.1 g/dl in Hb concentration was associated with a 1.0‐g increase in GWG.
Adjusted for acute phase protein.
Statistically significant, P < .05.
Marginally significant; P = .05–.07.
3.2.1. GWG per week nonadjusting and adjusting for GA
In the analysis not adjusting for GA, increasing concentrations of AGP (β = −.10, 95% CI [−0.17, −0.02]) and CRP (β = −.002, 95% CI [−0.006, −0.002]) were associated with decreasing GWG. When adjusting for GA, this was consistent in the analysis for AGP (β = −.1, 95% CI [−0.2, −0.02]) and CRP (β = −.03, 95% CI [−0.06, −0.01]), respectively. Higher haemoglobin concentration was associated with increased GWG when not adjusting for GA (β = .2, 95% CI [0.02, 0.4]) and after adjusting GA (β = .2, 95% CI [0.006, 0.40]), respectively.
3.2.2. Low GWG according to IOM guidelines, adjusting for GA
Women who reported being food secure (OR = 1.8, 95% CI [1.0, 3.4]), had higher AGP in grams per litre (OR = 1.7, 95% CI [1.1, 2.8]), higher sTfR in milligrams per litre (OR = 1.6, 95% CI [1.04, 2.5]), and higher HRP2 in nanograms per millilitre (OR = 1.72, 95% CI [1.01, 2.92]) concentration had increased odds of low GWG. Pregnant women who were taller in centimetres (OR = 0.96, 95% CI [0.92, 0.98]) and those with higher haemoglobin concentrations in grams per decilitre (OR = 0.29, 95% CI [0.10, 0.88]) had decreased odds of low GWG.
3.2.3. Low GWG according to the third INTERGROWTH‐21st standards not adjusting for GA
Higher plasma ferritin in micrograms per litre (OR = 1.5, 95% CI [1.1, 2.0]), higher AGP in grams per litre (OR = 1.9, 95% CI [1.2, 3.0]), and higher CRP in milligrams per litre (OR = 1.25, 95% CI [1.02, 1.50]) concentration were associated with increased odds of low GWG. Women who had any formal education (OR = 0.5, 95% CI [0.3, 0.9]) had decreased odds of low GWG compared with those who were not formally educated.
3.2.4. GWG < 50th centile of the INTERGROWTH‐21st standards not adjusting for GA
One increase in the number of pregnancies that a woman had (OR = 1.11, 95% CI, [1.03, 1.20]) was associated with increased odds of GWG below the 50th centile. Pregnant women who were taller (cm) had decreased odds of GWG below the 50th centile (OR = 0.94, 95% CI [0.91, 0.98]).
3.3. Determinants of low MUAC and continuous MUAC among pregnant women
The odds of low MUAC were higher among adolescent women (OR = 2.7, 95% CI [1.8, 04.0]) compared with adult women, women who identified themselves as housewives (OR = 1.97, 95% CI [1.36, 2.86]) compared with those who had other principal occupations, and among those who reported recent receipt of food assistance (OR = 1.8, 95% CI [1.04, 3.11]; Table 4). This was consistent in the analysis with continuous MUAC. One increase in the number of pregnancies that a woman had (OR = 0.90, 95% CI [0.83, 0.98]), one increase in the number of children that a woman had (OR = 0.87, 95% CI [0.78, 0.97]), greater height in centimetres (OR = 0.93, 95% CI, [0.91, 0.94]), and higher haemoglobin concentrations in grams per decilitre (OR = 0.21, 95% CI, [0.01, 0.42]) were associated with decreased odds of low MUAC. These were consistent in the analysis with continuous MUAC.
Table 4.
| Variables | Minimally adjusted mean difference (95% CI) | P value | Minimally adjusted odds ratio or minimally adjusted mean difference(95% CI) | P value |
|---|---|---|---|---|
| Adolescent | ||||
| Yes | −1.3 [−1.7, −0.9] | <.0001 | 2.7 [1.8, 4.0] | <.0001 |
| No | Ref | |||
| Education | ||||
| Any formal education | −0.3 [−0.7, 0.2] | .25 | 1.5 [0.95, 2.45] | .0807 |
| No education | Ref | Ref | ||
| Principal occupation | ||||
| Housewife | −0.7 [−1.1, −0.3] | .001 | 1.97 [1.36, 2.86] | .0006 |
| Nonhousewife | Ref | Ref | ||
| Number of pregnancies | 0.2 [0.1, 0.3] | <.0001 | 0.90 [0.83, 0.98] | .02 |
| Number of living children | 0.2 [0.1, 0.3] | <.0001 | 0.87 [0.78, 0.97] | .01 |
| Height, cm | 0.08 [0.06, 0.11] | <.0001 | 0.93 [0.91, 0.94] | <.0001 |
| Household livestock index | ||||
| Above the median | 0.16 (−0.17, 0.49) | .33 | 1.2 (0.9, 1.6) | .29 |
| At or below the median | Ref | Ref | ||
| Housing quality index | ||||
| Above the median | 0.4 [0.06, 0.74] | .02 | 0.85 [0.61, 1.20] | .33 |
| At or below the median | Ref | |||
| Household asset index | ||||
| Above the median | 0.15 [−0.1, 0.4] | .23 | 0.8 [0.6, 1.2] | .35 |
| At or below the median | Ref | |||
| Reported food intake | ||||
| Increased | −0.4 [−0.97, 0.16] | .11 | 1.4 [0.9, 2.2] | .09 |
| Decreased | −0.02 [−0.52, 0.49] | 0.9 [0.6, 1.4] | — | |
| No change | Ref | Ref | ||
| Number of meals | ||||
| Increased | −0.5 [−1.0, 0.1] | .11 | 1.6 [1.0, 2.6] | .06 |
| Decreased | −0.2 [−0.6, 0.3] | 1.2 [0.8, 1.8] | — | |
| No change | Ref | Ref | ||
| Levels of household food insecurity | ||||
| Food secure | 0.2 [−0.4, 0.8] | .52 | −0.1 [0.52, .147] | .91 |
| Mildly food insecure | 0.4 [−0.3, 1.1] | −0.1 [0.46, 1.91] | — | |
| Moderately food insecure | 0.1 [−0.4, 0.5] | 0.02 [0.66, 1.58] | — | |
| Severely food insecure | Ref | Ref | ||
| Adequate dietary diversity | 0.2 [−0.2, 0.6] | .28 | 0.9 [0.7, 1.3]. | .63 |
| Received food assistance | −0.9 [−1.6, −0.3] | .006 | 1.80 [1.04, 3.11] | .04 |
| Haemoglobin, g/dlc | 2.8 [1.4, 4.1] | <.0001 | 0.21 [0.01, 0.42] | .04 |
| Plasma folate concentration, nmol/L | 0.6 [−0.03, 1.26] | .06 | 0.6 [0.3, 1.1] | .09 |
| Plasma vitamin B12 concentration, pmol/L | −0.4 [−1.0, 0.2] | .19 | 1.20 [0.58, 2.48] | .63 |
| Retinol binding protein concentrationd, μmol/L | 0.26 [−0.37, 0.88] | .42 | 0.9 [0.5, 1.7] | .86 |
| Plasma ferritin concentrationd , μg/L | −0.1 [−0.3, 0.2] | .57 | 0.9 [0.7, 1.2] | .60 |
| Soluble transferrin receptor concentration, mg/L | −0.1 [−0.5, 0.4] | .78 | 1.4 [0.8, 2.3] | .23 |
| Plasma zinc concentrationd, μg/dl | 0.02 [−0.002, 0.04] | .07 | 0.98 [0.96, 1.00] | .06 |
| α‐1‐acid glycoprotein concentration, mg/L | −0.4 [−0.9, 0.2] | .20 | 1.7 [0.9, 3.3] | .10 |
| C‐reactive protein concentration, g/L | −0.01 [−0.2, 0.2] | .94 | 1.0 [0.9, 1.2] | .78 |
| Plasma histidine‐rich protein II concentration, ng/L | −0.2 [−0.6, 0.2] | .34 | 0.98 [0.69, 1.41] | .92 |
Sample size for MUAC analysis, n = 1,384.
Minimally adjusted model: adjusted for year, season, and village because of the nature of the study design.
A 0.1 g/dl in Hb concentration was associated with a 1.0‐cm increase in MUAC.
Adjusted for acute phase protein.
4. DISCUSSION
The prevalence of low GWG was high among pregnant women in Zinder irrespective of whether the IOM guidelines or the INTERGROWTH‐21st standards were used. More than one in two pregnant women (62.9 %) had low GWG (GWG per week <0.35 kg) according to the 2009 IOM guidelines, and more than one fourth of the pregnant women (27.4%) had low GWG (GWG per week less than the third centile) based on the INTERGROWTH‐21st standards. Similar results were reported in Ghana, where the estimated prevalence of low GWG based on the GWG over the whole pregnancy according to IOM guidelines was 62.7%, and the percentage of women with GWG less than the third centile of GWG according to the INTERGROWTH‐21st standards was 27% (Adu‐Afarwuah et al., 2017). In Ethiopia, the prevalence of low GWG based on the total GWG according to the IOM criteria was also high (69.3%; Asefa & Nemomsa, 2016). Given the adverse maternal and child health outcomes associated with low GWG, our findings indicate that low GWG is a concern in the study area and highlights the need for effective maternal health and nutrition interventions to influence these outcomes (Hamad, Cohen, & Rehkopf, 2016).
The two sets of cut‐offs used for GWG in the present study differ substantially. For example at 28 weeks of GA, the IOM cutoff for low GWG is 0.35 kg/week, and the third centile of the INTERGROWTH‐21st standard is 0.27 kg/week. Consequently, we found that the prevalence of low GWG according to the 2009 U.S. IOM guideline (GWG <0.35 kg/week) was about two times higher than the prevalence based on the third centile of the INTERGROWTH‐21st standards. The IOM guidelines were developed based on the available published literature as well as the reports of consultants, and the goal was to identify GWG values or range of GWG values in the U.S. population associated with lowest prevalence of adverse outcomes including caesarean delivery, postpartum weight retention, preterm birth, small‐ or large‐for‐GA birth, and childhood obesity (IOM and NRC, 2009). Conversely, the INTERGROWTH‐21st standards are derived from a prospective longitudinal, multicountry population study. They represent GWG of healthy, well‐nourished, and educated women who had a BMI between 18.5 and 24.9 in the first trimester of pregnancy with good maternal and perinatal outcomes (Cheikh Ismail et al., 2016). Interestingly, the INTERGROWTH project did not find any country‐specific differences, suggesting that these standards may be useful internationally. However, the third centile of the INTERGROWTH‐21st standards identifies women with severely low GWG as compared with the IOM guidelines, and using another, slightly higher centile may be more useful for identifying women at risk of low GWG.
In the present study, we were also interested in assessing the prevalence of low MUAC as another indicator of nutritional status during pregnancy, because MUAC reflects both past and current nutritional status (WHO expert Committee on physical status, 1995). We found that a significant proportion of pregnant women had low MUAC. In a study conducted in Ethiopia, a similar prevalence of low MUAC (31.8%) was reported among pregnant women (Mariyam & Dibaba, 2018), although this study applied a cutoff of 21 cm.
Our study revealed that higher concentrations of markers of systemic inflammation (AGP and CRP) and higher concentrations of sTfR (indicative of iron deficiency and/or increased erythropoiesis) were associated with decreased GWG per week and increased odds of low GWG. Inflammation and iron deficiency can be accompanied by loss of appetite, decreasing food intake, and possibly leading to low weight gain during pregnancy (Katona & Katona‐Apte, 2008; Raiten et al., 2015; Scrimshaw, 1977). However, it is interesting to note that higher plasma ferritins concentrations (indicative of iron sufficiency and/or inflammation) were associated with increased odds of GWG less than the third centile of INTERGROWTH. Although ferritin concentrations were adjusted for inflammation in the present study, it is possible that adjustments did not fully capture the effects of inflammation.
In the present study, pregnant women with higher haemoglobin concentrations had decreased odds of low GWG and decreased odds of low MUAC. Although we are unaware of previous studies that have reported direct associations between GWG and haemoglobin, previous studies have shown a similar relationship between haemoglobin concentration and MUAC (Addis Alene & Mohamed Dohe, 2014; Makhoul et al., 2012; Saaka, Oladele, Larbi, & Hoeschle‐Zeledon, 2017). Maternal anaemia (Figueiredo et al., 2018; Sukrat et al., 2013) and both anaemia and low GWG are known risk factors of LBW (Edwards et al., 1996; Hellerstedt et al., 1997; Hickey et al., 1997; Schieve et al., 1998), intrauterine growth restriction (Strauss & Dietz, 1999), and SGA (Hellerstedt et al., 1997; Hickey et al., 1997; Nielsen et al., 2006).
We found that taller women had lower odds of low GWG and lower odds of low MUAC. Similar findings were reported in the Philippines, where higher maternal height was associated with greater total weight gain (Siega‐Riz & Adair, 1993). In a study conducted in Tanzania, taller women (>159.5 cm) were also more likely to gain more weight than shorter women (<151.5 cm; Changamire et al., 2014). Maternal height reflects both genetic and environmental factors, as well as long‐term dietary intake and nutritional status (Perkins, Subramanian, Davey Smith, & Ozaltin, 2016), all of which have been also shown to influence GWG (IOM and NRC, 2009).
Increased gravidity was associated with increased odds of low GWG (<50th centile); similar findings have been reported in Tanzania (Changamire et al., 2014). It is possible that higher gravidity and parity, and frequent reproductive cycling, may result in maternal nutritional depletion in the context of high food insecurity and thus increasing the risk of SGA and LBW (Klerman, Cliver, & Goldenberg, 1998; Miller, 1991). However, increases in the number of pregnancies and children were associated with decreased odds of low MUAC. MUAC has been shown to be relatively stable throughout pregnancy (WHO expert Committee on physical status, 1995), except in adolescence; thus, the relationship between gravidity and MUAC may be mediated by age, reflecting both lower gravidity and increased odds of low MUAC among adolescents.
Pregnant adolescents had higher odds of low MUAC. This is consistent with the findings in Nepal and Malawi, where lower age was associated with decreased MUAC during pregnancy (Chithambo, May May 2017; Ghosh et al., 2017). Adolescence is a period of rapid growth, and when pregnancy occurs during this period, there is increased competition for nutrients with the fetus, which increases the risk of undernutrition among adolescent pregnant women (Das et al., 2017).
We found that pregnant women who reported food assistance during the present pregnancy had decreased MUAC and higher odds of low MUAC. Similar results on the relationship between food assistance and low MUAC have been reported in Ethiopia (Gebre, Biadgilign, Taddese, Legesse, & Letebo, 2018). Somewhat unexpectedly, however, we observed that women in food secure households had increased odds of GWG below IOM guidelines. It is possible that dietary patterns differed between women in food secure versus insecure households. Outside of economic constraints, it has been shown that food intake during pregnancy is largely driven by personal preferences and cravings, cultural beliefs, food taboos (i.e., prohibition against consuming certain foods), and beliefs surrounding pregnancy physiology (Kavle & Landry, 2018).
To our knowledge, this is the first study to examine GWG and undernutrition in pregnant woman in Niger and its determinants. The present study has several strengths. Due to the extensive data collected by highly trained and supervised field workers, we were able to examine numerous potential predictors of GWG and MUAC. All data were collected over >12 months allowing us to account for the effect of season on outcomes. Despite its strengths, this study has several limitations. First, the present study was limited to two health districts, and the findings are thus not representative of the population of the entire Zinder region, nor of Niger as a whole. Second, we performed analysis only for complete cases. This method reduces the sample size and may lead to loss of power to detect a significant association. Third, GWG assessment was based on only two weight measurements taken 1 month apart; longer term observation may more accurately describe the pattern of the true GWG of these women. Fourth, we classified pregnant women according to the IOM guidelines for GWG, which are based on pre‐pregnancy BMI, data which were not available in the present study. In addition, the INTERGROWTH‐21st standards are derived from healthy, well‐nourished, and educated women, and participants had normal BMI in the first trimester of pregnancy (Cheikh Ismail et al., 2016). Thus, it is likely that some women in the present study were misclassified according to the IOM guidelines and the INTERGROWTH‐21st standards. Nevertheless, by using both sets of cutoffs and running analyses with multiple definitions of low GWG, we were likely able to identify women at highest risk of low GWG and explore risk factors associated with low GWG. Lastly, a significant proportion of pregnant women in the study population (16.3%) were adolescent, but due to the lack of a specific MUAC recommendation for the adolescent age group, those women were classified using the same guidelines and standards as adult women. For GWG, the IOM did not see sufficient evidence to support a specific guideline for adolescents (IOM and NRC, 2009). Similarly, we did not find a higher risk of low GWG among adolescent in the present study, but further research is needed (Harper, Chang, & Macones, 2011).
5. CONCLUSION
The prevalence of low GWG was high among pregnant women in the Zinder region of Niger, and the odds of low GWG were consistently associated with higher concentrations of markers of inflammation (AGP and CRP). A considerable proportion of pregnant women also had low MUAC. Considering the importance of adequate GWG and MUAC for maternal health and pregnancy outcomes, effective and cost‐effective interventions (e.g., daily iron folic acid supplementation; Baltussen, Knai, & Sharan, 2004, behaviour change communication about nutrition; Lamstein et al., 2014, and balanced protein energy dietary supplementation; Imdad & Bhutta, 2012) to ensure adequate GWG and nutritional status should be considered.
CONFLICTS OF INTEREST
The authors declare that they have no conflicts of interest.
CONTRIBUTIONS
The NiMaNu Project was conceived and designed by SYH, KRW, and CTO. Data were analysed by CTO with the guidance from RRY and SYH. Results were interpreted by all co‐authors. CTO drafted the manuscript, and SYH edited the manuscript. All authors reviewed and approved the final version of the manuscript.
Supporting information
Figure S1:
Study participants' GWG per week compared to the INTERGROWTH‐21th standards
ACKNOWLEDGMENTS
We would like to thank the NiMaNu study team, the participants, the local communities, staff of the Zinder and Mirriah health districts, and the national steering committee. Our sincere appreciation goes to Professors Stephen A. Vosti and Daniel J. Tancredi (University of California, Davis, USA) for valuable comments on the manuscript and to Dr. Leila Cheikh Ismail, Dr. Eric O. Ohuma, and Professor José Villar (University of Oxford, Oxford, UK) for providing additional information on gestational weight gain of women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH‐21st Project. This study was funded by the Government of Canada through Global Affairs Canada and Nutrition International, Grant Number 201300662. Nutriset SAS provided financial support for CTO's PhD thesis.
Ouédraogo CT, Wessells KR, Young RR, Faye MT, Hess SY. Prevalence and determinants of gestational weight gain among pregnant women in Niger. Matern Child Nutr. 2020;16:e12887 10.1111/mcn.12887
REFERENCES
- Addis Alene, K. , & Mohamed Dohe, A. (2014). Prevalence of anemia and associated factors among pregnant women in an urban area of Eastern Ethiopia. Anemia, 2014, 561567 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25215230, 10.1155/2014/561567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adu‐Afarwuah, S. , Lartey, A. , Okronipa, H. , Ashorn, P. , Ashorn, U. , Zeilani, M. , … Dewey, K. G. (2017). Maternal supplementation with small‐quantity lipid‐based nutrient supplements compared with multiple micronutrients, but not with iron and folic acid, reduces the prevalence of low gestational weight gain in semi‐urban Ghana: a randomized controlled trial. The Journal of Nutrition, 147(4), 697–705. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28275100, 10.3945/jn.116.242909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asefa, F. , & Nemomsa, D. (2016). Gestational weight gain and its associated factors in Harari Regional State: Institution based cross‐sectional study, Eastern Ethiopia. Reproductive Health, 13, 101 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27576539, 10.1186/s12978-016-0225-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assefa, N. , Berhane, Y. , & Worku, A. (2012). Wealth status, mid upper arm circumference (MUAC) and antenatal care (ANC) are determinants for low birth weight in Kersa, Ethiopia. PLoS ONE, 7(6), e39957 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22792140, 10.1371/journal.pone.0039957 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baltussen, R. , Knai, C. , & Sharan, M. (2004). Iron fortification and iron supplementation are cost‐effective interventions to reduce iron deficiency in four subregions of the world. The Journal of Nutrition, 134(10), 2678–2684. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15465766, 10.1093/jn/134.10.2678 [DOI] [PubMed] [Google Scholar]
- Begum, F. , Buckshe, K. , & Pande, J. N. (2003). Risk factors associated with preterm labour. Bangladesh Medical Research Council Bulletin, 29(2), 59–66. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/14674621 [PubMed] [Google Scholar]
- Begum, K. , Ouedraogo, C. T. , Wessells, K. R. , Young, R. R. , Faye, M. T. , Wuehler, S. E. , & Hess, S. Y. (2018). Prevalence of and factors associated with antenatal care seeking and adherence to recommended iron‐folic acid supplementation among pregnant women in Zinder, Niger. Maternal & Child Nutrition, 14(Suppl 1), e12466 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29493896, 10.1111/mcn.12466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blencowe, H. , Cousens, S. , Oestergaard, M. Z. , Chou, D. , Moller, A. B. , Narwal, R. , … Lawn, J. E. (2012). National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet, 379(9832), 2162–2172. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22682464, 10.1016/S0140-6736(12)60820-4 [DOI] [PubMed] [Google Scholar]
- Caulfield, L. E. , Stoltzfus, R. J. , & Witter, F. R. (1998). Implications of the Institute of Medicine weight gain recommendations for preventing adverse pregnancy outcomes in black and white women. American Journal of Public Health, 88(8), 1168–1174. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9702142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Changamire, F. T. , Mwiru, R. S. , Msamanga, G. I. , Spiegelman, D. , Urassa, W. , Hertzmark, E. , … Peterson, K. E. (2014). Macronutrient and sociodemographic determinants of gestational weight gain among HIV‐negative women in Tanzania. Food and Nutrition Bulletin, 35(1), 43–50. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/24791578, 10.1177/156482651403500106 [DOI] [PubMed] [Google Scholar]
- Cheikh Ismail, L. , Bishop, D. C. , Pang, R. , Ohuma, E. O. , Kac, G. , Abrams, B. , … Villar, J. (2016). Gestational weight gain standards based on women enrolled in the Fetal Growth Longitudinal Study of the INTERGROWTH‐21st Project: a prospective longitudinal cohort study. BMJ, 352, i555 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26926301, 10.1136/bmj.i555 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chithambo, S. E. T. (May 2017). Factors associated with the rate of gestational weight gain in rural Mangochi district of Malawi. Master thesis in International Community Health. (Master in International Community Health), University of Oslo, Oslo, Norway. Retrieved from https://www.duo.uio.no/bitstream/handle/10852/58269/Shyreen-Chithambo-thesis.pdf?sequence=1
- Coates, J. , Swindale, A. , & Bilinsky, P. (2007). Household food insecurity access scale (HFIAS) for measurement of household food access: Indicator guide (v.3). Washington, DC, USA: FHI 360/FANTA. [Google Scholar]
- Das, J. K. , Salam, R. A. , Thornburg, K. L. , Prentice, A. M. , Campisi, S. , Lassi, Z. S. , … Bhutta, Z. A. (2017). Nutrition in adolescents: physiology, metabolism, and nutritional needs. Annals of the New York Academy of Sciences, 1393(1), 21–33. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28436102, 10.1111/nyas.13330 [DOI] [PubMed] [Google Scholar]
- Edwards, L. E. , Hellerstedt, W. L. , Alton, I. R. , Story, M. , & Himes, J. H. (1996). Pregnancy complications and birth outcomes in obese and normal‐weight women: effects of gestational weight change. Obstetrics and Gynecology, 87(3), 389–394. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8598961 [DOI] [PubMed] [Google Scholar]
- FAO and FHI 360 (2016). Minimum dietary diversity for women: A guide for the measurement. Rome, Italy: FAO. [Google Scholar]
- Figueiredo, A. , Gomes‐Filho, I. S. , Silva, R. B. , Pereira, P. P. S. , Mata, F. , Lyrio, A. O. , … Pereira, M. G. (2018). Maternal anemia and low birth weight: A systematic review and meta‐analysis. Nutrients, 10(5). Retrieved from). https://www.ncbi.nlm.nih.gov/pubmed/29757207, 10.3390/nu10050601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebre, B. , Biadgilign, S. , Taddese, Z. , Legesse, T. , & Letebo, M. (2018). Determinants of malnutrition among pregnant and lactating women under humanitarian setting in Ethiopia. BMC Nutrition, 4(1), 11 Retrieved from https://bmcnutr.biomedcentral.com/track/pdf/10.1186/s40795-018-0222-2, 10.1186/s40795-018-0222-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh, S. , Trevino, J. A. , Davis, D. , Shrestha, R. , Bhattarai, A. , Anusree, K. , … Webb, P. (2017). Factors associated with mid upper arm circumference in pregnant women in Banke, Nepal. Paper presented at the Experimental Biology, Chicago, IL, USA. Available at https://www.fasebj.org/doi/abs/10.1096/fasebj.31.1_supplement.960.10
- Goiburu, M. E. , Alfonzo, L. E. , Aranda, A. L. , Riveros, M. E. , & Ughelli, M. A. (2006). Clinical nutrition knowledge in health care members of university hospitals of Paraguay. Nutrition Hospital, 25(5), 591–595. [PubMed] [Google Scholar]
- Hamad, R. , Cohen, A. K. , & Rehkopf, D. H. (2016). Changing national guidelines is not enough: the impact of 1990 IOM recommendations on gestational weight gain among US women. International Journal of Obesity , 40(10), 1529‐1534. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/27200502. 10.1038/ijo.2016.97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper, L. M. , Chang, J. J. , & Macones, G. A. (2011). Adolescent pregnancy and gestational weight gain: Do the Institute of Medicine recommendations apply? American Journal of Obstetrics and Gynecology, 205(2), 140 e141‐148. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/21620365, 10.1016/j.ajog.2011.03.053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedderson, M. M. , Weiss, N. S. , Sacks, D. A. , Pettitt, D. J. , Selby, J. V. , Quesenberry, C. P. , & Ferrara, A. (2006). Pregnancy weight gain and risk of neonatal complications: Macrosomia, hypoglycemia, and hyperbilirubinemia. Obstetrics and Gynecology, 108(5), 1153–1161. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17077237, 10.1097/01.AOG.0000242568.75785.68 [DOI] [PubMed] [Google Scholar]
- Hellerstedt, W. L. , Himes, J. H. , Story, M. , Alton, I. R. , & Edwards, L. E. (1997). The effects of cigarette smoking and gestational weight change on birth outcomes in obese and normal‐weight women. American Journal of Public Health, 87(4), 591–596. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9146437 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hess, S. Y. , & Ouedraogo, C. T. (2016). NiMaNu project [Internet]. Open Science Framework. Available at: https://osf.io/4cenf
- Hickey, C. A. , Cliver, S. P. , Goldenberg, R. L. , McNeal, S. F. , & Hoffman, H. J. (1995). Relationship of psychosocial status to low prenatal weight gain among nonobese black and white women delivering at term. Obstetrics and Gynecology, 86(2), 177–183. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/7617346 [DOI] [PubMed] [Google Scholar]
- Hickey, C. A. , McNeal, S. F. , Menefee, L. , & Ivey, S. (1997). Prenatal weight gain within upper and lower recommended ranges: Effect on birth weight of black and white infants. Obstetrics and Gynecology, 90(4 Pt 1), 489–494. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9380302 [DOI] [PubMed] [Google Scholar]
- Hutcheon, J. A. , & Bodnar, L. M. (2018). Good practices for observational studies of maternal weight and weight gain in Pregnancy. Paediatric and Perinatal Epidemiology, 32(2), 152–160. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/29345321, 10.1111/ppe.12439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imdad, A. , & Bhutta, Z. A. (2012). Maternal nutrition and birth outcomes: Effect of balanced protein‐energy supplementation. Paediatric and Perinatal Epidemiology, 26(Suppl 1), 178–190. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22742610, 10.1111/j.1365-3016.2012.01308.x [DOI] [PubMed] [Google Scholar]
- Institut National de la Statistique (INS) & ICF International . (2007). Enquête démographique et de santé et à indicateurs multiples du Niger 2006. Calverton, Maryland, USA: Available at: https://dhsprogram.com/pubs/pdf/FR193/FR193-NI06.pdf
- IOM and NRC (2009). In Rasmussen K. M., & Yaktine A. L. (Eds.), The National Academies Collection: Reports funded by National Institutes of Health. Weight Gain During Pregnancy: Reexamining the Guidelines. Washington, DC: The National Academies Press; Available online. https://www.ncbi.nlm.nih.gov/pubmed/20669500, 10.17226/12584 [DOI] [PubMed] [Google Scholar]
- Kalanda, B. F. , Verhoeff, F. H. , Chimsuku, L. , Harper, G. , & Brabin, B. J. (2006). Adverse birth outcomes in a malarious area. Epidemiology and Infection, 134(3), 659–666. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16255832, 10.1017/S0950268805005285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karim, E. , & Mascie‐Taylor, C. G. (1997). The association between birthweight, sociodemographic variables and maternal anthropometry in an urban sample from Dhaka, Bangladesh. Annals of Human Biology, 24(5), 387–401. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9300116 [DOI] [PubMed] [Google Scholar]
- Katona, P. , & Katona‐Apte, J. (2008). The interaction between nutrition and infection. Clinical Infectious Diseases, 46(10), 1582–1588. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18419494, 10.1086/587658 [DOI] [PubMed] [Google Scholar]
- Kavle, J. A. , & Landry, M. (2018). Addressing barriers to maternal nutrition in low‐ and middle‐income countries: A review of the evidence and programme implications. Maternal & Child Nutrition, 14(1), e12508 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28836343, 10.1111/mcn.12508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klerman, L. V. , Cliver, S. P. , & Goldenberg, R. L. (1998). The impact of short interpregnancy intervals on pregnancy outcomes in a low‐income population. American Journal of Public Health, 88(8), 1182–1185. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9702144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamstein, S. , Stillman, T. , Koniz‐Booher, P. , Aakesson, A. , Collaiezzi, B. , Williams, T. , … Anson, M. (2014). Evidence of Effective Approaches to Social and Behavior Change Communication for Preventing and Reducing Stunting and Anemia: Report from a Systematic Literature Review. Arlington, VA: Retrieved from https://www.spring-nutrition.org/sites/default/files/publications/series/spring_sbcc_lit_review.pdf [Google Scholar]
- Lechtig, A. (1988). Predicting risk of delivering low birthweight babies: Which indicator is better? Journal of Tropical Pediatrics, 34(1), 34–41. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/3346940, 10.1093/tropej/34.1.34 [DOI] [PubMed] [Google Scholar]
- Lee, A. C. , Darmstadt, G. L. , Khatry, S. K. , LeClerq, S. C. , Shrestha, S. R. , & Christian, P. (2009). Maternal‐fetal disproportion and birth asphyxia in rural Sarlahi, Nepal. Archives of Pediatrics & Adolescent Medicine, 163(7), 616–623. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/19581544, 10.1001/archpediatrics.2009.75 [DOI] [PubMed] [Google Scholar]
- Makhoul, Z. , Taren, D. , Duncan, B. , Pandey, P. , Thomson, C. , Winzerling, J. , … Shrestha, R. (2012). Risk factors associated with anemia, iron deficiency and iron deficiency anemia in rural Nepali pregnant women. The Southeast Asian Journal of Tropical Medicine and Public Health, 43(3), 735–746. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23077854 [PubMed] [Google Scholar]
- Mariyam, A. F. , & Dibaba, B. (2018). Epidemiology of malnutrition among pregnant women and associated factors in central refit valley of Ethiopia, 2016. Journal of Nutritional Disorders and Therapy, 8(1). Retrieved from 10.4172/2161-0509.1000222 [DOI] [Google Scholar]
- Miller, J. E. (1991). Birth intervals and perinatal health: An investigation of three hypotheses. Family Planning Perspectives, 23(2), 62–70. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/2060613 [PubMed] [Google Scholar]
- Mohanty, C. , Prasad, R. , Srikanth Reddy, A. , Ghosh, J. K. , Singh, T. B. , & Das, B. K. (2006). Maternal anthropometry as predictors of low birth weight. Journal of Tropical Pediatrics, 52(1), 24–29. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/15980024, 10.1093/tropej/fmi059 [DOI] [PubMed] [Google Scholar]
- Mowe, M. , Bosaeus, I. , Rasmussen, H. H. , Kondrup, J. , Unosson, M. , & Irtun, O. (2006). Nutritional routines and attitudes among doctors and nurses in Scandinavia: A questionnaire based survey. Clinical Nutrition, 25(3), 524–532. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16701921, 10.1016/j.clnu.2005.11.011 [DOI] [PubMed] [Google Scholar]
- Mowe, M. , Bosaeus, I. , Rasmussen, H. H. , Kondrup, J. , Unosson, M. , Rothenberg, E. , … Scandinavian Nutrition, G. (2008). Insufficient nutritional knowledge among health care workers? Clinical Nutrition, 27(2), 196–202. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/18295936, 10.1016/j.clnu.2007.10.014 [DOI] [PubMed] [Google Scholar]
- Nielsen, J. N. , Gittelsohn, J. , Anliker, J. , & O'Brien, K. (2006). Interventions to improve diet and weight gain among pregnant adolescents and recommendations for future research. Journal of the American Dietetic Association, 106(11), 1825–1840. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17081834, 10.1016/j.jada.2006.08.007 [DOI] [PubMed] [Google Scholar]
- Perkins, J. M. , Subramanian, S. V. , Davey Smith, G. , & Ozaltin, E. (2016). Adult height, nutrition, and population health. Nutrition Reviews, 74(3), 149–165. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/26928678, 10.1093/nutrit/nuv105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raiten, D. J. , Sakr Ashour, F. A. , Ross, A. C. , Meydani, S. N. , Dawson, H. D. , Stephensen, C. B. , … Group, I. C. (2015). Inflammation and nutritional science for programs/policies and interpretation of research evidence (INSPIRE). The Journal of Nutrition, 145(5), 1039S–1108S. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/25833893, 10.3945/jn.114.194571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollins, N. C. , Coovadia, H. M. , Bland, R. M. , Coutsoudis, A. , Bennish, M. L. , Patel, D. , & Newell, M. L. (2007). Pregnancy outcomes in HIV‐infected and uninfected women in rural and urban South Africa. Journal of Acquired Immune Deficiency Syndromes, 44(3), 321–328. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/17195768, 10.1097/QAI.0b013e31802ea4b0 [DOI] [PubMed] [Google Scholar]
- Saaka, M. , Oladele, J. , Larbi, A. , & Hoeschle‐Zeledon, I. (2017). Dietary diversity is not associated with haematological status of pregnant women resident in rural areas of northern Ghana. Journal of Nutrition and Metabolism, 2017, 8497892 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28168052, 10.1155/2017/8497892 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schieve, L. A. , Cogswell, M. E. , & Scanlon, K. S. (1998). An empiric evaluation of the Institute of Medicine's pregnancy weight gain guidelines by race. Obstetrics and Gynecology, 91(6), 878–884. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/9610990 [DOI] [PubMed] [Google Scholar]
- Scrimshaw, N. S. (1977). Effect of infection on nutrient requirements. The American Journal of Clinical Nutrition, 30(9), 1536–1544. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/331935, 10.1093/ajcn/30.9.1536 [DOI] [PubMed] [Google Scholar]
- Sebayang, S. K. , Dibley, M. J. , Kelly, P. J. , Shankar, A. V. , Shankar, A. H. , & Group, S. S. (2012). Determinants of low birthweight, small‐for‐gestational‐age and preterm birth in Lombok, Indonesia: Analyses of the birthweight cohort of the SUMMIT trial. Tropical Medicine & International Health, 17(8), 938–950. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/22943372, 10.1111/j.1365-3156.2012.03039.x [DOI] [PubMed] [Google Scholar]
- Siega‐Riz, A. M. , & Adair, L. S. (1993). Biological determinants of pregnancy weight gain in a Filipino population. The American Journal of Clinical Nutrition, 57(3), 365–372. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/8438770, 10.1093/ajcn/57.3.365 [DOI] [PubMed] [Google Scholar]
- Stotland, N. E. , Cheng, Y. W. , Hopkins, L. M. , & Caughey, A. B. (2006). Gestational weight gain and adverse neonatal outcome among term infants. Obstetrics and Gynecology, 108(3 Pt 1), 635–643. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/16946225, 10.1097/01.AOG.0000228960.16678.bd [DOI] [PubMed] [Google Scholar]
- Strauss, R. S. , & Dietz, W. H. (1999). Low maternal weight gain in the second or third trimester increases the risk for intrauterine growth retardation. The Journal of Nutrition, 129(5), 988–993. Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/10222390, 10.1093/jn/129.5.988 [DOI] [PubMed] [Google Scholar]
- Sukrat, B. , Wilasrusmee, C. , Siribumrungwong, B. , McEvoy, M. , Okascharoen, C. , Attia, J. , & Thakkinstian, A. (2013). Haemoglobin concentration and pregnancy outcomes: a systematic review and meta‐analysis. BioMed Research International, 2013, 769057 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23984406, 10.1155/2013/769057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNICEF (2017). The state of the world's children: A fair chance for every child. In. New York, NY: UNICEF. [Google Scholar]
- United Nations (2008). Designing household survey samples: Pratical guidelines. New York, NY: Department of Economics and Social Affairs, Statistics Division, United Nations. [Google Scholar]
- Ververs, M. T. , Antierens, A. , Sackl, A. , Staderini, N. , & Captier, V. (2013). Which anthropometric indicators identify a pregnant woman as acutely malnourished and predict adverse birth outcomes in the humanitarian context? PLoS Currents, 5, June 7, Edition 1 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/23787989, 10.1371/currents.dis.54a8b618c1bc031ea140e3f2934599c8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Viswanathan, M. , Siega‐Riz, A. M. , Moos, M. K. , Deierlein, A. , Mumford, S. , Knaach, J. , … Lohr, K. N. (2008). Outcomes of maternal weight gain. Evidence Report/Technology Assessment, 168, 1–223. [PMC free article] [PubMed] [Google Scholar]
- Wessells, K. R. , Ouedraogo, C. T. , Young, R. R. , Faye, M. T. , Brito, A. , & Hess, S. Y. (2017). Micronutrient status among pregnant women in Zinder, Niger and risk factors associated with deficiency. Nutrients, 9(5), 430 Retrieved from https://www.ncbi.nlm.nih.gov/pubmed/28445440, 10.3390/nu9050430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO expert Committee on physical status . (1995). The use and interpretation of anthropometry. Retrieved from Geneva, Switzerland: Available at http://apps.who.int/iris/bitstream/handle/10665/37003/WHO_TRS_854.pdf?sequence=1&isAllowed=y
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1:
Study participants' GWG per week compared to the INTERGROWTH‐21th standards


