Abstract
Purpose:
Proton therapy can potentially improve the therapeutic ratio over conventional radiation therapy for oropharyngeal squamous cell cancer (OPSCC) by decreasing acute and late toxicity. We report our early clinical experience with intensity-modulated proton therapy (IMPT).
Materials and Methods:
We retrospectively reviewed patients with OPSCC treated with IMPT at our center. Endpoints include local regional control (LRC), progression-free survival (PFS), overall survival (OS), tumor response, and toxicity outcomes. Toxicity was graded as per the Common Terminology Criteria for Adverse Events v4.03. Descriptive statistics and Kaplan-Meier method were used.
Results:
We treated 46 patients from March 2015 to August 2017. Median age was 58 years, 93.5% were male, 67% were nonsmokers, 98% had stage III-IVB disease per the 7th edition of the AJCC [American Joint Committee on Cancer] Cancer Staging Manual, and 89% were p16 positive. Twenty-eight patients received definitive IMPT to total dose of 70 to 74.4 Gy(RBE), and 18 patients received postoperative IMPT to 60 to 66 Gy(RBE) following transoral robotic surgery (TORS). Sixty-four percent of patients received concurrent systemic therapy. There were no treatment interruptions or observed acute grade 4 or 5 toxicities. Eighteen patients had percutaneous endoscopic gastrostomy (PEG) tube placement; the majority (14) were placed prophylactically. The most common grade 3 acute toxicities were dermatitis (76%) and mucositis (72%). The most common late toxicity was grade 2 xerostomia (30%). At a median follow-up time of 19.2 months (interquartile range [IQR], 11.2-28.4), primary complete response was 100% and nodal complete response was 92%. One patient required a salvage neck dissection owing to an incomplete response at 4 months. There were no recorded local regional or marginal recurrences, PFS was 93.5%, and OS was 95.7%.
Conclusion:
Our early results for IMPT in OPSCC are promising with no local regional or marginal recurrences and a favorable toxicity profile. Our data add to a body of evidence that supports the clinical use of IMPT. Randomized comparative trials are encouraged.
Keywords: proton beam therapy, tonsil cancer, tongue base cancer, HPV/p16 positive, squamous cell cancer
Introduction
The application of proton beam therapy in oropharyngeal squamous cell cancer (OPSCC) has notably increased [1]. Over the past decade, there has been an increase in incidence of human papillomavirus (HPV)–positive tumors, with an improved prognosis when compared to HPV-negative tumors [2–4]. The demographic of this highly curable disease has shifted to a younger, highly functional population with fewer comorbidities and higher quality-of-life expectations [5]. The focus of contemporary trials has been to de-intensify treatment in the favorable-prognosis HPV-positive disease [6–11].
Intensity-modulated radiation therapy (IMRT) with or without concurrent systemic therapy remains the standard of care for most patients with OPSCC [12]. The development of pencil beam scanning implemented on a gantry allows for improved beam modulation, target conformality, and entrance dose reduction [13]. These characteristics can potentially facilitate greater dose sparing of important organs at risk (OARs). Multiple proton-photon dosimetric comparison studies [14–16] have demonstrated superiority of protons in terms of sparing the oral cavity, uninvolved mucosa, and major salivary glands. However, care must be taken with treatment planning to ensure that the steeper dose gradients with IMPT do not result in increase in marginal failures.
In 2005, Slater and colleagues [17] reported the first clinical use of proton therapy in the treatment of oropharyngeal cancer. More recently, the University of Pennsylvania [18], Memorial Sloan Kettering [19], and MD Anderson Cancer Center [20] reported on their experiences with IMPT. The MD Anderson Cancer Center also performed a nonrandomized case-matched analysis with IMRT in a more contemporary setting [21, 22]. In comparison with IMRT, patients treated with IMPT in these series showed encouraging results with favorable toxicity. This included a reduction in feeding tube dependency, severe weight loss, and osteoradionecrosis. Furthermore, changes in taste and appetite, xerostomia, and overall quality of life have all favored IMPT [23].
The purpose of the present study is to report on (1) initial oncologic outcomes including patterns of failure in patients with oropharyngeal cancer treated with IMPT at our center, (2) the associated acute and late treatment-related side effects, and (3) our treatment planning approach and achieved dosimetric results.
Materials and Methods
Study Design, Eligibility, and Ethics Approval
This is a single-institution retrospective review of patients treated at our center with IMPT from 2015-17. Eligibility criteria included (1) pathologic diagnosis of OPSCC, (2) curative intent treatment, and (3) no evidence of distant metastasis. Patients treated with proton therapy to either unilateral or bilateral neck were eligible. Patients treated with either primary radiation therapy or postoperative radiation therapy were eligible. Endpoints included local regional control (LRC), progression-free survival (PFS), overall survival (OS), tumor response, and toxicity outcomes. The study protocol was reviewed and approved by our institutional review board.
Patient Treatment
After initial consultation, examination, and multidisciplinary tumor board discussion, patients were treated with definitive radiation therapy or with transoral robotic surgery (TORS) followed by adjuvant radiation therapy with or without systemic therapy. Patients unable to tolerate high-dose cisplatin every 3 weeks received weekly cetuximab with or without altered fractionation radiation therapy. No patients received induction chemotherapy. Our follow-up evaluations did not routinely include positron emission tomography–computed tomography (PET/CT) scans. However, patients with a persistent neck node beyond 3 to 4 months post radiation therapy that was deemed borderline received a PET/CT scan at 5 to 6 months post radiation therapy. Salvage/consolidation neck dissection was performed only for PET-avid disease [24]. If the PET/CT scan finding was negative, the patient was subject to close surveillance [25].
Evaluation and Data Collection
Before initiating proton therapy, patients received dental clearance and a custom stent that deviates the tongue to the opposite side of the treated field in unilateral cases (34.8%) and depresses it in bilateral cases (65.2%) [26]. Patients receiving concurrent chemotherapy with bilateral neck radiotherapy were evaluated for prophylactic feeding tube placement. A reactive feeding tube was considered if patients lost significant weight (5%–10%) during treatment. After enrollment on our prospective registry, weekly evaluations were performed during treatment. Posttreatment evaluations were performed every 3 months for the first year, every 4 months for the second year, and every 6 to 12 months for 3 to 5 years. Data points were retrospectively collected from our local registry as well as the electronic medical record system. At each visit, toxicity endpoints were assessed by the treating physician and recorded according to the Common Terminology Criteria for Adverse Events version 4.03 [27]. Acute toxicity was defined from start of radiation therapy to 3 months post treatment, whereas late toxicity was defined from 3 months post radiation therapy and beyond. Primary tumor complete response was defined as no visible tumor on follow-up imaging and laryngoscopy, partial response was defined as >50% tumor reduction, and no response was defined as no reduction in tumor size. Neck nodal complete response was defined on imaging as a node reduction to <1.5 cm in maximal dimension without suspicious morphology (ie, central necrosis), a partial response was defined as >1.5-cm residual lymph node, and no response was defined as no reduction in size.
Treatment Planning
Treatment simulation for proton therapy was performed with a noncontrast and contrast CT scan with patients immobilized in a shoulder-length thermoplastic mask. Target volumes and OAR volumes were delineated by the treating radiation oncologist on each axial CT slice according to standard contouring guidelines. A proton therapy treatment planning system was used for IMPT planning (RayStation, version 6.0, RaySearch Laboratories, Stockholm, Sweden). The prescribed IMPT radiation doses used a relative biological effectiveness (RBE) value of 1.1. For the definitive cases, high-risk clinical target volumes (CTVs) including the gross disease received a dose of 66 to 69.96 Gy(RBE), while low- to intermediate-risk elective CTVs received a dose of 54 to 63 Gy(RBE). For postoperative cases, high-risk CTVs including the operative bed of the gross disease received a dose of 60 to 66 Gy(RBE), while elective low-risk CTVs received a dose of 54 to 57 Gy(RBE). Treatment plans with simultaneous integrated boost were generated by using pencil beam scanning with single-field optimization based on the planning target volumes (PTVs). Typically, a 3- to 5-mm margin was used for PTV expansions. Treatment plan robustness analysis was performed on CTVs and was based on ±3% range uncertainty and ±3 mm in all translational directions for geometric uncertainty. Additionally, we used robust optimization for challenging cases to improve target coverage and OAR sparing. Dental metal amalgam fillings and crowns proximal to the target volumes and in the path of the proton beam were replaced by low-density composite material. This reduced the dental CT artifact and minimized dose calculation uncertainties. For patients who could not have their dental amalgam fillings replaced, we contoured the dental CT artifact with a density override to tissue or bone, and also contoured the dental amalgam with a density override to brass of 11.6 g/cm3 [28]. Pencil beam algorithm was used for dose calculation except for 2 cases where Monte Carlo dose calculation algorithm was used. Bilateral neck irradiation plans were generated with 3 to 4 beams. Unilateral neck irradiation was used in selected appropriate cases (ie, early-stage well-lateralized tonsillar primary) and most commonly planned with a single anterior oblique beam [29]. Generally, plans were designed to spare the bilateral parotid glands and contralateral submandibular gland when feasible. Our clinical experience with this approach with IMRT is described elsewhere [30]. The oral cavity and pharyngeal constrictors were contoured as based on prior studies and were deemed as avoidance structures [31, 32]. The skin was not considered an avoidance structure as it was not part of our standard practice at the time. However, before planning, we crop 3 mm from PTVs that extend to the skin. Final plans were reviewed and approved during quality assurance rounds.
During treatment, daily orthogonal 2D kV images were used for patient localization. Quality assurance CT scans were routinely performed after 3 weeks into treatment to monitor setup and anatomical changes. Adaptive planning was performed at the discretion of the primary radiation oncologist when indicated.
Statistical Analysis
Descriptive statistics were used to characterize the baseline characteristics of the overall population and initial tumor response. Survival times were computed from radiation therapy end date to the occurrence of the first event. Events were death from any cause for OS and any recurrence or death for PFS. Local and/or regional recurrences were deemed as events for LRC analysis. Persistent neck disease requiring a salvage/consolidation neck dissection was not counted as a local regional event, since a planned neck dissection for a node-positive patient with persistent clinical/radiographic neck disease is part of a standard treatment algorithm. Survival rates were estimated by using the Kaplan-Meier method; patients were censored after a recorded event or last follow-up visit. Analyses were performed with IBM SPSS Statistics (version 25, IBM Corporation and its licensors [1989, 2017], Armonk, NY).
Results
Patient, Tumor, and Treatment Characteristics
A total of 46 patients with OPSCC were treated with IMPT from March 2015 to August 2017. Patient and tumor characteristics are listed in Table 1. Most patients had a pretreatment Karnofsky Performance Status score of ≥90. Five patients had a prior unrelated cancer diagnosis and none had received prior radiation therapy. The median time from diagnosis to proton therapy initiation was 53 days (IQR, 44-82). Treatment characteristics are listed in Table 2. Twenty-nine patients received definitive proton therapy, and 17 patients received adjuvant proton therapy post TORS. Four patients who received either cetuximab with radiotherapy, or radiotherapy alone, received a hyperfractionated accelerated twice-daily regimen of 72 to 74.4 Gy(RBE) in 60 to 62 fractions. One patient delayed definitive therapy to pursue naturopathic medicine before receiving treatment. Another patient declined adjuvant radiation therapy post TORS and developed a primary site recurrence several months later, requiring salvage therapy. Two thirds of the patients (n = 30) received treatment to bilateral neck, and one third (n = 16) to ipsilateral neck for lateralized tonsil cancers. No patient experienced an interruption or delay in treatment after initiating the IMPT course. Of patients who received concurrent chemotherapy (n = 30), 61% were able to complete all 3 cycles of the platin-based regimen and 86% were able to complete all 7 cycles of cetuximab.
Table 1.
Patient and tumor characteristics.
| Characteristic |
n |
Value |
| Median age, IQR, y | 58 | 36-80 |
| Sex, % | ||
| Male | 43 | 93.5 |
| Female | 3 | 6.5 |
| Race, white | 42 | 91.3 |
| Nonsmoker | 31 | 67.4 |
| Smoker <10 pack-year history | 13 | 28.3 |
| Smoker ≥10 pack-year history | 2 | 4.3 |
| Histology, % | ||
| Squamous cell carcinoma | 46 | 100 |
| Primary subsite, % | ||
| Tonsil | 27 | 58.7 |
| Tongue base | 19 | 41.3 |
| Tumor p16 status, % | ||
| Positive | 41 | 89.1 |
| Unknown | 5 | 10.9 |
| AJCC Cancer Staging Manual, 7th edition, % | ||
| Stage I-II | 1 | 2 |
| Stage III | 4 | 9 |
| Stage IVA-IVB | 41 | 89 |
| AJCC Cancer Staging Manual, 8th edition (p16+ only), % | ||
| Stage I-II | 33 | 71.7 |
| Stage III | 8 | 17.4 |
Abbreviations: IQR, interquartile range; AJCC, American Joint Committee on Cancer.
Table 2.
Treatment characteristics.
| Treatment characteristic |
n |
Value |
| Treatment strategy, % | ||
| Concurrent chemo-IMPT | 25 | 54.3 |
| IMPT alone | 4 | 8.7 |
| TORS→ concurrent chemo-IMPT | 5 | 10.9 |
| TORS→ IMPT alone | 12 | 26 |
| Pre-IMPT surgical procedures, % | ||
| TORS and neck dissection | 17 | 37 |
| Diagnostic tonsillectomy | 5 | 10.8 |
| Neck dissection | 4 | 8.7 |
| Post-TORS characteristics, % | 17 | |
| Positive margins (R1) | 0 | 0 |
| Extranodal extension | 4 | 22.2 |
| Perineural invasion | 3 | 16.7 |
| Lymphovascular invasion | 4 | 22.2 |
| Concurrent chemotherapy, % | ||
| Platin-based | 23 | 50 |
| Cetuximab | 7 | 15.2 |
| None | 16 | 34.8 |
| Neck radiation therapy volume, % | ||
| Bilateral | 30 | 65.2 |
| Unilateral | 16 | 34.8 |
| Median dose and range, Gy | 70 | 60-74.4 |
| Median IMPT duration, IQR, d | 44 | 39-44 |
Abbreviations: IMPT, intensity-modulated proton therapy; TORS, transoral robotic surgery; IQR, interquartile range.
Dosimetric Analysis
The median high-risk planning target volume (PTV-HR) was 388 cm3 (IQR, 297-497). The PTV-HR and CTV-HR D95 received ≥99% of prescribed dose in all cases. Pertinent OAR doses for both bilateral and unilateral IMPT plans are listed in Table 3. Five patients (10.8%) required adaptive planning. All 5 received definitive chemoradiotherapy.
Table 3.
Dosimetric report.
| Structure (n = 46) |
Median, cGy |
IQR, cGy |
| Parameter: max dose | ||
| Brainstem | 1591 | 706.5-2386 |
| Spinal cord | 2364 | 1380-2835 |
| Mandible – PTV-HR | 6519 | 6189-6980 |
| Parameter: mean dose | ||
| Bilateral plans (n = 30) | ||
| Mandible – PTV-HR | 1952 | 1464-2607 |
| Ipsilateral cochlea | 1252 | 472-1867 |
| Contralateral cochlea | 2 | 0-12 |
| Ipsilateral SMG | 6564 | 6101-6932 |
| Contralateral SMG | 2591 | 2324-3512 |
| Ipsilateral parotid | 2944 | 2197-3156 |
| Contralateral parotid | 962 | 693-1889 |
| Oral cavity | 1559 | 1168-2495 |
| Pharyngeal constrictors | 3855 | 3437-4256 |
| Larynx | 2990 | 1963-3718 |
| Esophagus | 518 | 366-1040 |
| Unilateral plans (n = 16) | ||
| Mandible – PTV-HR | 1448 | 1150-1821 |
| Ipsilateral cochlea | 1475 | 863-1991 |
| Contralateral cochlea | 0 | 0-5 |
| Ipsilateral SMG | 6223 | 6046-6628 |
| Contralateral SMG | 29 | 1-61 |
| Ipsilateral parotid | 4622 | 3520-5237 |
| Contralateral parotid | 0 | 0-1 |
| Oral cavity | 1003 | 832-1181 |
| Pharyngeal constrictors | 2517 | 2234-3522 |
| Larynx | 1642 | 885-2502 |
| Esophagus | 671 | 130-1312 |
Abbreviations: IQR, interquartile range; PTV-HR, high-risk planning target volume; SMG, submandibular gland.
Tumor Response and Survival Analysis
A complete tumor response at the primary site was achieved in all definitive IMPT cases within 3 to 6 months post treatment. A total of 4 patients received post–radiation therapy PET/CT scans on follow-up. Only 2 patients had a persistent neck node >1.5 cm at >4 months post treatment of which 1 was positive on PET/CT. That patient had a consolidation neck dissection and pathology revealed a 2-mm focus of residual disease and was not counted as a local regional event. The other patient had a negative PET/CT finding and continued on follow-up beyond 3 years post treatment with no evidence of recurrence. The median follow-up for the group was 19.2 months (IQR, 11.2-28.4) with 38 patients (82.6%) having more than 1 year of follow-up. At last follow-up, none developed a local regional recurrence, 2 patients developed a distant recurrence, and 2 patients died. One death related to distant spread occurred at 14 months post treatment. The other death was due to aspiration pneumonia (grade 5 event). The latter patient required a percutaneous endoscopic gastrostomy (PEG) tube immediately after TORS, and his swallowing function never recovered. At last follow-up, the LRC, PFS, and OS were 100%, 93.5%, and 95.7%, respectively. The Kaplan-Meier curves are displayed in Figure 1. Illustrations of representative bilateral neck and unilateral neck IMPT plans are shown in Figure 2.
Figure 1.
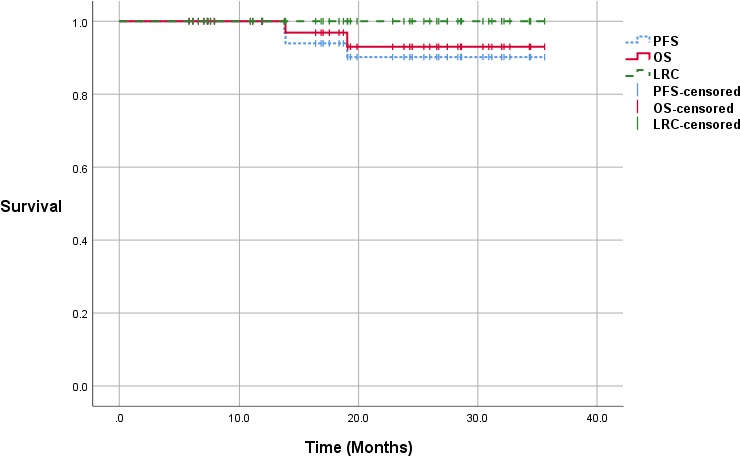
Kaplan-Meier curves (median follow-up, 19.2 months).
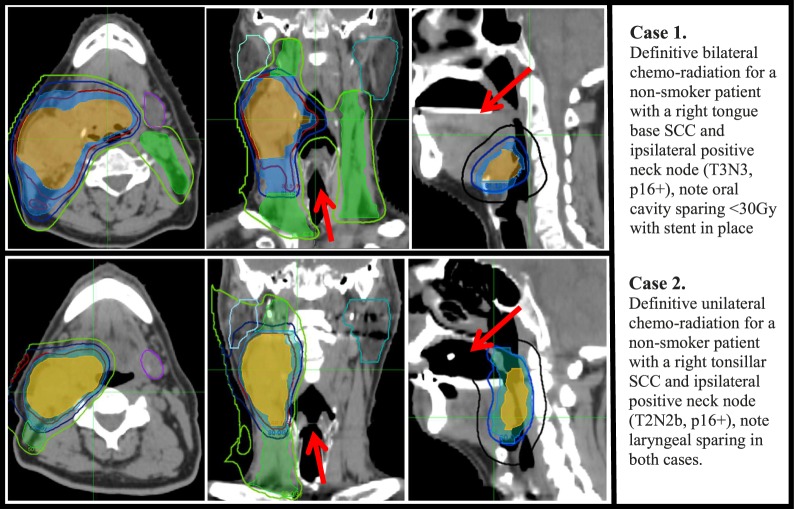
Figure 2.
IMPT plans for a bilateral neck irradiation case (top panel) and a unilateral radiation case (bottom panel). Contours = PTV70 (orange, color-wash), PTV63 (blue, color-wash), PTV54 (green, color-wash), right parotid (sky blue), left parotid (teal), submandibular gland (purple). Iso-dose lines = 70 Gy (red), 63 Gy (blue), 54 Gy (green), 30 Gy (black). A custom dental stent is seen. Abbreviations: IMPT, intensity-modulated proton therapy; PTV, planning target volume.
Toxicity
There were no treatment interruptions during the course of proton therapy. Two patients (4.3%) had emergency visits for pain and dehydration, both were hospitalized and subsequently discharged in 2 to 3 days. One patient (2.2%) had a documented weight loss >10% of body weight (grade 2). Prophylactic PEG tube insertion was performed for 14 patients (30.4%). Reactive PEG tube insertion during treatment was performed for 4 patients (8.7%), all of whom received definitive IMPT with concurrent cisplatin and 3 had bilateral neck treatments. One patient was PEG-tube dependent post TORS and before proton therapy. The median tube duration from treatment completion to PEG tube removal was 61.5 days (IQR, 35-80), all had their PEG tubes removed except for 1 PEG-tube–dependent patient. The peak incidence of treatment-related acute and late toxicities are listed in Table 4. The most common documented acute toxicities were radiation dermatitis grade 3 (76.1%), oral mucositis grade 3 (71.7%), xerostomia grade 1 (71.7%), and dysgeusia grade 1 (73.9%). The most common late toxicities were dysgeusia grade 1 (76%), xerostomia grade 1 (65.2%), and xerostomia grade 2 (30%). Grade 1 late dysphagia was documented in 9 patients (19.6%) and grade 2 in 1 patient, while 1 other patient had a grade 5 event as previously mentioned. Cisplatin chemotherapy–related hearing loss occurred in 2 patients. Six patients (13%) had a persistent nonhealing mucosal ulceration (versus soft tissue necrosis) beyond 3 months at the primary site. To be conservative, we coded them all as having soft tissue necrosis, defined as ulceration >6 weeks after completion of radiation therapy. Three of these 6 patients had prior TORS resection with (R0) margins and a median prescription dose of 60 Gy(RBE) to the primary site. One had a simple tonsillectomy with residual (R2) disease and 2 others had definitive radiotherapy (prescription dose 70-72 Gy-RBE in all 3). Three of the 6 patients received concurrent chemotherapy (cisplatin n = 1, cetuximab n = 2), including the 2 patients who received definitive radiotherapy and 1 post TORS with extracapsular extension. Two of these 6 patients required hyperbaric oxygen therapy (tonsillectomy with R2 residual disease and 70 Gy, n = 1; TORS radical tonsillectomy and 60 Gy, n = 1). Both had a smoking history of ≥30 pack years, and one had a significant mixed connective tissue disorder requiring medications. Neither had concurrent systemic therapy. The other 4 patients were nonsmokers and had a subcentimeter persistent mucosal ulcer that healed with conservative management. All 6 patients healed completely by 6 to 12 months post treatment.
Table 4.
Peak acute and chronic radiation toxicity grade recorded as per the Common Terminology Criteria for Adverse Events Version 4.03.
| Toxicity |
Grade 0 |
Grade 1 |
Grade 2 |
Grade 3a |
||||
|
n |
% |
n |
% |
n |
% |
n |
% |
|
| Acute | ||||||||
| Dermatitis | 0 | 0 | 3 | 6.5 | 8 | 17.4 | 35 | 76.1 |
| Mucositis | 3 | 6.5 | 1 | 2.2 | 9 | 19.6 | 33 | 71.7 |
| Weight loss | 24 | 52.2 | 21 | 45.7 | 1 | 2.2 | 0 | 0 |
| Xerostomia | 4 | 8.7 | 33 | 71.7 | 4 | 8.7 | 3 | 6.5 |
| Dysgeusia | 4 | 8.7 | 34 | 73.9 | 6 | 13 | ||
| Chronic | ||||||||
| Dysphagiab | 35 | 76 | 9 | 19.6 | 1 | 2.2 | 0 | 0 |
| Xerostomia | 2 | 4.3 | 30 | 65.2 | 14 | 30 | 0 | 0 |
| Dysgeusia | 5 | 10.8 | 35 | 76 | 3 | 6.5 | 0 | 0 |
| Trismus | 41 | 89.1 | 4 | 8.7 | 1 | 2.2 | 0 | 0 |
| Soft tissue necrosis | 40 | 87 | 4 | 8.7 | 2 | 4.3 | 0 | 0 |
No acute grade 4 or 5 events observed.
No late grade 4 events, only 1 reported grade 5 event (dysphagia).
Discussion
The role of IMPT for head and neck cancer continues to be defined by ongoing work at specialized centers. Preclinical and dosimetric studies hypothesized a potential decrease in both acute and long-term toxicity. A recent review article lists all the clinical data published so far with encouraging early results [33]. However, clinical data evaluating tumor control endpoints are still limited. The combination of proton beam range uncertainty, RBE variation, and sharp dose fall-off does argue for careful evaluation of clinical outcomes, including patterns of failure, to ensure there is not an increase in marginal misses [34, 35]. While there are questions regarding the cost effectiveness of using proton therapy for head and neck cancers, this topic is beyond the scope of this article [36, 37].
Our early clinical results with IMPT in OPSCC are encouraging. With 46 patients, a median follow-up of 19.2 months, and with most patients having more than a 1-year follow-up, there were no local regional failures. There were 2 distant failures and 2 deaths. One patient experienced distant failure 14 months post treatment then passed away from disease progression; his p16 status was unknown. Another patient who had distant failure 8 months post treatment delayed his definitive therapy for a year to pursue naturopathic medicine. One patient was PEG-tube dependent after TORS and died of aspiration pneumonia 22 months after treatment (grade 5 event) with no local regional or distant failure. Our early survival results are comparable to other series; at last follow-up the LRC, PFS, and OS were 100%, 93.5%, and 95.7%, respectively. The MD Anderson cohort consisted of patients receiving neoadjuvant chemotherapy. At a median follow-up of 29 months, their 2-year actuarial PFS and OS rates were 88.6% and 94.5% [23]. It is important to highlight that most of our patients were nonsmoking Caucasian males with HPV-positive disease and stages III to IV per the 7th edition of the AJCC [American Joint Committee on Cancer] Cancer Staging Manual [38]. By reclassification per the 8th edition of the staging manual, our cohort has mainly stage I-II disease [39]. Whether these outcomes may be extrapolated to HPV-negative smoking patients with poorer prognosis is not clear.
The acute and chronic toxicity profile for IMPT in OPSCC was overall favorable. There were no treatment interruptions and hospital admission rates were low. Overall, 18 patients had PEG tube placement (39%); most (14/18) were placed prophylactically and 4 patients needed a reactive PEG with a median tube duration of 61.5 days. The MD Anderson IMPT experience PEG rate was 24% with median tube duration of 82 days [23], while 2 other IMRT series reported rates of 47% to 58% [40, 41]. Ten patients (21.7%) developed late dysphagia, of whom only 1 had grade 2 dysphagia. Pharyngeal constrictor mean dose in our series was consistently below 40 Gy(RBE); this might explain our low dysphagia rates. We did not report on acute dysphagia rates to avoid confusion, as most patients who had a PEG received it prophylactically. Most of our patients (71.7%) did develop grade 3 acute mucositis, mostly confined near the targeted regions of the oropharynx (PTV-HR), with multiple patients experiencing a lesser degree of mucositis in the oral cavity and uninvolved oropharyngeal mucosa. However, it is difficult to report this accurately, as there are limited tools that capture this pattern of mucositis. The grade 2 chronic xerostomia (30%) and dysgeusia (6.5%) were low and could be explained by the consistently low oral cavity and contralateral salivary gland mean doses, especially for unilateral plans (Table 3). Compared to bilateral neck plans, unilateral plans had relatively high ipsilateral parotid mean dose; this is related to our routine use of a single anterior oblique beam. Planning unilateral neck cases on the gantry with multiple beam angles is expected to reduce the ipsilateral mean parotid dose closer to that obtained with bilateral neck plans. However, this would be more resource intensive, and even impractical, in centers with only a single gantry room like ours where the gantry is prioritized for patients requiring highly complex plans. As mentioned in the “Toxicity” section, a total of 6 patients (13%) developed persistent mucosal ulceration or soft-tissue necrosis that completely healed by 6 to 12 months post treatment. Lukens et al [42] described a subset of patients with OPSCC treated with TORS and postoperative radiotherapy who are at risk of developing surgical bed soft-tissue necrosis. Risk factors included tonsillar location, depth of resection, radiation dose fraction size (>2 Gy/d) to the surgical bed, and severe mucositis. In their retrospective series, the rate of soft-tissue necrosis with IMRT-based postoperative radiotherapy was 28%. From our experience, we now inform our patients, especially if they have a significant history of smoking, diabetes, or connective tissue disorder, about this uncommon consequence as part of informed consent.
Despite concerns over proton beam range uncertainty, sharp dose fall-off, and 2-dimensional image-guided radiotherapy capabilities, the patients in this series did not experience local regional failures or marginal misses. Our beam arrangement approach was anterior oblique beams with an additional 1 or 2 posterior beam angles for bilateral neck treatments. Some studies report that a posterior oblique approach with multifield optimization might better spare the major salivary glands and oral cavity [43]. We are currently using a multifield optimization approach with the same beam angles as described in “Materials and Methods.”
Most patients in this series were male (93.5%) and Caucasian (91.3%). It is well known that the rate of head and neck squamous cell carcinoma overall is significantly higher among men than women [2]. Additionally, recent epidemiologic data suggest that the risk of OPSCC is 4-fold higher among men than women. It also reported a significant increased risk among whites and a corresponding decreased risk among blacks between 2005 and 2014 [44]. In our institutional head and neck practice, patients with OPSCC were treated with either proton therapy or IMRT during the study period. In general, the more advanced tumors, and those patients whose insurance was not approved, were treated with IMRT. There were no other specific selection factors involved, and we believe the results reported here are also applicable to females, as well as people of all races.
This single-institution series has some limitations. First, although all patients were enrolled on a prospective registry before treatment, the data collected in the registry are limited. Hence, most of the survival and toxicity data were collected via the electronic medical records retrospectively. Second, despite the rigorous collection of toxicity data by the treating physician on a weekly basis during treatment, it was not measured with a formal patient reported outcome survey or other validated tool and is subject to potential reporting bias. Finally, the analysis is limited by the relatively short follow-up period, low event count, and a small number of patients.
Despite these limitations, this study is one of the few early reports on the efficacy of the clinical use of IMPT in OPSCC. Data from the forthcoming comparative randomized trials (NCT02923570 and NCT01893307) are expected to help evaluate the relative advantages of proton therapy.
Conclusion
Our early clinical and toxicity outcomes for IMPT are promising with no documented local regional or marginal recurrences to date and a favorable toxicity profile. We plan to continue to follow up our cohort and report long-term outcomes. Prospective data from multicenter randomized trials comparing IMPT with IMRT are encouraged.
ADDITIONAL INFORMATION AND DECLARATIONS
Conflicts of Interest: The authors have no relevant conflicts of interest to disclose.
Ethical Approval: All patient data have been collected under institutional review board–approved protocol.
References
- 1.Durante M, Orecchia R, Loeffler JS. Charged-particle therapy in cancer: clinical uses and future perspectives. Nat Rev Clin Oncol. 2017;14:483–95. doi: 10.1038/nrclinonc.2017.30. [DOI] [PubMed] [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2017. CA Cancer J Clin. 2017;67:7–30. doi: 10.3322/caac.21387. [DOI] [PubMed] [Google Scholar]
- 3.Gillison ML, Koch WM, Capone RB, Spafford M, Westra WH, Wu L, Zahurak ML, Daniel RW, Viglione M, Symer DE, Shah KV, Sidransky D. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J Natl Cancer Inst. 2000;92:709–20. doi: 10.1093/jnci/92.9.709. [DOI] [PubMed] [Google Scholar]
- 4.Ang KK, Harris J, Wheeler R, Weber R, Rosenthal DI, Nguyen-Tân PF, Westra WH, Chung CH, Jordan RC, Lu C, Kim H. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ringash J. Survivorship and quality of life in head and neck cancer. J Clin Oncol. 2015;33:3322–7. doi: 10.1200/JCO.2015.61.4115. [DOI] [PubMed] [Google Scholar]
- 6.Burtness B, Golemis EA. Molecular Determinants of Head and Neck Cancer. New York, NY: Springer; 2014. 585. [Google Scholar]
- 7.Holsinger FC, Ferris RL. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: robotics, lasers, and clinical trials. J Clin Oncol. 2015;33:3285–92. doi: 10.1200/JCO.2015.62.3157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marur S, Li S, Cmelak AJ, Gillison ML, Zhao WJ, Ferris RL, Westra WH, Gilbert J, Bauman JE, Wagner LI, Trevarthen DR, Balkrishna J, Murphy BA, Agrawal N, Colevas AD, Chung CH, Burtness B. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx—ECOG-ACRIN Cancer Research Group. J Clin Oncol. 2017;35:490–7. doi: 10.1200/JCO.2016.68.3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chera BS, Amdur RJ, Tepper JE, Tan X, Weiss J, Grilley-Olson JE, Hayes DN, Zanation A, Hackman TG, Patel S, Sheets N, Weissler MC, Mendenhall WM. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer. 2018;124:2347–54. doi: 10.1002/cncr.31338. [DOI] [PubMed] [Google Scholar]
- 10.Chen AM, Felix C, Wang PC, Hsu S, Basehart V, Garst J, Beron P, Wong D, Rosove MH, Rao S, Melanson H, Kim E, Palmer D, Qi L, Kelly K, Steinberg ML, Kupelian PA, Daly ME. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 2017;18:803–11. doi: 10.1016/S1470-2045(17)30246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee N, Schoder H, Beattie B, Lanning R, Riaz N, McBride S, Katabi N, Li D, Yarusi B, Chan S, Mitrani L, Zhang Z, Pfister DG, Sherman E, Baxi S, Boyle J, Morris LG, Ganly I, Wong R, Humm J. Strategy of using intratreatment hypoxia imaging to selectively and safely guide radiation dose de-escalation concurrent with chemotherapy for locoregionally advanced human papillomavirus–related oropharyngeal carcinoma. Int J Radiat Oncol Biol Phys. 2016;96:9–17. doi: 10.1016/j.ijrobp.2016.04.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nutting CM, Morden JP, Harrington KJ, Urbano TG, Bhide SA, Clark C, Miles EA, Miah AB, Newbold K, Tanay M, Adab F, Jefferies SJ, Scrase C, Yap BK, A'Hern RP, Sydenham MA, Emson M, Hall E. PARSPORT trial management group. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 2011;12:127–36. doi: 10.1016/S1470-2045(10)70290-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mohan R, Das IJ, Ling CC. Empowering intensity modulated proton therapy through physics and technology: an overview. Int J Radiat Oncol Biol Phys. 2017;99:304–16. doi: 10.1016/j.ijrobp.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Apinorasethkul O, Kirk M, Teo K, Swisher-McClure S, Lukens JN, Lin A. Pencil beam scanning proton therapy vs rotational arc radiation therapy: a treatment planning comparison for postoperative oropharyngeal cancer. Med Dosim. 2017;42:7–11. doi: 10.1016/j.meddos.2016.09.004. [DOI] [PubMed] [Google Scholar]
- 15.Holliday EB, Kocak-Uzel E, Feng L, Thaker NG, Blanchard P, Rosenthal DI, Gunn GB, Garden AS, Frank SJ. Dosimetric advantages of intensity-modulated proton therapy for oropharyngeal cancer compared with intensity-modulated radiation: a case-matched control analysis. Med Dosim. 2016;41:189–94. doi: 10.1016/j.meddos.2016.01.002. [DOI] [PubMed] [Google Scholar]
- 16.Kandula S, Zhu X, Garden AS, Gillin M, Rosenthal DI, Ang KK, Mohan R, Amin MV, Garcia JA, Wu R, Sahoo N, Frank SJ. Spot-scanning beam proton therapy vs intensity-modulated radiation therapy for ipsilateral head and neck malignancies: a treatment planning comparison. Med Dosim. 2013;38:390–4. doi: 10.1016/j.meddos.2013.05.001. [DOI] [PubMed] [Google Scholar]
- 17.Slater JD, Yonemoto LT, Mantik DW, Bush DA, Preston W, Grove RI, Miller DW, Slater JM. Proton radiation for treatment of cancer of the oropharynx: early experience at Loma Linda University Medical Center using a concomitant boost technique. Int J Radiat Oncol Biol Phys. 2005;62:494–500. doi: 10.1016/j.ijrobp.2004.09.064. [DOI] [PubMed] [Google Scholar]
- 18.Sharma S, Zhou O, Thompson R, Gabriel P, Chalian A, Rassekh C, Weinstein GS, O'Malley BW, Jr, Aggarwal C, Bauml J, Cohen RB, Lukens JN, Swisher-McClure S, Ghiam AF, Ahn PH, Lin A. Quality of life of postoperative photon versus proton radiation therapy for oropharynx cancer. Int J Part Ther. 2018;5:11–7. doi: 10.14338/IJPT-18-00032.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Romesser PB, Cahlon O, Scher E, Zhou Y, Berry SL, Rybkin A, Sine KM, Tang S, Sherman EJ, Wong R, Lee NY. Proton beam radiation therapy results in significantly reduced toxicity compared with intensity-modulated radiation therapy for head and neck tumors that require ipsilateral radiation. Radiother Oncol. 2016;118:286–92. doi: 10.1016/j.radonc.2015.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sio TT, Lin HK, Shi Q, Gunn GB, Cleeland CS, Lee JJ, Hernandez M, Blanchard P, Thaker NG, Phan J, Rosenthal DI, Garden AS, Morrison WH, Fuller CD, Mendoza TR, Mohan R, Wang XS, Frank SJ. Intensity modulated proton therapy versus intensity modulated photon radiation therapy for oropharyngeal cancer: first comparative results of patient-reported outcomes. Int J Radiat Oncol Biol Phys. 2016;95:1107–14. doi: 10.1016/j.ijrobp.2016.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Blanchard P, Garden AS, Gunn GB, Rosenthal DI, Morrison WH, Hernandez M, Crutison J, Lee JJ, Ye R, Fuller CD, Mohamed AS, Hutcheson KA, Holliday EB, Thaker NG, Sturgis EM, Kies MS, Zhu XR, Mohan R, Frank SJ. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer–a case matched analysis. Radiother Oncol. 2016;120:48–55. doi: 10.1016/j.radonc.2016.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhang W, Zhang X, Yang P, Blanchard P, Garden AS, Gunn B, Fuller CD, Chambers M, Hutcheson KA, Ye R, Lai SY, Radwan MAS, Zhu XR, Frank SJ. Intensity-modulated proton therapy and osteoradionecrosis in oropharyngeal cancer. Radiother Oncol. 2017;123:401–5. doi: 10.1016/j.radonc.2017.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gunn GB, Blanchard P, Garden AS, Zhu XR, Fuller CD, Mohamed AS, Morrison WH, Phan J, Beadle BM, Skinner HD, Sturgis EM, Kies MS, Hutcheson KA, Rosenthal DI, Mohan R, Gillin MT, Frank SJ. Clinical outcomes and patterns of disease recurrence after intensity modulated proton therapy for oropharyngeal squamous carcinoma. Int J Radiat Oncol Biol Phys. 2016;95:360–7. doi: 10.1016/j.ijrobp.2016.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yao M, Graham MM, Hoffman HT, Smith RB, Funk GF, Graham SM, Dornfeld KJ, Skwarchuk M, Menda Y, Buatti JM. The role of post–radiation therapy FDG PET in prediction of necessity for post–radiation therapy neck dissection in locally advanced head-and-neck squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2004;59:1001–10. doi: 10.1016/j.ijrobp.2004.01.040. [DOI] [PubMed] [Google Scholar]
- 25.Mehanna H, Wong WL, McConkey CC, Rahman JK, Robinson M, Hartley AG, Nutting C, Powell N, Al-Booz H, Robinson M, Junor E, Rizwanullah M, von Zeidler SV, Wieshmann H, Hulme C, Smith AF, Hall P, Dunn J. PET-NECK Trial Management Group. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N Engl J Med. 2016;374:1444–54. doi: 10.1056/NEJMoa1514493. [DOI] [PubMed] [Google Scholar]
- 26.Johnson B, Sales L, Winston A, Liao J, Laramore G, Parvathaneni U. Fabrication of customized tongue-displacing stents: considerations for use in patients receiving head and neck radiotherapy. J Am Dent Assoc. 2013;144:594–600. doi: 10.14219/jada.archive.2013.0170. [DOI] [PubMed] [Google Scholar]
- 27.US Department of Health and Human Services. National Cancer Institute. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.03. 2010 https://www.eortc.be/services/doc/ctc/CTCAE_4.03_2010-06-14_QuickReference_5x7.pdf Accessed October 2nd, 2017.
- 28.Richard P, Sandison G, Dang Q, Johnson B, Wong T, Parvathaneni U. Dental amalgam artifact: adverse impact on tumor visualization and proton beam treatment planning in oral and oropharyngeal cancers. Pract Radiat Oncol. 2015;5:e583–8. doi: 10.1016/j.prro.2015.04.007. [DOI] [PubMed] [Google Scholar]
- 29.Chronowski GM, Garden AS, Morrison WH, Frank SJ, Schwartz DL, Shah SJ, Beadle BM, Gunn GB, Kupferman ME, Ang KK, Rosenthal DI. Unilateral radiotherapy for the treatment of tonsil cancer. Int J Radiat Oncol Phys. 2012;83:204–9. doi: 10.1016/j.ijrobp.2011.06.1975. [DOI] [PubMed] [Google Scholar]
- 30.Gensheimer MF, Liao JJ, Garden AS, Laramore GE, Parvathaneni U. Submandibular gland-sparing radiation therapy for locally advanced oropharyngeal squamous cell carcinoma: patterns of failure and xerostomia outcomes. Radiat Oncol. 2014;9(1):255. doi: 10.1186/s13014-014-0255-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schwartz DL, Hutcheson K, Barringer D, Tucker SL, Kies M, Holsinger FC, Ang KK, Morrison WH, Rosenthal DI, Garden AS, Dong L, Lewin JS. Candidate dosimetric predictors of long-term swallowing dysfunction after oropharyngeal intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2010;78:1356–65. doi: 10.1016/j.ijrobp.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Eisbruch A, Schwartz M, Rasch C, Vineberg K, Damen E, Van As CJ, Marsh R, Pameijer FA, Balm AJ. Dysphagia and aspiration after chemoradiotherapy for head-and-neck cancer: which anatomic structures are affected and can they be spared by IMRT? Int J Radiat Oncol Biol Phys. 2004;60:1425–39. doi: 10.1016/j.ijrobp.2004.05.050. [DOI] [PubMed] [Google Scholar]
- 33.Blanchard P, Gunn GB, Lin A, Foote RL, Lee NY, Frank SJ. Proton therapy for head and neck cancers. Semin Radiat Oncol. 2018;28:53–63. doi: 10.1016/j.semradonc.2017.08.004. [DOI] [PubMed] [Google Scholar]
- 34.Mohan R, Peeler CR, Guan F, Bronk L, Cao W, Grosshans DR. Radiobiological issues in proton therapy. Acta Oncol. 2017;56:1367–73. doi: 10.1080/0284186X.2017.1348621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mohan R, Das IJ, Ling CC. Empowering intensity modulated proton therapy through physics and technology: an overview. Int J Radiat Oncol Biol Phys. 2017;99:304–16. doi: 10.1016/j.ijrobp.2017.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sher DJ, Tishler RB, Pham NL, Punglia RS. Cost-effectiveness analysis of intensity modulated radiation therapy versus proton therapy for oropharyngeal squamous cell carcinoma. Int J Radiat Oncol Biol Phys. 2018;101:875–82. doi: 10.1016/j.ijrobp.2018.04.018. [DOI] [PubMed] [Google Scholar]
- 37.Goitein M, Cox JD. Should randomized clinical trials be required for proton radiotherapy? J Clin Oncol. 2008;26:175–6. doi: 10.1200/JCO.2007.14.4329. [DOI] [PubMed] [Google Scholar]
- 38.Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17:1471–4. doi: 10.1245/s10434-010-0985-4. [DOI] [PubMed] [Google Scholar]
- 39.Lydiatt WM, Patel SG, O'Sullivan B, Brandwein MS, Ridge JA, Migliacci JC, Loomis AM, Shah JP. Head and neck cancers—major changes in the American Joint Committee on Cancer eighth edition cancer staging manual. CA Cancer J Clin. 2017;67:122–37. doi: 10.3322/caac.21389. [DOI] [PubMed] [Google Scholar]
- 40.Chen AM, Li BQ, Lau DH, Farwell DG, Luu Q, Stuart K, Newman K, Purdy JA, Vijayakumar S. Evaluating the role of prophylactic gastrostomy tube placement prior to definitive chemoradiotherapy for head and neck cancer. Int J Radiat Oncol Biol Phys. 2010;78:1026–32. doi: 10.1016/j.ijrobp.2009.09.036. [DOI] [PubMed] [Google Scholar]
- 41.Garden AS, Dong L, Morrison WH, Stugis EM, Glisson BS, Frank SJ, Beadle BM, Gunn GB, Schwartz DL, Kies MS, Weber RS, Ang KK, Rosenthal DI. Patterns of disease recurrence following treatment of oropharyngeal cancer with intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2013;85:941–7. doi: 10.1016/j.ijrobp.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 42.Lukens JN, Lin A, Gamerman V, Mitra N, Grover S, McMenamin EM, Weinstein GS, O'Malley BW, Jr, Cohen RB, Orisamolu A, Ahn PH, Quon H. Late consequential surgical bed soft tissue necrosis in advanced oropharyngeal squamous cell carcinomas treated with transoral robotic surgery and postoperative radiation therapy. Int J Radiat Oncol Biol Phys. 2014;89:981–8. doi: 10.1016/j.ijrobp.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 43.Ahn PH, Lukens JN, Teo BK, Kirk M, Lin A. The use of proton therapy in the treatment of head and neck cancers. Cancer J. 2014;20:421–6. doi: 10.1097/PPO.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 44.Fakhry C, Krapcho M, Eisele DW, D'Souza G. Head and neck squamous cell cancers in the United States are rare and the risk now is higher among white individuals compared with black individuals. Cancer. 2018;124(10):2125–33. doi: 10.1002/cncr.31322. [DOI] [PMC free article] [PubMed] [Google Scholar]