Abstract
To investigate the expression and clinical significance of mitogen-activated protein kinase (MAPK) and epidermal growth factor receptor (EGFR) in triple-negative breast cancer (TNBC), a total of 300 TNBC and 120 paired paracancerous tissues were examined. Immunohistochemistry was conducted to determine the expression levels of MAPK and EGFR, and the correlation between MAPK and EGFR expression was evaluated using Cramer's V test. The association between MAPK and EGFR expression, and various clinicopathological variables (such as lymph node metastasis, clinical stage, recurrence and metastasis) was also evaluated, using the χ2 test. MAPK and EGFR expression levels in TNBC tissues were significantly higher than in the paired paracancerous tissues. Moreover, MAPK expression was associated with that of EGFR in TNBC tissues. The positive expression rates of MAPK and EGFR in patients with lymph node metastasis, advanced clinical stage, tumor recurrence and metastasis were higher than those without. Patients with positive expression of MAPK and EGFR in TNBC tissues had poorer prognoses and lower overall survival times than those without expression. In summary, the expression of MAPK and EGFR is closely associated with tumor invasion and the metastasis of TNBC, and may therefore be used as an indicator of poor prognosis in patients with TNBC.
Keywords: TNBC, MAPK, EGFR, clinical significance
Introduction
Triple-negative breast cancer (TNBC) refers to a subtype in which the estrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor 2 (HER-2) are not expressed. TNBC exhibits high heterogeneity and invasiveness, and poor survival outcome, and is currently a key topic in the field of breast cancer research. Endocrine and targeted therapy for TNBC have not been successfully developed (1), and consequently, the most common treatment for TNBC is chemotherapy (2). However, long-term chemotherapy can lead to poor patient tolerance and side effects (3). Thus, the development of new targets for the treatment of TNBC is expected to further improve patient prognosis.
Kinase inhibitors have proven to be successful in improving the prognosis of TNBC (4). The expression of mitogen-activated protein kinases (MAPKs) is an independent risk factor for disease-free and overall survival in patients with TNBC (5). MAPKs play an important role in the development and progression of breast cancer (6), and consist of serine-threonine protein kinases that can be activated by various extracellular stimuli. They are also key components of numerous molecular signaling pathways, and play a central role in proliferation-related signaling pathways (7). MAPKs primarily regulate the functions of other proteins through phosphorylation, and play an important role in the occurrence, development and metastasis of multiple tumors (8). A total of three MAPK signaling pathways have been identified, among which the extracellular regulated kinase (ERK) and stress-activated protein kinase/c-Jun NH (2)-terminal kinase pathways are critically involved in the progression of TNBC (9).
Epidermal growth factor receptor (EGFR) is involved in various complex cellular signal transduction pathways (10). During tumor progression, EGFR can form homodimers or heterodimers by binding to ligands such as EGF, and subsequently activating multiple signal transduction pathways; these include the MAPK/ERK and phosphoinositide 3-kinase (PI3K)/Akt pathways, which promote tumor angiogenesis, proliferation, invasion and metastasis (11). Furthermore, EGFR plays a central role in cell proliferation and differentiation (12) and is closely associated with the growth of TNBC (11).
In the present study, the expression levels of MAPK and EGFR in TNBC tissues were investigated, and the relationship between their respective expression levels and certain clinicopathological features of patients with TNBC was further investigated.
Materials and methods
Patient samples
A total of 300 female patients with pathologically confirmed TNBC from the 3rd Affiliated Teaching Hospital of Xinjiang Medical University (Urumqi, China), were enrolled in the present study between January 2011 and December 2013. Their median age was 46.5 years old (range, 21–71 years old). The clinical data of the patients are exhibited in Table I. The inclusion criteria were as follows: i) The subjects exhibited no ER, PR or HER-2 expression; ii) subjects that were diagnosed with TNBC for the first time and received surgery for TNBC; and iii) the clinicopathological data were complete. The exclusion criteria were as follow: The subjects had carcinoma in situ or non-TNBC. Breast cancer tissues were collected during surgery. Paired breast para-cancerous tissues (n=120), obtained from the 300 enrolled patients with TNBC were selected as controls. Written informed consent was obtained from each patient and the present study was approved by the ethics review board of Xinjiang Medical University.
Table I.
Clinical data of patients with TNBC.
| MAPK | EGFR | |||||||
|---|---|---|---|---|---|---|---|---|
| Group age, years | + | − | χ2 value | P-value | + | − | χ2 value | P-value |
| <40 | 41 (46.1) | 48 (53.9) | 2.405 | 0.12 | 46 (51.5) | 43 (48.3) | 2.060 | 0.150 |
| ≥40 | 77 (36.5) | 134 (63.5) | 90 (42.7) | 121 (57.3) | ||||
| Ethnicity | ||||||||
| Han | 65 (40.1) | 97 (59.9) | 0.439 | 0.803 | 72 (44.4) | 90 (55.6) | 0.130 | 0.94 |
| Uighur | 31 (36.5) | 54 (63.5) | 39 (45.9) | 46 (54.1) | ||||
| Other | 22 (41.5) | 31 (58.5) | 25 (47.2) | 28 (52.8) | ||||
MAPK, mitogen-activated protein kinase; EGFR, epidermal growth factor receptor; TNBC, triple negative breast cancer. The percentage of the patient population is indicated in brackets.
Immunohistochemistry
The expression levels of MAPK and EGFR were determined using immunohistochemical staining. The tissues were fixed with 10% neutral formalin for 24 h at room temperature, embedded in paraffin and cut into 4-µm sections. The tissue sections were then dewaxed using xylene, and rehydrated in using a graded alcohol series. Subsequently, the sections were incubated with 3% hydrogen peroxide for 10 min at room temperature to inhibit endogenous peroxidase activity. After blocking with 10% BSA at 37°C for 40 min, the sections were incubated with primary antibodies against MAPK (1:200; cat. no. M-9692) and EGFR (1:100; cat. no. ZM-0093; both Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd.) at 37°C for 90 min. After washing with PBS, secondary antibody anti-mouse IgG (cat. no. ZDR-5006, Beijing Zhongshan Golden Bridge Biotechnology Co. Ltd) was added and incubated for 20 min at room temperature. Finally, the sections were treated with DAB chromogenic reagent and counterstained with hematoxylin. The tumor tissues with positive expression of MAPK and EGFR were used as positive controls. PBS was used instead of the primary antibody as a negative control.
Evaluation of staining results
The staining results were evaluated by two individuals in a double-blinded manner. Concerning MAPK expression; cells exhibiting yellow or brown staining in the cytoplasm and the nucleus were considered to be positively stained. A total of five fields were randomly selected under high magnification (magnification, ×200) using Olympus C-7070WZ light microscope (Olympus, Tokyo, Japan), and 100 cells per field were counted. The positive rate was the ratio of positively-stained cells to the total number of cells counted. The percentage of cells with positive staining corresponded with the following scores: 1, <25%; 2, 25–50%; 3, 50–75%; and 4, >75%. The staining intensity was evaluated as follows: 0, no staining; 1, light yellow; 2, brownish-yellow; and 3, tan. The degree of staining was calculated by multiplying the percentage of positive staining by the staining intensity. A total score of ≤3 points was defined as negative staining, and a total score >3 points was defined as positive staining.
EGFR results were based on the staining continuity of the cell membranes; the immunohistochemistry staining results were scored as follows: 0, no staining; 1, cell membrane staining was discontinuous and exhibited brown/tan staining >10%; 2, membrane staining was continuous with incomplete shape and exhibited brown/tan staining >10%; and 3, the membrane staining was continuous and with brown/tan staining >10%. The staining intensity was scored as follows: 1, light brown; 2, medium brown; and 3, dark brown. The degree of staining was calculated by adding the scores of the continuity of the cell membranes and the intensity of staining. A total score of <3 points was defined as negative staining, and a total score ≥3 points was defined as positive staining.
Follow-up
Follow-up was performed using hospital review and the telephone. The beginning of the follow-up period was defined as the date of surgery and follow-up occurred 5 years post-operation. December 1, 2018, or the date of patient death, was considered to be the deadline for follow-up. Overall survival time was defined as the time between first diagnosis and mortality of the patient. Local recurrence referred to recurrence of the tumor in the ipsilateral breast, chest wall or regional lymph nodes. Distant metastases were confirmed by clinical examination, imaging, and pathological diagnosis of tissue biopsy. The follow-up rate was 94.7%. Tumor-free survival was defined as the time from the start of surgery to the time of recurrence or metastasis.
Statistical analysis
The data were analyzed using SPSS 19.0 (IBM Corp.). The χ2 test was used to analyze differences in the data between the two groups, and the correlation between MAPK and EGFR expression in TNBC tissues were analyzed using Cramer's V test. The relationship between MAPK and EGFR expression with lymph node metastasis, clinical stage, recurrence and metastasis was analyzed using the χ2 test. Kaplan-Meier analysis was used for survival analysis and the log-rank test was used to examine differences in survival between groups. P<0.05 was considered to indicate a statistically significant difference.
Results
Expression levels of MAPK and EGFR in TNBC tissues
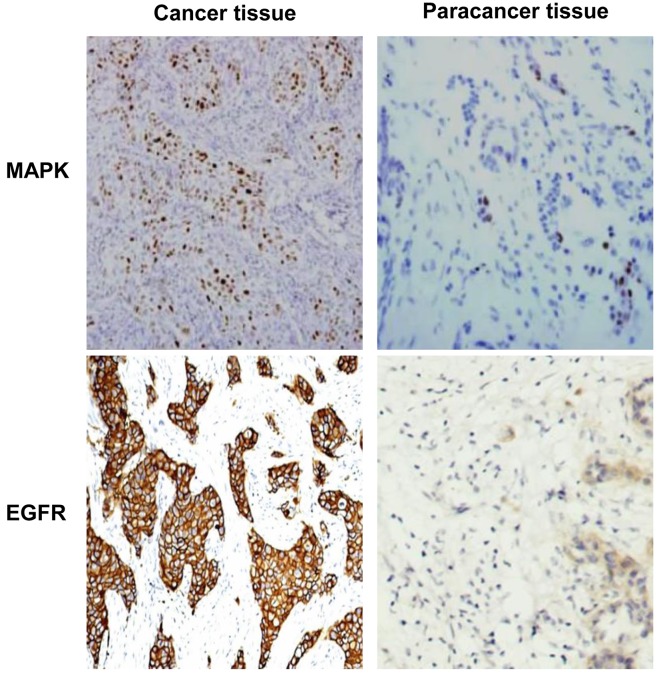
Expression levels of MAPK and EGFR in TNBC tissues were detected using immunohistochemistry. The representative staining results are displayed in Fig. 1. As shown in Table II, the number of cases exhibiting positive expression of MAPK and EGFR in TNBC tissues was 118/300 (39.3%) and 136/300 (45.3%), respectively, compared with paratumor tissues. In 120 paired paracancerous tissues, the number of cases with positive expression of MAPK and EGFR were 20 (16.7%) and 26 (21.7%), respectively. The expression levels of both MAPK and EGFR in the cancerous tissues were significantly higher, compared with paired paracancerous tissues (P<0.05). Clinical data analysis determined that there were no statistical differences in the expression levels between different ages or ethnic distributions (Table I). The results of the present study indicate that MAPK and EGFR expression levels are increased in TNBC tissues, potentially implicating them in the occurrence and progression of TNBC.
Figure 1.
Expression of MAPK and EGFR detected by immunohistochemistry. The expression levels of MAPK and EGFR in cancerous tend paired paracancerous tissues were detected using immunohistochemistry. Magnification, ×200. MAPK, mitogen-activated protein kinase; EGFR epidermal growth factor receptor.
Table II.
Expression of MAPK and EGFR in TNBC and paracancerous tissues.
| MAPK | EGFR | |||
|---|---|---|---|---|
| Groups | + | − | + | − |
| Cancer tissues (%) | 118 (39.3) | 182 (60.7) | 136 (45.3) | 164 (54.7) |
| Paracancer tissues (%) | 20 (16.7) | 100 (83.3) | 26 (21.7) | 94 (78.3) |
| χ2 value | 19.962 | 20.262 | ||
| P-value | <0.01 | <0.01 | ||
MAPK, mitogen-activated protein kinase; EGFR, epidermal growth factor receptor; TNBC, triple negative breast cancer.
Correlation between MAPK and EGFR expression levels in TNBC
To determine the degree of correlation between the expression levels of MAPK and EGFR in TNBC tissues, Cramer's V test was performed. As shown in Table III, there was a high degree of correlation between MAPK and EGFR expression in TNBC tissues (C=0.500, P<0.01). This result indicates that MAPK and EGFR are co-expressed in TNBC.
Table III.
Association between MAPK and EGFR expression in TNBC tissues.
| MAPK | |||||
|---|---|---|---|---|---|
| Positive | Negative | Total | C-value | P-value | |
| EGFR | |||||
| Positive | 90 | 46 | 136 | ||
| Negative 2 | 8 | 136 | 164 | 0.500 | <0.01 |
| Total | 118 | 182 | 300 | ||
MAPK, mitogen-activated protein kinase; EGFR, epidermal growth factor receptor; TNBC, triple negative breast cancer.
Association between MAPK and EGFR expression, and the clinicopathological features of patients with TNBC
The association between MAPK and EGFR expression levels with TNBC clinicopathological features, such as lymph node metastasis, clinical stage, recurrence and metastasis, was evaluated using the χ2 test. In patients with lymph node metastasis, the positive expression levels of MAPK and EGFR were 48.7 and 55.6%, respectively (P<0.05; Table IV), significantly higher than those of patients without lymph node metastasis. Similarly, an advanced clinical stage was associated with higher positive expression levels of both MAPK and EGFR (Table IV). At the end of follow-up, 85/300 patients (28.3%) experienced recurrence and metastasis. Of these, 46/85 (54.1%) exhibited positive MAPK expression and 52/85 (61.2%) exhibited positive EGFR expression. In the 215 cases without recurrence or metastasis, the positive expression level of MAPK was 33.5% (72/215), and that of EGFR was 39.1% (84/215); these differences were statistically significant. The results demonstrate that there is a significant association between MAPK and EGFR expression and lymph node metastasis, clinical stage, recurrence and metastasis in patients with TNBC.
Table IV.
Association between MAPK and EGFR expression, lymph node metastasis, and recurrence and metastasis in patients with TNBC.
| MAPK | EGFR | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Patients, n | Relative positive | Relative negative | χ2 value | P-value | Relative positive | Relative negative | χ2 value | P-value | ||
| Lymph node metastasis | Yes | 117 | 57 (48.7) | 60 (51.3) | 7.08 | 0.008 | 65 (55.6) | 52 (44.4) | 7.05 | 0.008 |
| No | 183 | 61 (33.3) | 122 (66.7) | 73 (39.9) | 110 (60.1) | |||||
| Clinical stage | I | 82 | 24 (29.3) | 58 (70.7) | 10.9 | 0.004 | 22 (26.8) | 60 (73.2) | 16.9 | 0.001 |
| II | 140 | 51 (36.4) | 89 (63.6) | 60 (42.9) | 80 (57.1) | |||||
| III | 78 | 42 (53.8) | 36 (46.2) | 46 (59.0) | 32 (41.0) | |||||
| Recurrence and metastasis | Yes | 85 | 46 (54.1) | 39 (45.9) | 10.86 | 0.001 | 52 (61.2) | 33 (38.8) | 12.01 | 0.001 |
| No | 215 | 72 (33.5) | 143 (66.5) | 84 (39.1) | 131 (60.9) | |||||
MAPK, mitogen-activated protein kinase; EGFR, epidermal growth factor receptor; TNBC, triple negative breast cancer. The percentage of the patient population is indicated in brackets.
Association between MAPK and EGFR expression levels, and patient survival time
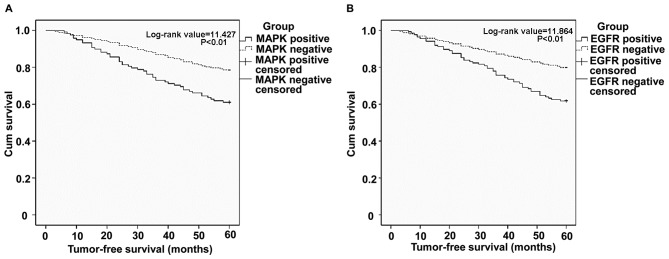
To understand the effect of MAPK and EGFR expression on the prognosis of TNBC patients, 5-year tumor-free survival analysis was performed on the enrolled patients. The survival curves of 85 patients with recurrence and metastasis were constructed using Kaplan-Meier analysis and the log-rank test. Compared with patients without MAPK expression, MAPK-positive patients exhibited a log-rank value of 11.427, where P<0.01 (Fig. 2A). Compared with EGFR-negative patients, those with positive EGFR expression indicated a log-rank value of 11.864 and P<0.01 (Fig. 2B). This indicates that the prognosis of patients with positive MAPK and EGFR expression was poor, compared with expression-negative individuals. The results of the present study indicate that the patients with positive MAPK and EGFR expression demonstrated shorter survival times than those negative for MAPK and EGFR expression.
Figure 2.
Relationship between MAPK and EGFR expression and survival rate in TNBC. Kaplan-Meier analysis was used for survival analysis and the log-rank test was used to analyze the differences in survival rate. Survival of patients with and without (A) MAPK and/or (B) EGFR expression. MAPK, mitogen-activated protein kinase; EGFR epidermal growth factor receptor; TNBC, triple negative breast cancer.
Discussion
TNBC is a subtype of breast cancer (13) that exhibits different clinicopathological features and prognosis compared with non-TNBC (9). In patients with TNBC, high expression levels of EGFR induce the activation of the MAPK signal-transduction pathway, stimulating the proliferation of malignant cells and causing the hormone-independent proliferation of TNBC cells (14). Therefore, the effect of EGFR on MAPK expression, and the effect of both proteins on the prognosis of patients with TNBC, requires further elucidation.
The MAPK signaling pathway is an important signal transduction pathway involved in the development of TNBC (15,16). MAPKs play a central role in the expression of ER, PR and HER-2, and are closely related to the invasion, metastasis and prognosis of TNBC (17). Higher MAPK activity is associated with shorter survival time in patients with TNBC, and may therefore be an indicator of a poor prognosis (15). Upregulation or continuous activation of EGFR causes the activation of MAPKs (18). During the progression of TNBC, EGFR forms homodimers or heterodimers by binding to its ligands (including EGF), and activating multiple signaling pathways (such as PI3K/Akt or MAPK/ERK) that promote tumor-cell proliferation, invasion and migration (19). In the present study, the positive expression rates of MAPK and EGFR in TNBC tissues were 39.3 and 45.3%, respectively, similar to previous studies [45%; (20)]. Correlation analysis determined that MAPK was significantly associated with EGFR expression, suggesting that MAPK and EGFR may act synergistically to promote TNBC progression.
Lymph node metastasis and TNM staging are important for evaluating clinical prognosis in patients with TNBC (21), and can provide a basis for the selection of treatment options (22). The present study determined that in patients with TNBC, both MAPK and EGFR expression were significantly associated with both lymph node metastasis and clinical stage. It has also been discovered that patients with high levels of MAPK and EGFR expression are more prone to early recurrence, metastasis and poor prognosis (23–25). However, two studies elucidated that there was no significant correlation between MAPK expression and lymph node metastasis (6,20), which was not consistent with the results of the present study. This may be attributable to study size. Further analysis in the present study determined that the expression levels of MAPK and EGFR were associated with recurrence and metastasis. Positive expression of MAPK and EGFR in TNBC patients with recurrence and metastasis was significantly higher than in those without. Furthermore, patients with high expression levels of MAPK and EGFR had a poorer prognosis and lower survival rates than those with low expression, consistent with previous studies (26,27). Moreover, the proliferation of TNBC cells may be inhibited by targeting the MAPK pathway, thus achieving therapeutic effects (17,28,29). Taken together, the results of the present study indicate that MAPK and EGFR play a primary role in TNBC development, and may be considered as independent prognostic indicators and new targets for the treatment of TNBC. Thus, the different statuses of TNBC patients should be considered, and individualized treatments should be employed to further improve prognosis.
The present study had certain limitations; firstly, it was a retrospective study analyzing a small sample population. Secondly, immunohistochemistry experiments were performed using antibodies with broad MAPK recognition, instead of antibodies specific to certain members of the MAPK family.
In conclusion, MAPK and EGFR expression levels were increased in TNBC patients (compared with paratumor tissues), and were associated with lymph node metastasis, advanced clinical stage, recurrence and metastasis, and poor survival rate. In the future, MAPK and EGFR may be used as independent prognostic indicators and novel targets for the treatment of TNBC, thus individualized treatment plans should be devised according to the different statuses of patients with TNBC, with the aim of further improving prognosis.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- MAPK
mitogen-activated protein kinase
- EGFR
epidermal growth factor receptor
- TNBC
triple-negative breast cancer
- ER
estrogen receptor
- PR
progesterone receptor
- HER-2
human epidermal growth factor receptor 2
Funding
The present study was supported by the Health Planning Commission Youth Science and Technology Talents Special Project of the Xingjian Uyghur Autonomous Region grant (grant no. 2016Y05) and the Natural Science Foundation of Xinjiang Uygur Autonomous Region (grant no. 2016D01C353).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
Experimental conception and design was conducted by WJ and LY. Data acquisition and analysis was performed by WJ, XW, CZ, LX and LY. WJ and XW drafted the manuscript, and all authors read and approved the final manuscript.
Ethics approval and consent to participate
Informed consent was obtained from all patients and the study was approved by the ethics review board of Xinjiang Medical University.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Corbex M, Bouzbid S, Traverse-Glehen A, Aouras H, McKay-Chopin S, Carreira C, Lankar A, Tommasino M, Gheit T. Prevalence of papillomaviruses, polyomaviruses, and herpesviruses in triple-negative and inflammatory breast tumors from algeria compared with other types of breast cancer tumors. PLoS One. 2014;9:e114559. doi: 10.1371/journal.pone.0114559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Collignon J, Lousberg L, Schroeder H, Jerusalem G. Triple-negative breast cancer: Treatment challenges and solutions. Breast Cancer (Dove Med Press) 2016;8:93–107. doi: 10.2147/BCTT.S69488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Maia AR, de Man J, Boon U, Janssen A, Song JY, Omerzu M, Sterrenburg JG, Prinsen MB, Willemsen-Seegers N, de Roos JA, et al. Inhibition of the spindle assembly checkpoint kinase TTK enhances the efficacy of docetaxel in a triple-negative breast cancer model. Ann Oncol. 2015;26:2180–2192. doi: 10.1093/annonc/mdv293. [DOI] [PubMed] [Google Scholar]
- 4.Sikov WM, Berry DA, Perou CM, Singh B, Cirrincione CT, Tolaney SM, Kuzma CS, Pluard TJ, Somlo G, Port ER, et al. Impact of the addition of carboplatin and/or bevacizumab to neoadjuvant once-per-week paclitaxel followed by dose-dense doxorubicin and cyclophosphamide on pathologic complete response rates in stage II to III triple-negative breast cancer: CALGB 40603 (Alliance) J Clin Oncol. 2015;33:13–21. doi: 10.1200/JCO.2014.57.0572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ali AM, Ansari JA, El-Aziz NMA, Abozeed WN, Warith AMA, Alsaleh K, Nabholtz JM. Triple negative breast cancer: A tale of two decades. Anticancer Agents Med Chem. 2017;17:491–499. doi: 10.2174/1871520616666160725112335. [DOI] [PubMed] [Google Scholar]
- 6.Qi MS, Elion EA. MAP kinase pathways. J Cell Sci. 2015;18:3569–3572. doi: 10.1242/jcs.02470. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Y, Wei L, Yu J, Li G, Zhang X, Wang A, He Y, Li H, Yin D. Targeting of the β6 gene to suppress degradation of ECM via inactivation of the MAPK pathway in breast adenocarcinoma cells. Oncol Rep. 2014;32:1787–1795. doi: 10.3892/or.2014.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cotrim CZ, Fabris V, Doria ML, Lindberg K, Gustafsson JÅ, Amado F, Lanari C, Helguero LA. Estrogen receptor beta growth-inhibitory effects are repressed through activation of MAPK and PI3K signalling in mammary epithelial and breast cancer cells. Oncogene. 2013;32:2390–2402. doi: 10.1038/onc.2012.261. [DOI] [PubMed] [Google Scholar]
- 9.Borah N, Gunawardana S, Torres H, McDonnell S, Van Slambrouck S. 5,6,7,3′,4′,5′-Hexamethoxyflavone inhibits growth of triple-negative breast cancer cells via suppression of MAPK and Akt signaling pathways and arresting cell cycle. Int J Oncol. 2017;51:1685–1693. doi: 10.3892/ijo.2017.4157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nalwoga H, Arnes JB, Wabinga H, Akslen LA. Expression of EGFR and c-kit is associated with the basal-like phenotype in breast carcinomas of African women. APMIS. 2008;116:515–525. doi: 10.1111/j.1600-0463.2008.01024.x. [DOI] [PubMed] [Google Scholar]
- 11.Normanno N, Campiglio M, Maiello MR, De Luca A, Mancino M, Gallo M, D'Alessio A, Menard S. Breast cancer cells with acquired resistance to the EGFR tyrosine kinase inhibitor gefitinib show persistent activation of MAPK signaling. Breast Cancer Res Treat. 2008;112:25–33. doi: 10.1007/s10549-007-9830-2. [DOI] [PubMed] [Google Scholar]
- 12.Abdelrahman AE, Rashed HE, Abdelgawad M, Abdelhamid MI. Prognostic impact of EGFR and cytokeratin 5/6 immunohistochemical expression in triple-negative breast cancer. Ann Diagn Pathol. 2017;28:43–53. doi: 10.1016/j.anndiagpath.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Shi Y, Yang F, Huang D, Guan X. Androgen blockade based clinical trials landscape in triple negative breast cancer. Biochim Biophys Acta Rev Cancer. 2018;1870:283–290. doi: 10.1016/j.bbcan.2018.05.004. [DOI] [PubMed] [Google Scholar]
- 14.Kim S, You D, Jeong Y, Yu J, Kim SW, Nam SJ, Lee JE. Berberine down-regulates IL-8 expression through inhibition of the EGFR/MEK/ERK pathway in triple-negative breast cancer cells. Phytomedicine. 2018;50:43–49. doi: 10.1016/j.phymed.2018.08.004. [DOI] [PubMed] [Google Scholar]
- 15.Gholami S, Chen CH, Gao S, Lou E, Fujisawa S, Carson J, Nnoli JE, Chou TC, Bromberg J, Fong Y. Role of MAPK in oncolytic herpes viral therapy in triple-negative breast cancer. Cancer Gene Ther. 2014;21:283–289. doi: 10.1038/cgt.2014.28. [DOI] [PubMed] [Google Scholar]
- 16.Giltnane JM, Balko JM. Rationale for targeting the Ras/MAPK pathway in triple-negative breast cancer. Discov Med. 2014;17:275–283. [PubMed] [Google Scholar]
- 17.Zhao M, Howard EW, Parris AB, Guo Z, Zhao Q, Yang X. Alcohol promotes migration and invasion of triple-negative breast cancer cells through activation of p38 MAPK and JNK. Mol Carcinog. 2017;56:849–862. doi: 10.1002/mc.22538. [DOI] [PubMed] [Google Scholar]
- 18.Oh AS, Lorant LA, Holloway JN, Miller DL, Kern FG, El-Ashry D. Hyperactivation of MAPK induces loss of ERalpha expression in breast cancer cells. Mol Endocrinol. 2001;15:1344–1359. doi: 10.1210/me.15.8.1344. [DOI] [PubMed] [Google Scholar]
- 19.Majorini MT, Manenti G, Mano M, De Cecco L, Conti A, Pinciroli P, Fontanella E, Tagliabue E, Chiodoni C, Colombo MP, et al. cIAP1 regulates the EGFR/Snai2 axis in triple-negative breast cancer cells. Cell Death Differ. 2018;25:2147–2164. doi: 10.1038/s41418-018-0100-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jing W. Expression and significance of MAPK protein in breast cancer. Journal of Ningxia Medical College. 2014;36:743–745. (In Chinese) [Google Scholar]
- 21.Zenzola V, Cabezas-Quintario MA, Arguelles M, Pérez-Fernández E, Izarzugaza Y, Correa A, García-Foncillas J. Prognostic value of Ki-67 according to age in patients with triple-negative breast cancer. Clin Transl Oncol. 2018;20:1448–1454. doi: 10.1007/s12094-018-1877-5. [DOI] [PubMed] [Google Scholar]
- 22.Walsh EM, Shalaby A, O'Loughlin M, Keane N, Webber MJ, Kerin MJ, Keane MM, Glynn SA, Callagy GM. Outcome for triple negative breast cancer in a retrospective cohort with an emphasis on response to platinum-based neoadjuvant therapy. Breast Cancer Res Treat. 2019;174:1–13. doi: 10.1007/s10549-018-5066-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Evans MK, Brown MC, Geradts J, Bao X, Robinson TJ, Jolly MK, Vermeulen PB, Palmer GM, Gromeier M, Levine H, et al. XIAP regulation by MNK links MAPK and NFκB signaling to determine an aggressive breast cancer phenotype. Cancer Res. 2018;78:1726–1738. doi: 10.1158/0008-5472.CAN-17-1667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rakha EA, El-Sayed ME, Green AR, Lee AH, Robertson JF, Ellis IO. Prognostic markers in triple-negative breast cancer. Cancer. 2007;109:25–32. doi: 10.1002/cncr.22381. [DOI] [PubMed] [Google Scholar]
- 25.Shah SP, Roth A, Goya R, Oloumi A, Ha G, Zhao Y, Turashvili G, Ding J, Tse K, Haffari G, et al. The clonal and mutational evolution spectrum of primary triple-negative breast cancers. Nature. 2012;486:395–399. doi: 10.1038/nature10933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Liu W, Zhang L, Shi J, Liu Y, Zhou L, Hou K, Qu X, Teng Y. Clinical significance of pAkt and pErk1/2 expression in early-stage breast cancer patients treated with anthracycline-based adjuvant chemotherapy. Oncol Lett. 2015;9:1707–1714. doi: 10.3892/ol.2015.2965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono H, Sowa Y, Horinaka M, Iizumi Y, Watanabe M, Morita M, Nishimoto E, Taguchi T, Sakai T. The histone deacetylase inhibitor OBP-801 and eribulin synergistically inhibit the growth of triple-negative breast cancer cells with the suppression of survivin, Bcl-xL, and the MAPK pathway. Breast Cancer Res Treat. 2018;171:43–52. doi: 10.1007/s10549-018-4815-x. [DOI] [PubMed] [Google Scholar]
- 28.Uehara N, Kanematsu S, Miki H, Yoshizawa K, Tsubura A. Requirement of p38 MAPK for a cell-death pathway triggered by vorinostat in MDA-MB-231 human breast cancer cells. Cancer Lett. 2012;315:112–121. doi: 10.1016/j.canlet.2011.07.032. [DOI] [PubMed] [Google Scholar]
- 29.Peng B, He R, Xu Q, Yang Y, Hu Q, Hou H, Liu X, Li J. Ginsenoside 20(S)-protopanaxadiol inhibits triple-negative breast cancer metastasis in vivo by targeting EGFR-mediated MAPK pathway. Pharmacol Res. 2019;142:1–13. doi: 10.1016/j.phrs.2019.02.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.