Abstract
Direct acting antivirals (DAA) have recently been developed to treat patients with hepatitis C virus (HCV) infection, and interferon-free DAA treatment has improved the cure rate of patients. However, the occurrence rate of hepatocellular carcinoma (HCC) following HCV eradication remains unknown. Therefore, the present study aimed to identify predictors of HCC occurrence following DAA treatment. Among 1,454 patients infected with HCV, 1,088 patients who achieved sustained virologic response and who had no history of HCC treatment were recruited between September 2014 and November 2018. The incidence of HCC in patients infected with HCV following DAA treatment, and the predictors contributing to HCC occurrence were identified using clinicopathological characteristics and blood test results. During the present study, 26 patients developed HCC. The incidence of HCC was 0.61, 1.88, 2.82 and 3.71% at 6, 12, 18 and 24 months after treatment with DAA, respectively. The results of multivariate analysis identified age [hazard ratio (HR), 1.0729; P=0.0044] and α-fetoprotein (AFP) level after DAA treatment (HR, 1.0486; P=0.0486) as independent factors that may contribute to HCC occurrence following DAA treatment. By using these two factors, a novel scoring system (0–2 points) was established to predict HCC occurrence following HCV eradication by DAA treatment. The incidence of HCC at 2 years was 0.3% in the 0 points group, 6.27% in the 1 point group and 18.37% in the 2 points group. In conclusion, AFP level after DAA treatment and age at DAA administration were identified as independent predictors of HCC occurrence in patients that were treated with DAA. The scoring system that was established in the present study is simple and easy, and using pre-treatment factors may be a convenient tool to predict the risk of HCC occurrence in HCV-free patients following DAA treatment.
Keywords: direct-acting antivirals, hepatocellular carcinoma, hepatitis C virus, α-fetoprotein, age
Introduction
Hepatitis C virus (HCV) infection is a risk factor for the occurrence of liver cirrhosis (LC) and hepatocellular carcinoma (HCC) (1). The combination of interferon (IFN) and ribavirin has been used as a standard treatment of HCV infection; however, the cure rate remains unsatisfactory (~50%) (2–4). Furthermore, IFN cannot be administered to elderly patients or to patients with LC and low platelet count, due to the risk of numerous adverse events, including high fever, general fatigue, depression, interstitial pneumonia and decreased platelet count (5). As a result, the number of patients who can benefit from IFN treatment is small. Direct acting antivirals (DAA) have been developed against HCV infection, and IFN-free DAA treatment has dramatically improved the cure rate of patients to >90% (6–11). In addition, this treatment is safe to use, due to mild adverse events compared with IFN treatment, for patients with HCV (6–11).
It has been previously reported that IFN can suppress hepatic carcinogenesis (12,13). However, since numerous elderly patients with HCV and patients with LC have received IFN-free DAA treatment, there is some concern regarding the potential increase in hepatic carcinogenesis occurrence following treatment. It has been demonstrated that DAA-induced sustained virological response (SVR) does not reduce the short-term occurrence of HCC in patients following successful DAA treatment (14). Conversely, a retrospective cohort study of 22,500 patients with HCV treated with DAA demonstrated that HCC occurrence is significantly diminished in patients with SVR compared with those without (15).
However, a method to predict the risk of HCC in HCV-infected patients achieving SVR by IFN-free DAA treatment remains to be determined. SVR was categorized as undetectable HCV RNA (target not detected) at week 12 after the end of treatment (SVR12) (6–11). The establishment of a simple scoring system using general clinical data to predict the risk of HCC after SVR12 is crucial. The present study proposed one scoring system for the prediction of HCC occurrence in patients who achieved SVR following IFN-free DAA treatment.
Materials and methods
Patients
This project was a multicenter study, which included six institutions (Kagawa University Hospital, Kagawa Prefectural Central Hospital, Takamatsu Red Cross Hospital, Mitoyo General Hospital, Kagawa Rosai Hospital and Yashima General Hospital) in Kagawa, Japan (All Kagawa Liver Disease Group Study). A total of 1,454 patients with HCV infection who received IFN-free DAA treatment between September 2014 and November 2018 were included in the present study. The study population included 742 men and 712 women. The median age of patients at the start of DAA treatment was 68 years (range, 59–76 years). The present study was conducted in accordance with the guidelines of the Declaration of Helsinki and was approved by the Ethics Committee of Kagawa University, Faculty of Medicine (approval no. Heisei-27-174). The requirement for informed consent from the participants was waived because of the retrospective nature of the study.
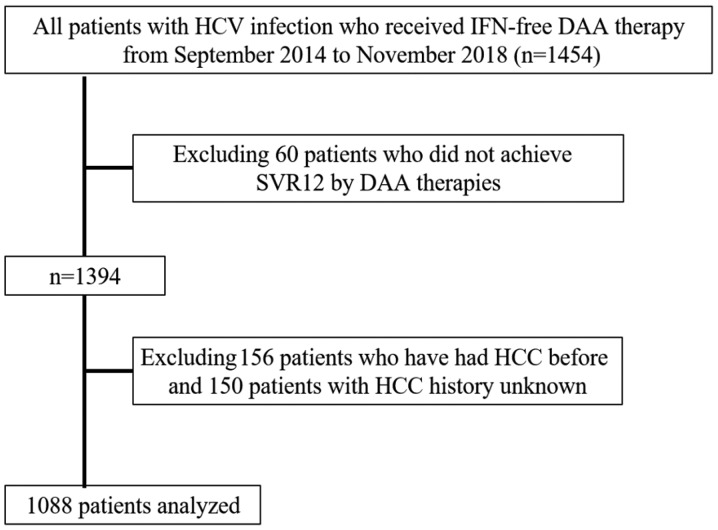
The exclusion criteria were as follows: i) Patients co-infected with hepatitis B virus or human immunodeficiency virus, or patients with other liver diseases, including primary biliary cholangitis and autoimmune hepatitis; ii) patients with decompensated cirrhosis, since IFN-free DAA treatment was not approved for these patients in Japan; and iii) patients with a previous history of HCC or patients who did not achieve SVR12 following DAA treatment. A total of 1,088 patients who achieved SVR12 following IFN-free DAA treatment were finally included in the present study (Fig. 1). Of the 1088 patients: 258 were treated with daclatasvir and asunaprevir for 24 weeks; 316 patients were treated with sofosbuvir (SOF) and ledipasvir for 12 weeks; 244 patients were treated with SOF and ribavirin for 12 weeks; 86 patients were treated with elbasvir and grazoprevir for 12 weeks; 91 patients were treated with ombitasvir, paritaprevir and ritonavir for 12 weeks; and the remaining 93 patients were treated with glecaprevir and pibrentasvir for 8–12 weeks. None of the patients enrolled in the present study received multiple courses of DAA treatment, due to recurrence or non-response to initial treatment. Each patient was examined by ultrasonography (US) or CT to exclude the presence of HCC at the onset of DAA administration. Serum samples for HCV RNA measurements were collected at screening; treatment weeks 1, 2, 4, 8 and 12 (or early discontinuation); and posttreatment weeks 4, 8, 12, and 24 (or early discontinuation). HCV-RNA was extracted from 200 µl of serum sample using the Qiagen mRNeasy serum-plasma kit (Qiagen) according to the manufacturer's instructions. By using a commercially available device, a TaqMan polymerase chain reaction method (COBAS AmpliPrep/COBAS TaqMan HCV test version 2; Roche Molecular Diagnostics; lower limit of quantification, 1.6 log10 IU/ml; lower limit of detection, 1.2 log10 IU/ml) was used to detect HCV RNA, according to the manufacturer's instructions. HCV Genotyping test was also detected with the commercial VERSANT HCV Genotype 2.0 assay (LiPA 2.0; Siemens Healthineers) and HCV sequencing for detection of six HCV genotypes 1a, 1b, 2, 3, 4, and 6 in the serum samples was conducted according to the manufacturer's instructions.
Figure 1.
Patient selection criteria. HCV, hepatitis C virus; IFN, interferon; DAA, direct-acting antiviral; HCC, hepatocellular carcinoma; SVR, sustained virological response.
HCC surveillance after obtaining SVR12
The blood was collected from patients before, during (at weeks 2, 4, 8 and 12), and after (at weeks 4, 8 and 12) treatment. The serum samples were prepared according to the manufacturer's instructions. The serum samples were not stored before analysis. US was performed at the time of SVR12 and twice a year after this. US examination was performed by experienced hepatologists or sonographers at each institution. The serum levels of α-fetoprotein (AFP) were measured every 1–6 months. If HCC was suspected following US examination or as a result of elevated AFP level, contrast enhanced US (CEUS), dynamic CT or gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid-enhanced magnetic resonance imaging (EOB-MRI) were performed for further evaluation of HCC. For the diagnosis of HCC, the 2005 Guidelines of the American Association for the Study of Liver Disease (16) were adopted. HCC was diagnosed based on the findings from dynamic CT, EOB-MRI and/or CEUS using perflubutane (Sonazoid™; Daiichi Sankyo Co., Ltd.) (17). Typical HCC pattern imaging criteria were defined as follows: i) Hypervascularity defined as focal lesion hyperattenuation relative to the liver during the arterial phase of each imaging method, and wash-out appearance was observed during the portal and parenchymal phase; and ii) tumors were revealed as defects in the post vascular phase of CEUS or the hepatobiliary phase of EOB-MRI. TNM stage was determined according to the HCC staging report in the Liver Cancer Study Group of Japan, 6th Edition (18). Diagnosis of LC was performed in accordance with clinical, experimental, abdominal US and/or histological findings (19). In addition, the fibrosis-4 (FIB-4) index was used to evaluate fibrosis clinically before and after DAA treatment. FIB-4 index was calculated using the following formula: FIB-4 index=[aspartate aminotransferase (AST; IU/l) × age (years) / platelet count (109/l) × alanine aminotransferase (ALT; IU/l)1/2]. A cut-off value of 3.25 was used for prediction of severe fibrosis (20,21).
Statistical analysis
Data are presented as the mean. Statistical analyses were performed using the Kaplan-Meier method, log-rank test, ROC analysis and Cox hazard analysis using JMP statistical software (version 11.0 for Windows; SAS Institute, Inc.). All P-values were derived from two-tailed tests, and P<0.05 was considered to indicate a statistically significant difference. Receiver operating characteristic (ROC) and area under the curve (AUC) values were calculated to define cut-off values for risk factors of HCC occurrence.
Results
Patient characteristics
A total of 1,454 patients started IFN-free DAA treatment during the study period and 1,088 patients were enrolled in the present study after applying the exclusion criteria. Baseline characteristics of the patients are presented in Table I. The study population included 545 men and 543 women. The median age of patients at the start of DAA treatment was 68 years (range, 58–75 years). The numbers of patients with chronic hepatitis (CH) and LC were 897 and 191, respectively. Only 683 cases were reported regarding the presence or absence of diabetes mellitus.
Table I.
Baseline characteristics of patients included in the present study.
| Characteristic | Value |
|---|---|
| Sex, n (male/female) | 545/543 |
| Age, years (range) | 68 (58–75) |
| CH/LC, n | 897/191 |
| HCV serotype, n (1/2 or 3) | 779/309 |
| AST, IU/l (range) | 37 (27–59) |
| ALT, IU/l (range) | 35 (23–60) |
| Total bilirubin, mg/dl (range) | 0.7 (0.6–0.9) |
| Albumin, g/dl (range) | 4.1 (3.8–4.3) |
| Hemoglobin, g/dl (range) | 13.5 (12.4–20.1) |
| White blood cell count,/µl (range) | 4,850 (3,820–5,950) |
| Platelet count, ×104/µl (range) | 15.8 (11.6–20.1) |
| AFP, ng/ml (range) | 4.4 (3–8) |
| Total cholesterol, mg/dl (range) | 168.5 (148.8–190) |
| FIB-4 (range) | 2.94 (1.85–4.63) |
| APRI (range) | 0.86 (0.50–1.55) |
| WFA-M2BP, COI (range) | 1.59 (0.99–2.42) |
| Diabetes mellitus, n, no/yes (n=683) | 572/111 |
| Body mass index, kg/m2 (range) | 22.7 (20.6–25.1) |
| HCV-RNA, log copies/ml (range) | 6.1 (5.5–6.5) |
| DAA therapy, n (ASV +DCV/SOF + LDV/SOF+RBV/ERB + GZR/OBV + PTV + r / GLE + PIB) | 258/316/244/86/91/93 |
AFP, α-fetoprotein; ALT, alanine aminotransferase; APRI, AST to platelet ratio index; AST, aspartate aminotransferase; ASV, asunaprevir; CH, chronic hepatitis; DAA, direct-acting antiviral; DCV, daclatasvir; ERB, Elbasvir; FIB-4, Fibrosis-4 index; GLE, Glecaprevir; GZR, Grazoprevir; HCV, hepatitis C virus; IU, international unit; LC, liver cirrhosis; LDV, ledipasvir; OBV, Ombitasvir; PIB, Pibrentasvir; PTV, paritaprevir; r, ritonavir; RBV, ribavirin; SOF, sofosbuvir; WFA-M2BP, floribunda agglutinin+-Mac-2 binding protein.
Cumulative incidence rate of HCC
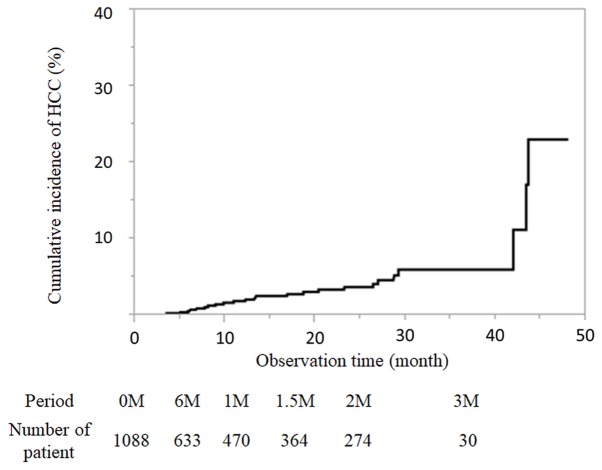
The median observation time was 13.8 months after the end of DAA treatment. There were many patients 3–6 months after the end of treatment, and 633 patients could be evaluated half a year later. During the present study, 26 patients developed HCC. The incidence of HCC was 0.61, 1.88, 2.82, 3.71 and 6% at 6, 12, 18, 24 and 36 months after DAA treatment by the Kaplan-Meier method, respectively (Fig. 2). The number of patients followed-up at each time point is presented in Fig. 2.
Figure 2.
Cumulative incidence of HCC after hepatitis C virus eradication using direct-acting antiviral treatment shown by a Kaplan-Meier curve and the number of patients at each observation time point. HCC, hepatocellular carcinoma; M, month.
Characteristics of patients who developed de novo HCC following DAA treatment
The characteristics of the 26 patients who developed de novo HCC after DAA treatment are presented in Table II. Among these patients, 15 were men and 11 were women (median age, 72 years; range, 66–79 years). Furthermore, the numbers of CH and LC cases were 16 and 10, respectively. Median values for serum levels of AFP and des-gamma-carboxy prothrombin were 6.5 ng/ml and 28 mAU/ml, respectively. Among the 26 patients, eight had non-alcoholic fatty liver disease, eight were alcohol consumers and seven had diabetes mellitus. Six of the patients with diabetes mellitus were treated for the condition. The median size of the maximum tumor diameter was 13 mm and the median number of tumors was one.
Table II.
Characteristics of patients who developed HCC newly after DAA treatment.
| Characteristic | Value |
|---|---|
| Sex, n (male/female) | 15/11 |
| Age, years (range) | 72 (66–79) |
| CH/LC, n | 16/10 |
| HCV serotype (1/2 or 3) | 24/2 |
| AFP, ng/ml (range) | 6.5 (5–22) |
| AFP-L3, % (range) | 0.85 (0.5–3.8) |
| DCP, mAU/ml (range) | 28 (17.5–66) |
| NAFLD, n, no/yes | 18/8 |
| Alcohol, n, none/drinking | 18/8 |
| Diabetes mellitus, n, no/yes | 19/7 |
| Drugs used in diabetes mellitus | |
| Dipeptidyl peptidase-4 inhibitor | 1 |
| Sulfonylurea | 2 |
| Metformin | 3 |
| Insulin preparation | 3 |
| No medication | 1 |
| Number of tumors | 1 (1–2) |
| Maximum tumor diameter, mm (range) | 13 (10.75–18) |
Data are presented as the mean. Values in parentheses represent interquartile ranges. AFP, α-fetoprotein; CH, chronic hepatitis; DCP, des-gamma-carboxy prothrombin; HCV, hepatitis C virus; LC, liver cirrhosis; NAFLD, non-alcoholic fatty liver disease.
Risk factors for HCC occurrence following DAA treatment
Potential prognostic factors for HCC occurrence included HCV serotype, sex, age, body mass index and blood test data at both the start and end of DAA treatment (ALT, AST, total bilirubin, albumin, platelet count, AFP, total cholesterol serum levels and FIB-4 index; Table III).
Table III.
Factors associated with HCC occurrence after DAA treatment in patients included in the present study (n=1,088).
| Univariate analysis | Multivariate analysis | |||||
|---|---|---|---|---|---|---|
| Factor | HR | 95% CI | P-value | HR | 95% CI | P-value |
| HCV serotype, 1 vs. others | 2.5198 | 0.8662–10.683 | 0.0950 | 1.3602 | 0.4127–6.1675 | 0.6363 |
| Sex, male vs. female | 1.6772 | 0.7732–3.7544 | 0.1905 | |||
| Age, for every year | 1.0729 | 1.0286–1.1253 | 0.0006 | 1.0814 | 0.4127–1.1517 | 0.0044 |
| Body mass index, for every kg/m2 | 1.0156 | 0.9390–1.0352 | 0.8617 | |||
| Diabetes mellitus, yes vs. no | 2.4271 | 0.7715–6.5522 | 0.1208 | |||
| History of interferon-based therapy, yes vs. no | 1.2026 | 0.5088–2.6610 | 0.6608 | |||
| Before DAA treatment | ||||||
| AST, for every IU/l | 1.0016 | 0.9899–1.0097 | 0.7640 | |||
| ALT, for every IU/l | 0.9966 | 0.9846–1.0038 | 0.4466 | |||
| Total bilirubin, for every mg/dl | 1.4651 | 0.6076–2.1760 | 0.3257 | |||
| Albumin, for every g/dl | 0.3022 | 0.1467–0.6677 | 0.0038 | 0.6493 | 0.1786–2.5285 | 0.0617 |
| Platelet count, for every 1×104/µl | 0.9873 | 0.9784–0.9966 | 0.0002 | 0.7966 | 0.6234–1.0109 | 0.5269 |
| AFP, for every ng/ml | 1.0004 | 0.9873–1.0052 | 0.9216 | |||
| FIB4, for every 1 | 1.1795 | 1.0725–1.2705 | 0.0016 | 0.7774 | 0.5294–1.0515 | 0.1119 |
| After DAA treatment | ||||||
| AST, for every IU/l | 0.9985 | 0.9985–1.0127 | 0.8660 | |||
| ALT, for every IU/l | 1.0008 | 0.9791–1.0136 | 0.9291 | |||
| Total bilirubin, for every mg/dl | 1.0709 | 0.6376–1.5731 | 0.4053 | |||
| Albumin, for every g/dl | 1.0042 | 0.8932–1.0160 | 0.8136 | |||
| Platelet count, for every 1×104/µl | 0.9099 | 0.8536–0.9674 | 0.0022 | 1.0560 | 0.8605–1.2188 | 0.5688 |
| AFP, for every ng/ml | 1.0497 | 1.0098–1.3828 | 0.0194 | 1.0486 | 1.0003–1.0900 | 0.0486 |
| FIB-4, for every 1 | 1.0896 | 0.9799–1.1489 | 0.0938 | 1.0254 | 0.8105–1.1309 | 0.7582 |
AFP, α-fetoprotein; AST, aspartate aminotransferase; ALT, alanine aminotransferase; DAA, direct-acting antiviral; FIB-4, fibrosis-4; HCC, hepatocellular carcinoma; HCV, hepatitis C virus; HR, hazard ratio; IU, international unit.
The univariate analysis of the log-rank test identified age (P=0.0006), albumin serum level before DAA treatment (P=0.0038), platelet count before DAA treatment (P=0.0002), FIB-4 before DAA treatment (P=0.0016), platelet count after DAA treatment (P=0.0022)and AFP serum level after DAA treatment (P=0.0194) as risk factors for HCC. In addition, these results identified serotype (P=0.0950) and FIB-4 after DAA treatment (P=0.0938) to have the tendency as potential risk factors. Cox hazard analysis was performed using HCV serotype, age, platelet count before and after DAA treatment, FIB-4 before and after DAA treatment, albumin before DAA treatment, and AFP serum level after DAA treatment. The results demonstrated that age [hazard ratio (HR), 1.0814; 95% CI, 1.0814–1.1517; P=0.0044] and AFP serum level after DAA treatment (HR, 1.0486; 95% CI, 1.0003–1.0900; P=0.0486) were significant risk factors that may contribute to HCC occurrence after DAA treatment (Table III).
ROC analysis and diagnostic value
According to the ROC value for HCC occurrence based on age noted in 1,088 patients (sensitivity, 0.500; specificity, 0.7372; AUC, 0.6416), the cut-off value for age was set as 75 years (data not shown). Furthermore, the cut-off value of AFP serum level after DAA treatment was set as 6.0 ng/ml, based on the ROC value for developing HCC based on AFP serum levels before DAA treatment noted in 1,088 patients (sensitivity, 0.8400; specificity, 0.6315; AUC, 0.743) (data not shown).
Determination of HCC risk score by combining sex and AFP serum level at the start of DAA treatment
The Cox hazard analysis was performed using age, platelet count before and after DAA treatment, FIB-4 before and after DAA treatment, albumin serum level before DAA treatment, AFP serum level after DAA treatment grouped by cut-off values, and HCV serotype (data not shown). Age ≥75 years (HR, 4.17; 95% CI, 1.5658–11.915; P=0.0043) and AFP serum level after DAA treatment (HR, 2.99; 95% CI, 1.1002–8.6251; P=0.0318) were significant risk factors contributing to HCC occurrence after DAA treatment (Table IV). Therefore, based on the results of the multivariate analysis, the scoring system to predict occurrence of HCC should be based on two factors: Age and post-treatment AFP serum level. The age groups of <75 and ≥75 years were set as 0 and 1 points, respectively and post-treatment AFP serum level (ng/ml) <6 and ≥6 were set as 0 and 1 points, respectively (Table IV). To simplify the score of HCC occurrence, the sum of the points given for each factor was considered as the score.
Table IV.
Independent factors associated with HCC occurrence after DAA treatment in Cox hazard analysis and scores for prediction of HCC occurrence.
| Factor | HR | 95% CI | P-value | Score for prediction of HCC occurrence (points) |
|---|---|---|---|---|
| Age | ||||
| ≥75 years | 4.17 | 1.5658–11.915 | 0.0043 | 1 |
| <75 years | 1.00 | 0 | ||
| AFP after DAA treatment | ||||
| <6 ng/ml | 1.00 | 0 | ||
| ≥6 ng/ml | 2.99 | 1.1002–8.6251 | 0.0318 | 1 |
AFP, α-fetoprotein; HR, hazard ratio; HCC, hepatocellular carcinoma; DAA, direct-acting antiviral.
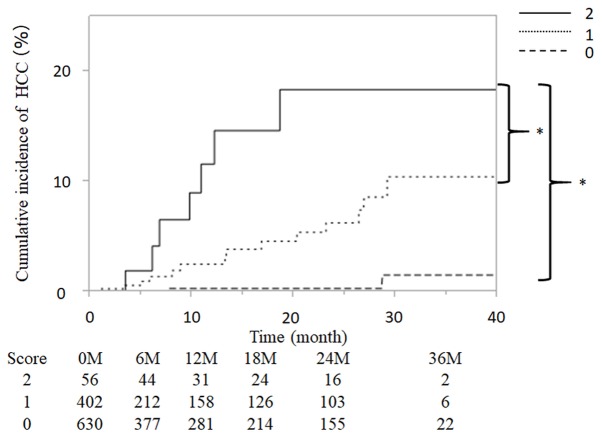
Patients included in the present study were scored based on the aforementioned risk factors, giving total scores of 0, 1 or 2 for each patient, and were subsequently grouped as follows: 0 points (n=630), 1 point (n=402) and 2 points (n=56). Fig. 3 presents the cumulative incidence curve of HCC occurrence for each group. The incidence of HCC was 0.00, 0.30, 0.30, 0.30 and 1.26% at 6, 12 18, 24 and 36 months, respectively, in the 0 points group. The incidence of HCC was 0.71, 1.05, 1.58, 6.27 and 10.45% in the 1 point group. The incidence of HCC was 2.88, 4.92, 11.61, 18.37 and 18.37%, respectively, in the 2 points group. In addition, the cumulative incidence of HCC was increased significantly with higher predictive scores (P<0.001; Fig. 3).
Figure 3.
Cumulative incidence of HCC in patients infected with hepatitis C virus with direct-acting antiviral treatment and scored by combining α-fetoprotein serum level after DAA treatment (<6 ng/ml, 0 points; ≥6 ng/ml, 1 point) and advanced age (<75 years, 0 points; ≥75, 1 point). Study patients were grouped based on these scores as follows: 0 point (n=630), 1 point (n=402) and 2 points (n=56). The cumulative HCC incidence increased significantly with the score (P<0.001). *P<0.001. HCC, hepatocellular carcinoma; M month.
Discussion
HCC frequently occurs in patients with chronic HCV infection who have received IFN-based treatment, even after achieving SVR. It has been previously reported that AFP and ALT levels after treatment are risk factors associated with HCC occurrence (12). Furthermore, other studies have demonstrated that risk factors for the occurrence of HCC in patients who achieved SVR after IFN-based treatment are elderly age, advanced liver fibrosis, fatty liver, male sex and alcohol consumption (22–24). Although IFN-based treatment has been the standard treatment for HCV-infected patients to date, it cannot be used in elderly patients and in patients with advanced liver fibrosis, due to the risks of IFN-related adverse events.
Recently, DAA has been introduced as a simple and safe antiviral oral treatment for HCV infection. The application of DAA has facilitated the eradication of HCV, even in elderly patients and patients with LC (6–11). In addition, it has been reported that eradication of HCV by DAA treatment reduces the risk of HCC (25). However, there are few reports on the incidence of HCC occurrence following DAA treatment in patients who have achieved SVR, compared with IFN therapy. Previous studies have demonstrated that IFN treatment reduces the incidence of HCC (26,27). The 5-year cumulative incidence of HCC is ~3% in patients who achieve SVR following IFN-based treatment (22,28). However, in patients >65 years, the 5-year cumulative incidence of HCC is 6%, whereas patients with cirrhosis presented with a high rate of 18.9% (12,29). Therefore, it is crucial to identify and predict the incidence of HCC after DAA treatment, which includes patients that are older and have cirrhosis.
There are two possible reasons for the occurrence of HCC after SVR. Firstly, HCC may have been missed following imaging diagnosis before DAA administration. Secondly, HCC could develop after DAA treatment. In the present study, all images obtained following US, CT or MRI were repeatedly reviewed to prevent any oversight and to exclude patients with HCC before starting DAA treatment. The results of the present study demonstrated that the 12- and 36-months cumulative incidences of HCC were 1.88 and 6.00%, respectively. Chang et al (30) reported that the 3-year cumulative incidence of HCC in patients who received IFN treatment was 1.2%. Furthermore, Watanabe et al (31) recently demonstrated that the 1- and 2-year cumulative incidences of HCC were 1.9 and 4.1%, respectively. In addition, in the high-risk groups for HCC occurrence, the 1- and 2-year cumulative incidences were 6.1 and 14.4%, respectively (31). The results of these studies supported the data from the present study demonstrating that the incidence of HCC at 3 years after DAA treatment was higher than that after IFN treatment (11,22,26–31). This difference in HCC incidence between DAA and IFN treatment may be due to the inclusion of patients with LC in the study group.
The present study demonstrated that the 26 cases of de novo HCC shared common characteristics (high age and early stage HCC). Fatty liver, alcohol consumption and diabetes mellitus have been suggested to be involved in HCC occurrence (24,31); however, the hazard ratio of these factors was not high in the present study. In addition, there was no difference observed in HCC occurrence in patients following diabetes treatment. Because the observation period until HCC occurrence was short (13.8 months), alcohol consumption and diabetes mellitus were not considered to be involved in HCC occurrence. Instead, the present study suggested a cytological origin of carcinogenesis before DAA treatment; however, long-term observations indicated that these factors would gradually contribute to liver carcinogenesis.
El-Serag et al demonstrated that older adults are at higher risk of developing HCC (32). In the present study, univariate analysis identified age, HCV serotype, albumin serum level, platelet count and FIB-4 index before DAA treatment as risk factors for HCC occurrence. In addition, platelet counts, AFP serum level and FIB-4 index after DAA treatment were considered as risk factors for HCC occurrence. Furthermore, following multivariate analysis, age and AFP serum level after DAA treatment were identified as independent risk factors for HCC occurrence. These results were consistent with previous studies, which reported that elderly patients have a higher risk of developing HCC after antiviral treatment (22,23,33,34).
Elevated AFP serum level is recognized as one of the common risk factors for HCC occurrence (31,35). In the present study, AFP serum level after DAA treatment was considered as a risk factor for HCC occurrence. Subsequently, the cutoff value of AFP serum level after DAA treatment was established as 6 ng/ml. In the present study, a simple scoring system was proposed to predict the risk of HCC occurrence after HCV eradication by DAA treatment. The HRs of age and AFP after DAA treatment were similar. In order to establish a simple scoring system, scores of 0 or 1 point for age <75 and ≥75 years, respectively, were designated. The identified risk factors of age (≥75 years) and AFP serum level after treatment (≥6.0 ng/ml) were scored as 1 point, and each patient was scored for these risk factors, giving a total of 0, 1 or 2 points. The 2-year incidence of HCC was 0.30, 6.27 and 18.37% in the 0, 1 and 2 points groups, respectively. The cumulative incidence of HCC increased significantly with higher scores (P<0.001). Previous studies have reported a stratification of HCC occurrence rate by scoring with FIB-4 and AFP after SVR or sex (31,34). However, compared with other reports, the scoring system established in the present study is simple and concise, and uses only two factors (advanced age and AFP levels after DAA treatment) to predict the risk of HCC occurrence after SVR.
The prognosis of patients with HCC is known to be dependent on tumor number, tumor size and liver function (36,37). It has been demonstrated that VEGF expression is increased following antiviral treatment, and may contribute to HCC occurrence after DAA treatment (38). However, because accurate quantification of VEGF is complicated, a simple method is required for the early detection of HCC, particularly in high-risk patients. Therefore, surveillance of HCC occurrence in patients who have achieved successful HCV eradication by DAA treatment is crucial in the presented simple scoring system. By identifying patients with high risk of HCC occurrence after SVR, early diagnosis could be more achievable. In the 2-points group identified by the present scoring system, it may be necessary to confirm non-hypervascular hyperpointense nodes with EOB-MRI before DAA treatment (39). However, the observation period was short in the present study, and a longer observation period is required to validate the conclusions.
In conclusion, age ≥75 years and AFP ≥6 ng/ml after DAA treatment were independent predictors for the development of HCC in patients who achieved SVR after DAA treatment. The proposed scoring system using these two factors may serve an important role in determining the risk of HCC occurrence after DAA treatment.
Acknowledgements
Not applicable.
Glossary
Abbreviations
- AFP
α-fetoprotein
- CEUS
contrast enhanced ultrasonography
- AUC
area under the curve
- ALT
alanine aminotransferase
- AST
aspartate aminotransferase
- CH
chronic hepatitis
- DAA
direct acting antivirals
- EOB-MRI
gadolinium-ethoxybenzyl-diethylenetriamine pentaacetic acid enhanced magnetic resonance imaging
- FIB-4
fibrosis-4
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HR
hazard ratio
- IFN
interferon
- LC
liver cirrhosis
- ROC
receiver operating characteristic
- SOF
sofosbuvir
- SVR
sustained virological response
- US
ultrasonography
- VEGF
vascular endothelial growth factor
Funding
No funding was received.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
JT designed the research and analyzed the patient data. JT drafted the manuscript. JT, AsM, TeS, KeT, MN, KF, KO, TT, TNo, HY, AT, ToS, TNa, CO, AkM and AD analyzed and interpreted the data. SM, HK, TH, KoT and TM interpreted the data and revised the manuscript critically for important intellectual content. All authors were involved in data interpretation and drafting the manuscript and have read and approved the final version of the manuscript.
Ethics approval and consent to participate
The present study conformed to the Clinical Research Guidelines and was approved by the Ethics Committee of Kagawa University, Faculty of Medicine (approval no. Heisei-27-174). The requirement for informed consent from the participants was waived because of the retrospective nature of the study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Lauer GM, Walker BD. Hepatitis C virus infection. N Engl J Med. 2001;345:41–52. doi: 10.1056/NEJM200107053450107. [DOI] [PubMed] [Google Scholar]
- 2.Fried MW, Shiffman ML, Reddy KR, Smith C, Marinos G, Gonçales FL, Jr, Häussinger D, Diago M, Carosi G, Dhumeaux D, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347:975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 3.Hadziyannis SJ, Sette H, Jr, Morgan TR, Balan V, Diago M, Marcellin P, Ramadori G, Bodenheimer H, Jr, Bernstein D, Rizzetto M, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: A randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140:346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 4.Manns MP, McHutchison JG, Gordon SC, Rustgi VK, Shiffman M, Reindollar R, Goodman ZD, Koury K, Ling M, Albrecht JK. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: A randomised trial. Lancet. 2001;358:958–965. doi: 10.1016/S0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 5.Fried MW. Side effects of therapy of hepatitis C and their management. Hepatology. 2002;36(5 Suppl 1):S237–S244. doi: 10.1053/jhep.2002.36810. [DOI] [PubMed] [Google Scholar]
- 6.Omata M, Nishiguchi S, Ueno Y, Mochizuki H, Izumi N, Ikeda F, Toyoda H, Yokosuka O, Nirei K, Genda T, et al. Sofosbuvir plus ribavirin in Japanese patients with chronic genotype 2 HCV infection: An open-label, phase 3 trial. J Viral Hepat. 2014;21:762–768. doi: 10.1111/jvh.12312. [DOI] [PubMed] [Google Scholar]
- 7.Ogawa E, Furusyo N, Nomura H, Dohmen K, Higashi N, Takahashi K, Kawano A, Azuma K, Satoh T, Nakamuta M, et al. NS5A resistance-associated variants undermine the effectiveness of ledipasvir and sofosbuvir for cirrhotic patients infected with HCV genotype 1b. J Gastroenterol. 2017;52:845–854. doi: 10.1007/s00535-016-1290-1. [DOI] [PubMed] [Google Scholar]
- 8.Poordad F, Felizarta F, Asatryan A, Sulkowski MS, Reindollar RW, Landis CS, Gordon SC, Flamm SL, Fried MW, Bernstein DE, et al. Glecaprevir and pibrentasvir for 12 weeks for hepatitis C virus genotype 1 infection and prior direct-acting antiviral treatment. Hepatology. 2017;66:389–397. doi: 10.1002/hep.29081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Toyoda H, Kumada T, Tada T, Shimada N, Takaguchi K, Senoh T, Tsuji K, Tachi Y, Hiraoka A, Ishikawa T, et al. Efficacy and tolerability of an IFN-free regimen with DCV/ASV for elderly patients infected with HCV genotype 1B. J Hepatol. 2017;66:521–527. doi: 10.1016/j.jhep.2016.11.012. [DOI] [PubMed] [Google Scholar]
- 10.Toyoda H, Chayama K, Suzuki F, Sato K, Atarashi T, Watanabe T, Atsukawa M, Naganuma A, Notsumata K, Osaki Y, et al. Efficacy and safety of glecaprevir/pibrentasvir in Japanese patients with chronic genotype 2 hepatitis C virus infection. Hepatology. 2018;67:505–513. doi: 10.1002/hep.29510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suda G, Kurosaki M, Itakura J, Izumi N, Uchida Y, Mochida S, Hasebe C, Abe M, Haga H, Ueno Y, et al. Safety and efficacy of elbasvir and grazoprevir in Japanese hemodialysis patients with genotype 1b hepatitis C virus infection. J Gastroenterol. 2019;54:78–86. doi: 10.1007/s00535-018-1495-6. [DOI] [PubMed] [Google Scholar]
- 12.Asahina Y, Tsuchiya K, Nishimura T, Muraoka M, Suzuki Y, Tamaki N, Yasui Y, Hosokawa T, Ueda K, Nakanishi H, et al. alpha-fetoprotein levels after interferon therapy and risk of hepatocarcinogenesis in chronic hepatitis C. Hepatology. 2013;58:1253–1262. doi: 10.1002/hep.26442. [DOI] [PubMed] [Google Scholar]
- 13.Hirakawa M, Ikeda K, Arase Y, Kawamura Y, Yatsuji H, Hosaka T, Sezaki H, Akuta N, Kobayashi M, Saitoh S, et al. Hepatocarcinogenesis following HCV RNA eradication by interferon in chronic hepatitis patients. Intern Med. 2008;47:1637–1643. doi: 10.2169/internalmedicine.47.1087. [DOI] [PubMed] [Google Scholar]
- 14.Conti F, Buonfiglioli F, Scuteri A, Crespi C, Bolondi L, Caraceni P, Foschi FG, Lenzi M, Mazzella G, Verucchi G, et al. Early occurrence and recurrence of hepatocellular carcinoma in HCV-related cirrhosis treated with direct-acting antivirals. J Hepatol. 2016;65:727–733. doi: 10.1016/j.jhep.2016.06.015. [DOI] [PubMed] [Google Scholar]
- 15.Kanwal F, Kramer J, Asch SM, Chayanupatkul M, Cao Y, El-Serag HB. Risk of hepatocellular cancer in HCV patients treated with direct-acting antiviral agents. Gastroenterology. 2017;153:996–1005.e1. doi: 10.1053/j.gastro.2017.06.012. [DOI] [PubMed] [Google Scholar]
- 16.Bruix J, Sherman M, Practice Guidelines Committee, American Association for the Study of Liver Diseases Management of hepatocellular carcinoma. Hepatology. 2005;42:1208–1236. doi: 10.1002/hep.20933. [DOI] [PubMed] [Google Scholar]
- 17.Hiraoka A, Hiasa Y, Onji M, Michitaka K. New contrast enhanced ultrasonography agent: Impact of Sonazoid on radiofrequency ablation. J Gastroenterol Hepatol. 2011;26:616–618. doi: 10.1111/j.1440-1746.2011.06678.x. [DOI] [PubMed] [Google Scholar]
- 18.Kudo M, Kitano M, Sakurai T, Nishida N. General rules for the clinical and pathological study of primary liver cancer, nationwide follow-up survey and clinical practice guidelines: The outstanding achievements of the liver cancer study group of Japan. Dig Dis. 2015;33:765–770. doi: 10.1159/000439101. [DOI] [PubMed] [Google Scholar]
- 19.Hung CH, Lu SN, Wang JH, Lee CM, Chen TM, Tung HD, Chen CH, Huang WS, Changchien CS. Correlation between ultrasonographic and pathologic diagnoses of hepatitis B and C virus-related cirrhosis. J Gastroenterol. 2003;38:153–157. doi: 10.1007/s005350300025. [DOI] [PubMed] [Google Scholar]
- 20.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, S Sulkowski M, Torriani FJ, Dieterich DT, Thomas DL, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 21.Vallet-Pichard A, Mallet V, Nalpas B, Verkarre V, Nalpas A, Dhalluin-Venier V, Fontaine H, Pol S. FIB-4: An inexpensive and accurate marker of fibrosis in HCV infection. comparison with liver biopsy and fibrotest. Hepatology. 2007;46:32–36. doi: 10.1002/hep.21669. [DOI] [PubMed] [Google Scholar]
- 22.Ikeda M, Fujiyama S, Tanaka M, Sata M, Ide T, Yatsuhashi H, Watanabe H. Risk factors for development of hepatocellular carcinoma in patients with chronic hepatitis C after sustained response to interferon. J Gastroenterol. 2005;40:148–156. doi: 10.1007/s00535-004-1519-2. [DOI] [PubMed] [Google Scholar]
- 23.Tokita H, Fukui H, Tanaka A, Kamitsukasa H, Yagura M, Harada H, Okamoto H. Risk factors for the development of hepatocellular carcinoma among patients with chronic hepatitis C who achieved a sustained virological response to interferon therapy. J Gastroenterol Hepatol. 2005;20:752–758. doi: 10.1111/j.1440-1746.2005.03800.x. [DOI] [PubMed] [Google Scholar]
- 24.Tanaka A, Uegaki S, Kurihara H, Aida K, Mikami M, Nagashima I, Shiga J, Takikawa H. Hepatic steatosis as a possible risk factor for the development of hepatocellular carcinoma after eradication of hepatitis C virus with antiviral therapy in patients with chronic hepatitis C. World J Gastroenterol. 2007;13:5180–5187. doi: 10.3748/wjg.v13.i39.5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ioannou GN, Green PK, Berry K. HCV eradication induced by direct-acting antiviral agents reduces the risk of hepatocellular carcinoma. J Hepatol. 2017 doi: 10.1016/j.jhep.2017.08.030. S0168-8278(17)32273-0. (Epub ahead of print) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cammà C, Giunta M, Andreone P, Craxì A. Interferon and prevention of hepatocellular carcinoma in viral cirrhosis: An evidence-based approach. J Hepatol. 2001;34:593–602. doi: 10.1016/S0168-8278(01)00005-8. [DOI] [PubMed] [Google Scholar]
- 27.Mazzaferro V, Romito R, Schiavo M, Mariani L, Camerini T, Bhoori S, Capussotti L, Calise F, Pellicci R, Belli G, et al. Prevention of hepatocellular carcinoma recurrence with alpha-interferon after liver resection in HCV cirrhosis. Hepatology. 2006;44:1543–1554. doi: 10.1002/hep.21415. [DOI] [PubMed] [Google Scholar]
- 28.Arase Y, Kobayashi M, Suzuki F, Suzuki Y, Kawamura Y, Akuta N, Kobayashi M, Sezaki H, Saito S, Hosaka T, et al. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatology. 2013;57:964–973. doi: 10.1002/hep.26087. [DOI] [PubMed] [Google Scholar]
- 29.Ogawa E, Furusyo N, Kajiwara E, Takahashi K, Nomura H, Maruyama T, Tanabe Y, Satoh T, Nakamuta M, Kotoh K, et al. Efficacy of pegylated interferon alpha-2b and ribavirin treatment on the risk of hepatocellular carcinoma in patients with chronic hepatitis C: A prospective, multicenter study. J Hepatol. 2013;58:495–501. doi: 10.1016/j.jhep.2012.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Chang KC, Hung CH, Lu SN, Wang JH, Lee CM, Chen CH, Yen MF, Lin SC, Yen YH, Tsai MC, et al. A novel predictive score for hepatocellular carcinoma development in patients with chronic hepatitis C after sustained response to pegylated interferon and ribavirin combination therapy. J Antimicrob Chemother. 2012;67:2766–2772. doi: 10.1093/jac/dks269. [DOI] [PubMed] [Google Scholar]
- 31.Watanabe T, Tokumoto Y, Joko K, Michitaka K, Horiike N, Tanaka Y, Tada F, Kisaka Y, Nakanishi S, Yamauchi K, et al. Predictors of hepatocellular carcinoma occurrence after direct-acting antiviral therapy in patients with hepatitis C virus infection. Hepatol Res. 2019;49:136–146. doi: 10.1111/hepr.13278. [DOI] [PubMed] [Google Scholar]
- 32.Asahina Y, Tsuchiya K, Tamaki N, Hirayama I, Tanaka T, Sato M, Yasui Y, Hosokawa T, Ueda K, Kuzuya T, et al. Effect of aging on risk for hepatocellular carcinoma in chronic hepatitis C virus infection. Hepatology. 2010;52:518–527. doi: 10.1002/hep.23691. [DOI] [PubMed] [Google Scholar]
- 33.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: Epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132:2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 34.Hiraoka A, Kumada T, Ogawa C, Kariyama K, Morita M, Nouso K, Toyoda H, Tada T, Ochi M, Murakami T, et al. Proposed a simple score for recommendation of scheduled ultrasonography surveillance for hepatocellular carcinoma after direct acting antivirals: Multicenter analysis. J Gastroenterol Hepatol. 2019;34:436–441. doi: 10.1111/jgh.14378. [DOI] [PubMed] [Google Scholar]
- 35.Toyoda H, Tada T, Takaguchi K, Senoh T, Shimada N, Hiraoka A, Michitaka K, Ishikawa T, Kumada T. Differences in background characteristics of patients with chronic hepatitis C who achieved sustained virologic response with interferon-free versus interferon-based therapy and the risk of developing hepatocellular carcinoma after eradication of hepatitis C virus in Japan. J Viral Hepat. 2017;24:472–476. doi: 10.1111/jvh.12665. [DOI] [PubMed] [Google Scholar]
- 36.Kudo M, Chung H, Osaki Y. Prognostic staging system for hepatocellular carcinoma (CLIP score): Its value and limitations, and a proposal for a new staging system, the Japan integrated staging score (JIS score) J Gastroenterol. 2003;38:207–215. doi: 10.1007/s005350300038. [DOI] [PubMed] [Google Scholar]
- 37.Hiraoka A, Kumada T, Kudo M, Hirooka M, Tsuji K, Itobayashi E, Kariyama K, Ishikawa T, Tajiri K, Ochi H, et al. Albumin-bilirubin (ALBI) grade as part of the evidence-based clinical practice guideline for HCC of the Japan society of hepatology: A comparison with the liver damage and child-pugh classifications. Liver Cancer. 2017;6:204–215. doi: 10.1159/000452846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Villani R, Facciorusso A, Bellanti F, Tamborra R, Piscazzi A, Landriscina M, Vendemiale G, Serviddio G. DAAs rapidly reduce inflammation but increase serum VEGF Level: A rationale for tumor risk during anti-HCV treatment. PLoS One. 2016;11:e0167934. doi: 10.1371/journal.pone.0167934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Toyoda H, Kumada T, Tada T, Mizuno K, Sone Y, Akita T, Tanaka J, Johnson PJ. The impact of HCV eradication by direct-acting antivirals on the transition of precancerous hepatic nodules to HCC: A prospective observational study. Liver Int. 2019;39:448–454. doi: 10.1111/liv.13987. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.