Abstract
A functional single-nucleotide polymorphism (SNP) in the catechol-O-methyltransferase (COMT) gene (rs4680) is a gene variant that has been shown to predict the ability to maintain cognitive agility during combat and competition. Critically, COMT Met (low-activity; high dopamine) allele carriers outperform Val (high-activity; low dopamine) homozygotes on a variety of cognitive tasks. However, the relationship between genotype and cognitive performance appears to reverse under stressful conditions. Stress increases pre-frontal cortex dopamine (PFC DA) levels, and Met allele carriers (with higher DA) show performance deficits relative to Val allele carriers. This pattern reflects the inverted U-shaped function of DA activity where too little (Val allele) or too much (Met allele carriers under stress) DA is associated with poor cognitive performance. The Val allele advantage for stress resiliency is referred to as the COMT “warrior/ worrier” model. In line with this model, we predicted that elite level mixed martial arts (MMA) fighters would be more likely than athlete controls to carry the GG (warrior) genotype compared to an athlete group and a non-athlete group. Based on findings in our previous studies, we also assessed the stress biomarkers cortisol and salivary alpha-amylase (sAA). There was an overall significant difference in genotype frequencies between groups (p =0.01) and the MMA group showed a significantly greater GG (warrior) genotype frequency than the non-athlete control group (p = 0.003). There was not a significant group x genotype interaction for the cortisol or sAA; however, the non-athlete GG group had significantly higher cortisol than the A/- group (p = 0.038). Combined, our findings suggest that the “warrior” genotype may play a participation role in combat sports.
Key points.
Dopamine signaling in the prefrontal cortex has been shown to moderate aggression levels.
It is currently unclear if genetic differences in dopamine levels relate to participation in combat sports.
A functional single-nucleotide polymorphism (SNP) in the catechol-O-methyltransferase (COMT) gene relates to dopamine levels and allele types considered the “warrior” and the “worrier” genotypes.
There is an increase in the “warrior” genotype in MMA fighters relative to control group.
This is the first study to report COMT genotype frequencies in combat and non-combat sport athletes.
Key words: Athlete, cortisol, dopamine, MMA, sAA
Introduction
Dopamine signaling in the prefrontal cortex has been shown to moderate aggression levels. In particular, dopamine signaling in the mesocorticolimbic system is involved in multiple facets of aggressive behaviors including the initiation, execution, and conclusion of aggressive acts (Ferrari et al., 2003; Haney et al., 1990; Mos, 1979; Van Erp and Miczek, 2000). Indeed, dopamine antagonists represent the most widely used pharmacological treatment in efforts to curb aggressive behavior in humans (McDougle et al., 1998). Dopamine has been shown to be a critical factor in individual differences in competitive ability and is associated with competitive behavior in males across many species. For example, zebra finches with higher tyrosine hydroxylase mRNA and higher D1 receptor (D1-R) mRNA show advantages in competitive ability (Eswine et al., 2019) In rats, learned aggression is associated with low D2 receptor densities (Suzuki et al., 2010). Increased activity of D2 receptors in the hypothalamus region increases aggressive defensive behavior in cats (Sweidan et al., 1991).
Catechol-O-methyltransferase (COMT) is one of two major enzymes responsible for catecholamine catabolism. The COMT gene is well expressed across the brain and regulates dopamine signaling in the prefrontal cortex. COMT variants can be used as molecular genetic markers associated with anxiety, pain, and stress responsivity. A functional single nucleotide polymorphism (SNP) in the COMT gene (rs4680) leads to an amino acid substitution of methionine (Met) in place of valine (Val) at position 158 (Val158Met). The Val158Met polymorphism is associated with decreased activity of the COMT enzyme in the prefrontal cortex and amygdala, increasing risk for early-onset major depression, panic disorders, and anxiety in adolescents and adults (Qiu et al., 2015). The COMT allele status has also been shown to functionally alter DA activity in the PFC wherein COMT Met (low-activity; high dopamine) allele carriers outperform Val (high-activity; low dopamine) allele carriers on a variety of cognitive tasks (Bruder et al., 2005; Diaz-Asper et al., 2008; Egan et al., 2001; Goldberg et al., 2003). Interestingly, the relationship between genotype and cognitive performance appears to reverse under stressful conditions. Stress increases PFC DA levels, and Met allele carriers (with higher DA) show performance deficits relative to Val allele carriers. This pattern reflects the inverted U-shaped function of DA activity where too little (Val allele) or too much (Met allele carriers under stress) DA is associated with poor cognitive performance (Goldman-Rakic et al., 2000).
Given the abundance of research that associates aggressive behavior with increased dopamine activity, combined with the COMT warrior/worrier model (Goldman-Rakic et al., 2000), we predicted that GG homozygote “warriors” would be more prevalent in fight sport athletes. In line with the warrior/worrier theory, increased physical and psychological stress during a match would push this genotype into a high dopamine level state. Whereas A allele carriers, who have high dopamine levels at baseline, would be pushed into the far right side of the inverted U- a level for suboptimal cognitive functioning. Given that our previous research showed a relationship between COMT genotypes and biomarkers of stress (Hill et al., 2018; Serrano et al., 2019), we also assessed these measures and their possible relationship to genotype status.
Methods
Participants
Participant descriptors by group can be seen in Table 1: Eighty-three male participants (MMA = 21, athlete = 21, non-athlete = 41) were tested in the current study (M age = 23.39 years, SD = 4.56). Self-reported ethnicities were as follows: White/Caucasian = 49, Black/African American = 17, Asian = 9, Multiracial = 9. A power calculation was not conducted since we aimed to test the maximum number of (relatively rare) participants we could recruit in the MMA group. All of the MMA athletes competed professionally under a variety of organizations including The Ultimate Fighting Championship, One Championship, and Bellator. Due to sample quality or technical error we were unable to genotype 2 participants and analyze sAA on one participant. To control for circadian fluctuations in cortisol secretion, participants were tested between 2:00-5:00 pm, when cortisol secretion, while not at the circadian nadir, is at a low, declining value (Bailey and Heitkemper, 2001). The Nova Southeastern University Institutional Review Board approved this study and written consent was acquired from all subjects before participating in the study procedure. Following consent, the height (M height =176.58 cm, SD = 21.68) and weight (M weight =81.09 kg, SD = 13.23) of participants were measured. 1 mL of saliva was collected into two 1.5 mL polyethylene centrifuge tubes using a passive salivation technique using a small sterile cylinder in order to measure selected biomarkers, outlined below. Saliva was immediately stored at -20°C.
Table 1.
Participant Characteristics and Biomarkers. Data are expressed as the mean (±standard deviation).
| Characteristics | MMA | Athlete | Non-Athlete |
|---|---|---|---|
| Age (years) | 25(6) | 23(4) | 23(4) |
| Height (m) | 1.84 (.06) | 1.74 (.03)* | 1.77 (.02) |
| Weight (kg) | 88.6 (15.5) | 75.3 (11.0)** | 81.1 (12.8)* |
| Cortisol (μg/dL) | .21 (.16) | .26 (.25) | .22 (.13) |
| sAA (U/mL) | 112.1 (96.4) | 110.0 (121.6) | 126.3 (95.2) |
* Indicates significantly different from MMA group at p< 0.05
** Indicates significantly different from MMA group at p< 0.001
Biomarkers
Cortisol and Alpha Amylase. Saliva samples were run in duplicate and quantified via a human cortisol enzyme immunoassay (EIA) kit and a sAA Kinetic Enzyme Assay Kit per the manufacturer’s instructions (Salimetrics LLC, USA). The samples were immediately read on a BioTek ELx800 plate reader (BioTek Instruments, Inc., USA) at 450 nm with a correction at 630 nm. All samples were within the detection ranges indicated in the immunoassay kits, and the variations of sample readings were within the expected limits. Final concentrations for the biomarkers were generated by interpolation from the standard curve in μg/dL for cortisol (sensitivity <0.007, range 0.012-3.000 ug/dL) and U/mL for sAA (sensitivity = 0.4 U/mL, range = 2-400 U/mL).
Genotyping. Each participant was genotyped for a functional G-to-A substitution SNP in the COMT gene. This results in an amino acid substitution of Met(A) in place of the wild-type Val(G). Genomic DNA was extracted in a QIAcube instrument following the manufacturer’s protocol (QIAGEN, Valencia, CA). Allelic discrimination for the COMT gene was determined via real-time polymerase chain reaction (PCR) using a TaqMan SNP genotyping assay using fluorogenic probes (Applied Biosystems, CA). Thermal cycling and fluorescence signal genotyping was performed on StepOne Real-Time PCR system (Applied Biosystems, CA) using negative controls and inter-assay positive controls. Samples were run in duplicate and in the case of a call discrepancy, samples were rerun. Genotype frequencies were as follows: AA = 10, AG = 43, GG = 28 and were consistent with the Hardy-Weinberg Equilibrium (X2 = 1.11, p = 0.29). In order to examine the greater frequency of the Val (G) allele in the MMA group, we collapsed across genotypes containing the Met allele since Met allele carriers have up to a 3-4-fold decrease in COMT enzymatic activity as compared to the wild-type Val/Val genotype (Lachman et al., 1996).
Statistical analyses
The distribution of allele frequencies was determined by the Hardy–Weinberg Exact (HWE) test. Group differences in genotype frequencies were examined through A Kruskal-Wallis H test and follow up test were conducted though Mann Whitney tests. Potential group differences in stress biomarkers was examined through analysis of variance (ANOVA) and interaction effects were examined through univariate ANOVA. All calculations were conducted using an SPSS statistical package (version 25, SPSS inc., IBM). All reported p-values are two-tailed with a priori significance level of p < 0.05.
Results
Genotype frequencies
The Kruskal-Wallis H test revealed a significant difference in genotype frequencies between groups (X2(2) = 9.20, p = 0.01). As shown in Figure 1, between group follow-up Mann Whitney tests showed that there was a significant difference in GG genotype frequencies between the MMA group (52.4%) and the non-athlete group (19.5%), (U = 240, p = 0.003). There was a statistical trend for a difference between the non-athlete group and the athlete group (U = 330, p = 0.05). There was not a significant difference between the MMA group (52.4%) and the athlete group (42.9%), (U = 169.500, p = 0.35).
Figure 1.
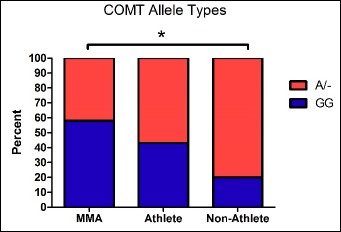
COMT genotype frequencies by group. There was a significant difference in allele frequencies between the MMA group and the non-athlete group. * indicates p < 0.05.
Biomarkers
Analysis of variance was conducted to examine possible group differences in cortisol and sAA. Figure 2 shows the biomarker results for each group. There were no between group differences in cortisol levels, F (2, 80) = 0.391, p = 0.68 or sAA levels, F(2, 79) = 0.434, p = 0.65. We also investigated a possible genotype x biomarker interaction through a univariate analysis of variance. There were no group x genotype interactions for cortisol F(2, 80) = 2.34, p = 0.10 or for sAA F (2, 79) = 0.80, p = 0.45. Based on our previous findings, we conducted planned analyses of cortisol differences by genotype in the non-athlete group. Consistent with our previous report, cortisol was significantly higher in the GG group (mean = 0.31, SD = 0.09) relative to the A/- group (mean = 0.20, SD = 0.24), p = 0.038.
Figure 2.
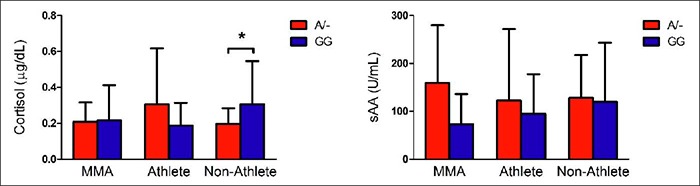
Cortisol and sAA levels by group and genotype. We did not observe any significant genotype x group interactions for sAA or cortisol. A planned analysis showed that the GG non-athlete group had significantly higher cortisol levels than the A/- group. * indicates p < 0.05.
Discussion
Results from this study show that MMA fighters are significantly more likely than non-athletes to carry the GG homozygous allele type on the COMT rs2650 SNP. Non-combat athletes’ GG allele type frequencies fell in the middle. They were more likely than non-athlete controls to carry this allele type, and less likely that the MMA athletes; however, none of these differences were statistically significant. Notably, the MMA GG genotype frequency (52.4%) is also higher than observed in our previous studies which were 31% (Serrano et al., 2019) and 28% (Hill et al., 2018). This is also a higher frequency than would be expected based on a publically available database from a North American population which was 29.2% (https://www.snpedia.com/index.php/SNPedia).
These findings support the warrior/worrier inverted U-shaped curve theory of dopamine proposed by Goldman-Rakic and colleagues (Goldman-Rakic et al., 2000). In particular, this idea suggests that too much or too little dopamine can impair performance (Cools and D'Esposito, 2011). MMA fighters, who have a high frequency of Val/Val homozygotes, have higher COMT activity in the PFC, which results in lower levels of dopamine. Conversely, the Met/- allele carriers have lower COMT activity and higher dopamine levels (Akil et al., 2003; Drabant et al., 2006; Weiss et al., 2014). Increased stress is known to increase dopamine levels (Kaneyuki et al., 1991; Nagano-Saito et al., 2013). This suggests that during a match, stress-induced increases in dopamine could push Met allele carrier (higher baseline PFC dopamine) outside of the optimum performance range, while pushing Val/Val genotypes (lower baseline PFC dopamine) into the optimal range. Accordingly, Val/Val genotypes would have an advantage in combat sports where they would need to perform aggressively under high stress conditions. In agreement with this idea, previous research showed that stress exposure results in better emotion processing in Val/Val genotypes relative to Met allele carriers (Mandelli et al., 2007). Previous findings also supports the role of dopamine in MMA activities. In particular, a variable-number tandem repeat (VNTR) polymorphism in DRD4 long allele genotype was more common in MMA fighters compared to a control group (Cherepkova et al., 2017). In general, MMA fighters benefit from a predilection towards aggressive reactions (Cherepkova et al., 2018), and controlled aggression in particular (Rosario et al., 2014).
We previously found that in a group of unstressed women cortisol levels were significantly lower in the Met/- group relative to the Val/Val group (Hill et al., 2018). Here we did not observe an interaction effect between cortisol and genotype. However, a planned analysis on the non-athlete group replicated our previous finding that the GG group had significantly higher cortisol levels at baseline. It is possible that these differences are not present in the athlete groups since physical exercise and training can alter cortisol levels (Hill et al., 2008; Skoluda et al., 2012). In a previous study looking at men only we found that following a stressor, sAA levels were significantly higher in the A/- group relative to the GG group (Serrano et al., 2019). We did not see any genotype x sAA concentration effects here. It will be interesting to carry out follow up work where stress biomarkers are captured in MMA fighters vs. control following a stressor.
One limitation in the current study is that our findings are limited to males and we cannot be certain if there is a higher than expected COMT GG genotype frequency in female fighters. We limited this initial study to male participants since there are known sexual dimorphisms in the DA pathway. For example, compared to men, women have significantly more DA cells within the mesocortical pathway, a major dopaminergic pathway projecting to PFC (50% vs. 30%, respectively) (Kritzer and Creutz, 2008; Swanson, 1982). It will be interesting for future work to investigate genotype frequencies in female fighters. An additional limitation is that while the results in the present study show that the MMA group is more likely to carry the warrior (GG) allele type, we cannot state if this is due to a a self-selection or an attrition process. In other words, it is uncertain if the GG genotypes are more likely to select a combat sport or if it confers a distinct performance advantage for perpetuation in the sport. However, since we tested MMA fighters who were elite performers (UFC, Bellator, One Championship), it is probable that the COMT GG genotype is working to increase their likelihood of advancement in competition level based on performance. Finally, due to the elite nature of the MMA group, we did not conduct an a priori power analysis; however, we had a large effect size (Cohen’s d = 0.82) and the post-hoc sensitivity analysis effect size was 1.03.
Conclusion
In summary, our results show that MMA fighters are significantly more likely than non-athletes to carry the GG “warrior” COMT rs4680 genotype. There was not an association between genotype and stress biomarkers, but in line with our previous results, we showed that non-athlete GGs have significantly higher cortisol levels than the A/- genotypes. Combined, these findings suggests that carrying the GG Val (high-activity; low dopamine) COMT genotype is advantageous for MMA fighters. We plan to carry out a follow up study that looks at the relationship between COMT genotype and performance (e.g. wins vs. losses) in MMA fighters.
Acknowledgements
This work was supported through a President’s Faculty Research and Development Grant from Nova Southeastern University awarded to JT, CP, and JA. The experiments comply with the current laws of the country in which they were performed. The authors have no conflict of interest to declare.
Biographies

Jaime TARTAR
Employment
Professor and Program Director of Behavioral Neuroscience at Nova Southeastern University
Degree
Ph.D.
Research interests
Affective Neuroscience, Sleep, Sports Neuroscience, Co-Founder and President of The Society for NeuroSports.
E-mail: tartar@nova.edu

Dominick CABRERRA
Employment
Graduate student in the Department of Psychology and Neuroscience at Nova Southeastern University
Degree
B.Sc.
Research interests
Neuroscience applied to sports performance and epigenetic modification in professional MMA fighters.
E-mail: dc1258@mynsu.nova.edu

Sarah KNAFO
Employment
Physical Therapy doctoral student at The University of Florida.
Degree
B.Sc.
Research interests
Physical Therapy, Sports Neuroscience
E-mail: sarahknafo@ufl.edu

Julius THOMAS
Employment
Pursuing his Psy.D. degree at Nova Southeastern University
Degree
B.Sc.
Research interests
Affective Neuroscience, Sports Neuroscience
E-mail: Jt1809@mynsu.nova.edu

Jose ANTONIO
Employment
Associate Professor and the Program Director of Exercise and Sport Science at Nova Southeastern University
Degree
Ph.D.
Research interests
Sports Nutrition, Sports Neuroscience, co-founder and CEO of the ISSN as well as the co-founder of the Society for NeuroSports.
E-mail: ja839@nova.edu

Corey PEACOCK
Employment
Assoc. Prof. at Nova Southeastern Univ.
Degree
Ph.D.
Research interests
Sports Science, Sports Neuroscience, director of sports science of the Fight Science Institute, co-founder of the Society for NeuroSports
E-mail: cpeacock@nova.edu
References
- Akil M., Kolachana B.S., Rothmond D.A., Hyde T.M., Weinberger D.R., Kleinman J.E. (2003) Catechol-O-methyltransferase genotype and dopamine regulation in the human brain. The Journal of Neuroscience 23, 2008-2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S.L., Heitkemper M.M. (2001) Circadian rhythmicity of cortisol and body temperature: morningness-eveningness effects. Chronobiology International 18, 249-261. [DOI] [PubMed] [Google Scholar]
- Bruder G.E., Keilp J.G., Xu H., Shikhman M., Schori E., Gorman J.M., Gilliam T.C. (2005) Catechol-O-methyltransferase (COMT) genotypes and working memory: associations with differing cognitive operations. Biological Psychiatry 58, 901-907. [DOI] [PubMed] [Google Scholar]
- Cherepkova E.V., Maksimov V.N., Kushnarev A.P., Shakhmatov I.I., Aftanas L.I. (2017) The polymorphism of dopamine receptor D4 (DRD4) and dopamine transporter (DAT) genes in the men with antisocial behaviou r and mixed martial arts fighters. The World Journal of Biological Psychiatry 1-14. [DOI] [PubMed] [Google Scholar]
- Cherepkova E.V., Maksimov V.V., Aftanas L.I. (2018) Polymorphism of serotonin transporter gene in male subjects with antisocial behavior and MMA fighters. Translational Psychiatry 8, 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R., D'Esposito M. (2011) Inverted-U–shaped dopamine actions on human working memory and cognitive control. Biological Psychiatry 69, e113-e125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diaz-Asper C.M., Goldberg T.E., Kolachana B.S., Straub R.E., Egan M.F., Weinberger D.R. (2008) Genetic variation in catechol-O-methyltransferase: effects on working memory in schizophrenic patients, their siblings, and healthy controls. Biological Psychiatry 63, 72-79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant E.M., Hariri A.R., Meyer-Lindenberg A., Munoz K.E., Mattay V.S., Kolachana B.S., Egan M.F., Weinberger D.R. (2006) Catechol O-methyltransferase val158met genotype and neural mechanisms related to affective arousal and regulation. Archives of General Psychiatry 63, 1396-1406. [DOI] [PubMed] [Google Scholar]
- Egan M.F., Goldberg T.E., Kolachana B.S., Callicott J.H., Mazzanti C.M., Straub R.E., Goldman D., Weinberger D.R. (2001) Effect of COMT Val108/158 Met genotype on frontal lobe function and risk for schizophrenia. Proceedings of the National Academy of Sciences 98, 6917-6922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eswine S. L., Pontinen J. K., Heimovics S. A. (2019). Competitive ability during mate competition relates to unique patterns of dopamine-related gene expression in the social decision-making network of male zebra finches. Neuroscience Letters 706, 30-35. [DOI] [PubMed] [Google Scholar]
- Ferrari P., Van Erp A., Tornatzky W., Miczek K. (2003) Accumbal dopamine and serotonin in anticipation of the next aggressive episode in rats. European Journal of Neuroscience 17, 371-378. [DOI] [PubMed] [Google Scholar]
- Goldberg T.E., Egan M.F., Gscheidle T., Coppola R., Weickert T., Kolachana B.S., Goldman D., Weinberger D.R. (2003) Executive subprocesses in working memory: relationship to catechol-O-methyltransferase Val158Met genotype and schizophrenia. Archives of General Psychiatry 60, 889-896. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic P.S., Muly E.C., III, Williams G.V. (2000) D1 receptors in prefrontal cells and circuits. Brain Research Reviews 31, 295-301 [DOI] [PubMed] [Google Scholar]
- Haney M., Noda K., Kream R., Miczek K.A. (1990) Regional serotonin and dopamine activity: sensitivity to amphetamine and aggressive behavior in mice. Aggressive Behavior 16, 259-270. [Google Scholar]
- Hill E., Zack E., Battaglini C., Viru M., Viru A., Hackney A. (2008) Exercise and circulating cortisol levels: the intensity threshold effect. Journal of endocrinological investigation 31, 587-591. [DOI] [PubMed] [Google Scholar]
- Hill L.D., Lorenzetti M.S., Lyle S.M., Fins A.I., Tartar A., Tartar J.L. (2018) Catechol-O-methyltransferase Val158Met polymorphism associates with affect and cortisol levels in women. Brain and Behavior 8, e00883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneyuki H., Yokoo H., Tsuda A., Yoshida M., Mizuki Y., Yamada M., Tanaka M. (1991) Psychological stress increases dopamine turnover selectively in mesoprefrontal dopamine neurons of rats: reversal by diazepam. Brain Research 557, 154-161. [DOI] [PubMed] [Google Scholar]
- Kritzer M.F., Creutz L.M. (2008) Region and sex differences in constituent dopamine neurons and immunoreactivity for intracellular estrogen and androgen receptors in mesocortical projections in rats. The Journal of Neuroscience 28, 9525-9535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lachman H.M., Papolos D.F., Saito T., Yu Y.-M., Szumlanski C.L., Weinshilboum R.M. (1996) Human catechol-O-methyltransferase pharmacogenetics: description of a functional polymorphism and its potential application to neuropsychiatric disorders. Pharmacogenetics 6, 243-250. [DOI] [PubMed] [Google Scholar]
- Mandelli L., Serretti A., Marino E., Pirovano A., Calati R., Colombo C. (2007) Interaction between serotonin transporter gene, catechol-O-methyltransferase gene and stressful life events in mood disorders. International Journal of Neuropsychopharmacology 10, 437-447. [DOI] [PubMed] [Google Scholar]
- McDougle C.J., Holmes J.P., Carlson D.C., Pelton G.H., Cohen D.J., Price L.H. (1998) A double-blind, placebo-controlled study of risperidone in adults with autistic disorder and other pervasive developmental disorders. Archives of General Psychiatry 55, 633-641. [DOI] [PubMed] [Google Scholar]
- Mos J. (1979) Specific effect on social stess and aggression on regionaldopamine metabolism in rat brain. Neuroscience Letters 15, 325-327. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A., Dagher A., Booij L., Gravel P., Welfeld K., Casey K.F., Leyton M., Benkelfat C. (2013) Stress-induced dopamine release in human medial prefrontal cortex—18F-Fallypride/PET study in healthy volunteers. Synapse 67, 821-830. [DOI] [PubMed] [Google Scholar]
- Qiu A., Tuan T.A., Ong M.L., Li Y., Chen H., Rifkin-Graboi A., Broekman B.F., Kwek K., Saw S.-M., Chong Y.-S. (2015) COMT haplotypes modulate associations of antenatal maternal anxiety and neonatal cortical morphology. American Journal of Psychiatry 172, 163-172. [DOI] [PubMed] [Google Scholar]
- Rosario D., Kerr J.H., Rhodius A. (2014) The experience of aggression among mixed martial arts athletes interpreted through reversal theory. International Journal of Sport Psychology 45, 79-99. [Google Scholar]
- Serrano J.M., Banks J.B., Fagan T.J., Tartar J.L. (2019) The influence of Val158Met COMT on physiological stress responsivity. Stress 22, 276-279. [DOI] [PubMed] [Google Scholar]
- Skoluda N., Dettenborn L., Stalder T., Kirschbaum C. (2012) Elevated hair cortisol concentrations in endurance athletes. Psychoneuroendocrinology 37, 611-617. [DOI] [PubMed] [Google Scholar]
- Suzuki H., Han S.D., Lucas L.R. (2010) Chronic passive exposure to aggression decreases D2 and 5-HT1B receptor densities. Physiology & Behavior 99, 562-570. [DOI] [PubMed] [Google Scholar]
- Swanson L. (1982) The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Research Bulletin 9, 321-353. [DOI] [PubMed] [Google Scholar]
- Sweidan S., Edinger H., Siegel A. (1991) D2 dopamine receptor-mediated mechanisms in the medial preoptic-anterior hypothalamus regulate affective defense behavior in the cat. Brain Research 549, 127-137. [DOI] [PubMed] [Google Scholar]
- Van Erp A.M., Miczek K.A. (2000) Aggressive behavior, increased accumbal dopamine, and decreased cortical serotonin in rats. Journal of Neuroscience 20, 9320-9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss E.M., Schulter G., Fink A., Reiser E.M., Mittenecker E., Niederstätter H., Nagl S., Parson W., Papousek I. (2014) Influences of COMT and 5-HTTLPR polymorphisms on cognitive flexibility in healthy women: inhibition of prepotent responses and memory updating. PLoS One 9, e85506. [DOI] [PMC free article] [PubMed] [Google Scholar]


