Abstract
Telomeres play an important part in aging and show relationships to lifetime adversity, particularly childhood adversity. Meta-analyses demonstrate reliable associations between psychopathology (primarily depression) and shorter telomere length, but the nature of this relationship has not been fully understood. Here, we review and evaluate the evidence for impaired telomere biology as a consequence of psychopathology or as a contributing factor, and the important mediating roles of chronic psychological stress and impaired allostasis. There is evidence for a triadic relationship among stress, telomere shortening, and psychiatric disorders that is positively reinforcing and unfolds across the life course and, possibly, across generations. We review the role of genetics and biobehavioral responses that may contribute to shorter telomere length, as well as the neurobiological impact of impaired levels of telomerase. These complex interrelationships are important to elucidate because they have implications for mental and physical comorbidity and, potentially, for the prevention and treatment of depression.
Keywords: depression, stress, early life adversity, psychopathology, telomeres, allostasis, telomerase
INTRODUCTION
The comorbidity of psychiatric and medical illness is common. Data from the National Comorbidity Survey Replication indicated that 68% of individuals with a psychiatric disorder also reported one or more general medical conditions. However, of those with a general medical condition, a much smaller proportion reported that they also had a psychiatric disorder (29%) (Alegria et al. 2003, Kessler et al. 2004). Prospective studies demonstrate that psychiatric disorders, such as schizophrenia and major depressive disorder, confer significant risk for the development of a myriad of age-related conditions, such as cardiovascular disease, type 2 diabetes, metabolic syndrome, and neurodegenerative diseases (Bioque et al. 2017, Eaton et al. 1996, Hennekens et al. 2005, Nicholson et al. 2006, Ownby et al. 2006, Rugulies 2002). The biological mechanisms that may underlie these relationships have been vigorously explored in the scientific literature; however, headway has been slow, in part due to the complexities at play in both physical disease and psychiatric illness. Converging animal and human evidence points to common and complementary sets of biochemical and cellular processes that are affected in psychiatrically disordered patients (Lindqvist et al. 2015). In particular, telomere length (TL), which is a marker of cellular aging and tightly related to psychological stress, has emerged as a biological correlate of many psychiatric disorders and on the pathway toward many of the medical disorders observed at greater frequency among individuals with psychiatric disorders.
Here, we review and evaluate the evidence for changes in telomere biology arising as a consequence of psychiatric disorders and/or as a possible contributor to psychopathology or simply as a reflection of common mediators. We also review how psychological stress may mediate the psychiatric disorder–TL link, both across the life course and across generations. We propose that there is a triadic relationship among chronic or traumatic psychological stress, telomere shortening, and psychiatric disorders that is positively reinforcing, iterative, and unfolds over the life course. Next, we review what is known about behavioral interventions that appear effective in modulating TL, and this is followed by a review of the emerging literature demonstrating the transgenerational effects of stress and psychiatric disorders on offspring TL. Last, we lay out current methodological challenges and important future directions for the field, including the integration of the National Institute of Mental Health’s Research Domain Criteria (RDoC) framework, which we believe will provide needed insights into likely mechanisms through which psychiatric disorders affect TL. Below, we start with a selective overview of how telomeres fit into the biology of aging (see Blackburn et al. 2015 for a more detailed technical discussion).
Overview of Telomeres and the Biology of Aging
Telomeres are DNA–protein complexes that cap chromosomal ends of eukaryotic cells, conferring chromosomal stability (Blackburn 1991, Blackburn et al. 2015, Effros 2009, McElhaney & Effros 2009). With each cellular division, telomeric DNA terminal regions are not fully replicated. If not counteracted by cellular repair mechanisms (e.g., telomerase), progressive telomere attrition can lead to cell death or cell cycle arrest, resulting in cellular senescence (Effros 2009). Telomerase is a critical enzyme produced by cells and it functions to add DNA sequence repeats to telomere regions, thereby preventing the constant telomere attrition incurred with each cell division or from biochemical stress mediators, as discussed below. There is telomere shortening in nondividing cells as well, for example, in postmitotic tissue, such as in parts of the brain, which is likely due to the biochemical environment. While our understanding of how short telomeres lead to cellular senescence continues to evolve, research suggests that critically short telomeres lead to activation of tumor suppressors, in particular p53, which results in the cell’s inability to divide (Campisi & d’Adda di Fagagna 2007). A cell that can no longer divide is called a senescent cell, and its functions change toward pro-aging. Telomere attrition and related p53 signaling lead to diminished mitochondrial function—that is, poor functioning of the cellular machinery that produces energy for the cell—and increased intracellular reactive oxygen species (ROS) (Sahin et al. 2011). While ROS are produced as a normal by-product of cell metabolism, at high levels ROS can lead to genetic mutations and eventual cell death.
Inflammation is also produced in excess by senescent cells, which is one pathway by which systemic levels of inflammation increase with age (known as inflammaging), building up from all types of senescent cells in the body, such as adipose tissue and immune cells. Notably, the link between inflammation and telomere biology appears bidirectional. For example, mice genetically modified to express low levels of telomerase (TERT−/−) show an upregulation in inflammatory gene expression over time (Jurk et al. 2014). Conversely, mice expressing an inflammatory phenotype show accelerated telomere dysfunction compared with wild-type mice, which can be ameliorated with anti-inflammatory blockade treatment (Jurk et al. 2014).
Telomeres provide a metric of cellular age that tracks with chronological age and has a reliable but typically small relationship, accounting for roughly 15% of the variance of age (depending on how wide the range of age is in a particular study). Unlike the epigenetic clock (a measure of genome methylation derived purposefully to correlate with age), which correlates with age at approximately 0.90 or higher, TL provides largely different information than calendar age, and thus TL has emerged as an intriguing pathway for understanding how exposures, including psychosocial stress, “get under the skin.” Many meta-analyses show that short telomeres in immune cells, the most frequently studied cell type in humans, predict earlier onset of the diseases of aging, such as heart disease and diabetes, after accounting for age and potential sociodemographic and lifestyle confounders (e.g., D’Mello et al. 2015). Although the effects of telomeres on disease are small, they now appear to be causal. Having the genes for long telomeres [based on findings in genomewide association studies (GWAS)] directly predicts protection from heart diseases and dementia (Codd et al. 2013, Zhan et al. 2015). In some cases, genetically long telomeres can predict a greater incidence of some types of cancer, such as melanoma and glioma.
The Triad of Stress, Psychopathology, and Telomere Attrition
Telomere science has become important in understanding physical health as well as mental health. There are now multiple reviews that demonstrate reliable relationships between short telomeres and adversity or psychopathology (primarily affective disorders), as reviewed below. On the surface, this may sound like another example of the cumulative biological toll that stress places on the body, a form of allostatic load, which is the physiological price of adaptation to stress (McEwen 2004). But turning these intuitive relationships on their head, animal studies and genetic research suggest that impaired telomere biology directly predicts greater risk for psychiatric disorders, namely major depression (Wei et al. 2016, Zhou et al. 2011). How genetic vulnerability for short telomeres confers this risk is discussed in more depth below.
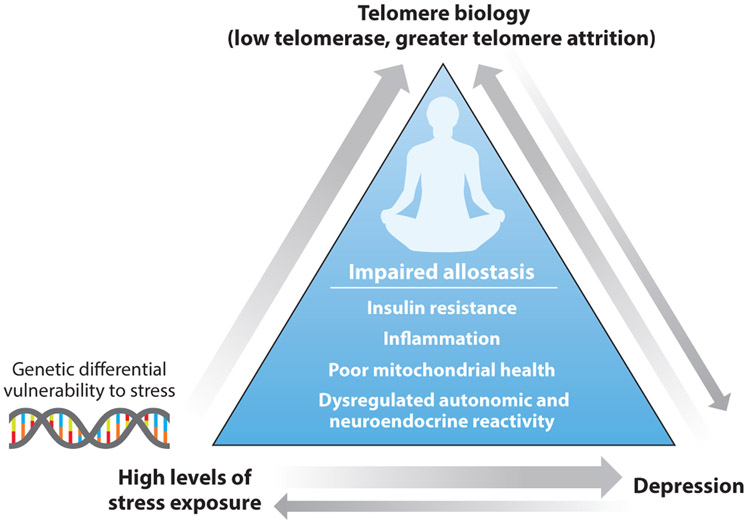
As displayed in Figure 1, there is growing evidence for the existence of a triad among stress exposure, psychiatric disorders, and telomere attrition. Given that the relationships between stress exposure and psychiatric disorders are already well established, we do not review these here. Instead, we provide an overview of what is known about associations between stress in early life and adulthood, as well as links between psychiatric disorders and TL. The bulk of research has centered on major depression, which is thus disproportionately reflected in this review.
Figure 1.
The triad of stress exposure, depression, and telomere biology. This triad illustrates the strength of predictive or causal relationships shown in the literature, represented by arrow thickness. The most consistent and well-established relationships, from both longitudinal studies and experimental animal studies, show that greater exposure to major life stressors, especially early in life, leads to a greater rate of telomere attrition (as well as likelihood of major depression). This is presumably exaggerated if one has genetic loading for greater vulnerability to stress. In turn, major depression is consistently related to shorter telomeres, although fewer studies find that depression leads to a greater rate of shortening over time. While aspects of having major depression can clearly lead to telomere shortening, it is likely that the greater telomere shortness in depression is at least in part the result of cumulative exposure to chronic stress and, consequently, impaired allostasis (stress causing both attrition and depression). People with greater reactivity tend to have faster telomere attrition, but no studies have tested whether telomere shortness predicts greater stress reactivity. There is also the possibility of reverse causation, as one study found that a genotype for low telomerase levels predicts depression. Last, chronic stress, depression, and telomere shortness have all been associated with indices of impaired allostasis.
STUDIES EXAMINING THE ASSOCIATION BETWEEN STRESS EXPOSURE AND TELOMERE LENGTH
Childhood Adversity
Childhood adversity encompasses many forms, from exposure to traumatic stressors (e.g., sexual assault, physical abuse) to social adversity (e.g., growing up in poverty or suffering neglect). Despite the heterogeneity of exposures, there is accruing evidence that childhood adversity is associated with shorter TL. Many studies that have examined relationships between childhood adversity and TL have done so using buccal (cheek) cells or leukocytes. The first study was carried out in healthy adults with no history of psychopathology, and it used a retrospective measure of early adversity (Tyrka et al. 2010). Since then, many studies have examined these associations in children directly. There have now been three meta-analyses of this literature, all showing similar patterns: More severe exposures, or exposures across a wide range of types of adversity, show stronger relations with shorter TL. Some meta-analyses included studies that examined stressors retrospectively and some focused on studies that included both children and adults. Hanssen et al. (2017) focused on traumatic stressors, excluding socioeconomic adversity, and reported a significant small effect (Cohen’s d=−0.08). Z. Li et al. (2017) found that separation events appeared highly impactful, and they found a similarly small significant effect size (Cohen’s d=−0.05). Tyrka and colleagues (Ridout et al. 2017) included a wider range of adversities—for example, socioeconomic status adversity, maternal depression, and prenatal stress exposures—among a total of 30,773 participants and found a larger effect size (Cohen’s d=−0.35). These meta-analyses demonstrate the robustness of the association across published studies. Not surprisingly, the relationship appeared stronger when there was a shorter duration between trauma exposure and measure of TL (Hanssen et al. 2017, Ridout et al. 2017). At the same time, despite this recency effect, there was also a larger effect for events that happened earlier in life (e.g., before 5 years old).
It is important to note that telomeres are impacted not only by individual exposures but also by the social environment, factors we do not typically measure in studies of psychopathology. For example, neighborhood characteristics—such as fear of crime, level of violence, and neighborhood disorder—have been associated with shorter TL even when controlling for individual characteristics (Park et al. 2015, Theall et al. 2017).
Coimbra and colleagues (2017) recently conducted a systematic review of studies that examined TL and adversity in children up to 15 years of age, finding 11 eligible studies, mostly cross-sectional. Various types of adversity—including violence, family disruption, and institutionalization—are associated with shorter TL. Since children exposed to one type of adversity tend to be exposed to many other types, it is difficult to identify isolated effects. It is notable that some studies found a relationship between maternal clinical depression or lower parental education and shorter child TL. These factors may have influenced parenting or the social environment, but it is also possible that the relationship may have been preexisting: These effects may have been determined even before birth, from stress or even direct transmission of shorter maternal TL. In accord with this idea, Wojcicki et al. (2016) found that lower maternal education predicted shorter TL in cord blood.
Finally, in the first longitudinal study, Shalev et al. (2013b), using the Dunedin longitudinal study, found a prospective relationship between traumatic violence exposures (bullying, victimization, maternal domestic violence, and physical abuse) and buccal cell TL over 5 years. A greater exposure to violence during the study period predicted greater TL attrition during that time (Shalev et al. 2013b).
Adult and Life Span Cumulative Adversity
In adults, there have been many studies showing that exposure to severe stress (violence, poverty, caregiving) is associated with shorter TL, as reviewed elsewhere (Oliveira et al. 2016, Shalev et al. 2013a). Newer studies have examined cumulative life span stress. For example, Puterman and colleagues (2016) examined TL in saliva samples and cumulative life-stressor exposures in approximately 4,600 men and women from the Health and Retirement Study. They found that although an index of cumulative life span adversity predicted shorter TL, this was mainly due to childhood adversity.
Despite this growing literature, there have been few longitudinal studies in adults. We examined telomere change during a 1-year period and found that experiencing a greater number of stressful life events predicted greater telomere shortening, but not among women who scored high on engaging in protective health behaviors (e.g., engaging in physical activity, eating a healthy diet, having good sleep quality) (Puterman et al. 2015). Van Ockenburg et. al. (2015) found that repeated measures of severe life events prospectively predicted telomere attrition over 6 years. In the Netherlands Study of Depression and Anxiety, long TL at baseline was the major determinant of decline during the next 6 years. After accounting for baseline TL, childhood trauma but not adult stressors, and not psychiatric disorders, predicted attrition over 6 years (Révész et al. 2016a). Thus, it appears that both major events in adulthood as well as early adversity can predict a faster rate of attrition.
These observational longitudinal studies in humans suggest a causal relationship, but experimental studies are needed to determine causality. Indeed, there is a growing number of stress–telomere studies in rodents, birds, and primates. Animal studies have shown that experimentally induced stress decreases TL over time, as reviewed elsewhere (Haussmann & Heidinger 2015). For example, in blackbirds repeated stressors during 1 year led to greater oxidative stress and shortened TL (Hau et al. 2015). Naturalistic stress manipulations—such as imposing competition for food and social isolation—have been linked to telomere shortening (Aydinonat et al. 2014, Nettle et al. 2015). In a small study, Suomi and colleagues (Schneper et al. 2016) examined TL in monkeys that were randomized at birth to be raised by their mother or without their mother for the first 7 months of life. At 7–10 years old, the monkeys raised without their mother had significantly shorter TL (Schneper et al. 2016).
In summary, prospective human and experimental animal studies together provide evidence suggesting that various forms of social stress or chronic adversity may causally accelerate telomere attrition. One of the limitations of the stress–telomere literature has been a tendency to include individuals with psychiatric disorders in research samples, making it difficult to understand independent effects. It is possible that early adversity may be one of the underlying causes of short TL in people with psychiatric disorders. For example, one small study found that post-traumatic stress disorder (PTSD) was not associated with shorter TL once early childhood adversity was taken into account (O’Donovan et al. 2011). However, some studies have found that the effects of childhood stress are smaller or not significant in samples of people with psychiatric disorders or once psychiatric disorders have been included in statistical models (Liu et al. 2017, Ridout et al. 2017), suggesting that it is partly the development of a chronic psychiatric disorder that causes shorter TL in those with childhood adversity. Last, high telomerase levels have been found in five of the nine studies of depression where telomerase was measured (Deng et al. 2016), and it is possible that elevated telomerase may compensate for short telomeres in affective disorders, dampening the magnitude of the relationship.
In the next section, we review the relationships between different types of psychiatric disorders and telomeres.
ASSOCIATIONS BETWEEN PSYCHOPATHOLOGY AND TELOMERE LENGTH
There is a substantial literature linking psychopathology to shorter telomeres, with a growing number of studies assessing cases versus controls, thus lending themselves to meta-analyses. While meta-analyses typically lack the ability to provide information about mechanisms or causality, they can test the reliability of a crude bivariate association across studies and moderators.
Depression has been studied the most frequently. Meta-analyses have found that people with depression have shorter TL than controls (Ridout et al. 2016, Schutte & Malouff 2015). Similarly, both men and women with PTSD have shorter TL versus controls, and the type of trauma exposure most related to TL was sexual assault (X. Li et al. 2017). In another meta-analysis that examined anxiety, people with an anxiety disorder had shorter TL (r=0.06) (Malouff & Schutte 2017).
Psychotic disorders present a more complicated picture because once diagnosed, most people are immediately given atypical antipsychotic medications and these may have potent effects on telomere biology. The results of studies on schizophrenia are conflicting. There was a trend for shorter TL in a meta-analysis (Polho et al. 2015), but some studies found longer TL, and this is thought to be due to the effects of atypical medications (Cui et al. 2017, Monroy-Jaramillo et al. 2017). Indeed, one study showed that lithium treatment was associated with longer telomeres (Martinsson et al. 2013). A small study that compared medication-naive people who had schizophrenia with those who were medicated found shorter TL in those who were medication naive (Fernandez-Egea et al. 2009), and, similarly, shorter TL was found in a study of nonmedicated patients who were at very high risk for psychosis (Maurya et al. 2017).
It may be that telomere shortness precedes schizophrenia, and any lengthening could be due to atypical antipsychotics. Notably, people at risk of schizophrenia have a higher rate of early trauma and dysregulated stress responses (Mayo et al. 2017), both of which are thought to be common dimensional risk factors for shorter TL. There also may be effects of sex and illness severity (Wolkowitz et al. 2017).
The largest meta-analysis of psychiatric disorders examined depression (unipolar and bipolar separately), anxiety, and psychotic disorders among 14,827 participants from 32 studies (Darrow et al. 2016). This meta-analysis compared cases and controls and found significantly shorter TL in cases for all disorders examined, with a nonsignificant, larger effect among those with depression and anxiety disorders (including PTSD).
Shalev et al. (2014) studied 1,037 men and women and found that depression, anxiety, and PTSD correlated with and predicted accelerated telomere attrition over 12 years in men but not women. The largest depression study was carried out by Cai et al. (2015), who examined approximately 10,000 Chinese women. In this study, measures of early childhood adversity were associated with salivary TL. However, this relationship was fully accounted for by a later incidence of depression. In this same Chinese sample, the earlier onset of depression and dysthymia (which has a chronic nature) was associated with shorter TL, suggesting a dose–response relationship throughout life, as found in other studies (Verhoeven et al. 2014). One might interpret the study by Cai et al. (2015) as showing that severe adversity puts people at greater risk of depression, and if they develop depression, they are then likely to show telomere shortening later in life. However, it is also possible that short TL preceded the onset of depression.
In summary, there are robust associations between psychiatric disorders, primarily depression and anxiety, and shorter TL. Longitudinal studies suggest either stable relationships over time or, in some cases, a worsening with depression or anxiety. There is considerable diversity among studies, suggesting the presence of moderators or individual differences in the extent, duration, and characteristics of the illnesses, as well as important confounds.
CAUSAL ASSOCIATIONS BETWEEN TELOMERE LENGTH BIOLOGY AND PSYCHIATRIC DISORDERS
The bulk of the existing literature about humans provides strong evidence of a cross-sectional link between psychiatric disorders and TL, with a growing number of studies also demonstrating prospectively that psychiatric disorders predict telomere shortening over time. However, less is known about whether short telomeres may directly contribute to the development of psychiatric disorders. There are two lines of inquiry that shed some light on this possibility. The first focuses on animal and some human research investigating telomeres and the telomerase maintenance system in the brain and the response to depression and the drugs used to treat psychiatric disorders. The second line of inquiry focuses on telomere genetics, which provides a unique opportunity to test causality in predicting risk for psychiatric disorders.
Neurogenesis, Cell Aging, and Depression
There is a growing body of research on telomerase in the brain. Telomerase appears to be involved in the development of stress-induced depression, as well as in neuroprotection from more acute insults and injuries, through its role in preventing oxidative stress, protecting telomeres, and promoting neurogenesis. Basic research has shown that telomeres have a role in regulating neuronal life, from neural stem cell proliferation to senescence and apoptosis (Mattson et al. 2008).
Telomerase consists of a protein, TERT, that helps to add back de novo base pairs during cell replication and also after oxidative damage. Telomerase and TERT are pleiotropic, meaning that they have many roles other than protecting telomeres, such as neuroprotection in models of brain damage and injury. TERT reduces oxidative stress, protects mitochondria in neurons, and prevents apoptosis (González-Giraldo et al. 2016).
Accelerated brain aging is postulated to occur in many psychiatric disorders. For example, patients with diagnosed anxiety disorders have been found to have less gray and white matter and poorer cognitive function compared with controls (Perna et al. 2016). Impaired telomeres and telomerase may impact brain neurogenesis through early replicative senescence or through inducing higher oxidative stress, as suggested by animal studies (see the section titled Animal Studies: Stress-Induced Depression and Telomere Biology). The hippocampus is one of the most critical tissues to examine since it is implicated in depression and dementia and has higher levels of neurogenesis. Numerous studies support the idea that across psychiatric disorders, including anxiety and depression, the hippocampus has smaller volume in those with psychiatric disorders than in controls. Lower hippocampal volume is associated with shorter TL in peripheral blood in some, but not all, studies (Nilsonne et al. 2015). The mechanism for this relationship is unknown, but it could involve the migration of monocytes to microglia. It may develop early in life due to common causes, such as early adversity. In a sample of clinically depressed participants, higher telomerase activity in immune cells was associated with higher hippocampal volume in depression, especially in participants who experienced early adversity, potentially acting as a protective mechanism against the neurotoxicity of depression (S.H. Chen et al. 2014, Wolkowitz et al. 2015). The enzyme activity of telomerase, levels of TERT, and TL have been examined directly in the brain in postmortem studies: Compared with controls, TERT levels are lower in the brain (in white matter oligodendrocytes but not astrocytes) of people with depression, and TL is shorter in the hippocampus (Mamdani et al. 2015, Szebeni et al. 2014).
Bersani, Wolkowitz, and colleagues (Bersani et al. 2015) have detailed the preclinical data pointing to the molecular mechanisms through which telomerase may be involved in the development and treatment of psychiatric disorders. Various psychopharmacological treatments impact serotonin modulation as well as increase brain-derived neurofactor (BDNF) and β-catenin, and reduce oxidative stress. These can alter two molecular pathways (the PI3K/Akt pathway and the Wnt/β-catenin pathway), both of which can increase TERT expression and telomerase activity (Bersani et al. 2015). In turn, higher telomerase activity can create telomere elongation, bolster mitochondrial health, and induce growth factors that promote neurogenesis, and, possibly, improve mental well-being.
Animal Studies: Stress-Induced Depression and Telomere Biology
Animal models provide an opportunity to test experimentally the effects of stress on the development of depression and on telomere biology. There have been four critical studies in this area. Cai et al. (2015) examined the effects of 4 weeks of unpredictable stress in mice. The mice developed increases in mitochondrial DNA copy number and shorter TL in liver, but there were no changes in the hippocampus. TL reverted to normal levels a month after the stressful period (Cai et al. 2015). Based on their pattern of findings in humans and mice, they conjectured that short-term adversity causes a transient, reversible profile of damage, whereas depression can create an irreversible pattern of cellular damage (in this case, higher mitochondrial DNA copy number and shorter TL). Surprisingly, despite the large number of studies of psychiatric disorders and TL in humans, we identified only a handful of animal studies that examined changes in TL in the brain. Thus, we still have a limited understanding of mechanisms.
A chronic mild stress (CMS) paradigm has been used in two studies to examine the relationship among stress, cell aging, and depression. Zhou et al. (2011) showed that TERT and telomerase in the hippocampus appear to be critically involved in depressive-like behavior in mice exposed to CMS. By either decreasing or increasing telomerase levels they were able to, respectively, induce or prevent the stress-induced depression phenotype and to show that these effects may be mediated by neurogenesis in the dentate gyrus (Zhou et al. 2011). They also showed that TERT-null mice have depression and aggression, which can be reversed with reactivation of TERT (Zhou et al. 2016).
In another rodent study, Xie and colleagues (2017) modeled CMS-induced depression for 4 weeks in rats. They found signs of liver damage, decreases in TERT messenger RNA and telomerase activity, and shorter TL in liver cells, but no change in the hippocampus. It is not clear why they did not find brain changes, but this was in a different type of rodent exposed to a different duration of stress than in Zhou et al.’s (2016) study. Beery et al. (2012) carried out 3 months of mild, unpredictable stress using a paradigm that does not induce a full depression phenotype and found significant elevations in immune cell telomerase levels. Further, telomerase levels increased in a dose–response fashion with increasing signs of anxiety. It is possible this reflected a healthy defensive response that might not have lasted or been robust had Beery and colleagues used a more severe stressor leading to depression.
Last, Wei et al. (2015) examined a rat model of depression, comparing the Flinders Sensitive Line rat with the Flinders Resistant Line rat. They found that the Sensitive rat had lower levels of BDNF, TERT, telomerase activity, and shorter telomeres in the hippocampus. They then treated the Flinders Sensitive Line rats with lithium and found increased β-catenin, TERT, and telomerase, but no changes in TL in the hippocampus.
In sum, certain brain changes in anxiety and depression may mimic accelerated aging. The biochemical changes in the periphery and brain (such as low BDNF, high oxidative stress, and inflammation) can impair TL in both the body and brain. However, if there is a direct role for telomerase and the telomere maintenance system in the pathogenesis of psychiatric disorders, one would expect to see a greater prevalence of psychiatric disorders in people with genetic disorders of telomerase or telomeres, or in those with a normal genetic propensity for short telomeres. This is the area of our inquiry in the next section.
Telomere Genetics and Psychiatric Disorders
In this section we evaluate the studies of both mutations in telomere biology, resulting in rare genetic conditions, and of the common variance in telomere-related genes to see whether these conditions are associated with a greater incidence of psychiatric disorders.
Genetic conditions.
There has been only one study of psychiatric disorders among people with dyskeratosis congenita, a genetic condition in which there is a mutation in one of the several genes related to telomerase that leaves people with only 50% of the normal dose of telomerase. In a study of 14 patients, both children and adults, 64% had been diagnosed with a psychiatric disorder, primarily depression or anxiety (Rackley et al. 2012). This is much higher than the expected 38% prevalence in a medically ill sample. As mentioned above, mice without telomerase show notable depressive and aggressive behaviors (Zhou et al. 2016).
Common gene variance in telomere-related genes.
Cross-sectional studies of TL and psychiatric disorders suffer from reverse causation bias. In contrast, Mendelian randomization studies, in which TL is partly determined at birth from genetic variants, can avoid the issue of reverse causation and allow the ability to conduct studies to test causal associations between genotypes and phenotypes and diseases of interest. This type of study has been done to show that variation in telomere genetics predicts the risk for cardiovascular disease (Rode et al. 2015), Alzheimer’s dementia (Zhan et al. 2015), and some common cancers, with long telomeres leading to protection from cardiovascular disease but a predisposition to some rare cancers (Stone et al. 2016). There have also been comparisons of genetic and observed TL on cognitive function, with some causal association found, especially among apolipoprotein E4 (APOE4) carriers, such that they were more vulnerable to the effect of genetically short TL on general cognitive function (Hägg et al. 2017). Since telomere genetics can predict a brain health outcome such as cognitive function, it could logically predict psychiatric disorders as well.
Only two studies have examined genetic determinants of psychiatric disorders, mainly depression. The first study analyzed 67,306 white Danish participants aged 20–100 years using a telomere genetic index that included three single-nucleotide polymorphisms (SNPs) shown to have the largest effect on TL in a prior GWA study (Wium-Andersen et al. 2017). Consistent with the literature described above, shorter measured TL was associated with a diagnosis in medical records of depression; however, shorter TL did not prospectively predict the incidence of depression up to 7.6 years later. The total allele score for shorter TL was not related to depression or antidepressant use.
In a second study, Wei et al. (2016) selected cases with depression (436 with depression, 368 with bipolar depression) and 1,590 age-matched controls from a Swedish population-based study who had completed a self-reported Major Depression Inventory or, in the case of bipolar disorder, had been diagnosed by a psychiatrist. They limited the sample to those younger than 55 years old because some studies have found that the depression–TL relationship is stronger in younger adults. They examined salivary TL and the common TERT SNP rs2736100. In this study, they replicated the association between depression and observed TL. However, they also found that having the rs2736100 minor allele was associated with depression only among those without experience of childhood adversity. This group of people with depression, who had not been exposed to severe early childhood adversity, were approximately 50% more likely to have the SNP, suggesting a more biological than environmental cause of their depression. This is intriguing as it is what one would expect in a group without high exposures to stress.
The literature on the genetics of telomeres and psychiatric disorders is in its infancy. However, it is possible that telomere shortness predisposes to depression. The Mendelian randomization study method is the only way to determine the causal effect of telomere biology in humans. It is expected that as scientists uncover additional variants that account for TL, researchers will be better equipped to causally test the possibility that TL predicts psychiatric disorder risk.
INTEGRATION OF FINDINGS: TRIAD MODEL OF STRESS, TELOMERE ATTRITION, AND PSYCHIATRIC DISORDERS
We have reviewed the associations among stress, psychiatric disorders, and TL, with a focus on depression because this is the disorder that is most well studied. The strength of the evidence is evaluated in Figure 1. As shown, the relationships are complex, including feedforward causation, reverse causation, and mediation.
Is Stress a Determinant of Psychiatric Disorder and of Telomere Length Attrition?
The most consistent and well-established relationships from both longitudinal human studies and experimental animal studies show that greater exposure to major life stressors, especially early in life, leads to a higher rate of telomere attrition. There are different types of depression with different etiologies, but here we focus on stress-induced depression. There is already an established relationship between major life events and the onset of major depression, as well as bipolar disorder and schizophrenia (Lex et al. 2017, Monroe & Harkness 2005). In turn, depression is consistently related to shorter telomeres; however, findings are mixed in prospective studies, with only a few studies finding that depression leads to greater shortening over time (Figure 1). As discussed earlier, people with depression tend to have higher telomerase levels, and this could help explain the lack of strong prospective findings of further telomere shortening.
Is Depression a Determinant of Telomere Length Attrition?
While aspects of depression can lead to telomere shortening, it is likely that the greater telomere shortening in states of major depression is at least in part the result of cumulative exposure to chronic stress and thus allostatic dysregulation, with stress causing both attrition and depression. Common underlying processes related to stress and present during psychiatric disorders may lead to TL attrition, such as dysregulation or exaggerated stress responses (Steptoe et al. 2017).
Is Telomere Length Attrition a Determinant of Depression?
The link between shorter TL and prediction of depression prospectively is weak and has been rarely studied. One study found that genes for short telomeres (TERT) predicted greater incidence of unipolar and bipolar depression in people without early childhood adversity, suggesting a more neurologically based vulnerability (Wei et al. 2016). In addition, the rodent studies described above suggest that lower telomerase levels in the hippocampus may have a causal role in depression.
TL measured in adulthood reflects both genetics and the wear and tear of exposures and life experiences. A genetic measure of TL based on the findings of GWAS is a very different measure than actual observed TL. The genetic index is related to actual TL in adults, but accounts for only roughly 1% of the variance (Codd et al. 2013). For these reasons, TL as measured in adulthood would not necessarily predict the same outcomes as a genetic index for TL.
Just as with stress-shortened telomeres, genetically determined telomere shortness can also lead to allostatic dysregulation, such as greater insulin resistance and inflammation (Zhan et al. 2017). Thus, both stress-induced telomere shortening and genetically short telomeres may have roles in vulnerability to depression.
Impaired Allostasis: The Triadic Link Among Stress, Psychiatric Disorders, and Telomere Attrition
As shown in Figure 1, we postulate that stress and depression can lead to impairments in allostatic function (insulin resistance, poor mitochondria health, dysregulation of the neuroendocrine and autonomic stress responses) that may damage telomere health. These allostatic changes are also linked to telomere shortness, completing the triad of interconnections. While some cellular changes may matter more than others (e.g., inflammation as a primary driver), these processes are highly interrelated, and thus we discuss them as an allostatic system of interdependent regulatory processes that maintains cellular homeostasis.
Many well-known biochemical changes occur with chronic stress and with depression, such as increases in systemic inflammation (e.g., increases in interleukin 6 and C-reactive protein), greater insulin resistance, poor mitochondria health, and dysregulation in autonomic and neuroendocrine functioning in response to stress or in basal states (McEwen 2007, Wolkowitz et al. 2011b). In depression, there are greater levels of inflammation, oxidative and nitrosative stress, and lower levels of antioxidants (Black et al. 2015,Maurya et al. 2016). There is some evidence linking shorter telomeres with greater stress reactivity and chronic stress arousal [reviewed in the section titled The Negative Valence System (Threat Appraisal, Negative Affect, Threat Reactivity)].
Thus, chronic stress and depression can create different types of allostatic dysregulation, which in turn can impair TL. It has been established that senescent cells create a proinflammatory milieu, so it should not be surprising that short telomeres are associated with allostatic dysregulation in many studies. Révész et al. (2016b) found that the cross-sectional link between shorter TL and psychiatric diagnosis was mediated in part by inflammatory factors and metabolic health (e.g., waist circumference, lipids), which accounted for more than 30% of the variance in TL. In a sample of more than 65,000 individuals, the relationship of TL with depression was partly mediated by elevated levels of C-reactive protein (Wium-Andersen et al. 2017).
Few longitudinal studies have examined the directionality of TL and allostatic dysregulation. In a large twin study, baseline insulin resistance failed to predict the rate of telomere attrition, but rather TL at baseline predicted increases in insulin resistance (Verhulst et al. 2016). In sum, these diverse studies support the idea that stress reactivity or allostatic dysregulation is a mediating link between short TL and psychiatric disorders, but this relationship could be bidirectional.
INTERVENTION EFFECTS ON TELOMERE BIOLOGY
One method for determining whether telomere biology contributes to depressive mood is via intervention. Indeed it is possible, albeit unlikely, that interventions to directly lengthen telomeres would improve mood or psychiatric disorders as the TL–major depressive disorder link is a relationship that emerges over years and, if it exists, is a small part of a causal relationship, if it has any part at all. However, rodent studies suggest that hippocampal telomerase has an important mediating role in depression, and telomerase activators have been suggested as a treatment for psychiatric disorders based on hypothesized molecular mechanisms (Bersani et al. 2015).
However, considering the converse, it is more feasible that reducing stress or depression may ameliorate the ongoing toxic stress mediators, improve allostasis and, through this, improve or stabilize TL. A growing number of interventions targeting psychological stress and depression have examined the effects of stress reduction on cell aging. In the first demonstrations, a small pilot study found that intensive lifestyle modification, including stress reduction, led to increases in telomerase levels and telomere lengthening 5 years after the intervention for those adhering to the program (Ornish et al. 2008, 2013). Since these studies, several interventions to reduce psychological stress also appeared to increase telomerase levels or stabilize telomeres in most studies (Carlson et al. 2015, Lengacher et al. 2014; with early studies reviewed in Schutte & Malouff 2014), but not in all (Wang et al. 2017).
Unlike the studies addressing stress, few studies have examined the treatment of depression. In an initial cohort, Wolkowitz et al. (2012) found that people with depression had elevated telomerase levels at baseline compared with healthy matched controls. After 8 weeks of treatment with a selective serotonin reuptake inhibitor (SSRI), they found that depression remitted in a dose–response fashion, with telomerase levels becoming even more elevated. Those with the lowest telomerase levels at baseline and those with the greatest treatment-associated increase in telomerase showed the most benefit to mood (Wolkowitz et al. 2012). However, shorter TL at baseline predicted worse response to an SSRI (Hough et al. 2016). None of the treatment responders had very short telomeres, but the nonresponders did.
Studies targeting inflammation may be promising for treating major depressive disorder and comorbidity with short TL. In one study, Rasgon et al. (2016) used pioglitazone, a peroxisome proliferator-activated receptor (PPAR) agonist, and found that it improved depression for those with long telomeres, but those with short TL had worse treatment response, similar to the findings in the study of SSRIs. These small clinical studies should be replicated in larger trials, especially because TL is a relatively easy measure to add to a study, even retrospectively, with well-preserved, high-quality DNA.
There is a continued need to test intervention strategies to mitigate the deleterious effects of stress and depression on telomere attrition (Bersani et al. 2015, Lindqvist et al. 2015). In addition to addressing stress and depression directly, there are many other possible targets, such as improving social connections and boosting healthy lifestyle factors (e.g., encouraging exercise, and ensuring optimal nutrition with an anti-inflammatory diet and high omega-3 levels). Pharmacological strategies should also be considered, such as using PPAR agonists or supplements that help mitochondria and reduce oxidative stress (e.g., coenzyme Q), all of which need further study. That said, any pharmacological intervention should be considered as an adjuvant, and it will be ineffective if an individual is under high stress and not also using psychological or behavioral means to enhance stress resilience.
INTERGENERATIONAL TRANSMISSION OF TELOMERE LENGTH
TL has high genetic heritability of approximately 50% (Broer et al. 2013), but new research suggests that it is also directly transmitted from germ line telomeres (i.e., sperm and eggs). Parents with very short telomeres tend to have offspring with short telomeres, independent of genetic transmission. Proof of this concept comes from families in which parents have a genetic disorder for low telomerase levels and their children who are unaffected (i.e., did not inherit the mutation) nevertheless have significantly shorter TL (Aubert et al. 2012, Collopy et al. 2015). This opens up another pathway that could lead to the observation of shorter telomeres being associated with psychiatric disorders. The parent may have transmitted both the risk of a psychiatric disorder and direct inheritance of shorter TL from the germ line.
A second route to transmitting shorter TL is through prenatal stress. There are several small studies showing that maternal stress experienced during pregnancy predicts shorter TL in the offspring. The first study of this type was retrospective; Entringer and colleagues (2011) found that maternal reports of serious life events predicted shorter TL in young, healthy adult offspring who did not have any psychiatric disorders. They then showed this prospectively by measuring prenatal anxiety and using cord blood TL (Entringer et al. 2013). Marchetto et al. (2016) found that stressful life events in the year preceding pregnancy predicted shorter TL in cord blood.
The first large-scale study of maternal stress and offspring TL examined 318 newborn and mother dyads (Send et al. 2017). The study found that perceived stress during pregnancy, but not psychiatric conditions, was associated with short TL in the newborn but not in the mother. Rather, for the mothers, their lifetime history of psychiatric conditions was related to shorter TL. This makes logical sense in that their own history of exposure to the long-term stress of psychiatric disorders rather than short-term pregnancy stress would have a chance to impact their own telomeres. Together, these studies of prenatal stress provide converging evidence that pregnancy may serve as a critical period during which stress can be transmitted and imprinted on offspring TL These studies are also consistent with animal studies in birds showing that either prenatal stress or injections of cortisol promote shorter offspring TL (Haussmann & Heidinger 2015).
Where are the impacts of stress stored in the body? Most studies reviewed find that shorter TL acquired from lifetime adversity is observable in peripheral cells, immune cells (in blood or saliva), or buccal cells. It is possible that exposure to severe stress may impact progenitor and, possibly, stem cell reserves. Stem cells have special protections, but they are still vulnerable to external stresses, especially oxidative stress (Tower 2012). Theoretically, the germ line should be more protected, although it depends on the same single circulating blood source, which houses and transmits the biochemical effects of stress. Animal studies find grandmother effects in that manipulating nutrition affects the germ line TL of the second generation (granddaughters), leaving this as an open possibility in humans (Aiken et al. 2015). This is a speculative and an important area for basic research.
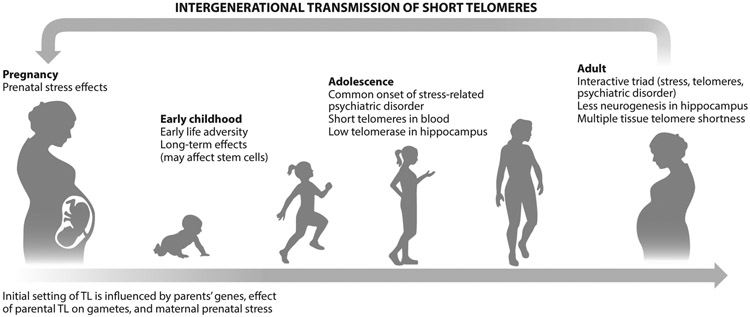
In summary, as shown in Figure 2, there are different mechanisms through which TL is determined at different developmental stages. During pregnancy, TL is determined by genetics, by direct epigenetic-like transmission from the germ line, and by the prenatal sociodemographic factors of environment and nutrition (Whiteman et al. 2017), as well as by stress exposures. Hematopoietic stem cells are determined during the first few months of development (Entringer et al. 2012). There are relations between cord blood TL and maternal prenatal stress exposures and responses. Stress-induced telomere attrition has been observed during childhood as well as with retrospective measures during adulthood, and it can leave a lifelong imprint, but this relationship is likely to be highly impacted by individual differences. For example, genetic sensitivity to stress may interact with stress exposure to impact TL, as suggested by one study (Mitchell et al. 2014). Early adversity may shape neural and peripheral stress response patterns, which can further influence TL throughout life. During adolescence, a preponderance of psychiatric disorders may arise, resulting from both inherited vulnerabilities and stress exposures. At adolescence, shorter TL may preexist the onset of psychiatric disorders (Gotlib et al. 2015). During adulthood, more cells reach critically short lengths and become senescent, contributing to systemic inflammation and thus further reinforcing the risk of psychiatric disorders. Poor bodily health contributes to allostatic load, which may further exacerbate TL and psychiatric disorders. Last, there is positive feedback across generations in that either prenatal stress shapes offspring TL or, possibly, short parental germ line TL is directly transmitted to offspring.
Figure 2.
A model of life span relations among stress exposure, telomeres, and psychiatric disorders. Stress has effects throughout life. It has imprinting effects during pregnancy, leading to shorter telomere length (TL) in cord blood. In addition to genetic transmission, parents also transmit directly to their offspring their own acquired TL in the germ line. After birth, TL is further shortened by early adversity, which can have lifelong effects, possibly by impacting stem cell reserves. Early signs of dysregulated mood and high-risk temperaments in childhood are already related to shorter TL at that time. These early exposures put children at risk for psychopathology emerging in adolescence. Hippocampal volume appears to be associated with telomerase activity or TL. Recurrent mood disorders can further impact TL, likely through stress-related pathways. This can be a lifelong, iterative, positive-feedback loop in which greater stress vulnerability and mood disorders impact TL. These interrelations, leading to a vicious cycle, can help explain the greater comorbidity with physical disease later in life.
FUTURE DIRECTIONS AND METHODOLOGICAL ISSUES
Innovations continue to accrue in our understanding of telomere biology and the psychosocial processes that may contribute to our biological aging. One of the most recent advances in the area of psychiatric disorders has been the shift from a focus on the categorization of mental health disorders to the use of common behavioral and biological processes that may help uncover common underlying etiological factors and mechanisms that cut across phenomenological diagnoses (i.e., transdiagnostic mechanisms). This RDoC framework may yield novel insights into how psychiatric disorders affect telomere biology. In the following sections we provide a selective review of areas in which we find that the RDoC constructs associate with TL. Admittedly, a great deal of research needs to be conducted to evaluate whether such a framework adds value to the discussion of psychiatric disorders, stress, and TL.
Transdiagnostic Mechanisms
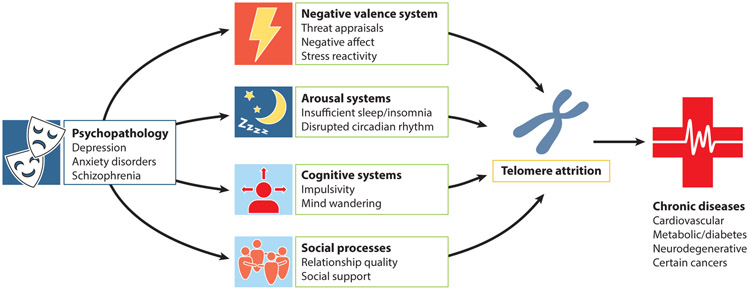
As displayed in Figure 3, there are several constructs within established RDoC that are associated with TL. The common environmental stress exposures that exaggerate these individual differences have already been discussed above, that is, early and lifetime exposure to traumatic or chronic adversity. These are categorized as measures of sustained threat in the RDoC (Natl. Inst. Ment. Health 2016). Other RDoC domains with measures that have been examined in relation to TL are summarized below. While it is easy to assume that impairments in basic function related to stress and stress resilience came before telomere shortening, the causal direction of these is not known.
Figure 3.
A model of Research Domain Criteria (RDoC) pathways linking psychopathology with telomere attrition. A move by mental health researchers to adopt the RDoC framework, which focuses on transdiagnostic processes present across psychiatric disorders, provides a new way to think about how psychopathology confers a biological influence on accelerated cellular aging, which in turn results in elevated rates of chronic disease. Some of the RDoC domains have been examined with respect to telomere attrition, and this is reflected in the figure. Additional research is needed to test many of the proposed constructs within each domain to determine which of these common pathways may account for the telomere attrition observed across multiple psychiatric illnesses.
The Negative Valence System (Threat Appraisal, Negative Affect, Threat Reactivity)
Exaggerated threat appraisals can be measured by the startle response, reaction time, or face-valid self-reported measures of threat versus challenge in anticipation of an acute stressor. In two studies using a standardized stressor, greater threat, or the threat-to-challenge ratio of appraisals, was associated with shorter TL (Epel et al. 2009, O’Donovan et al. 2012). Trait-like psychological constructs under the umbrella of negative affect have also been associated with TL. In this regard, hostility and pessimism (Ikeda et al. 2014, O’Donovan et al. 2009) have been linked with shorter TL, whereas optimism was associated with longer TL (Schutte et al. 2016). In a study of 3,432 adults using the Eysenck Personality Questionnaire-Revised, neuroticism predicted telomere attrition during 6 years (van Ockenburg et al. 2014). That said, there were inconsistent or null results in three larger studies using measures of neuroticism (Sadahiro et al. 2015, Savolainen et al. 2015b, Starnino et al. 2016).
Several studies have also used laboratory stressors to test associations between physiological stress reactivity and TL. In a sample of healthy premenopausal women, lower telomerase levels were related to greater vagal withdrawal to stress and a greater number of risk factors for cardiovascular disease (Epel et al. 2006). In addition, higher urinary cortisol and catecholamine levels were related to shorter TL. In a second small study of 23 healthy postmenopausal women, we observed that shorter TL was associated with a pattern of impaired allostasis, characterized by greater cortisol responses to stress, higher nocturnal urinary cortisol, and a flatter diurnal rhythm (Tomiyama et al. 2012).
Some studies have found a relationship between basal levels of autonomic arousal and TL. In the Netherlands Study of Depression and Anxiety, greater sympathetic activity, as indexed by the cardiac preejection period, was associated with shorter telomeres at baseline, although it did not predict attrition over time (Révész et al. 2016b).
In one of the largest studies of stress reactivity, Steptoe and colleagues (2017) examined the acute cortisol reactivity responses to a lab stressor in 411 men and women from the Whitehall II study. Cortisol reactors tended to have slightly shorter TL at baseline. Controlling for this baseline difference, the high reactors showed greater TL attrition during the next 3 years. This is remarkable given that baseline TL was strongly negatively related to subsequent change (as it tends to be in most studies), so this group should have otherwise remained more stable over time if not for their tendency for greater stress reactivity. In the same cohort, men with what we consider a profile of high adversity—high telomerase levels and short telomeres—were characterized by an impaired stress response: lower total cortisol, poor recovery of autonomic arousal, and impoverished psychosocial resources. They may have had a more progressed state of burnout from chronic stress (Zalli et al. 2014). Acute cortisol reactivity was also examined in the nearly 2,000 women and men in the Helsinki Birth Cohort Study, but stress-related changes in cortisol were not related to TL (Savolainen et al. 2015a).
In the first study of stress and TL in children, kindergartners with greater cortisol reactivity and sympathetic activity had shorter buccal cell TL (Kroenke et al. 2011). Gotlib et al. 2015 studied 97 healthy girls, half of whom were daughters of mothers with depression. Greater cortisol reactivity to the Trier Social Stress Test was associated with shorter leukocyte TL, and furthermore, this relationship was stronger in the girls at risk for depression. It may be that in addition to inherited risk, a greater tendency toward stress reactivity promotes both shorter telomeres and risk for later depression (Gotlib et al. 2015).
Arousal and regulatory systems (sleep).
Sleep disturbances are common to a range of psychiatric disorders, and there is some understanding that sleep disturbance can contribute to the risk of psychiatric disorders rather than serve only as a concurrent symptom. Several studies support associations between poor sleep and telomere attrition. For example, self-reported sleep quality, insomnia, shorter sleep duration, and sleep apnea are associated with shorter TL (Carroll et al. 2016, James et al. 2017, Prather et al. 2015). Sleep apnea in women is associated with shorter offspring TL at birth (Salihu et al. 2015). In humans and mice, telomerase activity in cells appears to have a circadian rhythm, and disruption of this rhythm in mice leads to shortened telomeres (W-D. Chen et al. 2014).
Cognitive systems (impulsivity).
The cognitive systems domain includes a number of cognitive processes that have not been examined with respect to telomere biology (e.g., declarative memory, language), largely because it is not clear how or why they would be linked. However, processes related to cognitive control, such as impulsivity, have been examined. In this regard, measures of impulsivity, delay discounting, and mind wandering have all been related to shorter TL. In a sample of 1,158 Chinese undergraduates, greater delay discounting was related to shorter TL, especially in women (Yim et al. 2016). In various clinical samples, indices of impulsivity, such as delay discounting (Kang et al. 2017) and hyperactive-type attention-deficit/hyperactivity disorder (Costa et al. 2015), have been related to shorter TL.
Social processes (attachment and relationships).
No studies we are aware of have examined basic measures of social affiliation or the perception of others in relation to TL. However, there have been associations between shorter TL and self-reported lower social support in the elderly (Carroll et al. 2013) and poorer-quality relationships in men (Liu et al. 2017). Being married is associated with longer TL (Yen & Lung 2013). The correlation between TL in couples is stronger in older couples (Broer et al. 2013). The reason for this age effect is unknown, but it is possible that the more decades spent together reflect a longer duration of shared influences on TL. Last, greater parental responsiveness is associated with longer TL in high-risk children (Asok et al. 2013).
Measurement Issues to Consider for Moving the Field Forward
Here, we discuss some of the common questions and methodological issues that arise around the stress–telomere–depression triad. However, there are many additional methodological issues in telomere research that have been addressed elsewhere (Barrett et al. 2015, Verhoeven et al. 2016).
As with many biomarkers, the assay and laboratory used have major impacts on the results. There have been many comparisons of different methods of measurement, and their well-documented differences, and the trade-off of accuracy versus cost. The Southern blot is considered the gold standard, but it is much more expensive than polymerase chain reaction (PCR). PCR is used in most population-based studies due to its lower cost and the need for only small amounts of blood, although the coefficient of variation is higher than with the Southern blot. PCR values have been associated with Southern blot values in an unbiased comparison, with a correlation of more than r=0.80 (Aviv et al. 2011). The most important issue to attend to is the coefficient of variability in a TL measure from PCR, as this can vary widely from lab to lab and should not be above 10%, but the lower, the better. The coefficient of variability will directly limit how small of an effect size is detectable. In the University of California, San Francisco’s Blackburn lab, one of the major labs that has produced TL values for several large studies, the coefficient of variability has improved over time, and it is now 3–4% (e.g., Lin et al 2016). It is important to rely on replication studies, and with telomere studies using PCR, there are now many replication studies and meta-analyses of the associations of TL with stress, psychiatric disorders, and diseases of aging, suggesting reliability in these PCR-based findings. However, with small clinical samples, one should consider using a method with higher accuracy, such as the Southern blot. The fluorescence imaging methods are more accurate for very short telomeres, but they rely on fresh cells rather than DNA and the high cost makes them less feasible for large, population-based studies.
Preclinical collection and DNA extraction methods are also important sources of error, a common topic in the literature (e.g., Raschenberger et al. 2016). Whole-genome sequencing methods are now being used (Lee et al. 2017), and they may become the measurement of choice when the cost decreases and the method is better validated for accuracy.
Most studies use whole blood to examine leukocyte TL. Buccal cells are related to blood measures (r=0.39, p < 0.01) even though they are different cell types (epithelial cells versus immune cells) (Finnicum et al. 2017). An important addition to future studies will be to include both types of samples when possible. Immune cell TL reflects the aging of the immune system, a major pathway for bodily aging. However, blood has the issue of containing mixed cell types that can vary over time, leading to pseudolengthening and other challenges to interpreting results (Epel 2012). Buccal cells, which can be collected noninvasively, offer a single cell type in which there are no cell redistribution effects. Although the significance of aged epithelial cells is questionable for diseases of aging, they appear to be informative barometric markers in studies of stress, nutrition, and maternal influences on infants.
The new ability to use a genetic index based on GWAS offers a measure of the pure biological effect of longer telomeres and is a great methodological advantage that should be used whenever possible. Indeed, in one of the few studies that used both genetic and observed TL, the authors noted that TL was associated with all of the potential confounders, whereas the allele score was not (Wium-Andersen et al. 2017). Using the genetic index should be helpful, but inferences about whether causal influences exist should be considered tentative, leaving room for type II errors because current, genetically based methods for determining TL account for only approximately 1% of actual TL.
Confounds and Moderators
Age is a necessary covariate to take into account, and it is rarely if ever meaningful to examine TL outcomes without simultaneously accounting for age, which is the biggest determinant of TL. Meta-analyses are powerful ways to detect confounds of the psychological factor–telomere relationship. Ridout and colleagues (2017) conducted the large meta-analysis described above that examined childhood adversity and TL, which included an examination of moderators that could confound the relationship between stress and TL. Indeed, they found that when participants were healthy, there was a stronger relationship but the relationship was significantly weaker in participants who had a medical condition or psychiatric disorder, or were taking medications or smoking. People with these conditions or behaviors should be excluded or the factor should be well quantified and controlled for statistically.
There may be small but important sex differences in the psychopathology–TL relationship (Shalev et al. 2014, Whisman & Richardson 2017) or the relationship may get stronger with age. The effects of both sex and age may be too small or too hard to detect in meta-analyses, which are often based on small clinical studies that usually include a limited range of these demographic variables.
Relying on large studies with less accurate measurements can also be misleading in this field in which TL effect sizes are small and many assays have coefficients of variation greater than 5%. The measurement of depression in large studies is also a source of error. Antidepressant use is often used as the indicator for depression in population-based studies; however, this is highly problematic because antidepressants may actually protect or lengthen telomeres, as several studies suggest (Kim et al. 2017, Wolkowitz et al. 2011a). As described earlier, lithium or antipsychotic medications may also have a lengthening effect.
In this review we did not discuss the role of unhealthy behaviors (e.g., poor nutrition, insufficient sleep, sedentariness), which are clearly other pathways through which stress and depression shape the biochemical milieu and telomere biology. These behaviors must be well measured or controlled for during studies, and they could present opportunities for fruitful adjuvant interventions for the triad of stress, psychiatric disorders, and telomere biology.
CONCLUSIONS
How could such a fundamental part of cell biology serve as a consequence and possibly a cause of psychiatric disorders? Emerging literature shows that telomeres have a role in bodily and brain aging. There is a vicious cycle between stress mediators that cause tissue aging—oxidative stress, inflammation, mitochondria—and telomere shortening. These mediators impact telomeres in both brain and body and, possibly, even in bone marrow and the more protected stem cell niches. Severe and chronic stress can lead to both telomere shortening and depression, contributing to their observed relationships. Conversely, there are also bidirectional relationships between depression and impaired telomere biology in the body and brain, suggesting the relationship is not simply due to stress exposure (Figure 1). Further, the timing of stress exposure also determines effects; pregnancy and early childhood are critical periods when toxic stress can create what has been called biological embedding of a lifelong risk for telomere shortness and psychiatric disorders. Measures aimed at preventing this collusion of interactive forces must focus on early life, the developmental period when risks become embedded. However, after this period, the management of stress and psychiatric disorders should also aim at ameliorating or slowing accelerated cell aging, preventing the common comorbidity of mental illness and chronic diseases.
Research into the triad of stress, psychiatric disorders, and TL is strong in descriptive relations and weaker in uncovering mechanisms, which are likely complex and intergenerational. Including TL measurement in both pregnancy and life span studies will help determine the significance of TL for lifelong mental and physical health. Further study of the common processes that lead to psychiatric illness and how these may impair telomere biology, or vice versa, will also be illuminating. A better understanding of the role of telomerase and telomeres in brain health and aging, and in resilience to stress, should make important contributions to prevention and interventions.
SUMMARY POINTS.
Telomere length is shortened after exposure to serious or traumatic stressful events. This appears to be especially true during the critical periods of development, such as childhood and, potentially, in utero. Biological stress mechanisms—including inflammation, insulin resistance, oxidative stress, and autonomic and neuroendocrine stress reactivity—appear to mediate these relationships, likely in part through dampening telomerase activity.
Psychopathology across diagnostic categories is consistently associated with shorter telomere length. This relationship is robust in cross-sectional studies, particularly in affective disorders. There is some inconsistent prospective evidence that experiencing a psychiatric disorder predicts telomere shortening. There is novel evidence from one genetic case–control study that genes for short telomere length predispose to depression (Wei et al. 2016). Whether telomere length has a causative role in other psychiatric disorders requires further study.
Stress exposure accounts for at least part of the relationship between psychopathology and shorter telomere length. Chronic and traumatic stress exposures are common precursors to telomere shortening and to psychiatric conditions. The telomere shortening observed in those with psychopathology may be due to earlier or concurrent chronic, stress-induced impairments in biochemical processes (i.e., dysregulated allostasis).
FUTURE ISSUES.
More research is needed that tracks cell aging through the life course and tracks intergenerational transmission. In animal models and humans, offspring exposed to greater maternal stress during pregnancy have shorter telomere length. More systematic research tracking high-risk families and next generations is necessary to understand the true causal relationships among the triad of stress, depression, and cell aging.
The use of the Research Domain Criteria (RDoC) framework may inform the link between psychiatric disorders and telomere length. Several RDoC domains that serve as transdiagnostic pathways across psychiatric conditions are also associated with shorter telomere length. Systematic investigation of the proposed RDoC constructs and telomere length may help explain heterogeneity and illuminate critical pathways linking psychiatric conditions and telomere attrition.
Enriched methods, such as the simultaneous measurement of observed telomere length and the genetic index of telomere length, will improve our ability to infer the direct effects of telomere length on outcomes. Measuring telomere length in blood along with immune cell type distribution, in tandem with a noncirculating cell type (e.g., buccal cells), will allow us to determine predictors of telomere length while accounting for cell redistribution effects. The use of more accurate or new methods for telomere assessment is needed when accuracy is most critical, as with small clinical samples. Animal and translational studies are critical to help determine mechanisms and interventions to impact mechanisms.
Telomeres: The protective caps at the ends of chromosomes made up of DNA; longer telomere length indicates better protection of the genes and lower probability that cells will become aged in the near future
Telomerase: The intracellular enzyme that protects telomeres by lengthening them and reducing oxidative stress
TERT (or hTERT for human): The telomerase reverse transcriptase gene, a critical part of the telomerase enzyme, which makes lengthening possible by adding telomeric base pairs back to the ends of telomeres
Genomewide association studies (GWAS): assess genetic variations (typically, single-nucleotide polymorphisms) across the whole genome to determine associations with a trait, phenotype, or disease
Replicative senescence: an irreversible state when a cell can no longer replicate to create new cells; this leads to inflammation
ACKNOWLEDGMENTS
We gratefully acknowledge helpful comments from Dr. Jue Lin and Dr. Owen Wolkowitz, and we thank Samantha Schilf for help with the illustrations. We also acknowledge the support of the National Institute on Aging (grant R01 AG030424 to E.S.E., and grant R24AG048024 to E.S.E. and Wendy Mendes) and the National Heart, Lung, and Blood Institute (grant K08HL112961 to A.A.P.).
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
LITERATURE CITED
- Aiken CE, Tarry-Adkins JL, Ozanne SE. 2015. Transgenerational developmental programming of ovarian reserve. Sci. Rep 5:16175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alegria M, Jackson JS, Kessler RC, Takeuchi D. 2003. National Comorbidity Survey Replication (NCS-R), 2001–2003. ICPSR 00189, Inter-Univ. Consort. Political Soc. Res., Ann Arbor, MI: https://www.icpsr.umich.edu/icpsrweb/DSDR/studies/00189 [Google Scholar]
- Asok A, Bernard K, Roth TL, Rosen JB, Dozier M. 2013. Parental responsiveness moderates the association between early-life stress and reduced telomere length. Dev. Psychopathol 25:577–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert G, Baerlocher GM, Vulto I, Poon SS, Lansdorp PM. 2012. Collapse of telomere homeostasis in hematopoietic cells caused by heterozygous mutations in telomerase genes. PLOS Genet. 8:e1002696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aviv A, Hunt SC, Lin J, Cao X, Kimura M, Blackburn E. 2011. Impartial comparative analysis of measurement of leukocyte telomere length/DNA content by Southern blots and qPCR. Nucleic Acids Res. 39:e134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aydinonat D, Penn DJ, Smith S, Moodley Y, Hoelzl F, et al. 2014. Social isolation shortens telomeres in African Grey parrots (Psittacus erithacus erithacus). PLOS ONE 9:e93839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett JH, Iles MM, Dunning AM, Pooley KA. 2015. Telomere length and common disease: study design and analytical challenges. Hum. Genet 134:679–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beery AK, Lin J, Biddle JS, Francis DD, Blackburn EH, Epel ES. 2012. Chronic stress elevates telomerase activity in rats. Biol. Lett 8:1063–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bersani FS, Lindqvist D, Mellon SH, Penninx BWJH, Verhoeven JE, et al. 2015. Telomerase activation as a possible mechanism of action for psychopharmacological interventions. Drug Discov. Today 20:1305–9 [DOI] [PubMed] [Google Scholar]
- Bioque M, García-Portilla MAP, García-Rizo C, Cabrera B, Lobo A, et al. 2017. Evolution of metabolic risk factors over a two-year period in a cohort of first episodes of psychosis. Schizophr. Res In press. 10.1016/j.schres.2017.06.032 [DOI] [PubMed] [Google Scholar]
- Black CN, Bot M, Scheffer PG, Cuijpers P, Penninx BWJH. 2015. Is depression associated with increased oxidative stress? A systematic review and meta-analysis. Psychoneuroendocrinology 51:164–75 [DOI] [PubMed] [Google Scholar]
- Blackburn EH. 1991. Structure and function of telomeres. Nature 350:569–73 [DOI] [PubMed] [Google Scholar]
- Blackburn EH, Epel ES, Lin J. 2015. Human telomere biology: a contributory and interactive factor in aging, disease risks, and protection. Science 350:1193–98 [DOI] [PubMed] [Google Scholar]
- Broer L, Codd V, Nyholt DR, Deelen J, Mangino M, et al. 2013. Meta-analysis of telomere length in 19,713 subjects reveals high heritability, stronger maternal inheritance and a paternal age effect. Eur. J. Hum. Genet 21:1163–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai N, Chang S, Li Y, Li Q, Hu J, et al. 2015. Molecular signatures of major depression. Curr. Biol 25:1146–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campisi J, d’Adda di Fagagna F. 2007. Cellular senescence: when bad things happen to good cells. Nat. Rev. Mol. Cell Biol 8:729–40 [DOI] [PubMed] [Google Scholar]
- Carlson LE, Beattie TL, Giese-Davis J, Faris P, Tamagawa R, et al. 2015. Mindfulness-based cancer recovery and supportive-expressive therapy maintain telomere length relative to controls in distressed breast cancer survivors. Cancer 121:476–84 [DOI] [PubMed] [Google Scholar]
- Carroll JE, Diez Roux AV, Fitzpatrick AL, Seeman T. 2013. Low social support is associated with shorter leukocyte telomere length in late life: multi-ethnic study of atherosclerosis. Psychosom. Med 75:171–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll JE, Esquivel S, Goldberg A, Seeman TE, Effros RB, et al. 2016. Insomnia and telomere length in older adults. Sleep 39:559–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen SH, Epel ES, Mellon SH, Lin J, Reus VI, et al. 2014. Adverse childhood experiences and leukocyte telomere maintenance in depressed and healthy adults. J. Affect. Disord 169:86–90 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W-D, Wen M-S, Shie S-S, Lo Y-L, Wo H-T, et al. 2014. The circadian rhythm controls telomeres and telomerase activity. Biochem. Biophys. Res. Commun 451:408–14 [DOI] [PubMed] [Google Scholar]
- Codd V, Nelson CP, Albrecht E, Mangino M, Deelen J, et al. 2013. Identification of seven loci affecting mean telomere length and their association with disease. Nat. Genet 45:422–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coimbra BM, Carvalho CM, Moretti PN, Mello MF, Belangero SI. 2017. Stress-related telomere length in children: a systematic review. J. Psychiatr. Res 92:47–54 [DOI] [PubMed] [Google Scholar]
- Collopy LC, Walne AJ, Cardoso S, de la Fuente J, Mohamed M, et al. 2015. Triallelic and epigenetic-like inheritance in human disorders of telomerase. Blood 126:176–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa DS, Rosa DVF, Barros AGA, Romano-Silva MA, Malloy-Diniz LF, et al. 2015. Telomere length is highly inherited and associated with hyperactivity-impulsivity in children with attention deficit/hyperactivity disorder. Front. Mol. Neurosci 8:28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui Y, Prabhu VV, Nguyen TB, Devi SM, Chung Y-C. 2017. Longer telomere length of T lymphocytes in patients with early and chronic psychosis. Clin. Psychopharmacol. Neurosci 15:146–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darrow SM, Verhoeven JE, Révész D, Lindqvist D, Penninx BWJH, et al. 2016. The association between psychiatric disorders and telomere length: a meta-analysis involving 14,827 persons. Psychosom. Med 78:776–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deng W, Cheung ST, Tsao SW, Wang XM, Tiwari AFY. 2016. Telomerase activity and its association with psychological stress, mental disorders, lifestyle factors and interventions: a systematic review. Psychoneuroendocrinology 64:150–63 [DOI] [PubMed] [Google Scholar]
- D’Mello MJJ, Ross SA, Briel M, Anand SS, Gerstein H, Paré G. 2015. Association between shortened leukocyte telomere length and cardiometabolic outcomes: systematic review and meta-analysis. Circ. Cardiovasc. Genet 8:82–90 [DOI] [PubMed] [Google Scholar]
- Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE. 1996. Depression and risk for onset of type II diabetes: a prospective population-based study. Diabetes Care 19:1097–102 [DOI] [PubMed] [Google Scholar]
- Effros RB. 2009. Kleemeier Award Lecture 2008—The canary in the coal mine: telomeres and human healthspan. J. Gerontol. A 64:511–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Buss C, Wadhwa PD. 2012. Prenatal stress, telomere biology, and fetal programming of health and disease risk. Sci. Signal 5:pt 12. [DOI] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Kumsta R, Lin J, Hellhammer DH, et al. 2011. Stress exposure in intrauterine life is associated with shorter telomere length in young adulthood. PNAS 108:E513–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Entringer S, Epel ES, Lin J, Buss C, Shahbaba B, et al. 2013. Maternal psychosocial stress during pregnancy is associated with newborn leukocyte telomere length. Am. J. Obstet. Gynecol 208:134.e1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES. 2012. How “reversible” is telomeric aging? Cancer Prev. Res 5:1163–68 [DOI] [PubMed] [Google Scholar]
- Epel ES, Daubenmier J, Moskowitz JT, Folkman S, Blackburn E. 2009. Can meditation slow rate of cellular aging? Cognitive stress, mindfulness, and telomeres. Ann. N. Y. Acad. Sci 1172:34–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, Lin J, Wilhelm FH, Wolkowitz OM, Cawthon R, et al. 2006. Cell aging in relation to stress arousal and cardiovascular disease risk factors. Psychoneuroendocrinology 31:277–87 [DOI] [PubMed] [Google Scholar]
- Fernandez-Egea E, Bernardo M, Heaphy CM, Griffith JK, Parellada E, et al. 2009. Telomere length and pulse pressure in newly diagnosed, antipsychotic-naive patients with nonaffective psychosis. Schizophr. Bull 35:437–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnicum CT, Dolan CV, Willemsen G, Weber ZM, Petersen JL, et al. 2017. Relative telomere repeat mass in buccal and leukocyte-derived DNA. PLOS ONE 12:e0170765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Giraldo Y, Forero DA, Echeverria V, Gonzalez J, Ávila-Rodriguez M, et al. 2016. Neuroprotective effects of the catalytic subunit of telomerase: a potential therapeutic target in the central nervous system. Ageing Res. Rev 28:37–45 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, LeMoult J, Colich NL, Foland-Ross LC, Hallmayer J, et al. 2015. Telomere length and cortisol reactivity in children of depressed mothers. Mol. Psychiatry 20:615–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hägg S, Zhan Y, Karlsson R, Gerritsen L, Ploner A, et al. 2017. Short telomere length is associated with impaired cognitive performance in European ancestry cohorts. Transl. Psychiatry 7:e1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanssen LM, Schutte NS, Malouff JM, Epel ES. 2017. The relationship between childhood psychosocial stressor level and telomere length: a meta-analysis. Health Psychol. Res 5:6378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hau M, Haussmann MF, Greives TJ, Matlack C, Costantini D, et al. 2015. Repeated stressors in adulthood increase the rate of biological ageing. Front. Zool 12:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haussmann MF, Heidinger BJ. 2015. Telomere dynamics may link stress exposure and ageing across generations. Biol. Lett 11:20150396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hennekens CH, Hennekens AR, Hollar D, Casey DE. 2005. Schizophrenia and increased risks of cardiovascular disease. Am. Heart J 150:1115–21 [DOI] [PubMed] [Google Scholar]
- Hough CM, Bersani FS, Mellon SH, Epel ES, Reus VI, et al. 2016. Leukocyte telomere length predicts SSRI response in major depressive disorder: a preliminary report. Mol. Neuropsychiatry 2:88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda A, Schwartz J, Peters JL, Baccarelli AA, Hoxha M, et al. 2014. Pessimistic orientation in relation to telomere length in older men: the VA normative aging study. Psychoneuroendocrinology 42:68–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- James S, McLanahan S, Brooks-Gunn J, Mitchell C, Schneper L, et al. 2017. Sleep duration and telomere length in children. J. Pediatr 187:247–52 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurk D, Wilson C, Passos JF, Oakley F, Correia-Melo C, et al. 2014. Chronic inflammation induces telomere dysfunction and accelerates ageing in mice. Nat. Commun 2:4172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang JI, Hwang SS, Choi JR, Lee S-T, Kim J, et al. 2017. Telomere length in alcohol dependence: a role for impulsive choice and childhood maltreatment. Psychoneuroendocrinology 83:72–78 [DOI] [PubMed] [Google Scholar]
- Kessler RC, Berglund P, Chiu WT, Demler O, Heeringa S,et al. 2004. The US National Comorbidity Survey Replication (NCS-R): design and field procedures. Int. J. Methods Psychiatr. Res 13:69–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TY, Kim SJ, Choi JR, Lee S-T, Kim J, et al. 2017. The effect of trauma and PTSD on telomere length: an exploratory study in people exposed to combat trauma. Sci. Rep 7:4375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroenke CH, Epel E, Adler N, Bush NR, Obradovic J, et al. 2011. Autonomic and adrenocortical reactivity and buccal cell telomere length in kindergarten children. Psychosom. Med 73:533–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee M, Napier CE, Yang SF, Arthur JW, Reddel RR, Pickett HA. 2017. Comparative analysis of whole genome sequencing-based telomere length measurement techniques. Methods 114:4–15 [DOI] [PubMed] [Google Scholar]
- Lengacher CA, Reich RR, Kip KE, Barta M, Ramesar S, et al. 2014. Influence of mindfulness-based stress reduction (MBSR) on telomerase activity in women with breast cancer (BC). Biol. Res. Nurs 16:438–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lex C, Bäzner E, Meyer TD. 2017. Does stress play a significant role in bipolar disorder? A meta-analysis. J. Affect. Disord 208:298–308 [DOI] [PubMed] [Google Scholar]
- Li X, Wang J, Zhou J, Huang P, Li J. 2017. The association between post-traumatic stress disorder and shorter telomere length: a systematic review and meta-analysis. J. Affect. Disord 218:322–26 [DOI] [PubMed] [Google Scholar]
- Li Z, He Y, Wang D, Tang J, Chen X. 2017. Association between childhood trauma and accelerated telomere erosion in adulthood: a meta-analytic study. J. Psychiatr. Res 93:64–71 [DOI] [PubMed] [Google Scholar]
- Lin J, Cheon J, Brown R, Coccia M, Puterman E,et al. 2016. Systematic and cell type–specific telomere length changes in subsets of lymphocytes. J. Immunol. Res 2016:5371050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindqvist D, Epel ES, Mellon SH, Penninx BW, Révész D, et al. 2015. Psychiatric disorders and leukocyte telomere length: underlying mechanisms linking mental illness with cellular aging. Neurosci. Biobehav. Rev 55:333–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu JJ, Wei YB, Forsell Y, Lavebratt C. 2017. Stress, depressive status and telomere length: Does social interaction and coping strategy play a mediating role? J. Affect. Disord 222:138–45 [DOI] [PubMed] [Google Scholar]
- Malouff JM, Schutte NS. 2017. A meta-analysis of the relationship between anxiety and telomere length. Anxiety Stress Coping 30:264–72 [DOI] [PubMed] [Google Scholar]
- Mamdani F, Rollins B, Morgan L, Myers RM, Barchas JD, et al. 2015. Variable telomere length across postmortem human brain regions and specific reduction in the hippocampus of major depressive disorder. Transl. Psychiatry 5:e636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchetto NM, Glynn RA, Ferry ML, Ostojic M, Wolff SM, et al. 2016. Prenatal stress and newborn telomere length. Am. J. Obstet. Gynecol 215:94.e1–8 [DOI] [PubMed] [Google Scholar]
- Martinsson L, Wei Y, Xu D, Melas PA, Mathé AA, et al. 2013. Long-term lithium treatment in bipolar disorder is associated with longer leukocyte telomeres. Transl. Psychiatry 3:e261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP, Zhang P, Cheng A. 2008. Telomere neurobiology. Methods Mol. Biol 438:185–96 [DOI] [PubMed] [Google Scholar]
- Maurya PK, Noto C, Rizzo LB, Rios AC, Nunes SOV, et al. 2016. The role of oxidative and nitrosative stress in accelerated aging and major depressive disorder. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 65:134–44 [DOI] [PubMed] [Google Scholar]
- Maurya PK, Rizzo LB, Xavier G, Tempaku PF, Zeni-Graiff M, et al. 2017. Shorter leukocyte telomere length in patients at ultra high risk for psychosis. Eur. Neuropsychopharmacol 27:538–42 [DOI] [PubMed] [Google Scholar]
- Mayo D, Corey S, Kelly LH, Yohannes S, Youngquist AL, et al. 2017. The role of trauma and stressful life events among individuals at clinical high risk for psychosis: a review. Front. Psychiatry 8:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. 2009. Immunosenescence: What does it mean to health outcomes in older adults? Curr. Opin. Immunol 21:418–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS. 2004. Protection and damage from acute and chronic stress: allostasis and allostatic overload and relevance to the pathophysiology of psychiatric disorders. Ann. N. Y. Acad. Sci 1032:1–7 [DOI] [PubMed] [Google Scholar]
- McEwen BS. 2007. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev 87:873–904 [DOI] [PubMed] [Google Scholar]
- Mitchell C, Hobcraft J, McLanahan SS, Siegel SR, Berg A, et al. 2014. Social disadvantage, genetic sensitivity, and children’s telomere length. PNAS 111:5944–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monroe SM, Harkness KL. 2005. Life stress, the “kindling” hypothesis, and the recurrence of depression: considerations from a life stress perspective. Psychol. Rev 112:417–45 [DOI] [PubMed] [Google Scholar]
- Monroy-Jaramillo N, Rodríguez-Agudelo Y, Aviña-Cervantes LC, Roberts DL, Velligan DI, Walss-Bass C. 2017. Leukocyte telomere length in Hispanic schizophrenia patients under treatment with olanzapine. J. Psychiatr. Res 90:26–30 [DOI] [PubMed] [Google Scholar]
- Natl. Inst. Ment. Health. 2016. Behavioral Assessment Methods for RDoC Constructs: A Report by the National Advisory Mental Health Council Workgroup on Tasks and Measures for Research Domain Criteria (RDoC). Bethesda, MD: Natl. Inst. Ment. Health [Google Scholar]
- Nettle D, Monaghan P, Gillespie R, Brilot B, Bedford T, Bateson M. 2015. An experimental demonstration that early-life competitive disadvantage accelerates telomere loss. Proc. R. Soc. B 282:20141610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson A, Kuper H, Hemingway H. 2006. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146 538 participants in 54 observational studies. Eur. Heart J 27:2763–74 [DOI] [PubMed] [Google Scholar]
- Nilsonne G, Tamm S, Månsson KNT, Åkerstedt T, Lekander M. 2015. Leukocyte telomere length and hippocampus volume: a meta-analysis. F1000Res. 4:1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Epel E, Lin J, Wolkowitz O, Cohen B, et al. 2011. Childhood trauma associated with short leukocyte telomere length in posttraumatic stress disorder. Biol. Psychiatry 70:465–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Lin J, Tillie J, Dhabhar FS, Wolkowitz OM, et al. 2009. Pessimism correlates with leukocyte telomere shortness and elevated interleukin-6 in post-menopausal women. Brain Behav. Immun 23:446–49 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Donovan A, Tomiyama AJ, Lin J, Puterman E, Adler NE, et al. 2012. Stress appraisals and cellular aging: a key role for anticipatory threat in the relationship between psychological stress and telomere length. Brain Behav. Immun 26:573–79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira BS, Zunzunegui MV, Quinlan J, Fahmi H, Tu MT, Guerra RO. 2016. Systematic review of the association between chronic social stress and telomere length: a life course perspective. Ageing Res. Rev 26:37–52 [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Chan JM, Epel E, Kemp C,et al. 2013. Effect of comprehensive lifestyle changes on telomerase activity and telomere length in men with biopsy-proven low-risk prostate cancer: 5-year follow-up of a descriptive pilot study. Lancet Oncol. 14:1112–20 [DOI] [PubMed] [Google Scholar]
- Ornish D, Lin J, Daubenmier J, Weidner G, Epel E, et al. 2008. Increased telomerase activity and comprehensive lifestyle changes: a pilot study. Lancet Oncol. 9:1048–57 [DOI] [PubMed] [Google Scholar]
- Ownby RL, Crocco E, Acevedo A, John V, Loewenstein D. 2006. Depression and risk for Alzheimer disease: systematic review, meta-analysis, and metaregression analysis. Arch. Gen. Psychiatry 63:530–38 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M, Verhoeven JE, Cuijpers P, Reynolds CF III, Penninx BWJH. 2015. Where you live may make you old: the association between perceived poor neighborhood quality and leukocyte telomere length. PLOS ONE 10:e0128460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perna G, Iannone G, Alciati A, Caldirola D. 2016. Are anxiety disorders associated with accelerated aging? A focus on neuroprogression. Neural Plast. 2016:8457612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polho GB, De-Paula VJ, Cardillo G, dos Santos B, Kerr DS. 2015. Leukocyte telomere length in patients with schizophrenia: a meta-analysis. Schizophr. Res 165:195–200 [DOI] [PubMed] [Google Scholar]
- Prather AA, Gurfein B, Moran P, Daubenmier J, Acree M, et al. 2015. Tired telomeres: poor global sleep quality, perceived stress, and telomere length in immune cell subsets in obese men and women. Brain Behav. Immun 47:155–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Gemmill A, Karasek D, Weir D, Adler NE, et al. 2016. Lifespan adversity and later adulthood telomere length in the nationally representative US Health and Retirement Study. PNAS 113:E6335–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puterman E, Lin J, Krauss J, Blackburn EH, Epel ES. 2015. Determinants of telomere attrition over 1 year in healthy older women: Stress and health behaviors matter. Mol. Psychiatry 20:529–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rackley S, Pao M, Seratti GF, Giri N, Rasimas JJ, et al. 2012. Neuropsychiatric conditions among patients with dyskeratosis congenita: a link with telomere biology? Psychosomatics 53:230–35 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschenberger J, Lamina C, Haun M, Kollerits B, Coassin S, et al. 2016. Influence of DNA extraction methods on relative telomere length measurements and its impact on epidemiological studies. Sci. Rep 6:25398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasgon N, Lin KW, Lin J, Epel E, Blackburn E. 2016. Telomere length as a predictor of response to Pioglitazone in patients with unremitted depression: a preliminary study. Transl. Psychiatry 6:e709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Révész D, Milaneschi Y, Terpstra EM, Penninx BWJH. 2016a. Baseline biopsychosocial determinants of telomere length and 6-year attrition rate. Psychoneuroendocrinology 67:153–62 [DOI] [PubMed] [Google Scholar]
- Révész D, Verhoeven JE, Milaneschi Y, Penninx BWJH. 2016b. Depressive and anxiety disorders and short leukocyte telomere length: mediating effects of metabolic stress and lifestyle factors. Psychol. Med 46:2337–49 [DOI] [PubMed] [Google Scholar]
- Ridout KK, Levandowski M, Ridout SJ, Gantz L, Goonan K, et al. 2017. Early life adversity and telomere length: a meta-analysis. Mol. Psychiatry In press. 10.1038/mp.2017.26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridout KK, Ridout SJ, Price LH, Sen S, Tyrka AR. 2016. Depression and telomere length: a meta-analysis. J. Affect. Disord 191:237–47 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rode L, Nordestgaard BG, Bojesen SE. 2015. Peripheral blood leukocyte telomere length and mortality among 64 637 individuals from the general population. J. Natl. Cancer Inst 107:djv074. [DOI] [PubMed] [Google Scholar]
- Rugulies R 2002. Depression as a predictor for coronary heart disease. Am. J. Prev. Med 23:51–61 [DOI] [PubMed] [Google Scholar]
- Sadahiro R, Suzuki A, Enokido M, Matsumoto Y, Shibuya N, et al. 2015. Relationship between leukocyte telomere length and personality traits in healthy subjects. Eur. Psychiatry 30:291–95 [DOI] [PubMed] [Google Scholar]
- Sahin E, Colla S, Liesa M, Moslehi J, Müller FL, et al. 2011. Telomere dysfunction induces metabolic and mitochondrial compromise. Nature 470:359–65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salihu HM, King L, Patel P, Paothong A, Pradhan A, et al. 2015. Association between maternal symptoms of sleep disordered breathing and fetal telomere length. Sleep 38:559–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savolainen K, Eriksson JG, Kajantie E, Lahti J, Räikkönen K. 2015a. Telomere length and hypothalamic–pituitary–adrenal axis response to stress in elderly adults. Psychoneuroendocrinology 53:179–84 [DOI] [PubMed] [Google Scholar]
- Savolainen K, Eriksson JG, Kajantie E, Pesonen A-K, Räikkönen K. 2015b. Associations between the five-factor model of personality and leukocyte telomere length in elderly men and women: the Helsinki Birth Cohort Study (HBCS). J. Psychosom. Res 79:233–38 [DOI] [PubMed] [Google Scholar]
- Schneper LM, Brooks-Gunn J, Notterman DA, Suomi SJ. 2016. Early-life experiences and telomere length in adult rhesus monkeys: an exploratory study. Psychosom. Med 78:1066–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. 2014. A meta-analytic review of the effects of mindfulness meditation on telomerase activity. Psychoneuroendocrinology 42:45–48 [DOI] [PubMed] [Google Scholar]
- Schutte NS, Malouff JM. 2015. The association between depression and leukocyte telomere length: a meta-analysis. Depression Anxiety 32:229–38 [DOI] [PubMed] [Google Scholar]
- Schutte NS, Palanisamy SKA, McFarlane JR. 2016. The relationship between positive psychological characteristics and longer telomeres. Psychol. Health 31:1466–80 [DOI] [PubMed] [Google Scholar]
- Send TS, Gilles M, Codd V, Wolf I, Bardtke S,et al. 2017. Telomere length in newborns is related to maternal stress during pregnancy. Neuropsychopharmacology 42:2407–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Entringer S, Wadhwa PD, Wolkowitz OM, Puterman E, et al. 2013a. Stress and telomere biology: a lifespan perspective. Psychoneuroendocrinology 38:1835–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Braithwaite AW, Danese A, Fleming NI, et al. 2014. Internalizing disorders and leukocyte telomere erosion: a prospective study of depression, generalized anxiety disorder and post-traumatic stress disorder. Mol. Psychiatry 19:1163–70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalev I, Moffitt TE, Sugden K, Williams B, Houts RM, et al. 2013b. Exposure to violence during childhood is associated with telomere erosion from 5 to 10 years of age: a longitudinal study. Mol. Psychiatry 18:576–81 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Starnino L, Busque L, Tardif J-C, D’Antono B. 2016. Psychological profiles in the prediction of leukocyte telomere length in healthy individuals. PLOS ONE 11:e0165482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steptoe A, Hamer M, Lin J, Blackburn EH, Erusalimsky JD. 2017. The longitudinal relationship between cortisol responses to mental stress and leukocyte telomere attrition. J. Clin. Endocrinol. Metab 102:962–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone RC, Horvath K, Kark JD, Susser E, Tishkoff SA, Aviv A. 2016. Telomere length and the cancer–atherosclerosis trade-off. PLOS Genet. 12:e1006144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szebeni A, Szebeni K, DiPeri T, Chandley MJ, Crawford JD, et al. 2014. Shortened telomere length in white matter oligodendrocytes in major depression: potential role of oxidative stress. Int. J. Neuropsychopharmacol 17:1579–89 [DOI] [PubMed] [Google Scholar]
- Theall KP, Shirtcliff EA, Dismukes AR, Wallace M, Drury SS. 2017. Association between neighborhood violence and biological stress in children. JAMA Pediatr. 171:53–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomiyama AJ, O’Donovan A, Lin J, Puterman E, Lazaro A, et al. 2012. Does cellular aging relate to patterns of allostasis? An examination of basal and stress reactive HPA axis activity and telomere length. Physiol. Behav 106:40–45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tower J 2012. Stress and stem cells. Wiley Interdiscip. Rev. Dev. Biol 1:789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyrka AR, Price LH, Kao HT, Porton B, Marsella SA, Carpenter LL. 2010. Childhood maltreatment and telomere shortening: preliminary support for an effect of early stress on cellular aging. Biol. Psychiatry 67:531–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ockenburg SL, Bos EH, de Jonge P, van der Harst P, Gans ROB, Rosmalen JGM. 2015. Stressful life events and leukocyte telomere attrition in adulthood: a prospective population-based cohort study. Psychol. Med 45:2975–84 [DOI] [PubMed] [Google Scholar]
- van Ockenburg SL, de Jonge P, van der Harst P, Ormel J, Rosmalen JGM. 2014. Does neuroticism make you old? Prospective associations between neuroticism and leukocyte telomere length. Psychol. Med 44:723–29 [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Lin J, Révész D, Wolkowitz OM, Penninx BWJH. 2016. Unresolved issues in longitudinal telomere length research: response to Susser et al. Am. J. Psychiatry 173:1147–49 [DOI] [PubMed] [Google Scholar]
- Verhoeven JE, Révész D, Epel ES, Lin J, Wolkowitz OM, Penninx BWJH. 2014. Major depressive disorder and accelerated cellular aging: results from a large psychiatric cohort study. Mol. Psychiatry 19:895–901 [DOI] [PubMed] [Google Scholar]
- Verhulst S, Dalgård C, Labat C, Kark JD, Kimura M, et al. 2016. A short leucocyte telomere length is associated with development of insulin resistance. Diabetologia 59:1258–65 [DOI] [PubMed] [Google Scholar]
- Wang X, Sundquist K, Hedelius A, Palmér K, Memon AA, Sundquist J. 2017. Leukocyte telomere length and depression, anxiety and stress and adjustment disorders in primary health care patients. BMC Psychiatry 17:148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YB, Backlund L, Wegener G, Mathé AA, Lavebratt C. 2015. Telomerase dysregulation in the hippocampus of a rat model of depression: normalization by lithium. Int. J. Neuropsychopharmacol 18:pyv002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei YB, Martinsson L, Liu JJ, Forsell Y, Schalling M, et al. 2016. hTERT genetic variation in depression. J. Affect. Disord 189:62–69 [DOI] [PubMed] [Google Scholar]
- Whisman MA, Richardson ED. 2017. Depressive symptoms and salivary telomere length in a probability sample of middle-aged and older adults. Psychosom. Med 79:234–42 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whiteman VE, Goswami A, Salihu HM. 2017. Telomere length and fetal programming: a review of recent scientific advances. Am. J. Reprod. Immunol 77:e12661. [DOI] [PubMed] [Google Scholar]
- Wium-Andersen MK, Ørsted DD, Rode L, Bojesen SE, Nordestgaard BG. 2017. Telomere length and depression: prospective cohort study and Mendelian randomisation study in 67 306 individuals. Br. J. Psychiatry 210:31–38 [DOI] [PubMed] [Google Scholar]
- Wojcicki JM, Olveda R, Heyman MB, Elwan D, Lin J, et al. 2016. Cord blood telomere length in Latino infants: relation with maternal education and infant sex. J. Perinatol 36:235–41 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Jeste DV, Martin AS, Lin J, Daly RE, et al. 2017. Leukocyte telomere length: effects of schizophrenia, age, and gender. J. Psychiatr. Res 85:42–48 [DOI] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Dhabhar FS, et al. 2011a. Leukocyte telomere length in major depression: correlations with chronicity, inflammation and oxidative stress—preliminary findings. PLOS ONE 6:e17837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Epel ES, Lin J, Reus VI, et al. 2012. Resting leukocyte telomerase activity is elevated in major depression and predicts treatment response. Mol. Psychiatry 17:164–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Mellon SH, Lindqvist D, Epel ES, Blackburn EH, et al. 2015. PBMC telomerase activity, but not leukocyte telomere length, correlates with hippocampal volume in major depression. Psychiatry Res. 232:58–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolkowitz OM, Reus VI, Mellon SH. 2011b. Of sound mind and body: depression, disease, and accelerated aging. Dialogues Clin. Neurosci 13:25–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie X, Chen Y, Ma L, Shen Q, Huang L, et al. 2017. Major depressive disorder mediates accelerated aging in rats subjected to chronic mild stress. Behav. Brain Res 329:96–103 [DOI] [PubMed] [Google Scholar]
- Yen Y-C, Lung F-W. 2013. Older adults with higher income or marriage have longer telomeres. Age Ageing 42:234–39 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yim O-S, Zhang X, Shalev I, Monakhov M, Zhong S, et al. 2016. Delay discounting, genetic sensitivity, and leukocyte telomere length. PNAS 113:2780–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalli A, Carvalho LA, Lin J, Hamer M, Erusalimsky JD, et al. 2014. Shorter telomeres with high telomerase activity are associated with raised allostatic load and impoverished psychosocial resources. PNAS 111:4519–24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan Y, Karlsson IK, Karlsson R, Tillander A, Reynolds CA, et al. 2017. Exploring the causal pathway from telomere length to coronary heart disease: a network Mendelian randomization study. Circ. Res 121:214–19 [DOI] [PubMed] [Google Scholar]
- Zhan Y, Song C, Karlsson R, Tillander A, Reynolds CA,et al. 2015. Telomere length shortening and Alzheimer disease—a Mendelian randomization study. JAMA Neurol. 72:1202–3 [DOI] [PubMed] [Google Scholar]
- Zhou Q-G, Hu Y, Wu D-L, Zhu L-J, Chen C, et al. 2011. Hippocampal telomerase is involved in the modulation of depressive behaviors. J. Neurosci 31:12258–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q-G, Wu H-Y, Zhou H, Liu M-Y, Lee H-W, et al. 2016. Reactivation of Tert in the medial prefrontal cortex and hippocampus rescues aggression and depression of Tert−/−mice. Transl. Psychiatry 6:e836. [DOI] [PMC free article] [PubMed] [Google Scholar]