Abstract
Background
Optic nerve hypoplasia (ONH), one of the most common causes of pediatric blindness in developed countries, has been difficult to directly quantify. We sought to measure optic nerve size in Alaskan pediatric patients with optic nerve hypoplasia using ultra-widefield fundus imaging.
Methods
Adult and pediatric patients underwent conventional ultra widefield fundus imaging (OPTOS, Dunfermline, Scotland) with manual image processing to determine optic nerve size validated against refractive error and nystagmus and compared to optical spectral domain tomography. De-identified cases were then compared relative to visual acuity and birth prevalence.
Results
In Alaska’s only pediatric ophthalmology outreach clinic, 108 cases of ONH less than 20 years old were clinically identified with 80 having ultra-widefield analysis. Median horizontal optic nerve diameter for 135 normals was 1.70 (95% C.I. 1.49, 2.14) whereas in patients clinically diagnosed with optic nerve hypoplasia was 1.23 (95% C.I 0.38, 1.45). Visual acuity (20/y) was related to horizontal optic nerve diameter (x) by y = 187 x-4.1. Horizontal nerve diameter h could be estimated from vertical nerve diameter v by h = 0.73v + 0.3 even in nystagmus patients. From 108 with ONH, 6 had threshold retinopathy of prematurity, 12 profound nystagmus, 32 legally blind, 6 with septo-optic dysplasia, and 5 with fetal alcohol syndrome. ONH is very prevalent in Alaska occurring at least 8–10 per 10,000 births.
Conclusion
Compared to vertical diameter, horizontal diameter was more distinctive of optic nerve hypoplasia and more perturbed by nystagmus. Both were independent of refractive error. When hand-held, spectral domain OCT is not convenient, ultra-widefield fundus analysis is recommended for direct estimation of optic nerve size in children and adults. Optic nerve hypoplasia is prevalent in Alaskan children.
Keywords: pediatric blindness, optic nerve, birth defect, dysmorphic, retinopathy of prematurity, septo-optic dysplasia, OPTOS
Video abstract
Point your SmartPhone at the code above. If you have a QR code reader the video abstract will appear. Or use:
Introduction
Optic nerve hypoplasia (ONH) has become one of the most common causes of pediatric blindness since new screening and treatment options have reduced the prevalence of Retinopathy of Prematurity (ROP) blindness.1 In Sweden as in other parts of the world, about 10/100,000 children suffer from ONH.1 In Olmstead Country, Minnesota ONH occurs 1 in 2287 births.2 The prevalence and severity of ONH are increased by environmental factors such as fetal alcohol syndrome.3 ONH can be associated with midline brain anomalies like septo-optic dysplasia.4 Given the degree of hypoplasia, the impact on visual acuity can range from near normal to no light perception. It remains very difficult to directly measure ONH and this can be further complicated by concomitant nystagmus.
The size and configuration of the optic nerve have important implications in pediatric ophthalmology.5 In the past, ONH was diagnosed by ophthalmoscopy6 and/or fundus photography, each of which is markedly influenced by the patient’s refractive error.7 An adjustment to retinal dimensions accounting for refractive error has been championed by Littman,7,8 however it has limitations. Optic nerve size can also be quantified directly by hand-held OCT or MRI if the patient is cooperative, or is under general anesthesia which will dampen nystagmus if present. ONH can also be quantified by spectral domain optical coherence tomography (SD-OCT)9 or B-wave OCT, however it is very challenging to obtain images in some uncooperative pediatric patients or in patients with nystagmus.
Handheld spectral domain OCT can get reliable optic nerve size and has shown that whites have smaller vertical diameters than blacks and Hispanics.10 Acuity deficits accompany all children with diameter less than 1000 microns whereas VEP anomalies accompanied all optic nerves smaller than 1200 microns horizontal diameter; these images were obtained even in the presence of nystagmus.10 Pilat et al also measured horizontal diameter in ONH with OCT.9 Similarly, Heidelberg retinal tomography disc area of ONH averaged 0.84 ± 0.35 mm2 with the upper limit area for ONH being 1.42 mm2.11 Retrobulbar optic nerve cross-sectional area on MRI under general anesthesia can differentiate optic nerve hypoplasia (<2.9 mm2) from normals.12 Refractive errors (hyperopia, myopia and anisometropia) can be much more common in patients with optic nerve hypoplasia.13
One way to determine ONH proposed by Dutton et al is to compare the optic nerve mean diameter (DD) with the nerve-to-macula (M-D) distance; a MD/DD ratio greater than 2.94 strongly suggests ONH.14,15 Re-evaluation with Heidelberg retinal tomography suggested ONH has a M-D/DD ratio greater than 3.20 corresponding to a 1.42 mm2 disc area.11
Using ultra-widefield (OPTOS, Dunfermline, Scotland) imaging, we had been able to capture images of awake infants and pediatric retinae16 with sufficient peripheral and posterior quality that the optic nerve could be measured in the apparently arbitrary units afforded in OPTOS image analysis software. Therefore, we pondered the potential for analysis of optic nerve head dimensions.
Methods
This observational, retrospective study has institutional review from Pacific University. The study is compliant with Health Insurance portability and Accountability Act and the Declaration of Helsinki. Data were de-identified before public storage and written consent was waived. De-identified raw data can be accessed through http://www.abcd-vision.org/references/ONH%20de-ident%20Alaska.pdf.
We developed a method for efficient acquisition of fundus images with OPTOS California model in children as young as newborns.16 Refractive error, age and clinical diagnoses were recorded and de-identified. Accompanying software allowed image zoom and direct linear measurement. A subset of cooperative, non-nystagmus ONH patients and 53 of our 135 normal subjects also underwent optical coherence tomography (OCT, Heidelberg, Spectralis) from which horizontal and vertical nerve diameters were derived for comparison with OPTOS.
We acquired horizontal measurements but then recognized limitations in patients with nystagmus and poor fixation. Therefore, for 12 ONH nystagmus patients who were able to gaze into the camera portal, multiple horizontal and vertical measurements were also taken from which horizontal and vertical optic nerve diameters, deviations and ranges were estimated. An additional measure from the center of the nerve to the center of the macula (M-D) was recorded. We attempted to collect OCT measurements in ONH patients with nystagmus, but none these was successful.
Inclusion criteria: Patients with optic nerve hypoplasia were clinically diagnosed if associated with fetal alcohol syndrome, deMorsier’s (septo-optic dysplasia) syndrome, asymmetric vision accompanying asymmetric nerve size, or decreased visual acuity not explained by other reasons, or associated nystagmus. Patients with amblyopia risk factors who responded sub-optimally to treatment were included. Exclusion criteria: no case with small nerves was deliberately excluded, but we recognize that we have not evaluated every child in Alaska and therefore probably have missed some cases with smaller nerves, but normal visual acuity. Optic nerve measurements were compared to best-corrected visual acuity. Our cases were compared to Alaskan birth statistics with an intent to make a point estimate of the minimum prevalence.
Results
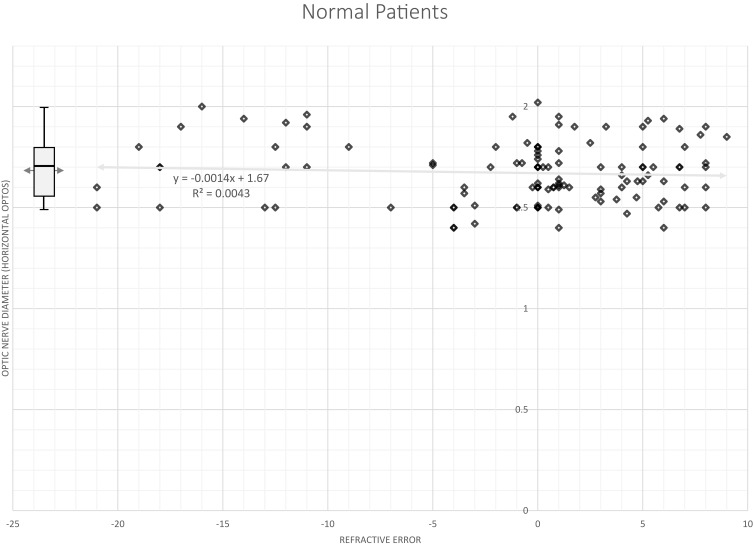
Three steps were taken to determine the reliability of OPTOS measurement of optic nerve hypoplasia; determining impact of refractive error, comparison with OCT and factoring in variability of scan measurement in patients with horizontal nystagmus. Fifty-four normal subjects had images for regression of horizontal optic nerve diameter regressed against refractive error: nerve size = 0.0002(spherical equivalent) + 1.66, r2= 0.0001. The relationship between our estimates of horizontal optic nerve diameter and spherical equivalent refractive error for normal subjects is shown in Figure 1.
Figure 1.
The relationship between horizontal optic nerve diameter estimated from ultra-widefield (OPTOS) fundus imaging and refractive error (spherical equivalent). Box plot surrounds quartiles of ordinal data with median indicated inside the box and the mean indicated by an arrow behind the box. Bars extend to the 5 and the 95 percentiles of the distribution. Optos is reported in arbitrary units.
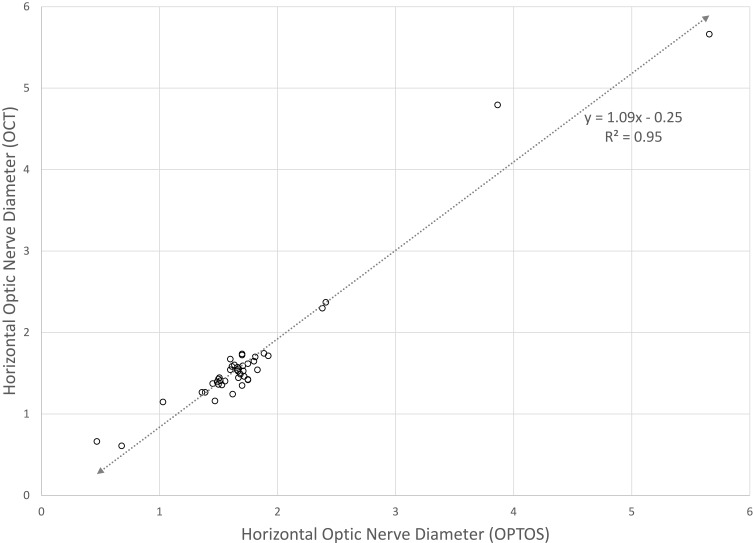
The strong correlation between OPTOS and OCT over a wide range of optic nerve size, non-nystagmus patients is shown for horizontal (Figure 2) calculations; vertical measurements were similar. From these, we derived a formula relating horizontal to vertical nerve dimensions. The near-unity slope of the correlation indicates that dimensions on OPTOS measurements closely resemble OCT measurement in millimeters.
Figure 2.
The correlation between OPTOS and OCT measurement of horizontal optic nerve dimensions in patients with normal nerves, optic nerve hypoplasia, large nerves. Optos is reported in arbitrary units; however, we found close direct correlation with OCT in millimeters.
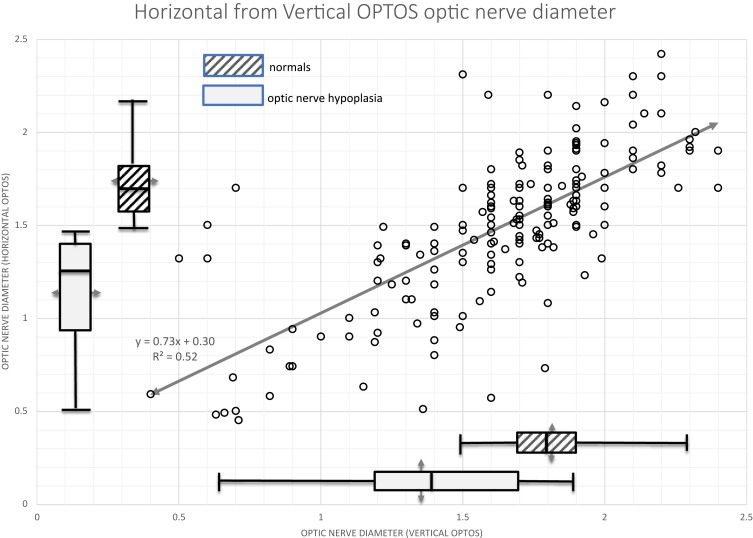
Since the widefield camera is a scanning laser device, horizontal nystagmus can result in magnified or minimized optic nerve horizontal diameter depending on whether the eye movement is with, or against the scanning direction of the laser. From 18 non-ONH patients with nystagmus (infantile nystagmus syndrome [INS], fusion maldevelopment nystagmus syndrome [FMDNS] and visual deprivation) ultra-widefield optic nerve repeat (3x to 6x) analysis gave mean horizontal diameter 1.24±0.75 with the mean deviation (S.D.) of 0.18 ± 0.22 whereas the mean vertical diameter 1.31 ± 0.56 had mean deviation 0.05 ± 0.03. Therefore, the vertical measurements have 3.3±3 times less variability than the horizontal measurements. Figure 3 shows the relationship between horizontal and vertical optic nerve head diameters with a formula estimating horizontal if vertical is known in cases of horizontal nystagmus.
Figure 3.
Horizontal optic nerve diameter from OPTOS as a function of vertical optic nerve diameter. Box plots indicate medians and ranges for normal subjects (cross hatched) and patients with a clinical diagnosis of optic nerve hypoplasia (light gray shading). Optos is reported in arbitrary units.
We identified 114 patients with optic nerve hypoplasia in ours, the only pediatric ophthalmology practice in Alaska. Our primary practice in Anchorage houses the OPTOS camera, but we also staff out-reach clinics to the communities of Wasilla, Kodiak and Fairbanks with additional ongoing consulting clinics and surgery in the Alaska Native Medical Center (ANMC) system. Of these, 108 patients were under age 20 years and 31 had vision of 20/200 or worse (legal blindness). Four had at least one eye with light perception while five had no light perception due to optic nerve hypoplasia. The optic nerve hypoplasia involved the right eye in 23, the left eye in 32 and both eyes in 59. Females constituted 55 of the 114 patients. Race was undeclared in 48 while in those declared were 53% white, 8% Hispanic, 29% Alaskan native, 8% African American and 3% Asian. We found 11 patients had ROP with 6 treated with laser, 5 had fetal alcohol exposure, 17 with amblyopia, 6 with deMorsier’s syndrome, and 10 with neurological disorders. Fifty-eight had vision less than 20/40 legal driving, 32 qualified for 20/200 legal blindness and 4 had no light perception.
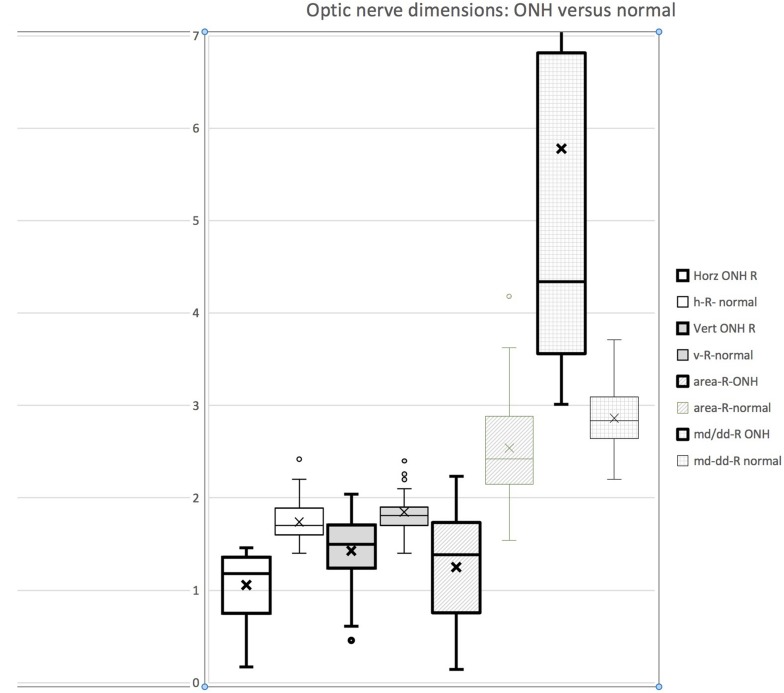
Table 1 gives our ultra-widefield estimates of horizontal and vertical optic nerve diameter for 135 normal patients and also for 80 patients with clinical optic nerve hypoplasia. Normal subjects had horizontal diameters with mean of 1.74 ± 0.22 units. Clinical cases of optic nerve hypoplasia had diameters of 1.14 ± 0.33 units. Figure 4 shows several metrics of optic nerve head OPTOS derived dimensions comparing optic nerve hypoplasia patients with normal subjects. The metrics that best distinguish hypoplasia from normal, in order, are horizontal diameter, M-D/DD ratio, surface area and finally vertical diameter.
Table 1.
Optic Nerve Metrics Derived from OPTOS Ultra-Widefield Fundus Images Showing Values for 80 Patients with Optic Nerve Hypoplasia Compared to 135 Normal Subjects: M-D Is Macula to Disk Distance, on Is Optic Nerve, Horiz Is Horizontal While Vert Is Vertical, Md/Dd Is the Macular-to-Disk Distance versus Horizontal Disk Diameter Ratio. S.D. Is Standard Deviation while 98% and 3% are Percentiles
| M-Dr | M-Dl | Horz ON R | Horz ON L | Vert ON R | Vert ON L | Area-R | Area-L | md/dd-R | md/dd-L | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| ONH | Mean | 4.95 | 4.89 | 1.06 | 1.14 | 1.43 | 1.38 | 1.25 | 1.30 | 5.69 | 4.75 |
| S.D. | 0.47 | 0.53 | 0.35 | 0.33 | 0.36 | 0.40 | 0.58 | 0.58 | 3.74 | 2.30 | |
| 98% | 5.97 | 6.12 | 1.45 | 1.46 | 1.93 | 1.93 | 2.13 | 2.00 | 13.42 | 11.71 | |
| 3% | 4.14 | 3.99 | 0.38 | 0.43 | 0.63 | 0.52 | 0.19 | 0.21 | 3.07 | 2.98 | |
| Normal | Mean | 4.87 | 4.81 | 1.74 | 1.74 | 1.85 | 1.87 | 2.54 | 2.58 | 2.86 | 2.80 |
| S.D. | 0.46 | 0.41 | 0.20 | 0.22 | 0.22 | 0.23 | 0.52 | 0.60 | 0.35 | 0.38 | |
| 98% | 5.88 | 5.64 | 2.106 | 2.215 | 2.3 | 2.215 | 3.54 | 3.49 | 3.54 | 3.4 | |
| 3% | 4.13 | 4.11 | 1.50 | 1.50 | 1.50 | 1.56 | 1.77 | 2 | 2.32 | 2.27 |
Figure 4.
Compared metrics of optic nerve head ultra-wide field OPTOS image derived dimensions comparing optic nerve hypoplasia with normal. From the left, horizontal optic nerve diameter, then vertical optic nerve diameter, optic nerve cross-sectional area and finally to the right ratio of macula-disc dimension divided by horizontal optic nerve diameter (M-D/DD). Optos is reported in arbitrary units.
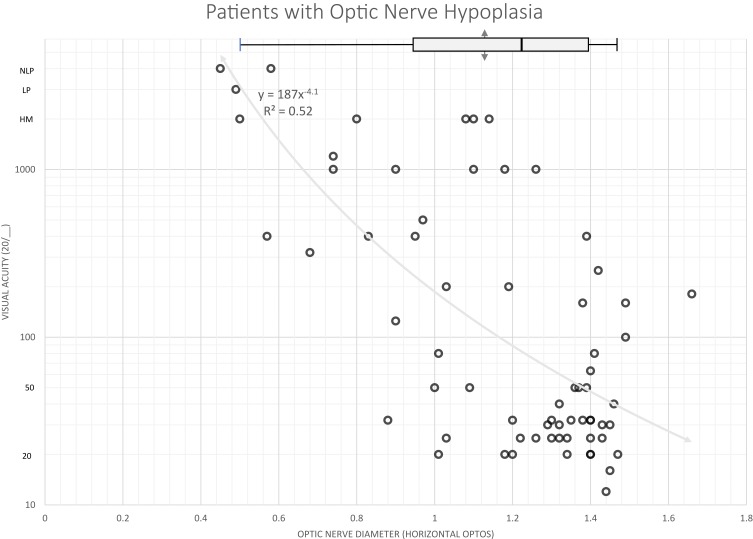
The relationship between horizontal optic nerve diameter and best-corrected visual acuity is shown in Figure 5. There was a strong correlation between acuity and horizontal nerve size (x) in ONH (Acuity = 20/y such that y = 187 (x−4.1)).
Figure 5.
The relationship between horizontal optic nerve diameter and visual acuity in patients with a clinical diagnosis of optic nerve hypoplasia. Optos is reported in arbitrary units.
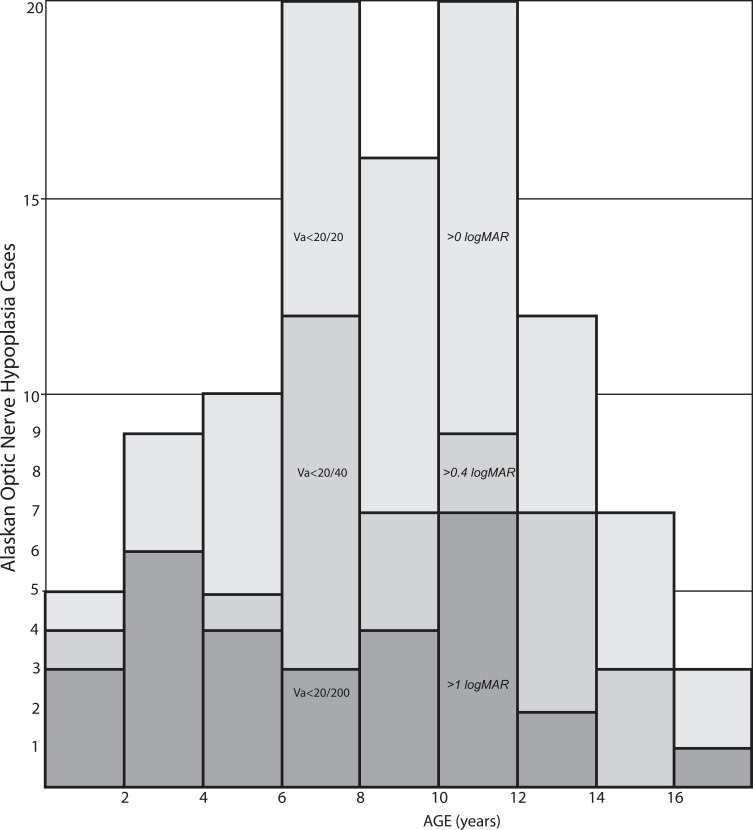
In Alaska since 2001, there has been a stable rate of about 11,000 babies born each year.17 We have identified 114 children born between 2000 and 2019 with a diagnosis of optic nerve hypoplasia- some due to associated vision, and some due to growth, neurologic and endocrine and others on the basis of retinal examination. The estimated minimum prevalence of ONH based on patient age is shown in Figure 6. For every 10,000 Alaskan births, the median number of cases is at least 6 with 95% confidence intervals 3–10 cases.
Figure 6.
A histogram showing the estimated minimum prevalence of optic nerve hypoplasia in Alaskan children presented in 2-year bins striated by vision worse than 20/200, vision worse than 20/40 and all patients.
Discussion
Ultra-widefield scanning laser imaging is optically different from conventional fundus imaging with respect to refractive error; we found independence. The ultra-widefield imaging correlated well with OCT and therefore is a reliable measure of optic nerve dimensions with OPTOS units closely matching OCT millimeters. Conventional retinal imagery requires adjustment to account for refractive error.18 We determined that, in the presence of horizontal nystagmus, it is more reliable to obtain several ultra-widefield images and then measure vertical optic nerve diameter and then apply a linear formula to estimate horizontal nerve diameter. Nerve metrics that best indicated clinical optic nerve hypoplasia were first horizontal diameter, then horizontal disk to macula disc ratio, then cross-sectional area, and finally vertical diameter. We found that 90% of normal optic nerve horizontal diameters fall between 1.49 and 2.14 Optos units. A horizontal optic nerve diameter on OPTOS less than 1.45 is likely hypoplasia.
Optic nerve hypoplasia seems to be particularly prevalent in Alaska. Due to the nature of our pediatric ophthalmology practice, children old enough to give reliable visual acuity screening, and still within the first decade amblyopia care would be most likely referred to us and retained. As such, our prevalence estimates from age 5 to 12 years would have the best chance of inclusion in our database whereas young children might not yet be detected, and older referred to other Alaskan clinics. For our patients aged 6–12 years with optic nerve hypoplasia, the prevalence is at least 8–10 cases per year of age translating to about 9 cases per 10,000 Alaskan children, an almost ten-fold increase over another northern country, Sweden1 and 2–3 times as was found in a small part of the state of Minnesota.2
We agree that an M-D/DD ratio greater than 3.2 is a good definition for ONH.11 We also confirmed that ROP is common with ONH.19 The OPTOS direct measurement arbitrary units closely resemble millimeters.
Limitations of Study
Not all Alaskan cases with septo-optic dysplasia were retrievable. Children with milder forms of ONH, or those with normal visual acuity and subnormal nerve size would not necessarily be imaged or referred to our practice resulting in an unknown degree of under-reporting; this limitation is shared by the Sweden and Minnesota studies as well. We lacked a direct calibrating method for the OPTOS camera concerning linear measurement; it would be ideal to place objects of known length (i.e. retinal inserts) in expendable eyes with a wide range of refractive error, and then imaged with and without induced horizontal and then vertical nystagmus.
An advantage of our study is the ability of our practice to include most patients with optic nerve hypoplasia due to our location within a remote, non-contiguous state. Very few patients transfer between Canada and Alaska. Most severe pediatric eye cases would be referred to us via pediatricians, public health clinics and low vision services for preschool and school-aged children. Since 2014 all Alaskan children have access to our clinic regardless of the patient’s type of medical insurance.
Conclusion
Quick ultra-widefield fundus imaging with simple image analysis affords a practical way to quantitate optic nerve size in awake children and adults with implications for optic nerve hypoplasia (2), amblyopia (3) coloboma and perhaps ROP. Optic nerve hypoplasia is particularly common in Alaskan children.
IRB
Pacific University, FWA 7392, IRB: 4173, IRB # 007-18 (approved 4/16/18). De-identified data: http://www.abcd-vision.org/references/ONH%20de-ident%20Alaska.pdf.
Disclosure
Dr. Robert W Arnold is the President of Glacier Medical software which markets NICU cloud-based ROP Check software and he coordinates the Alaska Blind Child Discovery which has received discount vision screening technology from several vendors. Dr. Arnold is a board member of Glacier Medical Software. Dr. Arnold is also an investigator and protocol planner for PEDIG. Dr. Arnold and Dr. Kyle Smith are board members of PDI Check which developed a vision screening game for Nintendo 3DS. Dr. Arnold has a patent pending with PDI Check. The authors report no other conflicts of interest in this work.
References
- 1.Blohme J, Bengtsson-Stigmar E, Tornqvist K. Visually impaired Swedish children. Longitudinal comparisons 1980–1999. Acta Ophthalmol Scand. 2000;78(4):416–420. doi: 10.1034/j.1600-0420.2000.078004416.x [DOI] [PubMed] [Google Scholar]
- 2.Mohney BG, Young RC, Diehl N. Incidence and associated endocrine and neurologic abnormalities of optic nerve hypoplasia. JAMA Ophthalmol. 2013;131(7):898–902. doi: 10.1001/jamaophthalmol.2013.65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ryabets-Lienhard A, Stewart C, Borchert M, Geffner ME. The optic nerve hypoplasia spectrum: review of the literature and clinical guidelines. Adv Pediatr. 2016;63(1):127–146. doi: 10.1016/j.yapd.2016.04.009 [DOI] [PubMed] [Google Scholar]
- 4.Birkebaek NH, Patel L, Wright NB, et al. Endocrine status in patients with optic nerve hypoplasia: relationship to midline central nervous system abnormalities and appearance of the hypothalamic-pituitary axis on magnetic resonance imaging. J Clin Endocrinol Metab. 2003;88(11):5281–5286. doi: 10.1210/jc.2003-030527 [DOI] [PubMed] [Google Scholar]
- 5.Dutton GN. Congenital disorders of the optic nerve: excavations and hypoplasia. Eye. 2004;18(11):1038–1048. doi: 10.1038/sj.eye.6701545 [DOI] [PubMed] [Google Scholar]
- 6.Kelly JP, Phillips JO, Weiss AH. VEP analysis methods in children with optic nerve hypoplasia: relationship to visual acuity and optic disc diameter. Doc Ophthalmol. 2016;133(3):159–169. doi: 10.1007/s10633-016-9566-6 [DOI] [PubMed] [Google Scholar]
- 7.Sampson DM, Gong P, An D, et al. Axial length variation impacts on superficial retinal vessel density and foveal avascular zone area measurements using optical coherence tomography angiography. Invest Ophthalmol Vis Sci. 2017;58(7):3065–3072. doi: 10.1167/iovs.17-21551 [DOI] [PubMed] [Google Scholar]
- 8.Yang B, Ye C, Yu M, Liu S, Lam DS, Leung CK. Optic disc imaging with spectral-domain optical coherence tomography: variability and agreement study with Heidelberg retinal tomograph. Ophthalmology. 2012;119(9):1852–1857. doi: 10.1016/j.ophtha.2012.02.033 [DOI] [PubMed] [Google Scholar]
- 9.Pilat A, Sibley D, McLean RJ, Proudlock FA, Gottlob I. High-resolution imaging of the optic nerve and retina in optic nerve hypoplasia. Ophthalmology. 2015;122(7):1330–1339. doi: 10.1016/j.ophtha.2015.03.020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Allingham MJ, Cabrera MT, O’Connell RV, et al. Racial variation in optic nerve head parameters quantified in healthy newborns by handheld spectral domain optical coherence tomography. J AAPOS. 2013;17(5):501–506. doi: 10.1016/j.jaapos.2013.06.014 [DOI] [PubMed] [Google Scholar]
- 11.Pang Y, Frantz KA. Comparison of Heidelberg retina tomograph with disc-macula distance to disc diameter ratio in diagnosing optic nerve hypoplasia. Ophthalmic Physiol Opt. 2016;36(3):317–323. doi: 10.1111/opo.12274 [DOI] [PubMed] [Google Scholar]
- 12.Birkebaek NH, Patel L, Wright NB, et al. Optic nerve size evaluated by magnetic resonance imaging in children with optic nerve hypoplasia, multiple pituitary hormone deficiency, isolated growth hormone deficiency, and idiopathic short stature. J Pediatr. 2004;145(4):536–541. doi: 10.1016/j.jpeds.2004.06.041 [DOI] [PubMed] [Google Scholar]
- 13.Pang Y, Frantz KA, Roberts DK. Association of refractive error with optic nerve hypoplasia. Ophthalmic Physiol Opt. 2015;35(5):570–576. doi: 10.1111/opo.2015.35.issue-5 [DOI] [PubMed] [Google Scholar]
- 14.Zeki SM, Dudgeon J, Dutton GN. Reappraisal of the ratio of disc to macula/disc diameter in optic nerve hypoplasia. Br J Ophthalmol. 1991;75(9):538–541. doi: 10.1136/bjo.75.9.538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alvarez E, Wakakura M, Khan Z, Dutton GN. The disc-macula distance to disc diameter ratio: a new test for confirming optic nerve hypoplasia in young children. J Pediatr Ophthalmol Strabismus. 1988;25(3):151–154. [DOI] [PubMed] [Google Scholar]
- 16.Arnold RW, Grendahl RL, Kevin Winkle R, Jacob J. Outpatient, wide-field, digital imaging of infants with retinopathy of prematurity. Ophthalmic Surg Lasers Imaging Retina. 2017;48(6):494–497. doi: 10.3928/23258160-20170601-08 [DOI] [PubMed] [Google Scholar]
- 17.Butler JC, SoA DHSS. Alaska vital statistics annual report. Available from: http://dhss.alaska.gov/dph/VitalStats/Documents/PDFs/VitalStatistics_Annualreport_2017.pdf. Published 2017. Accessed September30 2019.
- 18.Langenbucher A, Seitz B, Viestenz A. Computerised calculation scheme for ocular magnification with the Zeiss telecentric fundus camera. Ophthalmic Physiol Opt. 2003;23(5):449–455. doi: 10.1046/j.1475-1313.2003.00140.x [DOI] [PubMed] [Google Scholar]
- 19.Arnold RW. Optic nerve hypoplasia potentiates retinopathy of prematurity. J Pediatr Ophthalmol Strabismus. 2008;45(4):247–249. doi: 10.3928/01913913-20080701-02 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Citations
- Butler JC, SoA DHSS. Alaska vital statistics annual report. Available from: http://dhss.alaska.gov/dph/VitalStats/Documents/PDFs/VitalStatistics_Annualreport_2017.pdf. Published 2017. Accessed September30 2019.