Abstract
No difference in the gene methylation status of tumor-suppression genes between pancreatic cancer tissues and adjacent non-cancer tissues is observed. The present study investigated whether the promoter CpG islands of the cysteine dioxygenase 1 (CDO1), tachykinin precursor 1 (TAC1) and checkpoint with forkhead and ring finger domains (CHFR) genes were methylated in pancreatic cancer and adjacent non-cancerous pancreatic tissue in order to determine if they could be considered as markers for the detection of pancreatic cancer. A total of 38 Formalin-fixed and paraffin-embedded pancreatic adenocarcinoma tissues and their adjacent non-cancerous specimens from patients with pancreatic cancer, as well as 9 non-cancerous pancreatic samples from patients without pancreatic adenocarcinoma were obtained following surgical resection. The hypermethylation of CpG islands was detected using a methylation-specific quantitative PCR. The methylation values were calculated using the ∆Cq method and were expressed as 2−ΔCq. The 2−ΔCq value of the CDO1 promoter from pancreatic adenocarcinoma specimens was significantly higher compared with that of adjacent non-cancerous and tumor-free pancreatic tissues (P<0.0001 and P=0.0008, respectively). The 2−ΔCq value of the TAC1 promoter of pancreatic adenocarcinoma was also significantly higher compared with that of adjacent non-cancerous tissues and tumor-free pancreatic samples (both P<0.0001). However, there was no significant difference in the 2−ΔCq value of the CHFR promoter among the pancreatic cancer, adjacent non-cancer tissue and tumor-free pancreatic samples. Furthermore, 12 out of the 38 pancreatic adenocarcinoma cases (31.6%) presented some methylation in the CHFR promoter. The results from Kaplan-Meier analysis between CHFR promoter methylation values and the clinicopathological characteristics of patients with pancreatic adenocarcinoma demonstrated that CHFR promoter methylation was significantly associated with lymph node metastasis. The methylation values of CDO1 and TAC1 promoters in cancer tissues were higher compared with adjacent tissues. However, whether hypermethylation of CDO1 and TAC1 promoters may serve as a biomarker in the diagnosis of pancreatic adenocarcinoma remains unclear.
Keywords: pancreatic cancer, methylation, promoter, CDO1, TAC1, CHFR
Introduction
Pancreatic ductal cancer is the 7th leading cause of cancer-associated mortality worldwide (1). Although the treatment of pancreatic ductal cancer has progressed, the 5-year survival rate remains low (2–9%) (1,2). Numerous genetic alterations contribute to pancreatic cancer tumorigenesis. For example, mutation of the KRAS proto-oncogene, GTPase (Kras) gene is commonly observed in the early stage of pancreatic cancer (3). Furthermore, somatic mutations in the tumor protein p53 (TP53), SMAD family member 4 (SMAD4) and p16 genes can also contribute to the progression of pancreatic cancer (3–5). In addition to genetic mutations, modifications that are not due to changes in DNA sequence, including promoter hypermethylation, are often observed in pancreatic cancer cells (6). Epigenetic silencing and transcriptional inactivation due to hypermethylation in the 5′promoter regions of specific genes, including tumor-suppressor genes, for example hMLH1, BRCA1, p16INK4a, can contribute to cancer progression (7).
Hypermethylation of the promoter regions of the cysteine dioxygenase 1 (CDO1), tachykinin precursor 1 (TAC1) and checkpoint with forkhead and ring finger domains (CHFR) genes has been reported in various types of cancer (8–21), including colorectal cancer (12,15,19). The risk factors for pancreatic cancer are similar to those for colorectal cancer, and include cigarette smoking and alcohol consumption (22,23). Furthermore, patients with colorectal cancer have a significantly higher risk of developing pancreatic cancer compared with that of the general population (24,25). The present study hypothesized therefore that pancreatic and colorectal cancer may share some genes presenting similar methylation alterations in their CG-rich region in 5′end of the promoter, called CpG islands. This alteration leads to silencing gene expression. Although DNA methylation of various genes, including APC, BRCA1, p16INK4a, p15INK4b, RARβ, and p73, has been examined in pancreatic cancer (26), the CDO1, TAC1 and CHFR genes have not been fully described. Vedeld et al (12) demonstrated that the promoter region of CDO1 in pancreatic cancer, formalin-fixed, paraffin-embedded (FFPE) samples was hypermethylated. Furthermore, Henriksen et al (27,28) reported that the promoter of TAC1 in the plasmatic nucleic acids of patients with pancreatic cancer was hypermethylated, and the promoter of CHFR was not hypermethylated. However, the hypermethylation of these genes promoters in pancreatic cancer tissues was not compared with adjacent non-cancerous pancreatic tissues. Whether hypermethylation of these genes is already present in non-cancerous pancreatic tissues remains therefore unclear, as this was not examined by Henriksen et al (27,28). CDO1, TAC1, and CHFR methylation in pancreatic cancer tissues have not been compared with adjacent non-cancerous pancreatic tissues. The present study investigated, therefore, the methylation state of the promoter regions of the CDO1, TAC1 and CHFR genes in pancreatic cancer and adjacent non-cancerous pancreatic tissues from patients with pancreatic cancer. In addition, it has been reported that hypermethylation of CHFR is associated with tumor aggressiveness in gastric and colorectal cancer (29,30). The present study hypothesized that the promoter region of these three genes may be hypermethylated, and investigated whether these genes may be considered as suitable biomarker candidates for early detection of pancreatic cancer.
Materials and methods
Patients samples
FFPE pancreatic cancer specimens [pancreatic cancer (C) group] and adjacent non-cancerous pancreatic specimens [adjacent tissue (AT) group] were obtained from 38 patients with pancreatic cancer treated at the Juntendo University Shizuoka Hospital, Japan, between January 2011 and December 2016 (Table I). Furthermore, FFPE non-cancerous pancreatic samples from 9 patients with extra-hepatic biliary tract cancers [healthy non-adjacent tissue (HN) group] were also obtained between January 2011 and December 2016 and were used as controls (Table II). In the tables, histological findings were described using the World Health Organization classification of tumors of the digestive system from 2010 (31). Clinical stages were described using the Union for International Cancer Control 8th edition classification (32).
Table I.
Clinicopathological characteristics of the 38 patients with pancreatic ductal cancer.
| Variables | Median (range) or number |
|---|---|
| Total number | 38 |
| Sex | |
| Male | 16 |
| Female | 22 |
| Age, years, median (range) | 70 (56–82) |
| Tumor location | |
| Head | 24 |
| Body | 5 |
| Tail | 9 |
| Tumor size | |
| ≤4 cm | 24 |
| >4 cm | 14 |
| Node involvement | |
| Positive | 30 |
| Negative | 8 |
| Clinical stage (UICC 8th edition) | |
| IB | 5 |
| IIA | 3 |
| IIB | 11 |
| III | 14 |
| IV | 5 |
| Histology (WHO classification 2010)a | |
| Wel | 30 |
| Mod | 2 |
| Por | 6 |
| Follow-up, months median (range) | 14 (3–78) |
WHO classification 2010 corresponds to the World Health Organization for the classification of tumours of the digestive system (31). Mod, moderately differentiated carcinomas; Por, poorly differentiated ductal adenocarcinomas; Wel, well differentiated carcinomas; UICC, Union for International Cancer Control (32).
Table II.
Clinicopathological characteristics of the 9 patients with extra-hepatic bile tract cancer.
| Variables | Median (range) or number |
|---|---|
| Total number | 9 |
| Sex | |
| Male | 6 |
| Female | 3 |
| Age, years, median (range) | 72 (62–79) |
| Tumor location | |
| Distal bile duct | 3 |
| Papilla of Vater | 6 |
| Node involvement | |
| Positive | 5 |
| Negative | 7 |
| Clinical stage (UICC 8th edition) | |
| IA | 1 |
| IB | 3 |
| IIB | 3 |
| IIIA | 2 |
UICC; Union for International Cancer Control (32).
The study protocol was performed according to the ethical guidelines of the World Medical Association and the Declaration of Helsinki, and was approved by the Ethics Committee of Juntendo University Shizuoka Hospital (approval no. 463). Patients provided consent for the use of their samples for scientific research.
Extraction and bisulfite conversion of DNA from FFPE samples
FFPE tumor and non-cancerous samples from patients with pancreatic cancer, and FFPE normal samples from patients with extra-hepatic biliary tract cancer diagnosed using hematoxylin and eosin staining sections were analyzed.
All specimens were serially cut into 10-µm thick sections. To extract DNA, sections were deparaffinized twice with xylene for 15 min and rehydrated using 100% ethanol for 3 min twice at room temperature. Proteins were digested using proteinase K (cat. no. P8107S; New England BioLabs, Inc.) dissolved in digestion lysis buffer containing denaturing agents, including sodium dodecyl sulfate, at 55°C for 4 h. Subsequently, bisulfite conversion was performed using a Zymo EZ DNA Methylation kit (cat. no. D5002; Zymo Research Corp.) according to the manufacturer's instructions. Finally, bisulfite-modified DNA was eluted using distilled H2O with the column from the kit. All samples were stored at −20°C.
DNA methylation analysis
DNA methylation analysis was performed as previously described (33). The sequences of the primers (Integrated DNA Technologies, Inc.) used are presented in Table III. Following DNA bisulfite treatment, the methylation levels of the three genes CDO1, TAC1 and CHFR was measured by quantitative methylation-specific PCR (qMSP). The qMSP levels were normalized to the values of the internal control gene β-actin. Briefly, 2 µl bisulfite-converted DNA was added to a 23-µl PCR mixture. The final reaction mixture contained 1X buffer [16.6 mM (NH4)2SO4, 67 mM Tris pH 8.8, 6.7 mM MgCl2 and 10 mM β-mercaptoethanol in nuclease-free deionized water], 200 nM sense primer, 200 nM antisense primer, 80 nM TaqMan probe (Integrated DNA Technologies, Inc.), 10 nM fluorescein reference dye (Thermo Fisher Scientific, Inc.), 0.167 mM dNTPs (Invitrogen; Thermo Fisher Scientific, Inc.) and a 1U Platinum Taq® DNA Polymerase (Invitrogen; Thermo Fisher Scientific, Inc.). Amplification reaction of each sample was performed using MicroAmp® optical 96-well reaction plates (Applied Biosystems; Thermo Fisher Scientific, Inc.) in triplicate. The thermocycling conditions were as follows: 95°C for 5 min, 50 cycles at 95°C for 15 sec and 65°C for 1 min, and 72°C for 1 min. The StepOnePlus™ Real-Time PCR System (Applied Biosystems; Thermo Fisher Scientific, Inc.) was used.
Table III.
Primer sequences for quantitative methylation-specific PCR.
| Gene | Forward, 5′-3′ | Reverse, 5′-3′ | Probe 5′ | Product size, bp | Annealing temperature, °C |
|---|---|---|---|---|---|
| CDO1 | CGTTTTTTTTCGTTTTATTTTCGTCG | CCTCCGACCCTTTTTATCTACG | TGTGGTTCGCGACGTTGGGACGT | 69 | 65 |
| TAC1 | TCGGGTTATTTCGTTTCGTATTTGTTC | CACTATCCCTCGCCGCAACG | AGGTGGTCGCGTTGGGGGCGTCGT | 69 | 65 |
| CHFR | TTAGAGGTTTTTGCGTTTCGCG | CGACTCCGCTTTAACTACCG | TTGGTTGGCGGCGGCGTTTATTAAGAGCG | 70 | 65 |
| β-actin | TAGGGAGTATATAGGTTGGGGAAGTT | AACACACAATAACAAACACAAATTCAC | TGTGGGGTGGTGATGGAGGAGGTTTAG | 103 | 65 |
CDO1, cysteine dioxygenase 1; CHFR, checkpoint with forkhead and ring finger domains; TAC1, tachykinin precursor 1.
The methylation value for each sample was calculated using the ∆Cq method (34) according to the following formula: ∆Cq=Cqsample-Cqβ-actin. A sample was considered as positively amplified when amplification was detected in ≥2 of the triplicates. For replicates that were not detected, a Cq of 100 was used, which set a minimum methylation value 0, as previously described (33). All the Cqsamples were changed to 100 when only 1 of the 3 triplicates was amplified. The mean 2−ΔCq value was calculated as follows: Methylation value=(2−ΔCqreplicate 1 + 2−ΔCqreplicate 2 + 2−ΔCqreplicate 3)/3. For a methylation value >1, a value of 1 was used, which set the maximum methylation value at 1.
Statistical analysis
The results were expressed as median values (25 and 75th percentiles). Wilcoxon signed-rank test was used to compare pancreatic cancer samples with adjacent non-cancer pancreatic samples, while Mann-Whitney U test followed by Bonferroni's correction was used to compare pancreatic cancer samples with tumor-free pancreatic samples. All clinicopathological factors were analyzed with Mann-Whitney U or Kruskal-Wallis tests. The patients' survival rates were represented using the Kaplan-Meier method and were analyzed with the log-rank test for survival data. All analyses were conducted using Graph Pad Prism version 5 (GraphPad Software, Inc.) and JMP version 12.2.0 (SAS Institute, Inc.). P<0.05 was considered to indicate a statistically significant difference.
Results
Methylation values of the CDO1, TAC1 and CHFR promoter
The methylation values of the CDO1 gene promoter are presented in Fig. 1. The 2−ΔCq values of the CDO1 promoter in the AT and the HN groups from patients with extra-hepatic biliary tract cancer were significantly lower compared with the those in the C group [C, 0.28 (0.13–0.64); AT, 0.06 (0.04–0.09); HN, 0.06 (0.03–0.10), median (25 and 75th percentiles); C vs. AT, P<0.0001; C vs. HN, P=0.0008]. The methylation values of the TAC1 gene promoter are presented in Fig. 2. The 2−ΔCq values of TAC1 in the AT and HN groups were significantly lower compared with those in the C group [C, 0.13 (0.07–0.48); AT, 0.02 (0.004–0.03); HN, 0.01 (0.002–0.02), median (25 and 75th percentiles); C vs. AT, P<0.0001; C vs. HN, P<0.0001]. Conversely, the 2−ΔCq values of the CHFR gene promoter in the C, AT and HN groups were 5.28×10−22 (2.82×10−22−1.02×10−4), 6.52×10−22 (2.44×10−22−1.52×10−21) and 2.72×10−22 (2.15×10−22−3.78×10−21), median (25 and 75th percentiles), respectively (Fig. 3). When comparing the 2−ΔCq values of the CHFR promoter among pancreatic cancer specimens, no significant difference was observed among pancreatic cancer, adjacent non-cancer tissue and tumor-free pancreatic samples (Fig. 3). In addition, 12 of the 38 cases in the C group (31.6%) exhibited methylation values of the CHFR gene promoter >1.0×10−6.
Figure 1.
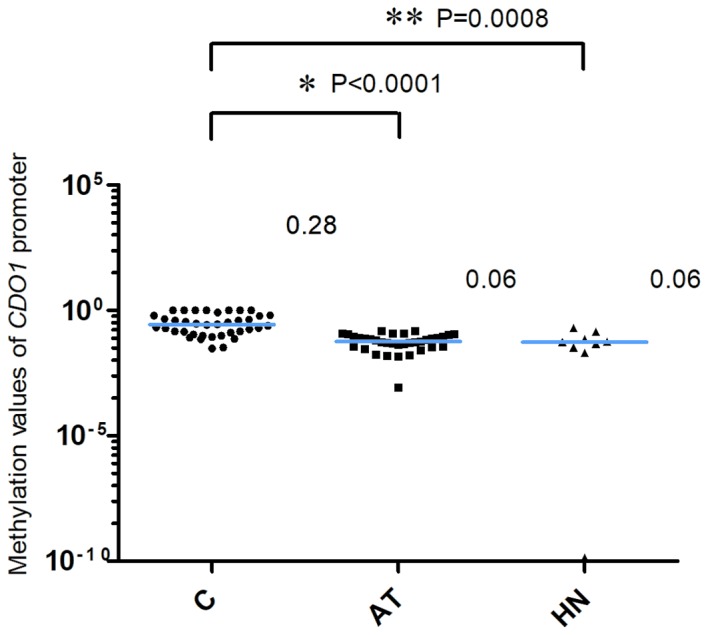
Methylation of the CDO1 promoter. The 2−ΔCq values of the CDO1 promoter in the AT and HN groups were significantly lower compared with those in the C group. *P<0.0001 and **P=0.0008, AT and HN groups vs. C group, respectively. The blue horizontal lines represent median values. One single data point in the HN group was outside the axis limits. C, cancer tissues; AT, adjacent tissues; HN, the healthy non-adjacent tissue from patients with extra-hepatic biliary tract cancer; CDO1, cysteine dioxygenase 1.
Figure 2.
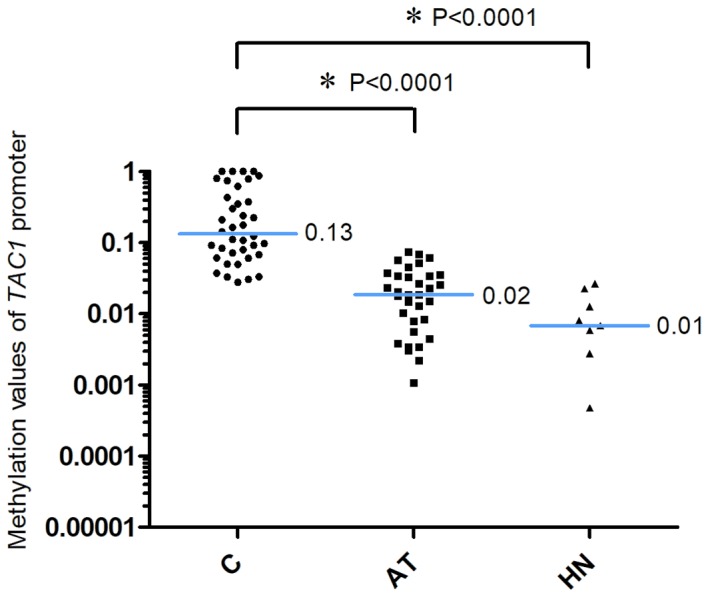
Methylation of the TAC1 promoter. The 2−ΔCq values of TAC1 promoter in the AT and HN groups were significantly lower compared with those in the C group. *P<0.0001, AT and HN groups vs. C group. The blue horizontal lines represent median values. Two data points in the AT group and one data point in the HN group were outside the axis limits. C, cancer tissues; AT, adjacent tissues; HN, the healthy non-adjacent tissue from patients with extra-hepatic biliary tract cancer; TAC1, tachykinin precursor 1.
Figure 3.
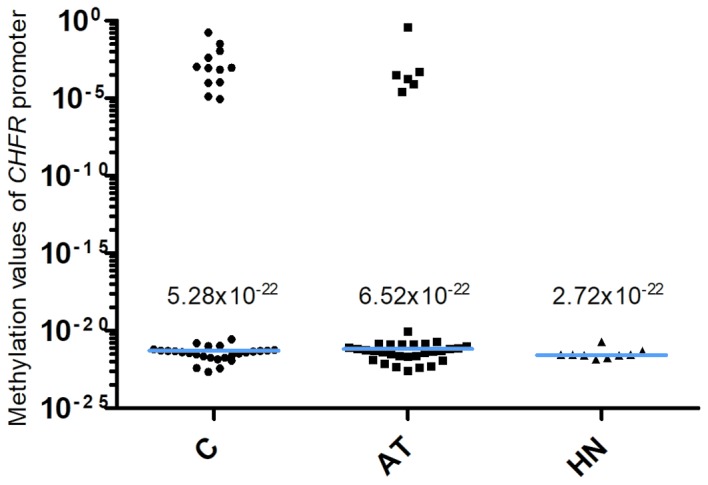
Methylation of the CHFR promoter. The 2−ΔCq values of the CHFR gene promoter in the C, AT and HN groups were 5.28×10−22, 6.52×10−22 and 2.72×10−22, respectively. There was no significant difference in the 2−ΔCq value among pancreatic cancer, adjacent non-cancer tissue and tumor-free pancreatic samples (P=0.5030, C vs. AT; P=0.1388, C vs. HN). The blue horizontal lines represent median values. C, cancer tissues; AT, adjacent tissues; HN, the healthy non-adjacent tissue from patients with extra-hepatic biliary tract cancer; CHFR, checkpoint with forkhead and ring finger domains.
Association between the patients' clinicopathological characteristics and the methylation values
The association between the patients' clinicopathological characteristics and the 2−ΔCq values of the CDO1, TAC1 and CHFR promoter regions in the cancer tissues was investigated (Table IV). No significant association was observed between the 2−ΔCq values of the three gene promoters and the clinicopathological variables tumor stage, tumor size or tumor differentiation. However, a significant association between the 2−ΔCq values of the CHFR promoter and node metastasis was observed. The 2−ΔCq values of the CHFR promoter in node metastasis-positive cases were significantly higher compared with those in node metastasis-negative cases (P=0.0484).
Table IV.
Comparison between the patients' clinicopathological characteristics and the methylation values of CDO1, CHFR and TAC1.
| Variable | CDO1 2−ΔCq value, median (25 and 75th percentiles) | TAC1 2−ΔCq value, median (25 and 75th percentiles) | CHFR 2−ΔCq value, median (25 and 75th percentiles) |
|---|---|---|---|
| Node metastasis | |||
| Positive | 0.23 (0.11–0.69) | 0.13 (0.06–0.65) | 1.01×10−21 (4.05×10−22−3.97×10−4) |
| Negative | 0.42 (0.22–0.64) | 0.15 (0.08–0.28) | 3.23×10−22 (6.56×10−22−5.37×10−22) |
| P-value | 0.3151 | 0.9857 | 0.0484a |
| Tumor size, cm | |||
| ≤4 | 0.33 (0.11–0.63) | 0.09 (0.06–0.40) | 6.17×10−22 (3.5×10−22−5.42×10−4) |
| >4 | 0.24 (0.16–0.82) | 0.21 (0.10–0.76) | 4.75×10−22 (1.65×10−22−1.32×10−21) |
| P-value | 0.7345 | 0.1805 | 0.2587 |
| Differentiation | |||
| Wel, mod | 0.23 (0.12–0.57) | 0.12 (0.07–0.42) | 5.87×10−22 (3.48×10−22−6.87×10−4) |
| Por | 0.91 (0.30–1.00) | 0.46 (0.04–0.84) | 3.37×10−22 (1.47×10−22−8.70×10−22) |
| P-value | 0.0680 | 0.5750 | 0.1223 |
| Stage | |||
| IB | 0.39 (0.23–0.73) | 0.09 (0.06–0.24) | 3.56×10−22 (1.63×10−22−5.34×10−4) |
| IIA | 0.61 (0.18–0.65) | 0.22 (0.12–0.80) | 1.47×10−22 (3.86×10−23−3.87×10−22) |
| IIB | 0.21 (0.08–0.40) | 0.14 (0.06–0.43) | 6.46×10−22 (4.62×10−22−9.23×10−4) |
| III | 0.22 (0.11–0.87) | 0.10 (0.04–0.58) | 2.83×10−21 (3.46×10−22−3.97×10−4) |
| IV | 0.44 (0.14–1.01) | 0.62 (0.12–0.83) | 5.22×10−22 (2.66×10−22−5.70×10−3) |
| P-value | 0.6009 | 0.5566 | 0.2562 |
P<0.05. Comparison between the three gene values and node metastasis, tumor size and differentiation was analyzed with Mann-Whitney U test. Comparison between the three gene values and tumor stage was analyzed with Kruskal-Wallis test. CDO1, cysteine dioxygenase 1; CHFR, checkpoint with forkhead and ring finger domains; TAC1, tachykinin precursor 1; Wel, well differentiated adenocarcinomas; Mod, moderately differentiated carcinomas; Por, poorly differentiated ductal adenocarcinomas.
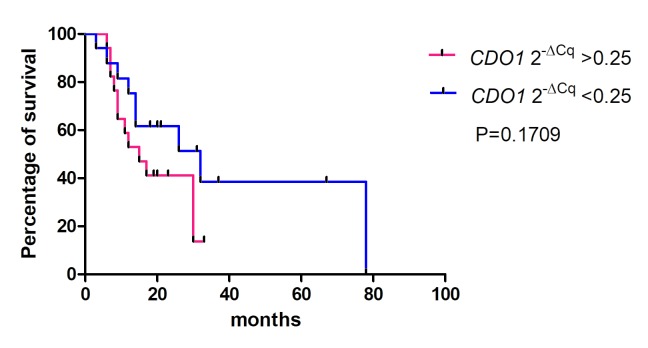
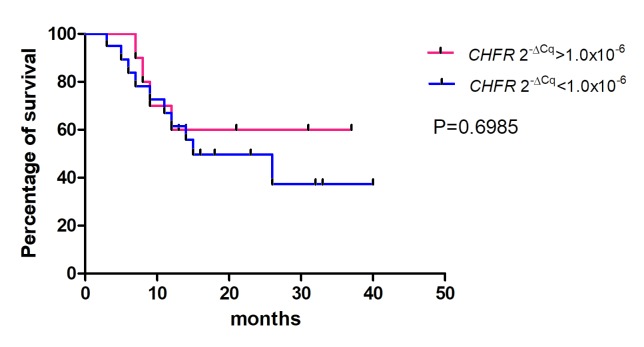
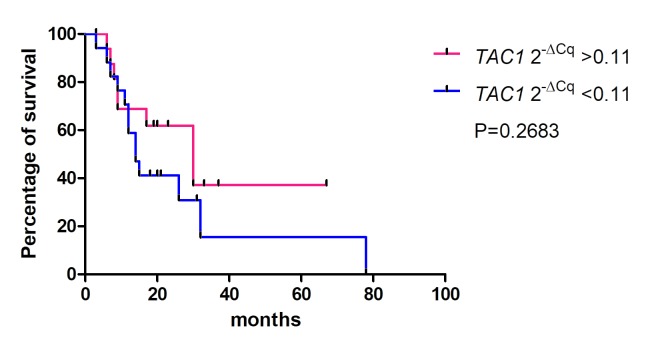
The association between the 2−ΔCq values of the CDO1, TAC1 and CHFR genes in pancreatic cancer tissues and the overall survival rates of patients was determined using Kaplan-Meier analysis (Figs. 4–6). The values from 5 cases were excluded because these patients had stage IV cancer and underwent palliative resection. The cut-off values were defined as the median of the CDO1 and TAC1 promoter 2−ΔCq values, as previously described (13), and were 0.25 and 0.11 for the CDO1 and TAC1 genes, respectively. The cut-off value for the CHFR 2−ΔCq value was 1.0×10−6. The results demonstrated that there was no significant association between the 2−ΔCq values of the CDO1, TAC1 and CHFR genes and the overall survival rates of patients with pancreatic cancer [P=0.1709 (Fig. 4), P=0.2683 (Fig. 5) and P=0.6985 (Fig. 6), respectively].
Figure 4.
Survival rates for high and low CDO1 promoter 2−ΔCq values estimated by the Kaplan-Meier method. There was no significant difference between the high and low hypermethylation groups of the CDO1 promoter. The cut-off value was defined as 0.25, which was the median 2−ΔCq value of the CDO1 promoter. In total, 5 patients with stage IV pancreatic cancer who underwent palliative resection were excluded. CDO1, cysteine dioxygenase 1.
Figure 6.
Survival rates for high and low CHFR promoter 2−ΔCq values estimated by the Kaplan-Meier method. There was no significant difference between the high and low hypermethylation groups of the CHFR promoter. The cut-off value was defined as 1.0×10−6, which was considered the positively hypermethylated. 2−ΔCq value of the CHFR promoter. In total, 5 patients with stage IV pancreatic cancer who underwent palliative resection were excluded. CHFR, checkpoint with forkhead and ring finger domains.
Figure 5.
Survival rates for high and low TAC1 promoter 2−ΔCq values estimated by the Kaplan-Meier method. There was no significant difference between the high and low hypermethylation groups of the TAC1 promoter. The cut-off value was defined as 0.11, which was the median 2−ΔCq value of the TAC1 promoter. In total, 5 patients with stage IV pancreatic cancer who underwent palliative resection were excluded. TAC1, tachykinin precursor 1.
Discussion
The epigenetic hypermethylation of the promoter CpG islands of tumor-suppressor genes, including APC, BRCA1, p16INK4a can induce transcription inactivation during tumorigenesis, which is often observed in pancreatic cancer (6). Previous studies in pancreatic cancer reported frequent genetic abnormalities in Kras gene activation, but also in the epigenetic inactivation of p16INK4a, p53 and SMAD4 in >50% of pancreatic ductal cancer cases (4,5). Guo et al (26) demonstrated that the promoters of the genes APC regulator of WNT signaling pathway, BRCA1 DNA repair associated, p16INK4a, p15INK4b, retinoid acid receptor-β and p73 were hypermethylated in patients with pancreatic ductal cancer. However, the promoter hypermethylation of TAC1 and CHFR remains unclear. Hypermethylation of the CDO1 gene promoter in only 20 pancreatic cancer tissues has been evaluated by Vedeld et al (12), who reported that promoter of CDO1 in 18 of the 20 pancreatic cancer tissues using FFPE samples is hypermethylated. However, the association between CDO1 gene promoter methylation status and clinicopathological characteristics of patients was not analyzed.
CDO1 is a protein that catalyzes the conversion of cysteine to cysteine sulfinic acid, which helps decreasing the levels of reactive oxygen species (ROS) in the cell (35). Furthermore, depletion of CDO1 increases oxidative stress in tumor cells, which induces tumor cell resistance to ROS and metastasis (9). Hypermethylation of the CDO1 CpG island promoter has been reported in various types of cancer, including breast (9,13), lung (non-small cell type) (14), colon (12), kidney (clear cell type) (11), esophageal (10) and pancreatic cancer (12). Vedeld et al (12) reported that CDO1 silencing occurs in early-stage tumorigenesis of colorectal cancer and that CDO1 hypermethylation is detected in normal colorectal mucosa samples. These results suggest that genetic methylation could occur prior to detection of any histological, anatomical or morphological changes. The results from the present study demonstrated that the 2−ΔCq values of the CDO1 promoter regions in the adjacent non-cancerous pancreatic tissues of patients with pancreatic cancer were lower compared with those of patients with pancreatic cancer tissues; however, methylation did occur in these histologically normal-appearing tissues. These results also suggested that CDO1 methylation may occur before detection of morphological changes in pancreatic cancer. The reason why the methylation value of CDO1 promoter was elevated in HN group remains unclear. CDO1 promoter hypermethylation in pancreatic cancer tumorigenesis appears therefore to be similar to that in colorectal cancer.
TAC1 encodes preprotachykinin-1, which is converted to neurokinin A or substance P (36). Since neurokinin A inhibits cell proliferation in normal cell (37), TAC1 is therefore considered a tumor-suppressor gene, and hypermethylation of the TAC1 CpG island promoter has been observed in various types of cancer, including lung (non-small cell type) cancer (14), colon cancer (15), head and neck cancer (16), uterus cancer (17) and pancreatic cancer (27,28). Patai et al (38) reported that TAC1 promoter is hypermethylated in the precancerous condition of colorectal sessile serrated adenomas. Subsequently, TAC1 gene methylation is likely to occur during the early stage of tumorigenesis in colorectal cancer (38). In the present study, TAC1 promoter methylation was higher in pancreatic cancer tissues compared with that in adjacent non-cancerous tissues. Similar to CDO1, hypermethylation of TAC1 promoter was also detected in adjacent non-cancerous tissues, suggesting that TAC1 promoter methylation may occur during the early stage of tumorigenesis in pancreatic cancer.
CHFR encodes a protein that regulates DNA synthesis and delays entry into mitosis during the G2 phase (39). Hypermethylation of the CHFR gene is crucial during esophageal and gastric cancer tumorigenesis (20,21,40). CHFR promoter methylation could also provide clinical information, including clinical response to taxane chemotherapy, since patients with gastric or esophageal cancer and with CHFR hypermethylation, or with CHFR gene silencing in gastric and esophageal cancer are thought to have good clinical responses to docetaxel and paclitaxel treatments (20,41). Pelosof et al (18) suggested therefore that docetaxel should be used for the treatment of patients with colorectal cancer who presented with CHFR promoter methylation. Cleven et al (19) reported that hypermethylation of CHFR in patients with colorectal cancer indicates poor prognosis of stage ll colorectal cancer. Subsequently, CHFR methylation may serve for selecting chemotherapy agents for cancers of the digestive tract system, and could be considered a putative prognostic indicator in cancer therapy. The results from the present study demonstrated that CHFR promoter hypermethylation only occurred in 12 out of 38 cases (31.6%) and did not predict pancreatic cancer tumorigenesis. Since the response rate to gemcitabine and nab-paclitaxel is 23% in the MPACT trial (42), the present study hypothesized that 31.6% as a CHFR hypermethylation frequency might be reasonable. The present study also demonstrated that patients with lymph node metastasis had higher 2−ΔCq values of CHFR gene promoter methylation compared with those of patients without lymph node metastasis. In gastric and colorectal cancer, CHFR methylation has been reported to be associated with lymph node metastasis and prognosis (29,30). Although the present study did not report the prognostic value of CHFR gene promoter methylation in patients with pancreatic cancer, it demonstrated that CHFR gene methylation was associated with lymph node metastasis in patients with pancreatic cancer. However, two populations presenting highly different CHFR methylation values in the C and AT groups were observed. These observations may be caused by cell contamination, such as tumor cells migration to non-tumor tissue, although absence of cancer was confirmed by histopathological analysis. However, the CHFR methylation levels were increased in the cancer-free pancreas or precancerous condition, which has been previously described (27).
This study presented some limitations. Firstly, the sample size was small. Secondly, evaluation of the methylation status of the three genes in normal pancreatic tissue or tissues from patients with chronic pancreatitis. Thirdly, the association between disease recurrence of patients treated with chemotherapy, in particular paclitaxel, and their overall survival rate was not assessed. In addition, further investigation on the role of CHFR as a prognostic and predictive marker is required.
Pancreatic cancer is characterized by virulent tumor and a low 5-year survival rate (6%) mainly because it is frequently diagnosed at a late stage (1,2). The present study demonstrated that CDO1 and TAC1 promoter methylation values were similar in all stages. These results suggest that the hypermethylation of CDO1 and TAC1 promoters may be related to early events in pancreatic cancer.
The methylation values of CDO1 and TAC1 promoters in cancer tissues were higher compared with adjacent tissues. However, whether the hypermethylation of CDO1 and TAC1 may serve as biomarkers for the diagnosis of pancreatic cancer remains unknown. The role of CHFR promoter methylation in pancreatic cancer remains unclear and requires further investigation.
Acknowledgements
The authors would like to thank Professor Ryo Wada (Department of Pathology, Juntendo University Shizuoka Hospital) for his histopathological diagnosis, and Ms. Junko Kawai (Department of Pathology, Juntendo University Shizuoka Hospital) for preparing FFPE sections.
Funding
This study was supported by a Grant-in-Aid from MEXT-Supported Program for the Strategic Research Foundation at Private Universities, 2015–2019 (grant no. S1511008L) and the Banks Family Foundation. Tomoaki Ito was supported by the TORAY Company.
Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Authors' contributions
HM, KS, TI and MB designed this study. HO, MS and TK collected FFPE samples and clinical information. TI and AH performed the experiments. HM and TI analyzed the data and drafted the manuscript. MB revised the manuscript. All authors reviewed and approved the final version of the manuscript.
Ethical approval and consent to participate
The study protocol followed the ethical guidelines of the World Medical Association and the Declaration of Helsinki, and was approved by the Ethical Committee of Juntendo University Shizuoka hospital. Patients provided informed consent for the use of their samples.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.McGuigan A, Kelly P, Turkington RC, Jones C, Coleman HG, McCain RS. Pancreatic cancer: A review of clinical diagnosis, epidemiology, treatment and outcomes. World J Gastroenterol. 2018;24:4846–4861. doi: 10.3748/wjg.v24.i43.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Urayama S. Pancreatic cancer early detection: Expanding higher-risk group with clinical and metabolomics parameters. World J Gastroenterol. 2015;21:1707–1717. doi: 10.3748/wjg.v21.i6.1707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Deramaudt T, Rustgi AK. Mutant KRAS in the initiation of pancreatic cancer. Biochim Biophys Acta. 2005;1756:97–101. doi: 10.1016/j.bbcan.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 4.Maitra A, Kern SE, Hruban RH. Molecular pathogenesis of pancreatic cancer. Best Pract Res Clin Gastroenterol. 2006;20:211–226. doi: 10.1016/j.bpg.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 5.Cowan RW, Maitra A. Genetic progression of pancreatic cancer. Cancer J. 2014;20:80–84. doi: 10.1097/PPO.0000000000000011. [DOI] [PubMed] [Google Scholar]
- 6.Silverman BR, Shi JQ. Alterations of epigenetic regulators in pancreatic cancer and their clinical implications. Int J Mol Sci. 2016;17(pii):E2138. doi: 10.3390/ijms17122138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Baylin SB, Herman JG. DNA hypermethylation in tumorigenesis: Epigenetics joins genetics. Trends Genet. 2000;16:168–174. doi: 10.1016/s0168-9525(99)01971-x. [DOI] [PubMed] [Google Scholar]
- 8.Brait M, Ling S, Nagpal JK, Chang X, Park HL, Lee J, Okamura J, Yamashita K, Sidransky D, Kim MS. Cysteine dioxygenase 1 is a tumor suppressor gene silenced by promoter methylation in multiple human cancers. Cancer Res. 2012;7:e44951. doi: 10.1371/journal.pone.0044951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jeschke J, O'Hagan HM, Zhang W, Vatapalli R, Calmon MF, Danilova L, Nelkenbrecher C, Van Neste L, Bijsmans IT, Van Engeland M, et al. Frequent inactivation of cysteine dioxygenase type 1 contributes to survival of breast cancer cells and resistance to anthracyclines. Clin Cancer Res. 2013;19:3201–3211. doi: 10.1158/1078-0432.CCR-12-3751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kwon J, Park M, Kim JH, Lee HW, Kang MC, Park JH. Epigenetic regulation of the novel tumor suppressor cysteine dioxygenase 1 in esophageal squamous cell carcinoma. Tumour Biol. 2015;36:7449–7456. doi: 10.1007/s13277-015-3443-x. [DOI] [PubMed] [Google Scholar]
- 11.Deckers IA, Schouten LJ, Van Neste L, van Vlodrop IJ, Soetekouw PM, Baldewijns MM, Jeschke J, Ahuja N, Herman JG, van den Brandt PA, van Engeland M. Promoter methylation of CDO1 identifies clear-cell renal cell cancer patients with poor survival outcome. Clin Cancer Res. 2015;21:3492–3500. doi: 10.1158/1078-0432.CCR-14-2049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vedeld HM, Andresen K, Eilertsen IA, Nesbakken A, Seruca R, Gladhaug IP, Thiis-Evensen E, Rognum TO, Boberg KM, Lind GE. The novel colorectal cancer biomarkers CDO1, ZSCAN18 and ZNF331 are frequently methylated across gastrointestinal cancers. Int J Cancer. 2015;136:844–853. doi: 10.1002/ijc.29039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Minatani N, Waraya M, Yamashita K, Kikuchi M, Ushiku H, Kojo K, Ema A, Nishimiya H, Kosaka Y, Katoh H, et al. Prognostic significance of promoter DNA Hypermethylation of cysteine dioxygenase 1 (CDO1) Gene in primary breast cancer. PLoS One. 2016;11:e0144862. doi: 10.1371/journal.pone.0144862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wrangle J, Machida EO, Danilova L, Hulbert A, Franco N, Zhang W, Glöckner SC, Tessema M, Van Neste L, Easwaran H, et al. Functional identification of cancer-specific methylation of CDO1, HOXA9, and TAC1 for the diagnosis of lung cancer. Clin Cancer Res. 2014;20:1856–1864. doi: 10.1158/1078-0432.CCR-13-2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tham C, Chew M, Soong R, Lim J, Ang M, Tang C, Zhao Y, Ong SY, Liu YQ. Postoperative serum methylation levels of TAC1 and SEPT9 are independent predictors of recurrence and survival of patients with colorectal cancer. Cancer. 2014;120:3131–3141. doi: 10.1002/cncr.28802. [DOI] [PubMed] [Google Scholar]
- 16.Misawa K, Mochizuki D, Imai A, Endo S, Mima M, Misawa Y, Kanazawa T, Carey TE, Mineta H. Prognostic value of aberrant promoter hypermethylation of tumor-related genes in early-stage head and neck cancer. Oncotarget. 2016;7:26087–26098. doi: 10.18632/oncotarget.8317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Liu MY, Zhang H, Hu YJ, Chen YW, Zhao XN. Identification of key genes associated with cervical cancer by comprehensive analysis of transcriptome microarray and methylation microarray. Oncol Lett. 2016;12:473–478. doi: 10.3892/ol.2016.4658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pelosof L, Yerram SR, Ahuja N, Delmas A, Danilova L, Herman JG, Azad NS. CHFR silencing or microsatellite instability is associated with increased antitumor activity of docetaxel or gemcitabine in colorectal cancer. Int J Cance. 2014;134:596–605. doi: 10.1002/ijc.28390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cleven AHG, Derks S, Draht MX, Smits KM, Melotte V, Van Neste L, Tournier B, Jooste V, Chapusot C, Weijenberg MP, et al. CHFR promoter methylation indicates poor prognosis in stage II microsatellite stable colorectal cancer. Clin Cancer Res. 2014;20:3261–3271. doi: 10.1158/1078-0432.CCR-12-3734. [DOI] [PubMed] [Google Scholar]
- 20.Li Y, Yang Y, Lu Y, Herman JG, Brock MV, Zhao P, Guo M. Predictive value of CHFR and MLH1 methylation in human gastric cancer. Gastric Cancer. 2015;18:280–287. doi: 10.1007/s10120-014-0370-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sepulveda JL, Gutierrez-Pajares JL, Luna A, Yao Y, Tobias JW, Thomas S, Woo Y, Giorgi F, Komissarova EV, Califano A, et al. High-definition CpG methylation of novel genes in gastric carcinogenesis identified by next-generation sequencing. Mod Pathol. 2016;29:182–193. doi: 10.1038/modpathol.2015.144. [DOI] [PubMed] [Google Scholar]
- 22.Ordóñez-Mena JM, Schöttker B, Mons U, Jenab M, Freisling H, Bueno-de-Mesquita B, O'Doherty MG, Scott A, Kee F, Stricker BH, et al. Quantification of the smoking-associated cancer risk with rate advancement periods: Meta-analysis of individual participant data from cohorts of the CHANCES consortium. BMC Med. 2016;14:62. doi: 10.1186/s12916-016-0607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.de Menezes RF, Bergmann A, Thuler LC. Alcohol consumption and risk of cancer: A systematic literature review. Asian Pac J Cancer Prev. 2013;14:4965–4972. doi: 10.7314/apjcp.2013.14.9.4965. [DOI] [PubMed] [Google Scholar]
- 24.Rahimi E, Batra S, Thosani N, Singh H, Guha S. Increased incidence of second primary pancreatic cancer in patients with prior colorectal cancer: A population-based US study. Dig Dis Sci. 2016;61:1652–1660. doi: 10.1007/s10620-016-4170-x. [DOI] [PubMed] [Google Scholar]
- 25.Chung JW, Chung MJ, Bang S, Park SW, Song SY, Chung JB, Park JY. Assessment of the risk of colorectal cancer survivors developing a second primary pancreatic cancer. Gut Liver. 2017;11:728–732. doi: 10.5009/gnl16526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guo M, Jia Y, Yu Z, House MG, Esteller M, Brock MV, Herman JG. Epigenetic changes associated with neoplasms of the exocrine and endocrine pancreas. Discov Med. 2014;17:67–73. [PMC free article] [PubMed] [Google Scholar]
- 27.Henriksen SD, Madsen PH, Larsen AC, Johansen MB, Drewes AM, Pedersen IS, Krarup H, Thorlacius-Ussing O. Cell-free DNA promoter hypermethylation in plasma as a diagnostic marker for pancreatic adenocarcinoma. Clin Epigenetics. 2016;8:117. doi: 10.1186/s13148-016-0286-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Henriksen SD, Madsen PH, Larsen AC, Johansen MB, Pedersen IS, Krarup H, Thorlacius-Ussing O. Promoter hypermethylation in plasma-derived cell-free DNA as a prognostic marker for pancreatic adenocarcinoma staging. Int J Cancer. 2017;141:2489–2497. doi: 10.1002/ijc.31024. [DOI] [PubMed] [Google Scholar]
- 29.Sun Z, Liu J, Jing H, Dong SX, Wu J. The diagnostic and prognostic value of CHFR hypermethylation in colorectal cancer, a meta-analysis and literature review. Oncotarget. 2017;8:89142–89148. doi: 10.18632/oncotarget.19408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ding Y, Lian HF, Du Y. Clinicopathological significance of CHFR promoter methylation in gastric cancer: A meta-analysis. Oncotarget. 2017;9:10083–10090. doi: 10.18632/oncotarget.23394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bosman FT, Carneiro F, Hruban RH, Theise ND. World Health Organization; 2010. WHO classification of tumours of the digestive system. [Google Scholar]
- 32.Brierley JD, Gospodarowicz M, Wittekind C. TNM classification of malignant tumors 8th edn. Wiley-Blackwell; 2017. UICC International union against cancer. [Google Scholar]
- 33.Hulbert A, Jusue-Torres I, Stark A, Chen C, Rodgers K, Lee B, Griffin C, Yang A, Huang P, Wrangle J, et al. Early detection of lung cancer using DNA promoter hypermethylation in plasma and sputum. Clin Cancer Res. 2017;23:1998–2005. doi: 10.1158/1078-0432.CCR-16-1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7:33. doi: 10.1186/1471-2199-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oien DB, Moskovitz J. Ablation of the mammalian methionine sulfoxide reductase A affects the expression level of cysteine deoxygenase. Biochem Biophys Res Commun. 2007;352:556–559. doi: 10.1016/j.bbrc.2006.11.063. [DOI] [PubMed] [Google Scholar]
- 36.Patak E, Pinto FM, Story ME, Pintado CO, Fleming A, Page NM, Pennefather JN, Candenas ML. Functional and molecular characterization of tachykinins and tachykinin receptors in the mouse uterus. Biol Reprod. 2005;72:1125–1133. doi: 10.1095/biolreprod.104.036814. [DOI] [PubMed] [Google Scholar]
- 37.Rameshwar P, Gascón P. Induction of negative hematopoietic regulators by neurokinin-A in bone marrow stroma. Blood. 1996;88:98–106. [PubMed] [Google Scholar]
- 38.Patai ÁV, Barták BK, Péterfia B, Micsik T, Horváth R, Sumánszki C, Péter Z, Patai Á, Valcz G, Kalmár A, et al. Comprehensive DNA methylation and mutation analyses reveal a methylation signature in colorectal sessile serrated adenomas. Pathol Oncol Res. 2017;23:589–594. doi: 10.1007/s12253-016-0154-6. [DOI] [PubMed] [Google Scholar]
- 39.Kang D, Chen J, Wong J, Fang G. The checkpoint protein Chfr is a ligase that ubiquitinates Plk1 and inhibits Cdc2 at the G2 to M transition. J Cell Biol. 2002;156:249–259. doi: 10.1083/jcb.200108016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shibata Y, Haruki N, Kuwabara Y, Ishiguro H, Shinoda N, Sato A, Kimura M, Koyama H, Toyama T, Nishiwaki T, et al. Chfr expression is downregulated by CpG island hypermethylation in esophageal cancer. Carcinogenesis. 2002;23:1695–1699. doi: 10.1093/carcin/23.10.1695. [DOI] [PubMed] [Google Scholar]
- 41.Yun T, Liu Y, Gao D, Linghu E, Brock MV, Yin D, Zhan Q, Herman JG, Guo M. Methylation of CHFR sensitizes esophageal squamous cell cancer to docetaxel and paclitaxel. Genes Cancer. 2015;6:38–48. doi: 10.18632/genesandcancer.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Von Hoff DD, Ervin T, Arena FP, Chiorean EG, Infante J, Moore M, Seay T, Tjulandin SA, Ma WW, Saleh MN, et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N Engl J Med. 2013;369:1691–1703. doi: 10.1056/NEJMoa1304369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.