Abstract
Background
Reduced circulating estrogen levels around the time of the menopause can induce unacceptable symptoms that affect the health and well‐being of women. Hormone therapy (both unopposed estrogen and estrogen/progestogen combinations) is an effective treatment for these symptoms, but is associated with risk of harms. Guidelines recommend that hormone therapy be given at the lowest effective dose and treatment should be reviewed regularly. The aim of this review is to identify the minimum dose(s) of progestogen required to be added to estrogen so that the rate of endometrial hyperplasia is not increased compared to placebo.
Objectives
The objective of this review is to assess which hormone therapy regimens provide effective protection against the development of endometrial hyperplasia or carcinoma.
Search methods
We searched the Cochrane Menstrual Disorders and Subfertility Group trials register (searched January 2012), The Cochrane Library (Issue 1, 2012), MEDLINE (1966 to January 2012), EMBASE (1980 to January 2012), Current Contents (1993 to May 2008), Biological Abstracts (1969 to 2008), Social Sciences Index (1980 to May 2008), PsycINFO (1972 to January 2012) and CINAHL (1982 to May 2008). Attempts were made to identify trials from citation lists of reviews and studies retrieved, and drug companies were contacted for unpublished data.
Selection criteria
Randomised comparisons of unopposed estrogen therapy, combined continuous estrogen‐progestogen therapy, sequential estrogen‐progestogen therapy with each other or placebo, administered over a minimum period of 12 months. Incidence of endometrial hyperplasia/carcinoma assessed by a biopsy at the end of treatment was a required outcome. Data on adherence to therapy, rates of additional interventions, and withdrawals owing to adverse events were also extracted.
Data collection and analysis
In this update, 46 studies were included. Odds ratios (ORs) were calculated for dichotomous outcomes. The small numbers of studies in each comparison and the clinical heterogeneity precluded meta‐analysis for many outcomes.
Main results
Unopposed estrogen is associated with increased risk of endometrial hyperplasia at all doses, and durations of therapy between one and three years. For women with a uterus the risk of endometrial hyperplasia with hormone therapy comprising low‐dose estrogen continuously combined with a minimum of 1 mg norethisterone acetate (NETA) or 1.5 mg medroxyprogesterone acetate (MPA) is not significantly different from placebo at two years (1 mg NETA: OR 0.04; 95% confidence interval (CI) 0 to 2.8; 1.5 mg MPA: no hyperplasia events).
Authors' conclusions
Hormone therapy for postmenopausal women with an intact uterus should comprise both estrogen and progestogen to reduce the risk of endometrial hyperplasia.
Keywords: Female; Humans; Drug Therapy, Combination; Drug Therapy, Combination/methods; Drug Therapy, Combination/standards; Endometrial Hyperplasia; Endometrial Hyperplasia/chemically induced; Endometrial Hyperplasia/prevention & control; Endometrial Neoplasms; Endometrial Neoplasms/chemically induced; Endometrial Neoplasms/prevention & control; Estrogen Replacement Therapy; Estrogen Replacement Therapy/adverse effects; Estrogen Replacement Therapy/standards; Estrogens; Estrogens/administration & dosage; Postmenopause; Progestins; Progestins/administration & dosage; Randomized Controlled Trials as Topic; Uterine Hemorrhage; Uterine Hemorrhage/chemically induced; Uterine Hemorrhage/prevention & control
Plain language summary
Hormone therapy for postmenopausal women with intact uterus
Hormone therapy may be used to manage troublesome menopausal symptoms, but is currently recommended to be given at the lowest effective dose and regularly reviewed by a woman and her doctor. In women with an intact uterus hormone therapy comprising estrogen and progestogen is desirable to minimise the risk of endometrial hyperplasia, which can develop into endometrial cancer. Low‐dose estrogen plus progestogen (minimum of 1 mg norethisterone acetate or 1.5 mg medroxyprogesterone acetate) taken daily (continuously) appears to be safe for the endometrium. For women who had their last menstrual period less than one year ago low‐dose estrogen combined sequentially with 10 days of progestogen (1 mg norethisterone acetate) per month appears to be safe for the endometrium.
Background
Description of the condition
Menopause means the cessation of menstruation and typically occurs in women aged between 45 and 55 years with a mean age of about 51 years. Women are said to be postmenopausal when menstruation has ceased for six to 12 months and blood serum levels of follicle‐stimulating hormone (FSH) increase to at least 49 IU/L. The decline in circulating estrogen around the time of the menopause can induce symptoms that affect the well‐being and health of women; hot flushes, insomnia, declining bone mass, night sweats, mood disturbances and vaginal dryness have all been reported. Estrogen therapy has been utilised for the treatment of many of the menopausal symptoms, particularly hot flushes and vaginal dryness. As life expectancy and the proportion of older adults in the population increase, there has been an increased focus on the effects of ageing.
Description of the intervention
Hormone therapy (HT) may consist of unopposed estrogen or a combination of estrogen plus progestogen (E+P).
Unopposed estrogens include conjugated equine estrogen (CEE), ethinyl estradiol (EE), micronised 17β‐estradiol (E2), estradiol valerate (EV), estrone sulphate (EIS) and esterified estrogens (ESE). These estrogens cannot be considered equal. They vary in their dose equivalency and have different metabolic effects on different tissues or end organs. In order to make meaningful comparisons estrogens were grouped into low, moderate and high doses according to the advice of experts (Ansbacher 1994; France 1998; MacLennan 1998; O'Connell 1998). See Table 9.
1. Classification of estrogen dosages.
| Oestrogen | Low dose (mg/day) | Moderate dose (mg/day) | High dose (mg/day) |
| Conjugated equine estrogens (CEE) | 0.15, 0.3, 0.4, 0.45 | 0.625 | 1.25 |
| Piperazine estrone sulphate (POS) | 0.3, 0.625 | 1.25, 1.5 | 2.5 |
| Ethinyl estradiol (EE) | < 0.010 | 0.010 | > 0.010 |
| 17β estradiol (E2) | 0.5, 1.0 | 1.5, 2 | 4 |
| Estradiol valerate (EV) | (EV)0.5 | 1 | 2 |
| Esterified estrogens (ESE) | 0.3 | 0.625 | 1.25 |
There are a number of different progestogens used in HT, which can be classified according to their structure and bioactivity, or both (Lawrie 2011). These include:
micronised progesterone (MP), dydrogesterone (DYG; a retroprogesterone), and progesterone derivatives such as medrogestone (MG);
pregnanes such as medroxyprogesterone acetate (MPA), megestrol acetate (MA), cyproterone acetate (CPA);
norpregnanes such as trimegestone (TMG), nestorone and promegestone;
estranges such as norethisterone, norethisterone acetate (NETA) and lynestrol, derived from testosterone and collectively known as first‐generation progestogens;
gonane progestogens, which can be divided into two categories ‐ the second‐generation progestogens such as norgestrel (NG), levonorgestrel (LNG) and the third‐generation progestogens, such as desogestrel (DG), gestodene, norgestimate (NGM) and dienogest (DNG);
drospirinone (DSP) ‐ a new progestogen derived from spironolactone not included in the generation classification.
Some progestogens are prodrugs that are metabolised by the liver into active compounds. Examples are promegestone which is converted to TMG, and NGM which is converted to NG.
Why it is important to do this review
Several studies have suggested a causal relationship between unopposed estrogen therapy (daily use of estrogen without the addition of progestogen) and the development of endometrial hyperplasia and carcinoma (Antunes 1979; Gardan 1977; Grady 1995; HOPE 2001; Smith 1977; Ziel 1975). Endometrial hyperplasia is regarded as a precursor of endometrial cancer but progression is dependent on the type of hyperplasia (Kurman 2000; Terakawa 1997). The estimated risk of hyperplasia progression to cancer is 1%, 3%, 8% and 29% for women with simple, complex, simple atypical and complex atypical hyperplasia, respectively (Kurman 1985). The risk of hyperplasia and carcinoma appears to increase with higher doses and increased duration of unopposed estrogen treatment (Speroff 2005). Adding a progestogen to estrogen therapy significantly reduces the risk of hyperplasia (Corson 1999; Cust 1990;Udoff 1995; Whitehead 1977), but can result in premenstrual symptoms that are problematic for some women. These symptoms and increased bleeding and spotting with both types of HT regimens are often given as a reason not to continue HT (Ellerington 1992; Rozenberg 2001). An additional concern is that the addition of progestogen to estrogen also appears to increase the risk of cardiovascular disease and breast cancer (WHI 2002).
As a result of evidence suggesting that HT is associated with an increased risk of cardiovascular disease and breast cancer in women aged over 55 years (WHI 2002), current advice is that HT not be used for chronic disease prevention. HT is an effective treatment for women with menopausal symptoms of hot flushes, night sweats and vaginal dryness and the duration of therapy should be decided for individual women based on an assessment of both benefits in terms of menopausal symptom management, and harms of therapy such as venous thromboembolism (Hickey 2005; Roberts 2007). The duration of treatment with HT should be reviewed by a woman with her doctor, because for most women hot flushes resolve within one year of onset of the menopause. About one third of women will continue to have vasomotor symptoms for up to five years and some women for even longer (Hickey 2005).
HT is usually taken orally, but there are also other modalities including transdermal (patches, gels and creams), subcutaneous (implants), intranasal, vaginal and intrauterine. This review will consider only oral HT and the other modalities will be covered in separate reviews.
The original version of this review investigated abnormal vaginal bleeding, and concluded as follows:
Regular withdrawal bleeding is expected with a sequential regimen of E+P but women appear to experience less irregular bleeding than with a continuous E+P regimen. Irregular bleeding or spotting is common in the first year of continuous E+P but following the first year of treatment, bleeding and spotting become more common in sequential E+P regimens. A large proportion of women taking continuous E+P become amenorrhoeic after one year of therapy while withdrawal bleeding continues for women taking sequential regimens.
Bleeding during the first year of continuous E+P therapy does not need investigation with vaginal ultrasound scan, endometrial thickness or endometrial biopsy (or a combination of these), but these investigations should be considered in the second and subsequent years of HT. Unscheduled bleeding on sequential HT should be investigated by hysteroscopy and endometrial biopsy. For the 2008 and subsequent updates of this review, we have addressed long‐term endometrial safety outcomes rather than bleeding patterns, and have amended the protocol accordingly
We aimed to assess which of the oral HT regimens, unopposed estrogen or E+P administered either continuously or sequentially, provides the best protection against the development of endometrial hyperplasia or carcinoma, has better adherence to therapy regimens and the lowest rate of unscheduled biopsies. In addition we aimed to compare the effects of different types, doses and duration of progestogen use on both endometrial hyperplasia and adherence to treatment, in order to determine the minimum dose and duration of progestogen required for endometrial protection.
Objectives
To assess which oral HT regimens provide effective protection against the development of endometrial hyperplasia or carcinoma.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials (RCTs) of oral estrogen or oral combined E+P therapy versus placebo, oral estrogen versus combined oral E+P (sequential or continuous therapy), oral E+P (continuous) versus oral E+P (sequential) with a minimum treatment period of 12 months.
Types of participants
Inclusion criteria
Postmenopausal women with an intact uterus, defined as women who have not menstruated for more than six months and who have a serum FSH of 40 IU/L or greater. It is recognised that this criterion is relatively liberal and that a common definition of postmenopausal status requires a minimum of 12 months to elapse since last menses, but the majority of trials use this more liberal criterion and postmenopausal status is often further confirmed by FSH levels. The definition includes women who have undergone a natural menopause and women who have had bilateral oophorectomy (removal of both ovaries). The participants could be recruited from any healthcare setting or from advertisements.
Exclusion criteria
Perimenopausal women (menstruation less than six months prior to study)
Intercurrent major disease
Previous HT within one month of commencement of the study
Any contraindication to HT (either unopposed estrogen or estrogen + progestogen therapy)
Current use of other sex steroids including tibolone
Types of interventions
Oral interventions administered for a period of 12 months or greater.
Unopposed estrogen versus placebo (low, moderate or high dose)
Combined estrogen + progestogen (continuous) versus placebo
Combined estrogen +progestogen (sequential) versus placebo
Unopposed estrogen versus combined E+P (continuous)
Unopposed estrogen versus combined E+P (sequential)
Combined estrogen + progestogen (continuous) versus E+P (sequential)
Continuous combined estrogen + progestogen (dose comparisons)
Sequential combined estrogen + progestogen (dose/regimen comparisons)
Estrogens vary in their dose equivalency and have different metabolic effects on different tissues or end organs. In order to make meaningful comparisons, estrogens were grouped into 'low', 'moderate' and 'high' dose subgroups. The allocations of different types and doses of estrogens to these groupings were made according to the literature and the advice of experts (Ansbacher 1994; France 1998; MacLennan 1998; O'Connell 1998) and are set out in Table 9. However, disagreement persists among clinical experts regarding categorisation of E2 in the moderate range and two different doses (1.5 mg and 2 mg) of this estrogen have sometimes been included in this category. From the information available (Ansbacher 1994) 0.010 mg EE can be considered equivalent to 0.625 mg CEE and can be considered a moderate dose. Consequently we have modified the categorisation of EE doses in this update of the review.
The progestogens also vary in dose equivalency and metabolic effects on different tissues. As all progestogens share the effect of inducing a structural change in estrogen‐primed endometrium, the potency of a progestogen can be expressed by the difference in dose required in order to achieve this transformation. Estimates of potency based on animal studies give a "transformation dose" per cycle (Kumar 2000). However, the serum level resulting from administration of progestogen to women is dependent on a number of variables including the mode of administration, absorption, metabolism, distribution and storage in fat and other tissues, binding to serum proteins, inactivation and conjugation. We were unable to find empirical evidence as to a 'transformation dose' per cycle in women. In this review we attempted to order the progestogens based on estimates of their relative potency from animal studies (Kumar 2000) as there are few data from studies in humans as to dose equivalency of progestogens used in HT.
In the analysis, continuous and sequential E+P regimens were evaluated separately. For each type of regimen, different doses were compared.
There is evidence that progestogens must be taken for at least 10 days per month to reduce the risk of endometrial hyperplasia and carcinoma (Whitehead 1981), though some studies suggest that progestogens should be given for at least 12 to 14 days (Archer 2001; Sturdee 1994; Whitehead 1987). It was planned to assess separately the effects of sequential progestogen given for less than and more than 10 days per cycle.
The effects of sequential therapy were evaluated separately for different doses and duration of progestogen treatment, and for different treatment regimens (monthly sequential, long cycle (three monthly sequential) and intermittent (three days estrogen only followed by three days E+P, repeated).
Types of outcome measures
Primary outcomes
Frequency of endometrial hyperplasia (of any type) or adenocarcinoma (assessed by endometrial biopsy)
Secondary outcomes
Requirements for other medical or surgical therapy (e.g. unscheduled endometrial biopsies, hysteroscopy)
Adherence/compliance to therapy
Withdrawal owing to adverse events
Included studies are those where endometrial assessment is planned for every participant at the end of the intervention. Endometrial assessment may be either an endometrial biopsy for all women or measurement of endometrial thickness by transvaginal ultrasound, followed by endometrial biopsy in those women whose endometrial thickness is 5 mm or greater. Studies that did not report rates of endometrial hyperplasia were excluded.
In this review we used the outcome withdrawal owing to adverse events as a proxy for the acceptability of the therapy regimens to women. It is defined as withdrawal owing to adverse events prior to the end of the planned therapy period whether or not the investigator considered that the event was related to the therapy.
Search methods for identification of studies
We searched for all publications that describe (or might describe) RCTs of:
estrogen versus E+P or placebo;
E+P versus placebo;
combined continuous E+P versus sequential E+P therapy;
sequential combined E+P where different regimens are compared;
and their impact on rates of endometrial hyperplasia or cancer in postmenopausal women.
Electronic searches
The original search was performed in 1998. Updated searches were completed in 2003, July 2007, May 2008 and January 2012.
(1) We searched the Menstrual Disorders and Subfertility Group's trials register for any trials (searched January 2003, July 2007, May 2008 and January 2012). See Menstrual Disorders and Subfertility Group for more details on the make‐up of the trial register. The following search strategy was used:
((Keywords = "*Menopaus*" or Keywords = "postmenopaus*" or #43= "menopaus*" or #43="postmenopausal" ) AND ( Keywords ="*Hormone Therap*" or Keywords = "HRT*" or Keywords = "HT* " ) AND (Keywords = "endometrial biops*" or Keywords ="endometrial hyperpla*" or Keywords ="endometrial response*" or Keywords = "endometrial proliferat*" or Keywords ="bleeding*" or #43= "bleeding pattern*" )) AND NOT (Keywords = "tibolone" or Keywords = "SERM" or Keywords ="raloxifene" or Keywords = "phytoestrogen*" ) Appendix 1 (2) We searched The Cochrane Library (Issue 1, 2012) , MEDLINE (1966 to January 2012) Appendix 2, EMBASE (1980 to January 2012) Appendix 3, Current Contents (1993 to May 2008), Biological Abstracts (1969 to 2008), Social Sciences Citation Index (1980 to May 2008), PsycINFO (1972 to January 2012) Appendix 4 and CINAHL (1982 to May 2008) Appendix 5.
These electronic databases were searched using the highly sensitive search strategy developed by The Cochrane Collaboration together with the following terms: 1. exp climacteric/ or exp menopause/ 2. (climacter$ or menopaus$).tw. 3. (postmenopaus$ or post‐menopaus$ or post menopaus$).tw. 4. or/1‐3 5. exp estrogens/ 6. exp Contraceptives, Oral, Combined/ 7. hormone replacement therapy/ or oestrogen replacement therapy/ 8. exp Progestins/ 9. (hormone replacement therapy or HRT).tw. 10. (oestrogen$ or progest$).tw. 11. or/5‐10 12. 4 and 11 13. endometrial hyperplasia/ 14. (endometri$ adj5 hyperplasia).tw. 15. (endometri$ adj5 carcinoma).tw. 16. (endometri$ adj5 (biops$ or histology)).tw. 17. (hysteroscop$ or hysterectomy).tw. 18. (adherence or compliance).tw. 19. or/13‐18 20.12 and 19
The output from these searches was transferred to a database, where duplicates were identified and removed. Titles and abstracts were read to identify publications of trials that were potentially eligible for inclusion. We obtained paper copies of all potentially eligible studies. Where there was insufficient information available electronically we obtained paper copies to establish whether a study met the inclusion criteria for this review.
Searching other resources
We searched citation lists of included trials, conference abstracts and relevant review articles. Relevant journals were handsearched for additional trials (see Menstrual Disorders and Subfertility Group details for more information) and drug companies were contacted for details of unpublished trials.
We made attempts to contact the corresponding author of included trials where data were not in a form suitable for extraction or where information relating to the study was not explicit.
Data collection and analysis
Selection of studies
The selection of trials for inclusion in the review was performed by at least two review authors working independently for each of the versions of this review, after employing the search strategy described previously. For the 2008 update of the review selection of included trials was undertaken independently by three review authors (JM, SF and AL) with any discrepancies resolved by discussion. For the 2012 update of the review selection of included trials was undertaken independently by two review authors (SF and JM) with any discrepancies resolved by discussion.
Data extraction and management
Included trials were assessed independently by at least two of the review authors for the following quality criteria and methodological details, with any discrepancies resolved by discussion:
Trial characteristics
Method of randomisation
Presence or absence of blinding to treatment allocation
Quality of allocation concealment
Number of women randomised, excluded or lost to follow‐up
Whether an intention‐to‐treat analysis was done
Whether a power calculation was done
Duration, timing and location of the study
Characteristics of the study participants
Age and any other recorded characteristics of women in the study
Other inclusion criteria
Exclusion criteria
Interventions used
Doses and types of unopposed estrogen therapy used
Doses, types and regimens of E+P therapy used
Duration of HT
Outcomes reported
Endometrial hyperplasia as assessed by endometrial biopsy
Endometrial cancer as confirmed by histology
Requirements for additional investigations to exclude endometrial pathology, including ultrasound, endometrial biopsy or hysteroscopy, or saline infusion sonography
Adherence/compliance to therapy
Withdrawals owing to adverse events
Data extraction and assessment of risk of bias were also performed independently by at least two review authors, using forms designed according to the Cochrane Handbook for Systematic Reviews of Interventions (Higgins 2011, Table 7.3), with any discrepancies resolved by discussion. Authors included clinical experts and those with statistical or methodological expertise. Where necessary, additional information on trial methodology or original trial data were sought from the corresponding author of any trials that appeared to meet the eligibility criteria.
Assessment of risk of bias in included studies
Risk of bias was assessed according to the method described by Higgins 2011; see Figure 1; Figure 2.
1.
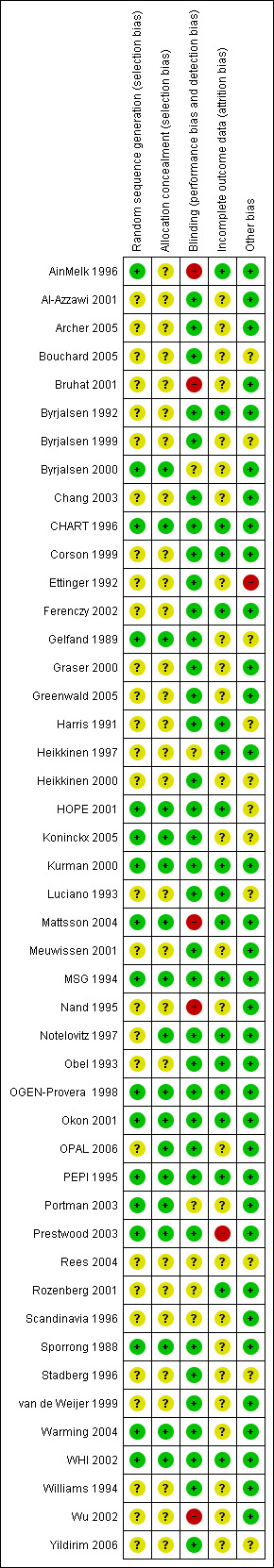
Summary of risk of bias: review authors' judgements about each 'Risk of bias' domain for each included study (Characteristics of included studies/risk of bias section, for details).
2.
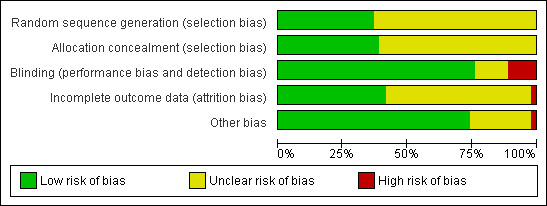
Risk of bias graph: review authors' judgements about each 'Risk of bias' item presented as percentages across all included studies.
In order to determine the likelihood of publication bias a funnel plot was planned.
Measures of treatment effect
For dichotomous outcomes, such as endometrial hyperplasia, endometrial cancer and withdrawal owing to adverse effects, we used the numbers of events in the intervention and control arm of each study to calculate Peto‐modified Mantel‐Haenszel odds ratios (ORs).
Where possible we took an intention‐to‐treat approach and used the number of women randomised to each group to calculate the rates of hyperplasia. This approach is based on the assumption that women who stopped HT and did not return for a biopsy did not have endometrial hyperplasia.
Unit of analysis issues
The unit of analysis was the woman randomised to treatment with HT.
Dealing with missing data
The data were analysed on an intention‐to‐treat basis as far as possible and attempts were made to obtain missing data from original trialists. No imputations were made where data were missing.
Assessment of heterogeneity
Heterogeneity between the results of different studies was examined by inspecting the scatter in the data points and the overlap in their confidence intervals (CIs) and, more formally, by checking the Q and I2 statistics (Higgins 2002). As a guide, a value of I2 of 25% or less can be considered a low degree of heterogeneity, a value of 50% or more considered as moderate heterogeneity and a value of 75% or more considered as high heterogeneity. A priori, it was planned to look at the possible contribution of differences in trial design to any heterogeneity identified in this manner.
Assessment of reporting biases
We undertook a comprehensive search for trials and placed no restrictions on language or publication status. Many of the studies included in this review have a number of associated publications. In this review we planned a priori to select only studies that reported the primary outcome of interest for this review; specifically harms of HT ‐ endometrial hyperplasia and endometrial carcinoma. Publication bias often results in the absence of data on the possible harms of treatment.
Data synthesis
Statistical analysis was performed in accordance with the guidelines for statistical analysis developed by the Menstrual Disorders and Subfertility Group. Where it was clinically appropriate, study outcomes were pooled statistically. It was planned to use a fixed‐effect model for calculation of summary effects in the meta‐analysis. Where significant heterogeneity was demonstrated it was planned to calculate summary effects using a random‐effects model to take account of the added uncertainty.
For dichotomous data (e.g. proportion of participants with hyperplasia or carcinoma), results for each study were expressed as an OR with 95% CIs and combined for meta‐analysis with RevMan software (RevMan 2011) using the Peto‐modified Mantel‐Haenszel method. All outcomes apart from adherence to therapy were categorised so that a high value represented a harm or negative consequence rather than a benefit of treatment. Thus, a negative consequence of treatment is in most cases represented in the graphs as an OR and CI on the right of the centre line.
In order to avoid double counting we chose to use subtotals only where there was a single reference group (e.g. unopposed estrogen or placebo) and two or more comparison groups in a trial.
Subgroup analysis and investigation of heterogeneity
Comparisons were subgrouped according to the HT regimen (estrogen only, E+P combined either continuously or sequentially) and also by estrogen dose (grouped as low, moderate or high dose ‐ see Table 9) and by progestogen type and dosage given.
Sensitivity analysis
We also planned a priori sensitivity analyses comparing the pooled results of all trials with:
trials with adequate allocation concealment,
trials with double blinding,
trials with < 20% withdrawals,
where there were sufficient studies to make this possible.
Results
Description of studies
Results of the search
Owing to the changes in the protocol for the 2008 update, 12 previously included studies were excluded from the review; three because they had less than 12 months of therapy (Blumel 1994; Pinto 2003;Von Holst 2002), and nine because the primary outcomes were bleeding patterns and these studies did not report endometrial hyperplasia as an outcome (Archer 1999; Hagen 1982;Limpaphayom 2000;Marslew 1991;Marslew 1992;Mizunuma 1997;Simon 2001;Simon 2003;Williams 1990). Three studies previously excluded because they were dose‐finding studies are now included (Bruhat 2001; Graser 2000;OGEN‐Provera 1998). An additional 28 studies were identified that met the inclusion criteria, to give a total of 45 studies included in the updated review.
The searches were again updated in January 2012, and resulted in 297 additional references. The titles and abstracts were screened by two review authors (SF, JM) and 290 were assessed as being not relevant to this review. We obtained full‐text copies of seven references that appeared to meet the inclusion criteria, and assessment of these papers resulted in one additional trial being included (OPAL 2006) and six being excluded (Bergeron 2010; Endrikat 2007; Steiner 2007; Stevenson 2010; Sturdee 2008; Veerus 2008; see Characteristics of excluded studies for details).
Included studies
The main analyses were based on 46 trials that involved a total of 39,409 participants randomised to treatment. Not all of the women randomised completed the total period of follow‐up or were included in an intention‐to‐treat analysis. Data extraction was independently performed by at least two review authors (SF, JM, AL).
Study design and setting
The included trials ranged in size from 32 (Nand 1995) to 16,608 participants (WHI 2002). The three largest trials were WHI 2002 (16,608 participants), HOPE 2001 (2673 participants) and MSG 1994 (1724 participants). Twenty eight were multicentre trials and in the remaining 18 trials women were recruited from a single centre. All had a parallel group design. Ten trials had performed a power calculation for sample size and analysis was by ITT (Bruhat 2001; Greenwald 2005; Kurman 2000; Mattsson 2004; OGEN‐Provera 1998; OPAL 2006; PEPI 1995; Portman 2003; Warming 2004; WHI 2002), three trials had power calculations and no ITT analysis (CHART 1996; HOPE 2001; MSG 1994), two studies did not provide power calculations but noted that the planned sample size "probably lacked power" (Ettinger 1992; Williams 1994) and another was a pilot study (Nand 1995). In 30 studies no mention of a power calculation was made. Twenty‐two studies used either a placebo control group or a placebo instead of one of the hormones in order to maintain blinding.
The trials took place in the US (15 studies: Archer 2005;CHART 1996;Corson 1999;Ettinger 1992;Greenwald 2005;Harris 1991;HOPE 2001; Kurman 2000;Luciano 1993;Notelovitz 1997;PEPI 1995;Portman 2003;Prestwood 2003;WHI 2002;Williams 1994); Denmark, Norway and Sweden (three studies: Mattsson 2004;Okon 2001;Scandinavia 1996); Denmark (five studies: Byrjalsen 1992; Byrjalsen 1999;Byrjalsen 2000;Obel 1993; Warming 2004); Finland (two trials: Heikkinen 1997;Heikkinen 2000); Sweden (two trials: Sporrong 1988;Stadberg 1996); the Netherlands (van de Weijer 1999); Europe (Meuwissen 2001); Europe and Scandinavia (Rozenberg 2001); Europe, Scandinavia and the UK (Bouchard 2005); Europe and the UK (three trials: Al‐Azzawi 2001; Koninckx 2005;Rees 2004); Europe and South Africa (Graser 2000); France and Germany (Bruhat 2001) and Turkey (Yildirim 2006). One large trial took place in 99 centres in the US and Europe (MSG 1994), one in 11 centres in the UK and Europe (OPAL 2006), one in Canada (AinMelk 1996) and two in both Canada and the Netherlands (Ferenczy 2002;Gelfand 1989). There was one included trial from China (Wu 2002), one from Taiwan (Chang 2003) and two from Australia (Nand 1995;OGEN‐Provera 1998).
Participants
Most of the included trials specified that women be postmenopausal and this was defined in 32 trials as cessation of bleeding for six months or more prior to entry into the study. In two trials postmenopausal was defined as serum FSH ≥ 40 IU/L (CHART 1996; Rozenberg 2001), in four trials postmenopausal was defined as serum FSH "in the postmenopausal range" (Harris 1991; Heikkinen 1997;Nand 1995;Warming 2004) and in one trial postmenopausal was defined as estradiol < 20 pg/mL (Greenwald 2005). Seven studies did not define postmenopausal (Byrjalsen 1992; Byrjalsen 1999;Mattsson 2004;Scandinavia 1996;Stadberg 1996;Warming 2004;Wu 2002) and in one trial the inclusion criteria specified that participants be older than 65 years and consequently postmenopausal (Prestwood 2003).
Participants ranged in age from 40 to 75 years although in most studies women were in the early menopause with the requirement that women should be within five or less years of their last spontaneous menstrual bleeding. Results can thus be generalised only to women in the early postmenopause rather than all postmenopausal women. Most of the trials also required that women have an intact uterus, or this was implied by the requirement for an endometrial biopsy at baseline, exclusion criterion of previous gynaecological operation or the nature of the primary outcomes. Full details of the inclusion and exclusion criteria for each study are found in the Characteristics of included studies. Common exclusion criteria were malignancy, chronic illness or use of contraindicated medications.
Interventions
A wide variety of unopposed estrogen or estrogen + progestogen combinations were used as interventions in the included trials. Unopposed estrogens included CEEs, EV, EIS, ESEs, micronised E2 and piperazine oestrone sulphate (POS). Two studies (CHART 1996; Portman 2003) compared placebo with unopposed EE at four different doses and with the same doses continuously combined with NETA. Most of the unopposed estrogen studies compared different doses of the same drug with placebo.
A number of different progestogens were used in the studies included in this review. In alphabetical order these included CPA, DG, DNG, DSP, DYG, gestodene, MPA, MA, NG, NETA, NGM, LNG and TMG.
In some trials, unopposed estrogen treatment was compared with estrogen plus progestogen (E+P) combined treatment, either continuous or sequential.
Sequential combined regimens of HT may be divided into four main types. The most common regimens use unopposed estrogen for part of the cycle followed by E+P for 10 to 15 days per monthly cycle (28 to 30 days). Alternatively there are experimental regimens that use intermittent progestogen (i.e. estrogen only for three days) followed by E+P for three days repeated for up to one year (Byrjalsen 2000; Corson 1999; Rozenberg 2001). Some studies have used regimens where progestogen is added for 10 to 12 days every two or three months (Heikkinen 1997; Scandinavia 1996; Williams 1994) or every six months (Prestwood 2003) (long‐cycle regimens). In one study (Rees 2004) a biphasic regimen comprising 11 days of unopposed estrogen and 10 days of E+P followed by seven days of placebo, was compared with a triphasic regimen comprising 12 days of unopposed estrogen followed by 10 days of E+P, followed by six days of lower‐dose estrogen only. The biphasic regimen in this study was similar to the cyclical regimens that were originally used in HT but were found to be unsatisfactory for women because the troublesome menopausal symptoms returned during the seven‐day placebo phase.
In this review, duration of progestogen therapy varied from 10 to 14 days and it was given at differing times in the treatment cycle.
Where there was only one comparison group of unopposed estrogen and two or more groups with different E+P doses (MSG 1994; PEPI 1995), we chose to use subtotals only, to avoid double counting the comparison group. Likewise, in evaluating the effects of combined E+P, both continuous and sequential, where there was only one comparison group the analyses use subtotals only.
Seventeen of the trials had a placebo control group. Five studies had an estrogen‐only group as the comparison with combined regimens (Archer 2005: Corson 1999;Gelfand 1989;MSG 1994;PEPI 1995). Eleven trials compared two or more different continuous combined regimens or dosages (AinMelk 1996: Bouchard 2005;Bruhat 2001;Graser 2000;Heikkinen 2000;Kurman 2000;Mattsson 2004;Nand 1995;OGEN‐Provera 1998;Wu 2002;Yildirim 2006). Seven studies compared two or more sequential regimens or dosages (Chang 2003; Meuwissen 2001;Okon 2001;Rees 2004;Scandinavia 1996;van de Weijer 1999;Williams 1994) and a further six studies compared a combined sequential regimen with a combined continuous regimen (Al‐Azzawi 2001; Koninckx 2005;Luciano 1993;Rozenberg 2001;Sporrong 1988;Stadberg 1996).
Duration of treatment in the included trials ranged from 12 months to six years but the majority of studies assessed treatment over either one or two years.
Outcomes
The primary outcomes were frequency of endometrial hyperplasia or carcinoma. Some studies assessed endometrial thickness by ultrasound and only performed a biopsy where endometrial thickness was greater than 5 mm. This would appear to be common clinical practice. Endometrial hyperplasia was invariably confirmed by endometrial biopsy and reported at 12, 24 and 36 months. Most studies included any type of hyperplasia as a hyperplasia outcome.
A subgroup of women from the Women's HOPE study were followed up for two years and there were no additional cases of endometrial hyperplasia in the second year except in the women on unopposed estrogen. In another study (Rozenberg 2001) participants were initially randomised to treatment for one year and then an open‐label extension study followed for a second year. Only 65% of those women initially randomised had endometrial biopsies at the end of the second year.
Incidence of endometrial carcinoma was measured as an outcome by 14 studies (Al‐Azzawi 2001;Byrjalsen 1999;Chang 2003;Ferenczy 2002;Koninckx 2005;Kurman 2000; Meuwissen 2001;MSG 1994;Notelovitz 1997;Obel 1993;OPAL 2006;PEPI 1995;Scandinavia 1996;WHI 2002) at different time points: after one, two, three or more than five years of treatment.
A small number of trials (Ettinger 1992; Harris 1991; Heikkinen 1997; Notelovitz 1997; OPAL 2006; Rozenberg 2001) assessed change in bone density, lipid profile, carotid artery or climacteric symptoms as the primary outcomes. Effects on the endometrium and frequency of unscheduled bleeding were secondary outcomes.
The frequency of unscheduled biopsies or dilation and curettage was measured in one large trial (PEPI 1995), and non‐adherence to treatment as measured by pill counts was assessed in only two studies (PEPI 1995; Rees 2004). However, there were significant numbers of participants in most of the trials who withdrew from the trial prior to completion (10% to 50%), owing to adverse events, lack of efficacy or other reasons.
Excluded studies
We retrieved paper copies of an additional 60 studies identified as potentially eligible for inclusion. These were evaluated and subsequently excluded. Twenty‐nine studies had primary outcomes of bleeding patterns or bone mineral density and did not include planned endometrial biopsy, or other means of diagnosing hyperplasia, as an outcome measure (Archer 1999;Archer 2001;Arrenbrecht 2004;Blumel 1994;Byrjalsen 1992a; Byrjalsen 1992b;Campodonico 1996;Chen 1999;Christensen 1982;Gulhan 2004;Hagen 1982;Jaisamrarn 2002;Jirapinyo 2003;Kazerooni 2004;Limpaphayom 2000;Liu 2005;Marslew 1991;Marslew 1992;Mizunuma 1997;Morabito 2004;Odmark 2001;Simon 2001;Simon 2003;Stevenson 2001;Veerus 2008;Wang 2006;Warming 2004a;Weinstein 1990;Williams 1990). Six studies were subsequently found to be non‐randomised (Bergeron 2010; Granberg 2002; Nachtigall 1979;Sturdee 2000;Wahab 2002; Wells 2002) and 11 had a treatment period less than one year (Endrikat 2007; Keil 2002;Luciano 1988;Pinto 2003;Stevenson 2010;Sturdee 2008;Symons 2000;Symons 2002;Utian 2002;Von Holst 2002;Yang 2001). Three studies included women with endometrial hyperplasia at baseline, (Campbell 1977;Popp 2006;Volpe 1986), which is an exclusion criteria for this review; in one study all the participants underwent transcervical resection of the endometrium at baseline (Istre 1996), and another publication (Steiner 2007) was a comparison of subgroups selected retrospectively from two previously conducted randomised studies that drew participants from very different populations. A study published in 1982 (Schiff 1982) compared a now obsolete cyclic estrogen‐only regimen with a continuous estrogen‐only regimen, and therefore did not meet the inclusion criteria for this review. A further eight studies were found by handsearching and published in abstract form only. These publications contained insufficient information to establish eligibility for inclusion in the review and attempts to obtain further information from the authors have been unsuccessful (Aoki 1990;Heytmanek 1990;Pickar 2003a; Sturdee 1996; Ulla Timonen 2002; van der Mooren 1996; van de Weijer 2002; Webster 1996).
Risk of bias in included studies
Allocation
Sequence generation
All 46 trials were randomised but in 29 no details were provided of the method of sequence generation. Four trials used random number tables (AinMelk 1996; Byrjalsen 2000; Sporrong 1988; Warming 2004), and 13 trials used computerised randomisation (CHART 1996; Gelfand 1989; HOPE 2001; Koninckx 2005; Kurman 2000; Mattsson 2004; MSG 1994; OGEN‐Provera 1998; Okon 2001; PEPI 1995; Portman 2003; Prestwood 2003; WHI 2002).
Allocation concealment
Twenty‐eight trials did not describe methods of allocation concealment and were assessed as unclear risk of bias for this domain. The remaining 18 trials were classified as low risk of bias because randomisation was conducted centrally (Byrjalsen 2000; CHART 1996; Gelfand 1989; HOPE 2001; Koninckx 2005; Kurman 2000; Mattsson 2004; MSG 1994; Notelovitz 1997; OGEN‐Provera 1998; Okon 2001; OPAL 2006; PEPI 1995; Portman 2003; Prestwood 2003; Sporrong 1988; Warming 2004; WHI 2002).
Blinding
Thirty‐three of the 46 included trials used double blinding and two trials were single blinded (Byrjalsen 1992; Yildirim 2006). These 35 trials were assessed at low risk of performance and detection bias. In three studies (Byrjalsen 2000; Rees 2004; Rozenberg 2001) some of the participants had open‐label treatment and in a further three trials blinding was not clear (Heikkinen 1997; Portman 2003; Scandinavia 1996) and these six trials were assessed as unclear risk of bias for this domain. Unblinding occurred in 38 women in the PEPI trial (31 of those receiving the unopposed estrogen regimen, four receiving one of the E+P regimens and three receiving placebo) because of endometrial biopsy results classified as complex hyperplasia, atypia or cancer (PEPI 1995). Unblinding also occurred in the WHI trial: 331 participants were unblinded and reassigned to the experimental group owing to the release of the PEPI trial results indicating long‐term adherence to unopposed estrogen was not feasible in women with a uterus. The WHI protocol was subsequently changed to randomise women with a uterus to only E+P or placebo in equal proportions. Five trials were assessed at high risk of performance and detection bias owing to the absence of blinding (AinMelk 1996; Bruhat 2001; Mattsson 2004; Nand 1995; Wu 2002).
Incomplete outcome data
Losses to follow‐up and withdrawals were common, particularly in the larger trials and trials with long duration. In the CHART study, 570 women (45%) had withdrawn (out of a total of 1265) by the conclusion of the trial at two years. In this trial, a priori stopping rules were applied for participants who developed hyperplasia and consequently a proportion of subjects in group 8 (10 μg daily of oral unopposed EE continuously) were terminated from the study early owing to a high rate of hyperplasia. All remaining treatment groups had similar rates of withdrawal that ranged from 22% to 30% and excluding the high‐dose estrogen group, over 73% of the subjects completed the study (CHART 1996). In five other trials more than 40% of the women randomised withdrew prior to the end of the trial (Byrjalsen 1999; Ettinger 1992; Gelfand 1989; Greenwald 2005; Rees 2004). Details of the numbers of and reasons for early withdrawals are in the included studies table.
Where more than 20% of women withdrew during the course of the study (owing to adverse events or other reasons such as change of address and unwillingness to continue to participate) there was a correspondingly low rate of endometrial biopsy. High rates of withdrawals and losses to follow‐up thus reduced the power of the study to detect the primary outcome of interest to this systematic review.
Other potential sources of bias
One publication (Fugh‐Berman 2010) has described how pharmaceutical companies have used medical education and communication companies to create publications as part of a marketing strategy for their products. Academics are then invited to become the 'authors' of these pre‐written articles that contain the desired marketing messages ‐ a practice known as 'ghost‐writing'. The extent of this practice is unclear, but there is evidence that three major articles published about the HOPE 2001 study, which provided data for this review (Archer 2001; Pickar 2003; Utian 2001) were 'ghost‐written' (Fugh‐Berman 2010). However, as 'ghost‐writing' has no influence on statistical data we consider that the likelihood of bias in this review owing to 'ghost‐writing' is low.
Effects of interventions
We extracted data on the cumulative incidence of endometrial hyperplasia at 12, 24 and 36 months. Not all of the studies reported the incidence rates at these time intervals. No differentiation was made in the analysis between the type of hyperplasia (simple, atypical or complex), although this was reported in some studies. Where a study had only one comparison or control group and several different doses in the experimental group, we used subgroups only and did not combine the results, in order to avoid counting the control group more than once.
We have created four additional tables to summarise the lowest dose of progestogen added to various doses of estrogen that result in endometrial hyperplasia rates that are:
statistically significantly reduced compared with unopposed estrogen (Table 10 continuous combined regimens and Table 11 sequentially combined regimens);
not statistically significantly increased compared with placebo (Table 12 continuous combined regimens and Table 13 sequentially combined regimens).
2. Continuous HT ‐ the lowest 'safe' dose; minimum progestogen dose for various estrogen types and doses compared to unopposed estrogen.
| Estrogen dose | P Dose | RCT evidence | Events estrogen alone | Events E+P |
OR (95% CI) |
Duration | Allocation concealment | |
| Low‐dose estrogen | 5 µg EE | 1 mg NETA | CHART 1996 | 4/221 | 0/65 | 3.70 (0.35 to 38.81) |
2 years | Adequate |
| 1 mg E2 | 0.5 DRSP | Archer 2005 | 4/113 | 0/231 | 7.80 (1.93 to 31.51) | 1 year | B | |
| 1 mg E2 | 0.1 mg NETA | Kurman 2000 | 36/247 | 2/249 | 6.98 (3.60 to 13.52) | 1 year | Adequate | |
| 0.3‐0.45 mg CEE | 1.5 mg MPA | HOPE 2001 | 6/65 | 0/144 | 26.97 (4.69 to 155.13) | 2 years | Adequate | |
| 0.45 mg CEE | 2.5 mg MPA | HOPE 2001 | 6/65 | 0/66 | 8.13 [1.59, 41.60] | 2 years | Adequate | |
| Moderate‐dose estrogen | 0.625 mg CEE | 2.5 mg MPA | HOPE 2001; PEPI 1995 | 69/174 | 1/182 | 11.99 (7.09 to 20.27) | 2 years | Adequate; Adequate |
| 0.625 mg CEE | 5 mg MPA | MSG 1994 | 57/283 | 0/274 | 8.92 (5.16 to 15.43) | 1 year | Adequate | |
| 10 µg EE | 1 mg NETA | CHART 1996 | 10/18* | 0/65 | 177.61 (36.08 to 874.43) | 2 years | Adequate |
*this group stopped hormone therapy early owing to high rate of endometrial hyperplasia
3. Sequential HT ‐ the lowest 'safe' dose of progestogen for various types and doses of estrogen compared to unopposed estrogen.
| Estrogen dose | P Dose | RCT evidence | Events estrogen alone | Events E+P |
OR (95% CI) |
Duration | Allocation concealment | |
| Low‐dose estrogen | 1 mg E2 | 30 µg NGM intermittent** | Corson 1999 | 74/265 | 16/260 | 5.91 (3.33 to 10.47) | 1 year | Unclear |
| Moderate‐dose estrogen | 0.625 mg CEE | 5 mg MPA (11‐12 days/month) | Gelfand 1989; MSG 1994 | 65/310 | 4/302 | 20.04 (7.16 to 56.07) | 1 year | Adequate; Adequate |
| 0.625 mg CEE | 10 mg MPA (12 days/month) | PEPI 1995 | 74/119 | 6/118 | 12.71 (7.43 to 21.76) | 3 years | Adequate | |
| 0.625 mg CEE | 200 mg progesterone (12 days/month) | PEPI 1995 | 74/119 | 6/120 | 12.90 (7.55 to 22.05) | 3 years | Adequate | |
| 0.625 mg CEE | 10 mg MPA (12 days/month) | MSG 1994; PEPI 1995 | 82/402 | 1/390 | 67.46 (13.26 to 343.08) | 1 year | Adequate | |
| High‐dose estrogen | 1.25 mg CEE | 5 mg MPA | Gelfand 1989 | 13/23 | 2/20 | 11.70 (2.19 to 62.62) | 1 year | Adequate |
** 3 days E+P followed by 3 days unopposed estrogen only, repeated.
4. Continuous HT ‐ the lowest 'safe' dose: minimum progestogen doses for various types and doses of estrogen compared to placebo.
| Estrogen dose | P Dose | RCT evidence | Events E+P | Events placebo |
OR (95% CI) |
Duration | Allocation concealment | |
| Low‐dose estrogen | 5 µg EE | 1 mg NETA | CHART 1996; Portman 2003 | 0/257 | 1/198 | 0.13 (0.00, 6.48) | 1 year | Adequate; Adequate |
| 5 µg EE | 1 mg NETA | CHART 1996 | 0/130 | 1/59 | 0.04 (0.00 to 2.79) | 2 years | Adequate | |
| 0.3‐0.45 mg CEE | 1.5 mg MPA | HOPE 2001 | 0/144 | 0/61 | not estimable | 2 years | Adequate | |
| 1 mg E2 | 1 mg DSP | Warming 2004 | 0/39 | 0/47 | not estimable | 2 years | Adequate | |
| 1 mg E2 | 25 µg gestodene | Byrjalsen 1999 | 0/34 | 0/43 | not estimable | 2 years | Unclear | |
| Moderate‐dose estrogen | 10 µg EE | 1 mg NETA | CHART 1996 | 0/65 | 1/59 | 0.12 (0.00 to 6.19) | 2 years | Adequate |
| 2 mg E2 | 1 mg NETA | Byrjalsen 2000; Greenwald 2005; Obel 1993 | 0/117 | 1/118 | 0.14 (0.00 to 6.82) | 2 years | Adequate; Unclear; Unclear | |
| 0.625 mg CEE | 2.5 mg MPA | PEPI 1995; HOPE 2001 | 1/182 | 0/180 | 7.33 (0.15 to 369.31) | 2 years | Adequate; Adequate | |
| 0.625 mg CEE | 2.5 mg MPA | PEPI 1995; OPAL 2006 | 1/356 | 2/360 | 0.51 (0.05 to 4.91) | 3 years | Adequate: Adequate |
5. Sequential HT ‐ the lowest 'safe' dose of progestogen for various doses and types of estrogen compared to placebo.
| Estrogen dose | Progestogen dose | RCT evidence | Events E+P | Events placebo |
OR (95% CI) |
Duration | Allocation concealment | |
| Low‐dose estrogen | 0.25 mg E2 | 100 mg progesterone (15 days/6 months) | Prestwood 2003 | 1/51 | 1/57 | 1.12 (0.07 to 18.20) | 3 years | Adequate |
| 1 mg E2 | 5 mg DYG (14 days/month) | Ferenczy 2002 | 0/100 | 0/63 | not estimable | 2 years | Unclear | |
| 1 mg E2 | 25 µg gestodene (12 days/month) | Byrjalsen 1999 | 0/34 | 0/43 | not estimable | 2 years | Unclear | |
| 0.75 mg POS | 0.35 mg NETA (intermittent)** | Byrjalsen 2000 | 0/32 | 0/25 | not estimable | 2 years | Adequate | |
| Moderate‐dose estrogen | 1.5 mg E2 | 150 µg DG (14 days/month) | Byrjalsen 1992 | 0/20 | 0/18 | not estimable | 2 years | Unclear |
| 1.5 mg POS*** | 0.7 mg NETA (intermittent)** | Byrjalsen 2000 | 0/26 | 0/25 | not estimable | 2 years | Adequate | |
| 0.625 mg CEE | 200 mg progesterone (12 days/month) | PEPI 1995 | 6/120 | 2/119 | 2.83 (0.69 to 11.54) | 3 years | Adequate | |
| 2 mg E2 | 1 mg NETA (10 days/month) | Obel 1993 | 0/45 | 0/45 | not estimable | 2 years | Unclear | |
| 2 mg E2 | 10 mg DYG (14 days/month) | Ferenczy 2002 | 0/88 | 0/63 | not estimable | 2 years | Unclear | |
| 2 mg E2 | 25 µg gestodene (12 days/month) | Byrjalsen 1999 | 0/27 | 0/43 | not estimable | 2 years | Unclear | |
| High‐dose estrogen | 2 mg EV | 10 mg MPA (10 days/month) | Heikkinen 1997; Byrjalsen 1992 | 3/41 | 1/43 | 3.19 (0.42 to 24.21) | 2 years | Unclear; Unclear |
| 2 mg EV | 10 mg MPA (14 days/6 months)‐long cycle | Heikkinen 1997 | 0/21 | 1/25 | 0.16 (0.00 to 8.12) | 2 years | Unclear |
**3 days E+P followed by 3 days estrogen only, repeated
*** Piperazine estrone sulphate
In Tables 2 to 5, allocation concealment was used as an indication of overall risk of bias. Allocation concealment is only one aspect of study validity, and has the objective of avoiding selection bias. However, adequate allocation concealment is also strongly associated with the presence of double‐blinding, and it may in addition be a marker for other bias‐reducing strategies (Wood 2008). For more details on individual aspects of study quality, see Characteristics of included studies and risk of bias Figure 1.
(1) Unopposed estrogen versus placebo
Low dose
After one year of therapy there was no statistically significant difference in the rate of endometrial hyperplasia between the group receiving unopposed estrogen and the placebo group (4 RCTs; OR 2.84; 95% CI 0.97 to 8.29) (Analysis 1.1). However, there was a statistically significant difference between the two groups in the rate of endometrial hyperplasia at 18 to 24 months, favouring the placebo group (6 RCTs; OR 2.42; 95% CI 1.19 to 4.92) (Analysis 1.2).
1.1. Analysis.
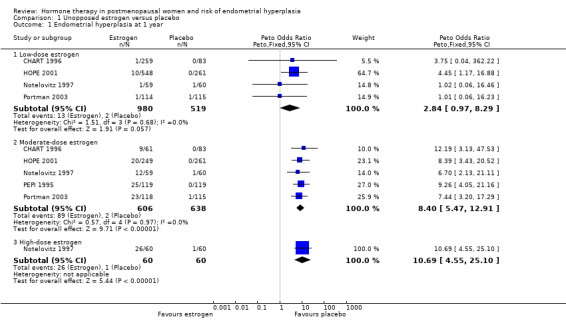
Comparison 1 Unopposed estrogen versus placebo, Outcome 1 Endometrial hyperplasia at 1 year.
1.2. Analysis.
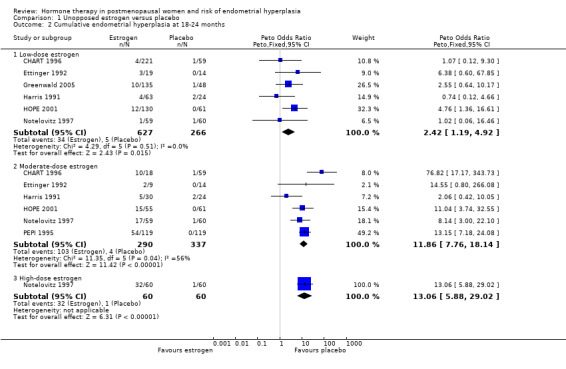
Comparison 1 Unopposed estrogen versus placebo, Outcome 2 Cumulative endometrial hyperplasia at 18‐24 months.
One trial with 591 participants (HOPE 2001) noted a statistically significant increase in the rate of endometrial hyperplasia when low doses of CEE (either 0.3 mg or 0.45 mg) were compared with placebo. After 12 months' follow‐up, there was a 1.8% rate of hyperplasia in the low‐dose unopposed groups and 0% rate and no hyperplasia in the placebo group. After two years these rates of endometrial hyperplasia increased to 9.2% for the combined low‐dose groups (3.2% in the 0.3 mg CEE group and 14.9% in the 0.45 mg CEE group) with no hyperplasia in the placebo group.
The other trial, with 490 participants (CHART 1996), noted a 0.4% overall rate of endometrial hyperplasia after one year in the low‐dose estrogen groups (1 to 5 µg EE), compared to no cases of hyperplasia in the placebo group. In the CHART study after two years of therapy the low‐dose estrogen groups showed a 1.8% rate of endometrial hyperplasia, which was not significantly different from the 1.7% placebo rate in this study.
There were no cases of endometrial cancer detected in the one small study that assessed this outcome after two years of therapy (Notelovitz 1997).
There was no statistically significant difference in the rate of withdrawal owing to adverse events between the low‐dose unopposed estrogen and placebo groups (4 RCTs; OR 1.1; 95% CI 0.7 to 1.7).
Moderate dose
There were statistically significant differences in the rates of endometrial hyperplasia at 12 months (5 RCTs; OR 8.4; 95% CI 5.5 to 12.9) (Analysis 1.1), 18 to 24 months (6 RCTs; OR 11.9; 95% CI 7.8 to 18.1) (Analysis 1.2) and three years (1 RCT; OR 16; 95% CI 9.3 to 27.5) (Analysis 1.3) in the studies that compared moderate‐dose unopposed estrogen therapy with placebo.
1.3. Analysis.
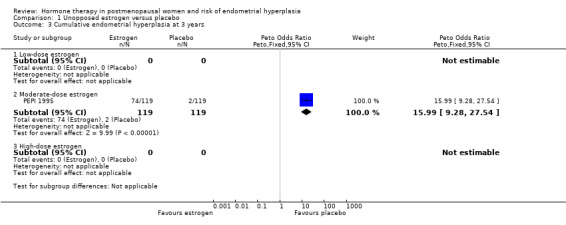
Comparison 1 Unopposed estrogen versus placebo, Outcome 3 Cumulative endometrial hyperplasia at 3 years.
After one year of therapy, there were 89 cases of endometrial hyperplasia in the 606 women from five RCTs randomised to moderate‐dose unopposed estrogen (14.7%) compared to two cases in the 638 women randomised to placebo (0.3%) (Analysis 1.1). After two years of therapy there were 103 cases of endometrial hyperplasia in the 290 women randomised to moderate‐dose unopposed estrogen therapy (35.5%) compared with four of the 337 women (1.2%) of those randomised to placebo (Analysis 1.2). After three years, the PEPI 1995 trial showed a 62% rate of endometrial hyperplasia associated with moderate‐dose unopposed estrogen compared to 1.7% in the placebo group (Analysis 1.3).
The only case of endometrial cancer in the two trials that assessed this outcome occurred in the placebo group (Analysis 1.4).
1.4. Analysis.
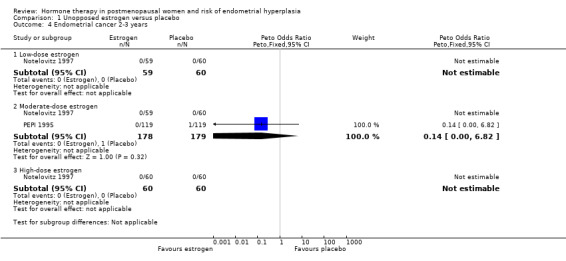
Comparison 1 Unopposed estrogen versus placebo, Outcome 4 Endometrial cancer 2‐3 years.
There was a small statistically significant increase in adherence to therapy in the placebo group compared to the unopposed estrogen group in the one study (PEPI 1995) that assessed medication compliance (OR 0.2; 95% CI 0.1 to 0.36) (Analysis 1.5). The only study that assessed the rate of unscheduled biopsies (PEPI 1995) found a significant increase associated with moderate‐dose unopposed estrogen therapy (1 RCT; OR 11.8; 95% CI 7.0 to 19.9) (Analysis 1.6).
1.5. Analysis.

Comparison 1 Unopposed estrogen versus placebo, Outcome 5 Adherence to therapy at 1 year.
1.6. Analysis.
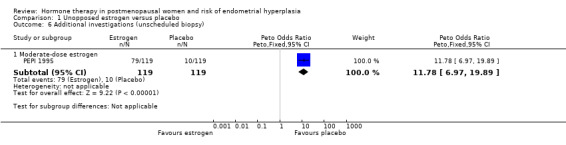
Comparison 1 Unopposed estrogen versus placebo, Outcome 6 Additional investigations (unscheduled biopsy).
There was no statistically significant difference in the rate of early withdrawal from treatment owing to adverse events in the moderate‐dose unopposed estrogen therapy group compared to the placebo group (Analysis 1.7).
1.7. Analysis.
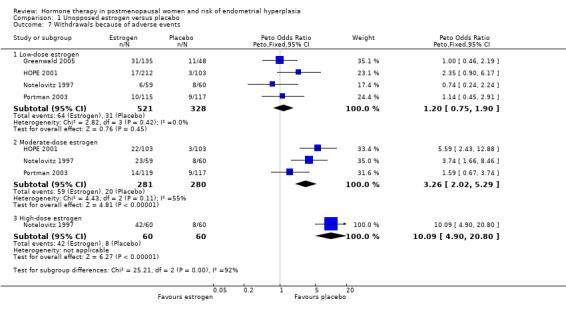
Comparison 1 Unopposed estrogen versus placebo, Outcome 7 Withdrawals because of adverse events.
High dose
In the only study (Notelovitz 1997) that compared high‐dose unopposed estrogen therapy with placebo the odds of developing endometrial hyperplasia at 12 months were significantly higher in the intervention group (OR 10.7; 95% CI 4.6 to 25.1) (Analysis 1.1) and increased further at 18 to 24 months of therapy (OR 13.1; 95% CI 5.9 to 29) (Analysis 1.2).
There were no cases of endometrial cancer in either the high‐dose unopposed estrogen or the placebo group after two years of therapy in this study (Analysis 1.4).
The odds of early withdrawal owing to adverse events were significantly higher in the high‐dose unopposed estrogen group than in the placebo group (OR 6.8; 95% CI 3.4 to 14.0) (Analysis 1.7). Vaginal bleeding and endometrial hyperplasia were the main reasons given for discontinuation in the high‐dose group.
(2) Estrogen plus progestogen (continuous) versus placebo
In the nine RCTs (Byrjalsen 2000; CHART 1996; Greenwald 2005; HOPE 2001; Obel 1993; OPAL 2006; PEPI 1995; Portman 2003; Warming 2004) that compared various doses and types of continuous combined E+P with placebo no statistically significant differences were found between any of the groups in the rates of endometrial hyperplasia after one, two or three years (Analysis 2.1; Analysis 2.2; Analysis 2.3). It should be noted that the OPAL 2006 study included some women who had had a hysterectomy. For the outcomes of endometrial hyperplasia and endometrial cancer we have considered the denominator to be the number of women in each group with an intact uterus at baseline (236 and 243 in CEE and placebo groups, respectively). However, for the outcome withdrawal owing to adverse effects we present the only data available that is for all women randomised (288 in each group).
2.1. Analysis.
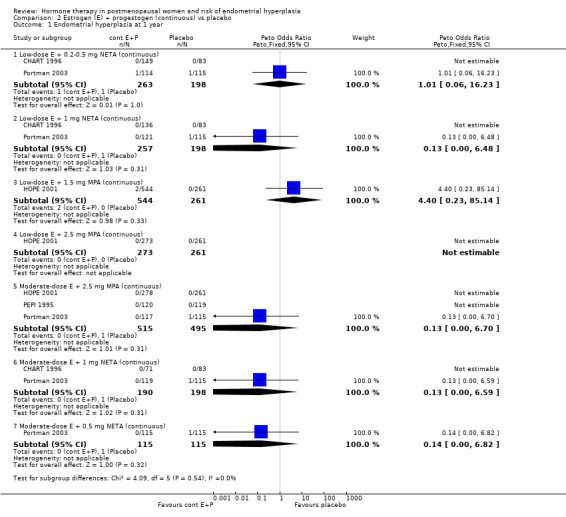
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 1 Endometrial hyperplasia at 1 year.
2.2. Analysis.
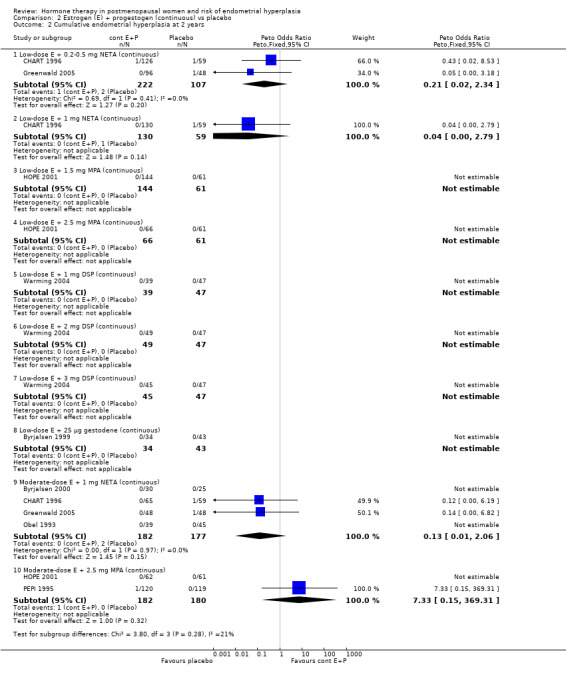
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 2 Cumulative endometrial hyperplasia at 2 years.
2.3. Analysis.
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 3 Endometrial hyperplasia at 3 years.
A summary of the lowest 'safe' dose of progestogens for endometrial protection for the various estrogen doses used can be found in Table 12.
Similarly, rates of endometrial carcinoma were very low in the five studies (Byrjalsen 1999; Obel 1993; OPAL 2006; PEPI 1995; WHI 2002) that reported this outcome, with no statistically significant difference between the groups receiving continuous combined regimens and those on placebo, even after 5+ years of follow‐up (WHI 2002) (Analysis 2.6).
2.6. Analysis.
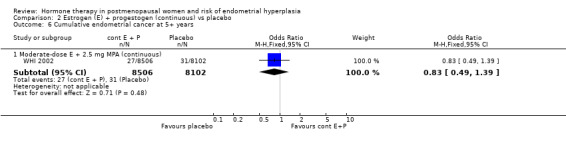
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 6 Cumulative endometrial cancer at 5+ years.
There was no evidence of a statistically significant difference between the groups in the odds of adherence to therapy, unscheduled biopsies or withdrawals owing to adverse events (Analysis 2.7; Analysis 2.8; Analysis 2.9).
2.7. Analysis.

Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 7 Adherence to therapy.
2.8. Analysis.
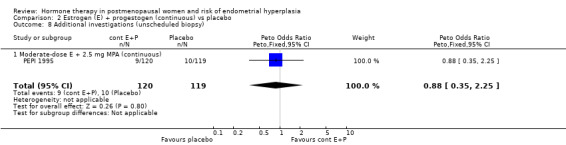
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 8 Additional investigations (unscheduled biopsy).
2.9. Analysis.
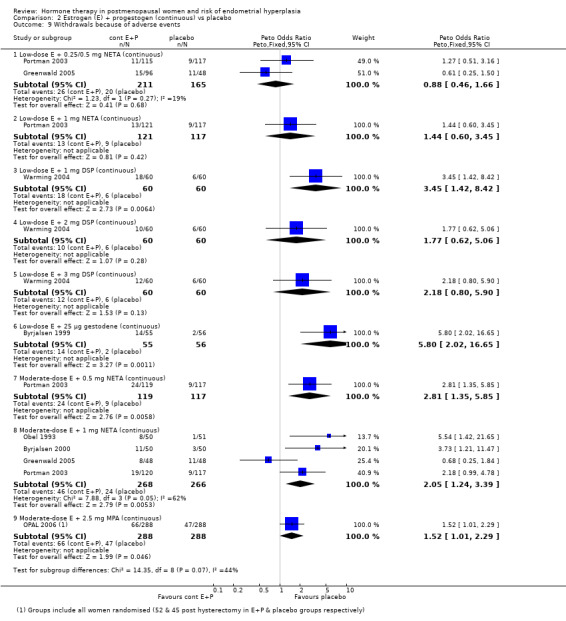
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 9 Withdrawals because of adverse events.
(3) Estrogen plus progestogen (sequential) versus placebo
There were eight RCTs included in this comparison (Byrjalsen 1992; Byrjalsen 1999; Byrjalsen 2000; Ferenczy 2002; Heikkinen 1997; Obel 1993; PEPI 1995, Prestwood 2003).
There were no cases of endometrial hyperplasia associated with the low‐dose estrogen sequential regimens in three RCTs (Byrjalsen 1999; Byrjalsen 2000; Ferenczy 2002) over two years of treatment (Analysis 3.2).
3.2. Analysis.
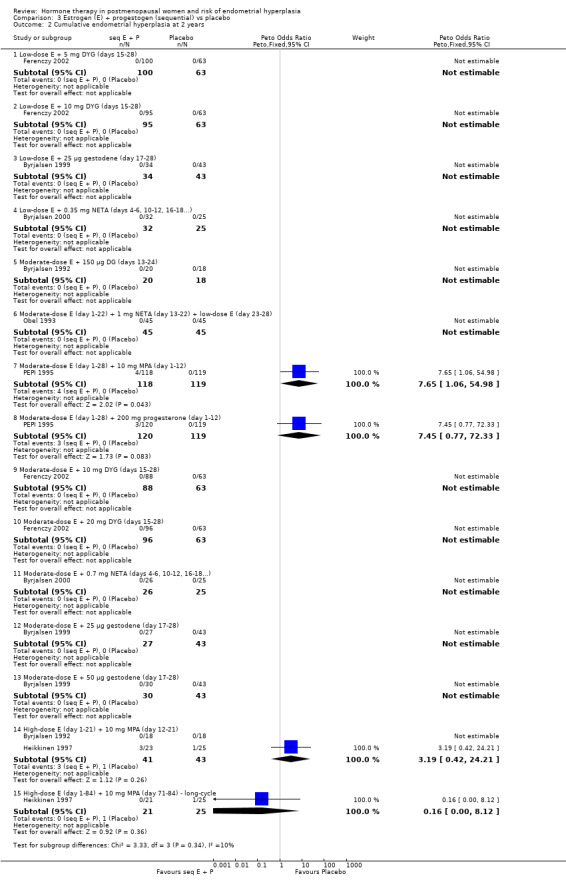
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 2 Cumulative endometrial hyperplasia at 2 years.
In five of the RCTs that compared moderate‐dose estrogen sequential regimens with placebo (Byrjalsen 1992; Byrjalsen 1999; Byrjalsen 2000; Ferenczy 2002; Obel 1993) no cases of endometrial hyperplasia were found in either group (Analysis 3.2). Regimens were as follows:
1.5 mg E2 plus 150 µg DG for 14 days per cycle (Byrjalsen 1992);
2 mg estradiol plus 1 mg NETA for 10 days per cycle (Obel 1993);
2 mg E2 plus 10 mg DYG for 14 days per cycle (Ferenczy 2002);
2 mg E2 plus 20 mg DYG for 14 days per cycle (Ferenczy 2002);
1.5 mg POS plus 0.7 mg norethisterone (intermittent three days unopposed estrogen, three days E+P) (Byrjalsen 2000);
2 mg estradiol plus 25 µg gestodene for 12 days per cycle (Byrjalsen 1999);
2 mg estradiol plus 50 µg gestodene for 12 days per cycle (Byrjalsen 1999).
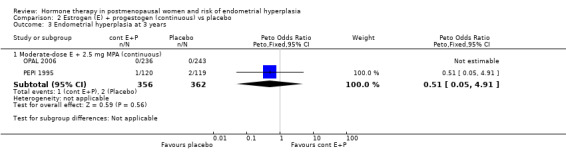
In the PEPI 1995 study, two moderate‐dose estrogen sequential regimens were compared with placebo.
This study found a difference in odds of endometrial hyperplasia between placebo and a sequential regimen comprising 0.625 mg CEEs plus 10 mg MPA for 12 days per cycle, which narrowly attained statistical significance after two years (OR 7.65; 95% CI 1.06 to 54.98). The absolute risk of hyperplasia in the sequential regimen group was 3.3% compared with 0% in the placebo group. At three years' follow‐up, there was no evidence of a statistically significant difference in rates of hyperplasia between the groups (OR 2.83; 95% CI 0.7 to 11.5) for any sequential regimen (absolute risk with the MPA sequential regimen had increased to 5.1% compared with 1.7% in the placebo group).
There was no statistically significant difference in the odds of developing endometrial hyperplasia after either two or three years of therapy in the group receiving the other sequential regimen utilised in PEPI 1995 (0.625 mg CEE plus 200 mg MP), compared to the placebo group (Analysis 3.3).
3.3. Analysis.
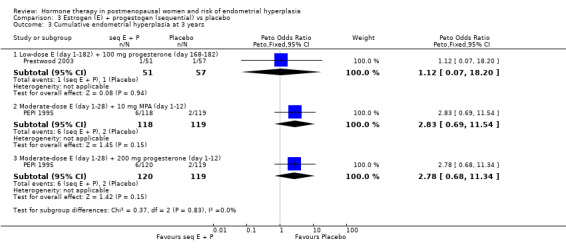
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 3 Cumulative endometrial hyperplasia at 3 years.
In the two studies that compared high‐dose estrogen sequential regimens with placebo (Byrjalsen 1992; Heikkinen 1997) there was no statistically significant difference between the groups in endometrial hyperplasia rates after two years of therapy (Analysis 3.3).
There were no statistically significant differences between the groups in any of the included studies in the rates of endometrial cancer (Analysis 3.5) or additional investigations (Analysis 3.6) when sequential regimens were compared with placebo after two or three years of therapy.
3.5. Analysis.
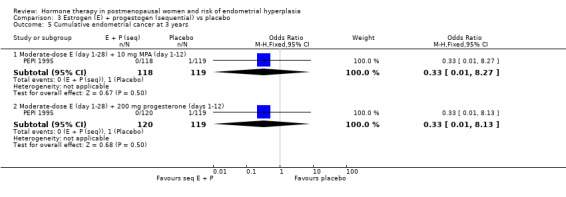
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 5 Cumulative endometrial cancer at 3 years.
3.6. Analysis.
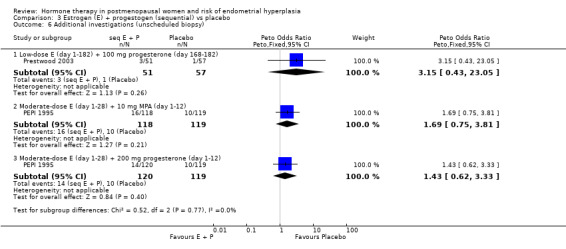
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 6 Additional investigations (unscheduled biopsy).
In the six RCTs that reported the outcome 'withdrawal owing to adverse events' (Analysis 3.7), most of the regimens showed no statistically significant difference between the intervention and placebo groups. However the odds of withdrawal owing to adverse events were higher in the groups receiving the following regimens:
3.7. Analysis.
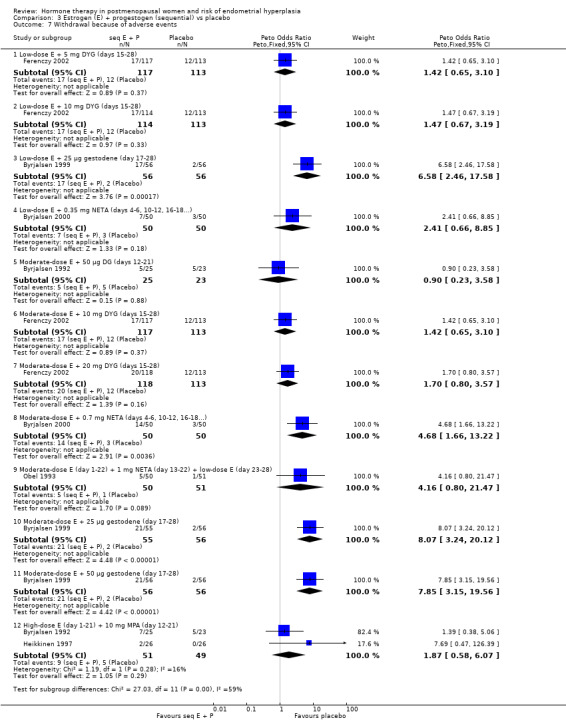
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 7 Withdrawal because of adverse events.
2 mg estradiol plus 25 µg or 50 µg gestodene for 12 days per cycle (Byrjalsen 1999);
1 mg estradiol plus 25 µg gestodene for 12 days per cycle (Byrjalsen 1999);
1.5 mg POS plus 0.35 mg NETA taken intermittently (three days on, three days off) (Byrjalsen 2000).
In both RCTs uterine bleeding was a common reason for early withdrawal.
(4) Unopposed estrogen versus estrogen plus progestogen (continuous)
In six trials (Archer 2005; CHART 1996; HOPE 2001; Kurman 2000; MSG 1994; PEPI 1995), rates of endometrial hyperplasia after one year of therapy were significantly higher for the group receiving low‐ or moderate‐dose unopposed estrogen therapy, compared with the group receiving continuous combined low‐ or moderate‐dose E+P treatment, for all of the 13 regimens compared, with ORs for the individual comparisons ranging between 3.8 and 9.4. The summary OR was not calculated because the same control group was used in more than one subgroup in these comparisons (Analysis 4.1).
4.1. Analysis.
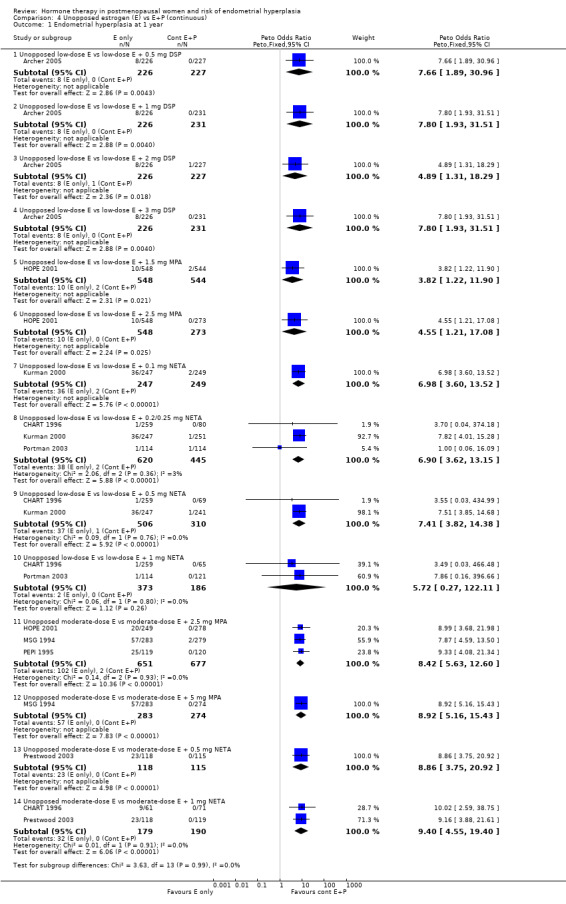
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 1 Endometrial hyperplasia at 1 year.
After one year of continuous combined therapy the groups receiving following regimens containing low‐dose estrogen:
1 mg E2 plus either 0.5, 1, 2 or 3 mg DSP (Archer 2005);
1 mg E2 plus 0.1, 0.25 or 0.5 mg NETA (Kurman 2000);
0.3 mg CEEs plus 1.5 mg MPA (HOPE 2001);
0.45 mg CEEs plus 2.5 mg MPA (HOPE 2001);
1 or 2.5 µg EE plus 0.2 or 0.5 mg NETA (CHART 1996);
showed a statistically significant reduction in the odds of hyperplasia compared to the groups receiving low‐dose unopposed estrogen.
After two years of therapy, the statistically significant decrease in rate of endometrial hyperplasia was still evident for groups receiving the following low‐dose estrogen regimens:
but there was insufficient statistical power to determine the endometrial safety of the 1 to 5 µg EE plus 0.2 to 1 mg NETA regimens (Analysis 4.1; Analysis 4.2).
4.2. Analysis.
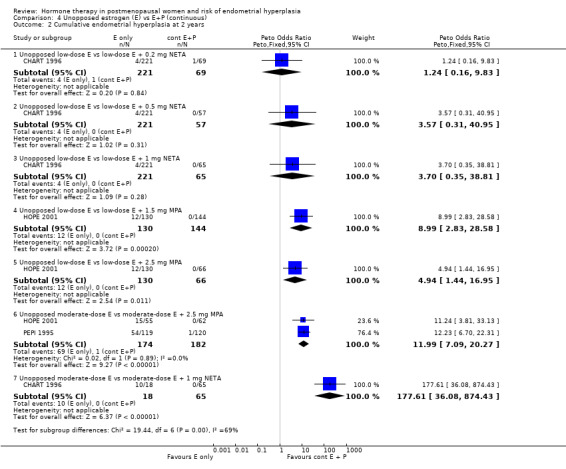
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 2 Cumulative endometrial hyperplasia at 2 years.
Groups receiving the following regimens containing a moderate dose of estrogen:
0.625 mg CEE plus either 2.5 or 5 mg MPA (HOPE 2001; MSG 1994; PEPI 1995);
10 µg EE plus 1 mg NETA (CHART 1996);
showed a statistically significant reduction in odds of endometrial hyperplasia compared to groups receiving moderate‐dose unopposed estrogen after one or two years of therapy (Analysis 4.2) and the PEPI 1995 study showed that the reduced odds of endometrial hyperplasia persisted after three years of therapy (Analysis 4.3).
4.3. Analysis.
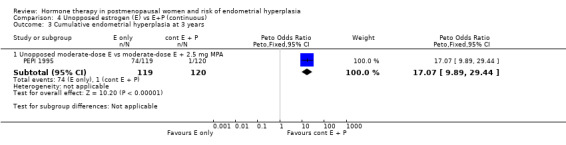
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 3 Cumulative endometrial hyperplasia at 3 years.
There were three RCTs that compared unopposed estrogen with continuous combined E+P with regard to the outcome of endometrial cancer. After one year of therapy there were no cases of endometrial cancer in either group in Kurman 2000, which compared low‐dose unopposed estrogen only with low‐dose estrogen plus 0.1 to 0.5 mg NETA, and there was only one case of endometrial cancer in MSG 1994 in the unopposed estrogen group. However, this study lacked sufficient power to show a statistically significant difference between the groups with regard to the outcome of endometrial cancer after one year of therapy. PEPI 1995 reported no cases of endometrial cancer in either the moderate‐dose estrogen only group or the moderate‐dose estrogen plus 2.5 mg MPA group after three years of therapy.
Adherence to therapy as measured by pill counts showed a small but statistically significant increase in the continuous combined E+P group compared to the groups receiving unopposed estrogen (1 RCT; OR 0.2; 95% CI 0.1 to 0.3) (Analysis 4.6) and unscheduled biopsies were more likely under unopposed estrogen treatment (1 RCT; OR 12.4; 95% CI 7.4 to 21.0) (Analysis 4.7).
4.6. Analysis.
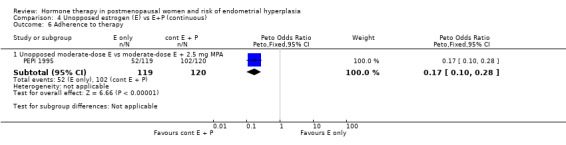
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 6 Adherence to therapy.
4.7. Analysis.
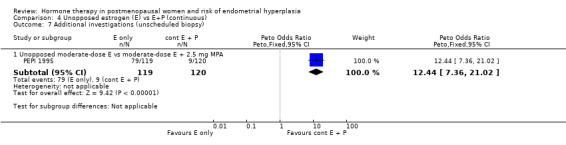
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 7 Additional investigations (unscheduled biopsy).
Withdrawals owing to adverse effects were significantly higher in the unopposed estrogen group (1 RCT; OR 2.3; 95% CI 1.4 to 4.0) (Analysis 4.8).
4.8. Analysis.
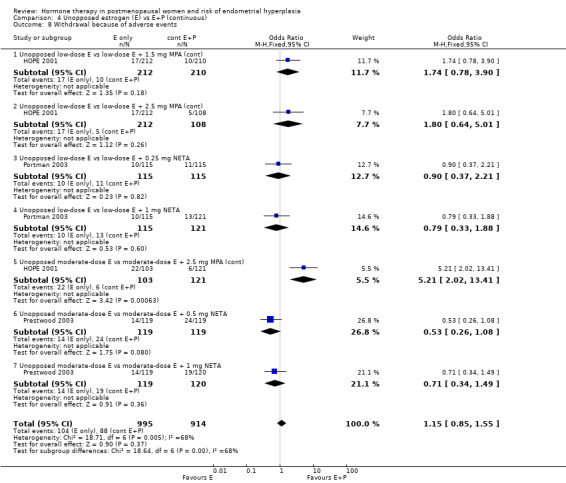
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 8 Withdrawal because of adverse events.
(5) Unopposed estrogen versus estrogen plus progestogen (sequential)
In this comparison sequential combined HT included intermittent regimens where women took estrogen alone for three days, followed by E+P for three days, then estrogen alone for three days (repeated for one year), and also the more common regimen where progestogen was added for 11 or 12 days per cycle.
Four RCTs (Corson 1999; Gelfand 1989; MSG 1994; PEPI 1995) compared sequential E+P regimens with unopposed estrogen using the following doses and regimens:
1 mg E2 plus 30 µg NGM (intermittent three days on/three days off) (Corson 1999);
1 mg E2 plus 90 µg NGM (intermittent three days on/three days off) (Corson 1999);
1 mg E2 plus 180 µg NGM (intermittent three days on/three days off) (Corson 1999);
0.625 mg CEEs plus 5 mg MPA (11 to 12 days per cycle) (Gelfand 1989; MSG 1994);
0.625 mg CEEs plus 10 mg MPA (12 days per cycle) (MSG 1994; PEPI 1995);
1.25 mg CEEs plus 5 mg MPA (11 days per cycle) (Gelfand 1989).
There were statistically significant differences in the odds of developing endometrial hyperplasia at one year between the groups taking unopposed estrogen and the groups taking sequential E+P in all of the regimens compared, favouring the sequential group (Analysis 5.1). These are summarised in Table 11.
5.1. Analysis.
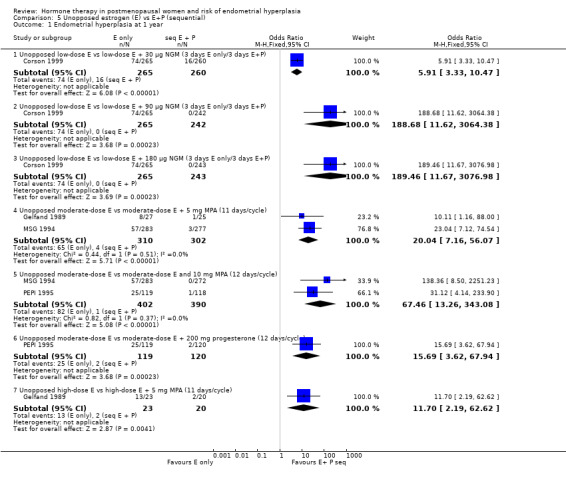
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 1 Endometrial hyperplasia at 1 year.
Only one study (PEPI 1995) followed women treated for more than one year and found that a sequential regimen with 10 mg of either MPA or MP given for 12 days per cycle was associated with a statistically significant decrease in the odds of endometrial hyperplasia compared to an unopposed estrogen regimen (OR 12.8; 95% CI 8.8 to 18.8) after three years of therapy Analysis 5.3.
5.3. Analysis.
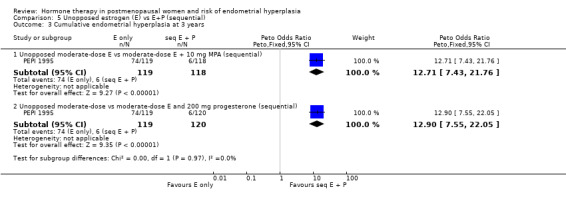
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 3 Cumulative endometrial hyperplasia at 3 years.
After one year of therapy there was no difference in the rate of endometrial cancer (one case in estrogen‐only group and one in the estrogen plus MPA group) in the one study that reported this outcome (MSG 1994). After three years of HT the PEPI 1995 study reported no cases of endometrial cancer in either the unopposed estrogen or the sequential combined groups.
Unscheduled biopsies were more frequent in the estrogen‐only group in the only trial that reported these outcomes (PEPI 1995) (Analysis 5.7) and adherence to therapy was greater in the sequential combined groups compared to the unopposed estrogen group in the same study (Analysis 5.6).
5.7. Analysis.
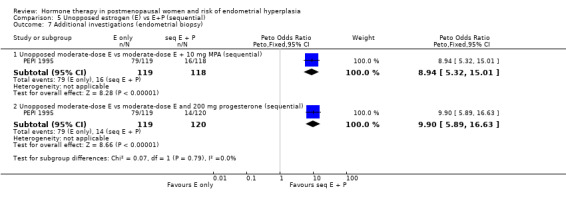
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 7 Additional investigations (endometrial biopsy).
5.6. Analysis.
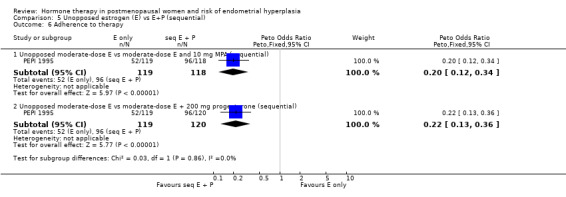
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 6 Adherence to therapy.
Women were more likely to withdraw because of an adverse event in the unopposed estrogen group in one study that compared low‐dose unopposed estrogen with three different doses of an intermittent three days on/three days off E+P regimen (Corson 1999). However, in the other study that reported this outcome (Gelfand 1989) where unopposed estrogen was compared with a sequential regimen where MPA was added for 11 days per cycle, withdrawal owing to adverse events were similar in both groups receiving moderate‐dose estrogen. In Gelfand 1989, withdrawal owing to adverse events was significantly more likely in the high‐dose sequential group compared to the unopposed high‐dose estrogen group (Analysis 5.8).
5.8. Analysis.
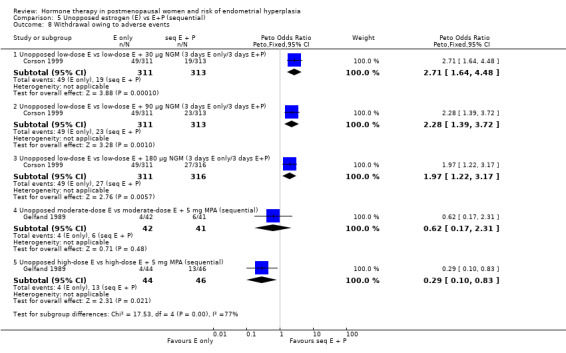
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 8 Withdrawal owing to adverse events.
(6) Estrogen plus progestogen (continuous) versus estrogen plus progestogen (sequential)
There were six RCTs that compared sequential combined therapy with continuous combined therapy (Byrjalsen 2000; Luciano 1993; MSG 1994; Obel 1993; PEPI 1995; Rozenberg 2001). The sequential therapy groups included regimens of 10 days' progestogen per cycle (Obel 1993), 12 days' progestogen per cycle (Luciano 1993; PEPI 1995), 14 days' progestogen per cycle (MSG 1994) and regimens where progestogen was taken for three days followed by a three‐day break throughout the cycle (Byrjalsen 2000; Rozenberg 2001; intermittent regimens). The odds of endometrial hyperplasia were not significantly different between the groups receiving continuous and the groups receiving sequential regimens of combined treatment at 12, 24 and 36 months for any of the comparisons included (Analysis 6.1; Analysis 6.2; Analysis 6.3).
6.1. Analysis.
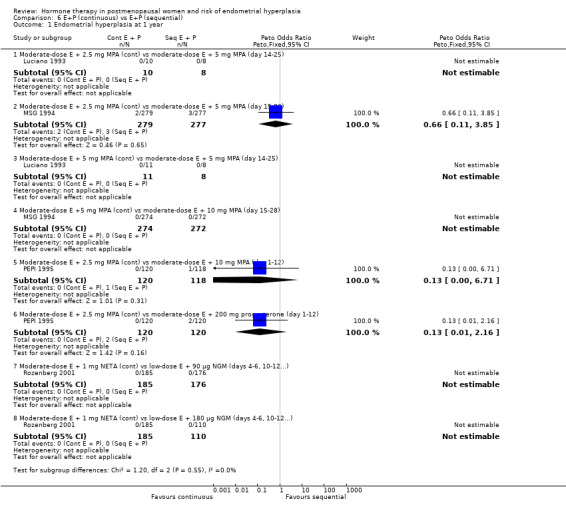
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 1 Endometrial hyperplasia at 1 year.
6.2. Analysis.
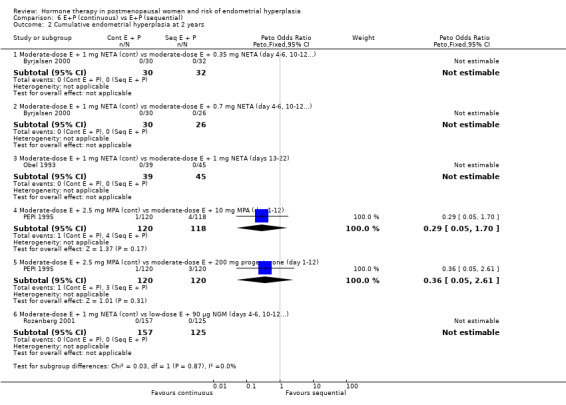
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 2 Cumulative endometrial hyperplasia at 2 years.
6.3. Analysis.
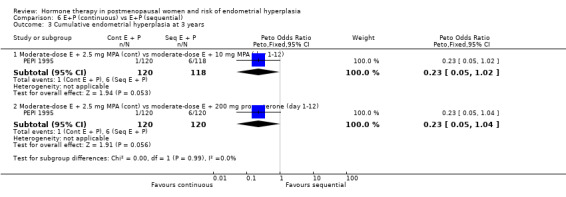
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 3 Cumulative endometrial hyperplasia at 3 years.
There were no statistically significant differences between the groups receiving continuous and sequential regimens in the rates of carcinoma (Analysis 6.4; Analysis 6.5; Analysis 6.6), adherence to therapy (Analysis 6.7), additional investigations (Analysis 6.8) or withdrawal owing to adverse events (Analysis 6.9).
6.4. Analysis.
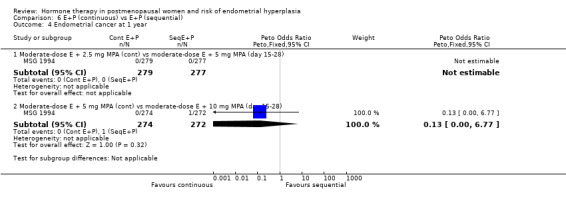
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 4 Endometrial cancer at 1 year.
6.5. Analysis.
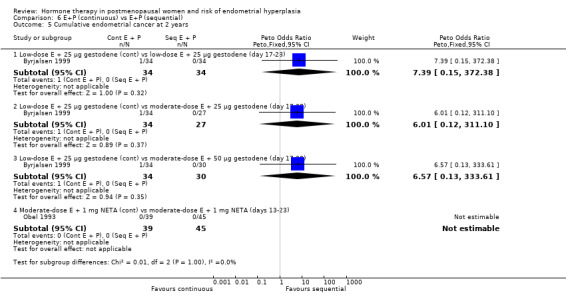
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 5 Cumulative endometrial cancer at 2 years.
6.6. Analysis.
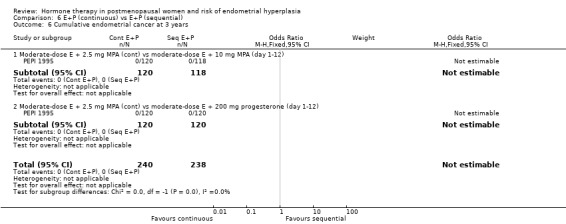
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 6 Cumulative endometrial cancer at 3 years.
6.7. Analysis.
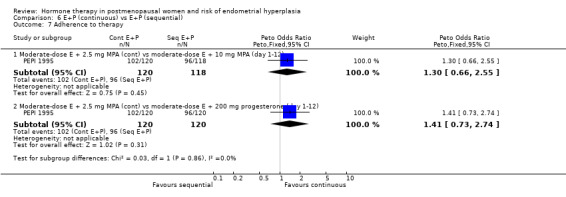
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 7 Adherence to therapy.
6.8. Analysis.
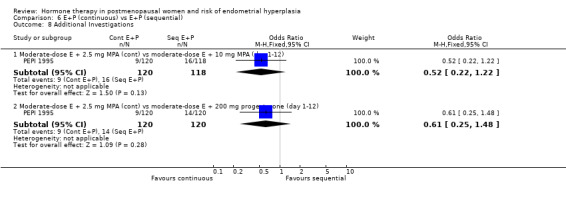
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 8 Additional Investigations.
6.9. Analysis.
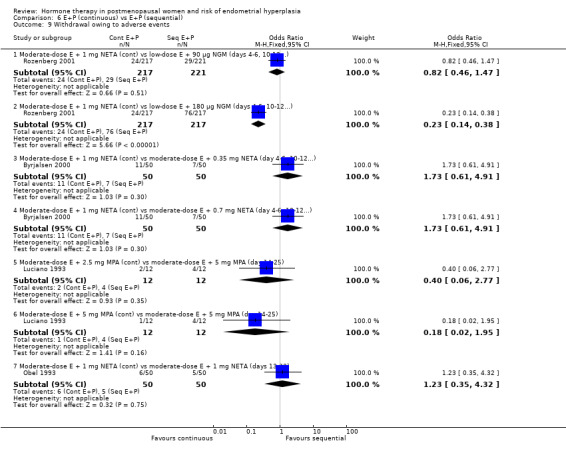
Comparison 6 E+P (continuous) vs E+P (sequential), Outcome 9 Withdrawal owing to adverse events.
(7) Continuous combined estrogen + progestogen (dose/regimen comparisons)
There were 15 RCTs that compared various dose combinations of continuous combined HT:
six RCTs (Archer 2005; Bouchard 2005; CHART 1996; HOPE 2001; Kurman 2000; Portman 2003) compared low‐dose estrogen combined with various doses of either DSP, TMG, medroxyprogesterone and NETA;
four RCTs (AinMelk 1996; Greenwald 2005; Stadberg 1996; Yildirim 2006) compared low‐dose estrogen combinations with moderate‐dose estrogen combinations;
three RCTs (MSG 1994; OGEN‐Provera 1998; Sporrong 1988) compared moderate‐dose estrogen combined with various doses of either META, MA or MPA;
two RCTs (Bruhat 2001; Graser 2000) compared moderate‐ to high‐dose estrogen combined with various doses of MPA, META or DNG.
After one or two years of therapy there were no statistically significant differences in the rates of endometrial hyperplasia between groups receiving any of the doses compared in the 15 RCTs included (Analysis 7.1; Analysis 7.2).
7.1. Analysis.
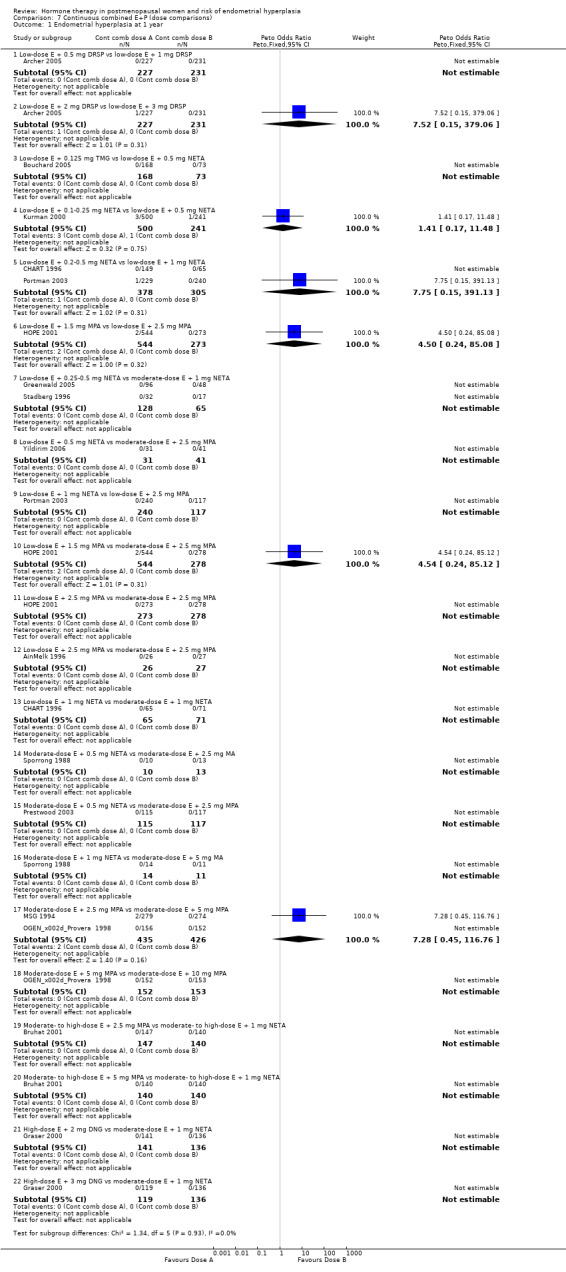
Comparison 7 Continuous combined E+P (dose comparisons), Outcome 1 Endometrial hyperplasia at 1 year.
7.2. Analysis.
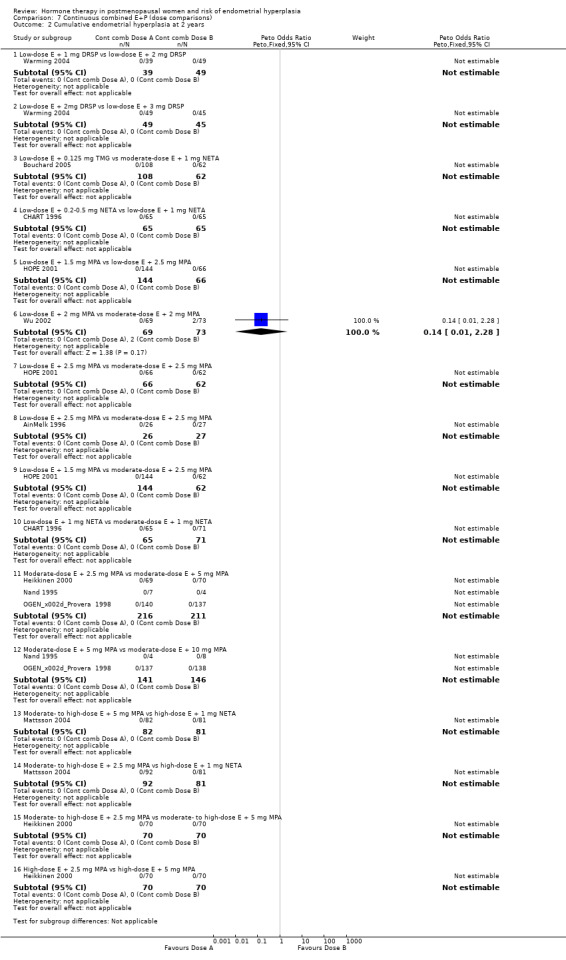
Comparison 7 Continuous combined E+P (dose comparisons), Outcome 2 Cumulative endometrial hyperplasia at 2 years.
Regimens included the following dose ranges:
low‐dose estrogen continuously combined with either 1 to 3 mg DSP, 0.1 to 1 mg NETA, 1.5 to 2.5 mg MPA or 0.125 mg TMG;
moderate‐dose estrogen continuously combined with 1 mg NETA, 2.5 to 10 mg MPA or 2.5 to 5 mg MA;
high‐dose estrogen continuously combined with 2 to 3 mg DNG.
All of the continuous combined regimens included were associated with low rates of hyperplasia (approximately 0.3% over one year).
In the two RCTs that reported endometrial cancer (Kurman 2000, MSG 1994), no cases were found after one year of therapy.
Withdrawal from the study owing to adverse events was reported by eight RCTs and no statistically significant difference was found between the groups receiving any of the continuous combined regimens compared (Analysis 7.4).
7.4. Analysis.
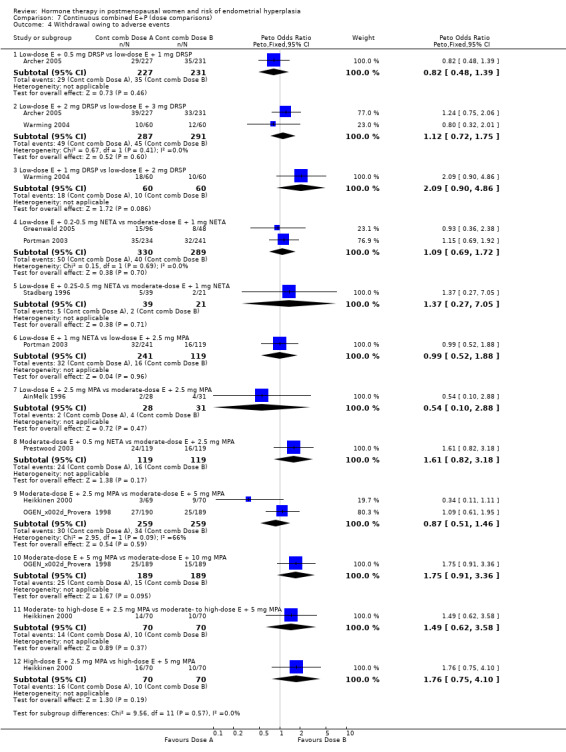
Comparison 7 Continuous combined E+P (dose comparisons), Outcome 4 Withdrawal owing to adverse events.
(8) Sequential combined estrogen plus progestogen (dose/regimen comparisons)
There were 12 RCTs that compared different sequential regimens and doses of HT. The regimens compared fall into five major groups:
five RCTs (Al‐Azzawi 2001; Chang 2003; Koninckx 2005; MSG 1994; van de Weijer 1999) compared different doses of progestogens (TMG, DYG, MPA) taken for 14 days per monthly cycle
two RCTs (Okon 2001; PEPI 1995) compared 12 days per monthly cycle of different progestogens;
two RCTs (Al‐Azzawi 2001; Meuwissen 2001) compared 14 days per cycle of TMG with 10 days of either NETA or NG per cycle;
three RCTs (Heikkinen 1997; Scandinavia 1996; Williams 1994) compared long‐cycle sequential progestogen (for 10 to 28 days every three months) with progestogen given for 10 to 14 days per month, and one RCT (Prestwood 2003) compared placebo with ultra‐low‐dose estrogen combined with progestogen given for 14 days every six months;
one RCT (Rees 2004) compared triphasic (nine days higher dose estrogen only, 12 days E+P, seven days estrogen lower dose) regimens with biphasic (11 days estrogen only, 10 days E+P, seven days placebo).
With regard to the outcome of endometrial hyperplasia, after one year of therapy only one RCT found a statistically significant difference between the groups (Scandinavia 1996; n = 240; Analysis 8.1). This study had a planned duration of five years but was stopped after an average of 2.8 years treatment in the long‐cycle group (moderate‐dose estrogen + 1 mg NETA for 10 days every three months) because the rate of endometrial hyperplasia was so unexpectedly high in the first year in this group (7.5%) (OR 8.7; 95% CI 1.93 to 39.27). This difference persisted in the second and third years of the trial with 15% endometrial hyperplasia after three years of therapy in the long‐cycle group compared to 2.0% in the monthly sequential group.
8.1. Analysis.
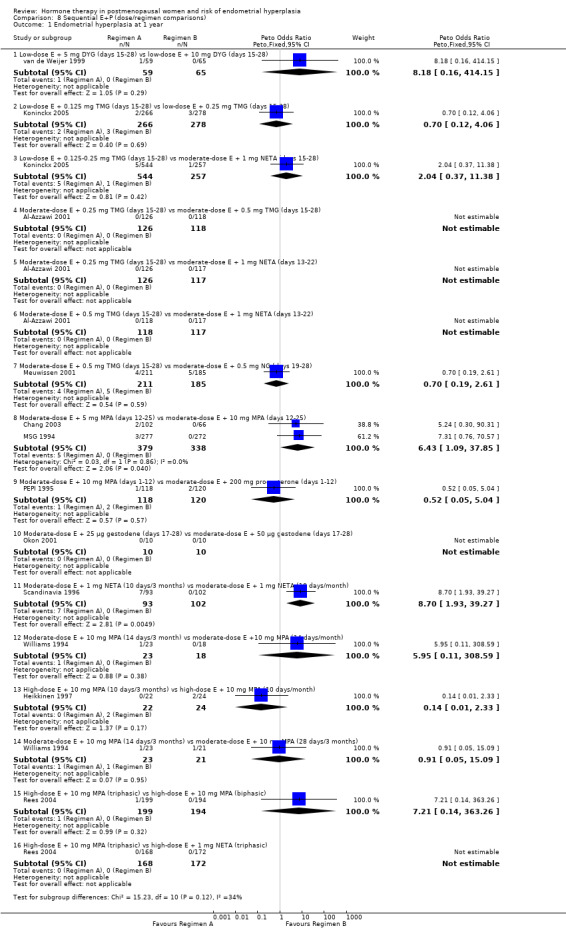
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 1 Endometrial hyperplasia at 1 year.
Two other small RCTs compared long‐cycle regimens with monthly sequential regimens (Heikkinen 1997; Williams 1994). Heikkinen 1997 randomised 78 women into three groups: (1) monthly cyclic, (2) long‐cycle and placebo and (3) used high‐dose estrogen combined with 10 mg MPA for 10 days per month or 14 days per three months. Williams 1994 randomised 80 women into three groups, with moderate‐dose estrogen (0.625 mg CEE) plus 10 mg MPA given for 14 days per month, 14 days per three months or 28 days per three months, respectively. There was no statistically significant difference between the groups in the rate of endometrial hyperplasia after one year in either study, and the Heikkinen 1997 study found no statistically significant difference between the groups after two years. Neither of these trials specified power calculations, but Williams 1994 stated that the study "probably lacked power to find a difference between the groups".
Another RCT (Prestwood 2003; 167 women) compared ultra‐low‐dose estrogen (0.25 mg E2 daily) plus 100 mg of progesterone for two weeks every six months, with placebo. One hundred and eight women completed three years of therapy and there was no statistically significant difference between the groups in the rate of endometrial hyperplasia (Analysis 8.3).
8.3. Analysis.
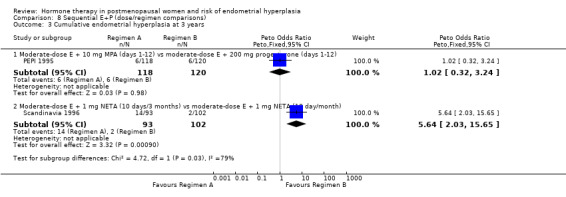
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 3 Cumulative endometrial hyperplasia at 3 years.
In the nine RCTs (Al‐Azzawi 2001; Byrjalsen 1999; Chang 2003; Ferenczy 2002; Koninckx 2005; Meuwissen 2001; MSG 1994; PEPI 1995; Scandinavia 1996) that reported the outcome of endometrial cancer there was no statistically significant difference between groups receiving any of the sequential regimens compared, after one, two or three years of HT (Analysis 8.4; Analysis 8.5; Analysis 8.6).
8.4. Analysis.
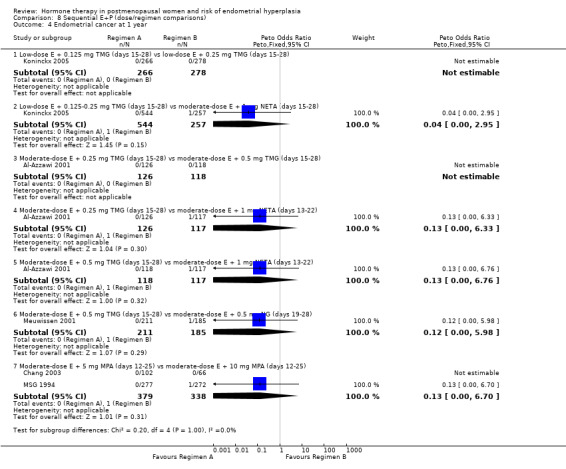
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 4 Endometrial cancer at 1 year.
8.5. Analysis.
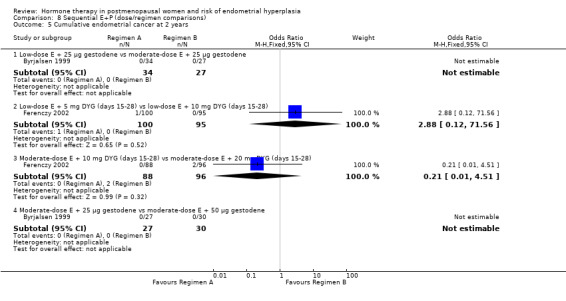
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 5 Cumulative endometrial cancer at 2 years.
8.6. Analysis.
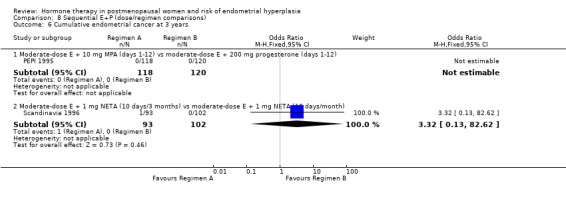
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 6 Cumulative endometrial cancer at 3 years.
The PEPI 1995 study found no statistically significant difference between groups receiving the sequential regimens in the rate of unscheduled biopsies. There was no statistically significant difference found in the odds of adherence to therapy in the sequential regimens compared in the two RCTs that reported this outcome (PEPI 1995; Rees 2004) (Analysis 8.7). Likewise there was no statistically significant difference found in odds of withdrawal owing to adverse events in the regimens compared in any of the five RCTs that reported this outcome (Analysis 8.9).
8.7. Analysis.
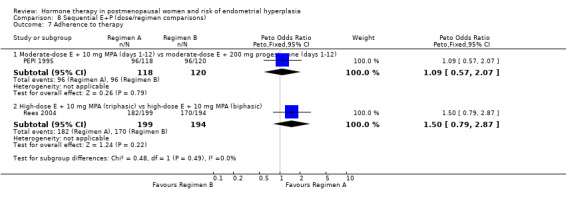
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 7 Adherence to therapy.
8.9. Analysis.
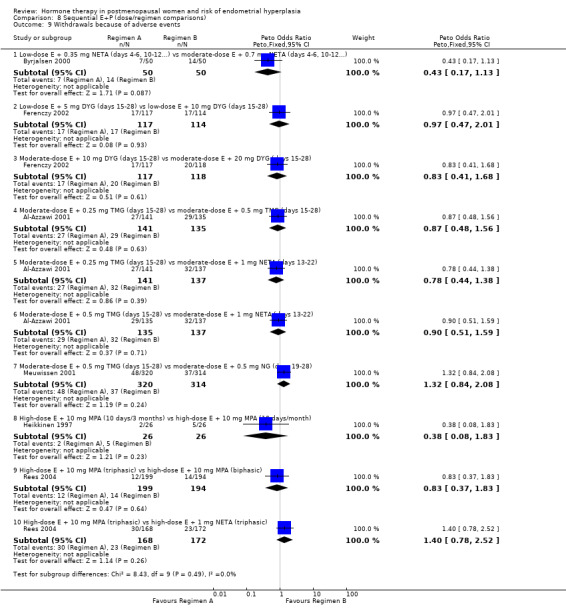
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 9 Withdrawals because of adverse events.
Sensitivity analyses
For many of the comparisons, the outcomes were recorded by only one or two trials and sensitivity analysis could not be performed.
Sensitivity analysis was conducted for comparisons 1.2.1 and 1.2.2 (Analysis 1.2) where unopposed estrogen therapy was compared with placebo and the outcome was endometrial hyperplasia after two years of therapy.
For low‐dose estrogen alone, the OR for trials with low risk of bias increased to 2.9 (95% CI 1.4 to 6.0) compared to OR 2.6 (95% CI 1.4 to 5.0) when all trials were included.
For moderate‐dose estrogen alone the OR increased to 11.5 (95% CI 7.2 to 18.3) for trials with low risk of bias from 10.1 (95% CI 6.5 to 15.7) for all trials.
Funnel plots
Although funnel plots were planned to investigate possible publication bias, there were so few studies in any of the meta‐analyses that it was considered that funnel plots were unlikely to have sufficient power to distinguish chance from meaningful asymmetry.
Heterogeneity
Throughout this systematic review there is a high level of clinical heterogeneity owing to variations in the populations of women included, for example in age, years since menopause, and the variety of HT doses and regimens compared. We have attempted to deal with this by separating out the regimens and doses, which has had the undesirable effect of reducing the potential for meta‐analysis. For example, when unopposed estrogen was compared with continuous estrogen and progesterone there were 13 different regimens used in six RCTs. We chose to display each regimen separately, which meant that only three of the 13 regimens had more than one trial contributing data. Where data were pooled, statistical heterogeneity was absent or low for all analyses reporting endometrial hyperplasia or cancer, except for analysis 1.2.2 (I2 = 56%). There was also moderate heterogeneity for the outcome 'withdrawals owing to adverse events' in three analyses (Analysis 1.7.2, Analysis 2.9.8, Analysis 7.4.9; I2 55% to 66%). There were no obvious design differences between the studies to account for the heterogeneity. When continuous E+P was compared with sequential E+P therapy for 'withdrawal owing to adverse events', significant heterogeneity was also evident from the forest plot. We did not calculate a pooled estimate here as only one of the four trials was considered to have a low risk of bias.
Discussion
Summary of main results
Unopposed estrogen therapy was associated with a significantly increased risk of endometrial hyperplasia at two and three years at all doses and there was evidence of a dose‐response relationship and a duration of treatment‐response relationship between unopposed estrogen and risk of hyperplasia. After one year of treatment, low‐dose unopposed estrogen was associated with a marginally non‐significant increase in endometrial hyperplasia compared to placebo (Analysis 1.1).
The addition of progestogen to unopposed estrogen therapy in women with intact uteri significantly reduced the risk of endometrial hyperplasia, when either sequential or continuous combined regimens were adopted. The results confirm that after one year of therapy, continuous combined E+P at any of the doses used in the trials is associated with a statistically significant reduction in risk of endometrial hyperplasia when compared to unopposed estrogen regimens. After two years of therapy, low‐dose estrogen plus either 1.5 or 2.5 mg MPA showed a statistically significant reduction in odds of hyperplasia compared to unopposed estrogen, but there was insufficient power to determine the endometrial safety of 1 to 5 µg EE combined with 0.2 to 1 mg NETA.
The review also found that when unopposed estrogen was compared to continuous E+P there was an increased odds of endometrial cancer in the unopposed estrogen group.
There was a statistically significant reduction in the odds of developing endometrial hyperplasia in all the sequential combined E+P regimens compared with unopposed estrogen therapy. The duration of progestogen in the included studies in this review ranged from 10 to 14 days. Our review confirms the finding that doses of progestogen in sequential therapy need to be given for at least 10 days, as reported by a large case‐control study (Pike 1997).
The two studies that reported the outcome of endometrial cancer (MSG 1994; PEPI 1995) showed no difference in odds of developing endometrial cancer between women receiving unopposed estrogen and those receiving sequential combined regimens. However these women were closely monitored throughout the trial and if a diagnosis of endometrial hyperplasia was made, study treatment was stopped and appropriate treatment was provided.
One large trial (WHI 2002) of continuous E+P versus placebo found that the annualised incidence of endometrial cancer in the E+P group was 0.06% compared with 0.07% in the placebo group after a mean 5.6 years of follow‐up (Analysis 2.6). The risk of endometrial cancer was not significantly different in the two groups but endometrial cancer is an uncommon event and an adequate assessment of this risk is unlikely to be made within the context of the limited time frame of the number of trials included in this review (maximum six years, WHI 2002). It should also be noted that if a woman in the WHI 2002 trial developed endometrial hyperplasia of any type, HT was stopped and treatment of hyperplasia undertaken. The low rates of endometrial cancer in both groups show the value of regular monitoring of women taking HT.
In the trials that compared continuous combined regimens directly with sequential regimens (comparison 6) there was no statistically significant difference in the odds of endometrial hyperplasia after one, two or three years. There was no statistically significant difference in the odds of endometrial cancer after up to three years. However, the sequential regimens included in these comparisons were quite varied and there were insufficient data to determine the relative merits of the different types of regimens used. There was no difference with regard to the outcome of adherence, withdrawal owing to adverse events or in the rate of unscheduled biopsies between the continuous and sequential regimens compared.
The comparisons between the various continuous combined regimens found no statistically significant differences with regard to endometrial hyperplasia because all the regimens included in these comparisons were associated with very low rates of hyperplasia. There were no cases of endometrial cancer in the two studies that reported this outcome but the follow‐up period in each was only one year.
Long‐ (or very long) cycle sequential therapy (progestogen given once every three months) was compared with short‐cycle sequential therapy (progestogen given once a month) in four trials (Heikkinen 1997; Prestwood 2003; Scandinavia 1996; Williams 1994). In one trial (Scandinavia 1996), where the long‐cycle group received moderate‐dose estrogen (2 mg E2 + 1 mg NETA for 10 days every three months, the rate of endometrial hyperplasia was so unexpectedly high in the first year (7.5%) that the trial was stopped early (mean duration of long‐cycle therapy 2.8 years compared with the planned five years).
This finding was not evident in two small studies (Heikkinen 1997; Williams 1994), which found no statistically significant difference between long‐cycle and short‐cycle groups in the rate of endometrial hyperplasia after one or two years of HT. A further study (Prestwood 2003), which compared placebo with ultra‐low‐dose estrogen plus 14 days of progesterone (0.25 mg E2 +100 mg MP) given every six months (very long cycle), showed no statistically significant difference between the groups with regard to endometrial hyperplasia after three years of therapy.
The PEPI 1995 study found a marginally statistically significant difference in the rate of endometrial hyperplasia at two years between women receiving sequential therapy and those receiving placebo, but this is unlikely to be clinically significant. After three years of treatment there was no statistically significant difference between these groups.
Adherence to therapy and the acceptability of therapy regimens to women are estimated and interpreted differently in the trials included in this review. We have chosen to define adherence to therapy as occurring when women take more than 80% of prescribed hormone doses as measured by counts of remaining tablets at clinic visits. Only one RCT (PEPI 1995) compared unopposed estrogen with combined regimens and reported adherence to therapy. Adherence was greater in both continuous and sequentially combined regimens than in unopposed estrogen regimens.
Withdrawals owing to adverse events and unscheduled biopsies were more likely in women receiving unopposed estrogen than in those receiving either continuous or sequential combined therapy. Unscheduled biopsies are more likely to be performed where there is concern about endometrial stimulation and consequent hyperplasia.
Overall completeness and applicability of evidence
The assessment of endometrial hyperplasia in this review is clinically important because it is associated with an overall increased risk of endometrial cancer, although this risk differs according to the type of hyperplasia diagnosed. There is evidence that untreated simple hyperplasia without atypia progresses infrequently over a 13‐year period to carcinoma while the risk of progression to carcinoma is greater in women with complex hyperplasia (Kurman 2000). Untreated hyperplasia with atypia is more likely to progress to cancer (Kurman 2000; Terakawa 1997). Most of the trials included in this review have distinguished 'hyperplastic' endometrium of any type from other types of endometrium.
This review is restricted to trials of oral HT. However it should be noted that intrauterine progestogen‐releasing systems are available, and can be used in combination with oral estrogen in HT. Intrauterine progestogen‐releasing systems induce profound endometrial suppression and may overcome many of the problems of sensitivity to systemic progestogens while also offering benefits such as control of heavy menstrual bleeding and contraception in perimenopausal women (Raudaskoski 2002). There is a need for trials to evaluate the benefits and harms of intrauterine progestogen‐releasing systems used in combination with low‐dose oral estrogen for both short‐ and longer‐term HT in peri‐ and postmenopausal women.
Quality of the evidence
One of the strengths of this review is the grouping of a number of different estrogenic preparations together with approximately similar endometrial effects. A further outcome of this review is a list of minimum progestogen doses that have been found to safely 'oppose' these estrogen doses with regard to preventing the development of endometrial hyperplasia (see Table 10; Table 11; Table 12; Table 13). Most of the studies included in this review (70%) are at unclear risk of bias, and in the majority of these, the reason is inadequate reporting of the method of sequence generation and allocation concealment. More than half the included studies are at unclear risk of attrition bias owing to the large numbers of withdrawals. This is a common problem in studies of HT. We have assumed that participants who withdrew from the studies during the treatment period were free from hyperplasia or endometrial cancer, because these would cause symptoms that would need to be investigated. Consequently the event rates reported in this review are a conservative estimate of these harms.
Potential biases in the review process
A potential bias in this review process concerns the decision to include only trials that reported the outcomes of endometrial hyperplasia and endometrial cancer. The harmful outcomes of HT are only one of many outcomes reported in trials of HT and we have been selective in our inclusion criteria for this review. In part this is a pragmatic response to the very large volume of literature on this topic. However in general there is evidence to suggest that biased reporting of the outcomes of treatment results in the under‐reporting of harmful outcomes, so we believe that in this review, the selection of included studies is an attempt to redress that balance.
We have noted that the practice of 'ghost‐writing' resulted in marketing messages being included in trials to help 'sell HRT' as a treatment for postmenopausal women. However we do not believe that 'ghost‐writing' is a threat to the validity of this review, as we are extracting data from the included studies but not taking their marketing messages into consideration.
In order to meet the objectives of this review we have presented separate analyses with little pooling of data. It is likely that this approach is associated with low power to detect the outcome of interest, unless and until further trials are conducted and can be added to this review in future updates.
Agreements and disagreements with other studies or reviews
Previous studies and reviews have documented an increased risk of endometrial hyperplasia associated with the use of unopposed estrogen, rising with higher doses and longer duration of treatment (Grady 1995; Mack 1976; Nelson 2002; Ziel 1975). Large RCTs found that combined therapy was not associated with increased risk of endometrial cancer (no increase in risk was associated with combined HT (Nelson 2002). Authors noted that prevention of endometrial hyperplasia requires adequate dose and duration of progestogen use (Grady 1995).
Authors' conclusions
Implications for practice.
The evidence that unopposed estrogen therapy increases the risk of endometrial hyperplasia shows a consistent association between the level of risk and the duration and strength of the dose. As low‐dose unopposed estrogen was associated with an increase in the risk of endometrial hyperplasia over placebo at one year's duration that bordered on statistical significance, clinicians utilising this dose for this short time frame should monitor the endometrial thickness by ultrasound. Best practice is to use both E+P in women with a uterus. There was no evidence of an increase in endometrial hyperplasia at three years associated with ultra‐low‐dose estrogen (0.25 mg E2) (Prestwood 2003) and progesterone for 15 days every six months. However there were no data to support that ultra‐low‐dose oral E2 would be adequate for symptom relief, as the primary outcome in this study was bone protection. Other research though has found a microdose of transdermal estrogen (0.014 mg/day) to be more effective than placebo for relief of hot flushes (Bachmann 2007).
Low‐dose EE (5 μg) required 1 mg NETA continuously to protect the endometrium (CHART 1996; Portman 2003). EE, commonly used in the oral contraceptive pill, has a pronounced effect on hepatic metabolism, and is now rarely used for HT in postmenopausal women.
Advice regarding HT for women with troublesome menopausal symptoms is to use the lowest effective dose and determine the duration of therapy based on an estimation of the benefits and harms for each individual woman. A previous meta‐analysis of 35,000 women found the median duration of menopausal flushes to be around four years (Politi 2008) with 10% of women reporting symptoms up to 12 years after the last period. A more recent cohort study of 4000 women followed for 13 years has suggested that the median duration may indeed be 10 years (Freeman 2011). For many women low‐dose HT gives adequate relief of symptoms (Peeyananjarassri 2005). However, at the time of writing not all countries have generally available packaged low‐dose sequential and combined continuous regimens. In this situation clinicians will need to separately prescribe the progestogen in doses that give endometrial protection.
The combined continuous regimen is suitable for women who are more than one to two years post menopause; use for women in early menopause can lead to unacceptable bleeding patterns (Archer 1999a).
This systematic review has shown that 1.5 mg of MPA given continuously with 0.3 mg of CEE will give endometrial protection (Table 12). However because this particular dose of MPA is not currently manufactured, clinicians will need to prescribe the 2.5 mg MPA tablet as this is the minimum dose available. For E2 this systematic review shows that 0.1 mg of NETA taken continuously will give endometrial protection (Table 10). However the commonly available product is currently one progestogen‐only pill containing 0.35 mg norethisterone.
Sequential regimens are suitable for women in early menopause. The RCTs included in this review with sequential regimens using low‐dose estrogen use regimens with types and doses of progestogens not commonly available as separate tablets (Table 13). This makes separate prescribing of low‐dose sequential HT problematic. A low‐dose sequential product containing 1 mg E2 together with 10 days of 1 mg NETA is manufactured, but may not be available in all countries.
Implications for research.
Many of the studies in this review are small and lack statistical power, and others recruited larger numbers of women but high rates of attrition for various reasons resulted in small numbers of women having endometrial biopsies after two or three years of treatment. Larger studies examining the endometrial safety of short‐term use (≤ one year) of unopposed low‐dose estrogen would help to determine the safety of this regimen.
Data are also lacking regarding endometrial safety with sequential regimens containing low doses of estrogen with the more commonly available progestogens, which would be the treatment of choice for perimenopausal women. Results from such studies would aid clinicians who wish to individually prescribe low‐dose hormone combinations in countries where these packaged regimens are not available.
What's new
| Date | Event | Description |
|---|---|---|
| 2 March 2012 | New search has been performed | Searches updated January 2012. One additional trial added but conclusions unchanged. |
| 2 March 2012 | New citation required but conclusions have not changed | One trial added, but no change to conclusions. |
History
Protocol first published: Issue 1, 1997 Review first published: Issue 2, 1999
| Date | Event | Description |
|---|---|---|
| 11 February 2009 | New citation required but conclusions have not changed | This review was updated July 2008 |
| 21 October 2008 | Review declared as stable | The conclusion of this review are now regarded as stable |
| 21 October 2008 | New search has been performed | Review update involved an extensive overhaul of the entire original review |
| 15 September 2008 | Amended | Title changed as irregular bleeding no longer a focus of the review and for simplicity removed in postmenopausal women |
| 16 July 2008 | New search has been performed | Protocol amended, new search done and review updated |
| 15 April 2008 | Amended | Converted to new review format. |
| 9 July 2007 | New citation required and conclusions have changed | Substantive amendment |
Acknowledgements
Thanks to Helen Nagels for her support in completing the 2012 update of this review.
Thanks to Martha Hickey for her substantial contributions to the 2008 update of this review.
Thanks are due to Jane Clarke, Review Group Coordinator to 2011, for her assistance with problem solving especially related to RevMan 5, Marian Showell, Trials Search Coordinator, for assistance with searching and locating copies of trials, Vanessa Jordan‐Cole for her assistance with RevMan 5 problems and Henrietta Wilkinson for her secretarial support.
Contributors to previous published versions of this review include Jane Suckling (past review author), who updated the review in 2004. She performed searches, selected trials for inclusion, assessed quality, performed data extraction, entered data and prepared the final review.
Arpine Sarkis (past review author) registered the title, prepared the protocol, selected trials for inclusion, assessed quality and performed data extraction for the first published version of this review. Ruth Jepson reviewed the protocol, performed searches, selected trials for inclusion, assessed quality and commented on the final draft of the review in 1999. David Barlow provided comment on the protocol and the final draft of the review in 1999.
The authors acknowledge the helpful comments of those who refereed previous versions of this review and we are especially grateful to those authors of included trials who provided additional data for this review. We also thank Professor John France and Professor Alastair MacLennan for their assistance in grouping estrogens according to approximate equivalence.
Contributors to previous versions of this review acknowledge the assistance of Mrs Michelle Proctor, Review Group Coordinator, for her professionalism and help with the inevitable problems that arise, to Mrs Ruth Buist, Trials Search Coordinator, for her assistance with identifying trials and to Mrs Sue Hall, Secretary of the Review Group, for her secretarial help.
Appendices
Appendix 1. MDSG register search strategy
((Keywords = "*Menopaus*" or Keywords = "postmenopaus*" or #43= "menopaus*" or #43="postmenopausal" ) and ( Keywords ="*Hormone Therap*" or Keywords = "HRT*" or Keywords = "HT* " )and (Keywords = "endometrial biops*" or Keywords ="endometrial hyperpla*" or Keywords ="endometrial response*" or Keywords = "endometrial proliferat*" or Keywords ="bleeding*" or #43= "bleeding pattern*" )) AND NOT (Keywords = "tibolone" or Keywords = "SERM" or Keywords ="raloxifene" or Keywords = "phytoestrogen*" )
Appendix 2. MEDLINE search strategy
1. exp climacteric/ or exp menopause/
2. (climacter$ or menopaus$).tw.
3. (postmenopaus$ or post‐menopaus$ or post menopaus$).tw.
4. or/1‐3
5. exp estrogens/
6. hormone replacement therapy/ or oestrogen replacement therapy/
7. exp Progestins/
8. (hormone replacement therapy or HRT).tw.
9. (oestrogen$ or progest$).tw.
10. endometrial hyperplasia/
11. (endometri$ adj5 hyperplasia).tw.
12. (endometri$ adj5 carcinoma).tw.
13. (endometri$ adj5 (biops$ or histology)).tw.
14. (hysteroscop$ or hysterectomy).tw.
15. (adherence or compliance).tw.
16. randomized controlled trial.pt.
17. controlled clinical trial.pt.
18. Randomized Controlled Trials/
19. Random allocation/
20. Double‐blind method/
21. Single‐blind method/
22. or/16‐21
23. clinical trial.pt.
24. exp clinical trials/
25. (clin$ adj25 trial$).ti,ab,sh.
26. ((singl$ or doubl$ or tripl$ or trebl$) adj25 (blind$ or mask$)).ti,ab,sh.
27. Placebos/
28. placebo$.ti,ab,sh.
29. random$.ti,ab,sh.
30. Research design/
31. or/23‐30
32. animal/ not (human/ and animal/)
33. 22 or 31
34. 33 not 32
35. review.pt.
36. letter.pt.
37. or/5‐9
38. or/10‐15
39. 4 and 37 and 38
40. 39 and 34
41. 40 not (35 or 36)
Appendix 3. EMBASE search strategy
1. exp climacteric/ or exp menopause/
2. (climacter$ or menopaus$).tw.
3. (postmenopaus$ or post‐menopaus$ or post menopaus$).tw.
4. or/1‐3
5. exp estrogens/
6. hormone replacement therapy/ or oestrogen replacement therapy/
7. exp Progestins/
8. (hormone replacement therapy or HRT).tw.
9. (oestrogen$ or progest$).tw.
10. endometrial hyperplasia/
11. (endometri$ adj5 hyperplasia).tw.
12. (endometri$ adj5 carcinoma).tw.
13. (endometri$ adj5 (biops$ or histology)).tw.
14. (hysteroscop$ or hysterectomy).tw.
15. (adherence or compliance).tw.
16. Controlled study/ or randomized controlled trial/
17. double blind procedure/
18. single blind procedure/
19. crossover procedure/
20. drug comparison/
21. placebo/
22. random$.ti,ab,hw,tn,mf.
23. latin square.ti,ab,hw,tn,mf.
24. crossover.ti,ab,hw,tn,mf.
25. cross‐over.ti,ab,hw,tn,mf.
26. placebo$.ti,ab,hw,tn,mf.
27. ((doubl$ or singl$ or tripl$ or trebl$) adj5 (blind$ or mask$)).ti,ab,hw,tn,mf.
28. (comparative adj5 trial$).ti,ab,hw,tn,mf.
29. (clinical adj5 trial$).ti,ab,hw,tn,mf.
30. or/16‐29
31. nonhuman/
32. animal/ not (human/ and animal/)
33. or/31‐32
34. 30 not 33
35. review.pt.
36. letter.pt.
37. or/5‐9
38. or/10‐15
39. 4 and 37 and 38
40. (39 and 34) not (35 or 36)
Appendix 4. PsycINFO search strategy
1. exp Menopause/
2. (climacteric or menopaus$ or postmenopaus$).tw.
3. 1 or 2
4. exp ESTROGENS/
5. exp Hormone Therapy/
6. hormone replacement therap$.mp. or hormone therap$.tw. [mp=title, abstract, heading word, table of contents, key concepts]
7. (oestrogen replacement therap$ or ert or hrt).mp. or ht.tw. [mp=title, abstract, heading word, table of contents, key concepts]
8. exp Progestational Hormones/ or exp Progesterone/
9. or/4‐8
10. (endometri$ adj5 hyperplas$).tw.
11. (endometri$ adj5 (biops$ or histolog$)).tw.
12. 10 or 11
13. 3 and 9 and 12
14. 3 and 9
15. 3 and 9
16. 2007$.mp. or 2008$.up. [mp=title, abstract, heading word, table of contents, key concepts]
17. 3 and 9
Appendix 5. CINAHL search strategy
1. exp climacteric/
2. (climacteri$ or menopaus$).tw.
3. (postmenopaus$ or post‐menopaus$).tw.
4. or/1‐3
5. exp Contraceptives, Oral/
6. exp Estrogens/
7. Hormone Replacement Therapy/
8. exp Progestational Hormones/
9. (hormone replacement therapy or hrt).tw.
10. (oestrogen$ or progest$ or oestrogen$).tw.
11. or/5‐10
12. 4 and 11
13. hyperplasia/ and endometri$.tw.
14. (endometria$ adj5 hyperplas$).tw.
15. ((bleed$ or spotting) adj5 vagina$).tw.
16. (endometri$ adj5 (Carcinoma$ or cancer$)).tw.
17. (endometri$ adj5 (biops$ or histol$ or thickness$)).tw.
18. exp Uterine Hemorrhage/
19. or/13‐18
20. 12 or 19
21. controlled study/ or randomized controlled trial/
22. (drug$ adj5 Compar$).tw.
23. placebo/
24. random$.tw.
25. latin square.tw.
26. (crossover or cross‐over).tw.
27. placebo$.tw.
28. ((doubl$ or singl$ or Tripl$ or trebl$) adj5 (blind$ or mask$)).tw.
29. (comparative adj5 trial$).tw.
30. (clinical adj5 trial$).tw.
31. or/21‐30
32. animal/ not (human/ and animal/)
33. 31 not 32
34. 33 and 20
Data and analyses
Comparison 1. Unopposed estrogen versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low‐dose estrogen | 4 | 1499 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.84 [0.97, 8.29] |
| 1.2 Moderate‐dose estrogen | 5 | 1244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.40 [5.47, 12.91] |
| 1.3 High‐dose estrogen | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.69 [4.55, 25.10] |
| 2 Cumulative endometrial hyperplasia at 18‐24 months | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose estrogen | 6 | 893 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.42 [1.19, 4.92] |
| 2.2 Moderate‐dose estrogen | 6 | 627 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.86 [7.76, 18.14] |
| 2.3 High‐dose estrogen | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 13.06 [5.88, 29.02] |
| 3 Cumulative endometrial hyperplasia at 3 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low‐dose estrogen | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Moderate‐dose estrogen | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 15.99 [9.28, 27.54] |
| 3.3 High‐dose estrogen | 0 | 0 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Endometrial cancer 2‐3 years | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low‐dose estrogen | 1 | 119 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Moderate‐dose estrogen | 2 | 357 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 4.3 High‐dose estrogen | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Adherence to therapy at 1 year | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Moderate‐dose estrogen | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.12, 0.35] |
| 6 Additional investigations (unscheduled biopsy) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Moderate‐dose estrogen | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.78 [6.97, 19.89] |
| 7 Withdrawals because of adverse events | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Low‐dose estrogen | 4 | 849 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.20 [0.75, 1.90] |
| 7.2 Moderate‐dose estrogen | 3 | 561 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.26 [2.02, 5.29] |
| 7.3 High‐dose estrogen | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.09 [4.90, 20.80] |
Comparison 2. Estrogen (E) + progestogen (continuous) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low‐dose E + 0.2‐0.5 mg NETA (continuous) | 2 | 461 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.01 [0.06, 16.23] |
| 1.2 Low‐dose E + 1 mg NETA (continuous) | 2 | 455 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.48] |
| 1.3 Low‐dose E + 1.5 mg MPA (continuous) | 1 | 805 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.40 [0.23, 85.14] |
| 1.4 Low‐dose E + 2.5 mg MPA (continuous) | 1 | 534 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Moderate‐dose E + 2.5 mg MPA (continuous) | 3 | 1010 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.70] |
| 1.6 Moderate‐dose E + 1 mg NETA (continuous) | 2 | 388 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.59] |
| 1.7 Moderate‐dose E + 0.5 mg NETA (continuous) | 1 | 230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.00, 6.82] |
| 2 Cumulative endometrial hyperplasia at 2 years | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose E + 0.2‐0.5 mg NETA (continuous) | 2 | 329 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.21 [0.02, 2.34] |
| 2.2 Low‐dose E + 1 mg NETA (continuous) | 1 | 189 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.04 [0.00, 2.79] |
| 2.3 Low‐dose E + 1.5 mg MPA (continuous) | 1 | 205 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Low‐dose E + 2.5 mg MPA (continuous) | 1 | 127 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Low‐dose E + 1 mg DSP (continuous) | 1 | 86 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Low‐dose E + 2 mg DSP (continuous) | 1 | 96 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Low‐dose E + 3 mg DSP (continuous) | 1 | 92 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Low‐dose E + 25 µg gestodene (continuous) | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Moderate‐dose E + 1 mg NETA (continuous) | 4 | 359 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.06] |
| 2.10 Moderate‐dose E + 2.5 mg MPA (continuous) | 2 | 362 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.33 [0.15, 369.31] |
| 3 Endometrial hyperplasia at 3 years | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Moderate‐dose E + 2.5 mg MPA (continuous) | 2 | 718 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.51 [0.05, 4.91] |
| 4 Cumulative endometrial cancer at 2 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low‐dose E + 25 µg gestodene (continuous) | 1 | 77 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.90 [0.15, 98.69] |
| 4.2 Moderate‐dose E + 1 mg NETA (continuous) | 1 | 84 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cumulative endometrial cancer at 3 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Moderate‐dose E + 2.5 mg MPA (continuous) | 2 | 718 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.03, 3.24] |
| 6 Cumulative endometrial cancer at 5+ years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Moderate‐dose E + 2.5 mg MPA (continuous) | 1 | 16608 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.83 [0.49, 1.39] |
| 7 Adherence to therapy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Moderate‐dose E + 2.5 mg MPA (continuous) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.35 [0.69, 2.65] |
| 8 Additional investigations (unscheduled biopsy) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.35, 2.25] |
| 8.1 Moderate‐dose E + 2.5 mg MPA (continuous) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.35, 2.25] |
| 9 Withdrawals because of adverse events | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Low‐dose E + 0.25/0.5 mg NETA (continuous) | 2 | 376 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.88 [0.46, 1.66] |
| 9.2 Low‐dose E + 1 mg NETA (continuous) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.44 [0.60, 3.45] |
| 9.3 Low‐dose E + 1 mg DSP (continuous) | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.45 [1.42, 8.42] |
| 9.4 Low‐dose E + 2 mg DSP (continuous) | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.77 [0.62, 5.06] |
| 9.5 Low‐dose E + 3 mg DSP (continuous) | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.18 [0.80, 5.90] |
| 9.6 Low‐dose E + 25 µg gestodene (continuous) | 1 | 111 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.80 [2.02, 16.65] |
| 9.7 Moderate‐dose E + 0.5 mg NETA (continuous) | 1 | 236 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.81 [1.35, 5.85] |
| 9.8 Moderate‐dose E + 1 mg NETA (continuous) | 4 | 534 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.05 [1.24, 3.39] |
| 9.9 Moderate‐dose E + 2.5 mg MPA (continuous) | 1 | 576 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.52 [1.01, 2.29] |
2.4. Analysis.
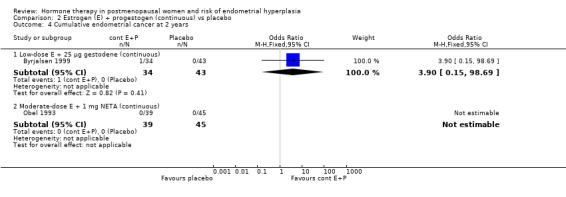
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 4 Cumulative endometrial cancer at 2 years.
2.5. Analysis.
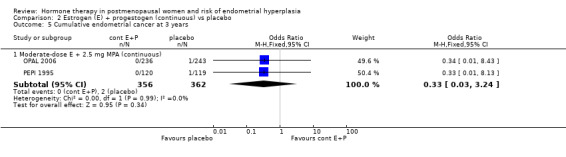
Comparison 2 Estrogen (E) + progestogen (continuous) vs placebo, Outcome 5 Cumulative endometrial cancer at 3 years.
Comparison 3. Estrogen (E) + progestogen (sequential) vs placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Moderate‐dose E (day 1‐28) + 10 mg MPA (day 1‐12) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.15, 375.57] |
| 1.2 Moderate‐dose E (day 1‐28) + 200 mg progesterone (day 1‐12) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.46, 118.84] |
| 1.3 High‐dose E (day 1‐21) + 10 mg MPA (day 12‐21) | 1 | 49 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [0.21, 21.13] |
| 1.4 High‐dose E (day 1‐84) + 10 mg MPA (day 71‐84) ‐ long‐cycle | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cumulative endometrial hyperplasia at 2 years | 7 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose E + 5 mg DYG (days 15‐28) | 1 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low‐dose E + 10 mg DYG (days 15‐28) | 1 | 158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Low‐dose E + 25 µg gestodene (day 17‐28) | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Low‐dose E + 0.35 mg NETA (days 4‐6, 10‐12, 16‐18...) | 1 | 57 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Moderate‐dose E + 150 µg DG (days 13‐24) | 1 | 38 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Moderate‐dose E (day 1‐22) + 1 mg NETA (day 13‐22) + low‐dose E (day 23‐28) | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.7 Moderate‐dose E (day 1‐28) + 10 mg MPA (day 1‐12) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.65 [1.06, 54.98] |
| 2.8 Moderate‐dose E (day 1‐28) + 200 mg progesterone (day 1‐12) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.45 [0.77, 72.33] |
| 2.9 Moderate‐dose E + 10 mg DYG (days 15‐28) | 1 | 151 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Moderate‐dose E + 20 mg DYG (days 15‐28) | 1 | 159 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Moderate‐dose E + 0.7 mg NETA (days 4‐6, 10‐12, 16‐18...) | 1 | 51 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Moderate‐dose E + 25 µg gestodene (day 17‐28) | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 Moderate‐dose E + 50 µg gestodene (day 17‐28) | 1 | 73 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.14 High‐dose E (day 1‐21) + 10 mg MPA (day 12‐21) | 2 | 84 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.19 [0.42, 24.21] |
| 2.15 High‐dose E (day 1‐84) + 10 mg MPA (day 71‐84) ‐ long‐cycle | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.16 [0.00, 8.12] |
| 3 Cumulative endometrial hyperplasia at 3 years | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low‐dose E (day 1‐182) + 100 mg progesterone (day 168‐182) | 1 | 108 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.07, 18.20] |
| 3.2 Moderate‐dose E (day 1‐28) + 10 mg MPA (day 1‐12) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.83 [0.69, 11.54] |
| 3.3 Moderate‐dose E (day 1‐28) + 200 mg progesterone (day 1‐12) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.78 [0.68, 11.34] |
| 4 Cumulative endometrial cancer at 2 years | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low‐dose E + 5 mg DYG (days 15‐28) | 1 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.10 [0.09, 285.72] |
| 4.2 Low‐dose E + 10 mg DYG (days 15‐28) | 1 | 158 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Low‐dose E + 25 µg gestodene (day 17‐28) | 1 | 77 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Moderate‐dose E + 10 mg DYG (days 15‐28) | 1 | 151 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.5 Moderate‐dose E + 20 mg DYG (days 15‐28) | 1 | 159 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.30 [0.31, 90.86] |
| 4.6 Moderate‐dose E + 1 mg NETA (sequential) | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.7 Moderate‐dose E + 25 µg gestodene (day 17‐28) | 1 | 70 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.8 Moderate‐dose E + 50 µg gestodene (day 17‐28) | 1 | 73 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.9 Moderate‐dose E (day 1‐22) + 1 mg NETA (day 13‐22) + low‐dose E (day 23‐28) | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5 Cumulative endometrial cancer at 3 years | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Moderate‐dose E (day 1‐28) + 10 mg MPA (day 1‐12) | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.27] |
| 5.2 Moderate‐dose E (day 1‐28) + 200 mg progesterone (days 1‐12) | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.33 [0.01, 8.13] |
| 6 Additional investigations (unscheduled biopsy) | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Low‐dose E (day 1‐182) + 100 mg progesterone (day 168‐182) | 1 | 108 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.15 [0.43, 23.05] |
| 6.2 Moderate‐dose E (day 1‐28) + 10 mg MPA (day 1‐12) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.69 [0.75, 3.81] |
| 6.3 Moderate‐dose E (day 1‐28) + 200 mg progesterone (day 1‐12) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.43 [0.62, 3.33] |
| 7 Withdrawal because of adverse events | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Low‐dose E + 5 mg DYG (days 15‐28) | 1 | 230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.65, 3.10] |
| 7.2 Low‐dose E + 10 mg DYG (days 15‐28) | 1 | 227 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.47 [0.67, 3.19] |
| 7.3 Low‐dose E + 25 µg gestodene (day 17‐28) | 1 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.58 [2.46, 17.58] |
| 7.4 Low‐dose E + 0.35 mg NETA (days 4‐6, 10‐12, 16‐18...) | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.41 [0.66, 8.85] |
| 7.5 Moderate‐dose E + 50 µg DG (days 12‐21) | 1 | 48 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.23, 3.58] |
| 7.6 Moderate‐dose E + 10 mg DYG (days 15‐28) | 1 | 230 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.42 [0.65, 3.10] |
| 7.7 Moderate‐dose E + 20 mg DYG (days 15‐28) | 1 | 231 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.70 [0.80, 3.57] |
| 7.8 Moderate‐dose E + 0.7 mg NETA (days 4‐6, 10‐12, 16‐18...) | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.68 [1.66, 13.22] |
| 7.9 Moderate‐dose E (day 1‐22) + 1 mg NETA (day 13‐22) + low‐dose E (day 23‐28) | 1 | 101 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.16 [0.80, 21.47] |
| 7.10 Moderate‐dose E + 25 µg gestodene (day 17‐28) | 1 | 111 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.07 [3.24, 20.12] |
| 7.11 Moderate‐dose E + 50 µg gestodene (day 17‐28) | 1 | 112 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.85 [3.15, 19.56] |
| 7.12 High‐dose E (day 1‐21) + 10 mg MPA (day 12‐21) | 2 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.87 [0.58, 6.07] |
3.1. Analysis.
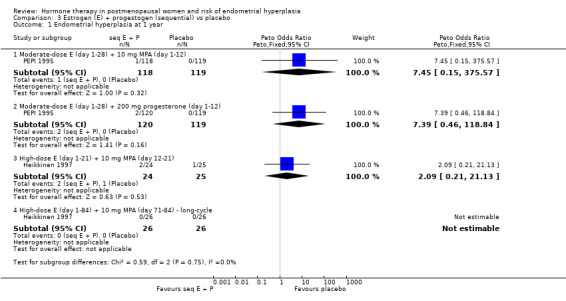
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 1 Endometrial hyperplasia at 1 year.
3.4. Analysis.
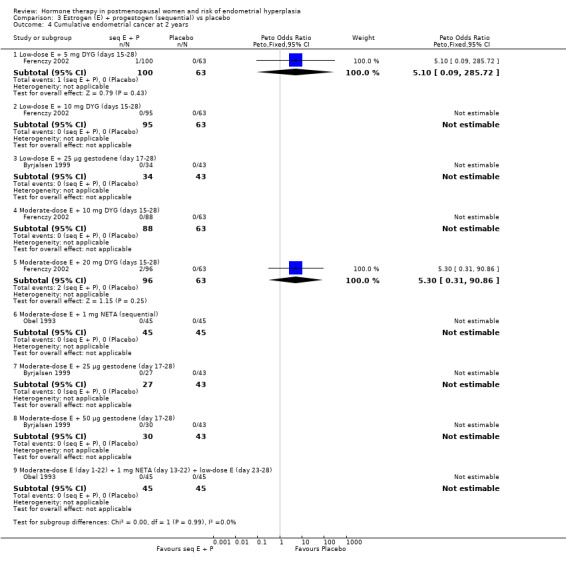
Comparison 3 Estrogen (E) + progestogen (sequential) vs placebo, Outcome 4 Cumulative endometrial cancer at 2 years.
Comparison 4. Unopposed estrogen (E) vs E+P (continuous).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 8 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Unopposed low‐dose E vs low‐dose E + 0.5 mg DSP | 1 | 453 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.66 [1.89, 30.96] |
| 1.2 Unopposed low‐dose E vs low‐dose E + 1 mg DSP | 1 | 457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.80 [1.93, 31.51] |
| 1.3 Unopposed low‐dose E vs low‐dose E + 2 mg DSP | 1 | 453 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.89 [1.31, 18.29] |
| 1.4 Unopposed low‐dose E vs low‐dose E + 3 mg DSP | 1 | 457 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.80 [1.93, 31.51] |
| 1.5 Unopposed low‐dose E vs low‐dose E + 1.5 mg MPA | 1 | 1092 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.82 [1.22, 11.90] |
| 1.6 Unopposed low‐dose E vs low‐dose E + 2.5 mg MPA | 1 | 821 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.55 [1.21, 17.08] |
| 1.7 Unopposed low‐dose E vs low‐dose E + 0.1 mg NETA | 1 | 496 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.98 [3.60, 13.52] |
| 1.8 Unopposed low‐dose E vs low‐dose E + 0.2/0.25 mg NETA | 3 | 1065 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.90 [3.62, 13.15] |
| 1.9 Unopposed low‐dose E vs low‐dose E + 0.5 mg NETA | 2 | 816 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.41 [3.82, 14.38] |
| 1.10 Unopposed low‐dose E vs low‐dose E + 1 mg NETA | 2 | 559 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.72 [0.27, 122.11] |
| 1.11 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA | 3 | 1328 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.42 [5.63, 12.60] |
| 1.12 Unopposed moderate‐dose E vs moderate‐dose E + 5 mg MPA | 1 | 557 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.92 [5.16, 15.43] |
| 1.13 Unopposed moderate‐dose E vs moderate‐dose E + 0.5 mg NETA | 1 | 233 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.86 [3.75, 20.92] |
| 1.14 Unopposed moderate‐dose E vs moderate‐dose E + 1 mg NETA | 2 | 369 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.40 [4.55, 19.40] |
| 2 Cumulative endometrial hyperplasia at 2 years | 3 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Unopposed low‐dose E vs low‐dose E + 0.2 mg NETA | 1 | 290 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.24 [0.16, 9.83] |
| 2.2 Unopposed low‐dose E vs low‐dose E + 0.5 mg NETA | 1 | 278 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.57 [0.31, 40.95] |
| 2.3 Unopposed low‐dose E vs low‐dose E + 1 mg NETA | 1 | 286 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 3.70 [0.35, 38.81] |
| 2.4 Unopposed low‐dose E vs low‐dose E + 1.5 mg MPA | 1 | 274 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.99 [2.83, 28.58] |
| 2.5 Unopposed low‐dose E vs low‐dose E + 2.5 mg MPA | 1 | 196 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.94 [1.44, 16.95] |
| 2.6 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA | 2 | 356 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 11.99 [7.09, 20.27] |
| 2.7 Unopposed moderate‐dose E vs moderate‐dose E + 1 mg NETA | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 177.61 [36.08, 874.43] |
| 3 Cumulative endometrial hyperplasia at 3 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 17.07 [9.89, 29.44] |
| 4 Endometrial cancer at 1 year | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Unopposed low‐dose E vs low‐dose E + 0.1 mg NETA | 1 | 496 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Unopposed low‐dose E vs low‐dose E + 0.2/0.25 mg NETA | 1 | 498 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.3 Unopposed low‐dose E vs low‐dose E + 0.5 mg NETA | 1 | 488 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 MPA | 1 | 562 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.29 [0.14, 367.19] |
| 4.5 Unopposed moderate‐dose E vs moderate‐dose E + 5 MPA | 1 | 557 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.16 [0.14, 360.91] |
| 5 Endometrial cancer at 3 years | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adherence to therapy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.17 [0.10, 0.28] |
| 7 Additional investigations (unscheduled biopsy) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.44 [7.36, 21.02] |
| 8 Withdrawal because of adverse events | 3 | 1909 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.15 [0.85, 1.55] |
| 8.1 Unopposed low‐dose E vs low‐dose E + 1.5 mg MPA (cont) | 1 | 422 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.74 [0.78, 3.90] |
| 8.2 Unopposed low‐dose E vs low‐dose E + 2.5 mg MPA (cont) | 1 | 320 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.80 [0.64, 5.01] |
| 8.3 Unopposed low‐dose E vs low‐dose E + 0.25 mg NETA | 1 | 230 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.90 [0.37, 2.21] |
| 8.4 Unopposed low‐dose E vs low‐dose E + 1 mg NETA | 1 | 236 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.79 [0.33, 1.88] |
| 8.5 Unopposed moderate‐dose E vs moderate‐dose E + 2.5 mg MPA (cont) | 1 | 224 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.21 [2.02, 13.41] |
| 8.6 Unopposed moderate‐dose E vs moderate‐dose E + 0.5 mg NETA | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.53 [0.26, 1.08] |
| 8.7 Unopposed moderate‐dose E vs moderate‐dose E + 1 mg NETA | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.71 [0.34, 1.49] |
4.4. Analysis.
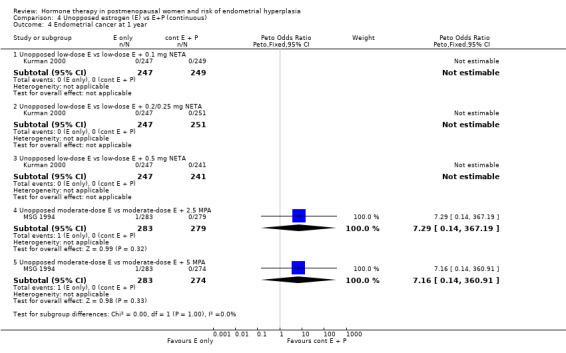
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 4 Endometrial cancer at 1 year.
4.5. Analysis.
Comparison 4 Unopposed estrogen (E) vs E+P (continuous), Outcome 5 Endometrial cancer at 3 years.
Comparison 5. Unopposed estrogen (E) vs E+P (sequential).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 1.1 Unopposed low‐dose E vs low‐dose E + 30 µg NGM (3 days E only/3 days E+P) | 1 | 525 | Odds Ratio (M‐H, Fixed, 95% CI) | 5.91 [3.33, 10.47] |
| 1.2 Unopposed low‐dose E vs low‐dose E + 90 µg NGM (3 days E only/3 days E+P) | 1 | 507 | Odds Ratio (M‐H, Fixed, 95% CI) | 188.68 [11.62, 3064.38] |
| 1.3 Unopposed low‐dose E vs low‐dose E + 180 µg NGM (3 days E only/3 days E+P) | 1 | 508 | Odds Ratio (M‐H, Fixed, 95% CI) | 189.46 [11.67, 3076.98] |
| 1.4 Unopposed moderate‐dose E vs moderate‐dose E + 5 mg MPA (11 days/cycle) | 2 | 612 | Odds Ratio (M‐H, Fixed, 95% CI) | 20.04 [7.16, 56.07] |
| 1.5 Unopposed moderate‐dose E vs moderate‐dose E and 10 mg MPA (12 days/cycle) | 2 | 792 | Odds Ratio (M‐H, Fixed, 95% CI) | 67.46 [13.26, 343.08] |
| 1.6 Unopposed moderate‐dose E vs moderate‐dose E + 200 mg progesterone (12 days/cycle) | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 15.69 [3.62, 67.94] |
| 1.7 Unopposed high‐dose E vs high‐dose E + 5 mg MPA (11 days/cycle) | 1 | 43 | Odds Ratio (M‐H, Fixed, 95% CI) | 11.7 [2.19, 62.62] |
| 2 Cumulative endometrial hyperplasia at 2 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Unopposed moderate‐dose E vs moderate‐dose E + 10 mg MPA (sequential) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.60 [5.32, 17.34] |
| 2.2 Unopposed moderate‐dose E vs moderate‐dose E and 200 mg progesterone (sequential) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 10.50 [5.80, 19.01] |
| 3 Cumulative endometrial hyperplasia at 3 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Unopposed moderate‐dose E vs moderate‐dose E + 10 mg MPA (sequential) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.71 [7.43, 21.76] |
| 3.2 Unopposed moderate‐dose vs moderate‐dose E and 200 mg progesterone (sequential) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 12.90 [7.55, 22.05] |
| 4 Endometrial cancer at 1 year | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Unopposed moderate‐dose E vs moderate‐dose E + 5 mg MPA (sequential) | 1 | 560 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.23 [0.14, 364.65] |
| 4.2 Unopposed moderate‐dose E vs moderate‐dose E and 10 mg MPA (sequential) | 1 | 555 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.96 [0.06, 15.41] |
| 5 Endometrial cancer at 3 years | 1 | 476 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.1 Unopposed moderate‐dose E vs moderate‐dose E and 10 mg MPA (sequential) | 1 | 237 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Unopposed moderate‐dose E vs moderate‐dose E + 200 mg progesterone (sequential) | 1 | 239 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Adherence to therapy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 6.1 Unopposed moderate‐dose E vs moderate‐dose E and 10 mg MPA (sequential) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.20 [0.12, 0.34] |
| 6.2 Unopposed moderate‐dose E vs moderate‐dose E + 200 mg progesterone (sequential) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.22 [0.13, 0.36] |
| 7 Additional investigations (endometrial biopsy) | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Unopposed moderate‐dose E vs moderate‐dose E + 10 mg MPA (sequential) | 1 | 237 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.94 [5.32, 15.01] |
| 7.2 Unopposed moderate‐dose E vs moderate‐dose E and 200 mg progesterone (sequential) | 1 | 239 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.90 [5.89, 16.63] |
| 8 Withdrawal owing to adverse events | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 8.1 Unopposed low‐dose E vs low‐dose E + 30 µg NGM (3 days E only/3 days E+P) | 1 | 624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.71 [1.64, 4.48] |
| 8.2 Unopposed low‐dose E vs low‐dose E + 90 µg NGM (3 days E only/3 days E+P) | 1 | 624 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.28 [1.39, 3.72] |
| 8.3 Unopposed low‐dose E vs low‐dose E + 180 µg NGM (3 days E only/3 days E+P) | 1 | 627 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.97 [1.22, 3.17] |
| 8.4 Unopposed moderate‐dose E vs moderate‐dose E + 5 mg MPA (sequential) | 1 | 83 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.62 [0.17, 2.31] |
| 8.5 Unopposed high‐dose E vs high‐dose E + 5 mg MPA (sequential) | 1 | 90 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.10, 0.83] |
5.2. Analysis.
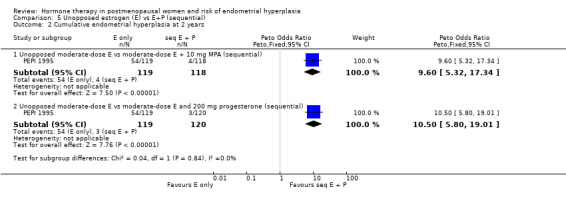
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 2 Cumulative endometrial hyperplasia at 2 years.
5.4. Analysis.
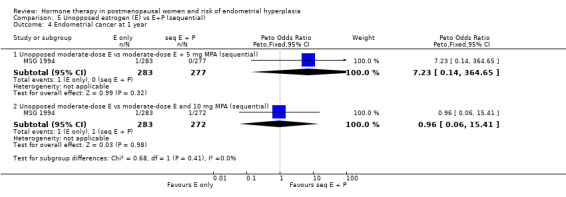
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 4 Endometrial cancer at 1 year.
5.5. Analysis.
Comparison 5 Unopposed estrogen (E) vs E+P (sequential), Outcome 5 Endometrial cancer at 3 years.
Comparison 6. E+P (continuous) vs E+P (sequential).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 5 mg MPA (day 14‐25) | 1 | 18 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 5 mg MPA (day 15‐28) | 1 | 556 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.66 [0.11, 3.85] |
| 1.3 Moderate‐dose E + 5 mg MPA (cont) vs moderate‐dose E + 5 mg MPA (day 14‐25) | 1 | 19 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Moderate‐dose E +5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 15‐28) | 1 | 546 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.71] |
| 1.6 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 200 mg progesterone (day 1‐12) | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 2.16] |
| 1.7 Moderate‐dose E + 1 mg NETA (cont) vs low‐dose E + 90 µg NGM (days 4‐6, 10‐12...) | 1 | 361 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Moderate‐dose E + 1 mg NETA (cont) vs low‐dose E + 180 µg NGM (days 4‐6, 10‐12...) | 1 | 295 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cumulative endometrial hyperplasia at 2 years | 4 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 0.35 mg NETA (day 4‐6, 10‐12...) | 1 | 62 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 0.7 mg NETA (day 4‐6, 10‐12...) | 1 | 56 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 84 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.29 [0.05, 1.70] |
| 2.5 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 200 mg progesterone (day 1‐12) | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.36 [0.05, 2.61] |
| 2.6 Moderate‐dose E + 1 mg NETA (cont) vs low‐dose E + 90 µg NGM (days 4‐6, 10‐12...) | 1 | 282 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Cumulative endometrial hyperplasia at 3 years | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.02] |
| 3.2 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 200 mg progesterone (day 1‐12) | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.23 [0.05, 1.04] |
| 4 Endometrial cancer at 1 year | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 5 mg MPA (day 15‐28) | 1 | 556 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Moderate‐dose E + 5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 15‐28) | 1 | 546 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.77] |
| 5 Cumulative endometrial cancer at 2 years | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 5.1 Low‐dose E + 25 µg gestodene (cont) vs low‐dose E + 25 µg gestodene (day 17‐28) | 1 | 68 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.39 [0.15, 372.38] |
| 5.2 Low‐dose E + 25 µg gestodene (cont) vs moderate‐dose E + 25 µg gestodene (day 17‐28) | 1 | 61 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.01 [0.12, 311.10] |
| 5.3 Low‐dose E + 25 µg gestodene (cont) vs moderate‐dose E + 50 µg gestodene (day 17‐28) | 1 | 64 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.57 [0.13, 333.61] |
| 5.4 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 1 mg NETA (days 13‐23) | 1 | 84 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cumulative endometrial cancer at 3 years | 1 | 478 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.1 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 1‐12) | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 200 mg progesterone (day 1‐12) | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 7 Adherence to therapy | 1 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.30 [0.66, 2.55] |
| 7.2 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 200 mg progesterone (day 1‐12) | 1 | 240 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.73, 2.74] |
| 8 Additional Investigations | 1 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 8.1 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 10 mg MPA (day 1‐12) | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.52 [0.22, 1.22] |
| 8.2 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 200 mg progesterone (day 1‐12) | 1 | 240 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.61 [0.25, 1.48] |
| 9 Withdrawal owing to adverse events | 4 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 9.1 Moderate‐dose E + 1 mg NETA (cont) vs low‐dose E + 90 µg NGM (days 4‐6, 10‐12...) | 1 | 438 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.82 [0.46, 1.47] |
| 9.2 Moderate‐dose E + 1 mg NETA (cont) vs low‐dose E + 180 µg NGM (days 4‐6, 10‐12...) | 1 | 434 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.23 [0.14, 0.38] |
| 9.3 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 0.35 mg NETA (day 4‐6, 10‐12...) | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.61, 4.91] |
| 9.4 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 0.7 mg NETA (day 4‐6, 10‐12...) | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.73 [0.61, 4.91] |
| 9.5 Moderate‐dose E + 2.5 mg MPA (cont) vs moderate‐dose E + 5 mg MPA (day 14‐25) | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.4 [0.06, 2.77] |
| 9.6 Moderate‐dose E + 5 mg MPA (cont) vs moderate‐dose E + 5 mg MPA (day 14‐25) | 1 | 24 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.18 [0.02, 1.95] |
| 9.7 Moderate‐dose E + 1 mg NETA (cont) vs moderate‐dose E + 1 mg NETA (days 13‐23) | 1 | 100 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.23 [0.35, 4.32] |
Comparison 7. Continuous combined E+P (dose comparisons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 16 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low‐dose E + 0.5 mg DRSP vs low‐dose E + 1 mg DRSP | 1 | 458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.2 Low‐dose E + 2 mg DRSP vs low‐dose E + 3 mg DRSP | 1 | 458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.52 [0.15, 379.06] |
| 1.3 Low‐dose E + 0.125 mg TMG vs low‐dose E + 0.5 mg NETA | 1 | 241 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.4 Low‐dose E + 0.1‐0.25 mg NETA vs low‐dose E + 0.5 mg NETA | 1 | 741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.41 [0.17, 11.48] |
| 1.5 Low‐dose E + 0.2‐0.5 mg NETA vs low‐dose E + 1 mg NETA | 2 | 683 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.75 [0.15, 391.13] |
| 1.6 Low‐dose E + 1.5 mg MPA vs low‐dose E + 2.5 mg MPA | 1 | 817 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.50 [0.24, 85.08] |
| 1.7 Low‐dose E + 0.25‐0.5 mg NETA vs moderate‐dose E + 1 mg NETA | 2 | 193 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.8 Low‐dose E + 0.5 mg NETA vs moderate‐dose E + 2.5 mg MPA | 1 | 72 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.9 Low‐dose E + 1 mg NETA vs low‐dose E + 2.5 mg MPA | 1 | 357 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.10 Low‐dose E + 1.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 822 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 4.54 [0.24, 85.12] |
| 1.11 Low‐dose E + 2.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 551 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.12 Low‐dose E + 2.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.13 Low‐dose E + 1 mg NETA vs moderate‐dose E + 1 mg NETA | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.14 Moderate‐dose E + 0.5 mg NETA vs moderate‐dose E + 2.5 mg MA | 1 | 23 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.15 Moderate‐dose E + 0.5 mg NETA vs moderate‐dose E + 2.5 mg MPA | 1 | 232 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.16 Moderate‐dose E + 1 mg NETA vs moderate‐dose E + 5 mg MA | 1 | 25 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.17 Moderate‐dose E + 2.5 mg MPA vs moderate‐dose E + 5 mg MPA | 2 | 861 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.28 [0.45, 116.76] |
| 1.18 Moderate‐dose E + 5 mg MPA vs moderate‐dose E + 10 mg MPA | 1 | 305 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.19 Moderate‐ to high‐dose E + 2.5 mg MPA vs moderate‐ to high‐dose E + 1 mg NETA | 1 | 287 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.20 Moderate‐ to high‐dose E + 5 mg MPA vs moderate‐ to high‐dose E + 1 mg NETA | 1 | 280 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.21 High‐dose E + 2 mg DNG vs moderate‐dose E + 1 mg NETA | 1 | 277 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.22 High‐dose E + 3 mg DNG vs moderate‐dose E + 1 mg NETA | 1 | 255 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cumulative endometrial hyperplasia at 2 years | 10 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose E + 1 mg DRSP vs low‐dose E + 2 mg DRSP | 1 | 88 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low‐dose E + 2mg DRSP vs low‐dose E + 3 mg DRSP | 1 | 94 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Low‐dose E + 0.125 mg TMG vs moderate‐dose E + 1 mg NETA | 1 | 170 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Low‐dose E + 0.2‐0.5 mg NETA vs low‐dose E + 1 mg NETA | 1 | 130 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.5 Low‐dose E + 1.5 mg MPA vs low‐dose E + 2.5 mg MPA | 1 | 210 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.6 Low‐dose E + 2 mg MPA vs moderate‐dose E + 2 mg MPA | 1 | 142 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.28] |
| 2.7 Low‐dose E + 2.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 128 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.8 Low‐dose E + 2.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 53 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.9 Low‐dose E + 1.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 206 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.10 Low‐dose E + 1 mg NETA vs moderate‐dose E + 1 mg NETA | 1 | 136 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.11 Moderate‐dose E + 2.5 mg MPA vs moderate‐dose E + 5 mg MPA | 3 | 427 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.12 Moderate‐dose E + 5 mg MPA vs moderate‐dose E + 10 mg MPA | 2 | 287 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.13 Moderate‐ to high‐dose E + 5 mg MPA vs high‐dose E + 1 mg NETA | 1 | 163 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.14 Moderate‐ to high‐dose E + 2.5 mg MPA vs high‐dose E + 1 mg NETA | 1 | 173 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.15 Moderate‐ to high‐dose E + 2.5 mg MPA vs moderate‐ to high‐dose E + 5 mg MPA | 1 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.16 High‐dose E + 2.5 mg MPA vs high‐dose E + 5 mg MPA | 1 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3 Endometrial cancer at 1 year | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Low‐dose E + 0.1‐0.25 mg NETA vs low‐dose E + 0.5 mg NETA | 1 | 741 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 3.2 Moderate‐dose E + 2.5 mg MPA vs moderate‐dose E + 5 mg MPA | 1 | 553 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4 Withdrawal owing to adverse events | 9 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low‐dose E + 0.5 mg DRSP vs low‐dose E + 1 mg DRSP | 1 | 458 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.82 [0.48, 1.39] |
| 4.2 Low‐dose E + 2 mg DRSP vs low‐dose E + 3 mg DRSP | 2 | 578 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.12 [0.72, 1.75] |
| 4.3 Low‐dose E + 1 mg DRSP vs low‐dose E + 2 mg DRSP | 1 | 120 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.09 [0.90, 4.86] |
| 4.4 Low‐dose E + 0.2‐0.5 mg NETA vs moderate‐dose E + 1 mg NETA | 2 | 619 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.69, 1.72] |
| 4.5 Low‐dose E + 0.25‐0.5 mg NETA vs moderate‐dose E + 1 mg NETA | 1 | 60 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.37 [0.27, 7.05] |
| 4.6 Low‐dose E + 1 mg NETA vs low‐dose E + 2.5 mg MPA | 1 | 360 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.99 [0.52, 1.88] |
| 4.7 Low‐dose E + 2.5 mg MPA vs moderate‐dose E + 2.5 mg MPA | 1 | 59 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.54 [0.10, 2.88] |
| 4.8 Moderate‐dose E + 0.5 mg NETA vs moderate‐dose E + 2.5 mg MPA | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.61 [0.82, 3.18] |
| 4.9 Moderate‐dose E + 2.5 mg MPA vs moderate‐dose E + 5 mg MPA | 2 | 518 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.51, 1.46] |
| 4.10 Moderate‐dose E + 5 mg MPA vs moderate‐dose E + 10 mg MPA | 1 | 378 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.75 [0.91, 3.36] |
| 4.11 Moderate‐ to high‐dose E + 2.5 mg MPA vs moderate‐ to high‐dose E + 5 mg MPA | 1 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.49 [0.62, 3.58] |
| 4.12 High‐dose E + 2.5 mg MPA vs high‐dose E + 5 mg MPA | 1 | 140 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.76 [0.75, 4.10] |
7.3. Analysis.
Comparison 7 Continuous combined E+P (dose comparisons), Outcome 3 Endometrial cancer at 1 year.
Comparison 8. Sequential E+P (dose/regimen comparisons).
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Endometrial hyperplasia at 1 year | 12 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 1.1 Low‐dose E + 5 mg DYG (days 15‐28) vs low‐dose E + 10 mg DYG (days 15‐28) | 1 | 124 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.18 [0.16, 414.15] |
| 1.2 Low‐dose E + 0.125 mg TMG (days 15‐28) vs low‐dose E + 0.25 mg TMG (days 15‐28) | 1 | 544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.12, 4.06] |
| 1.3 Low‐dose E + 0.125‐0.25 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 15‐28) | 1 | 801 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 2.04 [0.37, 11.38] |
| 1.4 Moderate‐dose E + 0.25 mg TMG (days 15‐28) vs moderate‐dose E + 0.5 mg TMG (days 15‐28) | 1 | 244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.5 Moderate‐dose E + 0.25 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.6 Moderate‐dose E + 0.5 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 235 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.7 Moderate‐dose E + 0.5 mg TMG (days 15‐28) vs moderate‐dose E + 0.5 mg NG (days 19‐28) | 1 | 396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.70 [0.19, 2.61] |
| 1.8 Moderate‐dose E + 5 mg MPA (days 12‐25) vs moderate‐dose E + 10 mg MPA (days 12‐25) | 2 | 717 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 6.43 [1.09, 37.85] |
| 1.9 Moderate‐dose E + 10 mg MPA (days 1‐12) vs moderate‐dose E + 200 mg progesterone (days 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.52 [0.05, 5.04] |
| 1.10 Moderate‐dose E + 25 µg gestodene (days 17‐28) vs moderate‐dose E + 50 µg gestodene (days 17‐28) | 1 | 20 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 1.11 Moderate‐dose E + 1 mg NETA (10 days/3 months) vs moderate‐dose E + 1 mg NETA (10 days/month) | 1 | 195 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 8.70 [1.93, 39.27] |
| 1.12 Moderate‐dose E + 10 mg MPA (14 days/3 month) vs moderate‐dose E +10 mg MPA (14 days/month) | 1 | 41 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.95 [0.11, 308.59] |
| 1.13 High‐dose E + 10 mg MPA (10 days/3 months) vs high‐dose E + 10 mg MPA (10 days/month) | 1 | 46 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.14 [0.01, 2.33] |
| 1.14 Moderate‐dose E + 10 mg MPA (14 days/3 months) vs moderate‐dose E + 10 mg MPA (28 days/3 months) | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.91 [0.05, 15.09] |
| 1.15 High‐dose E + 10 mg MPA (triphasic) vs high‐dose E + 10 mg MPA (biphasic) | 1 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 7.21 [0.14, 363.26] |
| 1.16 High‐dose E + 10 mg MPA (triphasic) vs high‐dose E + 1 mg NETA (triphasic) | 1 | 340 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2 Cumulative endometrial hyperplasia at 2 years | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 2.1 Low‐dose E + 0.35 mg NETA (days 4‐6, 10‐12...) vs moderate‐dose E + 0.7 mg NETA (days 4‐6, 10‐12...) | 1 | 58 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.2 Low‐dose E + 5 mg DYG (days 15‐28) vs low‐dose E + 10 mg DYG (days 15‐28) | 1 | 195 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.3 Moderate‐dose E + 10 mg DYG (days 15‐28) vs moderate‐dose E + 20 mg DYG (days 15‐28) | 1 | 184 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 2.4 Moderate‐dose E + 10 mg MPA (days 1‐12) vs moderate‐dose E + 200 mg progesterone (days 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.36 [0.30, 6.12] |
| 2.5 High‐dose E + 10 mg MPA (10 days/3 months) vs high‐dose E + 10 mg MPA (10 days/month) | 1 | 44 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.01, 1.37] |
| 2.6 Moderate‐dose E + 1 mg NETA (10 days/3 months) vs moderate‐dose E + 1 mg NETA (10 days/month) | 1 | 195 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.24 [1.27, 21.51] |
| 3 Cumulative endometrial hyperplasia at 3 years | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 3.1 Moderate‐dose E + 10 mg MPA (days 1‐12) vs moderate‐dose E + 200 mg progesterone (days 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.02 [0.32, 3.24] |
| 3.2 Moderate‐dose E + 1 mg NETA (10 days/3 months) vs moderate‐dose E + 1 mg NETA (10 day/month) | 1 | 195 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 5.64 [2.03, 15.65] |
| 4 Endometrial cancer at 1 year | 5 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 4.1 Low‐dose E + 0.125 mg TMG (days 15‐28) vs low‐dose E + 0.25 mg TMG (days 15‐28) | 1 | 544 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.2 Low‐dose E + 0.125‐0.25 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 15‐28) | 1 | 801 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.04 [0.00, 2.95] |
| 4.3 Moderate‐dose E + 0.25 mg TMG (days 15‐28) vs moderate‐dose E + 0.5 mg TMG (days 15‐28) | 1 | 244 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 4.4 Moderate‐dose E + 0.25 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 243 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.33] |
| 4.5 Moderate‐dose E + 0.5 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 235 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.76] |
| 4.6 Moderate‐dose E + 0.5 mg TMG (days 15‐28) vs moderate‐dose E + 0.5 mg NG (days 19‐28) | 1 | 396 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.12 [0.00, 5.98] |
| 4.7 Moderate‐dose E + 5 mg MPA (days 12‐25) vs moderate‐dose E + 10 mg MPA (days 12‐25) | 2 | 717 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.13 [0.00, 6.70] |
| 5 Cumulative endometrial cancer at 2 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 5.1 Low‐dose E + 25 µg gestodene vs moderate‐dose E + 25 µg gestodene | 1 | 61 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 5.2 Low‐dose E + 5 mg DYG (days 15‐28) vs low‐dose E + 10 mg DYG (days 15‐28) | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 2.88 [0.12, 71.56] |
| 5.3 Moderate‐dose E + 10 mg DYG (days 15‐28) vs moderate‐dose E + 20 mg DYG (days 15‐28) | 1 | 184 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.21 [0.01, 4.51] |
| 5.4 Moderate‐dose E + 25 μg gestodene vs moderate‐dose E + 50 μg gestodene | 1 | 57 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6 Cumulative endometrial cancer at 3 years | 2 | Odds Ratio (M‐H, Fixed, 95% CI) | Subtotals only | |
| 6.1 Moderate‐dose E + 10 mg MPA (days 1‐12) vs moderate‐dose E + 200 mg progesterone (days 1‐12) | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 0.0 [0.0, 0.0] |
| 6.2 Moderate‐dose E + 1 mg NETA (10 days/3 months) vs moderate‐dose E + 1 mg NETA (10 days/month) | 1 | 195 | Odds Ratio (M‐H, Fixed, 95% CI) | 3.32 [0.13, 82.62] |
| 7 Adherence to therapy | 2 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 7.1 Moderate‐dose E + 10 mg MPA (days 1‐12) vs moderate‐dose E + 200 mg progesterone (days 1‐12) | 1 | 238 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.09 [0.57, 2.07] |
| 7.2 High‐dose E + 10 mg MPA (triphasic) vs high‐dose E + 10 mg MPA (biphasic) | 1 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.50 [0.79, 2.87] |
| 8 Additional investigations | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.55, 2.56] |
| 8.1 Moderate‐dose E + 10 mg MPA (days 1‐12) vs moderate‐dose E + 200 mg progesterone (days 1‐12) | 1 | 238 | Odds Ratio (M‐H, Fixed, 95% CI) | 1.19 [0.55, 2.56] |
| 9 Withdrawals because of adverse events | 6 | Peto Odds Ratio (Peto, Fixed, 95% CI) | Subtotals only | |
| 9.1 Low‐dose E + 0.35 mg NETA (days 4‐6, 10‐12...) vs moderate‐dose E + 0.7 mg NETA (days 4‐6, 10‐12...) | 1 | 100 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.43 [0.17, 1.13] |
| 9.2 Low‐dose E + 5 mg DYG (days 15‐28) vs low‐dose E + 10 mg DYG (days 15‐28) | 1 | 231 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.97 [0.47, 2.01] |
| 9.3 Moderate‐dose E + 10 mg DYG (days 15‐28) vs moderate‐dose E + 20 mg DYG (days 15‐28) | 1 | 235 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.41, 1.68] |
| 9.4 Moderate‐dose E + 0.25 mg TMG (days 15‐28) vs moderate‐dose E + 0.5 mg TMG (days 15‐28) | 1 | 276 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.87 [0.48, 1.56] |
| 9.5 Moderate‐dose E + 0.25 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 278 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.78 [0.44, 1.38] |
| 9.6 Moderate‐dose E + 0.5 mg TMG (days 15‐28) vs moderate‐dose E + 1 mg NETA (days 13‐22) | 1 | 272 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.90 [0.51, 1.59] |
| 9.7 Moderate‐dose E + 0.5 mg TMG (days 15‐28) vs moderate‐dose E + 0.5 mg NG (days 19‐28) | 1 | 634 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.32 [0.84, 2.08] |
| 9.8 High‐dose E + 10 mg MPA (10 days/3 months) vs high‐dose E + 10 mg MPA (10 days/month) | 1 | 52 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.38 [0.08, 1.83] |
| 9.9 High‐dose E + 10 mg MPA (triphasic) vs high‐dose E + 10 mg MPA (biphasic) | 1 | 393 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 0.83 [0.37, 1.83] |
| 9.10 High‐dose E + 10 mg MPA (triphasic) vs high‐dose E + 1 mg NETA (triphasic) | 1 | 340 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 1.40 [0.78, 2.52] |
8.2. Analysis.
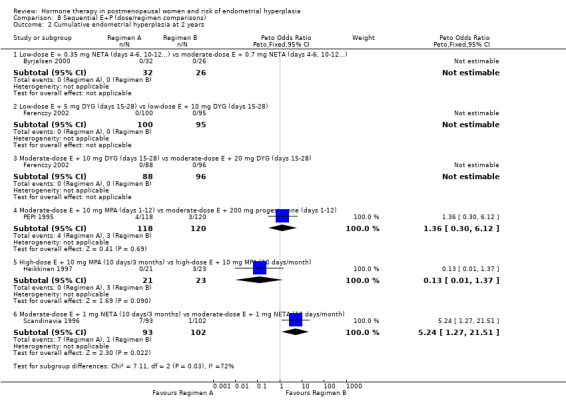
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 2 Cumulative endometrial hyperplasia at 2 years.
8.8. Analysis.
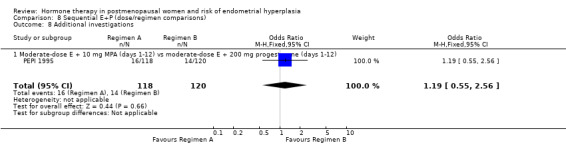
Comparison 8 Sequential E+P (dose/regimen comparisons), Outcome 8 Additional investigations.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
AinMelk 1996.
| Methods | Randomisation: random number table Open‐label single‐centre, parallel group trial Number of women randomised: 59 Number of women analysed: 53 Number of withdrawals: 6 (2 in group 1 owing to mastalgia, nausea, and bleeding and 2 from each group owing to bleeding) Power calculation and ITT analysis: not described Sources of funding: Upjohn (Canada), Wyeth‐Ayerst (Canada) and Ogen provided medication for the study | |
| Participants | Country: Canada Inclusion criteria: healthy postmenopausal at least 1‐year post natural menopause, with intact uterus, vasomotor symptoms and normal endometrial histology Exclusion criteria: previous use of HT | |
| Interventions | Continuous (1) 0.625 mg CEE + 2.5 mg MPA (2) 0.625 mg EIS + 2.5 mg MPA Duration: 2 years (104 weeks) | |
| Outcomes | Irregular bleeding, endometrial histology, amenorrhoea | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Not mentioned and results are based on data from the 53 women who completed the study (90% of those randomised) | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random number table |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Reasons for withdrawal described |
| Other bias | Low risk | 2 parallel groups, balanced at baseline |
Al‐Azzawi 2001.
| Methods | Randomisation: method not stated Randomised double‐blind multicentre study, (n = 42) Number of women randomised: 487 Number of women analysed: 349 Number of withdrawals: 138 of which 64% were because of AEs (specified) not including bleeding Source of funding: Joint development programme by Aventis and Wyeth‐Ayerst International | |
| Participants | Country: not specified ‐ Europe and UK Inclusion criteria: postmenopausal women with intact uterus, at least 6 months of amenorrhoea or at least 12 months on HT, FSH, LH and E2 levels in the postmenopausal range, and at least 3 hot flushes per day. There was a washout period of 4 weeks for those previously on HT Exclusion criteria: specified intercurrent disease, any contraindication to HT, endometrial hyperplasia |
|
| Interventions | Continuous versus sequential (1) 2 mg E2 + 0.25 mg TMG daily (2) 2 mg E2 + 0.5 mg TMG daily (3) 2 mg E2 daily for 12 days, then 2 mg E2 + 1 mg NETA daily for 10 days, then 1 mg E2 daily for 6 days Duration: 13 cycles | |
| Outcomes | Mean duration of withdrawal bleeding, severity of endometrial bleeding, endometrial hyperplasia, endometrial cancer | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Analysis described as ITT but denominator numbers for endometrial hyperplasia are the numbers of women completing the study | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind with treatments placed into capsules of identical appearance |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Reasons for early withdrawal described in Table 12 for the 28% of women who did not complete the study |
| Other bias | Low risk | 3 parallel groups, some difference between groups in menopause duration. |
Archer 2005.
| Methods | Randomisation: method not stated Multicentre, double‐blind, parallel group study Number of women randomised: 1147 Number of women analysed: 1142 Source of funding: Berlex Inc, New Jersey | |
| Participants | Country: US, women recruited from several centres Inclusion: postmenopausal women, aged 42 to 75 years, with 6 to 12 months' amenorrhoea, with intact uterus, with or without menopausal symptoms. Negative endometrial biopsy or endometrial thickness < 5 mm. Serum FSH > 50 IU/L, serum estradiol < 20 pg/mL Exclusion: abnormal endometrial histology, specified intercurrent illness | |
| Interventions | Continuous (1) E2 1 mg daily (2) E2 1 mg + 0.5 mg DSP daily (3) E2 1 mg + 1 mg DSP daily (4) E2 1 mg + 2 mg DSP daily (5) E2 1 mg + 3 mg DSP daily Duration: 1 year | |
| Outcomes | Endometrial hyperplasia, bleeding patterns | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Analysis described as by ITT | |
| Notes | Hot flush frequency, urogenital symptoms, quality of life | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Participants, medical staff and outcome assessors were blinded to treatment allocation |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 5 women who were randomised did not take any of the medication. Paper describes ITT analysis on 1142 women who took at least 1 dose of the study medication. 297 (26%) of those randomised prematurely discontinued the study medication for reasons described in Table 9. However, endometrial biopsy results are only available for the 966 (86%) of participants who underwent both a baseline and a post baseline biopsy |
| Other bias | Low risk | 5 parallel groups, well balanced at baseline |
Bouchard 2005.
| Methods | Randomisation: method not stated Double‐blind, multicentre (107 sites in 13 countries) randomised trial by the TMG 301 study group Number of women randomised: 911 Number of women analysed: ?? Number of withdrawals: approximately 167 women withdrew owing to AEs (74 from group 1, 56 from group 2, 37 from group 3) | |
| Participants | Countries: Israel, Denmark, Estonia, Finland, France, Germany, Italy,
Latvia, Norway, Spain, Sweden, the Netherlands, UK Inclusion criteria: healthy postmenopausal women with an intact uterus, more than 3 hot flushes per day, at least 6 months amenorrhoea or 12 months previous HT, BMI < 35 Exclusion criteria: intercurrent illness (specified), endometrial hyperplasia, contraindication to HT |
|
| Interventions | Continuous (1) 1 mg E2 +0.125 mg TMG (2) 2 mg E2 +1 mg NETA (3) 1 mg E2 + 0.5 mg NETA Duration: 2 years | |
| Outcomes | Amenorrhoea, mean number of bleeding/spotting days, endometrial hyperplasia | |
| Power calculation described | Yes ‐ described in the methods paper (see Gambacciani 2005 under Bouchard 2005) | |
| Analysis by Intention to Treat | Paper describes analysis by ITT but no denominator numbers given | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Numbers randomised to each part of the study unclear |
| Other bias | Unclear risk | At baseline some differences between groups in smoking status and duration of menopause, which were accounted for in the analysis |
Bruhat 2001.
| Methods | Randomisation: method not stated Design: multicentre (15 in France and 18 in Germany), open‐label parallel group Number of women randomised: 427 Number of women analysed: 340 Number of withdrawals: 87 (20%) mostly because of AEs including bleeding (15, 10, 3 women in groups 1, 2, 3, respectively), weight gain (7, 3, 1, respectively) mastalgia (12, 6, 5, respectively) Source of funding: not stated | |
| Participants | Countries: France and Germany Inclusion criteria: healthy postmenopausal women aged 45 to 65 years, at least 3 years past menopause, with BMI ≤ 31, intact uterus and normal gynaecological findings. Women on HT had a 1‐month washout period. Exclusion criteria: endometrial hyperplasia, specified serious intercurrent illness | |
| Interventions | Continuous (1) 1 mg E2 + 2.5 mg MPA daily for 6 months, then 2 mg E2 + 2.5 mg MPA daily (2) 1 mg E2 + 5 mg MPA daily for 6 months, then 2 mg E2 + 5 mg MPA daily (3) 2 mg E2 + 1 mg NETA daily Duration: 12 months | |
| Outcomes | Mean number of bleeding days per cycle at 1 year, Climacteric symptoms, lipid profile, endometrial hyperplasia, withdrawal because of AEs, adherence to protocol | |
| Power calculation described | Yes, "it was calculated that a total group size of 390 in 26 centres (with at least 15 women per centre) was sufficient to reveal a 20% difference in E2/MPA doses and E2/NETA in the mean number of bleeding days" | |
| Analysis by Intention to Treat | "The primary analysis was based on the intention to treat approach" | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open label |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All the women who withdrew from the study (20% of those randomised) are accounted for but there is no indication of how many women had endometrial biopsy taken at study completion |
| Other bias | Low risk | 3 groups balanced at baseline |
Byrjalsen 1992.
| Methods | Randomisation: method not stated Single‐blind, parallel‐group, placebo‐controlled, single centre Number of women randomised: 73 Number of women analysed: 66 Number of early withdrawals: 7 (3 because of vaginal bleeding, 1 because of oedema of the legs, 2 because of lack of time, 1 because of illness unrelated to study) | |
| Participants | Country: Denmark Inclusion criteria: healthy postmenopausal women aged 45 to 54 years who had experience natural menopause 9 to 40 months previously Exclusion criteria: diseases or concomitant medications known to influence study measurements | |
| Interventions | Sequential vs sequential vs placebo (A) 2 mg EV days 1 to 21 + 10 mg MPA daily days 12 to 21 (B) 1.5 mg E2 days 1 to 24 + 150 µg DG days 13 to 24 (C) placebo Duration: 2 years |
|
| Outcomes | Mean duration of withdrawal bleed, amenorrhoea, endometrial biopsy | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 73 women were randomised to 3 groups. Biopsies at the end of the study on 56 women (77% of those randomised) |
| Other bias | Low risk | 3 groups balanced at baseline |
Byrjalsen 1999.
| Methods | Randomisation: method not stated (randomisation mentioned in the conference paper but not in the full paper) Double‐blind, single‐centre, parallel‐groups Number of women included: 278 Number of women analysed: 168 Number of withdrawals: 110 (42 because of uterine bleeding, 34 because of medical AEs, 35 because of events unrelated to the trial) | |
| Participants | Country: Denmark Inclusion criteria: healthy postmenopausal women aged 45 to 63 years who had undergone a natural menopause Exclusion criteria: intercurrent diseases or concomitant medications known to influence study measurements | |
| Interventions | sequential versus continuous vs placebo (A) 2 mg E2 daily days 1 to 28 + 50 µg gestodene daily on days 17 to 28 (B) 2 mg E2 daily days 1 to 28 + 25 µg gestodene daily on days 17 to 28 (C) 1 mg E2 daily days 1 to 28 + 25 µg gestodene daily on days 17 to 28 (D) 1 mg E2 + 25 µg gestodene daily (continuous) (E) placebo Duration: 2 years |
|
| Outcomes | Endometrial biopsy at 2 years, bleeding patterns, compliance | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No, paper does not mention ITT and does not give numbers randomised to each group, endometrial hyperplasia reported as numbers and % of those who completed the end of study biopsy | |
| Notes | Numbers allocated to each group calculated from Table 9 and Table 10 | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind ‐ investigators and participants |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 110 of the 278 women randomised (40%) withdrew before the end of the study |
| Other bias | Unclear risk | 5 groups, not told how many randomised to each group, but baseline data only given for completers (46% of those randomised) |
Byrjalsen 2000.
| Methods | Randomisation: using random numbers 2 groups double‐blind and comparison group single‐blind, parallel group, single centre, allocation concealed during the study Number of women randomised: 200 Number of women analysed: 113 Reasons for withdrawal:
|
|
| Participants | Country: Denmark Inclusion criteria: healthy postmenopausal women aged 45 to 65 years who had undergone natural menopause at least 1 year before the study Exclusion criteria: diseases or medication known to influence study measurements. Endometrial dysplasia or malignancy | |
| Interventions | Intermittent vs continuous vs placebo (A) 1.5 mg POS daily + 0.7 mg NETA on days 4 to 6, 10 to 12, 16 to 18, 22 to 24, 28 to 30 (high E+P group) (B) 0.75 mg POS daily + 0.35 mg NETA on days 4 to 6, 10 to 12, 16 to 18, 22 to 24, 28 to 30 (low E+P group) (C) 2 mg E2 + 1 mg NETA daily (continuous combination) (D) Placebo |
|
| Outcomes | After 2 years of treatment blood samples, endometrial thickness and endometrial biopsy | |
| Power calculation described | No | |
| Analysis by Intention to Treat | ITT not mentioned with regard to analysis | |
| Notes | Part of this study is described as double blind and part as single blind. 3 groups had medication identical in appearance (high E+P, low E+P and placebo), while the continuous combined group had medication that looked different | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Random numbers used to generate sequence |
| Allocation concealment (selection bias) | Low risk | Allocation concealed during study |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | High E+P and low E+P sequential groups were double blinded (investigator and participant). The continuous E+P and placebo groups were described as single blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 78 women (39%) withdrew from the study prematurely, 9 refused a biopsy, 20 biopsies yielded insufficient tissue and 2 biopsy samples were lost. Endometrial hyperplasia data is from the remaining 91 women (46% of those randomised) |
| Other bias | Low risk | 4 parallel groups balanced at baseline for the parameters reported |
Chang 2003.
| Methods | Randomisation: method not stated. Groups were of uneven size (142, 84, 88, groups A, B, C, respectively) Single‐centre, double‐blind Number of women randomised: 314 Number of women analysed: varied between 81 and 241 (102, 66, 73, groups A, B, C, respectively) Number of withdrawals: 73 because of intolerable breast tenderness or loss to follow‐up Source of funding: grant from National Sciences Council, Taiwan | |
| Participants | Country: Taiwan Inclusion criteria: postmenopausal women with intact uterus, aged 45 to 65 years, referred to the Menopausal Special Clinic at National Taiwan University Hospital, Taipei. Women were amenorrhoeic for at least 1 year or 6 months + serum FSH > 40 IU/L and estradiol < 20 ng/L Exclusion criteria: history of endometrial or breast cancer | |
| Interventions | Sequential (A) 0.625 mg CEE daily days 1 to 25 + 5 mg MPA daily on days 12 to 25 (B) 0.625 mg CEE daily days 1 to 25 + 10 mg MPA daily on days 12 to 25 (C) 0.625 mg CEE daily days 1 to 25 + 20 mg DYG daily on days 12 to 25 Duration: 1 year |
|
| Outcomes | Endometrial thickness, endometrial biopsy | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | Approximately 30% of participants had 'concomitant diseases' including diabetes and hypertension | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23% of those randomised withdrew owing to "intolerable breast tenderness and loss to follow up". Numbers of withdrawals in each group not given |
| Other bias | Low risk | 3 groups well balanced at baseline |
CHART 1996.
| Methods | Randomised, double‐blind, placebo‐controlled, multicentre study Method of randomisation and allocation concealment: randomisation code prepared by Biometrics Department in blocks of 9 with computer‐generated random numbers Number of women randomised: 1265 Number of withdrawals: 570 (at 2 years) based on losses to follow‐up and stopping rule. Excluding the 10 μg EE group (treatment terminated owing to high incidence of endometrial hyperplasia), 73% of women completed the study Source of funding: Parke‐Davis Pharmaceutical Research | |
| Participants | Country: US (65 study centres participated) Participants: women aged 40 years or older who had undergone spontaneous menopause within the last 5 years and who had an intact uterus Inclusion criteria: healthy volunteers; FSH ≥ 40 IU/L; estradiol ≤ 73 pmol/L; atrophic endometrium; no major illnesses Exclusion criteria: baseline vaginal bleeding, baseline mammography suggestive of malignant disease, chronic use of medications that affect bone calcium metabolism or significant vasomotor symptoms that required medical treatment No participant was to have taken oral or transdermal estrogen therapy for 6 months prior to randomisation | |
| Interventions | Continuous vs placebo (1) 1 μg daily of oral EE + 0.2 mg of oral NETA continuously (2) 2.5 μg daily of oral EE + 0.5 mg of oral NETA continuously (3) 5 μg daily of oral EE + 1 mg of oral NETA continuously (4) 10 μg daily of oral EE + 1 mg of oral NETA continuously (5) 1 μg daily of oral EE continuously (6) 2.5 μg daily of oral EE continuously (7) 5 μg daily of oral EE continuously (8) 10 μg daily of oral EE continuously (9) Control group: placebo Duration: 2 years |
|
| Outcomes | Endometrial biopsy at baseline and after 6, 12, 18 and 24 months of therapy | |
| Power calculation described | Yes, "A sample size of 110 evaluable subjects per treatment group was needed at the end of month 24 to detect, at 0.05 significance level and power of 0.95, a difference in hyperplasia rate, assuming a 12% rate in the unopposed EE groups and 1% rate in the combination groups... A 22% drop out rate was estimated over the duration of the study therefore at least 135 women per treatment group were enrolled" | |
| Analysis by Intention to Treat | No | |
| Notes | Based on priori stopping rules, subjects in group 8 (10 μg daily of oral EE continuously) were terminated from the study early owing to a high rate of hyperplasia for that treatment group | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation code prepared by Biometrics Department in blocks of 9 with computer‐generated random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation code prepared by Biometrics Department, and held centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Subjects in the 10 μg EE group were terminated early. All the other groups had similar rates of withdrawal ranging between 22% and 30% |
| Other bias | Low risk | 9 parallel treatment groups well balanced at baseline |
Corson 1999.
| Methods | Randomisation: method not stated
Multicentre, parallel group design
with double blinding
Number of women randomised: n = 1253
Number of women analysed: 903
Number of withdrawals: 361
(E2
1 mg; 100 women, E2/NGM 30 μg 65 women, E2 1 mg/NGM 90 μg 97 women, E2 1
mg/NGM 180 μg 99 women. Reasons for early withdrawal provided in Table 10 Source of funding: not stated |
|
| Participants | Country: US Age: 40 to 65 years Inclusion criteria: postmenopausal for > 12 months, serum estradiol < 20 pg/mL and serum FSH > 30 mlU/mL, intact uterus, no HT in the 8 weeks before study entry, discontinue injectable or implantable sex steroids use 6 months before study entry, have no contraindications to estrogen or progestin therapy | |
| Interventions | Intermittent vs unopposed estrogen (1) 1 mg E2 for 3 days, then 1 mg E2 + 30 μg NGM for 3 days (sequential) (2) 1 mg E2 for 3 days, then 1 mg E2 + 90 μg NGM for 3 days (sequential) (3) 1 mg E2 for 3 days, then 1 mg E2 + 180 μg NGM for 3 days (sequential) (4) estrogen only group, 1 mg E2 daily (continuous) Duration: 12 months |
|
| Outcomes | Endometrial hyperplasia | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Yes | |
| Notes | Author contacted regarding randomisation method and exclusion criteria | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 361 women of the 1253 randomised (29%) withdrew from the study, but analysis is by ITT |
| Other bias | Low risk | 4 parallel treatment groups well balanced at baseline |
Ettinger 1992.
| Methods | Randomisation: method not stated Single‐centre, parallel group dose‐ranging design with double blinding in phase 1 Number of women randomised: 63 (52 with intact uterus) Number of women analysed for endometrial outcome: 28 Source of funding: Mead Johnson Laboratories, Division of Bristol Myers | |
| Participants | Country: US Postmenopausal mostly white women, aged 40 to 58 years, recruited from advertisements and physician referrals Inclusion criteria: within 5 years of menopause (confirmed by bilateral oophorectomy or no menses for ≥ 6 months; E2 deficiency (confirmed by FSH "> 40 U/L"); body weight within 20% of ideal for height (Metropolitan Life Company Tables) Exclusion criteria: presence of diseases/conditions known to affect skeletal health such as thyroid/parathyroid disorders or other disorders of calcium homeostasis; use of anticonvulsants or glucocorticoids; evidence of renal, hepatic, cardiac or malignant diseases | |
| Interventions | Unopposed estrogen vs placebo (1) 0.5 mg micronised E2 + calcium carbonate supplements daily on 23 of 28 days (2) 1.0 mg micronised E2 + calcium carbonate supplements daily on 23 of 28 days (3) 2.0 mg micronised E2 + calcium carbonate supplements daily on 23 of 28 days (4) Control: placebo + calcium carbonate supplements daily on 23 of 28 days Duration: 18 months |
|
| Outcomes | Frequency of hyperplasia (from endometrial biopsy) Frequency of unexpected bleeding | |
| Power calculation described | No, but statement in discussion (p.486) that this study lacked power to detect differences between the dosages | |
| Analysis by Intention to Treat | No | |
| Notes | 11 women (17.5%) had previous hysterectomy so above outcomes analysed in a subgroup of 52 women. Primary objective of the study was to evaluate bone loss | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Phase 1 double‐blind (18 months) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 28 women (54%) of those randomised had an endometrial biopsy at the end of the treatment |
| Other bias | High risk | Small study with 4 parallel groups not well balanced at baseline |
Ferenczy 2002.
| Methods | Randomisation: method not stated Multicentre, double‐blind placebo‐controlled study Number of women randomised: 595 Number of women analysed: 579 Number of withdrawals: 152 Source of funding: Solvay Pharmaceutical | |
| Participants | Non‐hysterectomised, postmenopausal women with amenorrhoea of at least 6 months or surgically postmenopausal (following bilateral oophorectomy without hysterectomy, > 3 months prior to enrolment), FSH within normal postmenopausal range Age: 45 to 65 years from Canada, the Netherlands Exclusion criteria: abnormal (uninvestigated bleeding) vaginal bleeding; the use of estrogens, progestogens, androgens, or a combination in the preceeding 6 months or more and any previous use of estradiol pellet/implant therapy | |
| Interventions | Sequential vs placebo (1) Placebo (2) 1 mg/day E2/5 mg DYG for the last 14 days of each 28 day cycle (3) 1 mg/day E2/10 mg DYG for the last 14 days of each 28 day cycle (4) 2 mg/day E2/10 mg DYG for the last 14 days of each 28 day cycle (5) 2 mg/day E2/20 mg DYG the last 14 days of each 28 day cycle Duration: 26 cycles (104 weeks) |
|
| Outcomes | Endometrial hyperplasia, endometrial cancer | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Yes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 442 women (74%) of the 595 randomised had an endometrial biopsy at the end of the treatment |
| Other bias | Low risk | 5 parallel groups well balanced at baseline |
Gelfand 1989.
| Methods | Computerised random assignment controlled by pharmacist Single‐centre, parallel‐group design, double blinding Number of women randomised: 173 Number of withdrawals: 78 (24 women: 1 year of treatment not complete when study terminated and not included in analysis; 54 women withdrew during the study for medical reasons but withdrawals not comparable between treatment groups (9.5%, 9%, 2% and 28% groups 1, 2, 3 and 4, respectively)) Source of funding: Ayerst, McKenna and Harrison | |
| Participants | Country: Quebec, Canada, the Netherlands Age: 45 to 65 years Non‐hysterectomised, postmenopausal women with amenorrhoea of at least 6 months or surgically postmenopausal (following bilateral oophorectomy without hysterectomy, > 3 months prior to enrolment), FSH within normal postmenopausal range Exclusion criteria: abnormal (uninvestigated bleeding) vaginal bleeding, the use of estrogens, progestogens, androgens, or a combination in the preceeding 6 months or more and any previous use of estradiol pellet/implant therapy | |
| Interventions | Unopposed estrogen vs sequential (1) 0.625 mg daily oral CEE for 25 days of a 30‐day cycle + placebo (2) 0.625 mg daily oral CEE for 25 days of a 30‐day cycle + 5 mg oral MPA added to the last 11 days of the CEE cycle (3) 1.25 mg daily oral CEE for 25 days of a 30‐day cycle + placebo (4) 1.25 mg daily oral CEE for 25 days of a 30‐day cycle + 5 mg oral MPA added to the last 11 days of the CEE cycle Duration: 1 year |
|
| Outcomes | Frequency of hyperplasia (endometrial biopsy at baseline, 6 and 12 months) Withdrawal because of AEs | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computerised random assignment controlled by pharmacist |
| Allocation concealment (selection bias) | Low risk | Computerised random assignment controlled by pharmacist |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 95 women of 173 randomised (55%) had endometrial biopsies at the end of the treatment |
| Other bias | Unclear risk | 4 groups with some imbalance at baseline in % smokers and alcohol consumption |
Graser 2000.
| Methods | Randomisation: method not stated Double‐blind, multicentre international trial (49 centres in Europe and South Africa) Number of women randomised: 595 Number of women analysed: 410 to 595 Number of withdrawals: 58, 67, 60 women withdrew from groups 1, 2, 3, respectively Source of Funding: Jenapharm GmbH & Co, and Schering AG, Germany | |
| Participants | Countries: Germany. Czech Republic, Poland, Hungary, Bulgaria, South Africa Inclusion criteria: postmenopausal women with troublesome vasomotor symptoms, aged ≤ 65 years; BMI 20 to 32; ≥ 24 months' amenorrhoea; serum estradiol in postmenopausal range; serum FSH ≥ 30 IU/L Exclusion criteria: previous HT use (sequential regimen within 4 weeks or continuous regimen within 6 months of start of trial), other treatment for hot flushes, previous or existing serious illness (specified), endometrial hyperplasia, contraindications to HT, concomitant medications (specified) | |
| Interventions | Continuous (1) 2 mg EV + 2 mg DNG daily "Climodien" (2) 2 mg EV + 3 mg DNG daily (3) 2 mg estrogen + 1 mg estriol + 1 mg NETA daily "Kliogest" first‐generation formula Duration: 1 year |
|
| Outcomes | Vaginal bleeding, endometrial biopsy, AEs | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | Efficacy, vasomotor symptoms, vaginal cytology | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 396 women of the 595 randomised (67%) had endometrial biopsies at the end of treatment |
| Other bias | Low risk | 3 parallel groups well balanced at baseline |
Greenwald 2005.
| Methods | Randomisation: method not stated Multicentre (17 sites) double‐blind, parallel‐group study Number of women randomised: 327 Number of women analysed: 189 Number of withdrawals: 138 of which 65 were because of AEs (not specified) Source of funding: grant from Novo Nordisk A/S, Bagsvaerd, Denmark | |
| Participants | Country: US Inclusion criteria: healthy women at least 45 years old, between 1 and 5 years postmenopause, with an intact uterus, serum E2 < 20 pg/mL, and normal bone mineral density Exclusion criteria: serious intercurrent disease (specified) bone disease; immobilisation; treatment with fluoride, calcitonin, bisphosphonate or corticosteroids |
|
| Interventions | Unopposed estrogen vs continuous vs placebo (A) placebo (B) 0.25 mg E2 daily (C) 0.5 mg E2 daily (D) 1 mg E2 daily (E) 1 mg E2 + 0.25 mg NETA daily (F) 1 mg E2 + 0.5 mg NETA daily (G) 2 mg E2 + 1 mg NETA daily Duration: 26 months |
|
| Outcomes | Bone mineral density, endometrial hyperplasia | |
| Power calculation described | Yes, "Assuming a patient dropout rate of 30% a sample size of 43 women per group was required to detect a 4% difference in BMD between the active treatment groups and placebo, with a power of 95% and a significance level of 5%" | |
| Analysis by Intention to Treat | Analysis by both ITT (bone mineral density outcome) and per protocol (hyperplasia outcome) | |
| Notes | Bone mineral density primary outcome | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Unclear how many women had endometrial biopsies at the end of the treatment period. 138 of the 327 women randomised (42%) withdrew and had last observation carried forward. |
| Other bias | Low risk | 7 parallel groups well balanced at baseline |
Harris 1991.
| Methods | Randomisation: method not reported but study medication identical and concealed in identical bottles Multicentre (3 study sites), parallel‐group design, placebo controlled with double blinding Number of women randomised: 156 Number of withdrawals: 36 (28 lacked TBD values, 2 did not comply and 6 had baseline measurements outside the time limits specified) Power calculation for sample size performed and analysis by ITT for the outcomes reported in this review Source of funding: not reported. | |
| Participants | Country: US Postmenopausal women with mean age 51 years recruited from 3 study sites Inclusion criteria: no use of sex hormones for previous 3 months; last normal period or bilateral oophorectomy for benign conditions > 2 months previously; FSH levels within the postmenopausal range; no contraindications to estrogen therapy; no drug treatment (except sex steroids or calcium) for osteoporosis < 1 year before entry into the study; resting supine blood pressure ≤ 160/90 mmHg at first visit; weight within 125% of upper limit of Metropolitan Insurance Company reference weights; spinal bone mineral content measured by tomography ≥ 80 mg/cm3 at first study visit Exclusion criteria: diseases or conditions that might affect bone or calcium metabolism or gastrointestinal absorption; requirement for medication that might interfere with estrogen metabolism or efficacy or calcium or bone metabolism; treatment with hypolipidaemic agents or ketoconazole; use of fluoride for osteoporosis at any time | |
| Interventions | Unopposed estrogen vs placebo (1) EIS 0.3 mg + 2.5 g calcium carbonate daily for 25 days, followed by 6 days without treatment (2) EIS 0.625 mg + 2.5 g calcium carbonate daily for 25 days, followed by 6 days with no treatment (3) EIS 1.25 mg + 2.5 g calcium carbonate daily for 25 days, followed by 6 days with no treatment (4) Control: placebo with same regimen Duration: 2 years |
|
| Outcomes | Withdrawal because of AEs, endometrial hyperplasia, non‐adherence to therapy | |
| Power calculation described | Calculated that "in order to have 88 assessable patients after 2 years would need to enrol 135 patients, and this would give 80% power (with one‐sided p<.05) to detect a difference of 5% in BMD between the two treatment groups at 2 years. However the observed difference was only 2.8% which would have required enrolment of 360 women (compared to the 156 actually enrolled) to give the desired power" | |
| Analysis by Intention to Treat | Yes | |
| Notes | Primary outcome osteoporosis prevention. Author contacted for clarification of data relating to adherence to therapy and reviewer was referred to Abbott Laboratories. Abbott Laboratories have not replied so adherence to therapy is not included in the review | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 36 women of the 156 randomised (23%) withdrew prior to the end of the study but endometrial biopsy data available on 98% of those randomised |
| Other bias | Unclear risk | 4 parallel groups with some imbalance in duration of menopause |
Heikkinen 1997.
| Methods | Randomisation: method not stated
Single‐centre, parallel group,
blinding unclear
Number of women randomised: 78
Number of
withdrawals: 9 Number of women excluded: 2 Source of funding: Orion Corporation |
|
| Participants | Country: Finland 78 healthy women, aged 49 to 55 years, in early menopause recruited from the city of Oulu in Finland. Inclusion criteria: 0.5 to 3 years postmenopausal (confirmed by FSH), without previous HT, without contraindications for HT Exclusion criteria: specified diseases | |
| Interventions | Monthly sequential vs long‐cycle sequential vs placebo (1) 2 mg EV on days 1 to 11 and 2 mg EV + 10 mg MPA then placebo for 7 days (2) 2 mg EV on days 1 to 70 and 2 mg EV + 20 mg MPA on days 71 to 84 then 7 days placebo (3) Control: placebo for 24 months Duration: 2 years |
|
| Outcomes | Hyperplasia at 2 years Irregular bleeding (data not published) Withdrawal because of AEs | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | Published trial supplied by drug company, Orion Corporation, which manufactures Tridestra, a long‐cycle sequential O + P treatment The primary outcomes of this study were effects of HT and exercise on bone density, muscle strength and lipid metabolism. Hyperplasia and adherence to treatment were secondary outcomes | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding unclear, though placebo used |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | At the end of treatment endometrial biopsies performed on 69 of the 78 women randomised (88%) |
| Other bias | Low risk | 3 parallel groups well balanced at baseline |
Heikkinen 2000.
| Methods | Randomisation: method not stated, single‐centre, parallel groups, described as double blind but 2 of the 6 groups had dose escalation after 6 months Number of women randomised: 419 Number of women analysed: 419 at 2 years, 296 at 4 years Number withdrawals: 56 at 12 months mainly owing to bleeding disturbances Source of funding: not stated | |
| Participants | Country: Oulu, Finland Inclusion Criteria: healthy postmenopausal women at least 3 years post last menstruation, with intact uterus and BMI ≤ 30 Exclusion criteria: contraindications to HT, serious intercurrent disease, smoker of more than 15 cigarettes per day, suspected alcohol abuse. 1‐month washout period for previous HT users | |
| Interventions | Continuous (1) 1 to 2 mg EV + 2.5 mg MPA daily (2) 1 to 2 mg EV + 5 mg MPA daily (3) 2 mg EV + 2.5 mg MPA daily (4) 2 mg EV + 5 mg MPA daily (5) 1 mg EV + 2.5 mg MPA daily (6) 1 mg EV + 5 mg MPA daily Duration: 24 months |
|
| Outcomes | Bone mineral density, bleeding patterns, endometrial hyperplasia | |
| Power calculation described | No | |
| Analysis by Intention to Treat | ITT for bone mineral density outcome but no data given on the numbers of women who had endometrial biopsy at the end of the study | |
| Notes | This study was a randomised comparison for the first 7 years, and outcomes from this period are included in this review. After 7 years the women in groups 1 and 3 stopped treatment because this HT regimen was no longer available. 198 of 279 women originally in the other groups were able to continue on 2/5, 1/2.5 or 1/5 doses of EV/MPA for a further 18 months and then all were given 1/2.5 for 6 months and then followed for a further year after HT was stopped | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind for the first 2 years and then single blind (participants only) thereafter; however' 2 groups had dose escalation after 6 months, which may have compromised blinding in these groups |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Moderate attrition but information on numbers and reasons for losses for each treatment group not clear |
| Other bias | Unclear risk | 6 parallel treatment groups, with some differences at baseline in % smokers and % previously used HT |
HOPE 2001.
| Methods | Randomisation: computer‐generated table, in blocks of 8 Multicentre, double‐blind, placebo‐controlled Number of women randomised: basic study 2673; 2‐year sub‐study 822 Number of women analysed: basic study 2153; 2‐year sub‐study 518 (analysed for the hyperplasia outcome) Number of withdrawals: 520 (lost to follow‐up) Source of funding: Wyeth‐Ayerst Research, Philadelphia, Pennsylvania | |
| Participants | Country: US
Postmenopausal women with intact uterus, absence of
menses for at least the past year, E2 levels < 184 pmol/L (50 pg/mL) and
FSH levels > 30 IU/L, within 20% of their normal body weight range
Age: 40 to 65 years old
Exclusion criteria: if taken
medication containing estrogens, progestins, or androgens within 8 weeks of
the pre‐study screening, endometrial hyperplasia diagnosed at baseline,
specified intercurrent disease, smoking > 15 cigarettes/day Sub‐study: intact uterus, 1 to 4 years' postmenopausal, baseline E2 ≤ 184 pmol/L and FSH levels ≥ 30 IU/L and body weight within 20% of desirable weight range |
|
| Interventions | Unopposed estrogen vs continuous vs placebo (1) CEE 0.625 mg daily (2) CEE 0.625 mg/MPA 2.5 mg/daily (3) CEE 0.45 mg (4) CEE 0.45 mg/MPA 2.5 mg/daily (5) CEE 0.45 mg/MPA 1.5 mg/daily (6) CEE 0.3 mg/daily (7) CEE 0.3 mg/MPA 1.5 mg/daily (8) Placebo/daily Duration: 1 year (13 cycles) | |
| Outcomes | Endometrial hyperplasia | |
| Power calculation described | Yes (details in WHI 2002 appendix J) | |
| Analysis by Intention to Treat | No | |
| Notes | The basic study was focused on menopausal symptoms, endometrial histology and bleeding profiles. A 2‐year sub‐study examined bone density and turnover, serum lipoproteins and carbohydrate metabolism | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated table, in blocks of 8 |
| Allocation concealment (selection bias) | Low risk | Randomisation performed centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double‐blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | ‐ |
| Other bias | Unclear risk | 8 parallel groups; baseline characteristics described for the 69% of those
randomised who completed the 2‐year study. Pharmaceutical company Wyeth employed a medical communication company to insert specific marketing messages in all 3 of the primary publications on the HOPE study, that are referenced in this review. However, as 'ghost‐writing' has no influence on statistical data we consider that the likelihood of bias in this review due to 'ghost‐writing' is low |
Koninckx 2005.
| Methods | Randomisation: by global randomisation scheme following an allocation within each country by investigators. Multicentre, double blind Analysis: primary efficacy variable (hot flushes) by efficacy evaluable population, remainder by ITT Number randomised: 1218 (531 remained in study for year 2) Number premature withdrawals: 352 (main reason owing to AEs) | |
| Participants | Country: 98 sites in 8 countries (Belgium, Czech Republic, France, Germany, Israel, Switzerland, the Netherlands and the UK Inclusion criteria: postmenopausal women with an intact uterus, be amenorrhoeic for at least 6 months or have had hormone replacement therapy for at least 12 months, serum FSH greater than lower limit of normal for postmenopausal women, serum estradiol level lower than upper limit of normal for postmenopausal women and at least 3 hot flushes per day over a 7‐day period Exclusion criteria: known/suspected estrogen‐dependent neoplasia, endometrial hyperplasia, polyp or carcinoma at screening, serious specified intercurrent disease | |
| Interventions | Sequential (A) 1 mg E2 daily + 0.125 mg TMG daily on days 15 to 28 (B) 1 mg E2 daily + 0.25 mg TMG daily on days 15 to 28 (C) 1 mg EV daily + 1 mg norethisterone daily on days 17 to 28 Duration: 13 cycles |
|
| Outcomes | Bleeding profile, endometrial histology, safety | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Analysis of hyperplasia outcome not ITT | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by global randomisation scheme following an allocation within each country by investigators |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Endometrial biopsies on 801 women of 1218 randomised (66%) at end of treatment |
| Other bias | Unclear risk | 3 parallel groups. Baseline characteristics given for efficacy evaluable population not all those randomised |
Kurman 2000.
| Methods | Randomisation: by computer using blocks of 8 Double‐masked, randomised, parallel, controlled study in 40 centres Number of women randomised: 1176 Number of withdrawals: 251 Source of funding: Novo Nordisk Pharmaceutical | |
| Participants | Country: US Inclusion criteria: healthy women ≥ 45 years with an intact uterus, minimum 12 months' postmenopause, and serum E2 levels up to 25 pg/mL Exclusion criteria: women who had been treated with estrogen in the previous 12 weeks or with E+P combinations within the last 8 weeks; had known, suspected, or history of hormone‐dependent tumours or cancers; had known or suspected endometrial hyperplasia at study entry biopsy; had vaginal bleeding of unknown cause; were more than 30% above ideal body weight; had known deep vein thrombosis, active thrombophlebitis, thromboembolic disorder, cerebrovascular accident or history of these conditions; had myocardial infarction or Ischaemic heart disease in the previous 6 months; had treated or untreated systolic blood pressure > 160 mmHg or diastolic blood pressure > 100 mmHg; had presence of any endocrine disorder except controlled thyroid disease; were known alcohol or drug abusers; or had a known smoking habit of one pack of cigarettes a day or more | |
| Interventions | Unopposed estrogen vs continuous (1) 1 mg E2 daily (2) 1 mg E2 + 0.1 mg NETA daily (3) 1 mg E2 + 0.25 mg NETA daily (4) 1 mg E2 + 0.5 mg NETA daily Duration: 1 year |
|
| Outcomes | Frequency of endometrial hyperplasia/carcinoma Non‐adherence to therapy | |
| Power calculation described | Yes, "the sample size calculation assumed that the incidence of hyperplasia would be no more than 1% in the continuous combined groups and 7% in the unopposed E2. On the basis of those assumptions, approximately 250 women per group would have provided 80% power to detect a statistical difference (at the 0.05 level) between treatment groups assuming a dropout rate of 25%, therefore a minimum of 1000 women required for this study." | |
| Analysis by Intention to Treat | "Women who completed follow up were analysed by intention to treat." Analysis of endometrial hyperplasia based on the women who completed the study and underwent a final biopsy | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer using blocks of 8 |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Endometrial biopsies on 988 of the 1176 women randomised (84%) |
| Other bias | Low risk | 4 parallel groups well balanced at baseline |
Luciano 1993.
| Methods | Randomisation: method not stated Single‐centre, parallel‐group design with double blinding Number of women randomised: 36 Number of withdrawals: 7 (in group 1 owing to recurrence of depression and 1 owing to exacerbation of migraine, in group 2, 1 owing to exacerbation of hypertension, and in group 3 1 because of exacerbation of migraine and 3 because of cyclic bleeding) Source of funding: Upjohn (in part) | |
| Participants | Postmenopausal women were recruited from newspaper advertisements and letters to general practionners in Farmington, Connecticut, US Inclusion criteria: intact uterus, no contraindication to HT, last menses at least 1 year prior, no HT for at least 6 months prior, oestradiol < 35 pg/mL and FSH "<50 mIU/mL" No exclusion criteria stated | |
| Interventions | Continuous vs sequential (1) 0.625 mg CEE + 2.5 mg MPA daily (2) 0.625 mg CEE + 5.0 mg MPA daily (3) 0.625 mg CEE days 1‐25 + 5.0 mg MPA days 14 to 25 Duration: 1 year |
|
| Outcomes | Frequency of irregular bleeding | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Endometrial biopsies on 30 of the 36 women randomised (83%) |
| Other bias | Unclear risk | Small study with 3 parallel groups, some imbalance at baseline |
Mattsson 2004.
| Methods | Randomisation: stratified and blocked by centre, all participants received treatment according to that allocated next consecutive study number Open randomised multicentre trial in 16 centres in Scandinavia Number of women randomised: 394 Number of women analysed: 393 Number of withdrawals: 1 woman who received no treatment Sources of funding: Orion Pharma, Finland | |
| Participants | Countries: Norway, Denmark, Sweden Inclusion criteria: postmenopausal women with intact uterus, aged 53 to 65 years, BMI ≤ 30, with at least 1 month since last dose of HT Exclusion criteria: endometrial hyperplasia, contraindications to HT, previous use of comparator (2 mg E2 +1 mg NETA 'Kliogest') | |
| Interventions | Continuous (1) 1 to 2 mg EV + 2.5 mg MPA daily (2) 1 to 2 mg EV + 5 mg MPA daily (3) 2 mg EV + 1 mg NETA daily Duration: 24 cycles |
|
| Outcomes | Mean number days of bleeding, amenorrhoea, endometrial biopsy, endometrial thickness | |
| Power calculation described | Yes. The study was powered to detect a difference in the primary outcome which was bleeding | |
| Analysis by Intention to Treat | Yes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation stratified and blocked by centre, all participants received treatment according to that allocated next consecutive study number |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 255 of the 394 women randomised (65%) had endometrial biopsy at the end of treatment |
| Other bias | Low risk | 3 parallel groups well balanced at baseline |
Meuwissen 2001.
| Methods | Randomisation: method not stated, into 2 parallel groups Double blind, multicentre Number of women randomised: 634 Number of premature withdrawals: 153 (74 in group 1 and 79 in group 2, owing to AEs (48 and 37, respectively, 7 from group 1 because of lack of efficacy, and 47 (group not specified) because of protocol violation | |
| Participants | Country: 56 centres in Europe, countries not specified Inclusion criteria: postmenopausal women with at least 6 months' amenorrhoea, or who had received HT for < 12 months after 6 months' amenorrhoea (washout period 2 months). At least 4 hot flushes per day and FSH and estradiol levels in postmenopausal range Exclusion criteria: hysterectomy, serious specified intercurrent illness, endometrial hyperplasia/carcinoma at baseline biopsy | |
| Interventions | Sequential (A) 2 mg 17 E2 daily + 0.5 mg TMG daily on days 15 to 28 (B) Placebo days 1 to 7, 2 mg EV daily days 8 to 28 + 0.5 NG daily on days 19 to 28 Duration: 1 year (13 cycles) |
|
| Outcomes | Endometrial histology, bleeding patterns | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Yes | |
| Notes | Mean daily number hot flushes = primary efficacy variable. Numbers randomised to each group calculated from percentages reported | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 153 of the 634 women randomised (24%) withdrew during the study. End of treatment biopsies on 396 women (62%) of those randomised |
| Other bias | Low risk | 2 parallel groups well balanced at baseline |
MSG 1994.
| Methods | Methods of randomisation and allocation: computer‐generated schedule with packaged coded medication Multicentre (99 sites), double‐blind with parallel group design, placebo controlled Number of women randomised: 1724 Number of withdrawals: 255 after 1 year of follow‐up; 339 after 2 years of follow‐up Source of funding: Wyeth‐Ayerst Research | |
| Participants | Countries: US and Europe
Healthy women aged 45 to 65 years with an
intact uterus were recruited from 99 sites
Inclusion criteria: last
natural menstrual cycle at least 12 months before the baseline screening;
FSH higher than the lower limit for postmenopausal women for the given
laboratory; no use of estrogen‐ or progestogen‐containing medication for at
least 2 weeks before the pre‐study screening Exclusion criteria: any contraindication for estrogen or progestogen use, or if they had used any estrogen‐containing medication within 3 months of entry; major medical illness, liver, kidney or diabetes; hypertension, systolic blood pressure > 160 mmHg or diastolic pressure > 90 mmHg; abnormal cervical cytology or endometrial hyperplasia at baseline biopsy |
|
| Interventions | Continuous vs sequential (1) 0.625 mg per day CEE + placebo (2) 0.625 mg per day CEE + 2.5 mg per day MPA continuous (3) 0.625 mg per day CEE + 5 mg per day MPA continuous (4) 0.625 mg per day CEE + 5 mg per day MPA last 14 days of the cycle (days 15 to 28), + placebo (days 1 to 14) (5) 0.625 mg per day CEE + 10 mg per day MPA last 14 days of the cycle (days 15 to 28), + placebo (days 1 to 14) Duration: 1 year (13 cycles) |
|
| Outcomes | Frequency of hyperplasia, carcinoma, or both (confirmed by endometrial biopsy) at 6 and 12 months (at the end of cycles 6 and 13) Frequency of irregular bleeding or spotting (number of cycles) | |
| Power calculation described | Yes, "With a hypothesized incidence of endometrial hyperplasia of 7.55 in the Unopposed group and 25 in the CEE/MPA groups it was calculated that 215 patients per treatment group would provide 80% power to detect at least one statistically significant difference at the 0.0125 level (includes Bonferroni adjustment for multiple comparisons)" (see Woodruff 1994 p. 1214 under MSG 1994) | |
| Analysis by Intention to Treat | No | |
| Notes | If hyperplasia was confirmed, the patient was withdrawn from the study and given appropriate treatment | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Methods of randomisation and allocation: computer‐generated schedule with packaged coded medication |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Endometrial biopsies after 1 year of treatment on 1385 of the 1724 women randomised (80%) |
| Other bias | Low risk | 5 parallel groups well balanced at baseline |
Nand 1995.
| Methods | Randomisation: method not stated Open study, no blinding Number of women randomised: 32 Number of withdrawals: 9 (group A 3, group B 3, group C 3) Source of funding: Upjohn Pty. |
|
| Participants | Country: Australia Inclusion criteria: 1‐year postmenopausal women with intact uterus and menopausal symptoms. Women already on HT had a 6‐week washout period Exclusion criteria: undiagnosed vaginal bleeding or specified serious intercurrent disease | |
| Interventions | continuous (A) 1.5 mg POS + 2.5 mg MPA daily (n = 9) (B) 1.5 mg POS + 5 mg MPA daily (n = 11) (C) 1.5 mg POS + 10 mg MPA daily (n = 11) Duration: 2 years |
|
| Outcomes | Bleeding pattern, endometrial biopsies, lipid levels, bone mineral density | |
| Power calculation described | No, pilot study | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Open study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 23 of the 32 women (72%) randomised had endometrial biopsies after 1 year |
| Other bias | Low risk | 3 parallel groups well balanced at baseline |
Notelovitz 1997.
| Methods | Randomisation: a single randomisation schedule was generated by the Department of Biometrics, Solvay Pharmaceuticals, with 4‐patient randomisation blocks of treatment distributed to each centre Multicentre, parallel group and placebo‐controlled design with double‐blinding Number of women randomised: 280 (with intact uterus) of a total of 406 Number of withdrawals: 42 (before follow up endometrial biopsy) Number of patients analysed: 238 (54% of this group completed the study) Source of funding: Solvay Pharmaceuticals | |
| Participants | Country: US Healthy postmenopausal women with a uterus, aged 40 to 62 years, were recruited from 29 centres Inclusion criteria: natural or surgical menopause (final menstrual period or oophorectomy between 6 months and 4 years prior to start of study; FSH ≥ 50 IU/L; non‐smokers; 45 to 54 years of age (> 21 years if documented bilateral oophorectomy; within 25% of ideal body weight Metropolitan Height and Weight tables) Exclusion criteria: bone mineral density ≥ 2 SD below normal peak for young adult women or evidence of vertebral compression fracture on screening radiography; treatment with estrogens or progestins within 8 weeks of enrolment; endometrial histology indicating either insufficient tissue in the presence of TVUS endometrial thickness of > 4 mm or proliferative, hyperplastic or secretory endometrium; previous endometrial ablation; undiagnosed vaginal bleeding; estrogen‐dependent cancers; abnormalities of Pap smear or mammogram | |
| Interventions | Unopposed estrogen vs placebo (1) ESE 0.3 mg daily (2) ESE 0.625 mg daily (3) ESE 1.25 mg daily (4) Control: placebo daily Duration: 2 years |
|
| Outcomes | Frequency of endometrial hyperplasia at 6 and 12 months follow‐up Non‐adherence to treatment Frequency of irregular bleeding Frequency of unscheduled endometrial biopsies (data not suitable for entry in the review) | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Yes | |
| Notes | We have reported the outcomes in this review for the 280 women with an intact uterus out of a total of 406 in the study | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | A single randomisation schedule was generated by the Department of Biometrics, Solvay Pharmaceuticals, with 4‐patient randomisation blocks of treatment distributed to each centre |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 218 of the 278 women randomised (78%) had endometrial biopsy after 2 years of treatment |
| Other bias | Low risk | 4 parallel groups well balanced at baseline |
Obel 1993.
| Methods | Randomisation: method and concealment of allocation not reported Single‐centre, parallel group and placebo‐controlled design with double blinding Number of women randomised: 151 Number of withdrawals: 22 (group 1: 5 because of AEs, 1 because of breast cancer, 5 for reasons unrelated to treatment, total 11; group 2: 3 because of AEs, 2 because of carcinoma, total 5; group 3: 1 because of AEs, 1 because of anxiety, 1 for reasons unrelated to treatment, total 3) Source of funding: not reported | |
| Participants | Country: Denmark Volunteers with early menopause (last spontaneous vaginal bleeding > 6 and < 24 months earlier) and with no use of HT during preceding 24 months recruited from Frederiksborg County Exclusion criteria: previous or current estrogen‐dependent neoplasia; thromboembolic disease, liver or pancreatic disease, diabetes mellitus, severe obesity, diseases with high or low bone turnover and medication known to influence bone metabolism or provoke induction of liver enzymes | |
| Interventions | Continuous vs sequential vs placebo (1) Continuous: 2 mg E2 + 1 mg NETA (Kliogest) daily (2) Sequential: 2 mg E2 days 1 to 12, E2 2 mg + 1 mg NETA days 13 to 22, 1 mg E2 days 23 to 28 (3) Control: placebo Duration: 2 years |
|
| Outcomes | Frequency of endometrial hyperplasia, carcinoma, or both, at 2 years Frequency of irregular bleeding Non‐adherence to therapy | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | Some of the data was read off the graphs | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Blinding |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Endometrial biopsies on 96 of the 151 women randomised (64%) after 2 years |
| Other bias | Low risk | 3 parallel groups described as having 'no inter‐group differences' |
OGEN‐Provera 1998.
| Methods | Randomisation: by computer code stratified by previous HT use, in blocks of 6 to facilitate even distribution of groups within each centre Multicentre (9 sites), double blind with regard to progestogen dose, parallel groups Number of women randomised: 568 Number of withdrawals: 146 (group A 50, group B 49, group C 47) Source of funding: Statistical support from Pharmacia and Upjohn. | |
| Participants | Country: Australia Inclusion criteria: healthy postmenopausal women with intact uterus and 1 to 10 years of amenorrhoea, with climacteric symptoms requiring therapy. Previous users of HT had at least 6‐week washout period. FSH > 40 IU/L Exclusion criteria: severe intercurrent disease | |
| Interventions | Continuous (A) 1.5 mg POS + 2.5 mg MPA daily (n = 189) (B) 1.5 mg POS + 5 mg MPA daily (n = 185) (C) 1.5 mg POS + 10 mg MPA daily (n = 183) Duration: 2 years |
|
| Outcomes | Primary outcome: cessation of vaginal bleeding Secondary outcomes: protection of endometrium, maintenance of acceptable blood lipid profile, maintenance of bone mineral density and improvement of climacteric symptoms | |
| Power calculation described | Yes, to detect a 10% difference in the incidence of bleeding, and assuming a drop‐out rate of 10% by 6 months, it was calculated that 157 patients per group would be required to achieve a power of 80% at a level of significance of 5% | |
| Analysis by Intention to Treat | Yes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer code stratified by previous HT use, in blocks of 6 to facilitate even distribution of groups within each centre |
| Allocation concealment (selection bias) | Low risk | Randomisation conducted centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Endometrial biopsies after 2 years on 415 of 568 women randomised (73%) |
| Other bias | Low risk | 3 parallel groups well balanced at baseline |
Okon 2001.
| Methods | Randomisation: computer‐generated codes, with allocation concealed in sealed envelopes Single‐centre double‐blind parallel‐group design Number of women randomised: 33 Number of women analysed: 23 Number of early withdrawals: 10 (main reasons were hormone related side effects, e.g. fluid retention, weight gain, depression, breast tenderness) Funding: Schering | |
| Participants | Countries: Denmark, Norway and Sweden. Inclusion criteria: women recruited aged 45 to 65 years (mean 52 ± 4 years) who had amenorrhoea for 12 to 72 months, serum FSH > 20 IU/L, plasma estradiol ≥ 30 pmol/L, intact uterus and requesting HT Exclusion criteria: women with a pathological biopsy at study entry, or abnormal liver or renal function test results | |
| Interventions | Sequential (1) 2 mg E2 daily + 25 µg gestodene added days 17 to 28 (2) 2 mg E2 daily + 50 µg gestodene added days 17 to 28 Duration: 1 year |
|
| Outcomes | Endometrial hyperplasia, bleeding patterns | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No per protocol only | |
| Notes | PP14 and CA125 levels in endometrial flushing solutions | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation using computer‐generated codes |
| Allocation concealment (selection bias) | Low risk | Allocation concealed in sealed envelopes |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | 23 of the 33 women randomised (70%) who completed the study had endometrial biopsies after 1 year of treatment |
| Other bias | Low risk | small trial 2 parallel groups, no differences at baseline |
OPAL 2006.
| Methods | Randomisation: method not stated Double blind, 11 centres, OPAL study Number of women randomised with intact uterus: 707 Number of women analysed (end point TVUS): 602 Number of withdrawals: approximately 105 women withdrew reasons not described (37 from group 1, 36 from group 2 and 32 from group 3) | |
| Participants | Countries: 6 centres in US and centres in the UK, Germany, the Netherlands (Utrecht and Rotterdam) and Sweden Inclusion criteria: healthy postmenopausal women (aged 45 to 79 years), BMI 19 to 32 kg/m2, amenorrhoea for at least 12 months or serum estradiol < 20 pg/mL and FSH at least 40 IU/L Exclusion criteria: abnormal Pap smear, endometrial thickness > 5 mm, endometrial hyperplasia, Intercurrent illness (specified), contraindication to HT | |
| Interventions | (1) oral tibolone 2.5 mg (2) continuous combined 0.625 CEE + 2.5 mg MPA (3) placebo Duration: 39 cycles of 28 days, 3 years All participants also received 500 mg/day oral calcium supplement |
|
| Outcomes | TVUS, at 52, 104 and 156 weeks, in US and UK centres. Endometrial biopsy at baseline and end of treatment | |
| Power calculation described | Yes, described in the methods paper (see Bots 2003 under OPAL 2006). 142 participants per group for 90% power at α = 0.05 to detect at difference in primary outcome carotid intima‐media thickness of 0.0185 mm/year | |
| Analysis by Intention to Treat | Bots 2003 paper describes analysis by ITT, as well as per protocol analysis but no denominator numbers given | |
| Notes | Some of the women included in this study had undergone hysterectomy. For this review we have used the data from participants with an intact uterus for the primary outcome (E+P 236 and placebo 243). For the outcome of withdrawal owing to AEs we have used the only data available, where the denominator is all the women randomised to E+P (288) or placebo (288) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Low risk | Central randomisation |
| Blinding (performance bias and detection bias) All outcomes | Low risk | "Double blind" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 707 women with a uterus were randomised, 665 (216, 219, 230) had a baseline TVUS, and only 602 (85%) (191, 200, 211) had an end of trial TVUS. Reasons not described but numbers excluded from each group are similar |
| Other bias | Low risk | Groups well balanced at baseline |
PEPI 1995.
| Methods | Randomisation and allocation: computer generated Treatment group assignment was stratified by clinical centre and uterine status (hysterectomy status) Multicentre (7 clinical centres), parallel group, placebo‐controlled design with double blinding Number of women randomised: 596 Number of women analysed: 596 (no exclusions post randomisation) Source of funding: Wyeth‐Ayerst Laboratories | |
| Participants | Country: US 875 healthy postmenopausal volunteers (596 with a uterus, 279 without a uterus), aged 45 to 65 years (average 56.2 years) were recruited via a national mass media campaign Inclusion criteria: good health; willing to accept random assignment to a HT or placebo; cessation of menses for at least 1 year, but not more than 10 years prior to enrolment; surgically menopausal at least 2 months after hysterectomy; FSH levels ≥ 40 IU/L; normal atrophic endometrial biopsy and mammography results at baseline Exclusion criteria: breast or endometrial cancer; any other cancer except non‐melanomatous skin cancer diagnosed < 5 years before baseline; serious medical illness (myocardial infarction within 6 months, congestive heart failure, stroke, transient ischaemic attack); severe menopausal symptoms; use of HT within previous 2 months; hyper‐ or hypothyroidism; normal pelvic examination, Papanicolaou test and endometrial biopsy | |
| Interventions | Participants randomised to equal numbers to 1 of the following oral
treatments in 28 day cycles: Unopposed estrogen vs continuous vs sequential vs placebo (1) 0.625 mg per day of CEE (2) 0.625 mg per day CEE + 10 mg per day of MPA for the first 12 days per month (sequential) (3) 0.625 mg per day CEE + 2.5 mg per day of MPA (continuous) (4) 0.625 mg per day CEE + 200 mg per day of cyclic MP for the first 12 days per month (sequential) (5) Control: placebo Duration: 3 years |
|
| Outcomes | Frequency of hyperplasia or carcinoma (confirmed by endometrial biopsy)
annually (at 12, 24 and 36 months)
Frequency of unscheduled biopsies
or dilatation and curettage
Non‐adherence to therapy Diary of symptoms, reports of vaginal bleeding, medication use and interim illnesses was reviewed Visits at 3, 6 and 12 months first year, 6 months thereafter for a total of 3 years |
|
| Power calculation described | Yes, sample size was based on a set of assumptions concerning attrition and loss to follow‐up over the 3 years of the study (See Table 12 rationale, design and conduct). A sample size of 840 women was calculated to provide a minimum power of 0.92 to detect differences of ≥ 5 mg/dL in high‐density lipoprotein cholesterol at the end of 3 years | |
| Analysis by Intention to Treat | Yes | |
| Notes | 39 women were unblinded because of endometrial biopsy results classified as complex hyperplasia, atypia or carcinoma. 32 of these were from the unopposed estrogen group (group 1), 3 were receiving placebo, and 4 were receiving 1 of the E+P regimens | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Computer generated |
| Allocation concealment (selection bias) | Low risk | Randomisation held centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Yes |
| Other bias | Low risk | 5 parallel groups well balanced at baseline |
Portman 2003.
| Methods | Randomisation: computer‐generated block randomisation in blocks of 8 Multicentre, blinding unclear ‐ participants in 6 treatment groups "blinded to placebo" but control group was open label Number of women randomised: 945 Number of women analysed: 936 (ITT population) Number of withdrawals: 288 (116 because of AEs ‐ details not specified) Source of funding: not stated |
|
| Participants | Country: US Inclusion criteria: at least 40 years old, with intact uterus and < 5 years post natural menopause, amenorrhoea for at least 6 months, FSH ≥ 50 IU/L and estradiol ≤ 20 pg/mL, BMI < 31, washout period required for those previously taking HT Exclusion criteria: any contraindication to HT, significant intercurrent disease (specified), use of bisphosphonate or calcitonin, use of other specified concomitant medications | |
| Interventions | Unopposed estrogen vs continuous (1) 5 µg E2 + 0.25 mg NETA daily (2) 5 µg E2 + 1 mg NETA daily (3) 10 µg E2 + 0.5 mg NETA daily (4)10 µg E2 + 1 mg NETA daily (5) 5 µg E2 unopposed (6) 10 µg E2 unopposed (7) 0.625 mg CEE + 2.5 mg MPA daily Duration: 12 cycles |
|
| Outcomes | Endometrial hyperplasia, withdrawal because of AEs | |
| Power calculation described | Yes, assuming an incidence of hyperplasia of 8% in group 5 and 0% in group 1, it was calculated that the a sample size of 124 would provide 90% power to detect this difference at a significance of 5% | |
| Analysis by Intention to Treat | Yes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation computer‐generated block randomisation in blocks of 8 |
| Allocation concealment (selection bias) | Low risk | Randomisation held centrally |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Participants 'blinded to placebo' except for group 7, which was an 'open label control group' |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | All participants in the ITT population had biopsy results reported (those who withdrew had last observation carried forward) |
| Other bias | Low risk | 8 parallel groups, well balanced at baseline |
Prestwood 2003.
| Methods | Randomisation: computer‐generated list held by the research pharmacist and concealed from investigators Design: parallel group, placebo‐controlled, double‐blind, single centre Number of women randomised: 167 (115 with intact uterus) Number of women analysed: 167 Number of withdrawals: 55 (24 in group A, 31 in placebo) Funding by grants from Claude Pepper Older Americans Independence Center, General Clinical Research Center and the Paul Beeson Physician Faculty Scholars in Aging Research program, and the calcium and vitamin D were provided by Mission Pharmacal | |
| Participants | Country: US Inclusion criteria: healthy community‐dwelling women over 65 years Exclusion criteria: specified past of intercurrent diseases and medications affecting bone metabolism, estrogen or calcitonin in previous 6 months, ever use of bisphosphonate or fluoride |
|
| Interventions | Long‐cycle sequential vs placebo (A) 0.25 mg E2 daily + 100 mg MP daily for 2 weeks every 6 months (B) placebo All participants took 1300 mg/day of calcium and 1000 IU/day of vitamin D Duration: 3 years |
|
| Outcomes | Bone mineral density (hip), endometrial hyperplasia, bleeding patterns | |
| Power calculation described | Yes | |
| Analysis by Intention to Treat | Yes for the primary outcome bone mineral density. Endometrial hyperplasia incidence assessed in those who completed the study | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation by computer‐generated list held by the research pharmacist and concealed from investigators |
| Allocation concealment (selection bias) | Low risk | Randomisation by computer‐generated list held by the research pharmacist and concealed from investigators |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | High risk | 112 of the 167 women randomised completed the study. Not clear how many of those with intact uterus completed the final endometrial biopsy |
| Other bias | Low risk | 2 parallel groups well balanced at baseline |
Rees 2004.
| Methods | Study 1 Randomisation: method not stated Multicentre, parallel group, double blind Number of women randomised: 399 Number of withdrawals: 6 (failed to take any medication) Source of funding: Orion Pharma, Espoo, Finland Study 2 Randomisation: method not stated Multicentre, parallel group, no blinding Number of women randomised: 341 Number of withdrawals: 1 (failed to take any medication) Source of funding: Orion Pharma, Espoo, Finland | |
| Participants | Postmenopausal women with intact uterus, at least 6 months since last menstruation or serum FSH "≥30 U/L", BMI not exceeding 32 ± 1, washout period of at least 1 month for those previously on HT Age: 40 to 65 years old from 31 centres in the UK (10), France (6) and Germany (15) Exclusion criteria: endometrial hyperplasia, endometriosis, endometrial fibroids > 1 cm, undiagnosed uterine bleeding, abnormal mammography and gynaecological status, thromboembolic disorders, uncontrolled hypertension Country: Germany and France (Study 1); UK (Study 2) | |
| Interventions | Study 1
Triphasic: 2 mg EV daily (9 days), then 2 mg EV + 10 mg MPA
daily (12 days), then 1 mg EV daily (7 days)
Biphasic: 2 mg EV daily
(11 days), then 2 mg EV + 10 mg MPA daily (10 days) then placebo (7 days)
Duration: 12 or 13 cycles Study 2 Triphasic: 2 mg EV daily (9 days), then 2 mg EV + 10 mg MPA daily (12 days), then 1 mg EV daily (7 days) Triphasic: 2 mg E2 + 1 mg estriol daily (12 days) then 2 mg E2 + 1 mg estriol + 1 mg NETA daily (10 days) then 1 mg E2 + 0.5 mg estriol daily (6 days) Duration: 12 or 13 cycles |
|
| Outcomes | Endometrial biopsy (primary), endometrial thickness, cycle control, climacteric symptoms | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Yes | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Only Study 1 was blinded |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | In Study 1, 202 of 399 women randomised completed the study (51%) but it is
not clear how many of these women had endometrial biopsy at treatment
end In Study 2, 167 of 341 women randomised, completed the study (49%) but it is not clear how many of these women had endometrial biopsy at treatment end |
| Other bias | Unclear risk | 3 parallel groups well balanced at baseline, but only Study 1 was blinded so bias is possible |
Rozenberg 2001.
| Methods | Randomisation: method not stated Multicentre, for the first year subjects and investigators were blinded to sequential therapy groups, continuous group not blinded, parallel design. An extension study continued open‐label treatment of 1 mg E2 + sequential intermittent 90 µg NGM compared to continuous 2 mg E2 + 1 mg NETA Number of women randomised: 657 Number of withdrawals: 186 (45 in group 1, 109 in group 2, 32 in group 3) Source of funding: not stated | |
| Participants | Postmenopausal women with serum E2 concentration < 20 pg/mL, FSH "≥40 mIU/ml", BMI within 30% of the average BMI for age and no contraindications to estrogen or progestogen therapy Age: 40 to 65 years old from 42 centres in Belgium, Finland, Sweden, the Netherlands Exclusion criteria: history of using other hormonal therapies prior to screening within 6 weeks, injectable or implantable steroids 6 months before screening | |
| Interventions | Intermittent sequential vs continuous (1) 1 mg E2 daily + 90 µg NGM days 4‐6, 10‐12, 16‐18 etc (sequential) (2) 2 mg E2 daily + 180 µg NGM days 4‐6, 10‐12, 16‐18 etc (sequential) (3) 2 mg E2 + 1 mg NETA daily (continuous) |
|
| Outcomes | Endometrial hyperplasia, withdrawal | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Yes | |
| Notes | Same study as Ylikorkala 2002 but different outcomes (see under Rozenberg 2001) | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding in the first year of treatment only |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | After 2 years 282 of the 322 women randomised (88%) had an endometrial biopsy |
| Other bias | Low risk | 3 parallel groups well balanced at baseline |
Scandinavia 1996.
| Methods | Randomisation: method not stated Multicentre, parallel group design, blinding not clear Number of women randomised: 40 Number of exclusions: not given Source of funding: not given | |
| Participants | Countries: Denmark, Norway and Sweden 240 women recruited aged 45 to 65 years (mean 52 ± 4 years) who had been postmenopausal for at least 1 year No other inclusion or exclusion criteria given | |
| Interventions | Long‐cycle sequential vs monthly sequential (1) HT with extended cycle of 84 days: 2 mg E2 for 68 days; 2 mg E2 and 1 mg of NETA for 10 days and 1 mg E2 for 6 days (2) HT with a regular cycle of 28 days (monthly cycle HT): 2 mg E2 for 12 days; 2 mg E2 and 1 mg NETA for 10 days, and 1 mg E2 for 6 days Duration: planned to be 5 years but terminated early after 4 years (mean duration of long‐cycle group 2.8 years) | |
| Outcomes | Endometrial hyperplasia or carcinoma | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | Authors contacted regarding randomisation method, blinding, number of withdrawals, whether a power calculation and ITT analysis was performed. Trial discontinued before 5 years owing to safety analysis The study was discontinued because of the unsatisfactory safety profile of the long‐cycle hormone regimen | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Unclear risk | Blinding not clear |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | After 1 year of treatment 195 of 240 women randomised (81%) had an endometrial biopsy. Trial was stopped early and numbers of women biopsied after 2 and 3 years of treatment are unclear |
| Other bias | Low risk | 2 parallel groups balanced at baseline for age, BMI and previous HT use |
Sporrong 1988.
| Methods | Randomisation: randomisation tables Single‐centre, double‐blind parallel group study Number of women randomised: 60 Number of withdrawals: 12 (7 because of gastrointestinal disturbances, 4 because of depression/irritability and 1 because of deep vein thrombosis) Source of funding: not stated | |
| Participants | Country: Sweden Inclusion criteria: postmenopausal women attending outpatient clinic for climacteric complaints, who were otherwise healthy, had intact uterus, no contraindications to hormone replacement therapy and had ceased menstruation at least 1 year prior to study. No hormone treatment was given for 6 weeks prior to the start of the study | |
| Interventions | Continuous (A) 2 mg E2 + 1 mg NETA daily (B) 2 mg E2 + 0.5 mg NETA daily (C) 2 mg E2 + 5 mg MA daily (D) 2 mg E2 + 2.5 mg MA daily Duration: 1 year |
|
| Outcomes | Endometrial hyperplasia, mean number days with bleeding, amenorrhoea at 4 months | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Randomisation tables were used |
| Allocation concealment (selection bias) | Low risk | Randomisation held centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 48 of the 60 women randomised (80%) had endometrial biopsy after 1 year |
| Other bias | Low risk | 4 parallel groups well balanced at baseline |
Stadberg 1996.
| Methods | Single‐centre double‐blind trial, randomised in blocks of 9, to 3 parallel treatment groups Number randomised: 60 Number analysed: 49 Number withdrawals: 11 (5, 2, 4 from groups 1, 2, 3, respectively) owing to insufficient effect on vasomotor symptoms (2 in group 1, 2 in group 3), spotting (2 in group 1), leg cramps (1 in group 1), urinary tract infections (2 in group 2), depression (1 in group 3), bleeding disorders (1 in group 3) Source of funding: grants from Hjalmar Svensson's fund and Goteborg Medical Society at University of Goteburg. NOVO industries A/S supplied the tablets | |
| Participants | Country: Sweden Inclusion criteria: postmenopausal women with climacteric complaints, either had a natural menopause or used HT for >3 years, an intact uterus, and 6‐week washout for previous users of HT Exclusion criteria: serious intercurrent illness, contraindications for HT | |
| Interventions | Continuous (1) 1 mg E2 + 0.25 mg NETA daily (2) 1 mg E2 + 0.5 mg NETA daily (3) 1 mg E2 + 1 mg NETA daily Duration: 1 year |
|
| Outcomes | Bleeding patterns at 12 months, endometrial hyperplasia | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | "Randomised in blocks of 9" method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Endometrial biopsies after 1 year were obtained in 49 of the 60 women randomised (82%) |
| Other bias | Unclear risk | 3 parallel groups with differences at baseline in mean age and weight of participants |
van de Weijer 1999.
| Methods | Randomisation: method not stated, medication separately packaged for each participant and labelled with code number Double‐blind parallel group study in 2 centres in the Netherlands Number women randomised: 151 1 post randomisation exclusion because of endometrial carcinoma at baseline Number of women analysed: 131 (64 in low‐dose group, 67 in high‐dose group) Number of women prematurely withdrawn: 17 owing to: bleeding problems (2 in group A, 1 in group B, total 3), minor AEs (11), lack of efficacy (1), gynaecological problems unrelated to study (1), lost to follow‐up (1); and 2 owing to non‐compliance. | |
| Participants | Country: the Netherlands Inclusion criteria: healthy, non‐hysterectomised women aged 45 to 65 years, with at least 6 months amenorrhoea, serum FSH > 35 IU/L and a baseline endometrial biopsy showing non‐secretory, non‐hyperplastic, non‐malignant endometrium Exclusion criteria: any contraindication to estrogen use, concomitant medications known to influence study measurements, HT within 4 weeks of first screening visit | |
| Interventions | Sequential (A) 1 mg micronised E2 daily + 5 mg DYG daily on days 15 to 28 of each cycle (B) 1 mg micronised E2 daily + 10 mg DYG daily on days 15 to 28 of each cycle Duration: 13 cycles |
|
| Outcomes | Bleeding pattern, endometrial biopsy | |
| Power calculation described | No | |
| Analysis by Intention to Treat | Outcome of endometrial biopsy based on those who completed the study including the final biopsy | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Randomisation method not stated, medication separately packaged for each participant and labelled with code number |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 124 of the 151 women randomised had endometrial biopsies after 1 year |
| Other bias | Low risk | 2 parallel groups balanced at baseline |
Warming 2004.
| Methods | Randomisation: block randomised using random numbers
Double‐blind
placebo‐controlled single centre study Number of women randomised: 240 Number of women analysed: ?180/?240 Number of withdrawals: 60 (lack of efficacy (2), HT related AEs (35), other AEs (11), breast cancer (1), pulmonary embolus (1), other (10)) Sources of funding: Schering AG, Berlin |
|
| Participants | Country: Denmark Inclusion criteria: 45 to 60 year women at least one year post natural menopause Exclusion criteria: systemic disease, diseases of bone metabolism, abnormal blood and urine laboratory tests, history of malignancy | |
| Interventions | Continuous vs placebo (1) 1 mg E2 + 1 mg DSP daily (2) 1 mg E2 + 2 mg DSP daily (3) 1 mg E2 + 3 mg DSP daily (4) placebo Duration: 24 months |
|
| Outcomes | Endometrial hyperplasia, withdrawals because of AEs, amenorrhoea, bleeding patterns | |
| Power calculation described | Yes, assuming a 40% attrition rate of randomised participants and a 5% difference between placebo group and verum in bone mineral density, and allowing for Bonferroni correction for multiple comparisons it was calculated that at least 40 women per group would be required to 90% power. Group size was increased to 60 to allow for the inclusion of non‐osteopenic women | |
| Analysis by Intention to Treat | Yes for the primary end point (bone mineral density), not clear how many women were included in the analysis of endometrial hyperplasia | |
| Notes | Bone mineral density, biochemical markers of bone metabolism, lipid profiles | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Block randomised using random numbers |
| Allocation concealment (selection bias) | Low risk | Randomisation held centrally |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 180 of the 240 women (75%) randomised had endometrial biopsies after 2 years |
| Other bias | Low risk | 4 parallel groups balanced at baseline |
WHI 2002.
| Methods | Randomisation: centrally randomised by permuted block algorithm, stratified
Stratification: by clinical centre site and age group
Allocation: by local access to study database
Blinding: all
participants, clinic staff and outcome assessors blinded, with the exception
of 331 participants who were unblinded and reassigned to experimental group
owing to change in protocol (see notes)
Unblinding: when required
for safety or symptom management, unblinding officer, unblinded clinic
gynaecologist, who was not involved with outcomes assessment. At average 5.2
year follow‐up, 3444 women in experimental group and 5448 women in placebo
group had been unblinded, mainly to manage persistent vaginal bleeding)
Number of women randomised: 16,608 (8506 to experimental group, 8102
to placebo group) Losses to follow‐up: 583 women (3.5%) ‐ i.e. no outcomes data for > 18 months: (307 in HT arm (3%), 276 in control arm (3.5%). Vital status known for 96.5% Non‐adherence to allocated treatment: by 5.2 years (median): 42% experimental arm, 10.7% placebo arm. 432 women, 248 in experimental arm, 183 in placebo arm, who had hysterectomy after randomisation (for reasons other than cancer) switched to unopposed estrogen or corresponding placebo Withdrawals: 583 lost to follow‐up (3.5%) or stopped providing outcomes information for more than 18 months |
|
| Participants | Inclusion criteria: postmenopausal women (no vaginal bleeding for 6 months, or for 12 months for 50 to 54 year olds; any use of postmenopausal hormones), with a uterus, aged 50 to 79 at initial screening, likely to reside in area for 3 years, provision of written informed consent Exclusion criteria: medical condition predictive of survival time < 3 years, invasive cancer in past 10 years (except non‐melanoma skin cancer), breast cancer at any time or suspicion of breast cancer at baseline screening, acute myocardial infarction, stroke, transient ischaemic attack in previous 6 months, known chronic active hepatitis or severe cirrhosis, blood counts indicative of disease, severe hypertension or current use of oral corticosteroids, femoral neck bone mineral density of > 3 SDs below the corresponding age‐specific mean, endometrial cancer or endometrial hyperplasia at baseline, malignant melanoma, pulmonary embolism or deep vein thrombosis that was non‐traumatic or that had occurred in the previous 6 months, bleeding disorder, lipaemic serum and hypertriglyceridaemia diagnosis, current use of anticoagulants or tamoxifen, Pap smear or pelvic abnormalities, unwillingness or inability to complete baseline study requirements, alcoholism, drug dependency, mental illness, dementia, severe menopausal symptoms inconsistent with assignment to placebo, inability or unwillingness to discontinue current HT use or oral testosterone use, inadequate adherence with placebo run‐in, unwillingness to have baseline or follow‐up endometrial aspirations, active participant in another RCT Mean age: 63 years (SD 7 years) Age range: 50 to 79 years. Age ratio of 33%:45%:21% for the baseline age categories of 50 to 59, 60 to 69, 70 to 79, respectively (enrolment targeted to achieve ratio of 30%:45%:25%) Recruitment: letter of invitation in conjunction with media awareness programme. Sampling method gave women from minority groups 6‐fold higher odds for selection than Caucasian women and resulted in sample with 84% racially/ethnically designated "white", 16% non‐"white" Screening: interested women screened by telephone or mail for eligibility, then attended 3 screening visits for history, clinical examination and tests. 3‐month washout period before baseline evaluation of women using postmenopausal hormones at baseline screening. Lead‐in placebo pills given for at least 4 weeks during screening process to establish compliance with pill taking Baseline equality of treatment groups: no substantive differences between study groups at baseline Country: US | |
| Interventions | Continuous vs placebo (1) 0.625 mg CEE + 2.5 mg MPA 1 daily tablet (2) Control group: matching placebo Duration: planned 8 years stopped after 5.2 Permanent discontinuation of medication: women who developed breast cancer, endometrial hyperplasia not responsive to treatment, endometrial atypia, endometrial cancer, deep vein thrombosis, pulmonary embolus, malignant melanoma, meningioma, triglyceride level over 1000 mg/dL, prescription of estrogen, testosterone or selective estrogen‐receptor modulators by their personal physician Temporary discontinuation of medication: women who had acute myocardial infarction, stroke, fracture, major injury involving hospitalisation, surgery involving anaesthesia, illness resulting in immobilisation for over 1 week, or other severe illness in which hormone use temporarily inappropriate |
|
| Outcomes | Cardiovascular disease: acute myocardial infarction, silent myocardial infarction, coronary death, stroke, pulmonary embolus Cancer: breast, colorectal, endometrial, other cancers Fractures: hip, vertebral, osteoporotic | |
| Power calculation described | Yes, sample gives 80% to 95% power for primary end point comparisons at 5% significance, assuming an intervention effect of 20% for coronary heart disease and 21% for combined fractures at 6 to 9 years' follow‐up, and an intervention effect of 22% for breast cancer at 14‐year follow up (risk ratio of 1.3 assumed for increased risk of breast cancer in intervention group) | |
| Analysis by Intention to Treat | Yes, analysed with and without unblinded group in experimental arm | |
| Notes | Power: 88% power to detect intervention effect of 21% for coronary heart disease over 9 years; 73% power to detect 21% difference in hip fractures over 9 years; 99% power to detect 20% difference in all fractures over 9 years; 79% power to detect 22% difference in breast cancer incidence over 9 years (all at 2‐sided P = 0.05) Planned 8.5 years follow‐up. Trial stopped after 5.2 years as test statistic for breast cancer exceeded stopping boundary After release of PEPI trial results indicating long‐term adherence to unopposed estrogen was not feasible in women with a uterus, the WHI protocol was changed to randomise women with a uterus to only estrogen + progestin or placebo in equal proportions. The 331 women previously randomised to unopposed estrogen were unblinded and re‐assigned to estrogen + progestin | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Centrally randomised by permuted block algorithm, stratified by clinical centre site and age group |
| Allocation concealment (selection bias) | Low risk | By local access to study database |
| Blinding (performance bias and detection bias) All outcomes | Low risk | ‐ |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Follow‐up data for 96.5% of those randomised |
| Other bias | Low risk | 2 parallel groups well balanced at baseline |
Williams 1994.
| Methods | Randomisation: method not stated Placebo‐controlled double‐blind study undertaken at 2 centres in the US Number of women randomised: 80 Number of women analysed: 62 Number of women withdrawn: 18. 8 following non‐serious medical events, 4 for personal reasons, 2 lost to follow‐up, 1 ineligible because of abnormal mammogram, 3 for 'other' reasons Funding: grant from Upjohn Company | |
| Participants | Country: US
Inclusion criteria: postmenopausal women with intact
uterus with amenorrhoea for at least 1 year, normal endometrial histology,
FSH "greater than 50 mIU/mL" and serum estradiol < 30 pg/mL. Women had
not had injectable HT in the year prior to the start of the study or oral
therapy in the month prior. All were within 30% of ideal body weight Exclusion: history of cancer, alcohol or drug abuse, current vaginal bleeding or serious intercurrent illness (specified) |
|
| Interventions | Sequential monthly vs long cycle (1) 0.625 mg CEE daily + 10 mg MPA on days 15 to 28 (14/28 group) (2) 0.625 mg CEE daily + 10 mg MPA on days 71 to 84 (14/84 group) (3) 0.625 mg CEE daily + 10 mg MPA on days 57 to 84 (28/84 group) In all groups a placebo tablet was given daily on the progestogen‐free days |
|
| Outcomes | Bleeding patterns, endometrial histology | |
| Power calculation described | Power calculation not given but there is a statement that the planned study size would probably lack power to distinguish between groups | |
| Analysis by Intention to Treat | No | |
| Notes | ||
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Double blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 62 women of the 80 randomised (78%) had endometrial biopsy after 12 cycles of treatment |
| Other bias | Low risk | 3 parallel groups balanced at baseline |
Wu 2002.
| Methods | Randomisation: method not stated No blinding of participants or researchers, study undertaken at 3 medical centres Number of women randomised: 236 Number of women analysed: 213 at 1 year, 176 at 2 years Number of withdrawals: 60 Source of funding: Wyeth‐White Hall Pharmaceuticals provided the CEE and vitamin D tablets | |
| Participants | Country: China Women 1 to 4 years postmenopause, with an intact uterus, endometrial thickness < 5 mm, BMI 18 to 28 Exclusion criteria: thrombotic diseases, coronary heart disease, scoliosis, L2‐4 bone mineral density < ‐2.5 SD, exogenic hormone use in previous 3 months, calcitonin or biphosphate in previous 6 months or fluoride in previous 12 months | |
| Interventions | Continuous vs no treatment (A) 0.625 mg CEE + 2 mg MPA + caltrate D (vitamin D) daily (B) 0.3 mg CEE + 2 mg MPA + caltrate D daily (C) Caltrate D only |
|
| Outcomes | Endometrial thickness and biopsy | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | Other outcomes bone mineral density, breast tenderness, bone metabolic markers, vaginal bleeding | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | High risk | Not mentioned |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 103 of the 236 women randomised (44%) had endometrial assessment after 2 years |
| Other bias | Low risk | 3 parallel groups balanced at baseline |
Yildirim 2006.
| Methods | Randomisation: method not stated Single‐blind, single‐centre randomised trial Number of women randomised: 246 Number of women analysed: 204 Number of withdrawals: group 1 27 (11 because of AEs including vaginal bleeding, mastodynia and headache); group 2 15 (9 because of AEs including vaginal bleeding, mastodynia and headache) Sources of funding: not mentioned | |
| Participants | Country: Turkey Inclusion criteria: healthy postmenopausal women aged 41 to 57 years, with vasomotor, psychological or atrophic symptoms, intact uterus and ovaries, normal endometrial thickness, at least 12 months of amenorrhoea, and vasomotor symptoms. women had to have normal blood biochemical levels, normal cervical smear and bilateral mammography Exclusion criteria: Women under 41 years of age, over 60 years of age, or who had had bilateral oopherectomy or hysterectomy | |
| Interventions | Continuous (1) 0.625 mg CEE + 2.5 mg MPA daily (2) 1 mg E2 + 0.5 mg NETA daily Duration: 12 months |
|
| Outcomes | Bleeding/spotting at 3, 6, 9, 12 months, amenorrhoea at 12 months | |
| Power calculation described | No | |
| Analysis by Intention to Treat | No | |
| Notes | ‐ | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Not described |
| Blinding (performance bias and detection bias) All outcomes | Low risk | Single blind |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 72 women of the 246 randomised (29%) had endometrial biopsy after 1 year |
| Other bias | Unclear risk | 2 parallel groups with unequal numbers and differences at baseline in duration of menopause and parity |
AE: adverse effect; BMI: body mass index; CEE: conjugated equine estrogen; DG: desogestrel; DNG: dienogest; DSP: drospirinone; DYG: dydrogestone; E+P: estrogen plus progestogen; E2: 17β estradiol; EE: ethinyl estradiol; EIS: estrone sulphate; ESE: esterified estrogen; EV: estradiol valerate; FSH: follicle‐stimulating hormone; HT: hormone therapy; ITT: intention to treat; LH: luteinising hormone; MA: megestrol acetate; MP: micronised progesterone; MPA: medroxyprogesterone acetate; NETA: norethisterone acetate; NG: norgestrel; NGM: norgestimate; POS: piperazine oestrone sulphate; RCT: randomised controlled trial; SD: standard deviation; TMG: trimegestone; TVUS: transvaginal ultrasound.
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Aoki 1990 | This trial has published in abstract form and located by handsearching. No indication was given whether women were randomised to treatment groups and attempts were made to contact the author for clarification but no reply was received |
| Archer 1999 | Outcomes are adherence to treatment and bleeding patterns. No data on endometrial hyperplasia |
| Archer 2001 | Excluded as amenorrhoea is primary outcome. No hyperplasia outcome data |
| Arrenbrecht 2004 | Primary outcomes related to bone density and bleeding. No data on endometrial hyperplasia |
| Bergeron 2010 | Not RCT, no control or comparison group |
| Blumel 1994 | Primary outcome is bleeding patterns after 6 months therapy. No hyperplasia outcome data |
| Byrjalsen 1992a | Bleeding outcomes only. No hyperplasia outcome data |
| Byrjalsen 1992b | No hyperplasia outcome data reported |
| Campbell 1977 | This trial was excluded because endometrial hyperplasia at baseline is not an exclusion criterion for entry into the trial (exclusion criterion for this review is any contraindication to HT) |
| Campodonico 1996 | Primary outcome is amenorrhoea |
| Chen 1999 | Primary outcomes are bleeding patterns. No hyperplasia data reported |
| Christensen 1982 | Primary outcomes are bleeding patterns. No hyperplasia data reported |
| Endrikat 2007 | Treatment period only 12 weeks |
| Granberg 2002 | Trial not randomised |
| Gulhan 2004 | Article in Turkish language with English language abstract. The outcome is endometrial thickness by ultrasound |
| Hagen 1982 | Primary outcomes are bleeding patterns. No hyperplasia data reported |
| Heytmanek 1990 | This trial was published in abstract form and was located by handsearching. No indication was given whether women were randomised to treatment groups and attempts were made to contact the author for clarification but no reply was received |
| Istre 1996 | Excluded because all participants underwent transcervical resection of endometrium prior to starting the study |
| Jaisamrarn 2002 | Excluded because bleeding outcomes only |
| Jirapinyo 2003 | Primary outcomes are bleeding patterns. No hyperplasia data reported |
| Kazerooni 2004 | Primary outcome is cumulative amenorrhoea rates |
| Keil 2002 | Study only 4 months in length. Protocol states studies should be at least 1 year in length |
| Limpaphayom 2000 | Primary outcomes are bleeding patterns and climacteric symptoms. No endometrial hyperplasia data |
| Liu 2005 | Primary outcomes are bone indices. No endometrial hyperplasia data |
| Luciano 1988 | Study only 4 months in length. Protocol states studies should be at least 1 year in length |
| Marslew 1991 | Primary outcomes are bleeding patterns and adherence to therapy |
| Marslew 1992 | Primary outcomes are bleeding patterns and adherence to therapy |
| Mizunuma 1997 | Primary outcomes are bleeding patterns |
| Morabito 2004 | This study (together with conference abstract by Crisafulli) had bone mineral density as primary outcome. Endometrial biopsy was not planned a priori, and were undertaken on only 8 women who had endometrial thickness > 5 mm |
| Nachtigall 1979 | Trial was based on 84 matched pairs that were randomised to treatment or placebo. However, participants were hospitalised with long‐term chronic disease, which is an exclusion criterion for this review |
| Odmark 2001 | Trial compared the bleeding patterns of women who had started HT for the first time with the bleeding patterns of those who 'switched' (without a washout period) from an alternative HT regimen. Washout period for previous hormone users is an inclusion criteria. No endometrial hyperplasia data |
| Pickar 2003a | This study was identified by handsearching and published as an abstract. There was insufficient information to determine whether it met the inclusion criteria for this review. No response to our email to the authors and no subsequent publication has been identified |
| Pinto 2003 | 9 months' therapy only given to frail elderly women |
| Popp 2006 | 5 of the 73 women randomised (7%) had endometrial hyperplasia at baseline. Protocol states that participants must have either normal endometrial histology by biopsy or endometrial thickness < 5 mm |
| Schiff 1982 | Comparison is estrogen (cyclic) vs estrogen continuous, an obsolete regimen which does not meet the inclusion criteria for studies included in this review |
| Simon 2001 | Primary outcomes are bleeding patterns. No data on endometrial hyperplasia |
| Simon 2003 | Primary outcomes are adverse events and withdrawals. No data on endometrial hyperplasia |
| Steiner 2007 | EPAT (estrogen in the prevention of atherosclerosis trial) was conducted from April 1994 to Nov 1998. Women recruited from newspaper advertisements targeting employees of universities and health maintenance organisations. 220 women randomised to estrogen only or placebo WELLHART (Women's Estrogen ‐ Progestin Lipid Lowering Hormone Atheroscelerosis Regression Trial) is a randomised trial of 448 women recruited from angiography clinics and randomised to usual care, estrogen only or estrogen + progestin Steiner et al combined the outcome data from the estrogen only groups of these trials and compared them to those from the WELLHART trial who took E+P, with regard to the outcome of endometrial hyperplasia This is retrospective analysis of participants from different populations, randomised at different times according to different criteria |
| Stevenson 2001 | Primary outcomes are bone mineral density and amenorrhoea. No data on endometrial hyperplasia |
| Stevenson 2010 | Treatment period only 13 weeks |
| Sturdee 1996 | This trial was published in abstract form and was located by handsearching. No indication was given whether women were randomised to treatment groups and attempts were made to contact the author for clarification but no reply was received |
| Sturdee 2000 | No indication was given whether women were randomised to treatment groups and attempts were made to contact the author for clarification but no reply was received |
| Sturdee 2008 | Treatment period only 6 months |
| Symons 2000 | Study only 4 months in length. Protocol states studies should be at least 6 months in length |
| Symons 2002 | Study duration only 6 months and outcomes bleeding patterns |
| Ulla Timonen 2002 | This trial was published in abstract form and was located by handsearching. No indication was given whether women were randomised to treatment groups and attempts were made to contact the author for clarification but no reply was received. |
| Utian 2002 | Study only 3 months in length. Protocol states studies should be at least 6 months in length |
| van de Weijer 2002 | This study was identified by handsearching and published as an abstract. There was insufficient information to determine whether it met the inclusion criteria for this review. No response to our email to the authors and no subsequent publication has been identified |
| van der Mooren 1996 | This study was identified by handsearching and published as an abstract. There was insufficient information to determine whether it met the inclusion criteria for this review. No response to our email to the authors and no subsequent publication has been identified |
| Veerus 2008 | No data on endometrial biopsy |
| Volpe 1986 | This trial is excluded because endometrial hyperplasia at baseline is not an exclusion criterion for entry into the trial (exclusion criterion for this review is any contraindication to HT). The numbers in each arm of the trial are small and the effects of treatment on the endometrium are evaluated by assessing the improvement in the endometrium from baseline as a result of treatment |
| Von Holst 2002 | 9 months of therapy only |
| Wahab 2002 | This study is excluded because it is a 6‐month non‐randomised extension of a previous dose finding study |
| Wang 2006 | The outcomes in this study are endometrial thickness by ultrasound and bleeding patterns only |
| Warming 2004a | Primary outcomes are bone related, secondary outcomes are bleeding. No data on endometrial hyperplasia |
| Webster 1996 | This study was identified by handsearching and published as an abstract. There was insufficient information to determine whether it met the inclusion criteria for this review. No response to our email to the authors and no subsequent publication has been identified |
| Weinstein 1990 | No data in published paper on the number of endometrial biopsies performed at end of treatment in each treatment group. First author was contacted and reports that data no longer available |
| Wells 2002 | This study is not an RCT |
| Williams 1990 | Primary outcomes are vaginal spotting and adherence to therapy. No endometrial hyperplasia data reported |
| Yang 2001 | Study only 4 months in length. Protocol states studies should be at least 6 months in length |
HT: hormone therapy; RCT: randomised controlled trial.
Differences between protocol and review
The original version of this review investigated abnormal vaginal bleeding, and concluded as follows:
Regular withdrawal bleeding is expected with a sequential regimen of E+P but women appear to experience less irregular bleeding than with a continuous E+P regimen. Irregular bleeding or spotting is common in the first year of continuous E+P but following the first year of treatment, bleeding and spotting become more common in sequential E+P regimens. A large proportion of women taking continuous E+P become amenorrhoeic after one year of therapy while withdrawal bleeding continues for women taking sequential regimens.
While bleeding during the first year of continuous E+P therapy does not need investigation with vaginal ultrasound scan, endometrial thickness, endometrial biopsy, or a combination, these investigations should be considered in the second and subsequent years of HT. Unscheduled bleeding on sequential HT should be investigated by hysteroscopy and endometrial biopsy.
For the 2008 update of this review, we addressed long‐term endometrial safety outcomes rather than bleeding patterns, and we have amended the protocol accordingly
We assessed which of the oral HT regimens, unopposed estrogen or E+P administered either continuously or sequentially, provided the best protection against the development of endometrial hyperplasia or carcinoma, has better adherence to therapy regimens and the lowest rate of unscheduled biopsies. In addition we compared the effects of different types, doses and duration of progestogen use on both endometrial hyperplasia and adherence to treatment, in order to determine the minimum dose and duration of progestogen required for endometrial protection.
Amendments to the original protocol
For the 2008 update of this review, the protocol was been amended to include only studies with a minimum of 12 months of therapy with either unopposed estrogen or combined E+P. The primary outcomes were endometrial hyperplasia, carcinoma or both, with secondary outcomes of adherence to therapy, withdrawal owing to adverse events and rate of unscheduled investigations for abnormal bleeding (hysteroscopy and endometrial biopsy). Bleeding patterns are no longer an outcome in this updated review.
For the 2012 update of this review there were no further changes to the protocol.
Contributions of authors
For the 2012 update of this review:
Sue Furness screened the results of the searches, agreed on the studies to be included, assessed risk of bias, extracted and entered data and incorporated this into the results section;
Jane Marjoribanks screened the results of the searches, agreed on the studies to be included, assessed risk of bias, extracted and entered data and incorporated this into the results section;
Helen Roberts updated the implications for practice section;
Anne Lethaby commented on the draft of the updated review.
For the 2008 update of this review:
Sue Furness modified the protocol, performed searches, selected trials for inclusion, assessed quality, performed data extraction, entered data, prepared the final review and incorporated suggested changes;
Helen Roberts modified the protocol, contributed to the design of the table of comparisons and results section of the text, and wrote the implications for practice and research sections;
Jane Marjoribanks selected trials for inclusion, assessed quality, performed data extraction and made detailed comments on many drafts of the text;
Anne Lethaby reviewed the protocol, contributed to the methods and results sections, and made detailed comments on several drafts of the text;
Martha Hickey reviewed the protocol, collated the data on the endometrial effects of progestogens and commented on the final review;
Cindy Farquhar reviewed the protocol and commented on the final draft of the review.
For the first published version of the review:
Anne Lethaby selected trials for inclusion, assessed quality, performed data extraction, entered data, prepared the final review and incorporated suggested changes;
Cindy Farquhar reviewed the protocol, performed data extraction and commented on the final draft of the review;
Helen Roberts assessed included trials for quality, performed data extraction and commented on the final draft of the review.
Sources of support
Internal sources
Dept of Obstetrics and Gynaecology, University of Auckland, New Zealand.
External sources
Health Research Council, New Zealand.
Declarations of interest
There was no conflict of interest.
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
AinMelk 1996 {published data only}
- AinMelk Y. Comparison of two continuous combined estrogen progestogen regimens in postmenopausal women: a randomised trial. Fertility & Sterility 1996;66(6):962‐8. [DOI] [PubMed] [Google Scholar]
Al‐Azzawi 2001 {published data only}
- Al‐Azzawi F, Wahab M, Thompson J, Pornel B, Hirvonen E, Ylikorkala O, et al. Acceptability and patterns of endometrial bleeding in estradiol‐based HRT regimens: a comparative study of cyclical sequential combinations of trimegestone or norethisterone acetate. Climacteric 2001;4(4):343‐54. [PubMed] [Google Scholar]
Archer 2005 {published data only}
- Archer DF, Thorneycroft IH, Foegh M, Hanes V, Glant MD, Bitterman P, et al. Long‐term safety of drospirinone‐estradiol for hormone therapy: a randomised, double‐blind, multicenter trial. Menopause 2005;12(6):716‐27. [DOI] [PubMed] [Google Scholar]
Bouchard 2005 {published data only}
- Bouchard P, Addo S, Spielmann D, The Trimegestone 301 Study Group. A comparison of the effects of continuous combined regimens of 1mg 17B‐estradiol and trimegestone with continuous combined estradiol and norethisterone acetate upon the bleeding profile and safety in postmenopausal women for up to 2 years. Climacteric. 2002; Vol. 5, issue Suppl 1:145.
- Bouchard P, Cicco‐Nardone F, Spielmann D, Garcea N, The Trimegestone 301 Study Group. Bleeding profile and endometrial safety of continuous combined regimens 1 mg 17beta‐estradiol/trimegestone versus 1 or 2 mg 17beta‐estradiol/norethisterone acetate in postmenopausal women. Gynecological Endocrinology 2005;21(3):142‐8. [DOI] [PubMed] [Google Scholar]
- Gambacciani M, Spielmann D, Genazzani AR. Efficacy on climacteric symptoms of a continuous combined regimen of 1 mg 17beta‐estradiol and trimegestone versus two regimens combining 1 or 2 mg 17beta‐estradiol and norethisterone acetate. Gynecological endocrinology : the official journal of the International Society of Gynecological Endocrinology 2005;21(2):65‐73. [DOI] [PubMed] [Google Scholar]
Bruhat 2001 {published data only}
- Bruhat M, Rudolf K, Vaheri R, Kainulainen P, Timonen U, Viitanen A. Effective bleeding control and symptom relief by lower dose regimens of continuous combined hormone replacement therapy: a randomized comparative dose‐ranging study. Maturitas 2001;40:259‐71. [DOI] [PubMed] [Google Scholar]
Byrjalsen 1992 {published data only}
- Byrjalsen I, Thormann L, Meinecke B, Riis BJ, Christiansen C. Sequential estrogen and progestogen therapy: assessment of progestational effects on the postmenopausal endometrium. Obstetrics & Gynecology 1992;79(4):523‐8. [PubMed] [Google Scholar]
Byrjalsen 1999 {published data only}
- Byrjalsen I, Bjarnason NH, Christiansen C. Progestational effects of combinations of gestodene on the postmenopausal endometrium during hormone replacement therapy. Acta Obsterica et Gynecologica Scandinavica 1997;76(Suppl):19. [DOI] [PubMed] [Google Scholar]
- Byrjalsen I, Bjarnason NH, Christiansen C. Progestational effects of combinations of gestodene on the postmenopausal endometrium during hormone replacement therapy. American Journal of Obstetrics & Gynecology 1999;180(3 I):539‐49. [DOI] [PubMed] [Google Scholar]
Byrjalsen 2000 {published data only}
- Byrjalsen I, Alexandersen P, Christiansen C. Piperazine oestrone sulphate and interrupted norethisterone: effects on the postmenopausal endometrium. BJOG: an International Journal of Obstetrics & Gynaecology 2000;107(3):347‐55. [DOI] [PubMed] [Google Scholar]
Chang 2003 {published data only}
- Chang TC, Chen M, Lien YR, Chen RJ, Chow SN. Comparison of the difference in histopathology and cell cycle kinetics among the postmenopausal endometrium treated with different progestins in sequential‐combined hormone replacement therapy. Menopause 2003;10(2):172‐8. [DOI] [PubMed] [Google Scholar]
CHART 1996 {published data only}
- Speroff L, Rowan J, Symons J, Genant H, Wilborn W. The comparative effect on bone density, endometrium and lipids of continuous hormones as replacement therapy (CHART study). A randomised controlled trial. JAMA 1996;276(7):1397‐403. [PubMed] [Google Scholar]
Corson 1999 {published data only}
- Corson S, Richart R, Caubel P, Lim P. Effect of a unique constant estrogen, pulsed progestin hormone replacement therapy containing 17B estradiol and norgestimate on endometrial histology. International Journal of Fertility 1999;44:279‐85. [PubMed] [Google Scholar]
- Sulak P, Caubel P, Lane R. Efficacy and safety of constant estrogen, pulsed progestin regimen in hormone replacement therapy. International Journal of Fertility 1999;44:286‐96. [PubMed] [Google Scholar]
Ettinger 1992 {published data only}
- Ettinger B, Genant HK, Steiger P, Madvig P. Low‐dosage micronized 17B‐estradiol prevents bone loss in postmenopausal women. American Journal of Obstetrics and Gynecology 1992;166:479‐88. [DOI] [PubMed] [Google Scholar]
Ferenczy 2002 {published data only}
- Ferenczy A, Gelfand MM, Weijer PHM, Rioux JE. Endometrial safety and bleeding patterns during a 2 year study of 1 or 2 mg of 17B‐estradiol combined with sequential 5‐20mg dydrogesterone. Climacteric 2002;5:26‐35. [PubMed] [Google Scholar]
Gelfand 1989 {published data only}
- Gelfand M, Ferenczy A. A prospective 1‐year study of estrogen and progestin in postmenopausal women: effects on the endometrium. Obstetrics & Gynecology 1989;74:398‐402. [PubMed] [Google Scholar]
Graser 2000 {published data only}
- Graser T, Koytchev R, Muller A, Oettel M. Comparison of the efficacy and endometrial safety of two estradiol valerate/dienogest combinations and Kliogest for continuous combined hormone replacement therapy in postmenopausal women. Climacteric 2000;3(2):109‐18. [DOI] [PubMed] [Google Scholar]
Greenwald 2005 {published data only}
- Greenwald MW, Gluck OS, Lang E, Rakov V. Oral hormone therapy with 17s‐estradiol and 17s‐estradiol in combination with norethindrone acetate in the prevention of bone loss in early postmenopausal women: dose‐dependent effects. Menopause 2005;12(6):741‐8. [DOI] [PubMed] [Google Scholar]
Harris 1991 {published data only}
- Harris ST, Genant HK, Baylink DJ, Gallagher C, Karp SK, McConnell MA, et al. The effects of estrone (OGEN) on spinal bone density of postmenopausal women. Archives of Internal Medicine 1991;151:1980‐4. [PubMed] [Google Scholar]
Heikkinen 1997 {published data only}
- Heikkinen J, Kyllonen E, Kurttila‐Matero E, Wilen‐Rosenqvist G, Lankinen KS, Rita H, et al. HRT and exercise: effects on bone density, muscle strength and lipid metabolism. A placebo controlled 2‐year prospective trial on two estrogen‐progestin regimens in healthy postmenopausal women. Maturitas 1997;26:139‐49. [DOI] [PubMed] [Google Scholar]
- Heikkinen JE, Finney JS, Lankinen KS, Wilen‐Rosenqvist GH. Comparison of bleeding patterns and endometrial histology between a three monthly and monthly cycle HRT (abstract). Acta Obstetricia et Gynecologica Scandinavica Supplement 1997;76(167):P76.33. [Google Scholar]
Heikkinen 2000 {published data only}
- Heikkinen J, Vaheri R, Kainulainen P, Timonen U. Long‐term continuous combined hormone replacement therapy in the prevention of postmenopausal bone loss: a comparison of high‐ and low‐dose estrogen‐progestin regimens. Osteoporosis International 2000;11(11):929‐37. [DOI] [PubMed] [Google Scholar]
- Heikkinen J, Vaheri R, Maenpaa J, Timonen U. Long‐term endometrial safety of continuous combined HRT. Results from a 7‐year randomised study. International Journal of Obstetrics & Gynecology 2003;83(Suppl 3):98‐9. [Google Scholar]
- Heikkinen J, Vaheri R, Timonen U. A 10‐year follow‐up of postmenopausal women on long‐term continuous combined hormone replacement therapy: update of safety and quality‐of‐life findings [erratum appears in Journal of the British Menopause Society 2006;12(4):174]. Journal of the British Menopause Society 2006; Vol. 12, issue 3:115‐25. [DOI] [PubMed]
- Heikkinen J, Vaheri R, Timonen U. Long‐term safety and tolerability of continuous‐combined hormone therapy in postmenopausal women: results from a seven‐year randomised comparison of low and standard doses. Journal of the British Menopause Society 2004;10(3):95‐102. [DOI] [PubMed] [Google Scholar]
- Heikkinen JE, Vaheri RT, Ahomaki SM, Kainulainen PM, Viitanen AT, Timonen UM. Optimizing continuous‐combined hormone replacement therapy for postmenopausal women: a comparison of six different treatment regimens. American Journal of Obstetrics & Gynecology 2000;182(3):560‐7. [DOI] [PubMed] [Google Scholar]
- Weijer P. Long‐term data on endometrial safety and bleeding control with a continuous combined HRT regimen. Climacteric 2002;5(Suppl 1):210. [Google Scholar]
HOPE 2001 {published data only}
- Archer D, Lobo R, Utian W, Pickar J. Improved amenorrhoea, favourable vasomotor and lipid effects, and endometrial safety with lower doses of conjugated estrogens (CE) and medroxyprogesterone acetate (MPA). Gynecological Endocrinology 2000;14(Suppl 2):239. [Google Scholar]
- Archer DF, Dorin M, Lewis V, Schneider DL, Pickar JH. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertility & Sterility 2001;75(6):1080‐7. [DOI] [PubMed] [Google Scholar]
- Pickar J, Tien Yeh I, Wheeler J, Cunnane M, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertility & Sterility 2001;76:25‐31. [DOI] [PubMed] [Google Scholar]
- Pickar J, Yeh I, Wheeler J, Cunnane M, Speroff L. Endometrial safety with lower doses of conjugated equine estrogens (CEE) and medroxyprogesterone acetate (MPA). Fertility & Sterility. 2001; Vol. 76, issue 3S:52. [DOI] [PubMed]
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two‐year substudy results. Fertility & Sterility 2003;80(5):1234‐40. [DOI] [PubMed] [Google Scholar]
- Utian WH, Gass ML, Pickar JH. Body mass index does not influence response to treatment, nor does body weight change with lower doses of conjugated estrogens and medroxyprogesterone acetate in early postmenopausal women. Menopause 2004;11(3):306‐14. [DOI] [PubMed] [Google Scholar]
- Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertility & Sterility 2001;75(6):1065‐79. [DOI] [PubMed] [Google Scholar]
Koninckx 2005 {published data only}
- Koninckx P, Spielmann D, The Trimegestone 302 Study Group. A comparative 2‐year study of two sequential regimens of 1mg estradiol and trimegestone with 1mg estradiol and norethisterone upon profiles of endometrial bleeding and safety in postmenopausal women. Climacteric 2002;5(Suppl 1):144. [DOI] [PubMed] [Google Scholar]
- Koninckx PR, Spielmann D. A comparative 2‐year study of the effects of sequential regimens of 1 mg 17beta‐estradiol and trimegestone with a regimen containing estradiol valerate and norethisterone on the bleeding profile and endometrial safety in postmenopausal women. Gynecological Endocrinology 2005;21(2):82‐9. [DOI] [PubMed] [Google Scholar]
- Pornel B, Spielman D, The Trimgestone 302 Study Group. A study of the control of climacteric symptoms in postmenopausal women following sequential regimens of 1mg 17 B‐estradiol and trimegestone compared with a regimen containing 1mg estradiol valerate and norethisterone over a 2 year period. Gynaecological Endocrinology 2005;21(2):74‐81. [DOI] [PubMed] [Google Scholar]
Kurman 2000 {published data only}
- Kurman J, Carlos Felix J, Archer D, Nanavati N, Arce JC, Moyer D. Norethindrone acetate and estradiol‐induced endometrial hyperplasia. Obstetrics & Gynecology 2000;96(3):373‐9. [DOI] [PubMed] [Google Scholar]
Luciano 1993 {published data only}
- Luciano AA, Souza MJ, Roy MP, Schoenfeld MJ, Nulsen JC, Halvorson CV. Evaluation of low‐dose estrogen and progestin therapy in postmenopausal women. Journal of Reproductive Medicine 1993;38(3):207‐14. [PubMed] [Google Scholar]
Mattsson 2004 {published data only}
- Mattsson LA, Skouby SO, Heikkinen J, Vaheri R, Maenpaa J, Timonen U. A low‐dose start in hormone replacement therapy provides a beneficial bleeding profile and few side‐effects: randomized comparison with a conventional‐dose regimen. Climacteric 2004;7(1):59‐69. [DOI] [PubMed] [Google Scholar]
- Vaheri R, Matsson LA, Skouby S, Heikkinen J, Maenpaa J. Bleeding control, endometrial safety and tolerability of various continuous combined HRT regimens (abstract). Climacteric 2002;5(Suppl 1):146. [Google Scholar]
Meuwissen 2001 {published data only}
- Meuwissen JH, Beijers‐De Bie L, Vihtamaki T, Tuimala R, Siseles N, Magaril C, et al. A 1‐year comparison of the efficacy and clinical tolerance in postmenopausal women of two hormone replacement therapies containing estradiol in combination with either norgestrel or trimegestone. Gynecological Endocrinology 2001;15(5):349‐58. [PubMed] [Google Scholar]
MSG 1994 {published data only}
- Archer D, Pickar J. Effects of progestin dose and time since menopause on endometrial bleeding with continuous combine hormone replacement therapy. International Journal of Obstetrics and Gynecology 2000;70(Suppl 3):120. [DOI] [PubMed] [Google Scholar]
- Archer DF, Pickar JH. Hormone replacement therapy: effect of progestin dose and time since menopause on endometrial bleeding. Obstetrics & Gynecology 2000;96(6):899‐905. [DOI] [PubMed] [Google Scholar]
- Archer DF, Picker JH, Bottiglioni F for the Menopause Study Group. Bleeding patterns in postmenopausal women taking continuous combined or sequential regimens of conjugated estrogens with medroxyprogesterone acetate. Obstetrics and Gynecology 1994;83(5):686‐92. [PubMed] [Google Scholar]
- Pickar JH, Archer DF. Is bleeding a predictor of endometrial hyperplasia in postmenopausal women receiving hormone replacement therapy. American Journal of Obstetrics and Gynecology 1997;177:1178‐83. [DOI] [PubMed] [Google Scholar]
- Woodruff JD, Pickar JH for the Menopause Study Group. Incidence of endometrial hyperplasia in postmenopausal women taking conjugated estrogens (Premarin) with medroxyprogesterone acetate or conjugated estrogens alone. American Journal of Obstetrics and Gynecology 1994;170(5):1213‐23. [DOI] [PubMed] [Google Scholar]
Nand 1995 {published data only}
- Nand SL, Webster MA, Wren BG. Continuous combined piperazine oestrone sulphate and medroxyprogesterone acetate hormone replacement therapy ‐ a study of bleeding pattern, endometrial response, serum lipid and bone density changes. Australian & New Zealand Journal of Obstetrics & Gynaecology 1995;35(1):92‐6. [DOI] [PubMed] [Google Scholar]
Notelovitz 1997 {published data only}
- Genant HK, Lucas J, Weiss S, Akin M, Emkey R, McNaney‐Flint H, et al. Low‐dose esterified estrogen therapy. Effects on bone, plasma estradiol concentrations, endometrium and lipid levels. Archives of Internal Medicine 1997;157:2609‐15. [DOI] [PubMed] [Google Scholar]
- Lucas J, Rebar R, Speroff L, Trabal J, Silfen S. A two‐year study with low dose, continuous, unopposed esterified estrogens: prevention of postmenopausal bone loss with minimal endometrial effects. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sydney, Australia, 1996:212.
- Notelovitz M, Varner RE, Rebar RW, Fleischmann R, McIlwain HH, Schwartz SL, et al. Minimal endometrial proliferation over a two‐year period in postmenopausal women taking 0.3mg of unopposed esterified estrogens. Menopause 1997;4(2):80‐8. [Google Scholar]
- Trabal JF, Lenihan JP, Melchione TE, Stoltz RR, Khairi S, Yang H‐M, et al. Low‐dose unopposed estrogens: preliminary findings on the frequency and duration of vaginal bleeding in postmenopausal women receiving esterified estrogens over a two‐year period. Menopause 1997;4(3):130‐8. [Google Scholar]
Obel 1993 {published data only}
- Obel EB, Munk‐Jensen N, Svenstrup B, Bennett P, Micic S, Henrik‐Nielsen R, et al. A two‐year double‐blind controlled study of the clinical effect of combined and sequential postmenopausal replacement therapy and steroid metabolism during treatment. Maturitas 1993;16:13‐21. [DOI] [PubMed] [Google Scholar]
OGEN‐Provera 1998 {published data only}
- Nand S, Webster M, Baber R, O'Connor V. Bleeding pattern and endometrial changes during continuous combined hormone replacement therapy. Obstetrics & Gynecology 1998;91:678‐84. [DOI] [PubMed] [Google Scholar]
- Webster M. Combination continuous therapy ‐ what is the optimal MPA dose? Effect on bleeding profiles and endometrial histology. Proceedings of the 8th International Congress on the Menopause, 1996 Nov 3‐7. Sydney, Australia, 1996:27.
Okon 2001 {published data only}
- Okon MA, Lee S, Laird SM, Li TC. A prospective randomised controlled study comparing two doses of gestodene in cyclic combined HRT preparations on endometrial physiology. Human Reproduction 2001;16(6):1244‐50. [DOI] [PubMed] [Google Scholar]
OPAL 2006 {published data only}
- Bots ML, Evans GW, Riley W, McBride KH, Paskett ED, Helmond FA, et al. The effect of tibolone and continuous combined conjugated equine oestrogens plus medroxyprogesterone acetate on progression of carotid intima‐media thickness: the Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study. European Heart Journal 2006;27(6):746‐55. [DOI] [PubMed] [Google Scholar]
- Bots ML, Evans GW, Riley W, Meijer R, McBride KH, Paskett ED, et al. The Osteoporosis Prevention and Arterial effects of tiboLone (OPAL) study: design and baseline characteristics. Controlled Clinical Trials 2003;24(6):752‐75. [DOI] [PubMed] [Google Scholar]
- Langer RD, Landgren BM, Rymer J, Helmond FA. Effects of tibolone and continuous combined conjugated equine estrogen/medroxyprogesterone acetate on the endometrium and vaginal bleeding: results of the OPAL study. American Journal of Obstetrics and Gynecology 2006;195(5):1320‐7. [DOI] [PubMed] [Google Scholar]
PEPI 1995 {published and unpublished data}
- Barrett‐Connor E, Espeland MA, Greendale GA, Trabal J, Johnson S, Legault C, et al. Postmenopausal hormone use following a 3‐year randomised clinical trial. Journal of Womens Health & Gender‐Based Medicine 2000;9(6):633‐43. [DOI] [PubMed] [Google Scholar]
- Bush TL, Espeland MA, Mebane‐Sims I. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. Overview. Controlled Clinical Trials 1995;16:1S‐2S. [DOI] [PubMed] [Google Scholar]
- Espeland MA, Bush TL, Mebane‐Sims I, Stefanick ML, Johnson S, Sherwin R, et al. Rationale, design, and conduct of the PEPI Trial. Controlled Clinical Trials 1995;16:3S‐19S. [DOI] [PubMed] [Google Scholar]
- Johnson S, Mebane‐Sims I, Hogan PE, Stoy DB. Recruitment of postmenopausal women in the PEPI Trial. Controlled Clinical Trials 1995;16:20S‐35S. [DOI] [PubMed] [Google Scholar]
- Lindenfield E, Langer R. Bleeding patterns of the hormone replacement therapies in postmenopausal estrogen and progestin interventions trial. Obstetrics and Gynecology 2002;100:853‐63. [DOI] [PubMed] [Google Scholar]
- Miller VT, Byington RL, Espeland MA, Langer R, Marcus R, Shumaker S, et al. Baseline characteristics of the PEPI participants. Controlled Clinical Trials 1995;16:54S‐65S. [DOI] [PubMed] [Google Scholar]
- Wood PD, Kessler G, Lippel K, Stefanick ML, Wasilauskas CH, Wells HB. Physical and laboratory measurements in the PEPI Trial. Controlled Clinical Trials 1995;16:36S‐53S. [DOI] [PubMed] [Google Scholar]
- Writing Group for the Pepi trial. Effects of hormone replacement therapy on endometrial histology in postmenopausal women. The Postmenopausal Estrogen/Progestin Interventions (PEPI) trial. JAMA 1996;275(5):370‐5. [DOI] [PubMed] [Google Scholar]
Portman 2003 {published data only}
- Portman DJ, Shumel BS, Symons JP. Hormone replacement therapy (HRT) with 1 mg norethindrone acetate (NA) / 5 mcg ethinyl estradiol (EE) (FemHRT) provides greater protection against breakthrough bleeding versus 0.625 mg combined equine estrogens (CEE) / 2.5 mg medroxyprogesterone (MPA) (Prempro). The 3rd World Congress on Controversies in Obstetrics, Gynecology & Infertility. 2002:82.
- Portman DJ, Symons JP, Wilborn W, Kempfert NJ. A randomised, double‐blind, placebo‐controlled, multicenter study that assessed the endometrial effects of norethindrone acetate plus ethinyl estradiol versus ethinyl estradiol alone. American Journal of Obstetrics & Gynecology 2003;188(2):334‐42. [DOI] [PubMed] [Google Scholar]
Prestwood 2003 {published data only}
- Prestwood KM, Kenny AM, Kleppinger A, Kulldorff M. Ultra‐low‐dose micronized 17beta‐estradiol and bone density and bone metabolism in older women: a randomized controlled trial. JAMA 2003;290(8):1042‐8. [DOI] [PubMed] [Google Scholar]
Rees 2004 {published data only}
- Rees MCP, Kuhl H, Engelstein M, Mattila L, Maenpaa J, Mustonen M. Endometrial safety and tolerability of triphasic sequential hormone replacement estradiol valerate/medroxyprogesterone acetate therapy regimen. Climacteric 2004;7(1):23‐32. [DOI] [PubMed] [Google Scholar]
Rozenberg 2001 {published data only}
- Rozenberg S, Caubel P, Lim PC. Constant estrogen, intermittent progestogen vs continuous combined hormone replacement: tolerability and effect on vasomotor symptoms. International Journal of Gynecology and Obstetrics 2001;72:235‐43. [DOI] [PubMed] [Google Scholar]
- Rozenberg S, Lim P. One‐year efficacy and safety of Prefect, a pulsed progestogen, constant estrogen hormone replacement therapy regimen. European Journal of Obstetrics, Gynecology, & Reproductive Biology 1999;86(Suppl):S18‐9. [Google Scholar]
- Ylikorkala O, Lim P, Caubel P. Effects on serum lipid profiles of continuous 17beta‐estradiol, intermittent norgestimate regimens versus continuous combined 17beta‐estradiol/norethisterone acetate hormone replacement therapy. Clinical Therapeutics 2000; Vol. 22, issue 5:622‐36. [DOI] [PubMed]
- Ylikorkala O, Wahlstrom T, Caubel P, Lane R. Intermittent progestin administration as part of hormone replacement therapy: long‐term comparison between estradiol 1 mg combined with intermittent norgestimate and estradiol 2 mg combined with constant norethisterone acetate. Acta Obstetricia et Gynecologica Scandinavica 2002;81:654‐9. [DOI] [PubMed] [Google Scholar]
Scandinavia 1996 {published and unpublished data}
- Bjarnason K, Cerin A, Lingren R, Weber T. Adverse endometrial effects during long cycle hormone replacement therapy. Maturitas 1999;32:161‐70. [DOI] [PubMed] [Google Scholar]
- Cerin A, Heldaas K, Moeller B for the Scandinavian Long Cycle Study Group. Adverse endometrial effects of long‐cycle estrogen and progestogen replacement therapy (letter). New England of Journal of Medicine 1996;334(10):668‐9. [DOI] [PubMed] [Google Scholar]
- Cerin A, Hjarnason K, Heldaas K, Thomsen HK, Boeller B. Adverse endometrial effects during long cycle hormone replacement therapy. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sydney, Australia, 1996:48.
Sporrong 1988 {published data only}
- Sporrong T, Hellgren M, Samsioe G, Mattsson LA. Comparison of four continuously administered progestogen plus oestradiol combinations for climacteric complaints. British Journal of Obstetrics and Gynaecology 1988;95(10):1042‐8. [DOI] [PubMed] [Google Scholar]
- Sporrong T, Samsioe G, Larsen S, Mattsson LA. A novel statistical approach to analysis of bleeding patterns during continuous hormone replacement therapy. Maturitas 1989;11(3):209‐15. [DOI] [PubMed] [Google Scholar]
Stadberg 1996 {published data only}
- Stadberg E, Mattsson LA, Uvebrant M. 17 beta‐estradiol and norethisterone acetate in low doses as continuous combined hormone replacement therapy. Maturitas 1996;23(1):31‐9. [DOI] [PubMed] [Google Scholar]
van de Weijer 1999 {published data only}
- Weijer PH, Scholten PC, Mooren MJ, Barentsen R, Kenemans P. Bleeding patterns and endometrial histology during administration of low‐dose estradiol sequentially combined with dydrogesterone. Climacteric 1999;2(2):101‐9. [DOI] [PubMed] [Google Scholar]
Warming 2004 {published data only}
- Warming L, Ravn P, Nielsen T, Christiansen C. Safety and efficacy of drospirinone used in a continuous combination with 17beta‐estradiol for prevention of postmenopausal osteoporosis. Climacteric 2004;7(1):103‐11. [DOI] [PubMed] [Google Scholar]
WHI 2002 {published data only}
- Anderson GL, Judd HL, Kaunitz AM, Barad DH, Beresford SAA, Pettinger M, et al. Effects of estrogen plus progestin on gynecologic cancers and associated diagnostic procedures: the Women's Health Initiative randomized trial.[see comment]. JAMA 2003; Vol. 290, issue 13:1739‐48. [DOI] [PubMed]
- Barnabei VM, Cochrane BB, Aragaki AK, Nygaard I, Williams RS, McGovern PG, et al. Menopausal symptoms and treatment‐related effects of estrogen and progestin in the Women's Health Initiative. Obstetrics & Gynecology 2005;105(5 Pt 1):1063‐73. [DOI] [PubMed] [Google Scholar]
- Writing group for the Women's Health Initiative Investigators. Risks and benefits of estrogen plus progestin in healthy postmenopausal women. JAMA 2002;288:321‐33. [DOI] [PubMed] [Google Scholar]
Williams 1994 {published data only}
- Williams DB, Voigt BJ, Fu YS, Schoenfeld MJ, Judd HL. Assessment of less than monthly progestin therapy in postmenopausal women given estrogen replacement. Obstetrics & Gynecology 1994;84(5):787‐93. [PubMed] [Google Scholar]
Wu 2002 {published data only}
- Wu Y, Liu J, Xing S, Xu R, Zhang Z, Wang Y. Comparative study on two different dosages of conjugated equine estrogen continuously combined with medroxyprogesterone in prevention of postmenopausal osteoporosis. Chinese Journal of Obstetrics & Gynecology 2002;37(5):267‐70. [PubMed] [Google Scholar]
- Xing S, Wu Y, Liu J, Xu R, Zhang Z, Wang Y. A comparison of two different dosages of conjugated equine estrogen in continuous combined hormone replacement therapy with progestin. Chinese Medical Journal 2003;116(4):584‐7. [PubMed] [Google Scholar]
Yildirim 2006 {published data only}
- Yildirim G, Tugrul S, Uslu H, Pekin O, Eren S. Effects of two different regimens of continuous hormone replacement therapy on endometrial histopathology and postmenopausal uterine bleeding. Archives of Gynecology & Obstetrics 2006;273(5):268‐73. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
Aoki 1990 {published data only}
- Aoki T, Asai T. Assessment of oestrogen replacement therapy with conjugated oestrogens by histological evaluation of the endometrium in 205 women. Proceedings of the 6th International Congress on the Menopause, Bangkok, Thailand, 1990, Oct 29‐Nov 3. Bangkok, Thailand, 1990:151.
Archer 1999 {published data only}
- Archer D, Dorin M, Heine W, Nanavati N, Arce J. Uterine bleeding in postmenopausal women on continuous therapy with estradiol and norethindrone acetate. Obstetrics and Gynecology 1999;94:323‐9. [DOI] [PubMed] [Google Scholar]
Archer 2001 {published data only}
- Archer D, Dorin M, Lewis V, Schneider D, Pickar J. Effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate on endometrial bleeding. Fertility & Sterility 2001;75(6):1080‐7. [DOI] [PubMed] [Google Scholar]
Arrenbrecht 2004 {published data only}
- Arrenbrecht S, Caubel P, Garnero P, Felsenberg D, Vogel N, Coenders A, et al. The effect of continuous oestradiol with intermittent norgestimate on bone mineral density and bone turnover in post‐menopausal women. Maturitas 2004;48(3):197‐207. [DOI] [PubMed] [Google Scholar]
Bergeron 2010 {published data only}
Blumel 1994 {published data only}
- Blumel JE, Roncagliolo ME, Gramegna G, Vasquez R, Estartus A, Tacla X, et al. Double‐blind study of the effect of continuous therapy with estradiol valerate and medroxyprogesterone acetate on menopausal symptoms, lipid profile and endometrial thickness [Estudio doble ciego del efecto sobre la sintomatologia menopausica, el perfil lipidico y el grosor endometrial de una terapia continua de valerato de estradiol mas acetato de medroxiprogesterona]. Revista Chilena Obstetricia y Ginecologia 1994;59(5):354‐60. [PubMed] [Google Scholar]
Byrjalsen 1992a {published data only}
- Byrjalsen I, Thormann L, Riis BJ, Christiansen. Secretory endometrial protein PP14 in serum from post‐menopausal women receiving continuous combined oestradiol‐cyproterone acetate: correlation with serum hormone concentrations and bleeding patterns. Maturitas 1992;15:39‐46. [DOI] [PubMed] [Google Scholar]
Byrjalsen 1992b {published data only}
Campbell 1977 {published data only}
- Campbell S, Whitehead M. Oestrogen therapy and the menopausal syndrome. Clinics in Obstetrics and Gynaecology 1977;4(1):31‐47. [PubMed] [Google Scholar]
Campodonico 1996 {published data only}
- Campodonico I, Valdivia I. Bleeding pattern in two combined continuous hormone replacement therapy (HRT) regimes in climacteric women. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sydney, Australia, 1996:191.
Chen 1999 {published data only}
- Chen FP, Teng LF. Effects of cyclic continuous and sequential post‐menopausal hormone replacement therapy on uterine bleeding and climacteric symptoms. Human Reproduction 1999;14:246. [Google Scholar]
Christensen 1982 {published data only}
- Christensen MS, Hagen C, Christiansen C, Transbol I. Dose‐response evaluation of cyclic estrogen/gestagen in postmenopausal women: placebo‐controlled trial of its gynaecologic and metabolic actions. American Journal of Obstetrics & Gynecology 1982;144(8):873‐9. [DOI] [PubMed] [Google Scholar]
Endrikat 2007 {published data only}
- Endrikat J, Graeser T, Mellinger U, Ertan K, Holz C, Endrikat J, et al. A multicenter, prospective, randomized, double‐blind, placebo‐controlled study to investigate the efficacy of a continuous‐combined hormone therapy preparation containing 1mg estradiol valerate/2mg dienogest on hot flushes in postmenopausal women. Maturitas 2007; Vol. 58, issue 2:201‐7. [DOI] [PubMed]
Granberg 2002 {published data only}
- Granberg S, Eurenius K, Lindgren R, Wilhelmsson L. The effects of oral estriol on the endometrium in postmenopausal women. Maturitas 2002;42:149‐56. [DOI] [PubMed] [Google Scholar]
Gulhan 2004 {published data only}
- Gulhan S, Dilek S. The effect of different postmenopausal hormone replacement treatment protocols on endometrial thickness. Gulhane Medical Journal 2004;46(3):205‐8. [Google Scholar]
Hagen 1982 {published data only}
- Hagen C, Christensen MS, Christiansen C, Stocklund K‐E, Transbol I. Effects of two years' estrogen‐gestagen replacement on climacteric symptoms and gonadotropins in the early postmenopausal period. Acta Obstetrica et Gynecologica Scandinavica 1982;61:237‐41. [DOI] [PubMed] [Google Scholar]
Heytmanek 1990 {published data only}
- Heytmanek G. Continuous hormone replacement therapy with oestradiol valerate alone and in combination with cyproterone acetate: clinical and endometrial findings. Proceedings of the 6th International Congress on the Menopause, Bangkok, Thailand, 1990, Oct 29‐Nov 3. Bangkok, Thailand, 1990:86.
Istre 1996 {published data only}
- Istre O, Holm Nielsen P, Bourne T, Forman A. Hormone replacement therapy after transcervical resection of the endometrium. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:100. [DOI] [PubMed]
- Istre O, Holm‐Nielsen P, Bourne T, Forman A. Hormone replacement therapy after transcervical resection of the endometrium. Obstetrics & Gynecology 1996;88(5):767‐70. [DOI] [PubMed] [Google Scholar]
Jaisamrarn 2002 {published data only}
- Jaisamrarn S, Sitravarin N, Taechakrichana K, Panyakamlert K, Limpapayom K. Bleeding patterns in menopausal women taking HRT. Climacteric 2002;F‐13‐03:75. [Google Scholar]
Jirapinyo 2003 {published data only}
- Jirapinyo M, Theppisai U, Manonai J, Suchartwatnachai C, Jorgensen LN. Effect of combined oral estrogen/progestogen preparation (Kliogest) on bone mineral density, plasma lipids and postmenopausal symptoms in HRT‐naive Thai women. Acta Obstetricia et Gynecologica Scandinavica 2003;82(9):857‐66. [PubMed] [Google Scholar]
Kazerooni 2004 {published data only}
- Kazerooni T, Zolghadri J. The comparison of bleeding patterns with high‐dose and low‐dose hormone replacement therapy in postmenopausal women. Gynecological Endocrinology 2004;19(2):64‐8. [DOI] [PubMed] [Google Scholar]
Keil 2002 {published data only}
- Holst TH, Keil D, Lang E. Comparison of the incidence of irregular bleeding during treatment with a low dose continuous combined or sequential hormone replacement therapy. The 1st scientific meeting of the Asia‐Pacific Menopause Federation. 2002; Vol. 02‐05:61.
Limpaphayom 2000 {published data only}
- Limpaphayom K, Bunyavejchevin S. Clinical effects of 17 beta‐estradiol and norethisterone acetate in postmenopausal Thai women. Journal of the Medical Association of Thailand 2000;83:407‐15. [PubMed] [Google Scholar]
Liu 2005 {published data only}
- Liu JH, Muse KN. The effects of progestins on bone density and bone metabolism in postmenopausal women: a randomized controlled trial. American Journal of Obstetrics & Gynecololgy 2005;192(4):1316‐24. [DOI] [PubMed] [Google Scholar]
Luciano 1988 {published data only}
- Luciano A, Turksoy N, Carleo J, Hendrix J. Clinical and metabolic responses of menopausal women to sequential versus continuous estrogen and progestin replacement therapy. Obstetrics and Gynecology 1988;71:39‐43. [PubMed] [Google Scholar]
Marslew 1991 {published data only}
- Marslew U, Riis BJ, Christiansen C. Desogestrel in hormone replacement therapy: long‐term effects on bone, calcium and lipid metabolism, climacteric symptoms, and bleeding. European Journal of Clinical Investigation 1991;21:601‐7. [DOI] [PubMed] [Google Scholar]
Marslew 1992 {published data only}
- Marslew U, Overgaard K, Riis BJ, Christiansen C. Two new combinations of estrogen and progestogen for prevention of postmenopausal bone loss: long‐term effects on bone, calcium and lipid metabolism, climacteric symptoms, and bleeding. Obstetrics and Gynecology 1992;79:202‐10. [PubMed] [Google Scholar]
- Marslew U, Riis BJ, Christiansen C. Bleeding patterns during continuous combined estrogen‐progestogen therapy. American Journal of Obstetrics & Gynecology 1991;164(5 Pt 1):1163‐8. [DOI] [PubMed] [Google Scholar]
Mizunuma 1997 {published data only}
- Mizunuma H, Okano H, Soda M, Kagami I, Miyamoto S, Tokizawa T, et al. Prevention of postmenopausal bone loss with minimal uterine bleeding using low dose continuous estrogen/progestin therapy: a 2‐year prospective study. Maturitas 1997;27:69‐76. [DOI] [PubMed] [Google Scholar]
Morabito 2004 {published data only}
- Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, et al. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double‐blind EPT‐ and placebo‐controlled study. Menopause 2004;11(4):400‐4. [DOI] [PubMed] [Google Scholar]
- Morabito N, Crisafulli A, Vergara C, Gaudio A, Lasco A, Frisina N, et al. Effects of genistein and hormone‐replacement therapy on bone loss in early postmenopausal women: a randomized double‐blind placebo‐controlled study. Journal of Bone & Mineral Research 2002;17(10):1904‐12. [DOI] [PubMed] [Google Scholar]
Nachtigall 1979 {published data only}
- Nachtigall LE, Nachtigall RH, Nachtigall RD, Beckman EM. Estrogen replacement therapy II: A prospective study in the relationship to carcinoma and cardiovascular and metabolic problems. Obstetrics and Gynecology 1979;54(1):74‐9. [DOI] [PubMed] [Google Scholar]
Odmark 2001 {published data only}
- Odmark I, Jonsson B, Backstrom T. Bleeding patterns in postmenopausal women using two types of continuous combined hormone replacement therapy. Gynecological Endocrinology 2000;14(Suppl 2):128. [Google Scholar]
- Odmark IS, Jonsson B, Backstrom T. Bleeding patterns in postmenopausal women using continuous combination hormone replacement therapy with conjugated estrogen and medroxyprogesterone acetate or with 17B‐estradiol and norethindrone acetate. American Journal of Obstetrics and Gynecology 2001;184(6):1131‐8. [DOI] [PubMed] [Google Scholar]
Pickar 2003a {published data only}
- Pickar J, Yeh IT, Cunnane M, Archer D. Impact of conjugated estrogens (CE)/trimegestone (TMG) on endometrial hyperplasia and bleeding profiles in a double‐blind, randomized, placebo‐controlled study. Fertility & Sterility 2003;80(Suppl 3):18. [Google Scholar]
Pinto 2003 {published data only}
- Pinto A, Binder E, Kohrt W, Bronder D, Williams D. Effects of trimonthly progestin administration on the endometrium in elderly postmenopausal women who receive hormone replacement therapy: a pilot study. American Journal of Obstetrics and Gynecology 2003;189:11‐5. [DOI] [PubMed] [Google Scholar]
Popp 2006 {published data only}
- Popp AWE, Bodmer C, Senn C, Fuchs G, Kraenzlin ME, Wyss H, et al. Prevention of postmenopausal bone loss with long‐cycle hormone replacement therapy. Maturitas 2006;53(2):191‐200. [DOI] [PubMed] [Google Scholar]
Schiff 1982 {published data only}
- Schiff I, Sela HK, Cramer D, Tulchinsky D, Ryan KJ. Endometrial hyperplasia in women on cyclic or continuous estrogen regimens. Fertility & Sterility 1982;37(1):79‐82. [DOI] [PubMed] [Google Scholar]
Simon 2001 {published data only}
- Simon J, Symons J. Unscheduled bleeding during initiation of continuous combined hormone replacement therapy:a direct comparison of two combinations of norethindrone acetate and ethinyl estradiol to medroxyprogesterone acetate and conjugated equine estrogens. Menopause 2001;8:321‐6. [DOI] [PubMed] [Google Scholar]
Simon 2003 {published data only}
- Simon J, Liu J, Speroff L, Speroff L, Shumel B, Symons J. Reduced vaginal bleeding in postmenopausal women who receive combined norethindrone acetate and low‐dose ethinyl estradiol therapy versus combined conjugated equine estrogens and medroxyprogesterone acetate therapy. American Journal of Obstetrics & Gynecology 2003;188:92‐9. [DOI] [PubMed] [Google Scholar]
Steiner 2007 {published data only}
- Steiner AZ, Xiang M. Unopposed estradiol therapy in postmenopausal women: results from two randomized trials. Obstetrics & Gynecology 2007;109(3):581‐7. [DOI] [PubMed] [Google Scholar]
Stevenson 2001 {published data only}
- Stevenson JC, Teter P, Lees B. 17beta‐Estradiol (1 mg/day) continuously combined with dydrogesterone (5, 10 or 20 mg/day) increases bone mineral density in postmenopausal women. Maturitas 2001;38(2):197‐203. [DOI] [PubMed] [Google Scholar]
Stevenson 2010 {published data only}
Sturdee 1996 {published data only}
- Sturdee DW. Bleeding patterns and endometrial histology with sequential HRT. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:S36.
Sturdee 2000 {published data only}
- Sturdee DW, Ulrich LG, Barlow DH, Wells M, Campbell MJ, Vessey MP, et al. The endometrial response to sequential and continuous combined oestrogen‐progestogen replacement therapy. British Journal of Obstetrics and Gynecology 2000;11:1392‐400. [DOI] [PubMed] [Google Scholar]
Sturdee 2008 {published data only}
Symons 2000 {published data only}
- Symons J, Kempfert N, Speroff L. Vaginal bleeding in postmenopausal women taking low dose norethindrone acetate and ethinyl estradiol combinations. Obstetrics & Gynecology 2000;96:366‐72. [DOI] [PubMed] [Google Scholar]
Symons 2002 {published data only}
- Symons J. Comparative effect of norethindrone acetate/ethinyl estradiol and 0.625 mg conjugated estrogen/2.5 mg medroxyprogesterone acetate on bleeding control: early results from a randomized placebo controlled trial.. Obstetrics & Gynecology 2002;Suppl:84. [Google Scholar]
Ulla Timonen 2002 {published data only}
- Ulla Timonen R, Heikkinen R, Vaheri P, Kainulainen P. Bleeding control, endometrial safety and adherence to continuous combined HRT after 5 years. Climacteric 2002;P‐07‐21:146. [Google Scholar]
Utian 2002 {published data only}
- Utian W, Leonard T, Davis A, Vega R. Efficacy and safety study of a new synthetic 10‐component, modified release conjugated estrogens (CE) tablet for treatment of vasomotor symptoms in postmenopausal women. Fertility & Sterility 2002;78(3 Suppl 1):S159. [Google Scholar]
van der Mooren 1996 {published data only}
- Mooren MJ, Hanselaar A, Schiff Ch PT, Girardin C. Sequential three‐monthly versus monthly HRT: a prospective double‐blind randomised study. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:47.
van de Weijer 2002 {published data only}
- Weijer P. Long‐term data on endometrial safety and bleeding control with a continuous combined HRT regimen. Climacteric. 10th World Congress on Menopause 2002;5(Suppl 1):210. [Google Scholar]
Veerus 2008 {published data only}
- Veerus P, Fischer K, Hovi SL, Karro H, Rahu M, Hemminki E. Symptom reporting and quality of life in the Estonian Postmenopausal Hormone Therapy Trial. BMC Women's Health 2008;26(8):5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Volpe 1986 {published data only}
- Volpe A, Facchinetti F, Grasso A, Petraglia F, Campanini D, Genazzani AR. Benefits and risks of different hormonal replacement therapies in post‐menopausal women. Maturitas 1986;6:327‐34. [DOI] [PubMed] [Google Scholar]
Von Holst 2002 {published data only}
- Holst T, Lang E, Winkler U, Keil D. Bleeding patterns in peri and postmenopausal women taking a continuous combined regimen of estradiol with norethisterone acetate or a conventional sequential regimen of conjugated equine estrogens with medrogestone. Maturitas 2002;43(4):265‐75. [DOI] [PubMed] [Google Scholar]
Wahab 2002 {published data only}
- Wahab M, Thompson J, Whitehead M, Al‐Azzawi F. The effect of a change in the dose of trimegestone on the pattern of bleeding in estrogen‐treated post‐menopausal women: 6 month extension of a dose‐ranging study. Human Reproduction 2002;17(5):1386‐90. [DOI] [PubMed] [Google Scholar]
Wang 2006 {published data only}
- Wang SH, Lin SQ, Gui QF, Jin MJ, Jiang Y. Profiles of irregular bleeding induced by low‐dose hormone therapy and Chinese formulated herb products. Acta Academiae Medicinae Sinicae 2006;28(2):256‐61. [PubMed] [Google Scholar]
Warming 2004a {published data only}
- Warming L, Ravn P, Spielman D, Delmas P, Christiansen C. Trimegestone in a low‐dose, continuous‐combined hormone therapy regimen prevents bone loss in osteopenic postmenopausal women. Menopause 2004;11(3):337‐42. [DOI] [PubMed] [Google Scholar]
Webster 1996 {published data only}
- Webster M. Combination continuous therapy ‐ what is the optimal MPA dose? Effect on bleeding profiles and endometrial histology. Proceedings of the 8th International Congress on the Menopause, 1996, Nov 3‐7. Sidney, Australia, 1996:27.
Weinstein 1990 {published data only}
- Weinstein LB. Evaluation of a continuous combined low‐dose regimen of estrogen‐progestin for treatment of the menopausal patient. American Journal of Obstetrics & Gynecology 1990;162(6):1534‐9. [DOI] [PubMed] [Google Scholar]
Wells 2002 {published data only}
- Wells M, Sturdee D, Barlow D, Ulrich L, O'Brien K, Campbell M, et al. Effect on endometrium of long term treatment with continuous combined oestrogen‐progestogen replacement therapy: follow up study. British Medical Journal 2002;325:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Williams 1990 {published data only}
- Williams SR, Frenchek B, Speroff T, Speroff L. A study of combined continuous ethinyl estradiol and norethindrone acetate for postmenopausal hormone replacement. American Journal of Obstetrics and Gynecology 1990;162:438‐46. [DOI] [PubMed] [Google Scholar]
Yang 2001 {published data only}
- Yang TS, Liang WH, Chang SP, Yuan CC. Effects of period free hormone replacement therapy in postmenopausal women in Taiwan. Chinese Medical Journal (Taipei) 2001;65:23‐8. [PubMed] [Google Scholar]
Additional references
Ansbacher 1994
- Ansbacher R. Estrogens; conjugated and esterified therapeutic substitution. Female Pat 1994;19:14‐20. [Google Scholar]
Antunes 1979
- Antunes CM, Strolley PD, Rosenshein NB, Davies JL, Tonascia JA, Brown C, Burnett L, et al. Endometrial cancer and estrogen use. Report of a large case‐control study. New England Journal of Medicine 1979;300(1):9‐13. [DOI] [PubMed] [Google Scholar]
Archer 1999a
- Archer DF, Dorin MH, Heine W, Nayan N, Arce JC. Uterine bleeding in postmenopausal women on continuous therapy with oestradiol and norethindrone acetate. Obstetrics & Gynecology 1999;94(3):323‐9. [DOI] [PubMed] [Google Scholar]
Bachmann 2007
Cust 1990
- Cust MP, Gangar KF, Hillard TC, Whitehead MI. A risk benefit assessment of oestrogen therapy in post menopausal women. Drug Safety 1990;5:345‐358. [DOI] [PubMed] [Google Scholar]
Ellerington 1992
- Ellerington MC, Whitecroft SIV, Whitehead MI. HRT: Developments in therapy. British Medical Bulletin 1992;48:401‐25. [DOI] [PubMed] [Google Scholar]
France 1998
- France J. Personal communication July 1998.
Freeman 2011
- Freeman EW, Sammel MD, Lin H, Liu Z, Gracia CR. Duration of menopausal hot flushes and associated risk factors. Obstetrics & Gynecology 2011; Vol. 117, issue 5:1095‐104. [1873‐233X: (Electronic)] [DOI] [PMC free article] [PubMed]
Fugh‐Berman 2010
- Fugh‐Berman, AJ. The haunting of medical journals: How ghostwriting sold "HRT". PLoS Medicine 2010;7(9):e1000335. [DOI: 10.1371/journal.pmed.1000335] [DOI] [PMC free article] [PubMed] [Google Scholar]
Gardan 1977
- Gardan J, Reagan JW, Finkle WD. Oestrogen and endometrial carcinoma: an independent pathology review supporting original size estimate. New England Journal of Medicine 1977;297:570. [DOI] [PubMed] [Google Scholar]
Grady 1995
- Grady D, Gebretsadik T, Kerlikowske K, Ernster V, Petitti D. Hormone replacement therapy and endometrial cancer risk: a meta‐analysis. Obstetrics & Gynecology 1995;85:304‐13. [DOI] [PubMed] [Google Scholar]
Hickey 2005
- Hickey M, Davis SR, Sturdee DW. Treatment of menopausal symptoms: what shall we do now?. Lancet 2005;366(9483):409‐21. [DOI] [PubMed] [Google Scholar]
Higgins 2002
- Higgins JP, Thompson SG. Quantifying heterogeneity in a meta‐analysis. Statistics in Medicine 2002;21:1539‐58. [DOI] [PubMed] [Google Scholar]
Higgins 2011
- Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane‐handbook.org.
Kumar 2000
- Kumar N, Koide SS, Tsong Y, Sundaram K. Nestorone: a progestin with a unique pharmacological profile. Steroids 2000;65(10‐1):629‐36. [DOI] [PubMed] [Google Scholar]
Kurman 1985
- Kurman RJ, Kaminshi PF, Norm HJ. The behaviour of endometrial hyperplasia. A long term study of 'untreated' hyperplasia in 170 patients. Cancer 1985;56:403‐12. [DOI] [PubMed] [Google Scholar]
Lawrie 2011
- Lawrie TA, Helmerhorst FM, Maitra NK, Kulier R, Bloemenkamp K, Gülmezoglu AM. Types of progestogens in combined oral contraception: effectiveness and side‐effects. Cochrane Database of Systematic Reviews 2011, Issue 5. [DOI: 10.1002/14651858.CD004861.pub2] [DOI] [PubMed] [Google Scholar]
Mack 1976
- Mack TM, Pike MC, Henderson BE, Pfeffer RI, Gerkins VR, Arthur M, et al. Estrogens and endometrial cancer in a retirement community. New England Journal of Medicine 1976;294:1262‐7. [DOI] [PubMed] [Google Scholar]
MacLennan 1998
- MacLennan AH. Personal communication 16 March 1998; 13 August 1998.
Nelson 2002
- Nelson H, Humphrey LL, Nygren P, Teutsch SM, Allan JD. Postmenopausal hormone replacement therapy. JAMA 2002;288(7):872‐81. [DOI] [PubMed] [Google Scholar]
O'Connell 1998
- O'Connell D, Robertson J, Henry D, Gillespie W. A systematic review of the skeletal effects of estrogen therapy in postmenopausal women. II An assessment of treatment effects. Climacteric 1998;1:112‐23. [DOI] [PubMed] [Google Scholar]
Peeyananjarassri 2005
- Peeyananjarassri K, Baber R. Effects of low‐dose hormone therapy on menopausal symptoms, bone mineral density, endometrium, and the cardiovascular system: a review of randomised clinical trials. Climacteric 2005;8(1):13‐23. [DOI] [PubMed] [Google Scholar]
Pickar 2003
- Pickar JH, Yeh IT, Wheeler JE, Cunnane MF, Speroff L. Endometrial effects of lower doses of conjugated equine estrogens and medroxyprogesterone acetate: two‐year substudy results. Fertility & Sterility 2003;80(5):1234‐40. [DOI] [PubMed] [Google Scholar]
Pike 1997
- Pike MC, Peters RK, Cozen W, Probst‐Hensch NM, Felix JC, Wan PC, et al. Estrogen‐progestin replacement therapy and endometrial cancer. Journal of the National Cancer Institute 1997;89:1110‐6. [DOI] [PubMed] [Google Scholar]
Politi 2008
- Politi MC, Schleinitz MD, Col NF. Revisiting the duration of vasomotor symptoms of menopause: a meta‐analysis. Journal of General Internal Medicine 2008; Vol. 23, issue 9:1507‐13. [1525‐1497: (Electronic)] [DOI] [PMC free article] [PubMed]
Raudaskoski 2002
- Raudaskoski T, Tapanainen J, Tomas E, Luotola H, Pekonen F, Ronni‐Sivula, H, et al. Intrauterine 10mug and 20mug levonorgestrel systems in postmenopausal women receiving oral oestrogen replacement therapy: Clinical, endometrial and metabolic response. BJOG: An International Journal of Obstetrics & Gynaecology 2002;109(2):136‐44. [DOI] [PubMed] [Google Scholar]
RevMan 2011 [Computer program]
- The Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager (RevMan). Version 5.1. Copenhagen: The Nordic Cochrane Centre, The Cochrane Collaboration, 2011.
Roberts 2007
- Roberts H. Managing the menopause. BMJ 2007;334(7596):736‐41. [DOI] [PMC free article] [PubMed] [Google Scholar]
Smith 1977
- Smith DC, Prentice R, Thompson DJ, Herrmann WL. Association of exogenous oestrogen and endometrial carcinoma. New England Journal of Medicine 1977;293:1164‐7. [DOI] [PubMed] [Google Scholar]
Speroff 2005
- Speroff L, Fritz MA. Clinical gynecologic endocrinology and infertility (7th edition). Lippincott Williams & Wilkins & Wilkins, 2005. [Google Scholar]
Sturdee 1994
- Sturdee DW, Barlow DH, Ulrich LG, Wells M, Gydesen H, Campbell M, et al. Is the timing of withdrawal bleeding a guide to endometrial safety during sequential oestrogen‐progestogen replacement therapy?. Lancet 1994;344(8928):979‐82. [DOI] [PubMed] [Google Scholar]
Terakawa 1997
- Terakawa N, Kigawa J, Taketani Y, Yoshikawa H, Yajima A, Noda K. The behaviour of endometrial hyperplasia: a prospective study. Journal of Obstetrics and Gynaecology Research 1997;28:223‐30. [DOI] [PubMed] [Google Scholar]
Udoff 1995
- Udoff L, Langenberg P, Adashi E. Combined continuous hormone replacement therapy: a clinical review. Obstetrics & Gynecology 1995;86:306‐16. [DOI] [PubMed] [Google Scholar]
Utian 2001
- Utian WH, Shoupe D, Bachmann G, Pinkerton JV, Pickar JH. Relief of vasomotor symptoms and vaginal atrophy with lower doses of conjugated equine estrogens and medroxyprogesterone acetate. Fertility & Sterility 2001;75(6):1065‐79. [DOI] [PubMed] [Google Scholar]
Whitehead 1977
- Whitehead MI, Queen J, Beard RN, Minardi J, Campbell S. The effects of cyclical oestrogen therapy and sequential oestrogen therapy on the endometrium of postmenopausal women. Acta Obstetrica et Gynecologica Scandinavica Supplement 1977;65:91‐101. [DOI] [PubMed] [Google Scholar]
Whitehead 1981
- Whitehead MI, Townsend PT, Pryse‐Davies J, Ryder TA, King RJB. Effects of estrogens and progestins on the biochemistry and morphology of the postmenopausal endometrium. New England Journal of Medicine 1981;305:1599‐605. [DOI] [PubMed] [Google Scholar]
Whitehead 1987
- Whitehead MI, Fraser D. Controversies concerning the safety of estrogen replacement therapy. American Journal of Obstetrics & Gynecology 1987;156:13113‐22. [DOI] [PubMed] [Google Scholar]
Wood 2008
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates in controlled trials with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336(7644):601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
Ziel 1975
- Ziel HK, Finkle WD. Increased risk of endometrial carcinoma amongst users of conjugated oestrogens. New England Journal of Medicine 1975;293:1167‐70. [DOI] [PubMed] [Google Scholar]


