Abstract
A recent study characterized the long non-coding RNA (lncRNA) ELF3-antisense RNA 1 (ELF3-AS1) as an oncogenic lncRNA in bladder cancer. The present study aimed to investigate the role of ELF3-AS1 in osteosarcoma (OS). It was found that ELF3-AS1 was upregulated in OS tissues, and ELF3-AS1 expression level increased with increasing clinical stage. In OS tissues, Kruppel-like factor 12 (KLF12) was positively correlated with ELF3-AS1, while microRNA (miR)-205 was negatively correlated with ELF3-AS1. ELF3-AS1 overexpression resulted in the upregulation of KLF12, but the downregulation of miR-205. Overexpression of miR-205 caused downregulation of KLF12, but had no significant effects on ELF3-AS1 expression. Overexpression of KLF12 showed no significant impact on ELF3-AS1 and miR-205. ELF3-AS1 and KLF12 overexpression resulted in an increased proliferation rate in OS cells, while miR-205 played an opposite role and attenuated the effects of ELF3-AS1 overexpression. ELF3-AS1 overexpression promoted the methylation of the miR-205 gene. Therefore, ELF3-AS1 may promote OS cell proliferation by upregulating KLF12 through the methylation of the miR-205 gene.
Keywords: osteosarcoma, Kruppel-like factor 12, long non-coding RNA ELF3-antisense RNA 1, microRNA-205
Introduction
Osteosarcoma (OS) is the most common primary sarcoma of the bone and mainly affects adolescents and children (1,2). In spite of the low incidence rate, OS cause a considerable number of cancer-related deaths due to its highly aggressive nature and systemic metastasis occurs at early stages (2,3). With the development of therapeutic approaches and diagnostic techniques, the overall survival rate has increased from 20 to 65–75% during the past century (4). However, cancer metastasis, such as lung metastasis can easily occur, and only less than 30% of OS patients with metastatic OS can survive. The high mortality rate is mainly because most OS patients with cancer metastasis inevitably develop resistance to currently available chemical drugs (5).
Genetic factors are critical players in the occurrence and development of OS (6,7). Non-coding RNAs, such as miRNAs and long (>200 nt) non-coding RNAs (lncRNAs) encode no proteins but participate in cancer development by regulating gene expression (8–10). ncRNA-targeted therapies have shown promising potentials in cancer diagnosis and prognosis (11), while the function of most ncRNAs is hardly known, which limits their clinical applications. LncRNA ELF3-AS1 has been reported to be an oncogenic lncRNA in bladder cancer (12). Our preliminary data showed that ELF3-AS1 was inversely correlated with miR-205, which plays tumor-suppressive or oncogenic roles in different types of cancer (13,14). It is known miR-205 can directly target KLF12 in basal-like breast carcinoma (15). The present study was carried out to investigate the interaction between miR-205, KLF12 and ELF3-AS1 in OS.
Materials and methods
Research subjects
The First Affiliated Hospital of Wannan Medical College (Wuhu, China) admitted 79 patients with OS during the period between December 2015 and December 2018. The current study selected 40 (25 males and 17 females; range, 19–48 years; mean age, 33.2±5.4 years) of these patients according to strict criteria. The inclusion criteria were as follows: i) Newly diagnosed patients with OS; and ii) all major organs showed normal functions. The exclusion criteria were as follows: i) therapies initiated before admission; ii) family history of malignancies; or iii) previous history of malignancies. Patients with OS were staged according to American Joint Committee on Cancer criteria (16), and there were 8, 13, 11 and 8 cases at stages I–IV, respectively. All patients were informed of experimental details and consented to the use of their samples in this study, and the Ethics Committee of the First Affiliated Hospital of Wannan Medical College approved the study.
Tissues
Patients with OS were diagnosed by biopsy. During a biopsy, OS tumor and non-tumor (within 2 cm of the tumor) tissues were collected from each patient. The weight of tissues were 0.08–0.12 g, and the tissue types were confirmed by histopathological examinations.
Cells and transient transfection
The human OS cell line U2OS (ATCC) was used in this study. Eagle's minimum essential medium (American Type Culture Collection) with 10% FBS (Sigma-Aldrich; Merck KGaA) was used as a cell culture medium, and cell culture conditions were 37°C and 5% CO2. KLF12 and ELF3-AS1 expression vectors were constructed by Sangon Biotech Co., Ltd. using the pcDNA3.1 vector. Negative control miRNA (5′-UGACGUCAGUCGUAGGUACGUG-3′) and miR-205 mimic (5′-UCCUUCAUUCCACCGGAGUCUG-3′) were purchased from Sigma-Aldrich (Merck KGaA). KLF12 and ELF3-AS1 expression vector (10 nM), or 10 nM empty pcDNA3 vector (negative control, NC1), 45 nM miR-205 mimic or negative control miRNA (NC2, targets to a non-human sequence) were transfected into 106 U2OS cells using Lipofectamine® 2000 reagent (Sigma-Aldrich; Merck KGaA). Untransfected cells were control cells (C). The subsequent experiments were performed using cells collected at 24 h after transfection.
RT-qPCR
RiboZol™ RNA extraction reagent (VWR International) was mixed with U2OS cells and tissue powder (ground in liquid nitrogen before use) to extract total RNA. SensiFAST™ cDNA Synthesis kit (Bioline) was used to perform RT. Temperature conditions for RT were 55°C for 30 min and 80°C for 10 min. SYBR Green Master mix (Bio-Rad Laboratories, Inc.) was used for preparation of qPCR mixtures. 18S rRNA and GAPDH were used as endogenous controls to detect the expression of KLF12 and ELF3-AS1, respectively. To detect miR-205 expression, an miRNA Isolation kit (Geneaid Biotech Ltd.) was used to extract miRNA. miRNA reverse transcription and qPCR were performed using All-in-One™ miRNA qRT-PCR Detection Kit (Genecopoeia, Inc.). U6 was used as an endogenous control for miR-205. Primer sequences were as follows: ELF3-AS1 forward 5′-TGAAGTCATCACGAACCG-3′ and reverse, 5′-GAGCCCCAAGTTAATGCG-3′; KLF12 forward 5′-GATGAATTGTTGCAGCCCCA-3′ and reverse, 5′-CATTTGAAATAATGACCAAG-3′; GADPH forward 5′-CCAGGGCTGCTTTTAACTCT-3′ and reverse, 5′-GGACTCCACGACGTACTCA-3′. The forward primer for miR-205 was 5′-UCCUUCAUUCCACCGGAGU-3′, and the reverse primer for miR-205 and both U6 primers were from the kit. Thermocycling conditions for all qPCR reactions were 95°C for 40 sec, followed by 95°C for 10 sec and 60°C for 30 sec for 40 cycles. Each sample was measured in triplicate, and the 2−ΔΔCq method was used to process data (17).
Methylation-specific PCR (MSP)
Genomic DNA was extracted from U2OS cells using a genomic DNA extraction kit (cat. no. ab156900; Abcam). All DNA samples were processed using a Methylation-Gold™ kit (Zymo Research Corp.). Routine Taq DNA polymerase (New England BioLabs, Inc.) was used to perform the subsequent PCR. Primer sequences were as follows: Methylation (M) forward, 5′-TTGTTTTGATGATTATGAAGGAATG-3′ and reverse, 5′-ACCCCTAAACTAACTAAACCCAAAC-3′; unmethylation (U) forward 5′-TCTGCCCTGATGATCATGAAGG-3′ and reverse, 5′-CCTGGGCTGACTGAACCCAAGCC-′. Thermocycling conditions were 95°C for 40 sec, followed by 95°C for 30 sec, 55°C for 30 sec and 72°C for 40 sec for 30 cycles. PCR products were subjected to 1.5% agarose gel electrophoresis (80 v for 15 min) and ethidium bromide staining (10 min at room temperature).
Measurement of cell proliferation ability
U2OS cells were harvested at 24 h after transfections, and 4×104 cells were mixed with 1 ml Eagle's minimum essential medium with 10% FBS to prepare single-cell suspensions. A 96-well cell culture plate was used to cultivate the cells at 37°C and in 5% CO2, and 10 µl Cell Counting Kit-8 reagent (Dojindo Molecular Technologies, Inc.) was added at 2 h Prior to cell collection. Cells were collected every 24 h for a total of 4 days. Subsequently, cell proliferation rates were calculated based on OD values measured at a wavelength of 450 nm.
Western blot analysis
RIPA solution (Sangon Biotech Co., Ltd.) was used to extract total protein from both tissues and transfected cells. Proteins were denatured in boiling water for 10 min, and SDS-PAGE was performed using 12% gels with 30 µg protein per lane. Proteins were transferred to PVDF membranes, followed by blocking in 5% non-fat milk for 2 h at 25°C. Subsequently, the membranes were incubated with rabbit polyclonal primary antibodies against GAPDH (1:1,300; cat. no. ab9485; Abcam) and KLF12 (1:1,200; cat. no. ab221602; Abcam) overnight at 4°C, followed by incubation with goat anti-rabbit IgG horseradish peroxidase-conjugated secondary antibody (1:2,000; cat. no. sc-2004; Santa Cruz Biotechnology, Inc.) for 2 h at room temperature. ECL (Sigma-Aldrich; Merck KGaA) was used to develop signals. ImageJ software (version 1.46; National Institutes of Health) was used to analyze data.
Statistical analysis
Mean values (± SEM) represent data from 3 biological replicates. GraphPad Prism 6 (GraphPad, Inc.) was used to perform all statistical analysis. Paired Student's t-test was used to compare the differences between 2 types of tissues from OS patients. One-way ANOVA and Tukey's post hoc test were used to explore the differences between different patient and cell groups. Correlations were analyzed by linear regression. P<0.05 was considered to indicate a statistically significant difference.
Results
ELF3-AS1 is upregulated in OS and affected by cancer development
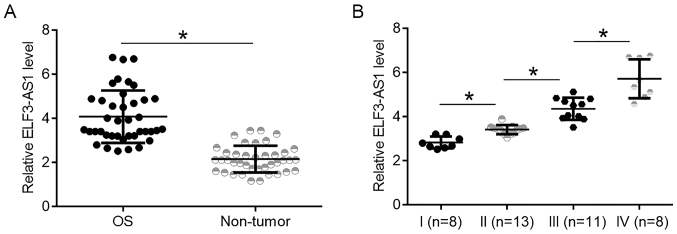
ELF3-AS1 expression was compared between tumor and non-tumor tissues. It was observed that expression levels of ELF3-AS1 were significantly higher in OS tissues compared with non-tumor tissues (Fig. 1A; P<0.05). ELF3-AS1 expression data in OS tissues were compared between patients at 4 clinical. It was observed that ELF3-AS1 expression levels in OS tissues increased with increasing clinical stages (Fig. 1B; P<0.05).
Figure 1.
ELF3-AS1 is upregulated in OS tissues compared with non-tumor tissues and is associated with cancer stage. (A) ELF3-AS1 expression in OS and non-tumor tissues. (B) ELF3-AS1 expression levels in OS tissues of patients based on each clinical stage *P<0.05. ELF3-AS1, ELF3-antisense RNA 1; OS, osteosarcoma.
KLF12 and miR-205 are significantly correlated with ELF3-AS1 in OS
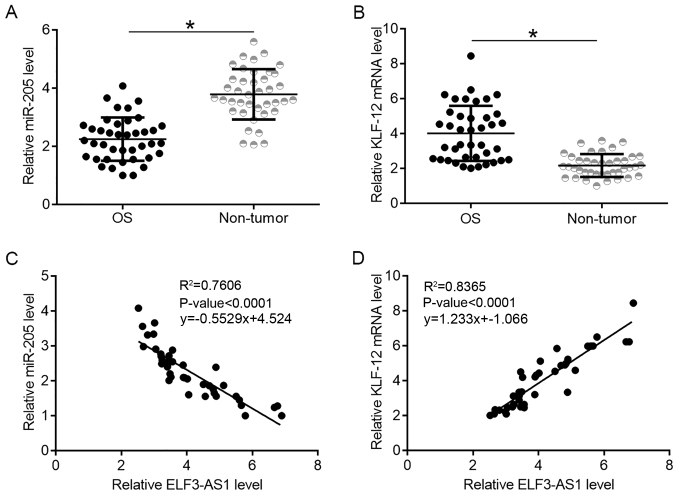
KLF12 and miR-205 expression were also detected in tumor and non-tumor tissues. It was observed that miR-205 was significantly downregulated (Fig. 2A; P<0.05), while KLF12 was upregulated (Fig. 2B; P<0.05) in OS tissues compared with non-tumor tissues. ELF3-AS1 expression was found to be negatively correlated with miR-205 expression (Fig. 2C), while ELF3-AS1was positively correlated with KLF12 (Fig. 2D).
Figure 2.
KLF12 and miR-205 expression is altered in OS and is correlated with ELF3-AS1 expression. (A) miR-205 and (B) KLF12 expression in OS and non-tumor tissues; *P<0.05. (C) Linear regression analysis of ELF3-AS1 expression and expression of (C) miR-205 and (D) KLF12. KLF12, Kruppel-like factor 12; miR, microRNA; ELF3-AS1, ELF3-antisense RNA 1; OS, osteosarcoma.
ELF3-AS1 upregulates KLF12 through miR-205
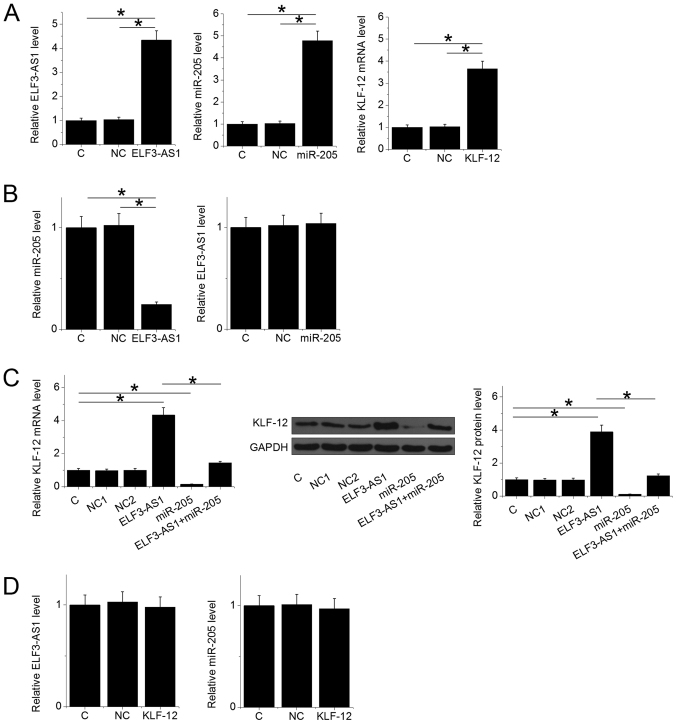
ELF3-AS1 and KLF12 expression vectors, as well as miR-205 mimic, were transfected into U2OS cells. Compared with C and NC groups, expression levels of ELF3-AS1, miR-205 and KLF12 were significantly upregulated at 24 h after transfection (Fig. 3A; P<0.05). ELF3-AS1 overexpression resulted in miR-205 downregulation (P<0.05), while miR-205 overexpression failed to affect ELF3-AS1 (Fig. 3B). ELF3-AS1 overexpression resulted in upregulated KLF12, while overexpression of miR-205 led to downregulated KLF12 expression at both mRNA and protein levels (Fig. 3C; P<0.05). Overexpression of miR-205 reduced the effects of ELF3-AS1 overexpression in cells transfected with both the ELF3-AS1 expression vector and miR-205 mimic (Fig. 3C; P<0.05). Additionally, overexpression of KLF12 exhibited no significant impact on ELF3-AS1 and miR-205 expression (Fig. 3D).
Figure 3.
Overexpression of ELF3-AS1 in osteosarcoma cells upregulates KLF12 potentially via downregulation of miR-205. (A) Expression levels of ELF3-AS1, KLF12 and miR-205 in cells transfected with ELF3-AS1, KLF12 expression vectors or miR-205 mimic. (B) miR-205 expression in cells with ELF3-AS1 overexpression (left), and ELF3-AS1 expression in cells with miR-205 overexpression (right). (C) KLF12 expression at mRNA and protein levels in cells with ELF3-AS1 and/or miR-205 overexpression. (D) ELF3-AS1 (left) and miR-205 (right) expression in cells with KLF12 overexpression. *P<0.05. C, untransfected control; NC1, empty vector negative control; NC2, miRNA negative control; KLF12, Kruppel-like factor 12; miR, microRNA; ELF3-AS1, ELF3-antisense RNA 1.
ELF3-AS1 promotes OS cell proliferation through KLF12 and miR-205
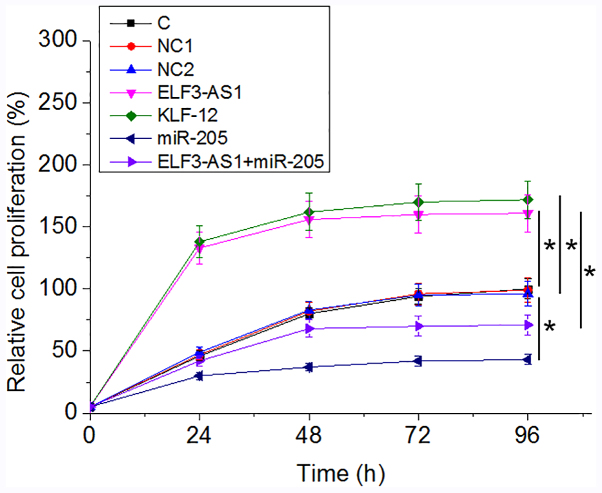
Analysis of cell proliferation revealed that, compared with the C group, ELF3-AS1 and KLF12 overexpression resulted in an increased proliferation rate in OS cells at 96 h after the initiation of cell culture, while overexpression miR-205 played an opposite role and attenuated the effects of ELF3-AS1 overexpression (Fig. 4; P<0.05).
Figure 4.
ELF3-AS1 promotes osteosarcoma cell proliferation through KLF12 and miR-205. *P<0.05. C, untransfected control; NC1, empty vector negative control; NC2, miRNA negative control; KLF12, Kruppel-like factor 12; miR, microRNA; ELF3-AS1, ELF3-antisense RNA 1.
ELF3-AS1 promotes the methylation of miR-205 gene
MSP was performed to analyze the effects of ELF3-AS1 overexpression on miR-205 gene methylation. It was observed that ELF3-AS1 overexpression led to increased methylation of the miR-205 gene (Fig. 5).
Figure 5.
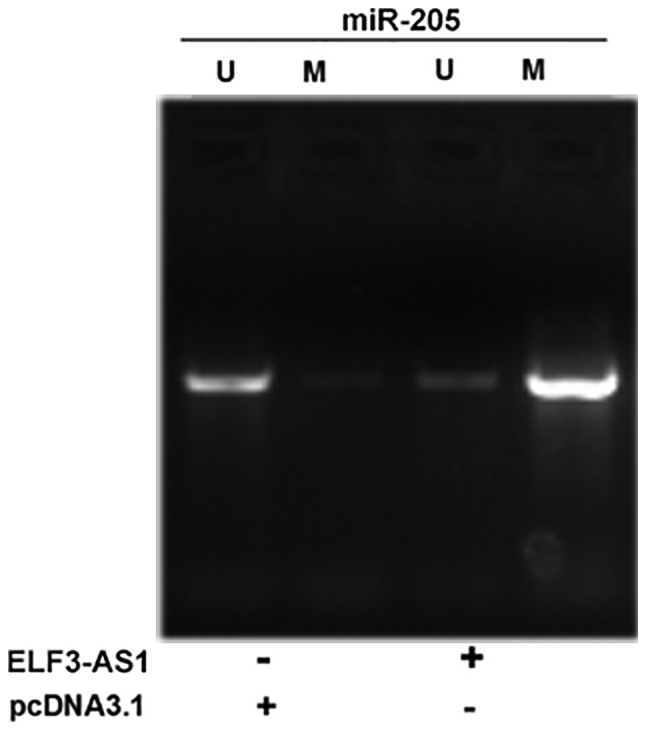
ELF3-AS1 promotes the methylation of the miR-205 gene, as determined by methylation-specific PCR. U, unmethylated; M, methylated; miR, microRNA; ELF3-AS1, ELF3-antisense RNA 1.
Discussion
The expression pattern and function of ELF3-AS1 have been investigated in the present study. ELF3-AS1 was fond to be upregulated in OS tissues compared with adjacent non-tumor tissue, and overexpression of ELF3-AS1 promoted the proliferation of OS cells by upregulating KLF12 through miR-205. A previous study found that miR-205 can directly target KLF12 in basal-like breast carcinoma (15).
miR-205 serves different roles in various types of cancer (13,14). In esophageal adenocarcinoma, miR-205 is downregulated and inhibits the development of cancer (13). By contrast, in squamous cell carcinoma of the esophagus and ovarian cancer, miR-205 is upregulated and promotes cancer progression by targeting tumor suppressors such as PTEN and SMAD4 (13,14). In a recent study, miR-205 was found to target KLF12 directly and to be involved in the regulation of cancer cell apoptosis and invasion in basal-like breast carcinoma (15). KLF12 is a zinc finger DNA-binding transcription factor that regulates gene expression (18). In many types of cancer, including colorectal cancer, KLF12 promotes the growth of the tumor by regulating cancer-related factors, such as early growth response protein 1 (18). In the present study, upregulated KLF12 and downregulated miR-205 were observed in OS tissues, and the miR-205 overexpression in OC cells resulted in downregulation of KLF12. Therefore, miR-205 may also directly target KLF12 in OS.
In the current study, a reduced cell proliferation rate was observed after miR-205 overexpression and an increased rate was observed after KLF12 overexpression. Therefore, miR-205 may be a tumor-suppressive miRNA, while KLF12 may be an oncogene in OS. In bladder cancer, ELF3-AS1 promotes cancer development by interacting with KLF8 (12). In the present study, ELF3-AS1 may upregulate KLF12 in OS cells via the downregulation of miR-205. Therefore, ELF3-AS1 may regulate different KLFs in various types of cancer, or ELF3-AS1 may regulate multiple KLFs. However, the mechanism underlying the inhibition of miR-205 by ELF3-AS1 is unclear. It is known that lncRNA can inhibit the function of miRNAs by serving as miRNA sponges (19,20). However, sponges do not downregulate miRNA expression, and in the current study, downregulated miR-205 was observed following ELF3-AS1 overexpression. In addition, no target site of miR-205 was found on ELF3-AS1. However, ELF3-AS1 overexpression led to increased methylation of the miR-205 gene. Therefore, ELF3-AS1 may downregulate miR-205 via methylation of its gene. It is worth noting that only one cell line was used in this study and therefore the findings should be further validated using other OS cell lines. In addition, the present study failed to perform KLF12 and miR-205 knockdown experiments. Future studies will try to include these experiments to further validate the findings. The effects of co-transfection of miR-205 mimic and KLF12 expression vector on the proliferation of OS cells should also be investigated to further analyze the interactions between miR-205 and KLF12 in regulating OS cell proliferation. The cell phenotypes were also analyzed after transfections, but no significant changes were observed (data not shown). Therefore, ELF3-AS1, miR-205 and KLF12 may have no significant effects on OS cell phenotypes.
In conclusion, ELF3-AS1 was found to be upregulated in OS, and ELF3-AS1 overexpression may promote the proliferation of OS cells by upregulating KLF12 potentially through methylation of the miR-205 gene.
Acknowledgements
Not applicable.
Funding
The present study was supported by Natural Science Foundation for Middle-aged and Young Scientists of Wannan Medical College for 2019 (grant no. WK2019F34) and Funding of ‘Peak’ Training Program and ‘Panfeng’ Innovation Team Project for Scientifc Research of Yijishan Hospital, Wannan Medical College (grant. no. PF2019005).
Availability of data and materials
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.
Authors' contributions
JY and JK designed and performed experiments. MY collected and analyzed data. JK drafted the manuscript. All authors approved this manuscript.
Ethics approval and consent to participate
The Ethics Committee of the First Affiliated Hospital of Wannan Medical College (Wuhu, China) approved the study. All patients consented to the use of their samples in this study.
Patient consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
References
- 1.Geller DS, Gorlick R. Osteosarcoma: A review of diagnosis, management, and treatment strategies. Clin Adv Hematol Oncol. 2010;8:705–718. [PubMed] [Google Scholar]
- 2.Messerschmitt PJ, Garcia RM, Abdul-Karim FW, Greenfield EM, Getty PJ. Osteosarcoma. J Am Acad Orthop Surg. 2009;17:515–527. doi: 10.5435/00124635-200908000-00005. [DOI] [PubMed] [Google Scholar]
- 3.Mirabello L, Troisi RJ, Savage SA. International osteosarcoma incidence patterns in children and adolescents, middle ages and elderly persons. Int J Cancer. 2009;125:229–234. doi: 10.1002/ijc.24320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jaffe N. Osteosarcoma: Review of the past, impact on the future. The American experience. Cancer Treat Res. 2009;152:239–262. doi: 10.1007/978-1-4419-0284-9_12. [DOI] [PubMed] [Google Scholar]
- 5.Daw NC, Billups CA, Rodriguez-Galindo C, McCarville MB, Rao BN, Cain AM, Jenkins JJ, Neel MD, Meyer WH. Metastatic osteosarcoma. Cancer. 2006;106:403–412. doi: 10.1002/cncr.21626. [DOI] [PubMed] [Google Scholar]
- 6.Kansara M, Thomas DM. Molecular pathogenesis of osteosarcoma. DNA Cell Biol. 2007;26:1–18. doi: 10.1089/dna.2006.0505. [DOI] [PubMed] [Google Scholar]
- 7.Hansen MF. Genetic and molecular aspects of osteosarcoma. J Musculoskelet Neuronal Interact. 2002;2:554–560. [PubMed] [Google Scholar]
- 8.Reddy KB. MicroRNA (miRNA) in cancer. Cancer Cell Int. 2015;15:38. doi: 10.1186/s12935-015-0185-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McManus MT. MicroRNAs and cancer. Semin Cancer Biol. 2003;13:253–258. doi: 10.1016/S1044-579X(03)00038-5. [DOI] [PubMed] [Google Scholar]
- 10.Yang G, Lu X, Yuan L. LncRNA: A link between RNA and cancer. Biochim Biophys Acta. 2014;1839:1097–1109. doi: 10.1016/j.bbagrm.2014.08.012. [DOI] [PubMed] [Google Scholar]
- 11.Esteller M. Non-coding RNAs in human disease. Nat Rev Genet. 2011;12:861–874. doi: 10.1038/nrg3074. [DOI] [PubMed] [Google Scholar]
- 12.Guo Y, Chen D, Su X, Chen J, Li Y. The lncRNA ELF3-AS1 promotes bladder cancer progression by interaction with Krüppel-like factor 8. Biochem Biophys Res Commun. 2019;508:762–768. doi: 10.1016/j.bbrc.2018.11.183. [DOI] [PubMed] [Google Scholar]
- 13.Hezova R, Kovarikova A, Srovnal J, Zemanova M, Harustiak T, Ehrmann J, Hajduch M, Sachlova M, Svoboda M, Slaby O. MiR-205 functions as a tumor suppressor in adenocarcinoma and an oncogene in squamous cell carcinoma of esophagus. Tumuor Biol. 2016;37:8007–8018. doi: 10.1007/s13277-015-4656-8. [DOI] [PubMed] [Google Scholar]
- 14.Li J, Hu K, Gong G, Zhu D, Wang Y, Liu H, Wu X. Upregulation of MiR-205 transcriptionally suppresses SMAD4 and PTEN and contributes to human ovarian cancer progression. Sci Rep. 2017;7:41330. doi: 10.1038/srep41330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guan B, Li Q, Shen L, Rao Q, Wang Y, Zhu Y, Zhou XJ, Li XH. MicroRNA-205 directly targets Krüppel-like factor 12 and is involved in invasion and apoptosis in basal-like breast carcinoma. Int J Oncol. 2016;49:720–734. doi: 10.3892/ijo.2016.3573. [DOI] [PubMed] [Google Scholar]
- 16.Steffner RJ, Jang ES. Staging of bone and soft-tissue sarcomas. J Am Acad Orthop Surg. 2018;26:e269–e278. doi: 10.5435/JAAOS-D-17-00055. [DOI] [PubMed] [Google Scholar]
- 17.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 18.Kim SH, Park YY, Cho SN, Margalit O, Wang D, DuBois RN. Krüppel-like factor 12 promotes colorectal cancer growth through early growth response protein 1. PLoS One. 2016;11:e0159899. doi: 10.1371/journal.pone.0159899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liang WC, Fu WM, Wong CW, Wang Y, Wang WM, Hu GX, Zhang L, Xiao LJ, Wan DC, Zhang JF, Waye MM. The lncRNA H19 promotes epithelial to mesenchymal transition by functioning as miRNA sponges in colorectal cancer. Oncotarget. 2015;6:22513–22525. doi: 10.18632/oncotarget.4154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liz J, Esteller M. lncRNAs and microRNAs with a role in cancer development. Biochim Biophys Acta. 2016;1859:169–176. doi: 10.1016/j.bbagrm.2015.06.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used and/or analyzed during the present study are available from the corresponding author on reasonable request.