Abstract
Objective:
Risk for prescription opioid addiction is an endemic public health concern, especially for adults with chronic pain. This study examined craving as a mediator from pain to opioid use outcomes during prescription opioid addiction treatment and tested whether counseling in pain coping skills moderated the effects of craving on treatment outcomes.
Method:
Secondary analysis on a sample (N = 148) randomized to standard or enhanced counseling for 12 weeks with adjunct opioid maintenance medication. Multilevel analyses examined mediated effects between weekly pain, craving, and opioid use, and tested the interaction between craving and a counseling module on pain coping skills.
Results:
Greater pain predicted greater craving (β = 0.25, p < .001), which predicted next-week opioid use (β = 0.17, p < .001). A statistically significant indirect effect of craving (β = 0.04, 95% CI [0.02, 0.06]) mediated 95% of the total effect from pain to opioid use. A significant interaction (b = −0.22, p < .01) revealed that after receiving the pain coping module, the association between craving and next-week opioid use was reduced, with greater exposure to the module associated with stronger effects (b = −0.12, p < .01).
Conclusion:
More severe pain predicts greater opioid use due to the association between pain and cravings. Pain coping skills counseling suppressed the association between cravings and opioid use. For adults with chronic pain receiving treatment for prescription opioid addiction, interventions that address cravings through behavioral pain coping skills may be crucial for achieving optimal treatment outcomes.
Keywords: prescription opioid addiction, craving, chronic pain, pain coping, intervention
Prescription opioid abuse is an endemic public health concern with the prescribing of opioids, opioid-related overdoses, and reported opioid use disorders increasing significantly over the past two decades (Substance Abuse and Mental Health Services Administration, 2014; Sullivan & Howe, 2013). Chronic pain patients are particularly vulnerable to developing prescription opioid addiction, as they are often prescribed opioids at high doses and for long durations (Garland, Froeliger, Zeidan, Partin, & Howard, 2013; Sullivan et al., 2008; Sullivan & Howe, 2013). Empirically supported interventions exist for treatment of prescription opioid addiction, but these treatments have low to moderate effect sizes, and often have low rates of retention and completion (Ehde, Dillworth, & Turner, 2014; Veilleux, Colvin, Anderson, York, & Heinz, 2010; Williams, Eccleston, & Morley, 2012). Research that identifies risk factors that contribute to suboptimal treatment outcomes should be conducted to help clinicians specify the best targets for prevention and treatment. Furthermore, if studies can identify specific elements of therapies that moderate the impact of such risk factors, it would assist in creating more useful and targeted treatments for individuals with prescription opioid addiction and chronic pain.
Craving is one proximal marker that may have a significant impact on treatment outcomes for individuals with prescription opioid addiction and chronic pain. Severity of craving is implicated in the diagnosis of substance use disorders (SUDs), severity of addiction, and risk of relapse across multiple substances of abuse (MacKillop et al., 2010; McHugh et al., 2014; Witkiewitz & Bowen, 2010). In adults who use prescription opioids, craving is related to a host of negative outcomes including increased fluctuations in mood, greater desire and intention to use prescription opioids, and increased risk of relapse during and following treatment (Ashrafioun & Carels, 2014; Huhn et al., 2016; McHugh et al., 2014; Northrup et al., 2015; Tsui, Anderson, Strong, & Stein, 2014). Similar negative effects of craving have been identified in chronic pain patients who are prescribed opiates for pain, such that increased craving is associated with increased preoccupation with next dose, greater rates of aberrant medication use, increased rates of opioid use, and pain catastrophizing (Martel, Jamison, Wasan, & Edwards, 2014; Wasan et al., 2009, 2012). For those with comorbid chronic pain and prescription opioid abuse, increased pain severity is associated with increased odds of subsequent week opioid use and is also associated with greater craving and increased risk of relapse during treatment (Griffin et al., 2016; Tsui et al., 2016). Interventions that explicitly target cravings or their antecedents are promising new interventions for treatment of SUDs (Witkiewitz & Bowen, 2010; Witkiewitz, Bowen, Douglas, & Hsu, 2013). In order to develop better treatments for prescription opioid addiction in chronic pain patients, the antecedents, consequences, and clinical impact of fluctuations in craving must be better understood.
For both researchers and clinicians, the identification of optimal treatment approaches for adults with prescription opioid addiction and chronic pain is a major priority. Buprenorphine-naloxone (BUP-NLX) is one recommended pharmacotherapy with empirical support for reducing opioid use and achieving adequate pain control for individuals with chronic pain who are diagnosed with an opioid use disorder (Neumann et al., 2013; Weiss et al., 2011, 2015). Some behavioral interventions, such as cognitive-behavioral therapy, have also been effective in decreasing pain severity and opioid misuse among chronic pain patients currently prescribed opioids (Ehde et al., 2014; Garland, Thomas, & Howard, 2014; Williams et al., 2012). These interventions typically target maladaptive thoughts and behaviors that perpetuate the pain cycle, such as pain catastrophizing and behavioral deactivation. Access to such evidence-based interventions has also been improved by the development of primary care-based interventions that target either substance use or chronic pain (Oslin et al., 2014; Wetherell et al., 2011), although a comprehensive standard of care for co-occurring chronic pain and prescription opioid addiction does not currently exist. Furthermore, relapse commonly occurs even in the midst of high-quality pharmacological and behavioral treatment, and continued pain and craving may be factors that impact the success of treatment outcomes (Ehde et al., 2014; Veilleux et al., 2010). In order to maximize the benefit of behavioral interventions for this population, a crucial goal for research is to examine the mechanisms involved in poor treatment outcomes and to identify aspects of treatment that may mitigate these factors.
The current study investigated a sample of adults treated for prescription opioid addiction with 12 weeks of BUP-NLX and counseling. Previous studies of this sample revealed that chronic pain did not predict opiate use outcomes or moderate the effect of treatment condition (Weiss et al., 2011, 2015). However, greater baseline pain severity did predict greater odds of opioid dependence at an 18-month follow up as well as predicted next-week opioid use in subjects with chronic pain (Griffin et al., 2016; Potter et al., 2015). Next-week opioid use in the full sample was also predicted by greater time-varying craving (McHugh et al., 2014). These prior studies demonstrated the significance of pain severity and craving in this sample but did not examine the relationship between pain and craving as well as their effects on opioid use for individuals with chronic pain within a unified model. Therefore, we conducted the current study which had two major aims. The first aim was to examine a hypothesized mediational relationship between higher levels of pain, craving, and opioid use to determine whether the mediated effects of pain through craving would explain worse treatment outcomes in this population. The second aim was to determine whether counseling in behavioral pain coping skills would moderate these effects. Specifically, we tested the hypothesis that receiving a counseling module on behavioral pain coping would attenuate the maladaptive effects of the pain-craving pathway. Given the neurobiological immediacy in which pain can initiate opioid craving (Evans & Cahill, 2016; Navratilova et al., 2012), we hypothesized that the intervention would facilitate extinction of the conditioned opioid use response via the use of behavioral pain coping strategies. We hypothesized that behavioral pain coping would solely attenuate the craving-opioid use pathway as pain coping strategies offered individuals an alternative to opioid use when they experienced pain.
Method
Study Design
This institutional review board-exempt study was a secondary analysis of public data from the Prescription Opioid Addiction Treatment Study (POATS), a multisite, randomized clinical trial for the treatment of prescription opioid abuse. From the original adaptive two-phase trial, we examined only Phase 2 in which participants who failed to sustain abstinence after a 4-week BUP-NLX detoxification were rerandomized to standard medical management (SMM) or enhanced medical management (EMM). For 12 weeks of treatment participants received adjunct BUP-NLX maintenance and attended weekly clinic visits to complete assessments, receive medication refills and adjustments, and attend counseling sessions. For a complete overview of the study design and main findings please refer to previous reports (Weiss et al., 2010, 2011). The current study only utilized data from the Phase 1 baseline visit, the Phase 2 randomization visit, and the 12 weeks of active treatment delivered in Phase 2.
Study Intervention
Individuals randomized to the SMM condition received manual-based SMM during physician visits (O’Connor et al., 1998; Weiss et al., 2011). Broadly, SMM consisted of physicians assessing substance use, craving, and BUP-NLX response, recommending abstinence, and referring patients to self-help groups. Those randomized to EMM received a manual-based intervention for opioid dependence counseling delivered by a trained substance abuse or mental health counselor including drug counselors, psychologists, social workers, or nurses (Pantalon, Fiellin, Schottenfeld, Gordon, & O’Connor, 1999; Weiss et al., 2011). EMM sessions involved education and discussion on one of the 13 available modules with topics selected collaboratively by counselor and participant and modules could be repeated if so desired. Examples of topics available included warning signs of relapse, handling triggers, and managing emotions. The pain skills module involved a brief introduction to cognitive-behavioral coping skills for managing physical pain including; relaxation, pacing, engagement in pleasurable activities, and cognitive restructuring of pain catastrophizing. Participants were also provided with a manual for reference of concepts discussed during this session.
Participants
Individuals enrolled in the original POATS study were at least 18 years old, diagnosed with Diagnostic and Statistical Manual of Mental Disorders (4th ed.; DSM–IV; American Psychiatric Association, 1994) current opioid dependence, physically dependent on opioids, not dependent on other substances, and had no unstable medical or psychiatric conditions. Key exclusion criteria included heroin use on over 4 days in the past month, opioid dependence for heroin alone, lifetime heroin injection, current participation in formal substance use treatment (other than self-help groups), or any major pain event within the past 6 months. A full list of inclusion/exclusion criteria and a detailed explanation of the study timeline are described in the primary study findings (Weiss et al., 2011). For Phase 1 of the trial 653 participants were randomized, and Phase 2 involved rerandomization of participants (N = 360) who had unsuccessful opioid use outcomes following Phase 1.
For the current study, our sample includes the participants from Phase 2 who reported having chronic pain at the original baseline assessment conducted prior to Phase 1 randomization (N = 148). The sample was evenly split between the SMM and EMM treatment conditions (n = 74 in each). Within this sample participants were predominantly male (55%), White (87.9%), and unmarried (69.8%), with an average of 12.8 (SD = 2.37) years of education. Lifetime use of heroin was reported by 24% of the sample, 34% had received prior prescription opioid treatment, and participants used prescription opioids for a mean of 27.9 (SD = 3.8) of the past 30 days. Additional demographic and sample characteristics by treatment condition are provided in Table 1.
Table 1.
Descriptive Statistics for Adults With Chronic Pain Enrolled in 12-Week Trial of Counseling and Adjunct Opioid Substitution
| Demographics | SMM (n = 74); M (SD) or % | EMM without pain coping (n = 45); M (SD) or % | EMM with pain coping (n = 30); M (SD) or % |
|---|---|---|---|
| Baseline | |||
| Sex (% male) | 54.1 | 61.4 | 46.6 |
| Race/ethnicity (% White/non-Hispanic) | 86.50 | 90.80 | 86.70 |
| Married (% currently married) | 25.70 | 27.30 | 43.30 |
| Lifetime heroin use (% never used) | 74.30 | 70.50 | 86.70 |
| Duration of chronic paina | 6.14 (1.10) | 6.18 (.97) | 6.52 (.69) |
| Duration of prescription opioid useb | 4.47 (1.38) | 4.59 (1.45) | 4.69 (1.34) |
| Days used opioids in past 30 | 28.23 (2.85) | 27.30 (5.10) | 27.97 (3.45) |
| Postbaseline | |||
| Past-week opiate use | .63 (1.35) | .44 (1.13) | .50 (1.37) |
| Current pain | 3.17 (2.52) | 3.21 (2.52) | 2.83 (2.45) |
| Craving | 2.62 (3.04) | 2.67 (2.65) | 2.04 (2.71) |
Note. N = 148. SMM = standard medication management; EMM = enhancement medication management.
Scale for measure is 1 (less than 6 months) to 7 (more than 10 years).
Scale for measure is 1 (less than 1 month) to 7 (4 years or greater).
Measures
Opioid use.
Opioid use was assessed at baseline and at weekly study visits using the Substance Use Report, a calendar-assisted interview similar to the timeline follow-back procedure (Sobell & Sobell, 1992). Raw data were used to code each available day from baseline to the Week 12 visit as positive if participants reported using any type of opioid (i.e., prescription analgesics, illicit opioids, methadone). Raw data were aggregated to 7-day intervals to compute the outcome variable of number of opioid use days computed for each 7-day interval. Urine drug screens were used to corroborate self-report, with weeks without drug screen results treated as missing. In the event the week contained a positive drug screen result but no use was reported, multiple imputation was used to compute the drug use variable. As compared with other secondary studies of this dataset that coded weeks as “use or “no use” (Griffin et al., 2016; McHugh et al., 2014), allowed analyses conducted to maximize the information available in the dataset by examining a dimensional outcome.
Pain and craving.
Participants reported their current pain rating from 0–10 using an abbreviated version of the Brief Pain Inventory (BPI)—Short Form (Keller et al., 2004). Current craving for opioids was assessed on a 0–10 scale using a visual analogue scale (VAS; Carlsson, 1983). Each variable was assessed at weekly study visits and used as time-varying predictors in this study. We utilized the single item from each measure that captures the rating of current severity as other items on the abbreviated BPI and VAS did not match in their frame of reference This approach provided the most pure and direct way to capture the association between craving and pain.
Counseling participation.
For each EMM session the module used in the session was recorded. We used this EMM module data to compute a dichotomous, time-varying variable for each week indicating whether the participant had received the pain coping module at that point in time. We also computed a time-varying count of the cumulative number of pain coping sessions received at each week and an ordinal variable indicating whether the module was received 0, 1, or ≥2 times. These separate indicators were created to allow us to separately test the effects of any exposure to the pain coping module as well as the dose of exposure.
Of the 74 subjects in the EMM condition, just over 40% (n =30) received the pain coping module. Of these 30 subjects, just over half (n = 16) received the module exactly once with the rest receiving it 2–6 times. The average week of first exposure to the module was halfway through treatment at Week 6 (M = 6.1, SD = 3.3), but the timing of first delivery of the module varied considerably with 33% receiving it in Weeks 1–4, 47% in Weeks 5–8, and 20% in Weeks 9–12.
Baseline covariates.
Items from several baseline measures were tested as covariates of opioid use and as potential confounders of person-level differences between participants who did and did not receive the pain coping module. We took a liberal approach in selecting a broad range of covariates to identify any potential pretreatment differences between participants who received the pain module and those who did not, so that statistical models could control for these factors. Baseline covariates included sex, race, and marital status from a demographics questionnaire, past-week opioid use from the Substance Use Report, opioid craving from the VAS, total withdrawal score from the Clinical Opiate Withdrawal Scale, initial BUP/NLX dose, and lifetime major depression from the Composite International Diagnostic Interview (Robins et al., 1988; Wesson & Ling, 2003). Covariates from the Brief Pain Inventory Short-From (BPI-SF) included the general pain interference item and current pain rating. From a structured pain and opioid interview developed for POATS covariates included past 30-day opioid use, prior heroin use, nonoral use of opioids, pain frequency, opioid of choice (short-acting, oxycontin, or other), duration of chronic pain, duration of nonmedical opioid use, and three items on self-reported expectancies to benefit from medication, to benefit from counseling, and for pain to improve.
Data Analysis
The study hypotheses were tested using multilevel models which allow repeated measures of both outcome variables and time-varying covariates, including of person-level and time-level covariates in the same model, and inclusion of all available data using maximum likelihood estimation (Schafer & Graham, 2002). Preliminary analyses did not reveal violations of the missing-at-random assumption, thus maximum likelihood estimation was used. All models used random subject-level intercepts. The primary outcome variable was self-reported opioid use assessed weekly during the treatment phase (Weeks 1 to 12). Preliminary analyses compared baseline covariates between EMM subjects who received the pain coping module (n = 30) and those who did not (n = 44) using one-way analysis of variance for continuous variables and chi-square tests for categorical variables. Effect sizes for these tests were also examined, given the small sample sizes of the groups. Using the full sample, preliminary models tested each baseline covariate as a predictor of weekly opioid use, with statistically significant covariates (p < .05) retained for final models. All multilevel models controlled for counseling condition, time, previous-week opioid use, and receipt of the pain coping module. Continuous data were transformed to mean-centered z scores prior to analyses and all statistical analyses were conducted in Stata 14.2 (StataCorp, 2016).
The main analyses, which included our full sample (N = 148), were conducted to examine two distinct hypotheses. The first hypothesis was that craving would mediate the effects of pain on opioid use. To examine this hypothesis we tested a multilevel mediation model (Krull & MacKinnon, 2001) with pain as a time-varying predictor of time-varying craving, and then a model with craving as a time-varying predictor of time-varying future opioid use, controlling for pain and other covariates. All models paired pain and craving with subsequent (i.e., “next week”) opioid use to establish temporal precedence of mediation. We tested statistical significance of mediation by estimating the indirect effect with 95% confidence intervals (CIs) estimated using bias-corrected bootstrap with 1,000 replications. The second hypothesis was that the pain coping module would moderate the effects of craving on opioid use. For this hypothesis, the model of next-week opioid use included baseline covariates, previous opioid use, pain, craving, the pain coping module indicator, and an interaction term between pain coping module and craving (Figure 1). Additional analyses were conducted to test additional mechanism of change criteria (Kazdin & Nock, 2003). To test dose-response effects the moderation model was repeated, replacing the dichotomous pain coping module variable with the count of pain coping module sessions. Specificity was examined by controlling for total therapy attendance to test whether moderation persisted when controlling for total treatment exposure. An additional interaction model was tested within the pain module recipient subsample (n = 30) which provided a direct test of the within-person interaction effect by removing nonrecipients from the model. For all interaction effects, simple slopes were estimated using the postmodel margins command in Stata to obtain average marginal effects.
Figure 1.
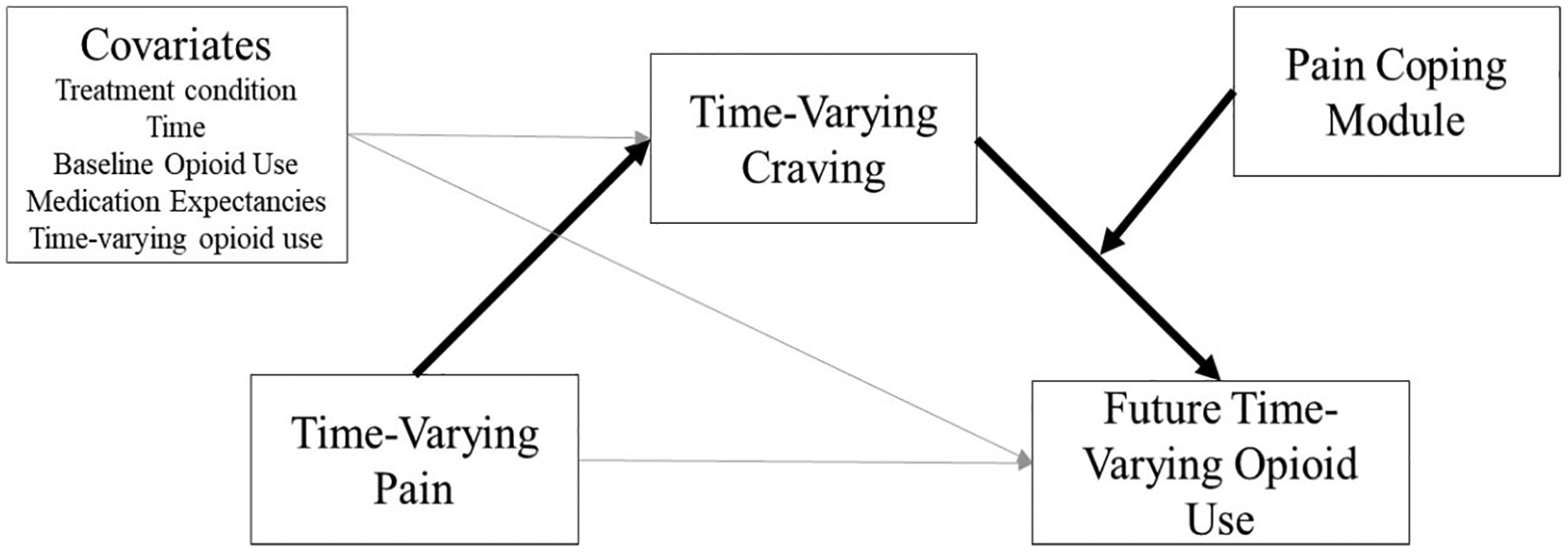
Depiction of proposed moderated mediation model of pain, craving, and opioid use, with emphasis on primary effects of interest
Results
Covariate Analyses
In preliminary analyses of 21 baseline covariates there were no significant differences between recipients and nonrecipients of the pain coping module on any variables, including initial BUP-NLX dose, pain rating, withdrawal score, duration of chronic pain, or duration of prescription opioid use. Only three variables (baseline opioid use, duration of chronic pain, baseline pain severity) had effect size differences greater than d = 0.20, and of these variables, only baseline opioid use predicted opioid use in the predictive models (b = 0.07, p < .001). Preliminary covariate analyses identified only one other significant baseline covariate with greater expectancies for study medication predicting more opioid use (b =0.07, p < .05). As such, previous-week opioid use (which included baseline opioid use) and medication expectancies were controlled for in all subsequent analyses.
Mediation of Prescription Opioid Use
In the model of time-varying craving (model R2 = 0.61), greater time-varying pain significantly predicted greater craving (β = 0.25, p < .001), indicating that pain and craving had a strong, positive association (Figure 2). Of the remaining model variables treatment week significantly predicted craving, with a significant reduction in craving over time (b = −0.04, p < .001), and greater past-week opioid use predicted greater craving (β = 0.33, p < .001). Craving was not significantly predicted by EMM condition, pain coping module, or medication help expectancy.
Figure 2.
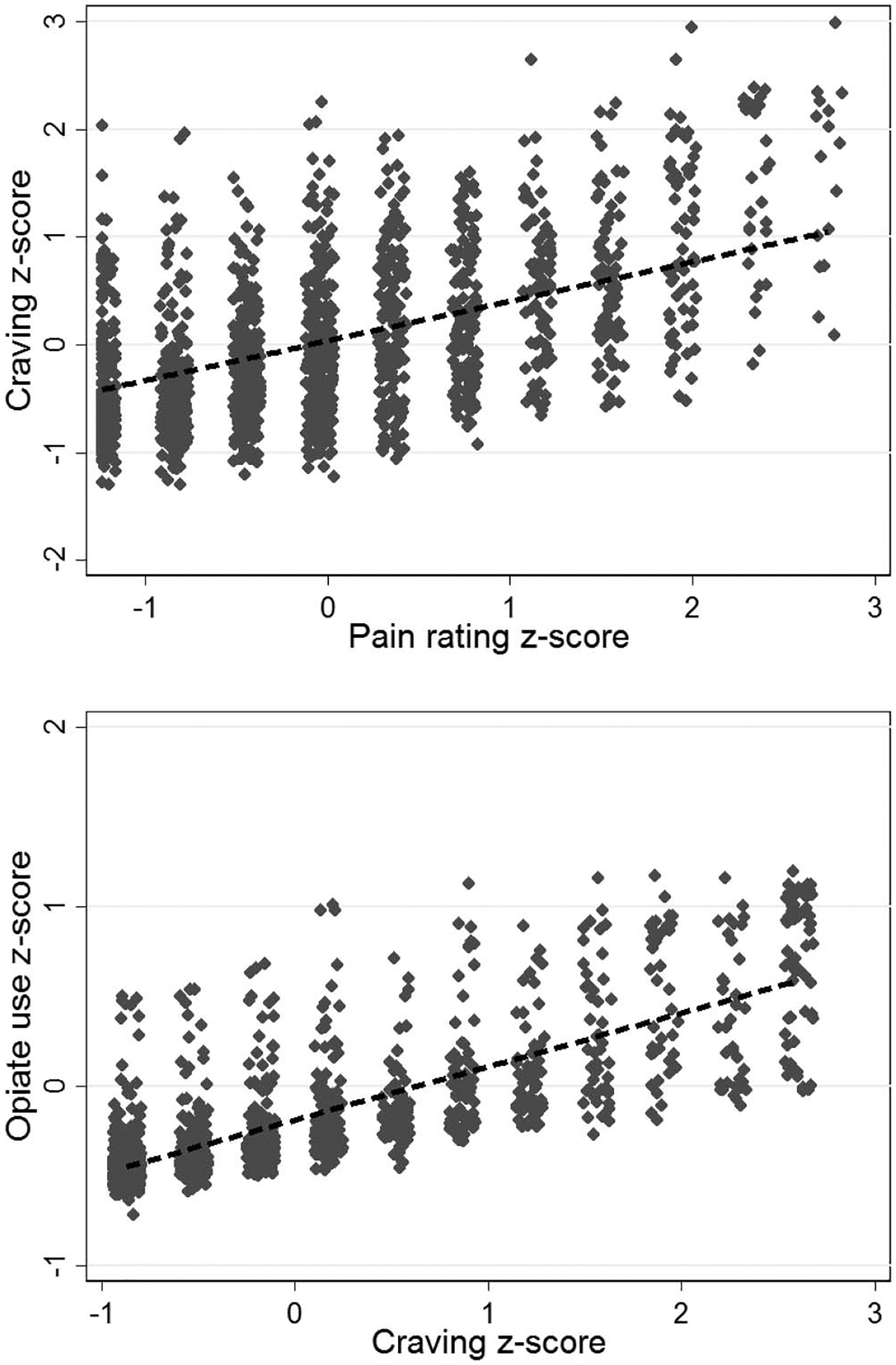
Standardized self-reported craving for opioids, pain, and opioid use in opioid-addicted chronic pain patients (N = 148). Greater pain rating was associated with greater reported opioid craving and greater opioid craving was associated with greater reported opioid use.
In the model of time-varying opioid use (model R2 = 0.63), greater time-varying craving significantly predicted greater next-week opioid use (β = 0.17, p < .001), indicating that craving had a strong, prospective effect on future opioid use (see Figure 2). The only other significant predictor of opioid use was past-week opioid use, which was positively associated with subsequent opioid use (β = 0.25, p < .001). Opioid use was not significantly predicted by other model variables, including time-varying pain (β = 0.005, p = .82), pain coping module (b = −0.07, p = .35), or treatment week (b = 0.01, p = .17).
In combination, the above results suggest craving mediated the effect of pain on opioid use, but a formal mediation test is recommended to provide statistical evidence of a significant indirect effect. Results of a bias-corrected bootstrap analysis indicated that the indirect effect from pain to craving to opioid use was statistically significant, β = 0.04, 95% CI [0.02, 0.06]. A summary of model results is provided in Table 2. Additionally, the proportion of the total effect explained by the indirect effect was 95%, suggesting that the relationship between pain and opioid use was almost completely mediated through craving. We estimated R2 = 0.02 for the total effect of pain and craving on opioid use, suggesting that approximately 1.9% of the variance in opioid use was explained by the indirect effect.
Table 2.
Summary of Model Results
| Outcome variable | Mediation models | Moderated mediation model: Past-week opioid use (β) | |
|---|---|---|---|
| Craving (β) | Past-week opioid use (β) | ||
| Treatment group (EMM vs. SMM) | −.03 | −.06 | −.08 |
| Treatment week | −.04*** | .01 | .01 |
| Medication help expectancy | .03 | .03 | .04 |
| Pain coping module | −.08 | −.02 | −.10 |
| Lagged opioid use | .33*** | .25*** | .16*** |
| Lagged pain | .25*** | −.001 | .01 |
| Lagged craving | .17*** | .24*** | |
| Lagged Craving × Pain Module | −.22* | ||
Note. EMM = enhancement medication management; SMM = standard medication management.
p < .05.
p < .001.
Moderation of Craving Effects on Prescription Opioid Use
Moderated mediation analyses tested whether the effects of craving on opioid use were attenuated by receiving the pain coping module. Results showed that the interaction between pain coping module and craving was negative and statistically significant (b = −0.22, p < .01, model R2 = 0.72). As shown in Figure 3, in weeks that occurred after subjects had received the pain module, there was a reduced association between craving and future opioid use. Simple slopes analysis revealed a significant effect of craving when the module had not been received (b = 0.24, p < .001), but after receiving the module there was no significant effect of craving (b = 0.02, p = .80). Additional analyses conducted within the pain module recipients only (n = 30) estimated a similar effect size and statistically significant interaction (b = −0.28, p < .001, model R2 = 0.75). Simple-slopes analysis revealed a significant effect of craving when the module had not been received (b = 0.48, p < .001), but after receiving the module there was no significant effect of craving (b = 0.20, p = .06). This analysis indicates the Pain Module × Craving interaction occurred within subjects and was not due to differences between module recipients and nonrecipients. Additionally, when we controlled for week of the first pain module session (main effect and interaction with craving) to explore the potential confound of differential timing of module exposure, the pain module interaction effect remained significant while the effects of session timing were not.
Figure 3.
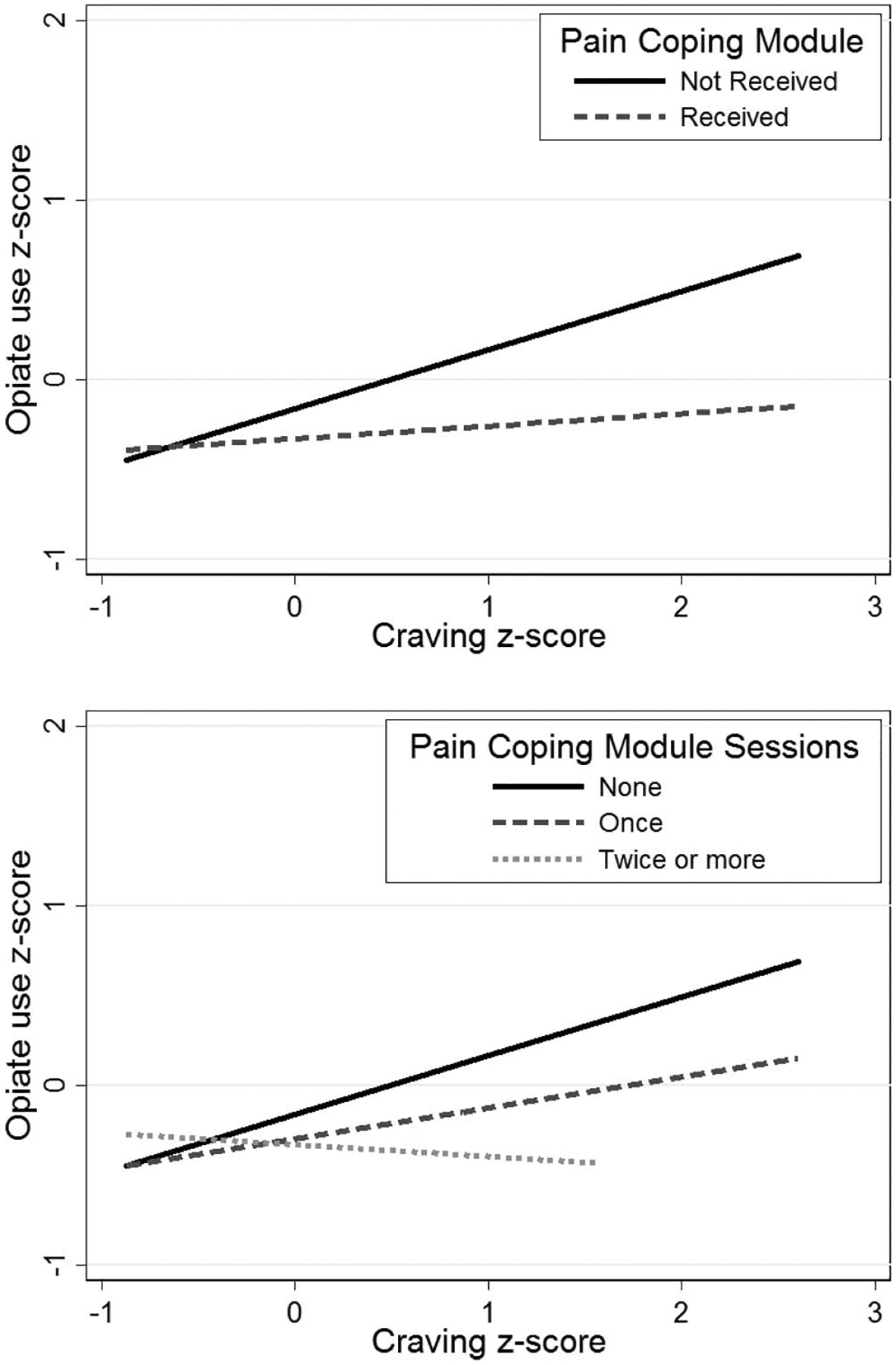
Standardized self-reported opiate use and craving for opioids by treatment condition and frequency of module engagement in opioid-addicted chronic pain patients (N = 148). Patients who received the pain coping module reported reduced opiate use and craving relative to those that did not receive the module and additional module repetitions increased these reductions.
Mechanism of Change: Dose-Response and Specificity
Further analyses tested additional criteria for mechanisms of change, including dose-response effects and specificity. Results revealed a significant interaction between the number of pain coping module sessions and craving (b = −0.12, p < .01, model R2 = 0.72), such that a larger “dose” of the pain coping skills counseling module was associated with a greater attenuation of craving’s effects on opioid use. As shown in Figure 3, similar results were observed when testing pain coping sessions as an ordinal variable indicating receipt of the model never, once, or more than once (b = −0.16, p < .01, model R2 = 0.72). We also examined specificity of the pain module effect by repeating the moderation analyses when controlling for total EMM session count to determine if findings could be attributed to a larger amount of EMM in general. When controlling for total EMM exposure, receipt of the pain coping module still attenuated the effects of craving on opioid use (b = −0.27, p < .01 model R2 = 0.70.), while greater total EMM exposure also significantly predicted lower levels of opioid use (b = −0.02, p < .05).
Discussion
This study examined relations between pain, craving, and a behavioral therapy that explained opioid use outcomes in adults with chronic pain and prescription opioid addiction. Treatment of prescription opioid addiction in adults with chronic pain is often challenging (Højsted & Sjøgren, 2007; Turk, Swanson, & Gatchel, 2008). Therefore, we conducted this study to identify mediators and moderators that explained variability in treatment outcomes in this population. Findings indicated that greater pain predicted greater subsequent use of opioids through the mediating effects of opioid craving. Furthermore, we found that the effects of craving on opioid use were attenuated after receiving a counseling module designed to improve behavioral pain coping skills. The effect of this interaction was so large that greater cravings no longer predicted opioid use after receiving the module, but greater cravings continued to predict opioid use if the pain coping skills module was never received. These findings provide the first known empirical evidence that, in adults with chronic pain and prescription opioid addiction, the risk for poor treatment outcomes associated with ongoing pain is largely due to cravings. Additionally, this maladaptive effect of craving could be mitigated by relatively brief counseling in behavioral pain coping skills.
Craving is a clinically significant aspect of SUD etiology and treatment. Prior studies of prescription opioid use found associations between pain, craving, severity of opioid use disorder, and relapse during treatment (Griffin et al., 2016; Northrup et al., 2015; Potter et al., 2015; Tsui et al., 2016). While prior studies of adults prescribed opioids for chronic pain have linked craving to a number of negative outcomes including aberrant drug behavior, abnormal urine drug screens, and pain catastrophizing (Martel et al., 2014; Wasan et al., 2009), our findings extend this knowledge to adults with chronic pain receiving treatment for prescription opioid addiction. Our study provides evidence that cravings mediate the effects of pain and present ongoing risk for opioid use even during opioid maintenance therapy. We observed a large mediated effect size between pain, craving, and future opioid use. This finding suggests that in chronic pain patients receiving treatment for opioid addiction, ongoing pain presents risk for future opioid use, and that this risk is primarily due to associated craving which has implications for treatment. For this population, ongoing pain during treatment may not impact future opioid use unless it is accompanied by ongoing opioid cravings. Ongoing pain accompanied by craving may be useful as an observable marker of poor treatment progress and should be addressed with tailored clinical approaches.
Prior studies provide empirical support for SUD interventions that target cravings either directly by reducing their frequency or intensity, or indirectly by attenuating the stimulus-response connection between craving and substance use (Bowen & Marlatt, 2009; Ehde et al., 2014; Wasan et al., 2012; Witkiewitz et al., 2013). The curriculum in the enhanced counseling condition of this sample included a session module on pain coping skills and we investigated whether the effects of craving on opioid use would be reduced after receiving this session. In the weeks following exposure to the pain coping module, the strong association between craving and future opioid use was nearly eliminated. Furthermore, additional repetitions of this module were associated with a greater reduction in the effects of craving, and this interaction was independent of greater attendance at counseling, in general. Unfortunately, we cannot draw causal inferences regarding the effects of the pain module, as exposure to this module was not randomized. However, the presence of a prospective, specific, dose-response effect of the pain coping module meets the most rigorous nonexperimental criteria established for evaluating a hypothesized mechanism of change (Kazdin & Nock, 2003; Longabaugh, 2007; Nock, 2007). These findings therefore provide preliminary empirical evidence to support behavioral pain coping skills as a beneficial treatment target for individuals with prescription opioid addiction and chronic pain. Interestingly, our findings do not imply behavioral pain coping will improve treatment outcomes via direct reduction in pain, as the pain coping module did not have significant direct effects on pain. Instead, the data suggest pain coping counseling may provide useful tools for this population to cope with cravings when they occur.
Although our findings should be considered preliminary, they may be useful in informing clinical practice and research in co-occurring prescription opioid addiction and chronic pain. In this sample the associations between pain and craving were relatively strong, comparable to a medium effect size. Still, the variance in these two measures did not overlap completely, and future studies may wish to investigate the processes that explain why some adults with chronic pain and opioid addiction experience stronger associations between pain and opioid cravings. In this study opioid maintenance therapy reduced overall cravings to a low level, but moderate cravings persisted in a subset of the sample. Future research may also seek to explain the underlying reasons that cravings persist in a subset of patients during opioid maintenance treatment. Our study suggests that when pain and cravings continue despite opioid substitution treatment, provision of counseling in pain coping skills may be useful in limiting risk for opioid use, with repeated exposure to such skills associated with greater reduction in risk. Prior research has also demonstrated the efficacy of delivering substance use or chronic pain interventions in primary care (Oslin et al., 2014; Wetherell et al., 2011), and our findings suggest future randomized studies may wish to test the efficacy of brief interventions focused on behavioral pain coping skills in adults with chronic pain and prescription opioid addiction. Such primary care-based interventions might be especially useful for both treatment and prevention in this population as chronic pain patients are predominantly treated within the primary-care setting (Guck, Burke, Rainville, Hill-Taylor, & Wallace, 2015; Haibach, Beehler, Dollar, & Finnell, 2014; Pade, Cardon, Hoffman, & Geppert, 2012). Finally, over half of the EMM group in this sample did not elect to receive the behavioral pain coping skills module, despite having chronic pain. This low level of exposure may indicate issues with desirability of behavioral pain treatment in this population which warrants additional examination.
Our findings should be considered in light of several limitations. This study was a secondary analysis to test aims that were not a primary focus of the original clinical trial. The most important limitation is that participants in EMM were not randomized to receive the pain module, and coverage of pain coping skills during treatment was subject to a collaborative decision by the counselor and participant. Therefore, although results from a number of different within-subjects analyses are supportive of the pain coping session having beneficial effects, it is possible that unobserved factors may have played some role in these results, and we cannot definitively conclude that receipt of the module itself caused moderation of craving effects. These findings should therefore be considered preliminary and require replication from an experimental design. The study sample did not have a broad range of ethnic diversity, which limits the generalizability of these findings. Pain and craving were both assessed using a single item assessed once per week. While effect sizes of results were promising given these single-item measures, both pain and craving are complex and dynamic, and future work in this area might benefit from measures that better capture the fluidity in these constructs. The original study was conducted in an established research network which may not accurately represent clinical practice in real-world settings, and the integrity of the treatments delivered is unknown. Finally, we found promising effects of a single behavioral pain coping skills module, but in the context of a multiweek counseling protocol along with weekly BUP-NLX management. Thus, further research is needed to confirm whether pain coping or other individual components of the intervention would be effective when delivered in isolation, brief formats, or without adjunctive pharmacotherapy.
In summary, findings from this study suggest that in adults with chronic pain receiving treatment for prescription opioid addiction, persistent and more severe pain is associated with opioid use during treatment primarily via the mechanism of increased cravings. In this population, counseling in pain coping skills may be effective in limiting the maladaptive impact of opioid cravings. Future research should confirm these findings by direct experimentation that tests the causal effects of interventions that target specific skills and comorbidities, which may be useful in deploying therapies to more accessible settings such as primary care. Dissemination of such optimized interventions will strengthen the overall public health capacity to prevent and treat prescription opioid addiction.
What is the public health significance of this article?
For persons with chronic pain receiving treatment for prescription opioid addiction, our study suggests pain-related cravings increase risk for continued opioid use. Results also suggest that counseling in behavioral pain coping skills reduces this risk.
Contributor Information
Bryan G. Messina, Veteran Affairs San Diego Healthcare System, San Diego, California
Matthew J. Worley, University of California, San Diego
References
- American Psychiatric Association. (1994). Diagnostic and statistical manual of mental disorders (4th ed.). Washington, DC: Author. [Google Scholar]
- Ashrafioun L, & Carels RA (2014). Prescription opioid use among university students: Assessment of post-cue exposure craving. Addictive Behaviors, 39, 586–592. 10.1016/j.addbeh.2013.11.012 [DOI] [PubMed] [Google Scholar]
- Bowen S, & Marlatt A (2009). Surfing the urge: Brief mindfulness-based intervention for college student smokers. Psychology of Addictive Behaviors, 23, 666–671. 10.1037/a0017127 [DOI] [PubMed] [Google Scholar]
- Carlsson AM (1983). Assessment of chronic pain. I. Aspects of the reliability and validity of the visual analogue scale. Pain, 16, 87–101. 10.1016/0304-3959(83)90088-X [DOI] [PubMed] [Google Scholar]
- Ehde DM, Dillworth TM, & Turner JA (2014). Cognitive-behavioral therapy for individuals with chronic pain: Efficacy, innovations, and directions for research. American Psychologist, 69, 153–166. 10.1037/a0035747 [DOI] [PubMed] [Google Scholar]
- Evans CJ, & Cahill CM (2016). Neurobiology of opioid dependence in creating addiction vulnerability. F1000 Research, 5, 1748 10.12688/f1000research.8369.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Froeliger B, Zeidan F, Partin K, & Howard MO (2013). The downward spiral of chronic pain, prescription opioid misuse, and addiction: Cognitive, affective, and neuropsychopharmacologic pathways. Neuroscience and Biobehavioral Reviews, 37, 2597–2607. 10.1016/j.neubiorev.2013.08.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garland EL, Thomas E, & Howard MO (2014). Mindfulness-oriented recovery enhancement ameliorates the impact of pain on self-reported psychological and physical function among opioid-using chronic pain patients. Journal of Pain and Symptom Management, 48, 1091–1099. 10.1016/j.jpainsymman.2014.03.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin ML, McDermott KA, McHugh RK, Fitzmaurice GM, Jamison RN, & Weiss RD (2016). Longitudinal association between pain severity and subsequent opioid use in prescription opioid dependent patients with chronic pain. Drug and Alcohol Dependence, 163, 216–221. 10.1016/j.drugalcdep.2016.04.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guck TP, Burke RV, Rainville C, Hill-Taylor D, & Wallace DP (2015). A brief primary care intervention to reduce fear of movement in chronic low back pain patients. Translational Behavioral Medicine, 5, 113–121. 10.1007/s13142-014-0292-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haibach JP, Beehler GP, Dollar KM, & Finnell DS (2014). Moving toward integrated behavioral intervention for treating multimorbidity among chronic pain, depression, and substance-use disorders in primary care. Medical Care, 52, 322–327. 10.1097/MLR.0000000000000098 [DOI] [PubMed] [Google Scholar]
- Højsted J, & Sjøgren P (2007). Addiction to opioids in chronic pain patients: A literature review. European Journal of Pain, 11, 490–518. 10.1016/j.ejpain.2006.08.004 [DOI] [PubMed] [Google Scholar]
- Huhn AS, Harris J, Cleveland HH, Lydon DM, Stankoski D, Cleveland MJ, … Bunce SC (2016). Ecological momentary assessment of affect and craving in patients in treatment for prescription opioid dependence. Brain Research Bulletin, 123, 94–101. 10.1016/j.brainresbull.2016.01.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kazdin AE, & Nock MK (2003). Delineating mechanisms of change in child and adolescent therapy: Methodological issues and research recommendations. Journal of Child Psychology and Psychiatry, 44, 1116–1129. 10.1111/1469-7610.00195 [DOI] [PubMed] [Google Scholar]
- Keller S, Bann CM, Dodd SL, Schein J, Mendoza TR, & Cleeland CS (2004). Validity of the brief pain inventory for use in documenting the outcomes of patients with noncancer pain. The Clinical Journal of Pain, 20, 309–318. 10.1097/00002508-200409000-00005 [DOI] [PubMed] [Google Scholar]
- Krull JL, & MacKinnon DP (2001). Multilevel modeling of individual and group level mediated effects. Multivariate Behavioral Research, 36, 249–277. 10.1207/S15327906MBR3602_06 [DOI] [PubMed] [Google Scholar]
- Longabaugh R (2007). The search for mechanisms of change in behavioral treatments for alcohol use disorders: A commentary. Alcoholism: Clinical and Experimental Research, 31, 21s–32s. 10.1111/j.1530-0277.2007.00490.x [DOI] [PubMed] [Google Scholar]
- MacKillop J, Miranda R Jr., Monti PM, Ray LA, Murphy JG, Rohsenow DJ, … Gwaltney CJ (2010). Alcohol demand, delayed reward discounting, and craving in relation to drinking and alcohol use disorders. Journal of Abnormal Psychology, 119, 106–114. 10.1037/a0017513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel MO, Jamison RN, Wasan AD, & Edwards RR (2014). The association between catastrophizing and craving in patients with chronic pain prescribed opioid therapy: A preliminary analysis. Pain Medicine, 15, 1757–1764. 10.1111/pme.12416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh RK, Fitzmaurice GM, Carroll KM, Griffin ML, Hill KP, Wasan AD, & Weiss RD (2014). Assessing craving and its relationship to subsequent prescription opioid use among treatment-seeking prescription opioid dependent patients. Drug and Alcohol Dependence, 145, 121–126. 10.1016/j.drugalcdep.2014.10.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navratilova E, Xie JY, Okun A, Qu C, Eyde N, Ci S, … Porreca F (2012). Pain relief produces negative reinforcement through activation of mesolimbic reward-valuation circuitry. Proceedings of the National Academy of Sciences of the United States of America, 109, 20709–20713. 10.1073/pnas.1214605109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann AM, Blondell RD, Jaanimägi U, Giambrone AK, Homish GG, Lozano JR, … Azadfard M (2013). A preliminary study comparing methadone and buprenorphine in patients with chronic pain and coexistent opioid addiction. Journal of Addictive Diseases, 32, 68–78. 10.1080/10550887.2012.759872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nock MK (2007). Conceptual and design essentials for evaluating mechanisms of change. Alcoholism: Clinical and Experimental Research, 31, 4s–12s. 10.1111/j.1530-0277.2007.00488.x [DOI] [PubMed] [Google Scholar]
- Northrup TF, Stotts AL, Green C, Potter JS, Marino EN, Walker R, … Trivedi M (2015). Opioid withdrawal, craving, and use during and after outpatient buprenorphine stabilization and taper: A discrete survival and growth mixture model. Addictive Behaviors, 41, 20–28. 10.1016/j.addbeh.2014.09.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Connor PG, Oliveto AH, Shi JM, Triffleman EG, Carroll KM, Kosten TR, … Schottenfeld RS (1998). A randomized trial of buprenorphine maintenance for heroin dependence in a primary care clinic for substance users versus a methadone clinic. The American Journal of Medicine, 105, 100–105. 10.1016/S0002-9343(98)00194-6 [DOI] [PubMed] [Google Scholar]
- Oslin DW, Lynch KG, Maisto SA, Lantinga LJ, McKay JR, Possemato K, … Wierzbicki M (2014). A randomized clinical trial of alcohol care management delivered in Department of Veterans Affairs primary care clinics versus specialty addiction treatment. Journal of General Internal Medicine, 29, 162–168. 10.1007/s11606-013-2625-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pade PA, Cardon KE, Hoffman RM, & Geppert CMA (2012). Prescription opioid abuse, chronic pain, and primary care: A co-occurring disorders clinic in the chronic disease model. Journal of Substance Abuse Treatment, 43, 446–450. 10.1016/j.jsat.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Pantalon MV, Fiellin DA, Schottenfeld RS, Gordon L, & O’Connor PG (1999). Manual for enhanced medical management of opioid dependence with buprenorphine. New Haven, CT: Yale University Press. [Google Scholar]
- Potter JS, Dreifuss JA, Marino EN, Provost SE, Dodd DR, Rice LS, … Weiss RD (2015). The multi-site Prescription Opioid Addiction Treatment Study: 18-month outcomes. Journal of Substance Abuse Treatment, 48, 62–69. 10.1016/j.jsat.2014.07.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robins LN, Wing J, Wittchen HU, Helzer JE, Babor TF, Burke J, … Towle LH (1988). The Composite International Diagnostic Interview. An epidemiologic Instrument suitable for use in conjunction with different diagnostic systems and in different cultures. Archives of General Psychiatry, 45, 1069–1077. 10.1001/archpsyc.1988.01800360017003 [DOI] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2014). Results from the 2013 National Survey on Drug Use and Heath: Vol. 1. Summary of national findings. Rockville, MD: Author. [Google Scholar]
- Schafer JL, & Graham JW (2002). Missing data: Our view of the state of the art. Psychological Methods, 7, 147–177. 10.1037/1082-989X.7.2.147 [DOI] [PubMed] [Google Scholar]
- Sobell LC, & Sobell MB (1992). Timeline follow-back: A technique for assessing self-reported alcohol consumption In Litten RZ & Allen JP (Eds.), Measuring alcohol consumption: Psychosocial and biochemical methods (pp. 41–72). Totowa, NJ: Humana Press; 10.1007/978-1-4612-0357-5_3 [DOI] [Google Scholar]
- StataCorp. (2016). Stata MP 14.2 for Windows 64-bit. College Station, TX: Author. [Google Scholar]
- Sullivan MD, Edlund MJ, Fan M-Y, Devries A, Braden JB, & Martin BC (2008). Trends in use of opioids for non-cancer pain conditions 2000–2005 in commercial and Medicaid insurance plans: The TROUP study. Pain, 138, 440–449. 10.1016/j.pain.2008.04.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan MD, & Howe CQ (2013). Opioid therapy for chronic pain in the U. S.: Promises and perils. Pain, 154, S94–S100. 10.1016/j.pain.2013.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Anderson BJ, Strong DR, & Stein MD (2014). Craving predicts opioid use in opioid-dependent patients initiating buprenorphine treatment: A longitudinal study. The American Journal of Drug and Alcohol Abuse, 40, 163–169. 10.3109/00952990.2013.848875 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsui JI, Lira MC, Cheng DM, Winter MR, Alford DP, Liebschutz JM, … Samet JH (2016). Chronic pain, craving, and illicit opioid use among patients receiving opioid agonist therapy. Drug and Alcohol Dependence, 166, 26–31. 10.1016/j.drugalcdep.2016.06.024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk DC, Swanson KS, & Gatchel RJ (2008). Predicting opioid misuse by chronic pain patients: A systematic review and literature synthesis. The Clinical Journal of Pain, 24, 497–508. 10.1097/AJP.0b013e31816b1070 [DOI] [PubMed] [Google Scholar]
- Veilleux JC, Colvin PJ, Anderson J, York C, & Heinz AJ (2010). A review of opioid dependence treatment: Pharmacological and psychosocial interventions to treat opioid addiction. Clinical Psychology Review, 30, 155–166. 10.1016/j.cpr.2009.10.006 [DOI] [PubMed] [Google Scholar]
- Wasan AD, Butler SF, Budman SH, Fernandez K, Weiss RD, Greenfield SF, & Jamison RN (2009). Does report of craving opioid medication predict aberrant drug behavior among chronic pain patients? The Clinical Journal of Pain, 25, 193–198. 10.1097/AJP.0b013e318193a6c4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wasan AD, Ross EL, Michna E, Chibnik L, Greenfield SF, Weiss RD, & Jamison RN (2012). Craving of prescription opioids in patients with chronic pain: A longitudinal outcomes trial. The Journal of Pain, 13, 146–154. 10.1016/j.jpain.2011.10.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, … Ling W (2011). Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: A 2-phase randomized controlled trial. Archives of General Psychiatry, 68, 1238–1246. 10.1001/archgenpsychiatry.2011.121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Griffin ML, Provost SE, Fitzmaurice GM, McDermott KA, … Carroll KM (2015). Long-term outcomes from the National Drug Abuse Treatment Clinical Trials Network Prescription Opioid Addiction Treatment Study. Drug and Alcohol Dependence, 150, 112–119. 10.1016/j.drugalcdep.2015.02.030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, … Ling W (2010). A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): Rationale, design, and methodology. Contemporary Clinical Trials, 31, 189–199. 10.1016/j.cct.2010.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wesson DR, & Ling W (2003). The Clinical Opiate Withdrawal Scale (COWS). Journal of Psychoactive Drugs, 35, 253–259. 10.1080/02791072.2003.10400007 [DOI] [PubMed] [Google Scholar]
- Wetherell JL, Afari N, Rutledge T, Sorrell JT, Stoddard JA, Petkus AJ, … Atkinson JH (2011). A randomized, controlled trial of acceptance and commitment therapy and cognitive-behavioral therapy for chronic pain. Pain, 152, 2098–2107. 10.1016/j.pain.2011.05.016 [DOI] [PubMed] [Google Scholar]
- de, C. Williams AC, , Eccleston C, & Morley S (2012). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database of Systematic Reviews, 11, CD007407 10.1002/14651858.CD007407.pub3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, & Bowen S (2010). Depression, craving, and substance use following a randomized trial of mindfulness-based relapse prevention. Journal of Consulting and Clinical Psychology, 78, 362–374. 10.1037/a0019172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witkiewitz K, Bowen S, Douglas H, & Hsu SH (2013). Mindfulness-based relapse prevention for substance craving. Addictive Behaviors, 38, 1563–1571. 10.1016/j.addbeh.2012.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]


