Abstract
Background
The potential benefit of cysteamine for Huntington’s disease has been demonstrated in HD animal models. Cysteamine and its derivate cystamine were shown to reduce neuropathology and prolong lifespan. Human studies have demonstrated safety, and suggestive results regarding efficacy. Despite all the studies available in vivo, there are only few in vitro studies, and the mechanism of action of cysteamine remains unclear.
Objective
The objective of this study is to assess the capacity of cysteamine for neuroprotection against mutant Huntingtin in vitro using cellular models of HD, and to provide initial data regarding mechanism of action.
Methods
We tested the neuroprotective properties of cysteamine in vitro in our primary neuron and iPSC models of HD.
Results
Cysteamine showed a strong neuroprotective effect (EC50 = 7.1 nM) against mutant Htt-(aa-1–586 82Q) toxicity, in a nuclear condensation cell toxicity assay. Cysteamine also rescued mitochondrial changes induced by mutant Htt. Modulation of the levels of cysteine or glutathione failed to protect neurons, suggesting that cysteamine neuroprotection is not mediated through cysteine metabolism. Taurine and Hypotaurine, which are metabolites of cysteamine can protect neurons against Htt toxicity, but the inhibition of the enzyme converting cysteamine to hypotaurine does not block either protective activity, suggesting independent protective pathways. Cysteamine has been suggested to activate BDNF secretion; however, cysteamine protection was not blocked by BDNF pathway antagonists.
Conclusions
Cysteamine was strongly neuroprotective with relatively high potency. We demonstrated that the main neuroprotective pathways that have been proposed to be the mechanism of protection by cysteamine can all be blocked and still not prevent the neuroprotective effect. The results suggest the involvement of other yet-to-be-determined neuroprotective pathways.
Keywords: Huntington’s disease, huntingtin toxicity, primary neurons, neurodegeneration, neuroprotection, cysteamine, patient-derived induced pluripotent stem cells
INTRODUCTION
Huntington’s disease (HD) is an inherited neurodegenerative disease characterized by motor dysfunction, behavioral impairments and cognitive dysfunction. The disease is progressive and fatal, and no disease-modifying therapeutic treatment currently available [1]. Cysteamine is a cell metabolite that has been used for decades in clinic to treat nephropathic cystinosis due to its cystine lowering properties [2]. Cysteamine has been shown protective in several mouse models of HD. Several studies demonstrated beneficial effects of cysteamine in the R6/2 mouse model [3–6]. In these studies, cysteamine caused amelioration of weight loss and motor abnormalities, and prolongation of survival. Another study has shown benefits in the R6/1 mouse model [7]. In this study, one week of cysteamine injection prevented the loss of DARP32 positive cells in the striatum of the HD mice. Finally, cysteamine has also been shown to be protective in a full-length transgenic model, the YAC128 mouse model [5]. In this study, cysteamine treatment ameliorated the striatal phenotype of the YAC128 mice by reducing the atrophy and the cell loss. However, the motor function of the treated mice remained impaired.
In human, the CYST-HD study group recently published the results of their trial with cysteamine [8]. In this study, cysteamine proved to be safe and well tolerated by patients with HD. After 18 months of treatment, the efficacy of the drug was not significant for the complete cohort. However, a post-hoc analysis of sub-groups of patients sorted by their initial Total Motor Scores, suggest that the treatment significantly reduced the rate of progression of the disease of patients with the most severe impairment at the beginning of the study.
A number of mechanisms have been proposed for the effects of cysteamine. Initial studies suggested that cysteamine action as a transglutaminase inhibitor (e.g., [3]) was responsible for the cell survival. However, Bailey and Johnson demonstrated that knocking out transglutaminase in the R6/2 mouse did not affect the protection by cysteamine thus proving that the mechanism must be independent of inhibition of transglutaminase [6]. It has also been suggested that cystamine may act through inhibition of caspases [9]. However, caspase activation is likely to be at relatively late stage in HD pathogenesis, and, while caspase cleavage of huntingtin has been proposed to be a pathogenic mechanism, this would not be relevant for the exon 1 fragment transgenics.
Another interesting hypothesis is that cysteamine or cystamine may act via BDNF. Alterations of BDNF are well documented in HD pathogenesis [10, 11] and cysteamine strikingly increased levels of BDNF [7]. When HD mice were crossed with mice heterozygous for a deletion of BDNF, cysteamine became less effective consistent with the idea that the mechanism of action involves BDNF. Interestingly, cysteamine increases blood levels of BDNF in both rodents and in a primate HD models, suggesting that peripheral BDNF could be used as a biomarker of treatment effect.
Finally, a possible mechanism for the effect of cysteamine might be a modulation of the cysteine metabolism. Cysteine has a number of potential beneficial effects, including increasing levels of the antioxidant glutathione (GSH) and hypotaurine [12]. It has been suggested by the Snyder group that cysteine itself can be neuroprotective in HD [13]. Cysteamine and cystamine increases levels of L-cysteine in R6/2 [14] and YAC128 mice [15].
In this study, we characterized the protective effect of cysteamine on cellular models of HD. We measured the effects of cysteamine treatments on primary neuronal survival, morphology and mitochondrial integrity. We were able to demonstrate a potent neuroprotection by both cysteamine and cystamine. By using further treatments to modulate the main pathways previously hypothesized to be responsible for the protective effect induced by cysteamine, we showed that the mechanism of action of cysteamine likely does not involve the mechanisms mentioned above, but may involve as yet unidentified pathways.
MATERIALS AND METHODS
Primary neuron preparation
Primary neurons from CD1 mice were prepared from E15 to E17 embryos. Cells from both cortex and striatum were independently prepared from the same embryos. and plated at 106 cells/mm2 in Neurobasal medium with 2% B27, 2mM Glutamax and 1% Pen/strep in 24-well plate. Cells were maintained at 37°C/5% CO2 throughout the experiments. All cell culture supplies were obtained from Corning and all the media were from ThermoFisher Scientific.
All the animals used in this study were taken care of according to the guidelines for animal experimentation. All experimental protocol involving animals were approved by the protocol approval by the Johns Hopkins Institutional Animal Care and Use Committee (Protocol number: MO15M341).
Transfection of primary neurons
Neurons were co-transfected using Lipofectamine 2000 (ThermoFisher Scientific) at day in vitro (DIV) 5 or 6. Per well of 24 well plates, 1 μg of Htt plasmid and 0.1 μg GFP was used with a ratio DNA/Lipofectamine of 1/1.75. DNA and Lipofectamine were diluted separately in 50 μL OptiMEM per well for each and incubated for 5 min. The combined transfecting solution is incubated for 90 min at room temperature before 100 μL of transfection solution is deposited in each well along with 400 μL of OptiMEM. After 4 h 30 min, the transfection medium is replaced with fresh Neurobasal medium.
Compound treatments
All the chemical compounds were purchased from Millipore-Sigma. For protection experiments, cells were treated with the described compounds at the time of the transfection. Working concentration for cysteamine and cystamine was 1 μM.
All the cysteine pathway metabolites: L-cysteine, D-cysteine, NAc-L-cysteine and Glutathione were used at 1 mM. For the cysteamine downstream pathway experiment, 8HQ was used at 5mM and Hypotaurine and Taurine were used at 1 μM. For the BDNF pathway experiment, ANA12 was used at 10 μM, K252a 1 μM and BDNF at 20 ng/mL.
Nuclear condensation cell death assay
The toxicity experiments were performed in primary cortical and primary striatal neurons according to our established protocol [16]. Nuclei were stained using Hoechst 3342 (Sigma, 0.2 μg/mL in PBS for 5 mn). Automated picture acquisition was performed using a Zeiss Axiovert 200 inverted microscope with a 10x objective. The integrality of the surface of the wells is imaged. Automatic quantification of the nuclear intensity of transfected cells was performed using Volocity. Cells were considered dead when their nuclear intensity was higher than the average intensity plus two standard deviations. Each condition was performed in quadruplicate within each experiment and each experiment was repeated in at least 4 independent neuronal preparations for each construct studied. Overall, a total of at least 6500 cells were counted in each condition.
Time-lapse imaging of cell death
Primary cortical neurons were transfected with GFP and Htt constructs as described above. 24 h after transfection, neurons expressing GFP were randomly chosen, and imaged every 20 min for 10 h. Morphology of every neuron at each frame was quantified blindly using Image J. Neurons were quantified as 100 when alive and 0 when dead. Cells were considered dead when most of the neurites detached from the soma or were fragmented, or when the soma became totally round. Results are expressed as mean ± sem. n = 200 cells analyzed in 3 independent experiments.
Mitochondrial function assays
Primary cortical neurons were co-transfected at DIV5 with the full-length Htt constructs and GFP, as described above. 24 h after transfection the cells were loaded with 20 nM Tetramethyl rhodamine methyl ester (TMRM) stain for 45 min at 37°C. Average measure of the TMRM intensity of transfected cells was quantified using Volocity. For mitochondrial size measurement of the mitochondria, primary cortical neurons were co-transfected at DIV5 with the full-length Htt constructs and GFP, as described above. 24 h after transfection, the cells were loaded with 500 nM Mitotracker Red (Molecular probes) for 45 min at 37°C. After 3 washes with PBS, the cells were fixed with 4% Paraformaldehyde/1x PBS for 30 min. Cells were imaged on a laser-scanning confocal Zeiss LSM 510-meta microscope. Average sizes of individual mitochondria of transfected cells were measured using Volocity. Results were normalized to the average size of mitochondria of cells transfected with Htt −82Q.
iPSC propagation and neural differentiation
Generation and characterization of HD 109Qn1 and Control 21Qn2 iPSC lines have been described before [17, 18]. For neural differentiation, iPSC colonies grown on Matrigel in TeSR media (feeder-free) were converted into non-adherent neural progenitor colonies termed “EZ-spheres” using previously established protocol [19]. Briefly, iPS colonies were lifted using 2 mg/ml Collagenase IV solution in DMEM and propagated in Stemline NSC medium (Sigma S3194) supplied with bFGF, EGF (100 ng/ml each, Peprotech) and 5 μg/ml in non-adherent tissue culture flasks. The “spheres” were mechanically chopped for propagation weekly. To induce neural differentiation the “spheres” were transferred into Neural Induction Medium (NIM: 1% N2 in DMEM:F12, Invitrogen) for 5 days. Then, BDNF (20 ng/ml; Peprotech 450–02) was added for 2 days. For the next 21 days, cells were differentiated in 20 ng/ml BDNF (20 ng/ml; Peprotech 450–02), rhSHH (200 ng/ml; Peprotech 100–45), and DKK1 (100 ng/ml; Peprotech 120–30) to promote a rostral forebrain fate. Finally, cells were matured in 20 ng/ml BDNF, dibutyryl cyclic AMP (dbcAMP, 0.5 mM; Sigma D0260) and valproic acid (VPA, 0.5 mM; Sigma P4546) for 14 days. Medium was half-changed three times per week or as needed. Cells were plated upon Matrigel coated coverslips for imaging or into 48-well Matrigel coated plates for toxicity assay on day 26 of differentiation.
For the induction of cell toxicity, differentiation medium was replaced with BDNF-free NIM for 48 h in 48-well plates after 42 days of differentiation. To test effect of cysteamine and other compounds on cell survival NIM was supplied with appropriate compounds for all time of BDNF withdrawal. 20 ng/ml BDNF were used as a positive control.
Statistical analysis
Quantitative data were obtained on at least three separate neuronal preparations. Statistically significant differences among groups were analyzed by ANOVA using SigmaPlot. A p value of 0.05 or lower was considered significant. Post-hoc comparisons were performed using the Bonferroni method.
RESULTS
Neuroprotective properties of cysteamine in primary cortical neurons
The toxicity of mHtt in vitro has been previously demonstrated in many cell types including primary neurons. We have established a primary neuron system of transfection to measure mHtt (using mHtt fragments as well as the full-length protein) toxicity by nuclear condensation assay [16, 20]. In our assay, we can monitor significant and reliable cell death, and determine protective properties of compounds and small molecules (for examples [21, 22]).
We first established the effects of cysteamine in our in vitro neuronal toxicity assay. Cortical primary neurons were transfected at with plasmids containing the fragment of Htt containing the first 586 amino acids of the protein (Htt N586) and containing either 22 glutamines (Htt N586 22Q) or 82 glutamines (Htt N586 82Q). After 48 h of transfection, Htt N586 82Q induces a strong toxicity. Neurons transfected with Htt N586 82Q showed a condensation of the nucleus as well as a neurite fragmentation as visualized by the GFP co-transfection and a disappearance of MAP2 (Fig. 1A). When the amount of cell death is quantified by nuclear condensation, we observed a strong toxicity of Htt N586 82Q after 48 h (Fig. 1B, left bars). Cysteamine caused striking, dose-dependent protection with an EC50 around 10 nM. Maximum protection is reached between 0.1 and 1 μM (Fig. 1B), and no toxicity of cysteamine itself was observed even at higher doses, up to 50 μM.
Fig. 1.
Cysteamine protects primary cortical neurons from mHtt toxicity. Primary cortical neurons were transfected at 7 DIV with either Htt N586 82Q or Htt N586 22Q and treated with different doses of cysteamine. After 48 h, neurons were fixed and stained with DAPI and MAP2 was immunodetected. A) Representative pictures of transfected neurons. Scale bar: 25 μm. B) mHtt toxicity measured by nuclear condensation assay was reduced by cysteamine, Results presented as means ± S.E. of the percentage of dead cells. *p < 0.05 vs Htt N586 82Q by ANOVA with Bonferroni post-hoc test. (n = 4 independent neuronal preparations).
Cysteamine protects neurons against functional and morphological changes
In addition to the toxicity assay, we also conduted a series of functional measurments of morphological integrity and mitochondrial parameters of transfected primary neurons. The results of protection by cysteamine measured by nuclear condensation assay were confirmed by time lapse imaging. Neurons were transfected according to the same protocol than for the toxicity assay, and live neurons were imaged over time (Fig. 2A). At the start of the experiment, 24 h after transfections, the cells are well expressing GFP and all present a normal morphology with multiple extended neurites and a bright soma. Cells that were transfected with mHtt showed a rapid degradation of their mohphology with a neurite retraction and fragmentation, whereas the neurons transfected with Htt 22Q remained stable throughout the experiments. Adding 1 μM of cysteamine to the medium was able to protect the cells from morphological changes induced by mHtt. Morphology of each cell was evaluated for each time point, and the neurons were considered dead when they presented retraction or breaks in a majority of the neurites and/or a rounding of the soma (Fig. 2A).
Fig. 2.
Cysteamine protects from morphological changes induced by mHtt. Primary cortical neurons were transfected at 7 DIV with either Htt N586 82Q, Htt N586 22Q or GFP alone and treated with 1 μM cysteamine. A) Representative images of GFP transfected neurons at 0, 5, and 10 h of live cell imaging. Scale bar: 50 μm. B) Quantification of survival over time by time lapse imaging morphological analysis. Transfected cells were identified live on the microscope and imaged every 15 min for 12 h. Cells are evaluated as 100 if alive and 0 dead. Results are presented as means ± S.E. of cell survival over time (n = 100 individual neurons from N = 5 independent experiments).
During cell death induced by mHtt, there is a loss of mitochondrial potential as well as a general swelling of the mitochondria. We previously showed that mHtt effects on mitochondria can be reverted [20]. Using TMRM, a mitochondrial dye which intensity is proportinal to the mitochondrial potential, we showed that primary cortical neurons transfected with N586 82Q are losing their potential 24 h after transfection as illustrated in Fig. 3A. In comparison, neurons transfected with N586 22Q presented a similar potential than when transfected with GFP alone. When quantified and normalized to control cells, N586 82Q induced a decrease of the potential by 30%. When treated with cysteamine, mitochondria are protected from mHtt toxicity and are able to maintain a normal potential (Fig. 3B).
Fig. 3.
Cysteamine protects primary striatum neurons from mitochondria impairments induced by mHtt. A, B) Measurement of mitochondrial potential using the potential sensitive mitochondrial dye TMRM staining. 24 h after transfections, cells were loaded with 100 nM TMRM for 45 min before individual transfected cells were imaged. A) Examples of cells stained with TMRM. Scale bar: 10 μm. B) Intensity quantification was done using Volocity and the data was normalized to the GFP only transfected cells. Results presented as means ± S.E. of normalized potential. *p < 0.05 vs Htt N586 22Q; #p < 0.05 vs Htt N586 82Q by ANOVA with Bonferroni post-hoc test. (n = 6 independent neuronal preparations). C, D) Measurement of mitochondrial size using Mitotracker. 24 h after transfections, cells were loaded for 45 min with 500 nM Mitotracker for 45 min before fixation and individual transfected cells were imaged. A) Examples of cells stained with Mitotracker. Scale bar: 10 μm. B) Size quantification was done using Volocity and the data was normalized to the GFP only transfected cells. Results presented as means ± S.E. of normalized size. *p < 0.05 vs Htt N586 22Q; #p < 0.05 vs Htt N586 82Q by ANOVA with Bonferroni post-hoc test. (n = 6 independent neuronal preparations).
Using Mitotracker to stain all the mitochondria, we showed that 24 h after transfection, the mitochondria of cells transfected with N586 82Q showed signs of swelling, the average size of the mitochondria being increased compared to control (Fig. 3C). When quantified and normalized to control, N586 82Q induced a increase of mitonchondiral size by about 25%. Cysteamine treatment can protect the cells from mHtt toxicity and cells do not show any sign of swelling (Fig. 3D).
Cysteamine protects primary striatal neurons in vitro
In addition to the primary cortical neurons, we also tested the effects of cysteamine on mHtt induced toxicity in primary striatal neurons. In a comparable fashion, Htt N586 82Q overexpressed in striatal neurons induced a significant cell death as observed by nuclear condensation assay (Fig. 4A). Cysteamine protected the neurons at concentrations as low as 10 nM, and no toxicity was observed for higher doses treatments. Similarly to cortical neurons, the protection by cysteamine was complete. The mitochondrial alterations observed in cortical neurons was also reproduced in striatal neurons. Expression of mHtt induced a loss of potential as well as a swelling of the mitochondria. A 1 μM cysteamine treatment was able to completely prevent the impairment of the mitochondria (Fig. 4B, C) suggesting that cysteamine not only prevents neuron from death but also maintains mitochondrial function.
Fig. 4.
Cysteamine protects primary striatum neurons from mHtt toxicity. Primary striatal neurons were transfected at 7 DIV with either Htt N586 82Q or Htt N586 22Q and treated with cysteamine. Htt toxicity was reduced by cysteamine treatment as measured by (A) nuclear condensation, (B) measurement of mitochondrial potential using TMRM staining and (C) measurement of mitochondrial size using Mitotracker. *p < 0.05 vs N86–22Q, #p < 0.05 vs N586–82Q by ANOVA with Bonferroni post-hoc test. (n = 4 independent neuronal preparations).
Cysteamine metabolites protection from mHtt toxicity
To test the mechanism of action of cysteamine, we first investigated the cellular metabolic pathways in which cysteamine is involved and that were previously described as potentially neurprotective.
First, we investigated the cysteine metabolism. We asked wether cysteamine can act through a direct increase of L-cysteine transport in the cells as suggested by the Snyder lab [13]. We performed the experiment of in vitro toxicity of Htt N586 82Q in presence of 1 mM of L-cysteine. The results (Fig. 5A) show that the addition of L-cysteine or the control D-cysteine did not reduce significantly the Htt toxicity. When tested at different concentrations, these compounds did not induce a significant protection or toxicity up to 100 mM (Supplementary Figure 1A and B).
Fig. 5.
Cysteamine metabolites protection from mHtt toxicity. Primary cortical neurons were transfected at 7 DIV with either Htt N586 82Q or Htt N586 22Q and treated with cell metabolites. A) From the cysteine pathway. Either 1 mM L-cysteine, D-cysteine, NAc-L-cysteine or GSH for 48 h. B) From the Taurine pathway. Either 1 μM cysteamine, hypotaurine or taurine in presence or absence of 5 mM 8HQ for 48 h. Cell death was measured by nuclear condensation assay. *p < 0.05 vs N586–22Q by ANOVA with Bonferroni post-hoc test. (n = 4 independent neuronal preparations).
Another possible level of action of cysteamine in the cysteine metabolism is through an increase of available glutathione (GSH) [2]. In order to test this hypothesis, we performed the nuclear condensation experiment using increasing doses of GSH or NAc-L-Cysteine to assay their protective capacity in our system. The results (Fig. 5A) show that neither GSH nor NAc-L-Cysteine were protective against Htt toxicity in our system. When tested at different concentrations, these compounds did not induce a significant protection or toxicity up to 100 mM (Supplementary Figure 1C and D).
Taken together, since cysteamine treatment in our system cannot be replaced by any compounds of the cysteine metabolism, it is not likely that the cysteamine neuroprotection goes through its effects on cysteine and its derivates.
Although cysteamine can be produced from several sources from the cysteine or the pyruvate metabolism, it is processed by only one enzyme. Cysteamine dioxygenase is the downstream enzyme that converts cysteamine into hypotaurine which can be further metabolized into taurine. Hypotaurine and taurine have been shown to be neuroprotective under many conditions [23]. We first tested if in our system, treatment with taurine or hypotaurine could protect against Htt toxicity at similar levels than cysteamine. As shown on Fig. 5B, both hypotaurine and taurine were capable of protecting the neurons against N586 Htt 82Q toxicity. This result suggests the possibility of a common pathway for protection by the 3 compounds. To test this hypothesis further, we reproduced the experiment in presence of an inhibitor (8-hydroxyquinoline (8 HQ)) of cysteamine dioxygenase, the enzyme that converts cysteamine to hypotaurine. A dose response of the inhibitor showed that the inhibitor became toxic to the cells at 10 mM and above (Supplementary Figure 2). At 5 mM, the inhibitor was tolerated by the cells and it was reported to be sufficient to achieve almost complete inhibition [24]. The addition of 5 mM of 8HQ alone did not change the toxicity of Htt 82Q. However, it did not change the rescue effect of the treatment with taurine, hypotaurine or cysteamine. This result suggests that the protective mechanisms of cysteamine is independent from the protective effect of taurine and hypotaurine.
BDNF pathway neuroprotective effect against mHtt toxicity
Another proposed mechanism of action for cysteamine is through an increase of BDNF production and the activation of its downstream pathway. To test this hypothesis, we performed the cysteamine protection experiment in presence of the TrkB antagonists ANA12 and K252a (Fig. 6). These small molecules can inhibit BDNF neuroprotection in vitro [25, 26]. Neither K252a nor ANA-12 on primary neurons alone modulated the toxicity induced by N586 82Q. The protection induced by BDNF was abolished by both inhibitors. Cysteamine protective effect, on the other hand was not changed when TrkB was inhibited either by K252a or ANA12. These experiments suggest that the protective effect of cysteamine does not go through an activation of TrkB receptor.
Fig. 6.
Cysteamine neuroprotection does not require BDNF pathway activation. Primary cortical neurons were transfected at 7 DIV with either Htt N586 82Q or Htt N586 22Q and treated with either 1 μM cysteamine or 20 ng/mL BDNF. Cell death was measured by nuclear condensation assay after 48 h with cotreatment with 10 μM ANA12 (A) or 1 μM K252a (B). *p < 0.05 vs N586–22Q by ANOVA with Bonferroni post-hoc test. (n = 6 independent neuronal preparations).
Cystamine is also neuroprotective
In previous studies, cysteamine and cystamine have been often used interchangeably, and rare are the studies comparing both. To determine if cysteamine and cystamine can produce the same neuroprotection, we repeated the experiments using cystamine (Fig. 7). Cystamine, just as cysteamine, showed neuroprotective properties against mHtt toxicity in primary cortical neurons. The efficiency of cystamine was comparable to (or slightly less than) the efficiency of cystamine, with a significant protection for concentrations around 10–50 nM (Fig. 7A). In presence of 8HQ to block the conversion of cysteamine to hypotaurine, cystamine was still neuroprotective, just like cysteamine (Fig. 7B). The protection by cystamine was not blocked by ANA12 either, suggesting that the BDNF pathway is not involved in cystamine neuroprotection (Fig. 7C). Taken together, these results suggest that cysteamine and cystamine have comparable same neuroprotective action.
Fig. 7.
Cystamine protects primary cortical neurons from mHtt toxicity. Primary cortical neurons were transfected at 7 DIV with either Htt N586 82Q or Htt N586 22Q with additional treatments. Cell death was measured at 48 h by nuclear condensation assay. A) Dose response of cysteamine neuroprotection. B) Cystamine protection in presence or absence of the cysteamine dioxygenase 8HQ (5 mM). C) Cystamine protection in presence or absence of the TrkB antagonist ANA12. *p < 0.05 vs Htt N586 82Q by ANOVA with Bonferroni post-hoc test. (n = 4 independent neuronal preparations).
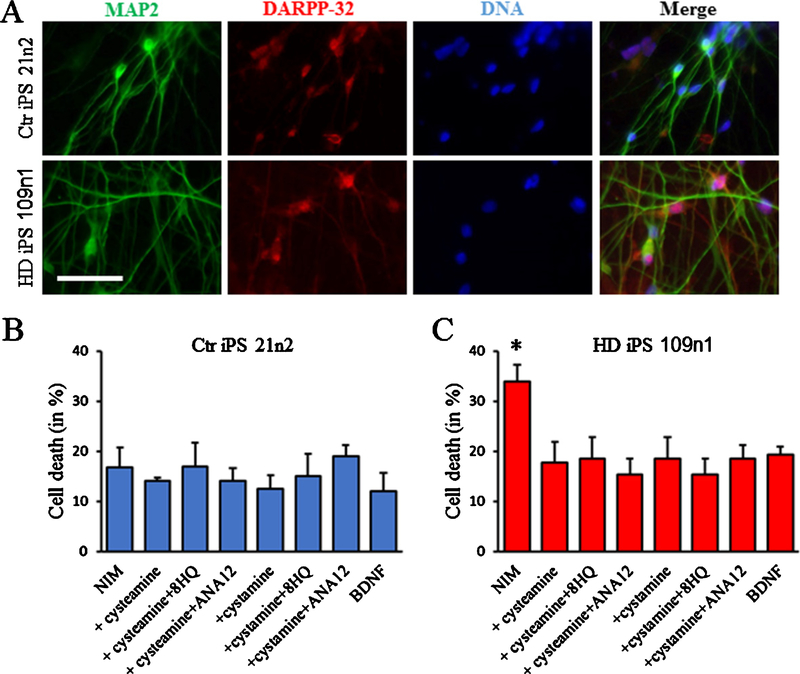
Cysteamine protects HD iPS cells from toxicity
In addition to the primary neurons, we also investigated the effects of cysteamine in our HD inducible Pluripotent Stem cells (HD iPSc). We differentiated control and HD iPS line toward a medium spiny neuron phenotype. In our earlier publication, using immunofluorescence and cell quantification in HD and non-disease lines we have shown expression of neuronal markers TUJ1 (26.5%) and MAP2ab (14.8%), and striatal marker DARPP-32 (14.3%) as an average among the lines [18]. Also, previous quantitative reverse transcriptase-PCR (qRT-PCR) data showed that GFAP, MAP2 and DARPP-32 genes were not significantly different between differentiated HD and control cultures [17].
After differentiation, as illustrated on Fig. 8A, the HD cells (HD iPS 109n2) and the control cells (HD iPS 22n2) express the neuronal marker MAP2 and the striatal marker DARP32. When the cells are challenged by changing the medium to Neuronal Induction Medium (NIM), which does not contain any BDNF for 48 h, HD iPS 109n2 cells showed a significant cell toxicity as measured by nuclear condensation assay whereas the control cells remain unchanged. As expected, the addition of BDNF back to the medium decreases the toxicity. Additionally, both cysteamine and cystamine were able to induce a significant protection of the iPS cells. The protective effect of cysteamine and cystamine could not be blocked by the addition of ANA12 to inhibit the BDNF pathway activation, or the addition of 8HQ to inhibit the conversion of cysteamine into hypotaurine (Fig. 8B).
Fig. 8.
Cysteamine neuroprotection in HD iPS cells. 21n2 control cells and 109n1 HD iPS cells were differentiated into medium spiny neurons according to our protocol. A) Examples of immunostaining of control and HD cells showing the expression of MAP2 and DARP32. To measure toxicity, differentiated 21n2 control cells (B) and 109n1 HD iPS cells (C) were cultivated in NIM medium for 48 h. Cysteamine and other compounds were added to NIM for all time of BDNF withdrawal. 20 ng/ml BDNF were used as a positive control. Cell death was quantified by nuclear condensation assay. *p < 0.05 vs BDNF by ANOVA with Bonferroni post-hoc test. (n = 4 independent neuronal preparations).
DISCUSSION
In this study, we have demonstrated the neuroprotective effects of cysteamine against mHtt in vitro. This protection is consistent with the beneficial effect that has been observed in several HD mouse models [3–7]. In primary cortical as well as in primary striatal neurons, cysteamine showed a strong protective effect at concentrations as low as 10 nM. The protective properties of cysteamine we observed were as potent or even more so compared to other potent neuroprotective compounds like the PPARδ activators KD3010 [21] and bexarotene [27], or the SIRT pathway activator ε-viniferin [28] in the exact same system. This strong potency of the protection of cysteamine was also observed by live cell imaging. The morphology of the neurons treated with cysteamine did not show sign of anomaly until the very end of the experiment. In addition to protecting the neuronal morphology, cysteamine protected the mitochondria against the depolarization and swelling induced by mHtt. This protection by cysteamine against mitochondria depolarization has also been reported in STHdhQ111 striatal cells treated 3-nitroproprionic acid [29]. Taken together, these results suggest that not only cysteamine can protect neurons from cell death but also may protect their functionality.
Cysteine metabolism and levels have been proposed to be important in HD—it has been hypothesized that cysteine levels may be low in HD models, and that correcting the putative low level of cysteine in HD mice is protective [13]. However, in our systems, none of the metabolites of the cysteine pathway were able to protect neurons against mHtt toxicity. The difference may be explained by the levels of cysteine in our system not being reduced, hence not triggering the ATF 4 deficit observed in HD striatal cells [30]. The previous studies were conducted in systems with media with low cysteine levels, so perhaps not surprising that raising cysteine levels to normal had beneficial effects in those systems. These results suggest that the main neuroprotection mechanism of cysteamine in our system is independent of cysteine.
In addition to be part of the cysteine metabolism, cysteamine is also the precursor of hypotaurine in neurons [31]. Both taurine and hypotaurine showed to be protective against mHtt in our system and blocking cysteamine dioxigenation into hypotaurine did not block their effects. This suggests that cysteamine and taurine/hypotaurine neuroprotective effects are independent. However, since we did not directly measure cysteamine dioxygenases in our system but based our experiments on literature, there is a small possibility that the dose of 8HQ used in the experiment might not have been enough to inhibit the enzyme.
Our experiments ruled out the main metabolic pathways in which cysteamine is involved, it does not however rule out completely a metabolic effect of cysteamine. To answer that question, specific and detailed metabolomic experiments of the effects of cysteamine are required.
In a previous study, Borrell-Pages and colleagues showed that cysteamine increases the levels of heat shock DnaJ-containing protein 1b (HSJ1b). In turn, HSJ1b increases BDNF levels and its overexpression is neuroprotective [7]. They also showed that the protective effect by cysteamine observed in R6/1 mice is absent in BDNF deficient mice. However, here we found that inhibiting the activation of the BDNF pathway was not enough to block the cysteamine neuroprotection. In presence of a Trk B inhibitor, ANA 12, no protection by BDNF was observed whereas cysteamine was still efficient. Taken together, these results suggest that cysteamine in vivo can have several modes of protection, some of which could be independent of the BDNF pathway.
In addition to be neuroprotective in HD models, cysteamine has been shown to be protective in several mouse model of Parkinson’s disease. In a 6-OHDA mouse model of Parkinson’s, cystamine reduced the dopaminergic neuronal loss and rescues behavioural impairments. Cystamine also showed neurorestorative properties on nigral dopaminergic neurons [32]. Cysteamine however did not improved the behavior of Levo-DOPA induced dyskinesia-related behavioral impairments [33]. Taken together with the results on HD, it shows the importance of cysteamine can have as a therapeutic agent. Further studies of the effect of cysteamine in vitro in both Huntington’s and Parkinson’s cell models may help to identified common neuroprotective pathways that could be targeted for the treatment of both disease. Our primary neuron toxicity assay was previously successfully used as a model of Parkinson’s [34] and could be used for these studies.
A remaining possible mechanism of action of cysteamine is through its effects on gene transcription. A study has suggested that cysteamine combined with mithramycin decreases the hypertrimethylation of histone H3 and of ESET/SETDB1, a histone H3-K9-specific methyltransferase observed, in HD patients and R6/2 mice. This effect in turn reduces gene silencing resulting from histone methylation [35].
In conclusion, our results show that cysteamine is a strong neuroprotective agent in vitro in both primary mouse neuronal and human iPS cell models of HD. The neuroprotection was independent from the cysteamine metabolic pathways as well as the well-documented BDNF neuroprotective pathway. Given the potency of cysteamine in vitro and the disease-modifying effects displayed in vivo, further investigation to determine the exact molecular mechanism of the neuroprotection is warranted, and additional human trials may be considered.
Supplementary Material
ACKNOWLEDGMENTS
This work was funded by Raptor Pharmaceuticals.
Footnotes
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
SUPPLEMENTARY MATERIAL
The supplementary material is available in the electronic version of this article: http://dx.doi.org/10.3233/JHD-180312.
REFERENCES
- [1].McColgan P, Tabrizi SJ. Huntington’s disease: A clinical review. Eur J Neurol. 2018;25(1):24–34. [DOI] [PubMed] [Google Scholar]
- [2].Besouw M, Masereeuw R, van den Heuvel L, Levtchenko E. Cysteamine: An old drug with new potential. Drug Discov Today. 2013;18(15–16):785–92. [DOI] [PubMed] [Google Scholar]
- [3].Dedeoglu A, Kubilus JK, Jeitner TM, Matson SA, Bogdanov M, Kowall NW, et al. Therapeutic effects of cystamine in a murine model of Huntington’s disease. J Neurosci. 2002;22(20):8942–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Karpuj MV, Becher MW, Steinman L. Evidence for a role for transglutaminase in Huntington’s disease and the potential therapeutic implications. Neurochem Int. 2002;40(1):31–6. [DOI] [PubMed] [Google Scholar]
- [5].Van Raamsdonk JM, Pearson J, Bailey CD, Rogers DA, Johnson GV, Hayden MR, et al. Cystamine treatment is neuroprotective in the YAC128 mouse model of Huntington disease. J Neurochem. 2005;95(1):210–20. [DOI] [PubMed] [Google Scholar]
- [6].Bailey CD, Johnson GV. The protective effects of cystamine in the R6/2 Huntington’s disease mouse involve mechanisms other than the inhibition of tissue transglutaminase. Neurobiol Aging. 2006;27(6):871–9. [DOI] [PubMed] [Google Scholar]
- [7].Borrell-Pages M, Canals JM, Cordelieres FP, Parker JA, Pineda JR, Grange G, et al. Cystamine and cysteamine increase brain levels of BDNF in Huntington disease via HSJ1b and transglutaminase. J Clin Invest. 2006;116(5):1410–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Verny C, Bachoud-Levi AC, Durr A, Goizet C, Azulay JP, Simonin C, et al. A randomized, double-blind, placebo-controlled trial evaluating cysteamine in Huntington’s disease. Mov Disord. 2017;32(6):932–6. [DOI] [PubMed] [Google Scholar]
- [9].Lesort M, Lee M, Tucholski J, Johnson GV. Cystamine inhibits caspase activity. Implications for the treatment of polyglutamine disorders. J Biol Chem. 2003;278(6):3825–30. [DOI] [PubMed] [Google Scholar]
- [10].Zuccato C, Ciammola A, Rigamonti D, Leavitt BR, Goffredo D, Conti L, et al. Loss of huntingtin-mediated BDNF gene transcription in Huntington’s disease. Science. 2001;293(5529):493–8. [DOI] [PubMed] [Google Scholar]
- [11].Ross CA, Tabrizi SJ. Huntington’s disease: From molecular pathogenesis to clinical treatment. Lancet Neurol. 2011;10(1):83–98. [DOI] [PubMed] [Google Scholar]
- [12].Maher P, Lewerenz J, Lozano C, Torres JL. A novel approach to enhancing cellular glutathione levels. J Neurochem. 2008;107(3):690–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Paul BD, Sbodio JI, Xu R, Vandiver MS, Cha JY, Snowman AM, et al. Cystathionine gamma-lyase deficiency mediates neurodegeneration in Huntington’s disease. Nature. 2014;509(7498):96–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Fox JH, Barber DS, Singh B, Zucker B, Swindell MK, Norflus F, et al. Cystamine increases L-cysteine levels in Huntington’s disease transgenic mouse brain and in a PC12 model of polyglutamine aggregation. J Neurochem. 2004;91(2):413–22. [DOI] [PubMed] [Google Scholar]
- [15].Pinto JT, Van Raamsdonk JM, Leavitt BR, Hayden MR, Jeitner TM, Thaler HT, et al. Treatment of YAC128 mice and their wild-type littermates with cystamine does not lead to its accumulation in plasma or brain: Implications for the treatment of Huntington disease. J Neurochem. 2005;94(4):1087–101. [DOI] [PubMed] [Google Scholar]
- [16].Watkin EE, Arbez N, Waldron-Roby E, O’Meally R, Ratovitski T, Cole RN, et al. Phosphorylation of mutant huntingtin at serine 116 modulates neuronal toxicity. PLoS One. 2014;9(2):e88284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Mattis VB, Tom C, Akimov S, Saeedian J, Ostergaard ME, Southwell AL, et al. HD iPSC-derived neural progenitors accumulate in culture and are susceptible to BDNF withdrawal due to glutamate toxicity. Hum Mol Genet. 2015;24(11):3257–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].HD iPSC Consortium. Developmental alterations in Huntington’s disease neural cells and pharmacological rescue in cells and mice. Nat Neurosci. 2017;20(5):648–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].HD iPSC Consortium. Induced pluripotent stem cells from patients with Huntington’s disease show CAG-repeat-expansion-associated phenotypes. Cell Stem Cell. 2012;11(2):264–78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Arbez N, Ratovitski T, Roby E, Chighladze E, Stewart JC, Ren M, et al. Post-translational modifications clustering within proteolytic domains decrease mutant huntingtin toxicity. J Biol Chem. 2017;292(47): 19238–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Dickey AS, Pineda VV, Tsunemi T, Liu PP, Miranda HC, Gilmore-Hall SK, et al. PPAR-delta is repressed in Huntington’s disease, is required for normal neuronal function and can be targeted therapeutically. Nat Med. 2015;22(1):37–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Bowie LE, Maiuri T, Alpaugh M, Gabriel M, Arbez N, Galleguillos D, et al. N6-Furfuryladenine is protective in Huntington’s disease models by signaling huntingtin phosphorylation. Proc Natl Acad Sci U S A. 2018;115(30):E7081–E90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Wu JY, Wu H, Jin Y, Wei J, Sha D, Prentice H, et al. Mechanism of neuroprotective function of taurine. Adv Exp Med Biol. 2009;643:169–79. [DOI] [PubMed] [Google Scholar]
- [24].Cavallini D, Dupre S, Scandurra R, Graziani MT, Cotta Rasusino F. Metal content of cysteamine oxygenase. Eur J Biochem. 1968;4(2):209–12. [DOI] [PubMed] [Google Scholar]
- [25].Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Graos MM, et al. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12(10):1329–43. [DOI] [PubMed] [Google Scholar]
- [26].Saba J, Turati J, Ramirez D, Carniglia L, Durand D, Lasaga M, et al. Astrocyte truncated tropomyosin receptor kinase B mediates brain-derived neurotrophic factor antiapoptotic effect leading to neuroprotection. J Neurochem. 2018;146(6):686–702. [DOI] [PubMed] [Google Scholar]
- [27].Dickey AS, Sanchez DN, Arreola M, Sampat KR, Fan W, Arbez N, et al. PPARdelta activation by bexarotene promotes neuroprotection by restoring bioenergetic and quality control homeostasis. Sci Transl Med. 2017;9(419). pii: eaal2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Fu J, Jin J, Cichewicz RH, Hageman SA, Ellis TK, Xiang L, et al. trans-(−)-epsilon-Viniferin increases mitochondrial sirtuin 3 (SIRT3), activates AMP-activated protein kinase (AMPK), and protects cells in models of Huntington Disease. J Biol Chem. 2012;287(29):24460–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Mao Z, Choo YS, Lesort M. Cystamine and cysteamine prevent 3-NP-induced mitochondrial depolarization of Huntington’s disease knock-in striatal cells. Eur J Neurosci. 2006;23(7):1701–10. [DOI] [PubMed] [Google Scholar]
- [30].Sbodio JI, Snyder SH, Paul BD. Transcriptional control of amino acid homeostasis is disrupted in Huntington’s disease. Proc Natl Acad Sci U S A. 2016;113(31): 8843–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Vitvitsky V, Garg SK, Banerjee R. Taurine biosynthesis by neurons and astrocytes. J Biol Chem. 2011;286(37):32002–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Cisbani G, Drouin-Ouellet J, Gibrat C, Saint-Pierre M, Lagace M, Badrinarayanan S, et al. Cystamine/cysteamine rescues the dopaminergic system and shows neurorestorative properties in an animal model of Parkinson’s disease. Neurobiol Dis. 2015;82:430–44. [DOI] [PubMed] [Google Scholar]
- [33].David LS, Saint-Pierre M, Lamontagne-Proulx J, Cicchetti F. The effects of cysteamine in a mouse model of levodopa-induced dyskinesias. Neurosci Lett. 2018;662:395–401. [DOI] [PubMed] [Google Scholar]
- [34].Yang D, Li T, Liu Z, Arbez N, Yan J, Moran TH, et al. LRRK2 kinase activity mediates toxic interactions between genetic mutation and oxidative stress in a Drosophila model: Suppression by curcumin. Neurobiol Dis. 2012;47(3):385–92. [DOI] [PubMed] [Google Scholar]
- [35].Ryu H, Lee J, Hagerty SW, Soh BY, McAlpin SE, Cormier KA, et al. ESET/SETDB1 gene expression and histone H3 (K9) trimethylation in Huntington’s disease. Proc Natl Acad Sci U S A. 2006;103(50):19176–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.