Abstract
Nonvisual arrestins (arrestin-2/arrestin-3) interact with hundreds of G protein-coupled receptor (GPCR) subtypes and dozens of non-receptor signaling proteins. Here we describe the methods used to identify the interaction sites of arrestin-binding partners on arrestin-3 and the use of monofunctional individual arrestin-3 elements in cells. Our in vitro pull-down assay with purified proteins demonstrates that relatively few elements in arrestin engage each partner, whereas cell-based functional assays indicate that certain arrestin elements devoid of other functionalities can perform individual functions in living cells.
Keywords: Arrestin, MAPK, Protein-protein interactions, JNK3 activation
1. Introduction
Mammals have only two nonvisual arrestins (arrestin-2 and arrestin-3, aka β-arrestin1 and β-arrestin2) [1], both of which demonstrate an amazing versatility: they interact with hundreds of GPCR subtypes and also numerous non-receptor signaling proteins [2]. The rich multifunctionality of these ~45 kDa proteins raises questions as to where these different partners bind and how many proteins can be brought together by a single arrestin molecule simultaneously. It also creates practical problems in studying biological functions of arrestins; for example, conventional protein overexpression or knockdown in the case of arrestins affects numerous cellular processes, resulting in pleiotropic effects [3]. One could envision an alternative approach using comprehensive mutagenesis of every residue in a ~400 amino acid protein to identify binding sites for interacting partners [4], but it is hardly practical to test dozens of distinct functions of hundreds of mutants. In addition, protein-protein interactions are often mediated by groups of residues, and mutation in any single residue might not affect an interaction significantly. There are a few known exceptions in arrestins, where one- or two-point mutations significantly changed receptor preference [5, 6], the binding of MEK1 [7], or c-Raf1 [8], but as a rule, greater perturbations are necessary.
If protein binding is mediated primarily through peptide-based interactions, one possible approach in the identification of elements involved in binding a partner is via separation of the protein in question into a series of peptides and identification of those elements that still bind a partner. This approach was used successfully to identify three arrestin-3-derived peptides that can interact with JNK3 [9]; however, this is not to suggest that other secondary elements in the intact structure do not play a role in the interaction. Interestingly, a 25-residue peptide termed T1A was found to bind JNK3 and each of the upstream kinases in the ASK1-MKK4/7-JNK3 cascade and facilitate JNK3 activation in cells, making it the smallest MAPK scaffold identified so far [10]. Here we describe two critical methods used in these studies: an in vitro pull-down with purified proteins that unambiguously shows that an interaction is direct and a cell-based functional assay, demonstrating the biological relevance of the interactions detected in vitro. Together, these techniques provide a powerful depiction of how arrestin interacts with downstream effectors.
1.1. In Vitro Pull-Down
The in vitro pull-down described here can be used to verify any protein-protein interaction when the proteins in question can be isolated with relatively high purity. The simplest way to perform this experiment is to have usable tags on both proteins (maltose-binding protein/MBP, glutathione S-transferase/GST, His, etc.), so that the pull-down can be performed both ways. If a protein can be expressed as a fusion with one of these tags, it is amenable to this technique and can provide convincing evidence for a direct interaction.
The main advantage of an in vitro pull-down with purified proteins is that any interaction detected by this method is direct, meaning that it does not require helpers and/or intermediaries. The limitations are mostly related to the affinity of the interaction: only relatively high-affinity interactions survive several washes necessary to remove non-specifically bound proteins. This affinity could also be impacted by the addition of another partner protein or cofactor; for example, ATP interaction with a protein kinase could change the kinase’s affinity for an interacting partner. Another drawback is that investigated proteins must be expressed and purified and need tags suitable for pull-down. Lastly, as far as scaffolding proteins are involved, their expression level determines the binding outcome; at higher than optimal concentrations, scaffolds suppress signaling in cells and in vitro [11, 12]. An overview of the in vitro pull-down method is provided (Fig. 1).
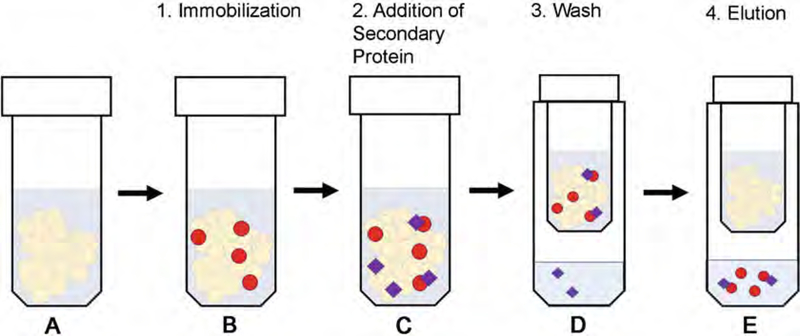
Fig. 1.
Schematic overview of the in vitro pull-down assay. This is described as a pull-down for a protein with an MBP affinity tag, but the procedure can be easily modified for a protein with any type of affinity tag. (a) Prepare MBP affinity resin (or an alternative resin appropriate for the tag) by washing the suspension in Wash buffer A. (b) Immobilize MBP-fused proteins on the resin by incubating for 1 h with gentle rotation at 4 °C. (c) Add the secondary protein (either tagged, or for which a validated commercial antibody is available) to the mixture, and incubate for an additional 2 h with gentle rotation at 4 °C. (d) Wash each sample three times quickly with Wash buffer B. This will remove any non-specific interactions. (e) Elute the bound proteins using buffer containing 50 mM maltose or an alternative appropriate elution buffer for proteins with other tags. The eluate is then analyzed by both Coomassie (to control even bait protein loading) and Western blot (to assess binding)
1.2. Cell-Based Activation
The cell-based activation or phosphorylation assay is suitable when the protein in question facilitates or suppresses the activation or phosphorylation of other proteins, which can be easily assessed experimentally. Here we present ASK1-dependent JNK3 phosphorylation as an example, but similar assays can show the effect of the protein of interest on other processes, such as MKK4- or MKK7-dependent JNK1/2/3 phosphorylation [13]. When the interaction is studied in a cellular context, one can also use co-immunoprecipitation as an alternative approach [14].
The main advantage of cell-based assays is that they demonstrate how the process occurs in an intact living cell. The main disadvantage is that one can never be sure whether additional proteins and/or metabolites are involved. Below is a schematic of the cell-based assay for detection of phosphorylated JNK3 in the presence of HA-ASK1, HA-JNK3, and GFP-tagged arrestins (Fig. 2). Our laboratory is specifically interested in JNK3 for its interaction with arrestin-3 and its role in neuronal apoptosis; however, this setup can be used to detect any arrestin-dependent activation, such as the other JNK isoforms, other MAP kinases, etc.
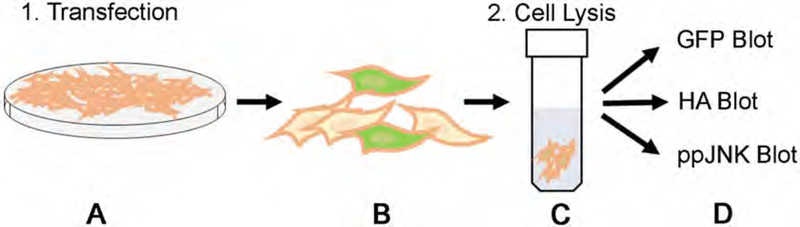
Fig. 2.
Schematic overview of the in-cell assay. (a) Transfect the cells at 90–100% confluency with HA-ASK1, HA-JNK3, and the appropriate Venus-arrestin construct. (b) Allow 48 h for protein expression with serum starvation overnight before the assay. The cells can be monitored using a fluorescent microscope. Cells that have been transfected will glow green because of the Venus (GFP) transfection. (c) Lyse the cells to release the protein using the lysis buffer described below. (d) Run a Western blot for arrestin expression (GFP), ASK1 and JNK3 expression (HA), and ppJNK
2. Materials
Solutions were prepared using double-distilled water and commercially available reagents. Unless otherwise stated, reagents were prepared and stored at room temperature. The listed antibodies were selected due to widespread use in our laboratory, but primary antibodies will depend on the proteins being tested. It is recommended to follow proper waste disposal for all materials.
2.1. In Vitro Pull-Down Assay
2.1.1. MBP-Fusion Protein Expression
Lysis buffer: 50 mM Tris–HCl, pH 8.0, 5 mM EGTA, 2 Mm benzamidine (BA), protease inhibitor cocktail (Sigma-Aldrich, Product #P2714).
Equilibration buffer: 20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 2 mM BA.
Elution buffer: 20 mM Tris–HCl, pH 7.5, 150 mM NaCl, 2 mM BA, and 50 mM maltose.
Sonicator, Fisher Scientific, Model 505.
MBPTrap HP 5 mL column, GE Healthcare Life Sciences (Product code: 28918779).
PD-10 desalting column with Sephadex G-25 resin from GE Healthcare Life Sciences.
AKTA protein purification chromatography systems, GE Healthcare Life Sciences.
2.1.2. In Vitro Pull-Down
Ultrafree®-MC DV centrifugal filters, Durapore® PVDF 0.65 μm.
Amylose resin: Stored in 20% ethanol, New England Biolabs Inc. (#E8021S, store at 4 °C).
Wash buffer A: 20 mM Tris–HCl, pH 7.5, and 150 mM NaCl.
Wash buffer B: 50 mM HEPES, pH 7.3, and 150 mM NaCl.
Elution buffer: 50 mM HEPES, pH 7.3, 150 mM NaCl, 50 mM maltose.
2.1.3. SDS Polyacrylamide Gel
Resolving gel buffer: 1.5 M Tris–HCl buffer, pH 8.8.
Stacking gel buffer: 0.5 M Tris–HCl buffer, pH 6.8.
Thirty percent acrylamide/bis solution 29:1: 3.3% cross-linker.
Ammonium persulfate: 10% solution in water.
N,N,N,N′-tetramethylethylenediamine (TEMED).
SDS solution 10% (w/v).
SDS-PAGE running buffer: TG-SDS 10× concentrate.
Sample buffer, Laemmli (2×) concentrate (store at −20 °C).
Bio-Safe Coomassie G-250 stain.
2.1.4. Immunoblotting
Transfer buffer: TG 10× concentrate with 15% methanol.
Immobilon-P transfer membrane (Merck Millipore Ltd.).
Blocking solution: 5% milk (w/v) in Tris-buffered saline and 0.05% Tween 20 (TBS-T) or 2% bovine serum albumin in TBS-T.
SuperSignal® West Pico Luminol/enhancer solution and stable peroxide solution.
Premium X-ray film.
2.1.5. Antibodies
SAPK/JNK and phospho-JNK rabbit (Cell Signaling, 1:1000 dilution, #9252P, #9251).
HA Rabbit (Cell Signaling, 1:1000 dilution, #3724).
GFP Mouse (Cell Signaling, 1:1000 dilution, #2526) (used for Venus constructs).
Secondary antibodies: Peroxidase-conjugated affiniPure goat anti-mouse IgG and anti-rabbit (Jackson Immuno Research Laboratories Inc., 1:10,000 dilution, store at 20 °C).
2.2. In-Cell Activation Assay
2.2.1. Cell Types
COS7 African green monkey cells (ATCC).
HEK 293 cells (ATCC).
2.2.2. Transfection
Transfection reagent: Trans-Hi™ Reagent (FormuMax, Lot 1029) or Lipofectamine® 2000 (Invitrogen by Life Technologies, Ref #11668–019).
Media: DMEM (1×) + GlutaMAX™−1, 4.5 g/L D-glucose, 110 mg/L sodium pyruvate, supplemented with 10% fetal bovine serum, and 5% penicillin/streptomycin.
0.05% Trypsin-EDTA (1×).
4. Corning® 100 mm × 20 mm style dish, cell culture treated.
5. Disposable six-well plate.
2.2.3. Cell Lysis
Lysis buffer: 50 mM Tris–HCl, pH 7.5, 2 mM EDTA, 250 mM NaCl, 10% glycerol, 0.5% NP-40, 20 mM NaF, 1 mM Na3VO4, 10 mM N-ethylmaleimide, 1 mM PMSF, 2 mM benzamide.
Phosphate-buffered saline (PBS).
Corning™ Falcon™ cell scrapers.
3. Methods
3.1. In Vitro Pull-Down Assay
3.1.1. Expression and Purification of Arrestins
We recently described in detail methods used for the expression and purification of untagged arrestins in E. coli [15]. Purification of MBP-tagged arrestin-3 and MBP fusions of arrestin-3-derived peptides was described in [9] and is detailed below.
3.1.2. Expression of MBP-Fusion Proteins
Express MBP-fusion proteins in E. coli (BL21-codon plus (DE3)-RIL). Inoculate cells in a 500 mL flask (100 mL LB) from a single colony or glycerol stock, and grow overnight at 30 °C at 205 rpm.
Use around 10–20 mL of the overnight culture to inoculate each of the four 1-L cultures (in 2 L flasks), and grow the cultures at 30 °C at 205 rpm to an optical density between 0.6 and 0.7 (approximately 3 h postinoculation). Remove the flasks from the shaker, and chill in an ice water bath for 20–30 min (see Note 1a).
Induce the cultures with 1 mM isopropyl β-D-thiogalactoside for an additional 5–6 h at 30 °C followed by centrifugation (15 min, 6000 rpm (4,031 rcf)). Freeze the pellets at −80 °C (see Note 1b).
Thaw protein pellets on ice, and resuspend in lysis buffer (approximately 30 mL per 1 L culture). Add a small amount of lysozyme to the suspension, and incubate on ice for 40 min.
Sonicate the suspension at 75% max amplitude for 2 min (5 s on, 10 s off). Incubate the lysate with 25 μL DNase I and 2 mM MgCl2 for 30 min (see Note 1c).
Centrifuge the lysate for 1 h at 18,000 rpm (38,360 rcf) (Sorvall SS-34 rotor in RC 5B plus centrifuge). Filter the supernatant through a 0.45 μM filter before loading on the MBP column.
Wash the MBP-trap column with five column volumes of equilibration buffer before loading the lysate. Filter the lysate, and load on the column at 3 mL/min (check system pressure to ensure that the column is not over-pressurized). After the entire lysate enters the column, wash the system with equilibration buffer until the UV absorption returns to baseline. Use a gradient elution (100% elution buffer, 0–50 mM maltose, flow rate 2 mL/min, 25 min) to elute the MBP-fusion protein off the column. This should result in a symmetric peak. Collect the fractions, and analyze the protein using SDS-PAGE (see Note 1d).
Pool the fractions containing the correct MBP-fusion protein, and concentrate to an appropriate volume for a PD-10 desalting column (less than 2.5 mL) (see Note 1e). Equilibrate the column by washing four times in equilibration buffer (approximately 25 mL buffer). This can be accomplished by gravity flow or by low-speed centrifugation (1000 × g). Apply 2.5 mL of protein sample to the column (add buffer if the sample is less than 2.5 mL), and allow the sample to completely enter the column bed. Discard all flow through. Elute the sample with 3.5 mL of buffer solution and concentrate as necessary. This step removes maltose from the protein sample.
3.1.3. Immobilization of MBP-Fusion Proteins
Remove MBP-fusion proteins and the protein being assayed as a potential-binding partner from the −80 °C freezer. Allow the proteins to thaw on ice before proceeding to the next step (see Note 1f).
Remove amylose resin from the 4 °C fridge, and shake at low speed for gentle resuspension. Allotting 25 μL resin per sample condition, cut the tip of a plastic pipette, and remove enough resin for each sample condition plus one additional (see Note 1g). Add 1 mL of Wash buffer A to the microcentrifuge tube containing the resin, and centrifuge at low speed (around 3200 rpm (1,147 rcf)) for 30 s. Repeat three times. Remove the supernatant without disturbing the resin, leaving enough volume to distribute 25 μL of resin per sample. Using a cut plastic pipette tip, resuspend the resin and aliquot to microcentrifuge tubes.
To immobilize the MBP-fusion proteins on the amylose resin, distribute 5–10 μg of an individual MBP-fusion construct diluted to 25 μL in Wash buffer A to one of the microcentrifuge tubes containing amylose resin. Incubate the proteins at 4 °C for 1 h using gentle rotation (see Notes 1h and 1i).
3.1.4. Addition of Secondary Protein
Add 5–10 μg of the prey protein to a total volume of 50 μL in Wash buffer A. For consistency, calculate the volume of protein and buffer needed for one sample, and multiply these values by the number of samples plus one additional to generate a master mix. From the master mix, distribute 50 μL to each microcentrifuge tube containing an immobilized MBP-fusion construct. Incubate for 2 h at 4 °C with gentle rotation.
3.1.5. Elution
Prior to removing samples from the 4 °C incubation, prepare a series of tubes for washing and elution. Label an Ultrafree centrifugal filter for each sample, in addition to a microcentrifuge tube with the cap removed and a microcentrifuge tube labeled to hold the final eluted sample. Place the centrifugal filter and the microcentrifuge tube with the cap removed into a 4 °C centrifuge to cool before adding the samples.
Remove the samples from the 4 °C fridge and place on ice. Transfer each sample into a centrifugal filter using a cut plastic pipette tip. Centrifuge the samples at 3200 rpm for 20 s.
Add 300 μL of Wash buffer B directly to each sample, and centrifuge at 3200 rpm for 20 s. Quickly remove the centrifugal filter, and place into the microcentrifuge tube with the cap removed (see Note 1j). Proceed with two additional 300 μL washes in Wash buffer B at 3200 rpm for 20 s.
Move the centrifugal filters into the final microcentrifuge tube, and add 100 μL elution buffer to each sample. Incubate at 4 °C for 5 min with gentle rotation. Elute the samples by centrifugation at 4 °C for 1 min at 3200 rpm.
3.1.6. Methanol Precipitation
Add 1 mL of 100% methanol to each microcentrifuge tube containing eluted sample. Centrifuge the samples at high speed (around 12,000–14,000 rpm (16,000–21,952 rcf)) for 10 min. Discard the supernatant, and let the samples air-dry for 30 min or until droplets are no longer visible (see Note 1k).
Resuspend in 30 μL SDS loading buffer with dye. Make sure to wash the sides of the tube up to 1 mL. Vortex each sample, and centrifuge the samples in preparation for SDS-PAGE.
3.1.7. SDS-PAGE and Western Analysis
Prepare two 10% SDS-PAGE gels (4% stacking). Run 12 μL of sample on a gel for Coomassie staining to verify equal loading of the MBP-fusion constructs on the amylose resin (Fig. 3 top panel). Run 8 μL of sample on another gel for Western blotting to assess protein interaction (Fig. 3 lower panel).
Use a primary antibody against the prey protein when performing the Western analysis. This will inform whether a protein interaction occurred.
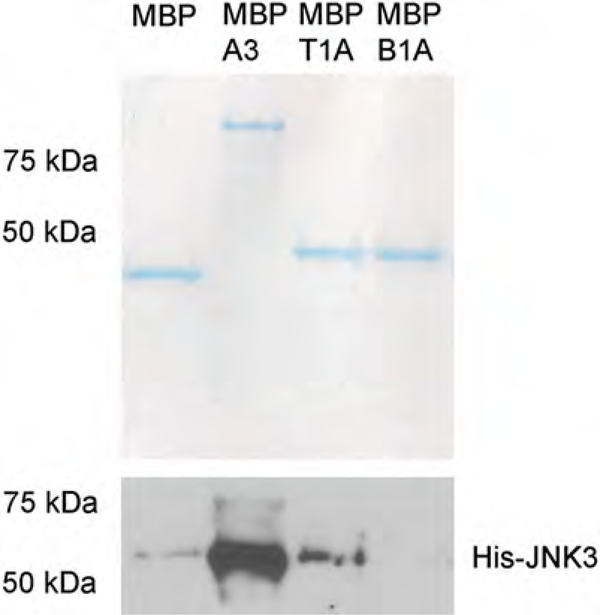
Fig. 3.
Representative Coomassie gel and Western blot for an MBP pull-down using His-JNK3 as prey protein. The Coomassie gel is used to assess even loading of the MBP-fusion constructs, while the Western blot to show relative binding of His-JNK3 (A3, arrestin3; T1A, first 25 amino acids of arrestin-3; B1A, first 25 amino acids of arrestin-2)
3.2. In-Cell Activation Assay
All cell culture experiments were performed in the class II laminar flow hood until the cell lysis step. Make sure to maintain lab etiquette when working with cells—clean all materials with 70% ethanol before entering the hood space; wear gloves and a lab coat.
3.2.1. Cell Seeding and Transfection
Grow COS7 African green monkey cells in DMEM solution containing 10% FBS and 5% penicillin/streptomycin at 37 °C and 5% CO2. Other cell lines can be used depending on the system being tested.
Remove cells from 10 cm plates using 2.5 mL 0.05% trypsin (~3–5 min), and seed into six-well plates at a density that ensures 90–95% confluency within 24 h (approximately 50,000 cells/mL) (see Note 2a).
After 24 h, replace the cell media with serum-free media, and transfect the cells with pcDNA3-HA-ASK1 (0.4 μg), pcDNA3-HA-JNK3α2 (0.2 μg), and pcDNA3-Venus-arrestin constructs (0.2–0.6 μg) at a 1:2 ratio of DNA:Lipofectamine 2000 (see Note 2b). The μg amounts of pcDNA3-Venus-arrestin constructs were previously optimized using Western analysis of different transfected amounts (see Note 2c). Equalize the DNA amounts using an empty pcDNA3 vector. After 5–6 h, replace the serum-free media with DMEM solution containing 10% FBS and 5% penicillin/streptomycin.
The night before cell lysis (approximately 12 h), replace the media with serum-free media to exclude any possible receptor stimulation from factors in the serum.
3.2.2. Cell Lysis
Forty-eight hours post-transfection, remove the cells from incubation, and wash three times with 1 mL cold phosphate-buffered saline (PBS). Place the cells on ice, and incubate with 1 mL phosphatase inhibitors (20 mM NaF and 1 mM Na3VO4) in PBS for 15 min prior to complete lysis (see Note 2d).
After treatment with phosphatase inhibitors, wash the cells twice with 1 mL cold PBS. Prepare lysis buffer and keep on ice. Add PMSF and BA to the lysis buffer immediately prior to adding the buffer to the cells (see Note 2e). Add 100 μL of lysis buffer to each well, and incubate the plate for 10 min on ice with gentle rotation.
Mechanically remove cells from the wells using a cell scraper, and resuspend by pipetting (see Note 2f). Remove the solutions from each well, and transfer to a 1.5 mL centrifuge tube. Centrifuge the cells for 10 min (4 °C, microcentrifuge at maximum speed) to pellet the debris, and take the top 80% of the lysate for analysis.
Measure protein concentration using a Bradford assay (or any alternative method). The yields typically range between 0.5 and 2 mg/mL (see Note 2g).
3.2.3. Gel Electrophoresis
Prepare samples for gel electrophoresis by combining 10 μL of 2× Laemmli buffer with the correct amount of protein for visualization by Western-ppJNK3 blot (8.5 μg), HA blot (5 μg), and Venus blot (1 μg) (see Note 2h). Boil the samples for 2–3 min to ensure that the proteins will enter the gel.
Prepare a 10% SDS-PAGE gel (4% stacking) for each respective blot (ppJNK3 blot (Fig. 4 upper panel), HA blot (Fig. 4 middle panel), and Venus/GFP blot (Fig. 4 lower panel). Load samples into wells at 80 mV for 15 min, and then run at 150 mV for another 45 min–1 h (until the dye front is close to the bottom of the gel).
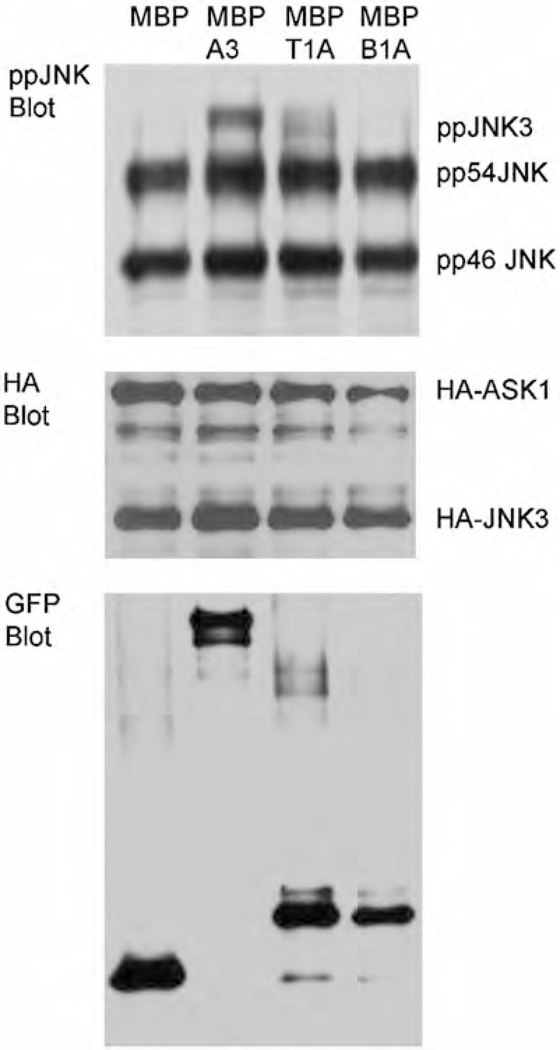
Fig. 4.
Representative Western blots for antibodies against ppJNK, HA, and GFP. The HA and GFP blots are used to confirm equal protein expression following transfection. The top band of the ppJNK blot represents ppJNK3, while the other bands are isoforms of ppJNK1/2 (pp54 JNK and pp46JNK). Quantification was performed on ppJNK3 bands and compared across Venus fusion constructs (not shown)
3.2.4. Transfer and Visualization
Remove gels from their casing after electrophoresis, and transfer to PDVF membrane for 45 min (100 V). After transfer, block the membranes with 5% milk (w/v) in TBS-T for 1 h (alternatively, you can use a 5% milk/2% BSA in TBS-T combination for enhanced blocking). Wash the membranes five times for 5 min in TBS-T before overnight incubation with the corresponding primary antibody (see Note 2i).
The next morning, wash the membranes five times for 5 min in TBS-T, and then incubate with HRP-conjugated secondary antibodies for 1 h. Detect bands by X-ray film using enhanced chemiluminescence (or an alternative method if you prefer). Perform densitometric quantification to assess the difference in ppJNK3 levels (see Note 2j).
4. Notes
- In vitro pull-down assay
- Chilling the flasks slows down cell growth prior to lowering the temperature for induction. This promotes proper folding of the protein.
- Can be stored at −80 °C after this step for several weeks.
- DNAse I treatment reduces the viscosity and helps with filtration of the lysate during later steps.
- Boil the samples at 100 °C for 5 min to make it easier for loading the gel. This helps if you have a lot of protein in the sample.
- Alternatives to the PD-10 desalting column include the HiTrap desalting column available from GE or buffer exchange using a concentrator with the correct cutoff to retain your protein of interest.
- Limit the number of times that you thaw a protein by freezing your samples in small aliquots.
- Cutting the pipette tip ensures successful pipetting of the resin.
- Be careful not to agitate the resin as it will stick to the walls of the tube and cannot be recovered.
- Optimization may be required when loading the MBP-fusion proteins onto the amylose resin. This is because some protein samples are cleaner than others, and competing proteins might reduce the binding.
- The washing step must be performed quickly to minimize change in equilibrium between the bound and unbound state. To assist in this, try not to process more than ten samples at a time.
- Methanol will prevent dissolution of the sample proteins in SDS-PAGE buffer.
- In-cell activation assay
- A 12-well plate can also be used, as this will typically yield enough protein for measurement. The greater the confluency of your cells prior to transfection, the greater the chance of cell survival. Transfection reagent and DNA are toxic to the cells.
- Lipofectamine 2000 is more effective for transfection with high DNA, while other transfection agents, such as Trans-Hi, are more effective with lower DNA amounts.
- Obtaining an even transfection of the pcDNA3-Venus-arrestin constructs is one of the more difficult aspects of this assay. Perform a titration using different μg amounts of each construct prior to experimentation to balance the constructs.
- This prevents the removal of phosphate groups from modified proteins in the cell lysate.
- These reagents are not stable over a prolonged period. Make sure to add them directly prior to administering the lysis buffer to the cells.
- Complete lysis is important for extraction of all the protein in the cell. Make sure to scrape the cells off so that you do not have any remaining on the dish.
- Low yields may indicate high cell death. Revisit the transfection amounts, and monitor your cell population over the 48 h, if you encounter this issue.
- These values are experimentally determined. If there is no detectable signal, increase the amounts used for Western analysis. Alternatively, if the signal is too high, decrease the amounts used for analysis.
- It is important to wash the milk away completely if the primary antibody solution is to be frozen and reused.
- Our preferred software package is Quantity One 1-D Analysis Software from Bio-Rad.
Acknowledgments
This work was supported by NIH R01 grants EY011500, GM109955 and GM077561 (the latter two were merged into R35 GM122491) (VVG), NS065868 and DA030103 (EVG), GM120569 (TMI), R21grant DA DA043680 (TMI/VVG), and an AHA Predoctoral Fellowship 16PRE30180007 (NAP).
References
- 1.Gurevich EV, Gurevich VV (2006) Arrestins are ubiquitous regulators of cellular signaling pathways. Genome Biol 7:236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Xiao K, McClatchy DB, Shukla AK, Zhao Y, Chen M, Shenoy SK, Yates JR, Lefkowitz RJ (2007) Functional specialization of beta-arrestin interactions revealed by proteomic analysis. Proc Natl Acad Sci U S A 104:12011–12016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gurevich VV, Gurevich EV (2015) Analyzing the roles of multi-functional proteins in cells: the case of arrestins and GRKs. Crit Rev Biochem Mol Biol 50:440–452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ostermaier MK, Peterhans C, Jaussi R, Deupi X, Standfuss J (2014) Functional map of arrestin-1 at single amino acid resolution. Proc Natl Acad Sci U S A 111:1825–1830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gimenez LE, Babilon S, Wanka L, Beck-Sickinger AG, Gurevich VV (2014) Mutations in arrestin-3 differentially affect binding to neuropeptide Y receptor subtypes. Cell Signal 26:1523–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gimenez LE, Vishnivetskiy SA, Baameur F, Gurevich VV (2012) Manipulation of very few receptor discriminator residues greatly enhances receptor specificity of non-visual arrestins. J Biol Chem 287:29495–29505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Meng D, Lynch MJ, Huston E, Beyermann M, Eichhorst J, Adams DR, Klusmann E, Houslay MD, Baillie GS (2009) MEK1 binds directly to betaarrestin1, influencing both its phosphorylation by ERK and the timing of its isoprenaline-stimulated internalization. J Biol Chem 284:11425–11435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coffa S, Breitman M, Spiller BW, Gurevich VV (2011) A single mutation in arrestin-2 prevents ERK1/2 activation by reducing c-Raf1 binding. Biochemistry 50:6951–6958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan X, Perez A, Gimenez LE, Vishnivetskiy SA, Gurevich VV (2014) Arrestin-3 binds the MAP kinase JNK3α2 via multiple sites on both domains. Cell Signal 26:766–776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhan X, Stoy H, Kaoud TS, Perry NA, Chen Q, Perez A, Els-Heindl S, Slagis JV, Iverson TM, Beck-Sickinger AG, Gurevich EV, Dalby KN, Gurevich VV (2016) Peptide mini-scaffold facilitates JNK3 activation in cells. Sci Rep 6:21025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kook S, Zhan X, Kaoud TS, Dalby KN, Gurevich VV, Gurevich EV (2013) Arrestin-3 binds c-Jun N-terminal kinase 1 (JNK1) and JNK2 and facilitates the activation of these ubiquitous JNK isoforms in cells via scaffolding. J Biol Chem 288:37332–37342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhan X, Kaoud TS, Dalby KN, Gurevich VV (2011) Nonvisual arrestins function as simple scaffolds assembling the MKK4-JNK3alpha2 signaling complex. Biochemistry 50:10520–10529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zhan X, Kook S, Kaoud TS, Dalby KN, Gurevich EV, Gurevich VV (2015) Arrestin-3-dependent activation of c-Jun N-terminal kinases (JNKs). Curr Protoc Pharmacol 68:1–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo J, Tsakem EL, Breitman M, Gurevich VV (2011) Identification of arrestin-3-specific residues necessary for JNK3 activation. J Biol Chem 286:27894–27901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vishnivetskiy SA, Zhan X, Chen Q, Iverson TM, Gurevich VV (2014) Arrestin expression in E. coli and purification. Curr Protoc Pharmacol 67:2.11.11–2.11.19 [DOI] [PMC free article] [PubMed] [Google Scholar]