Abstract
Purpose
Post-stroke hemiparesis has a significant impact on postural stability. The transversus abdominis (TrA) muscle contributes to the stability of the spine. The aim was to assess both the postural stability and the activity of the TrA muscle in overweight post-stroke patients.
Methods
A group of 56 participants (61.12 ± 11.5 years) was divided into the study group (n=28 post-stroke patients, 63.7 ± 10.9 years) and control group (n= 23 healthy participants (58.5 ± 12.2 years). The Berg Balance Scale (BBS) and the Timed Up and Go Test (TUG) were used to evaluate postural stability and risk of falls. The Pressure Bio-Feedback Stabilizer (PBFS) device was used to assess functional stability.
Results
Stroke had a significantly negative effect on the BBS (p < 0.001) and TUG (p = 0.001). The older age negatively affected the BBS (p = 0.001), TUG (p = 0.017), and the TrA muscle activity (p = 0.017). Higher values of body mass index (BMI) negatively affected the BBS (p = 0.028), however there were no changes of TUG results (p = 0.141), and the TrA muscle activity (p = 0.808). Also, BBS and TUG results were not associated with TrA muscle activity (p = 0.541 and p = 0.411, respectively). The results of the BBS, TUG, and PBFS did not differ according to gender (p < 0.05). Time from stroke negatively affected the TUG (p = 0.001), but had no effect on the TrA muscle activity (p < 0.05). The side of hemiparesis did not affect the postural stability (p < 0.05).
Conclusion
The consequences of a stroke have an essential negative effect on postural stability. Factors such as age, gender, time from stroke, and the side of the hemiparesis have not negatively affected postural stability in overweight post-stroke patients.
Keywords: post-stroke hemiparesis, postural control, postural stability, muscle activity, transversus abdominis muscle, risk of falls, body mass index
Introduction
Coronary heart disease and stroke are the leading causes of both lost potential life years and lost health years.1 In Poland, the above-mentioned complications of cardiovascular disease are at the top of the list of the leading causes of death of its citizens. Coronary heart disease and stroke are in the first two places in the list of diseases responsible for lost years in the lives of Poles.2
According to the World Stroke Organization (WSO) Report, 15 million people suffer from stroke every year; nearly 6 million of them die.3 Strokes are the leading cause of death in people over 60 years of age and the fifth leading cause of death in the group between 15 and 59 years of age: the incidence rates of stroke throughout the world range from 17 to 317 per 100,000 people.4 In Poland, for men and women, they are 177 per 100,000 and 125 per 100,000 persons, respectively.5 Mortality rates in comparison with those of other countries appear discouraging, especially in comparison with Western European countries. Forecasts for 2030 indicate that the number of strokes will double, which will make it one of the most severe problems of modern medicine.6
Studies show that as many as 70% of the patients who survive an acute period after a stroke are disabled.7 Motor functions are more severely affected, and patients have many long-term musculoskeletal disorders.8,9 The patients’ quality of life decreases rapidly due to the reduced possibilities of independent functioning, primarily mobility and self-service related to personal hygiene.10 This is primarily due to postural instability, which is understood not only as of the maintenance of balance in a standing position but also as the ability to recover balance despite the action of external destabilizing stimuli.11 The control of the stable posture of the body is the essence of independent movement and is extremely important to both perform arbitrary movements and to prevent the loss of balance during the performance of movement activities.12
Stroke leads to brain damage, including damage to the corticospinal tract, with one of the most common consequences being hemiparesis.13 This condition prevents the patient from making any movement without complete assurance. It is accompanied by spatial orientation and head alignment disorders, which directly translate into a total lack of postural control and/or significant disturbances in dynamic conditions.14
According to current data, 13% of adults between the ages of 65 and 69 report imbalances, and this percentage increases to 46% in people aged 85 and over.15 All neurological diseases, especially stroke, are a significant factor negatively affecting postural stability. It was proved that postural imbalance is a frequent activity limitation among post-stroke patients.16 In the acute phase of stroke, over 80% of subjects present a postural imbalance.17 The risk of falls is increased by 73% in the 6 months following a stroke. What is more, stroke is a major cause of falls in 42% of the patients.18 The risk of falls in post-stroke people is twice as high in comparison to the rest of the population, particularly within up to one year of a stroke.19
The transversus abdominis (TrA) muscle performs essential functions as both a supporting expiratory muscle and as one of the primary abdominal muscles.20 The diaphragm, pelvic floor and TrA regulate intra‐abdominal pressure and provide anterior lumbopelvic postural stability.21 It means that TrA co-creates the internal cylinder of the abdominal cavity, which, based on the feedforward principle, ensures the stability of the spine.22 Besides, the TrA muscle plays an essential role in performing functional movements and in stabilizing the trunk in both static and dynamic conditions, in which balance of the body can be affected.23 It has been demonstrated that the muscle contraction of TrA in patients with hemiparetic stroke is significantly reduced in the no-paretic side, as well as in the paretic side, compared to healthy individuals.24 Since the tension that appears before the movement is performed protects the spine against all displacements, thus contributing to the stability of the human posture, this is an essential function.25 Although other muscles such as the diaphragm, the lumbar muscle, the multifidus muscle, and the pelvic floor muscles all support the TrA muscle, because of its fascia trailers, it performs its role most efficiently.
Therefore, the primary aim of this study was to evaluate the activity of the TrA muscle in the context of postural stability in overweight post-stroke patients. We hypothesized that stroke negatively affects postural stability, which is the result of decreased activation of the TrA muscle. Correlations between the time from the onset of stroke, the side of the hemiparesis, the patients’ age, body mass index (BMI), gender, and residence, and the ability of the TrA muscle activation were additionally verified.
Methods
Ethics, Design and Settings
This perspective and observational study was conducted from October 2016 to April 2017 at the Department of Neurological Rehabilitation of the Provincial Specialist Hospital in Wroclaw, Poland. The STROBE guidelines (Strengthening the Reporting of Observational Studies in Epidemiology) were followed. The study was approved by the independent Bioethics Committee at the Wroclaw Medical University in Poland (no. KB–300/2016). The study was completely anonymous, and each of the nurses surveyed provided written informed consent. The study was conducted according to the Declaration of Helsinki and Good Clinical Practice guidelines.
Qualification Procedure
The inclusion criteria were: (1) history of stroke, (2) the ability to move independently with or without rehabilitation devices, (3) the absence of diseases that directly endanger the life and health of the patient, (4) full awareness of the patient, (5) the ability to communicate, and (6) consent to participate in the examination. The exclusion criteria were: (1) no possibility of independent movement with or without rehabilitation devices, (2) diseases directly threatening the health and life of the patient, (3) lack of full self-awareness of the patient, (4) no possibility of any communication with the patient, (5) no consent to participate in the study, and (6) advanced degenerative disease of the hip or knee joints.
All of the tests were also carried out on a control group to make a comparative assessment of the results. The control group participants met the following criteria: (1) age over 40 years, (2) no history of stroke, (3) no significant diseases affecting the nervous system, cardiovascular system, and musculoskeletal system., (4) no mental illness, (5) full self-awareness, and (6) voluntary consent to participate in the study.
Study Participants
The material for the study was a group of 56 people (25 women and 31 men) aged 61.12±11.5 years. The study group consisted of 28 patients after ischemic stroke (10 women and 18 men) aged 63.7±10.9 years. The mean time after stroke was 29.6±141.3 weeks (2.0–468.0 weeks). Ischaemic stroke was observed in 86% of subjects (n=24), and hemorrhagic stroke occurred in 14% of subjects (n=4). Left-sided hemiparesis was more frequent in 61% of patients (n=17) and right paresis in 39% of patients (n=11). The control group consisted of healthy persons (15 women and 13 men) in the middle age of 58.5±12.2 years. There were no differences in the range of selected features characterizing the subjects. The detailed characteristics are presented in Table 1.
Table 1.
Characteristics and Comparison of Groups
| Characteristic | Study Group (n = 28) | Control Group (n = 30) | p-value | |
|---|---|---|---|---|
| Age [years] | M | 63.70 | 58.50 | = 0.13* |
| Me | 67.53 | 54.50 | ||
| Min-Max | 6.0–77.0 | 41.0–81.0 | ||
| SD | 10.90 | 12.20 | ||
| BMI [kg/m2] | M | 25.00 | 25.00 | = 0.94* |
| Me | 25.00 | 25.60 | ||
| Min-Max | 28.80–32.30 | 20.90–28.90 | ||
| SD | 2.10 | 2.20 | ||
| Time from stroke [weeks] | M | 29.60 | – | – |
| Me | 4.50 | |||
| Min-Max | 2.00–468.00 | |||
| SD | 141.30 | |||
| Sex | Female | 36% (n = 10) | 53% (n = 16) | = 0.18** |
| Male | 18% (n = 64) | 47% (n = 14) | ||
| Type of stroke | Ischemic | 86% (n = 24) | – | – |
| Haemorrhagic | 14% (n = 4) | – | ||
| Side of hemiparesis | Left-sided | 61% (n = 17) | – | – |
| Right-sided | 39% (n = 11) | – | ||
Notes: *Mann–Whitney U-test; **χ2 test.
Abbreviations: n, number of participants; M, mean; SD, standard deviation; Me, mean; Min, minimum; Max, maximum; p, statistical significance.
Measurement Tools
Berg Balance Scale (BBS) was used to assess postural stability, Timed Up and Go test (TUG) was used to assess functional stability and the Pressure Bio-Feedback Stabilizer device (PBFS) was used to estimate the risk of falls.
Berg Balance Scale (BBS)
The BBS was developed during the 1980s in Canada to assess the balance and risk of falling among older people. Now a recognized clinical test, it is used primarily to evaluate the progress of rehabilitation and postural stability in post-stroke patients. The patient is evaluated in 14 different tasks, including (1) sitting to standing, (2) standing unsupported, (3) sitting unsupported, (4) standing to sitting, (5) transfers, (6) standing with eyes closed, (7) standing with feet together, (8) reaching forward with outstretched arm, (9) retrieving object from floor, (10) turning to look behind, (11) turning 360 degrees, (12) placing alternate foot on stool, (13) standing with one foot in front, and (14) standing on one foot.26
Each task is graded on a 5-point scale, where 0 means that the task cannot be completed, and 4 means that the tasks are completed in a smooth and self-contained manner. Depending on the score, the patient is classified in the appropriate group: At 0–20 points – the patient is dependent on a wheelchair, at 21–36 points – the patient should use a walker while walking, at 37–40 points – the patient should use a walking stick or other supporting devices while walking, and at 41–56 points – the person is independent of orthopedic aids and equipment.27
Timed Up and Go (TUG)
The TUG is an exceptionally valued clinical test that assesses stability and gait based on the time in which a patient can travel a tested distance. For this test, it is necessary to have a standard size chair, a stopwatch, and a 3-meter walking space. In the starting position, the patient is in a sitting position on a chair with his or her back resting against the back of the chair. The patient’s feet rest on the floor, spread out to the width of their hips. At the command of the investigator, the patient gets up (he or she can use their upper limbs if necessary), travels a distance of three meters, turns around, and heads back to the chair. The time is counted from the command to start the examination until the person sits on the chair again.28
During the examination, the patient should wear comfortable shoes that in no way slow down or impair walking. If a patient regularly uses rehabilitation equipment to move around, she/he can use it during the test. Stopping for rest is permitted. The interpretation of the results is as follows: ≤ 10 seconds – stands for standard; ≤ 20 seconds – the test person may go outdoors independently without using additional equipment, but an additional fall risk assessment is recommended; ≤ 30 seconds – high risk of falling, it is recommended that additional equipment be used.29
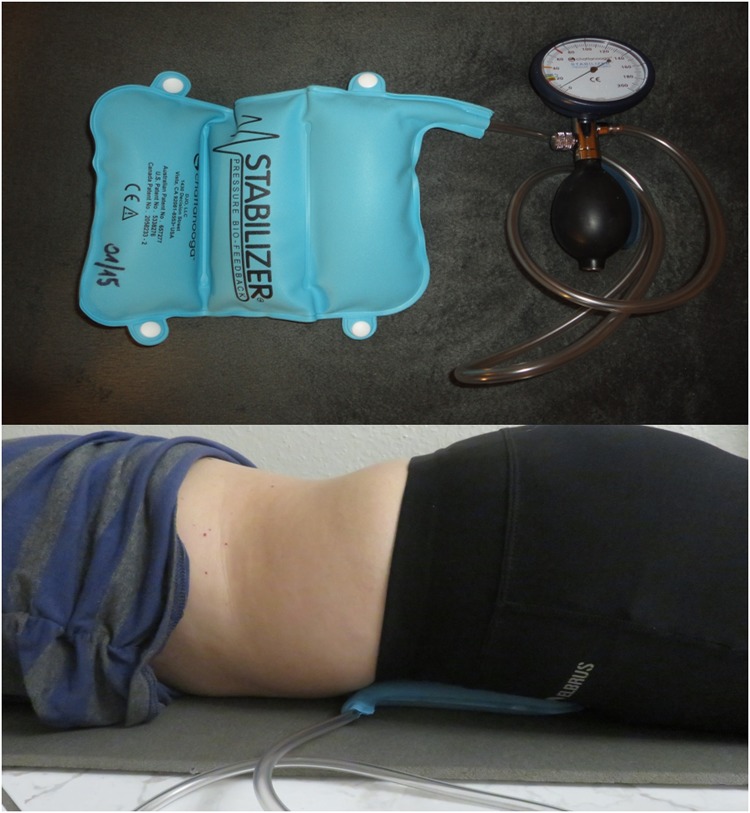
Pressure Bio-Feedback Stabilizer (PBFS)
The PBFS (Chattanooga, Guildford, UK) is one of the methods used to assess the correct activation of the TrA muscle objectively. It is a simple device, consisting of a pressure gauge and a chamber into which air is pumped, which is divided into three parts. The device works on the principle of analyzing changes in the pressure in chambers filled with air, which is under the influence of changes in spinal pressure. It provides the ability to control the position of the lumbopelvic complex and assess the activity of the TrA muscle.21 Recent studies consider the PBFS device to be the most useful tool for evaluating TrA muscle activation in clinical practice due to its low cost and ease in usage. The PBFS device has also proven to be reliable in healthy individuals, but also patients with chronic low back pain or other non-specific musculoskeletal conditions.30 A moderate to good reproducibility (intra-class correlation coefficients from 0.47 to 0.82) and acceptable construct validity (intra-class correlation coefficients from 0.48 to 0.90) was found in previous systematic review.31
During the examination, the patient is in a prone position, the head remains in the middle line of the body, and the face faces the ground. The patient’s upper limbs should be straightened and positioned alongside the trunk. The main part of the device is placed under the abdomen of the test subject and in such a way that the lower edge of the chambers is at the level of the upper anterior iliac spine and the middle chamber at the line of the navel.32
Before proceeding with the main part of the test, the device chambers shall be filled with air (using a pump) to 70 mmHg. The pressure value allows for both a precise examination and for the examination to be comfortable for the patient. Then the test person is asked to activate the TrA muscle throughout its contraction. The task is to pull in the lower part of the abdomen, move the navel toward the spine, and simultaneously activate the TrA muscle. Attention should be paid to ensure that the subjects do not change their respiratory patterns, withhold air, lift the pelvis upward, or move the spine. The correct result of the test is to reduce the pressure in the device chambers from 4 to 10 mmHg and to maintain the contraction of the TrA muscle for 10 seconds.33 It should be emphasized that each participant made three attempts at this test with an interval of one week. Subsequently, the arithmetic mean of the individual measurements was calculated, which represented the final result of the PBFS test. The same researcher performed all measurements to ensure maximum repeatability of data collection and to avoid the risk of bias (Figure 1).
Figure 1.
PBFS device and correct ventricular placement during the measurement of the TrA muscle activity.
Statistical Analysis
The obtained data was encoded and transferred to MS Office Excel 2017 and then subjected to statistical analysis using Statistica version 13.3 (TIBCO Inc. USA). During the preparation of the results, the basic description of quantitative variables (mean, median, standard deviation, maximum, minimum) was made. Qualitative variables (nominal and ranged) are described in percentages and numbers. The chi-squared test was used to compare qualitative variables. The t-test or U-Mann–Whitney test (dependence on fulfilling the assumptions) was used to compare quantitative variables. The correlation analysis of the studied variables was evaluated using the Spearman correlation coefficient (rho). The level of statistical significance was set at p<0.05.
Sample size analysis was performed in Statistica 13 (TIBCO Software Inc., United States). Based on the available results in our pilot study, it was assessed the difference in the results of the activation of the TrA muscle between post-stroke patients and healthy participants. Means and standard deviations of activation of the TrA muscle results in both group (post-stroke patients’ group vs healthy participants’ group) were used in the analysis of estimating the sample size. The estimated sample size for a two-sample unpaired-means test (unpaired t-test). Parameters: mean in post-stroke patients group was 7.1 mmHg (SD=5.5); mean in healthy participants group was 11.8 mmHg (SD=6.1). The alpha level was set at 0.05, and the power of the test at 0.8. It also assumed no correlation of evaluated variables and adopted a 2-sided null hypothesis. On the basis of the parameters, the estimated sample size has been obtained equal to 28 people in each group. Sample size calculations were also performed for clinimetric properties of BBS and TUG tests; however, the obtained estimation for TrA (n=28) indicated the larger sample size required for this study.
Results
Participant Characteristics
The study group consisted of 28 people (10 women and 18 men), aged 36 to 77 years (average age of 63.82 years). The distribution of the education of the respondents was as follows: 35.71% had secondary education, 32.14% had vocational, 21.42% had primary, and 10.71% had higher education. The majority of the surveyed persons lived in urban areas (75%). The control group also consisted of 28 people (15 women and 13 men), aged 45 to 81 years (average age 59.64 years). The distribution of education of the control group respondents was as follows: 42.86% vocational education, 32.14% secondary education, 17.85% primary education, and 7.14% higher. The majority of the surveyed persons in the control group also lived in urban areas (75%).
Analysis of the Results for Each Test
With a median of 47.5 points, the BBS results in the study group ranged from 29 to 54 points. The results in the control group ranged from 34 to 56 points (Me = 54 pts). The results of both groups were statistically significant (p < 0.001) (Table 2).
Table 2.
Results of BBS, TUG, and PBFS Tests
| Test | Study Group | Control Group | Statistical Test Results | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Me | Min | Max | Me | Min | Max | ||||
| BBS [pts] | 47.50 | 29.00 | 54.00 | 54.00 | 34.00 | 56.00 | U = 147.50 | ||
| p < 0.001 | |||||||||
| TUG [s] | 11.06 | 8.79 | 19.52 | 8.79 | 6.02 | 13.92 | U = 125.50 | ||
| p = 0.001 | |||||||||
| Test | Study Group | Control Group | Statistical Test Results | ||||||
| M | SD | M | SD | ||||||
| PBFS [mmHg] | 8.80 | 9.70 | 10.28 | 15.53 | t = −0.371 | ||||
| df = 38 | |||||||||
| p = 0.713 | |||||||||
Abbreviations: BBS, Berg Balance Scale; TUG, Timed Up and Go test; PBFS, Pressure Bio-Feedback Stabilizer; M, mean; SD, standard deviation; Me, mean; Min, minimum; Max, maximum; p, statistical significance; U, Mann–Whitney U-test; t, t-Student test; df, degrees of freedom.
The second (standing unsupported for 2 mins) and third task (sitting unsupported for 2 mins) turned out to be the least burdensome task for the patients from the study group, who obtained 3.93 and 4.0 points, respectively. However, the most challenging task in the study group was task fourteen (standing on one foot for as long as possible); here, the patients only reached 1.64 points.
The TUG results in the study group were higher than those in the control group (p = 0.001), which means that after a stroke, the patients need significantly more time to perform the task than do the healthy controls (Table 3).
Table 3.
Influence of BMI and Age on BBS, TUG, and PBFS Results in Both Groups
| Spearman Correlation Coefficient (BMI) | ||
|---|---|---|
| Variables | Study Group | Control Group |
| BBS [pts] |
rho = −0.415 p = 0.028 |
rho = −0.722 p < 0.001 |
| BMI [kg/m2] | ||
| TUG [s] |
rho = 0.285 p = 0.141 |
rho = 0.419 p = 0.021 |
| BMI [kg/m2] | ||
| PBFS [mmHg] |
rho = 0.05 p = 0.808 |
rho = 0.307 p = 0.099 |
| BMI [kg/m2] | ||
| Spearman correlation coefficient (age) | ||
| Variables | Study group | Control group |
| BBS [pts] |
rho = −0.585 p = 0.001 |
rho = −0.771 p < 0.001 |
| Age [years] | ||
| TUG [s] |
rho = 0.604 p = 0.017 |
rho = 0.321 p = 0.118 |
| Age [years] | ||
| PBFS [mmHg] |
rho = 0.604 p = 0.017 |
rho = 0.321 p = 0.118 |
| Age [years] | ||
| Spearman correlation coefficient (clinimetrics) | ||
| Variables | Study group | Control group |
| PBFS [mmHg] |
rho = −0.134 p = 0.541 |
rho = −0.358 p = 0.052 |
| BBS [pts] | ||
| PBFS [mmHg] |
rho = 0.180 p = 0.411 |
rho = 0.352 p = 0.056 |
| TUG [s] | ||
Abbreviations: BBS, Berg Balance Scale; TUG, Timed Up and Go test; PBFS, Pressure Bio-Feedback Stabilizer; BMI, Body Mass Index; p, statistical significance; rho, Spearman correlation coefficient.
Analysis of the PBFS results showed that, during the evaluation of the TrA muscle, the average increase in cuff pressure in the study group was 8.80 mmHg; in comparison, it was 10.28 mmHg in the control group (p = 0.713) (Table 2).
Analysis of the Results According to BMI
A statistically significant negative correlation between the higher BMI values and the lower BBS score was shown in the study group (p = 0.028, rho = −0.415). A stronger correlation was observed in the control group (p < 0.001, rho = −0.771) (Table 3).
A statistically significant positive correlation between the higher BMI values and increased TUG result was shown in both, the study group (p = 0.032, rho = 0.405) the control group (p = 0.021, rho = 0.419) in comparable level of strength (Table 3).
There was no correlation between higher BMI values and the TrA muscle activity performed with the PBFS in both, the study group (p = 0.808, rho = 0.05) and the control group (p = 0.099, rho = 0.307) (Table 3).
Analysis of the Results According to Age
A statistically significant negative correlation between the older age and the lower BBS score was shown in the study group (p = 0.001, rho = −0.585). A stronger correlation was observed in the control group (p < 0.001, rho = −0.771) (Table 3).
A statistically significant positive correlation between the older age and increased TUG result was shown in the study group (p = 0.017, rho = 0.604). There was no statistically significant correlation between these variables in the control group (p = 0.118, rho = 0.321) (Table 3).
A statistically significant positive correlation was revealed between the older age and the TrA muscle activity performed with the PBFS in the study group (p = 0.017, rho = 0.604). There was no statistically significant correlation between these variables in the control group (p = 0.117, rho = 0.614) (Table 3).
Analysis of the Results According to Gender
The results of the BSS test in the study group were similar for women (Me = 46.50 pts) and men (Me = 48 pts). On the other hand, the results of the BSS test among the women in the control group were higher (Me = 55.00 pts) than among the men (Me = 53 pts) at a statistically significant level (p = 0.023) (Table 4).
Table 4.
Results for BBS, TUG, and PBFS Tests According to Participants’ Sex
| Test | Female | Male | Statistical Test Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Me | Min | Max | Me | Min | Max | |||||
| BSS [pts] | Study group | 46.50 | 29.00 | 54.00 | 48.00 | 30.00 | 54.00 | U = 84.50 | ||
| p = 0.791 | ||||||||||
| Control group | 55.00 | 49.00 | 56.00 | 53.00 | 34.00 | 56.00 | U = 41.00 | |||
| p = 0.023 | ||||||||||
| TUG [s] | Study group | 12.29 | 8.79 | 16.00 | 11.15 | 8.88 | 34.49 | U = 69.00 | ||
| p = 0.868 | ||||||||||
| Control group | 8.57 | 6.53 | 12.50 | 9.50 | 7.52 | 13.92 | U = 53.00 | |||
| p = 0.678 | ||||||||||
| Test | Female | Male | Statistical Test Results | |||||||
| M | SD | M | SD | |||||||
| PBFS [mmHg] | Study group | 7.70 | 8.20 | 11.47 | 13.35 | t = –0.418 | ||||
| df = 42 | ||||||||||
| p = 0.531 | ||||||||||
| Control group | 4.85 | 13.14 | 16.17 | 16.28 | t = 1.920 | |||||
| df = 23 | ||||||||||
| p = 0.067 | ||||||||||
Abbreviations: BBS, Berg Balance Scale; TUG, Timed Up and Go test; PBFS, Pressure Bio-Feedback Stabilizer; M, mean; SD, standard deviation; Me, mean; Min, minimum; Max, maximum; p, statistical significance; U, Mann–Whitney U-test; t, t-Student test; df, degrees of freedom.
In the TrA muscle activity PBFS test performed in the study group, the women scored lower mean values than the men, 7.70 and 11.47 mmHg, respectively (p = 0.791). In comparison, when analyzing the results in the control group, it can be observed that the mean score for the TrA muscle activity measurement was lower in the women compared to the men, 4.85 and 16.17 mmHg, respectively (p = 0.067) (Table 4).
Analysis of the Results According to the Place of Residence
The median score obtained in the BBS test in the surveyed group among the people living in the city was 47.00 points, while it was 53.00 points among the people from villages (p = 0.021). In the control group, the opposite results were obtained: the people living in the city obtained 55.00 points, and the people from the villages obtained 52.50 points (p = 0.034) (Table 5).
Table 5.
Results for BBS, TUG, and PBFS Tests According to Participants’ Residence
| Test | City | Village | Statistical Test Results | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Me | Min | Max | Me | Min | Max | |||||
| BSS [pts.] | Study group | 47.00 | 29.00 | 54.00 | 53.00 | 41.00 | 54.00 | U = 30.00 | ||
| p = 0.021 | ||||||||||
| Control group | 55.00 | 46.00 | 56.00 | 52.50 | 37.00 | 56.00 | U = 43.50 | |||
| p = 0.034 | ||||||||||
| TUG [s.] | Study group | 12.23 | 9.00 | 44.00 | 10.85 | 9.00 | 20.00 | U = 34.00 | ||
| p = 0.036 | ||||||||||
| Control group | 8.50 | 7.00 | 22.00 | 9.25 | 8.00 | 25.00 | U = 37.00 | |||
| p = 0.356 | ||||||||||
| Test | City | Village | Statistical Test Results | |||||||
| M | SD | M | SD | |||||||
| PBFS [mmHg] | Study group | 7.20 | 6.61 | 12.00 | 14.56 | t = −0.702 | ||||
| df = 5 | ||||||||||
| p = 0.515 | ||||||||||
| Control group | 4.88 | 13.09 | 21.75 | 14.64 | t = −2.898 | |||||
| df = 23 | ||||||||||
| p = 0.008 | ||||||||||
Abbreviations: BBS, Berg Balance Scale; TUG, Timed Up and Go test; PBFS, Pressure Bio-Feedback Stabilizer; M, mean; SD, standard deviation; Me, mean; Min, minimum; Max, maximum; p, statistical significance; U, Mann–Whitney U-test; t, t-Student test; df, degrees of freedom.
The median of the TUG test in the study group among the people living in the city was 12.23 seconds, while it was 10.85 seconds in the group living in the village (p = 0.036). Likewise, the median of the TUG test in the control group of the people living in the city was 8.50 seconds, while it was 9.25 seconds in the group living in the village (Table 5).
The mean score for TrA muscle activity obtained with the PBFS in the study group of urban residents was 7.20 mmHg, in comparison to rural residents with a higher score of 12.00 mmHg. The results turned out to be statistically insignificant (p = 0.515). The average PBFS score in the control group of urban residents was 4.88 mmHg, in comparison to rural residents who scored significantly higher at 21.75 mmHg (p = 0.008) (Table 5).
Analysis of the Results According to the Time from Stroke
The results of the BSS balance test of people who were examined up to and including one month after a stroke ranged from 29 to 54 points (Me = 48.5 pts). In comparison, the results of the people who took part in the study more than one month after the stroke were limited in values: 30 and 49 points (Me = 44 pts) (p = 0.087) (Table 6).
Table 6.
Results for BBS, TUG, and PBFS Test According to the Time of Stroke in the Study Group
| Test | ≤ 1 Month Since a Stroke | > 1 Month Since a Stroke | Statistical Test Results | ||||
|---|---|---|---|---|---|---|---|
| Me | Min | Max | Me | Min | Max | ||
| BSS [pts.] | 48.50 | 29.00 | 54.00 | 44.00 | 30.00 | 49.00 | U = 46.50 |
| p = 0.087 | |||||||
| TUG [s.] | 10.29 | 8.79 | 15.53 | 17.76 | 11.11 | 34.34 | U = 10.00 |
| p = 0.001 | |||||||
| PBFS [mmHg] | 6.00 | −2.00 | 28.00 | 10.00 | 0.00 | 26.00 | U = 23.00 |
| p = 0.806 | |||||||
Abbreviations: BBS, Berg Balance Scale; TUG, Timed Up and Go test; PBFS, Pressure Bio-Feedback Stabilizer; Me, mean; Min, minimum; Max, maximum; p, statistical significance; U, Mann–Whitney U-test.
Median of TUG test of subjects who were tested up to 1 month after the stroke was 10.29 seconds. However, it was 17.76 seconds for those who took part in the study for more than one month after the stroke (p < 0.001) (Table 6).
The results of the TrA muscle activity test using the PBFS of the subjects who were examined up to and including one month after a stroke were between −2 and 28 mmHg (Me = 6 mmHg). In comparison, the results in the patients who were more than one month after the stroke were 0 and 26 mmHg (Me = 10 mmHg) (p = 0.806) (Table 6).
Analysis of the Results According to the Side of the Hemiparesis
The results of the BSS balance test for people with left-sided hemiparesis ranged from 30 to 54 points (Me = 47.00 pts). In comparison, the results of the right-sided hemiparesis were within the range from 29.00 to 54.00 points (Me = 49.00 pts). The results of the two groups do not differ significantly from each other statistically (p = 0.549) (Table 7).
Table 7.
Results for BBS, TUG, and PBFS Tests According to the Side of Hemiparesis in the Study Group
| Test | Left-Sided Hemiparesis | Right-Sided Hemiparesis | Statistical Test Results | ||||
|---|---|---|---|---|---|---|---|
| Me | Min | Max | Me | Min | Max | ||
| BSS [pts.] | 47.00 | 30.00 | 54.00 | 49.00 | 29.00 | 54.00 | U = 71.00 |
| p = 0.549 | |||||||
| TUG [s.] | 12.23 | 9.14 | 35.24 | 10.98 | 8.79 | 16.00 | U = 51.00 |
| p = 0.183 | |||||||
| PBFS [mmHg] | 2.00 | −2.00 | 28.00 | 17.00 | −2.00 | 26.00 | U = 11.50 |
| p = 0.330 | |||||||
Abbreviations: BBS, Berg Balance Scale; TUG, Timed Up and Go test; PBFS, Pressure Bio-Feedback Stabilizer; Me, mean; Min, minimum; Max, maximum; p, statistical significance; U, Mann–Whitney U-test.
The median TUG test result of the patients with left-sided hemiparesis was 12.23 seconds, and it was 10.98 seconds for people with right-sided hemiparesis (p = 0.549) (Table 7).
The results of the TrA muscle activity test on the PBFS in patients with left-sided hemiparesis ranged from −2.00 to 28.00 mmHg (Me =2.00 mmHg). The results of the people with right-sided hemiparesis ranged from −2.00 to 26 mmHg (Me = 17.00 mmHg) (p = 0.330) (Table 7).
Discussion
The limit values obtained in our study in the BBS balance test in the study group ranged from 29.00 to 54.00 points. The average score obtained by the respondents was 45.43 points (Me = 47.50 pts). While the number of points obtained does not qualify for a group with a higher risk of falls, it does signal caution during patients’ walk. Although the BBS test showed a higher average score of 51.92 points in the control group, this was not the maximum. The results of the next test, a TUG test, ranged from 8.79 to 19.52 seconds; the average time needed to perform this task was 11.06 seconds. The results of the subsequent TUG test ranged from 8.79 to 19.52 seconds. The average time needed to perform this task was 11.06 seconds.
Regarding associations between BMI values and subsequent study outcomes, only one significant correlation was found between the higher BMI values and the lower BBS score in both study and control groups (stronger correlation in controls). It confirms that overweight patients have a potentially more significant postural imbalance, which determines a higher risk of falls. We observed a significant relationship between higher BMI values and increased TUG results in both groups. This relationship was entirely predictable, as a higher degree of overweight will have a substantial impact on the quality and speed of gait, especially in patients after a stroke. Also, we found no correlations between higher BMI values and the PBFS results in both tested groups. The fact that BMI values do not affect the TrA muscle activity is somewhat surprising, as it would seem that people with higher levels of overweight might show less ability to activate the TrA muscle, which would indicate less stability of the lower trunk. However, to answer this question unequivocally, patients should be grouped into particular degrees of overweight and then correlated with PBFS results.
The above results are similar to the data obtained by Persson et al,34 where the maximum number of points awarded for the BBS equilibrium test was 56 (Me = 42 pts). In their TUG test, minimum and maximum scores of 8.00 and 59.00 seconds were obtained, respectively. In comparing these results to our research, it is clear that the minimum value is only 0.79 seconds slower. Both of the minimum values of the TUG test are within the same time range (up to 10 seconds) as the standard and range of safe, self-maneuverability. There is a more significant difference between the maximum values, which is most likely the result of qualifying for the study by Persson et al.34 The patients with severe functional status, which is also indicated by the minimal BBS test result, had zero points in the quoted studies.
Other studies conducted by Drużbicki and Przysada35 showed that people with a stroke with moderate levels of efficiency on the side of the hemiparesis obtain an average of 41.00 BBS points after a stroke. This score is the lower limit for the scoring of people who are considered independent in their day-to-day life. However, in Drużbicki and Przysada’s studies,35 the patients needed 24.00 seconds on average in the TUG test, in comparison to 13.26 seconds in our study. Further studies by Kalisz et al36 additionally indicate that post-stroke patients are mostly independent during everyday activities, as the average number of points awarded to them from the BBS test was 44.12 points. The worst patient results (the non-standard) during the TUG test were noted by Kalisz et al,36 where the meantime was 26.97 seconds.
A negative result was the most demanded result, proving the proper activation of TrA muscle – unfortunately, none of the studied groups managed to achieve this. The results of the study group indicate that, on average, the cuff pressure increased by 8.80 mmHg during the measurement. The value was even higher, amounting to 10.28 mmHg, in the healthy group. The results indicate a lack of ability to lead to conscious contraction of TrA, which may suggest a lack of adequate muscle tension forming the abdominal cylinder, which by ensuring the stability of the spine affects the maintenance of balance and safety during movement. Because of the lack of available literature on this subject, it is not possible to determine whether studies by other authors confirm our obtained results.
In summary, most post-stroke patients cope with simple everyday tasks such as getting up from a chair or lifting an object off the floor. The high mean score obtained by the BBS patients demonstrates this fact. Each time it was equal to or greater than 41.00 points. This number was a point threshold below which the patient was qualified to a group with a higher risk of falling during the above-mentioned activities, and it was/is recommended that they use additional rehabilitation equipment while moving.
Unfortunately, more significant difficulties occur in post-stroke patients during gait, which by definition, means temporary loss and restoration of equilibrium. Only properly cooperating nervous and musculoskeletal system centers can ensure the proper postural stability of a person while moving. The results of the TUG test in the studies by Kalisz et al36 and Drużbicki and Przysada35 indicate that, after a stroke, patients remain at increased or high risk of falls.
Study Limitations
There are several methodological limitations to be mentioned in our study. First, we used two clinimetric methods for assessment of balance (BBS) and falls (TUG) and one measurement tool (PBFS) for objective evaluation of functional stability. It should be emphasized that some other valuable devices could be used, such as surface electromyography (sEMG) for assessment of TrA muscle rest and functional activity (local assessment) or stabilometric platform for observation of postural sway during static and dynamic conditions (global assessment) which have to be considered in the future studies in this subject. Also, our study could present some potential bias during sample selection due to its non-randomized design. Participants in the control group took part in this study as volunteers; thus, self-selection could exist. In the case of the study group, convenience sampling was used. We avoided other biases such as susceptibility and attrition, as wee as an early termination a pre-screening of study participants, performing repeated experiments and reporting only the most favorable results, and presenting the most significant result.
Conclusion
The consequences of a stroke have an essential negative effect on postural stability, balance, and risk of falls. Place of residence was the only factor affecting postural stability after stroke. Remaining factors such as age, gender, time from stroke, and the side of the hemiparesis do not have a negative effect on postural stability in overweight post-stroke patients.
Acknowledgments
There were no contributors to the article other than the authors, and there was no writing assistance required. The certified English language services were provided by an academic highly qualified native speaker.
Abbreviations
BBS, Berg Balance Scale; PBFS, Pressure Bio-Feedback Stabilizer; STROBE, Strengthening the Reporting of Observational Studies in Epidemiology; TrA, transversus abdominis muscle; TUG, Timed Up and Go test; WSO, World Stroke Organization.
Funding
This study was conducted under a research project funded by the Ministry of Science and Higher Education of Poland as a part of a statutory grant of the Wroclaw Medical University for maintaining research potential (no. SUB.E.060.19.001).
Disclosure
The authors declare no potential conflict of interest with respect to the research, authorship, or publication of this article.
References
- 1.Benjamin EJ, Muntner P, Alonso A, et al. heart disease and stroke statistics-2019 update: a report from the American Heart Association. Circulation. 2019;139(10):e56–e528. doi: 10.1161/CIR.0000000000000659 [DOI] [PubMed] [Google Scholar]
- 2.Pająk A, Szafraniec K, Janion M, et al. The impact of the Polish national Programme of Cardiovascular Disease Prevention on the quality of primary cardiovascular disease prevention in clinical practice. Kardiol Pol. 2010;68(12):1332–1340. [PubMed] [Google Scholar]
- 3.Gubitz G, Saini M, Belson S, Sahathevan R, Sandercock P, WSO Education Committee and WSO Educational Needs Workshop participants. How can the World Stroke Organization (WSO) optimize education in stroke medicine around the world? Report of the 2018 WSO Global Stroke Stakeholder Workshop. Int J Stroke. 2019;1747493019874726. doi: 10.1177/1747493019874726 [DOI] [PubMed] [Google Scholar]
- 4.Sun H, Zou X, Liu L. Epidemiological factors of stroke: a survey of the current status in China. J Stroke. 2013;15(2):109–114. doi: 10.5853/jos.2013.15.2.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grabowska-Fudala B, Jaracz K, Górna K. Stroke incidence case fatality and mortality – current trends and future prognosis. Epidemiol Rev. 2010;64(3):439–442. [PubMed] [Google Scholar]
- 6.Howard G, Goff DC. Population shifts and the future of stroke: forecasts of the future burden of stroke. Ann N Y Acad Sci. 2012;1268:14–20. doi: 10.1111/j.1749-6632.2012.06665.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Coleman ER, Moudgal R, Lang K, et al. Early rehabilitation after stroke: a narrative review. Curr Atheroscler Rep. 2017;19(12):59. doi: 10.1007/s11883-017-0686-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Calvo-Lobo C, Useros-Olmo AI, Almazán-Polo J, et al. Rehabilitative ultrasound imaging of the bilateral intrinsic plantar muscles and fascia in post-stroke survivors with hemiparesis: a case-control study. Int J Med Sci. 2018;15(9):907–914. doi: 10.7150/ijms.25836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Calvo-Lobo C, Useros-Olmo AI, Almazán-Polo J, et al. Quantitative ultrasound imaging pixel analysis of the intrinsic plantar muscle tissue between hemiparesis and contralateral feet in post-stroke patients. Int J Environ Res Public Health. 2018;15(11):2519. doi: 10.3390/ijerph15112519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Donkor ES. Stroke in the 21st century: a snapshot of the burden, epidemiology, and quality of life. Stroke Res Treat. 2018;2018. doi: 10.1155/2018/3238165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baggio JAO, Mazin SSC, Alessio-Alves FF, et al. Verticality perceptions associate with postural control and functionality in stroke patients. PLoS One. 2016;11(3):e0150754. doi: 10.1371/journal.pone.0150754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ahn S-N. Differences in body awareness and its effects on balance function and independence in activities of daily living for stroke. J Phys Ther Sci. 2018;30(11):1386–1389. doi: 10.1589/jpts.30.1386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maraka S, Jiang Q, Jafari-Khouzani K, et al. Degree of corticospinal tract damage correlates with motor function after stroke. Ann Clin Transl Neurol. 2014;1(11):891–899. doi: 10.1002/acn3.132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Oliveira CB, Medeiros ÍRT, Greters MG, et al. Abnormal sensory integration affects balance control in hemiparetic patients within the first year after stroke. Clinics (Sao Paulo). 2011;66(12):2043–2048. doi: 10.1590/s1807-59322011001200008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osoba MY, Rao AK, Agrawal SK, Lalwani AK. Balance and gait in the elderly: a contemporary review. Laryngoscope Investig Otolaryngol. 2019;4(1):143–153. doi: 10.1002/lio2.252 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tyson SF, Hanley M, Chillala J, Selley A, Tallis RC. Balance disability after stroke. Phys Ther. 2006;86(1):30–38. doi: 10.1093/ptj/86.1.30 [DOI] [PubMed] [Google Scholar]
- 17.Hugues A, Di Marco J, Janiaud P, et al. Efficiency of physical therapy on postural imbalance after stroke: study protocol for a systematic review and meta-analysis. BMJ Open. 2017;7(1):e013348. doi: 10.1136/bmjopen-2016-013348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho K, Yu J, Rhee H. Risk factors related to falling in stroke patients: a cross-sectional study. J Phys Ther Sci. 2015;27(6):1751–1753. doi: 10.1589/jpts.27.1751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jørgensen L, Engstad T, Jacobsen BK. Higher incidence of falls in long-term stroke survivors than in population controls: depressive symptoms predict falls after stroke. Stroke. 2002;33(2):542–547. doi: 10.1161/hs0202.102375 [DOI] [PubMed] [Google Scholar]
- 20.Abraham KA, Feingold H, Fuller DD, Jenkins M, Mateika JH, Fregosi RF. Respiratory-related activation of human abdominal muscles during exercise. J Physiol. 2002;541(Pt 2):653–663. doi: 10.1113/jphysiol.2001.013462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kim E, Lee H. The effects of deep abdominal muscle strengthening exercises on respiratory function and lumbar stability. J Phys Ther Sci. 2013;25(6):663–665. doi: 10.1589/jpts.25.663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Allison GT. Abdominal muscle feedforward activation in patients with chronic low back pain is largely unaffected by 8 weeks of core stability training. J Physiother. 2012;58(3):200. doi: 10.1016/S1836-9553(12)70114-5 [DOI] [PubMed] [Google Scholar]
- 23.Crommert ME, Ekblom MM, Thorstensson A. Activation of transversus abdominis varies with postural demand in standing. Gait Posture. 2011;33(3):473–477. doi: 10.1016/j.gaitpost.2010.12.028 [DOI] [PubMed] [Google Scholar]
- 24.Kim HD, You JM, Han N, Eom MJ, Kim JG. Ultrasonographic measurement of transverse abdominis in stroke patients. Ann Rehabil Med. 2014;38(3):317–326. doi: 10.5535/arm.2014.38.3.317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Izzo R, Guarnieri G, Guglielmi G, Muto M. Biomechanics of the spine. Part I: spinal stability. Eur J Radiol. 2013;82(1):118–126. doi: 10.1016/j.ejrad.2012.07.024 [DOI] [PubMed] [Google Scholar]
- 26.Smith PS, Hembree JA, Thompson ME. Berg Balance Scale and Functional Reach: determining the best clinical tool for individuals post acute stroke. Clin Rehabil. 2004;18(7):811–818. doi: 10.1191/0269215504cr817oa [DOI] [PubMed] [Google Scholar]
- 27.Downs S, Marquez J, Chiarelli P. The Berg Balance Scale has high intra- and inter-rater reliability but absolute reliability varies across the scale: a systematic review. J Physiother. 2013;59(2):93–99. doi: 10.1016/S1836-9553(13)70161-9 [DOI] [PubMed] [Google Scholar]
- 28.Kear BM, Guck TP, McGaha AL. Timed Up and Go (TUG) Test. J Prim Care Community Health. 2017;8(1):9–13. doi: 10.1177/2150131916659282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barry E, Galvin R, Keogh C, Horgan F, Fahey T. Is the timed up and go test a useful predictor of risk of falls in community dwelling older adults: a systematic review and meta- analysis. BMC Geriatr. 2014;14:14. doi: 10.1186/1471-2318-14-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Oliveira IO, Pilz B, Santos RLG, Vasconcelos RA, Mello W, Grossi DB. Reference values and reliability for lumbopelvic strength and endurance in asymptomatic subjects. Braz J Phys Ther. 2018;22(1):33–41. doi: 10.1016/j.bjpt.2017.09.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.de Paula Lima PO, de Oliveira RR, Costa LOP, Laurentino GEC. Measurement properties of the pressure biofeedback unit in the evaluation of transversus abdominis muscle activity: a systematic review. Physiotherapy. 2011;97(2):100–106. doi: 10.1016/j.physio.2010.08.004 [DOI] [PubMed] [Google Scholar]
- 32.da Silva AP, Dos Santos RPM, Coertjens PC, Coertjens M. Clinimetric properties of the pressure biofeedback unit method for estimating respiratory pressures. Physiother Theory Pract. 2017;33(4):345–351. doi: 10.1080/09593985.2017.1289577 [DOI] [PubMed] [Google Scholar]
- 33.França FR, Burke TN, Hanada ES, Marques AP. Segmental stabilization and muscular strengthening in chronic low back pain ‐ a comparative study. Clinics (Sao Paulo). 2010;65(10):1013–1017. doi: 10.1590/S1807-59322010001000015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Persson CU, Hansson P-O, Sunnerhagen KS. Clinical tests performed in acute stroke identify the risk of falling during the first year: postural stroke study in Gothenburg (POSTGOT). J Rehabil Med. 2011;43(4):348–353. doi: 10.2340/16501977-0677 [DOI] [PubMed] [Google Scholar]
- 35.Drużbicki M, Przysada G. Assessment of the balance and risk of falls in the group of patients with hemiplegic paresis after stroke. Young Sport Sci Ukraine. 2011;3:120–125. [Google Scholar]
- 36.Kalisz K, Kalisz Z, Hagner-Derengowska M, Żukow W, Trela E. Assessment of balance in patients after a stroke on the basis of selected scales and tests. J Health Sci. 2012;2(4):141–177. [Google Scholar]