Abstract
Introduction
Platelets are one factor promoting tumor development. Conversely, lymphocytes are one factor for immune protection. The peripheral blood platelets–lymphocyte ratio (PLR) is useful as an inflammation/immune indicator to predict postoperative recurrence and prognosis of a variety of malignancies. The peripheral blood neutrophil–lymphocyte ratio (NLR) has also been reported as a useful inflammation/immune indicator. However, there are few studies evaluating the relationship between these peripheral blood indicators and the effectiveness of chemotherapy. Thus, we examined these relationships in gastric cancer patients.
Patients and Methods
Between 2005 and 2018, 41 gastric cancer patients treated with preoperative DCS therapy (docetaxel, cisplatin, and S-1) therapy followed by gastrectomy were evaluated. Data for peripheral blood tests prior to the initiation of chemotherapy were used. The effectiveness of chemotherapy was determined using Response Evaluation Criteria in Solid Tumors (RECIST) and the pathological response of primary lesions (Ef grade). The relationship between the blood test results and the effectiveness of chemotherapy was evaluated.
Results
Each optimal cut-off value of peripheral inflammation/immune indicators was calculated through ROC curves. Although the pathological responder (Ef grade 2 or 3) revealed significantly better prognosis than the non-responder (Ef grade 0-1b), no relationship was found between responder according to RECIST and prognosis (P=0.014, P=0.992). In univariate analysis, a low PLR (<180, P=0.005), low NLR (<2.6, P=0.019), high lymphocyte (≥1.43, P=0.019) and high PNI (≥40, P=0.032) were identified as prognostic markers, whereas PLR was the only marker correlated with pathological response (P=0.031).
Conclusion
PLR obtained prior to chemotherapy might be a useful indicator for predicting chemosensitivity owing to the simplicity of its procedure.
Keywords: gastric cancer, preoperative chemotherapy, chemosensitivity, platelet-lymphocyte ratio
Introduction
Gastric cancer is a major cause of cancer-related deaths in East Asia. Multidisciplinary therapy combining chemotherapy with surgery is considered to be important for treating this disease. The safety and efficacy of preoperative chemotherapy have been reported by multiple studies in recent years.1–3 The JCOG0405 (Japan Clinical Oncology Group) study reported the safety of S-1 plus cisplatin treatment as preoperative chemotherapy followed by D2 gastrectomy with para-aortic lymph node dissection. The 3- and 5-year overall survival rate of this approach is 59% and 53%, respectively, which suggests its effectiveness.3 However, some patients who show no response to chemotherapy have fatal outcomes because of delayed surgery. For this reason, a predictive biomarker for the effectiveness of chemotherapy is required.
Extravascular platelets deposited in the cancer microenvironment have been reported as a short-term prognostic factor and are related to anticancer drug resistance.4 These effects are thought to be a result of microparticles from activated platelets, such as vascular endothelial growth factor (VEGF) and transforming growth factor-β (TGF-β), both of which promote tumor development. Many reports identify platelets as cancer development factors,5–7 however, immunostaining is required for identifying extravascular platelets. Hence, new simple biomarkers should be explored.
Peripheral blood inflammation/immuno-nutrition indicators, such as the platelets–lymphocyte ratio (PLR), neutrophil–lymphocyte ratio (NLR), and prognostic nutritional index (PNI), are widely recognized as useful prognostic predictors in various malignancies.8–11 A low NLR or a high peripheral lymphocyte count could be predictors for a high efficacy of preoperative chemotherapy for breast cancer patients.12–14 To our knowledge, this is the first report to investigate whether a peripheral inflammation/immuno-nutrition indicator could predict the efficacy of preoperative chemotherapy in gastric cancer.
Patients and Methods
Ethical Approval
Prior to the research, written informed consent was obtained from each patient. The present study was in accordance with the ethical standards of the responsible committees on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions. This study was approved by the Institutional Review Board of Kanazawa University Graduate School of Medical Science (Study permission number 1840-1).
Patients
Forty-one patients with advanced gastric cancer between 2005 and 2018 in whom preoperative modified DCS (mDCS) therapy was administered were selected as the study subjects and were retrospectively analyzed. The eligibility criteria were as follows: all gastric cancer patients (either cStage III or cStage IV) with ≤3 peripheral liver metastases and para-aortic lymph node metastases. According to the Japanese Classification of Gastric Cancer (JCGC) 3rd English edition,15 para-aortic lymph node metastasis is defined as a swelling of ≥10 mm on a 2.5-mm slice contrast-CT scan. An absence of peritoneal dissemination was confirmed by intraperitoneal observation. Patients aged between 20 and 80 years with an Eastern Cooperative Oncology Group (ECOG) Performance Status (PS) rank from 0 to 1 were included. No other preoperative chemotherapy, radiation therapy, or gastric surgery were administered to any of the patients. No patient had any signs of hemorrhage from the primary lesion. The oral intake of the patients was favorable and hematopoietic, liver, and kidney functions were all maintained. The following patients were excluded from the study: patients with cardiovascular disease, pulmonary fibrosis, hemorrhaging tendencies, poorly controlled high blood pressure, diabetes mellitus, active malignancy, central nervous system disease, history of severe drug allergy, and pregnant, or breastfeeding patients. The blood test results used were obtained within 1 week prior to the initiation of chemotherapy. When multiple results within this 1-week period were available, the latest one was used.
Treatment
mDCS therapy that originated in Kanazawa University was used as preoperative chemotherapy with the goal of reducing the adverse effects and enhancing the effectiveness of the chemotherapy.16–18 The content of the protocol was as follows: 35 mg/m2 docetaxel as a 1-h IV drip on days 1 and 15, and 35 mg/m2 cisplatin as 2-h IV drip with simultaneous maintenance of hydration on days 1 and 15. S1 was administered orally at a dosage of 80 mg/m2 on days 1 through 14. One cycle lasted for 4 weeks, and only patients for whom a minimum of two cycles was administered were chosen as subjects. The surgical treatments were complete gastrectomy, D2 lymph node dissection, para-aortic lymph node dissection, and hepatectomy for R0 resection. The para-aortic lymph nodes were defined as lymph nodes 16a2 and b1 between the upper edge of the celiac artery and the lower edge of the inferior mesenteric artery.
Response Evaluation
Following two cycles of preoperative mDCS therapy, tumor regression was evaluated by a contrast-CT scan using RECIST.19 The evaluation categories were as follows: complete response (CR, complete disappearance the tumor), partial response (PR, reduction of tumor by ≥30% or more), progressive disease (PD, enlargement of tumor by ≥20%), and stable disease (SD, reduction less than PR or enlargement less than PD). Within these RECIST criteria, CR and PR cases were considered responders.
The pathological effectiveness of chemotherapy was also judged in accordance with the categories defined in the JCGC (3rd English Edition). The five classifications range from complete response (Grade 3) to no effect (Grade 0). The subjects were rated as follows depending on the degree of degeneration or necrosis of the invasive cancer cells: Grade 1a: ≥2/3 of remaining cancer cells, Grade 1b: 1/3 to 2/3 of remaining cancer cells, and Grade 2: <1/3 of remaining cancer cells. Grades 2 and 3 were considered pathological responses in this study. All specimens were evaluated by two independent pathologists.
Statistical Analyses
PLR, NLR, neutrophil-to-monocyte ratio (NMR), and lymphocyte-to-monocyte ratio (LMR) were calculated based on the peripheral blood test. PNI was calculated based on serum albumin values and peripheral blood lymphocyte count was calculated as [PNI = (10 × albumin) + (0.005 × TLC (total lymphocyte count))]. Through receiver operating characteristic (ROC) curve analysis, we obtained the optimal cut-off levels and areas under the curves (AUCs) of routine blood parameters and their ratios, as shown in Table 1. The Ef grade was applied to select the optimal cut-off points. In addition, we adopted the upper limit at our hospital as the cut-off value for CRP and tumor markers, such as CEA, CA19-9, CA125, and AFP. Fisher’s test was used to determine the difference in the clinicopathological parameters and chemotherapy response. The Kaplan–Meier method and the Log-rank test were used for survival analysis. Multivariate analysis was performed using the Cox hazard model as the prognostic parameters. P< 0.05 was considered statistically significant. SPSS version 23 (IBM Corp., Armonk, NY, USA) was used for the analyses.
Table 1.
Optimal Cut-Off Values Together with AUCs
| Variates | AUC | Cut-off Point |
|---|---|---|
| PLR | 0.682 | 180 |
| NLR | 0.626 | 2.4 |
| NMR | 0.535 | 1.1 |
| LMR | 0.462 | 4.01 |
| Platelet | 0.666 | 240×109/L |
| Lymphocyte | 0.493 | 1.43×109/L |
| Neutrophil | 0.562 | 4.1×109/L |
| Monocyte | 0.521 | 0.33×109/L |
| PNI | 0.502 | 40 |
| Albumin | 0.464 | 3.6 g/dL |
Abbreviations: PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to lymphocyte ratio; NMR, neutrophil-to-monocyte ratio; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index.
Results
Characteristics of the Patients
Forty-one patients who received mDCS therapy as preoperative chemotherapy treatment between 2005 and 2018 were included. The characteristics of the patients are shown in Table 2. Para-aortic lymph node metastasis was identified in 16 patients (39%), and liver metastasis was identified in 9 patients (22%). The preoperative clinical tumor stage was stage III in 14 patients and stage IV in 27 patients.
Table 2.
Patient Characteristics
| Characteristics | ||
|---|---|---|
| Number of patients | 41 | |
| Age: median (range) | 65 (30–78) | |
| Gender | Male | 34 |
| Female | 7 | |
| ECOG performance status | ≧1 | 13 |
| 0 | 28 | |
| Differentiation | Poorly | 19 |
| Moderate-well | 22 | |
| Clinical T stage | cT1 | 0 |
| cT2 | 4 | |
| cT3 | 13 | |
| cT4 | 24 | |
| Clinical N stage | cN0 | 2 |
| cN1 | 2 | |
| cN2 | 19 | |
| cN3 | 18 | |
| Clinical stage | 0 | 0 |
| I | 0 | |
| II | 0 | |
| III | 14 | |
| IV | 27 | |
| RECIST | CR | 0 |
| PR | 29 | |
| SD | 11 | |
| PD | 1 | |
| Histological evaluation criteria (Grade) | 3 | 2 |
| 2 | 19 | |
| 1b | 3 | |
| 1a | 16 | |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; RECIST, Response Evaluation Criteria in Solid Tumors; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease.
Response Rates
Among the patients receiving preoperative mDCS treatment, 29 patients (70.1%) were identified by RECIST as clinical responders and 21 patients (51.2%) were identified as pathological responders.
Relationships Between Overall Survival and Clinicopathological Parameters
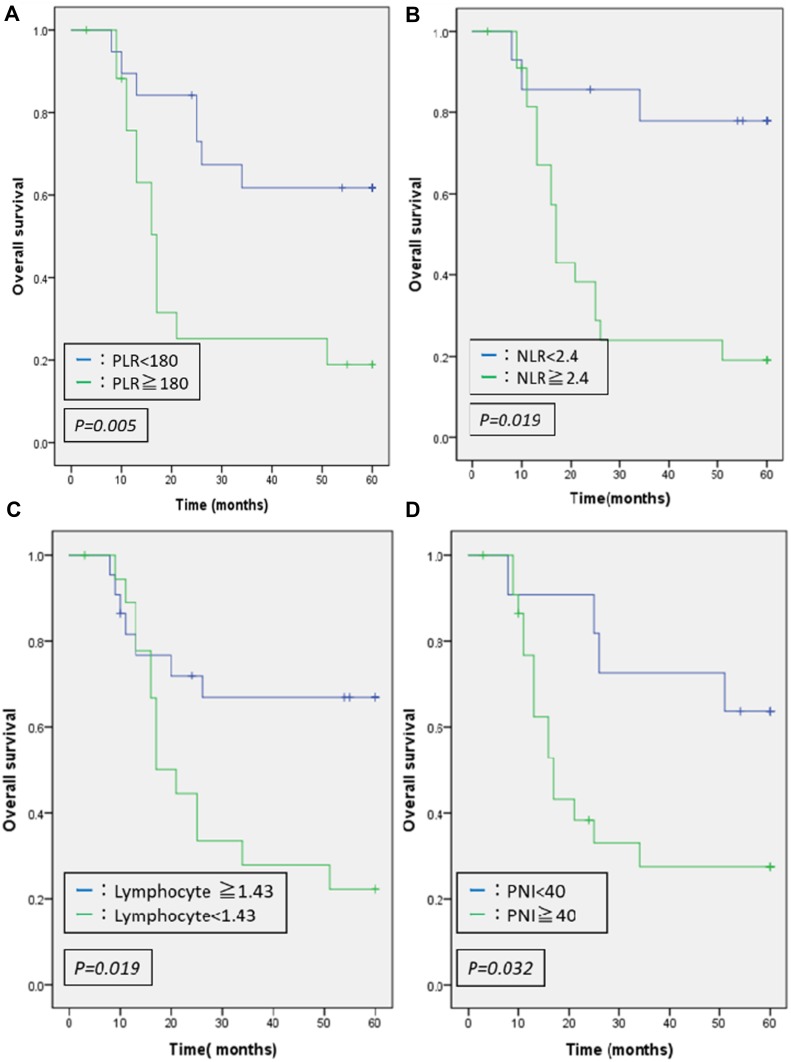
Table 3 shows the relationships between OS and each clinicopathological factors. In the univariate analysis, lower Ef grade was identified as a risk factor for OS (P=0.014). Furthermore, significant differences were identified in the blood test findings in the high PLR group (P=0.005), high NLR group (P=0.019), low lymphocyte group (P=0.019) and low PNI group (P=0.032) all of whom exhibiting shortened OS in the univariate analysis. No significant difference was identified in the multivariate analysis. As shown in Figure 1, Kaplan–Meier curves revealed significantly better survival in lower PLR, lower NLR, high lymphocytes, and high PNI group.
Table 3.
Univariate/Multivariate Analyses of Factors Associated with Prognosis
| Parameters (Cut-off Point) | Univariate P-value | Multivariate HR (95% CI) | P-value |
|---|---|---|---|
| Age | 0.329 | ||
| Gender | 0.037 | 0.379(0.092–1.555) | 0.178 |
| PS | 0.264 | ||
| Differentiation | 0.319 | ||
| TNM stage | 0.75 | ||
| T stage | 0.676 | ||
| N stage | 0.665 | ||
| RECIST | 0.992 | ||
| Histrogical evaluation criteria | 0.014 | 2.672(0.812–8.794) | 0.106 |
| PLR (180) | 0.005 | 4.778(0.984–23.205) | 0.052 |
| NLR (2.4) | 0.019 | 0.588(0.116–2.987) | 0.522 |
| NMR (1.1) | 0.661 | ||
| LMR (4.05) | 0.07 | ||
| Platelet (240) | 0.839 | ||
| Lymphocyte (1.43) | 0.019 | 1.360(0.328–5.639) | 0.672 |
| Neutrophil (4.1) | 0.533 | ||
| Monocyte (0.33) | 0.861 | ||
| PNI (40) | 0.032 | 3.680(0.981–13.808) | 0.054 |
| CEA (5) | 0.519 | ||
| CA19-9 (37) | 0.232 | ||
| CA125 (35) | 0.15 | ||
| AFP (10) | 0.621 | ||
| Albumin (3.6) | 0.118 | ||
| CRP (1.0) | 0.302 |
Abbreviations: HR, hazard ratio; PS, performance status; RECIST, Response Evaluation Criteria in Solid Tumors; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to lymphocyte ratio; NMR, neutrophil-to-monocyte ratio; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index.
Figure 1.
Overall survival curves for different inflammatory/immune-nutrition indicator. Significantly shorter survivals were shown in patients with high PLR (A), high NLR (B), low lymphocyte (C), and low PNI (D).
Relationship Between the Efficacy of Chemotherapy and Hematological Parameters
There was no relationship between RECIST and any factor (data not shown). Table 4 shows the relationship between Ef grade and each factor. Among the 20 patients in the high PLR group, a pathological response was identified in 6 patients (30%), while 14 patients (70%) were unresponsive. Of the 21 patients in the low PLR group, a pathological response was identified in 15 patients (71%), while 6 patients (29%) were unresponsive. A statistically significant correlation with chemotherapy resistance was identified in the high PLR group (P=0.031). Of the 22 patients in the high NLR group, a pathological response was identified in 9 patients (41%), while 13 patients (59%) were unresponsive. Of the 19 patients in the low NLR group, a pathological response was identified in 12 patients (63%), while 7 patients (37%) were unresponsive. No statistically significant correlation with the pathological response was identified in the values of NLR (P=0.155) and PNI (P=0.914).
Table 4.
Relationship Between Ef Grade† and Each Parameter
| Parameters | Total,N | Ef Grade 0-1b(N) | Ef Grade 2-3(N) | χ2 | Univariate P-value | Multivariate HR (95% CI) | P-value |
|---|---|---|---|---|---|---|---|
| Age, years | 0.196 | 0.658 | |||||
| <70 | 28 | 13 | 15 | ||||
| ≧70 | 13 | 7 | 6 | ||||
| Gender | 1.38 | 0.24 | |||||
| Male | 34 | 18 | 16 | ||||
| Female | 7 | 2 | 5 | ||||
| PS | 0.196 | 0.658 | |||||
| 0 | 28 | 13 | 15 | ||||
| 1 | 13 | 7 | 6 | ||||
| Differentiation | 4.193 | 0.041 | 0.480(0.183–1.256) | 0.135 | |||
| Poorly differentiated | 19 | 6 | 13 | ||||
| Moderate-well differentiated | 22 | 14 | 8 | ||||
| PLR | 4.659 | 0.031 | 1.993(0.793–5.008) | 0.143 | |||
| <180 | 21 | 6 | 15 | ||||
| ≧180 | 20 | 14 | 6 | ||||
| NLR | 2.02 | 0.155 | |||||
| <2.4 | 19 | 7 | 12 | ||||
| ≧2.4 | 22 | 13 | 9 | ||||
| NMR | 0.028 | 0.867 | |||||
| <1.1 | 19 | 9 | 10 | ||||
| ≧1.1 | 22 | 11 | 11 | ||||
| LMR | 0.605 | 0.437 | |||||
| <4.05 | 21 | 9 | 12 | ||||
| ≧4.05 | 20 | 11 | 9 | ||||
| Platelet | 3.064 | 0.08 | |||||
| <240 | 18 | 6 | 12 | ||||
| ≧240 | 23 | 14 | 9 | ||||
| Lymphocyte | 0.028 | 0.867 | |||||
| <1.43 | 19 | 9 | 10 | ||||
| ≧1.43 | 22 | 11 | 11 | ||||
| Neutrophil | 0.023 | 0.879 | |||||
| <4.1 | 20 | 10 | 10 | ||||
| ≧4.1 | 21 | 10 | 11 | ||||
| Monocyte | 0.266 | 0.606 | |||||
| <0.33 | 17 | 7 | 10 | ||||
| ≧0.33 | 24 | 13 | 11 | ||||
| PNI | 0.012 | 0.914 | |||||
| <40 | 23 | 13 | 10 | ||||
| 40- | 11 | 6 | 5 | ||||
| CEA | 0.286 | 0.593 | |||||
| <5 | 22 | 13 | 9 | ||||
| ≧5 | 14 | 7 | 7 | ||||
| CA19-9 | 0.6 | 0.439 | |||||
| <37 | 27 | 16 | 11 | ||||
| ≧37 | 9 | 4 | 5 | ||||
| CA125 | 1.905 | 0.167 | |||||
| <35 | 31 | 19 | 12 | ||||
| ≧35 | 4 | 1 | 3 | ||||
| AFP | 2.399 | 0.121 | |||||
| <10 | 28 | 15 | 13 | ||||
| ≧10 | 3 | 3 | 0 | ||||
| Albumin | 0.864 | 0.353 | |||||
| <3.6 | 7 | 5 | 2 | ||||
| ≧3.6 | 27 | 14 | 13 | ||||
| CRP | 3.16 | 0.075 | |||||
| <1.00 | 21 | 13 | 8 | ||||
| ≧1.00 | 8 | 2 | 6 |
Notes: †The histrogical evaluation criteria were classified into five categories ranging from the complete response (Grade 3) to no effect (Grade 0) in the JCGC (3rd English Edition) as Ef grade.
Abbreviations: HR, hazard ratio; PS, performance status; PLR, platelet-to-lymphocyte ratio; NLR, neutrophil-to-lymphocyte ratio; NMR, neutrophil-to-monocyte ratio; LMR, lymphocyte-to-monocyte ratio; PNI, prognostic nutritional index.
Discussion
S-1 is a standard anticancer drug used for the treatment of locally advanced gastric cancers in Japan.20 Some studies have indicated its effectiveness as preoperative chemotherapy.1–3,21–23 When choosing a treatment regimen, predicting the effectiveness of the drugs is crucial.
It is thought that systemic inflammation and tumor-related micro-environments play an important role in modulating chemotherapy resistance.24–26 However, the related mechanism of action remains largely unknown.4 Although PLR, NLR, and other hematological markers including nutrition indicators have been established as effective prognostic factors for numerous types of cancers,8–11 the relationship between these markers and chemotherapy resistance is also unclear.
In this study, only PLR was linked to chemotherapy resistance, but not NLR and PNI.
We have previously reported that a primary lesion with platelet infiltration as CD42b positive cells revealed chemo-resistance.4 We focused on the role of circulating platelets in this study. Circulating platelets attach and aggregate to the vascular wall via von Willebrand factor (vWF), which released from damaged endothelial cells by cisplatin-based chemotherapy.27
Aggregated and activated platelets release several chemical mediators, such as TGF-β and VEGF-A.28 TGF-β signaling contributes to epithelial–mesenchymal transition (EMT), which induces upregulation of metastatic ability and chemo-resistance in cancer cells.29
Recent studies have revealed that Foxp3+CD25+CD4+ regulatory T cells (Tregs), which are physiologically engaged in maintaining immunological self-tolerance, play critical roles in the control of antitumor immune responses.30 TGF-β also plays an important role in the induction and maintenance of Tregs via Foxp3 activation.31
Angiogenesis induced by VEGF-A reveals irregularly shaped and hyperpermeable vessels, which result in impaired oxygen and drug delivery within the tumor.32 VEGF-A can increase the recruitment of Treg and myeloid-derived suppressor cells (MDSCs), and hinder the differentiation and activation of dendritic cells.33
A reduction in the lymphocyte count in the peripheral blood might cause a restriction in tumor-infiltrating lymphocytes (TIL), which are involved in the antitumor activity.
In cancer patients, it has been reported that a reduction in the lymphocyte count can indicate an inadequate immune response to cancer cells.34 A number of reports have documented the association of a high peripheral blood lymphocytes (PBL) count and a favorable prognosis.35,36 In addition, an increase in TIL is reportedly associated with an improved prognosis in cancer patients.37 By contrast, the level of TILs is a strong predictor of the efficacy of NAC in breast cancer,38 and it is assumed that a high peripheral lymphocyte count is a useful predictor of pCR in breast cancer patients with NAC.14 A high peripheral lymphocyte count may play a crucial role not only in the systemic anticancer immunological response but also in direct effects on local tumor cells. However, the direct relationship between PBL and TIL has not been sufficiently understood.
These positive relationships between the PBL count and local immunity are justified by the cancer-immunity cycle.39 PBL continually enters and exits lymph nodes, resulting in priming and activation by antigen-presenting dendritic cells (DCs). These lymphocytes can migrate and infiltrate to the cancer microenvironments. However, it is essential that DCs can recognize neoantigens from cancer cells, which are induced by immunogenic chemotherapy.40 Thus, the efficacy of chemotherapy depends on systemic and local immunity can activate the adaptive immune system and sensitize tumor cells to T-cell-mediated killing. Accordingly, peripheral lymphocyte counts strongly affect TIL count, resulting in reflecting chemosensitivity.
This study has some limitations. First, the efficacy of only one chemotherapy regimen (mDCS therapy) evaluated the correlation with peripheral inflammation/immune indicators. However, PLR should be considered a reliable predictor of chemosensitivity, because mDCS therapy consists of three typical agents, taxane, platinum, and fluorouracil, which are widely used for gastric cancer treatment worldwide. Second, there was no relationship between PLR and RECIST in this study. RECIST criteria is based on CT image, and according to common cut-off values of metastatic lymph node, positive prediction values as 77% and sensitivity was 62%.41 Therefore, RECIST responder is not always true responder.42 In gastric cancer, overall survival also correlated with Ef grade, but not RECIST.43 These are why there was significant relationship between PLR and Ef grade, but not RECIST. Third, this study was conducted retrospectively on a small scale in subjects included from a single medical institution. Future multi-institutional joint research studies on a large scale are essential for corroboration of these results.
Conclusion
Our study suggested that PLR including both platelets as a negative factor and lymphocytes as a positive factor for cancer immunity is a simple and useful predictor for chemosensitivity, which can be measured at any facility.
Acknowledgments
We are grateful to members of the Department of Gastroenterological Surgery of Kanazawa University for their helpful suggestions. We also thank Frampton RJ, from Edanz Group for editing a draft of this manuscript.
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Yoshikawa T, Sasako M, Yamamoto S, et al. Phase II study of neoadjuvant chemotherapy and extended surgery for locally advanced gastric cancer. Br J Surg. 2009;96(9):1015–1022. doi: 10.1002/bjs.v96:9 [DOI] [PubMed] [Google Scholar]
- 2.Yoshikawa T, Tuburaya A, Morita S, et al. A comparison of multimodality treatment: two or four courses of paclitaxel plus cisplatin or S-1 plus cisplatin followed by surgery for locally advanced gastric cancer, a randomized phase II trial (COMPASS). Jpn J Clin Oncol. 2010;40(4):369–372. doi: 10.1093/jjco/hyp178 [DOI] [PubMed] [Google Scholar]
- 3.Tsuburaya A, Mizusawa J, Tanaka Y, et al. Neoadjuvant chemotherapy with S-1 and cisplatin followed by D2 gastrectomy with para-aortic lymph node dissection for gastric cancer with extensive lymph node metastasis. Br J Surg. 2014;101(6):653–660. doi: 10.1002/bjs.9484 [DOI] [PubMed] [Google Scholar]
- 4.Saito H, Fushida S, Miyashita T, et al. Potential of extravasated platelet aggregation as a surrogate marker for overall survival in patients with advanced gastric cancer treated with preoperative docetaxel, cisplatin and S-1: a retrospective observational study. BMC Cancer. 2017;17(1):294. doi: 10.1186/s12885-017-3279-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Miyashita T, Tajima H, Makino I, et al. Metastasis-promoting role of extravasated platelet activation in tumor. J Surg Res. 2015;193(1):289–294. doi: 10.1016/j.jss.2014.07.037 [DOI] [PubMed] [Google Scholar]
- 6.Ishikawa S, Miyashita T, Inokuchi M, et al. Platelets surrounding primary tumor cells are related to chemoresistance. Oncol Rep. 2016;36(2):787–794. doi: 10.3892/or.2016.4898 [DOI] [PubMed] [Google Scholar]
- 7.Miyashita T, Tajima H, Gabata R, et al. Impact of extravasated platelet activation and podoplanin-positive cancer-associated fibroblasts in pancreatic cancer stroma. Anticancer Res. 2019;39(10):5565–5572. doi: 10.21873/anticanres.13750 [DOI] [PubMed] [Google Scholar]
- 8.Krenn-Pilko S, Langsenlehner U, Thurner EM, et al. The elevated preoperative platelet-to-lymphocyte ratio predicts poor prognosis in breast cancer patients. Br J Cancer. 2014;110(10):2524–2530. doi: 10.1038/bjc.2014.163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou ZY, Liu HL, Ning N, et al. Clinical significance of pre-operative neutrophil lymphocyte ratio and platelet lymphocyte ratio as prognostic factors for patients with colorectal cancer. Oncol Lett. 2016;11(3):2241–2248. doi: 10.3892/ol.2016.4216 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lee S, Oh SY, Kim SH, et al. Prognostic significance of neutrophil lymphocyte ratio and platelet lymphocyte ratio in advanced gastric cancer patients treated with FOLFOX chemotherapy. BMC Cancer. 2013;13:350. doi: 10.1186/1471-2407-13-350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hirahara N, Tajima Y, Fujii Y, et al. Prognostic nutritional index as a predictor of survival in resectable gastric cancer patients with normal preoperative serum carcinoembryonic antigen levels: a propensity score matching analysis. BMC Cancer. 2018;18(1):285. doi: 10.1186/s12885-018-4201-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chen Y, Chen K, Xiao X, et al. Pretreatment neutrophil-to-lymphocyte ratio is correlated with response to neoadjuvant chemotherapy as an independent prognostic indicator in breast cancer patients: a retrospective study. BMC Cancer. 2016;16:320. doi: 10.1186/s12885-016-2352-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Asano Y, Kashiwagi S, Onoda N, et al. Predictive value of neutrophil/lymphocyte ratio for efficacy of preoperative chemotherapy in triple negative breast cancer. Ann Surg Oncol. 2016;23(4):1104–1110. doi: 10.1245/s10434-015-4934-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Quien Y, Tao J, Li X, et al. Peripheral inflammation/immune indicators of chemosensitivity and prognosis in breast cancer patients treated with neoadjuvant chemotherapy. Onco Targets Ther. 2018;11:1423–1432. doi: 10.2147/OTT.S148496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Japanese Gastric Cancer Association. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer. 2011;14:101–112. doi: 10.1007/s10120-011-0041-5 [DOI] [PubMed] [Google Scholar]
- 16.Fushida S, Fujimura T, Oyama K, et al. Feasibility and efficacy of preoperative chemotherapy with docetaxel, cisplatin and S-1 in gastric cancer patients with para-aortic lymph node metastases. Anticancer Drugs. 2009;20(8):752–756. doi: 10.1097/CAD.0b013e32832ec02b [DOI] [PubMed] [Google Scholar]
- 17.Oyama K, Fushida S, Kinoshita J, et al. Efficacy of pre-operative chemotherapy with docetaxel, cisplatin, am\nd S-1 (DCS therapy) and curative resection for gastric cancer with pathologically positive para-aortic lymph nodes. J Surg Oncol. 2012;105(6):535–541. doi: 10.1002/jso.22125 [DOI] [PubMed] [Google Scholar]
- 18.Kinoshita J, Fushida S, Tsukada T, et al. Efficacy of conversion gastrectomy following docetaxel, cisplatin, and S-1 therapy in potentially resectable stage IV gastric cancer. Eur J Surg Oncol. 2015;41(10):1354–1360. doi: 10.1016/j.ejso.2015.04.021 [DOI] [PubMed] [Google Scholar]
- 19.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 20.Sasako M, Sakuramoto S, Katai H, et al. Five-year outcomes of a randomized Phase III trial comparing adjuvant chemotherapy with S-1 versus surgery alone in stage II or III gastric cancer. J Clin Oncol. 2011;29(33):4387–4393. doi: 10.1200/JCO.2011.36.5908 [DOI] [PubMed] [Google Scholar]
- 21.Nakayama N, Koizumi W, Sasaki T, et al. A multicenter, Phase I dose-escalating study of docetaxel, cisplatin and S-1 for advanced gastric cancer (KDOG0601). Oncology. 2008;75(1–2):1–7. doi: 10.1159/000151613 [DOI] [PubMed] [Google Scholar]
- 22.Sato Y, Takayama T, Sagawa T, et al. Phase II study of S-1, docetaxel and cisplatin combination chemotherapy in patients with unresectable metastatic gastric cancer. Cancer Chemother Pharmacol. 2010;66(4):721–728. doi: 10.1007/s00280-009-1215-2 [DOI] [PubMed] [Google Scholar]
- 23.Hirakawa M, Sato Y, Ohnuma H, et al. A phase II study of neoadjuvant combination chemotherapy with docetaxel, cisplatin, and S-1 for locally advanced resectable gastric cancer: nucleotide excision repair (NER) as potential chemoresistance marker. Cancer Chemother Pharmacol. 2013;71(3):789–797. doi: 10.1007/s00280-013-2073-5 [DOI] [PubMed] [Google Scholar]
- 24.Grivennikov SI, Greten FR, Karin M. Immunity, inflammation and cancer. Cell. 2010;140(6):883–899. doi: 10.1016/j.cell.2010.01.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144(5):646–674. doi: 10.1016/j.cell.2011.02.013 [DOI] [PubMed] [Google Scholar]
- 26.de Visser KE, Jonkers J. Towards understanding the role of cancer-associated inflammation in chemoresistance. Current Pharm Des. 2009;15(16):1844–1853. doi: 10.2174/138161209788453239 [DOI] [PubMed] [Google Scholar]
- 27.Dieckmann KP, Strusss WJ, Budde U. Evidence for acute vascular toxicity of cisplatin-based chemotherapy in patients with germ cell tumour. Anticancer Res. 2011;31(12):4501–4506. [PubMed] [Google Scholar]
- 28.Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis. Cancer Cell. 2011;20(5):576–590. doi: 10.1016/j.ccr.2011.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu J, Lamouille S, Derynck R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009;19(2):156–172. doi: 10.1038/cr.2009.5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishikawa H, Sakaguchi S. Regulatory T cells in tumor immunity. Int J Cancer. 2010;127(4):759–767. doi: 10.1002/ijc.25429 [DOI] [PubMed] [Google Scholar]
- 31.Takimoto T, Wakabayashi Y, Sekiya T, et al. Smad2 and Smad3 are redundantly essential for the TGF-beta-mediated regulation of regulatory T plasticity and Th1 development. J Immunol. 2010;185(2):842–855. doi: 10.4049/jimmunol.0904100 [DOI] [PubMed] [Google Scholar]
- 32.Jain RK. Barriers to drug delivery in solid tumors. Sci Am. 1994;271(1):58–65. doi: 10.1038/scientificamerican0794-58 [DOI] [PubMed] [Google Scholar]
- 33.Yang J, Yan J, Liu B. Targeting VEGF/VEGFR to modulate antitumor immunity. Front Immunol. 2018;9:978. doi: 10.3389/fimmu.2018.00978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hoffmann TK, Dworacki G, Tsukihiro T, et al. Spontaneous apoptosis of circulating T lymphocytes in patients with head and neck cancer and its clinical importance. Clin Cancer Res. 2002;8(8):2553–2562. [PubMed] [Google Scholar]
- 35.Riesco A. Five-year cancer cure: relation to total amount of peripheral lymphocytes and neutrophils. Cancer. 1970;25(1):135–140. doi: 10.1002/(ISSN)1097-0142 [DOI] [PubMed] [Google Scholar]
- 36.He JR, Shen GP, Ren ZF, et al. Pretreatment levels of peripheral neutrophils and lymphocytes as independent prognostic factors in patients with nasopharyngeal carcinoma. Head Neck. 2012;34(12):1769–1776. doi: 10.1002/hed.22008 [DOI] [PubMed] [Google Scholar]
- 37.Grimm M, Feyen O, Hofmann H, et al. Immunophenotyping of patients with oral squamous cell carcinoma in peripheral blood and associated tumor tissue. Tumour Biol. 2016;37(3):3807–3816. doi: 10.1007/s13277-015-4224-2 [DOI] [PubMed] [Google Scholar]
- 38.Denkert C, Loibl S, Noske A, et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J Clin Oncol. 2010;28(1):105–113. doi: 10.1200/JCO.2009.23.7370 [DOI] [PubMed] [Google Scholar]
- 39.Chen DS, Mellman I. Oncology meets immunology: the cancer immunity cycle. Immunity. 2013;39(1):1–10. doi: 10.1016/j.immuni.2013.07.012 [DOI] [PubMed] [Google Scholar]
- 40.Kroemer G, Galluzzi L, Kepp O, et al. Immunogenic cell death in cancer therapy. Ann Rev Immunol. 2013;31:51–72. doi: 10.1146/annurev-immunol-032712-100008 [DOI] [PubMed] [Google Scholar]
- 41.Fukagawa T, Katai H, Mizusawa J, et al. A prospective multi-institutional validity study to evaluate the accuracy of clinical diagnosis of pathological stage III gastric cancer (JCOG1302A). Gastric Cancer. 2018;21:68–73. doi: 10.1007/s10120-017-0701-1 [DOI] [PubMed] [Google Scholar]
- 42.Tokunaga M, Sugisawa N, Tanizawa Y, et al. The impact of preoperative lymph node size on long-term outcome following curative gastrectomy for gastric cancer. Ann Surg Oncol. 2013;20:1598–1603. doi: 10.1245/s10434-012-2699-2 [DOI] [PubMed] [Google Scholar]
- 43.Kurokawa Y, Shibata T, Sasako M, et al. Validity of response assessment criteria in neoadjuvant chemotherapy for gastric cancer (JCOG0507-A). Gastric Cancer. 2014;17:514–521. doi: 10.1007/s10120-013-0294-2 [DOI] [PubMed] [Google Scholar]