Abstract
Background
This is an updated version of the original Cochrane Review published in 2010, Issue 9, and last updated in 2014, Issue 4. Non‐invasive brain stimulation techniques aim to induce an electrical stimulation of the brain in an attempt to reduce chronic pain by directly altering brain activity. They include repetitive transcranial magnetic stimulation (rTMS), cranial electrotherapy stimulation (CES), transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS) and reduced impedance non‐invasive cortical electrostimulation (RINCE).
Objectives
To evaluate the efficacy of non‐invasive cortical stimulation techniques in the treatment of chronic pain.
Search methods
For this update we searched CENTRAL, MEDLINE, Embase, CINAHL, PsycINFO, LILACS and clinical trials registers from July 2013 to October 2017.
Selection criteria
Randomised and quasi‐randomised studies of rTMS, CES, tDCS, RINCE and tRNS if they employed a sham stimulation control group, recruited patients over the age of 18 years with pain of three months' duration or more, and measured pain as an outcome. Outcomes of interest were pain intensity measured using visual analogue scales or numerical rating scales, disability, quality of life and adverse events.
Data collection and analysis
Two review authors independently extracted and verified data. Where possible we entered data into meta‐analyses, excluding studies judged as high risk of bias. We used the GRADE system to assess the quality of evidence for core comparisons, and created three 'Summary of findings' tables.
Main results
We included an additional 38 trials (involving 1225 randomised participants) in this update, making a total of 94 trials in the review (involving 2983 randomised participants). This update included a total of 42 rTMS studies, 11 CES, 36 tDCS, two RINCE and two tRNS. One study evaluated both rTMS and tDCS. We judged only four studies as low risk of bias across all key criteria. Using the GRADE criteria we judged the quality of evidence for each outcome, and for all comparisons as low or very low; in large part this was due to issues of blinding and of precision.
rTMS
Meta‐analysis of rTMS studies versus sham for pain intensity at short‐term follow‐up (0 to < 1 week postintervention), (27 studies, involving 655 participants), demonstrated a small effect with heterogeneity (standardised mean difference (SMD) ‐0.22, 95% confidence interval (CI) ‐0.29 to ‐0.16, low‐quality evidence). This equates to a 7% (95% CI 5% to 9%) reduction in pain, or a 0.40 (95% CI 0.53 to 0.32) point reduction on a 0 to 10 pain intensity scale, which does not meet the minimum clinically important difference threshold of 15% or greater. Pre‐specified subgroup analyses did not find a difference between low‐frequency stimulation (low‐quality evidence) and rTMS applied to the prefrontal cortex compared to sham for reducing pain intensity at short‐term follow‐up (very low‐quality evidence). High‐frequency stimulation of the motor cortex in single‐dose studies was associated with a small short‐term reduction in pain intensity at short‐term follow‐up (low‐quality evidence, pooled n = 249, SMD ‐0.38 95% CI ‐0.49 to ‐0.27). This equates to a 12% (95% CI 9% to 16%) reduction in pain, or a 0.77 (95% CI 0.55 to 0.99) point change on a 0 to 10 pain intensity scale, which does not achieve the minimum clinically important difference threshold of 15% or greater. The results from multiple‐dose studies were heterogeneous and there was no evidence of an effect in this subgroup (very low‐quality evidence). We did not find evidence that rTMS improved disability. Meta‐analysis of studies of rTMS versus sham for quality of life (measured using the Fibromyalgia Impact Questionnaire (FIQ) at short‐term follow‐up demonstrated a positive effect (MD ‐10.80 95% CI ‐15.04 to ‐6.55, low‐quality evidence).
CES
For CES (five studies, 270 participants) we found no evidence of a difference between active stimulation and sham (SMD ‐0.24, 95% CI ‐0.48 to 0.01, low‐quality evidence) for pain intensity. We found no evidence relating to the effectiveness of CES on disability. One study (36 participants) of CES versus sham for quality of life (measured using the FIQ) at short‐term follow‐up demonstrated a positive effect (MD ‐25.05 95% CI ‐37.82 to ‐12.28, very low‐quality evidence).
tDCS
Analysis of tDCS studies (27 studies, 747 participants) showed heterogeneity and a difference between active and sham stimulation (SMD ‐0.43 95% CI ‐0.63 to ‐0.22, very low‐quality evidence) for pain intensity. This equates to a reduction of 0.82 (95% CI 0.42 to 1.2) points, or a percentage change of 17% (95% CI 9% to 25%) of the control group outcome. This point estimate meets our threshold for a minimum clinically important difference, though the lower confidence interval is substantially below that threshold. We found evidence of small study bias in the tDCS analyses. We did not find evidence that tDCS improved disability. Meta‐analysis of studies of tDCS versus sham for quality of life (measured using different scales across studies) at short‐term follow‐up demonstrated a positive effect (SMD 0.66 95% CI 0.21 to 1.11, low‐quality evidence).
Adverse events
All forms of non‐invasive brain stimulation and sham stimulation appear to be frequently associated with minor or transient side effects and there were two reported incidences of seizure, both related to the active rTMS intervention in the included studies. However many studies did not adequately report adverse events.
Authors' conclusions
There is very low‐quality evidence that single doses of high‐frequency rTMS of the motor cortex and tDCS may have short‐term effects on chronic pain and quality of life but multiple sources of bias exist that may have influenced the observed effects. We did not find evidence that low‐frequency rTMS, rTMS applied to the dorsolateral prefrontal cortex and CES are effective for reducing pain intensity in chronic pain. The broad conclusions of this review have not changed substantially for this update. There remains a need for substantially larger, rigorously designed studies, particularly of longer courses of stimulation. Future evidence may substantially impact upon the presented results.
Keywords: Humans, Brain, Brain/physiology, Chronic Pain, Chronic Pain/therapy, Electric Stimulation Therapy, Electric Stimulation Therapy/adverse effects, Electric Stimulation Therapy/methods, Pain Management, Pain Management/methods, Randomized Controlled Trials as Topic, Transcranial Magnetic Stimulation, Transcranial Magnetic Stimulation/adverse effects, Transcranial Magnetic Stimulation/methods
Stimulating the brain without surgery in the management of chronic pain in adults
Bottom line
There is a lack of high‐quality evidence to support or refute the effectiveness of non‐invasive brain stimulation techniques for chronic pain.
Background
Electrical stimulation of the brain has been used to address a variety of painful conditions. Various devices are available that can electrically stimulate the brain without the need for surgery or any invasive treatment. There are five main treatment types: repetitive transcranial magnetic stimulation (rTMS) in which the brain is stimulated by a coil applied to the scalp, cranial electrotherapy stimulation (CES) in which electrodes are clipped to the ears or applied to the scalp, transcranial direct current stimulation (tDCS), reduced impedance non‐invasive cortical electrostimulation (RINCE) and transcranial random noise stimulation (tRNS) in which electrodes are applied to the scalp. These have been used to try to reduce pain by aiming to alter the activity of the brain. How effective they are is uncertain.
Study characteristics
This review update included 94 randomised controlled studies: 42 of rTMS, 11 of CES, 36 of tDCS two of RINCE, two of tRNS and one study which evaluated both tDCS and rTMS.
Key findings
rTMS applied to the motor cortex may lead to small, short‐term reductions in pain but these effects are not likely to be clinically important. tDCS may reduce pain when compared with sham but for rTMS and tDCS our estimates of benefit are likely to be exaggerated by the small number of participants in each of the studies and limitations in the way the studies were conducted. Low‐ or very low‐quality evidence suggests that low‐frequency rTMS and rTMS that is applied to prefrontal areas of the brain are not effective. Low‐quality evidence does not suggest that CES is an effective treatment for chronic pain. For all forms of stimulation the evidence is not conclusive and there is substantial uncertainty about the possible benefits and harms of the treatment. Of the studies that clearly reported side effects, short‐lived and minor side effects such as headache, nausea and skin irritation were usually reported both with real and sham stimulation. Two cases of seizure were reported following real rTMS. Our conclusions for rTMS, CES, tDCS, and RINCE have not changed substantially in this update.
Quality of the evidence
We rated the quality of the evidence from studies using four levels: very low, low, moderate, or high. Very low‐quality evidence means that we are very uncertain about the results. High‐quality evidence means that we are very confident in the results. We considered all of the evidence to be of low or very low quality, mainly because of bias in the studies that can lead to unreliable results and the small size of the studies, which makes them imprecise.
Summary of findings
Summary of findings for the main comparison.
Repetitive transcranial magnetic stimulation (rTMS) compared with sham for chronic pain
| rTMS compared with sham for chronic pain | ||||
|
Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active rTMS Comparison: sham rTMS | ||||
| Outcomes | Effect size |
Relative and absolute effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. |
No of participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales |
SMD ‐0.22 (‐0.29 to ‐0.16) | This equates to a 7% (95% CI 5% to 9%) reduction in pain intensity, or a 0.40 (95% CI 0.53 to 0.32) point reduction on a 0 to 10 pain intensity scale. | 655 (27) | ⊕⊕⊝⊝ low1 |
|
Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales |
SMD ‐0.29, 95% CI ‐0.87 to 0.29 | ‐ | 119 (5) | ⊕⊝⊝⊝ very low2 |
|
Quality of life (0 to < 1 week postintervention) measured using Fibromyalgia Impact Questionnaire |
MD ‐10.80, 95% CI ‐15.04 to ‐6.55 | ‐ | 105 (4) | ⊕⊕⊝⊝ low3 |
| CI: confidence interval; MD: mean difference; rTMS: repetitive transcranial magnetic stimulation; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
1Downgraded once for study limitations due to high or unclear risk of bias and once for inconsistency due to heterogeneity. 2Downgraded once for study limitations due to high or unclear risk of bias, once for inconsistency due to heterogeneity and once for imprecision due to low participant numbers. 3Downgraded once for study limitations due to high or unclear risk of bias and once for imprecision due to low participant numbers.
Summary of findings 2.
Cranial electrotherapy stimulation (CES) compared with sham for chronic pain
| CES compared with sham for chronic pain | ||||
|
Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active CES Comparison: sham CES | ||||
| Outcomes | Effect size |
Relative effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. |
No of participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales |
SMD ‐0.24 (‐0.48 to 0.01) | ‐ | 270 (5) | ⊕⊕⊝⊝ low1 |
|
Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales |
No data available | No data available | No data available | No data available |
|
Quality of life (0 to < 1 week postintervention) measured using Fibromyalgia Impact Questionnaire |
MD ‐25.05 (‐37.82 to ‐12.28) | ‐ | 36 (1) | ⊕⊝⊝⊝ very low2 |
| CI: confidence interval; CES: cranial electrotherapy stimulation; MD: mean difference; SMD: standardised mean difference | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
1Downgraded once for study limitations due to high or unclear risk of bias and once for imprecision due to low participant numbers. 2Downgraded once for study limitations due to high or unclear risk of bias, once for inconsistency (single study) and once for imprecision due to low participant numbers.
Summary of findings 3.
Transcranial direct current stimulation (tDCS) compared with sham for chronic pain
| tDCS compared with sham for chronic pain | ||||
|
Patient or population: adults with chronic pain Settings: laboratory/ clinic Intervention: active tDCS Comparison: sham tDCS | ||||
| Outcomes | Effect size |
Relative effect (average % improvement (reduction) in pain (95% CIs) in relation to post‐treatment score from sham group)* *Where 95%CIs do not cross the line of no effect. |
No of participants (studies) | Quality of the evidence (GRADE) |
|
Pain intensity (0 to < 1 week postintervention) measured using visual analogue scales or numerical rating scales |
SMD ‐0.43 (‐0.63 to ‐0.22) | This equates to a 17% (95% CI 9% to 25%) reduction in pain intensity or a 0.82 (95% CI 0.42 to 1.2) point reduction on a 0 to 10 pain intensity scale. | 747 (27) | ⊕⊝⊝⊝ very low1 |
|
Disability (0 to < 1 week postintervention) measured using self‐reported disability/pain interference scales |
SMD ‐0.01, (95% CI ‐0.28 to 0.26) | ‐ | 212 (4) | ⊕⊕⊝⊝ low2 |
|
Quality of life (0 to < 1 week postintervention) measured using different scales across studies |
SMD 0.66, 95% CI 0.21 to 1.11 | ‐ | 82 (4) | ⊕⊕⊝⊝ low2 |
| CI: confidence interval; MD: mean difference; SMD: standardised mean difference; tDCS: transcranial direct current stimulation | ||||
| GRADE Working Group grades of evidence High quality: we are very confident that the true effect lies close to that of the estimate of the effect; Moderate quality: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different; Low quality: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect; Very low quality: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect. | ||||
1Downgraded once for study limitations due to high or unclear risk of bias, once for inconsistency due to heterogeneity and once for evidence of possible publication bias. 2Downgraded once for study limitations due to high or unclear risk of bias and once for imprecision due to low participant numbers.
Background
This is an updated version of the original Cochrane Review published in 2010, Issue 9, on non‐invasive brain stimulation techniques for chronic pain (O'Connell 2010) and updated in 2014 (O'Connell 2014).
Description of the condition
Chronic pain is a common problem. When defined as pain of greater than three months' duration, prevalence studies indicate that up to half the adult population suffer from chronic pain, and 10% to 20% experience clinically significant chronic pain (Smith 2008; Van Hecke 2013). In Europe, 19% of adults experience chronic pain of moderate to severe intensity with serious negative implications for their social and working lives and many of these receive inadequate pain management (Breivik 2006; Van Hecke 2013). Chronic pain is a heterogeneous phenomenon that results from a wide variety of pathologies including chronic somatic tissue degeneration such as in arthritis, peripheral nerve injury and central nervous system injury, as well as a range of chronic pain syndromes such as fibromyalgia and complex regional pain syndrome. It is likely that different mechanisms of pain production underpin these different types of chronic pain (Ossipov 2006).
Description of the intervention
Electrical brain stimulation techniques have been used to address a variety of pathological pain conditions including fibromyalgia, chronic poststroke pain and complex regional pain syndrome (Cruccu 2017; Fregni 2007; Gilula 2007), and clinical studies of both invasive and non‐invasive techniques have produced preliminary data showing reductions in pain (Fregni 2007; Lefaucheur 2008b). Various types of brain stimulation, both invasive and non‐invasive, are currently in clinical use for the treatment of chronic pain (Cruccu 2017). Non‐invasive stimulation techniques require no surgical procedure and are therefore easier and safer to apply than invasive procedures.
Repetitive transcranial magnetic stimulation (rTMS) involves stimulation of the cerebral cortex (the outer layer of the brain) by a stimulating coil applied to the scalp. Electric currents are induced in the neurons (brain cells) directly using rapidly changing magnetic fields (Fregni 2007). Trains of these stimuli are applied to the target region of the cortex to induce alterations in brain activity both locally and in remote brain regions (Leo 2007). A recent meta‐analysis suggested that rTMS may be more effective in the treatment of neuropathic pain conditions (pain arising as a result of a lesion or a disease of the somatosensory nervous system, as in diabetes, traumatic nerve injury, stroke, multiple sclerosis, epilepsy, spinal cord injury and cancer) with a central compared to a peripheral nervous system origin (Leung 2009).
Transcranial direct current stimulation (tDCS), transcranial random noise stimulation (tRNS) and cranial electrotherapy stimulation (CES) involve the safe and painless application of low‐intensity (commonly ≤ 2 mA) electrical current to the cerebral cortex of the brain (Fregni 2007; Gilula 2007; Hargrove 2012a). tDCS has been developed as a clinical tool for the modulation of brain activity in recent years and uses relatively large electrodes that are applied to the scalp over the targeted brain area to deliver a weak constant current (Lefaucheur 2008a). Clinical studies have concluded that tDCS was more effective than sham stimulation at reducing pain in both fibromyalgia and spinal cord injury‐related pain (Fregni 2006a; Fregni 2006b). tRNS is similar to tDCS but the stimulating current is varied randomly. It has been found to increase cortical excitability (Paulus 2011). CES was initially developed in the USSR as a treatment for anxiety and depression in the 1950s and its use later spread to Europe and the USA, where it began to be considered and used as a treatment for pain (Kirsch 2000). The electrical current in CES is commonly pulsed and is applied via clip electrodes that are attached to the patient's earlobes. A Cochrane Review of non‐invasive treatments for headaches identified limited evidence that CES is superior to placebo in reducing pain intensity after six to 10 weeks of treatment (Bronfort 2004). Reduced impedance non‐invasive cortical electrostimulation (RINCE) similarly applies an electrical current via scalp electrodes but utilises specific stimulation frequencies, which are hypothesised to reduce electrical impedance from the tissues of the skin and skull, allowing deeper cortical penetration and modulation of lower‐frequency cortical activity (Hargrove 2012a).
How the intervention might work
Brain stimulation techniques primarily seek to modulate activity in brain regions by directly altering the level of brain activity. The aim of brain stimulation in the management of pain is to reduce pain by altering activity in the areas of the brain that are involved in pain processing.
Both tDCS and rTMS have been shown to modulate brain activity specific to the site of application and the stimulation parameters. As a general rule, low‐frequency rTMS (≤ 1 Hz) results in lowered cortical excitability at the site of stimulation, whereas high‐frequency stimulation (≥ 5 Hz) results in raised cortical excitability (Lefaucheur 2008a; Pascual‐Leone 1999). Similarly, anodal tDCS, wherein the anode electrode is placed over the cortical target, results in a raised level of excitability at the target, whereas cathodal stimulation decreases local cortical excitability (Nitsche 2008). It is suggested that the observed alterations in cortical excitability (readiness for activity) following rTMS and tDCS that last beyond the time of stimulation are the result of long‐term synaptic changes (Lefaucheur 2008a). Both RINCE and tRNS are applied in a similar way to tDCS, though the current is delivered differently to enhance, in theory, signal transmission to neural networks. Modulation of activity in brain networks is also proposed as the mechanism of action of CES therapy and it is suggested that the therapeutic effects are primarily achieved by direct action upon the hypothalamus, limbic system and/or the reticular activating system (Gilula 2007).
Imaging studies in humans suggest that motor cortex stimulation may reduce pain by modulating activity in networks of brain areas involved in pain processing, such as the thalamus, and by facilitating descending pain inhibitory mechanisms (Garcia‐Larrea 1997; Garcia‐Larrea 1999; Peyron 2007).
Sham credibility issues for non‐invasive brain stimulation studies
An issue regarding the credibility of sham conditions specifically for rTMS studies is whether the sham condition that is employed controls for the auditory (clicking sounds of various frequencies) and sensory stimulation that occurs during active stimulation (Lisanby 2001; Loo 2000). Various types of sham have been proposed including angling the coil away from the scalp (thus preserving the auditory cues but not the sensation of stimulation), using coils that mimic the auditory cues combined with gentle scalp electrical stimulation to mask the sensation and simple inert coils that reproduce neither the sound nor the sensation of active stimulation. Failure to control for such cues may impact negatively on participant blinding, particularly in cross‐over design studies. Lisanby 2001 and Loo 2000 suggest that an ideal sham condition for rTMS should:
not stimulate the cortex;
be the same as active stimulation in visual terms and in terms of its position on the scalp; and
not differ from active stimulation in terms of the acoustic and afferent sensory sensations that it elicits.
Strategies have been developed to try to meet these criteria (Borckardt 2008; Rossi 2007; Sommer 2006). There is evidence that simply angling the coil away from the scalp at an angle of less than 90° may still result in brain stimulation and not be truly inert (Lisanby 2001). This strategy is also easily detected by the recipient of stimulation. In these ways this type of sham might obscure or exaggerate a real clinical effect of active stimulation.
In studies of tDCS the sham condition commonly involves the delivery of a short initial period (30 seconds to one minute) of identical stimulation to the active condition, at which point the stimulation is ceased without the participant's knowledge. There is evidence that this achieves effective blinding of tDCS at stimulation intensities of 1 mA in naive participants (Ambrus 2012; Gandiga 2006), but at a stimulation intensity of 2 mA tDCS both participant and assessor blinding has been shown to be inadequate, since participants can distinguish the active condition more than would be expected by chance and a proportion of those receiving active stimulation develop a temporary but visible redness over the electrode sites (O'Connell 2012). At 1.5 mA there are detectable differences in the experience of tDCS that might compromise blinding (Kessler 2013), though a formal investigation of the adequacy of blinding at this intensity has not been published to date.
Why it is important to do this review
This approach to pain treatment is relatively novel. It is important to assess the existing literature robustly to ascertain the current level of supporting evidence and to inform future research and potential clinical use. Published reviews have addressed this area and concluded that non‐invasive brain stimulation can exert a significant effect on chronic pain, but they have restricted their findings to specific cortical regions, types of painful condition or types of stimulation and did not carry out a thorough assessment of study quality or risk of bias (Lefaucheur 2008b; Leung 2009; Lima 2008).
Objectives
To evaluate the efficacy of non‐invasive cortical stimulation techniques in the treatment of chronic pain.
Methods
Criteria for considering studies for this review
Types of studies
We included randomised controlled trials (RCTs) and quasi‐randomised trials (e.g. by order of entry or date of birth) that utilised a sham control group. We included parallel and cross‐over study designs. We included studies regardless of language.
Types of participants
We included studies involving male or female participants over the age of 18 years with any chronic pain syndrome (with a duration of more than three months). It was not anticipated that any studies were likely to exist in a younger population. Migraine and other headache studies were not included due to the episodic nature of these conditions.
Types of interventions
We included studies investigating the therapeutic use of non‐invasive forms of brain stimulation (tDCS, rTMS, CES, RINCE or tRNS). We did not include studies of electroconvulsive therapy (ECT), as its mechanism of action (the artificial induction of an epileptic seizure (Stevens 1996)) differs substantially from the other forms of brain stimulation. We also excluded invasive forms of brain stimulation involving the use of electrodes implanted within the brain, and indirect forms of stimulation, such as caloric vestibular stimulation and occipital nerve stimulation. In order to meet our second objective of considering the influence of varying stimulation parameters, we included studies regardless of the number of stimulation sessions delivered, including single‐dose studies.
Types of outcome measures
Primary outcomes
The primary outcome measure was change in pain intensity using validated measures of pain intensity such as visual analogue scales (VAS), verbal rating scales (VRS) or numerical rating scales (NRS).
Secondary outcomes
Secondary outcomes that we extracted when available were self‐reported disability data, quality‐of‐life measures and the incidence/nature of adverse events.
Search methods for identification of studies
Electronic searches
For the OVID MEDLINE search, we ran the subject search with the Cochrane Highly Sensitive Search Strategy for identifying randomised trials in MEDLINE: sensitivity maximising version (2008 revision) as referenced in Chapter 6 and detailed in box 6.4c of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (Lefebvre 2011). We have slightly adapted this filter to include the term 'sham' in the title or abstract. The search strategies for this update are presented in Appendix 1 and included a combination of controlled vocabulary (MeSH) and free‐text terms. We based all database searches on this strategy but appropriately revised them to suit each database.
Electronic databases
Previous updates searched all databases from their inception to July 2013. To identify studies for inclusion in this update we searched the following electronic databases from July 2013 to September 2016 to identify additional published articles and performed a further search update in October 2017:
the Cochrane Central Register of Controlled Trials (CENTRAL; 2017, Issue 10);
MEDLINE & MEDLINE in Process via OVID to 11 October 2017;
Embase via OVID to 11 October 2017;
PsycINFO via OVID to 11 October 2017;
CINAHL via EBSCO to 11 October 2017;
LILACS via Birme to 11 October 2017;
For full details of the search parameters including for this update see Appendix 1 and Appendix 2.
Searching other resources
Reference lists
We searched reference lists of all eligible trials, key textbooks and previous systematic reviews to identify additional relevant articles.
Unpublished data
For this update we searched ClinialTrials.gov (clinicaltrials.gov) and the World Health Organization International Clinical Trials Registry Platform (www.who.int/ictrp/en/) to October 2017 to identify research in progress and unpublished research.
Language
The search attempted to identify all relevant studies irrespective of language. We assessed non‐English papers and, if necessary, translated them with the assistance of a native speaker.
We sent a final list of included articles to two experts in the field of therapeutic brain stimulation with a request that they review the list for possible omissions.
Data collection and analysis
Selection of studies
Two review authors (NOC and BW) independently checked the search results and the reference lists of included eligible studies. Initially two review authors (NOC and BW) read the titles or abstracts (or both) of identified studies. Where it was clear from the study title or abstract that the study was not relevant or did not meet the selection criteria we excluded it. If it was unclear then we assessed the full paper, as well as all studies that appeared to meet the selection criteria. Disagreement was resolved through discussion between the two review authors. Where resolution was not achieved a third review author (LDS) considered the paper(s) in question.
Data extraction and management
Two review authors (NOC and BW) extracted data independently using a standardised form that was piloted by both authors independently on three randomised controlled trials of transcutaneous electrical nerve stimulation prior to the searches. We resolved discrepancies by consensus. The form included the following.
'Risk of bias' assessment results
Country of origin
Study design
Study population ‐ condition; pain type; duration of symptoms; age range; gender split; prior management
Sample size ‐ active and control groups
Intervention ‐ stimulation site, parameters and dosage (including number and duration of trains of stimuli and number of pulses for rTMS studies)
Type of sham
Credibility of sham (for rTMS studies ‐ see below)
Outcomes ‐ mean postintervention pain scores for the active and sham treatment groups at all follow‐up points
Results ‐ short, intermediate and long‐term follow‐up
Adverse effects
Conflict of interest disclosure
Assessment of risk of bias in included studies
We assessed risk of bias using the Cochrane 'Risk of bias' assessment tool outlined in Chapter 8 of the Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (Higgins 2011a).
The criteria assessed for parallel study designs (using low/high/unclear judgements) were: adequate sequence generation; adequate allocation concealment; adequate blinding of assessors; adequate blinding of participants; adequate assessment of incomplete outcome data; whether free of suggestion of selective outcome reporting; and whether free of other bias.
The criteria assessed for cross‐over study designs (using low/high/unclear judgements) were: adequate sequence generation; whether data were clearly free from carry‐over effects; adequate blinding of assessors; adequate blinding of participants; whether free of the suggestion of selective outcome reporting; and whether free of other bias.
As with the previous update, in compliance with new author guidelines from Cochrane Pain, Palliative and Supportive Care and the recommendations of Moore 2010 we added two criteria, 'study size' and 'study duration', to our 'Risk of bias' assessment using the thresholds for judgement suggested by Moore 2010:
size (we rated studies with fewer than 50 participants per arm as being at high risk of bias, those with between 50 and 199 participants per arm at unclear risk of bias, and 200 or more participants per arm at low risk of bias);
duration (we rated studies with follow‐up of less than two weeks as being at high risk of bias, two to seven weeks at unclear risk of bias and eight weeks or longer at low risk of bias).
Two review authors (NOC and BW) independently checked risk of bias. Disagreement between review authors was resolved through discussion between the two review authors. Where resolution was not achieved a third review author (LDS) considered the paper(s) in question.
Assessment of sham credibility
We rated the type of sham used in studies of rTMS for credibility: as optimal (the sham controls for the auditory and sensory characteristics of stimulation and is visually indistinguishable from real stimulation (Lisanby 2001; Loo 2000)) and suboptimal (fails to account for either the auditory and sensory characteristics of stimulation, or is visually distinguishable from the active stimulation, or fails on more than one of these criteria). We made a judgement of 'unclear' where studies did not adequately describe the sham condition.
In light of empirical evidence that tDCS may be inadequately blinded at intensities of 2 mA (O'Connell 2012), and of detectable differences in the experience of tDCS at 1.5 mA (Kessler 2013), for this update we assessed studies that used these stimulation intensities to be at unclear risk of bias for participant and assessor blinding. We chose 'unclear' instead of 'high' risk of bias as the available evidence demonstrates the potential for inadequate blinding rather than providing clear evidence that individual studies were effectively unblinded. We applied this rule to all newly identified studies and retrospectively to studies identified in the first version of this review.
Two independent review authors (NOC and BW) performed rating of sham credibility. We resolved disagreement between review authors through consensus. Where resolution was not achieved a third review author (LDS) considered the paper(s) in question. Where sham credibility was assessed as unclear or suboptimal we made a judgement of 'unclear' for the criterion 'adequate blinding of participants' in the 'Risk of bias' assessment.
Measures of treatment effect
We used standardised mean difference (SMD) to express the size of treatment effect on pain intensity measured with a VAS or NRS. In order to aid interpretation of the pooled effect size we back‐transformed the SMD to a 0 to 10 pain intensity rating scale on the basis of the mean standard deviation from trials using a 0 to 10 point VAS. We considered the likely clinical importance of the pooled effect size using the criteria proposed in the IMMPACT consensus statement (Dworkin 2008). Specifically, we judged a decrease in pain of less than 15% as no important change, of 15% or more as a minimally important change, of 30% or more as a moderately important change and of 50% or more as a substantially important change.
Unit of analysis issues
We entered cross‐over trials into a meta‐analysis where it was clear that these data were free of carry‐over effects. We combined the results of cross‐over studies with parallel studies using the generic inverse‐variance method as suggested in the Cochrane Handbook for Systematic Reviews of Interventions, section 16.4.6.2 (Higgins 2011b). We imputed the post‐treatment between‐condition correlation coefficient from an included cross‐over study that presented individual participant data and used this to calculate the standard error of the standardised mean difference (SE (SMD)). Where data from the same cross‐over trials were entered more than once into the same meta‐analysis we corrected the number of participants by dividing by the number of times data from that trial were entered in the meta‐analysis. We calculated the SMD (SE) for parallel studies in Review Manager 5 (RevMan 5) (RevMan 2014). For each study we entered the SMD (SE) into the meta‐analysis using the generic inverse‐variance method.
Dealing with missing data
Where insufficient data were presented in the study report to enter a study into the meta‐analysis, we contacted the study authors to request access to the missing data.
Assessment of heterogeneity
We conducted separate meta‐analysis for each type of brain stimulation. We assessed heterogeneity using the Chi2 test to investigate its statistical significance and the I2 statistic (Higgins 2003) to estimate the amount. We planned to investigate the influence of altered chronic pain condition or stimulation parameters through pre‐planned subgroup analyses (see Subgroup analysis and investigation of heterogeneity).
Assessment of reporting biases
We planned to consider the possible influence of publication/small study biases on review findings. The influence of small study biases were, in part, addressed by the risk of bias criterion 'study size'. We planned to use funnel plots to visually explore the likelihood of reporting biases when at least 10 studies were included in a meta‐analysis and included studies differed in size. For continuous outcomes, we planned to use Egger's test to detect possible small study bias and, for dichotomised outcomes, we planned to test for the possible influence of publication bias on each outcome by estimating the number of participants in studies with zero effect required to change the number needed to treat for an additional beneficial outcome (NNTB) to an unacceptably high level (defined as a NNTB of 10).
Data synthesis
We performed pooling of results where adequate data supported this using RevMan 5 software (RevMan 2014), with a random‐effects model. Where an analysis included parallel and cross‐over trials we used the generic inverse variance method (see Unit of analysis issues). We conducted separate meta‐analyses for different forms of stimulation intervention (i.e. rTMS, tDCS, CES, RINCE and tRNS) and for short‐term (0 to < 1 week postintervention), mid‐term (≥ 1 to 6 weeks postintervention) and long‐term (≥ 6 weeks postintervention) outcomes where adequate data were identified.
Where more than one data point was available for short‐term outcomes, we used the first poststimulation measure, and where multiple treatments were given we took the first outcome at the end of the treatment period. For medium‐term outcomes where more than one data point was available, we used the measure that fell closest to the mid‐point of this time period. We excluded studies from the meta‐analysis that we rated at high risk of bias on any criteria, excluding the criteria 'study size' and 'study duration'.
Two review authors (NOC, BW) independently rated the quality of the outcomes. We used the GRADE system to rank the quality of the evidence, and the guidelines provided in Chapter 12.2 of the Cochrane Handbook for Systematic Reviews of Interventions (Schünemann 2011). The GRADE approach uses five considerations (study limitations, consistency of effect, imprecision, indirectness and publication bias) to assess the quality of the body of evidence for each outcome. The GRADE system uses the following criteria for assigning grade of evidence.
High: we are very confident that the true effect lies close to that of the estimate of the effect.
Moderate: we are moderately confident in the effect estimate; the true effect is likely to be close to the estimate of effect, but there is a possibility that it is substantially different.
Low: our confidence in the effect estimate is limited; the true effect may be substantially different from the estimate of the effect.
Very low: we have very little confidence in the effect estimate; the true effect is likely to be substantially different from the estimate of effect.
The GRADE system uses the following criteria for assigning a quality level to a body of evidence (Chapter 12, Schünemann 2011).
High: randomised trials; or double‐upgraded observational studies
Moderate: downgraded randomised trials; or upgraded observational studies
Low: double‐downgraded randomised trials; or observational studies
Very low: triple‐downgraded randomised trials; or downgraded observational studies; or case series/case reports
Factors that may decrease the quality level of a body of evidence are:
limitations in the design and implementation of available studies suggesting high likelihood of bias;
indirectness of evidence (indirect population, intervention, control, outcomes);
unexplained heterogeneity or inconsistency of results (including problems with subgroup analyses);
imprecision of results (wide confidence intervals);
high probability of publication bias.
To ensure consistency of GRADE judgements we applied the following criteria to each domain equally for all key comparisons of the primary outcome.
Limitations of studies: downgrade once if less than 75% of included studies are at low risk of bias across all key 'Risk of bias' criteria.
Inconsistency: downgrade once if heterogeneity is significant (p<0.05) and the I2 value is more than 40%.
Indirectness: downgrade once if more than 50% of the participants were outside the target group.
Imprecision: downgrade once if there were fewer than 400 participants for continuous data and fewer than 300 events for dichotomous data (Guyatt 2011).
Publication bias: downgrade where there is direct evidence of publication bias.
We considered single studies to be both inconsistent and imprecise, unless more than 400 participants were randomised.
'Summary of findings' table
We included three 'Summary of findings' tables to present the main findings in a transparent and simple tabular format for the three main forms of non‐invasive brain stimulation techniques (rTMS, tDCS, CES) compared to sham. In particular, we included key information concerning the quality of evidence, the magnitude of effect of the interventions examined and the sum of available data on the outcomes pain, disability and quality of life at short‐term follow‐up (see Table 1; Table 3; Table 2).
Subgroup analysis and investigation of heterogeneity
Where heterogeneity (P < 0.1) was present we explored subgroup analyses. Pre‐planned comparisons included site of stimulation, frequency of rTMS stimulation (low ≤ 1 Hz, high ≥ 5 Hz), multiple‐dose versus single‐dose studies and the type of painful condition (central neuropathic versus peripheral neuropathic versus non‐neuropathic pain versus facial pain) for each stimulation type. Central neuropathic pain included pain due to identifiable pathology of the central nervous system (e.g. stroke, spinal cord injury), peripheral neuropathic pain included injury to the nerve root or peripheral nerves, facial pain included trigeminal neuralgia and other idiopathic chronic facial pains, and non‐neuropathic pain included all chronic pain conditions without a clear neuropathic cause (e.g. chronic low back pain, fibromyalgia, complex regional pain syndrome type I).
Sensitivity analysis
When sufficient data were available, we conducted sensitivity analyses on the following study factors: risk of bias, sham credibility (for rTMS studies) and cross‐over versus parallel‐group designs.
Results
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Results of the search
For a full description of our screening process, see the study flow diagram (Figure 1). For a summary of the search results for this update see Appendix 2 and Appendix 3. See Appendix 4; Appendix 5; Appendix 6; Appendix 7 and Appendix 8 for full details of the search results and strategies from earlier versions of this review.
Figure 1.
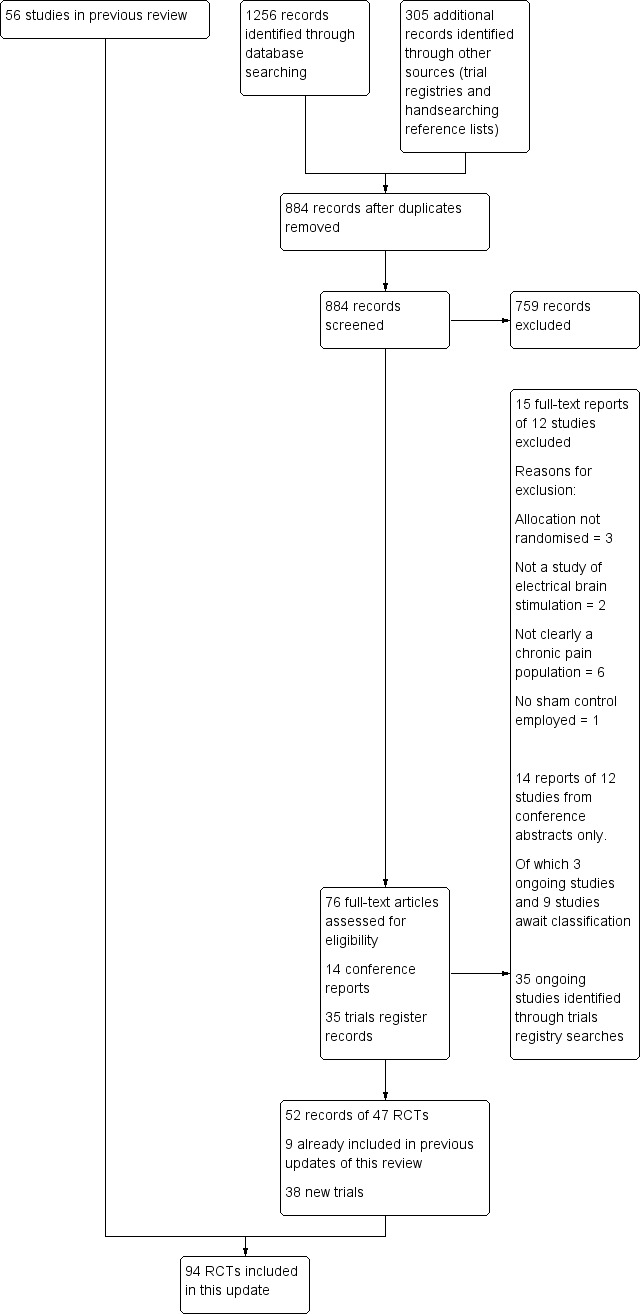
Study flow diagram
This 2017 update is based on a September 2016 search and a further search update in October 2017. For this update, the searches of the databases (see Electronic searches) retrieved 1256 records. Handsearching reference lists of included articles identified one additional RCT that met the inclusion criteria. Our searches of the trials registers identified 305 records. We therefore had a total of 1561 records. Once duplicates had been removed from the main searches and nonrelevant records were removed from the trials registry search results we had a total of 884 records. We excluded 759 records based on titles and abstracts leaving 76 full‐text papers, 14 conference reports and 35 trials register records. We obtained the full text of the remaining 76 records. We excluded 12 studies from 15 records, see Characteristics of excluded studies). Fourteen records were conference abstract reports relating to 12 RCTs. Of these we added nine records to Studies awaiting classification and classified three as Ongoing studies. Of the remaining 52 records (47 RCTs), nine RCTs had been included in previous versions of this update.
We included 38 new studies in this review. Of these, 12 studies (355 participants) investigated only rTMS (Boyer 2014; Dall'Agnol 2014; de Oliveira 2014; Jetté 2013; Malavera 2013; Medeiros 2016; Nardone 2017; Nurmikko 2016; Tekin 2014; Umezaki 2016; Yagci 2014; Yilmaz 2014), 22 studies (772 participants) investigated tDCS (Ahn 2017; Ayache 2016; Bae 2014; Brietzke 2016; Chang 2017; Donnell 2015; Fagerlund 2015; Hagenacker 2014; Harvey 2017; Hazime 2017; Jales Junior 2015; Khedr 2017; Kim 2013; Lagueux 2017; Luedtke 2015; Mendonca 2016; Ngernyam 2015; Oliveira 2015; Sakrajai 2014; Souto 2014; Thibaut 2017; Volz 2016) one study (36 participants) investigated tDCS and rTMS (Attal 2016), two studies (16 participants) investigated tRNS (Curatolo 2017; Palm 2016) and one study investigated RINCE (Deering 2017, 46 participants). Overall this updated review included 94 studies (2983 participants), with 42 trials of rTMS (1101 participants), 36 trials of tDCS (1073 participants), 11 studies of CES (572 participants), one study (36 participants) of both rTMS and tDCS, two studies of RINCE (137 participants) and two studies of tRNS (36 participants). We identified 13 conference abstract reports of 11 studies that were not related to full published studies (Ansari 2013; Fricová 2013; Deering 2017; Hwang 2015; Mattoo 2017; Moreno‐Duarte 2013a; Muniswamy 2016; Mylius 2013; Parhizgar 2011; Tanwar 2016; Williams 2014). We contacted the authors of these abstracts to try to ascertain whether they were unique studies or duplicates and to acquire full study reports. Of these, two authors confirmed that the studies were ongoing or had been submitted for publication (Ansari 2013; Muniswamy 2016) and they were subsequently included in Ongoing studies. The authors of one abstract (Deering 2017) shared a full unpublished study report and the study was included in this review. Where we were unable to obtain this information we placed these records in Studies awaiting classification. One report previously placed in Studies awaiting classification was identified as a full paper and included in this review (Yagci 2014).
We identified 35 new ongoing studies in total (see Characteristics of ongoing studies). We contacted the authors by email for any relevant data but no data were available for inclusion. Three studies, classified as ongoing after previous searches, had been published and were included in the review (Boyer 2014 NCT00697398; Luedtke 2015 ISRCTN89874874, Thibaut 2017 NCT01599767), one was terminated without results (NCT01608321). The remaining studies identified as ongoing in the last update of this review remain unpublished to our knowledge (NCT00815932; NCT00947622; NCT01112774; NCT01220323; NCT01402960; NCT01404052; NCT01575002; NCT01746355; NCT01747070).
Included studies
See Characteristics of included studies.
Country of origin and language of publication
All but one of the studies (Irlbacher 2006, written in German) were written in English. Studies were undertaken in Brazil, Canada, Colombia, Egypt, Europe (Austria, France, Germany, Italy, Spain, Norway, Russia and the UK), Israel, Japan, South Korea, Thailand, Australia and the USA. Most studies were based in a laboratory or outpatient pain clinic setting.
Type of stimulation, application and use
In total 43 studies investigated rTMS (Ahmed 2011; André‐Obadia 2006; André‐Obadia 2008; André‐Obadia 2011; Avery 2013; Borckardt 2009; Boyer 2014, Carretero 2009; Dall'Agnol 2014; Defrin 2007; de Oliveira 2014; Fregni 2005; Fregni 2011; Hirayama 2006; Hosomi 2013; Irlbacher 2006; Jetté 2013, Kang 2009; Khedr 2005; Lee 2012; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Malavera 2013; Medeiros 2016; Mhalla 2011; Nardone 2017; Nurmikko 2016; Onesti 2013; Passard 2007; Picarelli 2010; Pleger 2004; Rollnik 2002; Saitoh 2007; Short 2011; Tekin 2014; Tzabazis 2013; Umezaki 2016; Yagci 2014; Yilmaz 2014). Eleven studies investigated CES (Capel 2003; Cork 2004; Gabis 2003; Gabis 2009; Katsnelson 2004; Lichtbroun 2001; Rintala 2010; Tan 2000; Tan 2006; Tan 2011; Taylor 2013), 36 studies investigated tDCS (Ahn 2017; Antal 2010; Ayache 2016; Bae 2014; Boggio 2009; Brietzke 2016; Chang 2017; Donnell 2015; Fagerlund 2015; Fenton 2009; Fregni 2006a; Fregni 2006b; Hagenacker 2014; Harvey 2017; Hazime 2017; Jales Junior 2015; Jensen 2013; Khedr 2017; Kim 2013; Lagueux 2017; Luedtke 2015; Mendonca 2011; Mendonca 2016; Mori 2010; Ngernyam 2015; Oliveira 2015; Portilla 2013; Riberto 2011; Sakrajai 2014; Soler 2010; Souto 2014; Thibaut 2017; Valle 2009; Villamar 2013; Volz 2016; Wrigley 2014), two studies investigated RINCE (Deering 2017; Hargrove 2012a) two studies investigated tRNS (Curatolo 2017; Palm 2016) and one both rTMS and tDCS (Attal 2016).
Study designs
There was a mixture of parallel and cross‐over study designs. For rTMS there were 22 parallel studies (Ahmed 2011; Avery 2013; Boyer 2014; Carretero 2009; Dall'Agnol 2014; Defrin 2007; de Oliveira 2014; Fregni 2011; Khedr 2005; Lee 2012; Malavera 2013; Medeiros 2016; Mhalla 2011; Nardone 2017,Passard 2007; Picarelli 2010; Short 2011; Tekin 2014; Tzabazis 2013; Umezaki 2016; Yagci 2014; Yilmaz 2014), and 20 cross‐over studies (André‐Obadia 2006; André‐Obadia 2008; André‐Obadia 2011; Borckardt 2009; Fregni 2005; Hirayama 2006; Hosomi 2013; Irlbacher 2006; Jetté 2013; Kang 2009; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Nurmikko 2016; Onesti 2013; Pleger 2004; Rollnik 2002; Saitoh 2007). For CES there were eight parallel studies (Gabis 2003; Gabis 2009; Katsnelson 2004; Lichtbroun 2001; Rintala 2010; Tan 2006; Tan 2011; Taylor 2013), and three cross‐over studies (Capel 2003; Cork 2004; Tan 2000), of which we considered two as parallel studies, with only the opening phase of the study considered in this review because subsequent phases were unblinded (Capel 2003; Cork 2004). For tDCS there were 26 parallel studies (Ahn 2017; Bae 2014; Brietzke 2016; Chang 2017; Donnell 2015; Fagerlund 2015; Fregni 2006a; Fregni 2006b; Harvey 2017; Hazime 2017; Jales Junior 2015; Khedr 2017; Lagueux 2017; Kim 2013; Luedtke 2015; Mendonca 2011; Mendonca 2016; Mori 2010; Oliveira 2015; Riberto 2011; Sakrajai 2014; Soler 2010; Souto 2014; Thibaut 2017; Valle 2009; Volz 2016), and 10 cross‐over studies (Antal 2010; Ayache 2016; Boggio 2009; Fenton 2009; Hagenacker 2014; Jensen 2013; Ngernyam 2015; Portilla 2013; Villamar 2013; Wrigley 2014), of which we considered one as a parallel study with only the opening phase of the study considered in this review due to excessive attrition after the first phase (Antal 2010). One study of tRNS (Palm 2016) used a cross‐over design and one a parallel design (Curatolo 2017) and both RINCE studies used a parallel design (Deering 2017; Hargrove 2012a). The one study of both rTMS and tDCS employed a parallel design (Attal 2016).
Study participants
The included studies were published between 2000 and 2017. In rTMS studies sample sizes at the study outset ranged from four to 70 participants. In CES studies sample size ranged from 19 to 105 participants, in tDCS studies sample size ranged from three to 135 participants, the two RINCE studies recruited 91 and 46 participants and the two studies of tRNS included 16 and 20 participants.
Studies included a variety of chronic pain conditions. Ten rTMS studies included participants with neuropathic pain of mixed origin; of these, seven included a mix of participants with central, peripheral and facial neuropathic pain (André‐Obadia 2006; André‐Obadia 2008; André‐Obadia 2011; Hirayama 2006; Hosomi 2013, Lefaucheur 2004; Lefaucheur 2008), three included a mix of participants with central and peripheral neuropathic pain (Lefaucheur 2006; Nurmikko 2016; Saitoh 2007), of which two studies included one or more participants with phantom limb pain (Nurmikko 2016; Saitoh 2007). One study included a mix of participants with central neuropathic pain and phantom limb pain (Irlbacher 2006). One study included a mix of participants with central and facial neuropathic pain (Lefaucheur 2001a), six rTMS studies included only participants with central neuropathic pain (Defrin 2007; de Oliveira 2014; Jetté 2013; Kang 2009; Nardone 2017, Yilmaz 2014 ), one included only participants with peripheral neuropathic pain (Borckardt 2009), and one study included participants with burning mouth syndrome (Umezaki 2016). Sixteen studies included non‐neuropathic chronic pain including fibromyalgia (Boyer 2014; Carretero 2009; Lee 2012; Mhalla 2011; Passard 2007; Short 2011; Tekin 2014; Tzabazis 2013; Yagci 2014), chronic widespread pain (Avery 2013), chronic pancreatitis pain (Fregni 2005; Fregni 2011), chronic myofascial pain (Dall'Agnol 2014; Medeiros 2016) and complex regional pain syndrome type I (CRPSI) (Picarelli 2010; Pleger 2004). Two studies included only phantom limb pain (Ahmed 2011; Malavera 2013). Finally one study included a mix of peripheral neuropathic and non‐neuropathic chronic pain (Rollnik 2002), including one participant with phantom limb pain and one with osteomyelitis. The majority (21) of rTMS studies specified chronic pain that was refractory to current medical management (André‐Obadia 2006; André‐Obadia 2008, André‐Obadia 2011; Defrin 2007; Hirayama 2006; Hosomi 2013; Kang 2009; Khedr 2005; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Nardone 2017; Nurmikko 2016; Onesti 2013; Picarelli 2010; Rollnik 2002; Saitoh 2007; Yagci 2014; Yilmaz 2014). This inclusion criterion was varyingly described as intractable, resistant to medical intervention or resistant to drug management.
Of the studies investigating CES, one study included participants with pain related to osteoarthritis of the hip and knee (Katsnelson 2004), and two studied chronic back and neck pain (Gabis 2003; Gabis 2009). Of these, the later study also included participants with chronic headache but these data were not considered in this review. Three studies included participants with fibromyalgia (Cork 2004; Lichtbroun 2001; Taylor 2013), and three studies included participants with chronic pain following spinal cord injury (Capel 2003; Tan 2006; Tan 2011), although only one of these reports specified that the pain was neuropathic (Tan 2011). One study included participants with a mixture of "neuromuscular pain" excluding fibromyalgia, of which back pain was reportedly the most prevalent complaint (Tan 2000), although further details were not reported. One study included participants with chronic pain related to Parkinson's disease (Rintala 2010).
Of the studies of tDCS one study included participants with a mixture of central, peripheral and facial neuropathic pain (Boggio 2009), two studies included participants with neuropathic pain secondary to multiple sclerosis (Ayache 2016; Mori 2010), five included participants with central neuropathic pain following spinal cord injury (Fregni 2006a; Ngernyam 2015; Soler 2010; Thibaut 2017; Wrigley 2014), one with central poststroke pain (Bae 2014), one with neuropathic or non‐neuropathic pain following spinal cord injury (Jensen 2013), one with trigeminal neuralgia (Hagenacker 2014) and one with painful diabetic polyneuropathy (Kim 2013). Twenty studies included non‐neuropathic pain, specifically chronic pelvic pain (Fenton 2009), osteoarthritis (OA) of the knee (Ahn 2017; Chang 2017), fibromyalgia (Fagerlund 2015; Fregni 2006b; Jales Junior 2015; Khedr 2017; Mendonca 2011; Mendonca 2016; Riberto 2011; Villamar 2013), temporomandibular joint pain (Donnell 2015; Oliveira 2015), hepatitis C‐related chronic pain (Brietzke 2016), human T‐lymphotropic virus 1 (HTLV‐1) and viral hepatitis‐related chronic back or leg pain (Souto 2014), chronic nonspecific low back pain (Hazime 2017; Luedtke 2015), inflammatory bowel disease‐related pain (Volz 2016) or a mixed pain group (Antal 2010; Harvey 2017). One study included participants with neuropathic pain following burn injury (Portilla 2013) and one included participants with CRPS1 (Lagueux 2017). Four studies of tDCS specified recruiting participants with pain that was refractory to medical management (Antal 2010; Boggio 2009; Fenton 2009; Fregni 2006a). The studies relating to RINCE included participants with fibromyalgia (Deering 2017; Hargrove 2012a). The studies of tRNS included participants with multiple sclerosis‐related neuropathic pain (Palm 2016) and fibromyalgia (Curatolo 2017). The study of both tDCS and rTMS included participants with lumbar radicular pain (Attal 2016).
Most studies included both male and female participants except Fenton 2009 (chronic pelvic pain), Dall'Agnol 2014, Medeiros 2016 (chronic myofascial pain), Donnell 2015 (temporomandibular disorder), Curatolo 2017; Fregni 2006b; Jales Junior 2015; Lee 2012; Mhalla 2011; Riberto 2011; Valle 2009; Yagci 2014 (fibromyalgia) which recruited women only and Yilmaz 2014 (post‐spinal cord injury pain), which recruited only men. Three studies did not present data on gender distribution (Capel 2003; Fregni 2005; Katsnelson 2004).
Outcomes
Primary outcomes
All included studies assessed pain using self‐reported pain visual analogue scales (VAS) or numerical rating scales (NRS). There was variation in the precise measure of pain (for example, current pain intensity, average pain intensity over 24 hours) and in the anchors used particularly for the upper limit of the scale (e.g. "worst pain imaginable", "unbearable pain", "most intense pain sensation"). Several studies did not specify the anchors used.
All studies assessed pain at the short‐term (< 1 week post‐treatment) follow‐up stage. Thirty‐seven studies reported medium‐term outcome data (1 to 6 weeks post‐treatment) (Ahmed 2011; Ahn 2017,André‐Obadia 2008; Antal 2010; Ayache 2016; Bae 2014; Borckardt 2009; Carretero 2009; Defrin 2007; de Oliveira 2014; Fagerlund 2015; Fenton 2009; Fregni 2006a; Fregni 2006b; Fregni 2011; Gabis 2009; Kang 2009; Khedr 2005; Khedr 2017; Kim 2013; Lee 2012; Lefaucheur 2001a; Luedtke 2015; Mendonca 2016; Mori 2010; Nardone 2017; Nurmikko 2016; Passard 2007; Picarelli 2010; Short 2011; Soler 2010; Thibaut 2017; Tzabazis 2013; Valle 2009; Volz 2016; Wrigley 2014; Yagci 2014). Eight studies collected outcome data at long‐term (> 6 weeks post‐treatment) follow‐up (Avery 2013; Hazime 2017; Kang 2009; Luedtke 2015; Mendonca 2016; Passard 2007; Thibaut 2017; Yagci 2014).
Secondary outcomes
We considered secondary outcomes that distinctly measured self‐reported disability (that capture the extent of disability or functional limitation experienced, usually in relation to the pain) or quality of life (a multidimensional construct that includes domains related to physical, emotional and social functioning).
Sixteen studies used measures of disability (Ahn 2017; Attal 2016; Avery 2013; Chang 2017; Cork 2004; Hazime 2017; Kang 2009; Lagueux 2017; Luedtke 2015; Mhalla 2011; Passard 2007; Short 2011; Soler 2010; Tan 2000; Tan 2006; Umezaki 2016), and 27 studies collected measures of quality of life (Avery 2013; Boyer 2014; Curatolo 2017; de Oliveira 2014; Fregni 2006b; Jales Junior 2015; Lagueux 2017; Lee 2012; Lichtbroun 2001; Mendonca 2016; Mhalla 2011; Mori 2010; Oliveira 2015; Passard 2007; Picarelli 2010; Riberto 2011;Sakrajai 2014; Short 2011; Tan 2011; Taylor 2013; Tekin 2014; Thibaut 2017; Tzabazis 2013; Valle 2009; Villamar 2013; Volz 2016; Yagci 2014).
Twenty‐four studies did not report any information regarding adverse events (Ahmed 2011; André‐Obadia 2011; Bae 2014; Borckardt 2009; Brietzke 2016; Cork 2004; Curatolo 2017; Defrin 2007; Gabis 2009; Harvey 2017; Jales Junior 2015; Jensen 2013; Kang 2009; Katsnelson 2004; Khedr 2005; Lefaucheur 2006; Lefaucheur 2008; Lichtbroun 2001; Pleger 2004; Riberto 2011; Tan 2000; Tan 2006; Tekin 2014; Yilmaz 2014). Reporting of adverse events in the remaining studies varied substantially in terms of detail.
Studies of rTMS
See Table 9 for a summary of stimulation characteristics utilised in rTMS studies.
Table 1.
Repetitive transcranial magnetic stimulation (rTMS) studies ‐ characteristics of stimulation
| Study | Location of stimulation | Coil orientation | Frequency (Hz) | Intensity (% RMT) | Number of trains | Duration of trains | Inter‐train intervals (sec) | Number of pulses per session | Treatment sessions per group |
| Ahmed 2011 | M1 stump region | 45° angle from sagittal line | 20 | 80 | 10 | 10 sec | 50 | 2000 | 5, x 1 daily |
| Attal 2016 | M1 contralateral to painful side | Anteroposterior induced current | 10 | 80 | 30 | 10 | 20 | 3000 | 3, x1 daily |
| André‐Obadia 2006 | M1 contralateral to painful side | Posteroanterior | 20, 1 | 90 | 20 Hz: 20 1 Hz: 1 |
20 Hz: 4 sec 1 Hz: 26 min |
20 Hz: 84 | 1600 | 1 |
| André‐Obadia 2008 | M1 contralateral to painful side | Posteroanterior Medial‐lateral |
20 | 90 | 20 | 4 sec | 84 | 1600 | 1 |
| André‐Obadia 2011 | M1 hand area, not clearly reported but likely contralateral to painful side | Not specified | 20 | 90 | 20 | 4 sec | 84 | 1600 | 1 |
| Avery 2013 | Left DLPFC | Not specified | 10 | 120 | 75 | 4 | 26 | 3000 | 15 |
| Borckardt 2009 | Left PFC | Not specified | 10 | 100 | 40 | 10 sec | 20 | 4000 | 3 over a 5‐day period |
| Boyer 2014 | Left M1 | anteroposterior | 10 | 90 | 20 | 10 | 50 | 2000 | 14, 10 sessions in 2 weeks followed by maintenance phase of 1 session at weeks 4, 6, 8, and 10 |
| Carretero 2009 | Right DLPFC | Not specified | 1 | 110 | 20 | 60 sec | 45 | 1200 | Up to 20 on consecutive working days |
| Dall'Agnol 2014 | Left M1 | 45° angle from sagittal line | 10 | 80 | 16 | 10 | 26 | 1600 | 10, timescale not specified |
| Defrin 2007 | M1 midline | Not specified | 5 | 115 | 500 | 10 sec | 30 | ? 500* | 10, x 1 daily |
| de Oliveira 2014 | Left DLPFC/premotor | not specified | 10 | 120 | 25 | 5 sec | 25 | 1250 | 10, x 1 daily (working days) for 2 weeks |
| Fregni 2005 | Left and right SII | Not specified | 1 or 20 | 90 | Not specified | Not specified | Not specified | 1600 | 1 |
| Fregni 2011 | Right SII | Not specified | 1 | 70% maximum stimulator output intensity (not RMT) | 1 | Not specified | Not specified | 1600 | 10, x 1 daily (weekdays only) |
| Hirayama 2006 | M1, S1, PMA, SMA | Not specified | 5 | 90 | 10 | 10 sec | 50 | 500 | 1 |
| Hosomi 2013 | M1 corresponding to painful region | Not specified | 5 | 90 | 10 | 10 sec | 50 | 500 | 10, x 1 daily (weekdays only) |
| Irlbacher 2006 | M1 contralateral to painful side | Not specified | 5, 1 | 95 | Not specified | Not specified | Not specified | 500 | 1 |
| Jetté 2013 | M1 hand or leg area with neuro navigation | 45º postero‐lateral | 10 | 90 | 40 | 5 | 25 | 2000 | 1, per stimulation condition |
| Kang 2009 | Right M1 | 45º postero‐lateral | 10 | 80 | 20 | 5 sec | 55 | 1000 | 5, x 1 daily |
| Khedr 2005 | M1 contralateral to painful side | Not specified | 20 | 80 | 10 | 10 sec | 50 | 2000 | 5, x 1 daily |
| Lee 2012 | Right DLPFC (low‐frequency) Left M1 (high‐frequency) |
Not specified | 10, 1 | 10 Hz: 80 1 Hz: 110 |
10 Hz: 25 1 Hz: 2 |
10 Hz: 8 sec 1 Hz: 800 sec |
10 Hz: 10 1 Hz: 60 |
10 Hz: 2000 1 Hz: 1600 |
10, x 1 daily (weekdays only) |
| Lefaucheur 2001a | M1 contralateral to painful side | Not specified | 10 | 80 | 20 | 5 sec | 55 | 1000 | 1 |
| Lefaucheur 2001b | M1 contralateral to painful side | Posteroanterior | 10, 0.5 | 80 | 10 Hz: 20 0.5 Hz: 1 |
10 Hz: 5 sec 0.5 Hz: 20 min |
10 Hz: 55 | 10 Hz: 1000 0.5 Hz: 600 |
1 |
| Lefaucheur 2004 | M1 contralateral to painful side | Posteroanterior | 10 | 80 | 20 | 5 sec | 55 | 1000 | 1 |
| Lefaucheur 2006 | M1 contralateral to painful side | Posteroanterior | 10, 1 | 90 | 10 Hz: 20 1 Hz: 1 |
10 Hz: 6 sec 1 Hz: 20 min |
10 Hz: 54 | 10 Hz: 1200 1 Hz: 1200 |
1 |
| Lefaucheur 2008 | M1 contralateral to painful side | Posteroanterior | 10, 1 | 90 | 10 Hz: 20 1 Hz: 1 |
10 Hz: 6 sec 1 Hz: 20 min |
10 Hz: 54 | 10 Hz: 1200 1 Hz: 1200 |
1 |
| Malavera 2013 | M1 contralateral to painful side | 45° angle from sagittal line | 10 | 90 | 20 | 6 | 54 | 1200 | 10, x 1 daily (weekdays only) |
| Medeiros 2016 | Left M1 | 45° angle from sagittal line | 10 | 80 | not reported | not reported | not reported | 1600 | 10, x 1 daily |
| Mhalla 2011 | Left M1 | Posteroanterior | 10 | 80 | 15 | 10 sec | 50 | 1500 | 14, 5 x 1 daily (working days), then 3 x 1 weekly, then 3 x 1 fortnightly, then 3 x 1 monthly |
| Nardone 2017 | Left PFC | Posteroanterior | 10 | 120 | 25 | 5 sec | 25 | 1250 | 10, x5 per week for 2 weeks |
| Nurmikko 2016 | M1 hotspot contralateral to pain M1 in reorganised area contralateral to pain |
Posteroanterior | 10 | 90 | 20 | 10 sec | 60 | 2000 | 5, x 3‐5 times per week |
| Onesti 2013 | M1 deep central sulcus | H‐coil | 20 | 100 | 30 | 2.5 sec | 30 | 1500 | 5, x 1 daily on consecutive days |
| Passard 2007 | M1 contralateral to painful side | Posteroanterior | 10 | 80 | 25 | 8 sec | 52 | 2000 | 10, x 1 daily (working days) |
| Picarelli 2010 | M1 contralateral to painful side | Posteroanterior | 10 | 100 | 25 | 10 sec | 60 | 2500 | 10, x 1 daily (working days) |
| Pleger 2004 | M1 hand area | Not specified | 10 | 110 | 10 | 1.2 sec | 10 | 120 | 1 |
| Rollnik 2002 | M1 midline | Not specified | 20 | 80 | 20 | 2 sec | Not specified | 800 | 1 |
| Saitoh 2007 | M1 over motor representation of painful area | Not specified | 10, 5, 1 | 90 | 10 Hz; 5 5 Hz: 10 1 Hz: 1 |
10 Hz: 10 sec 5 Hz: 10 sec 1 Hz: 500 sec |
10 Hz: 50 5 Hz: 50 |
500 | 1 |
| Short 2011 | Left DLPFC | Parasagittal | 10 | 120 | 80 | 5 sec | 10 sec | 4000 | 10, x 1 daily (working days) for 2 weeks |
| Tekin 2014 | M1 midline | 45° angle from sagittal line | 10 | 100 | 30 | 5 | 12 | 1500 | 10, x 1 daily (not clear if only work days) |
| Tzabazis 2013 | Targeted to ACC | 4‐coil configuration | 1 Hz (10 Hz data excluded as not randomised) | 110 | Not reported | Not reported | Not reported | 1800 | 20, x 1 daily (working days) |
| Umezaki 2016 | Left DLPFC | Not specified | 10 | 100 | 10 | 5 | 10 | 3000 | 10, x1 daily (working days) |
| Yagci 2014 | Left M1 | Not specified | 1 | 90 | 20 | 60 | 45 | 1200 | 10, x1 daily (working days) |
| Yilmaz 2014 | M1 midline | Handle pointing posteriorly | 10 | 10 | 30 | 5 | 25 | 1500 | 10, x1 daily (working days) |
ACC: anterior cingulate cortex; DLPFC: dorsolateral prefrontal cortex; M1: primary motor cortex; PFC: prefrontal cortex; PMA: pre‐motor area; RMT: resting motor threshold; dS1: primary somatosensory cortex; SII: secondary somatosensory cortex; SMA: supplementary motor area
*Inconsistency between stimulation parameters and reported total number of pulses in study report. See Included studies section for mored detail.
Stimulation location
The parameters for rTMS application varied significantly between studies, including by site of stimulation, stimulation parameters and the number of stimulation sessions. The majority of rTMS studies targeted the primary motor cortex (M1) (Ahmed 2011; André‐Obadia 2006; André‐Obadia 2008; André‐Obadia 2011; Attal 2016; Boyer 2014; Dall'Agnol 2014; Defrin 2007; Hirayama 2006; Hosomi 2013; Irlbacher 2006; Jetté 2013; Kang 2009; Khedr 2005; Lee 2012, Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Malavera 2013; Medeiros 2016; Mhalla 2011; Nurmikko 2016; Onesti 2013; Passard 2007; Picarelli 2010; Pleger 2004; Rollnik 2002; Saitoh 2007; Tekin 2014;). Of these, one study specified stimulation of the right hemisphere (Kang 2009), five studies specified the left hemisphere (Boyer 2014; Dall'Agnol 2014; Medeiros 2016; Mhalla 2011; Yagci 2014), and four studies specified stimulation over the midline (Defrin 2007; Pleger 2004; Tekin 2014; Yilmaz 2014). One study used a novel H‐coil to stimulate the motor cortex of the leg representation situated deep in the central sulcus (Onesti 2013), and the remainder stimulated over the contralateral cortex to the side of dominant pain. One of these studies also investigated stimulation of the supplementary motor area (SMA), pre‐motor area (PMA) and primary somatosensory cortex (S1) (Hirayama 2006). Seven studies stimulated the dorsolateral prefrontal cortex (DLPFC) or prefrontal cortex (PFC), with five studies stimulating the left hemisphere (Borckardt 2009; de Oliveira 2014; Nardone 2017; Short 2011; Umezaki 2016), and two studies the right (Carretero 2009; Lee 2012). One study investigated stimulation of the left and right secondary somatosensory cortex (SII) as separate treatment conditions (Fregni 2005), and another investigated stimulation to the right SII area (Fregni 2011). One study used a four‐coil configuration to target the anterior cingulate cortex (Tzabazis 2013).
Stimulation parameters
Frequency
Twelve studies investigated low‐frequency (< 5 Hz) rTMS (André‐Obadia 2006; Carretero 2009; Fregni 2005; Fregni 2011; Irlbacher 2006; Lee 2012; Lefaucheur 2001b; Lefaucheur 2006; Lefaucheur 2008; Saitoh 2007; Tzabazis 2013; Yagci 2014). Of these, one study used a frequency of 0.5 Hz in one treatment condition (Lefaucheur 2001b), and the rest used a frequency of 1 Hz. Thirty‐nine studies investigated high‐frequency (≥ 5 Hz) rTMS (Ahmed 2011; André‐Obadia 2006; André‐Obadia 2008; André‐Obadia 2011; Attal 2016; Avery 2013; Borckardt 2009; Boyer 2014; Dall'Agnol 2014; Defrin 2007; de Oliveira 2014; Fregni 2005; Hirayama 2006; Hosomi 2013; Irlbacher 2006; Jetté 2013; Kang 2009; Khedr 2005; Lee 2012; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Malavera 2013; Medeiros 2016; Mhalla 2011; Nardone 2017; Nurmikko 2016; Onesti 2013; Passard 2007; Picarelli 2010; Pleger 2004; Rollnik 2002; Saitoh 2007; Short 2011; Tekin 2014; Umezaki 2016; Yilmaz 2014). While the study by Tzabazis 2013 did apply high‐frequency stimulation to some participants, the allocation of the high‐frequency groups was not randomised in that study (confirmed through correspondence with authors) and so those data will not be considered further in this review as they do not meet our inclusion criteria.
Other parameters
We observed wide variation between studies for various stimulation parameters. The overall number of rTMS pulses delivered varied from 120 to 4000. Defrin 2007 reported a total number of pulses of 500 although the reported stimulation parameters of 500 trains, delivered at a frequency of 5 Hz for 10 seconds would imply 25,000 pulses. Thirteen studies specified a posteroanterior or parasagittal orientation of the stimulating coil (André‐Obadia 2006; Attal 2016; Boyer 2014; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Nardone 2017; Nurmikko 2016; Passard 2007; Picarelli 2010; Short 2011; Yilmaz 2014), seven studies specified a coil orientation 45º to the midline (Ahmed 2011; Dall'Agnol 2014; Jetté 2013; Kang 2009; Malavera 2013; Medeiros 2016; Tekin 2014), one study compared a posteroanterior coil orientation with a medial‐lateral coil orientation (André‐Obadia 2008), one used an H‐coil (Onesti 2013), one used a four‐coil configuration (Tzabazis 2013), and the remaining studies did not specify the orientation of the coil. Within studies that reported the information, the duration and number of trains and the inter‐train intervals varied. Two studies did not report this information (Fregni 2005; Fregni 2011).
Type of sham
rTMS studies employed a variety of sham controls. In 13 studies the stimulating coil was angled away from the scalp to prevent significant cortical stimulation (Ahmed 2011; André‐Obadia 2006; André‐Obadia 2008; Carretero 2009; Hirayama 2006; Kang 2009; Khedr 2005; Lee 2012; Pleger 2004; Rollnik 2002; Saitoh 2007; Yagci 2014; Yilmaz 2014), of which two studies also simultaneously electrically stimulated the skin of the scalp in both the active and sham stimulation conditions in order to mask the sensations elicited by active rTMS and thus preserve participants' blinding (Hirayama 2006; Saitoh 2007). One study (Nurmikko 2016) applied active stimulation at the same parameters as for the active stimulation condition, but applied to the occipital fissure, which is a site at which stimulation is not hypothesised to induce analgesia. The remaining studies utilised sham coils. Of these, 13 studies specified that the sham coil made similar or identical sounds to those elicited during active stimulation (André‐Obadia 2011; Borckardt 2009; Boyer 2014; Defrin 2007; de Oliveira 2014; Irlbacher 2006; Malavera 2013; Mhalla 2011; Nardone 2017; Passard 2007; Picarelli 2010; Tekin 2014; Tzabazis 2013), and eight specified that the sham coil made similar sounds, looked the same and elicited similar scalp sensations as the real coil (Attal 2016; Avery 2013; Fregni 2011; Hosomi 2013; Jetté 2013; Onesti 2013; Short 2011; Umezaki 2016). Eight studies did not specify whether the sham coil controlled for the auditory characteristics of active stimulation (Dall'Agnol 2014; Fregni 2005; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Medeiros 2016).
Studies of CES
See Table 10 for a summary of stimulation characteristics utilised in CES studies.
Table 2.
Cranial electrotherapy stimulation (CES) studies ‐ characteristics of stimulation
| Study | Electrode placement | Frequency (Hz) | Pulse width (ms) | Waveform shape | Intensity | Duration (min) | Treatment sessions per group |
| Capel 2003 | Ear clip electrodes | 10 | 2 | Not specified | 12 μA | 53 | x 2 daily for 4 days |
| Cork 2004 | Ear clip electrodes | 0.5 | Not specified | Modified square‐wave biphasic | 100 μA | 60 | ? daily for 3 weeks |
| Gabis 2003 | Mastoid processes and forehead | 77 | 3.3 | Biphasic asymmetric | ≤ 4 mA | 30 | x 1 daily for 8 days |
| Gabis 2009 | Mastoid processes and forehead | 77 | 3.3 | Biphasic asymmetric | ≤ 4 mA | 30 | x 1 daily for 8 days |
| Katsnelson 2004 | Mastoid processes and forehead | Not specified | Not specified | 2 conditions: symmetric, asymmetric | 11 to 15 mA | 40 | x 1 daily for 5 days |
| Lichtbroun 2001 | Ear clip electrodes | 0.5 | Not specified | Biphasic square wave | 100 μA | 60 | x 1 daily for 30 days |
| Rintala 2010 | Ear clip electrodes | Not specified | Not specified | Not specified | 100 μA | 40 | x 1 daily for 6 weeks |
| Tan 2000 | Ear clip electrodes | 0.5 | Not specified | Not specified | 10 to 600 μA | 20 | 12 (timing not specified) |
| Tan 2006 | Ear clip electrodes | Not specified | Not specified | Not specified | 100 to 500 μA | 60 | x 1 daily for 21 days |
| Tan 2011 | Ear clip electrodes | Not specified | Not specified | Not specified | 100 μA | 60 | x 1 daily for 21 days |
| Taylor 2013 | Ear clip electrodes | 0.5 | Not specified | Modified square‐wave biphasic | 100 μA | 60 | x 1 daily for 8 weeks |
Stimulation device, parameters and electrode location
Seven studies of CES used the 'Alpha‐stim' CES device (Electromedical Products International, Inc, Mineral Wells, Texas, USA). This device uses two ear clip electrodes that attach to each of the participant's ears (Cork 2004; Lichtbroun 2001; Rintala 2010; Tan 2000; Tan 2006; Tan 2011; Taylor 2013), and these studies utilised stimulation intensities of 100 μA with a frequency of 0.5 Hz. One study (Capel 2003) used a device manufactured by Carex (Hemel Hempstead, UK) that also used earpiece electrodes and delivered a stimulus intensity of 12 μA.
Two studies used the 'Pulsatilla 1000' device (Pulse Mazor Instruments, Rehavol, Israel) (Gabis 2003; Gabis 2009). The electrode array for this device involved an electrode attached to each of the participant's mastoid processes and one attached to the forehead; current is passed to the mastoid electrodes. One study used the 'Nexalin' device (Kalaco Scientific Inc, Scottsdale, AZ, USA) (Katsnelson 2004). With this device current is applied to a forehead electrode and returned via electrodes placed behind the participant's ears. These three studies utilised significantly higher current intensities than those using ear clip electrodes with intensities of 4 mA (Gabis 2003; Gabis 2009), and 11 to 15 mA (Katsnelson 2004).
All CES studies gave multiple treatment sessions for each treatment group with variation between the number of treatments delivered.
Type of sham
Eight studies utilised inert sham units (Capel 2003; Cork 2004; Lichtbroun 2001; Rintala 2010; Tan 2000; Tan 2006; Tan 2011; Taylor 2013). These units were visually indistinguishable from the active devices. Stimulation at the intensities used is subsensation and as such it should not have been possible for participants to distinguish between the active and sham conditions.
Two studies utilised an "active placebo" treatment unit (Gabis 2003; Gabis 2009). This sham device was visually indistinguishable and delivered a current of much lower intensity (≤ 0.75 mA) than the active stimulator to evoke a similar sensation to ensure participant blinding. Similarly, Katsnelson 2004 utilised a visually indistinguishable sham device that delivered brief pulses of current of less than 1 mA. The placebo conditions used in these three studies delivered current at much greater intensities than those used in the active stimulation conditions of the other CES studies.
Studies of tDCS
See Table 11 for a summary of stimulation characteristics utilised in tDCS studies.
Table 3.
Transcranial direct current stimulation (tDCS) studies ‐ characteristics of stimulation
| Study | Location of stimulation (Anode) | Electrode pad size | Intensity (mA) | Anodal or cathodal? | Stimulus duration (min) | Treatment sessions per group |
| Ahn 2017 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Antal 2010 | M1 left hand area | 35 cm2 | 1 mA | Anodal | 20 | 5, x 1 daily |
| Ayache 2016 | Left DLPFC | 25 cm2 | 2mA | Anodal | 20 | 3, x 1 daily |
| Bae 2014 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | x 3 per week for 3 weeks |
| Boggio 2009 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 30 | 1 |
| Brietzke 2016 | Left M1 | 25‐35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Chang 2017 | M1 contralateral to painful side | 35 cm2 | 1 mA | Anodal | 20 | 16, x 2 weekly for 8 weeks |
| Donnell 2015 | M1 contralateral to painful side | HD‐tDCS | 2 mA | Anodal | 20 | 5, x 1 daily |
| Fagerlund 2015 | M1, side not specified | 35 cm2 | 2mA | Anodal | 20 | 5, x 1 daily |
| Fenton 2009 | M1 dominant hemisphere | 35 cm2 | 1 mA | Anodal | 20 | 2 |
| Fregni 2006a | M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Fregni 2006b | M1 and DLPFC contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Hagenacker 2014 | M1 contralateral to painful side | 40 cm2 | 1mA | Anodal | 20 | Daily, self‐administered for 14 days |
| Harvey 2017 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Hazime 2017 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 12, x 3 per week for 4 weeks |
| Jales Junior 2015 | Left M1 | 15 cm2 | 1mA | Anodal | 20 | x 1 weekly for 10 weeks |
| Jensen 2013 | M1 left | 35cm2 | 2 mA | Anodal | 20 | 1 |
| Khedr 2017 | M1 contralateral to painful side | 24 cm2 | 2 mA | Anodal | 20 | 10, x 1 daily, 5 days per week for 2 weeks |
| Kim 2013 | M1, side not specified DLPFC |
25 cm2 | 2mA | Anodal | 20 | 5, x 1 daily |
| Lagueux 2017 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 14, x 5 weekly for 2 weeks, x 1 weekly for 4 weeks |
| Luedtke 2015 | M1 left side not specified | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Mendonca 2011 | Group 1: anodal left M1 Group 2: cathodal left M1 Group 3: anodal supraorbital Group 4: cathodal supraorbital Group 5: sham |
35 cm2 | 2 mA | Anodal or cathodal | 20 | 1 |
| Mendonca 2016 | Left M1 | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Mori 2010 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Ngernyam 2015 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 1 |
| Oliveira 2015 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily, then x 2 weekly for 3 weeks, up to 10 sessions |
| Portilla 2013 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | x 1 per condition |
| Riberto 2011 | M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 10, x 1 weekly |
| Sakrajai 2014 | M1 contralateral to painful side | 35 cm2 | 1 mA | Anodal | 20 | 5, x 1 daily |
| Soler 2010 | M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 10, x 1 daily (weekdays only) |
| Souto 2014 | Left M1 | 25 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Thibaut 2017 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Valle 2009 | M1 and DLPFC contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Villamar 2013 | M1 left | HD‐tDCS 4 x 1‐ring montage | 2 mA | Anodal or cathodal | 20 | x 1 per condition |
| Wrigley 2014 | M1 contralateral to painful side or dominant hand | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
| Volz 2016 | M1 contralateral to painful side | 35 cm2 | 2 mA | Anodal | 20 | 5, x 1 daily |
DLPFC: dorsolateral prefrontal cortex; HD‐tDCS: high definition tDCS; M1: primary motor cortex
Stimulation parameters and electrode location
Four studies of tDCS stimulated the dorsolateral prefrontal cortex in one treatment group (Ayache 2016; Fregni 2006b; Kim 2013; Valle 2009). Thirty‐four studies stimulated the motor cortex (Ahn 2017; Antal 2010; Bae 2014; Boggio 2009; Brietzke 2016; Chang 2017; Donnell 2015; Fagerlund 2015; Fenton 2009; Fregni 2006a; Fregni 2006b; Hagenacker 2014; Harvey 2017; Hazime 2017; Jales Junior 2015; Jensen 2013; Khedr 2017; Kim 2013; Lagueux 2017; Luedtke 2015; Mendonca 2016; Mori 2010; Ngernyam 2015; Oliveira 2015; Portilla 2013; Riberto 2011; Sakrajai 2014; Soler 2010; Souto 2014; Thibaut 2017; Valle 2009; Villamar 2013; Volz 2016; Wrigley 2014). Of these, 23 stimulated the cortex contralateral to the side of worst pain (Ahn 2017; Bae 2014; Boggio 2009; Chang 2017; Donnell 2015; Fregni 2006a; Fregni 2006b; Hagenacker 2014; Harvey 2017; Hazime 2017; Khedr 2017; Lagueux 2017; Mori 2010; Ngernyam 2015; Oliveira 2015; Portilla 2013; Riberto 2011; Sakrajai 2014; Soler 2010; Thibaut 2017; Villamar 2013; Volz 2016; Wrigley 2014), of which six studies stimulated the opposite hemisphere to the dominant hand where pain did not have a unilateral dominance (Fregni 2006a; Fregni 2006b; Jensen 2013; Riberto 2011; Soler 2010; Wrigley 2014). Seven studies stimulated the left hemisphere for all participants (Antal 2010; Brietzke 2016; Jales Junior 2015; Mendonca 2016; Souto 2014; Valle 2009; Villamar 2013). One study of chronic pelvic pain stimulated the opposite hemisphere to the dominant hand in all participants (Fenton 2009). One study specifically investigated the use of tDCS in conjunction with transcutaneous electrical nerve stimulation (TENS) therapy (Boggio 2009). We extracted data comparing active tDCS and sham TENS with sham tDCS and sham TENS for the purposes of this review. One study applied anodal or cathodal stimulation to the left motor cortex or to the right supraorbital area (Mendonca 2011).
Eighteen studies delivered a current intensity of 2 mA for 20 minutes once a day for five days (Ahn 2017; Antal 2010; Brietzke 2016; Donnell 2015; Fagerlund 2015; Fregni 2006a; Fregni 2006b; Harvey 2017; Kim 2013; Luedtke 2015; Mendonca 2016; Mori 2010; Sakrajai 2014; Souto 2014; Thibaut 2017; Valle 2009; Volz 2016; Wrigley 2014). Across the remaining studies, dose, in terms of the number and frequency of stimulation sessions, varied considerably, from a single 20‐minute session to up to 10 weeks of stimulation with either one or multiple sessions of stimulation in a week. In one study (Hagenacker 2014) tDCS was self‐administered by participants, daily for 14 days. Six studies (Antal 2010; Chang 2017; Fenton 2009; Hagenacker 2014; Jales Junior 2015; Sakrajai 2014) delivered stimulation at a current intensity of 1 mA.
All studies of tDCS utilised a sham condition whereby active stimulation was ceased after 30 seconds without the participants' knowledge.
Excluded studies
See Characteristics of excluded studies.
In previous versions of this review we excluded 20 studies after consideration of the full study report. Of these, two were not studies of brain stimulation (Carraro 2010; Frentzel 1989), two did not assess self‐reported pain as an outcome (Belci 2004; Johnson 2006), seven were not restricted to participants with chronic pain or clearly in a chronic pain population (Avery 2007; Choi 2012a; Choi 2012b; Evtiukhin 1998; Katz 1991; Longobardi 1989; Pujol 1998), two were single case studies (Silva 2007; Zaghi 2009), one study presented duplicate data from a study already accepted for inclusion (Roizenblatt 2007, duplicate data from Fregni 2006b), one did not employ a sham control (Evtiukhin 1998), one was not a randomised controlled trial (O'Connell 2013), one reported uncontrolled long‐term follow‐up data from an included study (Hargrove 2012b), one employed an intervention that was not designed to alter cortical activity directly through electrical stimulation (Nelson 2010), and one included some participants who did not meet our criterion of chronic pain (Bolognini 2013). A final study was screened by a Russian translator and excluded on the basis that it did not employ a sham control for tDCS (Sichinava 2012).
In this update we excluded a further 14 reports of 12 studies. Three of these studies did not randomly allocate participants to groups (Cummiford 2016; Lindholm 2015; Yoon 2014). Six were not clearly in a chronic population (Bolognini 2015; Choi 2014; Khedr 2005; Ma 2015; Morin 2017; Schabrun 2014), two were not studies of electrical brain stimulation (Maestu 2013; Smania 2005), one did not employ a sham control (Seada 2013).
Studies awaiting classification
In this update we have 18 studies registered as awaiting classification. Of these 16 have been published as conference abstracts but we have not been able to obtain a full study report. We were unable to source the original study report for the remaining two. For further details see Characteristics of studies awaiting classification.
Ongoing studies
In this update we have identified 48 ongoing studies. These studies all investigate the effect of either tDCS or rTMS for chronic pain. For further details see Characteristics of ongoing studies.
Risk of bias in included studies
Risk of bias varied across studies for all of the assessment criteria. For summaries of 'Risk of bias' assessment across studies see Figure 2 and Figure 3.
Figure 2.
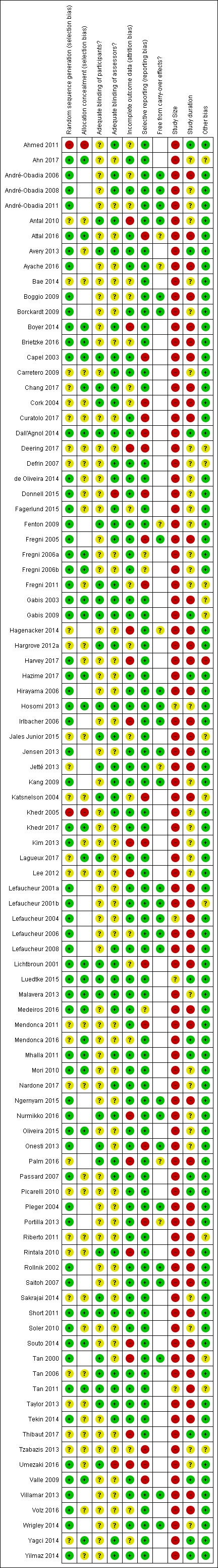
Methodological quality summary: review authors' judgements about each methodological quality item for each included study
Figure 3.
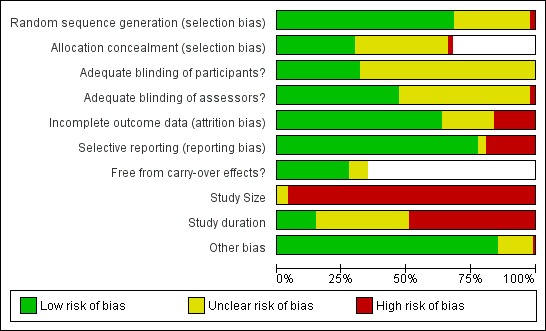
Risk of bias graph: review authors' judgements about each risk of bias item presented as percentages across all included studies
Sequence generation
For the criterion 'adequate sequence generation' we awarded cross‐over trials a judgement of 'low risk of bias' where the study report mentioned that the order of treatment conditions was randomised. Since this criterion has a greater potential to introduce bias in parallel designs we only awarded a judgement of 'low risk of bias' where the method of randomisation was specified and adequate.
We judged 28 trials as having an unclear risk of bias (Antal 2010; Bae 2014; Carretero 2009; Chang 2017; Cork 2004; Curatolo 2017; Deering 2017; Defrin 2007; Hagenacker 2014; Hargrove 2012a; Jales Junior 2015; Jetté 2013; Katsnelson 2004; Lagueux 2017; Lee 2012; Mendonca 2011; Mendonca 2016; Nardone 2017; Palm 2016; Picarelli 2010; Riberto 2011; Rintala 2010; Sakrajai 2014; Tan 2006; Taylor 2013; Thibaut 2017; Tzabazis 2013; Yagci 2014), as they did not specify the method of randomisation used or the description was not clear. We judged two studies as having a high risk of bias for this criterion (Ahmed 2011; Khedr 2005), as the reports suggested that participants were allocated depending on the day of the week on which they were recruited, which we did not judge as being genuinely random. We judged the remaining 64 studies as having a low risk of bias for this domain.
Allocation concealment
We only considered allocation concealment for parallel designs or cross‐over trials from which only data from the first cross‐over phase of the study was included (i.e. we considered them as parallel‐group studies). Thirty‐four studies did not clearly report concealment of allocation and we judged them as unclear (Antal 2010; Avery 2013; Bae 2014; Carretero 2009; Cork 2004; Curatolo 2017; de Oliveira 2014; Deering 2017; Defrin 2007; Donnell 2015; Fagerlund 2015; Fregni 2011; Hargrove 2012a; Harvey 2017; Jales Junior 2015; Katsnelson 2004; Kim 2013; Lee 2012; Mendonca 2011; Nardone 2017; Passard 2007; Picarelli 2010; Riberto 2011; Rintala 2010; Sakrajai 2014; Soler 2010; Tan 2006; Taylor 2013; Tekin 2014; Thibaut 2017; Tzabazis 2013; Umezaki 2016; Volz 2016; Yilmaz 2014), and we judged two studies as having a high risk of bias for this criterion since the method of randomisation employed would not have supported concealment of allocation (Ahmed 2011; Khedr 2005). We judged 28 studies as having a low risk of bias for this domain.
Blinding
Blinding of participants
All studies attempted to blind participants. However, due to the difficulties involved in producing a robust sham control in rTMS studies (see Assessment of risk of bias in included studies) we made an assessment of sham credibility. Where the coil was angled or angled and elevated away from the scalp, this is potentially distinguishable both visually and by the sensory effects of stimulation. Two studies simultaneously electrically stimulated the scalp during rTMS stimulation to mask the differences in sensation between conditions (Hirayama 2006; Saitoh 2007). However, by angling the coil away from the scalp, participants may have been able to visually distinguish between the conditions. Where sham coils were utilised they usually did not control for the sensory aspects of stimulation. We assessed most rTMS studies as having suboptimal sham control conditions and we therefore assessed them as having an 'unclear' risk of bias.
One study with a sham of this type presented a formal assessment of blinding that demonstrated blinding success (Malavera 2013) and was rated at low risk. Seven rTMS studies included in this update utilised sham coils that are visually indistinguishable, emit the same noise during stimulation and elicit similar scalp sensations (Avery 2013; Dall'Agnol 2014; Fregni 2011; Jetté 2013; Onesti 2013; Short 2011; Umezaki 2016). One study (Nurmikko 2016) applied active stimulation to a site of the brain not hypothesised to elicit analgesia as its sham condition. While there may be a risk of this stimulation having an effect we considered that this sham could be expected to be indistinguishable from real stimulation. These studies met the criteria for an optimal sham condition and as such we judged them at low risk of bias for participant blinding.
Similarly with tDCS studies, due to evidence that blinding of participants to the stimulation condition may be compromised at intensities of 1.5 mA and above, we judged the majority of tDCS studies at unclear risk of bias on this criterion (Ahn 2017; Attal 2016; Ayache 2016; Bae 2014; Boggio 2009; Brietzke 2016; Donnell 2015; Fagerlund 2015; Fregni 2006a; Fregni 2006b; Harvey 2017; Hazime 2017; Jensen 2013; Khedr 2017; Kim 2013; Mendonca 2011; Mendonca 2016; Mori 2010; Ngernyam 2015; Oliveira 2015; Portilla 2013; Riberto 2011; Soler 2010; Souto 2014; Thibaut 2017; Valle 2009; Villamar 2013; Volz 2016; Wrigley 2014) unless there was evidence of blinding success (Lagueux 2017; Luedtke 2015). We judged one study Hagenacker 2014 at unclear risk of bias as the method of blinding was not described.
We assessed all studies of CES and RINCE and the single study of tRNS as having a low risk of bias for this criterion.
Overall, we judged 27 studies at low risk of bias, and 57 studies at unclear risk of bias.
Blinding of assessors
While many studies used self‐reported pain outcomes we considered that the complex nature of the intervention, and the level of interaction this entails between participants and assessors, suggested that a lack of blinding of the researchers engaged in the collection of outcomes might potentially introduce bias. This is particularly the case when a VAS is used to measure pain intensity as this requires the assessor to measure the distance from the zero anchor point to the mark made by the participant. As such, where blinding of assessors was not clearly stated we made a judgement of 'unclear' for this criterion. We rated studies of tDCS that applied stimulation intensity of 2 mA and where no formal assessment of blinding success was presented as at unclear risk of bias, since there is evidence that assessor blinding may be compromised at the stimulation intensities used (O'Connell 2012).
We judged 48 studies to be at unclear risk of bias (Ahn 2017; André‐Obadia 2011; Attal 2016; Ayache 2016; Bae 2014; Boggio 2009; Borckardt 2009; Brietzke 2016; Curatolo 2017; Deering 2017; Fregni 2006a; Fregni 2006b; Hagenacker 2014; Harvey 2017; Hazime 2017; Hirayama 2006; Irlbacher 2006; Jensen 2013; Khedr 2017; Kim 2013; Lagueux 2017;Lee 2012; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Mendonca 2011; Mendonca 2016; Mori 2010; Ngernyam 2015; Oliveira 2015; Onesti 2013; Picarelli 2010; Pleger 2004; Portilla 2013; Riberto 2011; Rollnik 2002; Saitoh 2007; Sakrajai 2014; Soler 2010; Souto 2014; Tan 2000; Thibaut 2017; Tzabazis 2013; Valle 2009; Villamar 2013; Volz 2016; Wrigley 2014), two studies (Donnell 2015; Umezaki 2016) at high risk of bias, as they clearly reported that assessors were not blinded, and we rated the remaining studies at low risk of bias.
Incomplete outcome data
We assessed 19 studies as having an unclear risk of bias for this criterion (Ahmed 2011; André‐Obadia 2006; André‐Obadia 2011; Bae 2014; Brietzke 2016; Boggio 2009; Chang 2017; Cork 2004; Fagerlund 2015; Fregni 2011; Hargrove 2012a; Jales Junior 2015; Katsnelson 2004; Lefaucheur 2006; Lichtbroun 2001; Mendonca 2016; Tzabazis 2013; Volz 2016; Yagci 2014). Of these, Ahmed 2011; Bae 2014; Cork 2004; Fregni 2011; Jales Junior 2015; Katsnelson 2004; Lefaucheur 2006; Lichtbroun 2001; Tzabazis 2013 and Volz 2016 did not report the level of dropout from their studies. Tzabazis 2013 reported recruiting 16 participants in the full study report (Tzabazis 2013), but an earlier abstract report of the same study reported the recruitment of 45 participants. In the study of André‐Obadia 2006, two participants (17% of the study cohort) did not complete the study and this was not clearly accounted for in the data analysis. This was also the case for Boggio 2009, where two participants (25% of the cohort) failed to complete the study. Brietzke 2016 and Mendonca 2016 reported dropout of more than10% and used the last observation carried forward (LOCF) approach for imputation. Chang 2017 and Yagci 2014 reported dropout of more than 10% and conducted an available case analysis. Fagerlund 2015 had a high noncompletion rate for some outcomes and did not clearly report how many participants were analysed for each outcome.
We assessed fifteen studies as having a high risk of bias for this criterion (Antal 2010; Boyer 2014; Deering 2017; Hagenacker 2014; Harvey 2017; Irlbacher 2006; Kim 2013; Lee 2012; Nurmikko 2016; Palm 2016; Rintala 2010; Souto 2014; Tan 2000; Thibaut 2017; Umezaki 2016). In the Antal 2010 study, of 23 participants recruited only 12 completed the full cross over. Boyer 2014 reported dropout of more than 20% and, while an intention‐to‐treat approach was reported the details of this and any imputation of missing data were not reported. Deering 2017 excluded eight out of 15 participants randomised to the sham condition on the basis that "an unexpected signal source was discovered in EEG traces". Harvey 2017 reported a 25% dropout rate in the active stimulation arm only and those participants appear to have been excluded from the analysis. In the study by Irlbacher 2006, only 13 of the initial 27 participants completed all of the treatment conditions. Kim 2013 reported a 15% dropout rate and excluded those participants from the analysis. Nurmikko 2016 reported a 33% dropout rate with a per‐protocol analysis. Palm 2016 reported 13% dropout and excluded those participants from the analysis. Souto 2014 reported 20% dropout and used the LOCF method to impute missing data. In the studies of Hagenacker 2014; Lee 2012 and Rintala 2010, attrition exceeded 30% of the randomised cohort. In the study by Tan 2000, 17 participants did not complete the study (61% of the cohort) and this was not clearly accounted for in the analysis. Thibaut 2017 reported a 57% dropout rate. Umezaki 2016 reported dropout of more than 20% and conducted a per‐protocol analysis.
Selective reporting
We assessed studies as having a high risk of bias for this criterion where the study report did not produce adequate data to assess the effect size for all groups/conditions at all follow‐up time points, and these data were not made available upon request. We assessed 18 studies as having a high risk of bias for this criterion (Attal 2016; Capel 2003; Cork 2004; Curatolo 2017; Dall'Agnol 2014; Deering 2017; Donnell 2015; Fregni 2005; Fregni 2011; Katsnelson 2004; Kim 2013; Lichtbroun 2001; Mendonca 2011; Onesti 2013; Portilla 2013; Tzabazis 2013; Umezaki 2016; Valle 2009). We judged three studies as being at unclear risk of bias (Fregni 2006a; Fregni 2006b; Medeiros 2016). In the reports of Fregni 2006a and Fregni 2006b data were not presented in a format that could be easily interpreted. On request data were available from these two studies for the primary outcome at baseline and short‐term follow‐up but not for other follow‐up points. Medeiros 2016 reported pain VAS scores but not the results of pain diaries that were described in the methods. We assessed the remaining 73 studies as having a low risk of bias for this criterion. For this update, we first made requests for data (by email where possible). If any data are made available in time for future updates then we will revise judgements on this criterion accordingly.
Carry‐over effects in cross‐over trials
We judged seven studies (Attal 2016; Ayache 2016; Fenton 2009; Hagenacker 2014; Jetté 2013; Palm 2016; Portilla 2013) as unclear on this criterion as no formal investigation of carry‐over effects was discussed in the study report. In one cross‐over study baseline differences between the sham and the 10 Hz stimulation condition were notable (Saitoh 2007). A paired t‐test did not show a difference (P > 0.1) and we judged this study as having a low risk of bias for carry‐over effects. We rated 25 cross‐over studies at low risk of bias and the remaining 52 studies were not assessed due to their parallel design.
A number of studies were judged at unclear risk of bias as information regarding between group baseline comparability was not presented.
Study size
We rated four studies at unclear risk of bias (Hosomi 2013; Lefaucheur 2004; Luedtke 2015; Tan 2011), with all remaining studies rated at high risk of bias on this criterion.
Study duration
We rated 14 studies at low risk of bias on this criterion (Ahmed 2011; Avery 2013; Dall'Agnol 2014; Gabis 2009; Hazime 2017; Luedtke 2015; Mendonca 2016; Mhalla 2011; Passard 2007; Picarelli 2010; Thibaut 2017; Valle 2009; Yagci 2014; Yilmaz 2014), 34 studies at unclear risk of bias (Ahn 2017; André‐Obadia 2008; André‐Obadia 2011; Antal 2010; Bae 2014; Borckardt 2009; Carretero 2009; Deering 2017; Defrin 2007; de Oliveira 2014; Donnell 2015; Fagerlund 2015; Fenton 2009; Fregni 2006a; Fregni 2006b; Fregni 2011; Hosomi 2013; Kang 2009; Khedr 2005; Khedr 2017; Kim 2013; Lagueux 2017; Lee 2012; Malavera 2013; Mori 2010; Nardone 2017; Nurmikko 2016; Oliveira 2015; Onesti 2013; Sakrajai 2014; Soler 2010; Tzabazis 2013; Umezaki 2016; Wrigley 2014), and the remaining studies at high risk of bias (André‐Obadia 2006; Attal 2016; Ayache 2016; Boggio 2009; Boyer 2014; Brietzke 2016; Capel 2003; Chang 2017; Cork 2004; Curatolo 2017; Fregni 2005; Gabis 2003; Hagenacker 2014; Hargrove 2012a; Harvey 2017; Hirayama 2006; Irlbacher 2006; Jales Junior 2015; Jensen 2013; Jetté 2013; Katsnelson 2004; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Lichtbroun 2001; Medeiros 2016; Mendonca 2011; Ngernyam 2015; Palm 2016; Pleger 2004; Portilla 2013; Riberto 2011; Rintala 2010; Rollnik 2002; Saitoh 2007; Short 2011; Souto 2014; Tan 2000; Tan 2006; Tan 2011; Taylor 2013; Tekin 2014; Villamar 2013; Volz 2016).
Other potential sources of bias
Overall, we judged 13 studies at unclear risk of bias and one study at high risk of bias on this criterion. Five studies (Deering 2017; Fregni 2011; Jales Junior 2015; Katsnelson 2004; Tzabazis 2013) were judged at unclear risk of bias as they did not adequately report baseline values for the groups to allow assessment of baseline comparability. One of those studies (Deering 2017) was rated as unclear on the criteria as no formal baseline comparisons were presented and around half of those randomised to the sham group were excluded from the baseline score. We judged four studies (Ahn 2017; Defrin 2007; Riberto 2011; Tan 2011) at unclear risk of bias as baseline differences were apparent for pain‐related measures. We rated Harvey 2017 at high risk of bias on the basis of a greater than 3‐point difference between the active and sham groups in baseline pain levels on a 0 to 10 scale.
One study of CES also applied electrical stimulation to the painful body area as part of the treatment, which may have affected the final outcomes (Tan 2000). Two studies of CES used an "active placebo condition" that delivered a level of cortical stimulation that was greater than that used in the active arm of other CES studies (Gabis 2003; Gabis 2009). It is possible that delivering cortical stimulation in the sham group might mask differences between the sham and active condition. Also such a large difference in current intensity compared with other studies of CES might be a source of heterogeneity. We judged these three studies as 'unclear' on this criterion. We rated one study (Lefaucheur 2001b) at unclear risk of bias as the outcome of a planned statistical analysis was not reported. We judged 80 studies at low risk of bias for this criterion.
Effects of interventions
See: Table 1; Table 2; Table 3
For a summary of all core findings, see Table 1; Table 2; Table 3.
Primary outcome: pain intensity
Repetitive transcranial magnetic stimulation (rTMS): short‐term (0 to < 1 week postintervention)
The primary meta‐analysis (Analysis 1.1) pooled data from all rTMS studies with low or unclear risk of bias (excluding the risk of bias criteria 'study size' and 'study duration') where data were available (27 studies, n = 655), including cross‐over and parallel designs, using the generic inverse variance method (André‐Obadia 2006; André‐Obadia 2008; André‐Obadia 2011; Avery 2013; Borckardt 2009; Carretero 2009; Defrin 2007; de Oliveira 2014; Hirayama 2006; Hosomi 2013; Jetté 2013; Kang 2009; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Lefaucheur 2008; Medeiros 2016; Mhalla 2011; Nardone 2017; Passard 2007; Pleger 2004; Rollnik 2002; Saitoh 2007; Short 2011; Tekin 2014; Yagci 2014). We excluded the studies by Ahmed 2011; Boyer 2014; Dall'Agnol 2014; Khedr 2005; Irlbacher 2006; Lee 2012; Nurmikko 2016 and Umezaki 2016 as we classified them as having a high risk of bias on at least one criterion. We were unable to include data from six studies (Fregni 2005; Fregni 2011; Onesti 2013; Picarelli 2010; Tzabazis 2013; Umezaki 2016, combined n = 107) as the necessary data were not available in the study report or upon request by the submission date of this update. We could not include the data from Yilmaz 2014 as outcomes were only reported as a median (interquartile range). We imputed the correlation coefficient used to calculate the standard error (SE) (standardised mean difference (SMD)) for cross‐over studies (0.764) from data extracted from André‐Obadia 2008 (as outlined in Unit of analysis issues) and we entered the SMD (SE) for each study into a generic inverse variance meta‐analysis. We divided the number of participants in each cross‐over study by the number of comparisons made by that study included in the meta‐analysis. For parallel studies we calculated the standard error of the mean (SEM) from the 95% confidence intervals (CIs) of the standardised mean difference (SMD) and entered both the SMD and the SEM into the meta‐analysis. We then entered this into the meta‐analysis with the SMD using the generic inverse variance method.
Analysis 1.1.
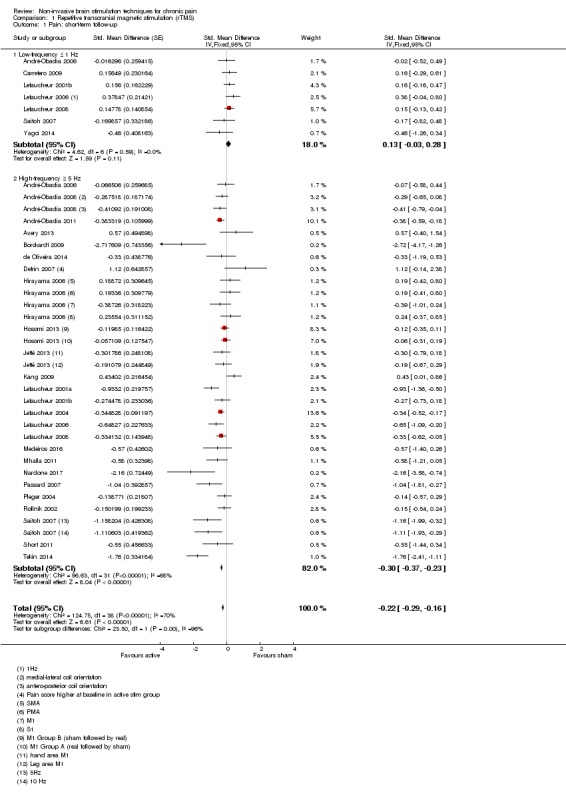
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 1 Pain: short‐term follow‐up.
The pooled SMD for this comparison was ‐0.22 (95% CI ‐0.29 to ‐0.16, P < 0.001). We back‐transformed the SMD to a mean difference using the mean standard deviation of the post‐treatment sham group scores of the studies included in this analysis (1.86). We then used this to estimate the real percentage change on a 0 to 10 pain intensity scale of active stimulation compared with the mean poststimulation score from the sham groups of the included studies (5.94). This equates to a 7% (95% CI 5% to 9%) reduction in pain, or a 0.40 (95% CI 0.53 to 0.32) point reduction on a 0 to 10 pain intensity scale, which does not meet the minimum clinically important difference threshold of 15% or more. Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once on the basis of inconsistency due to heterogeneity (see Table 1). We observed substantial heterogeneity (I2 = 70%, P < 0.001) and investigated this using pre‐planned subgroup analyses. Categorising studies by high (≥ 5 Hz) or low (< 5 Hz) frequency, rTMS demonstrated a difference between subgroups (P < 0.001) and reduced heterogeneity in the low‐frequency group (n = 106, I2 = 0%). In this group there was no evidence of an effect of low‐frequency rTMS for pain intensity (SMD 0.13, 95% CI ‐0.03 to 0.28, P = 0.11). While high‐frequency stimulation demonstrated an effect (n = 560, SMD ‐0.30, 95% CI ‐0.37 to ‐0.23, P < 0.001), we observed substantial heterogeneity in this analysis (P < 0.001, I2 = 68%). Separating studies that delivered a single treatment per condition from those that delivered multiple treatment sessions did not reduce heterogeneity substantially in multiple‐dose studies (n = 357, I2 = 80%, P < 0.001) or single‐dose studies (n = 319, I2 = 57%, P < 0.001) (Analysis 1.2).
Analysis 1.2.
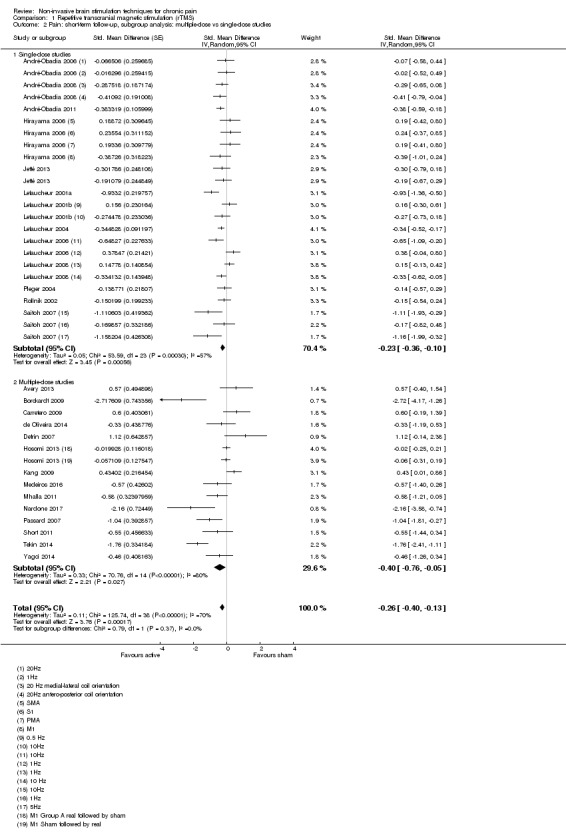
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies.
There were insufficient data to support the subgroup analysis by the type of painful condition as planned. However, when the analysis was restricted to studies including only well‐defined neuropathic pain populations (Analysis 1.3), there was little impact on heterogeneity (I2 = 69%, P < 0.001). In the subgroup of non‐neuropathic pain studies overall heterogeneity remained high (I2 = 77%, P < 0.001) (Analysis 1.4). Responder data were available from one study not judged at high risk of bias (Malavera 2013 n = 54, Analysis 1.14; Analysis 1.25). This demonstrated an effect in favour of active stimulation for 30% reduction in pain (risk ratio (RR) 2.11, 95% CI 1.17 to 3.80, P = 0.01).
Analysis 1.3.
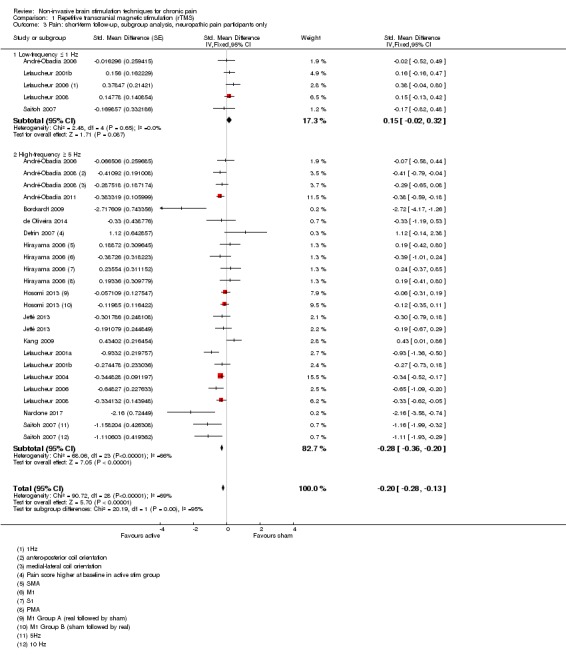
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only.
Analysis 1.4.
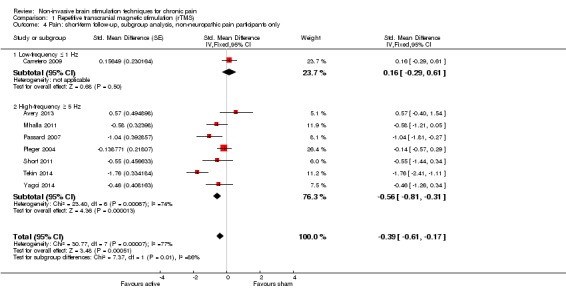
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only.
Analysis 1.14.
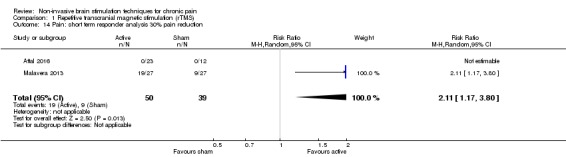
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 14 Pain: short term responder analysis 30% pain reduction.
Analysis 1.25.
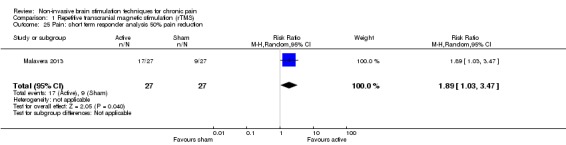
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 25 Pain: short term responder analysis 50% pain reduction.
rTMS motor cortex
Restricting the analysis to studies of high‐frequency stimulation of the motor cortex (Analysis 1.5) (21 studies, n = 505) the pooled SMD was ‐0.37 (‐0.51 to 0.22, P < 0.001) though heterogeneity was high (I2 = 67%, P < 0.001). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once on the basis of inconsistency due to heterogeneity (see Table 1).
Analysis 1.5.
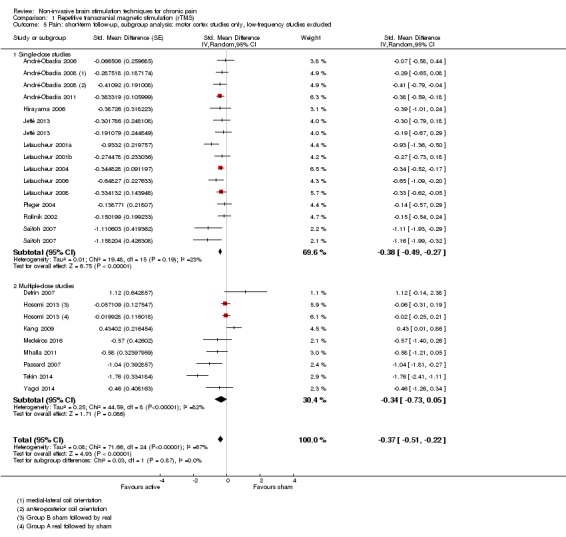
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.
Further restricting the analysis to single‐dose studies of high‐frequency stimulation of the motor cortex (n = 249) reduced heterogeneity (I2 = 23%, P = 0.19) (Analysis 1.5). The pooled SMD was ‐0.38 (95% CI ‐0.49 to ‐0.27, P < 0.001). We back‐transformed the SMD to a mean difference using the mean standard deviation of the post‐treatment sham group scores of the studies included in this analysis (2.04). We then used this to estimate the real percentage change on a 0 to 10 pain intensity scale of active stimulation compared with the mean poststimulation score from the sham groups of the included studies (6.2). This equated to a reduction of 0.77 (95% CI 0.55 to 0.99) points, or a percentage change of 12% (95% CI 9% to 16%) of the control group outcome. This estimate does not reach the pre‐established criteria for a minimal clinically important difference (≥ 15%). Of the included studies in this subgroup, nine did not clearly report blinding of assessors and we awarded them a judgement of 'unclear' risk of bias for this criterion (André‐Obadia 2011; Hirayama 2006; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Lefaucheur 2006; Pleger 2004; Rollnik 2002; Saitoh 2007). A sensitivity analysis removing these studies reduced heterogeneity to I2 = 0% although only three studies were preserved in the analysis (André‐Obadia 2006; André‐Obadia 2008; Lefaucheur 2008). There remained a difference between sham and active stimulation although the SMD reduced to ‐0.29 (95% CI ‐0.49 to ‐0.13). This equates to a percentage change of 9% (95% CI 4% to 14%) in comparison with sham stimulation. For multiple‐dose studies of high‐frequency motor cortex stimulation heterogeneity was high (n = 256, I2 = 82%, P < 0.001), and the pooled effect was not significant (SMD ‐0.34, 95% CI ‐0.73 to 0.05, P = 0.09).
When the analysis was restricted to studies of single‐dose, high‐frequency motor cortex stimulation in well‐defined neuropathic pain populations (excluding data from Pleger 2004 and Rollnik 2002), there was little effect on the pooled estimate (SMD ‐0.41, 95% CI ‐0.52 to ‐0.29) or heterogeneity (I2 = 23%, P = 0.20). When we applied the same process to multiple‐dose studies of high‐frequency motor cortex stimulation (excluding data from Medeiros 2016; Mhalla 2011; Passard 2007; Tekin 2014 and Yagci 2014 we found no pooled effect (SMD 0.12, 95% CI ‐0.16 to 0.40) and heterogeneity remained high.
Sensitivity analysis
To assess whether the imputation of standard errors for cross‐over studies was robust we repeated the analysis with the correlation coefficient reduced to 0.66 and increased to 0.86. This had no marked effect on the overall analysis (Analysis 1.6; Analysis 1.7). We applied the same process to the subgroup analysis of single‐dose studies of high‐frequency motor cortex stimulation (Analysis 1.8; Analysis 1.9). This had a negligible impact on the effect size or the statistical significance for this subgroup.
Analysis 1.6.
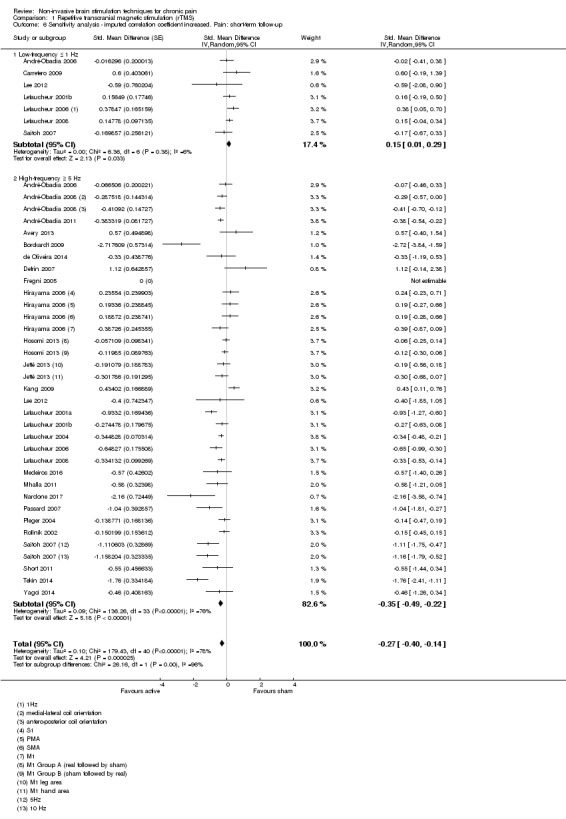
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up.
Analysis 1.7.
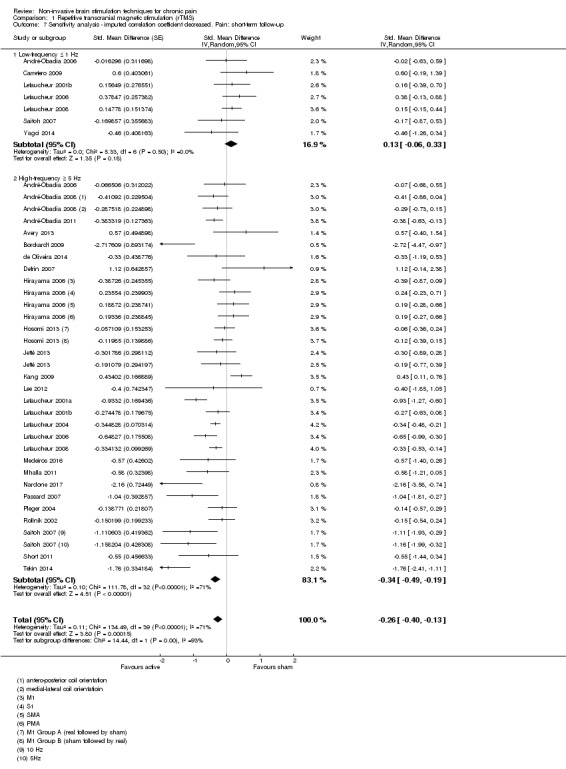
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up.
Analysis 1.8.
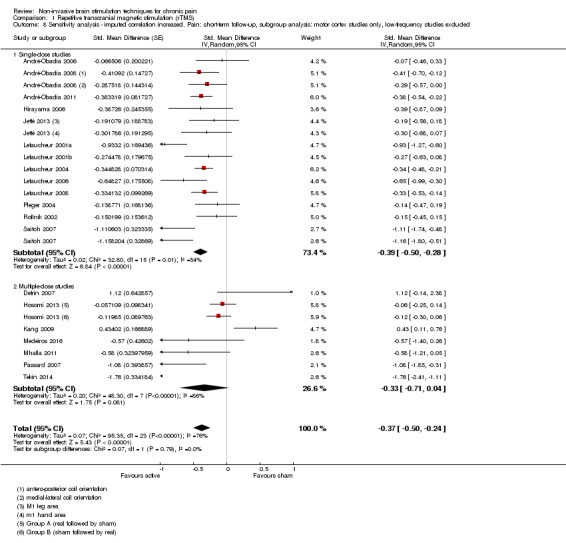
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.
Analysis 1.9.
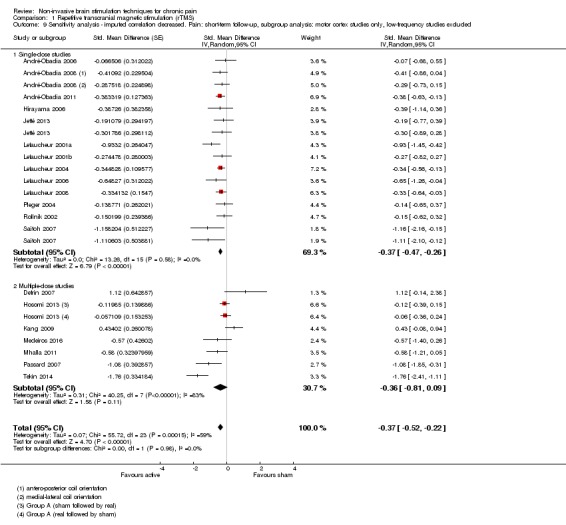
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.
To assess the impact of excluding the studies at high risk of bias we performed the analysis with data from these studies included (Analysis 1.10). While this produced a modest increase in the SMD it increased heterogeneity from 68% to 72%. Inclusion of high risk of bias studies to the multiple‐dose studies of high‐frequency motor cortex stimulation subgroup increased heterogeneity (I2 = 85%, P < 0.001), though the analysis demonstrated an effect (SMD ‐0.53, 95% CI ‐0.91 to ‐0.15, P = 0.006) (Analysis 1.11). Inclusion of the Irlbacher 2006 study in the single‐dose studies of high‐frequency motor cortex stimulation subgroup caused a slight decrease in the pooled effect size (SMD ‐0.35, 95% CI ‐0.46 to ‐0.24) with no impact on heterogeneity.
Analysis 1.10.
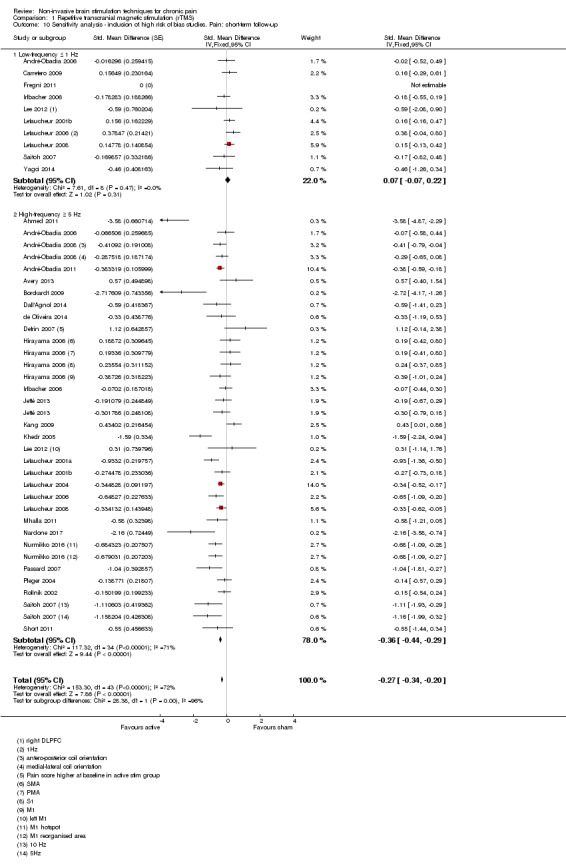
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up.
Analysis 1.11.
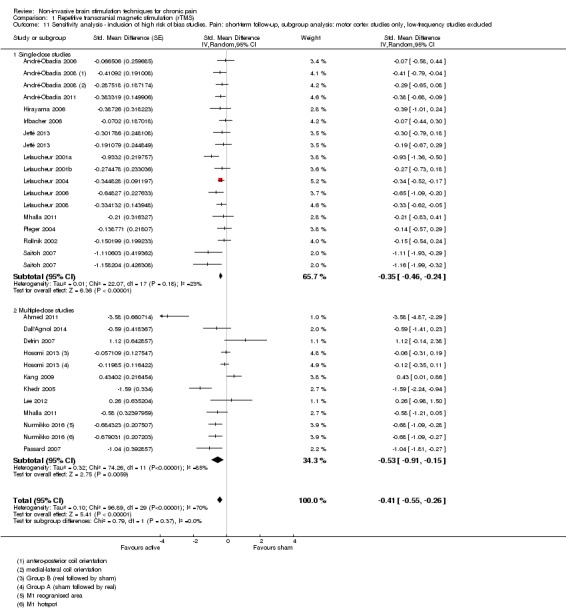
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded.
Small study effects
We investigated small study effects using Egger's test. The results are not suggestive of a significant influence of small study effects.
rTMS prefrontal cortex
Restricting the analysis to studies that stimulated the prefrontal cortex (PFC) included six studies (n = 103) (Avery 2013; Borckardt 2009; Carretero 2009; de Oliveira 2014; Nardone 2017; Short 2011) (Analysis 1.12). We excluded the study by Lee 2012 due to its high risk of bias. There was no clear pooled effect (P = 0.11) with substantial heterogeneity (I2 = 79%, P < 0.001). Restricting the analysis to high‐frequency studies (Avery 2013; Borckardt 2009; Nardone 2017; Short 2011), the results were unchanged (P = 0.12, I2 = 83%, P < 0.001).
Analysis 1.12.
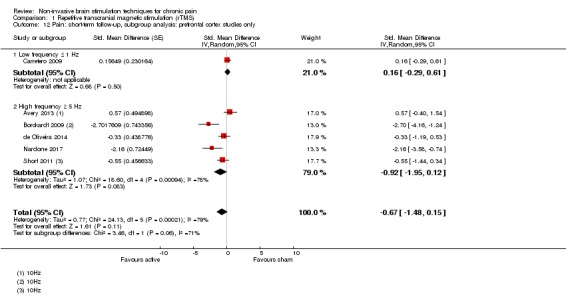
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only.
Sensitivity analysis
To assess the impact of excluding the study of Lee 2012, we performed the analysis with data from this study included (Analysis 1.13). The overall effect remained non‐significant (P = 0.08) with high heterogeneity (I2 = 75%, P < 0.001).
Analysis 1.13.
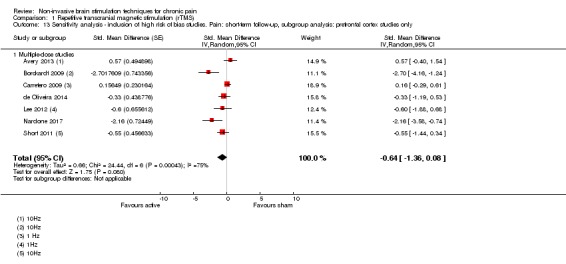
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only.
rTMS: medium‐term (≥ 1 to < 6 weeks postintervention)
Eleven studies provided data on medium‐term pain outcomes (Avery 2013; Carretero 2009; de Oliveira 2014; Hosomi 2013; Lefaucheur 2001a; Kang 2009; Malavera 2013; Nardone 2017; Passard 2007; Short 2011; Yagci 2014). We excluded the studies by Ahmed 2011; Khedr 2005; Lee 2012 and Nurmikko 2016 as we classified them as having a high risk of bias. The analysis included 293 participants (Analysis 1.16). Overall heterogeneity was high (I2 = 77%, P < 0.001) and no clear evidence of effect was observed (SMD ‐0.28, 95% CI ‐0.61 to 0.05, P = 0.09). Using GRADE we rated the quality of evidence for this comparison as very low, downgraded once on the basis of study limitations due to risk of bias, once on the basis of inconsistency due to heterogeneity and once for imprecision due to low participant numbers. Restricting the analysis to studies of prefrontal cortex stimulation (Avery 2013; Carretero 2009; de Oliveira 2014; Nardone 2017; Short 2011) demonstrated no clear effect (SMD ‐1.08, 95% CI ‐2.49 to 0.32, P = 0.13, I2 = 88%, P < 0.001, Analysis 1.19 ). Studies of motor cortex stimulation also demonstrated no effect (SMD ‐0.22, 95% CI ‐0.46 to 0.02, P = 0.08) although heterogeneity was high (I2 = 59%, P < 0.02) and remained high when only high‐frequency stimulation studies were included (SMD ‐0.23 (‐0.49 to 0.03, P = 0.08, I2 = 66%, P = 0.01) (Analysis 1.18). We performed sensitivity analysis to assess the impact of excluding the studies by Ahmed 2011; Khedr 2005; Lee 2012 and Nurmikko 2016 on the basis of risk of bias (Analysis 1.17). Including these studies did not substantially alter heterogeneity (I2 = 80%, P < 0.01) though the effect reached significance overall (SMD ‐0.50, 95% CI ‐0.80 to ‐0.20, P = 0.001).
Analysis 1.16.
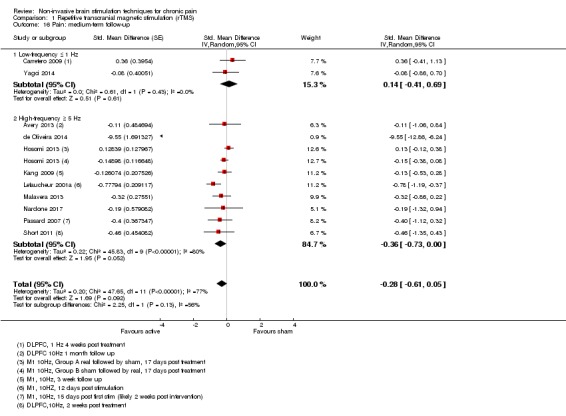
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 16 Pain: medium‐term follow‐up.
Analysis 1.19.
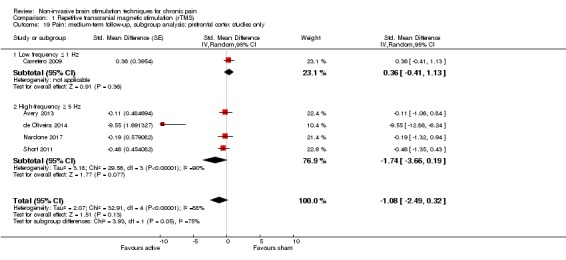
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only.
Analysis 1.18.
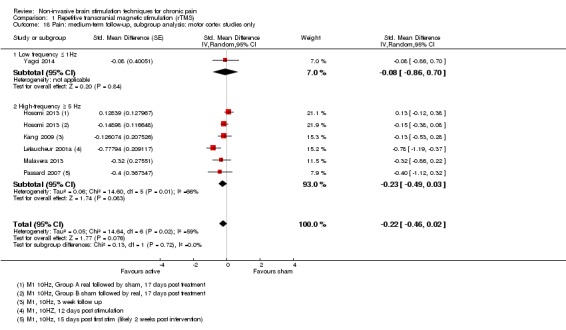
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only.
Analysis 1.17.
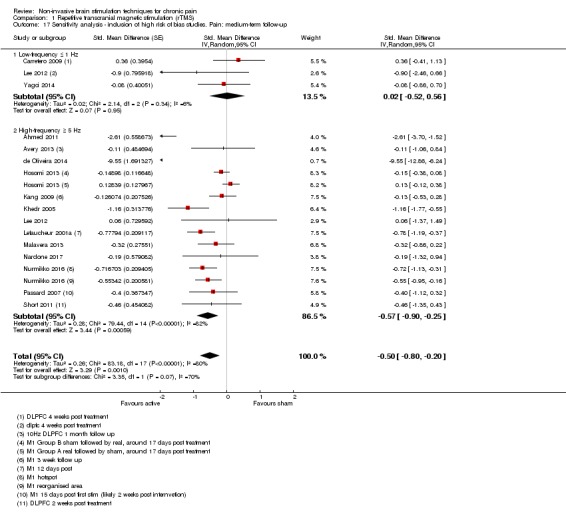
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up.
rTMS: long‐term (≥ 6 weeks postintervention)
Four studies provided data for long‐term pain relief (Avery 2013; Kang 2009; Passard 2007; Yilmaz 2014) (Analysis 1.20). The analysis included 75 participants. There was no heterogeneity (I2 = 0%, P = 0.99). The analysis demonstrated no effect (SMD ‐0.14, 95% CI ‐0.44 to 0.17, P = 0.39). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers. Sensitivity analysis to assess the impact of excluding the study of Ahmed 2011 due to its high risk of bias continued to demonstrate no evidence of effect, though heterogeneity was introduced (Analysis 1.21, I2 = 57%, P = 0.05).
Analysis 1.20.
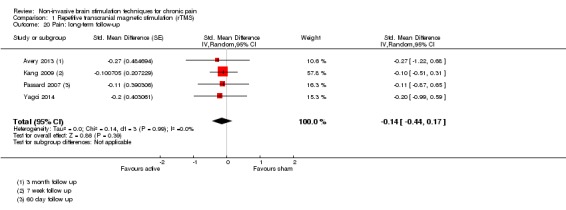
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 20 Pain: long‐term follow‐up.
Analysis 1.21.
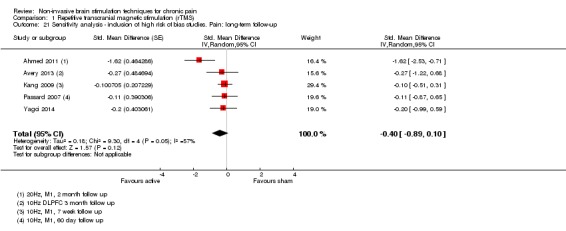
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up.
Cranial electrotherapy stimulation (CES): short‐term (0 to < 1 week postintervention)
Six studies provided data for this analysis (Gabis 2003; Gabis 2009;Tan 2006; Tan 2011; Taylor 2013) (Analysis 2.1, n = 270). We excluded the study by Rintala 2010 due to high risk of attrition bias. All studies utilised a parallel‐group design and so we used a standard inverse variance meta‐analysis using SMD. Four studies did not provide the necessary data to enter into the analysis (Capel 2003; Cork 2004; Katsnelson 2004; Lichtbroun 2001, combined n = 228) and we classified two studies as being at high risk of bias on criteria other than 'free of selective outcome reporting' (Katsnelson 2004; Tan 2000). The studies by Gabis 2003 and Gabis 2009 differed substantially from the other included studies on the location of electrodes and the intensity of the current provided. Despite this, there was no heterogeneity (I2 = 0%). No individual study in this analysis demonstrated superiority of active stimulation over sham and the results of the meta‐analysis do not demonstrate a clear effect (SMD ‐0.24, 95% CI ‐0.48 to 0.01, P = 0.06). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers (see Table 2). Sensitivity analysis, including the study by Rintala 2010, did not meaningfully affect the results (SMD ‐0.21, 95% CI ‐0.45 to 0.02, P = 0.07).
Analysis 2.1.
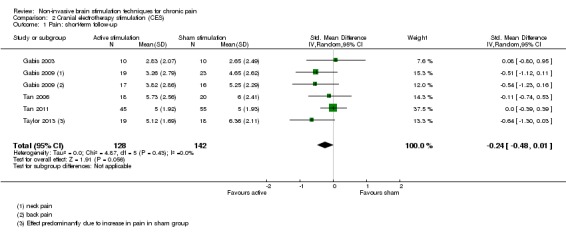
Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 1 Pain: short‐term follow‐up.
CES: medium‐term (≥ 1 to 6 weeks postintervention) and long‐term (≥ 6 weeks postintervention)
There were insufficient data to perform a meta‐analysis for medium‐ or long‐term pain outcomes for CES.
Transcranial direct current stimulation (tDCS): short‐term (0 to < 1 week postintervention)
Adequate data were available from 27 studies (Ahn 2017; Antal 2010; Ayache 2016; Bae 2014; Boggio 2009; Brietzke 2016; Chang 2017; Fagerlund 2015; Fenton 2009; Fregni 2006a; Fregni 2006b; Hazime 2017; Jales Junior 2015; Jensen 2013; Khedr 2017; Lagueux 2017; Luedtke 2015; Mendonca 2016; Mori 2010; Ngernyam 2015; Oliveira 2015; Riberto 2011; Sakrajai 2014; Soler 2010; Villamar 2013; Volz 2016; Wrigley 2014) for this analysis (n = 747). We were unable to include data from Donnell 2015; Mendonca 2011; and Valle 2009 (combined n = 95) as the necessary data were not reported in the study report or available upon request to the study authors. We analysed data using the generic inverse variance method. We imputed the correlation coefficient (0.635) used to calculate the SE (SMD) for cross‐over studies from data extracted from Boggio 2009 (see Unit of analysis issues). One study compared two distinct active stimulation conditions to one sham condition (Fregni 2006b). We considered that combining the treatment conditions would be inappropriate, as each involved stimulation of different locations and combination would hinder subgroup analysis. Instead we included both comparisons separately with the number of participants in the sham control group divided by the number of comparisons. We excluded data from Harvey 2017 as there was a baseline imbalance greater than 3 out of 10 in pain scores. We only included first‐stage data from the study of Antal 2010 (n = 12) due to the unsustainable level of attrition following this stage.
The overall meta‐analysis demonstrated an effect of active stimulation (SMD ‐0.43, 95% CI ‐0.63 to ‐0.22, P < 0.001) (Analysis 3.1), but heterogeneity was high (I2 = 60%, P < 0.001). We back‐transformed the SMD to a mean difference using the mean standard deviation of the post‐treatment sham group scores of the studies included in this analysis (1.91). We then used this to estimate the real percentage change on a 0 to 10 pain intensity scale of active stimulation compared with the mean post‐stimulation score from the sham groups of the included studies (4.77). This equates to a reduction of 0.82 (95% CI 0.42 to 1.2) points, or a percentage change of 17% (95% CI 9% to 25%) of the control group outcome, which meets our threshold for a clinically important difference, though the lower confidence interval is substantially below that threshold. Using GRADE we rated the quality of evidence for this comparison as very low, downgraded once on the basis of study limitations due to risk of bias, once for inconsistency due to heterogeneity and once for evidence of possible publication bias (see Table 3).
Analysis 3.1.
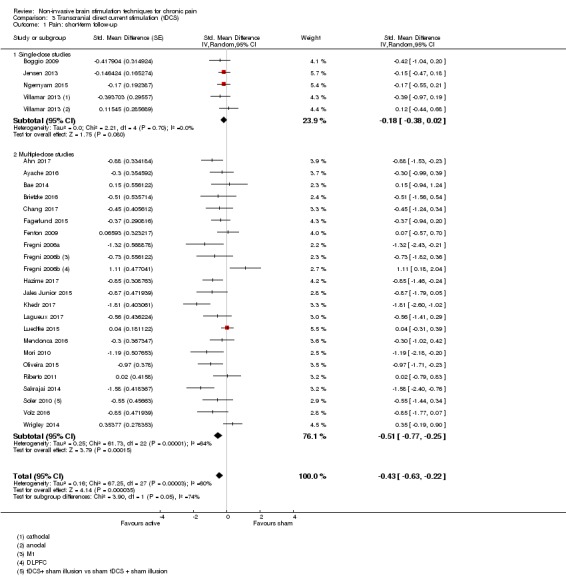
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 1 Pain: short‐term follow‐up.
Subgrouping studies by multiple or single dose decreased heterogeneity in the single‐dose subgroup (I2 = 0%, P = 0.70) but did not reduce heterogeneity in the multiple‐dose subgroup (I2 = 64%, P < 0.001). Inclusion of studies at high risk of bias (Analysis 3.4; Antal 2010; Hagenacker 2014; Kim 2013; Souto 2014; Thibaut 2017) slightly increased the effect size (SMD ‐0.48, 95% CI ‐0.67 to ‐0.29, P < 0.001, I2 = 60%, P < 0.001). Analysis restricted to comparisons of active motor cortex stimulation (single‐ and multiple‐dose studies) (n = 655, Analysis 3.5) did not reduce heterogeneity substantially (I2 = 58%, P < 0.001) and demonstrated an effect (SMD ‐0.47, 95% CI ‐0.67 to ‐0.28, P < 0.001).
Analysis 3.4.
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies.
Analysis 3.5.
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only.
There were insufficient data to support the planned subgroup analysis by the type of painful condition as planned. However, a modified subgroup analysis by neuropathic or non‐neuropathic pain conditions (Analysis 3.8) demonstrated no subgroup difference (P = 0.41) though heterogeneity was reduced in the neuropathic pain group (I2 = 40%, P = 0.10).
Analysis 3.8.
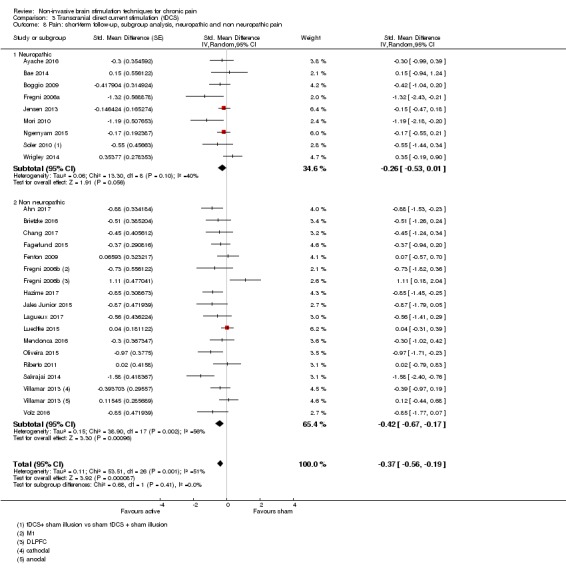
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain.
Responder data were only available from a small number of studies, all that were considered at high risk of bias. As such we did not conduct a formal meta‐analysis but the data can be seen in Analysis 3.9; Analysis 3.10; Analysis 3.12 and Analysis 3.13.
Analysis 3.9.
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 9 Pain: short term follow‐up responder analysis 30% pain reduction.
Analysis 3.10.
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 10 Pain: short term follow‐up responder analysis 50% pain reduction.
Analysis 3.12.
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 12 Pain: medium term follow‐up responder analysis 30% pain reduction.
Analysis 3.13.
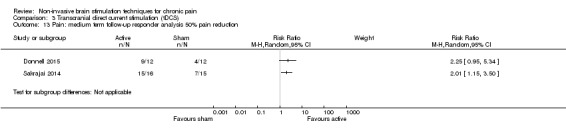
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 13 Pain: medium term follow‐up responder analysis 50% pain reduction.
To assess whether the imputation of standard errors for cross‐over studies was robust we repeated the analyses with the imputed correlation coefficient reduced and increased by a value of 0.1 (Analysis 3.2; Analysis 3.3; Analysis 3.6; Analysis 3.7). This had no meaningful impact upon the results.
Analysis 3.2.
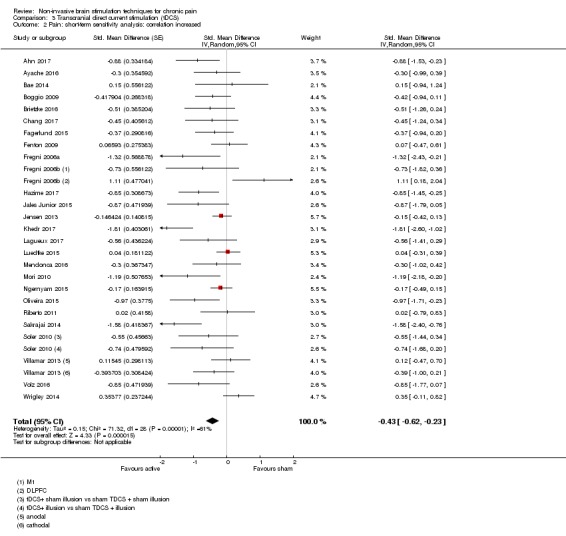
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 2 Pain: short‐term sensitivity analysis: correlation increased.
Analysis 3.3.
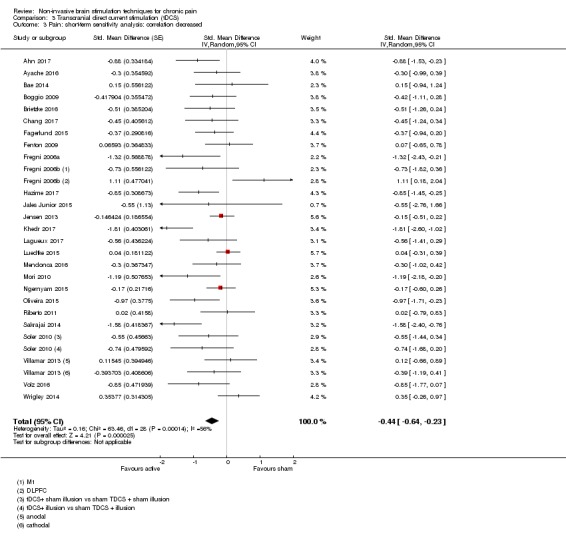
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 3 Pain: short‐term sensitivity analysis: correlation decreased.
Analysis 3.6.
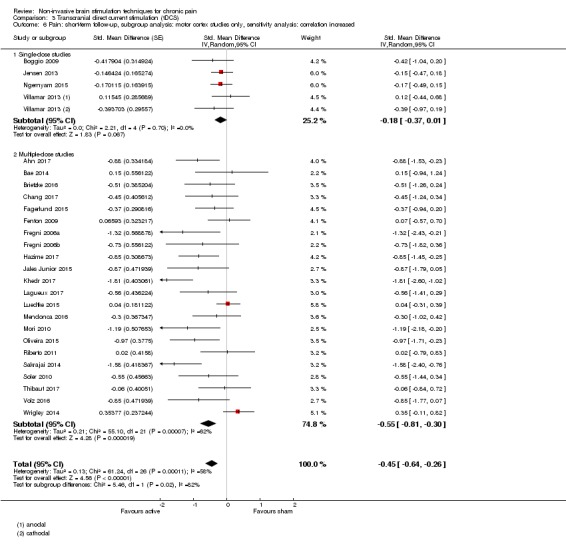
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased.
Analysis 3.7.
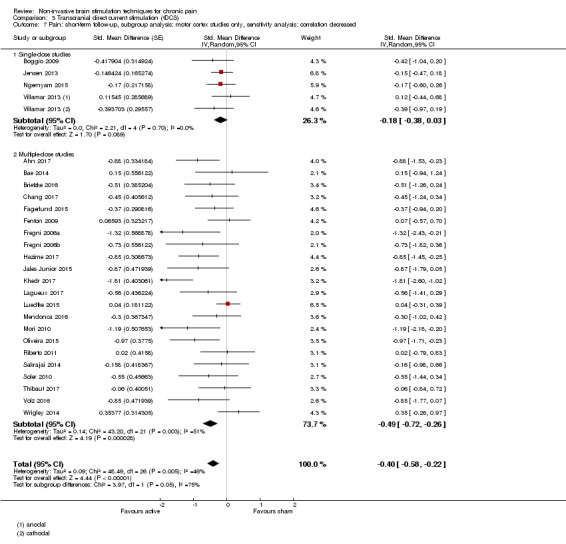
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased.
Small study effects
We investigated small study effects using Egger's test. Funnel plot asymmetry was apparent and Egger's test indicated small study effects for the overall comparisons (Figure 4, P = 0.019) and the subgroups of motor cortex stimulation studies (Figure 5, P = 0.002).
Figure 4.
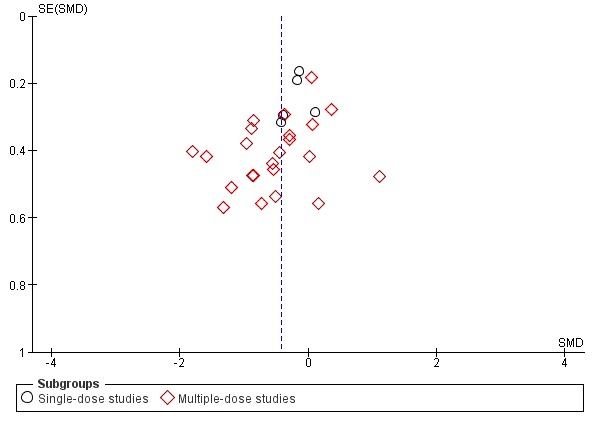
Funnel plot of comparison 3. Transcranial direct current stimulation (tDCS), outcome 3.1. Pain: short‐term follow‐up
Figure 5.
Funnel plot of comparison 3. Transcranial direct current stimulation (tDCS), outcome 3.5. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only
tDCS: medium‐term (1 to < 6 weeks post‐treatment)
Fourteen studies provided adequate data for this analysis (Ahn 2017; Ayache 2016 ; Bae 2014; Fagerlund 2015; Fenton 2009; Khedr 2017; Lagueux 2017; Luedtke 2015; Mendonca 2016; Mori 2010; Sakrajai 2014; Soler 2010, Volz 2016; Wrigley 2014, pooled n = 443) (Analysis 3.11). There was heterogeneity (I2 = 60%, P = 0.003) and the pooled results demonstrated an effect of tDCS (SMD ‐0.43, 95% CI ‐0.72 to ‐0.13, P = 0.004). Using GRADE we rated the quality of evidence for this comparison as very low, downgraded once on the basis of study limitations due to risk of bias, once for inconsistency and once for evidence of publication bias.
Analysis 3.11.
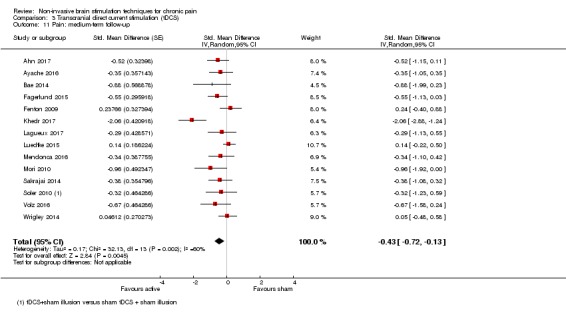
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 11 Pain: medium‐term follow‐up.
Small study effects
We investigated small study effects using Egger's test. Funnel plot asymmetry was apparent and Egger's test indicated small study effects (P = 0.013).
tDCS: long‐term (> 6 weeks post‐treatment)
Three studies provide data for this analysis (Hazime 2017; Luedtke 2015; Mendonca 2016, pooled n = 137). There was no heterogeneity (I2 = 36%, P = 0.21) and no effect of tDCS was observed (SMD ‐0.01, 95% CI ‐0.43 to 0.41, P = 0.97) (Analysis 3.15). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers.
Analysis 3.15.
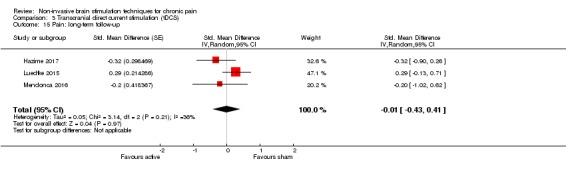
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 15 Pain: long‐term follow‐up.
Reduced impedance non‐invasive cortical electrostimulation (RINCE): short‐term (0 to < 1 week postintervention)
The one study not at high risk of bias that investigated RINCE demonstrated a positive effect on pain intensity (n = 77, mean difference (0 to 10 pain scale) ‐1.41, 95% CI ‐2.48 to ‐0.34, P < 0.01) (Analysis 4.1; Hargrove 2012a). Using GRADE we rated the quality of evidence as very low, downgraded once on the basis of study limitations due to risk of bias, once for inconsistency (single study) and once for imprecision due to low participant numbers. Sensitivity analysis including the study at high risk of bias (Deering 2017) did not increase heterogeneity (pooled n = 115, SMD ‐0.59, 95% CI ‐0.99 to ‐0.18, P = 0.004).
Analysis 4.1.
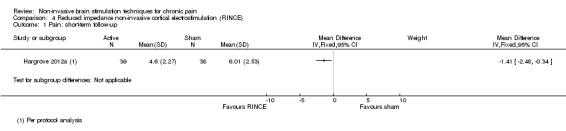
Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 1 Pain: short‐term follow‐up.
Transcranial random noise stimulation (tRNS): short‐term (0 to < 1 week postintervention)
One study at high risk of bias Palm 2016 offered data for tRNS. This study did not report a difference between active and sham stimulation (Analysis 5.1). Using GRADE we rated the quality of evidence as very low, downgraded once on the basis of study limitations due to risk of bias, once for inconsistency (single study) and once for imprecision due to low participant numbers. Curatolo 2017 did not report outcome data in a numeric format at any postintervention time point but the authors reported a statistically significant difference in favour of tRNS. It was not possible to extract an estimate of effect size from this high‐risk‐of‐bias study.
Analysis 5.1.
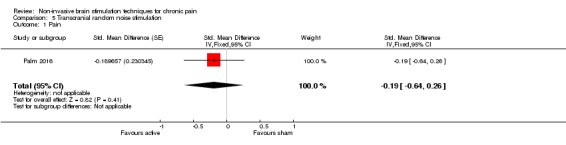
Comparison 5 Transcranial random noise stimulation, Outcome 1 Pain.
tRNS: medium‐term (≥1 to 6 weeks postintervention) and long‐term (≥ 6 weeks postintervention)
No data were available for medium‐ or long‐term pain outcomes for tRNS.
Secondary outcome: disability
rTMS: short‐term (0 to < 1 week postintervention) disability
Five studies provided data on disability at short‐term follow‐up (Avery 2013; Kang 2009; Mhalla 2011; Passard 2007; Short 2011). Pooling of these studies (Analysis 1.22; n = 119) demonstrated no effect (SMD ‐0.29, 95% CI ‐0.87 to 0.29, P = 0.33) with substantial heterogeneity (I2 = 71%, P = 0.007). All of these studies delivered multiple doses of high‐frequency stimulation. Using GRADE we rated the quality of evidence for this comparison as very low, downgraded once on the basis of study limitations due to risk of bias, once on the basis of inconsistency due to heterogeneity and once for imprecision due to low participant numbers (see Table 1). Two studies stimulated the DLPFC (Avery 2013; Short 2011) and three stimulated the motor cortex (Kang 2009; Mhalla 2011; Passard 2007). Subgrouping studies by stimulation site had no impact on heterogeneity. Sensitivity analysis including studies at high risk of bias (Umezaki 2016, n = 20) increased heterogeneity but did not substantially change the outcome (pooled n = 139, SMD ‐0.36, 95% CI ‐0.72 to 0.12, P = 0.16, I2 = 59%, P = 0.02).
Analysis 1.22.
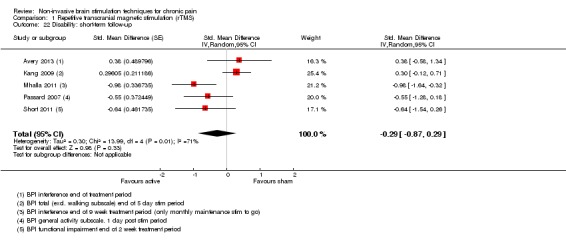
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 22 Disability: short‐term follow‐up.
rTMS:medium‐term (1 to < 6 weeks postintervention) disability
Four studies provided data on disability at medium‐term follow‐up (Avery 2013; Kang 2009; Mhalla 2011; Passard 2007). Pooling of these studies (Analysis 1.24; n = 99) demonstrated no effect (SMD ‐0.37, 95% CI ‐1.07 to 0.33, P = 0.3) with heterogeneity (I2 = 78%, P = 0.004). Using GRADE we rated the quality of evidence for this comparison as very low, downgraded once on the basis of study limitations due to risk of bias, once on the basis of inconsistency due to heterogeneity and once for imprecision due to low participant numbers (see Table 1).
Analysis 1.24.
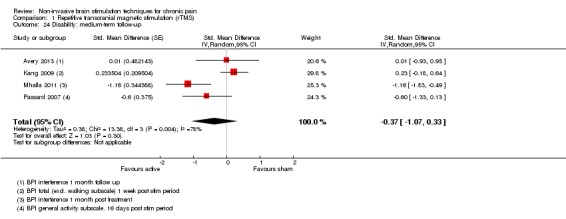
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 24 Disability: medium‐term follow‐up.
All studies delivered multiple sessions of high‐frequency stimulation. Of these, one study stimulated the DLPFC (Avery 2013) and the remaining studies stimulated the motor cortex (Kang 2009; Mhalla 2011; Passard 2007). Removing the study of Avery 2013 did not decrease heterogeneity (I2 = 85%, P = 0.001). Sensitivity analysis including studies at high risk of bias (Umezaki 2016, n = 20) increased heterogeneity but did not substantially change the outcome (pooled n = 119, SMD ‐0.42, 95% CI ‐1.01 to 0.17, P = 0.17, I2 = 72%, P < 0.001).
rTMS: long‐term (≥ 6 weeks postintervention) disability
Three studies provided data on disability at long‐term follow‐up (Avery 2013; Kang 2009; Passard 2007). Pooling of these studies demonstrated no effect (pooled n = 63, SMD ‐0.23, 95% CI ‐0.62 to 0.16, P = 0.24) without heterogeneity (I2 = 15%, P = 0.31) (Analysis 1.26). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers. Sensitivity analysis including studies at high risk of bias (Umezaki 2016, n = 20) did not substantially change the outcome (pooled n = 83, SMD ‐0.41, 95% CI ‐0.87 to 0.05, P = 0.08, I2 = 39%, P = 0.18).
Analysis 1.26.
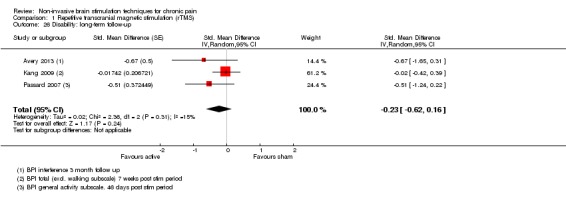
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 26 Disability: long‐term follow‐up.
tDCS: short‐term (0 to < 1 week postintervention) disability
Four studies (Ahn 2017; Chang 2017; Luedtke 2015; Soler 2010) provided data on disability in the short term. While Ayache 2016 reported disability, this was a cross‐over study and we were unable to source a representative correlation coefficient for this outcome in order to calculate the standard error (SMD) for cross‐over studies. No effect was seen (pooled n = 212, SMD ‐0.01, 95% ‐0.28 to 0.26, P = 0.84) and there was no heterogeneity (I2 = 0%, P = 0.59, Analysis 3.16). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers (see Table 3).
Analysis 3.16.
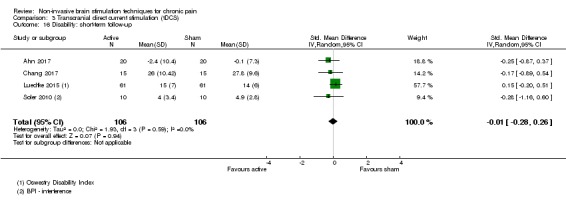
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 16 Disability: short‐term follow‐up.
tDCS: medium‐term (1 to < 6 weeks post‐treatment) disability
One study (Luedtke 2015) provided data on disability in the medium term. This study demonstrated no effect of tDCS (RMDQ mean difference 0.00 (95% CI ‐0.38 to 0.38).
Secondary outcome: quality of life
rTMS: short‐term (0 to < 1 week postintervention) quality of life
Four studies provided data on quality of life at short‐term follow‐up (Mhalla 2011; Passard 2007; Short 2011; Yagci 2014). We were unable to include data from Tzabazis 2013, as the size of the treatment groups was not clear from the study report. All studies used the Fibromyalgia Impact Questionnaire (FIQ) so we were able to use the mean difference as the measure of effect. Pooling data from these studies (Analysis 1.28; n = 105) demonstrated an effect in favour of active stimulation (mean difference (MD) ‐10.80, 95% CI ‐15.04 to ‐6.55, P < 0.001) with no heterogeneity (I2 = 0%, P = 0.96). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers (see Table 1). Tekin 2014 measured quality of life using the World Health Organization Quality of Life (WH‐QoL) scale but only reported data from individual subdomains. They reported a statistically significant difference in favour of active stimulation for the physical subdomain but not the psychological, social, environmental or national domains.
Analysis 1.28.
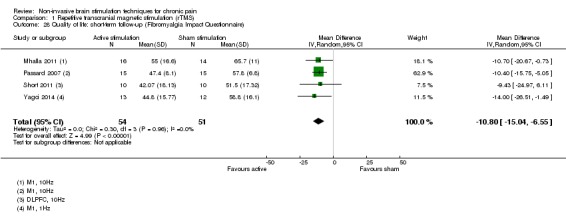
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire).
rTMS: medium‐term (1 to < 6 weeks postintervention) quality of life
The same four studies provided data on quality of life at medium‐term follow‐up (Mhalla 2011; Passard 2007; Short 2011; Yagci 2014). All studies used the FIQ so we were able to use the mean difference as the measure of effect. Pooling data from these studies (Analysis 1.29; n = 105) demonstrated an effect (MD ‐11.49, 95% CI ‐16.73 to ‐6.25, P < 0.001) with no heterogeneity (I2 = 0%, P = 0.82). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers. Sensitivity analysis including studies at high risk of bias (Boyer 2014) did not meaningfully alter the result (pooled n = 143, MD ‐8.93, 95% CI ‐13.49 to ‐4.37, P < 0.001, I2 = 15%, P = 0.32).
Analysis 1.29.
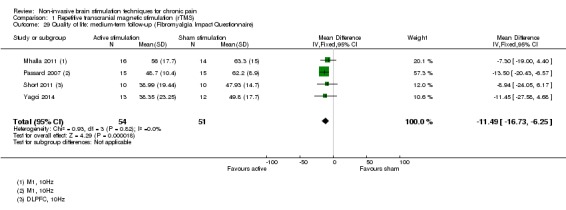
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire).
rTMS: long‐term (≥ 6 weeks postintervention) quality of life
Data were available from two studies (Passard 2007, Yagci 2014, pooled n = 51) for quality of life at long‐term follow‐up. The analysis demonstrated an effect in favour of active stimulation (FIQ total score: MD ‐6.78, 95% CI ‐13.43 to ‐0.14, I2 = 0%, P = 0.56) (Analysis 1.31). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers. Sensitivity analysis including studies at high risk of bias (Boyer 2014) did not meaningfully alter the result (pooled n = 89, MD ‐8.58, 95% CI ‐13.84 to ‐3.33, P < 0.001, I2 = 0%, P = 0.58).
Analysis 1.31.
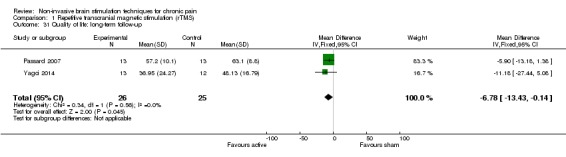
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 31 Quality of life: long‐term follow‐up.
CES: short‐term (0 to < 1 week postintervention) quality of life
Two studies provided quality of life data for this analysis (Tan 2011; Taylor 2013). One study used the physical component score of the SF‐12 and the other used the FIQ. However, one study demonstrated a baseline imbalance of the SF‐12 that exceeded in size any pre‐poststimulation change (Tan 2011), therefore we considered it inappropriate to enter this into a meta‐analysis. The study by Taylor 2013 (n = 36) demonstrated a positive effect on this outcome (MD ‐25.05,95%CI ‐37.82, ‐12.28, Analysis 2.2). Using GRADE we rated the quality of evidence for this comparison as very low, downgraded once on the basis of study limitations due to risk of bias, once for inconsistency (single study) and once for imprecision due to low participant numbers (see Table 2).
Analysis 2.2.
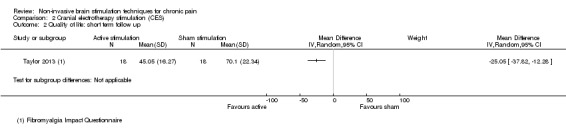
Comparison 2 Cranial electrotherapy stimulation (CES), Outcome 2 Quality of life: short term follow up.
tDCS: short‐term (0 to < 1 week postintervention) quality of life
Four studies provided adequate data for this analysis (Jales Junior 2015; Mori 2010; Riberto 2011; Volz 2016; pooled n = 82). Of these, Jales Junior 2015 used the FIQ, Mori 2010 used the Multiple Sclerosis Quality of Life 54 scale (MS‐QoL‐54), Riberto 2011 used the SF‐36 (total score) and Volz 2016 used the Inflammatory Bowel Disease Questionnaire Quality of Life scale. The pooled effect was in favour of active stimulation (SMD 0.66, 95% CI 0.21 to 1.11, P = 0.004) with no heterogeneity (I2 = 0%, P = 0.62) (Analysis 3.18). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers. Lagueux 2017, Mendonca 2016 and Oliveira 2015 reported quality of life using the or SF‐36 and WH‐QoL scales but did not report composite scores that we could enter into the meta‐analysis. All three studies reported no statistically significant differences across the different quality‐of‐life domains. We excluded Thibaut 2017 from the analysis due to high risk of bias. They measured quality of life using the Patient Health Questionnaire (PHQ‐9) but reported no significant difference between groups.
Analysis 3.18.
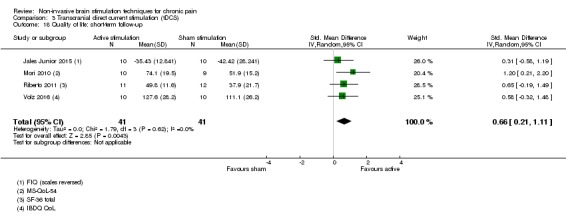
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 18 Quality of life: short‐term follow‐up.
tDCS: medium‐term (1 to < 6 weeks post‐treatment) quality of life
At medium‐term follow‐up Fagerlund 2015; Mori 2010 and Volz 2016 (pooled n = 87) provided data and demonstrated no clear effect of tDCS on quality of life (SMD 0.34, 95% CI ‐0.09 to 0.76, P = 0.12, I2 = 0%, P = 0.54, Analysis 3.19). Using GRADE we rated the quality of evidence for this comparison as low, downgraded once on the basis of study limitations due to risk of bias and once for imprecision due to low participant numbers.
Analysis 3.19.
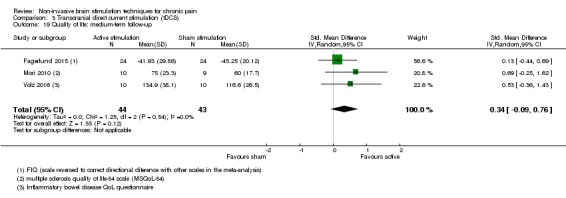
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 19 Quality of life: medium‐term follow‐up.
RINCE: short‐term (0 to < 1 week postintervention) quality of life
One study of RINCE therapy (Hargrove 2012a, n = 77) demonstrated no effect on quality of life (FIQ, MD ‐6.50, 95% CI ‐15.21 to 2.21, Analysis 4.3). Using GRADE we rated the quality of evidence as very low, downgraded once on the basis of study limitations due to risk of bias, once for inconsistency (single study) and once for imprecision due to low participant numbers. Sensitivity analysis including studies at risk of bias (the addition of Deering 2017, n = 38) did not alter the outcome (SMD ‐0.45, 95% CI ‐0.91 to 0.02, P = 0.06, I2 = 10%, P = 0.33).
Analysis 4.3.
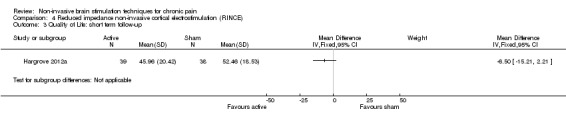
Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 3 Quality of Life: short term follow‐up.
Secondary outcome: adverse events
rTMS
Minor
Thirty‐one of 42 studies of rTMS reported on adverse events. Of these, 10 studies reported none (André‐Obadia 2006; André‐Obadia 2008; Boyer 2014; Fregni 2005; Hirayama 2006; Lefaucheur 2001a; Lefaucheur 2001b; Lefaucheur 2004; Onesti 2013; Saitoh 2007). Attal 2016 reported similar proportions of side effects between stimulation conditions with no serious events. Avery 2013 reported a range of reported sensations including headache, pain at the stimulation site, muscle aches/fatigue, dizziness and insomnia, though there were no clear differences in the frequency of these events between the two groups. Carretero 2009 reported neck pain or headache symptoms in six out of 14 participants in the active stimulation group compared with two out of 12 in the sham group. One participant in the active stimulation group reported worsening depression and four participants in the sham group reported symptoms of nausea and tiredness. Dall'Agnol 2014 reported that they did not observe moderate or severe adverse effects but did not report any details on the incidence of mild effects. de Oliveira 2014 reported mild headaches in three participants (27.3%) receiving active rTMS and in one participant receiving sham rTMS. In the study by Fregni 2011, the incidence of headache and neck pain was higher in the active stimulation group than in the sham group. Forty‐one participants reported headache after active stimulation compared to 19 after sham and 18 participants reported neck pain after active stimulation compared with three after sham. Hosomi 2013 reported no difference between real and sham rTMS for minor adverse events. Jetté 2013 reported that seven participants receiving rTMS reported mild discomfort related to scalp pressure and facial twitching. Malavera 2013 reported no serious adverse effects but reports of headache, neck pain and sleepiness without differences between groups, while Medeiros 2016 simply reported that they did not observe serious or moderate side effects from the treatment, with no further detail. Mhalla 2011 reported that nine participants (five following active stimulation and four following sham stimulation) reported transient headache, and one participant reported transient dizziness after active stimulation. Nardone 2017 reported that two participants undergoing active rTMS reported uncomfortable twitching of facial muscles during stimulation but that rTMS was tolerated well. Nurmikko 2016 reported that rTMS was well tolerated. Minor adverse effects observed during active stimulation included headache (25%), sleepiness (38%), transient increase in pain (31%) and dizziness (15%). Passard 2007 reported incidence of headaches (four out of 15 participants in the active group versus five out of 15 in the sham group), feelings of nausea (one participant in the active group), tinnitus (two participants in the sham group) and dizziness (one participant in the sham group). Picarelli 2010 found six reports of headache following active stimulation and four following sham stimulation, and two reports of neck pain following active stimulation with four reports following sham stimulation. Rollnik 2002 reported that one participant experienced headache, but it is unclear in the report whether this was following active or sham stimulation. Short 2011 reported that there were few side effects.Following four‐coil rTMS, Tzabazis 2013 reported no serious adverse events. The incidence of scalp pain, headache, lightheadedness, back pain, otalgia, hot flashes and pruritis was more commonly reported following sham stimulation than active stimulation. Neck pain (14% of participants following active stimulation versus no participants following sham) and nausea (19% of participants following active stimulation versus 11% following sham) were more common with active stimulation. Umezaki 2016 reported headaches in seven (58%) participants in the active stimulation and five (62%) in the sham stimulation group that were mild and resolved in one to two days. Yagci 2014 reported that three (23%) participants in the active group and one (8%) in the sham group reported adverse events. They only described those in the active group, which were two cases of transient headache and one of "daily tinnitus".
Major
Both Lee 2012 and Picarelli 2010 reported one incidence of seizure following high‐frequency active stimulation. The seizures occurred after the 6th and 7th session of active stimulation respectively. Nurmikko 2016 reported that one participant experienced a permanent reduction of hearing during an active stimulation phase. Investigations ruled out cochlear damage leading the study authors to conclude that an association with rTMS was unlikely.
CES
Four out of 11 studies of CES reported the incidence of adverse events (Capel 2003; Gabis 2003; Rintala 2010; Tan 2011). In these studies no serious adverse events were reported. Rintala 2010 reported that in the active stimulation group participants reported incidences of pulsing, tingling and tickling in the ears (three participants), tender ears (one participant) and a pins and needles feeling near the bladder (one participant). In the sham group they reported drowsiness (one participant), warm ears (one participant) and headache after one session (one participant). Tan 2011 reported only mild adverse events with a total of 41 reports in the active stimulation group and 56 in the sham group. Of note, sensations of ear pulse/sting/itch/electric sensations or ear clip tightness seemed more common in active group than the sham group (12 versus six incidents). Through correspondence with the authors of Taylor 2013, we confirmed that there were no adverse events reported.
tDCS
Thirty out of 36 studies of tDCS reported the incidence of adverse events with varying degrees of detail. Of these, five studies reported none (Fregni 2006a; Hagenacker 2014; Mendonca 2011; Mori 2010; Portilla 2013). Attal 2016 reported similar proportions of side effects between stimulation conditions with no serious events. Most studies reported similar rates of mild and transient effects. Ahn 2017 reported six incidents of pain at the stimulation site; two in the sham group and four in the active group. One participant in the active group reported change in visual perception. Thirteen participants reported tingling, itching or burning sensations at the stimulation site. The severity of these symptoms was rated as low. Tingling was more common during active stimulation. Antal 2010 recorded reports of tingling, moderate fatigue, tiredness, headache and sleep disturbances, though there were no large differences in the frequency of these between the active and sham stimulation groups. Ayache 2016 reported that headache occurred in three participants after active stimulation and one after sham but that otherwise rates were similar between active and sham stimulation and there was no difference in discomfort rates. Boggio 2009 reported that one participant experienced headache with active stimulation. Chang 2017 reported two adverse reactions to tDCS, one participant reported a headache after active stimulation and one participant reported a single incident of painful sensation under the electrode that resolved on cessation of stimulation. Donnell 2015 reported only mild adverse events with higher rates of skin redness in the active group (16.6% in active group versus 3.3% in the sham group) but similar rates for all others. Fagerlund 2015 found no difference in adverse events between active and sham stimulation except for acute mood change, which was higher in the sham group. However trouble concentrating was higher after active stimulation (18% of total sessions after active stimulation versus 5% of sessions after sham), as was scalp pain (18% of sessions versus 9%) and headache (18% of sessions versus 12%).The study by Fenton 2009 reported three cases of headache, two of neck ache, one of scalp pain and five of a burning sensation over the scalp in the active stimulation group versus one case of headache in the sham stimulation group. Fregni 2006b reported one case of sleepiness and one of headache in response to active stimulation of the DLPFC, three cases of sleepiness and three of headache with active stimulation of M1 and one case of sleepiness and two of headache in response to sham stimulation. Hazime 2017 reported the incidence of a variety of adverse effects but did not separate them into active and sham stimulation groups. These included headache, neck pain, scalp pain, back pain, tingling, itching, redness, burning sensations, sleepiness, trouble concentrating and largely reported as mild or moderate in severity. Khedr 2017 reported that all participants tolerated stimulation well with three cases of itching and redness seen in the active stimulation group. Kim 2013 reported that all participants tolerated tDCS well without "significant adverse events". Headache was reported in three participants, all in an active stimulation group, and skin itching was reported by three participants, one in each active stimulation group and one in the sham group. Lagueux 2017 reported that three participants in the active stimulation group and two in the sham group reported minor transient headaches. One participant reported skin redness and itching after active stimulation. Two participants in the active group and one in the sham group reported feelings of tiredness. Four participants in the active stimulation group are reported to have declared "being indisposed" by a stinging/ burning sensation under the electrodes. Luedtke 2015 briefly reported that the stimulation was tolerated well with minimal transitory side effects but gave no further detail. Mendonca 2016 reported just that all adverse events were mild and did not differ between groups, with no further detail. Ngernyam 2015 reported that all participants tolerated stimulation well, seven (of 20) in the active group experienced erythematous skin rash at the cathode placement site. Oliveira 2015 also did not formally report all events but reported that one of the participants suffered burns due to an electrode being placed on a skin site with acne, the skin healed but left a small scar. Similarly Sakrajai 2014 reported no adverse events in either group except transient skin redness in 13% of the active group. Soler 2010 recorded three reports of headache, all following active stimulation. Souto 2014 recorded adverse events in nine out of 10 participants in the sham group and all 10 participants in the active group. Thibaut 2017 reported that all participants tolerated stimulation well and that the majority reported mild to moderate itching and tingling during both active and sham stimulations. These were all mild and transient. Villamar 2013 reported that the vast majority of participants reported a mild to moderate tingling or itching sensation during both active and sham stimulation that faded over a few minutes but no other adverse effects. Valle 2009 reported "minor and uncommon" side effects, such as skin redness and tingling, which were equally distributed between active and sham stimulation. Volz 2016 reported no differences in side effects between stimulation groups except that skin redness was more common in the active group. Wrigley 2014 reported only "mild to moderate" side effects with no difference between active and sham over the 24‐hour poststimulation period. These included sleepiness (70% of participants following active, 60% following sham), fatigue, inertia (60% of participants following active, 30% following sham), lightheadedness (20% of participants during active and sham treatment) and headache (10% of participants during active and sham treatment).
Four studies monitored for possible effects on cognitive function using the Mini Mental State Examination questionnaire (Boggio 2009; Fregni 2006a; Fregni 2006b; Valle 2009) and three of these also used a battery of cognitive tests including the digit‐span memory test and the Stroop word‐colour test (Boggio 2009; Fregni 2006a; Fregni 2006b) and simple reaction time tasks (Fregni 2006a). No studies demonstrated any negative influence of stimulation on these outcomes. No studies of tDCS reported severe or lasting side effects. Bae 2014; Brietzke 2016; Harvey 2017; Jales Junior 2015; Jensen 2013 and Riberto 2011 did not consider adverse events in their study reports.
tRNS
Curatolo 2017 did not report on adverse events. Palm 2016 reported similar rates of adverse events between the active and sham groups with no suggestion of higher rates of any in the active group. Phosphenes were reported by one participant after sham treatment but none after active treatment. Six participants reported insomnia after sham treatment compared to five after tRNS, nausea occurred in four participants after sham treatment and in two after tRNS. Severe headache was reported by one participant after sham treatment but no participants reported severe headache after active stimulation.
RINCE
Hargrove 2012a reported a low incidence of side effects from RINCE including short‐lived headache (two participants in the active group, one in the sham group), eye movement/flutter during stimulation (one active, one sham), restlessness (one active and none sham) and nausea (one active and none sham). Deering 2017 reported an average of two adverse events per participant, of which 47% were reported to be mild and 50% moderate in severity. Thirty‐seven per cent of adverse events were reported to be related to study treatments. The authors reported that compared to sham, RINCE may be associated with small increases in the risk of mild to moderate headaches, nausea, dizziness/vertigo, and localised skin reactions, possibly due to the electrode gel. All events were short lived and resolved without further intervention.
The study by Attal 2016 delivered both rTMS and tDCS. They reported that the proportion of participants displaying side effects was low and similar between active rTMS or tDCS and sham stimulations. Three (out of 35) participants withdrew from the study because of side effects, after the second day of stimulation in the second treatment block.
Discussion
Summary of main results
This update has included a substantial number of new studies. Despite this our findings have not altered substantially from the previous version of this review.
Repetitive transcranial magnetic stimulation (rTMS) for chronic pain
Meta‐analysis of all rTMS studies in chronic pain demonstrated substantial heterogeneity. Predetermined subgroup analysis suggests a short‐term effect of single‐dose, high‐frequency rTMS applied to the motor cortex on chronic pain. This effect is small and does not conclusively exceed the threshold of minimal clinical importance. The evidence from multiple‐dose studies of rTMS demonstrates conflicting results with substantial heterogeneity both overall and when the analysis is confined to high‐frequency motor cortex studies. Low‐frequency rTMS does not appear to be effective. rTMS applied to the prefrontal cortex does not appear to be effective. That the majority of studies in this analysis are at unclear risk of bias, particularly for participant blinding, suggests that the observed effect sizes might be exaggerated. While there is substantial unexplained heterogeneity the available evidence does not strongly suggest an effect of rTMS in the medium term. The limited evidence at long‐term follow‐up consistently suggests no effect of rTMS. The evidence for all comparisons or rTMS is considered to be of low to very low quality.
Cranial electrotherapy stimulation (CES) for chronic pain
The evidence from trials where it is possible to extract data is not clearly suggestive of a beneficial effect of CES on chronic pain. While there are substantial differences within the trials in terms of the populations studied and the stimulation parameters used, there is no measurable heterogeneity and no trial shows a clear benefit of active CES over sham stimulation. The evidence for all comparisons or CES is considered to be of low to very low quality.
Transcranial direct current stimulation (tDCS) for chronic pain
Meta‐analysis of all tDCS studies in chronic pain demonstrated heterogeneity but did demonstrate an effect versus sham interventions. Predetermined subgroup analyses did not reduce heterogeneity. This effect may be exaggerated by study biases and small study effects. The evidence available at the medium term also demonstrates an effect but with substantial heterogeneity. Evidence from long follow‐up does not suggest an effect of tDCS. We consider the evidence for all comparisons for tDCS to be of low to very low quality.
Reduced impedance non‐invasive cortical electrostimulation (RINCE) stimulation for chronic pain
We analysed one small trial suggesting a positive effect of RINCE over sham for chronic pain. This trial is at unclear risk of bias due to possible attrition bias. As such, further high‐quality research is needed to confirm this exploratory finding.
Transcranial random noise stimulation (tRNS) for chronic pain
We identified two small studies of tRNS, both at high risk of bias. We are unable to draw any conclusions about the effectiveness or lack of effectiveness of tRNS for chronic pain.
Secondary outcome measures
The available evidence does not suggest an effect of rTMS or tDCS on disability levels at any follow‐up point. There is insufficient evidence from which to draw conclusions regarding CES for disability.
Limited, low‐quality to very low‐quality evidence suggests that rTMS and tDCS may have positive effects on quality of life. Given the limited amount of data available to inform these analyses, the risks of bias in the evidence base and the small effects observed in pain for both rTMS and tDCS we would recommend that this finding should be interpreted with caution. Limited evidence suggest that RINCE has no effect on quality of life.
rTMS, CES, tDCS, RINCE, tRNS and sham stimulation are associated with transient adverse effects such as headache, scalp irritation and dizziness, but reporting of adverse effects was inconsistent and did not allow for a detailed analysis. There were two incidences of seizure following active rTMS, which occurred in separate studies. For all forms of stimulation, adverse events reporting is inconsistent across studies.
Overall completeness and applicability of evidence
For rTMS we were unable to include pain intensity data from six full published studies (Fregni 2005; Fregni 2011, Onesti 2013; Picarelli 2010; Tzabazis 2013; Umezaki 2016, combined n = 107). In addition, we identified 11 studies of rTMS published in abstract format for which we have not been able to acquire full study reports. A conservative estimate of the combined number of participants that those studies might add is 438, assuming that some reports refer to the same study.
We were unable to extract the relevant data from four studies of CES (Capel 2003; Cork 2004; Katsnelson 2004; Lichtbroun 2001). This may have impacted upon the results of our meta‐analysis although one of those studies would have been excluded from the meta‐analysis as we judged it as being at risk of bias on criteria other than selective outcome reporting (Katsnelson 2004).
We were also unable to extract the relevant data from three studies of tDCS (Donnell 2015; Mendonca 2011; Valle 2009), and these data were not made available upon request to the study authors. These data would have contributed a further 95 participants to our analysis and may have altered our conclusions. In addition, we identified five studies of tDCS (Acler 2012; Albu 2011; Knotkova 2011; Moreno‐Duarte 2013a; Mylius 2013) published in abstract format that appear clearly relevant for which we have not been able to acquire full study reports.
For both rTMS and tDCS there are a number of ongoing studies identified through the trials registry searches. Of note, eight trials were registered prior to 2012, seven of which are of tDCS and have not yet been published to our knowledge. Given our finding of small study effects in tDCS studies this gives cause for concern regarding the risk of potential publication bias and this is reflected in our GRADE judgements. We hope that future updates of this review will include the aforementioned data.
Quality of the evidence
Using the GRADE criteria we judged the quality of evidence for all comparisons as low or very low, meaning that our confidence in the effect estimate is limited or we have very little confidence in the effect estimate and the true effect is likely to be substantially different from the estimated effect. In large part this is due to issues of blinding and of precision. The majority of studies of rTMS were at unclear risk of bias. The predominant reason for this was the use of suboptimal sham controls that were unable to control for all possible sensory cues associated with active stimulation. A number of studies did not clearly report blinding of assessors and sensitivity analysis excluding those studies reduced both heterogeneity and the pooled effect size. It could be reasonably argued that the presence of a subgroup of single‐dose studies of high‐frequency stimulation specific to the motor cortex that does demonstrate superiority over sham with acceptable levels of heterogeneity is evidence for a specific clinical effect of rTMS. It should be considered, however, that high‐frequency rTMS is associated with more intense sensory and auditory cues that might plausibly elicit a larger placebo response, and many of the included studies were unable to control conclusively for these factors. Furthermore, the pooled effect size for the high‐frequency studies of motor cortex rTMS does not meet our predetermined threshold for clinical significance. This estimate is based solely on studies that delivered a single dose of rTMS. It is feasible that a single dose may be insufficient to induce clinically meaningful improvement. These single‐dose studies included in the analysis are best characterised as proof of principle studies, which sought to test whether rTMS could modulate pain, rather than full‐scale clinical studies with the aim of demonstrating clinical utility. The combined evidence from studies of high‐frequency rTMS to the motor cortex that delivered multiple doses, so better reflecting the likely clinical delivery of rTMS (excluding studies judged as being at high risk of bias), demonstrate no effect, but with substantial heterogeneity.
There are multiple sources of potential heterogeneity within the rTMS literature, relating to stimulation parameters, dose and population. We have explored, through pre‐planned subgroup analyses the influence of cortical target, stimulation frequency and dose at the crude level of single versus multiple dose. However we did not plan to formally explore the influence of all of the potential sources of variation in terms of stimulation parameters. As an example it is possible that some studies delivered suboptimal stimulation in terms of the numbers of pulses delivered, which ranged in our review from 120 to more than 2000 per treatment session. In addition, for studies of motor cortex stimulation there was variation in the somatotopic target of stimulation and this may be an important factor. While some studies used imaging‐based neuro‐navigation techniques to more precisely locate targeted brain regions most did not. There were not adequate data to meaningfully explore the influence of using neuro‐navigation on outcomes. There is evidence that approaches to identifying prefrontal targets that do not use neuronavigation are inaccurate (Ahdab 2010; Herwig 2001). Should neuro‐navigation be found to be crucial to effectiveness it would have implications for the costs and availability of this intervention.
Similarly, we judged no study of tDCS as having a low risk of bias on all criteria. While there is evidence that the sham control used in tDCS does achieve effective blinding of participants at stimulation intensities of 1 mA (Gandiga 2006), evidence has emerged since the first version of this review that indicates that at 1.5 mA the sensory profile of stimulation differs between active and sham stimulation (Kessler 2013), and at 2 mA participant and assessor blinding may be compromised (Ezquerro 2014; Horvath 2014; O'Connell 2012; Wallace 2016). Meta‐epidemiological evidence demonstrates that incomplete blinding in controlled trials that measure subjective outcomes may exaggerate the observed effect sizes (Savovic 2012; Wood 2008). It is therefore reasonable to expect that incomplete blinding may have exaggerated the effect sizes seen in the current analyses of rTMS and tDCS. It is noteworthy that the largest study of tDCS (Luedtke 2015), also judged at low risk of bias for all criteria except study size, demonstrated no effect of tDCS versus sham.
No study of CES could be judged as having a low risk of bias across all criteria. Despite this, no study from which data were available demonstrated a clear advantage of active over sham stimulation. There was substantial variation in the stimulation parameters used between studies. Notably three studies utilised an 'active placebo' control, in which stimulating current was delivered but at much lower intensities (Gabis 2003; Gabis 2009; Katsnelson 2004). These intensities well exceed those employed in the active stimulation condition of other studies of CES devices and as such it could be hypothesised that they might induce a therapeutic effect themselves. This could possibly disadvantage the active stimulation group in these studies. However, the data available in the meta‐analysis do not suggest such a trend and statistical heterogeneity between studies entered into the analysis was low.
All of the included studies may be considered to be small in terms of sample size and we reflected this in our 'Risk of bias' assessment. The prevalence of small studies increases the risk of small study bias and the related issue of publication bias, wherein there is a propensity for small negative studies to not reach full publication. There is evidence that this might lead to an overly positive picture for some interventions (Dechartres 2013; Nüesch 2010). In a review of meta‐analyses, Dechartres 2013 demonstrated that trials with fewer than 50 participants, which reflects the majority of studies included in this review, returned effect estimates that were on average 48% larger than the largest trials and 23% larger than estimates from studies with sample sizes of more than 50. Similarly, in Cochrane Reviews of amitriptyline for neuropathic pain and fibromyalgia (Moore 2015a; Moore 2015b), smaller studies were associated with substantially lower numbers needed to treat for an additional beneficial outcome (NNTBs) for treatment response than larger studies. In their recommendations for establishing best practice in chronic pain systematic reviews, the authors of Moore 2010 suggest that study size should be considered an important source of bias. It is therefore reasonable to consider that the evidence base for all non‐invasive brain stimulation techniques is at risk of bias on the basis of sample size. In this update we found evidence of small study effects affecting the tDCS evidence, but not for rTMS or CES. However, it is accepted that existing approaches to detecting publication bias are unsatisfactory and lack sensitivity. It should therefore be noted that even where a pooled estimate includes a large number of participants, if it is dominated by small studies, as are all comparisons in this review, then it is prone to small study effects. Funnel plot asymmetry may be explained by reasons other than publication bias, such as methodological quality, or simple chance (Sterne 2011), but for tDCS there is an association between study size and effect size, with smaller studies demonstrating larger effects.
Potential biases in the review process
There is substantial variation between the included studies of rTMS and tDCS. Studies varied in terms of the clinical populations included, the stimulation parameters and location, the number of treatment sessions delivered and in the length of follow‐up employed. This heterogeneity is reflected in the I2 statistic for the overall rTMS and tDCS meta‐analyses. However, pre‐planned subgroup investigation reduced this heterogeneity in some instances.
Many of the rTMS and tDCS studies specifically recruited participants whose symptoms were resistant to current clinical management and most rTMS studies specifically recruited participants with neuropathic pain. As such it is important to recognise that this analysis in large part reflects the efficacy of rTMS and tDCS for refractory chronic pain conditions and may not accurately reflect their efficacy across all chronic pain conditions.
One study included in the analysis of rTMS studies demonstrated a difference in pain levels between the two groups at baseline that exceeded the size of the difference observed at follow‐up (Defrin 2007). Specifically, the group that received sham stimulation reported less pain at baseline than those in the active stimulation group. The use in the current analysis of a between‐groups rather than a change‐from‐baseline comparison is likely to have affected the results although the study contributes only 1.5% weight to the overall meta‐analysis and the study itself reported no difference in the degree of pain reduction between the active and sham stimulation groups.
The method used to back‐transform the pooled standardised mean difference (SMD) to a 0‐10 pain intensity scale and subsequent calculation of the effect as a percentage improvement rests upon the assumption that the standard deviation and the pain levels used are representative of the wider body of evidence and should be considered an estimate at best. Representing average change scores on continuous scales is problematic in chronic pain studies since response to pharmacological treatments has been found to display a bimodal distribution (Moore 2013). More plainly, some participants demonstrate a substantial improvement with pain therapies while many demonstrate little or no change, with few individual participants demonstrating a change similar to the average. As a consequence the meaning of the average effect sizes seen in this review is difficult to interpret. This had led to the recommendation that chronic pain trials employ responder analyses based on predetermined cut‐offs for a clinically important response (≥ 30% reduction in pain for a moderate benefit, ≥ 50% reduction for a substantial benefit) (Dworkin 2008; Moore 2010). Very few studies identified in this review presented the results of responder analyses and so this type of meta‐analysis was not possible. However, where effects were observed in this review they were small, which would indicate that if there is a subgroup of 'responders' to active stimulation who demonstrate moderate or substantial benefits it is likely to include only a small number of participants. We are not aware of any direct evidence that participant outcomes are commonly bimodally distributed following these interventions and a recent analysis of data from trials of various non‐surgical interventions for spinal pain did not find evidence for bimodal distribution of outcomes (O'Connell 2017). It is also worth noting that when the effect estimates were back‐transformed to a 0 to 10 pain intensity scale they were also below theminimal clinically important difference threshold for the between‐group difference of 1 point recently recommended by the OMERACT‐12 group (Busse 2015).
Agreements and disagreements with other studies or reviews
The European Academy of Neurology published guidelines on the use of neurostimulation therapy for chronic neuropathic pain in 2017 (Cruccu 2017). Based on a narrative synthesis of the evidence gave "weak recommendations" for the use of rTMS in neuropathic pain and fibromyalgia and "inconclusive recommendations" in CRPS. They offered "inconclusive recommendations" regarding tDCS for fibromyalgia and "weak recommendations" for the use of tDCS for peripheral neuropathic pain. The 'weak' descriptor term used to describe the positive recommendations was based on the low quality of the supporting evidence. Another recent guideline specific to the use of rTMS (Lefaucheur 2014) concluded that there was "level A evidence", which represents "definite efficacy" for the analgesic effect of high frequency rTMS applied to the motor cortex contralateral to the side of pain. In light of our findings we suggest that this assessment of the evidence may not adequately reflect the numerous limitations of the evidence base.
Leung 2009 performed a meta‐analysis of individual participant data from studies of motor cortex rTMS for neuropathic pain conditions. Whilst the analysis was restricted to studies that clearly reported the neuroanatomical origin of noxious input (and therefore excluded some of the studies included in the current analysis) the overall analysis suggests a similar effect size of 13.7% improvement in pain (excluding the study of Khedr 2005). The study authors also performed an analysis of the influence of the neuroanatomical origins of noxious input on the effect size. They noted a trend suggestive of a larger treatment effect in central compared with peripheral neuropathic pain states although this did not reach statistical significance. While the data in the current review were not considered sufficient to support a detailed subgroup analysis by neuro‐anatomical origin of noxious input, the exclusion of studies that did not specifically investigate neuropathic pain did not significantly affect the overall analysis and the two multiple‐dose studies of motor cortex rTMS for central neuropathic pain that were included failed to demonstrate superiority of active over sham stimulation (Defrin 2007; Kang 2009).
All but one of the included studies in the review by Leung 2009 delivered high‐frequency (≥ 5 Hz) rTMS and no clear influence of frequency variations was observed within this group. The authors suggest that the number of doses delivered may be more crucial to the therapeutic response than the frequency (within the high‐frequency group), based on the larger therapeutic response seen in the study of Khedr 2005, that was excluded from the current analysis. This review preceded the studies by Defrin 2007 and Kang 2009 that did not demonstrate superiority of active over sham stimulation. While there are limited data to test this proposition robustly the result of our subgroup analysis of studies of high‐frequency motor cortex rTMS does not suggest a benefit of active stimulation over sham.
Lima and Fregni undertook a systematic review and meta‐analysis of motor cortex stimulation for chronic pain (Lima 2008). They pooled data from rTMS and tDCS studies. While the report states that data were collected on mean between‐group pain scores they are not presented. The authors present the pooled data for the number of responders to treatment across studies. They conclude that the number of responders is higher following active stimulation compared with sham (risk ratio 2.64, 95% CI 1.63 to 4.30). In their analysis the threshold for treatment response is defined as a global response according to each study's own definition and as such it is difficult to interpret and may not be well standardised. They note a greater response to multiple doses of stimulation, an observation that is not reliably reflected in the current review. Additionally they included the study of Khedr 2005 (excluded from this review due to high risk of bias) and Canavero 2002 (excluded on title and abstract as it is not a randomised or quasi‐randomised study). The current review also includes a number of motor cortex rTMS studies in the main analysis published since that review (André‐Obadia 2008; Defrin 2007; Hosomi 2013; Jetté 2013; Kang 2009; Lefaucheur 2006; Lefaucheur 2008; Medeiros 2016; Mhalla 2011; Passard 2007; Saitoh 2007; Tekin 2014; Yagci 2014). Neither the review of Leung 2009 nor Lima 2008 applied a formal quality or 'Risk of bias' assessment. While the current review also suggests a small, short‐term benefit of high‐frequency motor cortex rTMS in the treatment of chronic pain the effect is small, appears short‐term and although the pooled estimate approaches the threshold of minimal clinical significance it is possible that it might be inflated by methodological biases in the included studies.
A systematic review of tDCS and rTMS for the treatment of fibromyalgia concluded that the evidence demonstrated reductions in pain similar to US Food and Drug Administration (FDA)‐approved pharmaceuticals for this condition and recommended that rTMS or tDCS should be considered, particularly where other therapies have failed (Marlow 2013). This review included randomised and non‐randomised studies, did not undertake meta‐analysis and took a 'vote‐counting' approach to identifying effects based primarily on each included study's report of statistical testing. While our analysis did not specifically investigate a subgroup of studies in fibromyalgia participants, we would suggest that the methodology chosen by Marlow 2013 does not offer the most rigorous approach to establishing effect size, particularly in light of the inconsistency seen among the included studies of that review. Indeed, given the degree of uncertainty that remains regarding the efficacy of these interventions, it could be suggested that the application of tDCS or rTMS for this or other conditions would ideally be limited to the clinical research situation.
Luedtke 2012 systematically reviewed studies of tDCS for chronic pain and experimental pain. Unlike our review they excluded the study by Fenton 2009, as it was judged to be at high risk of bias on the grounds of unclear randomisation procedure and due to a lack of clarity of participant withdrawal, and Boggio 2009 due to the level of dropout. The results of their meta‐analysis are broadly consistent with those presented here in that the authors conclude that the evidence is insufficient to allow definite conclusions but that there is low‐level evidence that tDCS may be effective for chronic pain. Moreno‐Duarte 2013 recently reviewed the evidence for a variety of electrical and magnetic neural stimulation techniques for the treatment for chronic pain following spinal cord injury, including rTMS, tDCS and CES, including both randomised and non‐randomised studies. They found that the results varied across studies, though trials of tDCS were consistently positive, and concluded that further research is needed and that there is a need to develop methods to decrease the variability of treatment response to these interventions. However, it is worth noting that this review did not include the recent negative study of tDCS for postspinal cord injury pain by Wrigley 2014, and also that variability in observed treatment 'responses' may simply represent the play of chance rather than evidence of a specific group of responders.
Kirsch 2000 reviewed studies of CES in the management of chronic pain and concluded in favour of its use. The review did not report any formalised search strategy, inclusion criteria or quality assessment and discussed a number of unpublished studies that remain unpublished at the time of the current review. Using a more systematic methodology and including papers published since that review, we found that the data that were available for meta‐analysis did not suggest a clinically important benefit of active CES over sham. Our analysis included 270 participants. While this is not particularly large it does suggest that if there is an effect of CES on chronic pain it is either small, or that the number of responders is likely to be small.
A recent review of rTMS for chronic pain (Galhardoni 2015) concluded that rTMS has potential utility. This review reported that rTMS was frequently associated with greater that 30% pain relief when compared with a control treatment, though no meta‐analysis was reported and no formal assessment of study quality or risk of bias was presented. Our results suggest that, compared with sham, rTMS is associated with somewhat smaller effects and that the effect estimate may be exaggerated by various biases in the literature.
While many reviews have concluded positively regarding the effectiveness and early promise of non‐invasive brain stimulation techniques this is frequently based on markers of statistical significance and arguably does not adequately consider the influence of the various biases at play in the literature.
Authors' conclusions
For people with chronic pain
There is a lack of high‐quality evidence to support or refute the effectiveness of non‐invasive brain stimulation techniques for chronic pain. Due to the small size of included studies and limitations in the way that many studies were conducted, future studies may have a substantial impact upon the estimates of effects presented.
For clinicians
Low‐ or very low‐quality evidence suggests that low‐frequency repetitive transcranial magnetic stimulation (rTMS), or rTMS applied to the prefrontal cortex, may not be effective for the treatment of chronic pain. Subgroup analysis suggests that single doses of high‐frequency rTMS of the motor cortex may have small, short‐term effects on chronic pain that do not meet our threshold of minimum clinical importance (low‐quality evidence) and may be exaggerated by the dominance of small studies and other sources of bias. The pooled evidence from multiple‐dose studies of high‐frequency rTMS to the motor cortex is heterogeneous but does not demonstrate an effect (very low‐quality evidence). Very low‐quality evidence suggests that transcranial direct current stimulation (tDCS) may have short‐term effects on chronic pain but these observed effects may be exaggerated by the dominance of small studies and other sources of bias. Low‐quality evidence suggests that cranial electrotherapy stimulation (CES) is not effective. Due to this uncertainty, clinical application of non‐invasive brain stimulation techniques would be most appropriate within a clinical research setting rather than in routine clinical care and it is not currently clear if any form of non‐invasive brain stimulation is a useful clinical tool.
For policy makers and funders of the intervention
There is a lack of high‐quality evidence to support or refute the effectiveness of non‐invasive brain stimulation techniques when compared to sham stimulation for people with chronic pain. The short‐term effects observed for rTMS and tDCS on pain may be exaggerated by the dominance of small studies and limitations in study methods. There is not currently a strong evidence base for routinely offering these options for the treatment of chronic pain.
General
The existing evidence across all forms of non‐invasive brain stimulation is dominated by small studies with unclear risk of bias and there is a need for larger, rigorously controlled trials. It is noteworthy that in the seven years since our original review the number of included studies has risen substantially but our conclusions have not changed. Contrasting the large number of trials included in this review with the persisting lack of certainty over its effectiveness speaks to a problem of research waste.
After our first review of this evidence was completed in 2010 we recommended that there was a need to examine the more promising findings within the existing data through more robust, large, rigorous, adequately blinded trials that deliver a reasonable dose and investigate effects over a meaningful timescale (O'Connell 2011). Until a body of this type of research is generated there will continue to be uncertainty over the clinical utility of any form of non‐invasive brain stimulation for chronic pain. This recommendation is relevant to all other types of non‐invasive brain stimulation. The ongoing studies, identified from searching trials registers, predominantly consist of more, relatively small trials and it is unlikely that the results will meaningfully change the findings of this review. A recent consensus statement (Klein 2015) has produced guidelines for future rTMS research on clinical pain with the goal of improving quality and these recommendations should be taken under consideration.
The proliferation of small heterogeneous trials presents a challenge to evidence synthesis. A robust, large scale trial of rTMS or tDCS might fail to reduce uncertainty if included in the same analysis as the existing trials. For future reviews of this evidence base, that seek to answer the question of clinical effectiveness, there may be a case for excluding single‐dose trials on the basis of inadequate dose and trials below a threshold size on the basis of imprecision. There is also a case for not updating the current review until trials of adequate size have been added to the evidence base, since an update characterised by the inclusion of more, small heterogeneous trials will sufficiently reduce uncertainty.
Design
Future rTMS research should consider employing recently developed sham coils that control for all of the sensory aspects of stimulation. Such coil systems should be robustly validated as valid sham controls. Future studies should have a strong theoretical basis underpinning the choice of stimulation location and parameters and ensure that stimulation delivered to high technical standards. Future studies of tDCS should give consideration to the integrity of participant blinding, particularly when utilising stimulation intensities that exceed 1 mA. The field should seek to generate consensus on optimal stimulation parameters and procedures.
Outcome measurement
Future trials should also consider the IMMPACT recommendations for the design of trials in chronic pain (Dworkin 2008; Dworkin 2009; Dworkin 2010; Turk 2008), to ensure that outcomes, thresholds for clinical importance and study designs are optimal, and should endeavour to ensure that published study reports are compliant with the CONSORT statement (Schulz 2010). All studies of non‐invasive brain stimulation techniques should measure, record and clearly report adverse events from both active and sham stimulation.
Acknowledgements
For this update
We would like to extend particular thanks to Cochrane Pain, Palliative and Supportive Care for their assistance throughout the review, in particular Anna Erskine (nee Hobson) and Joanne Abbott. We would also like to thank the following authors for generously providing additional data for this review upon request: Dr Paradee Auvichayapa, Dr Abrahão Fontes Baptista, Dr Jeffrey Hargrove, Dr Catherine Mercier. We would like to thank Professor Turo Nurmikko and Janet Wale for their valuable peer review comments.
For 2014 update
We would like to extend particular thanks to the Cochrane Pain, Palliative and Supportive Care Group for their assistance throughout the review, in particular Anna Erskine (née Hobson) and Joanne Abbott. We would also like to thank the following authors for generously providing additional data for this review upon request: Dr David Avery, Dr Andrea Antal, Professor Mark Jensen, Dr Francesco Mori, Dr Marcelo Riberto, Prof Youichi Saitoh and Ann Gillian Taylor.
For 2010 version of review
The authors would like to thank James Langridge of the Brunel University Library for sharing his expertise in the use of electronic databases, Arturo Lawson, Ana Bela Nascimento, Andrea Wand, Pete and Maria Heine and Dr Evgeny Makarov for assistance with interpretation.
We would also like to thank the following authors for generously providing additional data for this review upon request: Dr Nathalie André‐Obadia, Dr Didier Bouhassira, Dr Ruth Defrin, Dr Bradford Fenton, Dr Felipe Fregni, Dr Linda Gabis/Dr Ranann Raz, Dr Eman Khedr, Prof. Jean‐Pascale Lefaucheur, Dr Burkhard Pleger, Prof. Jens Rollnik, Prof Youichi Saitoh.
Cochrane Review Group funding acknowledgement: this project was supported by the National Institute for Health Research, via Cochrane Infrastructure funding to Cochrane Pain, Palliative and Supportive Care (PaPaS). The views and opinions expressed therein are those of the authors and do not necessarily reflect those of the Systematic Reviews Programme, NIHR, NHS or the Department of Health.
Appendices
Appendix 1. Main database search strategies for current update
CENTRAL (CRSO)
#1 MESH DESCRIPTOR pain EXPLODE ALL TREES 32731
#2 (((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*)):TI,AB,KY 15073
#3 ((sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*))):TI,AB,KY 6757
#4 #1 OR #2 OR #3 45871
#5 MESH DESCRIPTOR Transcranial Magnetic Stimulation 974
#6 MESH DESCRIPTOR Electronarcosis 33
#7 (((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*)):TI,AB,KY 4072
#8 (((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*))):TI,AB,KY 64
#9 (((non‐invasive or non*invasive) adj4 stimulat*)):TI,AB,KY 337
#10 ((theta burst stimulat* or iTBS or cTBS)):TI,AB,KY 150
#11 ((transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy)):TI,AB,KY 2912
#12 ((electrosleep or electronarco*)):TI,AB,KY 47
#13 #5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12 4355
#14 #4 AND #13 310
#15 31/07/2013 TO 30/09/2016:DL 264060
#16 #14 AND #15 176
MEDLINE (OVID)
1 exp Pain/ (283010)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (74023)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (28679)
4 or/1‐3 (325946)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (6328)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (25872)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (147)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (822)
9 (theta burst stimulat* or iTBS or cTBS).tw. (575)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (7423)
11 (electrosleep or electronarco*).tw. (357)
12 or/5‐11 (28316)
13 randomized controlled trial.pt. (337806)
14 controlled clinical trial.pt. (84996)
15 randomized.ab. (241501)
16 placebo.ab. (134421)
17 drug therapy.fs. (1571905)
18 randomly.ab. (173459)
19 trial.ab. (248492)
20 groups.ab. (1134392)
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (2928552)
22 exp animals/ not humans.sh. (3751730)
23 21 not 22 (2487755)
24 4 and 12 and 23 (295)
25 (200911* or 200912* or 2010* or 2011* or 2012* or 2013*).ed. (2428299)
26 24 and 25 (112)
Embase (OVID)
1 exp Pain/ (1006798)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (158849)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (52041)
4 or/1‐3 (1044575)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (18453)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (50617)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (237)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (2843)
9 (theta burst stimulat* or iTBS or cTBS).tw. (1549)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (17745)
11 (electrosleep or electronarco*).tw. (383)
12 or/5‐11 (57298)
13 random$.tw. (1121981)
14 factorial$.tw. (28563)
15 crossover$.tw. (58949)
16 cross over$.tw. (26241)
17 cross‐over$.tw. (26241)
18 placebo$.tw. (244121)
19 (doubl$ adj blind$).tw. (172110)
20 (singl$ adj blind$).tw. (18218)
21 assign$.tw. (295873)
22 allocat$.tw. (107828)
23 volunteer$.tw. (211373)
24 Crossover Procedure/ (48595)
25 double‐blind procedure.tw. (236)
26 Randomized Controlled Trial/ (419274)
27 Single Blind Procedure/ (23071)
28 or/13‐27 (1749640)
29 (animal/ or nonhuman/) not human/ (5110486)
30 28 not 29 (1554658)
31 4 and 12 and 30 (1112)
32 (201307* or 201308* or 201309* or 201310* or 201311* or 201312* or 2014* or 2015* or 2016*).dd. (5443542)
33 31 and 32 (527)
34 limit 33 to embase (487)
PsycINFO (OVID)
1 exp Pain/ (48364)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (25922)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (4998)
4 or/1‐3 (56650)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (5956)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (17936)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (89)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (983)
9 (theta burst stimulat* or iTBS or cTBS).tw. (791)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (7884)
11 (electrosleep or electronarco*).tw. (139)
12 or/5‐11 (18853)
13 clinical trials/ (9724)
14 (randomis* or randomiz*).tw. (62274)
15 (random$ adj3 (allocat$ or assign$)).tw. (35100)
16 ((clinic$ or control$) adj trial$).tw. (52603)
17 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (22429)
18 (crossover$ or "cross over$").tw. (8346)
19 random sampling/ (699)
20 Experiment Controls/ (856)
21 Placebo/ (4606)
22 placebo$.tw. (35030)
23 exp program evaluation/ (18184)
24 treatment effectiveness evaluation/ (20144)
25 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (70971)
26 or/13‐25 (221762)
27 4 and 12 and 26 (180)
28 limit 27 to yr="2013 ‐Current" (82)
CINAHL (EBSCO)
S26 S25 Limiters ‐ Published Date from: 20130701‐20160914
S25 S15 AND S24
S24 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 (allocat* random*)
S22 (MH "Quantitative Studies")
S21 (MH "Placebos")
S20 placebo*
S19 (random* allocat*)
S18 (MH "Random Assignment")
S17 (Randomi?ed control* trial*)
S16 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S15 S4 AND S14
S14 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 TI ( (electrosleep OR electronarco*) ) OR AB ( (electrosleep OR electronarco*) )
S12 TI ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") ) OR AB ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") )
S11 TI ( ("theta burst stimulat*" OR iTBS OR cTBS) ) OR AB ( ("theta burst stimulat*" OR iTBS OR cTBS) )
S10 TI ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) ) OR AB ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) )
S9 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S8 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S7 TI ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) ) OR AB ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) )
S6 (MH "Electric Stimulation")
S5 (MH "Electronarcosis")
S4 S1 OR S2 OR S3
S3 TI ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") ) OR AB ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") )
S2 TI ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*). ) OR AB ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*))
S1 (MH "Pain+")
LILACS
1. Pain$ or dolor$ or intractabl$ or neuropath$ or phantom or fantom or myofasc$ or temp$romandibular or sciatic$ or back‐ache or backache or ache or lumbago or fibromyalg$ or neuralg$ or dystroph$ or atroph$ or causalgi$ or whip‐lash or whiplash or polymyalg$ [Words]¬
2. ((Estimulaci$ or stimulat$) and (cerebra$ or brain$ or cortex or cortical or crania$ or transcranial$ or magneti$)) or electrostim$ or electrotherapy$ or electro‐therap$ or “theta burst stimul$” or iTBS or Ctbs or “transcrani$ magnet$ stimulat$” or rTMS or “transcrani$ direct current stimulat$” or tDCS or “cranial electrostimulat$” or “cranial electrotherapy$ or electrosleep or electronarco$ [Words]¬
3. ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
Appendix 2. Trials register search results for current update
| Register | Date of search | Search terms | Number of records |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
91 |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
1 |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
0 |
| Clinical trials.gov | 20 September 2016 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
0 |
| WHO ICTRP | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain 01/01/2009 to 07/02/2013 adult |
60 |
| WHO ICTRP | 20 September 2016 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
|
| WHO ICTRP | 20/9/16 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
2 |
| WHO ICTRP | 20 September 2016 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
| Register | Date of search | Search terms | Number of records |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
6 |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
3 |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
3 |
| Clinical trials.gov | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
0 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain 01/01/2009 to 07/02/2013 adult |
36 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp*romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
8 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
0 |
| WHO ICTRP | 18 Octoberr 2017 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
0 |
Appendix 3. Search results summary table for current update
| Database searched | Date last searched | Number of results |
| CENTRAL (CRSO) 31/07/2013 TO 30/09/2016 | 11/10/17 | 243 |
| MEDLINE (OVID) July 2013 to Aug week 5 2016 | 11/10/17 | 217 |
| Embase (OVID) July 2013 to 2016 week 37 | 11/10/17 | 595 |
| PsycINFO (OVID) 2013 to July week 4 2016 | 11/10/17 | 117 |
| CINAHL (EBSCO) July 2013 to Sept 2016 | 11/10/17 | 42 |
| LILACS (Birme) 2013 to Sept 2016 | 11/10/17 | 42 |
| Total | 1256 | |
Appendix 4. Main database search strategies for 2014 update
CENTRAL (years 2009 to 2013 searched)
#1 MeSH descriptor: [Pain] explode all trees
#2 (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint" or "temperomandib* joint" or "tempromandib* joint" or central or (post next stroke) or complex or regional or "spinal cord") near/4 pain*:ti,ab,kw (Word variations have been searched)
#3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* near/2 neuralg*) or (herp* near/2 neuralg*) or (diabet* near/2 neuropath*) or (reflex near/4 dystroph*) or (sudeck* near/2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back near/4 surg*) or (failed back near/4 syndrome*)):ti,ab,kw (Word variations have been searched)
#4 #1 or #2 or #3
#5 MeSH descriptor: [Transcranial Magnetic Stimulation] this term only
#6 MeSH descriptor: [Electronarcosis] explode all trees
#7 (brain* or cortex or cortical or transcranial* or cranial or magneti*) near/4 stimulat*:ti,ab,kw (Word variations have been searched)
#8 (transcrani* or crani* or brain*) near/4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*):ti,ab,kw (Word variations have been searched)
#9 (non‐invasive or non*invasive) near/4 stimulat*:ti,ab,kw (Word variations have been searched)
#10 "theta burst stimulat*" or iTBS or cTBS:ti,ab,kw (Word variations have been searched)
#11 "transcranial magnetic stimulation" or rTMS or "transcranial direct current stimulat*" or tDCS or "cranial electrostimulation" or "cranial electrotherap*":ti,ab,kw (Word variations have been searched)
#12 (electrosleep* or electronarco*):ti,ab,kw (Word variations have been searched)
#13 #5 or #6 or #7 or #8 or #9 or #10 or #11 or #12
#14 #4 and #13 from 2009 to 2013
MEDLINE and MEDLINE IN PROCESS (OVID)
1 exp Pain/ (283010)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (74023)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (28679)
4 or/1‐3 (325946)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (6328)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (25872)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (147)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (822)
9 (theta burst stimulat* or iTBS or cTBS).tw. (575)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (7423)
11 (electrosleep or electronarco*).tw. (357)
12 or/5‐11 (28316)
13 randomized controlled trial.pt. (337806)
14 controlled clinical trial.pt. (84996)
15 randomized.ab. (241501)
16 placebo.ab. (134421)
17 drug therapy.fs. (1571905)
18 randomly.ab. (173459)
19 trial.ab. (248492)
20 groups.ab. (1134392)
21 13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (2928552)
22 exp animals/ not humans.sh. (3751730)
23 21 not 22 (2487755)
24 4 and 12 and 23 (295)
25 (200911* or 200912* or 2010* or 2011* or 2012* or 2013*).ed. (2428299)
26 24 and 25 (112)
Embase (OVID)
1 exp Pain/ (729490)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (112128)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (41462)
4 or/1‐3 (759765)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (11875)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (35587)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (194)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (1314)
9 (theta burst stimulat* or iTBS or cTBS).tw. (770)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (10413)
11 (electrosleep or electronarco*).tw. (375)
12 or/5‐11 (39959)
13 4 and 12 (3078)
14 random$.tw. (793677)
15 factorial$.tw. (20700)
16 crossover$.tw. (46383)
17 cross over$.tw. (21096)
18 cross‐over$.tw. (21096)
19 placebo$.tw. (189884)
20 (doubl$ adj blind$).tw. (140353)
21 (singl$ adj blind$).tw. (13272)
22 assign$.tw. (220119)
23 allocat$.tw. (74677)
24 volunteer$.tw. (170305)
25 Crossover Procedure/ (36109)
26 double‐blind procedure.tw. (224)
27 Randomized Controlled Trial/ (338884)
28 Single Blind Procedure/ (16955)
29 or/14‐28 (1300700)
30 (animal/ or nonhuman/) not human/ (4566449)
31 29 not 30 (1146950)
32 13 and 31 (574)
33 (200911* or 200912* or 2010* or 2011* or 2012* or 2013*).dd. (4384183)
34 32 and 33 (303)
PsycINFO (OVID)
1 exp Pain/ (33859)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).tw. (17914)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).tw. (3654)
4 or/1‐3 (39372)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (3412)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).tw. (9508)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).tw. (55)
8 ((non‐invasive or non*invasive) adj4 stimulat*).tw. (401)
9 (theta burst stimulat* or iTBS or cTBS).tw. (441)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).tw. (4745)
11 (electrosleep or electronarco*).tw. (6)
12 or/5‐11 (9914)
13 4 and 12 (481)
14 clinical trials/ (6486)
15 (randomis* or randomiz*).tw. (39676)
16 (random$ adj3 (allocat$ or assign$)).tw. (22629)
17 ((clinic$ or control$) adj trial$).tw. (33763)
18 ((singl$ or doubl$ or trebl$ or tripl$) adj3 (blind$ or mask$)).tw. (15332)
19 (crossover$ or "cross over$").tw. (5478)
20 random sampling/ (445)
21 Experiment Controls/ (435)
22 Placebo/ (2892)
23 placebo$.tw. (23869)
24 exp program evaluation/ (12521)
25 treatment effectiveness evaluation/ (11860)
26 ((effectiveness or evaluat$) adj3 (stud$ or research$)).tw. (45199)
27 or/14‐26 (142131)
28 13 and 27 (95)
29 limit 28 to yr="2009 ‐Current" (60)
CINAHL (EBSCO)
S26 S25 Limiters ‐ Published Date from: 20091101‐20130231
S25 S15 AND S24
S24 S16 OR S17 OR S18 OR S19 OR S20 OR S21 OR S22 OR S23
S23 (allocat* random*)
S22 (MH "Quantitative Studies")
S21 (MH "Placebos")
S20 placebo*
S19 (random* allocat*)
S18 (MH "Random Assignment")
S17 (Randomi?ed control* trial*)
S16 (singl* blind* ) or (doubl* blind* ) or (tripl* blind* ) or (trebl* blind* ) or (trebl* mask* ) or (tripl* mask* ) or (doubl* mask* ) or (singl* mask* )
S15 S4 AND S14
S14 S5 OR S6 OR S7 OR S8 OR S9 OR S10 OR S11 OR S12 OR S13
S13 TI ( (electrosleep OR electronarco*) ) OR AB ( (electrosleep OR electronarco*) )
S12 TI ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") ) OR AB ( ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy") )
S11 TI ( ("theta burst stimulat*" OR iTBS OR cTBS) ) OR AB ( ("theta burst stimulat*" OR iTBS OR cTBS) )
S10 TI ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) ) OR AB ( (("non‐invasive brain" OR "non*invasive brain") AND stimulat*) )
S9 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S8 TI ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) ) OR AB ( ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)) )
S7 TI ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) ) OR AB ( ((brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti*) AND stimulat*) )
S6 (MH "Electric Stimulation")
S5 (MH "Electronarcosis")
S4 S1 OR S2 OR S3
S3 TI ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") ) OR AB ( (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*") )
S2 TI ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*). ) OR AB ( ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*))
S1 (MH "Pain+")
LILACS (7 February 2013)
1. (chronic$ or back or musculoskel$ or intractabl$ or neuropath$ or phantom limb or fantom limb or neck or myofasc$ or temporomandib$ or temperomandib$ or tempromandib$ or central or (post stroke) or complex or regional or spinal cord sciatica or back‐ache or back ache or lumbago or fibromyalg$ or trigemin$ neuralg$ or herp$ neuralg$ or diabet$ neuropath$ or reflex dystroph$ or sudeck$ atrophy$ or causalg$ or whip‐lash or whip$lash or polymyalg$ or failed back) 69863
2. (brain$ or cortex or cortical or transcrani$ or cranial or magneti$ stimulat$ or electrostim$ or electro‐stim$ or electrotherapy$ or electro‐therap$ or non‐invasive or non invasive or stimul$ or theta burst stimulat$ or iTBS or cTBS or transcranial magnetic stimulat$ or rTMS or transcranial direct current stimulat$ or tDCS or cranial electrostimulation or cranial electrotherapy$ or electrosleep$ or electronarco$) 24787
3. 1&2 5559
4. (randomized controlled trial or controlled clinical trial or placebo or sham or randomly or trial or groups) 31227
5. 3&4 545
6. REMOVE ANY PRE 2009 (removed 292) 253
Appendix 5. Trials register search results for 2014 update
| Register | Date of search | Search terms | Number of records | Number of relevant records |
| NRR archive | 7 February 2013 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp*romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*) al fields AND (2009 OR 2010 OR 2011 OR 2012 OR 2013) date started | 2 | 0 |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain 01/01/2009 to 07/02/2013 adult |
89 | 10 |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
20 | |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
2 | |
| Clinical trials.gov | 7 February 2013 | Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
0 | |
| HSRProj | 11 February 2013 | ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*)) | 152 | 0 |
| Current controlled trials (excl clinicatrials.gov) | 11 February 2013 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (cranial electrotherapy OR electrosleep OR electronarco*) | 0 | 1 |
| Current controlled trials (excl clinicatrials.gov) | 11 February 2013 | (sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (Ctbs OR transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation) | 0 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | TRANSCRANIAL and PAIN | 1 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | CRANIAL AND PAIN | 4 | |
| Current controlled trials (excl clinicatrials.gov) | 25/2/13 | STIMULATION AND PAIN | 75 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | (Cortex or cortical) and pain | 8 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | Brain and pain | 33 | |
| Current controlled trials (excl clinicatrials.gov) | 25 February 2013 | (Electro or electrical) and pain | 46 | |
| Total current controlled trials | 25 February 2013 | 167 | ||
| Total relevant trial records, all databases | 11 | |||
Appendix 6. Search results summary table for 2014 update
| Database searched | Date searched | Number of results |
| CENTRAL Issue 6 of 12, 2013 (The Cochrane Library) | 24 July 2013 | 2 |
| MEDLINE (OVID) June 2013 to 19/7/2013 MEDLINE In Process (OVID) – current week |
24 July 2013 24 July 2013 |
5 19 |
| Embase (OVID) June 2013 to 2013 week 29 | 24 July 2013 | 8 |
| PsycINFO (OVID) June 2013 to July week 3 2013 | 24 July 2013 | 1 |
| CINAHL (EBSCO) June 2013 to July 2013 | 24 July 2013 | 4 |
| Total | 39 | |
| After de‐duplication | 35 | |
| After title abstract screening | 0 | |
| After expert checking | 2 | |
Appendix 7. Full list of searches and results for 2009 version of review
1. Cochrane PaPaS Group Specialised Register, saved search: 177 results
“electric* stimulat* therap*” or “brain* stimulat*” or “cort* stimulat*” or “transcranial* stimulat*” or “cranial stimulat*” or “magneti* stimulat*” or “direct current stimulat*” or “electric* stimulat*” or electrostim* or electrotherapy* or electro‐therap* or “theta burst stimulat*” or “transcran* magnet* stimulat*” or iTBS or cTBS or rTMS or “transcran* direct current stimulat*” or tDCS or electrosleep or electronarco*
2. CENTRAL in The Cochrane Library
| #1 | MeSH descriptor Pain explode all trees | 25049 |
| #2 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint" or "temperomandib* joint" or "tempromandib* joint" or central or (post NEXT stroke) or complex or regional or "spinal cord") near/4 pain*:ti,ab,kw | 7785 |
| #3 | (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* near/2 neuralg*) or (herp* near/2 neuralg*) or (diabet* near/2 neuropath*) or (reflex near/4 dystroph*) or (sudeck* near/2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back near/4 surg*) or (failed back near/4 syndrome*)):ti,ab,kw | 3040 |
| #4 | (#1 OR #2 OR #3) | 30353 |
| #5 | MeSH descriptor Transcranial Magnetic Stimulation explode all trees | 328 |
| #6 | MeSH descriptor Electronarcosis explode all trees | 34 |
| #7 | (brain* or cortex or cortical or transcranial* or cranial or magneti*) near/4 stimulat*:ti,ab,kw | 1388 |
| #8 | (transcrani* or crani* or brain*) near/4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*):ti,ab,kw | 45 |
| #9 | (non‐invasive or non*invasive) near/4 stimulat*:ti,ab,kw | 55 |
| #10 | "theta burst stimulat*" or iTBS or cTBS:ti,ab,kw | 9 |
| #11 | "transcranial magnetic stimulation" or rTMS or "transcranial direct current stimulat*" or tDCS or "cranial electrostimulation" or "cranial electrotherap*":ti,ab,kw | 747 |
| #12 | (electrosleep* or electronarco*):ti,ab,kw | 45 |
| #13 | (#5 OR #6 OR #7 OR #8 OR #9 OR #10 OR #11 OR #12) | 1505 |
| #14 | (#4 AND #13) | 106 |
3a. MEDLINE
Database: Ovid MEDLINE(R) <1950 to November Week 3 2009>
1 exp Pain/ (252061)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (61945)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (25802)
4 1 or 3 or 2 (288507)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (4240)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (21248)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (116)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (526)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (359)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (5306)
11 (electrosleep or electronarco*).ab,ti. (357)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (23212)
13 4 and 12 (1069)
14 randomised controlled trial.pt. (291031)
15 controlled clinical trial.pt. (82962)
16 randomized.ab. (196258)
17 (placebo or sham).ab,ti. (164609)
18 drug therapy.fs. (1385685)
19 randomly.ab. (141449)
20 trial.ab. (203139)
21 groups.ab. (961704)
22 or/14‐21 (2562312)
23 exp animals/ not humans.sh. (3518581)
24 22 not 23 (2157467)
25 24 and 13 (219)
3b. Database: Ovid MEDLINE(R) In‐process & Other non‐indexed citations
<25 November 2009>
1 exp Pain/ (6)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (4772)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (1251)
4 1 or 3 or 2 (5661)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (0)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (1057)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (5)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (42)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (38)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (375)
11 (electrosleep or electronarco*).ab,ti. (0)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (1113)
13 4 and 12 (39)
4. Database: Embase
<1980 to 2009 Week 47>
1 exp Pain/ (394924)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or "temporomandib* joint*" or "temperomandib* joint*" or "tempromandib* joint*" or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (57196)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (21356)
4 1 or 3 or 2 (410258)
5 Transcranial Magnetic Stimulation/ or Electronarcosis/ (5841)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (18227)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (74)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (498)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (330)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (5259)
11 (electrosleep or electronarco*).ab,ti. (20)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (19954)
13 4 and 12 (1331)
14 random*.ti,ab. (415216)
15 factorial*.ti,ab. (8708)
16 (crossover* or cross over* or cross‐over*).ti,ab. (40788)
17 placebo*.ti,ab. (114266)
18 (doubl* adj blind*).ti,ab. (87525)
19 (singl* adj blind*).ti,ab. (7775)
20 assign*.ti,ab. (113729)
21 allocat*.ti,ab. (36179)
22 volunteer*.ti,ab. (102464)
23 CROSSOVER PROCEDURE.sh. (21985)
24 DOUBLE‐BLIND PROCEDURE.sh. (74829)
25 RANDOMIZED CONTROLLED TRIAL.sh. (176320)
26 SINGLE BLIND PROCEDURE.sh. (8721)
27 or/14‐26 (691134)
28 ANIMAL/ or NONHUMAN/ or ANIMAL EXPERIMENT/ (3551150)
29 HUMAN/ (6702208)
30 28 and 29 (569432)
31 28 not 30 (2981718)
32 27 not 31 (601828)
33 32 and 13 (234)
5. Database: PsycINFO
<1806 to November Week 4 2009>
1 exp Pain/ (26560)
2 ((chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib* joint or central or post*stroke or complex or regional or spinal cord) adj4 pain*).ab,ti. (14094)
3 (sciatica or back‐ache or back*ache or lumbago or fibromyalg* or (trigemin* adj2 neuralg*) or (herp* adj2 neuralg*) or (diabet* adj2 neuropath*) or (reflex adj4 dystroph*) or (sudeck* adj2 atroph*) or causalg* or whip‐lash or whip*lash or polymyalg* or (failed back adj4 surg*) or (failed back adj4 syndrome*)).ab,ti. (2649)
4 1 or 3 or 2 (30822)
5 Transcranial Magnetic Stimulation/ or Electrosleep treatment/ (1830)
6 ((brain* or cortex or cortical or transcranial* or cranial or magneti*) adj4 stimulat*).ab,ti. (7832)
7 ((transcrani* or crani* or brain*) adj4 (electrostim* or electro‐stim* or electrotherap* or electro‐therap*)).ab,ti. (47)
8 ((non‐invasive or non*invasive) adj4 stimulat*).ab,ti. (144)
9 (theta burst stimulat* or iTBS or cTBS).ab,ti. (259)
10 (transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy).ab,ti. (2652)
11 (electrosleep or electronarco*).ab,ti. (140)
12 8 or 6 or 11 or 7 or 10 or 9 or 5 (8307)
13 4 and 12 (277)
14 (random* or placebo* or sham or trial or groups).ti,ab. (391590)
15 13 and 14 (64)
6. CINAHL
<Search run 11 January 2010>
| 1 | exp PAIN/ | 64959 |
| 2 | ((chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR "temporomandib* joint*" OR "temperomandib* joint*" OR "tempromandib* joint*" OR central OR post*stroke OR complex OR regional OR spinal cord) AND pain*).ti,ab | 25127 |
| 3 | (sciatica OR back‐ache OR back*ache OR lumbago OR fibromyalg* OR "trigemin* neuralg*" OR "herp* neuralg*" OR "diabet* neuropath*" OR "reflex dystroph*" OR "sudeck* atroph*" OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR "failed back surg*" OR "failed back syndrome*").ti,ab | 4111 |
| 4 | 1 OR 2 OR 3 | 75018 |
| 5 | ELECTRONARCOSIS/ | 1 |
| 6 | ELECTRIC STIMULATION/ | 3829 |
| 7 | ((brain* OR cortex OR cortical OR transcranial* OR cranial OR "magneti*) AND stimulat*).ti,ab | 545 |
| 8 | ((transcrani* OR crani* OR brain*) AND (electrostim* OR electro‐stim* OR electrotherap* OR electro‐therap*)).ti,ab | 26 |
| 9 | (("non‐invasive brain" OR "non*invasive brain") AND stimulat*).ti,ab | 12 |
| 10 | ("theta burst stimulat*" OR iTBS OR cTBS).ti,ab | 16 |
| 11 | ("transcranial magnetic stimulation" OR rTMS OR "transcranial direct current stimulation" OR tDCS OR "cranial electrostimulation" OR "cranial electrotherapy").ti,ab | 437 |
| 12 | (electrosleep OR electronarco*).ti,ab | 1 |
| 13 | 5 OR 6 OR 7 OR 8 OR 9 OR 10 OR 11 OR 12 | 4387 |
| 14 | 4 AND 13 | 836 |
| 15 | exp CLINICAL TRIALS/ | 79642 |
| 16 | (clinical AND trial*).af | 148411 |
| 17 | ((singl* OR doubl* OR trebl* OR tripl*) AND (blind* OR mask*)).ti,ab | 11736 |
| 18 | (Randomi?ed AND control* AND trial*).af | 65515 |
| 19 | RANDOM ASSIGNMENT/ | 22506 |
| 20 | (Random* AND allocat*).ti,ab | 3666 |
| 21 | placebo*.af | 34556 |
| 22 | PLACEBOS/ | 5386 |
| 23 | QUANTITATIVE STUDIES/ | 5131 |
| 24 | 15 OR 16 OR17 OR 18 OR 19 OR 20 OR 21 OR 22 OR 23 | 176918 |
| 25 | 14 AND 24 | 226 |
7. SCOPUS
We did not search this database as it includes all of MEDLINE, all of Embase and some of CINAHL, which have been searched separately.
8. Search strategy for LILACS
http://bases.bireme.br/cgi‐bin/wxislind.exe/iah/online/
1. Pain$ or dolor$ or intractabl$ or neuropath$ or phantom or fantom or myofasc$ or temp$romandibular or sciatic$ or back‐ache or backache or ache or lumbago or fibromyalg$ or neuralg$ or dystroph$ or atroph$ or causalgi$ or whip‐lash or whiplash or polymyalg$ [Words]
2. ((Estimulaci$ or stimulat$) and (cerebra$ or brain$ or cortex or cortical or crania$ or transcranial$ or magneti$)) or electrostim$ or electrotherapy$ or electro‐therap$ or “theta burst stimul$” or iTBS or Ctbs or “transcrani$ magnet$ stimulat$” or rTMS or “transcrani$ direct current stimulat$” or tDCS or “cranial electrostimulat$” or “cranial electrotherapy$ or electrosleep or electronarco$ [Words]
3. ((Pt randomized controlled trial OR Pt controlled clinical trial OR Mh randomized controlled trials OR Mh random allocation OR Mh double‐blind method OR Mh single‐blind method) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Pt clinical trial OR Ex E05.318.760.535$ OR (Tw clin$ AND (Tw trial$ OR Tw ensa$ OR Tw estud$ OR Tw experim$ OR Tw investiga$)) OR ((Tw singl$ OR Tw simple$ OR Tw doubl$ OR Tw doble$ OR Tw duplo$ OR Tw trebl$ OR Tw trip$) AND (Tw blind$ OR Tw cego$ OR Tw ciego$ OR Tw mask$ OR Tw mascar$)) OR Mh placebos OR Tw placebo$ OR (Tw random$ OR Tw randon$ OR Tw casual$ OR Tw acaso$ OR Tw azar OR Tw aleator$) OR Mh research design) AND NOT (Ct animal AND NOT (Ct human and Ct animal)) OR (Ct comparative study OR Ex E05.337$ OR Mh follow‐up studies OR Mh prospective studies OR Tw control$ OR Tw prospectiv$ OR Tw volunt$ OR Tw volunteer$) AND NOT (Ct animal AND NOT (Ct human and Ct animal))) [Words]
4. 1 and 2 and 3 (68)
Appendix 8. Trials register search results for 2009 version of review
| Database | Date of search | Search strategy | No. hits | Agreed potential studies |
| National Research Register (NRR) Archive (NIHR) | 23 October 2009 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*) IN “TITLE” Field | 366 | 2 |
| Clinicaltrials.gov | 23 October 2009 Search 1 |
Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
62 | |
| Clinicaltrials.gov | 23 October 2009 Search 2 |
Field ‐ Interventional studies CONDITION: chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck OR myofasc* OR temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica OR back‐ache OR back*ache OR lumbago INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
8 (all also picked up in search 1) | |
| Clinicaltrials.gov | 23 October 2009 Search 3 |
Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OUTCOME: pain |
0 | |
| Clinicaltrials.gov | 23 October 2009 Search 4 |
Field ‐ Interventional studies CONDITION: fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph* OR sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash or polymyalg* OR failed back surg* OR failed back syndrome INTERVENTION: transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco* OUTCOME: pain |
0 | |
| TOTAL UNIQUE RESULTS FOR CLINICAL TRIALS.GOV | 62 | 7 | ||
| HSRProj (Health Services Research Projects in Progress) | 23 October 2009 | (chronic* or back or musculoskel* or intractabl* or neuropath* or phantom limb or fantom limb or neck or myofasc* or temp?romandib joint or central or post*stroke or complex or regional or spinal cord or sciatica or back‐ache or back*ache or lumbago or fibromyalg* or trigem* neuralg* or herp* neuralg* or diabet* neuropath* or reflex dystroph* or sudeck* atroph* or causalg* or whip‐lash or whip*lash or polymyalg* or failed back surg* or failed back syndrome) AND (brain* or cortex or cortical or transcranial* or cranial or magneti* or direct current or DC or electric or crani* or electrostim* or electrotherap* or electro‐therap* or non‐invasive or non*invasive or theta burst stimulat* or iTBS or Ctbs or transcranial magnetic stimulation or rTMS or transcranial direct current stimulation or tDCS or cranial electrostimulation or cranial electrotherapy or electrosleep or electronarco*) | 77 | 0 |
| Current Controlled Trials | 23 October 2009 Search 1 |
(sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (cranial electrotherapy OR electrosleep OR electronarco*) | 0 | |
| Current Controlled Trials | 23 October 2009 Search 2 |
(sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (Ctbs OR transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation) | 0 | |
| Current Controlled Trials | 23 October 2009 Search 3 |
(sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS) | 4 | |
| Current Controlled Trials | 23 October 2009 Search 4 |
(sudeck* atroph* OR causalg* OR whip‐lash OR whip*lash OR polymyalg* OR failed back surg* OR failed back syndrome) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC) | 13 | |
| Current Controlled Trials | 23 October 2009 Search 5 |
(back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 0 | |
| Current Controlled Trials | 23 October 2009 Search 6 |
(back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (Ctbs OR transcranial magnetic stimulation OR rTMS OR transcranial direct current stimulation OR tDCS ) | 9 | |
| Current Controlled Trials | 3 November 2009 Search 7 |
(back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (crani* OR electrostim* OR electrotherap* OR electro‐therap*) | 36 | |
| Current Controlled Trials | 23 October 2009 Search 8 |
(back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS) | 53 | |
| Current Controlled Trials | 3 November 2009 Search 9 |
(back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (cranial OR magneti* OR direct current OR DC) | 52 | |
| Current Controlled Trials | 3 November 2009 Search 10 |
(back‐ache OR back*ache OR lumbago OR fibromyalg* OR trigem* neuralg* OR herp* neuralg* OR diabet* neuropath* OR reflex dystroph*) AND (brain* OR cortex OR cortical OR transcranial*) | 63 | |
| Current Controlled Trials | 3 November 2009 Search 11 |
(temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 0 | |
| Current Controlled Trials | 3 November 2009 Search 12 |
(temp?romandib joint OR central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (transcranial direct current stimulation OR tDCS) | 11 | |
| Current Controlled Trials | 3 November 2009 Search 13 |
(central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (iTBS OR cTBS OR transcranial magnetic stimulation OR rTMS) | 48 | |
| Current Controlled Trials | 3 November 2009 Search 14 |
(central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat*) | 199 | |
| Current Controlled Trials | 3 November 2009 Search 15 |
(central OR post*stroke OR complex OR regional OR spinal cord OR sciatica) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR crani* OR electrostim*) | 1905 | |
| Current Controlled Trials | 3 November 2009 Search 16 |
(temp?romandib joint) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap* OR electro‐therap*) | 0 | |
| Current Controlled Trials | 3 November 2009 Search 17 |
(temp?romandib joint) AND (iTBS OR cTBS OR transcranial magnetic stimulation OR rTMS) | 0 | |
| Current Controlled Trials | 3 November 2009 Search 18 |
(temp?romandib joint) AND (non‐invasive OR non*invasive OR theta burst stimulat*) | 0 | |
| Current Controlled Trials | 3 November 2009 Search 19 |
(chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 16 | |
| Current Controlled Trials | 3 November 2009 Search 20 |
(chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (Ctbs OR transcranial magnetic stimulation OR Rtms) |
55 | |
| Current Controlled Trials | 3 November 2009 Search 21 |
(chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (crani* OR electrostim* OR electrotherap* OR electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS) | 557 | |
| Current Controlled Trials | 3 November 2009 Search 22 |
(chronic* OR back OR musculoskel* OR intractabl* OR neuropath* OR phantom limb OR fantom limb OR neck) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC) |
2385 | |
| Current Controlled Trials | 3 November 2009 Search 23 |
(temp*romandibular joint) AND (brain* OR cortex OR cortical OR transcranial* OR cranial OR magneti* OR direct current OR DC OR electric OR crani* OR electrostim* OR electrotherap*) | 8 | |
| Current Controlled Trials | 3 November 2009 Search 24 |
(temp*romandibular joint) AND (electro‐therap* OR non‐invasive OR non*invasive OR theta burst stimulat* OR iTBS OR Ctbs OR transcranial magnetic stimulation) | 1 | |
| Current Controlled Trials | 3 November 2009 Search 25 |
(temp*romandibular joint) AND (rTMS OR transcranial direct current stimulation OR tDCS OR cranial electrostimulation OR cranial electrotherapy OR electrosleep OR electronarco*) | 0 | |
| TOTAL RESULTS FOR CURRENT CONTROLLED TRIALS | 5415 | 14 | ||
| TOTAL RESULTS FROM ALL DATABASES | 23 | |||
| DUPLICATES BETWEEN DATABASES | 7 | |||
| FINAL TOTAL FROM TRIALS REGISTERS SEARCHES | 16 |
Data and analyses
Comparison 1.
Repetitive transcranial magnetic stimulation (rTMS)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: short‐term follow‐up | 27 | Std. Mean Difference (Fixed, 95% CI) | ‐0.22 [‐0.29, ‐0.16] | |
| 1.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | 0.13 [‐0.03, 0.28] | |
| 1.2 High‐frequency ≥ 5 Hz | 25 | Std. Mean Difference (Fixed, 95% CI) | ‐0.30 [‐0.37, ‐0.23] | |
| 2 Pain: short‐term follow‐up, subgroup analysis: multiple‐dose vs single‐dose studies | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| 2.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.36, ‐0.10] | |
| 2.2 Multiple‐dose studies | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.76, ‐0.05] | |
| 3 Pain: short‐term follow‐up, subgroup analysis, neuropathic pain participants only | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.20 [‐0.28, ‐0.13] | |
| 3.1 Low‐frequency ≤ 1 Hz | 5 | Std. Mean Difference (Fixed, 95% CI) | 0.15 [‐0.02, 0.32] | |
| 3.2 High‐frequency ≥ 5 Hz | 17 | Std. Mean Difference (Fixed, 95% CI) | ‐0.28 [‐0.36, ‐0.20] | |
| 4 Pain: short‐term follow‐up, subgroup analysis, non‐neuropathic pain participants only | 8 | Std. Mean Difference (Fixed, 95% CI) | ‐0.39 [‐0.61, ‐0.17] | |
| 4.1 Low‐frequency ≤ 1 Hz | 1 | Std. Mean Difference (Fixed, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 4.2 High‐frequency ≥ 5 Hz | 7 | Std. Mean Difference (Fixed, 95% CI) | ‐0.56 [‐0.81, ‐0.31] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.51, ‐0.22] | |
| 5.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.38 [‐0.49, ‐0.27] | |
| 5.2 Multiple‐dose studies | 8 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.73, 0.05] | |
| 6 Sensitivity analysis ‐ imputed correlation coefficient increased. Pain: short‐term follow‐up | 29 | Std. Mean Difference (Random, 95% CI) | ‐0.27 [‐0.40, ‐0.14] | |
| 6.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.15 [0.01, 0.29] | |
| 6.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.49, ‐0.22] | |
| 7 Sensitivity analysis ‐ imputed correlation coefficient decreased. Pain: short‐term follow‐up | 28 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.40, ‐0.13] | |
| 7.1 Low‐frequency ≤ 1 Hz | 7 | Std. Mean Difference (Random, 95% CI) | 0.13 [‐0.06, 0.33] | |
| 7.2 High‐frequency ≥ 5 Hz | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.34 [‐0.49, ‐0.19] | |
| 8 Sensitivity analysis ‐ imputed correlation increased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.50, ‐0.24] | |
| 8.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.39 [‐0.50, ‐0.28] | |
| 8.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.33 [‐0.71, 0.04] | |
| 9 Sensitivity analysis ‐ imputed correlation decreased. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded | 20 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.52, ‐0.22] | |
| 9.1 Single‐dose studies | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.47, ‐0.26] | |
| 9.2 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.81, 0.09] | |
| 10 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up | 31 | Std. Mean Difference (Fixed, 95% CI) | ‐0.27 [‐0.34, ‐0.20] | |
| 10.1 Low‐frequency ≤ 1 Hz | 10 | Std. Mean Difference (Fixed, 95% CI) | 0.07 [‐0.07, 0.22] | |
| 10.2 High‐frequency ≥ 5 Hz | 28 | Std. Mean Difference (Fixed, 95% CI) | ‐0.36 [‐0.44, ‐0.29] | |
| 11 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, low‐frequency studies excluded | 24 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.55, ‐0.26] | |
| 11.1 Single‐dose studies | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.35 [‐0.46, ‐0.24] | |
| 11.2 Multiple‐dose studies | 10 | Std. Mean Difference (Random, 95% CI) | ‐0.53 [‐0.91, ‐0.15] | |
| 12 Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.67 [‐1.48, 0.15] | |
| 12.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.16 [‐0.29, 0.61] | |
| 12.2 High frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.92 [‐1.95, 0.12] | |
| 13 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up, subgroup analysis: prefrontal cortex studies only | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 13.1 Multiple‐dose studies | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.64 [‐1.36, 0.08] | |
| 14 Pain: short term responder analysis 30% pain reduction | 2 | 89 | Risk Ratio (M‐H, Random, 95% CI) | 2.11 [1.17, 3.80] |
| 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐1.01, 0.17] | |
| 16 Pain: medium‐term follow‐up | 11 | Std. Mean Difference (Random, 95% CI) | ‐0.28 [‐0.61, 0.05] | |
| 16.1 Low‐frequency ≤ 1 Hz | 2 | Std. Mean Difference (Random, 95% CI) | 0.14 [‐0.41, 0.69] | |
| 16.2 High‐frequency ≥ 5 Hz | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.36 [‐0.73, 0.00] | |
| 17 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up | 15 | Std. Mean Difference (Random, 95% CI) | ‐0.50 [‐0.80, ‐0.20] | |
| 17.1 Low‐frequency ≤ 1 Hz | 3 | Std. Mean Difference (Random, 95% CI) | 0.02 [‐0.52, 0.56] | |
| 17.2 High‐frequency ≥ 5 Hz | 13 | Std. Mean Difference (Random, 95% CI) | ‐0.57 [‐0.90, ‐0.25] | |
| 18 Pain: medium‐term follow‐up, subgroup analysis: motor cortex studies only | 6 | Std. Mean Difference (Random, 95% CI) | ‐0.22 [‐0.46, 0.02] | |
| 18.1 Low frequency ≤ 1Hz | 1 | Std. Mean Difference (Random, 95% CI) | ‐0.08 [‐0.86, 0.70] | |
| 18.2 High‐frequency ≥ 5 Hz | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.49, 0.03] | |
| 19 Pain: medium‐term follow‐up, subgroup analysis: prefrontal cortex studies only | 5 | Std. Mean Difference (Random, 95% CI) | ‐1.08 [‐2.49, 0.32] | |
| 19.1 Low frequency ≤ 1 Hz | 1 | Std. Mean Difference (Random, 95% CI) | 0.36 [‐0.41, 1.13] | |
| 19.2 High‐frequency ≥ 5 Hz | 4 | Std. Mean Difference (Random, 95% CI) | ‐1.74 [‐3.66, 0.19] | |
| 20 Pain: long‐term follow‐up | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.14 [‐0.44, 0.17] | |
| 21 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: long‐term follow‐up | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.89, 0.10] | |
| 22 Disability: short‐term follow‐up | 5 | Std. Mean Difference (Random, 95% CI) | ‐0.29 [‐0.87, 0.29] | |
| 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up | 7 | Std. Mean Difference (Random, 95% CI) | ‐0.30 [‐0.72, 0.12] | |
| 24 Disability: medium‐term follow‐up | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐1.07, 0.33] | |
| 25 Pain: short term responder analysis 50% pain reduction | 1 | 54 | Risk Ratio (M‐H, Random, 95% CI) | 1.89 [1.03, 3.47] |
| 26 Disability: long‐term follow‐up | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.23 [‐0.62, 0.16] | |
| 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.41 [‐0.87, 0.05] | |
| 28 Quality of life: short‐term follow‐up (Fibromyalgia Impact Questionnaire) | 4 | 105 | Mean Difference (IV, Random, 95% CI) | ‐10.80 [‐15.04, ‐6.55] |
| 29 Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) | 4 | 105 | Mean Difference (IV, Fixed, 95% CI) | ‐11.49 [‐16.73, ‐6.25] |
| 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire) | 5 | 143 | Mean Difference (IV, Fixed, 95% CI) | ‐8.93 [‐13.49, ‐4.37] |
| 31 Quality of life: long‐term follow‐up | 2 | 51 | Mean Difference (IV, Fixed, 95% CI) | ‐6.78 [‐13.43, ‐0.14] |
| 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up | 3 | 89 | Mean Difference (IV, Fixed, 95% CI) | ‐8.58 [‐13.84, ‐3.33] |
Analysis 1.15.
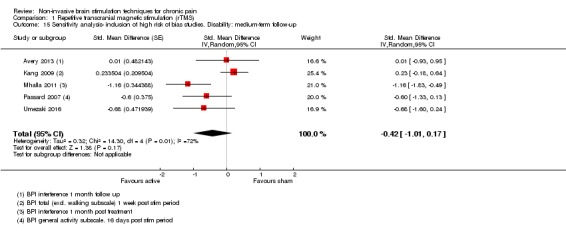
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 15 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: medium‐term follow‐up.
Analysis 1.23.
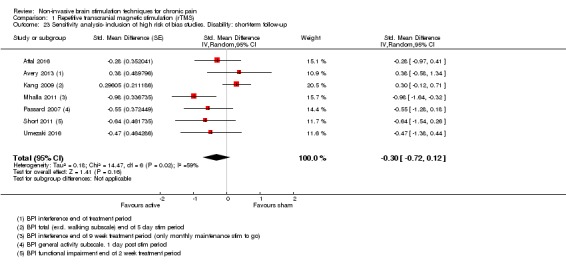
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 23 Sensitivity analysis‐ inclusion of high risk of bias studies. Disability: short‐term follow‐up.
Analysis 1.27.
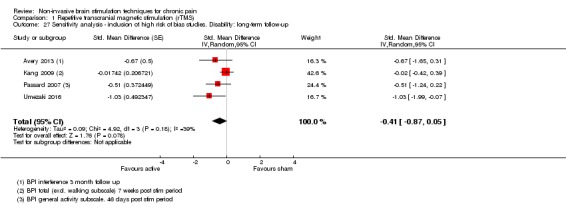
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 27 Sensitivity analysis ‐ inclusion of high risk of bias studies. Disability: long‐term follow‐up.
Analysis 1.30.
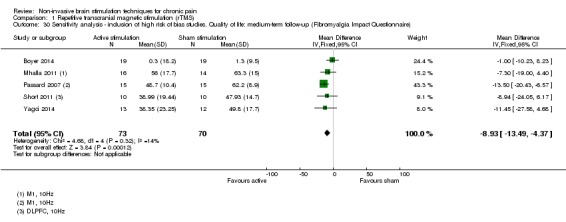
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 30 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: medium‐term follow‐up (Fibromyalgia Impact Questionnaire).
Analysis 1.32.
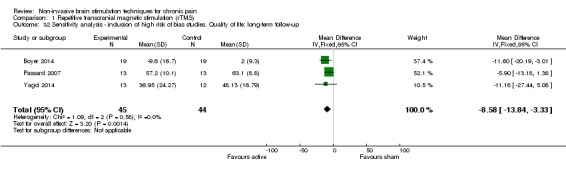
Comparison 1 Repetitive transcranial magnetic stimulation (rTMS), Outcome 32 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: long‐term follow‐up.
Comparison 2.
Cranial electrotherapy stimulation (CES)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: short‐term follow‐up | 5 | 270 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.24 [‐0.48, 0.01] |
| 2 Quality of life: short term follow up | 1 | Mean Difference (IV, Random, 95% CI) | Subtotals only |
Comparison 3.
Transcranial direct current stimulation (tDCS)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: short‐term follow‐up | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.63, ‐0.22] | |
| 1.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 1.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.51 [‐0.77, ‐0.25] | |
| 2 Pain: short‐term sensitivity analysis: correlation increased | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.62, ‐0.23] | |
| 3 Pain: short‐term sensitivity analysis: correlation decreased | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.44 [‐0.64, ‐0.23] | |
| 4 Pain: short term sensitivity analysis, inclusion of high risk of bias studies | 31 | Std. Mean Difference (Random, 95% CI) | ‐0.48 [‐0.67, ‐0.29] | |
| 4.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 4.2 Multiple‐dose studies | 27 | Std. Mean Difference (Random, 95% CI) | ‐0.56 [‐0.79, ‐0.32] | |
| 5 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.47 [‐0.67, ‐0.28] | |
| 5.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.02] | |
| 5.2 Multiple‐dose studies | 21 | Std. Mean Difference (Random, 95% CI) | ‐0.58 [‐0.84, ‐0.33] | |
| 6 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation increased | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.64, ‐0.26] | |
| 6.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.37, 0.01] | |
| 6.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.55 [‐0.81, ‐0.30] | |
| 7 Pain: short‐term follow‐up, subgroup analysis: motor cortex studies only, sensitivity analysis: correlation decreased | 26 | Std. Mean Difference (Random, 95% CI) | ‐0.40 [‐0.58, ‐0.22] | |
| 7.1 Single‐dose studies | 4 | Std. Mean Difference (Random, 95% CI) | ‐0.18 [‐0.38, 0.03] | |
| 7.2 Multiple‐dose studies | 22 | Std. Mean Difference (Random, 95% CI) | ‐0.49 [‐0.72, ‐0.26] | |
| 8 Pain: short‐term follow‐up, subgroup analysis, neuropathic and non neuropathic pain | 25 | Std. Mean Difference (Random, 95% CI) | ‐0.37 [‐0.56, ‐0.19] | |
| 8.1 Neuropathic | 9 | Std. Mean Difference (Random, 95% CI) | ‐0.26 [‐0.53, 0.01] | |
| 8.2 Non neuropathic | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.42 [‐0.67, ‐0.17] | |
| 9 Pain: short term follow‐up responder analysis 30% pain reduction | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 10 Pain: short term follow‐up responder analysis 50% pain reduction | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 11 Pain: medium‐term follow‐up | 14 | Std. Mean Difference (Random, 95% CI) | ‐0.43 [‐0.72, ‐0.13] | |
| 12 Pain: medium term follow‐up responder analysis 30% pain reduction | 1 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 13 Pain: medium term follow‐up responder analysis 50% pain reduction | 2 | Risk Ratio (M‐H, Random, 95% CI) | Subtotals only | |
| 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up | 16 | Std. Mean Difference (Random, 95% CI) | ‐0.45 [‐0.72, ‐0.18] | |
| 15 Pain: long‐term follow‐up | 3 | Std. Mean Difference (Random, 95% CI) | ‐0.01 [‐0.43, 0.41] | |
| 16 Disability: short‐term follow‐up | 4 | 212 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.01 [‐0.28, 0.26] |
| 17 Disability: medium‐term follow‐up | 1 | Std. Mean Difference (IV, Random, 95% CI) | Subtotals only | |
| 18 Quality of life: short‐term follow‐up | 4 | 82 | Std. Mean Difference (IV, Random, 95% CI) | 0.66 [0.21, 1.11] |
| 19 Quality of life: medium‐term follow‐up | 3 | 87 | Std. Mean Difference (IV, Random, 95% CI) | 0.34 [‐0.09, 0.76] |
Analysis 3.14.
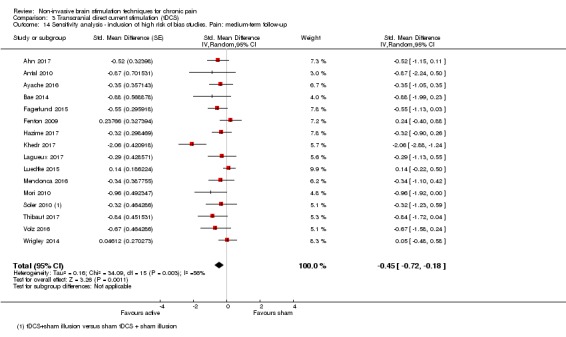
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 14 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: medium‐term follow‐up.
Analysis 3.17.
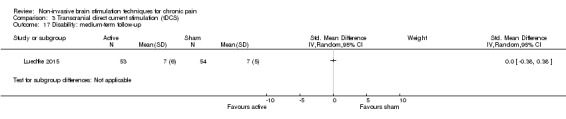
Comparison 3 Transcranial direct current stimulation (tDCS), Outcome 17 Disability: medium‐term follow‐up.
Comparison 4.
Reduced impedance non‐invasive cortical electrostimulation (RINCE)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain: short‐term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.59 [‐0.99, ‐0.18] |
| 3 Quality of Life: short term follow‐up | 1 | Mean Difference (IV, Fixed, 95% CI) | Subtotals only | |
| 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up | 2 | 115 | Std. Mean Difference (IV, Random, 95% CI) | ‐0.45 [‐0.91, 0.02] |
Analysis 4.2.
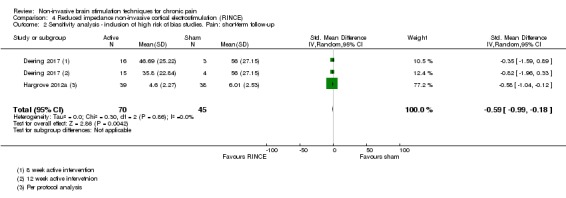
Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 2 Sensitivity analysis ‐ inclusion of high risk of bias studies. Pain: short‐term follow‐up.
Analysis 4.4.
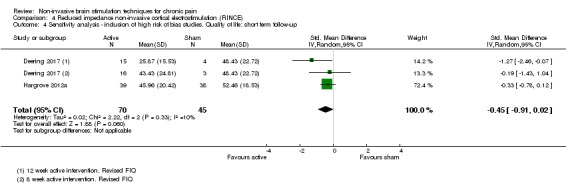
Comparison 4 Reduced impedance non‐invasive cortical electrostimulation (RINCE), Outcome 4 Sensitivity analysis ‐ inclusion of high risk of bias studies. Quality of life: short term follow‐up.
Comparison 5.
Transcranial random noise stimulation
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Pain | 1 | Std. Mean Difference (Fixed, 95% CI) | ‐0.19 [‐0.64, 0.26] |
What's new
Last assessed as up‐to‐date: 11 October 2017.
| Date | Event | Description |
|---|---|---|
| 7 November 2017 | New citation required but conclusions have not changed | We have updated all analyses and GRADE quality assessments for all core comparisons. The addition of data has not substantially altered our conclusions that there remains substantial uncertainty regarding the effectiveness of non invasive brain stimulation techniques for chronic pain. |
| 11 October 2017 | New search has been performed | We have performed a full update of the searches (October 2017). This involved the inclusion of 38 new trials with an additional 1225 participants. |
History
Protocol first published: Issue 1, 2010 Review first published: Issue 9, 2010
| Date | Event | Description |
|---|---|---|
| 11 February 2013 | New search has been performed | For this update we have altered the 'Risk of bias' assessment to reflect new evidence regarding the adequacy of blinding of studies of tDCS and we have included the following new 'Risk of bias' criteria: sample size and study duration. Details of this can be found in the sections: Assessment of risk of bias in included studies and Description of the intervention. We have also applied the GRADE approach to assessing the quality of evidence. |
| 13 September 2010 | Amended | We amended the 'Risk of bias' tables so that the criterion "allocation concealment" is not assessed for studies with cross‐over designs and the criterion "free from carry‐over effects?" is not assessed for studies with parallel designs. These changes are now reflected in Figure 2, where those criteria now appear as empty boxes for the appropriate studies. This is in line with the original review protocol and the changes are necessary due to a copy‐editing error rather than any change to the review methods. |
Differences between protocol and review
For this update
For this update we searched ClinialTrials.gov and the World Health Organization International Clinical Trials Registry Platform, as these searches offer superior coverage to those outlined in our original protocol, and because the meta‐register of controlled trials is no longer operational. We assessed the quality of the body of evidence using GRADE and added three 'Summary of findings' tables.
For the 2014 update
We did not search the database Scopus in the 2014 update or this update as the other searches had covered the full scope of this database.
In compliance with new author guidelines from Cochrane Pain, Palliative and Supportive Care and the recommendations of Moore 2010 we added two criteria, 'study size' and 'study duration', to our 'Risk of bias' assessment using the thresholds for judgement suggested by Moore 2010:
size (we rated studies with fewer than 50 participants per arm as being at high risk of bias, those with between 50 and 199 participants per arm at unclear risk of bias, and 200 or more participants per arm at low risk of bias);
duration (we rated studies with follow‐up of less than two weeks as being at high risk of bias, two to seven weeks at unclear risk of bias and eight weeks or longer at low risk of bias).
For the 2010 update
As described in detail in Unit of analysis issues, on advice from a Cochrane statistician we meta‐analysed parallel and cross‐over studies using the generic inverse variance method rather than combining them without this statistical adjustment as was specified in the protocol. Subsequently the planned sensitivity analysis investigating the influence of study design was not deemed necessary. However on advice from a Cochrane statistician we performed a sensitivity analysis to assess the impact of our approach to imputation of standard errors for cross‐over studies.
In order to meet our second objective of considering the influence of varying stimulation parameters, we included studies regardless of the number of stimulation sessions delivered, including single‐dose studies.
The following decision was taken on encountering multiple outcomes within the same time period: for short‐term outcomes where more than one data point was available, we used the first post‐stimulation measure; where multiple treatments were given, we took the first outcome at the end of the treatment period. For medium‐term outcomes where more than one data point was available we used the measure that was closest to the mid‐point of this time period. We decided to pool data from studies with a low or unclear risk of bias as we felt that the analysis specified in the protocol (including only those studies with an overall low risk of bias) was too stringent and would not allow any statistical assessment of the data.
We did not use overall risk of bias in sensitivity analyses as we found that it lacked sensitivity. Instead we considered individual criteria in the 'Risk of bias' assessment for sensitivity analyses. However, we excluded studies with a 'high' risk of bias for any criterion from the meta‐analysis except study size and study duration.
For this update we have altered the 'Risk of bias' assessment to reflect new evidence regarding the adequacy of blinding of studies of tDCS. Details of this can be found in Assessment of risk of bias in included studies and Description of the intervention.
Characteristics of studies
Characteristics of included studies [ordered by study ID]
| Methods | Parallel, quasi‐RCT | |
| Participants | Country of study: Egypt Setting: Dept of Neurology, hospital‐based Condition: chronic phantom limb pain Prior management details: unresponsive to various pain medications n = 27, 17 active and 10 sham Age, mean (SD): active group 52.01 (12.7) years, sham group 53.3 (13.3) years Duration of symptoms, mean (SD) months: active group 33.4 (39.3), sham group 31.9 (21.9) Gender distribution: active group 13 M, 4 F; sham group 6 M, 4 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 20 Hz; coil orientation not specified, number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 2000 Stimulation location: M1 stump region Number of treatments: x 5, daily Control type: sham ‐ coil angled away from scalp |
|
| Outcomes | Primary: pain VAS (anchors not reported), LANNS When taken: poststimulation session 1 and 5 and at 1 month and 2 months post‐treatment Secondary: none relevant |
|
| Notes | AEs: not reported COI: not reported Sources of support: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Comment: not true randomisation Quote: "patients were randomly assigned to 2 groups depending on the day of the week on which they were recruited" |
| Allocation concealment (selection bias) | High risk | Comment: given method of randomisation allocation concealment not viable |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ suboptimal. Coil angled away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "The second author evaluated these measures blindly, without knowing the type of TMS" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: levels of dropout not reported |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes presented in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | > 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: OA knee Prior management details: not reported n = 41 randomised, 40 analysed Age, mean (SD): active group 60.6 (9.8) years, sham group 59.3 (8.6) years Duration of symptoms: not reported Gender distribution: 19 M, 21 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS 2mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 1 daily for 5 days Control type: sham tDCS |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain imaginable When taken: 1 d postintervention, 3 weeks postintervention Secondary: WOMAC function score AEs |
|
| Notes | Funding source: supported in part by the Claude D. Pepper Older American's Independence Center (P30 AG028740), the Universityof Florida Center for Cognitive Aging and Memory, and NIA Grants K07AG04637 and K01AG050707, and R01AG054077. This Work was also partially supported by VA HSR&D Houston Center for Innovations in Quality, Effectiveness and Safety (CIN# 13‐413), Michael E. DeBakey VA Medical Center, Houston, TX. COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “randomly assigned with a ratio of 1 to 1 to either the active tDCS (n ¼ 20) or sham tDCS group (n ¼ 20) using a covariate adaptive randomization procedure so that the two groups had approximately equal distribution regarding age, gender and race.” |
| Allocation concealment (selection bias) | Low risk | Quote “Allocation concealment was ensured as the randomization codes were released only after all the interventions and assessments were completed.” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that participant blinding can be inadequate at intensity of 2 mA. No assessment of blinding success. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No assessment of blinding success. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only one participant withdrew. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | Unclear risk | Comment: 3‐week follow‐up |
| Other bias | Unclear risk | Comment: statistically significant between‐group difference in pain NRS scores at baseline |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management, candidates for invasive MCS n = 14 Age: 31‐66 years; mean 53 (SD 11) Duration of symptoms: mean 6.9 years (SD 4) Gender distribution: 10 M, 4 F |
|
| Interventions | Stimulation type: rTMS figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 20 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Condition 2: frequency 1 Hz; coil orientation lateromedial; number of trains 1; duration of trains 26 min, total number of pulses 1600 Condition 3: sham ‐ same as for condition 2 with coil angled away perpendicular to scalp Stimulation location: M1 contralateral to painful side Number of treatments: 1 for each condition |
|
| Outcomes | Primary: VAS 0‐10 cm, anchors "no pain" to "unbearable pain" When taken: immediately poststimulation then daily for 1 week Secondary: none |
|
| Notes | Data requested from study authors and received Sources of support: Supported in part by a Grant from the Fondation pour la Recherche Médicale (FRM), France COI: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Participants were consecutively assigned to a randomization scheme generated on the web site Randomization.com (Dallal GE, http://www.randomization.com, 2008). We used the second generator, with random permutations for a 3‐group trial. The randomization sequence was concealed until interventions were assigned." |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment 'suboptimal'. Coil angled away from scalp and not in contact in sham condition. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "To ensure the double‐blind evaluation effects, the physician applying magnetic stimulation was different from the one collecting the clinical data, who in turn was not aware of the modality of rTMS that had been used in each session." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | 2 participants lost to follow‐up and not accounted for in the data analysis. Given the small sample size it may influence the results |
| Selective reporting (reporting bias) | Low risk | Pain outcomes reported for all participants. Change from baseline figures given; point measures requested from study authors and received |
| Free from carry‐over effects? | Low risk | Comment: a 2‐week washout period was observed between stimulation conditions and possible carry‐over effects were checked and ruled out in the analysis |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: France Setting: laboratory‐based Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management, candidates for invasive MCS n = 30 Age: 31‐72 years, mean 55 (SD 10.5) Duration of symptoms: mean 5 years (SD 3.9) Gender distribution: 23 M, 7 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 20 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Condition 2: frequency 20 Hz, coil orientation lateromedial; number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Condition 3: sham ‐ same as for active conditions with coil angled away perpendicular to scalp Stimulation location: M1 contralateral to painful side Number of treatments: 1 for each condition |
|
| Outcomes | Primary: 0‐10 NRS (anchors "no pain" to "unbearable pain") When taken: daily for 2 weeks poststimulation Secondary: none |
|
| Notes | Data requested from study authors Sources of support: supported in part by a Grant from the Fondation pour la Recherche Médicale (FRM), France COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the order of sessions was randomised (by computerized random‐number generation)" |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled away from scalp and not in contact in sham condition. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "The physician who applied the procedure received from a research assistant one sealed envelope containing the order of the rTMS sessions for a given patient. The order remained unknown to the physician collecting clinical data." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 participants apparently lost to follow‐up and not obviously accounted for in the analysis. However, this is less than 10% and is unlikely to have strongly influenced the results |
| Selective reporting (reporting bias) | Low risk | Comment: medial‐lateral coil orientation condition data not presented but provided by study authors on request |
| Free from carry‐over effects? | Low risk | Comment: a 2‐week washout period was observed between stimulation conditions and possible carry‐over effects were checked and ruled out in the analysis |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory‐based Condition: chronic neuropathic pain (mixed) Prior management details: resistant to conventional pharmacological treatment n = 45 Age: 31‐72 years (mean 55) Duration of symptoms: "chronic" Gender distribution: 28 M, 17 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 20 Hz; coil orientation not specified, number of trains 20; duration of trains 4 s; ITI 84 s; total number of pulses 1600 Stimulation location: M1 hand area Number of treatments: 1 per group Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = unbearable pain When taken: daily for 2 weeks following each stimulation Secondary: none relevant |
|
| Notes | AEs: not reported Funding source: charity‐funded COI: declaration ‐ no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less likely to introduce bias in a cross‐over design Quote: "separated into 2 groups determined by the randomization" |
| Adequate blinding of participants? | Unclear risk | Comment: the study authors state "Because the first step of the procedure (motor hotspot and motor threshold determination) that induced motor contractions was identical in placebo and active sessions and the stimulation differed only when intensities below motor threshold were applied, no patient perceived any difference between the 2 types of rTMS" However, the sensation on the scalp may differ and no formal evaluation of blinding presented |
| Adequate blinding of assessors? | Unclear risk | Comment: no mention of blinded assessors |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of dropout/withdrawal |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported for all groups and further data made available upon request to authors |
| Free from carry‐over effects? | Low risk | Comment: 2‐week washout period observed |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: laboratory setting Condition: mixed chronic pain, neuropathic and non‐neuropathic Prior management details: therapy‐resistant n = 23, 10 in parallel (6 active, 4 sham), 13 crossed over Age: active‐only group 28‐70 years, sham‐only group 50‐70 years, cross‐over group 41‐70 years Duration of symptoms: chronic 1.5‐25 years (mean 7.4) Gender distribution: 6 M, 17 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ L M1 hand area, cathode right supraorbital Number of treatments: x 5, daily Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS 0‐10; VAS anchors 0 = no pain, 10 = the worst pain possible When taken: x 3, daily ‐ averaged for daily pain Secondary: none relevant |
|
| Notes | Funding: government funding COI: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "Randomization was performed using the order of entrance into the study." Comment: may not be truly random from description |
| Allocation concealment (selection bias) | Unclear risk | Comment: not mentioned though unlikely given the randomisation technique. This is a potentially significant source of bias given that only the parallel results were used in this review due to high levels of attrition after the first phase |
| Adequate blinding of participants? | Low risk | Comment: see above |
| Adequate blinding of assessors? | Low risk | Comment: 1 mA intensity and operator blinded Quote: "The stimulators were coded using a five letter code, programmed by one of the department members who otherwise did not participate in the study. Therefore neither the investigator not the patient knew the type of the stimulation" |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: the high level of dropout renders the cross‐over results at high risk of bias. This is less of an issue where only the parallel results from the first phase were used ‐ first‐phase data only used in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: while not all outcomes at all time points were included in the study report the authors have provided all requested data |
| Free from carry‐over effects? | Low risk | Comment: participants were excluded if pain had not returned to normal. This, however, represents a threat with regard to attrition bias |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other sources of bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: hospital pain units Condition: lumbar radicular pain Prior management details: stable pharmacological treatment for pain and sleep disorders for at least 1 month prior to study n = 36 Age, mean (SD): active group 53.4 (8) years, sham group 51.5 (13) years Duration of symptoms: not reported Gender distribution: 17 F 18 M |
|
| Interventions | Stimulation type: rTMS and tDCS (order randomised in active group) Stimulation parameters: rTMS frequency 10 Hz; coil orientation anteroposterior induced current; 80% RMT; number of trains 30; duration of trains 10 s; ITI 20 s; total number of pulses 3000 tDCS: 2 mA intensity, 30 min Stimulation location: M1 contralateral to painful side Number of treatments: 3 stimulation visits on 3 consecutive days for each stimulation type. 3 week washout period. Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = maximal pain imaginable When taken: postintervention Secondary: BPI interference scale AEs |
|
| Notes | Funding source: The study received financial support from the Institut National de la Sante´ et de la Recherche Médicale (INSERM) COI: the authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The 2 successive randomisations were prepared by a study nurse not involved in the running of the study or in data analysis, using validated software and a centralised randomisation schedule.” |
| Allocation concealment (selection bias) | Low risk | Quote: “The treatment allocation code was kept in a sealed envelope until the completion of the study.” |
| Adequate blinding of participants? | Unclear risk | Comment: rTMS sham described as controlling for sensory, auditory and visual cues. tDCS 2 mA intensity ‐ evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | tDCS 2 mA intensity ‐ evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: ITT analysis used and low dropout |
| Selective reporting (reporting bias) | High risk | Comment: point estimates for pain scores not provided ‐ only a responder analysis was presented |
| Free from carry‐over effects? | Unclear risk | Comment: the order of active stimulation types was randomised but it is not clear that there were not baseline differences between pre‐rTMS and pre tDCS from the presented data |
| Study Size | High risk | n = 36 |
| Study duration | High risk | Comment: 5 days post intervention was the longest follow up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: unclear Condition: chronic widespread pain Prior management details: not reported n = 19 Age mean (SD): active 54.86 (7.65) years, sham 52.09 (10.02) years Duration of symptoms (months mean (SD)): active group 11 (4.26), sham group 15.64 (6.93) Gender distribution: all F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation not specified; 120% RMT; number of trains 75; duration of trains 4 s; ITI 26 s; total number of pulses 3000 Stimulation location: L DLPFC Number of treatments: 15 sessions over 4 weeks Control type: sham coil ‐ controls for visual, auditory and scalp sensory cues |
|
| Outcomes | Primary: pain NRS 0‐10 anchors not reported When taken: end of treatment period, 1 month following and 3 months following Secondary: pain interference BPI QoL SF‐36 AEs: multiple minor; no clear difference in incidence between active and sham stimulation |
|
| Notes | Government‐funded study, manufacturer loaned stimulators COI: funded by the National Institute for Arthritis, Musculoskeletal and Skin Diseases, R21 ART053963 and the Bipolar Illness Fund Neuronetics, Inc. loaned the TMS machine to the study Dr. Avery was a consultant for Neuronetics, Inc. for one day, is a member of the Data and Safety Monitoring Board for Cerval Neuortech, Inc., was on the speakers bureau for Eli Lilly and Takeda, was a consultant for Takeda and received a grant from the National Institute of Mental Health. Dr. Roy‐Byrne is editor for Journal Watch, Depression and Anxiety, and UpToDate and has stock in Valant Medical Systems. None of the other authors has potential COI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "At the completion of the baseline assessment, patients were randomly assigned to either real TMS or sham stimulation using a computerized randomization program that uses an adaptive randomization and stratification strategy." |
| Allocation concealment (selection bias) | Unclear risk | Quote: "Based on the randomization, a "smart card" which determined whether the real TMS or sham coil would be administered was assigned to a particular patient. The card had only a code number that did not reveal the randomization." "The research coordinator blind to the randomization repeated the baseline assessments" Comment: not entirely clear whether the personnel overseeing randomisation was separate from that performing the screening assessment. |
| Adequate blinding of participants? | Low risk | Quote: "... sham stimulation with the electromagnet blocked within the coil by a piece of metal so the cortex was not stimulated. The coils appeared identical. Electrodes were attached to the left side of the forehead for each subject for each session. Those receiving the sham stimulation received an electrical stimulus to the forehead during the sham stimulation. Those receiving the real TMS received no electrical stimulation to the electrodes. Both groups experienced a sensation in the area of the left forehead. In addition, all subjects were given special earplugs and received an audible noise during the stimulation to mask any possible sound differences between the TMS and sham conditions." Comment: optimal sham ‐ controls for visual, sensory and auditory cues Formal testing ‐ blinding appears robust |
| Adequate blinding of assessors? | Low risk | Quote: "The research coordinator blind to the randomization repeated the baseline assessments of pain, functional status, depression, fatigue, and sleep before the 1st and after the 5th, the 10th, and the 15th TMS sessions as well as 1 week, 1 month, and 3 months after the last TMS treatment except for the SF‐36, neuropsychological tests, audiometry and the dolorimetry which were only done at baseline and one week after the 15th TMS session." Comment: while TMS physicians guessed beyond chance the raters were separate from this process |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "To examine differences in changes in outcomes over time between TMS and comparison group subjects, we estimated random coefficient models following the intent‐to‐treat principle." "11 were randomized to the sham group and 8 were randomized to the TMS group. However, one subject randomized to the TMS had a baseline BIRS score of 4 which was well below the BIRS score of 8 required for randomization. Because of this incorrect randomization, this subject was excluded from the efficacy analyses, but was included in the analysis of side effects. The clinical characteristics of those correctly randomized are in Table 1. One subject in the TMS dropped out after the 10th session because of lack of response and is included in the analyses." Comment: of 2 dropouts from the TMS group, 1 was excluded (reasons given) |
| Selective reporting (reporting bias) | Low risk | Comment: all outcomes presented in full in study report |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: > 8 weeks' follow‐up |
| Other bias | Low risk | No other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: MS‐related neuropathic pain Prior management details: concomitant medication intake stable throughout protocol n = 16 Age, mean (SD) 48.9 (10) years Duration of symptoms: mean (SD) 11.8 (9.4) months Gender distribution: 13 F, 3 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25 cm2 electrodes, duration 20 min Stimulation location: anode ‐ L DLPFC, cathode right supraorbital Number of treatments: x 3, daily Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS 0 ‐10; VAS anchors not reported When taken: Postintervention, 7 days postintervention Secondary: AEs |
|
| Notes | COI: "AC gave expert testimony for CSL Behring, Novartis, received grants from Biogen, Novartis, CSLBehring, GENeuro, Octapharma, and gave lectures for Genzyme. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships "that could be construed as potential conflict of interest" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “The randomization schedule was generated by U.P. prior to the beginning of the study using a dedicated software (“true”random number generation without any restriction, stored in a computer until the patient was assigned to the intervention).” |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity, particularly in cross‐over designs. Results of guessing mode of stimulation not reported |
| Adequate blinding of assessors? | Unclear risk | Quote: "Only the performing physician (S.S.A) was aware of the stimulation mode (real or sham tDCS). The evaluators (U.P and M.A.C) and the patients were blind to it.” Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition reported |
| Selective reporting (reporting bias) | Low risk | Comment: results reported in full |
| Free from carry‐over effects? | Unclear risk | Comment: baseline scores for each period not reported. No formal analysis for carry‐over effects presented |
| Study Size | High risk | Comment: n = 16 |
| Study duration | High risk | Comment: longest follow‐up 7 days after stimulation |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: South Korea Setting: laboratory Condition: CPSP Prior management details: not reported n = 14 Age, mean (SD): active group 51.1 (3.1) years, sham group 52.3 (2.8) years Duration of symptoms, mean (SD): active group 14.5 (3.2) months, sham group 14.7 (2.7) Gender distribution: 7 M, 7 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 contralateral to painful side, cathode right supraorbital Number of treatments: x 3 per week for 3 weeks Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS anchors 0 = no pain, 10 = unbearable When taken: "immediacy", 1 week, 3 weeks (unclear if from end of intervention) Secondary: None relevant |
|
| Notes | COI: study authors declared no COI Sources of support: none declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment procedures |
| Adequate blinding of participants? | Unclear risk | Comment: blinding not reported. Evidence that blinding can be inadequate at intensity of 2 mA |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding not reported. Evidence that blinding can be inadequate at intensity of 2 mA |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: unable to clearly verify if there was any attrition |
| Selective reporting (reporting bias) | Low risk | Comment: adequate reporting of outcomes |
| Study Size | High risk | Comment: total n = 14 |
| Study duration | Unclear risk | Comment: 3‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: Brazil Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management n = 8 Age: 40‐82 years; mean 63.3 (SD 5.6) Duration of symptoms: 1‐20 years; mean 8.3 (SD 5.6) Gender distribution: 2 M, 6 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 30 min Condition 1: active tDCS/active TENS Condition 2: active tDCS/sham TENS Condition 3: sham tDCS/sham TENS Stimulation location: M1 contralateral to painful side Number of treatments: 1 for each condition Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: VAS 0‐10 anchors "no pain" to "worst possible pain" When taken: pre and post each stimulation Secondary: none |
|
| Notes | Sources of support: not declared COI: not declared |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All the patients received the 3 treatments.... in a randomised order (we used a computer generated randomisation list with the order of entrance)." |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Quote: "All evaluations were carried out by a blinded rater" Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 2 participants lost to follow‐up. It is unclear how these data were accounted for as there were no missing data apparent in the results tables. However, this may have an impact given the small sample size |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome data presented clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: a 48‐h washout period was observed between stimulation conditions and possible carry‐over effects were checked and ruled out in the analysis Quote: "To analyze whether there was a carryover effect, we initially performed and showed that the baselines for the 3 conditions were not significantly different (P = 0.51). We also included the variable order in our model and this model also showed that order is not a significant term (P = 0.7)." |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT; 2 conditions | |
| Participants | Country of study: USA Setting: laboratory Condition: peripheral neuropathic pain Prior management details: not specified n = 4 Age: 33‐58 years; mean 46 (SD 11) Duration of symptoms: 5‐12 years; mean 10.25 (SD 3.5) Gender distribution: 1 M, 3 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation not specified; 100% RMT; number of trains 40; duration of trains 10 s; ITI 20 s; total number of pulses 4000 Stimulation location: L PFC Number of treatments: 3 over a 5‐d period Control type: neuronetics sham coil (looks and sounds identical) |
|
| Outcomes | Primary: average daily pain 0‐10 Likert scale, anchors "no pain at all" to "worst pain imaginable" When taken: post‐stimulation for each condition (unclear how many days post) and daily for 3 weeks poststimulation Secondary: none |
|
| Notes | AEs: not reported Sources of support: no separate statement provided COI: "Dr. Borckardt receives research funding from the National Institute for Neurological Disorders and Stroke at NIH, Cyberonics Inc, the Neurosciences Institute at MUSC, and is a consultant for Neuropace; however, he has no equity ownership in any device or pharmaceutical company. Dr. George receives research funding from the National Institute for Mental Health, NIDA, and NIAAA at NIH, Jazz Pharmaceuticals, GlaxoSmithKline, and Cyberonics Inc. He is a consultant for Aspect Biomedical, Argolyn, Aventis, Abbott, Bristol‐Meyers Squibb, Cephos, Cyberonics, and Neuropace; however, he has no equity ownership in any device or pharmaceutical company. Dr. Nahas receives research funding from the National Institute for Mental Health at NIH and Cyberonics Ind, and is a consultant for Neuropace. Dr. Kozel receives research funding from the National Institute for Mental Health at NIH and the U.S. Department of Defense. MUSC has filed six patents or invention disclosures in one or more of the authors’ names regarding brain imaging and stimulation." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order (real first or sham first) was randomised" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Quote: "Two of the four participants (50%) correctly guessed which treatment periods were real and sham, which is equal to chance. All four of the participants initially said that they did not know which was which, and it was not until they were pushed to "make a guess" that they were able to offer an opinion about which sessions were real and which were sham." Comments: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Unclear risk | Comment: not specified |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout |
| Selective reporting (reporting bias) | Low risk | Comment: all results reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: a 3‐week washout period was observed. Presented average pain values were very similar pre‐ each condition |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: specialised pain treatment centre Condition: fibromyalgia Prior management details: stable treatment for more than 1 month before enrolment n = 38 Age, mean (SD): active group 49.1(10.6) years, sham group 47.7 (10.4) years Duration of symptoms, mean (SD): active group 3.7 (4.5) years, sham group 3.6 (3.8) Gender distribution: 37 F, 1 M |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation anteroposterior; 90% RMT; number of trains 20; duration of trains 10 s; ITI 50 s; total number of pulses 2000 Stimulation location: L M1 Number of treatments: 14 sessions. 10 sessions in 2 weeks followed by maintenance phase of 1 session at weeks 4, 6, 8 and 10 Control type: sham coil ‐ did not control for sensory cues |
|
| Outcomes | Primary: pain VAS 0 = no pain, 10 = maximal pain imaginable When taken: 2 weeks, 11 weeks Secondary: FIQ AEs |
|
| Notes | Funding source: Supported by Inserm (Centre d’Investigation Clinique, CIC, Hôpital de la Conception, Marseille) and AP‐HM (AORC 2008/01) COI: the study authors report no disclosures relevant to the manuscript |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Individuals were randomized by a computer‐generated list…” |
| Allocation concealment (selection bias) | Low risk | Quote: “...which was maintained centrally so no investigators knew the treatment allocation of any patient.” |
| Adequate blinding of participants? | Unclear risk | Quote: “Sham stimulation was conducted with a sham coil of identical size, color, and shape, emitting a sound similar to that emitted by the active coil. Stimulations were administered by the same technologist.” Comments: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Low risk | Quote: “Patients and clinical raters were blinded to treatment” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Quote “All patients completed the induction phase, but 9 (23.7%) were excluded during the maintenance phase (3 in the active rTMS group and 6 in the sham rTMS group)“ Comment: dropout high, ITT analysis used but no information with regards imputation approach taken (or not) |
| Selective reporting (reporting bias) | Low risk | Comment: all results reported clearly and in full |
| Study Size | High risk | Comment: n = 38 |
| Study duration | High risk | Comment: no follow‐up after end of maintenance phase |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: hepatitis C‐related chronic pain Prior management details: not reported n = 28 Age, mean (SD): active group 53.86 (5.76) years, sham group 56.57 (8.52) years Duration of symptoms: not reported Gender distribution: 21 M, 7 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25‐35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 L, cathode right supraorbital Number of treatments: daily, x 5 Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS; anchors 0 = no pain, 10 = worst possible pain When taken: end of intervention Secondary: none relevant |
|
| Notes | Funding from Brazilian funding agencies: (i) Committee for the Development of Higher Education Personnel (ii) National Council for Scientific and Technological Development‐CNPq (iii) Postgraduate Program in Medical Sciences of Medical School of the Federal University of Rio Grande do Sul. (iv) Postgraduate Research Group at the Hospital de Clínicas de Porto Alegre (v) Laboratory of Neuromodulation & Center for Clinical Research Learning (vi) Foundation for Support of Research at Rio Grande do Sul |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomized numbers in a 1:1 ratio were generated using appropriate software (www.randomization.com) to assign each Participant to either active or sham‐placebo group.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Envelopes were prepared for randomization process and sealed. After subject’s agreement to participate in the trial, one investigator who was not involved with either stimulation or assessments opened the envelope. The allocation concealment was reached since no investigator (stimulators nor accessors) was aware of treatment allocations and had no control over the order of patients randomized.” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA |
| Adequate blinding of assessors? | Unclear risk | Quote: “Two independent blinded examiners were trained to apply the pain scales and to conduct the psychological tests. Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 3 participants dropped out (> 10%) reasons not given. ITT analysis with LOCF |
| Selective reporting (reporting bias) | Low risk | Comment: outcome data adequately reported |
| Study Size | High risk | Comment n = 28 |
| Study duration | High risk | Comment: no follow‐up after immediate postintervention period. |
| Other bias | Low risk | No other bias detected |
| Methods | Partial cross‐over RCT. NB: we only considered first‐phase results therefore we considered the trial as having a parallel design | |
| Participants | Country of study: UK Setting: residential educational centre Condition: post‐SCI pain (unclear whether this was neuropathic or otherwise) Prior management details: unclear n = 30 Age: unclear Duration of symptoms: unclear Gender distribution: unclear |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 10 Hz; pulse width 2 ms; intensity 1 2 μA; duration 53 min Stimulation location: ear clip electrodes Number of treatments: x 2, daily for 4 days Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: 0‐10 VAS 'level of pain', anchors not specified When taken: daily during the treatment period Secondary: none |
|
| Notes | COI: no declaration made Sources of support: Laing Foundation (charity) "financial assistance" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method equivalent to picking out of a hat Quote: "Subjects would be randomly assigned into two groups according to their choice of treatment device... The devices were numbered for identification, but neither the administrators nor the recipients of the treatment could distinguish between the devices." |
| Allocation concealment (selection bias) | Low risk | Comment: this is achieved through the method of randomisation |
| Adequate blinding of participants? | Low risk | Quote: "neither the administrators nor the recipients of the treatment could distinguish between the devices." |
| Adequate blinding of assessors? | Low risk | Quote: "neither the administrators nor the recipients of the treatment could distinguish between the devices." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 3 participants withdrew (not voluntarily) and while the data were not clearly accounted for in the data analysis this constituted 10% of the overall cohort and was unlikely to have strongly influenced the results Quote: "Three of the 30 subjects included were withdrawn from the study after commencement, one of whom developed an upper respiratory infection, and two others were withdrawn from the study because their medication (either H2 antagonist anti‐ulcer or steroidal inhalant) were interacting with the TCET treatment." |
| Selective reporting (reporting bias) | High risk | Comment: pain score values were not provided for any time point |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel randomised clinical trial | |
| Participants | Country of study: Spain Setting: outpatient clinic Condition: fibromyalgia (with major depression) Prior management details: unclear n = 26 Age: active group 47.5 (SD 5.7) years, sham group 54.9 (SD 4.9) years Duration of symptoms: unclear "chronic" Gender distribution: 2 M, 24 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 1 Hz; coil orientation not specified; 110% RMT; number of trains 20; duration of trains 60 s; ITI 45 s; number of pulses 1200 Stimulation location: R DLPFC Number of treatments: up to 20 on consecutive working days Control type: coil angled 45º from the scalp |
|
| Outcomes | Primary: Likert pain scale 0‐10, anchors "no pain" to "extreme pain" When taken: 2 weeks, 4 weeks and 8 weeks from commencement of study Secondary: none |
|
| Notes | COI: no declaration made Sources of support: IUNICS Institute, Research Institute of Health Sciences |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled 45º away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "patients and raters (but not the treating physician) were blind to the procedure" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 participant in each group did not complete the study. Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes presented clearly and in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Australia Setting: laboratory Condition: knee OA Prior management details: not reported n = 30 Age, mean (SD): active group 59.8 (9.1) years, sham group 64.1 (11.1) years Duration of symptoms mean (SD) years: active group: 7.2 (5.3), sham group 9.0 (7.3) Gender distribution: 10 M, 19 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 1 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 2 weekly for 8 weeks prior to a 30‐min supervised strengthening exercise session. 16 sessions Control type: sham tDCS |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain imaginable When taken: postintervention Secondary: WOMAC function AEs |
|
| Notes | Funding source: Trial funded by Arthritis Australia (The Zimmer Australia Grant). W‐JC (1094434), PWH (1002190), KLB (1058440), MBL (1059116) and SMS (1105040) receive salary support from the National Health and Medical Research Council of Australia, RSH from the Australian Research Council (FT#130100175) and VB from a Western Sydney University Postgraduate Research Award. COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomisation schedule was concealed in consecutively numbered, sealed opaque envelopes. An investigator not involved in recruitment and assessment prepared and provided the envelopes to the treating physiotherapists who revealed group allocation.” |
| Adequate blinding of participants? | Low risk | Comment: blinding likely maintained at 1 mA intensity |
| Adequate blinding of assessors? | Low risk | Quote: "A single investigator (W‐JC), blinded to the group allocation of the participants, performed participant recruitment, screening, and testing." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 2 (13% dropout from active group), 3 (20%) from control group. ITT analysis with no imputation of missing values. |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 30 |
| Study duration | High risk | Comment: postintervention follow‐up only (within 1 week) |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT (to be considered as parallel ‐ first treatment phase only as 2nd unblinded) | |
| Participants | Country of study: USA Setting: pain clinic Condition: fibromyalgia Prior management details: unclear n = 74 Age: 22‐75 years; mean 53 Duration of symptoms: 1‐21 years; mean 7.3 Gender distribution: 4 M, 70 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; pulse width unclear; intensity 100 μA; waveform shape modified square wave biphasic 50% duty cycle; duration 60 min Stimulation location: ear clip electrodes Number of treatments: ? daily for 3 weeks Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: 0 ‐5 pain NRS, anchors "no pain" to "worst pain imaginable" When taken: immediately following the 3‐week treatment period Secondary: Oswestry Disability Index When taken: immediately following the 3‐week treatment period |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: "Supported by a grant from the Department of Anesthesiology, LSU Health Sciences Center. No financial support was received from the makers of the Alpha‐Stim™; however, Electromedical Products International, Inc. did loan the authors the Alpha‐Stim™ units necessary to do the study." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Low risk | Quote: "All staff, the physicians, and the patient were blind to the treatment conditions." |
| Adequate blinding of assessors? | Low risk | Quote: "All staff, the physicians, and the patient were blind to the treatment conditions." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout rate not reported |
| Selective reporting (reporting bias) | High risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point |
| Study Size | High risk | Comment: < 50 participants per treatment arm (considered as a parallel trial ‐ 1st phase only) |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Italy Setting: laboratory Condition: fibromyalgia Prior management details: not reported n = 20 Age, mean (SD): active group 41.4 (10.25) years, sham group 44.2 (9.81) years Duration of symptoms, mean (SD) years: active group 4.3 (2.62), sham group 5 (5.04) Gender distribution: all F |
|
| Interventions | Stimulation type: tRNS Stimulation parameters: tDCS: 1.5 mA intensity, 20 min (randomly oscillating in frequency range 101‐640 Hz for 10 min, offset set to 0 ma sham ‐ stimulation turned on for 30 s only) Stimulation location: M1 (side not reported) Number of treatments: x 1 daily, 5 days a week for 2 weeks (x 10 sessions) Control type: sham tRNS |
|
| Outcomes | Primary: pain NRS anchors not reported When taken: postintervention Secondary: FIQ AEs not reported |
|
| Notes | Funding source: not reported COI: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not described |
| Adequate blinding of participants? | Unclear risk | Comment: method of blinding not reported |
| Adequate blinding of assessors? | Unclear risk | Comment: method of blinding not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout reported |
| Selective reporting (reporting bias) | High risk | Comment: no numeric reporting of primary outcomes |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: not specified Condition: chronic myofascial pain in the upper body Prior management details: not reported n = 24 Age, mean (SD): active group 45.83 ( 9.63) years, sham group 44.83 (14.09) years Duration of symptoms: not reported Gender distribution: all F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45º from midline, 80% RMT, number of trains 16; duration of trains 10 s; ITI 26 s; total number of pulses 1600 Stimulation location: L M1 Number of treatments: 10 sessions, timescale not specified Control type: sham coil ‐ same sound and appearance and sensation |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: postintervention Secondary: AEs |
|
| Notes | Funding source: grants and material support from the following Brazilian agencies: Brazilian Innovation Agency (FINEP), process number 1245/13; Committee for the Development of Higher Education Personnel—PNPD/CAPES, process number 023‐11, and material support; National Council for Scientific and Technological Development—CNPq (grants WC‐301256/2013‐6 and ILST‐ 302345/2011‐6 ); Postgraduate Program in Medical Sciences at the School of Medicine of the Federal University of Rio Grande do Sul (material support); Postgraduate Research Group at the Hospital de Clınicas de Porto Alegre (grant number 120343 and material support); and Foundation for Support of Research at Rio Grande do Sul (FAPERGS). COI: study authors declared that there was no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer random number generator assigned patients to 1 of 2 groups: rTMS or placebo‐sham using a block randomization strategy.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Before the recruitment phase, opaque envelopes containing the protocol materials were prepared. Each opaque envelope was sealed and numbered sequentially, containing 1 intervention allocation.” |
| Adequate blinding of participants? | Low risk | Quote “we used an inactive rTMS coil (MagPro X100; MagVenture Company, Lucernemarken, Denmark) as a sham method by placing it in the identical area as the active coil. Thus, sham patients underwent similar rTMS experience (including rTMS sound) as those receiving active stimulation.....The patient recorded identical experiences (including sound effects and somatic sensations caused by contraction of the muscles of the scalp) as during active stimulation” Comment: assessment indicates that blinding was successful. |
| Adequate blinding of assessors? | Low risk | Quote “Two independent evaluators who were blinded to the group assignments(W.C. and another) were trained to apply the pain scales and conduct psychophysical and psychological tests.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 dropout |
| Selective reporting (reporting bias) | High risk | Comment: point estimates for outcomes only reported at one time point |
| Study Size | High risk | n = 24 |
| Study duration | Low risk | 12‐week follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: neurology dept Condition: CPSP Prior management details: stable medication for 30 d preceding baseline n = 23 Age, mean (SD): active group 55 (9.67) years, sham group SD 57.8 (11.86) years Duration of symptoms, mean (SD): active group 64.18 (49.27) months, sham group 50.1 (28.04) Gender distribution:active group 45% M, sham group 50% M |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation not specified, 120% RMT, number of trains 25; duration of trains 5 s; ITI 25s; total number of pulses 1250 Stimulation location: L premotor/DLPFC Number of treatments: 10 sessions daily for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain NRS anchors not reported When taken: end of intervention, 1, 2 and 4 weeks postintervention Secondary: AEs, QoL (SF‐36) |
|
| Notes | Funding source: study was supported by the Pain Center of the Department of Neurology and by the Transcranial Magnetic Stimulation Laboratory of the Psychiatry Institute, University of Sao Pau COI: the study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “Participants were randomly assigned into 2 groups, active stimulation (a‐rTMS) and sham stimulation (s‐rTMS), according to a list automatically generated by an internet‐based tool (www.random.org)” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Quote “Sham stimulation was carried out with a sham coil of identical size color and shape emitting a sound similar to that emitted by the active coil (MC‐P‐B70).” Comment: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Low risk | Quote: “Pain intensity (VAS) was assessed daily, right before and immediately after each rTMS session, from D1 to D10 by an investigator (M.M.) blinded to the type of rTMS patients were receiving. All clinical assessments were performed by a physician and a neuropsychologist (T.L., M.L.M) who were blinded to the type of treatment and had no other role in the study.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 dropout per group |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | n = 21 |
| Study duration | Unclear risk | Comment: 4‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: "single clinical location" Condition: fibromyalgia Prior management details: FDA‐approved fibromyalgia drugs and centrally active analgesics or stimulants "prohibited". n = 46 Age mean (SD) active 12‐week programme group 55.7 (8.7) active 8‐week programme group 46.6 (10.3), sham group 47.9 (11.2) Duration of symptoms: not reported Gender distribution: reported for completers only 35 F, 3 M |
|
| Interventions | Stimulation type: RINCE Stimulation parameters: not reported Stimulation location: parietal region (international 10/20 site PZ),"positioned to create a conduction pathway that includes the primary somatosensory and motor cortex". Number of treatments: Active 12‐week group: 24 treatments of 12 weeks Active 8‐week group: 16 treatments over 8 weeks followed by 8 sham sessions in 4 weeks Sham group: 24 sham sesssions over 12 weeks Control type: nonactivated identical stimulation unit |
|
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = worst pain imaginable When taken: end of treatment period, 4 weeks post‐treatment Secondary: total FIQ score AEs |
|
| Notes | Sources of support: all funding for this study was provided by Cerephex Corporation who manufacture the device. COI: no formal declaration. 5 study authors affiliated to funder ‐ who manufacture the RINCE technology |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of random sequence generation unclear |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not clearly established |
| Adequate blinding of participants? | Unclear risk | Quote: “patients cannot feel the RINCE signal and are therefore blinded to receiving treatment or not….no element of hardware or software gave any indication of group assignment” |
| Adequate blinding of assessors? | Unclear risk | Quote: “The investigators were blinded to these codes and no element of hardware or software gave any indication of group assignment, thus maintaining a double blinded sham controlled condition.” |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 7/14 participants not analysed in the sham group due to “exposure to unexpected signal source”. These participants not included in sham analysis. Details on how this was confirmed or what the exposure was are not clear. |
| Selective reporting (reporting bias) | High risk | Comment: point estimates with measures of variance not provided for all groups at all time points |
| Study Size | High risk | n = 46, divided into 3 groups |
| Study duration | Unclear risk | Comment: 4‐week follow‐up period |
| Other bias | Unclear risk | Comment: full baseline data not tested and only data with 8 excluded sham participants removed were presented |
| Methods | Parallel RCT | |
| Participants | Country of study: Israel Setting: outpatient department Condition: post‐SCI central neuropathic pain Prior management details: refractory to drug, physical therapy and complementary therapy management n = 12 Age: 44‐60 years; mean 54 (SD 6) Duration of symptoms: > 12 months Gender distribution: 7 M, 4 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 5 Hz; coil orientation not specified; 115% RMT; number of trains 500; duration of trains 10 s; ITI 30 s; total number of pulses 500 reported, likely to have been 25,000 judging by these parameters Stimulation location: M1 ‐ midline Number of treatments: x 10, x 1 daily on consecutive days Control type: sham coil ‐ visually the same and makes similar background noise |
|
| Outcomes | Primary: 15 cm 0‐10 VAS pain intensity, anchors "no pain sensation" to "most intense pain sensation" When taken: pre and post each stimulation session Secondary: McGill pain questionnaire When taken: 2‐ and 6‐week follow‐up period |
|
| Notes | AEs: not reported Sources of support: supported by the National Association of the insurance companies. COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified Quote: "Patients were randomised into 2 groups that received either real or sham rTMS" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Quote: "Two coils were used; real and sham, both of which were identical in shape and produced a similar background noise." Comment: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation, but did not control for sensory characteristics of active stimulation over the scalp. Given that stimulation was delivered at 110% RMT active stimulation, but not sham, it is likely to have elicited muscle twitches in peripheral muscles |
| Adequate blinding of assessors? | Low risk | Quote: "The patients as well as the person conducting the outcome measurements were blind to the type of treatment received." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 participant withdrew for "logistic reasons". Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: while group means/SD were not presented in the study report, the study authors provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline differences observed in pain intensity levels (higher in active group) |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: chronic temperomandibular disorder Prior management details: pain not adequately controlled by previous therapies for more than 1 year n = 24 Age range, mean (SD): active group 34.8 (13.7) years, sham group 35.6 (16.7) years Duration of symptoms: not reported Gender distribution: all F |
|
| Interventions | Stimulation type: HD‐tDCS Stimulation parameters: intensity 2 mA, 4 electrodes arranged at the corners of a 4 x 4 cm square centred over M1 Stimulation location: anode ‐ M1 contralateral to painful side Number of treatments: daily, x 5 Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS; anchors not reported ‐ responder analysis only reported When taken: 1‐month follow‐up Secondary: AEs |
|
| Notes | Sources of funding: this project was funded by grants from the American Academy of Orofacial Pain and the University of Michigan Rackham Graduate School. Potential undisclosed COI: 1 study author (Biksom) worked for stimulation device manufacturer Soterix |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “participants were randomized to the treatment or placebo group using the Taves covariate adaptive randomization method.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: no mention of allocation concealment procedures |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA intensity. Evidence that blinding can be inadequate at intensity of 2 mA |
| Adequate blinding of assessors? | High risk | Comment: study described as single blinded |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participant dropout |
| Selective reporting (reporting bias) | High risk | Comment: pain outcomes not presented for all follow‐up time points |
| Study Size | High risk | n = 24 |
| Study duration | Unclear risk | 1‐month follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Norway Setting: university hospital Condition: fibromyalgia Prior management details: prescription medication stable for 3 months prior to inclusion n = 50 Age, mean (SD): active group 49/04 (8.63) years, sham group 48.17 (10.56) years Duration of symptoms, mean (SD) sham group 17.73 (7.54) years, sham group 18.50 (11.48) Gender distribution: 47 F, 3 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 side not reported, cathode supraorbital contralateral to anode Number of treatments: daily, x 5 Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS, anchors not reported When taken: postintervention, mean 30 days postintervention Secondary: FIQ, SF‐36, AEs |
|
| Notes | Sources of funding: study was funded by a grant from the Norwegian Extra Foundation for Health and Rehabilitation through the Norwegian Fibromyalgia Association Study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “The codes were associated with the active or sham tDCS condition and randomized using the online Web service www.randomize.org. The ratio of active and sham codes was 1:1.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: not clearly stated that the sequence generation was separated and concealed |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. Not formal assessment of blinding success |
| Adequate blinding of assessors? | Low risk | Comment: outcomes collected through text message with little potential for assessors to influence process |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: high noncompletion rate for some outcomes and there is not full clarity on how many participants were analysed |
| Selective reporting (reporting bias) | Low risk | Comment: full reporting of key outcomes |
| Study Size | High risk | n = 50 |
| Study duration | Unclear risk | Comment: follow‐up 30 days postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: unclear Condition: chronic pelvic pain Prior management details: refractory to treatment n = 7 Age: mean 38 years Duration of symptoms: mean 80 months Gender distribution: all F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1 dominant hemisphere Number of treatments: 2 Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: VAS overall pain, pelvic pain, back pain, migraine pain, bladder pain, bowel pain, abdomen pain and pain with intercourse. Anchors not specified When taken: daily during stimulation and then for 2 weeks post‐each condition Secondary: none |
|
| Notes | Sources of support: no declaration made COI: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Low risk | Quote: "All other personnel in the study, including the investigators, study coordinators, participants, and their families, and all primary medical caregivers, were blinded." |
| Adequate blinding of assessors? | Low risk | Quote: "All other personnel in the study, including the investigators, study coordinators, participants, and their families, and all primary medical caregivers, were blinded." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout reported |
| Selective reporting (reporting bias) | Low risk | Comment: variance measures not presented for group means poststimulation but data provided by study author on request |
| Free from carry‐over effects? | Unclear risk | Comments: pre‐stimulation data not presented and no formal investigation for carry‐over effects discussed |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: chronic pancreatitis pain Prior management details: not specified n = 5 Age: 44 (SD 11) Duration of symptoms: not specified, "chronic" Gender distribution: not specified |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 1 Hz or 20 Hz; coil orientation not specified; 90% RMT; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 1600 Stimulation location: L and R SII Number of treatments: 1 for each condition Control type: sham, "specially designed sham coil". No further details |
|
| Outcomes | Primary: pain VAS, anchors not specified When taken: after each stimulation session Secondary: none |
|
| Notes | COI: no declaration made Sources of support: National Pancreas Foundation/ NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The order of stimulation was randomised and counterbalanced across patients using a Latin square design." |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment "unclear". Type of sham coil not specified |
| Adequate blinding of assessors? | Low risk | Quote: "Patients were blinded to treatment condition, and a blinded rater evaluated analgesic use, patient's responses in a Visual Analogue Scale (VAS) of pain.... immediately after each session of rTMS." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout reported |
| Selective reporting (reporting bias) | High risk | Comment: pain NRS values not provided clearly with measures of variance for any time point for the sham condition |
| Free from carry‐over effects? | Low risk | Quote: "Importantly, baseline pain scores were not significantly different across the six conditions of stimulation... speaking against carryover effect." |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: post‐SCI central neuropathic pain Prior management details: refractory to drug management n = 17 Age: mean 35.7 (SD 13.3) years Duration of symptoms: chronic > 3/12 Gender distribution: 14 M, 3 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 5, x 1 daily on consecutive days Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: pain VAS 0‐10 cm, anchors "no pain" to "worst pain possible" When taken: before and after each stimulation and at 16‐day follow‐up Secondary: none |
|
| Notes | COI: no declaration made Sources of support: support from Harvard Medical School Scholars in Clinical Science programme |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entrance in the study and a previous randomisation list generated by a computer using random blocks of six (for each six patients, two were randomised to sham and four to active tDCS) in order to minimize the risk of unbalanced group sizes." |
| Allocation concealment (selection bias) | Low risk | Comment: the use of a pre‐generated randomisation list should ensure this |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "... we analyzed the primary and secondary endpoints using the intention‐to‐treat method including patients who received at least one dose of the randomised treatment and had at least one post‐baseline efficacy evaluation. We used the last evaluation carried out to the session before the missed session, assuming no further improvement after the dropout, for this calculation." |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain score numerical values not provided clearly in the study report with measures of variance for any time point. On request data were available for the primary outcome at one follow‐up point but not for other follow‐up points |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT; 3 conditions | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: unclear n = 32 Age: 53.4 (SD 8.9) years Duration of symptoms: condition 1: 8.4 (SD 9.3) years; condition 2: 10.0 (SD 7.8) years; condition 3: 8.1 (SD 7.5) years Gender distribution: 32 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: condition 1: DLPFC; condition 2: M1; condition 3: sham M1. All conditions contralateral to most painful side or dominant hand Number of treatments: 5, x 1 daily on consecutive days Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: pain VAS 0‐10 cm, anchors not specified When taken: at the end of the stimulation period and at 21‐day follow‐up Secondary: QoL: FIQ |
|
| Notes | COI: no declaration made Sources of support: support from Harvard Medical School Scholars in Clinical Science programme/ NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entry into the study and a previous computer‐generated randomisation list, using random blocks of 6 patients (for each 6 patients, 2 were randomised to each group) in order to minimize the risk of unbalanced group sizes." |
| Allocation concealment (selection bias) | Low risk | Comment: the use of a pre‐generated randomisation list should have adequately ensured this |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "One patient (in the M1 group) withdrew, and the few missing data were considered to be missing at random. We analyzed data using the intent‐to‐treat method and the conservative last observation carried forward approach." |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain score numerical values not provided clearly with measures of variance for most time points in the study report. On request data were available for the primary outcome at 1 follow‐up point but not for other follow‐up points |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: chronic visceral pain (chronic pancreatitis) Prior management details: most on continuous opioid therapy, most had received surgery for their pain n = 17, 9 in active group, 8 in sham group Age mean (SD): active group 41.11 (11.27) years, sham group 46.71 (13.03) years Duration of symptoms: > 2 years Gender distribution: 14 F, 3 M |
|
| Interventions | Stimulation type: rTMS Stimulation parameters:frequency 1 Hz; coil orientation not specified, number of trains 1; duration of trains not specified; intensity 70% maximum stimulator output, total number of pulses 1600 Stimulation location: SII Number of treatments: 10, x 1 daily (weekdays only) Control type: sham rTMS coil |
|
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = most intense pain imaginable When taken: daily pain logs for 3 weeks pre‐intervention, daily post‐stimulation during intervention period and at 3‐week follow‐up Secondary: none relevant |
|
| Notes | COI: no declaration made Sources of support: support from Harvard Thorndike Clinical Research Center/ NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomised (using a computer generated list with blocks of 4)" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Low risk | Quote "The sham and real TMS coils looked identical and were matched for weight and acoustic artefact. This sham coil induces a similar tapping sensation and generates the same clicking noise as the real TMS coil, but without induction of a significant magnetic field and secondary current." Comment: sham appears optimal |
| Adequate blinding of assessors? | Low risk | Quote: "The pain evaluation was carried out by a blinded assessor" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout/withdrawal not reported |
| Selective reporting (reporting bias) | High risk | Comment: reporting of pain scores incomplete across all time points |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline values not presented by group for key outcome variables |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: pain clinic Condition: chronic back and neck pain Prior management details: unclear n = 20 Age: 20‐77 years Duration of symptoms: 0.5‐40 years Gender distribution: 9 M, 11 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 77 Hz; pulse width 3.3 ms; intensity ≤ 4 mA; waveform shape biphasic asymmetric; duration 30 min Stimulation location: 3 electrodes, 1 attached to either mastoid process and 1 to the forehead Number of treatments: 8, x 1 daily on consecutive days Control type: "active placebo" units visually indistinguishable. Delivered 50 Hz frequency, intensity ≤ 0.75 mA. Note: may not be inert |
|
| Outcomes | Primary: pain VAS, anchors not specified When taken: pre and post each stimulation Secondary: none |
|
| Notes | COI: no declaration made Sources of support: grant by Pulse Mazor instruments, Israel |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list." |
| Allocation concealment (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list. At enrolment in the study, the investigator assigned the next random number in that patient’s category. The investigator did not have access to the randomisation list until after the study was completed." |
| Adequate blinding of participants? | Low risk | Quote: "The active placebo device was indistinguishable to the patient and medical team from the real TCES device ‐ it was designed to give the patient the feeling of being treated, inducing an individual sensation of skin numbness or muscle contraction" |
| Adequate blinding of assessors? | Low risk | Quote: "The active placebo device was indistinguishable to the patient and medical team." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all participants completed the study |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values were not provided clearly with measures of variance for most time points in the study report, the study authors have provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: an active placebo that delivers current may not be inert and may bias against between group differences (0.75 mA exceeds the intensity of the active arms of other CES trials) |
| Methods | Parallel RCT | |
| Participants | Country of study: Israel Setting: pain clinic Condition: chronic back and neck pain Prior management details: unclear n = 75 (excluding headache participants) Age: mean 53.9 years, range 22‐82 Duration of symptoms: 0.5‐40 years Gender distribution: 35 M, 40 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 77 Hz; pulse width 3.3 ms; intensity ≤ 4 mA; waveform shape biphasic asymmetric; duration 30 min Stimulation location: 3 electrodes, 1 attached to either mastoid process and 1 to the forehead Number of treatments: 8, x 1 daily on consecutive days Control type: "active placebo" units visually indistinguishable. Delivered 50 Hz frequency, intensity ≤ 0.75 mA. Note: may not be inert |
|
| Outcomes | Primary: pain VAS, anchors not specified When taken: pre and post each stimulation; 3 weeks and 3 months following treatment Secondary: none |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list" |
| Allocation concealment (selection bias) | Low risk | Quote: "The paramedic administered treatments based on a computer‐elicited randomisation list. At enrolment, the investigator assigned the next random number in that patient’s category. The investigator did not have access to the randomisation list until study completion." |
| Adequate blinding of participants? | Low risk | Quote: "The placebo device was indistinguishable from the active device" |
| Adequate blinding of assessors? | Low risk | Quote: "The investigator did not have access to the randomisation list until study completion" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout is indicated, comparing the results with the number enrolled |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: > 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: an active placebo that delivers current may not be inert and may bias against between group differences (0.75 mA exceeds the intensity of the active arms of other CES trials) |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: laboratory Condition: trigeminal neuralgia Prior management details: stable medication for 6 months prior to study, no invasive procedures prior to study n = 17 Age range: 32‐72 years Duration of symptoms: range 2‐27 years, mean 13 Gender distribution: 7 M, 10 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 40 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 contralateral to painful side, cathode supraorbital contralateral to anode Number of treatments: daily, self‐administered for 14 days Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS When taken: postintervention Secondary: AEs |
|
| Notes | Study authors' COI statement: "Tim Hagenacker has received research support from Astellas and CSL Behring. Vera Bude, Steffen Naegel have nothing to disclose. Dagny Holle has received research support from Grünental and Allergan. Mark Obermann has received scientific support and/or honoraria from Biogen Idec, Novartis, Sanofi‐Aventis, Genzyme, Pfizer, Teva. He received research grants from Allergan, Electrocore, and the German Ministry for Education and Research (BMBF). Hans‐Christoph Diener has received honoraria for participation in clinical trials, contribution to advisory boards or lectures from Addex Pharma, Allergan, Almirall, AstraZeneca, Bayer Vital, Berlin Chemie, Coherex Medical, CoLucid, Böhringer Ingelheim, Bristol‐Myers Squibb, GlaxoSmithKline, Grünenthal, Janssen‐Cilag, Lilly, La Roche, 3M Medica, Minster, MSD, Novartis, Johnson & Johnson, Pierre Fabre, Pfizer, Schaper and Brümmer, SanofiAventis, and Weber & Weber; received research support from Allergan, Almirall, AstraZeneca, Bayer, Galaxo‐Smith‐Kline, Janssen‐Cilag, and Pfizer. Sources of support: "Headache research at the Department of Neurology in Essen is supported by the German Research Council (DFG), the German Ministry of Education and Research (BMBF), and the European Union." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Adequate blinding of participants? | Unclear risk | Comment: method of blinding not clearly stated |
| Adequate blinding of assessors? | Unclear risk | Comment: method of blinding not clearly stated |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 7/17 participants discontinued trial. Details of when not clear. Per‐protocol analysis |
| Selective reporting (reporting bias) | Low risk | Comment: all key outcomes reported |
| Free from carry‐over effects? | Unclear risk | No formal assessment of baseline equivalence reported |
| Study Size | High risk | Comment: n = 17, 10 after attrition |
| Study duration | High risk | Comment: only immediate postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: "professional clinical setting" Condition: fibromyalgia Prior management details: no recent remission of symptoms n = 91 Age: active group 48‐54.7 years, sham group 51‐57 years Duration of symptoms: active group mean 17.12 years, sham group mean 17.5 years Gender distribution: reported for completers only 71 F, 6 M |
|
| Interventions | Stimulation type: RINCE Stimulation parameters: current density 0.3 mA/cm2, stimulation duration 11 min, frequency 10 kHz carrier signal delivered at 40 Hz Stimulation location: parietal region (international 10/20 site PZ), ground leads fixed to earlobes Number of treatments: x 2 weekly for 11 weeks Control type: non‐activated identical stimulation unit |
|
| Outcomes | Primary: FIQ pain VAS; 0 = no pain, 10 = unbearable pain When taken: end of treatment period Secondary: total FIQ score |
|
| Notes | Lead author declared an intellectual property interest in the technology and is a shareholder in a company seeking to develop the technology for commercialisation | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Low risk | Quote: "The combined involvement of low driving potentials and high carrier frequencies creates a signal that is subthreshold for perceptibility.....Subjects could not feel the signal regardless of group, and therefore could not tell if they were receiving treatment or not" |
| Adequate blinding of assessors? | Low risk | Quote: "The investigators were blinded to the settings, and no element of hardware or software gave any indication as to which setting had been assigned to the subject." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: per‐protocol analysis used, dropout rate 6/45 (13%) in active group and 8/46 (17%) in sham group |
| Selective reporting (reporting bias) | Low risk | Comment: data reported on all outcomes and supplementary data made available by the study author |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Canada Setting: laboratory Condition: mixed chronic pain (in the over 60s) Prior management details: not reported n = 16 Age, mean (SD): active group 72 (6) years, sham group 71 (8) years Duration of symptoms mean (SD) years: active group 26 (24), sham group 15 (11) Gender distribution: 11 F, 3 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 1 daily for 5 days Control type: sham tDCS |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain 10 = worst imaginable pain When taken: postintervention Secondary: none relevant AEs not reported |
|
| Notes | Funding source: G Léonard is supported by the Fonds de Recherche en Santé (FRQ‐S, Montréal, QC, Canada). This project was partially supported by the Neuroscience Centre of Excellence of the Université de Sherbrooke (CeNUS, Sherbrooke, QC, Canada) and an internal start‐up fund from the Research Centre on Aging (Initiatives stratégiques du Centre de recherche sur le vieillissement, Sherbrooke, QC, Canada). COI: study authors report no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization to sham or active tDCS was performed using a random numbers table with a ratio of 1:1, based on order of entry of the participants in the study.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2/8 (25%) in active group withdrew. Data appear to have been excluded from analysis |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 14 |
| Study duration | High risk | Comment: 1 week postintervention follow‐up |
| Other bias | High risk | Comment: baseline imbalance in average daily pain |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: chronic low back pain Prior management details: not reported n = 92, relevant to this review 46 Age, mean (SD): active group 51.9 (9.9) years, sham group 54.1 (9.8) years Duration of symptoms mean (SD) months: active group 91.6 (108.3) sham group 69.2 (92.7) months Gender distribution: 10 M, 36 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 3 per week for 4 weeks. 12 sessions in total Control type: sham tDCS |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain 10 = worst pain possible When taken: postintervention, 3 months, 6 months Secondary: disability (RMDQ) AEs |
|
| Notes | Funding source: none COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The patients were allocated to one of the four treatment groups by means of random‐number‐generating software." |
| Allocation concealment (selection bias) | Low risk | Quote: "The randomization and allocation concealment were carried out by an external collaborator, not a research participant, who organized patients and their previously allocated treatments in individual opaque envelopes." |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2mA. No assessment of blinding success. No formal assessment of blinding success. |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: minimal loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 46 |
| Study duration | Low risk | Comment: 6‐month follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT; 5 conditions | |
| Participants | Country of study: Japan Setting: laboratory Condition: intractable deafferentation pain (mixed central, peripheral and facial) Prior management details: intractable n = 20 Age: 28‐72 years Duration of symptoms: 1.5‐24.3 years, mean 6.4 (SD 6) Gender distribution: 13 M, 7 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 5 Hz; coil orientation not specified; 90% RMT; number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 500 Stimulation location: condition 1: M1; condition 2: primary sensory cortex; condition 3: pre‐motor area; condition 4: supplementary motor area; condition 5: sham Number of treatments: 1 for each condition Control type: coil angled 45º from scalp with synchronised electrical scalp stimulations to mask sensation |
|
| Outcomes | Primary: pain intensity VAS, anchors not specified When taken: 0, 30, 60, 90, 180 min poststimulation Secondary: none |
|
| Notes | COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "All targets were stimulated in random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Quote: "The patients were unable to distinguish sham stimulation from actual rTMS, because the synchronized electrical stimulation applied to the forehead made the forehead spasm, as was the case with actual TMS" Comment: sham credibility assessment ‐ suboptimal. Sensory and auditory aspects controlled for but angulation of coil away from the scalp may be visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 20 patients underwent all planned sessions of navigation‐ guided rTMS" |
| Selective reporting (reporting bias) | Low risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point but data provided upon request |
| Free from carry‐over effects? | Low risk | Comment: study authors provided requested data. Appears free of carry‐over effects |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Japan Setting: multicentre, laboratory‐based Condition: mixed neuropathic pain Prior management details: pain persisted despite "adequate treatments" n = 70 of whom 64 analysed Age mean (SD): 60.7 (10.6) years Duration of symptoms: 58.2 (10.6) months Gender distribution: 40 M, 24 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 5 Hz; coil orientation parasagittal, number of trains 10; duration of trains 10 s; ITI 50 s, intensity 90% RMT, total number of pulses per session 500 Stimulation location: M1 corresponding to painful region Number of treatments: 10, x 1 daily (consecutive working days) Control type: sham coil |
|
| Outcomes | Current daily pain 0‐100 VAS (anchors not reported), SF McGill AEs |
|
| Notes | COI: study authors declared no COI Sources of support: "funded by the Japanese Ministry of Health, Labour and Welfare with a Health and Labour Sciences Research Grant. This research was partly supported by Japanese MEXT SRPBS" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Before the patient enrolment, the independent data center developed a randomization program to assign each patient to one of 2 treatment groups (1:1). A real rTMS period was followed by a sham period in group A, and a real rTMS period came after a sham period in group B. We used Pocock and Simon’s minimization method to stratify treatment groups according to institution, age (< 60 or P60 years), sex, and underlying disease (a cerebral lesion or not), and the Mersenne twister for random number generation." |
| Allocation concealment (selection bias) | Low risk | Quote: "After confirmation of patient eligibility, the data center received a registration form from an assessor who collected questionnaires and assessed adverse events, and then sent an assignment notice to an investigator who conducted the rTMS intervention. Patients were identified by sequential numbers that were assigned by the data center. Patients and assessors were blind to group assignment until the study was completed. The data center was responsible for assigning patients to a treatment group, data management, central monitoring, and statistical analyses." |
| Adequate blinding of participants? | Low risk | Quote: "Realistic sham stimulation [32] was implemented in this study. Ten trains of electrical stimuli at 2 times the intensity of the sensory threshold (one train, 50 stimuli at 5 Hz; inter train interval, 50 s) were delivered with a conventional electrical stimulator through the electrodes fixed on the head. The cortical effect of the cutaneous electrical stimulation was considered to be negligible at this intensity because of the high electrical resistance of the skull and brief duration of the stimulation [32]. A figure‐8 coil, which did not connect to a magnetic stimulator, was placed on the head in the same manner as a real rTMS session. Another coil, which discharged simultaneously with the electrical stimuli, was placed near the unconnected coil to produce the same sound as real rTMS, but not to stimulate the brain." Comment: sham controls for sensory auditory and visual cues |
| Adequate blinding of assessors? | Low risk | Quote: "Patients and assessors were blind to group assignment until the study was completed." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout low (total 6 from recruited 70 participants) Quote: "Seventy patients were enrolled and randomly assigned to 2 groups. Of these patients, one patient never came to the hospital after the registration, and a suicidal wish became apparent before the start of the intervention in another patient. Sixty‐eight patients received the interventions and 64 patients were included in the intention‐to‐treat analysis after excluding 4 patients without any data collection." |
| Selective reporting (reporting bias) | Low risk | Comment: while full numerical means and SDs were not reported for all time points all data were made available upon request to the study authors |
| Free from carry‐over effects? | Low risk | Quote: "To evaluate carry‐over effects, Grizzle’s test for carry‐over effect was applied to the values at day 0 for each period ... Grizzle's test showed no carry‐over effects in VAS and SF‐MPQ" |
| Study Size | Unclear risk | Comment: > 50 but < 200 participants per treatment condition |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT; 3 conditions | |
| Participants | Country of study: Germany Setting: laboratory Condition: PLP and CNP Prior management details: unclear n = 27 Age: (median) PLP 46.6 years, CNP 51.1 years Duration of symptoms: mean PLP 15.2 (SD 14.8), CNP 3.9 (SD 4.1) years. Gender distribution: 16 M, 11 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 1 Hz; coil orientation not specified; 95% RMT; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 500 Condition 2: frequency 5 Hz; coil orientation not specified; 95% RMT; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 500 Condition 3: sham frequency 2 Hz; coil orientation not specified; number of trains not specified; duration of trains not specified; ITI not specified; total number of pulses 500 Stimulation location: M1, contralateral to painful side Number of treatments: x 1 for each condition Control type: sham coil; mimics sight and sound of active treatment |
|
| Outcomes | Primary: 0‐100 mm VAS pain intensity, anchors "no pain" and "most intense pain imaginable" When taken: pre‐ and post‐stimulation Secondary: none |
|
| Notes | Sources of support: no reporting of source of support COI: study authors decare no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 13 of 27 participants did not complete all treatment conditions and this dropout is not clearly accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome data presented clearly and in full |
| Free from carry‐over effects? | Low risk | Quote: "The VAS values before the stimulation showed no significant differences in the various types of treatment" |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: continued using pharmacological and nonpharmacological therapies. n = 20 Age mean (SD): 46.4 (10.62) years Duration of symptoms: not reported Gender distribution: all F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 15 cm2 electrodes, duration 20 min Stimulation location: anode ‐ M1 L, cathode right supraorbital Number of treatments: x 1 per week for 10 weeks Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS; anchors not reported When taken: postintervention Secondary: FIQ, SF‐36 |
|
| Notes | No reporting of sources of support or COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: no reporting of concealment procedures |
| Adequate blinding of participants? | Low risk | Quote “Patients, as well as investigator in charge and evaluators, were blind to the nature of applied stimulation” Comment: blinding likely at 1 mA intensity |
| Adequate blinding of assessors? | Low risk | Quote “Patients, as well as investigator in charge and evaluators, were blind to the nature of applied stimulation” Comment: blinding likely at 1 mA intensity |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: attrition not reported |
| Selective reporting (reporting bias) | Low risk | Comment: results reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Unclear risk | Comment: no reporting of baseline comparability |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: post‐SCI pain (neuropathic and non‐neuropathic) Prior management details: not reported n = 31 randomised Age: 22‐77 years Duration of symptoms (months): > 6 months Gender distribution: 22 M, 8 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1 contralateral to painful side or on L where pain bilateral Number of treatments: 1 Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: 0‐10 NRS; 0 = no pain, 10 = most intense pain sensation imaginable. An average of current, least, worst and average pain scores When taken: poststimulation Secondary: none relevant |
|
| Notes | AEs not reported Government‐funded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote "The remaining 31 individuals were randomly assigned to receive the five procedure conditions in one of five orders, using a Latin square design." |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: of 31 randomised there were data from 28 following active tDCS and 27 following sham |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Free from carry‐over effects? | Low risk | Comment: baseline pain levels pre active and sham tDCS session appear equivalent |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Canada Setting: outpatient rehabilitation centre Condition: post‐SCI neuropathic pain Prior management details: almost all participants in various medications n = 18 Age: range 31‐69 years, mean (SD) 50 (9) Duration of symptoms: not reported Gender distribution: 11 M, 5 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45º posterolateral, 90% RMT for hand, 110% RMTA for leg, number of trains 40; duration of trains 5 s; ITI 25 s; total number of pulses 2000 Stimulation location: M1 hand or leg area with neuronavigation Number of treatments: single session per condition, 1 session of sham Control type: sham coil ‐ same sound and appearance and sensation |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: immediately poststimulation, 20 min poststimulation Secondary: AEs ‐ though no formal assessment reported |
|
| Notes | Funding source: supported by the Canadian Institutes of Health Research (CIHR), Grant Number MOP‐79370. C. Mercier was supported by salary awards from the CIHR and the Fonds de recherche du Québec, Santé (FRQS). F. Jetté was supported by a fellowship from Université Laval and H. B. Meziane by a fellowship from the Réseau Provincial de Recherche en Adaptation‐Réadaptation (REPAR‐FRQS). Support was provided by the Consortium d’Imagerie en Neuroscience et Santé Mentale de Québec (CINQ) for MRI acquisition COI: the study authors declared no potential COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote “2 active rTMS sessions (hand/leg M1 area) and 1 sham rTMS session in a randomized, counterbalanced order.” Comment: method of randomisation not described |
| Adequate blinding of participants? | Low risk | Quote “Sham rTMS, using a sham coil (mimicking the noise and scalp sensations), was applied over the hand area using the same parameters |
| Adequate blinding of assessors? | Low risk | Quote “The researcher running the pre‐post assessment (as well as data analysis) was blind relative to the applied rTMS protocol(as was the participant), with the rTMS application being performed by a different researcher |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: dropout levels low ‐ 2 in total |
| Selective reporting (reporting bias) | Low risk | Comment: data provided upon author request |
| Free from carry‐over effects? | Unclear risk | Comment: 2‐week washout period observed but no analysis or data presented to confirm baseline comparability |
| Study Size | High risk | Comment: n = 16 |
| Study duration | High risk | Comment: immediate poststimulation measurement only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: South Korea Setting: university hospital outpatient setting Condition: post‐SCI central neuropathic pain Prior management details: resistant to drug, physical or complementary therapies n = 11 Age: 33‐75 years, mean 54.8 Duration of symptoms: chronic Gender distribution: 6 M, 5 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation angled 45º posterolaterally; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number pulses 1000 Stimulation location: R M1, hand area Number of treatments: 5, x 1 daily Control type: coil elevated and angled away from the scalp |
|
| Outcomes | Primary: NRS average pain over last 24 h, anchors "no pain sensation" to "most intense pain sensation imaginable" When taken: immediately after the 3rd and 5th treatments and 1, 3, 5 and 7 weeks after the end of the stimulation period Secondary: BPI ‐ pain interference (surrogate measure of disability) When taken: as for the NRS |
|
| Notes | AEs: not reported COI: studu authors declared no COI Sources of support: supported by the Seoul National University Bundang Hospital |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The real and sham rTMS stimulations were separated by 12 weeks and performed in a random order according to the prepared allocation code." Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled away from scalp and not in contact in sham condition. Didnot control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "... a different researcher collected the clinical data; the latter researcher was not aware of the type of rTMS (real or sham)" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no participants withdrew after receiving the first treatment condition |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: a 12‐week washout period was observed. The pre‐stimulation baseline scores closely match |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT; 3 conditions | |
| Participants | Country of study: Russia Setting: unclear Condition: hip and knee OA Prior management details: unclear n = 64 Age: unclear Duration of symptoms: unclear Gender distribution: unclear |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 11‐15 mA; waveform shape: condition 1 symmetric, condition 2 asymmetric; duration 40 min Stimulation location: appears to be 1 electrode attached to either mastoid process and 1 to the forehead Number of treatments: 5, x 1 daily for 5 consecutive Control type: sham unit ‐ visually indistinguishable from active units |
|
| Outcomes | Primary: 0‐10 NRS, anchors "no pain" to "very painful" When taken: unclear. Likely to be pre and post each stimulation session and then daily for 1 week after Secondary: none |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "If subjects passed all criteria they were randomly assigned to one of the two active treatments or the sham treatment." Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: not specified |
| Adequate blinding of participants? | Low risk | Quote: "The physicians, like all other participants in the study, were unaware of which treatment each subject received." |
| Adequate blinding of assessors? | Low risk | Quote: "The physicians, like all other participants in the study, were unaware of which treatment each subject received." |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: dropout level not specified |
| Selective reporting (reporting bias) | High risk | Comment: it is unclear in the report which time points were reported for primary outcomes |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: the reporting of baseline group characteristics is insufficient |
| Methods | Parallel RCT | |
| Participants | Country of study: Egypt Setting: university hospital neurology department Condition: neuropathic pain, mixed central (poststroke) and facial (trigeminal neuralgia) pain Prior management details: refractory to drug management n = 48 Age: poststroke 52.3 (SD 10.3) years, trigeminal neuralgia 51.5 (SD 10.7) years Duration of symptoms: poststroke 39 months (SD 31), trigeminal neuralgia 18 months (SD 17) Gender distribution: 8 M, 16 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 20 Hz; coil orientation not specified; 80% RMT; number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 2000 Stimulation location: M1 contralateral to the side of worst pain Number of treatments: 5, x 1 on consecutive days Control type: coil elevated and angled away from scalp |
|
| Outcomes | Primary: pain VAS, anchors not specified When taken: post 1st, 4th and 5th stimulation session and 15 days after the last session Secondary: none |
|
| Notes | AEs: not reported COI: study authors declared no COI Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | High risk | Quote: "Patients were randomly assigned to one of the two groups, depending on the day of the week on which they were recruited." Comment: not truly random |
| Allocation concealment (selection bias) | High risk | Comment: the method of sequence generation makes concealment of allocation unlikely |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled away from scalp and not in contact in sham condition. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Low risk | Quote: "The second author evaluated these measures blindly—that is, without knowing the type of rTMS" |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values were not provided clearly with measures of variance for all time points in the study report, the study authors have provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Egypt Setting: laboratory Condition: fibromyalgia Prior management details: not reported n = 40, 36 after attrition Age, mean (SD): active group 31.3 (10.99) years, sham group 33.89 (11.18) years Duration of symptoms, mean (SD) months, active group 6.1 (2.65), sham group 6.05 (2.5) Gender distribution: 34 F, 2 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: L M1 Number of treatments: x 1 daily for 5 days per week for 2 weeks ‐ 10 sessions in total Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS anchors not reported When taken: postintervention, 2 weeks and 1 month postintervention Secondary: none relevant AEs |
|
| Notes | Funding source: no funding reported COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Each patient was given a serial number from a computer generated randomization table, and was placed in the appropriate group after opening the corresponding sealed envelope." |
| Allocation concealment (selection bias) | Low risk | Quote: "Allocation concealment was done using serially numbered closed, opaque envelopes." |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 10% dropout per group |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 per group |
| Study duration | Unclear risk | Comment: 1 month postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: South Korea Setting: laboratory Condition: chronic painful diabetic polyneuropathy Prior management details: persistent pain after taking medications. Stable doses of analgesics for 2 months prior to commencement n = 72, 60 after dropout, outcome data only given on this 60 Age, mean (SD): active M1 group 59.60 (13.15) years, active DLPFC group 63.5 (8.75) years, sham group 61.6 (10.27) years Duration of symptoms: all participants had had pain for > 2 years Gender distribution: 25 M, 35 F (after dropout) |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25‐35 cm2 electrodes, duration 20 min Stimulation location: group 1: anode ‐ M1, side not specified, group 2 anode DLPFC side not specified, group 3 M1 sham, cathode contralateral supraorbital Number of treatments: daily, x 5 Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = "worst possible pain" When taken: end of intervention, 2 weeks, 4 weeks Secondary: AEs |
|
| Notes | Funding: supported by Eulji University COI: study authors declared no potential COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entry into the study and a computer‐generated randomization chart with random blocks of six patients each." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment procedure not described |
| Adequate blinding of participants? | Unclear risk | Comment: blinding can be compromised at intensities of 2 mA, no formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding can be compromised at intensities of 2 mA, no formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 15% dropout, even across groups, analysis appears to be per‐protocol. |
| Selective reporting (reporting bias) | High risk | Comment: point estimates and measures of variance for primary outcome only reported at selected time points |
| Study Size | High risk | Comment: n = 72, 3 groups |
| Study duration | Unclear risk | Comment: 4‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Canada Setting: laboratory Condition: CRPS type I Prior management details: not reported n = 22 Age, mean (SD): active group 40.9 (8.8) years, sham group 52.7 (10.5) years Duration of symptoms, mean (SD) months: active group 36.3 (25.6), sham group 36.6 (25.8) Gender distribution: 14 F, 8 M |
|
| Interventions | Stimulation type: tDCS (combined with graded motor imagery) Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x 5 weekly for 2 weeks, x 1 weekly for 4 weeks ‐ 14 sessions in total over 6 weeks Control type: sham tDCS (combined with grade motor imagery) |
|
| Outcomes | Primary: average pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: 1 month post intervention Secondary: physical function (BPI pain interference), QoL (SF36‐SF) AEs |
|
| Notes | Funding source: this study was supported by grants from the Canadian Pain Society (CPS), the Quebec Pain Research Network (QPRN), as well as the Inflammation and Pain Axis and the Faculty of Medicine and Health Sciences from the Université de Sherbrooke COI: the study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: precise method for randomisation not reported |
| Allocation concealment (selection bias) | Low risk | Quote: “order to avoid a potential concealment bias, the randomization sequence was concealed from the investigators, where only an independent research agent held the allocation list.” |
| Adequate blinding of participants? | Low risk | Comment: 2 mA can affect blinding negatively but formal assessment of participant blinding suggests success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: results reported adequately |
| Study Size | High risk | Comment: n = 22 |
| Study duration | Unclear risk | Comment: 1 month postinterventionfollow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Korea Setting: outpatient clinic Condition: fibromyalgia Prior management details: none reported n = 22 Age mean (SD): low‐frequency group 45.6 (9.6) years, high‐frequency group 53 (4.2) years, sham group 51.3 (6.2) years Duration of symptoms (months mean (SD)): low‐frequency group: 47.2 (20.1), high‐frequency group 57.1 (6.4), sham group 44.7 (10.3) Gender distribution: all F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: Low‐frequency group: frequency 1 Hz; coil orientation not specified, number of trains 2; duration of trains 800 s; ITI 60 s; total number of pulses 1600 High‐frequency group: frequency 10 Hz; coil orientation not specified, number of trains 25; duration of trains 8 s; ITI 10 s; total number of pulses 2000 Stimulation location: right DLPFC (low‐frequency), L M1 (high‐frequency) Number of treatments: 10, x 1 daily (weekdays only) for 2 weeks Control type: sham ‐ coil orientated away from scalp |
|
| Outcomes | Primary: 0‐100 mm pain VAS; 0 = none, 100 = an extreme amount of pain When taken: post‐treatment and at 1 month follow‐up Secondary: FIQ |
|
| Notes | Comment: no information on AEs given relating to those participants who did not complete all sessions COI: study authors declared no COI Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ suboptimal. Coil angled away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not specified |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: no ITT analysis described ‐ appears to be per protocol. 3/8 in low‐frequency group, 2/5 in high‐frequency group and 2/5 in sham group |
| Selective reporting (reporting bias) | Low risk | Comment: point measures presented in full for all outcomes |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: intractable neuropathic pain (mixed central and facial) Prior management details: refractory to drug management n = 14 Age: 34‐80 years, mean 57.2 Duration of symptoms: not specified "chronic" Gender distribution: 6 M, 8 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation not specified; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number of pulses 1000 Stimulation location: M1, contralateral to painful side Number of treatments: x 1 for each condition Control type: sham coil used (? inert) |
|
| Outcomes | Primary: 0‐10 VAS, anchors not specified When taken: daily for 12 days poststimulation Secondary: none |
|
| Notes | COI: no declaration made Sources of support: grant from the ‘Institut UPSA de la douleur’ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Two different sessions of rTMS separated by 3 weeks at least were randomly performed in each patient." Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham coil as that used in Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point in the report but were provided by study authors on request |
| Free from carry‐over effects? | Low risk | Comment: 3/52 washout period makes carry‐over effects unlikely |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central and peripheral) Prior management details: refractory to drug management n = 18 Age: 28‐75 years, mean 54.7 Duration of symptoms: not specified "chronic" Gender distribution: 11 M, 7 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation posteroanterior; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number of pulses 1000 Condition 2: frequency 0.5 Hz; coil orientation posteroanterior; number of trains 1; duration of trains 20 min; total number of pulses 600 Condition 3: sham ‐ same as for condition 1 with sham coil Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition |
|
| Outcomes | Primary: 0‐10 VAS pain, anchors not specified When taken: 5‐10 min poststimulation Secondary: none |
|
| Notes | COI: no declaration made Sources of support: grant from the ‘Institut UPSA de la douleur’ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "To study the influence of the frequency of stimulation, three different sessions of rTMS separated by three weeks at least were randomly performed in each patient" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham coil as that used in Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: 3‐week washout observed and no clear imbalance in pre‐stimulation pain scores between conditions |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: the results of some of the planned data analysis (ANOVA of group differences after each condition) not reported. However, adequate data were available for inclusion in the meta‐analysis |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management n = 60 Age: 27‐79 years, mean 54.6 Duration of symptoms: not specified "chronic" Gender distribution: 28 M, 32 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior; 80% RMT; number of trains 20; duration of trains 5 s; ITI 55 s; total number of pulses 1000 Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition Control type: sham coil |
|
| Outcomes | Primary: 0‐10 VAS pain, anchors not specified When taken: 5 min poststimulation Secondary: none |
|
| Notes | COI: study authors declared no COI Sources of support: grant from the ‘Institut UPSA de la douleur’ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "one of the following two protocols was applied in a random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Quote: "ideal sham...which should be performed by means of a coil similar to the real one in shape, weight, and location on the scalp, producing a similar sound and similar scalp skin sensation, but generating no electrical field within the cortex. Such a sham coil has not yet been designed, and at present, the sham coil used in this study is to our knowledge the more valid for clinical trials." Comments: sham credibility assessment ‐ suboptimal |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: results for primary outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: 3‐week washout observed and no clear imbalance in pre‐stimulation pain scores between conditions |
| Study Size | Unclear risk | Comment: > 50 but < 200 participants per treatment condition |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT, 3 conditions | |
| Participants | Country of study: France Setting: laboratory Condition: unilateral chronic neuropathic pain (mixed central and peripheral) Prior management details: refractory to drug management n = 22 Age: 28‐75 years, mean 56.5 (SD 2.9) Duration of symptoms: 2‐18 years, mean 5.4 (SD 4.1) Gender distribution: 12 M, 10 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 6 s; ITI 54 s; total number of pulses 1200 Condition 2: frequency 1 Hz; coil orientation posteroanterior; 90% RMT; number of trains 1; duration of trains 20 min; total number of pulses 1200 Condition 3: sham coil Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition |
|
| Outcomes | Primary: 0‐10 VAS pain, anchors not specified When taken: pre‐ and poststimulation Secondary: none |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: grant from the ‘Institut UPSA de la douleur’ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Three sessions of motor cortex rTMS, separated by at least 3 weeks, were performed in random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham as Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors only reported for measures of cortical excitability |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: level of dropout not reported and unclear from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: pain score numerical values not provided clearly with measures of variance for any time point in the study report but were provided by the study authors on request |
| Free from carry‐over effects? | Low risk | Quote: "Post hoc tests did not reveal any differences between the three pre‐rTMS assessments regarding excitability values or pain levels" |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT, 3 conditions | |
| Participants | Country of study: France Setting: laboratory Condition: neuropathic pain (mixed central, peripheral and facial) Prior management details: refractory to drug management for at least 1 year n = 46 Age: 27‐79 years, mean 54.2 Duration of symptoms: chronic > 1 year Gender distribution: 23 M, 23 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation posteroanterior; 90% RMT; number of trains 20; duration of trains 6 s; ITI 54 s; total number of pulses 1200 Condition 2: frequency 1 Hz; coil orientation posteroanterior; 90% RMT; number of trains 1; duration of trains 20 min; total number of pulses 1200 Condition 3: sham coil Stimulation location: M1 contralateral to painful side Number of treatments: x 1 for each condition |
|
| Outcomes | Primary: 0‐10 VAS, anchors not specified When taken: pre‐ and poststimulation Secondary: none |
|
| Notes | AEs: not reported COI: study authors declared no COI Sources of support: grant from the ‘Institut UPSA de la douleur’ |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Three different sessions of rTMS..... were performed in a random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. This study used the same sham coil as that used in Lefaucheur 2004, which in that paper was stated as not meeting the criteria for an ideal sham |
| Adequate blinding of assessors? | Low risk | Quote: "In all cases, the examiner was blinded to the type of rTMS administered." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 2 participants dropped out but this is < 5% of the cohort. Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: results for all outcomes reported clearly and in full |
| Free from carry‐over effects? | Low risk | Comment: 3‐week washout observed and no clear imbalance in pre‐stimulation pain scores between conditions |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: outpatient fibromyalgia clinic Condition: fibromyalgia Prior management details: unclear n = 60 Age: 23‐82 years, mean 50 Duration of symptoms: 1‐40 years, mean 11 Gender distribution: 2 M, 58 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; 50% duty cycle; intensity 100 μA; waveform shape biphasic square wave; duration 60 min Stimulation location: ear clip electrodes Number of treatments: 30, x 1 daily for consecutive days Control type: sham unit ‐ indistinguishable from active unit |
|
| Outcomes | Primary: 10‐point self‐rating pain scale, anchors not specified When taken: poststimulation (not precisely defined) Secondary: QoL: 0‐10 VAS scale (data not reported) |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the subjects were randomly assigned into three separate groups by an office secretary who drew their names, which were on separate sealed slips of paper in a container" |
| Allocation concealment (selection bias) | Low risk | Comment: probably, given the quote above |
| Adequate blinding of participants? | Low risk | Comment: see previous quote |
| Adequate blinding of assessors? | Low risk | Quote: "All subjects, staff, the examining physician and the psychometrician remained blind to the treatment conditions" |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Dropout levels not specified in the report. ITT analysis not discussed in the report |
| Selective reporting (reporting bias) | High risk | Comment: pain score numerical values not provided clearly with measures of variance for any time points in the study report |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Germany Setting: back pain clinic Condition: chronic nonspecific low back pain Prior management details: excluded if had spinal surgery in previous 6 months n = 135 Age range: 26‐64 years, mean (SD) active group 45(9), sham group 44 (10) Duration of symptoms, mean (SD) active group 45 (9) months, sham group 44 (10) Gender distribution: 63 F, 72 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode L M1, cathode right supraorbital area Number of treatments: x1 daIly for 5 d Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS anchors not reported When taken: end of intervention, 4, 12 and 24 weeks postintervention Secondary: Oswestry Disability Index |
|
| Notes | Sources of support: "This study was funded by the Deutsche Forschungsgemeinschaft DFG (MA 1862/10‐1)." Competing interests: "AM, TJ, KL, and AP had financial support from DFG (MA 1862/10‐1) and NeuroImageNord for the submitted work." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “We randomised 160 stimulation codes (80 triggering active stimulation, 80 triggering sham stimulation) by custom written software into two separate lists.” |
| Allocation concealment (selection bias) | Low risk | Quote: “An independent researcher created the randomisation lists. To achieve allocation concealment the recruiter provided participants with the next unused stimulation code from the randomised lists. The recruiter had no access to the randomisation list.” |
| Adequate blinding of participants? | Low risk | Quote: “Blinding of participants and the treating physiotherapist was achieved by using a sham paradigm identical to the anodal stimulation procedure…. “ kappa agreement ‐0.120. Comment While 2 mA intensity can be inadequately blinded, assessment suggests blinding successful |
| Adequate blinding of assessors? | Low risk | Comment: while 2 mA intensity can be inadequately blinded, formal assessment suggests blinding successful |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 3 in each group discontinued in stimulation period. ITT approach |
| Selective reporting (reporting bias) | Low risk | Comment: reporting of all core outcomes |
| Study Size | Unclear risk | Comment: n = 67 and 68 per group |
| Study duration | Low risk | Comment: 24‐week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Colombia Setting: rehabilitation department Condition: phantom limb pain Prior management details: no difference across groups in use of NSAIDS, physical rehabilitation or psychological therapy n = 54 Age, mean (SD): active group 33.1 (6.6) years, sham group 8.2 (6.3) years Duration of symptoms: not reported Gender distribution: 50 M, 4 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45° angle from midline, 90% RMT number of trains 20; duration of trains 6 s; ITI 54 s; total number of pulses 1200 Stimulation location: M1 contralateral to painful side, no neuronavigation Number of treatments: 10 sessions x 1 per work day for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain possible When taken: 15 d and 30 d after treatment Secondary: AEs |
|
| Notes | Funding source: study was partially supported by a grant from the Colombian Science and Technology Institute (COLCIENCIAS, project code: 6566‐49‐326169). Felipe Fregni is the principal investigator at Spaulding Rehabilitation Hospital of a research grant funded by NIH (5R01HD082302‐02). COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “a computer‐generated randomization method with a permuted block size of 6 was used to allocate subjects to the sham or active rTMS interventions” |
| Allocation concealment (selection bias) | Low risk | Quote: “The randomization code was only given to the treating investigator on the first day of treatment session by an independent investigator not involved with any other aspect of the trial.” |
| Adequate blinding of participants? | Low risk | Comment: while sham coil did not control for scalp sensation blinding assessment suggested adequate blinding Quote: “Subjects and investigators did not guess correctly the treatment allocation beyond chance (P = .704; P = .571).” |
| Adequate blinding of assessors? | Low risk | Quote: “All evaluations were performed by an investigator blinded to treatment allocation.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 1 participant per group dropped out at 15 days and 2 per group at 30 days. ITT analysis performed Quote “We analyzed the end point of the study using the intention‐to‐treat method including patients who attended at least 1 of the rTMS sessions. The missing data were considered at random, thus we used a regression imputation method to handle this issue.” |
| Selective reporting (reporting bias) | Low risk | Comment: key outcomes presented at all follow‐up points |
| Study Size | High risk | Comment: n = 27 per group |
| Study duration | Unclear risk | Comment: 15‐day follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Factorial RCT | |
| Participants | Country of study: Brazil Setting: not specified Condition: chronic myofascial pain syndrome Prior management details: not reported n = 46, of which 23 relevant to this review Age, mean (SD): active group 45.83 (9.63) years, sham group 46.73 (13.09) years Duration of symptoms: not reported Gender distribution: all F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45° from midline, 80% RMT, number of trains not reported; duration of trains not reported; ITI s not reported; total number of pulses 1600 Stimulation location: L M1 Number of treatments: 10 days of stimulation Control type: sham coil ‐ no details provided |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst possible pain When taken: at end of intervention Secondary: none relevant |
|
| Notes | Funding source: supported by Brazilian funding agencies: National Council for Scientific and Technological Development—CNPq (Dr. I.L.S. Torres, W. Caumo, L.F. Medeiros; J. Dussan‐Sarria, A. Souza, V.L. Scarabelot); Graduate Research Group (GPPG) of Hospital de Clı´nicas de Porto Alegre (Dr W. Caumo— Grant # 100196 and Dr. I.L.S. Torres # 100276); Coordination for the Improvement of Higher Education Personnel—CAPES (A. Deitos); International Cooperation Program—CAPES (n8023/11). COI: authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “Participants were randomized to one of the four groups, using a stratified blocked randomization scheme and appropriate statistical Random Allocation Software.” |
| Allocation concealment (selection bias) | Low risk | Quote: “Each envelope was sealed and numbered sequentially and contained the allocated treatment. During the entire protocol timeline, two investigators who were not involved in patient evaluation were responsible for then blinding and randomization procedures” |
| Adequate blinding of participants? | Unclear risk | Quote: “A sham coil was used” Comment: insufficient description to know whether it controlled for all aspects on the experience. No formal assessment of blinding provided |
| Adequate blinding of assessors? | Low risk | Quote: “All participants were instructed not to discuss their group assignment during the treatment sessions or with the project staff collecting outcomes data, all of them were also blind to the group assignments. Independent evaluators’ blind to the group assignments were trained to apply the pain scales and cortical excitability parameter.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: low levels of dropout (2 participants in total) |
| Selective reporting (reporting bias) | Unclear risk | Comment: pain diary data not reported in the results with no clear explanation offered for the omission |
| Study Size | High risk | Comment: group sizes ranged from 11‐12 participants |
| Study duration | High risk | Comment: only follow‐up immediately postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil/USA Setting: laboratory Condition: fibromyalgia Prior management details: not reported n = 30 (6 per group) Age, mean (SD): 43.2 (9.8) years Duration of symptoms: not reported Gender distribution: 28 F, 2 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: simulation intensity 2 mA, 20 min duration Stimulation location: Group 1 cathodal M1; Group 2 cathodal supraorbital; Group 3 anodal M1; Group 4 anodal supraorbital; Group 5 sham Number of treatments: 1 session Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = worst possible pain When taken: immediately poststimulation Secondary: none relevant |
|
| Notes | COI: study authors declared no COI Sources of support: NIH |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that participant blinding may be suboptimal at this intensity |
| Adequate blinding of assessors? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that assessor blinding may be suboptimal at this intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts occurred |
| Selective reporting (reporting bias) | High risk | No numerical data provided for any post‐treatment clinical outcome. Data not provided upon request to study authors |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | No other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: excluded if undergoing physical treatment or were on stable pain control medication for "less than 2 months" n = 45 (of which 30 relevant to this review) Age, mean (SD): active group 44.5 (14) years, sham group 48 (11.8) years Duration of symptoms, mean (SD): active group 140.6 (72.2) months, sham group 149.3 (111.1) Gender distribution: 29 F, 1 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode L M1, cathode right supraorbital area Number of treatments: x 1 daIly for 5 days Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = worst pain imaginable When taken: postintervention, 1 month postintervention, 2 months postintervention Secondary: QoL SF‐36 AEs |
|
| Notes | Study authors declared that there were no COI | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: “Randomization was performed by a blinded therapist using sealed envelopes for each individual.” Comment: no description of the actual allocation sequence generation |
| Allocation concealment (selection bias) | Low risk | Quote: “Randomization was performed by a blinded therapist using sealed envelopes for each individual.” Comment: likely to be a concealed process |
| Adequate blinding of participants? | Unclear risk | Quote: “Participants were blinded to the intervention groups, as were the therapists who performed the evaluation.” Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Quote: “Participants were blinded to the intervention groups, as were the therapists who performed the evaluation.” Comment: Evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: ITT analysis using LOCF. Low for postintervention (< 10%) and high for 2/12 follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: adequate reporting of core outcomes |
| Study Size | High risk | n = 45 in 3 groups of which n = 30 relevant to this review |
| Study duration | Low risk | 2‐month postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: laboratory Condition: fibromyalgia Prior management details: not reported but concomitant treatments allowed n = 40 Age, mean (SD): active group 51.8 (11.6) years, sham group 49.6 (10) years Duration of symptoms (mean (SD) years): active group 13 (12.9), sham group 14.1 (11.9) Gender distribution: all F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior, number of trains 15; duration of trains 10 s; ITI 50 s, intensity 80% RMT, total number of pulses 1500 Stimulation location: L M1 Number of treatments: 14, x 1 daily for 5 days, x 1 weekly for 3 weeks, x 1 every two weeks for 6 weeks, x 1 monthly for 3 months Control type: sham coil, did not control for sensory cues |
|
| Outcomes | Primary: pain NRS; 0 = no pain, 10 = maximal pain imaginable When taken: day 5, 3 weeks, 9 weeks, 21 weeks, 25 weeks Secondary: BPI interference scale, FIQ |
|
| Notes | COI: study authors declared no COI Sources of support: Grants from the ‘‘Fondation APICIL’’ and the ‘‘Fondation de France |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned to 2 groups...with equal numbers in each group. A study nurse prepared the concealed allocation schedule by computer randomisation of these 2 treatment groups to a consecutive number series; the nurse had no further participation in the trial. Patients were assigned in turn to the next consecutive number." |
| Allocation concealment (selection bias) | Low risk | Comment: see quote above |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ sham coil controls for sound and appearance but not the skin sensation of stimulation |
| Adequate blinding of assessors? | Low risk | Quote: "Both patients and investigators were blind to treatment group. Cortical excitability measurements and transcranial stimulation were performed by an independent investigator not involved in the selection or clinical assessment of the patients." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: 25% dropout at long‐term follow‐up but intention‐to‐treat analysis used with BOCF imputation |
| Selective reporting (reporting bias) | Low risk | Comment: no numeric point measures provided for the primary outcome but provided upon request to the authors |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: > 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Italy Setting: laboratory Condition: neuropathic pain secondary to multiple sclerosis Prior management details: refractory to drug management and medication discontinued over previous month n = 19 Age: 23‐69 years, mean 44.8 (SD 27.5) Duration of symptoms: 1‐10 years, mean 2.79 (SD 2.64) Gender distribution: 8 M, 11 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: M1, contralateral to painful side Number of treatments: 5, x 1 daily on consecutive days Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: 0‐100 mm VAS pain, anchors "no pain" to "worst possible pain" When taken: end of treatment period and x 1 weekly over 3‐week follow‐up Secondary: QoL, multiple sclerosis QoL‐54 scale (MSQoL‐54) When taken: as for primary outcome |
|
| Notes | AEs: none COI: no declaration made Sources of support: "Italian National Ministero dell’Universita` e della Ricerca, by the Italian National Ministero della Salute, by the Fondazione Italiana Sclerosi Multipla (FISM) to DC, and by the Agenzia Spaziale Italiana to GB" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entrance in the study and a previous randomization list generated by a computer." |
| Allocation concealment (selection bias) | Low risk | Comment: likely given that the randomisation list was generated pre‐study |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts observed Quote: "... none of the patients enrolled discontinued the study." |
| Selective reporting (reporting bias) | Low risk | Comment: between‐group means not presented clearly to allow meta‐analysis but data provided on request |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Italy and Austria Setting: laboratory Condition: below level post SCI, predominantly neuropathic pain Prior management details: > 4/10 pain despite rehabilitation and pharmacological treatment. All participants previously treated with antidepressant, anticonvulsants and analgesics for a minimum period of 6 months n = 12 Age, mean (range): active group 43.7 (26‐56) years, sham group 42.5 (24‐62) years Duration of symptoms: not reported Gender distribution: 9 M, 3 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation AP direction, 120% RMT, number of trains 25; duration of trains 5 s; ITI 25s; total number of pulses 1250 Stimulation location: L PFC (no neuronavigation) Number of treatments: 10 sessions daily x 5 per week for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain VAS anchors not reported When taken: postintervention, 1 month postintervention Secondary: none relevant AEs |
|
| Notes | Funding source: no statement provided regarding funding COI: the study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Quote: “Sham stimulation was carried out with a sham coil of identical size color and shape emitting a sound similar to that emitted by the active coil.” Comment: Sham suboptimal ‐ no control for cutaneous sensation associated with stimulation |
| Adequate blinding of assessors? | Low risk | Quote “Pain was assessed by an investigator blinded to the type of rTMS subjects were receiving.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: data reported adequately |
| Study Size | High risk | Comment: n = 12 |
| Study duration | Unclear risk | Comment: 1 month postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Thailand Setting: laboratory Condition: neuropathic pain associated with SCI Prior management details: refractory to medication including antidepressants, antiepileptics and opioids n = 20 Age, mean (SD) 44.5 (9.16) years Duration of symptoms: 50.1 (37.05) months Gender distribution: 15 M 5 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to most painful side, cathode supraorbital area contralateral to anode Number of treatments: x 1 session Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = the most possible pain When taken: immediately poststimulation Secondary: AEs |
|
| Notes | No author declaration of COI made Sources of support "This work was supported by an invitation research grant, Faculty of Medicine, Khon Kaen University, Thailand (Grant number I 55229), the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission and Faculty of Social Science, Naresuan University, Phitsanulok, Thailand." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “participants were randomized to receive either active tDCS followed by sham tDCS, or sham tDCS stimulation followed by active tDCS in a 1:1 ratio using a computer generated list of random numbers in blocks of four randomizations.” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: < 10% dropout rate |
| Selective reporting (reporting bias) | Low risk | Comment: numeric data on pain outcomes not presented in the paper. All data provided by study authors upon request |
| Free from carry‐over effects? | Low risk | Comment: preliminary ANOVA analyses yielded no significant main or interaction effects involving condition order |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: maximum follow‐up 1 week postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: UK Setting: laboratory Condition: mixed refractory neuropathic pain Prior management details: no benefit from medication or other stimulation approaches n = 40 (27 after loss to follow‐up) Age, range: 27‐79 years Duration of symptoms: not reported Gender distribution: 23 M, 17 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation AP direction, 90% RMT, number of trains 20; duration of trains 10 s; ITI 1 min; total number of pulses 2000 Stimulation location: Site A: M1 hotspot, Site B M1 reorganised area, Site C (sham) occipital fissure Number of treatments: 3‐5 sessions per week for 5 sessions Control type: sham active stimulation of occipital fissure |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain 10 = worst pain imagined When taken: postintervention, 3 weeks postintervention Secondary: none relevant AEs |
|
| Notes | Funding source: research funded by the National Institute for Health Research (NIHR) under Research for Patient Benefit (RfPB) Programme (Grant Reference Number PB‐PG‐0110‐20321). COI: Prof. Nurmikko has received travel sponsorship from Nexstim Ltd. None of the other authors report any COI. |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients were randomly allocated to receive three cycles of rTMS in 5 sessions at sites A, B, and SHAM. Randomization order was computer generated." |
| Adequate blinding of participants? | Low risk | Comment: sham was active stimulation of a non target brain area‐ likely indistinguishable from active stimulation |
| Adequate blinding of assessors? | Low risk | Comment: outcomes self‐reported via pain diaries |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 40 randomised, 38 received rTMS, 27 included in per‐protocol analysis (33% attrition). Responder analysis n = 33 (17% dropout) Reasons for dropout not reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Free from carry‐over effects? | Low risk | Comment: 3 weeks washout period observed. Baseline pain levels for each condition appear equivalent |
| Study Size | High risk | Comment: n = 40, 27 after loss to follow‐up |
| Study duration | Unclear risk | Comment: 3 week follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: chronic temporomandibular disorder Prior management details: excluded if received any type of physiotherapy in preceding month n = 32 Age, mean (SD): active group 23.80 (7.3) years sham group 25.5 (6.3) years Duration of symptoms, months mean (SD): active group 29.8 (17.1), sham group 33.7 (22.8) Gender distribution: 3 M, 29 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to painful side, cathode supraorbital area, contralateral to anode Number of treatments: daily sessions for 5 consecutive days. Then twice a week for 3 weeks, up to 10 sessions Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS, anchors not reported When taken: 5 months postintervention, no data reported from formal study period Secondary: QoL WHO‐QoL, AEs |
|
| Notes | Sources of support: study was carried out without funding COI: study authors decare no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “After the first comprehensive evaluation, the secretary of the clinical facility, who was not involved with any other procedures of the study, randomised participants who fulfilled the inclusion criteria for treatment and accepted to participate in the study. Randomisation occurred by the simple random method, in which each subject was invited to remove a small sealed envelope from a larger opaque envelope indicating two treatment groups.” |
| Allocation concealment (selection bias) | Low risk | Quote: “After the first comprehensive evaluation, the secretary of the clinical facility, who was not involved with any other procedures of the study, randomised participants who fulfilled the inclusion criteria for treatment and accepted to participate in the study." |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. 15 guessed stimulation condition correctly in active group vs 7 in sham group |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no attrition noted for core follow‐up points |
| Selective reporting (reporting bias) | Low risk | Comment: no numeric reporting of point estimates for most outcomes but data provided upon request |
| Study Size | High risk | Comment: n = 32 |
| Study duration | Unclear risk | Comment: formal follow‐up for 3 weeks postintervention |
| Other bias | Low risk | Commet: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Italy Setting: laboratory n = 25 Condition: neuropathic pain from diabetic neuropathy Prior management details: resistant to standard therapies for at least 1 year Age mean (SD): 70.6 (8.5) years Duration of symptoms (months mean (SD)): not reported Gender distribution: 9 F, 14 M |
|
| Interventions | Stimulation type: rTMS using H‐coil Stimulation parameters: frequency 20 Hz; coil orientation H coil, number of trains 30; duration of trains 2.5 s; ITI 30 s, intensity 100% RMT, total number of pulses 1500 Stimulation location: M1 lower limb (deep in central sulcus) Number of treatments: 5 per condition on consecutive days Control type: sham coil, controlled for scalp sensory, auditory and visual cues |
|
| Outcomes | Primary: pain VAS 0‐100, no pain to worst possible pain When taken: immediately poststimulation, 3 weeks poststimulation Secondary: none relevant |
|
| Notes | COI: 2 authors have links to the manufacturer of the H‐coil Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "After enrolment, patients were randomly assigned in a 1:1 ratio to two counterbalanced arms by receiving a sequential number from a computer‐generated random list." |
| Adequate blinding of participants? | Low risk | Quote: "Sham stimulation was delivered with a sham coil placed in the helmet encasing the active rTMS coil. The sham coil produced a similar acoustic artefact and scalp sensation as the active coil and could also mimic the facial muscle activation induced by the active coil. It induced only a negligible electric field inside the brain because its non‐tangential orientation on the scalp and components cancelling the electric field ensured that it rapidly reduced the field as a function of distance" Comment: controlled for visual auditory and sensory aspects of stimulation |
| Adequate blinding of assessors? | Unclear risk | Comment: while study described as "double blind" there was no specific mention of blinding assessors |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 2 participants lost to follow‐up |
| Selective reporting (reporting bias) | High risk | Comment: data not presented by stimulation condition ‐ rather they were grouped by the order in which interventions were delivered. No SDs presented. Data requested |
| Free from carry‐over effects? | Low risk | Comment: 5‐week washout period observed with no difference at T3 |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: France Setting: laboratory Condition: MS‐related neuropathic pain Prior management details: stable pharmacological and physical therapies for at least 1 month n = 16 Age, mean (SD) 47.4 (8.9) years Duration of symptoms: not reported for pain Gender distribution: 13 F, 3 M |
|
| Interventions | Stimulation type: tRNS Stimulation parameters: Intensity 1 mA, 25 cm2 electrodes, duration 20 min, VARIANCE 650/2 μA Stimulation location: M1 contralateral to most painful side Number of treatments: x 1 daily for 3 days Control type: sham tRNS |
|
| Outcomes | Primary: pain VAS, anchors not reported When taken: average for 7 days postintervention Secondary: BPI interference, AEs |
|
| Notes | COI: "FP has received grants from neuroConn GmbH, Ilmenau, Germany. The other authors declare no conflict" Sources of support: not reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Adequate blinding of participants? | Low risk | Quote: "Neither the patients nor the evaluators were aware about the nature of the stimulation block." Comment: assessment of participant blinding integrity suggests success |
| Adequate blinding of assessors? | Low risk | Quote: "Neither the patients nor the evaluators were aware about the nature of the stimulation block." |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2 participants (13%) withdrew and data were excluded |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Free from carry‐over effects? | Unclear risk | Comment: 3‐week washout period observed but no formal assessment of carry‐over effects |
| Study Size | High risk | Comment: n = 16 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: France Setting: laboratory Condition: fibromyalgia Prior management details: unclear n = 30 Age: active group: 52.6 (SD 7.8) years, sham group 55.3 (SD 8.9) years Duration of symptoms: active group: 8.1 (SD 7.9), sham group: 10.8 (SD 8.6) Gender distribution: 1 M, 29 F |
|
| Interventions | Stimulation type: rTMS, figure‐of‐8 coil Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior; 80% RMT; number of trains 25; duration of trains 8 s; ITI 52 s; total number of pulses 2000 Stimulation location: M1 contralateral to painful side Number of treatments: 10, x 1 daily for 10 working days Control type: sham rTMS coil. Mimics sight and sound of active treatment |
|
| Outcomes | Primary: 0‐10 NRS of average pain intensity over last 24 h, anchors "no pain" to "maximal pain imaginable" When taken: daily during treatment period and at 15, 30 and 60 days post‐treatment follow‐up Secondary: FIQ When taken: as for primary outcome |
|
| Notes | COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "patients who met all inclusion criteria were randomly assigned, according to a computer‐generated list, to two groups" |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Unclear risk | Quote: "Sham stimulation was carried out with the 'Magstim placebo coil system', which physically resembles the active coil and makes similar sounds." Comment: sham credibility assessment ‐ suboptimal. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation over the scalp |
| Adequate blinding of assessors? | Low risk | Quote: "... investigators were blind to treatment group." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: equal dropout in each group and appropriately managed in the data analysis Quote: "All randomized patients with a baseline and at least one post‐baseline visit with efficacy data were included in the efficacy analyses (intent to treat analysis)." "All the patients received the full course of treatment and were assessed on D15 and D30. Four patients (two in each treatment group) withdrew from the trial between days 30 and 60." |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values not provided clearly with measures of variance for all time points in the study report, the study authors provided the requested data |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: ≥ 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: laboratory Condition: CRPS type I Prior management details: refractory to best medical treatment n = 23 Age mean (SD): active group 43.5 (12.1) years, sham group 40.6 (9.9) years Duration of symptoms (months mean (SD)): active group 82.33 (34.5), sham group 79.27 (32.1) Gender distribution: 14 F, 9 M |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation posteroanterior, number of trains 25; duration of trains 10 s; ITI 60 s, intensity 100% RMT, total number of pulses 2500 Stimulation location: M1 contralateral to painful limb Number of treatments: 10, x 1 daily on consecutive weekdays Control type: sham coil ‐ did not control for sensory cues |
|
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "most severe pain" When taken: after first and last session then 1 and 3 months post‐treatment Secondary: QoL SF‐36, not reported |
|
| Notes | COI: study authors declared no COI Sources of support: University of Sao Paolo, Brazil |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: while stated "randomized" the method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: sham suboptimal as it did not control for scalp sensation. Study reported that number who guessed the condition correctly was similar but no formal data or analysis reported |
| Adequate blinding of assessors? | Unclear risk | Comment: study described as "double‐blinded" but assessor blinding not specifically reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 participant dropped out at follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: data presented for primary outcome. While this was not adequate for meta‐analysis it did not really constitute selectivity. No response received to request for full data access |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: ≥ 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: laboratory Condition: CRPS type I Prior management details: drug management ceased for 48 h prior to study n = 10 Age: 29‐72 years, mean 51 Duration of symptoms: 24‐72 months, mean 35 Gender distribution: 3 M, 7 F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation unspecified; 110% RMT; number of trains 10; duration of trains 1.2 s; ITI 10 s; total number of pulses 120 Stimulation location: M1 hand area Number of treatments: 1 for each condition Control type: coil angled 45º away from scalp |
|
| Outcomes | Primary: 0‐10 VAS current pain intensity, anchors "no pain" to "most extreme pain" When taken: 30 s, 15, 45 and 90 min poststimulation Secondary: none When taken: 30 s, 15, 45 and 90 min poststimulation |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: "grant from the BMBF (NR. 01EM0102) and by a grant of the Scientific Research Council of BG‐Kliniken Bergmannsheil, Bochum." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Using a computerized random generator, five patients were first assigned to the placebo group (sham rTMS), while the others were treated using verum rTMS" |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled 45º away from scalp. Did not control for sensory characteristics of active stimulation and was visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout apparent from the data presented |
| Selective reporting (reporting bias) | Low risk | Comment: while sham group results not presented in the study report, the study authors provided the requested data |
| Free from carry‐over effects? | Low risk | Quote: "The initial pain intensities (VAS) were similar prior to verum and sham rTMS (Student’s paired t‐test, P = 0.47). The level of intensity was also independent of whether the patients were first subjected to sham or verum rTMS (P > 0.05)." |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: postburn neuropathic pain Prior management details: varied n = 3 Age range: 34‐52 years Duration of symptoms: > 6 months Gender distribution: 2 F, 1 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 contralateral to most painful side Number of treatments: 1 per condition Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst pain ever felt" When taken: before and after stimulation Secondary: none relevant |
|
| Notes | COI: study authors declared no COI Sources of support: departmentally funded |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "subjects were randomized to either active tDCS or sham stimulation." Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: all 3 participants completed study |
| Selective reporting (reporting bias) | High risk | Comment: no numeric data provided for pain outcomes |
| Free from carry‐over effects? | Unclear risk | Comment: 1‐week washout observed but no data reported for pain outcome so unable to assess this issue |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: rehabilitation clinic Condition: fibromyalgia Prior management details: none reported n = 23 Age mean (SD): active group 58.3 (12.1) years, sham group 52.4 (11.5) years Duration of symptoms, months (mean (SD)): active group 9.9 (11.8), sham group 6.4 (10.3) Gender distribution: all F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 10, x 1 weekly for 10 weeks Control type: sham tDCS (switched off after 30 s stimulation) Both groups received 4 months rehabilitation programme |
|
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst pain" When taken: immediately at end of 4‐month rehabilitation programme Secondary: QoL SF36, FIQ |
|
| Notes | AEs: not reported COI: study authors declared no COI Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated simple randomisation method but method not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA used, which may threaten assessor blinding, though formal analysis of blinding appears acceptable |
| Adequate blinding of assessors? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that assessor blinding may be suboptimal at this intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropouts |
| Selective reporting (reporting bias) | Low risk | Comment: while numeric data on the primary outcome not reported in study report the authors made it available upon request |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: there were group imbalances at baseline on the duration of pain, education, age and economic activity |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: outpatient clinic, participants took device home Condition: pain related to Parkinson's disease Prior management details: not reported n = 19 (reduced to 13 through dropout) Age mean (SD): active group 74.7 (7.8) years, sham group 74.4 (8.3) years Duration of symptoms: > 6 months Gender distribution: 15 M, 4 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 100 μA; waveform shape not specified; duration 40 min per session Stimulation location: earlobe clips Number of treatments: 42, x 1 daily for 42 days Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: pain VAS 0 ‐10, anchors not reported When taken: at the end of the treatment period Secondary: none |
|
| Notes | Sources of support: equipment provided by CES manufacturer as an "unrestricted gift" COI: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: stated randomised but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Low risk | Comment: see above comment |
| Adequate blinding of assessors? | Low risk | Comment: participants and the study co‐ordinator were blinded to group assignment and the code sheet indicating which devices were active and which were sham was kept by another person who was not in contact with the participants |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: > 30% dropout |
| Selective reporting (reporting bias) | Low risk | Comment: mean (SD) pain scores reported for both groups pre‐ and poststimulation |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Germany Setting: pain clinic Condition: chronic pain (mixed musculoskeletal and neuropathic) Prior management details: "intractable" n = 12 Age: 33‐67 years, mean 51.3 (SD 12.6) Duration of symptoms: mean 2.7 (SD 2.4) Gender distribution: 6 M, 6 F |
|
| Interventions | Stimulation type: rTMS, circular coil for arm symptoms, double cone coil for leg symptoms Stimulation parameters: frequency 20 Hz; coil orientation not specified; 80% RMT; number of trains 20; duration of trains 2 s; ITI not specified; total number of pulses 800; treatment duration 20 min Stimulation location: M1 (midline) Number of treatments: x 1 for each condition Control type: coil angled 45º away from the scalp |
|
| Outcomes | Primary: 0‐100 mm VAS pain intensity, anchors "no pain" to "unbearable pain" When taken: 0, 5, 10 and 20 min post‐stimulation Secondary: none |
|
| Notes | Sources of support: supported by Deutsche Forschungsgemeinschaft COI: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "sham and active stimulation were given in a random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comments: sham credibility assessment ‐ suboptimal. Coil angled 45º away from scalp. Did not control for sensory characteristics of active stimulation over the scalp and was visually distinguishable. Given that stimulation was delivered at 110% RMT active stimulation, but not sham, likely to have elicited muscle twitches in peripheral muscles |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 participant withdrew due to "headaches". Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values not provided clearly with measures of variance for all time points in the study report, the study authors provided the requested data |
| Free from carry‐over effects? | Low risk | Comment: not clearly demonstrated in the study report but clear from unpublished data provided by the study authors (baseline mean group pain scores: active stimulation 65.1 (SD 16), sham stimulation 66.9 (SD 17.4)) |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT, 4 conditions | |
| Participants | Country of study: Japan Setting: laboratory Condition: neuropathic pain (mixed central and peripheral) Prior management details: intractable n = 13 Age: 29‐76 years, mean 59.4 Duration of symptoms: 2‐35 years, mean 10.2 (SD 9.7) Gender distribution: 7 M, 6 F |
|
| Interventions | Stimulation type: rTMS figure‐of‐8 coil Stimulation parameters: Condition 1: frequency 10 Hz; coil orientation not specified; 90% RMT; number of trains 5; duration of trains 10 s; ITI 50 s; total number of pulses 500 Condition 2: frequency 5 Hz; coil orientation not specified; 90% RMT; number of trains 10; duration of trains 10 s; ITI 50 s; total number of pulses 500 Condition 3: frequency 1 Hz; coil orientation not specified; 90% RMT; number of trains 1; duration of trains 500 s; total number of pulses 500 Condition 4: sham, coil angled 45º from scalp with synchronised electrical scalp stimulations to mask sensation Stimulation location: M1 over the representation of the painful area Number of treatments: 1 for each condition |
|
| Outcomes | Primary: VAS pain, anchors not specified When taken: 0, 15, 30, 60, 90 and 180 minutes poststimulation Secondary: none |
|
| Notes | Sources of support: no declaration made COI: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "rTMS was applied to all the patients at frequencies of 1, 5, and 10 Hz and as a sham procedure in random order" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comment: sham credibility assessment ‐ suboptimal. Sensory and auditory aspects controlled for but angulation of coil away from the scalp may be visually distinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Quote: "All 13 patients participated in all planned sessions of navigation‐guided rTMS" Comment: no dropouts observed |
| Selective reporting (reporting bias) | Low risk | Comment: while pain score numerical values not provided clearly with measures of variance for all time points in the study report, the study authors provided the requested data |
| Free from carry‐over effects? | Low risk | Comment: not clearly demonstrated in the study report but paired t‐tests on unpublished baseline data provided by the study authors suggest that carry‐over was not a significant issue |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Thailand Setting: laboratory Condition: myofascial pain syndrome (affecting shoulder) Prior management details: stable analgesic use for 3 months preceding study n = 31 Age mean (SD): active group 49.94 (8.25) years, sham group 45.93 (10.24) years Duration of symptoms, mean(SD) active group 5.91 (2.55) months, sham group 45.93 (10.24) Gender distribution: 22 F, 9 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 1 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to most painful side, cathode supraorbital area contralateral to anode Number of treatments: x 1 daily for 5 days Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = the most possible pain When taken: post‐treatment, average of daily score in week 1 postintervention, week 2, 3, 4 postintervention. Only responder analysis presented Secondary: QoL WHO‐QoL, data not reported AEs |
|
| Notes | COI: "M.P.J. is a consultant to Noninvasive Brain Stimulation Research Group of Thailand. The remaining authors declare no conflict of interest." Sources of support: "Supported in part by Grant Number R21 HD058049 from the National Institutes of Health, National Institute of Child Health and Human Development, Rockville, MD; and National Center for Medical Rehabilitation Research, Rockville, MD." |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of sequence generation not described |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment procedures not described |
| Adequate blinding of participants? | Low risk | Comment: “The tDCS device was designed to allow for masked (sham) stimulation. Specifically, the control switch was in front of the instrument, which was covered by an opaque adhesive during stimulation. The power indicator was on the front of the machine, which lit up during the time of stimulation both in active and sham stimulations.” |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not described |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 dropout |
| Selective reporting (reporting bias) | Low risk | Comment: no numeric reporting of pain score or QoL point estimates in the paper. All data provided by study authors upon request |
| Study Size | High risk | Comment: n = 31 |
| Study duration | Unclear risk | Comment: 4‐week follow‐up postintervention |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: fibromyalgia Prior management details: naive to TMS n = 20 Age mean (SD): active group 54.2 (8.28) years, sham group 51.67 (18.19) years Duration of symptoms, years mean (SD): active group 12.1 (7.75), sham group 10.10 (12.81) Gender distribution: 84% F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation parasagittal, number of trains 80; duration of trains 5 s; ITI 10 s, intensity 120% RMT, total number of pulses per session 4000 Stimulation location: L DLPFC Number of treatments: 10, x 1 daily (working days) for 2 weeks Control type: sham coil |
|
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst pain" When taken: after 1 and 2 weeks of treatment, then 1 week and 2 weeks posttreatment Secondary: FIQ, BPI function scale |
|
| Notes | AEs: no data provided COI: 1 researcher received research grants from the device manufacturer and holds patents for TMS technology Sources of support: Multidisciplinary Clinical, Research Center Grant P60 AR049459 The Office of the Provost and Vice President for Research |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Patients were randomly assigned (random generator software developed by JJB in the Brain Stimulation Laboratory)" |
| Allocation concealment (selection bias) | Low risk | Quote: "A co investigator not directly involved in ratings or treatment released treatment condition to the TMS operator" |
| Adequate blinding of participants? | Low risk | Quote: "A specially designed sham TMS coil is used for all sham conditions that produces auditory signals identical to active coils but shielded so that actual stimulation does not occur. However, subjects do experience sensory stimulation that is difficult to distinguish from real rTMS" Comment: sensory, auditory and visual cues controlled for |
| Adequate blinding of assessors? | Low risk | Quote: "A masked continuous rater assessed patients at baseline, at the end of each treatment week, and at the 2 follow‐up weeks. Importantly the continuous rater did not administer the TMS, minimizing the chances of unmasking due to events during the TMS treatment session." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: full reporting of primary outcomes |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Spain Setting: laboratory Condition: post‐SCI neuropathic pain Prior management details: stable pharmacological treatment for at least 2 weeks prior to start of treatment. Unresponsive to medication n = 39 Age mean (SD): 45 (15.5) years Duration of symptoms: not reported Gender distribution: 30 M, 9 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 10, x 1 daily (working days) for 2 weeks Control type: 4 groups, tDCS + visual illusion, sham tDCS + visual illusion, tDCS + control illusion, sham tDCS + control illusion |
|
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = unbearable pain; mean over previous 24 h When taken: end of treatment period, 12 and 24 d post‐treatment Secondary: BPI pain interference scale |
|
| Notes | COI: no declaration made Sources of support: "grants from a BBVA Translational Research Chair in Biomedicine, the International Brain Research Foundation (IBRF) and National Institutes of Health grant K 24 RR018875 to A.P.L., the Foundation La Marato´ TV3 (071931) and grant PI082004 and TERCEL funds from the Instituto de Salud Carlos III" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "We used a computer generated list as randomisation strategy." |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: 2 mA may threaten blinding but assessment of blinding seemed OK |
| Adequate blinding of assessors? | Unclear risk | Comment: 2 mA intensity used ‐ empirical evidence that assessor blinding may be suboptimal at this intensity |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 3 dropouts, 1 in each group |
| Selective reporting (reporting bias) | Low risk | Comment: all main outcomes reported |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other biases detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Brazil Setting: reference centre for integrated and multidisciplinary treatment for human T‐lymphotropic virus 1 (HTLV‐1) and viral hepatitis Condition: JTLVI‐infected patients with chronic low back or lower limb pain Prior management details: stable pharmacotherapy in the preceding month n = 20 Age, mean (SD): active group 48.8 (11.6) years, sham group 56.2 (14) years Duration of symptoms: not reported Gender distribution: 15 F, 5 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 25 cm2 electrodes, duration 20 min Stimulation location: anode L M1, cathode right supraorbital area Number of treatments: x 1 daIly for 5 days Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS; 0 = no pain, 10 = worst possible pain When taken: postintervention, responder analysis 30%, 50% pain relief Secondary: AEs |
|
| Notes | COI: the study authors declared no COI Sources of support: "G.S.G. was funded by FAPESB, Salvador, BA/Brazil (Fundação de Amparo à Pesquisa do Estado da Bahia) and M.E.M by CAPES, Brasília, DF/Brazil (Coordenação de Aperfeiçoamento Pessoal de Nível Superior)" |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Participants were randomized using a stratified randomization strategy with pain as the stratification factor.” |
| Allocation concealment (selection bias) | Low risk | Quote “A previously generated randomization list was used to allocate the patients to each stratum, in accordance with the order of their entrance into the study. A researcher who was not involved with assessments or interaction with participants randomized and allocated the patients” |
| Adequate blinding of participants? | Unclear risk | Comment: evidence that blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Comment: evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2 dropouts (20%) from sham group, imputation with LOCF |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: postintervention follow‐up only |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: tertiary care teaching hospital Condition: neuromuscular pain (excluding fibromyalgia) Prior management details: unclear n = 28 Age: 45‐65 years, mean 55.6 Duration of symptoms: 4‐45 years, mean 15 Gender distribution: 25 M, 3 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; pulse width not specified; intensity 10‐600 μA; waveform shape not specified Stimulation location: ear clip electrodes Number of treatments: 12, frequency of treatment not specified Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: VAS 0‐5 pain intensity When taken: pre‐ and post‐ each treatment Secondary: life interference scale, sickness impact profile ‐ Roland Scale When taken: not specified |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "each subject was randomly assigned to receive either the active or the sham treatment first" Comment: method of randomisation not specified but less critical in cross‐over design |
| Adequate blinding of participants? | Low risk | Quote: "sham treatment was made possible by having the treatment delivered via a black box" Comment: sham and active stimulators visually indistinguishable |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding of assessors not reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: only 17 participants completed the study and this dropout (over 50%) is not clearly accounted for in the analysis |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcome data presented clearly |
| Free from carry‐over effects? | Low risk | Quote: "Note that there were no significant differences in pain ratings pre‐post changes between the active and sham groups" |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: participants also received local stimulation to the painful area that may have elicited a therapeutic effect |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: medical centre Condition: post‐SCI pain (not clearly neuropathic) Prior management details: unclear n = 40 Age: 38‐82 years Duration of symptoms: chronic > 6 months Gender distribution: all M |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 100‐500 μA; waveform shape not specified; duration 1 h per session Stimulation location: ear clip electrodes Number of treatments: 21, x 1 daily for consecutive days Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: BPI (0‐10 NRS), anchors "no pain" to "pain as bad as you can imagine" When taken: post‐treatment period Secondary: pain interference subscale of BPI When taken: as for primary outcome |
|
| Notes | AEs: not reported COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Quote: "The participants were then randomly assigned to either the active or sham CES treatment groups" Comment: method of randomisation not specified |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not specified |
| Adequate blinding of participants? | Low risk | Comment: see quote above |
| Adequate blinding of assessors? | Low risk | Quote: "The investigators,research assistant (RA), and participants were blinded to treatment type until the end of the initial phase." |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 2 (5%) participants withdrew from the study. Unlikely to have strongly influenced the findings |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes presented clearly and in full |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: 4 Veterans Affairs medical centres and 1 private rehabilitation clinic Condition: post‐SCI neuropathic pain Prior management details: not reported n = 105 Age mean (SD): active group 52.1 (10.5) years, sham group 52.5 (11.7) years Gender distribution: 90 M, 15 F |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency not specified; pulse width not specified; intensity 100 μA; waveform shape not specified; duration 1 h per session Stimulation location: earlobe clips Number of treatments: 21, x 1 daily Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: BPIpain intensity VAS 0‐100, anchors not reported When taken: at end of treatment period Secondary: QoL SF‐12 physical and mental component subscales |
|
| Notes | COI: study authors declared no COI Sources of support: funded by Veterans Affairs rehabilitation research and development service |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "The equipment was set up for a double‐blind study by the manufacturer such that the participants could not differentiate active from sham CES devices. Research staff members who interacted with the participants (e.g. recruited and trained participants, administered questionnaires, followed up by telephone) did not know which devices were sham and which were active. Randomization was achieved by selecting a device from a box initially containing equal numbers of active and sham devices." Comment: whilst unconventional it appeared to avoid a systematic bias |
| Allocation concealment (selection bias) | Low risk | Comment: see quote/comment above |
| Adequate blinding of participants? | Low risk | Comment: stimulation subsensory and units indistinguishable |
| Adequate blinding of assessors? | Low risk | Comment: stimulation subsensory and units indistinguishable |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: available case analysis with small loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: key outcomes fully reported |
| Study Size | Unclear risk | Comment: > 50 but < 200 participants per treatment condition |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline between‐group imbalances on BPI pain interference, SF‐36 pain subscale and coping strategies |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: community rheumatology practices Condition: fibromyalgia Prior management details: not reported but continued stable medication usage n = 57 (46 after dropout) Age mean (SD): active group 51 (10.6) years, sham group 51.5 (10.9) years, usual care group 48.6 (9.8) years Duration of symptoms: not reported Gender distribution: 43 F, 3 M (data reported on completers) |
|
| Interventions | Stimulation type: CES Stimulation parameters: frequency 0.5 Hz; pulse width not specified; intensity 100 μA; waveform shape square wave biphasic, duration 1 h per session Stimulation location: earlobe clip electrodes Number of treatments: x 1 daily for 8 weeks Control type: sham CES unit indistinguishable from active unit |
|
| Outcomes | Primary: pain VAS, anchors not reported When taken: at the end of each week of treatment period Secondary: FIQ |
|
| Notes | COI: no declaration made Sources of support: University of Virginia. Center for the study of Complementary and Alternative Therapies. Devices loaned by Electromedical Products International |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: described as randomised but method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Low risk | Comment: identical devices given to sham and active group with subsensory stimulation parameters |
| Adequate blinding of assessors? | Low risk | Comment: participants self‐rated at home |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: of 57, 11 did not complete ‐ unclear if ITT analysis employed. However, only 2‐4 per group and balanced, mostly due to assessment burden |
| Selective reporting (reporting bias) | Low risk | Comment: while no numeric data were provided on primary outcomes in the study report, these data were provided upon request to the authors |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other source of bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Turkey Setting: Rehabilitation outpatient unit Condition: fibromyalgia Prior management details: no analgesic use for 1 month prior to enrolment n = 51 Age mean (SD): active group 42.4 (78.63) years, sham group 46.5 (8.36) years Duration of symptoms: mean (SD) active group 10.81 (6.31) years, sham group 13.33 (6.65) Gender distribution: 47 F, 4 M |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation 45º angle from the midline, 100% RMT number of trains 30; duration of trains 5 s; ITI 12 s; total number of pulses 1500 Stimulation location: M1 midline, no neuronavigation Number of treatments: 10 sessions daily ‐ unclear whether only work days Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = most severe pain When taken: end of intervention Secondary: WHQoL‐BREF |
|
| Notes | Funding source: none reported COI: the study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote “Randomisation was completed with the help of a software programme that produces random allocation” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: placebo coil did not control for the sensory aspects of stimulation. No formal assessment of blinding success reported |
| Adequate blinding of assessors? | Low risk | Quote: “the investigator who conducted the clinical evaluation received no information about patient admission, randomisation or mode of treatment” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 participant lost to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: no suggestion of selective outcome reporting |
| Study Size | High risk | Comment: 25 and 27 participants in each group |
| Study duration | High risk | Comment: only immediate postintervention follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: post‐SCI neuropathic pain (sublesion) Prior management details: not reported n = 33 (14 after loss to follow‐up in phase one) Age, mean (SD): active group 51.38 (14.89) years, sham group 51 (10.11) years Duration of symptoms: not reported Gender distribution: 24 M, 9 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: tDCS: 2 mA intensity, 20 min Stimulation location: M1 contralateral to painful side Number of treatments: x1 daily for 5 days in phase one. Phase 2 not relevant to this review Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS anchors 0 = no pain 10 = pain as bad as you can imagine When taken: postintervention, 1 week postintervention, 3 months postintervention Secondary: QoL (PHQ‐9) AEs |
|
| Notes | Funding source: this project was supported by the National Institute on Disability, Independent Living, and Rehabilitation Research (NIDILRR grant numbers 90DP0035 and H133N110010). COI: study authors declared no COI |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not reported |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Adequate blinding of assessors? | Unclear risk | Comment: blinding can be compromised at 2 mA intensity. No formal blinding assessment reported |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: while ITT analysis reported with multiple imputation, at the end of phase one, dropout was 57% |
| Selective reporting (reporting bias) | Low risk | Comment: data reported adequately |
| Study Size | High risk | Comment: n = 33 (14 after loss to follow‐up) |
| Study duration | Low risk | Comment: 3‐month follow‐up for phase 1 |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Unclear, likely parallel RCT (for 1 Hz only), 10 Hz data open‐label therefore excluded from this review | |
| Participants | Country of study: USA Setting: not reported, likely laboratory Condition: fibromyalgia Prior management details: "moderate to severe despite current and stable treatment regime" n = unclear, abstract report (Schneider 2012 (see Tzabazis 2013)) stated 45, but full paper stated 16 Age mean (SD): 53.2 (8.9) years Duration of symptoms, years mean (SD): not reported Gender distribution: 14 F, 2 M |
|
| Interventions | Stimulation type: rTMS 4‐coil configuration Stimulation parameters: frequency 1 Hz; no of trains not reported; duration of trains not reported; ITI not reported, intensity 110% RMT, total number of pulses per session 1800, stimulation duration 30 min Stimulation location: targeted to the anterior cingulate cortex Number of treatments: 20, x 1 daily (working days) for 4 weeks Control type: sham coil |
|
| Outcomes | Primary: BPI average pain last 24 h, NRS, anchors not reported When taken: end of treatment, 4 weeks post‐treatment Secondary: FIQ |
|
| Notes | COI: 3 study authors have acted as paid consultants to the manufacturer of the stimulation device, of which 2 hold stock in the company and 1 founded the company, is its chief medical officer and has intellectual property rights Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: no description of the sequence generation process used |
| Allocation concealment (selection bias) | Unclear risk | Comment: no description of allocation concealment |
| Adequate blinding of participants? | Unclear risk | Comment: no description of blinding of participants for clinical part of study. Sham coil controlled for auditory cues and was visually indistinguishable from active stimulation but did not control for sensory characteristics of active stimulation over the scalp |
| Adequate blinding of assessors? | Unclear risk | Comment: no description or mention of blinding assessors for clinical part of study |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: no mention of the degree of dropout or how it was managed. However, 45 participants with fibromyalgia reported in the abstract of the same study (Schneider 2012 (Tzabazis 2013)), but only 16 reported in the full paper |
| Selective reporting (reporting bias) | High risk | Comment: no presentation of numeric pain data with measures of variance |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Unclear risk | Comment: baseline and demographic data not presented for clinical group |
| Methods | Parallel RCT | |
| Participants | Country of study: USA Setting: not reported Condition: burning mouth syndrome Prior management details: not reported n = 26 Age mean (SD): active group 63.36 (10.78) years, sham group 64.42 (8.35) years Duration of symptoms, mean (SD): active group 61.57 (32.10) months, sham group 65.58 (55.52) Gender distribution: active group 93% F, sham group 92% F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation not specified, 100% RMT, number of trains 10; duration of trains 5 s; ITI 10 s; total number of pulses 3000 Stimulation location: L DLPFC Number of treatments: 10 x 1 daily on work days Control type: sham coil ‐ same sound and appearance and sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = extreme amount When taken: end of stimulation and 15, 30, 60 days after start of treatment Secondary: AEs |
|
| Notes | Funding source: no information provided COI: no information provided |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Patients who met all inclusion criteria were randomly assigned to one of two groups – one given active and the other sham stimulation – using a web‐based randomization generator” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment procedures not reported |
| Adequate blinding of participants? | Low risk | Comment: sham controls for all aspects of stimulation Quote: “The coil used in the sham group was the same configuration as that used with the real group but shielded so that actual stimulation does not occur. All subjects had ECT electrodes placed under the TMS coil. For those receiving active TMS, the electrodes were disconnected, such that there was no current flowing through during stimulation. In contrast, the electrodes were connected during sham, so participants received a small electrical stimulation through the electrodes, precisely when the TMS was being triggered.” “Ten of 12 (83%) patients in the real group and 4 of 8 (50%) patients in the sham group thought that they were in the real group. There was no significant difference for the belief of the allocated group between two groups (χ2 = 2.54,1, NS), suggesting that blinding for the subjects in this study was kept. The high percentage of correct guessing in the active group is concerning. However, when asked why they guessed the way they did, it was based on whether they had BMS symptom reduction. If this occurred, then they guessed the active group. There were no instances of patient unblinding.” |
| Adequate blinding of assessors? | High risk | Comment: assessor was not blinded to group allocation |
| Incomplete outcome data (attrition bias) All outcomes | High risk | Comment: 2/14 (14%) randomised did not receive active stim, 4/12 (33%) randomised to sham did not receive sham. Excluded from the analysis |
| Selective reporting (reporting bias) | High risk | Comment: pain intensity data only presented in graphical form without numeric point estimates/precision estimates |
| Study Size | High risk | Comment: combined n = 26 (per protocol = 20) |
| Study duration | Unclear risk | Comment: 7‐week follow‐up |
| Other bias | Low risk | Comment: no other risks of bias detected |
| Methods | Parallel RCT, 3 conditions | |
| Participants | Country of study: Brazil Setting: laboratory Condition: fibromyalgia Prior management details: refractory to medical intervention n = 41 Age: mean 54.8 (SD 9.6) years Duration of symptoms: condition 1: 7.54 (SD 3.93) years; condition 2: 8.39 (SD 2.06) years; condition 3: 8.69 (SD 3.61) years Gender distribution: 0 M, 41 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: condition 1: L DLPFC; condition 2: L M1, condition 3; sham L M1 Number of treatments: 10, x 1 daily on consecutive working days Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: pain VAS 0‐10 cm, anchors not specified When taken: immediately post‐treatment, averaged over 3 d post‐treatment, 30 and 60 d post‐treatment Secondary: QoL; FIQ |
|
| Notes | COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "Randomization was performed using the order of entrance in the study and a previous randomisation list generated by a computer" |
| Allocation concealment (selection bias) | Low risk | Comment: the use of a pregenerated randomisation list should have adequately ensured this |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no dropout occurred |
| Selective reporting (reporting bias) | High risk | Comment: pain score numerical values not provided clearly with measures of variance for any post‐treatment time point in the study report |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Low risk | Comment: ≥ 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no significant other bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: USA Setting: laboratory Condition: fibromyalgia Prior management details: pain refractory to common analgesics and muscle relaxants n = 18 randomised of which 17 allocated Age mean (SD): 50.3 (8.5) years Duration of symptoms (years) mean (SD): 10.7 (6.8) Gender distribution: 15 F, 3 M |
|
| Interventions | Stimulation type: HD‐tDCS Stimulation parameters: intensity 2 mA, duration 20 min, anodal/cathodal/sham 4 x 1‐ring configuration Stimulation location: L M1 Number of treatments: x 1 per condition Control type: sham tDCS |
|
| Outcomes | Primary: pain visual numerical scale; 0 = complete absence of pain, 10 = worst pain imaginable When taken: baseline, immediately poststimulation, 30 min poststimulation Secondary: adapted QoL scale for persons with chronic illness (7 points: 1 = terrible, 7 = delighted) |
|
| Notes | COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: "the order of stimulation was counterbalanced and randomly assigned for each individual" Comment: method of randomisation not specified but less likely to introduce bias in a cross‐over design |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only 1 loss to follow‐up and multiple imputation used |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported in full |
| Free from carry‐over effects? | Low risk | Comment: 7‐day washout periods observed. Data similar at baseline |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | High risk | Comment: < 2 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Germany Setting: laboratory Condition: chronic abdominal pain with inflammatory bowel disease Prior management details: participants allowed to continue anti‐inflammatory drugs and acute pain medication n = 20 Age, mean (SD) active group 40.6 (12.5) years, sham group 34.4 (13.2) years Duration of symptoms: active group 10 (8.9) years, sham group 34.4 (13.2) Gender distribution: 13 F, 7 M |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, 35 cm2 electrodes, duration 20 min Stimulation location: anode M1 contralateral to painful side, cathode supraorbital area, contralateral to anode Number of treatments: x 1 daIly for 5 days Control type: sham tDCS |
|
| Outcomes | Primary: pain VAS, anchors 0 = no pain, 10 = the worst pain possible When taken: postintervention, 1 week postintervention Secondary: inflammatory bowel disease QoL questionnaire AEs |
|
| Notes | COI: study authors declared no COI Sources of support: "This study has been supported by the grant “Patientenorientierte Forschung bei CED 2014” of the “Deutsche Morbus Crohn/Colitis ulcerosa Vereinigung e.V.” (Not industry) |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “Randomization was performed by the unblinded researcher (A.F.) in blocks of 4 generated from a computer‐based random allocation.” |
| Allocation concealment (selection bias) | Unclear risk | Quote: “Quote: “Randomization was performed by the unblinded researcher (A.F.)” Comment: no apparent steps to conceal allocation |
| Adequate blinding of participants? | Unclear risk | Evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Adequate blinding of assessors? | Unclear risk | Evidence that assessor blinding can be inadequate at intensity of 2 mA. No formal assessment of blinding success |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: levels of dropout, if any, not reported |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes reported adequately |
| Study Size | High risk | Comment: n = 20 |
| Study duration | High risk | Comment: 1‐week postintervention maximum follow‐up. |
| Other bias | Low risk | Comment: no further bias detected |
| Methods | Cross‐over RCT | |
| Participants | Country of study: Australia Setting: laboratory Condition: chronic neuropathic pain post‐SCI Prior management details; none n = 10 Age mean (SD): 56.1 (14.9) years Duration of symptoms: 15.8 (11.3) years Gender distribution: 8 M, 2 F |
|
| Interventions | Stimulation type: tDCS Stimulation parameters: intensity 2 mA, duration 20 min Stimulation location: M1 (contralateral to most painful side or dominant hand) Number of treatments: 5, x 1 daily 5 days Control type: sham tDCS (switched off after 30 s stimulation) |
|
| Outcomes | Primary: pain VAS; 0 = "no pain", 10 = "worst possible pain" When taken: at end of treatment, 4 weeks post‐treatment Secondary: none relevant |
|
| Notes | COI: no declaration made Sources of support: no declaration made |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Comment: method of randomisation not specified but less important for cross‐over design Quote: "A randomized crossover design was used so that all subjects participated in an active treatment (transcranial direct current stimulation) and sham treatment period. Both the subject and the response assessor were blinded to the randomization sequence." |
| Adequate blinding of participants? | Unclear risk | Comment: there is evidence that participant blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Adequate blinding of assessors? | Unclear risk | Comment: there is evidence that assessor blinding of tDCS may be inadequate at 2 mA intensity (see Assessment of risk of bias in included studies) |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: no loss to follow‐up |
| Selective reporting (reporting bias) | Low risk | Comment: primary outcomes reported in full |
| Free from carry‐over effects? | Low risk | Comment: 4‐week washout period observed and data appear free of carry‐over effects |
| Study Size | High risk | Comment: < 50 participants per treatment arm |
| Study duration | Unclear risk | Comment: ≥ 2 weeks but < 8 weeks' follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Turkey Setting: not reported Condition: fibromyalgia Prior management details: no improvement in cases of using medical treatment for fibromyalgia for at least 3 months n = 28 Age mean (SD): active group 45.25 (9.33) years, sham group 43 (7.63) years Duration of symptoms, mean(SD): active group 53 (29.15) months, sham group 54.92 (30.44) Gender distribution: all F |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 1 Hz; coil orientation not reported, 90% RMT, number of trains 20; duration of trains 60 s; ITI 45 s; total number of pulses 1200 Stimulation location: L M1, no neuronavigation Number of treatments: 10 sessions, weekdays for 2 weeks Control type: sham coil ‐ same sound and appearance, no control for sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = maximum pain imaginable When taken: end of intervention, 1 month, 3 months Secondary: FIQ AEs |
|
| Notes | Funding source: the study authors declared that this study received no financial support COI: no COI was declared by the authors |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Unclear risk | Comment: method of randomisation not outlined Quote: “patients were randomly assigned to be in either a real stimulation group or a sham stimulation group by another clinician” |
| Allocation concealment (selection bias) | Low risk | Quote: “masked clinician evaluated the patients clinically and provided the diagnosis of FM. The patients were randomly assigned to be in either a real stimulation group or a sham stimulation group by another clinician.” |
| Adequate blinding of participants? | Unclear risk | Comment: sham coil did not control for sensory aspects of stimulation. Quote: “Sham stimulation was carried out with the same parabolic coil, which was placed at 90° angles to the motor cortex area” |
| Adequate blinding of assessors? | Low risk | Quote: “A masked clinician evaluated the patients clinically and provided the diagnosis of FM [fibromyalgia]” |
| Incomplete outcome data (attrition bias) All outcomes | Unclear risk | Comment: 3 participants dropped out though this exceeds 10% of total number, the group they withdrew from and point of withdrawal were not clear |
| Selective reporting (reporting bias) | Low risk | Comment: outcomes adequately reported |
| Study Size | High risk | N = 28 (per protocol 25) |
| Study duration | Low risk | Comment: 3‐month follow‐up |
| Other bias | Low risk | Comment: no other risk of bias detected |
| Methods | Parallel RCT | |
| Participants | Country of study: Turkey Setting: rehabilitation unit Condition: post‐SCI below lesion neuropathic pain Prior management details: pain that is resistant to pharmacological (anticonvulsants, antidepressants, narcotics) and interventional treatments n = 17 Age mean (SD): active group: 40 (5.1) years, sham group 36.94 (8) years Duration of symptoms mean (SD): active group 32.3 (25.9) months, sham group 35.4 (17.9) Gender distribution: all M |
|
| Interventions | Stimulation type: rTMS Stimulation parameters: frequency 10 Hz; coil orientation handle pointing posteriorly, number of trains 30; duration of trains 5 s; ITI 25 s; total number of pulses 1500 Stimulation location: M1 midline Number of treatments: daily for 10 weekdays Control type: coil angled away ‐ same sound and appearance, did not control for visual or sensory cues |
|
| Outcomes | Primary: pain NRS anchors 0 = no pain, 10 = worst pain imaginable When taken: end of intervention, 6 weeks, 6 months postintervention Secondary: none relevant |
|
| Notes | Funding source: no information reported COI: no information reported |
|
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Random sequence generation (selection bias) | Low risk | Quote: “A computer‐generated randomization schedule was used.” |
| Allocation concealment (selection bias) | Unclear risk | Comment: allocation concealment not reported |
| Adequate blinding of participants? | Unclear risk | Comment: sham condition did not control for visual or sensory aspects of stimulation |
| Adequate blinding of assessors? | Low risk | Quote: “The patients and the researcher evaluating the patients were blinded to type of rTMS.” |
| Incomplete outcome data (attrition bias) All outcomes | Low risk | Comment: only one participant dropped out |
| Selective reporting (reporting bias) | Low risk | Comment: key outcomes adequately reported |
| Study Size | High risk | Comment: n = 16 |
| Study duration | Low risk | Comment: 6‐month follow‐up |
| Other bias | Low risk | Comment: no other bias detected |
AE: adverse event; ANOVA: analysis of variance; BIRS: Gracely Box Intensity Scale (BIRS); BOCF: baseline observation carried forward; BPI: Brief Pain Inventory; CES: cranial electrotherapy stimulation; CNP: central neuropathic pain; COI: conflict of interest; CPSP: central poststroke pain; CRPS: complex regional pain syndrome; DLPFC: dorsolateral prefrontal cortex; F: female; FIQ: Fibromyalgia Impact Questionnaire; HD‐tDCS: High definition tDCS; ITI: inter‐train interval; ITT: intention‐to‐treat; L: left; LANSS: Leeds Assessment of Neuropathic Symptoms and Signs pain scale; LOCF: last observation carried forward; M: male; M1: primary motor cortex; MCS: motor cortex stimulation (MCS); NIH: National Institutes of Health; NRS: numerical rating scale; NSAIDS: nonsteroidal anti‐imflammatory drugs; OA: osteoarthritis; PFC: prefrontal cortex; PLP: phantom limb pain; QoL: Quality of Life; R: right; RCT: randomised controlled trial; RINCE: reduced impedance non‐invasive cortical electrostimulation; RMDQ: Roland Morris Disability Questionnaire; RMT: resting motor threshold; rTMS: repetitive transcranial magnetic stimulation; SCI: spinal cord injury; SII: secondary somatosensory area; SD: standard deviation; TCES: transcranial electrical stimulation; tDCS: transcranial direct current stimulation; TENS: transcutaneous electrical nerve stimulation; TMS: transcranial magnetic stimulation; VAS: visual analogue scale; WOMAC: Western Ontario and McMaster Universities Arthritis Index
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Avery 2007 | The duration of painful symptoms is unclear. May not be exclusively chronic pain |
| Belci 2004 | Pain is not measured as an outcome |
| Bolognini 2013 | Inclusion of acute and chronic pain patients |
| Bolognini 2015 | Not clearly a chronic population |
| Carraro 2010 | Not a study of electrical brain stimulation |
| Choi 2012b | Study of acute pain |
| Choi 2012a | Study of acute pain |
| Choi 2014 | Not clearly a chronic population |
| Cummiford 2016 | Allocation not randomised |
| Evtiukhin 1998 | A study of postoperative pain. No sham control employed |
| Frentzel 1989 | Not a study of brain stimulation |
| Hargrove 2012b | Uncontrolled long‐term follow‐up data from Hargrove 2012a |
| Johnson 2006 | Self‐reported pain is not measured |
| Katz 1991 | Study not confined to chronic pain |
| Khedr 2015 | Not clearly a chronic population |
| Lindholm 2015 | Allocation not randomised |
| Longobardi 1989 | Not clearly studying chronic pain |
| Ma 2015 | Not clearly a chronic population |
| Maestu 2013 | Not electrical brain stimulation ‐ magnetic fields unlikely to induce electrical currents |
| Morin 2017 | Not clearly a chronic pain population ‐ provoked vestibulodynia |
| Nelson 2010 | Intervention not designed to alter cortical activity directly by electrical stimulation ‐ a neuro feedback intervention |
| O'Connell 2013 | Not a RCT or quasi‐RCT ‐ no randomisation specifically to treatment group or order |
| Pujol 1998 | Participants are a mixture of acute and chronic pain patients |
| Schabrun 2014 | Not clearly a chronic population |
| Seada 2013 | No sham control employed |
| Sichinava 2012 | No sham control employed |
| Silva 2007 | A single case report |
| Smania 2005 | Not a study of brain stimulation |
| Yoon 2014 | Allocation not randomised |
| Zaghi 2009 | Single case study |
RCT: randomised controlled trial
Characteristics of studies awaiting assessment [ordered by study ID]
| Methods | Parallel RCT |
| Participants | Post‐polio patients, n = 32 |
| Interventions | tDCS, bi‐anodal, bilateral motor cortex, 1.5 mA, 20 min, daily for 5 days |
| Outcomes | Pain, QoL |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled study, unclear whether randomised |
| Participants | Post‐SCI chronic neuropathic pain, n = 30 |
| Interventions | tDCS motor cortex, 2 mA, 10 sessions |
| Outcomes | Pain intensity |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled trial, unclear whether randomised |
| Participants | Chronic neurogenic orofacial pain, n = 26 |
| Interventions | rTMS motor cortex, frequency unclear, appears to be a single session of stimulation per condition |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled trial, unclear whether randomised, likely to be a cross‐over design |
| Participants | Chronic neurogenic orofacial pain, n = 26 |
| Interventions | rTMS motor cortex, frequency unclear, appears to be a single session of stimulation per condition |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Likely to be a duplicate report of Fricova 2009. Attempts to contact study authors currently unsuccessful |
| Methods | Sham‐controlled parallel trial ‐ unclear if randomised |
| Participants | Chronic orofacial pain n = 59 |
| Interventions | rTMS, 10 Hz and 20 Hz, location not clear |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | CRPS type I, n = 18 |
| Interventions | rTMS, 10 Hz, 10 treatment sessions |
| Outcomes | Pain, disability, QoL |
| Notes | Published as conference abstract only. Attempts to contact study author currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Neuropathic orofacial pain, n = 29 |
| Interventions | rTMS, motor cortex, 10 Hz, 5 treatment sessions |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Neuropathic orofacial pain, medication resistant, n = 29 |
| Interventions | rTMS, motor cortex, 10 Hz, 5 treatment sessions |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Likely to be a duplicate report of Klirova 2010. Attempts to contact authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | CRPS type I, n = 25 |
| Interventions | tDCS, motor cortex, 2 mA, 20 min per session, daily for 5 days |
| Outcomes | Pain, QoL, physical activity |
| Notes | Currently published as conference abstract only. Correspondence with study authors ‐ data unavailable as currently being re‐analysed |
| Methods | Parallel RCT |
| Participants | Fibromyalgia n = 50 |
| Interventions | Low‐frequency rTMS DLPFC |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Cross‐over RCT |
| Participants | Post‐SCI pain, n = 6 |
| Interventions | tDCS and visual illusion |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Chronic neuropathic pain |
| Interventions | Low‐frequency rTMS, M1 or DLPFC |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Parallel RCT |
| Participants | Current and former opioid abusers ‐ pain status unclear. n = 60 |
| Interventions | tDCS M1, number of sessions unclear |
| Outcomes | Not clear whether pain intensity was measured |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Cross‐over RCT |
| Participants | Parkinson's disease with related pain, n = 19 |
| Interventions | rTMS 20 Hz motor cortex, ? whether single session |
| Outcomes | Pain VAS |
| Notes | Published as conference abstract only. Attempts to contact study authors currently unsuccessful |
| Methods | Unable to retrieve study report |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Parallel RCT |
| Participants | Fibromyalgia n = 48 |
| Interventions | Low‐frequency rTMS DLPFC |
| Outcomes | Pain |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
| Methods | Unable to retrieve study report |
| Participants | — |
| Interventions | — |
| Outcomes | — |
| Notes | — |
| Methods | Parallel RCT |
| Participants | Fibromyalgia n = 20 |
| Interventions | rTMS, L DLPFC, 10 treatment sessions |
| Outcomes | ? whether pain intensity measured as an outcome |
| Notes | Published as conference abstract only. Attempt to contact study authors currently unsuccessful |
CRPS: complex regional pain syndrome; DLPFC: dorsolateral prefrontal cortex; FIQ: Fibromyalgia Impact Questionnaire: L: left; M1: primary motor cortex; QoL: quality of life; RCT: randomised controlled trial; rTMS: repetitive transcranial magnetic stimulation; SCI: spinal cord injury; tDCS: transcranial direct current stimulation; VAS: visual analogue scale
Characteristics of ongoing studies [ordered by study ID]
| Trial name or title | Investigating the role of transcranial direct current stimulation for pain relief in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia syndrome Myalgic encephalomyelitis/chronic fatigue syndrome |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain, fatigue, FIQ, stimulation condition |
| Starting date | |
| Contact information | Ms Hannah Bereznicki, hannah.bereznicki@deakin.edu.au |
| Notes | TRIAL WITHDRAWN |
| Trial name or title | The effectiveness of repetitive transcranial magnetic stimulation in the treatment of fibromyalgia |
| Methods | Parallel RCT |
| Participants | Fibromyalgia |
| Interventions | rTMS to DLPFC 10 Hz sham rTMS |
| Outcomes | Pain severity QoL |
| Starting date | 17 May 2013 |
| Contact information | Dr Bernadette Fitzgibbon, bernadette.fitzgibbon@monash.edu |
| Notes | Correspondence with authors 21 December 2016 ‐ data collection ongoing |
| Trial name or title | Modulation of chronic pain perception with noninvasive central and peripheral nervous system stimulation |
| Methods | RCT |
| Participants | Chronic musculoskeletal pain |
| Interventions | Intervention group 1: participants receive tDCS and TENS only Comparator group 1: participants receive tDCS and sham TENS only Comparator group 2: participants receive TENS and sham tDCS only Control group 1: participants receive sham tDCSand sham TENS only |
| Outcomes | Pain VAS WHO‐QOL |
| Starting date | 11 November 2013 |
| Contact information | Prof Allan Abbott, aabbott@bond.edu.au |
| Notes | Correspondence with authors 22 December 2016‐ trial did not go ahead due to "changes in project personnel and funding." |
| Trial name or title | The effects of non‐invasive brain stimulation on chronic arm pain |
| Methods | RCT |
| Participants | Neuropathic pain in the upper limb |
| Interventions | tDCS Sham |
| Outcomes | Arm pain Upper limb function |
| Starting date | 16 April 2014 |
| Contact information | A/Prof Gwyn Lewis, gwyn.lewis@aut.ac.nz |
| Notes | Correspondence with authors 21 December 2016, data collection ongoing |
| Trial name or title | The impact of non‐invasive brain stimulation on motor cortex excitability and cognition in chronic lower back pain |
| Methods | RCT |
| Participants | Chronic low back pain |
| Interventions | tDCS Sham |
| Outcomes | Pain, HR‐QoL |
| Starting date | 9 March 2015 |
| Contact information | Dr Andrea Loftus, andrea.loftus@curtin.edu.au |
| Notes | Correspondence with authors 3 January 2017, data collection ongoing |
| Trial name or title | Safety and feasibility of transcranial direct current stimulation (tDCS) combined with sensorimotor retraining in chronic low back pain: a pilot randomised controlled trial |
| Methods | RCT |
| Participants | Chronic nonspecific low back pain |
| Interventions | tDCS + sensorimotor training sham tDCS + sensorimotor training |
| Outcomes | Pain severity Physical function |
| Starting date | 8 August 2016 |
| Contact information | Dr Siobhan Schabrun, s.schabrun@westernsydney.edu.au |
| Notes | Correspondance with authors 22 December 2016, trial beginning recruitment |
| Trial name or title | |
| Methods | Parallel RCT |
| Participants | Fibromyalgia, n = 118 |
| Interventions | rTMS right DLPFC, low‐frequency, 20 sessions |
| Outcomes | Unclear whether self‐reported pain scores were collected |
| Starting date | |
| Contact information | |
| Notes | Published as conference abstract only. Correspondance with study authors ‐ paper currently in press awaiting publication |
| Trial name or title | Transcranial magnetic stimulation induced motor evoked potential in the expression of brain‐derived neurotrophic factor BDNF, pathological pain and quality of life in patients with spinal cord injury |
| Methods | Parallel RCT |
| Participants | Post‐SCI pain, n = 60 |
| Interventions | rTMS |
| Outcomes | Pain, QoL |
| Starting date | 01 July 2017 |
| Contact information | Dr Shi Jiajia 707529535@qq.com |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Effect of transcranial magnetic stimulation on pain modulation status in fibromyalgia patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia |
| Interventions | rTMS |
| Outcomes | Pain |
| Starting date | 01 September 2013 |
| Contact information | Dr Rathmi Mashur, mathurashmi@yahoo.co.in |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | |
| Methods | Parallel RCT |
| Participants | Mixed chronic pain |
| Interventions | tDCS, M1, DLPFC, number of sessions not clear |
| Outcomes | Pain, QoL |
| Starting date | |
| Contact information | |
| Notes | Published as conference abstract only. Correspondence with study authors ‐ study ongoing |
| Trial name or title | The effect of transcranial direct current stimulation (t‐DCS) On the P300 component of event‐related potentials in patients with chronic neuropathic pain due to CRPS or diabetic neuropathy |
| Methods | Cross‐over RCT |
| Participants | Chronic neuropathic pain due to CRPS or diabetic neuropathy |
| Interventions | tDCS or sham, 2 mA, 20 min, x 1 session, location not specified |
| Outcomes | Pain intensity |
| Starting date | February 2009 |
| Contact information | Dr Pesach Schvartzman, spesah@bgu.ac.il |
| Notes | Contact in 2010 ‐ study ongoing, recent attempts to contact for update unsuccessful |
| Trial name or title | Occipital transcranial direct current stimulation in fibromyalgia |
| Methods | Cross‐over RCT |
| Participants | Fibromyalgia |
| Interventions | tDCS or sham, parameters not specified |
| Outcomes | Pain VAS and FIQ |
| Starting date | July 2009 |
| Contact information | Dr Mark Plazier, mark.plazier@uza.be |
| Notes | Attempts to contact study authors currently unsuccessful |
| Trial name or title | Application of transcranial direct current stimulation in patients with chronic pain after spinal cord injury |
| Methods | Parallel RCT |
| Participants | Chronic pain after SCI, proposed n = 60 |
| Interventions | tDCS 2 mA, 10 sessions |
| Outcomes | Pain VAS, QoL |
| Starting date | April 2010 |
| Contact information | Dr Felipe Fregni, ffregni@neuromodulationlab.org, Kayleen Weaver, kmweaver@partners.org |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Transcranial direct current stimulation for chronic pain relief |
| Methods | Cross‐over RCT |
| Participants | Chronic pain patients, proposed n = 100 |
| Interventions | tDCS, motor cortex, 2 mA, daily for 5 days |
| Outcomes | Pain relief |
| Starting date | November 2010 |
| Contact information | Dr Silvio Brill, Tel Aviv Sourasky Medical Centre |
| Notes | Correspondence with study authors: study ongoing |
| Trial name or title | Exploration of parameters of transcranial direct current stimulation in chronic pain |
| Methods | Parallel RCT |
| Participants | Chronic pain following traumatic SCI, n = 60 |
| Interventions | tDCS or sham, 2 mA, motor cortex, 20 min, x 1 daily for 5 days |
| Outcomes | Pain |
| Starting date | April 2010 |
| Contact information | Dr Felipe Fregni, ffregni@partners.org; Kayleen Weaver, kmweaver@partners.org |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Effects of transcranial direct current stimulation and transcranial ultrasound on osteoarthritis pain of the knee |
| Methods | Parallel RCT |
| Participants | Chronic knee OA pain, n = 30 |
| Interventions | tDCS or sham, 20 min, 2 mA, motor cortex, 5 sessions |
| Outcomes | Pain |
| Starting date | January 2011 |
| Contact information | Dr Felipe Fregni, ffregni@partners.org; Kayleen Weaver, kmweaver@partners.org |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Effects of transcranial direct current stimulation in chronic corneal pain |
| Methods | Cross‐over RCT |
| Participants | Chronic corneal pain |
| Interventions | tDCS, active or sham, 1 session of each, parameters not reported |
| Outcomes | Pain VAS |
| Starting date | January 2012 |
| Contact information | Dr Felipe Fregni, ffregni@partners.org; Kayleen Weaver, kmweaver@partners.org |
| Notes | Contact with study author ‐ study at "to be analysed and reported" stage |
| Trial name or title | Assessment and treatment patients with atypical facial pain through repetitive transcranial magnetic stimulation |
| Methods | Parallel RCT |
| Participants | Atypical facial pain, n = 40 |
| Interventions | rTMS or sham, parameters not reported, 5 sessions |
| Outcomes | Pain VAS |
| Starting date | March 2011 |
| Contact information | Ricardo Galhardoni |
| Notes | Correspondence with study authors: study near completion |
| Trial name or title | Effect of cranial stimulation and acupuncture on pain, functional capability and cerebral function in osteoarthritis |
| Methods | Parallel RCT |
| Participants | Chronic OA pain, n = 80 |
| Interventions | 4 groups, real tDCS + electroacupuncture sham; sham tDCS + electroacupuncture sham, sham tDCS + electroacupuncture, real tDCS + electroacupuncture tDCS 2 mA motor cortex. All single session |
| Outcomes | Daily pain intensity, WOMAC |
| Starting date | January 2012 |
| Contact information | Dr Wolnei Caumo, caumo@cpovo.net |
| Notes | Correspondence with study authors: study ongoing |
| Trial name or title | The effects of transcranial direct current stimulation on central pain in patients with spinal cord injury |
| Methods | RCT |
| Participants | Central neuropathic pain post‐SCI |
| Interventions | tDCS Sham |
| Outcomes | Pain, average 24 h Pain interference |
| Starting date | March 2008 |
| Contact information | Hyung‐Ik Shin, Associate Professor, Seoul National University Bundang Hospital |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Effects of transcranial direct current stimulation (tDCS) on neuropathic symptoms following burn injury |
| Methods | RCT |
| Participants | Burn injury |
| Interventions | tDCS Sham |
| Outcomes | Pain QoL |
| Starting date | January 2013 |
| Contact information | Dr Felipe Fregni, ffregni@partners.org |
| Notes | Contact with authors unsuccessful |
| Trial name or title | tDCS for the management of chronic visceral pain in patients with chronic pancreatitis (tDCS) |
| Methods | RCT |
| Participants | Chronic pancreatitis pain |
| Interventions | tDCS Sham |
| Outcomes | Pain QoL |
| Starting date | March 2013 |
| Contact information | Steven Freedman, MD PhD |
| Notes | Contact with study author 20 December 2016 ‐ stated all results published but did not respond to request to identify the published paper. Trial register record implies the study was withdrawn prior to enrolment |
| Trial name or title | tDCS effects on chronic low back pain |
| Methods | RCT |
| Participants | Chronic nonspecific low back pain, n = 45 |
| Interventions | Real‐tDCS + back school Sham tDCS + back school |
| Outcomes | Pain |
| Starting date | January 2012 |
| Contact information | Sofia Straudi, MD |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Functional neuroimaging in fibromyalgia patients receiving tDCS |
| Methods | RCT |
| Participants | Fibromyalgia, n = 34 |
| Interventions | tDCS + pregabalin Sham tDCS + pregabalin |
| Outcomes | Pain FIQ WH‐QoL |
| Starting date | March 2013 |
| Contact information | Wolnei Caumo, MD, caumo@cpovo.net |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Deep rTMS in central neuropathic pain syndromes (DRTMS) |
| Methods | RCT |
| Participants | Central pain, n = 90 |
| Interventions | rTMS double cone coil rTMS H‐coil Sham rTMS |
| Outcomes | Pain VAS |
| Starting date | March 2011 |
| Contact information | Daniel Ciampi, MD, PhD, ciampi@usp.br |
| Notes | Correspondence with authors 22 December 2016, data collection complete, analysis ongoing |
| Trial name or title | Investigation of the efficacy of tDCS in the treatment of complex regional pain syndrome (CRPS) Type 1 |
| Methods | RCT |
| Participants | CRPS type 1, n = 22 |
| Interventions | tDCS + GMI |
| Outcomes | sham tDCS + GMI |
| Starting date | April 2013 |
| Contact information | Yannick Tousignant‐Laflamme, PT Ph.D, Université de Sherbrooke |
| Notes | Correspondence with study authors ‐ manuscript under review for publication |
| Trial name or title | Long‐term effects of transcranial direct current stimulation (tDCS) on patients with phantom limb pain (PLP) |
| Methods | Cross‐over RCT |
| Participants | Phantom limb pain, n = 24 |
| Interventions | Anodal tDCS Cathodal tDCS Sham TDCS |
| Outcomes | Pain AEs |
| Starting date | May 2015 |
| Contact information | Itzhak Siev‐Ner, MD |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Analgesic dffect of repetitive transcranial magnetic stimulation (rTMS) for central neuropathic pain in multiple sclerosis (STIMASEP) |
| Methods | RCT |
| Participants | Central neuropathic pain due to multiple sclerosis, n = 66 |
| Interventions | rTMS Theta burst TMS Sham rTMS |
| Outcomes | Pain |
| Starting date | February 2014 |
| Contact information | Patrick Lacarin placarin@chu‐clermontferrand.fr |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Transcranial magnetic stimulation for low back pain |
| Methods | Cross‐over RCT |
| Participants | Chronic low back pain |
| Interventions | rTMS ? comparator |
| Outcomes | Pain |
| Starting date | January 2014 |
| Contact information | Sean Mackey, Chief, Division of Pain Medicine, Stanford University |
| Notes | Contact with study authors unsuccessful. Register record states this study was withdrawn prior to enrolment. Reasons not given |
| Trial name or title | The effect of tDCS in the treatment of chronic pelvic pain associated with endometriosis (tDCS) |
| Methods | Parallel RCT |
| Participants | Painful endometriosis, n = 30 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain AEs QoL |
| Starting date | June 2014 |
| Contact information | Wolnei Caumo, MD, PhD, caumo@cpovo.net |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Efficacy of transcranial magnetic stimulation (TMS) in central post stroke pain (CPSP) |
| Methods | RCT |
| Participants | Central poststroke pain, n = 20 |
| Interventions | Navigated rTMS Sham rTMS |
| Outcomes | Pain intensity QoL AEs |
| Starting date | June 2013 |
| Contact information | Eija Kalso, PhD, Helsinki University Central Hospital |
| Notes | Register record notes "The recruitment status of this study is unknown. The completion date has passed and the status has not been verified in more than two years." Correspondence with study authors 05 January 2017: data analysis ongoing |
| Trial name or title | Effects of tDCS and tUS on pain perception in OA of the knee |
| Methods | Parallel RCT |
| Participants | OA of the knee, n = 28 |
| Interventions | Active tDCS + active tUS Sham tDCS + sham tUS |
| Outcomes | Pain AEs QoL |
| Starting date | March 2015 |
| Contact information | Felipe Fregni, Principal Investigator, Spaulding Rehabilitation Hospital |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Repetitive transcranial magnetic stimulation in central neuropathic pain |
| Methods | Cross‐over RCT |
| Participants | Central neuropathic pain, n = 50 |
| Interventions | rTMS Sham rTMS |
| Outcomes | Pain VAS, average and responder analysis |
| Starting date | November 2015 |
| Contact information | Charles Quesada, Roland Peyron |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | A novel non invasive brain stimulation based treatment for chronic low back pain (CLBP) |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 80 |
| Interventions | tDCS/tACS stimulation Sham tDCS |
| Outcomes | Pain |
| Starting date | May 2015 |
| Contact information | Dr Silviu Brill, paincenter@tlvmc.gov.il |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | The effects of cognitive behavioral therapy and transcranial current stimulation (tDCS) on chronic lower back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 120 |
| Interventions | tDCS of DLPFC + CBT Sham tDCS + CBT |
| Outcomes | Pain |
| Starting date | January 2015 |
| Contact information | Jeffrey Borckardt, Professor, Medical University of South Carolina |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Optimizing rehabilitation for phantom limb pain using mirror therapy and transcranial direct current stimulation (tDCS) |
| Methods | Factorial RCT |
| Participants | Chronic phantom limb pain, n = 132 |
| Interventions | Active tDCS and active mirror therapy Active tDCS and sham mirror therapy Sham tDCS and active mirror therapy Sham tDCS and sham mirrory therapy |
| Outcomes | Pain QoL (short version SF‐36) AEs |
| Starting date | July 2015 |
| Contact information | Dr Felipe Fregni ffregni@partners.org |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Non invasive brain stimulation treatment for CLBP (NIBSTCLBP) |
| Methods | Cross‐over RCT |
| Participants | Chronic low back pain, n = 60 |
| Interventions | tDCS Sham tDCS "partially active‐ first 2.5 weeks will receive sham treatment followed by active" |
| Outcomes | Pain Disability |
| Starting date | January 2016 |
| Contact information | Iftach Dolev, PhD |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Home‐based transcranial direct current stimulation in fibromyalgia patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia, n = 32 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain Functional capacity |
| Starting date | January 2016 |
| Contact information | Wolnei Caumo caumo@cpovo.net Aline Brietzke aline_brietzke@yahoo.com.br |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Adjunctive transcranial direct current stimulation (tDCS) |
| Methods | Parallel RCT |
| Participants | Chronic pain, n = 36 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain Physical activity |
| Starting date | January 2016 |
| Contact information | Alok Madan, PhD amadan@menninger.edu Gladys Jimenez, PhD gjtorres@menninger.edu |
| Notes | Correspondance with study authors 20 December 2016 ‐ data collection ongoing |
| Trial name or title | Imaging the effects of rTMS on chronic pain |
| Methods | Parallel RCT |
| Participants | Chronic neuropathic pain, n = 60 |
| Interventions | Active rTMS, prefrontal Sham rTMS |
| Outcomes | Pain QoL |
| Starting date | March 2016 |
| Contact information | Diana Martinez, MD, dm437@cumc.columbia.edu |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | The effects of CBT and (tDCS) on fibromyalgia patients |
| Methods | Parallel RCT |
| Participants | Fibromyalgia, n = 72 |
| Interventions | tDCS + CBT Sham tDCS + CBT |
| Outcomes | Pain QoL |
| Starting date | November 2014 |
| Contact information | Jeffrey Borckardt, Ph.D. borckard@musc.edu |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Effects of tDCS and tUS on pain perception in OA of the knee |
| Methods | Parallel RCT |
| Participants | OA knee, n = 64 |
| Interventions | Active tDCS/active tUS Sham tDCS/sham tUS |
| Outcomes | Pain |
| Starting date | September 2016 |
| Contact information | Felipe Fregni, Spaulding Rehabilitation Hospital |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Transcranial direct current stimulation for chronic low back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 60 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain |
| Starting date | November 2014 |
| Contact information | Butler Hospital, individual not specified |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | tDCS for chronic low back pain |
| Methods | Parallel RCT |
| Participants | Chronic low back pain, n = 40 |
| Interventions | tDCS Sham tDCS |
| Outcomes | Pain |
| Starting date | October 2013 |
| Contact information | Frederick Burgess, MD, PhD Benjamin Greenberg, MD, PhD Providence VA Medical Center |
| Notes | Correspondence with study authors 21 December 2017, study in progress |
| Trial name or title | tDCS associated with peripheral electrical stimulation for pain control in individuals with sickle cell disease (tDCS/PES_SCD) |
| Methods | Parallel RCT |
| Participants | Sickle cell disease, n = 80 |
| Interventions | ss‐tDCS (active) plus PES (active) ss‐tDCS (active) plus PES (simulated) ss‐tDCS (simulated) plus PES (active) ss‐tDCS (simulated) plus PES (simulated) sc‐tDCS (active) plus PES (active) sc‐tDCS (active) plus PES (simulated) sc‐tDCS (simulated) plus PES (active) sc‐TDCS (simulated) plus PES (simulated) |
| Outcomes | Pain Function |
| Starting date | March 2016 |
| Contact information | Prof. Abrahão F Baptista, afbaptista@ufba.br Tiago S. Lopes, Sr, tiago.lopes56@yahoo.com |
| Notes | Contact with study authors unsuccessful |
| Trial name or title | Analgesic effect of non invasive stimulation: transcranial direct current stimulation of opercular‐insular cortex |
| Methods | Parallel RCT |
| Participants | CRPS, n = 40 |
| Interventions | tDCS of operculo‐insular cortex |
| Outcomes | Pain |
| Starting date | November 2016 |
| Contact information | luis.garcia‐larrea@univ‐lyon1.fr |
| Notes |
| Trial name or title | TMS for complex regional pain syndrome |
| Methods | Parallel RCT |
| Participants | CRPS, n = 40 |
| Interventions | Theta‐burst rTMS |
| Outcomes | Pain |
| Starting date | 24 April 2017 |
| Contact information | vsalmasi@stanford.edu |
| Notes |
| Trial name or title | Effectiveness of transcranial direct current stimulation combined with kinesiotherapy in patients with chronic temporomandibular disorders (TMJ): clinical, randomized, double‐blind, placebo controlled trial |
| Methods | Parallel RCT |
| Participants | Chronic temporomandibular pain |
| Interventions | tDCS + kinesiotherapy Sham tDCS + kinesiotherapy |
| Outcomes | Pain |
| Starting date | December 2013 |
| Contact information | Maitê de Freitas, maite_famaral@hotmail.com |
| Notes | Correspondence with study authors 31 December 2016 ‐ study report under peer review for publication |
AE: adverse events; CBT: cognitive behavioural therapy; CRPS: complex regional pain syndrome; DLPFC: dorsolateral prefrontal cortex; FIQ: Fibromyalgia Impact Questionnaire; GMI: graded motor imagery; HR‐QoL: health‐related quality of life; OA: osteoarthritis; PES: peripheral electrical stimulation; QoL: quality of life; RCT: randomised controlled trial; rTMS: repetitive transcranial magnetic stimulation; SCI: spinal cord injury; tACS: transcranial alternating current stimulation; tDCS: transcranial direct current stimulation; TENS: transcutaneous electrical nerve stimulation; tUS: transcranial ultrasound; VAS: visual analogue scale; WHO‐QOL: World Health Organization‐QoL; WOMAC: Western Ontario and McMaster Universities Arthritis Index
Contributions of authors
For this update
NOC: co‐implemented the search strategy alongside the Cochrane PaPaS Group Information Specialist, applied eligibility criteria, assessed studies, extracted and analysed data, and led the write‐up of the review.
BW: acted as the second review author, applied eligibility criteria, assessed studies, extracted data and assisted with the write‐up of the review.
LM: provided statistical advice and support throughout the review.
LDS: acted as a third review author for conflicts in applying eligibility criteria and assessing included studies.
SS: aupported the implementation and reporting of the review throughout.
All review authors read and commented upon the systematic review and commented on and approved the final manuscript.
For previous versions of this review
NOC: conceived and designed the review protocol, co‐implemented the search strategy alongside the Cochrane PaPaS Group Information Specialist, applied eligibility criteria, assessed studies, extracted and analysed data, and led the write‐up of the review.
BW: closely informed the protocol design and acted as the second review author, applied eligibility criteria, assessed studies, extracted data and assisted with the write‐up of the review.
LM: provided statistical advice and support throughout the review and contributed to the design of the protocol.
LDS: was involved in the conception and design of the review and acted as a third review author for conflicts in applying eligibility criteria and assessing included studies.
SS: informed the design of the protocol and has supported the implementation and reporting of the review throughout.
All review authors read and commented upon the systematic review and commented on and approved the final manuscript.
Sources of support
Internal sources
-
Brunel University London, UK.
Salary for authors NOC, LDS
-
Edge Hill University, UK.
Salary for author SS
-
University College London, UK.
Salary for author LM
-
University of Notre Dame Australia, Australia.
Salary for author BMW
External sources
No sources of support supplied
Declarations of interest
NOC: none known
LM: none known
SS: none known
LHD: none known
BW: none known
New search for studies and content updated (no change to conclusions)
References
References to studies included in this review
- Ahmed MA, Mohamed SA, Sayed D. Long‐term antalgic effects of repetitive transcranial magnetic stimulation of motor cortex and serum beta‐endorphin in patients with phantom pain. Neurological Research 2011;33(9):953‐8. [DOI] [PubMed] [Google Scholar]
- Ahn H, Wood, Adam J, Kunik ME, Bhattacharjee A, Chen Z, et al. Efficacy of transcranial direct current stimulation over primary motor cortex (anode) and contralateral supraorbital area (cathode) on clinical pain severity and mobility performance in persons with knee osteoarthritis: an experimenter‐ and participant‐blinded, randomized, sham‐controlled pilot clinical study. Brain Stimulation (in press). [DOI] [PMC free article] [PubMed]; Ahn H, Woods AJ, Choi E, Padhye N, Fillingim R. Transcranial direct current stimulation and mobility functioning in older adults with knee osteoarthritis pain: a double‐blind, randomized, sham‐controlled pilot clinical study. Brain Stimulation 2017;10:E21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- André‐Obadia N, Peyron R, Mertens P, Mauguière F, Laurent B, Garcia‐Larrea L. Transcranial magnetic stimulation for pain control. Double‐blind study of different frequencies against placebo, and correlation with motor cortex stimulation efficacy. Clinical Neurophysiology 2006;117(7):1536‐44. [DOI] [PubMed] [Google Scholar]
- André‐Obadia N, Mertens P, Gueguen A, Peyron R, Garcia‐Larrea L. Pain relief by rTMS: differential effect of current flow but no specific action on pain subtypes. Neurology 2008;71(11):833‐40. [DOI] [PubMed] [Google Scholar]
- Andre‐Obadia N, Magnin M, Garcia‐Larrea L. On the importance of placebo timing in rTMS studies for pain relief. Pain 2011;152(6):1233‐7. [DOI] [PubMed] [Google Scholar]
- Antal A, Terney D, Kühnl S, Paulus W. Anodal transcranial direct current stimulation of the motor cortex ameliorates chronic pain and reduces short intracortical inhibition. Journal of Pain & Symptom Management 2010;39(5):890‐903. [DOI] [PubMed] [Google Scholar]
- Attal N, Ayache S, Ciampi De Andrade D, Baudic S, Jazat F, Mhalla A, et al. Comparison of the analgesic effects of RTMS and TDCS in painful radiculopathy: a randomized double blind placebo controlled study. Journal of the Neurological Sciences 2015;357:e357. [Google Scholar]; Attal N, Ayache SS, Ciampi De Andrade D, Mhalla A, Baudic S, Jazat F, et al. Repetitive transcranial magnetic stimulation and transcranial direct‐current stimulation in neuropathic pain due to radiculopathy: a randomized sham‐controlled comparative study. Pain 2016;157(6):1224‐31. [DOI] [PubMed] [Google Scholar]
- Avery DH, Zarkowski P, Krashin D, Rho W, Wajdik C, Joesch J, et al. Transcranial magnetic stimulation in the treatment of chronic widespread pain: a randomized, controlled study. Unpublished study. [DOI] [PMC free article] [PubMed]; Avery DH, Zarkowski P, Krashin D, Rho WK, Wajdik C, Joesch JM, et al. Transcranial magnetic stimulation in the treatment of chronic widespread pain. Journal of ECT 2015;31(1):57‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayache SS, Palm U, Chalah MA, Al‐Ani T, Brigno A, Abdellaoui M, et al. Prefrontal tDCS decreases pain in patients with multiple sclerosis. Frontiers in Neuroscience 2016;10:147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae SH, Kim GD, Kim KY. Analgesic effect of transcranial direct current stimulation on central post‐stroke pain. Tohoku Journal of Experimental Medicine 2014;234(3):189‐95. [DOI] [PubMed] [Google Scholar]
- Boggio PS, Amancio EJ, Correa CF, Cecilio S, Valasek C, Bajwa Z, et al. Transcranial DC stimulation coupled with TENS for the treatment of chronic pain: a preliminary study. Clinical Journal of Pain 2009;25(8):691‐5. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Smith AR, Reeves ST, Madan A, Shelley N, Branham R, et al. A pilot study investigating the effects of fast left prefrontal rTMS on chronic neuropathic pain. Pain Medicine 2009;10(5):840‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyer L, Dousset A, Roussel P, Dossetto N, Cammilleri S, Piano V, et al. rTMS in fibromyalgia: a randomized trial evaluating QoL and its brain metabolic substrate. Neurology 2014;82(14):1231‐8. [DOI] [PubMed] [Google Scholar]; Guedj E, Dousset A, Boyer L, Roussel P, Cammilleri S, Mundler O, et al. Transcranial magnetic stimulation in fibromyalgia: a randomized trial evaluating quality of life and its brain metabolic substrate. European Journal of Nuclear Medicine and Molecular Imaging 2013;40:S217. [Google Scholar]
- Brietzke AP, Rozisky JR, Dussan‐Sarria JA, Deitos A, Laste G, Hoppe PFT, et al. Neuroplastic effects of transcranial direct current stimulation on painful symptoms reduction in chronic hepatitis C: a phase II randomized, double blind, sham controlled trial. Frontiers in Neuroscience 2016;9:498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Capel ID, Dorrell HM, Spencer EP, Davis MW. The amelioration of the suffering associated with spinal cord injury with subperception transcranial electrical stimulation. Spinal Cord 2003;41(2):109‐17. [DOI] [PubMed] [Google Scholar]
- Carretero B, Martin MJ, Juan A, Pradana ML, Martin B, Carral M, et al. Low‐frequency transcranial magnetic stimulation in patients with fibromyalgia and major depression. Pain Medicine 2009;10(4):748‐53. [DOI] [PubMed] [Google Scholar]
- Chang WJ, Bennell KL, Hodges PW, Hinman RS, Young CL, Buscemi V, et al. Addition of transcranial direct current stimulation to quadriceps strengthening exercise in knee osteoarthritis: a pilot randomised controlled trial. PLoS ONE2017; Vol. 12, issue 6:e0180328. [DOI] [PMC free article] [PubMed]
- Cork RC, Wood P, Ming N, Shepherd C, Eddy J, Price L. The effect of cranial electrotherapy stimulation (CES) on pain associated with fibromyalgia. Internet Journal of Anesthesiology 2004;8(2):15. [Google Scholar]
- Curatolo M, Bianca G, Cosentino G, Baschi R, Salemi G, Talotta R. Motor cortex tRNS improves pain, affective and cognitive impairment in patients with fibromyalgia: preliminary results of a randomised sham‐controlled trial. Clinical and Experimental Rheumatology2017; Vol. 35, issue Suppl 105:100‐105. [PubMed]
- Dall'Agnol L, Medeiros LF, Torres ILS, Deitos A, Brietzke A, Laste G, et al. Repetitive transcranial magnetic stimulation increases the corticospinal inhibition and the brain‐derived neurotrophic factor in chronic myofascial pain syndrome: an explanatory double‐blinded, randomized, sham‐controlled trial. Journal of Pain 2014;15(8):845‐55. [DOI] [PubMed] [Google Scholar]
- Deering DE, Ahlers LR, Gendreau M, Gendreau JF, Deering SK, Hargrove JB. The safety and efficacy of Reduced Impedance Noninvasive Cortical Electrostimulation for the treatment of patients with fibromyalgia: a double‐blinded, randomized, sham‐controlled, feasibility study. Unpublished study report: shared upon request by study authors. ; Gendreau RM, Deering D, Gendreau J, Hargrove J. Treatment of fibromyalgia with neurostimulation: a randomized, double‐blinded, sham‐controlled trial. Arthritis and Rheumatology 2014;66:S483. [Google Scholar]
- Defrin R, Grunhaus L, Zamir D, Zeilig G. The effect of a series of repetitive transcranial magnetic stimulations of the motor cortex on central pain after spinal cord injury. Archives of Physical Medicine and Rehabilitation 2007;88(12):1574‐80. [DOI] [PubMed] [Google Scholar]
- Oliveira RAA, Andrade DC, Mendonça M, Barros R, Luvisoto T, Myczkowski ML, et al. Repetitive transcranial magnetic stimulation of the left premotor/dorsolateral prefrontal cortex does not have analgesic effect on central poststroke pain. Journal of Pain 2014;15(12):1271‐81. [DOI] [PubMed] [Google Scholar]
- Donnell A, Nascimento TD, Lawrence M, Gupta V, Zieba T, Truong DQ, et al. High‐definition and non‐invasive brain modulation of pain and motor dysfunction in chronic TMD. Brain Stimulation 2015;8(6):1085‐92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagerlund AJ, Hansen OA, Aslaksen PM. Transcranial direct current stimulation as a treatment for patients with fibromyalgia: a randomized controlled trial. Pain 2015;156(1):62‐71. [DOI] [PubMed] [Google Scholar]
- Fenton BW, Palmieri PA, Boggio P, Fanning J, Fregni F. A preliminary study of transcranial direct current stimulation for the treatment of refractory chronic pelvic pain. Brain Stimulation 2009;2(2):103‐7. [DOI] [PubMed] [Google Scholar]
- Fregni F, DaSilva D, Potvin K, Ramos Estebanez C, Cohen D, Pascual‐Leone A, et al. Treatment of chronic visceral pain with brain stimulation. Annals of Neurology 2005;58(6):971‐2. [DOI] [PubMed] [Google Scholar]
- Fregni F, Boggio PS, Lima MC, Ferreira MJ, Wagner T, Rigonatti SP, et al. A sham‐controlled, phase II trial of transcranial direct current stimulation for the treatment of central pain in traumatic spinal cord injury. Pain 2006;122(1‐2):197‐209. [DOI] [PubMed] [Google Scholar]
- Fregni F, Gimenes R, Valle AC, Ferreira MJ, Rocha RR, Natalle L, et al. A randomized, sham‐controlled, proof of principle study of transcranial direct current stimulation for the treatment of pain in fibromyalgia. Arthritis and Rheumatism 2006;54(12):3988‐98. [DOI] [PubMed] [Google Scholar]; Roizenblatt S, Fregni F, Gimenez R, Wetzel T, Rigonatti SP, Tufik S, et al. Site‐specific effects of transcranial direct current stimulation on sleep and pain in fibromyalgia: a randomized, sham‐controlled study. Pain Practice 2007;7(4):297‐306. [DOI] [PubMed] [Google Scholar]
- Fregni F, Potvin K, Dasilva D, Wang X, Lenkinski RE, Freedman SD, et al. Clinical effects and brain metabolic correlates in non‐invasive cortical neuromodulation for visceral pain. European Journal of Pain 2011;15(1):53‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabis L, Shklar B, Geva D. Immediate influence of transcranial electrostimulation on pain and beta‐endorphin blood levels: an active placebo‐controlled study. American Journal of Physical Medicine & Rehabilitation 2003;82(2):81‐5. [DOI] [PubMed] [Google Scholar]
- Gabis L, Shklar B, Baruch YK, Raz R, Gabis E, Geva D. Pain reduction using transcranial electrostimulation: a double blind "active placebo" controlled trial. Journal of Rehabilitation Medicine 2009;41(4):256‐61. [DOI] [PubMed] [Google Scholar]
- Hagenacker T, Bude V, Naegel S, Holle D, Katsarava Z, Diener HC, et al. Patient‐conducted anodal transcranial direct current stimulation of the motor cortex alleviates pain in trigeminal neuralgia. Journal of Headache and Pain 2014;15(1):78. [DOI] [PMC free article] [PubMed] [Google Scholar]; Obermann M, Bude V, Holle D, Hagenacker T, Diener H, Katsarava Z. Therapeutic efficacy of transcranial direct current stimulation in trigeminal neuralgia. Neurology 2014;82(10):(Suppl 1). [DOI] [PMC free article] [PubMed] [Google Scholar]; Obermann M, Bude V, Naegel S, Holle D, Diener HC, Hagenacker T. Anodal transcranial direct current stimulation alleviates pain in trigeminal neuralgia. Journal of Neurology 2014;261:S39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hargrove JB, Bennett RM, Simons DG, Smith SJ, Nagpal S, Deering DE. A randomized placebo‐controlled study of noninvasive cortical electrostimulation in the treatment of fibromyalgia patients. Pain Medicine 2012;13(1):115‐24. [DOI] [PubMed] [Google Scholar]; Hargrove JB, Bennett RM, Simons DG, Smith SJ, Nagpal S, Deering DE. Non‐invasive cortical electrostimulation in the treatment of fibromyalgia. Arthritis and Rheumatism 2010;62:647. 20187160 [Google Scholar]
- Harvey MP, Lorrain D, Martel M, Bergeron‐Vezina K, Houde F, Seguin M. Can we improve pain and sleep in elderly individuals with transcranial direct current stimulation? ‐ Results from a randomized controlled pilot study. Clinical Interventions in Aging2017; Vol. 12:937‐47. [DOI] [PMC free article] [PubMed]
- Hazime FA, Baptista AF, Freitas DG, Monteiro RL, Maretto RL, Hasue RH. Treating low back pain with combined cerebral and peripheral electrical stimulation: a randomized, double‐blind, factorial clinical trial. European Journal of Pain2017; Vol. 21, issue 7:1132‐43. [DOI] [PubMed]
- Hirayama A, Saitoh Y, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain by navigation‐guided repetitive transcranial magnetic stimulation of the primary motor cortex. Pain 2006;122(1‐2):22‐7. [DOI] [PubMed] [Google Scholar]
- Hosomi K, Shimokawa T, Ikoma K, Nakamura Y, Sugiyama K, Ugawa Y, et al. Daily repetitive transcranial magnetic stimulation of primary motor cortex for neuropathic pain: a randomized, multicenter, double‐blind, crossover, sham‐controlled trial. Pain 2013;154(7):1065‐72. [DOI] [PubMed] [Google Scholar]
- Irlbacher K, Kuhnert J, Röricht S, Meyer BU, Brandt SA. Central and peripheral deafferent pain: therapy with repetitive transcranial magnetic stimulation [Zentrale und periphere deafferenzierungs schmerzen. Therapie mit der repetitiven transkraniellen magnetstimulation?]. Der Nervenarzt 2006;77(10):1196, 1198‐203. [DOI] [PubMed] [Google Scholar]
- Jales Junior LH, Costa MD, Jales Neto LH, Ribeiro JPM, Freitas WJ, Teixeira MJ. Transcranial direct current stimulation in fibromyalgia: effects on pain and quality of life evaluated clinically and by brain perfusion scintigraphy [Estimulação elétrica transcraniana por corrente contínua em fibromialgia: efeitos sobre a dor e a qualidade de vida, avaliados clinicamente e por cintilografia de perfusão cerebral]. Revista Dor Sao Paolo 2015;16(1):37‐42. [Google Scholar]
- Jensen MP, Sherlin LH, Askew RL, Fregni F, Witkop G, Gianas A, et al. Effects of non‐pharmacological pain treatments on brain states. Clinical Neurophysiology 2013;124(10):2016‐24. [DOI] [PMC free article] [PubMed] [Google Scholar]; Jensen MP, Sherlin LH, Fregni F, Gianas A, Howe JD, Hakimian S. Baseline brain activity predicts response to nneuromodulatory pain treatment. Pain Medicine 2014;15(12):2055‐63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jetté F, Côté I, Meziane HB, Mercier C. Effect of single‐session repetitive transcranial magnetic stimulation applied over the hand versus leg motor area on pain after spinal cord injury. Neurorehabilitation & Neural Repair 2013;27(7):636‐43. [DOI] [PubMed] [Google Scholar]
- Kang BS, Shin HI, Bang MS. Effect of repetitive transcranial magnetic stimulation over the hand motor cortical area on central pain after spinal cord injury. Archives of Physical Medicine & Rehabilitation 2009;90(10):1766‐71. [DOI] [PubMed] [Google Scholar]
- Katsnelson Y, Khokhlov A, Tsvetkov V, Bartoo G, Bartoo M. Temporary pain relief using transcranial electrotherapy stimulation: results of a randomized, double‐blind pilot study. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine & Biology Society 2004;6:4087‐90. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Kotb H, Kamel NF, Ahmed MA, Sadek R, Rothell JC. Longlasting antalgic effects of daily sessions of repetitive transcranial magnetic stimulation in central and peripheral neuropathic pain. Journal of Neurology Neurosurgery & Psychiatry 2005;76:833‐8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khedr EM, Omran EAH, Ismail NM, El‐Hammady DH, Goma SH, Kotb H. Effects of transcranial direct current stimulation on pain, mood and serum endorphin level in the treatment of fibromyalgia: a double blinded, randomized clinical trial. Brain Stimulation2017; Vol. 10, issue 5:893‐901. [DOI] [PubMed]
- Kim YJ, Ku J, Kim HJ, Im DJ, Lee HS, Han KA, et al. Randomized, sham controlled trial of transcranial direct current stimulation for painful diabetic polyneuropathy. Annals of Rehabilitation Medicine 2013;37(6):766‐76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lagueux E, Bernier M, Bourgault P, Whittingstall K, Mercier C, Leonard G. The effectiveness of transcranial direct current stimulation as an add‐on modality to graded motor imagery for treatment of complex regional pain syndrome: a randomized proof of concept atudy. Clinical Journal of Pain (in press). [DOI] [PubMed]
- Lee SJ, Kim DY, Chun MH, Kim YG. The effect of repetitive transcranial magnetic stimulation on fibromyalgia: a randomized sham‐controlled trial with 1‐mo follow‐up. American Journal of Physical Medicine & Rehabilitation 2012;91(12):1077‐85. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Nguyen JP. Interventional neurophysiology for pain control: duration of pain relief following repetitive transcranial magnetic stimulation of the motor cortex [Neurophysiologie interventionnelle dans le contrôle de la douleur: la durée du soulagement de la douleur après la stimulation magnétique transcranienne répétitive du cortex moteur]. Neurophysiologie Clinique 2001;31(4):247‐52. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Keravel Y, Nguyen JP. Pain relief induced by repetitive transcranial magnetic stimulation of precentral cortex. Neuroreport 2001;12(13):2963‐5. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Zerah F, Bendib B, Cesaro P, et al. Neurogenic pain relief by repetitive transcranial magnetic cortical stimulation depends on the origin and the site of pain. Journal of Neurology, Neurosurgery & Psychiatry 2004;75(4):612‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Ménard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS restores defective intracortical inhibition in chronic neuropathic pain. Neurology 2006;67(9):1568‐74. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, Drouot X, Menard‐Lefaucheur I, Keravel Y, Nguyen JP. Motor cortex rTMS in chronic neuropathic pain: pain relief is associated with thermal sensory perception improvement. Journal of Neurology, Neurosurgery & Psychiatry 2008;79(9):1044‐9. [DOI] [PubMed] [Google Scholar]
- Lichtbroun AS, Raicer M‐MC, Smith RB, Katz RS. The treatment of fibromyalgia with cranial electrotherapy stimulation. Journal of Clinical Rheumatology 2001;7(2):72‐8. [DOI] [PubMed] [Google Scholar]
- Luedtke K, Rushton A, Wright C, Jürgens T, Polzer A, Mueller G, et al. Effectiveness of transcranial direct current stimulation preceding cognitive behavioural management for chronic low back pain: sham controlled double blinded randomised controlled trial. BMJ (Clinical Research Ed.) 2015;350:h1640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malavera M, Silva F, Garcia R, Quiros J, Dallos M, Pinzon A. Effects of transcranial magnetic stimulation in the treatment of phantom limb pain in landmine victims: a randomized clinical trial. Journal of the Neurological Sciences 2013;333:e534. [Google Scholar]
- Medeiros LF, Caumo W, Dussán‐Sarria J, Deitos A, Brietzke A, Laste G, et al. Effect of deep intramuscular stimulation and transcranial magnetic stimulation on neurophysiological biomarkers in chronic myofascial pain syndrome. Pain Medicine 2016;17(1):122‐35. [DOI] [PubMed] [Google Scholar]
- Mendonca ME, Santana MB, Baptista AF, Datta A, Bikson M, Fregni F, et al. Transcranial DC stimulation in fibromyalgia: optimized cortical target supported by high‐resolution computational models. Journal of Pain 2011;12(5):610‐7. [DOI] [PubMed] [Google Scholar]
- Mendonca ME, Simis M, Grecco LC, Battistella LR, Baptista AF, Fregni F. Transcranial direct current stimulation combined with aerobic exercise to optimize analgesic responses in fibromyalgia: a randomized placebo‐controlled clinical trial. Frontiers in Human Neuroscience 2016;10:68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attal N, Mhalla A, Baudic S, Andrade DC, Perrot S, Texeira MJ, et al. Long term analgesic efficacy of transcranial magnetic stimulation of the motor cortex in patients with fibromyalgia. Clinical Neurophysiology 2010;121((Suppl 1)):S121. [Google Scholar]; Baudic S, Attal N, Mhalla A, Ciampi de Andrade D, Perrot S, Bouhassira D. Unilateral repetitive transcranial magnetic stimulation of the motor cortex does not affect cognition in patients with fibromyalgia. Journal of Psychiatric Research 2013;47(1):72‐7. [DOI] [PubMed] [Google Scholar]; Mhalla A, Baudic S, Ciampi de Andrade D, Gautron M, Perrot S, Teixeira MJ, et al. Long‐term maintenance of the analgesic effects of transcranial magnetic stimulation in fibromyalgia. Pain 2011;152(7):1478‐85. [DOI] [PubMed] [Google Scholar]
- Mori F, Codecà C, Kusayanagi H, Monteleone F, Buttari F, Fiore S, et al. Effects of anodal transcranial direct current stimulation on chronic neuropathic pain in patients with multiple sclerosis. Journal of Pain 2010;11(5):436‐42. [DOI] [PubMed] [Google Scholar]
- Nardone R, Höller Y, Langthaler PB, Lochner P, Golaszewski S, Schwenker K. rTMS of the prefrontal cortex has analgesic effects on neuropathic pain in subjects with spinal cord injury. Spinal Cord2017; Vol. 55, issue 1:20‐5. [DOI] [PubMed]
- Ngernyam N, Jensen MP, Arayawichanon P, Auvichayapat N, Tiamkao S, Janjarasjitt S, et al. The effects of transcranial direct current stimulation in patients with neuropathic pain from spinal cord injury. Clinical Neurophysiology 2015;126(2):382‐90. [DOI] [PubMed] [Google Scholar]
- Nurmikko T, MacIver K, Bresnahan R, Hird E, Nelson A, Sacco P. Motor vortex reorganization and repetitive transcranial magnetic stimulation for pain ‐ a methodological study. Neuromodulation2016; Vol. 19, issue 7:669‐78. [DOI] [PubMed]
- Oliveira LB, Lopes TS, Soares C, Maluf R, Goes BT, Sa KN, et al. Transcranial direct current stimulation and exercises for treatment of chronic temporomandibular disorders: a blind randomised‐controlled trial. Journal of Oral Rehabilitation 2015;42(10):723‐32. [DOI] [PubMed] [Google Scholar]
- Onesti E, Gabriele M, Cambieri C, Ceccanti M, Raccah R, Stefano G, et al. H‐coil repetitive transcranial magnetic stimulation for pain relief in patients with diabetic neuropathy. European Journal of Pain 2013;17(9):1347‐56. [DOI] [PubMed] [Google Scholar]; Onesti E, Tartaglia G, Gabriele M, Gilio F, Frasca V, Pichiorri F, et al. The effect of H‐coil repetitive transcranial magnetic stimulation on painful diabetic neuropathy: a randomized placebo‐controlled crossover study. Journal of the Peripheral Nervous System 2012;17:S41. [Google Scholar]; Tartaglia G, Gabriele M, Frasca V, Pichiorri F, Giacomelli E, Cambieri C, et al. Pain relief by deep repetitive transcranial magnetic stimulation applied with the H‐coil. Clinical Neurophysiology 2011;122:S144. [Google Scholar]
- Palm U, Chalah MA, Padberg F, Al‐Ani T, Abdellaoui M, Sorel M, et al. Effects of transcranial random noise stimulation (tRNS) on affect, pain and attention in multiple sclerosis. Restorative Neurology and Neuroscience 2016;34(2):189‐99. [DOI] [PubMed] [Google Scholar]
- Passard A, Attal N, Benadhira R, Brasseur L, Saba G, Sichere P, et al. Effects of unilateral repetitive transcranial magnetic stimulation of the motor cortex on chronic widespread pain in fibromyalgia. Brain 2007;130:2661‐70. [DOI] [PubMed] [Google Scholar]
- Picarelli H. [Os efeitos da estimulação magnética transcraniana repetitiva (EMTr) aplicada sobre o córtex motor de pacientes com síndrome complexa de dor regional]. The effects of repetitive transcranial magnetic stimulation (rTMS) over the motor cortex on complex regional pain syndrome patients [PhD thesis]. Sao Paolo: Universidade de São Paulo, 2009. [Google Scholar]; Picarelli H, Teixeira MJ, Andra DC, Myczkowski ML, Luvisotto TB, Yeng LT, et al. Repetitive transcranial magnetic stimulation is efficacious as an add‐on to pharmacological therapy in complex regional pain syndrome (CRPS) type I. Journal of Pain 2010;11(11):1203‐10. [DOI] [PubMed] [Google Scholar]
- Pleger B, Janssen F, Schwenkreis P, Volker B, Maier C, Tegenthoff M. Repetitive transcranial magnetic stimulation of the motor cortex attenuates pain perception in complex regional pain syndrome type I. Neuroscience Letters 2004;356(2):87‐90. [DOI] [PubMed] [Google Scholar]
- Portilla AS, Bravo GL, Miraval FK, Villamar MF, Schneider JC, Ryan CM, et al. A feasibility study assessing cortical plasticity in chronic neuropathic pain following burn injury. Journal of Burn Care & Research 2013;34(1):e48‐52. [DOI] [PubMed] [Google Scholar]
- Riberto M, Alfieri FM, Benedetto Pacheco, Leite VD, Kaihami HN, Fregni F, et al. Efficacy of transcranial direct current stimulation coupled with a multidisciplinary rehabilitation program for the treatment of fibromyalgia. Open Rheumatology Journal 2011;5(1):45‐50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rintala DH, Tan G, Willson P, Bryant MS, Lai ECH. Feasibility of using cranial electrotherapy stimulation for pain in persons with Parkinson's disease. Parkinson's Disease 2010;2010:569154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rollnik JD, Wustefeld S, Dauper J, Karst M, Fink M, Kossev A, et al. Repetitive transcranial magnetic stimulation for the treatment of chronic pain ‐ a pilot study. European Neurology 2002;48(1):6‐10. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Hirayama A, Kishima H, Shimokawa T, Oshino S, Hirata M, et al. Reduction of intractable deafferentation pain due to spinal cord or peripheral lesion by high‐frequency repetitive transcranial magnetic stimulation of the primary motor cortex. Journal of Neurosurgery 2007;107(3):555‐9. [DOI] [PubMed] [Google Scholar]
- Sakrajai P, Janyacharoen T, Jensen MP, Sawanyawisuth K, Auvichayapat N, Tunkamnerdthai O, et al. Pain reduction in myofascial pain syndrome by anodal transcranial direct current stimulation combined with standard treatment: a randomized controlled study. Clinical Journal of Pain 2014;30(12):1076‐83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Short EB, Borckardt J, Beam W, Anderson B, Nahas Z, George MS. Transcranial magnetic stimulation for fibromyalgia. Biological Psychiatry 2010;1:151S. [Google Scholar]; Short EB, Borckardt JJ, Anderson BS, Frohman H, Beam W, Reeves S, et al. 10 sessions of adjunctive left prefrontal rTMS significantly reduces fibromyalgia pain: a randomized controlled trial. Biological Psychiatry 2011;1:136S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler D, Kumru H, Vidal J, Fregni F, Tormos JM, Navarro X, et al. Transcranial direct current stimulation (TDCS) and virtual reality (VR) techniques for treatment neuropathic central pain in spinal cord injury (NP‐SCI). European Journal of Pain Supplements 2010;4(1):105‐6. [Google Scholar]; Soler D, Kumru H, Vidal J, Fregni F, Tormos JM, Navarro X, et al. Transcranial direct current stimulation (TDCS) and virtual reality (VR) techniques for treatment neuropathic central pain in spinal cord injury (NP‐SCI). European Journal of Pain Supplements 2010;41(1):105‐6. [Google Scholar]
- Souto G, Borges IC, Goes BT, Mendonça ME, Gonçalves RG, Garcia LB, et al. Effects of tDCS‐induced motor cortex modulation on pain in HTLV‐1: a blind randomized clinical trial. Clinical Journal of Pain 2014;30(9):809‐15. [DOI] [PubMed] [Google Scholar]
- Tan G, Monga T, Thornby J. Efficacy of microcurrent electrical stimulation on pain severity, psychological distress, and disability. American Journal of Pain Management 2000;10(1):35‐44. [Google Scholar]
- Tan G, Rintala DH, Thornby JI, Yang J, Wade W, Vasilev C. Using cranial electrotherapy stimulation to treat pain associated with spinal cord injury. Journal of Rehabilitation Research and Development 2006;43(4):461‐74. [DOI] [PubMed] [Google Scholar]
- Tan G, Rintala DH, Jensen MP, Richards JS, Holmes SA, Parachuri R, et al. Efficacy of cranial electrotherapy stimulation for neuropathic pain following spinal cord injury: a multi‐site randomized controlled trial with a secondary 6‐month open‐label phase. Journal of Spinal Cord Medicine 2011;34(3):285‐95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AG, Anderson JG, Riedel SL, Lewis E, Kinser PA, Bourguignon C. Cranial electrical stimulation improves symptoms and functional status in individuals with fibromyalgia. Pain Management Nursing 2013;14(4):327‐35. [DOI] [PubMed] [Google Scholar]; Taylor AG, Anderson JG, Riedel SL, Lewis JE, Bourguignon C. A randomized, controlled, double‐blind pilot study of the effects of cranial electrical stimulation on activity in brain pain processing regions in individuals with fibromyalgia. Explore: The Journal of Science and Healing 2013;9(1):32‐40. [DOI] [PubMed] [Google Scholar]
- Tekin A, Ozdil E, Guleken MD, Iliser R, Bakim B, Oncu J, et al. Efficacy of high frequency [10 Hz] repetitive transcranial magnetic stimulation of the primary motor cortex in patients with fibromyalgia syndrome: a randomized, double blind, sham‐controlled trial. Journal of Musculoskeletal Pain 2014;22(1):20‐6. [Google Scholar]
- Thibaut A, Carvalho S, Morse LR, Zafonte R, Fregni F. Delayed pain decrease following M1 tDCS in spinal cord injury: a randomized controlled clinical trial. Neuroscience Letters2017; Vol. 658:19‐26. [DOI] [PubMed]
- Schneider MB, Yang S, Aparici CM, Brocklin H, Seo Y, Etkin A, et al. Steerable electrical currents using multi‐coil RTMS: clinical effects of modifying current direction. Biological Psychiatry 2012;1:282S. [Google Scholar]; Tzabazis A, Aparici CM, Rowbotham MC, Schneider MB, Etkin A, Yeomans DC. Shaped magnetic field pulses by multi‐coil repetitive transcranial magnetic stimulation (rTMS) differentially modulate anterior cingulate cortex responses and pain in volunteers and fibromyalgia patients. Molecular Pain 2013;9:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umezaki Y, Badran BW, Devries WH, Moss J, Gonzales T, George MS. The efficacy of daily prefrontal repetitive transcranial magnetic stimulation (rTMS) for burning mouth syndrome (BMS): a randomized controlled single‐blind study. Brain Stimulation 2016;9(2):234‐42. [DOI] [PubMed] [Google Scholar]
- Valle A, Roizenblatt S, Botte S, Zaghi S, Riberto M, Tufik S, et al. Efficacy of anodal transcranial direct current stimulation (tDCS) for the treatment of fibromyalgia: results of a randomized, sham‐controlled longitudinal clinical trial. Journal of Pain Management 2009;2(3):353‐62. [PMC free article] [PubMed] [Google Scholar]
- Villamar MF, Wivatvongvana P, Patumanond J, Bikson M, Truong DQ, Datta A, et al. Focal modulation of the primary motor cortex in fibromyalgia using 4x1‐ring high‐definition transcranial direct current stimulation (HD‐tDCS): immediate and delayed analgesic effects of cathodal and anodal stimulation. Journal of Pain 2013;14(4):371‐83. [DOI] [PubMed] [Google Scholar]
- Volz MS, Farmer A, Siegmund B. Reduction of chronic abdominal pain in patients with inflammatory bowel disease through transcranial direct current stimulation: a randomized controlled trial. Pain 2016;157(2):429‐37. [DOI] [PubMed] [Google Scholar]
- Wrigley PJ, Gustin SM, McIndoe LN, Chakiath RJ, Henderson LA, Siddall PJ. Long standing neuropathic pain following spinal cord injury is refractory to transcranial direct current stimulation: a randomized controlled trial. Pain 2014;154(10):2178‐84. [DOI] [PubMed] [Google Scholar]
- Yagci I, Agirman M, Ozturk D, Eren B. Is the transcranial magnetic stimulation an adjunctive treatment in fibromyalgia patients?. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2014;60(3):206‐11. [Google Scholar]; Yaĝci I, Atirman M, Ozturk D, Eren B. Effect of low‐frequency transcranial magnetic stimulation of the motor cortex area in fibromyalgia patients [Fibromiyalji hastalarinda motor kortekse dusuk frekansli transkraniyal magnetik stimulasyonun etkinlitinin arastirilmasi]. Turkiye Fiziksel Tip ve Rehabilitasyon Dergisi 2013;59(232):[CRSREF: 2710974]. [Google Scholar]
- Yilmaz B, Kesikburun S, Yasar E, Tan AK. The effect of repetitive transcranial magnetic stimulation on refractory neuropathic pain in spinal cord injury. Journal of Spinal Cord Medicine 2014;37(4):397‐400. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to studies excluded from this review
- Avery DH, Holtzheimer PE, Fawaz W, Russo J, Neumaier J, Dunner DL, et al. Transcranial magnetic stimulation reduces pain in patients with major depression: a sham‐controlled study. Journal of Nervous and Mental Disease 2007;195(5):378‐81. [DOI] [PubMed] [Google Scholar]
- Belci M, Catley M, Husain M, Frankel HL, Davey NJ. Magnetic brain stimulation can improve clinical outcome in incomplete spinal cord injured patients. Spinal Cord 2004;42(7):417‐9. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Olgiati E, Maravita A, Ferraro F, Fregni F. Motor and parietal cortex stimulation for phantom limb pain and sensations. Pain 2013;154(8):1274‐80. [DOI] [PubMed] [Google Scholar]
- Bolognini N, Spandri V, Ferraro F, Salmaggi A, Molinari ACL, Fregni F, et al. Immediate and sustained effects of 5‐day transcranial direct current stimulation of the motor cortex in phantom limb pain. Journal of Pain 2015;16(7):657‐65. [DOI] [PubMed] [Google Scholar]
- Carraro ER de O, Frazao ACDD, Soares KVB de C, Silva VF. Cerebral stimulation by photo and auditory synthesis associated to imagery and muscle therapy: reducing pain in women with fibromyalgia [Estimulação cerebral por sintetização fótica e auditiva associada àimagética e massoterapia: minimização de dor em mulheres portadoras de fibromialgia]. Motriz, Rio Claro 2010;16(2):359‐69. [Google Scholar]
- Choi YH, Lee SU. The effect of transcranial direct current stimulation on myofascial trigger pain syndrome. Journal of Bodywork & Movement Therapies 2012;16(3):401. [Google Scholar]
- Choi YH. Synergistic effects of transcranial direct current stimulation and trigger point injection for treatment of myofascial pain syndrome. Journal of Rehabilitation Medicine 2012;Suppl 52:0521FP34. [DOI] [PubMed] [Google Scholar]
- Choi Yoon‐Hee, Jung Sung‐Jin, Lee Chang Han, Lee Shi‐Uk. Additional effects of transcranial direct‐current stimulation and trigger‐point injection for treatment of myofascial pain syndrome: a pilot study with randomized, single‐blinded trial. Journal of Alternative & Complementary Medicine 2014;20(9):698‐704. [DOI] [PubMed] [Google Scholar]; Lee SU, Lee CH, Choi YH. Synergistic effects of transcranial direct current stimulation and trigger point injection for treatment of myofascial pain syndrome: a pilot study with randomized, single‐blinded trial. Arthritis and Rheumatism. 2013; Vol. 65:S464. [DOI] [PubMed] [Google Scholar]
- Cummiford CM, Nascimento TD, Foerster BR, Clauw DJ, Zubieta JK, Harris RE, et al. Changes in resting state functional connectivity after repetitive transcranial direct current stimulation applied to motor cortex in fibromyalgia patients. Arthritis Research and Therapy 2016;18(1):40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evtiukhin AI, Dunaevskii IV, Shabut AM, Aleksandrov VA. The use of transcranial electrostimulation for pain relief in cancer patients. Voprosy Onkologii 1998;44:229‐33. [PubMed] [Google Scholar]
- Frentzel N, Kleditzsch J, Konrad B. A comparative assessment of pain alleviating therapy using middle frequency currents. Voprosy Kurortologii, Fizioterapii 1989;26(1):23‐7. [Google Scholar]
- Hargrove JB, Bennett RM, Clauw DJ. Long‐term outcomes in fibromyalgia patients treated with noninvasive cortical electrostimulation. Archives of Physical Medicine & Rehabilitation 2012;93(10):1868‐71. [DOI] [PubMed] [Google Scholar]
- Johnson S, Summers J, Pridmore S. Changes to somatosensory detection and pain thresholds following high frequency repetitive TMS of the motor cortex in individuals suffering from chronic pain. Pain 2006;123(1‐2):187‐92. [DOI] [PubMed] [Google Scholar]
- Katz J, Melzack R. Auricular transcutaneous electrical nerve stimulation (TENS) reduces phantom limb pain. Journal of Pain & Symptom Management 1991;6(2):73‐84. [DOI] [PubMed] [Google Scholar]
- Khedr EM, Kotb HI, Mostafa MG, Mohamad MF, Amr SA, Ahmed MA, et al. Repetitive transcranial magnetic stimulation in neuropathic pain secondary to malignancy: a randomized clinical trial. European Journal of Pain 2015;19(4):519‐27. [DOI] [PubMed] [Google Scholar]
- Jaeaeskelaeinen S, Lindholm P, Lamusuo S, Lahti A, Pesonen U, Taiminen T, et al. S2 cortex‐a promising novel target for the treatment of neuropathic pain with rTMS. Clinical Neurophysiology 2014;125:S229. [Google Scholar]; Lindholm P, Lamusuo S, Lahti A, Pesonen U, Taiminen T, Virtanen A, et al. Right S2 cortex: an effective target for the treatment of neuropathic pain with transcranial magnetic stimulation. Journal of Neurology 2013;260:S200‐1. [Google Scholar]; Lindholm P, Lamusuo S, Taiminen T, Pesonen U, Lahti A, Virtanen A, et al. Right secondary somatosensory cortex‐a promising novel target for the treatment of drug‐resistant neuropathic orofacial pain with repetitive transcranial magnetic stimulation. Pain 2015;156(7):1276‐83. [DOI] [PubMed] [Google Scholar]
- Longobardi AG, Clelland JA, Knowles CJ, Jackson JR. Effects of auricular transcutaneous electrical nerve stimulation on distal extremity pain: a pilot study. Physical Therapy 1989;69(1):10‐8. [DOI] [PubMed] [Google Scholar]
- Ma SM, Ni JX, Li XY, Yang LQ, Guo YN, Tang YZ. High‐frequency repetitive transcranial magnetic stimulation reduces pain in postherpetic neuralgia. Pain Medicine 2015;16(11):2162‐70. [DOI] [PubMed] [Google Scholar]
- Maestu C, Blanco M, Nevado A, Romero J, Rodriguez‐Rubio P, Galindo J, et al. Reduction of pain thresholds in fibromyalgia after very low‐intensity magnetic stimulation: a double‐blinded, randomized placebo‐controlled clinical trial. Pain Research & Management 2013;18(6):e101‐6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morin A, Léonard G, Gougeon V, Cyr MP, Waddell G, Bureau YA et al. Efficacy of transcranial direct‐current stimulation in women with provoked vestibulodynia. Americal Journal of Obstetrics and Gynecology2017; Vol. 216, issue 6:e1‐584.e11. [DOI] [PubMed]
- Nelson DV, Bennett RM, Barkhuizen A, Sexton GJ, Jones KD, Esty ML, et al. Neurotherapy of fibromyalgia?. Pain Medicine 2010;11(6):912‐9. [DOI] [PubMed] [Google Scholar]
- O'Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Souza LH, et al. Transcranial direct current stimulation of the motor cortex in the treatment of chronic nonspecific low back pain: a randomized, double‐blind exploratory study. Clinical Journal of Pain 2013;29(1):26‐34. [DOI] [PubMed] [Google Scholar]
- Pujol J, Pascual‐Leone A, Dolz C, Delgado E, Dolz JL, Aldomà J. The effect of repetitive magnetic stimulation on localized musculoskeletal pain. Neuroreport 1998;9(8):1745‐8. [DOI] [PubMed] [Google Scholar]
- Schabrun SM, Jones E, Elgueta Cancino EL, Hodges PW. Targeting chronic recurrent low back pain from the top‐down and the bottom‐up: a combined transcranial direct current stimulation and peripheral electrical stimulation intervention. Brain Stimulation 2014;7(3):451‐9. [DOI] [PubMed] [Google Scholar]
- Seada Yi Nofel R, Sayed HM. Comparison between trans‐cranial electromagnetic stimulation and low‐level laser on modulation of trigeminal neuralgia. Journal of Physical Therapy Science 2013;25(8):911‐4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sichinava NV, Gorbunov FE, Stel'nikov AV, Lukianova TV. The correction of cognitive and psychological disorders in the patients presenting with vertebrogenic pain syndrome. Vaprosy Kurtortologii, Fizioterapii, i Lechebnoi, Fizicheskoi Kultury 2012;4:307. [PubMed] [Google Scholar]
- Silva G, Miksad R, Freedman SD, Pascual‐Leone A, Jain S, Gomes DL, et al. Treatment of cancer pain with noninvasive brain stimulation. Journal of Pain and Symptom Management 2007;34(4):342‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smania N, Corato E, Fiaschi A, Pietropoli P, Aglioti SM, Tinazzi M. Repetetive magnetic stimulation: a novel therapeutic approach for myofascial pain syndrome. Journal of Neurology 2005;252:307‐14. [DOI] [PubMed] [Google Scholar]
- Yoon EJ, Kim YK, Kim H‐R, Kim SE, Lee Y, Shin HI. Transcranial direct current stimulation to lessen neuropathic pain after spinal cord injury: a mechanistic PET study. Neurorehabilitation & Neural Repair 2014;28(3):250‐9. [DOI] [PubMed] [Google Scholar]
- Zaghi S, DaSilva AF, Acar M, Lopes M, Fregni F. One‐year rTMS treatment for refractory trigeminal neuralgia. Journal of Pain & Symptom Management 2009;38(4):e1‐5. [DOI] [PubMed] [Google Scholar]
References to studies awaiting assessment
- Acler M, Valenti D, Tocco P, Monaco S, Bertolasi L. Effects of non‐invasive cortical stimulation on fatigue and quality of life in post‐polio patients: a double blind real sham study. European Journal of Neurology 2012;19(Suppl 1):580. [Google Scholar]
- Albu S. Effectiveness of transcranial direct current stimulation in the treatment of chronic neuropathic pain in spinal cord injured patients. European Journal of Neurology 2011;18(Suppl 2):199. [Google Scholar]
- Fricova J, Klirova M, So P, Tilerova B, Masopust V, Hackel M, et al. Repetitive transcranial stimulation in chronic neurogenic pain. Pain Practice 2009;9(Suppl 1):38. [Google Scholar]
- Fricova J. Repetitive transcranial stimulation in chronic orofacial neurogenic pain treatment. Fundamental and Clinical Pharmacology 2011;25(Suppl 1):31. [Google Scholar]
- Fricová J, Klírová M, Masopust V, Novák T, Vérebová K, Rokyta R. Repetitive transcranial magnetic stimulation in the treatment of chronic orofacial pain. Physiological Research 2013;62 Suppl 1:S125‐34. [DOI] [PubMed] [Google Scholar]
- Hwang B, Lee S, Paik N. Repetitive transcranial magnetic stimulation in complex regional pain syndrome of stroke patients. Neuromodulation 2015;18(2):e18. [Google Scholar]
- Klirova M, Fricova J, Sos P, Novak T, Tislerova B, Haeckel M, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of neuropathic pain. European Neuropsychopharmacology 2010;20(Suppl 3):S227‐8. [Google Scholar]
- Klirova M, Fricova J, Sos P, Novak T, Kohutova B, Masopust V, et al. Repetitive transcranial magnetic stimulation (rTMS) in the treatment of medication‐resistant neuropathic pain. European Psychiatry. 2011;26(Suppl 1):549. [Google Scholar]
- Knotkova H, Nafissi A, Sibirceva U, Feldman D, Dvorkin E, Sundaram A, et al. A randomized, sham‐controlled, two‐parallel‐arm study of transcranial direct current stimulation (tDCS for the treatment of neuropathic pain in complex regional pain syndrome‐type I (CRPS/RSD). Pain Medicine 2011;12(3):516. [Google Scholar]
- Mattoo B, Tanwar S, Jain S, Kumar U, Bhatia R. Transcranial magnetic stimulation of dorsolateral prefrontal cortex in chronic pain management. Brain Stimulation. Conference: 2nd International Brain Stimulation Conference. Spain 2017;10(2):434‐5. [Google Scholar]
- Moreno‐Duarte I, Doruk D, Bravo G, Miraval F, Moura L, Simis M, et al. Investigation of the mechanisms of transcranial direct current stimulation of motor cortex coupled with visual illusion for the treatment of chronic pain in spinal cord injury. Topics in Spinal Cord Injury Rehabilitation 2013;19(1):9‐10. 23678281 [Google Scholar]
- Mylius V, Ayache SS, Farhat WH, Zouari HG, Passeri E, Aoun‐Sebaiti M, et al. Robotized‐navigated low‐frequency repetitive transcranial magnetic stimulation over the right motor and prefrontal cortex improved pain and fatigue in patients with macrophagic myofasciitis. Clinical Neurophysiology 2013;124(10):e116. [Google Scholar]
- Parhizgar SE, Ekhtiari H. Modulation of primary motor cortex with transcranial direct current stimulation (tDCS) for reduction of opioid induced hyperalgesia: a double‐blinded, sham‐controlled study. European Journal of Medical Research 2011;16:59. [Google Scholar]
- Pellaprat J, Gerdelat Mas A, Simonetta Moreau M, Dellapina E, Thalamas C, Ory‐Magne F, et al. Effect of high‐frequency repetitive transcranial magnetic stimulation (rTMS) applied on the primary motor cortex, on pain threshold in patients with Parkinson's disease: a physiopathological study. Movement Disorders 2012;27(Suppl1):S210. [Google Scholar]
- Shklar B, Gabis L, Stain A. Transcranial electrostimulation as a treatment of head and back pain: immediate analgesic effect. Presented at the meeting of the International Pain Congress, Barcelona, Spain1997.
- Tanwar S, Mattoo B, Jain S, Kumar U, Dada R, Bhatia R. Transcranial magnetic stimulation in reducing chronic pain and the related symptoms in patients with fibromyalgia. Indian Journal of Physiology and Pharmacology. Conference: 62nd Annual Conference of Physiologists and Pharmacologists of India, APPI 2016. India 2016;60(5 Supplement 1):67‐8. [Google Scholar]
- Vatashsky E. Transcranial electrical stimulation (TCES) of the brain for neck pain (whiplash injury). Israel Journal of Medical Science 1997;33:5. [Google Scholar]
- Williams EN, Borckardt JJ, Reeves ST, George MS, Short EB. The effects of daily RTMs on c‐reactive protein in patients with fibromyalgia. Biological Psychiatry 2014;75(9 SUPPL. 1):387S. [Google Scholar]
References to ongoing studies
- ACTRN12612001155886. Investigating the role of transcranial direct current stimulation for pain relief in fibromyalgia and myalgic encephalomyelitis/chronic fatigue syndrome patients. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=362490 (first received 14 May 2012).
- ACTRN12613000561785. Repetitive transcranial magnetic stimulation in the treatment of fibromyalgia [The effectiveness of repetitive transcranial magnetic stimulation in the treatment of fibromyalgia]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=364236 (first received 13 May 2013).
- ACTRN12613001232729. Modulation of chronic pain perception with noninvasivecentral and peripheral nervous system stimulation [The effect of transcranial direct current stimulation and transcutaneous electrical nerve stimulation on improving pain intensity, physical functioning, mental health and quality of life in a chronic pain population awaiting pain clinic intervention]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=365199 (first received 7 November 2013).
- ACTRN12614001247662. The effects of non‐invasive brain stimulation on chronic arm pain [The effects of non‐invasive brain stimulation on pain and the nociceptive system in people with chronic neuropathic pain]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367396 (first received 12 November 2014).
- ACTRN12615000110583. The impact of non‐invasive brain stimulation on motor cortex excitability and cognition in chronic lower back pain [In individuals with chronic lower back pain, does anodal transcranial direct current stimulation, compared to sham transcranial direct current stimulation, impact on motor cortex excitability and cognition?]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=367643 (first received 27 January 2015).
- ACTRN12616000624482. Combined application of brain stimulation and sensorimotor retraining for low back pain [Safety and feasibility of transcranial direct current stimulation (tDCS) combined with sensorimotor retraining in chronic low back pain: a pilot randomised controlled trial]. anzctr.org.au/Trial/Registration/TrialReview.aspx?id=370567 (first received 9 May 2016).
- Ansari A, Mathur R, Jain S, Bhattacharjee M. Study of effect of slow frequency repeated transcranial magnetic field on modulation of pain in fibromyalgia patients. Journal of Pain 2013;1:S67. [Google Scholar]; Ansari AH, Mathur R, Jain S, Mukherjee K. Repeated transcranial magnetic stimulation relieves pain in fibromyalgia patients: an electrophysiological approach to evaluate pain. Acta Physiologica (Oxford, England) 2014;210:179. [Google Scholar]
- ChiCTR‐INR‐17011706. Transcranial magnetic stimulation induced motor evoked potential in the expression of brain‐derived neurotrophic factor BDNF, pathological pain and quality of life in patients with spinal cord injury [Transcranial magnetic stimulation induced motor evoked potential in the expression of brain‐derived neurotrophic factor BDNF, pathological pain and quality of life in patients with spinal cord injury]. chictr.org.cn/showprojen.aspx?proj=19983 (date of registration 20 June 2017).
- CTRI/2013/12/004228. Pain relieving strategies in fibromyalgia patients [Effect of Transcranial Magnetic Stimulation on Pain Modulation Status in Fibromyalgia Patients]. ctri.nic.in/Clinicaltrials/ (first received 19 December 2013).
- Muniswamy VK, Powell E, Sloan P, Sawaki L. Modulating neuropathic pain with transcranial direct current stimulation: preliminary findings from an ongoing study (10265). Neuromodulation 2016;19 (3):e4. [Google Scholar]
- NCT00815932. The Effect of Transcranial Direct Current Stimulation (t‐DCS) On the P300 Component of Event‐Related Potentials in Patients With Chronic Neuropathic Pain Due To CRPS or Diabetic Neuropathy. clinicaltrials.gov/ct2/show/NCT00815932 (first received 31 December 2008).
- NCT00947622. Occipital Transcranial Direct Current Stimulation in Fibromyalgia. clinicaltrials.gov/ct2/show/NCT00947622 (first received 28 July 2009).
- NCT01112774. Application of Transcranial Direct Current Stimulation (tDCS) in Patients With Chronic Pain After Spinal Cord Injury [Investigation of the Mechanisms of Transcranial Direct Current Stimulation of Motor Cortex for the Treatment of Chronic Pain in Spinal Cord Injury]. clinicaltrials.gov/ct2/show/record/NCT01112774 (first received 20 April 2010).
- NCT01220323. Transcranial Direct Current Stimulation for Chronic Pain Relief. clinicaltrials.gov/ct2/show/record/NCT01220323 (first received 6 October 2010).
- NCT01402960. Exploration of Parameters of tDCS in Chronic Pain Patients [Exploration of Parameters of Transcranial Direct Current Stimulation (tDCS) in Chronic Pain]. clinicaltrials.gov/ct2/show/record/NCT01402960 (first received 29 June 2011).
- NCT01404052. Effects of Transcranial Direct Current Stimulation (tDCS) and Transcranial Ultrasound on Osteoarthritis Pain of the Knee [Effects of Transcranial Direct Current Stimulation and Transcranial Ultrasound on the Perception of Pain Due to Osteoarthritis of the Knee]. clinicaltrials.gov/ct2/show/record/NCT01404052 (first received 29 June 2011).
- NCT01575002. Effects of Transcranial Direct Current Stimulation (tDCS) in Chronic Corneal Pain [Neural Correlates of Pain‐related Cognitive Processing in Chronic Pain of the Cornea: an ERP and Electrical Stimulation Study.]. clinicaltrials.gov/ct2/show/record/NCT01575002 (first received 8 February 2012).
- NCT01746355. Assessment and Treatment Patients With Atypical Facial Pain Trough Repetitive Transcranial Magnetic Stimulation. clinicaltrials.gov/ct2/show/record/NCT01746355 (first received 27 November 2012).
- NCT01747070. Effect of Cranial Stimulation and Acupuncture on Pain, Functional Capability and Cerebral Function in Osteoarthritis [Effect of Transcranial Direct Current Stimulation and Electro Acupuncture in Pain, Functional Capability and Cortical Excitability in Patients With Osteoarthritis]. clinicaltrials.gov/ct2/show/record/NCT01747070 (first received 15 October 2012).
- NCT01781065. The Effects of Transcranial Direct Current Stimulation on Central Pain in Patients With Spinal Cord Injury [The purpose of this study is to evaluate the analgesic effect of transcranial direct current stimulation (tDCS) applied on motor cortex in patients with spinal cord injury who have chronic neuropathic pain.]. clinicaltrials.gov/ct2/show/NCT01781065 29 January 2013.
- NCT01795079. Effects of Transcranial Direct Current Stimulation (tDCS) on Neuropathic Symptoms Following Burn Injury [Boston‐Harvard Burn Injury Model System: Cortical Modulation With Transcranial Direct Current Stimulation (tDCS) for Neuropathic Symptoms Following Burn Injury]. clinicaltrials.gov/ct2/show/NCT01795079 (first received February 15 2013).
- NCT01857492. tDCS for the Management of Chronic Visceral Pain in Patients With Chronic Pancreatitis. clinicaltrials.gov/ct2/show/NCT01857492 (first received 16 May 2013).
- NCT01875029. tDCS Effects on Chronic Low Back Pain [The Effects of Transcranial Direct Current Stimulation (tDCS)Combined With Back School in Subjects With Chronic Low Back Pain. Randomised Control Trial Study.]. clinicaltrials.gov/ct2/show/NCT01875029 (first received 21 May 2018).
- NCT01904097. Functional Neuroimaging in Fibromyalgia Patients Receiving tDCS [Study of the Brain With Optic Functional Neuroimaging in Patients With Chronic Pain Using Transcranial Direct Current Stimulation]. clinicaltrials.gov/ct2/show/NCT01904097 (first received 8 July 2013).
- NCT01932905. Deep rTMS in Central Neuropathic Pain Syndromes. clinicaltrials.gov/ct2/show/NCT01932905 (first received 27 August 2017).
- NCT01960400. Investigation of the Efficacy of tDCS in the Treatment of Complex Regional Pain Syndrome (CRPS) Type 1 [Investigation of the Efficacy of Transcranial Direct Current Stimulation (tDCS) Added to the Graded Motor Imagery (GMI) in the Treatment of Complex Regional Pain Syndrome (CRPS) Type 1]. clinicaltrials.gov/ct2/show/NCT01960400 (first received 24 September 2013).
- NCT02051959. Long‐term Effects of Transcranial Direct Current Stimulation (tDCS) on Patients With Phantom Limb Pain (PLP) [Long‐Term Treatment of Patients Experiencing Phantom Limb Pain With Transcranial Direct Current Stimulation (tDCS)]. clinicaltrials.gov/ct2/show/NCT02051959 (first received 28 January 2014).
- NCT02059096. Analgesic Effect of Repetitive Transcranial Magnetic Stimulation (rTMS) for Central Neuropathic Pain in Multiple Sclerosis. clinicaltrials.gov/ct2/show/NCT02059096 (first received 6 February 2014).
- NCT02070016. Transcranial Magnetic Stimulation for Low Back Pain [Clinical Applicaitons of Non‐Invasive Brain Stimulation for the Treatment of Chronic Pain]. clinicaltrials.gov/ct2/show/NCT02070016 (first received 4 February 2014).
- NCT02161302. The effect of tDCS in the treatment of chronic pelvic pain associated with endometriosis. clinicaltrials.gov/ct2/show/NCT02161302 (first received 10 June 2014).
- NCT02277912. Efficacy of Transcranial Magnetic Stimulation (TMS) in Central Post Stroke Pain ( CPSP) [Efficacy of Navigated Repetitive Transcranial Magnetic Stimulation in Treatment of Central Post Stroke Pain]. clinicaltrials.gov/ct2/show/NCT02277912 (first received 22 October 2014).
- NCT02330315. Effects of tDCS and tUS on Pain Perception in OA of the Knee [Effects of Transcranial Direct Current Stimulation (tDCS) and Transcranial Ultrasound (TUS) on the Perception of Pain and Functional Limitations Due to Osteoarthritis of the Knee]. clinicaltrials.gov/ct2/show/NCT02330315 (first received 12 December 2014).
- NCT02386969. Repetitive Transcranial Magnetic Stimulation in Central Neuropathic Pain [Long‐term Efficacy of Repetitive Transcranial Magnetic Stimulation on the Primary Motor Cortex (M1) in Central Neuropathic Pain]. clinicaltrials.gov/ct2/show/NCT02386969 (first received 5 March 2015).
- NCT02393391. A Novel Non Invasive Brain Stimulation Based Treatment for Chronic Low Back Pain (CLBP). clinicaltrials.gov/ct2/show/NCT02393391 (first received 8 March 2015).
- NCT02483468. The Effects of Cognitive Behavioral Therapy and Transcranial Current Stimulation (tDCS) on Chronic Lower Back Pain. clinicaltrials.gov/ct2/show/NCT02483468 (first received 12 June 2015).
- NCT02487966. Optimizing Rehabilitation for Phantom Limb Pain Using Mirror Therapy and Transcranial Direct Current Stimulation (tDCS). clinicaltrials.gov/ct2/show/NCT02487966 (first received 22 June 2015).
- NCT02615418. Non Invasive Brain Stimulation Treatment for CLBP [A Novel Non Invasive Brain Stimulation,tDCS Based Treatment for Chronic Low Back Pain (CLBP)]. clinicaltrials.gov/ct2/show/NCT02615418 (first received 23 November 2015).
- NCT02652988. Home‐based Transcranial Direct Current Stimulation in Fibromyalgia Patients [Home‐based Transcranial Direct Current Stimulation in Fibromyalgia Patients. Phase II, Randomized, Double‐blind, Single‐center Clinical Trial]. clinicaltrials.gov/ct2/show/record/NCT02652988 (first received 28 October 2015).
- NCT02665988. Adjunctive Transcranial Direct Current Stimulation [A Single‐Blind, Randomized Control Trial of Adjunctive Transcranial Direct Current Stimulation (tDCS) for Chronic Pain Among Patients Receiving Specialized, Inpatient Multi‐Modal Pain Management]. clinicaltrials.gov/ct2/show/NCT02665988 (first received 22 January 2016). [DOI] [PubMed]
- NCT02687360. Imaging the Effects of rTMS on Chronic Pain. clinicaltrials.gov/ct2/show/NCT02687360 (first received 23 December 2015).
- NCT02723175. The Effects of CBT and (tDCS) on Fibromyalgia Patients [The Effects of Cognitive Behavioral Therapy and Transcranial Direct Current Stimulation (tDCS) on Fibromyalgia Patients]. clinicaltrials.gov/ct2/show/NCT02723175 (first received 14 December 2015).
- NCT02723929. Effects of tDCS and tUS on Pain Perception in OA of the Knee [Effects of Transcranial Direct Current Stimulation (tDCS) and Transcranial Ultrasound (TUS) on the Perception of Pain and Functional Limitations Due to Osteoarthritis of the Knee]. clinicaltrials.gov/ct2/show/NCT02723929 (received 26 February 2016).
- NCT02768129. Transcranial Direct Current Stimulation for Chronic Low Back Pain [Transcranial Direct Current Stimulation for Chronic Low Back Pain]. clinicaltrials.gov/ct2/show/NCT02768129 (first received 9 May 2016).
- NCT02771990. tDCS for Chronic Low Back Pain [tDCS for Chronic Low Back Pain: A Study Examining the Effect of Transcranial Direct Current Stimulation on the Emotional Response to Chronic Low Back Pain]. clinicaltrials.gov/ct2/show/NCT02771990 (first received 12 May 2016).
- NCT02813629. tDCS Associated With Peripheral Electrical Stimulation for Pain Control in Individuals With Sickle Cell Disease [Transcranial Direct Current Stimulation Associated With Peripheral Electrical Stimulation for Pain Control in Individuals With Sickle Cell Disease]. clinicaltrials.gov/ct2/show/NCT02813629 (first received 10 May 2016).
- NCT03015558. Analgesic Effect of Non Invasive Stimulation : Transcranial Direct Current Stimulation of Opercular‐insular Cortex [Transcranial Direct Current Stimulation (tDCS) for Neuropathic Chronic Pain : Study of the Opercular‐insular Cortex Stimulation]. clinicaltrials.gov/ct2/show/NCT03015558 (first received 22 December 2016).
- NCT03137472. TMS for Complex Regional Pain Syndrome [Transcranial Magnetic Stimulation (TMS) for Complex Regional Pain Syndrome (CRPS)]. clinicaltrials.gov/ct2/show/NCT03137472 (first received 28 April 2018).
- RBR‐9dxp3k. Effectiveness of transcranial direct current stimulation combined with kinesiotherapy in patients with chronic temporomandibular disorders (TMJ): clinical, randomized, double‐blind, placebo controlled [Eficácia da estimulação transcraniana por corrente contínua combinada à cinesioterapia em pacientes portadores de disfunção temporomandibular (DTM) crônica: ensaio clínico, aleatorizado, duplo‐cego, placebo controlado]. ensaiosclinicos.gov.br/rg/RBR‐9dxp3k/ (date registered 28 March 2014).
Additional references
- Ahdab R, Ayache SS, Brugières P, Goujon C, Lefaucheur JP. Comparison of "standard" and "navigated" procedures of TMS coil positioning over motor, premotor and prefrontal targets in patients with chronic pain and depression. Neurophysiologie Clinique 2010;40(1):27‐36. [DOI] [PubMed] [Google Scholar]
- Ambrus GG, Al‐Moyed H, Chaieb L, Sarp L, Antal A, Paulus W. The fade‐in‐short stimulation‐fade out approach to sham tDCS‐ reliable at 1 mA for naïve and experienced subjects, but not investigators. Brain Stimulation 2012;5(4):499‐504. [DOI] [PubMed] [Google Scholar]
- Borckardt JJ, Walker J, Branham RK, Rydin‐Gray S, Hunter C, Beeson H, et al. Development and evaluation of a portable sham TMS system. Brain Stimulation 2008;1:52‐9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breivik H, Collett B, Ventafridda V, Cohen R, Gallacher D. Survey of chronic pain in Europe: prevalence, impact on daily life and treatment. European Journal of Pain 2006;10:287‐333. [DOI] [PubMed] [Google Scholar]
- Bronfort G, Nilsson N, Haas M, Evans RL, Goldsmith CH, Assendelft WJJ, et al. Non‐invasive physical treatments for chronic/recurrent headache. Cochrane Database of Systematic Reviews 2004, Issue 3. [DOI: 10.1002/14651858.CD001878.pub2] [DOI] [PubMed] [Google Scholar]
- Busse JW, Bartlett SJ, Dougados M, Johnston BC, Guyatt GH, Kirwan JR, et al. Optimal strategies for reporting pain in clinical trials and systematic reviews: recommendations from an OMERACT 12 workshop. Journal of Rheumatology 2015;42(10):1962‐70. [DOI] [PubMed] [Google Scholar]
- Canavero S, Bonicalzi V, Dotta M, Vighetti S, Asteggiano G, Cocito D. Transcranial magnetic cortical stimulation relieves central pain. Stereotactic Functional Neurosurgery 2002;78:192‐6. [DOI] [PubMed] [Google Scholar]
- Cruccu G, Garcia‐Larrea L, Hansson P, Keindl M, Lefaucheur JP, Paulus W, et al. EAN guidelines on central neurostimulation therapy in chronic pain conditions. European Journal of Neurology 2017;23(10):1489‐99. [DOI] [PubMed] [Google Scholar]
- Dechartres A, Trinquart L, Boutron I, Ravaud P. Influence of trial sample size on treatment effect estimates: meta‐epidemiological study. BMJ 2013;346:f2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Wyrwich KW, Beaton D, Cleeland CS, Farrar JT, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. Journal of Pain 2008;9(2):105‐21. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, McDermott MP, Peirce‐Sandner S, Burke LB, Cowan P, et al. Interpreting the clinical importance of group differences in chronic pain clinical trials: IMMPACT recommendations. Pain 2009;146(3):238‐44. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Turk DC, Peirce‐Sandner S, Baron R, Bellamy N, Burke LB, et al. Research design considerations for confirmatory chronic pain clinical trials: IMMPACT recommendations. Pain 2010;149(2):177‐93. [DOI] [PubMed] [Google Scholar]
- Ezquerro F, Moffa AH, Bikson M, Khadka N, Aparicio LV, Sampaio‐Junior B, et al. The influence of skin redness on blinding in transcranial direct current stimulation studies: a crossover trial. Neuromodulation 2016;20(3):248‐55. [DOI] [PubMed] [Google Scholar]
- Fregni F, Freedman S, Pascual‐Leone A. Recent advances in the treatment of chronic pain with non‐invasive brain stimulation techniques. Lancet Neurology 2007;6:188‐91. [DOI] [PubMed] [Google Scholar]
- Galhardoni R, Correia GS, Araujo H, Yeng LT, Fernandes DT, Kaziyama HH, et al. Repetitive transcranial magnetic stimulation in chronic pain: a review of the literature. Archives of Physical Medicine and Rehabilitation 2015;96(4 Suppl):S156‐72. [DOI] [PubMed] [Google Scholar]
- Gandiga PC, Hummel FC, Cohen LG. Transcranial DC stimulation (tDCS): a tool for double‐blind sham‐controlled clinical studies in brain stimulation. Clinical Neurophysiology 2006;117(4):845‐50. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Bonnefoi F, et al. Positron emission tomography during motor cortex stimulation for pain control. Stereotactic and Functional Neurosurgery 1997;68(1‐4):141‐8. [DOI] [PubMed] [Google Scholar]
- Garcia‐Larrea L, Peyron R, Mertens P, Gregoire MC, Lavenne F, Bars D, et al. Electrical stimulation of motor cortex for pain control: a combined PET‐scan and electrophysiological study. Pain 1999;83(2):259‐73. [DOI] [PubMed] [Google Scholar]
- Gilula MF. Cranial electrotherapy stimulation and fibromyalgia. Experimental Review of Medical Devices 2007;4(4):489‐95. [DOI] [PubMed] [Google Scholar]
- Guyatt GH, Oxman AD, Kunz R, Brozek J, Alonso‐Coello P, Rind D, et al. GRADE guidelines 6. Rating the quality of evidence‐‐imprecision. Journal of Clinical Epidemiology 2011;64(12):1283‐93. [DOI] [PubMed] [Google Scholar]
- Herwig U, Padberg F, Unger J, Spitzer M, Schönfeldt‐Lecuona C. Transcranial magnetic stimulation in therapy studies: examination of the reliability of "standard" coil positioning by neuronavigation. Biological Psychiatry 2001;50(1):58‐61. [DOI] [PubMed] [Google Scholar]
- Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta‐analyses. BMJ 2003;327:557‐60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higgins JPT, Altman DG, Sterne JAC (editors). Chapter 8: Assessing risk of bias in included studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Higgins JPT, Deeks JJ, Altman DG (editors). Chapter 16: Special topics in statistics. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Horvath JC, Carter O, Forte JD. Transcranial direct current stimulation: five important issues we aren't discussing (but probably should be). Frontiers of Systems Neuroscience 2014;8:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kessler SK, Turkeltaub PE, Benson JG, Hamilton RH. Differences in the experience of active and sham transcranial direct current stimulation. Brain Stimulation 2012;5(2):155‐62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch DL, Smith RB. The use of cranial electrotherapy stimulation in the management of chronic pain: a review. NeuroRehabilitation 2000;14:85‐94. [PubMed] [Google Scholar]
- Klein MM, Treister R, Raij T, Pascual‐Leone A, Park L, Nurmikko T. Transcranial magnetic stimulation of the brain: guidelines for pain treatment research. Pain 2015;156(9):1601‐14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefaucheur JP. Principles of therapeutic use of transcranial and epidural cortical stimulation. Clinical Neurophysiology 2008;119(10):2179‐84. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP. Use of repetitive transcranial magnetic stimulation in pain relief. Expert Reviews in Neurotherapeutics 2008;8(5):799‐808. [DOI] [PubMed] [Google Scholar]
- Lefaucheur JP, André‐Obadia N, Antal A, Ayache SS, Baeken C, Benninger DH, et al. Evidence‐based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clinical Neurophysiology 2014;125(11):2150‐206. [DOI] [PubMed] [Google Scholar]
- Lefebvre C, Manheimer E, Glanville J. Chapter 6: Searching for studies. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Leo RJ, Latif T. Repetitive transcranial magnetic stimulation (rTMS) in experimentally induced and chronic neuropathic pain: a review. Journal of Pain 2007;8(6):453‐9. [DOI] [PubMed] [Google Scholar]
- Leung A, Donohue M, Xu R, Lee R, Lefaucheur JP, Khedr EM, et al. rTMS for suppressing neuropathic pain: a meta‐analysis. Journal of Pain 2009;10(12):1205‐16. [DOI] [PubMed] [Google Scholar]
- Lima MC, Fregni F. Motor cortex stimulation for chronic pain: systematic review and meta‐analysis of the literature. Neurology 2008;70:2329‐37. [DOI] [PubMed] [Google Scholar]
- Lisanby SH, Gutman D, Luber B, Schroeder C, Sackeim HA. Sham TMS: intracerebral measurement of the induced electrical field and the induction of motor‐evoked potentials. Biological Psychiatry 2001;49(5):460‐3. [DOI] [PubMed] [Google Scholar]
- Loo CK, Taylor JL, Gandevia SC, McDarmont BN, Mitchell PB, Sachdev PS. Transcranial magnetic stimulation (TMS) in controlled treatment studies: are some "sham" forms active?. Biological Psychiatry 2000;47(4):325‐31. [DOI] [PubMed] [Google Scholar]
- Luedtke K, Rushton A, Wright C, Geiss B, Juergens TP, May A. Transcranial direct current stimulation for the reduction of clinical and experimentally induced pain: a systematic review and meta‐analysis. Clinical Journal of Pain 2012;28(5):452‐61. [DOI] [PubMed] [Google Scholar]
- Marlow NM, Bonilha HS, Short EB. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Practice 2013;2:131‐45. [DOI] [PubMed] [Google Scholar]
- Moore RA, Eccleston C, Derry S, Wiffen P, Bell RF, Straube S, et al. ACTINPAIN Writing Group of the IASP Special Interest Group on Systematic Reviews in Pain Relief, Cochrane Pain, Palliative and Supportive Care Systematic Review Group Editors. "Evidence" in chronic pain‐‐establishing best practice in the reporting of systematic reviews. Pain 2010;150:386‐9. [DOI] [PubMed] [Google Scholar]
- Moore RA. What works for whom? Determining the efficacy and harm of treatments for pain. Pain 2013;154(Suppl 1):S77‐86. [DOI] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for neuropathic pain in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [CENTRAL: CD008242; DOI: 10.1002/14651858.CD008242.pub3] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore RA, Derry S, Aldington D, Cole P, Wiffen PJ. Amitriptyline for fibromyalgia in adults. Cochrane Database of Systematic Reviews 2015, Issue 7. [CENTRAL: CD011824; DOI: 10.1002/14651858.CD011824] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno‐Duarte I, Morse L, Alam M, Bikson M, Zafonte R, Fregni F. Targeted therapies using electrical and magnetic neural stimulation for the treatment of chronic pain in spinal cord injury. Neuroimage 2013;85(3):1003‐13. [DOI] [PubMed] [Google Scholar]
- Nitsche M, Cohen l, Wasserman E, Priori A, Lang N, Antal A, et al. Transcranial direct current stimulation: state of the art 2008. Brain Stimulation 2008;1:206‐23. [DOI] [PubMed] [Google Scholar]
- Nüesch E, Trelle S, Reichenbach S, Rutjes AWS, Tschannen B, Altman DG, et al. Small study effects in meta‐analyses of osteoarthritis trials: meta‐epidemiological study. BMJ 2010;341(7766):241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell NE, Wand BM. Repetitive transcranial magnetic stimulation for chronic pain: time to evolve from exploration to confirmation?. Pain 2011;152(11):2451‐2. [DOI] [PubMed] [Google Scholar]
- O'Connell NE, Cossar J, Marston L, Wand BM, Bunce D, Moseley GL, et al. Rethinking clinical trials of transcranial direct current stimulation: participant and assessor blinding is inadequate at intensities of 2mA. PLoS One 2012;10:e47514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell NE, Kamper SJ, Stevens ML, Li Q. Twin peaks? No evidence of bimodal distribution of outcomes in clinical trials of non‐surgical interventions for spinal pain: an exploratory analysis. Journal of Pain 2017;March:Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- Ossipov MH, Porecca F. Chronic pain: multiple manifestations, multiple mechanisms. Drug Discovery Today: Disease Mechanisms 2006;3(3):301‐3. [Google Scholar]
- Pascual‐Leone A, Tarazona F, Keenan J, Tormos JM, Hamilton R, Catala MD. Transcranial magnetic stimulation and neuroplasticity. Neuropsychologia 1999;37:207‐17. [DOI] [PubMed] [Google Scholar]
- Paulus W. Transcranial electrical stimulation (tES ‐ tDCS; tRNS, tACS) methods. Neuropsychological Rehabilitation 2011;21:602‐17. [DOI] [PubMed] [Google Scholar]
- Peyron R, Faillenot I, Mertens P, Laurent B, Garcia‐Larrea L. Motor cortex stimulation in neuropathic pain. Correlations between analgesic effect and hemodynamic changes in the brain. A PET study. NeuroImage 2007;34(1):310‐21. [DOI] [PubMed] [Google Scholar]
- Nordic Cochrane Centre, The Cochrane Collaboration. Review Manager 5 (RevMan 5). Version 5.3. Copenhagen: Nordic Cochrane Centre, The Cochrane Collaboration, 2014.
- Rossi S, Ferro M, Cincotta M, Ulivelli M, Bartalini S, Miniussi C, et al. A real electro‐magnetic placebo (REMP) device for sham transcranial magnetic stimulation (TMS). Clinical Neurophysiology 2007;118:709‐16. [DOI] [PubMed] [Google Scholar]
- Savović J, Jones H, Altman D, Harris R, Jűni P, Pildal J, et al. Influence of reported study design characteristics on intervention effect estimates from randomised controlled trials: combined analysis of meta‐epidemiological studies. Health Technology Assessments 2012;16(35):1‐82. [DOI] [PubMed] [Google Scholar]
- Schulz KF, Altman DG, Moher D, for the CONSORT Group. CONSORT 2010 Statement: updated guidelines for reporting parallel group randomised trials. BMJ 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schünemann HJ, Oxman AD, Vist GE, Higgins JPT, Deeks JJ, Glasziou P, et al. Chapter 12: Interpreting results and drawing conclusions. In: Higgins JPT, Green S (editors), Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Smith BH, Torrance N. Epidemiology of chronic pain. In: McQuay HJ, Kalso E, Moore RA editor(s). Systematic Reviews in Pain Research: Methodology Refined. Seattle: IASP Press, 2008:233‐46. [Google Scholar]
- Sommer J, Jansen A, Dräger B, Steinsträter O, Breitenstein C, Deppe M, et al. Transcranial magnetic stimulation ‐ a sandwich coil design for a better sham. Clinical Neurophysiology 2006;117:440‐6. [DOI] [PubMed] [Google Scholar]
- Sterne JAC, Egger M, Moher D (editors). Chapter 10: Addressing reporting biases. In: Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions. Version 5.1.0 (updated March 2011). The Cochrane Collaboration, 2011. Available from handbook.cochrane.org.
- Stevens A, Fischer A, Bartels A, Buchkremer G. Electroconvulsive therapy: a review on indications, methods, risks and medication. European Psychiatry 1996;11:165‐74. [DOI] [PubMed] [Google Scholar]
- Turk DC, Dworkin RH, Revicki D, Harding G, Burke LB, Cella D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008;137(2):276‐85. [DOI] [PubMed] [Google Scholar]
- Hecke O, Torrance N, Smith BH. Chronic pain epidemiology and its clinical relevance. British Journal of Anaesthesia 2013;111(1):13‐18. [DOI] [PubMed] [Google Scholar]
- Wallace D, Cooper NR, Paulmann S, Fitzgerald PB, Russo R. Perceived comfort and blinding efficacy in randomised sham‐controlled transcranial direct current stimulation (tDCS) trials at 2 mA in young and older healthy adults. PLoS One 2016;11(2):e0149703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood L, Egger M, Gluud LL, Schulz KF, Juni P, Altman DG, et al. Empirical evidence of bias in treatment effect estimates with different interventions and outcomes: meta‐epidemiological study. BMJ 2008;336:601‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
References to other published versions of this review
- O'Connell NE, Wand BM, Marston L, Spencer S, DeSouza LH. Non‐invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews 2010, Issue 9. [DOI: 10.1002/14651858.CD008208.pub2] [DOI] [PubMed] [Google Scholar]
- O’Connell NE, Wand BM, Marston L, Spencer S, DeSouza LH. Non‐invasive brain stimulation techniques for chronic pain. Cochrane Database of Systematic Reviews 2014, Issue 4. [DOI: 10.1002/14651858.CD008208.pub3] [DOI] [PubMed] [Google Scholar]


